Highlights
-
•
This ESMO Clinical Practice Guideline provides key recommendations for managing cancer-related cachexia.
-
•
It covers screening, assessment and multimodal management of cancer cachexia.
-
•
All recommendations were compiled by a multidisciplinary group of experts.
-
•
Recommendations are based on available scientific data and the author's expert opinion.
Key words: cachexia, cancer, Clinical Practice Guidelines, assessment, treatment, nutrition
Introduction
Cachexia remains an underdiagnosed and undertreated, complex condition which includes ‘objective’ components (e.g. inadequate food intake, weight loss, inactivity, loss of muscle mass and metabolic derangements, inducing catabolism)1,2 and ‘subjective’ components (e.g. anorexia, early satiety, taste alterations, chronic nausea, distress, fatigue and loss of concentration). Approximately half of all patients with advanced cancer experience cachexia.
Comprehensive treatment requires a multitargeted and multidisciplinary approach aimed at evaluating the objective signs and relieving the symptoms. The primary goal is to meet the physiological and psychological needs of the patient. This includes providing energy, nutritional substrates and anabolic stimuli, as well as compassionate support to address dysfunctions associated with the emotional and social aspects of eating. Nutritional and metabolic interventions range from dietary counselling to pharmacological agents and parenteral nutrition (PN). The invasiveness of an intervention needs to be chosen and tailored, weighing the benefits and risks for each individual patient. This is of increasing importance with advancing disease and when approaching end of life. In this sense, nutrition is an essential component of supportive, rehabilitative and palliative care. During the patient's trajectory towards end of life, however, the focus of nutritional care needs to change. During anticancer treatment, patients should be offered all available nutritional therapeutic options, if required, whereas during the last weeks of life, care should focus increasingly on immediate symptomatic relief (Figure 1).
Figure 1.
Invasiveness of interventions relative to expected survival.
ONS, oral nutritional supplement.
In general, if anticancer treatment is effective, this often results in an improvement in cachectic signs and symptoms,3 while ineffective anticancer treatment may increase catabolism and aggravate cachexia.4
This European Society for Medical Oncology (ESMO) Clinical Practice Guideline (CPG) on cancer cachexia has been designed for medical oncologists who frequently care for patients with cancer cachexia in their clinical practice. The goal is to provide answers to questions regarding the diagnosis and treatment of cachexia-related physical and psychological problems, relying on evidence-based information whenever possible. A similar approach has recently been published by the American Society of Clinical Oncology (ASCO).5
This CPG provides recommendations on overt cachexia as well as at-risk settings. Evidence to support these recommendations has been derived from trials studying the evolution of the signs and symptoms of cachexia. Whereas today we define cachexia on a pathophysiological basis to be malnutrition in the presence of disease-related metabolic alterations,6, 7, 8 historically, clinical trials used varying and inconsistent combinations of inclusion criteria. A summary of inclusion criteria for all clinical trials, guidelines and systematic review articles reported in this CPG is therefore provided for reference in Supplementary Tables S1, S2 and Supplementary Figures S1A–S1C, available at https://doi.org/10.1016/j.esmoop.2021.100092.
Recommendations
-
•
Regular nutritional screening and nutritional support, including (if necessary) enteral nutrition or PN, is recommended in all patients receiving anticancer treatment and in those with an expected survival of more than a few months [V, B].
-
•
In patients with an expected survival of less than a few months, a decrease in the invasiveness of nutritional interventions is recommended, with dietary counselling and oral supplements preferred, if possible [V, B].
-
•
In patients with an expected survival of less than a few weeks, comfort-directed care is the recommended approach, including alleviating thirst, eating-related distress and other debilitating symptoms [V, B].
Definition and impact of cachexia
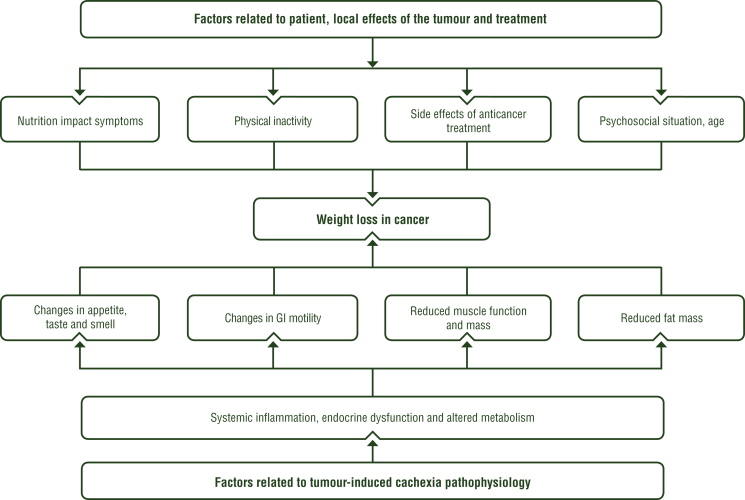
Weight loss with depletion of fat stores and muscle mass frequently develop in patients with advanced cancer and may be the first signs leading to the diagnosis of a malignancy. A number of pathophysiological derangements may result in weight loss and several factors often occur at the same time, including impaired food intake, reduction in physical activity and its associated anabolic effects as well as metabolic changes leading to systemic inflammation and activation of catabolism (see Figure 2). Patients with depleted resources are at an increased risk of anticancer treatment-related toxicity and a lower quality of life (QoL); toxicity results in shorter treatment times, lower dose intensity, lower response rates, increased surgical complications and higher mortality.7
Figure 2.
The complexity of causes contributing to weight loss in patients with cancer.
GI, gastrointestinal.
Although used since the 19th century, a disconcerting number of definitions have been proposed for the term ‘malnutrition’. To avoid confusion, we recommend following the recent suggestion of the Global Leadership Initiative in Malnutrition (GLIM)8 that defines malnutrition by the presence of a positive malnutrition screening test, one of a list of phenotypical and one of two aetiological criteria (see Table 1). Aetiological criteria are used to differentiate starvation-type (with protein-sparing metabolism9) from cachexia-type or disease-associated malnutrition, characterised by accelerated protein breakdown and the hallmark of muscle loss driven by metabolic changes, most notably systemic inflammation.6,10,11
Table 1.
Definitions of major terms
| Term | Definition and criteria | |
|---|---|---|
| Malnutrition | Defined by three criteria: a positive malnutrition screening test combined with one phenotypical and one aetiological criterion:8 | |
| Mandatory screening | Malnutrition risk predicted by a validated screening test, e.g. NRS-2002, MUST, SNAQ, MST or other | |
| Phenotypical criteria | Loss of or low body mass as defined by at least one of the following: A1: weight loss >5% in 6 months A2: body mass index below 20 kg/m2 A3: low muscle mass |
|
| Aetiological criteria | Reduced food availability (B1) and/or increased catabolism (B2) B1 (starvation type): reduction in food availability B1a: food intake <50% for >1 week B1b: any reduction in food intake for >2 weeks B1c: chronic malabsorption B2 (cachexia type): increased acute or chronic systemic inflammation |
|
| Cachexia | A disease-related subtype of malnutrition identified by malnutrition screening, at least one phenotypical criterion and systemic inflammation:8,11 | |
| Malnutrition screening | As described above | |
| Phenotypical criteria | As described above | |
| Aetiological criterion | B2 (systemic inflammation; described above) | |
| Sarcopenia | Defined by two criteria: low muscle strength combined with low muscle mass or quality:17 | |
| Optional screening | SARC-F questionnaire132 | |
| Criterion A | Low muscle strength | |
| Criterion B | Low muscle mass or quality | |
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS-2002, Nutrition Risk Screening 2002; SARC-F, Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls; SNAQ, Short Nutritional Assessment Questionnaire.
Tissue injury induces inflammation;12 in cancer, malignant and stromal immune cells may contribute to a chronic inflammatory state13 leading to complex catabolic sequelae.14 Systemic inflammation has been extensively and reliably associated with poor clinical outcome. A widely validated and simple score to categorise systemic inflammation is the modified Glasgow Prognostic Score, based on C-reactive protein and serum albumin (C-reactive protein normal: 0; raised C-reactive protein and normal albumin: 1; raised C-reactive protein and low albumin: 2). This score is highly prognostic of clinical outcome.15
Thus, the pathophysiology of cachexia is currently understood as host-tumour interactions redirecting metabolism and driving the brain to reduce appetite, cause alterations in taste and smell, impact gastrointestinal (GI) autonomic function, induce fatigue and decrease daily physical activity (see Section 2 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2021.100092). While inadequate food intake is a major driver of weight loss,7 metabolic changes and reduced activity contribute to loss of muscle mass.16
During the last decade, low muscle mass (sarcopenia; see definition in Table 117) has been identified as a central factor impacting clinical outcome, and anticancer agents have been recognised as an important cause of sarcopenia.18 In clinical practice, it is highly relevant that loss of muscle strength and muscle mass may appear early and before the occurrence of a clinically apparent weight loss and that it may coexist with obesity and hence be present in patients with a high body mass index (sarcopenic obesity).19
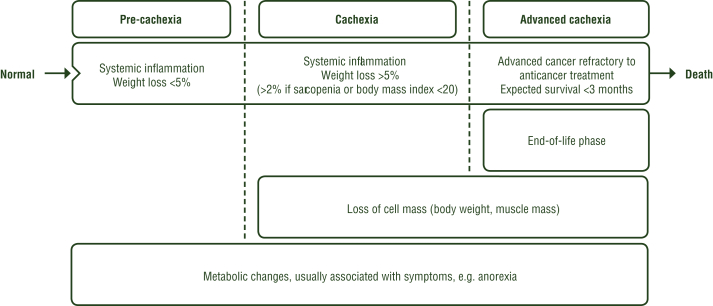
Cachexia may evolve over time and it has been proposed to differentiate early phases without discernible weight loss (pre-cachexia) from advanced or refractory stages6 (see Figure 3). In cachectic patients, the most common GI symptoms are anorexia and early satiety, nausea, bloating, taste alterations, xerostomia, dysphagia and constipation. In addition, other secondary nutrition impact symptoms may occur, such as breathlessness, severe fatigue, etc. Nutrition impact symptoms are commonly experienced and are associated with a poor QoL and performance status (PS).20
Recommendation
-
•
Defining cachexia as disease-related malnutrition based on the GLIM definition of malnutrition and the presence of systemic inflammation is recommended [V, A].
Screening and assessment of cachexia
To ensure access to adequate nutritional and metabolic care for all patients, it is important to:
-
•
Detect at-risk patients by routinely implementing a standardised screening procedure.
-
•
Assess all at-risk patients for their nutritional and metabolic status as well as all impairments endangering this status.7
Malnutrition risk screening
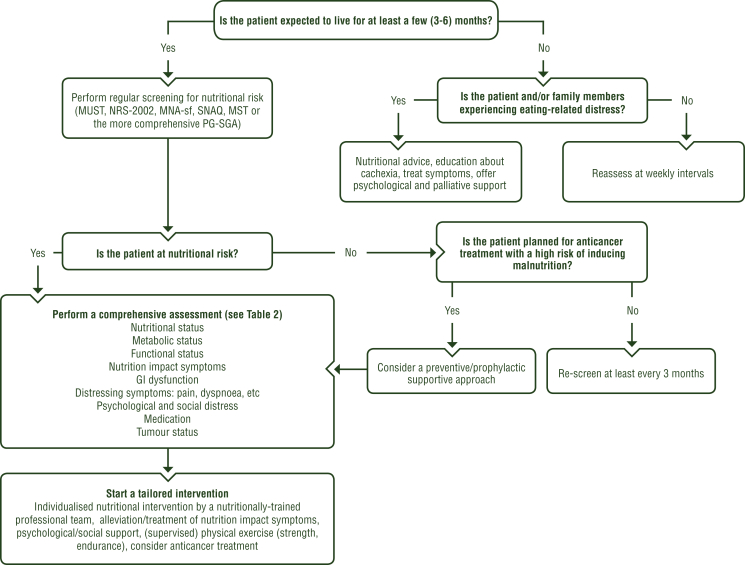
Nutritional risk screening should be carried out regularly in all cancer patients undergoing anticancer treatment and in those with an expected survival of more than a few (i.e. 3-6) months. In patients with an expected survival of less than a few months, screening for eating-related distress should be carried out (Figure 4).
Figure 4.
Screening and assessment of nutritional and metabolic risk for cachexia.
GI, gastrointestinal; MNA-sf, Mini Nutritional Assessment short form; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS-2002, Nutrition Risk Screening 2002; PG-SGA, Patient-Generated Subjective Global Assessment; SNAQ, Short Nutritional Assessment Questionnaire.
While there is no general agreement on the ‘best’ screening tool,21,22 the following are suggested: Malnutrition Universal Screening Tool (MUST), Nutrition Risk Screening 2002 (NRS-2002), Short Nutritional Assessment Questionnaire (SNAQ) and the Malnutrition Screening Tool (MST).21 Other more complex tools like the Patient-Generated Subjective Global Assessment (PG-SGA) may be included in assessment procedures.
Assessment of nutritional status
All patients diagnosed as being at-risk following malnutrition screening should be referred to a nutrition expert for assessment of nutritional and metabolic status and evaluation of food intake impairment and GI function.7 Assessing nutritional status should include objective assessment of the following (see Table 2 for recommended parameters):
-
•
Body weight (BW).
-
•
Weight change during the preceding months.
-
•
Body composition with a focus on muscle mass.6
-
•
Food intake with a focus on energy and protein.
-
•
PS [Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO)].
-
•
Information regarding the presence and degree of systemic inflammation.7
Table 2.
Parameters of comprehensive cachexia assessment and recommended tools
| Category | Parameter | Recommended tool(s) |
|---|---|---|
| Nutritional status | Whole body status | BW |
| Weight loss | % of usual healthy weight | |
| Food intake | % of required amount | |
| Energy and protein intakea | Kcal/kg/day, g/kg/day | |
| Micronutrient or macronutrient deficienciesa | Food diary or 24-hour recall and software-based analysis | |
| Body compositiona | Anthropometry BIA, CT or DEXA133 |
|
| Metabolic status | Systemic inflammation | Modified Glasgow Prognostic Score15 |
| Energy expenditurea | Indirect calorimetry | |
| Functional status | PS | ECOG/WHO index |
| Physical activity | ADL134 | |
| Dependency | Northwick Park Dependency Score135 | |
| Grip strengtha | Dynamometry | |
| Gait speeda | 4-metre gait speed test136 | |
| Nutritional barriers | Nutrition impact symptoms | PG-SGA137 Nutritional impact checklist23 |
| GI dysfunctions | Chewing, taste, swallowing, gut motility, constipation, diarrhoea, stenosis, malabsorption | Diagnostic interview, imaging tests, functional tests, visual analogue scales |
| Distressing symptoms | Symptom assessment and risk factors (cognition, emotion, depression) | ESAS138 |
| Psychological and social distress | Psychosocial assessment139 | FAACT140 EORTC QLQ-CAX24141 |
| Adverse events of medication | Possible adverse effects on appetite, GI tract, central nervous system, fatigue | Pharmacological counselling |
| Tumour status | Extent and activity of cancer disease, likelihood of response to anticancer treatment | Oncological counselling |
ADL, activities of daily living; BIA, bioelectrical impedance analysis; BW, body weight; CT, computed tomography; DEXA, dual X-ray absorptiometry; ECOG, Eastern Cooperative Oncology Group; ESAS, Edmonton Symptom Assessment System; EORTC, European Organisation for Research and Treatment of Cancer; FAACT, Functional Assessment of Anorexia/Cachexia Treatment; GI, gastrointestinal; PG-SGA, Patient-Generated Subjective Global Assessment; PS, performance status; QLQ-CAX24, cachexia-specific quality of life questionnaire; WHO, World Health Organization.
If available and appropriate, depending on resources available and the patient's capability.
An assessment of factors that are impeding or that might interfere with maintaining nutritional status should include evaluation of:
-
•
Nutrition impact symptoms, such as anorexia, nausea, taste and smell alterations, mucositis, constipation, dysphagia, chronic pain, abdominal pain (e.g. cramping) and diarrhoea, as well as aspects of GI function potentially responsible for these symptoms.
-
•
Fatigue, physical activity, shortness of breath and psychosocial distress.7
Assessment should focus on modifiable factors that can be addressed by an intervention. The systematic use of a nutritional impact checklist has been shown to trigger more therapeutic interventions, leading to better symptom control and subsequently to a better nutritional intake.23 Based on these findings, a tailored intervention can be started, including nutritional advice, alleviating nutritional impact factors and targeting any other factors that may hinder an adequate nutritional intake (such as social support or financial difficulties).
Recommendations
-
•
Standardised screening for nutritional risk at regular intervals is recommended for all patients undergoing anticancer treatment and those with a life expectancy of at least a few (i.e. 3-6) months; a validated screening tool should be applied [V, B].
-
•
Offering supportive nutritional advice and education about cachexia, as well as psychological and palliative support, is recommended for all patients experiencing eating-related distress [V, B].
-
•
Patients found to be at no immediate risk of malnutrition by screening should be re-screened at regular intervals (typically every 3 months or at staging for anticancer treatment) or, in cases where anticancer treatment with a high risk of inducing malnutrition is planned (e.g. combined-modality treatments, high-dose chemotherapy, highly emetogenic agents), prophylactic nutritional support should be considered [V, B].
-
•
For patients identified as being at nutritional risk, an objective assessment of nutritional and metabolic status (including weight, weight loss, body composition, inflammatory state, nutritional intake and physical activity) and examination for the presence of factors interfering with the maintenance or improvement of this status (including nutrition impact symptoms, GI dysfunction, chronic pain and psychosocial distress) is recommended. Repeating nutritional assessments at regular intervals, typically monthly, is also recommended to guide multi-component anti-cachexia treatment [V, B].
Deciding on cachexia treatment
The relative importance of cachexia-related subjective and objective signs and symptoms may change during the trajectory of the disease; changes in body resources and metabolic pattern as well as impairment of physical performance are essential targets in patients undergoing anticancer treatment, but they lose their importance near end of life. Debilitating symptoms, however, need to be treated and alleviated as much as possible throughout the life of every patient with the involvement of his/her family members and caregivers.
Predicting the overall survival (OS) and end of life of individual patients is inherently difficult, inaccurate and often overly optimistic.24 Probability estimates (i.e. prediction of the chances of a patient being alive at a certain time point) are more accurate,25 as are simple scores based on inflammatory markers.26
Dealing with uncertainties in prognosis requires continuous, honest and empathic communication with the patient and his/her caregivers as well as comprehensive discussions among all members of the medical team to recognise and repeatedly re-evaluate the indication for individual anti-cachexia interventions, given that each intervention is associated with different risks and burdens. Table 3 provides a summary of key criteria to consider when discussing the initiation of nutritional interventions with the patient and family/caregivers.
Table 3.
Criteria to consider when deciding on nutritional and metabolic interventions
| Benefit possible | Benefit uncertain |
|---|---|
| Ongoing anticancer treatment | Approaching end of life |
| No or only minimal inflammation or inflammation responsive to treatment | Persistent, severe and unresponsive inflammation |
| No or only slow and mild weight loss | Rapid and severe weight loss refractory to anticancer treatment |
| Stable or only slowly progressing cancer | Rapidly progressing cancer without reasonable treatment options |
| Good chance of intervention to improve the patient's well-being | No realistic chance that the intervention will improve the situation of the patient |
| Patient is aware of the prognosis and of the positive/negative effects of the intervention | Patient is not fully aware of the prognosis or the positive/negative effects of the intervention |
| Strong wish of the patient to accomplish or reach an individual goal | Patient is preparing for dying |
| Patient is motivated and feels very little inconvenience considering the planned nutritional intervention | Patient feels the nutritional intervention to be burdensome and is unmotivated/unwilling to start the intervention |
| Patient is able and motivated to be physically active | Immobilised patient without urge to be or to become active |
| Severely impaired food tolerance | Only mildly impaired food intake |
Choosing anti-cachexia treatment options: prioritising multimodal care
Given the complex and multifaceted contributors to cachexia, anti-cachexia treatment must be based on a comprehensive assessment of the patient's situation and an evaluation of reasonable, available treatment options, resulting in a personalised, multitargeted and multimodal approach.27
Food intake may be compromised by many factors and secondary to nutrition impact symptoms, some of which may be amenable to treatment. If, after alleviating these factors, food intake is still inadequate, nutrition-based interventions should be initiated.
Compared with providing energy and nutrients by nutritional interventions, modulating metabolic derangements is more complex. The evolution of insulin resistance28 and anabolic resistance29 impair the maintenance of whole-body muscle mass. Thus, interventions to decrease catabolism and increase anabolic pathways include the provision of adequate amounts of energy and proteins; muscle training; pharmacological agents to increase appetite, diminish systemic inflammation and stimulate muscle growth; and psychosocial interactions to alleviate distress.
When anticancer treatment is offered to a cachectic patient, in addition to carefully adjusting the dosing, the intensity of multimodal supportive management needs to be enhanced, encompassing nutrition, physical exercise, anti-catabolic and anti-inflammatory treatment, as well as psychological and social support. In the cachectic cancer patient who is physically unfit for further oncological therapy, a key challenge is to decide whether to maintain or reduce the intensity of multimodal supportive management. Nutritional support and physiotherapy may be offered on an individual basis while carefully monitoring individual goals and QoL. During the last weeks of life, it is essential to provide relief from eating-related distress and weight loss-related distress, strategies to cope with impending death and compassionate communication with patients and family.
Recommendations
-
•
Every patient with cachexia should be offered interventions with the goal of either improving or alleviating the consequences of cachexia [II, B].
-
•
Cachexia treatment requires a multimodal approach aimed at relieving symptoms impacting on food intake, ensuring adequate energy and nutrient intake, minimising catabolic alterations, supporting muscle training and offering psychological and social support [II, B].
-
•
During anticancer treatment and in patients with a life expectancy of more than a few (i.e. 3-6) months, interventions to both antagonise deterioration of body resources and metabolism, and to alleviate debilitating symptoms, are recommended [IV, B].
-
•
If expected survival is less than a few (i.e. 3-6) weeks, focussing on anti-cachexia interventions aimed at alleviating distressing symptoms like thirst, nausea, vomiting and dysphagia, and psychological and existential distress, as well as distress to family members, is recommended [IV, B].
-
•
In situations where it is difficult to decide on appropriate anti-cachexia intervention strategies, a tentative intervention for a limited period may be considered to evaluate the likelihood of improvement [IV, C].
Nutritional interventions
In patients undergoing anticancer therapy and/or with an expected survival of at least a few months, ensuring an adequate energy and nutrient intake should be pursued vigorously (see section on nutritional requirements). Cancer patients who cannot eat adequate amounts of food should receive nutritional support as an essential component of best supportive care to improve food intake, BW and QoL.30, 31, 32 Nutritional support in patients able to eat should be based on dietary counselling, guidance on choosing high-energy, high-protein foods, enriching foods (e.g. by adding fat/oils, protein powder) and use of oral nutritional supplements (ONSs). If this proves inadequate, tube feeding should be offered if the lower GI tract is working, otherwise PN is the method of choice. Separate routes of feeding may be combined for optimal effect7 (Figure 5).
Figure 5.
Choosing nutritional intervention options.
Purple: symptom; turquoise: nutritional interventions; white: other aspects of management.
EN, enteral nutrition; GI gastrointestinal; NTF, nasogastric tube feeding; ONS, oral nutritional supplement; PEG, percutaneous endoscopic gastrostomy; PN, parenteral nutrition.
In patients not receiving anticancer therapy with an expected survival of less than a few months, nutritional interventions with low risks/burdens for the patient (e.g. counselling and ONSs) are preferred. Very few trials have compared different modes or amounts of nutritional support. In one trial, which randomised patients with severely compromised food intake and a limited survival of 1-4 months to supplemental PN or oral feeding, PN did not improve QoL or survival but increased adverse events.33 Similarly, another trial which randomised patients in the end-of-life setting to PN or fluids only showed that PN did not affect median survival.34 During the last weeks of life, nutritional interventions are rarely indicated.7 Given the potential risks of enteral nutrition and PN (see sections on tube feeding and PN), these interventions should be considered as high-risk compared with low-risk interventions such as counselling and ONSs.
Nutritional requirements
The aim of nutritional support is to ensure adequate intake of energy and nutrients by enabling the patient to eat normal food, enjoy eating and participate in meals with others as a component of social life.7 It may be difficult or even impossible to achieve tissue accretion without physical activity and within the context of active systemic inflammation; therefore, these problems need to be addressed simultaneously.
While resting energy expenditure may be increased in cachexia, total energy expenditure is often normal (25-30 kcal/kg BW/day) because of corresponding reductions in physical activity,7 but may be unpredictably low or high in some patients.35 Even increased energy and protein intake may not be able to attenuate weight loss in all patients. Given the presence of anabolic resistance in older subjects and in chronic diseases, higher than normal amounts of protein (at least 1.2 and possibly up to 2 g/kg BW/day) may be required to balance protein synthesis.36, 37, 38
Fat utilisation in weight-losing cancer patients is very efficient and may cover a major part of resting energy expenditure,39,40 whereas carbohydrate utilisation is impaired in the presence of systemic inflammation and insulin resistance. In addition, fats are energy-dense and allow for feeding of smaller volumes. Compared with standard food, an isonitrogenous, isocaloric, ketogenic diet low in carbohydrates maintains nitrogen balance and whole-body protein turnover rates.41 In a randomised controlled trial (RCT) carried out in malnourished cancer patients, a high-fat diet improved weight control, fat-free mass and body mass compared with normal food.42
Dietary counselling and ONSs
It is unreasonable to expect an increase or stabilisation in weight if nutritional needs are not met. As a good example, two systematic reviews have shown that dietary counselling is generally effective in increasing dietary intake, BW and QoL in patients undergoing radiotherapy, with some suggestion that dietary counselling may also improve nutrition impact symptoms, complications, response to anticancer treatment and survival.43,44
ONSs are a balanced mixture of macro- and micro-nutrients available as liquid feeds, puddings and powdered formulations reconstituted with milk or water. They are available in a range of different presentations, flavours and formulations, including fibre-containing and milk-, juice- or yoghurt-like products. In general, dietary counselling with ONSs, when necessary, is effective for inducing weight gain and increasing dietary intake. However, most trials on this topic were hampered by poor methodological quality, specifically from inadequate reporting of actual dietary intake and not reaching recommended dietary intakes.32,45 Three systematic reviews reported that providing standard ONSs without dietary counselling was not effective.43,44,46 As such, ONSs are best used as an adjunct to a therapeutic diet and counselling by a professional dietician.46 A meta-analysis of patients undergoing chemotherapy showed positive effects of dietary counselling on weight gain with or without ONSs.32 Two systematic reviews focusing on dietary counselling and ONSs in malnourished patients reported positive effects on energy intake, weight gain and some aspects of QoL (e.g. emotional functioning, loss of appetite and global QoL) but noted that evidence was weak due to the poor methodological quality of included trials.30,47
In cancer cachexia, n-3 fatty acids have been studied, particularly for their anti-inflammatory properties, and are available as a component of specialised ONSs, usually also enriched in protein (N3P-ONSs). Several randomised trials have been published on the effects of N3P-ONSs in cancer patients.32,47, 48, 49, 50 Overall, studies were heterogeneous and inadequately powered to show effects on treatment toxicity or survival. No negative effects of the supplements were reported. Most trials suggested benefits of N3P-ONSs on weight, lean body mass and some aspects of QoL when given to patients receiving radiotherapy, chemotherapy or chemoradiotherapy. However, when given to patients not receiving anticancer therapy, no benefit of N3P-ONSs was detected.51
Tube feeding
Dysphagia due to obstruction, motility dysfunction or mucosal inflammation may compromise or prevent normal food intake and thus is an indication for tube feeding to circumvent the defect. Patients with head and neck or upper GI cancers are at particular risk of dysphagia due to obstructing tumours as well as severe mucositis induced by aggressive treatment (e.g. combined-modality treatment). It is critical to recognise the emergence of dysphagia early and to respond in a timely and individually appropriate way to safeguard adequate feeding. This may include diagnostic procedures to classify and grade swallowing deficits, involving a speech therapist, specialised dietary counselling and products either via nasogastric tube feeding (NTF) or percutaneous tube feeding [e.g. percutaneous endoscopic gastrostomy (PEG)].7,52
Tube feeding may be associated with potentially serious complications, including mechanical (e.g. tube blockage), GI (e.g. diarrhoea), infectious (e.g. aspiration pneumonia) and metabolic (e.g. refeeding syndrome) complications.53
Short-term RCTs have shown that the metabolic efficacy and complication rates of enteral nutrition and PN are similar.54,55 As the enteral route is more physiological, safer and less expensive, it represents the first option if there is no severe impairment of GI function (Figure 5). In some settings, supplemental PN should be preferred over tube feeding; for example, if patients are suffering from nausea, vomiting, abdominal discomfort or severe diarrhoea. Tube feeding may be ineffective due to frequent dislodging; in particular, tube feeding may not prevent aspirations in patients with dysphagia. Given the lack of reliable clinical evidence of superior outcome for either method, it has been proposed that patient preference be considered when deciding on the feeding method.56 Some patients strongly prefer an intravenous route over tube feeding,56,57 especially if a patient has already had a central venous catheter inserted.
Results from observational trials and RCTs comparing early tube feeding to oral nutrition in patients with head and neck cancer are heterogeneous, possibly due to the different oral nutrition regimens used in the control groups as well as a lack of stratification of patients by risk scores for malnutrition and dysphagia.58 Appropriate prediction and careful monitoring of food intake in order to identify the need for initiation of enteral feeding are recommended by European and Canadian guidelines.7,59
Several RCTs have compared NTF and PEG in head and neck cancer patients requiring nutritional support for ≥1 month. PEG resulted in better nutritional parameters after 6 weeks of treatment but not later.60,61 Meta-analyses have also reported no significant differences in the overall complication rates between NTF and PEG,62 even though tube dislodgement was more frequent with NTF and dysphagia was more frequent with PEG.63 Resolution of dysphagia is impaired with long-term tube feeding.
PN
In patients with severely compromised GI function, it may be impossible to ensure adequate nutrition by the oral or enteral route. PN and home PN are being widely used in patients with advanced cancer, both in patients still receiving or no longer receiving anticancer treatments, although evidence to support PN in patients with advanced cancer is weak.33,64, 65, 66 It appears obvious that a prolonged, severely reduced tolerance of food may compromise clinical outcome, and in these settings, PN might improve QoL and possibly survival. A recent systematic review assessing the effectiveness of home PN in people with malignant bowel obstruction included only observational studies, reported a high risk of bias and graded the certainty of evidence to be very low for improving survival and QoL.67 Another systematic review found that PN in patients with advanced cancer was understudied and that the level of evidence was weak.68 The PS and Glasgow Prognostic Score impact strongly on survival in patients with advanced cancer receiving home PN.69 From this, it has been suggested that PN should be avoided if the ECOG/WHO PS is 3 or 4.70 More complex scores and nomograms have been developed to estimate the probability of survival in patients with advanced cancer receiving home PN, e.g. based on Glasgow Prognostic Score, PS, presence of metastatic disease and cancer entity.65 While these tools may separate groups of patients with similar survival, predictions for individual patients are imprecise.
However, absence of evidence is not identical to evidence of absence of an effect,71 and a decision to forego the option of intravenous nutrition should not only be based on the lack of high-quality trials. Rather, the decision to initiate PN should be individualised based on the extent of disease, physical and psychological resources of the patient and on a case-by-case risk/benefit assessment (see Table 3).
PN carries the risk of potentially severe complications, including (but not limited to) catheter-related infection, occlusion and thrombosis, derangements of substrate and electrolyte levels, refeeding syndrome, exsiccosis, fluid overload and chronic hepatopathy and osteopathy.72
PN may be offered to patients who do not tolerate any oral food or those who still tolerate some but inadequate amounts of oral food. The latter has been termed ‘supplemental PN’, although there is no agreement on the amount of food tolerated to justify this designation. A number of prospective observational studies have reported the effects of PN in patients with advanced cancer suffering from either severe GI obstruction or malnutrition.66,73, 74, 75 OS was reported as 57%-75% after 1 month, 34%-67% after 3 months and 12%-34% after 6 months, but this was dependent on the type of patients included.66,73,75 Only one of these observational trials reported a small, but not clinically relevant, improvement in QoL.73 Obling et al.76 randomised 47 patients with incurable GI cancer to dietary counselling plus either ONSs or supplemental PN. The authors observed improvements in fat-free mass and QoL in favour of the supplemental PN group at 12 weeks but no difference in 6-month survival; however, the statistical analysis was flawed by the very large number of statistical tests carried out.76 Bouleuc et al.33 randomised 111 patients with advanced cancer to optimised nutritional care with or without additional supplemental PN for several months; supplemental PN did not improve either QoL or survival but increased severe adverse events. Thus, the potential clinical benefit of PN needs to be balanced against relevant risks (e.g. metabolic derangements, septic complications) and burdens (e.g. connection to an intravenous line for up to 14 hours per day).33
None of the published trials reported whether patients were aware of their prognosis or the uncertainty about the benefits of PN.
An open question is how to manage the withdrawal of home PN at end of life. Although there has been no formal study on this issue, clinical experience shows that general criteria to withhold home PN when deemed no longer helpful should be considered early at the time when home PN is first offered and thereafter be discussed repeatedly to minimise distress when it is required to phase out PN at end of life.
Recommendations
Nutritional interventions
-
•
In patients with inadequate food intake, nutritional interventions are recommended. In patients with an expected survival of more than several months and in those receiving anticancer therapy, these interventions should be escalated, as required. In other situations, low-risk interventions (e.g. counselling and ONSs) are preferred [II, A].
-
•
If safe, the oral route should be the first option for nutritional support. Enteral tube feeding may be used in cases of dysphagia if the small bowel function is preserved. PN should be considered if oral intake and tube feeding are not tolerated or remain inadequate [II, A].
Nutritional requirements
-
•
Nutritional interventions should aim to fulfil energy and nutrient requirements [III, B].
-
•
Nutritional interventions should be accompanied by muscle training and efforts to normalise metabolic state (diminish systemic inflammation, alleviate distress) [III, B].
-
•
To maintain nutritional status, at least 25-30 kcal/kg BW/day is recommended, adjusting the regimen as required [V, B].
-
•
At least 1.2 g protein/kg BW/day should be provided [V, B].
-
•
In patients with cachexia, regimens with fat accounting for half of the non-protein calories are recommended [II, B].
Dietary counselling and ONSs
-
•
Dietary counselling should be the first choice of nutritional support offered to improve oral intake and possibly weight gain in cachectic or at-risk patients who are able to eat. Dietary counselling should emphasise protein intake, an increased number of meals per day, treatment of nutrition impact symptoms and offering nutritional supplements when necessary. An adequately trained professional should guide this advice [II, B].
-
•
ONSs can be supplied as part of dietary counselling to improve energy intake and induce weight gain [II, B].
-
•
Patients receiving chemotherapy, radiotherapy or chemoradiotherapy may be offered N3P-ONSs to increase BW, attenuate loss of lean body mass and improve QoL [II, C].
Tube feeding
-
•
For patients with head and neck or upper GI cancers, especially those undergoing anticancer treatment, tube feeding to maintain BW or to reduce weight loss is recommended if oral feeding including ONSs is expected to remain inadequate for more than a few days [I, A].
-
•
In patients requiring >4 weeks of enteral feeding, PEG rather than NTF is recommended [II, C].
-
•
In patients requiring tube feeding, screening for and management of dysphagia is recommended along with encouragement and education to patients regarding how to maintain their swallowing function [II, B].
PN
-
•
Home PN should be offered to patients if their QoL and/or length of survival is expected to be severely compromised by progressive malnutrition. Indicators of a potential benefit are ECOG/WHO PS 0-2, a low level of systemic inflammation (normal levels of serum albumin, modified Glasgow Prognostic Score <2) and the absence of metastatic disease [V, B].
-
•
There is insufficient evidence to routinely recommend supplemental PN in hypophagic, malnourished patients receiving chemotherapy to improve QoL and nutrition parameters [V, B].
Muscle strength and endurance training to support anabolism
Current evidence shows that physical exercise is safe and provides benefit in QoL and in muscular and aerobic fitness for people with cancer, both during and after treatment.77 However, so far, reviews on exercise in cancer cachexia have generally been narrative or based on animal models. A systematic review and meta-analysis of RCTs focusing on exercise training in cachexia found no trials which met the inclusion criteria.78 An RCT comparing 8 weeks of exercise training with usual care in 231 patients with advanced cancer reported improved physical performance but no effect on subjective fatigue.79 However, despite limited robust data, multimodal rehabilitation programmes incorporating exercise and nutritional interventions have been reported to improve many outcomes, most notably those relating to physical endurance and depression scores.80
While many patients with advanced cancer may drop out of exercise programmes due to progressive disease, when carefully supervised, the intervention appears safe for patients with advanced cancer, even in the hospice setting.79,81,82 It is suggested that exercise techniques be chosen based on the individual risk of falls and of skeletal instabilities, and to suspend training when patients experience a fever >38°C, infection, platelet count <20 000 g/l, haemoglobin <8 g/dl or if they display other contraindications to exercising.83
Exercise has been hypothesised to attenuate the effects of cancer cachexia by modulating muscle metabolism, insulin sensitivity, anaemia, hypogonadism and systemic inflammation.84 Physical activity may increase muscle strength and maintain a patient's functional ability, especially when a combination of moderate- to high-intensity resistance and aerobic exercise is undertaken.84 Exercises of moderate intensity are described as those which fall in between 5-8 metabolic equivalents (METS; a procedure to quantify the energy cost of activities). These include activities that take as much effort as brisk walking (5 km/h), a stationary bike with light effort and home-based exercises.85 Resistance exercises should alternate between upper and lower limbs, focus on movement quality and use defined sets of repetitions. Aerobic training should be accompanied by continuous or intermittent heart rate monitoring.
Recommendations
-
•
When guided by professional experts, moderate physical exercise is safe in patients with cancer cachexia and is recommended to maintain and improve muscle mass [II, B].
-
•
Resistance exercise two to three times per week as well as moderate aerobic (endurance) training should be offered to all patients with cachexia. The exercise prescription should involve a physiotherapist or an adequately trained professional and comprise a structured approach, including mode (aerobic, resistance, flexibility), frequency, intensity and duration as well as defined time points for reassessment [II, B].
Pharmacological interventions
Several drugs have been investigated for their potential use to treat or ameliorate the consequences of cancer cachexia. However, only corticosteroids and progestins have consistently shown beneficial effects on appetite and/or BW, though at the expense of substantial side-effects, while for other agents, the data are heterogeneous or disappointing.
The following section presents drugs that are approved for clinical use and have shown some anti-cachexia effect in clinical trials.
Corticosteroids
Corticosteroids include several agents with variable glucocorticoid, mineralocorticoid and anti-inflammatory potency. Prednisolone, methylprednisolone and dexamethasone are used most frequently. Symptomatic relief appears to be mainly achieved by their potent anti-inflammatory activity.86 Toxicity is usually minor when used for only a few weeks, whereas during prolonged intake, corticosteroids cause a rapid loss of muscle mass, insulin resistance and increased likelihood of infections, such as candida and stomatitis, contributing to a deterioration of cachectic patients.87 Corticosteroids are recommended for the control of cancer-related fatigue.88 Several RCTs investigating the effects of corticosteroids on appetite in patients with advanced cancer have been published. Most trials reported a temporary benefit in appetite and well-being, whereas there were no effects on BW or survival.7,89 The anti-anorectic effect of corticosteroids is transient and often disappears after a few weeks.90 There are limited data available to recommend one corticosteroid over another.
Progestins
Medroxyprogesterone acetate and megestrol acetate (MA) have been studied widely to treat weight loss and anorexia in cancer patients. In preclinical models, progestins stimulate appetite and inhibit the synthesis of pro-inflammatory cytokines. A Cochrane review91 including 23 RCTs on the use of MA in patients with cancer (median duration of 8 weeks) found a significant improvement in appetite (relative risk 2.57) and weight gain (relative risk 1.55). However, no consistent improvement in QoL was observed and no data on muscle mass or physical function were reported. In the analysed trials, MA was used in doses of 160-800 mg/day and weight improvement appeared higher for doses >160 mg/day, while no dose effect was observed for appetite.
Treatment with MA is associated with an increased risk of thromboembolism, fluid retention, adrenal insufficiency and hypogonadism in male patients.92 While the aforementioned Cochrane review reported that MA treatment was associated with an increased mortality rate,91 an update concluded that MA does not increase the rates of adverse events or death.93
Although progestins have been studied in many RCTs, confirming modest stimulation of appetite, their clinical use is limited because of the significant risk of potentially serious side-effects.
Cannabinoids
Cannabis sativa is a medical plant containing multiple cannabinoids, including tetrahydrocannabinol (THC). Medical cannabis is available in various formulations, e.g. tablets/capsules, vaporiser or mouth spray.
In patients with cancer cachexia, when studied in small trials and case series, THC appeared to improve appetite and attenuate weight loss. However, larger randomised trials comparing THC with either MA94 or placebo95 could not detect a significant effect on appetite or QoL; in these trials, toxicity was low. In a small, placebo-controlled, 8-week RCT in 47 anorectic patients with advanced non-small-cell lung cancer, the synthetic THC derivative, nabilone, resulted in low toxicity but no significant effects on appetite or QoL.96 Current safety data for medical cannabis in cancer cachexia is based on only a few trials that likely under-dosed patients and so safety concerns remain.
Androgens
In cancer patients, hypogonadism is related to advanced cancer status, weight loss and, most likely, the use of opioid therapy. Anabolic-androgenic steroids have been shown to ameliorate loss of muscle mass and strength in patients with wasting associated with acquired immune deficiency syndrome. The use of androgens has not been studied extensively in patients with cancer cachexia. In an RCT of 37 lung cancer patients, the analogue nandrolone did not improve BW compared with placebo.97 In a three-armed RCT including 496 patients with cachexia, fluoxymesterone 10 mg b.i.d. was significantly inferior to MA 800 mg/day in terms of appetite improvement.98
Olanzapine
Olanzapine is an atypical antipsychotic drug acting on multiple receptors, including dopamine and serotonin receptors, both of which are potentially relevant in cachexia.99 In clinical use, olanzapine causes more weight gain than other antipsychotic drugs.100 In a single arm, dose escalation study in 39 weight-losing patients with advanced cancer, olanzapine could not attenuate weight loss.101 However, in a trial randomising 80 patients with advanced cancer to receive MA or MA and olanzapine, the combination arm yielded significant improvements in appetite and BW.99 In a recent RCT, olanzapine significantly reduced non-chemotherapy-induced nausea in 30 patients with advanced cancer compared with placebo.102 Thus, olanzapine may be considered for treating chronic nausea in patients with advanced cancer.
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) block the cyclooxygenase pathways and reduce inflammation by inhibiting prostaglandin production. NSAIDs have been studied to reduce the catabolic drive of systemic inflammation in patients with advanced cancer and cachexia. In a systematic review including six controlled trials and seven observational trials,103 11 of these trials reported an increase or stabilisation of BW or lean body mass with few side-effects reported. The cumulative evidence, however, was weak due to the low methodological quality of the analysed trials. Thus, in cachectic patients requiring pain control, NSAIDs could be considered with the potential additional benefit of improving BW.
Prokinetics
Metoclopramide and domperidone are widely used to treat early satiety and chronic nausea104 as well as dyspepsia syndrome and gastroparesis.105 However, no large RCT has investigated the role of prokinetic agents in cachexia. While one RCT in patients with advanced cancer showed that metoclopramide may improve nausea but not appetite,106 there are no similar studies with domperidone.
Metoclopramide and domperidone can cause serious, mainly neurological, side-effects, e.g. tardive dyskinesia, spasms, depression, dizziness and urinary retention.107
Ghrelin receptor agonists
Anamorelin has recently been approved in Japan for cancer cachexia in patients with non-small-cell lung cancer, gastric cancer, pancreatic cancer and colorectal cancer, but it is not approved in Europe based on findings from the ROMANO studies which showed a more modest improvement in lean body mass compared with that seen in the Japanese trials.
Combination therapy
Published trials investigating potential synergies among pharmacological agents like progestins, antioxidants, L-carnitine, thalidomide, n-3 fatty acids and NSAIDs were unsuccessful or unreliable due to methodological deficiencies.108,109
Recommendations
-
•
Corticosteroids may be used to increase appetite for a short period of up to 2-3 weeks. Effects on appetite usually disappear with longer treatment [I, B].
-
•
Progestins may be used to increase appetite and BW, but not muscle mass, QoL or physical function in patients with cancer cachexia [I, B]. The risk of serious side-effects, including thromboembolic events, must be considered.
-
•
There is insufficient evidence to support the use of medical cannabis or its derivatives to alleviate anorexia or early satiety in patients with cancer cachexia [II, C].
-
•
As there is evidence of no beneficial effect in terms of improvement in muscle mass, androgens are not recommended [II, D].
-
•
There is moderate evidence to suggest considering the use of olanzapine to treat appetite and nausea in patients with advanced cancer [II, B].
-
•
There is insufficient evidence to recommend the use of NSAIDs alone to treat cancer cachexia [III, C].
-
•
There is insufficient evidence to recommend the use of metoclopramide or domperidone alone to treat cancer cachexia [II, C].
-
•
There is insufficient evidence to recommend specific combination regimens due to the lack of evidence from large, well-designed, randomised trials [II, C].
Communication with patients and their families
Addressing cachexia-related psychosocial distress
In the presence of cachexia-related symptoms such as anorexia and fatigue, patients and their families experience stressful changes in eating habits and challenging social interactions.110,111 Patients report wanting and trying to eat but being unable to do so, while family members often misunderstand the complex and powerful derangements responsible for anorexia and food aversion in cachexia and pressure their relative to eat, thus increasing tension and conflict in the patient–family unit.112,113 In addition, continued loss of weight and function alters the patient's appearance with consequences on their self-image and self-esteem.113
Early identification of psychosocial distress and the impact of cachexia allows for timely interventions to manage distressing symptoms and improve QoL.114
Cachexia-centred communication
Focus groups and semi-structured interviews with 34 Irish health care professionals revealed that doctors, nurses and dieticians tend to avoid the problem of cancer cachexia because of difficulties in communicating its complex and often irreversible nature and negative prognosis, and for fear of lowering the patient's hope.115 A missed acknowledgement, however, has made family members feel misinformed and isolated.116 Poor communication by health care professionals may weaken the confidence of patients and families in their knowledge and understanding of cachexia; thus, transparent information is clearly preferred.116 A large survey (response rate 76%) of 702 bereaved family members of cancer patients in Japan suggested that health care professionals may relieve psychological and emotional distress by explaining the mechanisms of cancer cachexia as simply as possible.117
Tailored information about the role of nutritional support according to the stage of cachexia (see Figure 3) is fundamental to achieving agreement between health care professionals, patients and families on treatment goals. A systematic review including 19 studies investigating eating-related distress in patients with cachexia found that the main causes of negative psychosocial effects are a lack of knowledge regarding the nature of cancer cachexia and unsuccessful attempts to increase BW. A structured and informative intervention prevents families from feeling overwhelmed by their loved one's disease and alone in managing weight and eating problems.118
Figure 3.
Evolution of cancer cachexia.
Adapted from Fearon et al.6
These findings suggest that patients and their families need honest and problem-centred communication tailored to the disease stage.
Psychosocial interventions
The aim of psychosocial interventions is to reduce the emotional burden associated with cancer cachexia by empowering patients and families to cope with the dysfunctions and derangements of cachexia, thus improving QoL.118, 119, 120 Health care professionals can adopt different strategies (e.g. DVDs, stories, open questions) to help patients and families to share their perspectives about food-related issues.120
A small, randomised, exploratory trial evaluating psychosocial intervention on weight and eating-related distress in 50 patients with advanced cancer found that treated patients reported lower levels of distress compared with the control group.118 Qualitative analysis suggested that psychosocial intervention was helpful for carers as it provided information, reassurance and support for self-management.119 In a small, mixed-methods, qualitative research study, a family-centred psychosocial intervention was developed and delivered by a single nurse researcher to help patients with advanced cancer and their families/caregivers to cope with the patient's involuntary weight loss and worsening appetite. The intervention was delivered during face-to-face consultations and 15 out of 16 patient–caregiver dyads reported benefits.120
Recommendations
-
•
Health care professionals should routinely assess patients and their families to permit timely identification of any psychosocial distress [V, B].
-
•
Health care professionals should provide tailored information according to the stage of cachexia and empower patients and their families to understand its nature, course and biological mechanisms, and to acknowledge its negative effects (e.g. weight loss, reduced appetite, early satiety), thereby promoting greater awareness about the clinical condition and the need for early multidisciplinary intervention [IV, B].
-
•
Psychosocial interventions should be considered as early as possible in cachexia management. They should be conducted by trained health care professionals and aim to help patients and their families to cope with involuntary weight loss and to strengthen the dyadic coping resources [III, B].
Multimodal treatment
Multimodal interventions against cancer cachexia have been advocated for more than a decade based on the complex underlying pathophysiology [see Section 2 (text and Supplementary Figure S2) of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2021.100092] and the wealth of contributing factors impacting on BW, muscle mass, food intake and physical function.121 Components of such multimodal support may target calorific intake, physical activity, psychosocial and spiritual functions, as well as key factors in cachexia pathophysiology, such as inflammation. The concept of using synergies of supportive interventions has been described to achieve ‘anabolic competence’.122 Examples of multimodal care in daily clinical practice have been assembled by Maddocks et al.123 However, so far, few trials combining separate treatment modalities have been reported; ongoing investigations primarily focus on simultaneously targeting nutritional support, muscle training and anti-inflammatory concepts. Importantly, in healthy subjects, bouts of physical exercise significantly prolong the increase in muscle protein synthesis induced by feeding.124
The randomised, 6-week, MENAC pilot trial compared a multimodal combination of NSAIDs, nutritional advice, oral supplements enriched in eicosapentaenoic acid and physical exercise to standard treatment in 46 patients with solid tumours starting chemotherapy.82 This trial showed that the intervention improved BW and is now being followed by a phase III trial recruiting patients with lung and pancreatic cancer. Another small trial, randomising 58 patients with advanced cancer to usual care or 12 weeks of an exercise training programme combined with repeated nutritional counselling, showed a significant increase in protein intake and a decrease in nausea and vomiting.125 Recently, a large RCT including 328 patients with previously untreated metastatic oesophago-gastric cancer received either standard care or additional nutritional and psychological interventions; combined-modality support resulted in improved OS in the intention-to-treat analysis.126
Recommendation
-
•
In patients with cachexia, combining nutritional support with exercise training and psychological support is proposed [II, B]. Anti-inflammatory interventions should also be considered [V, C].
Organising successful cachexia care in modern oncology
Critical points for cachexia care are to implement screening, assessment and treatment in routine cancer care. Initiating and maintaining these efforts, including a quality control process, requires support by the institutional leadership. Evidence for the effectiveness of cachexia clinics is scarce due to a lack of RCTs.127 Extrapolation from pain interventions128 or specialised palliative care129 suggests its potential effectiveness. Typically, a registered dietician, physiotherapist, palliative care nurse, psychologist and a palliative/supportive/rehabilitative care specialist—who ideally would also be a medical oncologist130—could build the ‘inner circle’ of a cachexia clinic or team (Figure 6). A close integration of these professionals within the cancer clinic (e.g. case discussions, tumour boards, clinical rounds, education, clinical trials), as well as access to specialised professionals such as gastroenterologists (e.g. for vent, stent, gastrostomy, jejunostomy), head and neck specialists, logopaedic experts and invasive pain specialists, is highly recommended.
Figure 6.
Responsibilities and interactions of members of the multimodal care team.
GI, gastrointestinal.
Solid line arrows depict responsibilities. Dashed line arrows depict interactions. Further cooperation (e.g. with gastroenterologists, surgeons, head and neck specialists) will frequently be necessary.
Recommendations
-
•
Screening for cachexia should be integrated into routine cancer care, supported by accountable professionals and linked to immediate access to cachexia care interventions [V, B].
-
•
Cachexia care should be delivered utilising a combination of nutrition, physical activity, psychological, oncological, palliative/supportive/rehabilitative care and oncologist competencies [V, B].
Methodology
This CPG was developed in accordance with the ESMO standard operating procedures for CPG development (https://www.esmo.org/guidelines/esmo-guidelines-methodology). The relevant literature has been selected by the expert authors. Levels of evidence and grades of recommendation have been applied using the system shown in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100092.131 Statements without grading were considered justified standard clinical practice by the experts.
Acknowledgements
Manuscript editing support was provided by Angela Corstorphine of Kstorfin Medical Communications Ltd; this support was funded by ESMO.
Funding
No external funding has been received for the preparation of these guidelines. Production costs have been covered by ESMO from central funds.
Disclosure
FS reports receipt of unrestricted industry grants to institute for clinical research from Celgene, Fresenius and Helsinn, participation in company-led clinical cachexia trials for Novartis and participation in an advisory role for Helsinn, Mundipharma, Novartis, Fresenius and Kaiku Health (reimbursements to institute); TSS reports receipt of honoraria from Fresenius Kabi; MC reports receipt of payment from Tetra Biopharmaceuticals for helping to write research protocols; JA reports receipt of consultancy fees from Baxter and Danone and honoraria from Berg-Apotheke, Falk, Fresenius Kabi, Nutricia and Roche; CIR reports receipt of funding for independent educational events from Inpharm, Kyowa Kirin, Amgen Europe and Molteni SpA; MAEdvdS reports receipt of honoraria for independent lectures at educational and scientific events organised by Nutricia, Fresenius Kabi and Baxter; all other authors have declared no conflicts of interest.
Footnotes
☆Note: Approved by the ESMO Guidelines Committee: February 2021.
Supplementary data
References
- 1.Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 2.Schcolnik-Cabrera A., Chávez-Blanco A., Domínguez-Gómez G. Understanding tumor anabolism and patient catabolism in cancer-associated cachexia. Am J Cancer Res. 2017;7(5):1107–1135. [PMC free article] [PubMed] [Google Scholar]
- 3.Mannion E., Gilmartin J.J., Donnellan P. Effect of chemotherapy on quality of life in patients with non-small cell lung cancer. Support Care Cancer. 2014;22(5):1417–1428. doi: 10.1007/s00520-014-2148-9. [DOI] [PubMed] [Google Scholar]
- 4.Prigerson H.G., Bao Y., Shah M.A. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1(6):778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roeland E.J., Bohlke K., Baracos V.E. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38(21):2438–2453. doi: 10.1200/JCO.20.00611. [DOI] [PubMed] [Google Scholar]
- 6.Fearon K., Strasser F., Anker S.D. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 7.Arends J., Bachmann P., Baracos V. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Cederholm T., Jensen G.L., Correia M. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Cahill G.F., Jr. Starvation in man. N Engl J Med. 1970;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 10.Arends J. Mangelernährung bei Tumorpatienten. Onkologe. 2008;14:9–14. [Google Scholar]
- 11.Jensen G.L., Mirtallo J., Compher C. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin Nutr. 2010;29(2):151–153. doi: 10.1016/j.clnu.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baracos V.E., Martin L., Korc M. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 14.Straub R.H. Evolutionary medicine and chronic inflammatory state – known and new concepts in pathophysiology. J Mol Med (Berl) 2012;90(5):523–534. doi: 10.1007/s00109-012-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan D.C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Fearon K.C., Glass D.J., Guttridge D.C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft A.J., Bahat G., Bauer J. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozzetti F. Chemotherapy-induced sarcopenia. Curr Treat Options Oncol. 2020;21(1):7. doi: 10.1007/s11864-019-0691-9. [DOI] [PubMed] [Google Scholar]
- 19.Prado C.M., Lieffers J.R., McCargar L.J. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 20.Caillet P., Liuu E., Raynaud Simon A. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr. 2017;36(6):1473–1482. doi: 10.1016/j.clnu.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 21.van Bokhorst-de van der Schueren M.A., Guaitoli P.R., Jansma E.P. Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr. 2014;33(1):39–58. doi: 10.1016/j.clnu.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Isenring E., Elia M. Which screening method is appropriate for older cancer patients at risk for malnutrition? Nutrition. 2015;31(4):594–597. doi: 10.1016/j.nut.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Omlin A., Blum D., Wierecky J. Nutrition impact symptoms in advanced cancer patients: frequency and specific interventions, a case-control study. J Cachexia Sarcopenia Muscle. 2013;4(1):55–61. doi: 10.1007/s13539-012-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan M., Temel J.S., Wright A.A. Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol. 2013;11(2):68–74. doi: 10.12788/j.suponc.0004. [DOI] [PubMed] [Google Scholar]
- 25.Hui D., Paiva C.E., Del Fabbro E.G. Prognostication in advanced cancer: update and directions for future research. Support Care Cancer. 2019;27(6):1973–1984. doi: 10.1007/s00520-019-04727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons C.P.L., McMillan D.C., McWilliams K. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53(5):962–970.e10. doi: 10.1016/j.jpainsymman.2016.12.330. [DOI] [PubMed] [Google Scholar]
- 27.Arends J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment-focusing on metabolism and supportive care. Ann Oncol. 2018;29(suppl_2):ii27–ii34. doi: 10.1093/annonc/mdy093. [DOI] [PubMed] [Google Scholar]
- 28.Dev R., Bruera E., Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol. 2018;29(suppl_2):ii18–ii26. doi: 10.1093/annonc/mdx815. [DOI] [PubMed] [Google Scholar]
- 29.Haran P.H., Rivas D.A., Fielding R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3(3):157–162. doi: 10.1007/s13539-012-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin C., Spiro A., Ahern R. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104(5):371–385. doi: 10.1093/jnci/djr556. [DOI] [PubMed] [Google Scholar]
- 31.Bourdel-Marchasson I., Blanc-Bisson C., Doussau A. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9(9):e108687. doi: 10.1371/journal.pone.0108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de van der Schueren M.A.E., Laviano A., Blanchard H. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29(5):1141–1153. doi: 10.1093/annonc/mdy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouleuc C., Anota A., Cornet C. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. 2020;25(5):e843–e851. doi: 10.1634/theoncologist.2019-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh S.Y., Jun H.J., Park S.J. A randomized phase II study to assess the effectiveness of fluid therapy or intensive nutritional support on survival in patients with advanced cancer who cannot be nourished via enteral route. J Palliat Med. 2014;17(11):1266–1270. doi: 10.1089/jpm.2014.0082. [DOI] [PubMed] [Google Scholar]
- 35.Purcell S.A., Elliott S.A., Walter P.J. Total energy expenditure in patients with colorectal cancer: associations with body composition, physical activity, and energy recommendations. Am J Clin Nutr. 2019;110(2):367–376. doi: 10.1093/ajcn/nqz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozzetti F., Bozzetti V. Is the intravenous supplementation of amino acid to cancer patients adequate? A critical appraisal of literature. Clin Nutr. 2013;32(1):142–146. doi: 10.1016/j.clnu.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Winter A., MacAdams J., Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012;31(5):765–773. doi: 10.1016/j.clnu.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe R.R. The 2017 Sir David P Cuthbertson lecture. Amino acids and muscle protein metabolism in critical care. Clin Nutr. 2018;37(4):1093–1100. doi: 10.1016/j.clnu.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Cao D.X., Wu G.H., Zhang B. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr. 2010;29(1):72–77. doi: 10.1016/j.clnu.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Körber J., Pricelius S., Heidrich M. Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur J Clin Nutr. 1999;53(9):740–745. doi: 10.1038/sj.ejcn.1600843. [DOI] [PubMed] [Google Scholar]
- 41.Fearon K.C., Borland W., Preston T. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr. 1988;47(1):42–48. doi: 10.1093/ajcn/47.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Breitkreutz R., Tesdal K., Jentschura D. Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wien Klin Wochenschr. 2005;117(19-20):685–692. doi: 10.1007/s00508-005-0455-3. [DOI] [PubMed] [Google Scholar]
- 43.Garg S., Yoo J., Winquist E. Nutritional support for head and neck cancer patients receiving radiotherapy: a systematic review. Support Care Cancer. 2010;18(6):667–677. doi: 10.1007/s00520-009-0686-3. [DOI] [PubMed] [Google Scholar]
- 44.Langius J.A., Zandbergen M.C., Eerenstein S.E. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32(5):671–678. doi: 10.1016/j.clnu.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Balstad T.R., Solheim T.S., Strasser F. Dietary treatment of weight loss in patients with advanced cancer and cachexia: a systematic literature review. Crit Rev Oncol Hematol. 2014;91(2):210–221. doi: 10.1016/j.critrevonc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.L.C., Leong L.P., Lim S.L. Nutrition intervention approaches to reduce malnutrition in oncology patients: a systematic review. Support Care Cancer. 2016;24(1):469–480. doi: 10.1007/s00520-015-2958-4. [DOI] [PubMed] [Google Scholar]
- 47.Blackwood H.A., Hall C.C., Balstad T.R. A systematic review examining nutrition support interventions in patients with incurable cancer. Support Care Cancer. 2020;28(4):1877–1889. doi: 10.1007/s00520-019-04999-4. [DOI] [PubMed] [Google Scholar]
- 48.de Aguiar Pastore Silva J., Emilia de Souza Fabre M., Waitzberg D.L. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clin Nutr. 2015;34(3):359–366. doi: 10.1016/j.clnu.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Cereda E., Cappello S., Colombo S. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol. 2018;126(1):81–88. doi: 10.1016/j.radonc.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 50.van der Meij B.S., van Bokhorst-de van der Schueren M.A., Langius J.A. n-3 PUFAs in cancer, surgery, and critical care: a systematic review on clinical effects, incorporation, and washout of oral or enteral compared with parenteral supplementation. Am J Clin Nutr. 2011;94(5):1248–1265. doi: 10.3945/ajcn.110.007377. [DOI] [PubMed] [Google Scholar]
- 51.Dewey A., Baughan C., Dean T. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007;2007(1):Cd004597. doi: 10.1002/14651858.CD004597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bossola M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients. 2015;7(1):265–276. doi: 10.3390/nu7010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blumenstein I., Shastri Y.M., Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20(26):8505–8524. doi: 10.3748/wjg.v20.i26.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altintas N.D., Aydin K., Türkoğlu M.A. Effect of enteral versus parenteral nutrition on outcome of medical patients requiring mechanical ventilation. Nutr Clin Pract. 2011;26(3):322–329. doi: 10.1177/0884533611405790. [DOI] [PubMed] [Google Scholar]
- 55.Chow R., Bruera E., Arends J. Enteral and parenteral nutrition in cancer patients, a comparison of complication rates: an updated systematic review and (cumulative) meta-analysis. Support Care Cancer. 2020;28(3):979–1010. doi: 10.1007/s00520-019-05145-w. [DOI] [PubMed] [Google Scholar]
- 56.Scolapio J.S., Picco M.F., Tarrosa V.B. Enteral versus parenteral nutrition: the patient's preference. JPEN J Parenter Enteral Nutr. 2002;26(4):248–250. doi: 10.1177/0148607102026004248. [DOI] [PubMed] [Google Scholar]
- 57.King P.C., Barrimore S.E., Pulle R.C. “I wouldn't ever want it”: a qualitative evaluation of patient and caregiver perceptions toward enteral tube feeding in hip fracture inpatients. JPEN J Parenter Enteral Nutr. 2019;43(4):526–533. doi: 10.1002/jpen.1444. [DOI] [PubMed] [Google Scholar]
- 58.Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 2013;87(2):172–200. doi: 10.1016/j.critrevonc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Canadian Agency for Drugs and Technologies in Health Nasogastric feeding tubes versus percutaneous endoscopic gastrostomy for patients with head or neck cancer: a review of clinical effectiveness and guidelines. 2014. https://www.ncbi.nlm.nih.gov/books/NBK253809/ Available at. Accessed September 7, 2020. [PubMed]
- 60.Corry J., Poon W., McPhee N. Randomized study of percutaneous endoscopic gastrostomy versus nasogastric tubes for enteral feeding in head and neck cancer patients treated with (chemo)radiation. J Med Imaging Radiat Oncol. 2008;52(5):503–510. doi: 10.1111/j.1440-1673.2008.02003.x. [DOI] [PubMed] [Google Scholar]
- 61.Sadasivan A., Faizal B., Kumar M. Nasogastric and percutaneous endoscopic gastrostomy tube use in advanced head and neck cancer patients: a comparative study. J Pain Palliat Care Pharmacother. 2012;26(3):226–232. doi: 10.3109/15360288.2012.702199. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z., Zhu Y., Ling Y. Comparative effects of different enteral feeding methods in head and neck cancer patients receiving radiotherapy or chemoradiotherapy: a network meta-analysis. Onco Targets Ther. 2016;9:2897–2909. doi: 10.2147/OTT.S101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J., Liu M., Liu C. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J Radiat Res. 2014;55(3):559–567. doi: 10.1093/jrr/rrt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bozzetti F., Santarpia L., Pironi L. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: a multi-centre observational study with prospective follow-up of 414 patients. Ann Oncol. 2014;25(2):487–493. doi: 10.1093/annonc/mdt549. [DOI] [PubMed] [Google Scholar]
- 65.Bozzetti F., Cotogni P., Lo Vullo S. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann Oncol. 2015;26(11):2335–2340. doi: 10.1093/annonc/mdv365. [DOI] [PubMed] [Google Scholar]
- 66.Cotogni P., De Carli L., Passera R. Longitudinal study of quality of life in advanced cancer patients on home parenteral nutrition. Cancer Med. 2017;6(7):1799–1806. doi: 10.1002/cam4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sowerbutts A.M., Lal S., Sremanakova J. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst Rev. 2018;8(8):Cd012812. doi: 10.1002/14651858.CD012812.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tobberup R., Thoresen L., Falkmer U.G. Effects of current parenteral nutrition treatment on health-related quality of life, physical function, nutritional status, survival and adverse events exclusively in patients with advanced cancer: a systematic literature review. Crit Rev Oncol Hematol. 2019;139:96–107. doi: 10.1016/j.critrevonc.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 69.Keane N., Fragkos K.C., Patel P.S. Performance status, prognostic scoring, and parenteral nutrition requirements predict survival in patients with advanced cancer receiving home parenteral nutrition. Nutr Cancer. 2018;70(1):73–82. doi: 10.1080/01635581.2018.1380206. [DOI] [PubMed] [Google Scholar]
- 70.Bozzetti F., Cozzaglio L., Biganzoli E. Quality of life and length of survival in advanced cancer patients on home parenteral nutrition. Clin Nutr. 2002;21(4):281–288. doi: 10.1054/clnu.2002.0560. [DOI] [PubMed] [Google Scholar]
- 71.Altman D.G., Bland J.M. Absence of evidence is not evidence of absence. BMJ. 1995;311(7003):485. doi: 10.1136/bmj.311.7003.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pironi L., Arends J., Bozzetti F. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247–307. doi: 10.1016/j.clnu.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 73.Culine S., Chambrier C., Tadmouri A. Home parenteral nutrition improves quality of life and nutritional status in patients with cancer: a French observational multicentre study. Support Care Cancer. 2014;22(7):1867–1874. doi: 10.1007/s00520-014-2164-9. [DOI] [PubMed] [Google Scholar]
- 74.Girke J., Seipt C., Markowski A. Quality of life and nutrition condition of patients improve under home parenteral nutrition: an exploratory study. Nutr Clin Pract. 2016;31(5):659–665. doi: 10.1177/0884533616637949. [DOI] [PubMed] [Google Scholar]
- 75.Vashi P.G., Dahlk S., Popiel B. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer. 2014;14:593. doi: 10.1186/1471-2407-14-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Obling S.R., Wilson B.V., Pfeiffer P. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr. 2019;38(1):182–190. doi: 10.1016/j.clnu.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Segal R., Zwaal C., Green E. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290–e315. doi: 10.3747/co.24.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grande A.J., Silva V., Maddocks M. Exercise for cancer cachexia in adults: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2015;6(3):208–211. doi: 10.1002/jcsm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oldervoll L.M., Loge J.H., Lydersen S. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16(11):1649–1657. doi: 10.1634/theoncologist.2011-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hall C.C., Cook J., Maddocks M. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: a systematic review. Support Care Cancer. 2019;27(7):2371–2384. doi: 10.1007/s00520-019-04749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malcolm L., Mein G., Jones A. Strength in numbers: patient experiences of group exercise within hospice palliative care. BMC Palliat Care. 2016;15(1):97. doi: 10.1186/s12904-016-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solheim T.S., Laird B.J.A., Balstad T.R. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(5):778–788. doi: 10.1002/jcsm.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oechsle K., Aslan Z., Suesse Y. Multimodal exercise training during myeloablative chemotherapy: a prospective randomized pilot trial. Support Care Cancer. 2014;22(1):63–69. doi: 10.1007/s00520-013-1927-z. [DOI] [PubMed] [Google Scholar]
- 84.Hardee J.P., Counts B.R., Carson J.A. Understanding the role of exercise in cancer cachexia therapy. Am J Lifestyle Med. 2019;13(1):46–60. doi: 10.1177/1559827617725283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jetté M., Sidney K., Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 86.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schakman O., Kalista S., Barbé C. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45(10):2163–2172. doi: 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 88.Fabi A., Bhargava R., Fatigoni S. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 89.Yavuzsen T., Davis M.P., Walsh D. Systematic review of the treatment of cancer-associated anorexia and weight loss. J Clin Oncol. 2005;23(33):8500–8511. doi: 10.1200/JCO.2005.01.8010. [DOI] [PubMed] [Google Scholar]
- 90.Moertel C.G., Schutt A.J., Reitemeier R.J. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer. 1974;33(6):1607–1609. doi: 10.1002/1097-0142(197406)33:6<1607::aid-cncr2820330620>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 91.Ruiz Garcia V., López-Briz E., Carbonell Sanchis R. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;2013(3):Cd004310. doi: 10.1002/14651858.CD004310.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dev R., Del Fabbro E., Bruera E. Association between megestrol acetate treatment and symptomatic adrenal insufficiency with hypogonadism in male patients with cancer. Cancer. 2007;110(6):1173–1177. doi: 10.1002/cncr.22924. [DOI] [PubMed] [Google Scholar]
- 93.Ruiz-García V., López-Briz E., Carbonell-Sanchis R. Megestrol acetate for cachexia-anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444–452. doi: 10.1002/jcsm.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jatoi A., Windschitl H.E., Loprinzi C.L. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20(2):567–573. doi: 10.1200/JCO.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- 95.Strasser F., Luftner D., Possinger K. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24(21):3394–3400. doi: 10.1200/JCO.2005.05.1847. [DOI] [PubMed] [Google Scholar]
- 96.Turcott J.G., Del Rocío Guillen Núñez M., Flores-Estrada D. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. Support Care Cancer. 2018;26(9):3029–3038. doi: 10.1007/s00520-018-4154-9. [DOI] [PubMed] [Google Scholar]
- 97.Chlebowski R.T., Herrold J., Ali I. Influence of nandrolone decanoate on weight loss in advanced non-small cell lung cancer. Cancer. 1986;58(1):183–186. doi: 10.1002/1097-0142(19860701)58:1<183::aid-cncr2820580131>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 98.Loprinzi C.L., Kugler J.W., Sloan J.A. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17(10):3299–3306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 99.Navari R.M., Brenner M.C. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer. 2010;18(8):951–956. doi: 10.1007/s00520-009-0739-7. [DOI] [PubMed] [Google Scholar]
- 100.Parsons B., Allison D.B., Loebel A. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110(1-3):103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 101.Naing A., Dalal S., Abdelrahim M. Olanzapine for cachexia in patients with advanced cancer: an exploratory study of effects on weight and metabolic cytokines. Support Care Cancer. 2015;23(9):2649–2654. doi: 10.1007/s00520-015-2625-9. [DOI] [PubMed] [Google Scholar]
- 102.Navari R.M., Pywell C.M., Le-Rademacher J.G. Olanzapine for the treatment of advanced cancer-related chronic nausea and/or vomiting: a randomized pilot trial. JAMA Oncol. 2020;6(6):895–899. doi: 10.1001/jamaoncol.2020.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solheim T.S., Fearon K.C., Blum D. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol. 2013;52(1):6–17. doi: 10.3109/0284186X.2012.724536. [DOI] [PubMed] [Google Scholar]
- 104.Del Fabbro E., Hui D., Dalal S. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med. 2011;14(9):1004–1008. doi: 10.1089/jpm.2011.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Camilleri M., Parkman H.P., Shafi M.A. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37. doi: 10.1038/ajg.2012.373. quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruera E., Belzile M., Neumann C. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19(6):427–435. doi: 10.1016/s0885-3924(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 107.European Medicine Agency Metoclopramide-containing medicines. https://www.ema.europa.eu/en/medicines/human/referrals/metoclopramide-containing-medicines Available at: Accessed March 31, 2021.
- 108.Mantovani G., Macciò A., Madeddu C. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15(2):200–211. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mantovani G., Madeddu C. Cancer cachexia: medical management. Support Care Cancer. 2010;18(1):1–9. doi: 10.1007/s00520-009-0722-3. [DOI] [PubMed] [Google Scholar]
- 110.Oberholzer R., Hopkinson J.B., Baumann K. Psychosocial effects of cancer cachexia: a systematic literature search and qualitative analysis. J Pain Symptom Manage. 2013;46(1):77–95. doi: 10.1016/j.jpainsymman.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 111.Strasser F., Binswanger J., Cerny T. Fighting a losing battle: eating-related distress of men with advanced cancer and their female partners. A mixed-methods study. Palliat Med. 2007;21(2):129–137. doi: 10.1177/0269216307076346. [DOI] [PubMed] [Google Scholar]
- 112.Orrevall Y., Tishelman C., Herrington M.K. The path from oral nutrition to home parenteral nutrition: a qualitative interview study of the experiences of advanced cancer patients and their families. Clin Nutr. 2004;23(6):1280–1287. doi: 10.1016/j.clnu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 113.Reid J., McKenna H., Fitzsimons D. The experience of cancer cachexia: a qualitative study of advanced cancer patients and their family members. Int J Nurs Stud. 2009;46(5):606–616. doi: 10.1016/j.ijnurstu.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 114.Reid J. Psychosocial, educational and communicative interventions for patients with cachexia and their family carers. Curr Opin Support Palliat Care. 2014;8(4):334–338. doi: 10.1097/SPC.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Millar C., Reid J., Porter S. Healthcare professionals' response to cachexia in advanced cancer: a qualitative study. Oncol Nurs Forum. 2013;40(6):E393–E402. doi: 10.1188/13.ONF.E393-E402. [DOI] [PubMed] [Google Scholar]
- 116.Reid J., McKenna H.P., Fitzsimons D. An exploration of the experience of cancer cachexia: what patients and their families want from healthcare professionals. Eur J Cancer Care (Engl) 2010;19(5):682–689. doi: 10.1111/j.1365-2354.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 117.Amano K., Maeda I., Morita T. Eating-related distress and need for nutritional support of families of advanced cancer patients: a nationwide survey of bereaved family members. J Cachexia Sarcopenia Muscle. 2016;7(5):527–534. doi: 10.1002/jcsm.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hopkinson J.B., Fenlon D.R., Okamoto I. The deliverability, acceptability, and perceived effect of the Macmillan approach to weight loss and eating difficulties: a phase II, cluster-randomized, exploratory trial of a psychosocial intervention for weight- and eating-related distress in people with advanced cancer. J Pain Symptom Manage. 2010;40(5):684–695. doi: 10.1016/j.jpainsymman.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 119.Hopkinson J.B., Fenlon D.R., Foster C.L. Outcomes of a nurse-delivered psychosocial intervention for weight- and eating-related distress in family carers of patients with advanced cancer. Int J Palliat Nurs. 2013;19(3):116. doi: 10.12968/ijpn.2013.19.3.116. 118-23. [DOI] [PubMed] [Google Scholar]
- 120.Hopkinson J.B., Richardson A. A mixed-methods qualitative research study to develop a complex intervention for weight loss and anorexia in advanced cancer: the family approach to weight and eating. Palliat Med. 2015;29(2):164–176. doi: 10.1177/0269216314556924. [DOI] [PubMed] [Google Scholar]
- 121.Fearon K.C. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44(8):1124–1132. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 122.Reckman G.A.R., Gomes-Neto A.W., Vonk R.J. Anabolic competence: assessment and integration of the multimodality interventional approach in disease-related malnutrition. Nutrition. 2019;65:179–184. doi: 10.1016/j.nut.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 123.Maddocks M., Hopkinson J., Conibear J. Practical multimodal care for cancer cachexia. Curr Opin Support Palliat Care. 2016;10(4):298–305. doi: 10.1097/SPC.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Atherton P.J., Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol. 2012;590(5):1049–1057. doi: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uster A., Ruehlin M., Mey S. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. 2018;37(4):1202–1209. doi: 10.1016/j.clnu.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 126.Lu Z., Fang Y., Liu C. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39(7):748–756. doi: 10.1200/JCO.20.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dev R., Hui D., Chisholm G. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J Cachexia Sarcopenia Muscle. 2015;6(1):95–98. doi: 10.1002/jcsm.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fallon M., Walker J., Colvin L. Pain management in cancer center inpatients: a cluster randomized trial to evaluate a systematic integrated approach – the Edinburgh Pain Assessment and management tool. J Clin Oncol. 2018;36(13):1284–1290. doi: 10.1200/JCO.2017.76.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haun M.W., Estel S., Rücker G. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6(6):Cd011129. doi: 10.1002/14651858.CD011129.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hui D., Finlay E., Buss M.K. Palliative oncologists: specialists in the science and art of patient care. J Clin Oncol. 2015;33(20):2314–2317. doi: 10.1200/JCO.2014.60.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dykewicz C.A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. (Adapted from: Gross PA, Barrett TL, Dellinger EP et al. Purpose of quality standards for infectious diseases. Clin Infect Dis. 1994;18:421) [DOI] [PubMed] [Google Scholar]
- 132.Malmstrom T.K., Miller D.K., Simonsick E.M. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mourtzakis M., Prado C.M., Lieffers J.R. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 134.Edemekong P., Bomgaars D., Sukumaran S., Levy S.B. StatPearls [Internet] Treasure Island, FL; StatPearls Publishing: 2020. Activities of daily living.https://www.ncbi.nlm.nih.gov/books/NBK470404/ Available at: Updated June 26, 2020. Accessed January 22, 2021. [PubMed] [Google Scholar]
- 135.Turner-Stokes L., Tonge P., Nyein K. The Northwick Park Dependency Score (NPDS): a measure of nursing dependency in rehabilitation. Clin Rehabil. 1998;12(4):304–318. doi: 10.1191/026921598669173600. [DOI] [PubMed] [Google Scholar]
- 136.Ministry of Health Province of British Columbia. Frailty in older adults – early identification and management. BCGuidelines.ca. 2017. https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/frailty-gaitspeed.pdf Available at. Accessed January 27, 2021.
- 137.Ottery F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(Suppl):S15–S19. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 138.Hui D., Bruera E. The Edmonton symptom assessment system 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017;53(3):630–643. doi: 10.1016/j.jpainsymman.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nicholas D., Veach T. The psychosocial assessment of the adult cancer patient. Prof Psychol Res Pr. 2000;31:206–215. [Google Scholar]
- 140.FACIT Group Functional assessment of anorexia/cachexia treatment (version 4) 2007. https://www.facit.org/measure-english-downloads/faact-english-downloads Available at. Accessed January 27, 2021.
- 141.Wheelwright S.J., Hopkinson J.B., Darlington A.S. Development of the EORTC QLQ-CAX24, a questionnaire for cancer patients with cachexia. J Pain Symptom Manage. 2017;53(2):232–242. doi: 10.1016/j.jpainsymman.2016.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.