Abstract
Background: Giant axonal neuropathy (GAN) is a very rare fatal neurodegenerative disorder with clinical and allelic heterogeneity. The disease is caused by mutations in the GAN (gigaxonin) gene. Herein, we reported the clinical presentations and results of genetic analysis of the first Iranian GAN case.
Methods: Phenotypic data were obtained by neurologic examination, brain magnetic resonance imaging (MRI), electromyography (EMG), electroencephalography (EEG), and sonography from the proband. Deoxyribonucleic acid (DNA) was isolated from peripheral blood leucocytes and whole exome sequencing (WES) was performed. The candidate variant was screened by Sanger sequencing in the proband and her family members.
Results: The proband was a 7-year-old girl who was admitted with a chief complaint of ataxia, muscle weakness, delayed developmental milestones, and history of psychiatric disorders. She was very moody and had clumsy gait, decreased deep tendon reflexes (DTRs) of lower limbs, and kinky hair. The brain MRI revealed white matter abnormality. The EMG revealed that her disease was compatible with the chronic axonal type of sensorimotor polyneuropathy; however, her EEG was normal. Results of the WES revealed a homozygous variant; c.G778T:p.E260* in the GAN gene, indicating the GAN disorder.
Conclusion: The present study affirmed GAN allelic heterogeneity and resulted in the expansion of the phenotypic spectrum of GAN pathogenic variants. Identification of more families with mutations in GAN gene helps to further understand the molecular basis of the disease and provides an opportunity for genetic counseling especially in the populations with a high degree of consanguineous marriage such as the Iranian population.
Key Words: GAN Protein, Giant Axonal Neuropathy, Menkes Kinky Hair Syndrome, Whole Exome Sequencing
Introduction
Giant axonal neuropathy [GAN, Online Mendelian Inheritance in Man (OMIM): 256850] is a rare fatal neurodegenerative disorder caused by mutations in the GAN gene. The gene encodes 67-kD protein, which is highly expressed in the nervous system.1 Recent evidence has shown that GAN mutations are usually associated with the accumulation of pre-neural filaments, giant axon formation, and consequently, the inhibition of neural cell signaling.2
GAN is a progressive disorder in which the patients will be wheelchair-bound by the end of the second decade of life and death will occur in the third decade because of respiratory failure. Curly kinky hair, cerebellar signs, and early-onset predominantly sensory neuropathy are the most common phenotypic features in the patients with classical GAN disorder.
Here, we describe details of the clinical features of the first Iranian GAN case, explain the results of genetic analysis, and overview the literature.
Materials and Methods
The research was performed in accordance with the Declaration of Helsinki and with approval of the Ethics Board of University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. All participants were informed of the nature of research and the consent form was signed.
The proband was affected with a type of neuropathy and referred to us for molecular analysis. Her medical history revealed that she was the first child of the family with consanguineous marriage. The mode of inheritance was consistent with autosomal recessive pattern, as the proband was born to unaffected parents.
Genetic analysis : Deoxyribonucleic acid (DNA) was extracted from peripheral blood of the proband according to the salting-out method. Whole exome sequencing (WES) by Illumina HiSeq 2500 system was performed. Sequence alignment and variant calling were performed against human reference genome UCSC NCBI37/hg19. Early filtering was done to identify all splice sites, exonic, and exonic splice variations. Synonymous variants were put aside. Finally, all homozygous single-nucleotide polymorphisms (SNPs) with a reported minor allele frequency (MAF) less than 0.01 in the public genome databases were considered. The candidate disease-causing variants were confirmed and co-segregated with the disease status by the Sanger method (BigDye kit and the Prism 3130 sequencer; Applied Biosystems, Foster City, CA, USA).
Results
A 7-year-old girl (proband) was admitted to the neurology ward of Hazrat-e Rasoul Hospital (associated with Iran University of Medical Sciences, Tehran) with complaints of gait difficulties and history of psychiatric disorders presented with hallucination and night terrors along with crying and screaming. She had also a chief complaint of reflux accompanied by severe vomiting on admission time. She was born from a cesarean delivery and her birth weight was 3 kg. She had also a history of delayed developmental milestones and started walking by 24 months old. Her parents had a history of infertility problems for five years and she thereby was born after a period of infertility treatment.
On neurological examinations, she presented ataxia and lower limb weakness. She was very moody or bad-tempered. Deep tendon reflexes (DTRs) of lower limbs were decreased.
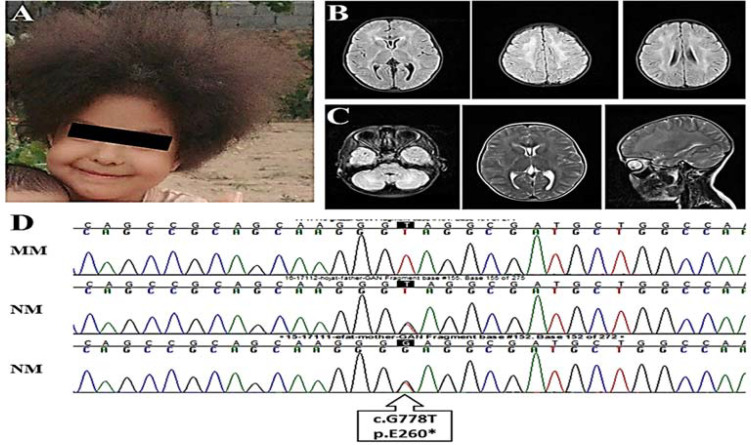
Her head circumference was 49 cm. Results of the cerebellar test, cranial nerve exam, and eye movements were normal. She had also clumsy gait. Phenotypically, she had curly and kinky hair (Figure 1, A). The cranial magnetic resonance imaging (MRI) revealed cavum vergae (CV) (Figure 1, B). The white matter demonstrated high signal intensity at the cerebellar and deep white matter regions (Figure 1, C). The routine laboratory tests were normal.
Figure 1.
(A) Curly kinky/frizzy hair in the proband affected by giant axonal neuropathy (GAN); (B) axial fluid-attenuated inversion recovery (FLAIR) sequences of magnetic resonance imaging (MRI) revealing posterior limb of internal capsul and deep white matter hyperintensity; (C) axial FLAIR sequence of MRI revealing cerebellar white matter hyperintensity (left), axial T2 sequence of MRI revealing posterior limb of internal capsul and deep white matter hyperintensity (middle), sagittal T2 sequence of MRI revealing deep and cerebellar white matter hyperintensity (right); (D) sequence chromatograms showing genotypes pertaining to c.G778T in GAN, causing p.E260* in members of GAN pedigree
N: Normal allele; M: Mutant allele
Due to urinary disorders such as hesitancy, a renal and bladder sonography has been performed which revealed the possibility of the neurogenic bladder. To rule out the possibility of GAN and neuroaxonal dystrophy, electromyography (EMG) and nerve conduction velocity (NCV) were performed which revealed that the disease was compatible with chronic axonal type of sensorimotor polyneuropathy. Electroencephalography (EEG) was also performed which showed normal results.
The result of WES revealed a previously-reported homozygous variant; c.G778T:p.E260* in exon 4 of the GAN gene. This variant was considered as a pathogenic variant by the American College of Medical Genetics and Genomics (ACMG) and was not found in any of the genome databases. The candidate variant, c.G778T, in the GAN gene was confirmed in the proband and co-segregated with the disease status in the family members (Figure 1, D).
Discussion
In the present study, we described an Iranian GAN case that manifested ataxia, delayed milestones, and a history of psychiatric disorders. GAN initially involves the peripheral nervous system (PNS) that is accompanied with walking disability, loss of sensation, and areflexia. It later affects the central nervous system (CNS) and causes mental dysfunction, movement disability, ataxia, and seizures and pyramidal and cerebellar signs are also reported.3 Pale and curly kinky hair is frequently observed in GAN cases (not in all cases) (Table 1).4,5 For example, Incecik et al. reported kinky hair in all eight Turkish GAN cases.4 Similarly, our case had curly kinky/frizzy hair (Figure 1, A).
The brain MRI of patients with GAN often reveals multiple abnormalities in white matter, parieto-occipital, frontal, and parietal white matter involvement, and diffuse white matter lesions in anterior and posterior periventricular regions. Corpus callosum thinning and posteromedial thalamus involvement are also reported.6 The brain MRI of our case revealed CV and high signal intensity at the cerebellar and periventricular regions of the white matter (Figure 1, B).
The absence or decrease of sensory action potentials, severe polyneuropathy, axonal neuropathy, chronic reinnervation, and decrease in motor NCV were also reported in several cases.5,7,8 We found normal EEG for our patient; however, the EMG revealed compatible with chronic axonal type of sensorimotor polyneuropathy. GAN disorder is caused by mutations in the GAN gene, and until now, more than 80 mutations have been reported in the GAN gene [The Human Gene Mutation Database (HGMD), http://www.hgmd.cf.ac.uk/ac/all.php], which affect the cytoskeletal protein gigaxonin (Table 1). P.E260* variant is a nonsense mutation that would result in the creation of a premature stop codon and production of a truncated protein.
Table 1.
All reported variations in the giant axonal neuropathy (GAN) gene and clinical presentations of the patients with these variations (Part I)
|
Variation in
|
Variation in
|
Exon | Population | Consanguinity |
AAO
(year) |
Present
age (year) |
Sex |
Kinky
hair |
Motor capacity |
Motor
milestone |
|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid | cDNA | |||||||||
| p.V7Sfs*3 | c.18_19insA | 1 | ||||||||
| p.P10Lfs*50 rearrangement |
c.27delC NA |
1NA | China | - | 3 | 10 | + | |||
| p.S8_L20del p.Y299C |
c.20_58del c.896A>G |
15 | Italy | 5 | 22 | F | - | Difficulties in climbing and stair running |
Normal | |
| p.R15S | c.43C>A | 1 | Italy | 6 | 11 | M | + | Difficulties in climbing |
Normal | |
| 6 | 16 | F | + | stairs and running | Normal | |||||
| p.H33P p.P53L |
c.98A>C c.158C>T |
1 | China | - | 6 | 18 | M | - | Walking without aid | Normal |
| p.V35F | c.103G>T | 1 | Israel | + | 4 | M | + | Crouched gait | Delayed | |
| + | 6 | 15 | M | - | Ambulatory | Delayed | ||||
| + | 6 | 15 | M | - | Ambulatory | Delayed | ||||
| + | 1st decade | 33 | M | - | Wheelchair-bound | |||||
| + | 1st decade | 39 | M | - | Wheelchair-bound | |||||
| p.Q44* p.G474R |
c.130C>T c.1429C>T |
19 | - | 1.5 | 12 | M | - | Walker | Normal | |
| p.A49T p.P315L |
c.145G>A c.944C>T |
15 | Italy | - | 2 | 18 | M | - | Wheelchair-bound | Delayed |
| p.A49E | c.146C>A | 1 | ||||||||
| p.A51P | c.151G>C | 1 | Pakistan | + | 4 | + | ||||
| p.S52G p.C393* |
c.154A>G c.1179T>A |
17 | ||||||||
| p.S52N | c.155G>A | 1 | Algeria | + | 3 | 12 | F | + | Normal | |
| p.R56fs*59 | c.168-1G>A | Intron 1 | Algeria | + | 3 | 9 | M | + | Walking with aid | NA |
| p.Y71* | c.213T>A | 2 | Scotland | - | 1.5 | F | + | |||
| p.L75H p.R545H |
c.224T>A c.1634G>A |
211 | - | 3 | F | + | Abnormal waddling gait |
|||
| p.S79L | c.236C>T | 2 | ||||||||
| p.V82F | c.244G>T | 2 | ||||||||
| p.I86F p.L114_T119del |
c.256A>T c.340–357del18 |
23 | Germany | - | 3 | M | + | |||
| p.Y89C p.G368R |
c.266A>G c.1102G>A |
27 | Serbia | - | 2 | 6 | M | + | Walking without aid | Normal |
| p.Y89C p.G368R |
c.266A>G c.1102G>A |
27 | Serbia | - | 2 | 6 | M | + | Walking without aid | Normal |
| p.G93R p.E260* |
c.277G>A c.778G>T |
24 | ||||||||
| NA | c.282+3A>C | Intron 2 | ||||||||
| p.I102T | c.305T>C | 3 | 7 | 59 | M | |||||
| p.F124C | c.371T>G | 3 | China | + | 6 | 19 | F | - | Walking with aid | Normal |
| p.R138H | c.413G>A | 3 | ||||||||
| p.R162* | c.484C>T | 3 | Japan | + | 3 | 24 | M | + | Wheelchair-bound | Delayed |
| p.E169L | c.505G>A | 3 | + | 3 | 13 | M | - | |||
| p.E180Rfs*6 | c.534_539insA | 3 | + | 5 | 12 | F | + | Walking with support | ||
| p.I182N | c.545T>A | 3 | ||||||||
| p.V195F NA |
c.583G>T c.633+1G>T |
3Intron 3 | Germany | - | 2 | 6.5 | F | + | Walking without aid | Delayed |
| p.R201* p.I423T |
c.601C>T c.1238T>C |
38 | Germany | - | 13 | M | + | Wheelchair-bound | Delayed | |
| p.R242* | c.724C>T | 4 | Turkey | + | 4 | M | + | |||
| + | 2 | F | + | |||||||
| p.I244Mfs*33p.R269W | c.731_732delT c.805C>T |
4 | 2 | 2 | F | + | Ambulatory | |||
| 4 | 7 | F | + | Walker | ||||||
| p.R269W p.R545H |
c.805C>T c.1634G>A |
411 | China | - | 1.5 | 11 | F | - | Clumsy gait | Delayed |
| p.R269Q | c.806G>A | 4 | Germany | -/the same | 4 | M | + | |||
| village | 4 | M | + | |||||||
| p.G270S p.A576E |
c.808G>A c.1727C>A |
411 | Japan | - | 12 | M | - | Walking with crutch | Normal till 15 months |
|
| p.R293* p.C570Y |
c.877C>T c.1709G>A |
511 | ||||||||
| p.L309R | c.926T>G | 5 | ||||||||
| p.A324V p.C464Y |
c.971C>T c.1391G>A |
59 | - | 3 | 22 | M | - | Wheelchair-bound | ||
| p.E325K | c.973G>A | 5 | Israel | + | 4 | 13 | F | + | Wheelchair-bound | Delayed |
| + | 3 | 9 | F | + | Wheelchair-bound | Delayed | ||||
| + | NA | 6 | F | + | Wheelchair-bound | Delayed | ||||
| + | 2 | 11 | F | + | Wheelchair-bound | Delayed | ||||
| p.G332R | C.994G>A | 6 | North Africa |
+ | 4 | 20 | F | + | Loss of ambulation | |
| p.K338* NA |
c.1012A>T NA |
66 to 8 | France | - | 2 | F | + | |||
| p.E362= | c.1086G>A | 6 | France | + | 3 | 7 | F | + | Normal | |
| + | 2 | 4 | F | + | Normal | |||||
| p.G368R | c.1102G>A | 7 | Sri Lanka | - | 4 | M | + | |||
| p.E392K p.R545H |
c.1174G>A c.1634G>A |
711 | China | - | 13 | F | + | Wide-based gait with foot drop |
Normal before the age of 3 |
|
| p.W401* p.E486K |
c.1203G>A c.1456G>A |
79 | France and Tunisia |
|||||||
| p.I413Vfs*22 | c.1237-1G>A | Intron 7 | - | 4 | 18 | + | Bed-bound | |||
| p.V438I | c.1312G>A | 8 | 4 | 5 | M | |||||
| p.W448L | c.1342G>T | 8 | China | + | 4 | 25## | F | - | Wheelchair-bound | Delayed |
| p.R458W | c.1372A>T | 8 | Algeria | + | 5 | 4 | M | + | Normal | |
| + | 3 | 12&& | M | + | Normal | |||||
| + | 4 | 4 | F | + | Normal | |||||
| p.A461V p.R545L |
c.1382C>T c.1634G>T |
911 | Mexico | - | 2 | 8 | F | + | Walker | |
| p.G474R p.R477* |
c.1429C>T c.1420G>C |
9 | Spain | - | Early infancy |
32^ | M | - | Wheelchair-bound | Normal |
| - | 3 | 25$ | F | - | Wheelchair-bound | Normal | ||||
| p.R477* | c.1429C>T | 9 | Algeria | + | NA | F | + | |||
| + | 5 | F | + | |||||||
| + | 3 | F | + | |||||||
| + | 3 | M | + | |||||||
| + | 3 | M | + | |||||||
| p.Q483* | c.1447C>T | 9 | ||||||||
| p.E486K 57–131 kb microdeletion |
c.1456G>A NA |
9 (2/3) to 11 |
- | M | + | Delayed | ||||
| p.E493L | c.1477G>A | 9 | France | + | 3 | 5 | F | + | Normal | |
| + | 3 | 15 | F | + | Normal | + | ||||
| + | 1 | 1 | F | + | Normal | + | ||||
| p.E493L p.A576E |
c.1477G>Ac.1727C>A | 911 | - | Early childhood |
12 | F | + | Sitting with support | ||
| NA | c.1502+1G>T | Intron 9 | Turkey | + | 3.5 | 12 | M | + | Normal | |
| p.W502*& | c.1505G>A | 10 | Pakistan | + | 5 | + | ||||
| p.F518* p.P315L |
c.1553_1554delTT c.944C>T |
105 | England | - | 3 | M | + | |||
| p.R545C | c.1633C>T | 11 | ||||||||
| p.T553_P597del | c.1657ALUYa5ins | 11 | India | 10# | + | Delayed | ||||
| 6@ | + | Normal | ||||||||
| NA p.L510* |
c.1088+1G>C c.1677T>G |
611 | Italy | 2 | 12 | + | Delayed | |||
| p.P562A | c.1684C>G | 11 | 6 | 16 | F | + | Normal | |||
| 6 | 11 | M | + | Normal | ||||||
| NA | NA | 2 to 9 | Algeria | + | + | Normal | ||||
| NA | c.1647_8680del2 58 |
10 and 11 |
AAO: Age at onset; F: Female; M: Male; NA: Not available; ID: Intellectual disability; EMG: Electromyography; MRI: Magnetic resonance imaging; cDNA: Complementary deoxyribonucleic acid
This table is based on the first report of the mutations.
Other symptoms were dementia and seizures,
Other symptoms were respiratory insufficiency and optic atrophy,
Other symptoms included spasticity, dysarthria, difficulties with breathing and swallowing,
Other symptoms were difficulties with swallowing, spasticity, and dysarthria,
Other symptoms were dysphagia, visual problems, and optic atrophy,
Other symptoms included dullness, apathy, urinary incontinence, thick curved eyelashes and eyebrows, depressed nasal bridge, dental malocclusion, dry skin, fasciculations of the tongue, and generelized hypotonia,
Other symptoms included thick curved eyelashes and eyebrows and dry skin
Table 1.
All reported variations in the giant axonal neuropathy (GAN) gene and clinical presentations of the patients with these variations (Part II)
|
Muscle
|
Foot
deformity |
Areflexia | Ataxia | Nystagmus |
Facial
weakness |
ID |
Kypho
scoliosis |
Scoliosis |
Sensory
impairment |
EMG
|
MRI
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| atrophy | weakness | Abnormality | ||||||||||
| Flat feet | + | + | ||||||||||
| + | Pes cavus | - | + | |||||||||
| + | Pes equinovarus |
+ | ||||||||||
| + | Pes equinovarus |
+ | + | |||||||||
| + | + | Pes cavus | + | - | + | + | + | + | ||||
| + | Pes planus | - | - | - | - | - | - | - | ||||
| + | Pes planus | - | - | - | - | - | - | |||||
| + | Pes planus | - | - | - | - | - | - | |||||
| + | + | - | - | - | - | + | - | NA | ||||
| + | + | - | - | - | - | - | NA | |||||
| + | Pes planus | + | + | - | Kyphosis | - | - | |||||
| + | Pes planovalgus |
+ | - | + | - | |||||||
| NA | + | |||||||||||
| + | + | + | + | + | ||||||||
| - | + | Pes valgus | + | + | + | NA | NA | NA | NA | + | ||
| + | + | + | - | + | ||||||||
| + | NA | - | + | + | - | |||||||
| - | + | Pes planovalgus, genu valgum |
+ | - | - | + | + | + | - | |||
| + | + | |||||||||||
| + | Pes cavus | + | - | + | + | + | - | |||||
| + | Ankle sprains | + | + | + | + | + | + | |||||
| + | + | + | + | - | + | + | + | |||||
| + | - | - | - | + | ||||||||
| - | - | Fixed supination deformity |
+ | - | - | - | - | NA | NA | |||
| - | + | + | + | + | + | + | + | + | ||||
| + | + | - | + | + | + | |||||||
| + | + | - | + | - | ||||||||
| + | + | Pes cavus | + | - | + | + | + | |||||
| + | + | + | + | + | - | |||||||
| + | + | + | + | + | - | |||||||
| + | Pes cavus, pes equinovarus |
+ | - | + | + | - | ||||||
| + | + | Varus | + | + | + | - | ||||||
| + | + | Pes equinovalgu, pes planus |
+ | + | - | - | - | + | + | + | + | |
| + | + | Pes equinovalgu, pes planus |
+ | + | - | + | + | + | + | |||
| - | + | - | + | + | - | - | + | + | + | |||
| + | + | Pes equinovalgu, pes planus |
+ | + | + | - | - | + | + | + | + | + |
| + | + | + | + | + | + | |||||||
| - | - | + | NA | + | + | |||||||
| + | + | + | + | + | ||||||||
| + | + | + | + | + | ||||||||
| + | + | NA | + | - | ||||||||
| + | Pes cavus | + | + | + | + | + | + | |||||
| + | + | + | + | + | + | + | + | |||||
| + | + | + | + | |||||||||
| + | + | Pes cavus | + | + | + | + | + | + | + | |||
| - | + | + | + | |||||||||
| + | + | + | + | |||||||||
| - | + | + | + | + | ||||||||
| + | + | Pes equinovarus |
+ | + | + | + | Hyperlordosis | + | ||||
| + | + | Club feet | + | + | - | - | - | + | + | + | ||
| - | + | + | + | + | ||||||||
| + | + | Pes equinovarus |
+ | + | + | + | Hyperlordosis | + | ||||
| + | + | Club feet | + | + | - | - | - | + | + | + | ||
| + | + | Pes cavus | + | + | - | - | + | - | + | + | ||
| + | + | NA | + | + | NA | |||||||
| + | + | + | + | + | + | |||||||
| + | + | NA | NA | + | NA | |||||||
| + | + | NA | + | NA | ||||||||
| - | + | + | - | + | NA | |||||||
| + | + | + | + | + | ||||||||
| + | + | + | + | + | ||||||||
| + | + | + | + | + | ||||||||
| + | - | + | + | + | + | |||||||
| + | + | + | + | + | ||||||||
| + | + | + | +, Ptosis | + | + | + | + | + | ||||
| + | + | + | + | - | - | - | + | - | ||||
| NA | ||||||||||||
| + | + | + | +, Ptosis | + | + | + | + | |||||
| + | Pes planovalgus |
+ | + | + | + | |||||||
| + | + | + | - | + | + | |||||||
| + | Pes cavus | + | + | + | ||||||||
| + | Pes equinovarus |
+ | ||||||||||
| + | Pes equinovarus |
+ | ||||||||||
| + | + | + | + | |||||||||
AAO: Age at onset; F: Female; M: Male; NA: Not available; ID: Intellectual disability; EMG: Electromyography; MRI: Magnetic resonance imaging; cDNA: Complementary deoxyribonucleic acid
##Other symptoms were dementia and seizures, &&Other symptoms were respiratory insufficiency and optic atrophy, ^Other symptoms included spasticity, dysarthria, difficulties with breathing and swallowing, $Other symptoms were difficulties with swallowing, spasticity, and dysarthria, &Other symptoms were dysphagia, visual problems, and optic atrophy, #Other symptoms included dullness, apathy, urinary incontinence, thick curved eyelashes and eyebrows, depressed nasal bridge, dental malocclusion, dry skin, fasciculations of the tongue, and generelized hypotonia, @Other symptoms included thick curved eyelashes and eyebrows and dry skin
This table is based on the first report of the mutations.
Absence or abnormality of the protein may result in the presence of giant axons with large axonal swelling, disorganization of neurofilaments (NFs), reduction of myelin thickness, and subsequently, degeneration and dysfunction of the PNS and CNS.9 This variant has been just reported in a Chinese family in a compound heterozygous state, but its phenotypic features were not available for comparison with our case.10
Conclusion
GAN is a very rare disease with high clinical heterogeneity likely due to the allelic heterogeneity. Also, other genetic or environmental associated risk factors may affect the disease variability. Identification of novel cases and expansion of the mutation spectrum of GAN is valuable to find a genotype/phenotype correlation and provide an opportunity for genetic counseling and prevention strategies for individuals and families at risk.
Acknowledgments
We acknowledge the University of Social Welfare and Rehabilitation Sciences for funding the research (grant number: 1969) and thank the patient and her family members for participating in the study.
Notes:
How to cite this article: Vafaee-Shahi M, Ghasemi S, Ghahvechi-Akbar M, Tahernia L, Davarzani A, Hajati R, et al. Giant axonal neuropathy: The first Iranian case with a variation in the gigaxonin gene and a glance to the other cases. Curr J Neurol 2020; 19(4): 200-10.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Cleveland DW, Yamanaka K, Bomont P. Gigaxonin controls vimentin organization through a tubulin chaperone-independent pathway. Hum Mol Genet. 2009;18(8):1384–94. doi: 10.1093/hmg/ddp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang JJ, Liu IY, Wang MB, Srivatsan ES. A review of gigaxonin mutations in giant axonal neuropathy (GAN) and cancer. Hum Genet. 2016;135(7):675–84. doi: 10.1007/s00439-016-1659-5. [DOI] [PubMed] [Google Scholar]
- 3.Kamate M, Ramakrishna S, Kambali S, Mahadevan A. Giant axonal neuropathy: A rare inherited neuropathy with simple clinical clues. BMJ Case Rep. 2014;2014:bcr2014204481. doi: 10.1136/bcr-2014-204481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Incecik F, Herguner OM, Ceylaner S, Zorludemir S, Altunbasak S. Giant axonal disease: Report of eight cases. Brain Dev. 2015;37(8):803–7. doi: 10.1016/j.braindev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Nalini A, Gayathri N, Yasha TC, Ravishankar S, Urtizberea A, Huehne K, et al. Clinical, pathological and molecular findings in two siblings with giant axonal neuropathy (GAN): report from India. Eur J Med Genet. 2008;51(5):426–35. doi: 10.1016/j.ejmg.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Ravishankar S, Goel G, Rautenstrauss CP, Nalini A. Spectrum of magnetic resonance imaging findings in a family with giant axonal neuropathy confirmed by genetic studies. Neurol India. 2009;57(2):181–4. doi: 10.4103/0028-3886.51290. [DOI] [PubMed] [Google Scholar]
- 7.Demir E, Bomont P, Erdem S, Cavalier L, Demirci M, Kose G, et al. Giant axonal neuropathy: clinical and genetic study in six cases. J Neurol Neurosurg Psychiatry. 2005;76(6):825–32. doi: 10.1136/jnnp.2003.035162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tandan R, Little BW, Emery ES, Good PS, Pendlebury WW, Bradley WG. Childhood giant axonal neuropathy. Case report and review of the literature. J Neurol Sci. 1987;82(1-3):205–28. doi: 10.1016/0022-510x(87)90019-0. [DOI] [PubMed] [Google Scholar]
- 9.Johnson-Kerner BL, Roth L, Greene JP, Wichterle H, Sproule DM. Giant axonal neuropathy: An updated perspective on its pathology and pathogenesis. Muscle Nerve. 2014;50(4):467–76. doi: 10.1002/mus.24321. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Ma Q, Cai Q, Liu Y, Wang W, Ren Z. Two novel pathogenic mutations of GAN gene identified in a patient with giant axonal neuropathy. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016;33(3):292–5. doi: 10.3760/cma.j.issn.1003-9406.2016.03.003. [In Chinese] [DOI] [PubMed] [Google Scholar]