Abstract
Rationale
Effector memory T lymphocytes (TEM cells) exacerbate hypertension in response to repeated hypertensive stimuli. These cells reside in the bone marrow for prolonged periods and can be reactivated upon re-exposure to the hypertensive stimulus.
Objective
Because hypertension is associated with increased sympathetic outflow to the bone marrow, we hypothesized that sympathetic nerves regulate accumulation and reactivation of bone marrow residing hypertension-specific TEM cells.
Methods and Results
Using unilateral superior cervical ganglionectomy in wild-type C57BL/6 mice, we showed that sympathetic nerves create a bone marrow environment that supports residence of hypertension-specific CD8+ T cells. These cells, defined by their proliferative response upon co-culture with dendritic cells from angiotensin II infused mice, were reduced in denervated compared to innervated bone of angiotensin II-infused mice. Adoptively transferred CD8+ T cells from angiotensin II-infused mice preferentially homed to innervated compared to denervated bone. In contrast, ovalbumin responsive T cells from OT-I mice did not exhibit this preferential homing. Increasing superior cervical ganglion activity by activating Gq-coupled DREADD (designer receptor exclusively activated by designer drug) augmented CD8+ TEM bone marrow accumulation. Adoptive transfer studies using mice lacking β2 adrenergic receptors (β2AR) indicate that β2AR in the bone marrow niche, rather than T cell β2AR is critical for TEM cell homing. Inhibition of global sympathetic outflow using Gi-coupled DREADD injected into the rostral ventrolateral medulla or treatment with a β2AR antagonist reduced hypertension specific CD8+ TEM cells in the bone marrow and reduced the hypertensive response to a subsequent response to low dose angiotensin II.
Conclusions
Sympathetic nerves contribute to the homing and survival of hypertension-specific TEM cells in the bone marrow after they are formed in hypertension. Inhibition of sympathetic nerve activity and β2AR blockade reduces these cells and prevents the blood pressure elevation and renal inflammation upon re-exposure to hypertension stimuli.
Keywords: Beta adrenergic receptor blocker, sympathetic, T cells, angiotensin II, dendritic cells, hypertension, inflammation, immunology
Subject Terms: Animal Models of Human Disease, Autonomic Nervous System, Basic Science Research, Inflammation, Hypertension
INTRODUCTION
Accumulating evidence from the past decade indicates that adaptive immunity, and especially T lymphocytes, plays a crucial role in the development of hypertension. Various hypertensive stimuli, such as angiotensin II, high salt, catecholamines and chronic psychological stress, lead accumulation of activated T cells with an effector phenotype in the kidney and vasculature.1–4 Cytokines released from these cells, including interferon-γ and interleukin-17A promote both renal and vascular dysfunction and damage, leading to enhanced sodium retention and increased systemic vascular resistance.5
The majority of activated T cells ultimately die after antigen withdrawal and resolution of an immune response, however a few remaining cells become memory T cells that can persist for years in humans. Upon antigen re-exposure, these memory cells can be rapidly reactivated. Memory T cells have been subdivided into (CD62Lhi/CD44hi) central memory (TCM) cells that predominantly reside in secondary lymphoid organs, (CD62Llo/CD44hi) effector memory (TEM) cells that remain in the circulation and patrol between peripheral tissues and resident memory cells that reside and regenerate in peripheral tissues.
The bone marrow plays a central role in the maintenance of long-term T cell memory. It provides a dedicated niche for memory CD8+ T cells to maintain a non-proliferative quiescent state and/or self-renewal in the absence of differentiation.6 After immunization or viral infection, a higher percentage of memory CD8+ T cells proliferate in the bone marrow than in the spleen or lymph nodes.7, 8 Estimates of cell numbers suggest that the bone marrow contributes a large proportion of proliferating memory CD8+ T cells compared with the other secondary lymphoid organs.
Since many hypertensive stimuli are intermittent and reoccurring, including sleep apnea, repeated episodes of dietary indiscretion or emotional stress, it is likely that memory T cells play a role in hypertension. We recently showed that TEM cells accumulate in the kidney and bone marrow following repeated hypertensive challenges, using either N(ω)-nitro-L-arginine methyl ester hydrochloride (L-NAME) followed by high salt or repeated angiotensin II stimulation. 9 In the kidney, memory T cells are predominant sources of interferon-γ and interleukin-17A.9 In the L-NAME/high-salt mouse model of hypertension, we found that bone marrow-residing TEM cells proliferate and redistribute to the kidney in response to repeated salt feeding.9 In this study, we also showed that mice that cannot form memory cells are protected against repeated hypertensive stimuli.
The sympathetic nervous system provides efferent input to the bone marrow, and modulates hematopoiesis and the stem-cell niche.10 Adrenergic nerves play a key role in the circadian recruitment of leukocytes to tissues including the bone marrow.11 In hypertension, sympathetic tone is elevated but its circadian rhythmicity is reduced.12 In the current study, we tested the hypothesis that sympathetic nerves regulate accumulation and reactivation of hypertension-specific memory T lymphocytes in the bone marrow. Our data suggest new therapeutic interventions to reduce the propensity for homing and survival of hypertension-specific T cells in the bone marrow will protect against blood pressure elevation and end-organ damage in response to repeated hypertensive stimuli.
METHODS
An extended methods section is available in the Online Data Supplement. The authors declare that all supporting data are available within the article and its online supplementary files. All methods have corresponding literature reference. Additional protocol information is available from the corresponding author upon reasonable request.
Animals studied
Wild type male C57BL/6 mice, B6 Cd45.1, Adrb2−/−, and OT-I mice on a C57Bl/6 background were originally obtained from Jackson Laboratories and were studied at 3 months of age. Hypertension was induced by subcutaneous infusion of angiotensin II (490 ng/kg/min) via mini-osmotic pumps for two weeks unless otherwise indicated. For unilateral superior cervical ganglionectomy (SCGx), mice were anesthetized by intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg). The left superior cervical ganglion (SCG) was identified underneath the left carotid bifurcation and was removed. For unilateral DREADD (designer receptor exclusively activated by designer drug) gene transduction in the SCG, an adeno-associated viral (AAV) vector (6×107particles in 50 nL) was injected into the SCG on one side with a 34-gauge needle attached to a 2.5-μL micro syringe. The expression of Gq-coupled hM3D DREADD fused with mCherry was under the control of neuron-specific human synapsin promoter. One week after SCGx or DREADD gene transduction, osmotic minipumps were implanted subcutaneously for infusion of angiotensin II or vehicle for 2 weeks. To perform T cell adoptive transfer, splenic pan T cells were obtained from donor mice by magnetic separation with a negative selection kit. Ten million cells were suspended in 200 μL PBS and adoptively transferred to naïve mice by tail vein injection. For OT-I immunization, mice were injected intraperitoneally with the ovalbumin peptide SIINFEKL (0.5 μg/μl in 200 μL alum adjuvant). For DREADD gene transduction in the rostral ventrolateral medulla (RVLM), mice were anesthetized and mounted in a stereotaxic frame as previously described.2 An AAV vector encoding Gi-coupled hM4D DREADD fused with mCherry under the control of human synapsin promoter was used, and 12×107 particles in 100 nL were injected into the RVLM bilaterally with a 30-gauge needle attached to a 2.5-μL micro syringe. Stereotaxic coordinates were −6.64 mm posterior to bregma, 1.15 mm left and right of the midline, and 5.80 mm ventral to the superior surface of the skull. At study termination, mice were euthanized by exposure to CO2. The Institutional Animal Care and Use Committee approved all experimental protocols.
Data presentation and analysis
Data are expressed as mean ± SEM. When local bone marrow sympathetic nervous activity was unilaterally manipulated by SCGx or DREADD, the effects were compared to the contralateral control limb by paired t tests as indicated. For other comparisons of two variables, unpaired t tests were employed. Data normality was confirmed using Anderson-Darling, D’Agostino-Pearson omnibus, Shapiro-Wilk, and Kolmogorov-Smirnov tests before t test was applied. To determine the effect of SCGx and β2AR deficiency, two-way ANOVA was used as indicated. For telemetry blood pressure measurements over time, two-way ANOVA with repeated-measures was employed, followed with a Bonferroni post hoc test when significance was indicated. P values (or Bonferroni-adjusted p values if applicable) are reported in the figures and a value less than 0.05 was considered statistically significant. Data were analyzed using GraphPad Prism 8 for Windows 64-bit (San Diego, CA)
RESULTS
Sympathetic innervation in bone marrow
The bone marrow is a highly innervated organ, and sympathetic nerves modulate hematopoiesis and the stem-cell niche.13 In initial experiments, expression of tyrosine hydroxylase in the bone marrow, a marker of sympathetic innervation, was analyzed by Western blot. In mice with angiotensin II-induced hypertension, tyrosine hydroxylase was increased in the bone marrow (Figure 1A). To ablate local sympathetic nerves in the bone marrow of forelimbs, we performed unilateral SCGx. The SCG innervates one side of the head and the front limb in mice. Successful removal of SCG resulted in ptosis on the surgical side of mice at conscious state (Figure 1B). Denervation of bone marrow was confirmed by a significant decrease of tyrosine hydroxylase expression in the ipsilateral forelimb by Western blot (Figure 1C). The calvaria of SCGx animals were also collected for confocal fluorescence microscopy, and the geometry of sympathetic nerve fibers in these flat bones could be visualized by tyrosine hydroxylase staining. We observed that sympathetic nerves travels along blood vessels as identified by endothelial marker CD31, and tyrosine hydroxylase staining diminished with SCGx (Figure 1D). These data indicate SCGx as an effective model for studying the effect of local sympathetic nerves in bone marrow.
Figure 1: Sympathetic innervation of the bone marrow and the effects of superior cervical ganglionectomy (SCGx).
(A) Western blot analysis of tyrosine hydroxylase (TH) expression in the forelimb bone marrow in mice with two weeks of sham or angiotensin II infusion. Data are expressed as mean ± SEM (Sham: 1.000±0.086 vs. Ang II: 2.065±0.224), n=14 in each group, p=0.0004 for the effect of angiotensin II was calculated by unpaired t test with Welch’s correction. (B) Example of ptosis resulting from unilateral SCGx. (C) Western blot analysis of TH expression in the innervated (In.) and denervated (De.) bone marrow. Protein samples were extracted from forelimb bone marrow of humerus, ulna and radius, and β-tubulin was probed as a loading control. Each set of connected symbols represent paired bone marrow samples from the same animal, n=8, p=0.0013 for effect of denervation analyzed by paired t test. (D) Expression of TH and its co-localization with the vascular marker CD31 were detected in the calvaria from mice that had received unilateral SCGx by confocal fluorescence microscopy. White bars indicate 100 micrometers. **p<0.01, ***P<0.001
Effect of unilateral SCGx on hypertension-specific T Cells in the bone marrow
Memory T cells formed in hypertension comprise only a small minority of the total T cells population in the bone marrow. Our data indicate about 1% of cells are CD8+ T cells and about 0.5% are CD4+ T cells in the bone marrow, and about one tenth of the T cells are TEM cells. To detect the response of memory T cells that accumulated in response to hypertension, we developed an assay in which we co-cultured the bone marrow cells with dendritic cells (DCs) isolated from the spleen of another mouse that received sham or angiotensin II infusion at a 1:10 ratio (Figure 2A). DCs from hypertensive mice present antigens formed in hypertension and can drive proliferation of hypertension-specific T cells.14 After seven days of culture the CD3+ T lymphocytes and specifically the CD8+ T cells amplified by DCs from the hypertensive mice were less when obtained from the denervated compared to the innervated bone marrow (Figure 2B to 2D). Moreover, CFSE dilution indicated that fewer CD8+ T cells proliferated from the denervated as compared to innervated bone marrow in response to hypertension specific antigens (Figure 2E and Online Figure I). Dilution pattern modeling indicated that the denervated bone marrow contained fewer precursor CD8+ T cells (Online Figure IB), but these precursor CD8+ T cells underwent similar numbers of divisions regardless of whether the bone marrow was denervated or not (Online Figure IC). These results indicate that sympathetic innervation promotes residence of memory CD8+ T cells in the bone marrow after they are formed in hypertension. The data also suggest that these memory T cells can be reactivated and proliferate upon re-exposure to antigens formed in hypertension.
Figure 2: Effects of sympathetic nerves on the accumulation of hypertension-specific T cells in the bone marrow.
(A) Dendritic cells isolated from sham or angiotensin II infused mice were cultured with bone marrow cells obtained from either denervated or innervated forelimbs of other hypertensive mice. Bone marrow cells were pre-labeled with CFSE. After 7 days in culture, CD3+, CD4+, CD8+ T cells were quantified by flow cytometry (B to D). Differences in the proliferation of CD8+ T cells from control and denervated bone marrow were determined by CFSE dilution and flow cytometry (E). Each set of connected symbols represent paired bone marrow samples from the same animal, n=5 to 6 in each group, p<0.0001 for the effect of denervation calculated by paired t test is shown.
Effect of local sympathetic nerves on T cells homing in the bone marrow after hypertension
To test the hypothesis that sympathetic nerves contribute to bone marrow homing of memory T cells after hypertension, we performed adoptive transfer of T cells as shown in figure 3A. We tracked the bone marrow homing of donor CD45.2+ T cells into either innervated or denervated bone marrow of recipient B6 Cd45.1 mice (Figure 3A and B). We found the numbers of adoptively transferred CD8+ TEM cells were consistently lower in the denervated bone marrow as compared to the innervated marrow. This pattern was not observed for total CD3+, CD4+, or naïve CD8+ lymphocytes from the donors, or for central memory T cells (Online Figure II). As the recipient B6 Cd45.1 mice were not hypertensive, these results indicate that sympathetic nerves regulate CD8+ TEM homing in the bone marrow even in the absence of hypertension.
Figure 3: Effects of sympathetic nerves on the accumulation of CD8+ effector memory T cells in the bone marrow after hypertension.
(A) Splenic pan-T cells were isolated from hypertensive wild type CD45.2 donor and adoptively transferred to CD45.1 recipient that either had unilateral SCGx, or AAV expressing either a control or Gq-DREADD injected into the SCG. In the case of the Gq-DREADD experiments, Clozapine-N-oxide (CNO) was administered in the drinking water for a week after adoptive transfer. One week later, recipient forelimb bone marrow was analyzed by flow cytometry. (B) A representative sample showing the gating strategy of central memory T cells (TCM) and effector memory T cells (TEM) in both CD4+ and CD8+ population from donor mice. CD8+ TEM cells are emphasized in red. After adoptive transfer, CD45.2+/CD8+ TEM cells were detected in the recipient mice with unilateral SCGx or those that had unilateral Gq-DREADD activation in SCG. The cells were quantified respectively in panel C (Innervated vs. Denervated, p=0.0073) and panel D (control vs. Gq-DREADD, p=0.0009), n=7 in each experiment. Expression of mCherry tagged Gq-DREADD was detected by confocal fluorescence microscopy in the SCG-innervated bone marrow and was co-localized with sympathetic nerve marker tyrosine hydroxylase (TH) (panel E, White bars indicate 50 micrometers). Levels of norepinephrine (NE) and epinephrine (Epi) in the bone marrow were measured by HPLC (panel F), n=9 in both groups. Each set of connected symbols represent paired bone marrow samples from the same animal, p=0.0040 in norepinephrine and p=0.0255 in epinephrine for the effect of Gq-DREADD as calculated by paired t test. * P<0.05, **P<0.01.
In additional experiments, we increased local sympathetic activity by injecting an AAV vector encoding Gq DREADD into SCG unilaterally (Figure 3A). Successful induction of the AAV gene product was confirmed by co-expression of an mCherry-fused transgene in post-ganglionic fibers of the bone marrow. These co-localized with tyrosine hydroxylase (Figure 3E). After T cell adoptive transfer, the DREADD specific ligand clozapine-N oxide (CNO) was given in the drinking water to augment local sympathetic nerve activity. CNO treatment was accompanied by an increase in tissue norepinephrine levels as measured by HPLC (Figure 3F). In contrast to our results with denervation, augmenting sympathetic nerve activity in bone marrow promoted CD8+ TEM homing (Figure 3D and Online Figure III). Thus, by manipulating local sympathetic outflow to the bone marrow, we found that sympathetic tone modulates CD8+ TEM cell homing to the bone marrow even under baseline conditions.
Role of beta 2 adrenergic receptors in t cell bone marrow homing
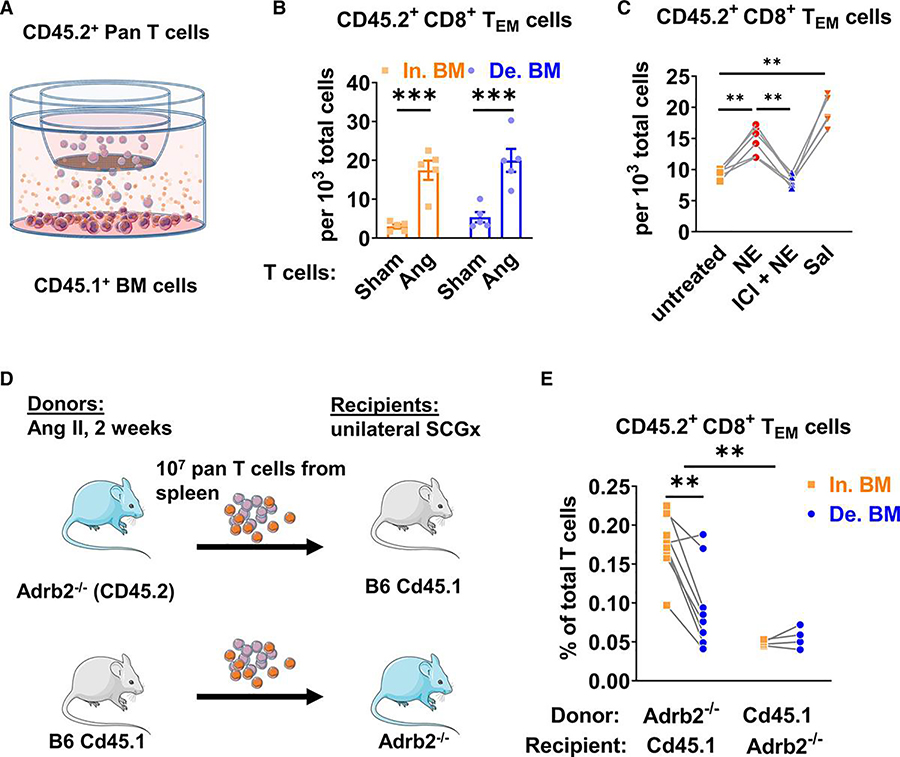
β-adrenergic signaling, and especially β2 adrenergic receptors, has been previously shown to regulate multiple cellular processes that contribute to the physiological function of bone and bone marrow.11, 15 To address a role of β adrenergic receptors in T cell homing, bone marrow cells from either sham or angiotensin II-infused B6 Cd45.1 mice were placed in the lower chamber of a transwell device and pan T cells isolated from wild type (CD45.2) mice were placed in the upper chamber (Figure 4A). In initial experiments, we observed that a significantly higher number of CD8+ TEM cells migrated to bone marrow derived from angiotensin II-infused compared to sham-infused mice (Figure 4B and Online Figure IV). We performed additional transmigration assays in which we added either norepinephrine (1 μmol/L), norepinephrine and the β2AR antagonist ICI118,551 (10 nmol/L) or the β2AR agonist salbutamol (1 μmol/L) to the medium. Norepinephrine enhanced CD8+ TEM migration to the bone marrow cells, and this was blocked by ICI118,551 (Figure 4C and Online Figure V). The β2AR agonist salbutamol potently enhanced CD8+ TEM cell transmigration to the bone marrow cells. These effects of norepinephrine and salbutamol were identical for bone marrow obtained from either innervated or denervated bones (data not shown).
Figure 4: Role of β2 adrenergic signaling in the bone marrow homing of CD8+ effector memory T cells after hypertension.
(A) Pan T cells were isolated for the spleen of wild type (CD45.2) mice that had two weeks of either sham or angiotensin II infusion. Bone marrow cells were isolated from both innervated and denervated forelimbs of B6 Cd45.1 mice that had unilateral SCGx. (B) Accumulation of transmigrated T cells isolated from innervated (In.) and denervated (De.) bone marrow (BM). Data are expressed as mean ± SEM (Sham In. BM: 3.00±0.53 vs. Ang In. BM: 17.46±2.47, p=0.0003; Sham De. BM: 5.27±1.31 vs. Ang De BM: 20.00±2.96, p=0.0002), P values for the differences between each treatments was calculated by two-way ANOVA with repeated measurements followed by a Bonferroni post hoc test, n=5 in each group. (C) Accumulation of T cells from mice after angiotensin II infusion with bone marrow cells from naïve B6 Cd45.1 mice in the presence of no treatment, or 1 μmol/L norepinephrine (NE), norepinephrine (NE) and the β2 adrenergic receptor antagonist ICI118,551 (10 nmol/L) added 30 minutes before NE (ICI + NE), or the selective β2 adrenergic receptor agonist salbutamol (Sal, 1 μmol/L). P values for the differences between each two groups were calculated by one-way ANOVA with repeated measurement followed by a Tukey’s post hoc test, n=6 in each group. (D) Adoptive transfer of splenic pan-T cells was performed between Adrb2−/− mice and B6 Cd45.1 mice. The donor received two weeks of angiotensin II infusion and the recipients received unilateral SCGx prior to the adoptive transfer. (E) CD8+ TEM cells in the innervated and denervated forelimb bone marrow were quantified by flow cytometry. For experiements shown in panel C and E, each set of connected symbols represent paired bone marrow samples from the same animal, the p values for the effect of Adrb2 gene deficiency in T cells vs. bone marrow was calculated by two-way ANOVA followed by a Bonferroni post hoc test, n=4 to 8 in each group. ** P<0.01, *** P<0.001.
To further determine if β2 adrenergic receptors promote memory T cell homing in vivo, we performed T cell adoptive transfer between B6 Cd45.1 mice and mice that were deficient of β2 adrenergic receptors (Adrb2−/−) as shown in Figure 4D. Similar to the experiments in Figure 3, the T cell donors received angiotensin II infusion for two weeks, and the recipients underwent unilateral SCGx prior to adoptive transfer. When T cells were isolated from Adrb2−/− donors and adoptively transferred to B6 Cd45.1 recipients we observed a pattern identical to that observed in mice with intact β2 adrenergic receptors; i.e. CD8+ TEM homed to both the innervated and denervated bone marrow, albeit to a lesser extent to the denervated limb. Interestingly, if the T cells from B6 Cd45.1 donors were transferred to Adrb2−/− mice, CD8+ TEM homing was virtually eliminated, whether the bone marrow was denervated or not (Figure 4E). Of note, this phenomenon was only seen for CD8+ TEM cells but not in other T cell populations (Online Figure VI). These studies indicate that β2ARs in the bone marrow niche, but not in T cells, mediate the effects of sympathetic tone on CD8+ TEM cell migration.
Specificity of sympathetic regulation of T Cells homing in the bone marrow
T cell migration is a multistep process initiated by selectin-mediated rolling on the endothelium. Subsequently, C-C chemokine receptor 7 (CCR7) binding C-C motif ligand (CCL) chemokines CCL19 and CCL21a leads to the activation of cell-surface integrin adhesion molecules in T cells, which binds to its ligands ICAM-1 and VCAM-1. We therefore examined bone marrow expression of CCL19 and CCl21 and found that denervation reduced mRNA expression of both of these ligands. In contrast, ICAM-1 and VCAM-1 expression were not affected by denervation (Figure 5).
Figure 5: Effects of sympathetic nerves on bone marrow chemokine expression.
Bone marrow samples were collected from both the innervated (In) and denervated (De) bones of mice one week after unilateral SCGx, and mRNA expression of C-C chemokine ligands (CCL)-19, CCL-21, vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) were measured by real-time PCR. Each set of connected symbols represent paired bone marrow samples from the same individual animal. The p values for the effect of denervation were calculated by paired t test, n=5 in each group. * p=0.0128 in CCL19, ** p=0.0032 in CCL21a.
To examine specificity for homing of CD8+ TEM cells from hypertensive mice, we performed adoptive transfer using T cells from OT-I transgenic mice that express a transgenic T cell receptor that recognizes the ovalbumin residues 257–264 (SIINFEKL) in the context of MHC class 1 H2Kb. One week after injection of SIINFEKL, we observed robust expansion CD8+Vβ5+ T cells observed in the blood and spleen (Online Figure VII). At this time splenic pan-T cells were isolated and injected into the tail vein of a recipient mouse that had undergone unilateral SCGx (Figure 6A). In contrast to CD8+ TEM cells that developed in response to hypertension, bone marrow homing of the OT1 CD8+Vβ5+ T cells was not affected by SCGx (Figure 6B–D). Thus, sympathetic tone is not required for all memory cell homing to the bone marrow but enhances homing of hypertension-specific TEM cells.
Figure 6:
Effects of sympathetic nerves on the accumulation of OT-I memory T cells in the bone marrow after immunization with the OVA257–264 (SIINFEKL) peptide. Splenic pan-T cells were isolated from immunized OT-I mice and transferred to B6 Cd45.1 recipient that had unilateral SCGx a week earlier. One week later, the recipient bone marrow in was analyzed by flow cytometry. (A) Representative sample showing the gating strategy. Total TCR Vβ5.1/5.2+ OT-I T cells, Vβ5.1/5.2+/CD8+ T cells as well as subsets of central and effector memory T cells in CD8+ T cell population were quantified by flow cytometry. Each set of connected symbols represent paired bone marrow samples from the same individual animal. No statistically significant difference was detected by paired t test, n=8 in each group.
Role of sympathetic outflow during T cell homing in hypertension
The above experiments indicate that sympathetic nerves and β2ARs play a role in TEM cell migration to the bone marrow. We hypothesized that this sympathetic mediation of TEM cell homing contributes to the development of recurrent hypertension. To address this, we performed bilateral microinjection of an AAV vector encoding inhibitory Gi-DREADD into the rostral ventrolateral medulla (RVLM), which contains the pre-sympathetic neurons of the brain stem (Figure 7A and 7B). Global sympathetic tone was temporarily inhibited by adding CNO to the drinking water during T cell adoptive transfer. This temporary sympatho-inhibition was confirmed by decreased blood pressure and heart rate, as well as power spectrum analysis from telemetry recordings (Online Figure VIII). Twenty days after adoptive transfer, we then infused a generally subpressor dose of angiotensin II and monitored blood pressure with radiotelemetry. As shown in Figure 7C, this dose of angiotensin II caused hypertension in mice that had received a control vector and adoptive transfer of T cells from hypertensive donors. In contrast, in mice in which sympathetic outflow was inhibited by Gi-DREADD in the RVLM during the time of T cell adoptive transfer, low dose angiotensin II infusion had no effect on blood pressure. We have previously shown that bone marrow residing CD8+ T cells can be reactivated to transmigrate to the kidney.9 In keeping with this, flow cytometry analyses of single cell suspensions of kidneys from these mice showed fewer total leukocyte, total T cells, both CD4+ and CD8+ T cell infiltration in mice that had received Gi-DREADD injection into the RVLM, indicating the role of sympathetic nerves in the potentiation of future hypertension and renal inflammation.
Figure 7:
Effects of systemic sympatho-inhibition during memory T cell homing on future hypertension development in response to low dose angiotensin II infusion. (A) Experimental paradigm employed. (B) Bilateral rostral ventrolateral medulla (RVLM) microinjection targets shown in coronal section of brainstem (marked in red circles). White bars indicate 100 micrometers. (C) Three-day measurements of systolic blood pressure were obtained by radiotelemetry at baseline and during the first and second week of angiotensin II infusion. After two weeks of angiotensin II infusion, kidneys from mice was harvested and infiltrating inflammatory cells were quantified by flow cytometry. Mean data for total leukocytes (CD45+), total T cells (CD3+), CD8+ and CD4+ T cells are shown in (D) to (G). Central and effector memory T cell subsets in CD8+ and CD4+ cells were further quantified as shown in (H) to (K). Blood pressure data were analyzed with 2-way ANOVA with repeated measurements, p=0.0124 between the two groups during angiotensin II infusion, n=5 in each group. Flow cytometry data were analyzed by unpaired t tests and p values between two groups were calculated (CD45+: p=0.0102, CD3+: p=0.0248, CD8+: p=0.0051, CD4+: p=0.0005, CD8+ TEM: p=0.0015, CD4+ TEM: p=0.0014, CD8+ TCM: p=0.4621, CD4+ TCM: p=0.3216), n=7 and 8 in each group, *P<0.05, **P<0.01, ***P<0.001 in the figure.
Role of beta 2 adrenergic blockade on T cell homing and future hypertension development
Based on previous results, it is likely that blockade of β2ARs after a blood pressure surge is protective from developing repeated hypertension. To test this hypothesis, wild type C57BL/6 mice were given pressor dose of angiotensin II infusion for two weeks, and then two-week infusion of either vehicle or β2AR antagonist ICI118,551 as shown in Figure 8A. The bone marrow was collected from a subset of mice after euthanasia, and bone marrow cells were co-cultured with DCs isolated from mice after two weeks of angiotensin II infusion (Figure 8B). After seven days of culture, we found that the total T cells (CD3+) proliferated less from mice treated with ICI118,551, and this was due to lower numbers of both CD4+ and CD8+ T cells (Figure 8C–E). We also confirmed that the differences in T cells were primarily due to the changes in the number of CD4+ and CD8+ TEM cells (Figure 8F and G). Another subset of mice received radiotelemetry implant to monitor their response to a two-week infusion of angiotensin II infusion at the same subpressor dose at used in Figure 7. Consistent with our earlier study,9 mice that had previously received high-dose angiotensin II infusion exhibited potentiated hypertension in response to this generally subpressor dose of angiotensin II compared to mice receiving only vehicle infusion (Figure 8H). Of note this second hypertensive response was blunted in mice that had ICI118,551 infusion between the two infusions of angiotensin II. These results suggest temporary blockade of β2AR may be useful as a potential treatment to improve the prognosis of hypertension and associated end-organ damage.
Figure 8:
Effects of β2 blockade on bone marrow memory T cells and future hypertension development. (A) After 2 weeks of angiotensin II infusion at pressor dose (490 ng/kg/min), mini-osmotic pumps for angiotensin II infusion were removed, and the mice received another pump for either vehicle infusion or selective β2 adrenergic receptor antagonist ICI118,551 infusion (200 ng/kg/min) for two weeks. (B) In a subset of mice, bone marrow was harvested for T cell proliferation assay. After 7 days of co-culture with DCs, CD3+, CD4+, CD8+ T cells and two TEM cell subsets in the bone marrow were quantified by flow cytometry as shown respectively from panels (C) to (G). CD3+: Veh: 20.5±0.8% vs. ICI:14.8±4.1%, p=0.0024; CD8+: Veh: 19.0±0.8% vs. ICI:14.0±1.4%, p=0.0031; CD4+: Veh: 1.08±0.06% vs. ICI:0.70±0.08%, p=0.0012; CD8+TEM: Veh: 15.6±0.6% vs. ICI:10.1±0.9%, p<0.0001; CD8+TEM: Veh: 0.91±0.17% vs. ICI:0.58±0.08%, p=0.0023. In another subset of mice, the ICI118,551 minipump was removed and a 3rd minipump inserted to administer a subpressor dose of angiotensin II (140 ng/kg/min). BP was measured using radiotelemetry (panel H). Data are expressed as mean ± SEM. Flow cytometry data were analyzed by unpaired t tests, n=8 in each group. Blood pressure was analyzed using 2-way ANOVA with repeated measurements, n=5 in each group, p=0.030 between the two groups during angiotensin II infusion, n=5 in each group. *P<0.05, **P<0.01, ***P<0.001.
DISCUSSION
In this study, we show that sympathetic nerves in the bone marrow play a critical role in the homing process of CD8+ effector memory T cells after they are formed in hypertension. These CD8+ TEM cells “remember” a previous surge of blood pressure and can be rapidly activated and divide upon re-exposure to antigens formed in hypertension. Sympathetic nerves provide a tonic control of the T cell migration to the bone marrow at baseline condition, and this effect is profoundly enhanced when sympathetic nerve activity is elevated. In addition, experiments with T cell adoptive transfer further indicate that this effect of sympathetic innervation is mediated by β2 adrenergic receptors in the bone marrow, which lead to upregulation of chemokines such as CCL19 and CCL21. More interestingly, sympathetic innervation of bone marrow does not affect the migration of OT-I memory T cells, indicating a distinct interaction between the sympathetic nerves and CD8+ TEM cells formed in hypertension.
Immunological memory has been recently identified to play a critical role in repetitive hypertension, but the mechanisms involved in the maintenance and reactivation of memory T cells in the bone marrow were poorly understood. Sympathetic innervation of the bone marrow is well established for several decades, and it plays a crucial role in modulating the circadian rhythm of hematopoietic and immune cell function in the bone marrow.11, 16, 17 Consistent with our current findings, spontaneously hypertensive rats have increased sympathetic nerve activity and impaired circadian rhythm and exhibit imbalanced production of endothelial progenitor cells and inflammatory cells in hypertension.12
Our adoptive transfer studies and experiments examining transwell transmigration clearly establish a role sympathetic tone and β2 stimulation in directing homing of CD8+ T cells to the bone marrow. Our findings are also compatible with the concept that sympathetic tone provides an environment that maintains hypertension-specific CD8+ T cells once they have homed to the marrow. The treatment of mice with ICI118,551 after a period of pressor dose angiotensin II exposure reduced the presence of T cells that proliferate in response to DCs from a hypertensive mouse. There is substantial discussion as to whether TEM cells survive in the bone marrow because these cells are truly quiescent, that they are maintained by sustained antigen exposure or that they undergo low-level homeostatic proliferation. Our studies cannot differentiate between these conditions. For CD8+ T cells, current evidence supports the concept that these cells are truly quiescent.18 In preliminary experiments, we examined the presence of isolevuglandin adducts in DCs within the bone marrow, as these could potentially support a low level of proliferation of hypertension-specific T cells, but we found that these are not altered by denervation. It is possible that specific antigenic peptides are altered by isolevuglandin, and these might be affected by β2 adrenergic stimulation. Our findings that T cells with adoptive transfer of T cells from OT-I mice suggest that sympathetic innervation within the bone marrow specifically promotes homing and maintenance of T cells related to hypertension, and not simply all TEM cells. This further supports the concept that features specific for hypertension, like the presentation of antigen, or perhaps unique conditions of the stroma with which the memory cells interact.
In the current study, we found that sympathetic innervation plays a predominant role in homing of CD8+ T cells to the bone marrow. CD8+ T cells seem to have a particularly important role in hypertension. We and others have previously shown that CD8+ T cells have a particularly important role in hypertension. We found that mice lacking these cells were protected against angiotensin II mediated hypertension, while mice lacking CD4+ T cells were not.19 Likewise, Youn et al have shown that activated, immunosenescent-like CD8+ T cells are increased in hypertensive individuals.20 We have also found that DCs of hypertensive mice present isolevuglandin modified peptides in the class 1 major histocompatibility complexes, and that these seem to selectively drive CD8+ T cell proliferation.14 Thus, the role of sympathetic nerves in modulating CD8+ T cell homing and residence in the bone marrow is likely important in the pathophysiology of hypertension. We have previously shown that repeated hypertensive stimuli promote accumulation of both CD4+ and CD8+ T cells in the bone marrow,9 and in the present study we demonstrated that ICI118,551 administration following a period of hypertension reduced both CD4+ and CD8+ T cells that proliferate in response to DCs from a hypertensive mouse. Thus, CD4+ T cells are likely also influence by sympathetic stimulation of the bone marrow.
With regard to the above considerations, we found that sympathetic innervation modulates expression of the chemokines CCL21 and CCL19, which are ligands for the chemokine receptor CCR7. CCR7 is expressed on both innate and adaptive immune cells, and it is conceivable that this promotes homing of both T cells and innate immune cells to the bone marrow. In contrast, we found no differences in expression of VCAM1 or ICAM1, ligands for LFA4 and VLA4, in innervated or denervated bone marrow. The precise receptor ligand pairs governing TEM cell homing and residence in the bone marrow remains to be defined, however the interactions of CCR7 with CCL19 and CCL21 are likely important.
Our findings might have important clinical implications. Historically, β-adrenergic blockade was considered first line therapy for hypertension, dating to the first report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure in 1977.21 Later randomized clinical trials, including the ASCOT and LIFE trials showed that atenolol is inferior to amlodipine and losartan.22, 23 Several meta-analyses have indicated that atenolol is at best neutral, and in many cases worsens all-cause mortality, compared to inhibitors of the renin-angiotensin system, calcium channel blockers and diuretics.24 This has led to the current ACC/AHA recommendation that beta-receptor antagonists be used only as add-on therapy except in special populations.25 Given that the randomized clinical trials have employed the selective β1 antagonist atenolol, and that this drug has been used in over 75% of other studies, the efficacy of non-selective beta-blockers has not been adequately studied. Our findings suggest that β2 adrenergic receptors are involved in allowing homing and survival and suggest that either non-selective β antagonists or perhaps β2 blockade might be efficacious in preventing accumulation of hypertension-specific TEM cells in sites like the bone marrow. As shown in our experiments with ICI118,551, even a short-term course of beta blockade, or perhaps drugs that reduce sympathetic outflow like β-methyldopa or β2 adrenergic agonists might create an environment hostile to survival of such cells, causing their ultimate death and thus alleviating the risk of subsequent blood pressure elevation upon repeated hypertensive challenges.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Adaptive immunity contributes to the etiology of hypertension and associated end organ damage.
Effector memory T cells reside in the bone marrow and can be reactivated by antigen re-exposure.
Bone marrow sympathetic drive is increased in hypertension.
What New Information Does This Article Contribute?
β2 adrenergic signaling preferentially mediates the accumulation of hypertension specific effector memory T cells.
β2 adrenergic blockade prevents sensatization to repeated hypertensive stimuli by creating a bone marrow environment that is hostile to survival of hypertension-specific memory T cells.
Effector memory T (TEM) cells play a crucial role in the blood pressure elevation and the renal dysfunction caused by repeated hypertensive stimuli. Formed during an initial immune challenge, TEM cells reside in the bone marrow (BM) in a quiescent state for prolonged periods, and can be reactivated upon re-exposure to the hypertensive stimulus. Hypertension is associated with increased sympathetic outflow. We performed sympathetomy and utilized DREADD (designer receptors exclusively activated by designer drugs) methodollogy to manipulate local and systemic sympathetic drive, and showed that the bone marrow homing of CD8+ TEM cells is guided by sympathetic innervation. We further found that β2 adrenergic receptors in the bone marrow are critical in mediating this process. Genetic deletion or pharmacological blockade of β2 adrenergic receptors protects mice from repeated hypertensive stimuli. These data define a novel role of sympathetic nerves in regulating memory T cell trafficking in hypertension. We propose that even a short course of sympatholytics, non-selective β -blockers or β2 antagonists could create an environment hostile to the survival of TEM cells and thus protect against future episodes of hypertension and the long-term end-organ damage that accompanies this disease.
ACKNOWLEDGEMENTS
We are grateful to the Translational Pathology Shared Resource and Cell Imaging Shared Resource at Vanderbilt for the preparation and imaging the immunostaining slides, and to Vanderbilt Hormone Assay & Analytical Services Core for the measurements of catecholamines. We thank Dr. Florent Elefteriou for providing the Adrb2−/− mice on a C57BL/6 background.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health Grants R35 HL140016 and Program Project Grant P01 HL129941 to D.G.H., and American Heart Association Scientist Development Grant 17SDG33670829 to L.X.
Nonstandard Abbreviations and Acronyms
- AAV
adeno-associated virus
- Adrb2−/−
beta 2 adrenergic knockout
- ANOVA
analysis of variance
- APC
allophycocyanin
- β2AR
β2 adrenergic receptor
- CCL
ligand chemokine containing cysteine-cysteine motifs
- CCR
C-C chemokine receptor
- CD
cluster of differentiation
- CFSE
carboxyfluorescein succinimidyl ester
- CNO
clozapine-N-oxide
- DC
dendritic cell
- DREADD
designer receptor exclusively activated by designer drug
- FITC
fluorescein isothiocyanate
- GAPDH
clyceraldehyde 3-phosphate dehydrogenase
- HPLC
high performance liquid chromatography
- ICAM
intracellular adhesion molecule
- L-NAME
N(ω)-nitro-L-arginine methyl ester hydrochloride
- PCR
polymerase chain reaction
- RVLM
rostral ventrolateral medulla
- SCG
superior cervical ganglion
- SCGx
superior cervical ganglionectomy
- SIINFEKL
ovalbumin peptide containing serine, isoleucine, isoleucine, phenylalanine, glutamate, leucine, leucine
- TCM
central memory T cells
- TEM
effector memory T cells
- TCR
T cell receptor
- VCAM
vascular cell adhesion molecule
- 7-AAD
7-aminoactinomycin D
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ and Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM and Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marvar PJ and Harrison DG. Stress-dependent hypertension and the role of T lymphocytes. Exp Physiol. 2012;97:1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norlander AE, Madhur MS and Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Rosa F Two Niches in the Bone Marrow: A Hypothesis on Life-long T Cell Memory. Trends Immunol. 2016;37:503–12. [DOI] [PubMed] [Google Scholar]
- 7.Becker TC, Coley SM, Wherry EJ and Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–73. [DOI] [PubMed] [Google Scholar]
- 8.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J and Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–64. [DOI] [PubMed] [Google Scholar]
- 9.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE and Harrison DG. CD70 Exacerbates Blood Pressure Elevation and Renal Damage in Response to Repeated Hypertensive Stimuli. Circ Res. 2016;118:1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanoun M, Maryanovich M, Arnal-Estape A and Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M and Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM and Raizada MK. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zubcevic J, Santisteban MM, Pitts T, Baekey DM, Perez PD, Bolser DC, Febo M and Raizada MK. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension. 2014;63:e129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd and Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang T, Ahmari N, Schmidt JT, Redler T, Arocha R, Pacholec K, Magee KL, Malphurs W, Owen JL, Krane GA, Li E, Wang GP, Vickroy TW, Raizada MK, Martyniuk CJ and Zubcevic J. Shifts in the Gut Microbiota Composition Due to Depleted Bone Marrow Beta Adrenergic Signaling Are Associated with Suppressed Inflammatory Transcriptional Networks in the Mouse Colon. Front Physiol. 2017;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felten DL, Felten SY, Carlson SL, Olschowka JA and Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 17.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, N AG, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A and Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang HD, Tokoyoda K and Radbruch A. Immunological memories of the bone marrow. Immunol Rev. 2018;283:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y and Harrison DG. Oligoclonal CD8+ T Cells Play a Critical Role in the Development of Hypertension. Hypertension. 2014;64:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC and Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–33. [DOI] [PubMed] [Google Scholar]
- 21.Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. A cooperative study. JAMA. 1977;237:255–61. [PubMed] [Google Scholar]
- 22.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J and Investigators A. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 23.Fossum E, Moan A, Kjeldsen SE, Devereux RB, Julius S, Snapinn SM, Edelman JM, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Dahlof B and Group LS. The effect of losartan versus atenolol on cardiovascular morbidity and mortality in patients with hypertension taking aspirin: the Losartan Intervention for Endpoint Reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;46:770–5. [DOI] [PubMed] [Google Scholar]
- 24.Ripley TL and Saseen JJ. beta-blockers: a review of their pharmacological and physiological diversity in hypertension. Ann Pharmacother. 2014;48:723–33. [DOI] [PubMed] [Google Scholar]
- 25.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017. [Google Scholar]
- 26.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE and Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension. 2014;64:573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL and Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lob HE, Schultz D, Marvar PJ, Davisson RL and Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J, Osborn JW 2nd, Itani HA and Harrison DG. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circ Res. 2015;117:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G and Franklin K. The Mouse Brain in Stereotaxic Coordinates Second ed. San Diego, CA: Academic Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.