Abstract
Purpose of Review
To provide updated evidence on the endoscopic procedures for weight loss and to bring personal insights on the future of endobariatrics.
Recent Findings
Intragastric balloons promote significant improvement in histologic and radiologic aspects of non-alcoholic steatohepatitis; the endoscopic sleeve gastroplasty is effective up to 5 years and seems particularly beneficial to patients with BMI≤40kg/m2; distal POSE is a promising technique but still lacks adequate clinical data; aspiration therapy triggers remarkable weight loss, but data on weight trends after removal of the device are still lacking; the satiety-inducing device, the sleeveballoon, the gastric mucosal devitalization, and the endoscopic magnetic partial jejunal diversion are promising procedures still under study and refinements.
Summary
Several therapeutic options are necessary during obesity’s natural history. Therefore, endobariatrics should act in harmony with lifestyle interventions, diet modification, psychological treatment, pharmacotherapy, and bariatric surgery seeking the best outcome in the long term.
Keywords: Endoscopy, Obesity, Overweight, Bariatric, Type 2 diabetes, Weight loss, Duodenal mucosal resurfacing, Jejunal diversion, Intragastric balloon, Duodenal liner
Introduction
The escalation of obesity has long become one of the worst pandemics human society has ever faced [1, 2]. Indeed, obesity is a silent, progressive, and relapsing disease that reduces both quality of life and life expectancy [3, 4].
Besides being a central risk factor for potentially lethal diseases such as acute myocardial infarction and cerebrovascular accident, it has also been identified as a predictor of poor COVID-19 outcomes [5]. That is probably due to the overlapping of its inflammatory baseline condition and the virus-induced acute inflammatory syndrome [6].
The surgical treatment is the most effective therapy to address moderate and severe stages of the disease [7, 8]. However, there is an enormous gap between patients with an indication for surgery and patients undergoing treatment [9]. Furthermore, a non-negligible portion of patients suffers from excess weight and overweight-related comorbidities but do not reach formal surgery indications. Nonetheless, those individuals could still benefit from more conservative weight loss therapies.
Thus, less invasive alternatives to the operative modality are timely as they may extend the reach of bariatric therapy to unfit-for-surgery individuals. Endoscopic bariatric therapies (EBTs) are one such alternative that presented a rapid growth during the last decades. This article aims to discuss the most up-to-date evidence on EBTs and to bring personal insights on the future of the endobariatrics.
New Data on Well-Established Procedures
Intragastric Balloons (IGB)
The IBG is the most ancient EBT. Drs Lloyd and Mary Garren firstly described an implantable gastric-occupying device in the early 1980s [10]. Since then, several refinements have been made to the device, and there is sound evidence supporting the use of the available IGBs.
Currently, there are three Food and Drug Administration-approved (FDA-approved) balloons: the Orbera (Apollo Endosurgery Inc, Austin, TX, USA), the Obalon (Obalon Therapeutics, Carlsbad, CA, USA), and the Reshape DuoTM. However, Apollo company purchased the latter one in 2018 and removed it from the market.
The Orbera is a liquid-filled 6-month or 12-month indwelling device and is most employed IGB worldwide. It has been marketed for more than 20 years in numerous countries and continents. That consistency allowed production and publication of reliable evidence, including a Brazilian consensus pooling experience from more than 40,000 procedures [11]. Several meta-analyses have already demonstrated the efficacy and safety of Orbera compared to placebo [12] and diet alone [13] in addressing severe obesity [14], obesity-related comorbidities [15], and non-alcoholic steatohepatitis (NASH) [16].
In the treatment of NASH, specifically, an interesting study recently demonstrated that a 6-month Orbera treatment leads to histologic and metabolic improvement. Twenty-one patients were enrolled and received preprocedural and postintervention liver biopsies and magnetic resonance elastography (MRE). Non-alcoholic fatty liver disease score (NAS) improved in 90% of patients. Fibrosis improved in 50% of individuals in MRE and 15% of histologic specimens. Mean serum liver enzymes also decreased significantly. Intriguingly, total body weight loss (TBWL) did not correlate with reductions in NAS and fibrosis [17••]. These data suggest an unknown positive effect of the IGB in NASH physiopathology that warrants further investigation. Finally, such a study led the FDA to grant Breakthrough Device Designation for Orbera IGB to treat NASH. That is particularly interesting as it will expand patients’ access to endoluminal therapy.
Beyond organic improvement, Gadd et al. evaluated the impact of IGB treatment on mental health and quality of life. Pooling 9 different studies via meta-analysis with a total sample of 371 patients, the authors demonstrated a considerable improvement in the postprocedural quality of life. As to mental health, five studies were pooled and showed amelioration of depressive symptoms and anxiety [18•].
Concerning patient selection, Lopez-Nava et al. recently proposed a personalized approach based on preprocedural gastric emptying. Since one of the main mechanisms of action of the IGB is delaying gastric emptying, the authors hypothesized that patients with already delayed gastric emptying at baseline would have excessive adaptation symptoms. Contrariwise, patients with faster emptying would benefit the most from the therapy. The authors employed either baseline scintigraphy or a breath test and stratified the cohort of patients in quartiles. The findings corroborated this rationale: individuals in the lowest quartile had a 6-time higher likelihood ratio for intolerance leading to early removal. That result may help determine which patient is unfit for IGBs. Additionally, individuals with delayed gastric emptying at 3 months lost significantly more weight at 6 and 12 months than patients with unchanged results. This finding confirmed that delayed gastric emptying is a critical weight loss promoter during IGB treatment. Also, it may be used as an early-on predictor of poor outcomes and may indicate the need for adjunct therapy in this subset of patients [19•].
The main drawback of the IGB treatment concerns durability. Even though long-term data are somewhat limited, a few studies reveal an overall trend towards weight regain after the IGB removal [20, 21]. Considering obesity is a chronic relapsing disease, one should not expect it differently as it may also happen after bariatric surgery [8]. Chan et al. recently published a 10-year data from a double-blind RCT. The initial trial compared IGB (Orbera balloon) plus placebo versus pharmacotherapy (sibutramine) plus sham procedure in non-morbidly obese individuals (BMI<35kg/m2) [22]. The authors reported a superior weight loss in the IGB group at 2 years, but no difference at 10 years. The groups only diverged in terms of willingness for further bariatric intervention, which favored the IGB (81% vs. 56%, p<0.01) [23•].
Such data indicate that the benefit of IGBs is transient. Nevertheless, it carries adequate control and improvement of organic and mental comorbidities until the relapse occurs. That usually happens between the second and fifth postprocedural year. Since the therapy is repeatable, combinable, and allows sequential treatment, a personalized step-up approach might mitigate this durability shortcoming. Finally, the IGB does not preclude future bariatric procedures. On the contrary, it seems to stimulate the patient to undergo further intervention if needed. Those characteristics led the American Medical Association to designate a specific Current Procedural Terminology (CPT) code for the IGB in the USA, which will expand the clinical use of this device nationwide.
As to novel devices, the Elipse (Allurion Technologies, Wellesley, MA, USA) is a liquid-filled IGB that foregoes endoscopy for both implantation and removal. After 4 months in place, it deflates and passes naturally through the GI tract. Although it is not Food and Drug Administration-approved (FDA-approved) yet, the Allurion company recently announced the completion of its pivotal study and the premarket approval submission. As to medical data, a recent systematic review pooling more than 2000 patients demonstrated that the Elipse balloon promotes an average of 12% TBWL and 49% excess weight loss (EWL). There are also accompanying reductions in waist circumference and triglyceride levels [24]. This device could be revolutionary as it may reduce costs and substantially increase access to weight loss therapies.
The most common side effects of IGBs are nausea and vomiting, especially during the first 2 weeks—the adaptation period. Nausea occurs in approximately 2 out of 3 patients undergoing treatment, and the majority of those also experience vomiting [25]. A combination of full-dose antiemetics is routinely recommended to avoid early removal. Ondansetron and dimenhydrinate are the central drugs in this context. More recently, the netupitant/palonosetron hydrochloride, a potent combination antiemetic drug commonly used to prevent nausea from chemotherapy, has also been shown efficacious in preventing nausea, vomiting, and gastric pain during the IGB adaptation period [26].
On the other hand, serious adverse events (SAEs) are rare but include gastric and esophageal perforations and small bowel obstruction due to migration. The clinical status at presentation should guide the decision between operative and non-operative management. If diffuse peritonitis or clinical instability is present, emergency surgery is needed. In the absence of those signs, one may attempt the non-operative management combining medications and local endoscopic treatment (closure for perforations, removal of migrated devices) [27].
TransPyloric Shuttle
The TPS (BARONova Inc, San Carlos, CA) has recently been approved by the FDA to treat adults with mild to moderate obesity. The device is composed of two smooth bulbs connected by a flexible silicone tether. After an endoscopic deployment, the large bulb attaches to the pylorus, while the small one hangs in the duodenum’s descending portion. During the antral pump contractions, the TPS intermittently obstructs the gastric outlet, ultimately delaying gastric emptying and enhancing satiation.
An initial trial published in 2014 gathered data from 20 procedures in an open-label non-randomized study. At 3 months, patients experienced a mean %TBWL of 8.9 ± 5.0% and a mean % EWL of 25.1 ± 14.0%. At 6 months, the mean %TBWL and %EWL rose to 14.5 ± 5.8% and 41.0 ± 21.1%, respectively. As to safety, there were two cases of persistent gastric ulcers requiring early removal and additional 8 cases of asymptomatic ulcerations [28]. Such a high incidence of gastric ulcers fostered refinements in the device.
A large multicenter sham-controlled trial investigated the new version of the TPS, and the results led the FDA to approve it for clinical use. Two-hundred and seventy subjects (181 TPS and 89 control) were enrolled. Additional 32 individuals from the control group received TPS in the open-label phase after they were unblinded. At 12 months, the intervention group had higher %TBWL and BMI reduction compared to sham (9.5% [8.2, 10.8] vs. 2.8% [1.1, 4.5], p<0.0001 and 3.5 vs. 1.01, p<0.0001). Nausea, vomiting, dyspepsia, and upper abdominal pain were present in more than 50% of treated subjects but were typically mild in severity. Twenty-one (10.3%) patients had at least one ulcer, and a total of 46 in the TPS group exited the study and had the device retrieved before the 12-month follow-up. Nonetheless, the serious adverse events (SAEs) rate was considerably lower than the overall AE: 2.8%, among which gastric impaction was the most frequent event (1.97%) [29].
The TPS seems effective but still carries unpleasant and persistent symptoms during the treatment. This characteristic probably hampered the worldwide spread since its approval in 2019. Further refinements are warranted to grant this device a central role in the war against obesity.
Endoscopic Sleeve Gastroplasty (ESG)
Abu Dayyeh et al. firstly described the ESG in 2013 [30]. This procedure, also called “the Apollo method,” consists in employing a full-thickness endoscopic suturing device (Apollo Overstitch) to create apposition of the anterior, greater curvature, and posterior wall of the gastric body. The fundus remains intact and acts as a pouch with delayed emptying [31]. Since the first description in 2013, the ESG technique suffered several technical refinements. That includes using the helix to grasp tissue, per protocol CO2 insufflation, increasing the number of bites per suture (from 6 up to 12), and adding reinforcement sutures in between the first suturing line [32]. With the current technique, the ESG has been proven reproducible and safe worldwide [33, 34], not only in academic centers but also in community health units [35]. A Brazilian consensus recently gathered 47 experts and provided practical guidelines to previously unattended technical and clinical issues related to the ESG [36]. Table 1 summarizes the main points of consensus in such a study.
Table 1.
Summary of practical recommendations to perform the endoscopic sleeve gastroplasty. Adapted from Galvao MG et al. [33]. BMI body mass index, DVT deep venous thrombosis, CO2 carbon dioxide, ESG endoscopic sleeve gastroplasty, PPI proton pump inhibitor
| Indications | |
| There is no age or BMI limit, but the minimum recommended BMI is 27kg/m2 | |
| The ideal indication is for patients with grade I obesity (BMI 30–35kg/m2) | |
| Contraindications | |
| Active gastric ulcers in the body or fundus, portal hypertensive gastropathy, gastric polyposis, gastric or esophageal varices, uncontrolled/untreated psychiatric disorders | |
| Preparation | |
| Preoperative upper endoscopy and laboratory workup are mandatory | |
| DVT prophylaxis should be initiated according to clinical criteria | |
| A surgeon, a dietitian, and a psychologist should be part of the multidisciplinary team | |
| Technique | |
| General anesthesia is mandatory | |
| Antibiotic prophylaxis is recommended | |
| CO2 insufflation is mandatory | |
| Whether to perform stomach marking or not, the kind of suture pattern and the number of sutures employed are at the discretion of the endoscopist | |
| The main goal is to shrink the greater curvature, but the antrum should never be sutured | |
| Most endoscopists try to reduce the distal part of the gastric fundus | |
| ESG is eminently an outpatient procedure | |
| Postprocedural care | |
| Recommended symptomatic drugs: PPI, ondansetron, hyoscine/scopolamine, steroid (dexamethasone), analgesics | |
| PPI should be maintained for 1–3 months | |
Interestingly, although some authors recently described improved outcomes employing different suturing patterns [37], other data suggest that weight loss outcomes are unrelated to the suture pattern [38]. Still concerning technical refinements, Itani et al. proposed association with argon plasma coagulation to enhance fibrosis in the plication line, resulting in a superior sleeve-like lumen [39]. Albeit clinical data are lacking, the rationale seems appropriate as adequate endoscopic anatomy correlates with better outcomes during follow-up [40]. Nevertheless, one should expect that the imbricated gastric mucosal will eventually unfold. Pizzicannella et al. demonstrated that only 49% of ESGs were fully intact at a 6-month upper endoscopy [40]. Therefore, family history or individual increased risk of adenocarcinoma should not raise further concerns in the context of an ESG. Despite the mucosal unfolding, experts advocate that a fibrotic process in the gastric wall occurs secondary to the full-thickness stitching that permanently hampers gastric accommodation.
Several systematic reviews assessing the efficacy of the ESG were published in 2020. Due-Petersson et al. [41], de Miranda Neto et al. [42], Singh et al. [43], and Hedjoudje et al. [44], in four different articles, pledged the same: ESG is effective in the short-term and promotes average %TBWL up to 20% at 24 months. Although controlled studies are still lacking, the consistency of their findings indicates that the demonstrated efficacy is reliable.
As to original data, Sharaiha et al. published a recent study showing 5-year outcomes of the ESG to treat obesity. From a series of 216 patients, 38 were eligible for a 5-year follow-up assessment. Among them, 18% were lost to follow-up. The remaining individuals presented a mean %total body weight loss (%TBWL) of 15.9% (95% CI, 11.7–20.5). Sixty-one percent of patients sustained at least 10% TBWL at 5 years. Although it is small-sampled, this study suggests that ESG outcomes are also durable in the long term [45••].
Concerning new indications, another recent study investigated the effect of ESG on obesity in children and adolescents. Alqahtani et al. reported a series of 109 consecutive mildly obese patients aged 10 to 21 years old undergoing ESG. At 12 and 24 months, the mean %TBWL was 16.2% ± 8.3% and 13.7% ± 8.0%, respectively. Fourteen patients (12.8%) required ambulatory visits for analgesia, and one underwent endoscopic removal of the stitches due to refractory abdominal pain. However, there were no emergency admissions, deaths, or significant morbidity. This data suggest that ESG is also effective and safe to treat young individuals suffering from obesity [46].
Another recent article assessed the impact of adjunct pharmacotherapy with liraglutide. This glucagon-like peptide-1 agonist amplifies insulin secretion, delays gastric emptying, and upregulates satiety by a central effect on the hypothalamus [47, 48]. In an international cohort study, Badurdeen et al. employed a propensity score analysis to match 26 patients receiving GLP-1 at month 5 after ESG to 26 individuals declining it. At 12 months (7 months after introducing pharmacotherapy), patients from the GLP-1 group presented superior weight loss and greater reduction in percent body fat [49••]. This study is of paramount importance as it proves synergism between endoscopic and pharmacological approaches. Possibly, the combination of such therapies will become the standard of care soon.
Finally, one of the potential negative implications of the ESG would be increased surgical risk due to peritoneal adhesions and gastric imbrication. Although the need for rescue bariatric surgery after ESG is negligible—around 0.8%—surgical conversion had not been proven safe until recently [50]. Two studies demonstrated that a previous ESG does not pose substantial technical issues to a surgical conversion. That applies to Roux-en-Y gastric bypass (RYGB) and laparoscopic sleeve gastrectomy (LSG) [51, 52].
As to comparative data, interesting articles have already been published, but most are non-controlled and non-matched retrospective cohorts. Non-matched studies frequently present groups with unbalanced baseline characteristics due to the intrinsic selection bias [53–55]. Dissimilar demographics handicaps interpretation of outcomes. One such study recently demonstrated that ESG is superior to IGB in weight loss, but baseline data typically differed between groups [56].
Consequently, some authors have employed case-matched designs to mitigate the negative impact of selection bias. In this sense, a case-matched cohort comparing ESG to high-intensity diet plus lifestyle therapy recently showed that the former promotes more significant weight loss within 12 months [57].
As to comparison against surgery, another case-matched study with 137 patients compared the LSG to the ESG. The authors demonstrated that the LSG carries superior weight loss at a 6-month follow-up (23.6% ± 7.6% vs 17.1% ± 6.5%, p<0.01). Conversely, it also poses a higher risk for adverse events (AEs) and new-onset GERD (16.9% vs 5.2%, p<0.05 and 14.5% vs 1.9%, p<0.05, respectively) [58].
Finally, Marincola et al. compared those modalities through a meta-analysis design and confirmed that the surgical approach is superior in weight loss. However, no head-to-head study was included as few cohorts, and no controlled studies comparing those two modalities were available [59]. Moreover, another meta-analysis demonstrated identical results, but the limitations persist as they are related to the literature itself and not to the methodology of the study [60].
Arguably, endoscopic and surgical sleeves fit better to different types of patients, which explains the absence of comparative head-to-head studies. Instead, future controlled trials should focus on assessing the efficacy and safety of those strategies according to the severity of obesity. In overweight and mild obesity, one should compare ESG to non-operative modalities (e.g., IGB, diet, medications). In class II patients, ESG should be tested against LSG. However, current data allows anticipating the inferiority of ESG in terms of weight loss and durability for class III obesity [54, 58]. Considering the evident benefit of bariatric surgery for severe obesity [8] and the high cost added to the complex logistics of conducting an RCT [61], one might not need to test ESG against LSG for class III individuals. In this situation, current non-comparative data already support ESG for patients declining or unfit for surgery [62].
Pose and Pose 2.0 (Distal POSE)
The primary obesity surgery endoluminal (POSE) involves the employment of the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, CA, USA). The IOP is a 4-component device that allows endoscopic control of a full-thickness plication system. It delivers sequential anchors in the stomach to promote gastric imbrication [63].
The initial technique proposed impairing gastric accommodation by shrinking the fundus. Several non-controlled studies demonstrated the efficacy and safety of the POSE procedure [64, 65] and led to the development of a large sham-controlled trial. The ESSENTIAL trial, however, failed to demonstrate the long-term efficacy [66].
This failure was credited to technique and not to the device itself, which prompted modifications in the plication sequence. Instead of focusing on the fundus, the new technique, called distal POSE or POSE 2.0, aims to reduce gastric volume and alter motility. The operator creates two transverse plication rows, one in the distal body and the other in the proximal body. Then, they are connected through two longitudinal rows, one in the anterior and the other in the posterior wall. The transverse rows are the belts, while the longitudinal rows are suspenders [67•]. The final aspect reduces the gastric length and alters the motility of the stomach [68].
Two case series describing the distal POSE have been published to date. Jirapinyo et al. reported results from 10 subjects undergoing this novel procedure. All patients received the distal POSE with an average of 21 ± 4 plications per case and experienced a mean shortening by 11.0 ± 5cm in the gastric body. There were no SAEs. At 6 months, the mean %TBWL was 15.0% ± 7.1%, and 8 patients (80%) presented at least 25% EWL [69]. Lopez-Nava et al. reported outcomes of 46 patients at 1 year. Mean %TBWL was 17.8 ± 9.5%, and BMI reduction was 7 ± 4.3kg/m2. Interestingly, endoscopy at 12 months showed intact sutures and sustained reduction in gastric length compared with baseline (26.9 ± 5.3cm vs. 35.7 ± 3.5cm, p<0.001) [70].
The distal POSE seems promising, but open-label followed by sham-controlled trials are warranted to confirm such high expectations. In the meantime, physiology studies could provide valuable information on how POSE 2.0 promotes weight loss. That information would allow better patient selection and even further refinements in this still-evolving technique.
Aspiration Therapy
The AspireAssistTM is a novel FDA-approved device for the treatment of obesity. The index procedure is similar to a standard percutaneous endoscopic gastrostomy. The gastric portion of the device is a thick multi-fenestrated tube (A-tube) directed to the fundus. The outer part is a button-like implant that attaches to an external portable aspiration machine. After eating, the patient aspirates and disposes of around 30% of the ingested food [71]. Adjunct lifestyle interventions and nutritional counseling are also recommended as in all obesity-directed therapy.
The PATHWAY trial, a large open-label multicenter study, enrolled 171 patients to compare the aspiration therapy (n=111) versus lifestyle counseling (n=60). At 12 months, the mean %EWL and %TBWL were 31.5±26.7% and 12.1±9.6%, respectively, in the intervention group versus 9.8±15.5% and 3.5±6.0% in the control group (p<0.001). Aspiration patients also presented significant reductions in triglycerides, HbA1c, low-density lipoprotein, systolic and diastolic blood pressure, and an increase in high-density cholesterol. The SAEs rate was 3.6% (4/111) and entailed severe abdominal pain, peritonitis, gastric ulcer, and port malfunctioning requiring tube replacement [72]. Among the 111 individuals from the aspiration group, 58 continued in the study for a 4-year evaluation. Interestingly, the patients presented a progressive weight loss throughout follow-up: 14.2%, 15.3%, 16.6%, and 18.7% TBWL at 1, 2, 3, and 4 years, respectively (p<0.01). There were two persistent fistulas (2%) requiring surgical repair after tube removal [73].
The low rate of persistent fistula contradicts data from a post-market European registry study published in 2018. Despite similar weight loss throughout a 4-year follow-up, Nystrom et al. reported four such cases among the 47 removals (8.5%) and suggested that the risk escalates after 2 years post-gastrostomy. Additionally, there were 12 cases of gastric leakages and several other device-related adverse events (stomal irritation/granulation tissue, infection, buried bumper, and tube malrotation) [74].
Such data suggest that the AspireAssistTM is effective in the short term, but unwanted complications arise in the long term. Undoubtedly, aspiration is not a definitive therapy, and information on clinical management after device removal is lacking. Furthermore, the ideal indwelling period remains unknown. Future studies should focus on filling those gaps to minimize adverse events and exploit the therapy to its best.
Upcoming Procedures
Intragastric Satiety-Inducing Device (Full-sense®)
The Full-sense® device (FSD) (Baker, Foote, Kemmeter, Walburn, LLC, Grand Rapids, MI, USA) resembles a metallic esophageal stent attached to a wide transversal disk. The operator deploys the FSD at the esophagogastric junction through an upper endoscopy and under fluoroscopic control. The tubular part is the esophageal side, and the disk is the gastric side of the device.
The FSD is capable of applying continuous pressure to the EGJ while distending the gastric fundus. Consequently, it may stimulate vagal afferent receptors to induce satiety [75] and downregulate circulating ghrelin, thus alleviating hunger [76, 77].
Three animal studies concerning the FSD have already been published, and the first-in-human study is currently ongoing in India. Initially, Park et al. [78] and Luo et al. [79] investigated different types of devices to reduce the high migration rates. Their results supported the third animal study that focused on the efficacy and physiology of weight loss. Bakheet et al. implanted the FSD in 5 juvenile pigs and compared them to three control ones. Despite previous refinements in the device, the migration rate was still 40% (2/5). The intervention group presented lower weight gain rates compared to the control group during a 6-week follow-up. Moreover, pigs receiving the FSD presented lower ghrelin levels and fewer gastric interstitial cells of Cajal in a microscopic post-mortem examination. This latter finding suggests that the FSD might also alter gastric motility, eventually improving weight loss [80].
Sleeveballoon
The Sleeveballoon consists of an intragastric balloon attached to a duodenal liner. The balloon occupies 2/3 of the gastric chamber, while the liner bypasses the duodenum as it delivers food directly to the mid-jejunum. The device mimics of the surgical RYGB effect by combining restriction, malabsorption, and hormonal changes.
An animal study assessing the physiology and metabolic effect of the Sleeveballoon has already been published. Casella-Mariolo et al. developed a three-group study involving 30 rats undergoing RYGB, Sleeveballoon, or a sham operation. Rats from both Sleeveballoon and RYGB groups presented sustained weight loss and similar improvement in hepatic and whole-body insulin sensitivity, reduction of visceral and subcutaneous fat, and an equivalent postprandial peak of GLP1 [81].
However, there are no ongoing human studies as the owner company is currently working on capitalization. Nevertheless, the rationale and the preliminary physiology results are fascinating. If further clinical studies confirm the rationale, the Sleeveballoon might become one of the first EBTs to target both the stomach and the small bowel.
Gastric Mucosal Devitalization (GMD)
The endoscopist should ablate the gastric mucosa using the standard Argon Plasma Coagulation (APC) to perform the GMD. This procedure’s rationale is based on the role of gastric signaling in controlling hunger and satiety, which seems critical after the surgical sleeve gastrectomy [82]. Some experts initially hypothesized that ablating around 70% of the gastric mucosal surface would trigger weight loss. Further animal studies confirmed that the GMD could promote a reduction in body weight, adiposity, and hepatic steatosis. Furthermore, it could ameliorate lipid metabolism, blood pressure, renin, and cardiovascular lipid deposition in rat models [83, 84].
More recently, Kumbhari et al. developed a porcine model comparing GMD, sleeve gastrectomy, and a sham operation. All procedures were technically successful with no adverse events. The endoscopic procedure elicited a more pronounced weight loss at 4 and 8 weeks compared to sham. Weight loss was similar between GMD and SG at 4 weeks, but SG pigs presented a more significant loss at 8 weeks (p<0.05).
These studies suggest GMD might be temporarily effective with few related adverse events. Nonetheless, it seems repeatable, which could mitigate its transitory trait. Human studies are probably the next step needed to transform GMD into another therapeutic option for obesity.
Magnetic Anastomosis System (GI Windows Inc.)
The endoscopic magnetic partial jejunal diversion (EMPJD) employs self-assembling magnets initially developed to address gastric outlet obstruction [85]. Two magnets should be deployed: the first in the proximal jejunum by upper endoscopy and the other in the terminal ileum by a deep colonoscopy. After deployment, each of them assembles in a ring-shaped octagon as they couple across the intestinal wall in the antimesenteric border. The magnetic force leads to local ischemic and subsequent necrosis. After 2 weeks, the magnets detach from the wall as the tissue mortifies and are naturally expelled during defecation. The result is a calibrated side-to-side jejunoileal anastomosis. This procedure diverges from the abandoned traditional surgical jejunoileal bypass once the original intestinal pathway remains intact. Accordingly, only a portion of food bypasses the small bowel, mitigating the risk for excessive malabsorption.
Ryou et al. published the animal proof-of-concept study in 2016 [86], and soon after, survival models demonstrated that the procedure was safe [87]. The first-in-man study was conducted in the Czech Republic and included 11 patients. Technical success was 83% (10/12—as one patient required two attempts). However, all but two deliveries required laparoscopic assistance, which was per protocol after 40 min attempting fully endoscopic coupling. Patients presented a gradual progressive weight loss throughout follow-up: mean %TBWL of 8.2%, 10.6%, and 14.6% at 3, 6, and 12 months, respectively. Interestingly, diabetic patients experienced a reduction by 1.9% in HbA1c levels at 12 months. There were no device-related SAEs, and all minor AEs were managed non-operatively [88].
A currently ongoing study (NCT03130244) confirmed that the fully endoscopic coupling is extremely challenging despite the operator’s experience. Therefore, the proposed technique has been modified to laparoscopic-assisted. Further studies are needed to establish the effectiveness of the EMPJD in the long term and demonstrate the combined endoscopic-surgical approach’s technical viability.
Ideas and Insights for the Future
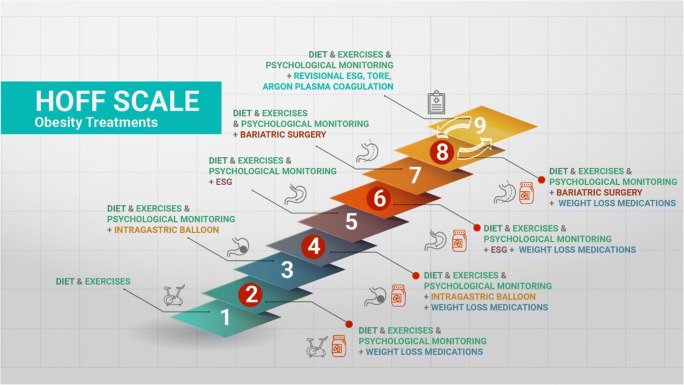
Obesity is a chronic relapsing disease. As such, several therapeutic options are therefore necessary during its natural history and considering all its grades and particularities. A personalized step-up approach seeking enhanced and sustained results is highly appropriate in this setting. Lifestyle interventions, diet modification, psychological treatment, pharmacotherapy, EBTs, and bariatric surgery are not mutually excluding. On the contrary, they must act in harmony, recognizing the seriousness of the disease. Endoscopic bariatric procedures may be consistently associated with pharmacotherapy (GLP-1 analogues, for instance), to enhance effectiveness and durability of the results [49••]. Figure 1 represents a proposal for step-up treatment of obesity, known as “the Hoff scale”.
Figure 1.
A proposed step-up therapeutic approach for obesity combining all available therapies. ESG, endoscopic sleeve gastroplasty; TORE, transoral outlet reduction. Courtesy of Dr. Ana Carolina Hoff.
As to the future of endobariatrics alone, more effective procedures will probably address two or three different GI tract targets. To date, some scattered articles have already demonstrated such a trend. Shah et al. performed same-session ESG and transoral incisionless fundoplication in a patient with obesity and gastroesophageal reflux disease [89]. Sawas et al. reported a simultaneous laparoscopic hiatus hernia repair and fundoplication, followed by ESG [90]. Ghoz et al. described sequential duodenal liner and IGB in porcine models [91], while Sartoretto et al. reported a series of 3 individuals receiving concurrent IGB after reaching weight loss plateau with the duodenal liner [92].
Conclusion
Endobariatrics is an ever-growing field with endless evolving possibilities. As such, it will certainly play an essential role in any of the possible future scenarios of the war against obesity.
Footnotes
This article is part of the Topical Collection on Health Services and Programs
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief United States. 2017:1–8. [PubMed]
- 2.Organization WH. Overweight and obesity - global observatory data [Internet]. 2016 [cited 2019 Aug 1]. Available from: https://www.who.int/gho/ncd/risk_factors/overweight_text/en/
- 3.Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. a position statement of the World Obesity Federation. Obes. Rev. an Off. J. Int. Assoc. Study Obes. England; 2017. p. 715–23. [DOI] [PubMed]
- 4.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet (London, England) 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14:655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes. 2020;44:1790–1792. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med United States. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson LMS, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Svensson P-A, Taube M, Carlsson B, Peltonen M. Life Expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535–1543. doi: 10.1056/NEJMoa2002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. United States; 2018 [DOI] [PubMed]
- 10.Brunaldi VO, Galvao Neto M. Gastric space-occupying devices for management of obesity and metabolic disease. Tech Innov Gastrointest Endosc [Internet]. Elsevier; 2020;22:130–5. Available from: 10.1016/j.tige.2020.05.001
- 11.Neto MG, Silva LB, Grecco E, de Quadros LG, Teixeira A, Souza T, Scarparo J, Parada AA, Dib R, Moon R, Campos J. Brazilian Intragastric Balloon Consensus Statement (BIBC): practical guidelines based on experience of over 40,000 cases. Surg Obes Relat Dis United States. 2018;14:151–159. doi: 10.1016/j.soard.2017.09.528. [DOI] [PubMed] [Google Scholar]
- 12.Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis Obes Surg. 2008;18:841–846. doi: 10.1007/s11695-007-9331-8. [DOI] [PubMed] [Google Scholar]
- 13.Moura D, Oliveira J, De Moura EGH, Bernardo W, Galvao Neto M, Campos J, et al. Effectiveness of intragastric balloon for obesity: a systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis United States. 2016;12:420–429. doi: 10.1016/j.soard.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 14.Yorke E, Switzer NJ, Reso A, Shi X, de Gara C, Birch D, Gill R, Karmali S. Intragastric Balloon for management of severe obesity: a systematic review. Obes Surg United States. 2016;26:2248–2254. doi: 10.1007/s11695-016-2307-9. [DOI] [PubMed] [Google Scholar]
- 15.Popov VB, Ou A, Schulman AR, Thompson CC. The impact of intragastric balloons on obesity-related co-morbidities: a systematic review and meta-analysis. Am J Gastroenterol United States. 2017;112:429–439. doi: 10.1038/ajg.2016.530. [DOI] [PubMed] [Google Scholar]
- 16.Popov VB, Thompson CC, Kumar N, Ciarleglio MM, Deng Y, Laine L. Effect of Intragastric balloons on liver enzymes: a systematic review and meta-analysis. Dig Dis Sci United States. 2016;61:2477–2487. doi: 10.1007/s10620-016-4178-2. [DOI] [PubMed] [Google Scholar]
- 17.••.Bazerbachi F, Vargas EJ, Rizk M, Maselli DB, Mounajjed T, Venkatesh SK, et al. Intragastric balloon placement induces significant metabolic and histologic improvement in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc United States. 2021;19:146–154.e4. Important series demonstrating that a 6-month treatment with intragastric balloon promotes metabolic and histological improvement in NASH parameters. [DOI] [PMC free article] [PubMed]
- 18.•.Gadd N, McIntosh A, Fear-Keen B, Hoult J, Maimone IR, Marshall S. Do endoscopic bariatric procedures improve postprocedural quality of life and mental health? A Systematic Review and Meta-analysis. Obes Surg United States. 2020;30:4091–100. An interesting meta-analysis showing metal status improvement after IGB treatment. [DOI] [PubMed]
- 19.•.Lopez-Nava G, Jaruvongvanich V, Storm AC, Maselli DB, Bautista-Castano I, Vargas EJ, et al. Personalization of endoscopic bariatric and metabolic therapies based on physiology: a prospective feasibility study with a single fluid-filled intragastric balloon. Obes Surg. United States; 2020. A recent study investigating baseline gastric emptying characteristics in patients undergoing IGB treatment. This study provides valuable information that could be used to better select patients for EBTs. [DOI] [PubMed]
- 20.Sander B, Arantes VN, Alberti L, Neto MG, Grecco E, Souza TF. 550 Long-term effect of intragastric balloon in the management of obesity. Gastrointest Endosc [Internet]. Elsevier; 2017;85:AB83. Available from: 10.1016/j.gie.2017.03.113
- 21.Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg United States. 2012;22:896–903. doi: 10.1007/s11695-012-0607-2. [DOI] [PubMed] [Google Scholar]
- 22.Ng E, Mui W, Tsung B, Yung M, Lam C, Wong S, et al. 424 A prospective, randomized, double-blind, controlled trial on efficacy of weight reduction of endoscopic intragastric balloon versus oral sibutramine in patients with non-morbid obesity. Gastroenterology. 2009;136:A–73. doi: 10.1016/S0016-5085(09)60326-7. [DOI] [Google Scholar]
- 23.•.Chan DL, Cruz JR, Mui WL, Wong SKH, Ng EKW. Outcomes with intra-gastric balloon therapy in BMI < 35 non-morbid obesity: 10-year follow-up study of an RCT. Obes Surg. United States; 2020. This is a 10-year follow-up study on intragastric balloon therapy. [DOI] [PubMed]
- 24.Ramai D, Singh J, Mohan BP, Madedor O, Brooks OW, Barakat M, et al. Influence of the Elipse intragastric balloon on obesity and metabolic profile: a systematic review and meta-analysis. J Clin Gastroenterol. United States; 2020; Publish Ah. [DOI] [PubMed]
- 25.Trang J, Lee SS, Miller A, Cruz Pico CX, Postoev A, Ibikunle I, Ibikunle CA. Incidence of nausea and vomiting after intragastric balloon placement in bariatric patients - a systematic review and meta-analysis. Int J Surg England. 2018;57:22–29. doi: 10.1016/j.ijsu.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Ienca R, Giardiello C, Scozzarro A, di Cola RS, Di Lorenzo N, Juneja G, et al. Improving nausea and vomiting post-Elipse balloon: a novel single-dose regimen of 300 mg netupitant/0.5 mg palonosetron hydrochloride. Obes Surg United States. 2019;29:2952–2956. doi: 10.1007/s11695-019-03937-x. [DOI] [PubMed] [Google Scholar]
- 27.Stavrou G, Tsaousi G, Kotzampassi K. Life-threatening visceral complications after intragastric balloon insertion: is the device, the patient or the doctor to blame? Endosc Int open Germany. 2019;7:E122–E129. doi: 10.1055/a-0809-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marinos G, Eliades C, Raman Muthusamy V, Greenway F. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg Obes Relat Dis United States. 2014;10:929–934. doi: 10.1016/j.soard.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Rothstein R, Woodman G, Swain J, Cruz-Munoz N de la Kushnir V, Pryor A, et al. Weight reduction in patients with obesity using the TransPyloric SHUTTLE®: ENDObesity® II Study [Internet]. 2018. p. 1. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180024D.pdf
- 30.Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530–535. doi: 10.1016/j.gie.2013.04.197. [DOI] [PubMed] [Google Scholar]
- 31.Abu Dayyeh BK, Acosta A, Camilleri M, Mundi MS, Rajan E, Topazian MD, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol United States. 2017;15:37–43.e1. doi: 10.1016/j.cgh.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, Galvao Neto MP, Sahdala NP, Shaikh SN, Hawes RH, Gostout CJ, Goenka MK, Orillac JR, Alvarado A, Jirapinyo P, Zundel N, Thompson CC. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc Germany. 2018;32:2159–2164. doi: 10.1007/s00464-017-5869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhandari M, Jain S, Mathur W, Kosta S, Neto MG, Brunaldi VO, et al. Endoscopic sleeve gastroplasty is an effective and safe minimally invasive approach for treatment of obesity: first Indian experience. Dig Endosc. 2020;32:541–546. [DOI] [PubMed]
- 34.Sartoretto A, Sui Z, Hill C, Dunlap M, Rivera AR, Khashab MA, Kalloo AN, Fayad L, Cheskin LJ, Marinos G, Wilson E, Kumbhari V. Endoscopic sleeve gastroplasty (ESG) is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: a large, international multicenter study. Obes Surg United States. 2018;28:1812–1821. doi: 10.1007/s11695-018-3135-x. [DOI] [PubMed] [Google Scholar]
- 35.James TW, Reddy S, Vulpis T, McGowan CE. Endoscopic sleeve gastroplasty is feasible, safe, and effective in a non-academic setting: short-term outcomes from a community gastroenterology practice. Obes Surg United States. 2020;30:1404–1409. doi: 10.1007/s11695-019-04331-3. [DOI] [PubMed] [Google Scholar]
- 36.Neto MG, Silva LB, de Quadros LG, Grecco E, Filho AC, de Amorim AMB, et al. Brazilian consensus on endoscopic sleeve gastroplasty. Obes Surg United States. 2021;31:70–78. doi: 10.1007/s11695-020-04915-4. [DOI] [PubMed] [Google Scholar]
- 37.Graus Morales J, Crespo Perez L, Marques A, Marin Arribas B, Bravo Arribas R, Ramo E, et al. Modified endoscopic gastroplasty for the treatment of obesity. Surg Endosc Germany. 2018;32:3936–3942. doi: 10.1007/s00464-018-6133-0. [DOI] [PubMed] [Google Scholar]
- 38.Espinet-Coll E, Nebreda-Durán J, Galvao-Neto M, Bautista-Altamirano C, Diaz-Galán P, Gómez-Valero JA, Vila-Lolo C, Guirola-Puche MA, Fernández-Huélamo A, Bargalló-Carulla D, Juan-Creix Comamala A. Suture pattern does not influence outcomes of endoscopic sleeve gastroplasty in obese patients. Endosc Int open. 2020;8:E1349–E1358. doi: 10.1055/a-1221-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itani MI, Farha J, Sartoretto A, Abbarh S, Badurdeen D, de Moura DTH, et al. Endoscopic sleeve gastroplasty with argon plasma coagulation: a novel technique. J Dig Dis Australia. 2020;21:664–667. doi: 10.1111/1751-2980.12939. [DOI] [PubMed] [Google Scholar]
- 40.Pizzicannella M, Lapergola A, Fiorillo C, Spota A, Mascagni P, Vix M, Mutter D, Costamagna G, Marescaux J, Swanström L, Perretta S. Does endoscopic sleeve gastroplasty stand the test of time? Objective assessment of endoscopic ESG appearance and its relation to weight loss in a large group of consecutive patients. Surg Endosc Germany. 2020;34:3696–3705. doi: 10.1007/s00464-019-07329-1. [DOI] [PubMed] [Google Scholar]
- 41.Due-Petersson R, Poulsen IM, Hedbäck N, Karstensen JG. Effect and safety of endoscopic sleeve gastroplasty for treating obesity - a systematic review. Dan Med J Denmark. 2020;67. [PubMed]
- 42.de Miranda Neto AA, de Moura DTH, Ribeiro IB, Khan A, Singh S, da Ponte Neto AM, et al. Efficacy and safety of endoscopic sleeve gastroplasty at mid term in the management of overweight and obese patients: a systematic review and meta-analysis. United States: Obes Surg; 2020. [DOI] [PubMed] [Google Scholar]
- 43.Singh S. Hourneaux de Moura DT, Khan A, Bilal M, Ryan MB, Thompson CC. Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: a systematic review and meta-analysis. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2020;16:340–351. doi: 10.1016/j.soard.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, Adam A, Neto MG, Badurdeen D, et al. Efficacy and safety of endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc United States. 2020;18:1043–1053.e4. doi: 10.1016/j.cgh.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 45.••.Sharaiha RZ, Hajifathalian K, Kumar R, Saunders K, Mehta A, Ang B, et al. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin Gastroenterol Hepatol. 2021;19:1051–1057.e2. This study provides 5-year efficacy data on ESG for weight loss. [DOI] [PubMed]
- 46.Alqahtani A, Elahmedi M, Alqahtani YA, Al-Darwish A. Endoscopic sleeve gastroplasty in 109 consecutive children and adolescents with obesity: two-year outcomes of a new modality. Am J Gastroenterol United States. 2019;114:1857–1862. doi: 10.14309/ajg.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 47.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol United States. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 49.••.Badurdeen D, Hoff AC, Hedjoudje A, Adam A, Itani MI, Farha J, et al. Endoscopic sleeve gastroplasty plus liraglutide versus endoscopic sleeve gastroplasty alone for weight loss. Gastrointest Endosc. 2021;93:1316–1324.e1. This is an interesting study demonstrating that the association of liraglutide enhances weight loss after ESG weight loss plateau. [DOI] [PubMed]
- 50.Alqahtani A, Al-Darwish A, Mahmoud AE, Alqahtani YA, Elahmedi M. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest Endosc. United States; 2018 [DOI] [PubMed]
- 51.Beitner M, Hopkins G. Conversion of endoscopic sleeve gastroplasty to laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis Off J Am Soc Bariatr Surg United States. 2020;16:590–591. doi: 10.1016/j.soard.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 52.Alqahtani AR, Elahmedi M, Alqahtani YA, Al-Darwish A. Laparoscopic sleeve gastrectomy after endoscopic sleeve gastroplasty: technical aspects and short-term outcomes. Obes Surg United States. 2019;29:3547–3552. doi: 10.1007/s11695-019-04024-x. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz AG, Breviere GL-N, Coll EE, Duran JN, Neto MG, Gebelli JP. Endoscopic gastroplasty vs. sleeve gastrectomy and laparoscopic gastric plication. A comparative study. Surg Obes Relat Dis [Internet]. Elsevier; 2017;13:S9. Available from: 10.1016/j.soard.2017.09.022
- 54.Novikov AA, Afaneh C, Saumoy M, Parra V, Shukla A, Dakin GF, Pomp A, Dawod E, Shah S, Aronne LJ, Sharaiha RZ. Endoscopic Sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic band for weight loss: how do they compare? J Gastrointest Surg United States. 2018;22:267–273. doi: 10.1007/s11605-017-3615-7. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Nava G, Asokkumar R, Bautista-Castaño I, Laster J, Negi A, Fook-Chong S, et al. Endoscopic sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic greater curve plication: do they differ at 2 years? Endoscopy Germany. 2021;53:235–243. doi: 10.1055/a-1224-7231. [DOI] [PubMed] [Google Scholar]
- 56.Fayad L, Cheskin LJ, Adam A, Badurdeen DS, Hill C, Agnihotri A, et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: efficacy, durability, and safety. Endoscopy. 2019;51:532–539. [DOI] [PubMed]
- 57.Cheskin LJ, Hill C, Adam A, Fayad L, Dunlap M, Badurdeen D, et al. Endoscopic sleeve gastroplasty versus high-intensity diet and lifestyle therapy: a case-matched study. Gastrointest Endosc United States. 2020;91:342–349.e1. doi: 10.1016/j.gie.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 58.Fayad L, Adam A, Schweitzer M, Cheskin LJ, Ajayi T, Dunlap M, et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest Endosc. 2019;89:782–788. [DOI] [PubMed]
- 59.Marincola G, Gallo C, Hassan C, Raffaelli M, Costamagna G, Bove V, et al. Laparoscopic sleeve gastrectomy versus endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Endosc Int open. 2021;9:E87–E95. doi: 10.1055/a-1300-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohan BP, Asokkumar R, Khan SR, Kotagiri R, Sridharan GK, Chandan S, Ravikumar NP, Ponnada S, Jayaraj M, Adler DG. Outcomes of endoscopic sleeve gastroplasty; how does it compare to laparoscopic sleeve gastrectomy? A systematic review and meta-analysis. Endosc Int open. 2020;8:E558–E565. doi: 10.1055/a-1120-8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Speich B, von Niederhäusern B, Schur N, Hemkens LG, Fürst T, Bhatnagar N, Alturki R, Agarwal A, Kasenda B, Pauli-Magnus C, Schwenkglenks M, Briel M, MAking Randomized Trials Affordable (MARTA) Group Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J Clin Epidemiol United States. 2018;96:1–11. doi: 10.1016/j.jclinepi.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Nava G, Laster J, Negi A, Fook-Chong S, Bautista-Castaño I, Asokkumar R. Endoscopic sleeve gastroplasty (ESG) for morbid obesity: how effective is it? Surg Endosc. Germany; 2021 [DOI] [PubMed]
- 63.Brunaldi VO, Galvao NM. Endoscopic techniques for weight loss and treating metabolic syndrome. Curr Opin Gastroenterol United States. 2019;35:424–431. doi: 10.1097/MOG.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 64.Espinos JC, Turro R, Mata A, Cruz M, da Costa M, Villa V, et al. Early experience with the Incisionless Operating Platform (IOP) for the treatment of obesity : the Primary Obesity Surgery Endolumenal (POSE) procedure. Obes Surg United States. 2013;23:1375–1383. doi: 10.1007/s11695-013-0937-8. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Nava G, Bautista-Castano I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The Primary Obesity Surgery Endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis United States. 2015;11:861–865. doi: 10.1016/j.soard.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 66.Sullivan S, Swain JM, Woodman G, Antonetti M, De La Cruz-Munoz N, Jonnalagadda SS, et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: the ESSENTIAL trial. Obesity (Silver Spring). 2017;25:294–301. [DOI] [PubMed]
- 67.•.Jirapinyo P, Thompson CC. Gastric plications for weight loss: distal primary obesity surgery endoluminal through a belt-and-suspenders approach. VideoGIE. 2018;3:296–300. This report is a technical description of the novel distal POSE procedure. [DOI] [PMC free article] [PubMed]
- 68.Lopez-Nava G, Asokkumar R, Turró Arau R, Neto MG, Dayyeh BA. Modified primary obesity surgery endoluminal (POSE-2) procedure for the treatment of obesity. VideoGIE. 2020;5:91–93. [DOI] [PMC free article] [PubMed]
- 69.Jirapinyo P, Thompson CC. Endoscopic gastric body plication for the treatment of obesity: technical success and safety of a novel technique (with video). Gastrointest Endosc. 2020;91:1388–1394. [DOI] [PMC free article] [PubMed]
- 70.Lopez Nava G, Asokkumar R, Laster J, Negi A, Normand E, Fook-Chong S, Bautista-Castaño I. Primary obesity surgery endoluminal (POSE-2) procedure for treatment of obesity in clinical practice. Endoscopy. 2020. 10.1055/a-1324-8498. [DOI] [PubMed]
- 71.Sullivan S, Stein R, Jonnalagadda S, Mullady D, Edmundowicz S. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology United States. 2013;145:1245. doi: 10.1053/j.gastro.2013.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson CC, Abu Dayyeh BK, Kushner R, Sullivan S, Schorr AB, Amaro A, Apovian CM, Fullum T, Zarrinpar A, Jensen MD, Stein AC, Edmundowicz S, Kahaleh M, Ryou M, Bohning MJ, Ginsberg G, Huang C, Tran DD, Glaser JP, Martin JA, Jaffe DL, Farraye FA, Ho SB, Kumar N, Harakal D, Young M, Thomas CE, Shukla AP, Ryan MB, Haas M, Goldsmith H, McCrea J, Aronne LJ. Percutaneous gastrostomy device for the treatment of class II and class III obesity: results of a randomized controlled trial. Am J Gastroenterol United States. 2017;112:447–457. doi: 10.1038/ajg.2016.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson CC, Abu Dayyeh BK, Kushnir V, Kushner RF, Jirapinyo P, Schorr AB, et al. Aspiration therapy for the treatment of obesity: 4-year results of a multicenter randomized controlled trial. Surg Obes Relat Dis. 2019;15:1348–1354. [DOI] [PubMed]
- 74.Nystrom M, Machytka E, Noren E, Testoni PA, Janssen I, Turro Homedes J, et al. Aspiration therapy as a tool to treat obesity: 1- to 4-year results in a 201-patient multi-center post-market European registry study. Obes Surg United States. 2018;28:1860–1868. doi: 10.1007/s11695-017-3096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148:1219–1233. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goitein D, Lederfein D, Tzioni R, Berkenstadt H, Venturero M, Rubin M. Mapping of ghrelin gene expression and cell distribution in the stomach of morbidly obese patients--a possible guide for efficient sleeve gastrectomy construction. Obes Surg United States. 2012;22:617–622. doi: 10.1007/s11695-011-0585-9. [DOI] [PubMed] [Google Scholar]
- 77.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 78.Park J-H, Bakheet N, Na HK, Jeon JY, Yoon SH, Kim KY, Zhe W, Kim DH, Jung HY, Song HY. A novel full sense device to treat obesity in a porcine model: preliminary results. Obes Surg United States. 2019;29:1521–1527. doi: 10.1007/s11695-018-03692-5. [DOI] [PubMed] [Google Scholar]
- 79.Luo Y, Zhang X, Tsauo J, Jung H-Y, Song H-Y, Zhao H, et al. Intragastric satiety-inducing device reduces food intake and suppresses body weight gain in a rodent model. Surg Endosc. Germany; 2020 [DOI] [PubMed]
- 80.Bakheet N, Na HK, Park J-H, Ryu DS, Jeon JY, Khashab MA, et al. A novel intragastric satiety-inducing device to inhibit weight gain in juvenile pigs: a pilot study. Obes Surg United States. 2020;30:4643–4651. doi: 10.1007/s11695-020-04930-5. [DOI] [PubMed] [Google Scholar]
- 81.Casella-Mariolo J, Castagneto-Gissey L, Angelini G, Zoli A, Marini P, Bornstein SR, Pournaras DJ, Rubino F, le Roux CW, Mingrone G, Casella G. Simulation of gastric bypass effects on glucose metabolism and non-alcoholic fatty liver disease with the Sleeveballoon device. EBioMedicine. 2019;46:452–462. doi: 10.1016/j.ebiom.2019.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oberbach A, Schlichting N, Heinrich M, Kullnick Y, Retschlag U, Lehmann S, et al. Gastric mucosal devitalization reduces adiposity and improves lipid and glucose metabolism in obese rats. Gastrointest Endosc United States. 2018;87:288–299.e6. doi: 10.1016/j.gie.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 83.Oberbach A, Schlichting N, Heinrich M, Kullnick Y, Retschlag U, Lehmann S, et al. Gastric mucosal devitalization reduces adiposity and improves lipid and glucose metabolism in obese rats. Gastrointest Endosc. 2018;87:288–299.e6. [DOI] [PubMed]
- 84.Oberbach A, Schlichting N, Kullnick Y, Heinrich M, Lehmann S, Retschlag U, Friedrich M, Fayad L, Dietrich A, Khashab MA, Kalloo AN, Kumbhari V. Gastric mucosal devitalization improves blood pressure, renin and cardiovascular lipid deposition in a rat model of obesity. Endosc Int open. 2019;7:E1605–E1615. doi: 10.1055/a-0990-9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Myers C, Yellen B, Evans J, DeMaria E, Pryor A. Using external magnet guidance and endoscopically placed magnets to create suture-free gastro-enteral anastomoses. Surg Endosc Germany. 2010;24:1104–1109. doi: 10.1007/s00464-009-0735-5. [DOI] [PubMed] [Google Scholar]
- 86.Ryou M, Agoston AT, Thompson CC. Endoscopic intestinal bypass creation by using self-assembling magnets in a porcine model. Gastrointest Endosc United States. 2016;83:821–825. doi: 10.1016/j.gie.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 87.Ryou M, Aihara H, Thompson CC. Minimally invasive entero-enteral dual-path bypass using self-assembling magnets. Surg Endosc Germany. 2016;30:4533–4538. doi: 10.1007/s00464-016-4789-x. [DOI] [PubMed] [Google Scholar]
- 88.Machytka E, Buzga M, Zonca P, Lautz DB, Ryou M, Simonson DC, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc United States. 2017;86:904–912. doi: 10.1016/j.gie.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Shah SL, Dawod S, Dawod Q, Sharaiha RZ. Same-session endoscopic sleeve gastroplasty and transoral incisionless fundoplication: a possible solution to a growing problem. VideoGIE an Off video J Am Soc Gastrointest Endosc. 2020;5:468–469. doi: 10.1016/j.vgie.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sawas T, Marya NB, Storm AC, Blackmon SH, Abu Dayyeh BK. Laparoscopic hernia repair and fundoplication with endoscopic sleeve gastroplasty for complex hernia and GERD management in morbid obesity. VideoGIE an Off video J Am Soc Gastrointest Endosc. 2020;5:555–556. doi: 10.1016/j.vgie.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghoz H, Jaruvongvanich V, Matar R, Beran A, Maselli DB, Storm AC, Abu Dayyeh BK. A Preclinical animal study of combined intragastric balloon and duodenal-jejunal bypass liner for obesity and metabolic disease. Clin Transl Gastroenterol. 2020;11:e00234. doi: 10.14309/ctg.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sartoretto A, Marinos G, Sui Z. Concurrent placements of a duodenal-jejunal bypass liner and an intragastric balloon among severely obese patients: a case series. ACG Case Rep J. 2019;6:e00101. [DOI] [PMC free article] [PubMed]