Abstract
Background
Peripheral T-cell lymphomas (PTCLs) are uncommon and their frequency is regionally heterogeneous. Several studies have been conducted to evaluate the clinical features and treatment outcomes of this disease entity, but the majority of these were conducted in limited areas, making it difficult to comprehensively analyze their relative frequency and clinical features. Furthermore, no consensus treatment for PTCLs has been established. Therefore, we conducted an Asia-specific study to understand the relative frequency of PTCLs and assess treatments and their outcomes in Asian patients.
Methods
We performed a multinational, multicenter, prospective registry of adult patients with PTCLs that was named as the International Cooperative non-Hodgkin T-cell lymphoma prospective registry study where thirty-two institutes from six Asian countries and territories (Korea, China, Taiwan, Singapore, Malaysia, and Indonesia) participated.
Findings
A total of 486 patients were registered between April 2016 and February 2019, and more than a half of patients (57%) had stage III or IV. Extranodal natural killer (NK)/T- cell lymphoma was the most common subtype (n = 139,28.6%), followed by angioimmunoblastic T-cell lymphoma (AITL, n = 120,24.7%), PTCL-not otherwise specified (PTCL-NOS, n = 101,20.8%), ALK-positive anaplastic large cell lymphoma (ALCL, n = 34,6.9%), and ALK-negative ALCL (n = 30,6.2%). The median progression-free survival (PFS) and overall survival (OS) were 21.1 months (95% CI,10.6–31.6) and 83.6 months (95% CI, 56.7–110.5), respectively. Upfront use of combined treatment with chemotherapy and radiotherapy showed better PFS than chemotherapy alone in localized ENKTL whereas consolidation with upfront autologous stem cell transplantation (SCT) provided longer PFS in advance stage ENKTL. In patients with PTCLs other than ENKTL, anthracycline-containing chemotherapies were widely used, but the outcome of those regimens was not satisfactory, and upfront autologous SCT was not significantly associated with survival benefit, either. The treatment outcome of salvage chemotherapy was disappointing, and none of the salvage strategies showed superiority to one another.
Interpretation
This multinational, multicenter study identified the relative frequency of each subtype of PTCLs across Asian countries, and the survival outcomes according to the therapeutic strategies currently used.
Funding
Samsung Biomedical Research Institute
Keywords: Lymphoma, T-Cell, Peripheral, Extranodal NK-T-Cell, Frequency, Therapeutics, Survival, Prognosis
Research in context.
Evidence before this study: We searched PubMed for reports published between 2000 and 2020, using the search terms“peripheral T-cell lymphoma” and “survival outcome”. There were more than 600 articles related to survival outcomes and frequency of neoplasms of mature T-cell and NK-cells. However, those results could not represent that of neoplasms of mature T-cell and NK-cells in Asian countries because patients from Asian countries accounted for only a small portion of the study population in most studies. Moreover, there were limited information about the treatment patterns and outcomes in newly diagnosed and relapsed or refractory patients with neoplasms of mature T-cell and NK-cells. Thus, we conducted a multinational and multicenter prospective registry study to assess the frequency of neoplasms of mature T-cell and NK-cells in Asia and organize currently preferred treatment modalities in neoplasms of mature T-cell and NK-cells.
Added-value of this study: We found out that the CHOP-like or CHOEP-like regimens are the main treatment for newly diagnosed neoplasms of mature T-cell and NK-cells in Asia, and there was no difference in efficacy between CHOP-like or CHOEP-like regimens. The survival outcomes for patients with relapsed or refractory disease were quite disappointing, no matter what salvage chemotherapy regimens were used. We also analyzed to identify the role of autologous stem cell transplantation(auto-SCT). Patients who underwent upfront auto-SCT after achieving complete response or partial response after first-line chemotherapy showed better survival outcomes.
Implications of all the available evidence: This study contains the only data for patients with neoplasms of mature T-cell and NK-cells across Asia without any western data. Our data shows the relative frequency and treatment schemes fully reflecting the situation in Asia, so far, it might be an excellent opportunity to identify neoplasms of mature T-cell and NK-cells in Asia. Besides, we expect our results to help set up various clinical trials targeting neoplasms of mature T-cell and NK-cells in Asia for the future.
Alt-text: Unlabelled box
1. Introduction
Neoplasms of mature T-cell and NK-cells are uncommon malignancies that represent 10–15% of all lymphomas [1], [2], [3]. The 2016 World Health Organization classification contains 27 different subtypes of PTCL, including angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL), PTCL-not otherwise specified (PTCL-NOS), enteropathy-associated T-cell lymphoma (EATL), and extranodal NK/T-cell lymphoma (ENKTL) [4]. The low frequency and complexity of the histologic classification of neoplasms of mature T-cell and NK-cells present a challenge to systematic research [5]. Furthermore, the frequency of neoplasms of mature T-cell and NK-cells differs by subtype across Western and Asian countries such as higher frequency of ENKTL in East Asian countries than Western countries and predominant occurrence of ATLL in Japan [6], [7], [8]. The International T-cell Lymphoma Project showed the relative frequency of each subtype by geographic region [9]. The major lymphoma subtype in North America and Europe was PTCL-NOS, whereas the most common neoplasms of mature T-cell and NK-cells in Asia were adult T-cell leukemia/lymphoma (ATLL) and ENKTL. However, those results might not represent the landscape of T-cell lymphoma in Asia because the number of patients enrolled from Asian countries accounted for only a small portion of all patients registered into the International T-cell Lymphoma Project.
As for the treatment of patients with neoplasms of mature T-cell and NK-cells, there is still no clear consensus on primary therapy for newly diagnosed as well as relapsed or refractory patients [10]. Currently, there is no consensus on the standard of primary treatment for newly diagnosed neoplasms of mature T-cell and NK-cells. Accordingly, anthracycline-containing chemotherapies such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens are still the main treatment for newly diagnosed neoplasms of mature T-cell and NK-cells, although they do not have satisfactory outcomes for neoplasms of mature T-cell and NK-cells [11,12]. On the other hand, nonanthracycline-based chemotherapies incorporating the non-P-glycoprotein-dependent drugs, methotrexate, ifosfamide, and L-asparaginase are commonly used for ENKTL patients [13,14]. For relapsed or refractory patients who failed after primary therapy, each institution uses different principles to choose the next treatment option, and the types of clinical trials being conducted at each institution vary. Nevertheless, the survival outcome of patients with peripheral T-cell and NK/T-cell lymphomas who relapsed after first-line treatment was reported as extremely poor in previous retrospective studies [15,16].
Therefore, we conducted a multinational, multicenter prospective registry study to assess the relative frequencies of each subtype of neoplasms of mature T-cell and NK-cells in Asian countries. In addition, considering these heterogeneity of primary and salvage treatments across subtypes of neoplasms of mature T-cell and NK-cells, our prospective registry study collected the treatments currently used for newly diagnosed and relapsed/refractory patients in clinical practice of Asian countries, and analyze their outcomes in real-world context.
2. Methods
2.1. Study design and data collection
We performed a multicenter, prospective registry study for adult patients with T-cell lymphoma called the International Cooperative non-Hodgkin T-cell Lymphoma Prospective Registry study (ICT study). Thirty-two institutes in Asian countries and territories (Korea, China, Taiwan, Singapore, Malaysia, and Indonesia) participated in the ICT study between April 2016 and February 2019 (Supplementary Table 1). This registry was approved by the Institutional Review Boards or Scientific Review Committees as required by the policies of individual institutions. Written informed consent was obtained from each patient before study enrollment. We enrolled patients age 19 years and older who were diagnosed with a neoplasms of mature T-cell and NK-cells according to the 2008 World Health Organization classification [17]. We excluded patients with Hodgkin lymphoma, non-Hodgkin B-cell lymphoma, or precursor T-lymphoblastic lymphoma/leukemia after a histological review in each center. If the diagnoses of patients with neoplasms of mature T-cell and NK-cells were confirmed by histological findings that satisfied the registration criteria, the pathologic report was uploaded to an electronic case report form (eCRF), and the patients were enrolled in the study. When a review of the pathology was deemed necessary by an investigator, a scanned file of the H&E and immunostaining slides were sent to the central institution Pathology Department as image files. The primary objective of the ICT study was to estimate the relative frequencys of neoplasms of mature T-cell and NK-cells across Asia. In addition, we investigated existing treatment strategies currently used in each country at diagnosis and in released/refractory cases. The secondary objectives were to assess the overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) following first-line therapy or salvage therapy. The ORR is presented as a proportion by calculating the number of patients with a complete response (CR) or partial response (PR). The occurrence of relapse or disease progression was defined as the development of a new lesion or progression of the lesion by physical or radiographic examination.
2.2. Variables
Clinical information including sex, age, diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, Ann-Arbor stage, bone marrow involvement, presence of hemophagocytic lymphohistiocytosis (HLH) in bone marrow, and number of extranodal involvement sites, were collected prospectively. We also collected crucial laboratory data: complete blood cell counts, serum Lactate dehydrogenase (LDH), detection of Epstein-Barr virus DNA, and viral markers (anti-HBs, anti-HBc antibody, anti-HCV, anti-HTLV-1, anti-HIV). Abnormalities in the laboratory data were evaluated according to the standards of each institution. Also, we collected the results of the bone marrow examination conducted according to the clinician's discretion or institution's criteria, and the results were confirmed as the presence or absence of lymphoma invasion. The prognostic risks of PTCL-NOS, AITL, and ALCL were measured using the International Prognostic Index (IPI) [18], Prognostic Index for PTCL-U (PIT) [19], modified-PIT (mPIT), including Ki-67≥80% [20], and the International PTCL Project [21]. ENKTL was evaluated using the Prognostic Index for Natural Killer Cell Lymphoma (PINK) [22]. Frontline and salvage chemotherapies were determined by clinicians at each center. We collected information about autologous stem cell transplantation (auto-SCT), allogeneic stem cell transplantation (allo-SCT), and radiation therapy. The response to treatment was evaluated according to the clinical practice for T-cell lymphoma at each institute using computed tomography scans and/or 18F-fluorodeoxyglucose positron emission tomography/computed tomography scans. Evaluation of the response to treatment was assessed according to the Lugano Classification [23]. The final update of survival data was performed in December 2019.
2.3. Statistical analysis
Categorical variables were described by proportion, and continuous variables were summarized by calculating the median and interquartile range. The OS was defined as the time from the initial diagnosis to the last follow-up date or the date of death by any cause. If patients were alive at last contact, they were censored at the date of confirmed last contact. The PFS was defined as the time from the initial diagnosis including, the date of lymphoma confirmation by biopsy or the study registration date, to the date of relapse, progression, last follow-up, or death from any cause. We defined and measured PFS as two cases: (1) PFS1; the time from initial treatment to progression, relapse, or death from any cause among all T-cell lymphoma patients; (2) PFS2; the time from second-line treatment to progression, relapse, or death from any cause among patients who received second-line chemotherapy. Both PFS and OS estimates were analyzed using the Kaplan–Meier method. All data were analyzed using the Statistical Package for Social Sciences software, version 24.0 (IBM Corp, Armonk, NY, USA).
2.4. Role of the funding source
This study was supported by a grant from the Samsung Biomedical Research Institute (GFO1150161). The funding source had no role in the following fields: study design, collection, analysis, and interpretation of data, writing the report, and access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
3. Results
3.1. Distribution and characteristics of T cell lymphoma in Asia
Among a total of 490 patients who were registered from 32 centers across Asia, 4 patients with precursor T cell acute lymphoblastic leukemia were excluded, and 486 patients were analyzed. The most prevalent subtype was ENKTL (n = 139,28.6%), and the second most common subtype was AITL (n = 120,24.7%), followed by PTCL-NOS (n = 101,20.8%), ALK-positive ALCL (n = 34,6.9%), and ALK-negative ALCL (n = 30,6.2%). T-cell lymphoma subtypes affecting the intestines, such as EATL type 1 (N = 7) and type 2 (N = 8) and intestinal T-cell lymphoma, NOS (N = 2) accounted for about 3.4% of cases (n = 17). Thirty-seven patients (7.5%) had T-cell lymphoma affecting cutaneous tissues, including subcutaneous panniculitis-like T-cell lymphoma (N = 9), Mycosis fungoides (N = 5), and Sezary syndrome (N = 3). (Fig. 1, Supplementary Table 2).
Fig. 1.
Histologic subtype distribution of 486 cases. EATL, enteropathy-associated T-cell lymphoma; SPTL, subcutaneous panniculitis-like T-cell lymphoma; MF, mycosis fungoides; SS, Sezary syndrome; NOS, not otherwise specified.
3.2. Characteristics of whole PTCLs and subtypes
The clinical characteristics found among the various subtypes of all T-cell lymphoma patients are shown in Table 1. Among all subtypes, the median patient age was 57 (range 19–89) years, 60% of the patients were men, and 61% of patients had B-symptoms at the time of diagnosis. Laboratory findings showed neutropenia affecting 10% of patients, anemia 16%, and thrombocytopenia 18%. Of the 77% of patients with lymphoma involvement in the bone marrow, 16% also had HLH. Stage 3 or 4 was seen in 57% of patients, and stage 1 or 2 in 43%.
Table 1.
Total patient characteristics (N = 486).
| Parameters | N (%) | |
|---|---|---|
| Sex | Male | 291 (60) |
| Female | 195 (40) | |
| Age | Median (range) | 57 (19–89)† |
| ECOG PS | 0–1 | 439 (90) |
| ≥2 | 47 (10) | |
| B-symptoms | Present | 298 (61) |
| Absent | 188 (39) | |
| Anemia | <10g/dL | 79 (16) |
| Thrombocytopenia | <150 × 109/L | 91 (19) |
| Neutropenia | <1000/dL | 51 (11) |
| LDH | > UNL | 282 (58) |
| HBsAg | Positive | 31 (6) |
| Anti-HBs antibody | Positive | 222 (46) |
| Anti-HBc antibody | Positive | 123 (25) |
| Anti-HCV | Positive | 8 (1.6) |
| EBV DNA | Positive | 88 (18) |
| BM invasion | Presence | 102 (21) |
| Absence | 384 (79) | |
| HLH presence in BM | Presence | 76 (16) |
| Stage | I/II | 207 (43) |
| III/IV | 279 (57) | |
| Events | Disease progression or death | 255 (53) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, Lactate Dehydrogenase; HBs, hepatitis B surface; HBc, Hepatitis B core; EBV, Epstein-Barr Virus; BM, Bone marrow; HLH, hemophagocytic lymphohistiocytosis.
†Inter Quartile Range (IQR, median, 25th, 75th centiles): 57 (44-66)
A more detailed analysis of the prevalent T-cell lymphomas subtypes found in Asia (ENKTL, AITL, PTCL-NOS, ALCL) was performed. The median age of patients with ALK-positive ALCL (median 42, range 21–76) or ENKTL (median 49, range 15–86) was younger than that of patients with ALK-negative ALCL (median 57, range 32–80), PTCL-NOS (median 60, range 16–85) or AITL (median 64, range 27–89). All five subtypes were found more commonly in male patients, and B-symptoms presented in more than 50% of all subtypes. Lymphoma involvement in bone marrow was frequently found in AITL (38%) and PTCL-NOS (26%), and it was infrequent in ALK-negative ALCL (17%), ALK-positive ALCL (15%), and ENKTL (7%) (Table 2). In terms of the site involved by subtype, ENKTL mainly invaded the nasal cavity and nasopharynx (92%), and 35.8% of AITL patients had spleen involvement (Supplementary Table 3).
Table 2.
Clinical characteristics by histologic type.
| DX | Age <65 | Male | B-SX | Extra nodal ≥ 2 | Stage III/IV | BM involved | HLH in BM | IPI 4/5 | Event† | |
|---|---|---|---|---|---|---|---|---|---|---|
| ENKTL (n=139) | N (%) | 119 (86) | 89 (64) | 86 (62) | 76 (55) | 35 (25) | 9 (7) | 6 (4) | 10 (7*) | 64 (46) |
| AITL (n=120) | N (%) | 66 (55) | 75 (63) | 68 (57) | 55 (46) | 102 (85) | 45 (38) | 34 (28) | 30 (25) | 66 (65) |
| PTCL NOS (n=101) | N (%) | 66 (65) | 59 (58) | 66 (65) | 42 (42) | 71 (70) | 26 (26) | 19 (19) | 18 (18) | 63 (53) |
| ALCL, ALK- (n=30) | N (%) | 21 (70) | 23 (55) | 30 (71) | 12 (29) | 20 (48) | 5 (17) | 3 (7) | 5 (12) | 15 (50) |
| ALCL, ALK+ (n=34) | N (%) | 32 (94) | 20 (59) | 19 (56) | 15 (44) | 19 (56) | 5 (15) | 6 (18) | 2 (6) | 12 (35) |
Abbreviations: DX, diagnosis; B-SX, B-symptoms; BM, bone marrow; HLH, hemophagocytic lymphohistiocytosis; IPI, International Prognostic Index; ENKTL, extranodal NK/T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; PTCL, peripheral T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma receptor tyrosine kinase.
*: assessed by the Prognostic Index for Natural Killer Cell Lymphoma
† Events: disease progression or/and Death.
3.3. Survival outcomes according to subtypes
With a median follow up of 31.6 months (95% CI 30.0–33.1), the median PFS of neoplasms of mature T-cell and NK-cells was 21.1 months (95% CI 10.6–31.6, Figure 2A), and the median OS was 83.6 months (95% CI 66.7–110.5). The PFS and OS according to the five most-common subtypes (ENKTL, PTCL-NOS, AITL, ALK-positive ALCL, and ALK-negative ALCL) are presented in Supplementary Figures 2B and 3B. ALK-positive ALCL patients had better PFS (P-value = 0.03) and OS (P-value < 0.01) rates, compared to PTCL-NOS patients who had the shortest survival rates. The PFS (PTCL-NOS, P-value < 0.01; ALCL, ALK-negative, P-value = 0.02; ALCL, ALK-positive, P-value < 0.01) was poor in patients with stage III/IV disease except ENKTL (P-value = 0.06), and AITL (P-value = 0.41, Supplementary Figures 1). Furthermore, the OS (ENKTL, P-value = 0.02; ALCL, ALK-positive, P-value = 0.05) was inferior in patients with stage III/IV disease except PTCL-NOS (P-value = 0.06), AITL (P-value = 0.46), and ALCL ALK-negative (P-value = 0.09, Supplementary Figures 2). The survival outcome comparison according to the four prognostic indexes validated in Caucasian population (IPI, PIT, mPIT, and the International PTCL Project) found no significant differences among four of the common subtypes (PTCL-NOS, AITL, ALK-negative ALCL, and ALK-positive ALCL) (Supplementary Fig. 3).
Fig. 3.
(A) Overall survival (OS) of 486 patients, (B) OS according to each subtype.
Fig. 2.
(A) Progression-free survival (PFS) of 486 patients, (B) PFS according to each subtype.
3.4. Survival outcomes according to treatment strategies
Frontline chemotherapy and second-line chemotherapy were given to 465 and 187 patients, respectively. However, 21 patients were not treated and received the best supportive care only (Supplementary Table 6). Consolidation with auto-SCT upon the first remission was given to 68 patients, while 23 patients received other salvage chemotherapy or passed away upon relapse. Eight patients underwent allo-SCT after frontline chemotherapy (n = 4) or salvage chemotherapy (n = 4) (Figure 4).
Fig. 4.
Overview of treatment strategies for all enrolled patients.
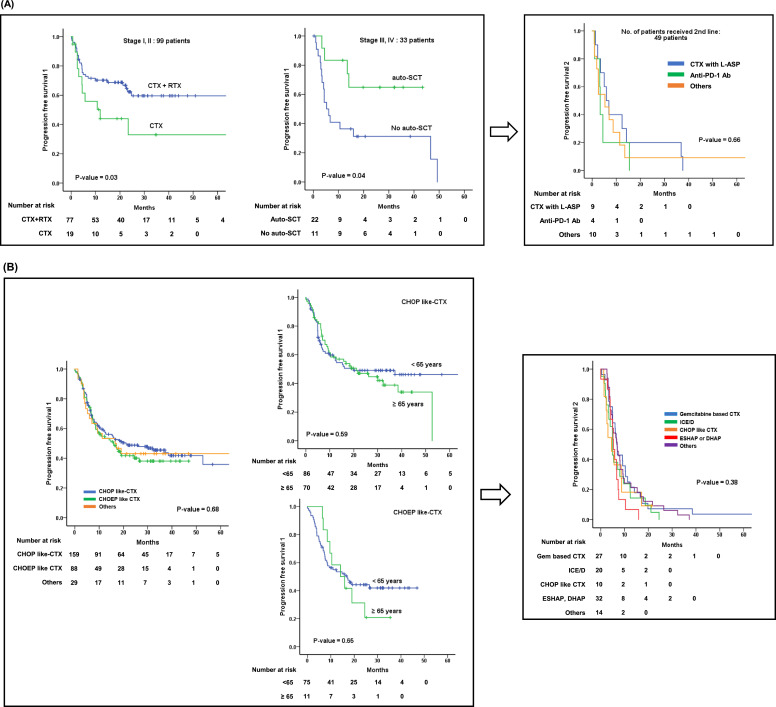
Among the 139 patients with ENTKL, 99 (71%) patients with localized disease received either combination of chemotherapy and radiation (n = 78) or chemotherapy alone (n = 21). The patients who received a combination of chemotherapy and radiotherapy had an ORR of 80%, which was superior to that with chemotherapy alone (ORR of 48%) (Table 3). The PFS1 of patients who received chemotherapy combined with radiation was also superior to that of patients who received chemotherapy alone (CTX+RTX versus CTX; NR versus 68.1 months, 95% CI 2.1–134, P-value = 0.03). The 33 patients with stage III/IV disease who underwent upfront auto-SCT after frontline cytotoxic chemotherapy showed better PFS outcomes than those who did not. (NR versus 5.3 months, 95% CI 2.9–7.7, P-value = 0.04). The number of patients who received chemotherapy without L-asparaginase was 21% (7/33), which was not enough patients to compare their results with those who received chemotherapy combined with L-asparaginase. However, the CR (50%, 13/26) and ORR (62%,16/26) for patients who received L-asparaginase containing chemotherapy were better than those of patients who received chemotherapy without L-asparaginase (CR 43%, and ORR 43%). Unfortunately, 49 patients experienced relapse or disease progression after first-line therapy, and none of the second-line treatments used offered an effective survival outcome, including re-administration of L-asparaginase combination cytotoxic chemotherapy, Anti-PD-1 antibody (pembrolizumab, nivolumab), other salvage chemotherapy, and novel clinical trial medications (Fig. 5A, Table 3).
Table 3.
Comparison of the overall response rate to frontline chemotherapy in PTCLs
| Line | Stage (N) | Regimens | N | ORR, (CR+PR) | CR | PR | SD | PD | NE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ENKTL | Frontline | I/II (99) | CTX+RTX | 78 | 80% (63/78) | 57 | 6 | 4 | 6 | 5 | |
| CTX | 21 | 48% (10/21) | 8 | 2 | 0 | 3 | 8 | ||||
| III/IV (33) | CTX with L-ASP | 26 | 62% (16/26) | 13 | 3 | 1 | 5 | 4 | |||
| CTX without L-ASP | 7 | 43% (3/7) | 3 | 0 | 0 | 3 | 1 | ||||
| Salvage | Relapse/ refractory (49) | CTX with L-ASP | 18 | 72% (13/18) | 9 | 4 | 0 | 4 | 1 | ||
| Anti-PD-L1 Ab | 7 | 28% (2/7) | 2 | 0 | 0 | 4 | 1 | ||||
| Other | 24 | 58% (15/24) | 14 | 1 | 1 | 5 | 3 | ||||
| PTCL-NOS, AITL, ALCL | Frontline | I/II/III/IV (279) | CHOP-like | 160 | 65 % (104/160) | 82 | 22 | 8 | 27 | 21 | |
| CHOEP-like | 89 | 60% (53/89) | 34 | 19 | 4 | 22 | 10 | ||||
| Others | 30 | 60% (18/30) | 13 | 5 | 1 | 7 | 4 | ||||
| Salvage | Relapse/ refractory (108) | GDP, GemOX | 28 | 43% (12/28) | 8 | 4 | 1 | 13 | 2 | ||
| ICE | 21 | 33% (7/21) | 4 | 3 | 3 | 10 | 1 | ||||
| CHOP-like | 11 | 27% (3/11) | 1 | 2 | 0 | 6 | 2 | ||||
| ESHAP, DHAP | 15 | 47% (7/15) | 3 | 4 | 2 | 4 | 2 | ||||
| Other | 33 | 52% (17/33) | 12 | 5 | 2 | 12 | 2 | ||||
| Other (33) | Chidamide | 6 | 40% (2/6) | 1 | 1 | 0 | 3 | 1 | |||
| Brentuximab vedotin | 5 | 80% (4/5) | 4 | 0 | 0 | 0 | 1 | ||||
| Bendamustine | 3 | 100% (3/3) | 3 | 0 | 0 | 0 | 0 | ||||
| Azathitidine | 3 | 0% (0/3) | 0 | 0 | 0 | 3 | 0 | ||||
| Other | 16 | 25% (4/16) | 4 | 4 | 2 | 9 | 0 | ||||
Abbreviations: ENKTL, extranodal NK/T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; PTCL, peripheral T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma receptor tyrosine kinase; CTX, chemotherapy; RTX, radiotherapy; L-ASP, L-asparaginase; ORR, overall response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluated.
Fig. 5.
(A) PFS1 and PFS2 of ENKTL patients who received frontline therapy and salvage therapy (B) PFS1 and PFS2 of PTCL, AITL, and ALCL patients who received frontline therapy and salvage therapy, respectively.
Additionally, we summarized the preferred systemic chemotherapies used in the management of intestinal T-cell lymphoma and cutaneous T-cell lymphoma (CTCL) in Supplementary Tables 4 and 5. Among 17 patients diagnosed with intestinal T-cell lymphoma, 15 patients received anthracycline-based chemotherapy (CHOP- or CHOEP-like chemotherapy). The data about local treatment, including phototherapy, topical steroid, radiation, was insufficient as the primary treatment for CTCL patients, so the analysis could not be performed. However, the majority of patients (N=20/30, 66.7%) with advanced-stage or local treatment failure received anthracycline-based chemotherapy.
In the 279 patients diagnosed with PTCL-NOS, AITL, and ALCL, there was no difference in CR (51% vs. 38%, P-value = 0.31) and ORR (65.1% vs. 59.5%, P-value = 0.65) according to CHOP-like chemotherapy (CHOP21, CHOP14, mini-CHOP) and CHOEP-like chemotherapy (EPOCH, DA-EPOCH, CHOEP). Also, CHOP-like chemotherapy and CHOEP-like chemotherapy showed no significant difference in PFS1 across all age groups (21.1 months versus 15.9 months, P-value = 0.68) as well as in age-dependent groups (< 65years or ≥ 65 years). Unfortunately, no salvage chemotherapy was found to overcome the poor survival of PTCLs following relapse or disease progression. As salvage chemotherapy, the re-administration of CHOP-like chemotherapy produced an ORR of 27.3%, gemcitabine-based chemotherapy achieved an ORR of 42.9%, and ICE (ifosfamide, carboplatin, etoposide, and mesna) achieved an ORR of 33.3%. However, PFS2 was quite disappointing (median 6.0 months, 95% CI 4.8–7.2, P-value = 0.38), without any difference between chemotherapy regimens (Fig. 5B, Table 3).
When we compared survival outcomes of patients who received upfront auto-SCT (n = 44) or not (n = 235), we found that the timing of auto-SCT did not affect PFS1 (P-value = 0.16, Supplementary figure 4A). However, among patients who underwent upfront auto-SCT (n = 40), patients who achieved CR or PR after first-line chemotherapy showed better PFS1 compared to those who did not (P-value < 0.01, Supplementary figure 4B). In addition, auto-SCT had a positive impact on the OS (Supplementary Figure 4C).
Since there were only 8 patients who received allo-SCT (3 PTCL, 3 ENKTL, 1 CTCL, 1 AITL), the number of patients who underwent allo-SCT was not representative to estimate the survival. Based on ICT study, the survival rate of 8 patients who received all-SCT was 60.7 months (95% CI 11.2–110.2; Supplementary Figure 5). Moreover, although the number of patients who received allo-SCT was so small that specific comparisons were difficult, there was no difference in the survival outcome, whether patients received allo-SCT upfront or salvage aim (Figure is not presented).
4. Discussion
4.1. Overview of the background and conclusions of ICT study
Although we evaluated the relative frequency of neoplasms of mature T-cell and NK-cells across Asia, mainly in the far east of Asia, our data analysis lacks data from Japan, India, and the near eastern region. In our cohort, ENKTL (28.6%) and AITL (24.7%) cases were more frequent than that of PTCL-NOS (20.8%). The ICT study produced results slightly different regarding to the estimated frequency of mature T-cell and NK-cell lymphomas in Asian countries; this likely related to the fact that the geographic regions involved in this cohort study deferred to the ones included in previous reports (Table 4). Since the ICT study findings included only Asian patients, we believe our results are more suitable for confirming the frequency of Pan-Asian neoplasms of mature T-cell and NK-cells. For treatment of limited-stage ENKTL, chemotherapy with radiotherapy is a critical strategy [24], and we confirmed that radiation plus chemotherapy is superior to chemotherapy alone. Therefore, our observation that radiation and chemotherapy are more effective than chemotherapy alone in the early stage support previously reported results [24]. Cytotoxic chemotherapy plus L-asparaginase produced a higher proportion of CR and ORR than chemotherapy without L-asparaginase, making it an essential regimen in the advanced stages of this disease. Among neoplasms of mature T-cell and NK-cells other than ENTKL, CHOP-like and CHOEP-like regimens had similar efficacy. Also, we could not conclude the efficacy of Upfront auto-SCT in patients with PTCL-NOS, AITL, ALCL, or advanced-stage ENKTL.
Table 4.
Overview of frequency in PTCLs reported by the International T-cell Lymphoma Project, T-cell Project, and ICT study.
| International T-cell Lymphoma Project [9] | T-cell Project [45] | ICT study | |
|---|---|---|---|
| Geographic regions | North America, Europe, Asia | South America, Europe, Asia | Asia |
| Asian countries and territories among enrolled regions | Bangkok, Hong Kong, Singapore, Japan, Korea | Hong Kong, Korea | China, Korea, Taiwan, Singapore, Indonesia, Malaysia |
| Number of cases | 1,314 cases | 737 cases | 486 cases |
| Pathology reading standards | WHO 2008 | WHO 2008 | WHO 2008 |
| Overall major lymphoma subtypes, % (N) | |||
| PTCL-NOS | 25.9% (340) | 38.4% (283) | 20.8% (101) |
| AITL | 18.5% (243) | 16.7% (123) | 24.7% (120) |
| ALK-negative ALCL | 5.5% (72) | 13.4% (99) | 6.2% (30) |
| ALK-positive ALCL | 6.6% (87) | 6.6% (49) | 6.9% (34) |
| ENKTL | 10.4% (136) | 12.5% (92) | 28.6% (139) |
| ATLL | 9.6% (126) | NE | 0.4% (2) |
Abbreviations: NE, not evaluated.
4.2. Treatment strategies and limitations of immune checkpoint inhibitors for ENKTL
Immuno-oncology is expected to be the next important strategy for ENKTL [25,26]. However, we know that the efficacy of immune checkpoint inhibitors such as pembrolizumab and nivolumab is not the same for all ENKTL [27,28]. Therefore, several types of research have been performed to find out why the effects of immune checkpoint inhibitors differ, even though PD-L1 expression is similar among patients diagnosed with ENKTL. Unfortunately, we could not collect the data of PD-1 and PD-L1 staining, as well as include a few patients treated with immune checkpoint inhibitors. Thus, our results are not suitable for testing whether PD-1 or PD-L1 expression results are meaningful biomarkers. However, we recognize the unmet need to discuss a new and powerful predictive marker that overcomes the limitations of PD-1 and PD-L1 before starting treatment for advanced and relapsed/refractory ENTKL. Cho et al. used immunohistochemistry (IHC) and gene expression to study the tumor immune microenvironment (TIME) of ENKTL [29]. Jie Xiong et al. performed a study to identify the molecular subtypes of ENKTL based on genomic structural alterations in the RNA helicase family, tumor suppressors, the JAK-STAT pathway, epigenetic modifiers, and the RAS-MAPK pathway [30]. Thus, they recommended choosing a PD1 blocker while considering NK cell-associated immunity, in addition to the PD-L1 marker, in an opinion similar to that of Cho et al.
4.3. Discussing effective chemotherapy strategies for neoplasms of mature T-cell and NK-cells
Because neoplasms of mature T-cell and NK-cells exhibit extraordinary and heterogeneous properties, standard treatments have not yet been established. Most patients with T-cell lymphoma receive CHOP as the standard first-line treatment [31]. In general, the response rate to CHOP chemotherapy was more than 50% for PTCL-NOS, AITL, and ALCL [32]. Intensive chemotherapy regimens are sometimes considered as the first-line therapy for T-cell lymphoma patients [33], many clinicians also add etoposide to CHOP. In a previous analysis comparing CHOP and CHOEP, their ORR, PFS, and OS for PTCLs did not differ significantly {34]. The ICT study supports the finding that the therapeutic advantages of CHOP and CHOEP are essentially the same. Gleeson et al. conducted a randomized phase 2 trial to compare the efficacy of GEM-P (gemcitabine, cisplatin, and methylprednisolone) and CHOP. They found that gemcitabine-based chemotherapy was not more beneficial than CHOP [35]. As a more biologic approach, brentuximab vedotin (BV), which targets the CD30 commonly found in PTCL, is gaining attention. Although the number of patients using BV monotherapy in our study was very limited to 5 patients, each CR and ORR was 80%. Based on a handful of our data, we could not determine the efficacy of BV in PTCLs, but the therapeutic response is very encouraging. A randomized phase 3 trial comparing CHOP versus CHP-BV (brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone), showed significant improvement of PFS in neoplasms of mature T-cell and NK-cells (P-value = 0.0110). However, the notable benefit is coming from ALCL (CD 30 high expression peripheral T-cell lymphomas). The CHP-BV benefit in PTCL-NOS is marginal (HR 0.75, 95% CI 0.41–1.37) and even reversed in AITL (HR 1.40, 95% CI 0.64–3.07) [36]. Therefore, CHOP is still considered as standard care in many countries, and this is also echoed in the ICT data. However, the outcomes remain poor with this approach. Therefore, we are conducting a randomized phase 2 study investigating the effectiveness of CHOP versus platinum-based ICE that is also ongoing (Randomized phase 2 study to compare the efficacy of CHOP versus fractionated ICED in transplant-eligible patients with previously untreated peripheral T-cell lymphoma: ROSE study, NCT02445404), and results will be released as soon as they have been solidified. The results of the ROSE study are expected to serve as a guide to more clearly locate CHOP as the standard chemotherapy for neoplasms of mature T-cell and NK-cells.
4.4. Identification of the role of auto-SCT for patients with neoplasms of mature T-cell and NK-cells
Although the role of high-dose chemotherapy with auto-SCT after achieving CR has been discussed in several studies, it remains challenging to clarify the role of upfront auto-SCT in clinical practice [37], [38], [39]. According to previously conducted research, high-dose chemotherapy followed by auto-SCT improved the long-term survival outcomes of patients with neoplasms of mature T-cell and NK-cells [40], [41], [42]. However, the results from the LYSA study did not support the opinion on the effectiveness of upfront auto-SCT [43]. Tang et al. also reported similar results to those published by LYSA on upfront auto- SCT [44]. In our study, ALK-negative ALCL, AITL, and PTCL-NOS patients who received upfront auto-SCT did not attain a significant survival benefit. Therefore, we considered that the role of upfront auto-SCT is not yet decisive.
Surprisingly, in our study, T-cell lymphomas such as ATLL, commonly seen in HTLV-1 endemic areas, were identified in very low frequencies, something different to previously reported Asian studies (mainly in regions of Japan and China). This low frequency of ATLL cases, which is known to have a dismal prognosis, could have potentially influenced the overall outcomes of our study population. Moreover, the lack of a centralized pathology review is a limitation of the study.
As the first multinational, multicenter joint study in Asia, the ICT study will give an opportunity to understand better the geographic difference of neoplasms of mature T-cells and NK-cells in the world. Besides, through an integrated analysis of the therapeutic strategies currently used in each country and institute, we have presented the preferred treatments and their survival outcomes across Asia. According to our findings, patients with relapsed/refractory disease still have an unmet need for the next therapeutic approaches to improve their prognosis. Thus, more efforts should continuously need to find better treatment approaches and therapy for relapsed/refractory disease patients in the future.
Authors’ contributions
Z.J., and W.S.K. conceived and designed the study.
S.E.Y., Y.S., S.J.K., D.H.Y, T.Y.C., Y.K., K.W.K., H.S.L., K.K.W.T., S.T.L., M.P., C.I., W.Z.,Y.R.D., M.H.L., S.C.N., W.S.L., Y.G., H.Z., H.J.K., H.J.Y., H.J.K., D.T.C.L., J.Y.K., J.J.H., Y.C.M., S.Y.O., H.S., J.H.K., B.S.S., S.K.P., J.C.J., Y.H.K., Z.J, and W.S.K did the prospective study, accrued patients to the study, and collected clinical data.
S.E.Y., Y.S., S.J.K., Z.J, and W.S.K anlysed the data and wrote the manuscript.
All authors edited the manuscript and all authors approved the final version of the manuscript.
Data sharing
All data will become publicly available upon request from the corresponding authors.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgements
This study was supported by grants from Mundipharma. The grants were used only for study protocol development, electronic Case Report Form (eCRF) development, eCRF operation, and data management.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100126.
Contributor Information
Zhu Jun, Email: zhu-jun2017@outlook.com.
Won Seog Kim, Email: wskimsmc@skku.edu.
Appendix. Supplementary materials
References
- 1.A clinical evaluation of the international lymphoma study group classification of non-Hodgkin's lymphoma. The non-Hodgkin's lymphoma classification project. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 2.Smith A, Crouch S, Lax S, Li J, Painter D, Howell D. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112(9):1575–1584. doi: 10.1038/bjc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federico M, Manni M, Civallero M, Skrypets T. The T cell project 2.0: the more we register, the more we learn. Hematol Oncol. 2019;37(S2):164–165. [Google Scholar]
- 4.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi MA, Evens AM, Tallman MS, Nelson BP, Rosen ST. T-cell non-Hodgkin lymphoma. Blood. 2006;107(4):1255–1264. doi: 10.1182/blood-2005-03-1306. [DOI] [PubMed] [Google Scholar]
- 6.Pathologists LSGoJ. The world health organization classification of malignant lymphomas in Japan: Incidence of recently recognized entities. Pathol Int. 2000;50(9):696–702. doi: 10.1046/j.1440-1827.2000.01108.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Ko YH, Lee SS, Huh J, Kang CS, Kim CW. WHO classification of malignant lymphomas in Korea: report of the third nationwide study. J Pathol Transl Med. 2011;45(3):254–260. [Google Scholar]
- 8.Liu W, Ji X, Song Y, Wang X, Zheng W, Lin N. Improving survival of 3760 patients with lymphoma: Experience of an academic center over two decades. Cancer Med. 2020;9(11):3765–3774. doi: 10.1002/cam4.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 10.de Leval L, Gaulard P. Pathology and biology of peripheral T-cell lymphomas. Histopathology. 2011;58(1):49–68. doi: 10.1111/j.1365-2559.2010.03704.x. [DOI] [PubMed] [Google Scholar]
- 11.Briski R, Feldman AL, Bailey NG, Lim MS, Ristow K, Habermann TM. The role of front-line anthracycline-containing chemotherapy regimens in peripheral T-cell lymphomas. Blood Cancer J. 2014;4:e214. doi: 10.1038/bcj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish lymphoma registry. Blood. 2014;124(10):1570–1577. doi: 10.1182/blood-2014-04-573089. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-cell tumor study group study. J Clin Oncol. 2011;29(33):4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 14.Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia lymphoma study group. Blood. 2012;120(15):2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 15.Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 16.Lim SH, Hong JY, Lim ST, Hong H, Arnoud J, Zhao W. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017;28(9):2199–2205. doi: 10.1093/annonc/mdx316. [DOI] [PubMed] [Google Scholar]
- 17.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 19.Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 20.Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24(16):2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- 21.Vose JM, Project TIP. International peripheral T-cell lymphoma (PTCL) clinical and pathologic review project: poor outcome by prognostic indices and lack of efficacy with anthracyclines. Blood. 2005;106(11) 811- [Google Scholar]
- 22.Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389–400. doi: 10.1016/S1470-2045(15)00533-1. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CP, Civallero M, Ko YH, Manni M, Skrypets T, Pileri S. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol. 2020;7(4):e284–ee94. doi: 10.1016/S2352-3026(19)30283-2. [DOI] [PubMed] [Google Scholar]
- 25.Kwong Y-L, Chan TSY, Tan D, Kim SJ, Poon L-M, Mow B. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11(1) doi: 10.1186/s13045-018-0559-7. 15- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Lim JQ, Laurensia Y, Cho J, Yoon SE, Lee JY. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020 doi: 10.1182/blood.2020007247. [DOI] [PubMed] [Google Scholar]
- 29.Somasundaram N, Lim JQ, Ong CK, Lim ST. Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL) J Hematol Oncol. 2019;12(1) doi: 10.1186/s13045-019-0717-6. 28- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong J, Cui B-W, Wang N, Dai Y-T, Zhang H, Wang C-F. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell. 2020;37(3) doi: 10.1016/j.ccell.2020.02.005. 403-19.e6. [DOI] [PubMed] [Google Scholar]
- 31.Kohrt H, Advani R. Extranodal natural killer/T-cell lymphoma: current concepts in biology and treatment. Leuk Lymphoma. 2009;50(11):1773–1784. doi: 10.3109/10428190903186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15(10):1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Yang M, Wu M, Zheng W, Xie Y, Zhu J. A retrospective study of the CHOP, CHOPE, and CHOPE/G regimens as the first-line treatment of peripheral T-cell lymphomas. Cancer Chemother Pharmacol. 2019;83(3):443–449. doi: 10.1007/s00280-018-3744-z. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, Metzner B. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German high-grade non-Hodgkin lymphoma study group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 35.Gleeson M, Peckitt C, To YM, Edwards L, Oates J, Wotherspoon A. CHOP versus GEM-P in previously untreated patients with peripheral T-cell lymphoma (CHEMO-T): a phase 2, multicentre, randomised, open-label trial. Lancet Haematol. 2018;5(5):e190–e200. doi: 10.1016/S2352-3026(18)30039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet North Am Ed. 2019;393(10168):229–240. doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 38.Reimer P, Rüdiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 39.Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20(9):1533–1538. doi: 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 40.Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR, Rosen ST. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019;125(9):1507–1517. doi: 10.1002/cncr.31861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang T, Khoo LP, Lim C, Ham JS, Kim SJ, Hong H. Outcomes of patients with peripheral T-cell lymphoma in first complete remission: data from three tertiary Asian cancer centers. Blood Cancer J. 2017;7(12) doi: 10.1038/s41408-017-0030-y. 653- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M, Wang X, Xie Y, Liu W, Zhang C, Ping L. Outcome and prospective factor analysis of high-dose therapy combined with autologous peripheral blood stem cell transplantation in patients with peripheral T-cell lymphomas. Int J Med Sci. 2018;15(9):867–874. doi: 10.7150/ijms.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fossard G, Broussais F, Coelho I, Bailly S, Nicolas-Virelizier E, Toussaint E. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol. 2018;29(3):715–723. doi: 10.1093/annonc/mdx787. [DOI] [PubMed] [Google Scholar]
- 44.Tang T, Khoo LP, Lim C, Ham JS, Kim SJ, Hong H. Outcomes of patients with peripheral T-cell lymphoma in first complete remission: data from three tertiary Asian cancer centers. Blood Cancer J. 2017;7(12) doi: 10.1038/s41408-017-0030-y. [Internet]2017/12//[653 p.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellei M, Chiattone CS, Luminari S, Pesce EA, Cabrera ME, de Souza CA. T-cell lymphomas in South america and europe. Revista brasileira de hematologia e hemoterapia. 2012;34(1):42–47. doi: 10.5581/1516-8484.20120013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.