Abstract
Chemotherapy is indispensable for gastric cancer. For unresectable and/or recurrent gastric cancer, first‐line chemotherapy consists of multidrug regimens including oral 5‐FU agents such as S1/Xeloda and platinum preparations, as well as Trastuzumab, which is effective in HER2‐positive cases. Second‐ and third‐line chemotherapy regimens include taxanes, Ramucirumab (R‐mab), and Nivolumab (N‐mab), which have different mechanisms of action from first‐line chemotherapy. R‐mab is molecularly targeted to vascular endothelial growth factor receptor 2 in the host cells, but its indication is not conditional. For resectable gastric cancer, in Eastern countries, postoperative adjuvant chemotherapy has been successful, including S1, Docetaxel/S1 (DS), and Xeloda/Oxaliplatin (Xelox) regimens, whereas, in Western countries, the 5‐FU/Leucovorin/Oxaliplatin/Docetaxel (FLOT) regimen was recently shown to be effective in the perioperative chemotherapy setting. Most recently, however, in Eastern countries, perioperative SOX was demonstrated to be effective in specific advanced gastric cancer. For stage IV gastric cancer, new therapeutic strategies have been proposed such as neoadjuvant chemotherapy and conversion surgery, and cures can be conditionally obtained. Recent genomic understanding of gastric cancer proposed a diversity of molecular targets by molecular profiling. Such optimized chemotherapy regimens, according to the specific clinical situations, have been rigorously established for the best survival of advanced gastric cancer.
Keywords: chemotherapy, resectable gastric cancer, stage IV, unresectable gastric cancer
History and emerging chemotherapy regimens in first‐line chemotherapy for unresectable and/or recurrent gastric cancer. Japan‐directed evidence recommends SOX as well as CS regimens the best. Extra‐Japan‐directed evidence recommends Xelox as well as XP or EOX.

1. INTRODUCTION
Gastric cancer is increasing worldwide and is the second leading cause of death among human cancers. 1 Early gastric cancer showed excellent prognosis following surgery alone, 2 , 3 whereas advanced gastric cancer exhibits poor prognosis with surgery alone in Western countries 4 , 5 (overall survival [5‐year OS] 20%‐30%, Figure 1A,B) and in Eastern countries 6 , 7 (5‐year OS 60%‐70%, Figure 1C,D).
FIGURE 1.

Overall survival (OS) in resectable advanced gastric cancer following surgery alone in phase III clinical trials. A, 5‐year OS in the United States of America was 27% following surgery alone in the INT116 trial. B, 5‐year OS in Europe was 24% following surgery alone in the MAGIC trial. C, 5‐year OS in Japan was 61% following surgery alone in the ACTS‐GC trial. D, 5‐year OS in Korea was 69% following surgery alone in the CLASSIC trial. These figures are used after modification of the original references 4 , 5 , 6 , 7
The different prognoses between Western and Eastern countries may be due to a different composition of advanced disease (the former included a curative rate of 66% following surgery, 4 whereas the latter was pathologically confirmed as stage II/III with a negative cytology test 8 ). As prognostic outcomes are geographically quite different, therapeutic strategies vary among countries. However, the most optimal therapeutic strategy, including chemotherapy for the specific clinical situation, should be selected based on the scientific evidence allowing for clinical benefits and toxicity profiles.
In this review, the history of development of chemotherapy for unresectable and/or recurrent advanced gastric cancer will be initially described. Then, perioperative adjuvant chemotherapy for resectable gastric cancer will be reviewed. Chemotherapy regimens included singlet, doublet, and triplet ones, including molecular targeted therapy as well as immune checkpoint inhibitors (ICIs).
2. AGENTS ACTIVE FOR GASTRIC CANCER AND MECHANISM OF ACTION
In the history of gastric cancer chemotherapy, anti‐metabolite (5‐fluorouracil‐5‐FU) and platinum preparations (cisplatin‐CDDP) as well as trastuzumab (HER2 neutralizing antibody, T‐mab) and nivolumab (N‐mab), an ICI, were critical and were adopted as first‐line chemotherapy regimens for unresectable and/or recurrent gastric cancer worldwide (Figure 2, red color).
FIGURE 2.
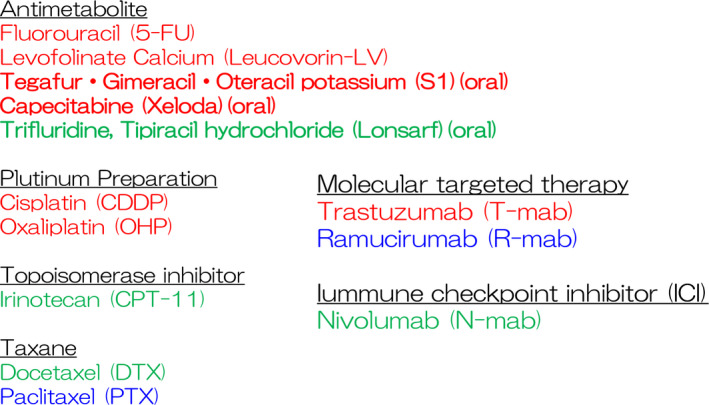
Active chemotherapeutic agents for gastric cancer. Active agents for gastric cancer are classified into antimetabolite, platinum preparation, molecular‐targeted therapy, topoisomerase inhibitor, taxane, and immune checkpoint inhibitor (ICIs). Red letters represent first‐line chemotherapy regimens, blue letters represent second‐line chemotherapy regimens, and green letters represent third‐line singlet chemotherapy drugs. Bold letters represent currently available for oral medicines
5‐FU inhibits cytoplasmic thymidylate synthase (TS) activity to suppress thymine synthesis, followed by inhibition of DNA synthesis. 9 Levofolinate calcium (Leucovorin/LV) can also directly bind to TS to augment 5‐FU activity, 10 and it is thus used as a part of the chemotherapeutic regimens such as FOLFOX 11 , 12 or FLOT. 13 Fluorinated pyrimidine preparations such as S1 (Taiho Pharmacoceutical Corp, Tokyo) and Xeloda (Roche Corp, France) represented by Capecitabine are available for oral delivery of 5‐FU (Figure 2, bold).
S1 includes tegafur (5‐FU derivative) with gimeracil and oteracil potassium to suppress its metabolic degradation in the liver and adverse events (AEs) in the gut, respectively, 14 whereas Xeloda includes capecitabine (5‐FU prodrug activated by thymidine phosphorylase specifically overexpressed in cancer cells). 15 As a result, the 5‐FU concentration in the blood with the use of Xeloda is theoretically lower while obtaining similar anti‐tumor effects in comparison to S1.
Platinum preparations can be covalently cross‐linked with adenine/guanine 16 to suppress DNA synthesis independently of 5‐FU antagonism. 17 Thus, the combination regimen of 5‐FU and platinum preparations has become the most popular first‐line chemotherapy. Recently, CDDP is exchanged for oxaliplatin (OXP), 18 because CDDP is not convenient, requiring massive hydration to reduce renal toxicity.
In gastric cancer, first‐line chemotherapy also included molecular targeted agents such as Trastuzumab (T‐mab) in cases of HER2‐positive gastric cancer. 19 , 20 HER2 oncogene (alternatively designated as ErbB2) is a membrane‐bound tyrosine kinase expressed on cancer cells.
HER2 can form a heterodimeric complex with other ErbB family members such as HER1 (epidermal growth factor receptor‐EGFR), HER3 (ErbB3), and HER4 (ErbB4), and their numerous cognate ligands (EGF, transforming growth factor‐alpha, amphiregulin, betacellulin, heparin binding‐EGF, epiregulin, neuregulin1‐4) can transmit cancer cell proliferation signaling through cell surface receptors. 21 Most intriguingly, HER2 DNA is frequently amplified in gastric cancer. 22 , 23 Thus, HER2 antagonism has therapeutic rationale in gastric cancer control.
For second‐line chemotherapy, paclitaxel (PTX)/ramucirumab (R‐mab) doublet regimen is effective in gastric cancer patients refractory to first‐line chemotherapy (Figure 2, blue color). 24 PTX is a taxane that inhibits depolymerization of tubulin and subsequent cell division. 25 On the other hand, R‐mab is a specific inhibitor of vascular endothelial growth factor (VEGF) receptor‐2 (VEGFR2) expressed on the host cells. 26 Ligands of VEGFR2 are VEGF‐A and VEGF‐C/D derived from the tumor cells, 27 which are involved in angiogenesis and lymphangiogenesis, 28 respectively, to suppress tumor progression.
For third‐line chemotherapy (Figure 2, green color), Nivolumab (N‐mab) is effective in gastric cancer patients refractory to conventional chemotherapy. 29 N‐mab theoretically antagonizes programmed death‐1 (PD‐1) expressed on stimulated T cells 30 , 31 and exhibits anti‐tumor activity by modulating the host immune system against cancer cells. 32 , 33 Oral anticancer drug Lonsarf would be convenient for such compromised patients with drug resistance. Lonsarf is a combination of two agents: trifluridine, a nucleoside analog, and tipiracil, a thymidine phosphorylase inhibitor that prevents rapid metabolism of trifluridine.
3. HISTORICAL SIGNIFICANCE OF FIRST‐LINE CHEMOTHERAPY REGIMENS FOR HER2‐POSITIVE UNRESECTABLE AND/OR RECURRENT GASTRIC CANCER
The Japanese Gastric Cancer Association (JGCA) gastric cancer treatment guidelines amended in 2018 (5th edition) clearly defined the recommended chemotherapy regimens for unresectable and/or recurrent gastric cancer (Figure 3A). 34
FIGURE 3.

History and emerging chemotherapy regimens in first‐line chemotherapy for unresectable and/or recurrent gastric cancer. A, The JGCA gastric cancer guidelines recommended chemotherapy regimens for first‐line, second‐line, and third‐line chemotherapy in unresectable and/or recurrent gastric cancer. B, Japan‐directed evidence in the JGCA gastric cancer guidelines at present recommend SOX as well as CS regimens. C, Extra‐Japan‐directed evidence in the JGCA gastric cancer guidelines at present recommends Xelox as well as XP, because epirubicine, which is used in Europe, is not available in Japan (yellow lines). Red colors represent level A evidence, and blue colors represent level B evidence in the JGCA gastric cancer guidelines. Publication years are shown in yellow letters in the black box
The recommended chemotherapy regimens are composed of the following three categories: (a) regimens that had confirmed superiority over or non‐inferiority to the conventional standard treatment in terms of OS in phase III clinical trials; (b) regimens demonstrating a reproducible clinical benefit in phase II clinical trial for a specific patient group; and (c) regimens that served as a control arm in multiple phase III clinical trials and were considered a standard treatment. The strength of evidence level is defined as A to D, and recommended treatments are composed of both evidence levels A and B.
For example, the ToGA trial 19 , 35 explored the efficacy for OS of T‐mab (XPT regimen) in addition to the Xeloda/CDDP (XP) regimen, and XPT was demonstrated its prognostic efficacy in HER2‐positive gastric cancer. On the other hand, the AVAGAST trial explored the efficacy and safety of T‐mab in addition to XP in gastric cancer irrespective of HER2 status, where its efficacy for OS was not confirmed. 20 Both were phase III clinical trials, and the control regimen was the XP regimen as first‐line standard chemotherapy for unresectable and/or recurrent gastric cancer. Hence, the XP regimen was considered an alternatively recommended first‐line chemotherapy regimen (evidence A) in unresectable and/or recurrent gastric cancer.
In the ToGA trial, median OS of the XPT group was 13.8 months, whereas that of the XP group was 11.1 months (P = .0046), 19 and thus XPT was effective for OS improvement only in HER2‐positive gastric cancer patients, 19 , 20 and the HER2 status must be initially examined for decision making.
S1/CDDP/T‐mab (SPT) regimen also showed good prognosis (median OS were 16.0 and 14.6 months) in the two independent phase II clinical trials for HER2‐positive gastric cancer, 36 , 37 and is thus considered as recommended first‐line chemotherapy (evidence B) as well.
4. FIRST‐LINE CHEMOTHERAPY REGIMENS FOR HER2‐NEGATIVE UNRESECTABLE AND/OR RECURRENT GASTRIC CANCER
In the JCOG9902 trial, S1 alone was non‐inferior to 5‐FU alone for OS in unresectable and/or recurrent gastric cancer, 38 and the SPIRITS trial confirmed superiority of the CS doublet regimen to S1 alone for OS. 39 Until recently in Japan, the CS regimen has long been considered the standard first‐line chemotherapy, because no regimen is superior to the CS regimens for OS based on S1 oral medication at present (Figure 3B).
On the other hand, S1/OXP (SOX) exhibited non‐inferiority to CS for progression‐free survival (PFS) similarly to OS in the G‐SOX trial. 18 In the JGCA gastric cancer treatment guideline, the SOX regimen was not level A, but level B, because non‐inferiority of the SOX regimen for PFS was the primary endpoint in the G‐SOX trial (Figure 3B). Nevertheless, the SOX regimen has become popular due to its convenient handling of AEs.
In Europe, the Epirubicin/CDDP/5‐FU (ECF) regimen was demonstrated early to be superior to the 5‐FU/Doxorubicin/Methotrexate (FAMTAX) regimen for OS in unresectable and/or recurrent gastric cancer, 40 where FAMTAX is considered to be equivalent to the CF regimen (Figure 3C). 41
Afterwards, as OS was longer in the Epirubicin/OXP/Xeloda (EOX) group (median 11.2 months) than in the ECF group (median 9.9 months, P = .02) in the REAL2 trial, 42 the Xeloda/OXP (Xelox) combination is believed to be at least non‐inferior to the CF combination in Epirubicin‐based triplet multidrug regimens (Figure 3C).
In Korea, the XP regimen actually showed non‐inferiority to the CF regimen for PFS of unresectable and/or recurrent gastric cancer, 43 and was used as control arms in the ToGA trial 19 and the AVAGAST trial 20 as previously described (evidence A)(Figure 3B). The Xeloda/OXP (Xelox) regimen is also approved as recommended first‐line chemotherapy (evidence B) in the JGCA gastric cancer treatment guideline, allowing for the subset analysis of the REAL‐2 trial. 42 Moreover, in the phase II NCT00985556 trial, both the SOX and Xelox regimens were equally active. 44
FOLFOX was also used for unresectable and/or recurrent gastric cancer as a control regimen of first‐line chemotherapy in several phase III clinical trials, 45 , 46 and was additionally listed as a recommended regimen of first‐line chemotherapy (evidence B).
In the Checkmate‐649 trial, recently, N‐mab plus chemotherapy (CDDP‐containing) clearly showed OS benefit as a first‐line chemotherapy in comparison to chemotherapy alone irrespective of PD‐L1 expression in HER2‐negative unresectable and/or recurrent gastric cancer patients. 47 This result is also followed by the Attraction‐4 trial, where N‐mab in addition to the standard first‐line chemotherapy (OXP‐containing) prolonged PFS (10.5 months, P = .0007) in the interim analysis of the Attraction‐4 phase II/III trial. 48 Thus, first‐line chemotherapy will be changed in the near future.
5. SECOND‐LINE AND THIRD‐LINE CHEMOTHERAPY
Second‐line chemotherapy is administered to patients with treatment failure of first‐line chemotherapy, and the RAINBOW trial demonstrated that the Paclitaxel/Ramucirumab (PTX/R‐mab) regimen significantly increases OS in comparison with PTX alone 24 (Figure 3A). Interestingly, The Cancer Genome Atlas (TCGA) molecular profile revealed frequent genomic amplification of VEGF as well as HER2 in chromosomal instable (CIN) gastric 22 and esophageal adenocarcinoma. 23 However, the relationship between the VEGF status and clinical efficacy of the PTX/R‐mab regimen remains elusive at present, and its use is not conditional differently from T‐mab, putatively because its target host cells include vascular and lymphatic endothelial cells.
The recommended third‐line chemotherapy includes singlet agents such as N‐mab or Irinotecan (Figure 3A). In the ATTRACTION‐2 trial, N‐mab, an ICI, drastically improves OS in comparison to best supportive care (BSC) in advanced gastric cancer that is refractory to chemotherapy. 29 The TAGS trial also demonstrated that Lonsarf, an oral anticancer drug, alone can improve OS in comparison with BSC in the third‐line chemotherapy setting. 49
T‐mab Deruxtecan (DS‐8201a) is an antibody‐drug conjugate (ADC) consisting of an anti‐HER2 antibody, a cleavable tetrapeptide‐based linker, and cytotoxic topoisomerase inhibitor exatecan mesylate. In the DECTINY‐Gastric01 trial, DS‐8201a led to significant improvements in response and OS, as compared with conventional therapies, among patients with HER2‐positive gastric cancer that had progressed while they were receiving at least two previous therapies (as third‐line chemotherapy), including T‐mab. 50 For DS‐8201a, myelosuppression and interstitial lung disease were the notable toxic effects.
6. POSTOPERATIVE ADJUVANT CHEMOTHERAPY FOR RESECTABLE GASTRIC CANCER
Adjuvant chemotherapy development has differed greatly between countries; however, it is associated with first‐line chemotherapy regimen (Figure 1A‐D). In Japan, postoperative 1‐year S1 adjuvant chemotherapy was confirmed to improve OS in comparison to surgery alone in pathological stage II/III gastric cancer 8 (Figure 4C, see S1‐1).
FIGURE 4.

Recurrence‐free survival (RFS) of resectable advanced gastric cancer following surgery plus postoperative adjuvant chemotherapy in phase III clinical trials. A, 5‐year RFS was 79% in pathological stage II (the JGCA 13th edition) in the ACTS GC trial. B, 3‐year RFS in pathological stage III was 67% following surgery alone in the JACCRO‐GC7 trial. C, S1 adjuvant chemotherapy was initially shown to be effective in pathological stage II/III in 2007 (S1‐1), and was re‐analyzed with sufficient follow‐up terms in 2011 (S1‐2). CS adjuvant chemotherapy was shown to be superior to S1 adjuvant chemotherapy in 2019. Xelox adjuvant chemotherapy was initially shown to be effective in pathological stage II/III in 2012 (Xelox‐1), and was re‐analyzed with sufficient follow‐up terms in 2014 (Xelox‐2). These figures are used after modification of the original references 6 , 52
Postoperative 1‐year S1 adjuvant chemotherapy showed better RFS than surgery alone (Figure 1C), especially in pathological stage II. 6 The 5‐year RFS rate of the pathological stage II gastric cancer patients was 79.2% in the S1 group and 64.4% in the surgery alone group (Figure 4A).
Postoperative half‐year S1 adjuvant chemotherapy was then compared to the standard 1‐year S1 administration in stage II gastric cancer, and the half‐year S1 chemotherapy did not demonstrate non‐inferiority to the standard 1‐year chemotherapy. 51 Thus, long‐term 1‐year chemotherapy is strongly recommended in stage II gastric cancer.
On the other hand, in the JACCRO GC‐7 trial, in pathological stage III gastric cancer, postoperative Docetaxel (DTX)/S1 (DS) regimen increased RFS in comparison to the 1‐year standard adjuvant S1 chemotherapy, 52 the success of which is associated with that in first‐line chemotherapy. 53 The 3‐year RFS rate of pathological stage III gastric cancer patients was 67% in the DS group and 50% in the S1 group (Figure 4B).
In Korea, a half‐year doublet postoperative chemotherapy (Xelox) improved disease‐free survival (DFS) in comparison to surgery alone 19 (Figure 4C, see Xelox‐1), and long‐term follow‐up recapitulated the initial analysis of prognosis, 7 as in the AGTS‐GC trial.
Direct comparison between S1‐based regimen and Xeloda‐based regimen was defective, and thus which regimen is better for pathological stage II/III gastric cancer is yet to be established. Anyway, postoperative adjuvant chemotherapy regimens for pathological stage II/III gastric cancer are available for S1, DS, and Xelox at present.
In ARTIST2 trial, the most recent postoperative adjuvant SOX or SOX radiotherapy (SOXRT) was proved to be effective in prolonging DFS, when compared to S1 monotherapy in patients with curatively D2‐resected, stage II/III gastric cancer. 54
7. NEOADJUVANT CHEMOTHERAPY FOR RESECTABLE GASTRIC CANCER
In European countries, the ECF regimen for perioperative chemotherapy was demonstrated to improve OS in comparison to surgery alone in resectable advanced gastric cancer (Figure 1B). 5 The 5‐year OS with perioperative chemotherapy plus surgery was 37%, which is a 13% improvement over that with surgery alone. Perioperative chemotherapy includes preoperative chemotherapy designated as neoadjuvant chemotherapy, which was considered to be a promising strategy for aggressive gastric cancer.
Because the prognosis of resectable advanced gastric cancer without adjuvant therapy between Western and Eastern counties is so different (~30% and ~70% for 5‐year OS, respectively), in Japan, neoadjuvant chemotherapy is limited to clinically aggressive gastric cancer.
JCOG0001 and JCOG0405 showed promising efficacy of CS neoadjuvant chemotherapy for gastric cancer with extensive lymph node metastasis, which included both curable stage (bulky lymph node disease in the regional lymph nodes) as well as paraaortic lymph node metastasis (stage IV). Hence, the recent gastric cancer treatment guideline described that neoadjuvant chemotherapy is weakly recommended for this special disease.
In the JCOG0501 trial, on the other hand, additional neoadjuvant chemotherapy with the CS regimen was compared only in macroscopically aggressive gastric cancer (type IV and giant type III) patients who received standard 1‐year postoperative S1 chemotherapy 55 ; however, no prognostic improvement was seen in the additional neoadjuvant group. 56 At present, in Japan, neoadjuvant therapy is considered to be limited to specific gastric cancer and can be largely performed only in clinical studies. The SOX regimen in the neoadjuvant setting is also being tested in advanced gastric cancer with cT3‐4N1‐3 in the JCOG1509 (NAGISA) trial.
Recently, in the RESOLVE trial in China, perioperative SOX improved 3‐year DFS compared with postoperative XELOX (P = .045), whereas postoperative SOX was non‐inferior to postoperative Xelox in gastric or gastro‐esophageal junction adenocarcinoma patients with cT4a/N + M0 or cT4bNxM0. 57 These findings suggested that perioperative SOX is effective in the specific advanced gastric cancer even in Eastern countries.
The novel Docetaxel/Oxaliplatin/S1 (DOS) neoadjuvant chemotherapy is being challenged in recent clinical trials in Eastern countries for resectable gastric cancer (PRODIGY trial), 58 gastric cancer with extensive nodal metastasis, 59 and esophagogastric cancer. 60 In the PRODIGY trial, addition of neoadjuvant DOS to D2 gastrectomy and postoperative adjuvant S1 chemotherapy led to significant tumor downstaging and improved PFS with acceptable safety, 61 and this treatment strategy is also promising as a treatment option for resectable advanaced gastric cancer.
In Western countries, perioperative chemotherapy has progressed outstandingly as shown in the FLOT4 trial, 13 in which the perioperative 5‐FU/LV/OXP/DTX (FLOT) chemotherapy improved OS (5‐year OS of 45%) in comparison to ECF/EOX (the standard perioperative chemotherapy)(5‐year OS of 36%). The FLOT regimen is corresponding to the DOS regimen developing in Eastern countries. Development of novel successful adjuvant chemotherapy took over 10 years in both Eastern and Western countries (Figure 4C).
8. CHEMOTHERAPEUTIC STRATEGIES FOR STAGE IV GASTRIC CANCER
Even in stage IV gastric cancer, surgery has become promising for a cure along with recently emerging therapeutic strategies such as neoadjuvant chemotherapy or conversion surgery.
For the first time, in the JCOG0405 trial, stage IV gastric cancer with paraaortic lymph node metastasis can be cured with neoadjuvant chemotherapy of the CS regimen (two or three courses) followed by surgery. 62 The 5‐year OS was 43.5% in gastric cancer patients with regional bulky N (−)/PAND (+) who underwent D2 + paraaortic lymph node dissection 63 (Figure 5A).
FIGURE 5.

OS of stage IV gastric cancer following surgery plus chemotherapy. A, 5‐year OS was 44% in patients with advanced gastric cancer with paraaortic lymph node (PAN) metastasis alone (blue line) who underwent irinotecan/CDDP or CS neoadjuvant chemotherapy followed by gastrectomy with D3 lymph node dissection in the JCOG0001 and JCOG0405 trials, respectively. B, 5‐year OS was 35% in patients with stage IV gastric cancer (yellow line) who underwent DCS neoadjuvant chemotherapy followed by gastrectomy in the National Cancer Center East Hospital, where stage IV gastric cancer included 18 patients with PAN metastasis. 11 C, Patients with gastric cancer with CY1 and/or P1a who underwent gastrectomy with postoperative chemotherapy such as S1 (blue), CS (yellow), and other chemotherapeutic regimens (green) showed 5‐y OS of 27%, 24%, and 23%, respectively, in contrast to no chemotherapy group (~0%). These figures are used after modification of the original references 63 , 64 , 66
On the other hand, potent chemotherapy followed by conversion surgery also recently produced a cure for stage IV gastric cancer that included paraaortic lymph node metastasis; conversion surgery after DCS chemotherapy produced 5‐year OS of over 30%‐40% in stage IV gastric cancer 64 , 65 (Figure 5B). As almost all cases of conversion surgery can be done after first‐line chemotherapy, emerging new first‐line chemotherapy is greatly anticipated to increase the chance for conversion surgery followed by a cure in the future.
For peritoneal disease of gastric cancer, postoperative chemotherapy after gastrectomy that leaves no macroscopically visible disease has survival benefits for gastric cancer patients with CY1 and/or P1a, 66 where patients who received any chemotherapy showed 5‐year OS of 20%‐30%, and those who received no chemotherapy exhibited 5‐year OS below 10% (Figure 5C).
9. FUTURE PROSPECTIVE BASED ON GENOMIC CLASSIFICATION OF GASTRIC CANCER
Gastric cancer was classified by TCGA (The Cancer Genome Atlas) into molecular profiles including Epstein‐Barr virus (EBV)‐integrated gastric cancer, microsatellite instability‐high (MSH‐H) gastric cancer, chromosomally instable (CIN) gastric cancer, and genomic stable (GS) gastric cancer. 22
Both EBV and MSI‐H gastric cancers are uniquely characterized by epigenetic carcinogenesis (gene silencing of p16 and MLH1, respectively, mainly by gene promoter DNA cytosine methylation). 22 N‐mab is recommended for EBV‐positive gastric cancer and MSI‐H gastric cancer, because both subtypes demonstrated PD‐L1 + immune cells with tumor‐infiltrating patterns. 67
Recent genomic profiles also revealed that unique ARID1A mutations are frequently found in both EBV and MSI‐H gastric cancer, 68 and such tumors with ARID1A mutations are highly sensitive to glutathione inhibition because of altered metabolism of cysteine via synthetic lethality mechanism. 69
On the other hand, almost all CIN gastric cancers harbor TP53 mutation with intestinal type histology. TP53 mutation leads to inactivation of tumor suppressive function as well as gain of function (GOF), 70 , 71 which could be molecularly targeted because mutated TP53 protein is overexpressed in human cancers. Mutated TP53 can bind specifically with TP63 and many other onco‐proteins, gaining an oncogenic potential, and CIN gastric cancer is considered to be addicted to TP53 GOF. 72
Recently, the number of patients with TP53 mutations in metastatic tumors was demonstrated to be significantly higher among those with liver metastasis (87%) in contrast to those without liver metastasis (40%), and moreover TP53 mutations in metastatic liver tumors and corresponding primary tumors were almost identical in 97% of cases. 73 These data suggested that TP53 mutations could be outstanding molecular targets in CIN gastric cancer.
GS gastric cancer is characterized by diffuse‐type gastric cancer, 22 which is characterized by emerging peritoneal disease. 74 However, even the most powerful intraperitoneal/intravenous chemotherapy does not produce satisfactory clinical outcomes. 75 Diffuse‐type gastric cancer is characterized by CDH1/RhoA mutations and is addicted to RhoA signal. 76 , 77 In the new era of gastric cancer clinics, specific molecular target would be promising based on the genome classification above.
In conclusion, history and emerging trends in chemotherapy for gastric cancer have been reviewed. Conventional anticancer drugs non‐specifically suppressed DNA synthesis and/or cell division, whereas emerging therapeutic trends included molecular‐targeted as well as immune‐targeted therapy, which is well consistent with molecular profiles. 22 , 23 Thus, further deep understanding of the genetic profiles in gastric cancer will expand novel therapeutic strategies in the near future.
CONFLICT OF INTEREST
There is no conflict of interest in this article.
Yamashita K, Hosoda K, Niihara M, Hiki N. History and emerging trends in chemotherapy for gastric cancer. Ann Gastroenterol Surg. 2021;5:446–456. 10.1002/ags3.12439
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, et al. Survival outcomes after laparoscopy‐assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non‐inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142–51. [DOI] [PubMed] [Google Scholar]
- 3. Yamashita K, Hosoda K, Moriya H, Mieno H, Katada N, Watanabe M. Long‐term prognostic outcome of cT1 gastric cancer patients who underwent laparoscopic gastrectomy after 5‐year follow‐up. Langenbecks Arch Surg. 2016;401:333–9. [DOI] [PubMed] [Google Scholar]
- 4. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 6. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93. [DOI] [PubMed] [Google Scholar]
- 7. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5‐year follow‐up of an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96. [DOI] [PubMed] [Google Scholar]
- 8. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. [DOI] [PubMed] [Google Scholar]
- 9. Longley DB, Harkin DP, Johnston PG. 5‐fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. [DOI] [PubMed] [Google Scholar]
- 10. Rustum YM. Modulation of fluoropyrimidines by leucovorin: rationale and status. J Surg Oncol Suppl. 1991;2:116–23. [DOI] [PubMed] [Google Scholar]
- 11. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. [DOI] [PubMed] [Google Scholar]
- 12. De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infusional 5‐fluorouracil and folinic acid (FOLFOX‐4) as first‐line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92:1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57. [DOI] [PubMed] [Google Scholar]
- 14. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5‐fluorouracil derivative (S‐1) directed to the potentiation of the tumor selective cytotoxicity of 5‐fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57. [DOI] [PubMed] [Google Scholar]
- 15. Lamont EB, Schilsky RL. The oral fluoropyrimidines in cancer chemotherapy. Clin Cancer Res. 1999;5:2289–96. [PubMed] [Google Scholar]
- 16. Liedert B, Pluim D, Schellens J, Thomale J. Adduct‐specific monoclonal antibodies for the measurement of cisplatin‐induced DNA lesions in individual cell nuclei. Nucleic Acids Res. 2006;34:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelland L. The resurgence of platinum‐based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. [DOI] [PubMed] [Google Scholar]
- 18. Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S‐1 with cisplatin plus S‐1 in chemotherapy‐naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8. [DOI] [PubMed] [Google Scholar]
- 19. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- 20. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first‐line therapy in advanced gastric cancer: a randomized, double‐blind, placebo‐controlled phase III study. J Clin Oncol. 2011;29:3968–76. [DOI] [PubMed] [Google Scholar]
- 21. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): a double‐blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- 25. Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–59. [DOI] [PubMed] [Google Scholar]
- 26. Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z, et al. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97:393–9. [DOI] [PubMed] [Google Scholar]
- 27. Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF‐C, is a ligand for the Flt4 (VEGFR‐3) and KDR (VEGFR‐2) receptor tyrosine kinases. EMBO J. 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. VEGF‐C receptor binding and pattern of expression with VEGFR‐3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–37. [DOI] [PubMed] [Google Scholar]
- 29. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 30. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, et al. Expression of the PD‐1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72. [DOI] [PubMed] [Google Scholar]
- 32. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti‐PD‐1 antibody, nivolumab, in patients with platinum‐resistant ovarian cancer. J Clin Oncol. 2015;33:4015–22. [DOI] [PubMed] [Google Scholar]
- 33. Hatae R, Kim YH, Sonomura K, Taneishi K, Kawaguchi S, et al. Combination of host immune metabolic biomarkers for the PD‐1 blockade cancer immunotherapy. JCI Insight. 2020;5:e133501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Japanese gastric cancer treatment guidelines 2018 (5th ed.). Gastric Cancer, 2020. [DOI] [PMC free article] [PubMed]
- 35. Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, et al. Efficacy of trastuzumab in Japanese patients with HER2‐positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012;15:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S‐1 plus cisplatin in HER2‐positive gastric cancer (HERBIS‐1). Br J Cancer. 2014;110:1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chua C, Tan IB, Yamada Y, Rha SY, Yong WP, Ong WS, et al. Phase II study of trastuzumab in combination with S‐1 and cisplatin in the first‐line treatment of human epidermal growth factor receptor HER2‐positive advanced gastric cancer. Cancer Chemother Pharmacol. 2015;76:397–408. [DOI] [PubMed] [Google Scholar]
- 38. Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S‐1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–9. [DOI] [PubMed] [Google Scholar]
- 39. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S‐1 plus cisplatin versus S‐1 alone for first‐line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. [DOI] [PubMed] [Google Scholar]
- 40. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7. [DOI] [PubMed] [Google Scholar]
- 41. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high‐dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–57. [DOI] [PubMed] [Google Scholar]
- 42. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. [DOI] [PubMed] [Google Scholar]
- 43. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5‐fluorouracil/cisplatin as first‐line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73. [DOI] [PubMed] [Google Scholar]
- 44. Kim GM, Jeung HC, Rha SY, Kim HS, Jung I, Nam BH, et al. A randomized phase II trial of S‐1‐oxaliplatin versus capecitabine‐oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48:518–26. [DOI] [PubMed] [Google Scholar]
- 45. Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip PA, Cohn AL, et al. Ramucirumab combined with FOLFOX as front‐line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double‐blind, multicenter Phase II trial. Ann Oncol. 2016;27:2196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2‐negative, MET‐positive gastroesophageal adenocarcinoma: the metgastric randomized clinical trial. JAMA Oncol. 2017;3:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first‐line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol. 2020;31:S1142–S1215. [Google Scholar]
- 48. Boku N, Ryu MH, Dea OH. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION‐4 (ONO‐4538‐37) study. ESMO Congress LBA7_PR. 2020.
- 49. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–48. [DOI] [PubMed] [Google Scholar]
- 50. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2‐positive gastric cancer. N Engl J Med. 2020;382:2419–30. [DOI] [PubMed] [Google Scholar]
- 51. Yoshikawa T, Terashima M, Mizusawa J, Nunobe S, Nishida Y, Yamada T, et al. Four courses versus eight courses of adjuvant S‐1 for patients with stage II gastric cancer (JCOG1104 [OPAS‐1]): an open‐label, phase 3, non‐inferiority, randomised trial. Lancet Gastroenterol Hepatol. 2019;4:208–16. [DOI] [PubMed] [Google Scholar]
- 52. Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC‐07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, et al. Addition of docetaxel to S‐1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park SH, Zang DY, Han B, Ji JH, Kim TG, Oh SY, et al. ARTIST 2: Interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2‐gastrectomy in stage II/III gastric cancer (GC). J Clin Oncol. 2019;37:4001. [Google Scholar]
- 55. Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S‐1 plus cisplatin for type 4 or large type 3 gastric cancer, the short‐term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019;22:1044–52. [DOI] [PubMed] [Google Scholar]
- 56. Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Gastrectomy with or without neoadjuvant S‐1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open‐label, phase 3, randomized controlled trial. Gastric Cancer. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 57. Ji J, Shen L, Li Z, Zhang X, Liang H, Xue Y, et al. Perioperative Chemotherapy of Oxaliplatin Combined with S‐1 (SOX) versus Postoperative Chemotherapy of SOX or Oxaliplatin with Capecitabine (XELOX) in Locally Advanced Gastric Adenocarcinoma with D2 Gastrectomy: a Randomized Phase III Trial (RESOLVE Trial). Ann Oncol. 2019;30:v851–v934. [Google Scholar]
- 58. Kang Y, Yook JH, Yea P.A Phase III open label randomized study of neoadjuvant chemotherapy with docetaxel, oxaliplatin and S‐1 (DOS) followed by surgery and adjuvant S‐1, vs surgery and adjuvant S‐1 for resectable advanced gastric cancer (PRODIGY Study). ESMO Congress 2111, 2019.
- 59. Sato Y, Kurokawa Y, Doki Y, Mizusawa J, Tanaka K, Katayama H , et al. A Phase II study of preoperative chemotherapy with docetaxel, oxaliplatin and S‐1 in gastric cancer with extensive lymph node metastasis (JCOG1704). Future Oncol. 2020;16:31–8. [DOI] [PubMed] [Google Scholar]
- 60. Hosoda K, Azuma M, Katada C, Ishido K, Niihara M, Ushiku H, et al. A phase I study of docetaxel/oxaliplatin/S‐1 (DOS) combination neoadjuvant chemotherapy for patients with locally advanced adenocarcinoma of the esophagogastric junction. Int J Clin Oncol. 2020;25:1090–7. [DOI] [PubMed] [Google Scholar]
- 61. Kang Y‐K, Yook JH, Park YK, Kim YW, Kim J, Ryu MH, et al. LBA41 ‐ Phase III randomized study of neoadjuvant chemotherapy (CT) with docetaxel(D), oxaliplatin(O) and S‐1(S) (DOS) followed by surgery and adjuvant S‐1, vs surgery and adjuvant S‐1, for resectable advanced gastric cancer (GC) (PRODIGY). Ann Oncol. 2019;30:v876–v877. [Google Scholar]
- 62. Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, et al. Neoadjuvant chemotherapy with S‐1 and cisplatin followed by D2 gastrectomy with para‐aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60. [DOI] [PubMed] [Google Scholar]
- 63. Katayama H, Tsuburaya A, Mizusawa J, Nakamura K, Katai H, Imamura H, et al. An integrated analysis of two phase II trials (JCOG0001 and JCOG0405) of preoperative chemotherapy followed by D3 gastrectomy for gastric cancer with extensive lymph node metastasis. Gastric Cancer. 2019;22:1301–7. [DOI] [PubMed] [Google Scholar]
- 64. Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I , et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S‐1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41:1354–60. [DOI] [PubMed] [Google Scholar]
- 65. Mieno H, Yamashita K, Hosoda K, Moriya H, Higuchi K, Azuma M, et al. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S‐1 (DCS) for far‐advanced gastric cancer. Surg Today. 2017;47:1249–58. [DOI] [PubMed] [Google Scholar]
- 66. Yamaguchi T, Takashima A, Nagashima K, Makuuchi R, Aizawa M, Ohashi M, et al. Efficacy of postoperative chemotherapy after resection that leaves no macroscopically visible disease of gastric cancer with positive peritoneal lavage cytology (CY1) or localized peritoneum metastasis (P1a): a multicenter retrospective study. Ann Surg Oncol. 2020;27:284–92. [DOI] [PubMed] [Google Scholar]
- 67. Kelly RJ. Immunotherapy for esophageal and gastric cancer. Am Soc Clin Oncol Educ Book. 2017;37:292–300. [DOI] [PubMed] [Google Scholar]
- 68. Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–23. [DOI] [PubMed] [Google Scholar]
- 69. Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the vulnerability of glutathione metabolism in ARID1A‐deficient cancers. Cancer Cell. 2019;35:177–190e8. [DOI] [PubMed] [Google Scholar]
- 70. Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li‐Fraumeni syndrome. Cell. 2004;119:861–72. [DOI] [PubMed] [Google Scholar]
- 71. Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA. Mutant p53 gain of function in two mouse models of Li‐Fraumeni syndrome. Cell. 2004;119:847–60. [DOI] [PubMed] [Google Scholar]
- 72. Tanaka T, Watanabe M, Yamashita K. Potential therapeutic targets of TP53 gene in the context of its classically canonical functions and its latest non‐canonical functions in human cancer. Oncotarget. 2018;9:16234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ikari N, Serizawa A, Mitani S, Yamamoto M, Furukawa T. Near‐comprehensive resequencing of cancer‐associated genes in surgically resected metastatic liver tumors of gastric cancer. Am J Pathol. 2019;189:784–96. [DOI] [PubMed] [Google Scholar]
- 74. Yamashita K, Sakuramoto S, Katada N, Futawatari N, Moriya H, Hirai K, et al. Diffuse type advanced gastric cancer showing dismal prognosis is characterized by deeper invasion and emerging peritoneal cancer cell: the latest comparative study to intestinal advanced gastric cancer. Hepatogastroenterology. 2009;56:276–81. [PubMed] [Google Scholar]
- 75. Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S‐1 versus cisplatin plus S‐1 in patients with gastric cancer with peritoneal metastasis: PHOENIX‐GC trial. J Clin Oncol. 2018;36:1922–9. [DOI] [PubMed] [Google Scholar]
- 76. Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, et al. Recurrent gain‐of‐function mutations of RHOA in diffuse‐type gastric carcinoma. Nat Genet. 2014;46:583–7. [DOI] [PubMed] [Google Scholar]
- 77. Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, et al. Whole‐genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–82. [DOI] [PubMed] [Google Scholar]


