Abstract
Background
Trichomonas vaginalis is the most prevalent nonviral sexually transmitted infection. We evaluated the efficacy and safety of secnidazole vs placebo in women with trichomoniasis.
Methods
Women with trichomoniasis, confirmed by a positive T. vaginalis culture, were randomized to single-dose oral secnidazole 2 g or placebo. The primary endpoint was microbiological test of cure (TOC) by culture 6–12 days after dosing. At the TOC visit, participants were given the opposite treatment. They were followed for resolution of infection afterward and offered treatment at subsequent visits, if needed. Fifty patients per group (N = 100) provided approximately 95% power to detect a statistically significant difference between treatment groups.
Results
Between April 2019 and March 2020, 147 women enrolled at 10 sites in the United States. The modified intention-to-treat (mITT) population included 131 randomized patients (secnidazole, n = 64; placebo, n = 67). Cure rates were significantly higher in the secnidazole vs placebo group for the mITT population (92.2% [95% confidence interval {CI}: 82.7%–97.4%] vs 1.5% [95% CI: .0%–8.0%]) and for the per-protocol population (94.9% [95% CI: 85.9%–98.9%] vs 1.7% [95% CI: .0%–8.9%]). Cure rates were 100% (4/4) in women with human immunodeficiency virus (HIV) and 95.2% (20/21) in women with bacterial vaginosis (BV). Secnidazole was generally well tolerated. The most frequently reported treatment-emergent adverse events (TEAEs) were vulvovaginal candidiasis and nausea (each 2.7%). No serious TEAEs were observed.
Conclusions
A single oral 2 g dose of secnidazole was associated with significantly higher microbiological cure rates vs placebo, supporting a role for secnidazole in treating women with trichomoniasis, including those with HIV and/or BV.
Clinical Trials Registration
Keywords: Trichomonas vaginalis, trichomoniasis, secnidazole, women
A single oral dose of secnidazole vs placebo significantly increased microbiological cure rates in women with trichomoniasis, including those with human immunodeficiency virus and/or bacterial vaginosis.
Trichomoniasis is the most prevalent nonviral sexually transmitted infection (STI) worldwide, affecting 3.7 million people in the United States (US) [1]. Women with Trichomonas vaginalis have a 2- to 3-fold increased risk for acquiring human immunodeficiency virus (HIV) [2] and other STIs [3]. Trichomoniasis is also associated with infertility [4] and adverse birth outcomes [5]. National guidelines recommend annual screening of women with HIV for trichomoniasis [6].
The 2015 Centers for Disease Control and Prevention’s (CDC) sexually transmitted disease treatment guidelines recommend a single 2 g dose of oral metronidazole (MTZ) or tinidazole (TDZ) for T. vaginalis–infected women without HIV; for infected women with HIV, MTZ 500 mg orally twice daily for 7 days is preferred [6]. The 2020 American College of Obstetricians and Gynecologists’ (ACOG) practice bulletin on vaginitis has recently recommended multidose MTZ for all nonpregnant women [3], based on new clinical trial data [7]. Updated 2021 CDC STI treatment guidelines are pending.
Secnidazole (SEC) is a potent 5-nitroimidazole antibiotic with a longer half-life than MTZ and TDZ [8]. In 2017, SEC was approved by the US Food and Drug Administration (FDA) as the first, single-dose oral treatment for bacterial vaginosis (BV) in women [9, 10]. The 2020 ACOG guidance includes SEC 2 g as an alternative treatment for BV, based on a randomized controlled trial, finding it comparable to 7-day MTZ for BV [3].
As a treatment for trichomoniasis, single-dose SEC had microbiological cure rates of 93%–96% after 2–3 days of treatment and up to 100% for ≤20 days of treatment in studies conducted outside the US [11]. We aimed to evaluate the efficacy and safety of single-dose SEC in US-based women with trichomoniasis. We hypothesized that microbiological cure rates would be high after treatment of T. vaginalis–infected women with SEC, similar to studies outside the US.
METHODS
Study Design
This phase 3, randomized, double-blind, placebo-controlled, delayed-treatment study received institutional review board approval (IntegReview, Austin, Texas; Western Institutional Review Board, Puyallup, Washington; Office of Human Research Ethics, Chapel Hill, North Carolina) for 10 clinical sites in the US (ClinicalTrials.gov identifier NCT03935217). It was designed and monitored in accordance with FDA recommendations and the sponsor’s standard operating procedures, which comply with the International Conference on Harmonisation’s Good Clinical Practice Guidelines and the Declaration of Helsinki. All patients provided written informed consent or parental/legal guardian consent (when appropriate) before engaging in study-related procedures.
In contrast to the FDA approval of tinidazole for trichomoniasis, which was based on a literature-focused New Drug Application submission, in this circumstance, the FDA required a single, placebo-controlled trial for the approval of SEC to treat trichomoniasis. To ensure patient safety, the investigators, in collaboration with the FDA, adopted a delayed-treatment study design, similar to FDA guidance on uncomplicated urinary tract infection studies [12]. Because there is no evidence that a 1- to 2-week delay in treatment for trichomoniasis is associated with significant health risks, the study design was approved by both local (academic) and central institutional review boards. Out of an abundance of caution, the investigators agreed on a trial protocol that was more stringent than the current standard of care, which recommends retesting for T. vaginalis at 3 months [6]. In the current trial, test of cure (TOC) was performed 6–12 days after treatment, with additional follow-up if needed to ensure successful treatment. In addition, the investigators counseled patients on the need for partner therapy and the importance of abstinence during the study to prevent reinfection.
Participants
Eligible patients were adult females or postmenarchal adolescent girls ≥12 years of age in general good health. An initial diagnosis of T. vaginalis was determined by a positive wet mount, positive OSOM Trichomonas Rapid Test (Sekisui Diagnostics, Burlington, Massachusetts), or a positive T. vaginalis nucleic acid amplification test (NAAT; within the past 30 days) for which treatment had not yet been initiated. Patients diagnosed with T. vaginalis based on a Pap smear were not excluded; however, these patients were required to have T. vaginalis confirmed with 1 of the other diagnostic tests mentioned above. Patients agreed to abstain from vaginal intercourse, vaginal penetration (eg, sex toys), or use of any vaginal products (eg, spermicides, tampons, vaginal douches, lubricants) until after study completion. Patients were counseled to notify all sexual partners within the past 60 days to be treated as contacts to trichomoniasis. Based on data from in vitro studies [13], and as noted in the package insert [14], SEC does not have an alcohol consumption restriction. Patients were excluded if they were pregnant (owing to placebo-controlled study design) or lactating, had symptomatic vulvovaginal candidiasis or an active genital herpes outbreak, received a course of antibacterial or antifungal therapy within the past 14 days, or had a known allergy to nitroimidazoles. Patients with chlamydia or gonorrhea via positive NAAT test at enrollment were included in the safety population; however, they were not included in the modified intention-to-treat (mITT) analysis, as per the direction of the FDA. Women with HIV and those with BV (based on all 4 Amsel criteria per FDA trial guidance: presence of homogenous, white/gray vaginal discharge; ≥20% clue cells per high-power field on wet mount; positive whiff test; vaginal pH ≥4.7) [15] were not excluded.
Procedures
Eligible patients were randomly assigned (1:1) to SEC 2 g or matching placebo in a double-blinded manner. Before study start, biostatisticians generated a randomization list assigning kit numbers to 1 of the 2 treatment groups. This list was used by the study sponsor to package study drug into treatment kits. Randomization was stratified by site and clinical symptoms of trichomoniasis at baseline (present/absent).
At visit 1 (baseline), patients completed assessments including demographics, medical history (including presence of genital symptoms), vital signs, urine pregnancy testing, and a physical examination. A pelvic examination was performed to assess genital signs and to collect vaginal specimens for pH, wet mount, potassium hydroxide whiff test, InPouch T. vaginalis culture (BioMed Diagnostics, White City, Oregon), and gonorrhea/chlamydia NAAT (Roche HC/Chlamydia Collection Kit [Cobas PCR Media Uni Swab]). SEC oral granules or matching placebo oral granules were mixed in approximately 4 ounces of unsweetened applesauce and administered under direct observation. The matching placebo contained the same ingredients as the active formulation with the exception of SEC. Both SEC and the placebo were packaged in white packets with blinded packaging and labeling so they were indistinguishable. Patients were evaluated 6–12 days later at visit 2 for TOC.
At visit 2 (TOC), patients were queried about treatment-emergent adverse events (TEAEs) and assessed for clinical symptoms of trichomoniasis. A provider obtained an additional vaginal specimen for T. vaginalis culture. At this visit, patients received the opposite treatment from baseline (ie, SEC if they had received placebo at baseline or placebo if they had received SEC at baseline) under direct observation by study staff. Patients with a microbiological cure, defined as negative T. vaginalis by InPouch culture at visit 2, were subsequently discharged from the study. Patients with a positive T. vaginalis culture from visit 2 were asked to return for a third visit, 7–12 days later, for an additional assessment, including determination of the need for additional therapy. A fourth visit (7–12 days after visit 3) was scheduled at the investigator’s discretion if a repeat T. vaginalis culture at visit 3 was positive. Patients who had a positive culture at visit 3 and visit 4 were offered treatment, based upon the investigator’s discretion and per standard of care [6]. InPouch cultures were examined daily for 5 days over a 7-day period to reduce the possibility of false-negative results [16].
The primary efficacy endpoint was microbiological cure at visit 2 (TOC). The trial was designed to limit the possibility of unprotected sex resulting in reinfection, which would become more likely the longer the interval between treatment and TOC. The InPouch culture was selected as the diagnostic TOC based on its relatively high sensitivity (81%–94%) [17, 18], allowing for a TOC visit within 1 week. We did not perform a T. vaginalis NAAT for TOC as the optimal timing of this highly sensitive test is 3–4 weeks after treatment. Use of this test within 3 weeks after treatment may have detected remnant trichomonal nucleic acid, leading to false-positive test results [19]. In addition, the use of T. vaginalis NAAT for TOC would have required patients to remain sexually inactive for as much as 4 weeks, which was considered impractical.
Statistical Analysis
Assuming microbiological cure rates of 75% [7] and 40% [20] with SEC and placebo, respectively, a sample size of 100 patients (50 patients/group) provided approximately 95% power to demonstrate a statistically significant between-treatment-group difference using a 2-sided, 2-sample comparison of proportions at the α = .05 level of significance. Assuming an attrition rate of 30%, 144 patients needed to be randomly assigned to SEC or placebo.
The primary efficacy endpoint was compared between the active and placebo treatment groups using a 2-sided Cochran-Mantel-Haenszel test (stratified by the presence/absence of clinical symptoms of trichomoniasis at baseline, HIV status, and BV status) at the α = .05 level of significance. As required by the FDA and determined a priori, the primary efficacy analysis was based on the mITT population, defined as all randomized patients who had a positive T. vaginalis culture at baseline and a negative chlamydia and gonorrhea NAAT at baseline. Secondary efficacy endpoint analysis was based on the per protocol (PP) population, defined as patients in the mITT population who received their assigned study medication and had a TOC visit. Post hoc efficacy analyses of the microbiological cure rate at TOC compared SEC- vs placebo-treated patients (1) with trichomoniasis who were symptomatic and those who were asymptomatic, (2) infected with HIV, and (3) with BV at baseline. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
Safety endpoints included the incidence, severity, and relationship to study medication of TEAEs, serious TEAEs, and TEAEs leading to discontinuation. Vital signs, physical examinations, and clinical laboratory tests also were assessed. Safety endpoints were evaluated using the safety population, defined as all randomized patients who received any study-related medication.
RESULTS
Patient Disposition and Characteristics
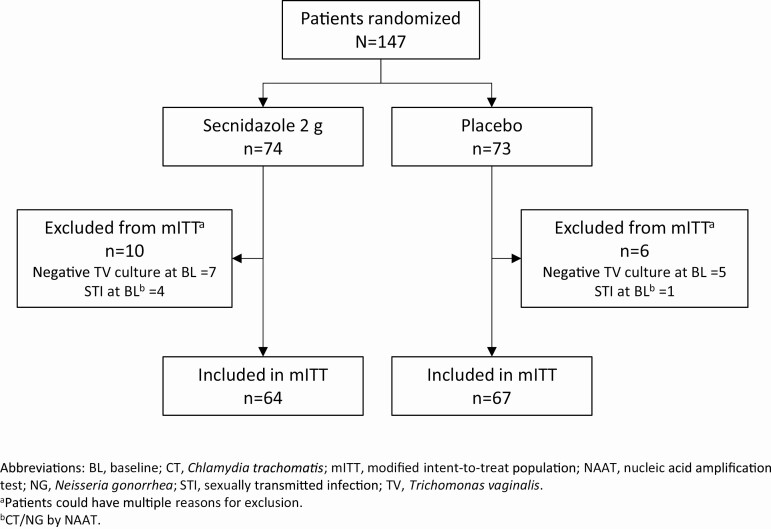
Between 23 April 2019 and 18 March 2020, 147 women with trichomoniasis (SEC = 74, placebo = 73), identified by a positive wet mount (n = 119), OSOM Trichomonas Rapid Test (n = 112), or T. vaginalis NAAT (n = 60) were enrolled at 10 clinical sites in the United States. One hundred thirty-one women (SEC = 64, placebo = 67) were included in the mITT population (Figure 1). In the mITT population, 57 patients had positive T. vaginalis InPouch test results at visit 2, and 54 of these 57 patients had negative T. vaginalis InPouch test results at visit 3. This corresponds to a 94.7% success rate for SEC.
Figure 1.
Modified intention-to-treat population and reasons for exclusion. aPatients could have multiple reasons for exclusion. bChlamydia trachomatis/Neisseria gonorrhoeae by nucleic acid amplification test. Abbreviations: BL, baseline; mITT, modified intention-to-treat; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
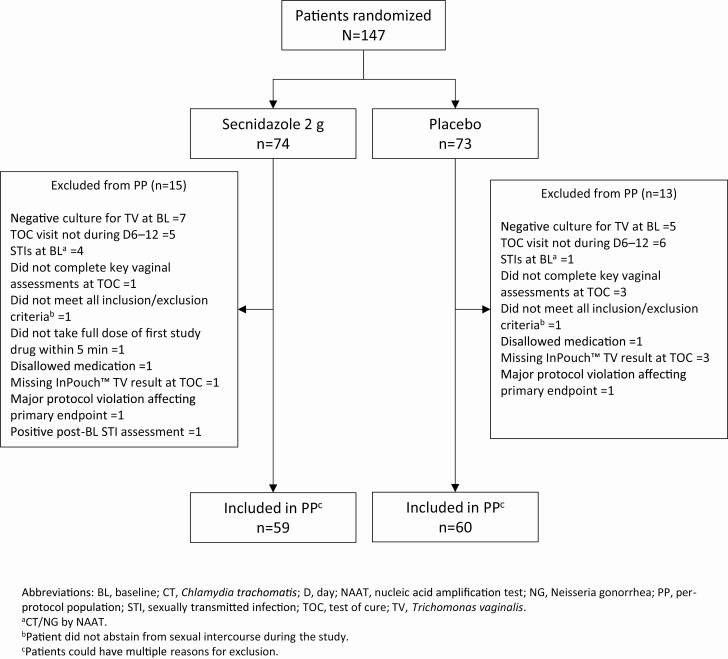
Eight percent (12/147) of randomized patients were excluded due to a negative T. vaginalis culture at baseline; 3.4% (5/147) of patients had a positive gonorrhea/chlamydia NAAT. One hundred nineteen patients were included in the PP population (SEC = 59, placebo = 60) (Figure 2). In addition to the above-mentioned exclusions, 7.5% (11/147) of patients were excluded from the PP population because their TOC visit did not occur within the 6- to 12-day window (Figure 2). Of the 12 patients excluded from both the mITT and PP populations due to a negative T. vaginalis culture at baseline, 6 had a positive NAAT (within 30 days of visit 1) at enrollment, 2 had positive wet mounts and positive OSOMs, 1 had a positive wet mount, and 1 had a positive wet mount and positive NAAT. Four patients did not return for TOC at visit 2. One patient withdrew owing to a TEAE, 2 withdrew consent, and 1 was contacted by phone multiple times and sent a certified letter in the mail. She acknowledged receipt of this letter but chose not to return. In the mITT population, the mean age was 37.7 years (standard deviation, 11.19 years; range, 15–65 years), and 90.8% were African American (Table 1). The majority of patients (84.7%) had genital symptoms, and among those, 43.5% had abnormal genital itching, 78.6% had abnormal vaginal discharge, and 63.4% had abnormal odor.
Figure 2.
Per-protocol population and reasons for exclusion. aChlamydia trachomatis/Neisseria gonorrhoeae by nucleic acid amplification test. bPatient did not abstain from sexual intercourse during the study. cPatients could have multiple reasons for exclusion. Abbreviations: BL, baseline; PP, per protocol; STI, sexually transmitted infection; TOC, test of cure; TV, Trichomonas vaginalis.
Table 1.
Baseline Characteristics (Modified Intention-to-Treat Population)a
| Characteristic | Secnidazole 2 g (n = 64) |
Placebo (n = 67) |
Overall (N = 131) |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 36.9 (11.3) | 38.4 (11.12) | 37.7 (11.19) |
| Median (range) | 34.5 (19–65) | 39.0 (15–65) | 36.0 (15–65) |
| Race, No. (%) | |||
| American Indian/Alaska Native | 1 (1.6) | 1 (1.5) | 2 (1.5) |
| Asian | 1 (1.6) | 0 | 1 (0.8) |
| Black/African American | 59 (92.2) | 60 (89.6) | 119 (90.8) |
| Native Hawaiian/Other Pacific Islander | 0 | 0 | 0 |
| White | 3 (4.7) | 6 (9.0) | 9 (6.9) |
| Other | 0 | 0 | 0 |
| Ethnicity, No. (%) | |||
| Not Hispanic or Latino | 62 (96.9) | 65 (97.0) | 127 (96.9) |
| Hispanic or Latino | 2 (3.1) | 2 (3.0) | 4 (3.1) |
| Weight, kg, mean ± SD | 90.4 (25.9) | 92.4 (26.7) | 91.4 (26.3) |
| Weight, kg, median (range) | 87.1 (45.4–167.8) | 86.2 (49.9–180.5) | 86.6 (49.9–180.5) |
| BMI, kg/m2, mean ± SD | 33.8 (9.4) | 34.1 (9.3) | 34.0 (9.3) |
| BMI, kg/m2, median (range) | 32.2 (17.7–63.5) | 31.2 (19.5–62.3) | 31.6 (17.7–63.5) |
| Trichomoniasis symptomsb, No. (%) | |||
| Present | 56 (87.5) | 55 (82.1) | 111 (84.7) |
| Absent | 8 (12.5) | 12 (17.9) | 20 (15.3) |
| Bacterial vaginosis, No. (%) | 21 (32.8) | 17 (25.4) | 38 (29.0) |
| HIV positive, No. (%) | 5 (7.8) | 4 (6.0) | 9 (6.9) |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; SD, standard deviation.
aData are means ± SDs or n (%), unless otherwise specified.
bVaginal itching, discharge, and/or odor.
Efficacy
In the mITT population, the microbiologic cure rate at TOC was significantly higher (P < .001) in the SEC vs placebo group (92.2% [95% confidence interval {CI}: 82.7%–97.4%] vs 1.5% [95% CI: .0–8.0%]) (Table 2). In the PP population, the cure rate at TOC also was significantly higher (P < .001) in the SEC vs placebo group (94.9% [95% CI: 85.9%–98.9%] vs 1.7% [95% CI: .0–8.9%]). There were no significant demographic differences between patients who were not cured (ie, at visit 2 in the SEC group and at visit 3 in the placebo group) and those who were cured (data not shown).
Table 2.
Microbiological Cure at Test-of-Cure Visit (Modified Intention-to-Treat Population)
| Status at TOC Visit | Secnidazole 2 g (n = 64) | Placebo (n = 67) |
|---|---|---|
| Microbiological curea, No. (%) | 59 (92.2)b | 1 (1.5)b |
| 95% exact binomial CI | 82.70–97.41 | .04–8.04 |
| P valuec | <.001 |
Abbreviation: CI, confidence interval; TOC, test of cure.
aInPouch Trichomonas vaginalis test negative.
bPatients with no test results were assumed to be positive (numbers imputed: secnidazole = 1; placebo = 3).
cP value vs placebo from a Cochran-Mantel-Haenszel test adjusted for clinical symptoms (present/absent) of trichomoniasis at baseline.
In the subgroup of patients with vaginal symptoms at baseline, the microbiological cure rate at TOC was 92.9% (95% CI: 82.7%–98.0%) in the SEC group; no patient in the placebo group had a negative T. vaginalis culture at TOC (P < .001; Table 3). In women with HIV, 100% (4/4) had a microbiological cure at TOC; no patient in the placebo group had a negative T. vaginalis culture at TOC. In patients with BV at baseline, the microbiological cure rate at TOC was 95.2% (95% CI: 76.2%–99.9%) in the SEC group; no patient in the placebo group had a negative T. vaginalis culture at TOC (P < .001).
Table 3.
Microbiological Cure by Presence of Clinical Symptoms and Human Immunodeficiency Virus or Bacterial Vaginosis Status at Baselinea (Modified Intention-to-Treat Population)
| Status at Baseline | Secnidazole 2 g (n = 64) | Placebo (n = 67) | |||
|---|---|---|---|---|---|
| Symptoms Present | Symptoms Absent | Symptoms Present | Symptoms Absent | P Valueb | |
| Microbiological curec, % (no./No.) | 92.9 (52/56) | 87.5 (7/8)d | 0 (0/55)d | 8.3 (1/12) | <.001 |
| 95% CI | 82.7–98.0 | 47.4–99.7 | .0–6.5 | .2–38.5 | <.001 |
| Microbiological curec, HIV, % (no./No.) | 100 (4/4) | … | 0 (0/4) | … | NC |
| Microbiological curec, BV, % (no./No.) | 95.2 (20/21) | … | … | 0 (0/17)d | <.001 |
| 95% CI | 76.2–99.9 | … | … | .0–19.5 | |
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; HIV, human immunodeficiency virus; NC, not calculated.
aPost hoc analysis.
bP value vs placebo from a Cochran-Mantel-Haenszel test adjusted for clinical symptoms (present/absent) of trichomoniasis at baseline.
cInPouch Trichomonas vaginalis test negative.
dPatients with no test results were assumed to be positive (numbers imputed: secnidazole [symptoms absent] = 1; placebo [symptoms present] = 3; placebo [symptoms absent] = 1).
Safety
In the safety population, TEAE rates were lower in the SEC vs placebo group. All TEAEs were mild. Only 1 patient in the SEC group discontinued due to mild nausea and productive cough. There were no serious TEAEs (Table 4).
Table 4.
Summary of Treatment-Emergent Adverse Eventsa (Safety Population)
| TEAE | Secnidazole 2 g (n = 74) | Placebo (n = 73) | ||
|---|---|---|---|---|
| Patientsb, No. (%) | Events, No. | Patientsb, No. (%) | Events, No. | |
| Any TEAE | 11 (14.9) | 13 | 16 (21.9) | 20 |
| Nausea | 2 (2.7) | 2 | 3 (4.1) | 3 |
| Abdominal pain | 1 (1.4) | 1 | 1 (1.4) | 1 |
| Diarrhea | 1 (1.4) | 1 | 2 (2.7) | 2 |
| Vomiting | 1 (1.4) | 1 | 1 (1.4) | 1 |
| Vulvovaginal candidiasis | 2 (2.7) | 2 | 0 | 0 |
| Vulvovaginal mycotic infection | 1 (1.4) | 1 | 0 | 0 |
| Trichomoniasis | 0 | 0 | 2 (2.7) | 2 |
| Productive cough | 1 (1.4) | 1 | 0 | 0 |
| Upper-airway cough syndrome | 1 (1.4) | 1 | 0 | 0 |
| Myalgia | 1 (1.4) | 1 | 0 | 0 |
| Back pain | 0 | 0 | 1 (1.4) | 1 |
| Headache | 1 (1.4) | 1 | 5 (6.8) | 5 |
| Vulvovaginal pruritus | 1 (1.4) | 1 | 0 | 0 |
| Dysmenorrhea | 0 | 0 | 2 (2.7) | 2 |
| Irregular menstruation | 0 | 0 | 1 (1.4) | 1 |
| Thirst | 0 | 0 | 1 (1.4) | 1 |
| Pruritus | 0 | 0 | 1 (1.4) | 1 |
Abbreviation: TEAE, treatment-emergent adverse event.
aIncludes all TEAEs during the primary phase (start date on or before the test-of-cure visit).
bPatients experiencing multiple TEAEs are counted only once within a given cell.
Discussion
The results of this study demonstrate the superiority of SEC vs placebo for the treatment of trichomoniasis. Importantly, SEC was superior to placebo in patients with trichomoniasis and vaginal symptoms and in those with HIV and/or BV. Although prior studies have shown trichomoniasis is asymptomatic in 70%–85% of cases [21], patients can present with symptoms including yellow-to-green frothy vaginal discharge, abnormal vaginal odor, pruritus, irritation, and/or dysuria [3]. Thus, single-dose SEC may be an effective option for symptomatic and asymptomatic trichomoniasis in women.
National guidelines have traditionally recommended a single, 2 g dose of oral MTZ or TDZ as the preferred treatment of trichomoniasis in women, with oral MTZ 500 mg twice daily (BID) for 7 days as an alternative regimen [6]. In 2010, results of a randomized, open-label trial of a single oral dose of MTZ 2 g vs the 7-day MTZ 500 mg BID regimen in women with HIV and trichomoniasis demonstrated microbiological cure rates of 83.2% vs 91.5%, 6–12 days after treatment [22]. These results led to the CDC recommendation to use the multidose MTZ regimen for trichomoniasis in women with HIV [6]. In 2018, similar results were reported from a randomized, open-label trial of single- vs multidose MTZ for trichomoniasis in women without HIV. Microbiological cure rates at TOC were 81.4% and 89.1% in the single- and multidose MTZ groups [7]. Hence, the 2020 ACOG practice bulletin on vaginitis now recommends the multidose MTZ regimen as the preferred regimen for trichomoniasis in nonpregnant women [3]. Updated 2021 CDC STI treatment guidelines are anticipated later this year.
As providers move toward multidose MTZ as the preferred regimen for all women with trichomoniasis, our findings also support the use of single-dose SEC. With efficacy rates for the treatment of trichomoniasis comparable to those of multidose MTZ [7, 22], SEC has favorable attributes that may provide advantages over other options. In vitro studies of T. vaginalis isolates [14] showed that the minimal lethal concentration of SEC is 56% lower compared with MTZ. SEC also differs from other 5-nitroimidazoles with a notably longer half-life (17 hours) vs MTZ (7–8 hours) and TDZ (12 hours) [23]. Single-dose options are convenient and likely to improve adherence overall, especially in populations at risk for noncompliance [24]. Of note, compliance with multidose MTZ has been low (50%–63%) in some studies [25]. Factors that may impact adherence include gastrointestinal complaints, treatment duration, and lifestyle restrictions. In 1 study, 23% of patients reported nausea in the single-dose and multidose MTZ groups [7]. In our study, 2.7% of patients reported nausea with SEC. Additionally, SEC does not have an alcohol restriction based on data from in vitro studies [13, 14].
In our study, cure rates were 92%–100% in the overall population and in subgroups of women with genital symptoms, HIV, and/or BV. Previous research showed that trichomoniasis treatment significantly decreases HIV viral load and viral shedding and may reduce HIV transmission [6, 26–28]. Although only 4 patients had HIV, our finding that all were cured is reassuring, and larger clinical trials in this patient population are warranted to confirm these results. Moreover, coinfection of BV and T. vaginalis is common, with rates of 60%–80% [29]. Thus, a single treatment for BV and trichomoniasis may have multiple benefits.
A potential limitation of this study was the use of T. vaginalis culture to achieve a shorter timeframe to TOC. Although InPouch may be less sensitive than NAAT [21], the accuracy in this study was high as supported by the low placebo responder rate (~1%). Other limitations include the lack of long-term follow-up and the exclusion of men and pregnant women. However, 2 non-US-based studies of SEC for trichomoniasis in patients, including a small subgroup of men, showed cure rates of 91.7%–100% at 2–20 days after treatment [30, 31]. Although our study did not include pregnant women, and data on the use of SEC during pregnancy are limited, reproductive preclinical toxicology studies have shown no evidence of toxicity, and SEC is not contraindicated during pregnancy [14, 32]. The CDC recommends that trichomoniasis treatment be considered for symptomatic pregnant women, regardless of pregnancy stage [6]. Finally, 5-nitroimidazole susceptibility testing was not performed on trichomonas isolates in the study; thus, we cannot comment on any resistance that potentially may have been present.
Study strengths include high cure rates for SEC (92%–95%), low placebo responder rates, minimal dropouts, a favorable TEAE profile, and treatment success in women with and without clinical symptoms, HIV, and/or BV.
In conclusion, this study demonstrated that a single dose of SEC 2 g was efficacious and well tolerated in women with trichomoniasis and in those with trichomoniasis and comorbid HIV and/or BV. If approved by the FDA for the treatment of trichomoniasis, SEC will be the only single oral-dose medication available for the treatment of BV and T. vaginalis. Future studies should be considered in pregnant women and those with persistent T. vaginalis infection, including the use of SEC multidose regimens.
Supplementary Material
Notes
Acknowledgments. The authors thank Angela Pontius, CRNP; Sanquetta McClendon; Joy Lewis; Stephanie Clevenger, RN; Arlene Seña, MD, MPH; Susan Blevins, RN; Stephanie Lee, RN; C. Paige Brainard, MD; Paula Maclish; Jennifer Brumfield; Monica Moore, MD; LaToya Hinton; Sirena Tucker; B. Todd Chappell, MD; Blair Longserre; Viviana Gutierrez; Toree Gomez; Alfred Moffett, MD; Suzanne Baratta, RN; Franklin Morgan, MD; April Rusch; and Jesslyn Payne for their assistance in recruiting and/or enrolling patients for this study.
Financial support. This study was sponsored by Lupin Pharmaceuticals, Inc.
Potential conflicts of interest. C. A. M. has received research grant support from the National Institute of Allergy and Infectious Diseases, the Centers for Disease Control and Prevention, and Lupin Pharmaceuticals; serves as a consultant for Lupin Pharmaceuticals and BioFire Diagnostics; and has received honoraria from Elsevier, Abbott Molecular, PhagoMed, DynaMed, Atom Consulting, Manitoba Medical Foundation, Cepheid, Becton Dickinson, Roche Diagnostics, and Lupin Pharmaceuticals. C. P. M. reports payments as a principal investigator for Lupin Pharmaceuticals from the Carolina Institute of Clinical Research, during the conduct of the study. J. R. S. has received research grant support and consultant fees from StarPharma, BD Diagnostics, Talis, and Hologic, and has received grant support, medical writing, and consulting fees from Lupin Pharmaceuticals. P. N. has received consulting fees from Lupin Pharmaceuticals, Hologic Corporation, and BD Diagnostic Systems; grants from Curatek Pharmaceuticals and Hologic; and grants and personal fees from Mycovia Pharmaceuticals and Scynexis, outside the submitted work. G. K., J. S., and B. P. are employees of Lupin Pharmaceuticals. L. A. M. has received grants and personal fees from Gilead Sciences, ViiV Healthcare, Merck, and Roche Molecular; and grants from Binx Health, Evofem, Visby Medical, Click Diagnostic, Janssen Pharmaceutical, Prosoft Clinical, ThaiMed, GSK, and SpeedDx Pty Ltd. S. E. and S. E. C. have received consulting fees from Lupin Pharmaceuticals. S. E. C. reports speaker’s bureau fees and consulting fees for secnidazole for bacterial vaginosis and was a principal investigator on the phase 3 clinical trial. G. K. reports a patent application filed (Secnidazole use for trichomoniasis). All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 2020 Infectious Diseases Society of Gynecology Annual Meeting, Virtual, 14–15 August 2020. Oral presentation 15.
References
- 1.Flagg EW, Meites E, Phillips C, Papp J, Torrone EA. Prevalence of trichomonas vaginalis among civilian, noninstitutionalized male and female population aged 14 to 59 years: United States, 2013 to 2016. Sex Transm Dis 2019; 46:e93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClelland RS, Sangare L, Hassan WM, et al. Infection with trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 2007; 195:698–702. [DOI] [PubMed] [Google Scholar]
- 3.Committee on Practice Bulletins-Gynecology. Vaginitis in nonpregnant patients: ACOG practice bulletin, number 215. Obstet Gynecol 2020; 135:e1–17. [DOI] [PubMed] [Google Scholar]
- 4.Mielczarek E, Blaszkowska J. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection 2016; 44:447–58. [DOI] [PubMed] [Google Scholar]
- 5.Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014; 41:369–76. [DOI] [PubMed] [Google Scholar]
- 6.Workowski KA, Bolan GA; Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 7.Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyirjesy P, Schwebke JR. Secnidazole: next-generation antimicrobial agent for bacterial vaginosis treatment. Future Microbiol 2018; 13:507–24. [DOI] [PubMed] [Google Scholar]
- 9.Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol 2010; 2010:705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwebke JR, Morgan FG Jr, Koltun W, Nyirjesy P. A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis. Am J Obstet Gynecol 2017; 217:678.e1–9. [DOI] [PubMed] [Google Scholar]
- 11.Gillis JC, Wiseman LR. Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs 1996; 51:621–38. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. Uncomplicated urinary tract infections: developing drugs for treatment guidance for industry. Rockville, MD: FDA, 2019. [Google Scholar]
- 13.Pentikis HS, Adetoro N, Kaufman G. In vitro metabolic profile and drug-drug interaction assessment of secnidazole, a high-dose 5-nitroimidazole antibiotic for the treatment of bacterial vaginosis. Pharmacol Res Perspect 2020; 8:e00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupin Pharmaceuticals Inc. Solosec (secnidazole) [package insert]. Baltimore, MD: Lupin Pharmaceutical Inc, 2017. [Google Scholar]
- 15.US Food and Drug Administration. Bacterial vaginosis: developing drugs for treatment guidance for industry. Report No.: FDA-2016-D-1659. Rockville, MD: FDA, 2019. [Google Scholar]
- 16.Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol 2013; 51:3875–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beverly AL, Venglarik M, Cotton B, Schwebke JR. Viability of Trichomonas vaginalis in transport medium. J Clin Microbiol 1999; 37:3749–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998; 22:205–8. [DOI] [PubMed] [Google Scholar]
- 19.Craig-Kuhn MC, Granade C, Muzny CA, et al. Optimal timing for trichomonas vaginalis test of cure using nucleic acid amplification testing. Sex Transm Dis 2019; 46:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanoff MA, Carey JC, Hauth JC, et al. ; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units . Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001; 345:487–93. [DOI] [PubMed] [Google Scholar]
- 21.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45:1319–26. [DOI] [PubMed] [Google Scholar]
- 22.Kissinger P, Mena L, Levison J, et al. A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J Acquir Immune Defic Syndr 2010; 55:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pentikis HS, Adetoro N. Two phase 1, open-label, single-dose, randomized, crossover studies to assess the pharmacokinetics, safety, and tolerability of orally administered granules of secnidazole (2 g) in healthy female volunteers under different administration conditions. Clin Pharmacol Drug Dev 2018; 7:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzny CA, Kardas P. A narrative review of current challenges in the diagnosis and management of bacterial vaginosis. Sex Transm Dis 2020; 47:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartley JB, Ferris DG, Allmond LM, Dickman ED, Dias JK, Lambert J. Personal digital assistants used to document compliance of bacterial vaginosis treatment. Sex Transm Dis 2004; 31:488–91. [DOI] [PubMed] [Google Scholar]
- 26.Cotch MF, Pastorek JG 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 1997; 24:353–60. [DOI] [PubMed] [Google Scholar]
- 27.Minkoff H, Grunebaum AN, Schwarz RH, et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol 1984; 150:965–72. [DOI] [PubMed] [Google Scholar]
- 28.Moodley P, Connolly C, Sturm AW. Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. J Infect Dis 2002; 185:69–73. [DOI] [PubMed] [Google Scholar]
- 29.Sobel JD, Subramanian C, Foxman B, Fairfax M, Gygax SE. Mixed vaginitis-more than coinfection and with therapeutic implications. Curr Infect Dis Rep 2013; 15:104–8. [DOI] [PubMed] [Google Scholar]
- 30.Siboulet A, Catalan F, Videau D, Niel G. La trichomonase urogénitale. Essais d’un imidazole à demi-vie longue: le secnidazole. Méd Mal Infect 1977; 7:400–4. [Google Scholar]
- 31.Videau D, Niel G, Siboulet A, Catalan F. Secnidazole. A 5-nitroimidazole derivative with a long half-life. Br J Vener Dis 1978; 54:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pentikis H, Eder S, Kaufman G, Chavoustie S. Secnidazole, an approved single dose drug for bacterial vaginosis, does not cause reproductive toxicity in animals. In: American Academy of Obstetricians and Gynecologists, Seattle, Washington, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.