Abstract
Hypoxia tolerance of the organism depends on many factors, including age. High newborn organisms tolerance and high level of oxidative stress throughout aging were demonstrated by many studies. However, there is lack of investigations reflecting the expression of key hypoxia-inducible factor HIF in different age organisms in correlation to levels of pro-inflammatory and anti-inflammatory cytokines. Liver is a sensitive to hypoxia organ, and is an important organ in providing an acute reaction to infections – it synthesizes acute inflammation phase proteins, in particular, C-reactive protein. The aim of study was to determine relationship between age-related tolerance to hypoxia and HIF-1 and PHD2 (prolyl hydroxylase domain protein) expression levels in the liver and the production of cytokines in the spleen in newborn, prepubertal and adult Wistar rats. Newborn rats are characterized by high mRNA Hif-1α expression level in the liver, accompanied by a low content of HIF-1 protein and high level of PHD2. The growth in HIF-1α protein level throughout age is accompanied by the growth of pro-inflammatory cytokines level. Prepubertal animals are the least hypoxia resistant and their HIF-1α mRNA expression level was higher than in adult animals. The PHD2 activity in prepubertal animals was significantly reduced in comparison to newborn rats, and the HIF-1α protein level did not change. Further studies require the identification of additional mechanisms, determining the regulation of the HIF-1α level in prepubertal animals.
Keywords: Hypoxia tolerance, HIF-1, PHD2, Age-related differences, Newborn, Prepubertal age
Hypoxia tolerance, HIF-1, PHD2, Age-related differences, Newborn, Prepubertal age.
1. Introduction
Hypoxia, or lack of oxygen, is one of the main mechanisms regulating the functional state of organism in normal conditions and in various diseases. At the molecular level, oxygen deficiency initiates a transcriptional program targeted to oxygen homeostasis and cell survival maintenance. Adaptation to hypoxia of cells and tissues is determined by the activation of genes involved in angiogenesis, glucose and iron metabolism, such as glucose transporter GLUT1, vascular endothelial growth factor (VEGF), erythropoietin (EPO). Key factors that realize cellular responses as a reaction to oxygen deficiency are the HIF transcription factors family (hypoxia-inducible factor) (Semenza, Wang, 1992; Kaelin, Ratcliffe, 2008; Semenza, 2010; Hirota, 2020). The expression of the oxygen-dependent subunit HIF-α is regulated primarily through hydroxylation. It is performed by special proteins on proline residues by prolyl hydroxylases (PHDs) and on asparagine residues via the factor that inhibits HIF – FIH (Jaakkola et al., 2001; Mahon et al., 2001). PHD2 is known as the dominant prolyl-4-hydroxylase (Kaelin, 2005). In spite of the fact that all PHDs are commonly expressed, nonetheless they are differently expressed in various tissues. PHD2, being the most expressed enzyme, could be found almost in all tissues. Considering HIF signaling, PHD2 performs the major role as the oxygen sensor (Lieb et al., 2002; Wong et al., 2013). In addition, there are many other mechanisms of HIF activity regulation. HIF-1α is activated in response to reactive oxygen species (ROS), through the mTOR pathway (Mammalian Target Of Rapamycin), inflammatory cytokines, NF-κB (Nuclear Factor-κB), etc (Bonello et al., 2007; Rius et al., 2008; van Uden et al., 2008, 2011; Smolková et al., 2011).
Age plays an important role in organism hypoxia tolerance. In 1870 P. Bert (1870) already found out that newborn animals are more resistant to low oxygen level in comparison to adults. The mechanisms of high tolerance of newborns to hypoxia, observed both in clinical and experimental studies, are not fully understood (Volpe, 1995; Singer, 1999; Bickler, 2004). Vital organs differ according to the hypoxia sensitivity – brain is the most susceptible, which ultimately determines the tolerance of the whole organism to a lack of oxygen (Burtscher et al., 2012). A possible mechanism of the nervous tissue higher tolerance to hypoxia in newborn rats could be a decrease in the HIF-1 cell content due to the active synthesis of PHD2 in the brain immediately after the hypoxic exposure and reoxygenation (Jones et al., 2006). Most studies in this field focus on biochemical characteristics of the newborns brain, allowing them to adapt to acute hypoxic effects. It was previously observed that under hypoxic conditions, newborns demonstrate slower decrease rate in the tissue ATP level than in adult mice and rats (Thurston, McDougal, 1969; Duffy et al., 1975; Singer, 1999). Under hypoxia obviously postponed depolarization and further growth in potassium extracellular level in newborn mammalian neurons compared to adult mammalian neurons were detected in some physiological studies (Hansen, 1977; Trippenbach et al., 1990).
Nevertheless, little is known about HIF expression in organisms of different ages. It was demonstrated that expression and concentration of proteins HIF-1α and HIF-2α changed with age in Tibetan Sheep (He et al., 2019). The myocardium level of HIF-1α was higher in 2-year old yaks, than in younger and older ones. However, the myocardium HIF-dependent VEGF level increased throughout the animals’ life and the oldest yaks demonstrated the highest levels (Duan et al., 2012; Zhou et al., 2013; He et al., 2016).
It is known that in early postnatal rats hypoxia tolerance level hesitates significantly and after 2 months of ontogenesis it reaches stable values (Korneev et al., 1993). Moreover, some studies found out less or more decrease rate in hypoxia resistance throughout aging (Glass et al., 1944; Britton, Kline, 1945; Adolph, 1969). It was demonstrated that in comparison to older children (13–18 years), younger children (8–12 years) utilize more oxygen and produce less lactate during high-intensity exercise than older children and adults. The research may be due to the lower anaerobic dependence that was detected in younger children (Chen et al., 2018). Muscle oxygen uptake was greater and blood lactate concentration was lower in children in comparison to adults after normalization to the amount of work performed (Beneke et al., 2005). However, recent meta-analysis (Wu et al., 2018) revealed no relationship between age (in humans between the ages of 10 and 76 years) and the risk of acute mountain sickness development.
Adult animals are divided into tolerant and susceptible to hypoxia according to gasping time assessment in the decompression chamber. Tolerance to hypoxia performs a significant part in the inflammation development (Dzhalilova et al., 2019a,b), since any disturbances of the reactions to a lack of oxygen molecular mechanisms lead to pathological processes that contribute to many inflammatory diseases development (Watts, Walmsley, 2019) It was demonstrated that prepubertal animals have more pronounced SIRS (systemic inflammatory response syndrome) in comparison to adults (Kosyreva et al., 2020). The key pathogenesis factors of SIRS are circulatory disorders and hypoxia. Possible age-related mechanisms of the predisposition to inflammatory diseases could be connected with the variability of hypoxia tolerance.
It is known that liver is a sensitive to hypoxia organ. In adult animals, it was demonstrated that basal HIF-1α level in the neocortex and other oxygen-dependent organs, especially in the liver, varies depending the individual hypoxia tolerance of the organism and is higher in less resistant to hypoxia rats (Kirova et al., 2013; Dzhalilova et al., 2019a). Moreover, liver is a target organ for the lipopolysaccharide, and, what is more, is an important organ in providing an acute reaction to infections – it synthesizes acute inflammation phase proteins, in particular, C-reactive protein. Furthermore, during SIRS, we established age-related differences in pathological changes in hepatocytes – in prepubertal Wistar rats, the area of necrosis was maximal compared to newborn and adult males (Kosyreva et al., 2019).
The concept of the study was to reveal the relationship between age-related tolerance to hypoxia and HIF-1 and PHD2 proteins expression levels in the liver and the production of pro-inflammatory and anti-inflammatory cytokines in the spleen in newborn, prepubertal, and adult Wistar rats.
2. Material and methods
2.1. Experimental animals
Study was made on male (n = 36) Wistar rats of three age groups - neonatal period (age – two days, body weight 8–10 g; n = 12), prepubertal (age – 10 days, body weight 25–30 g; n = 12) and adult (age – 3 months, body weight 220–250 g; n = 12). The Bioethics Committee of the Research Institute of Human Morphology (Protocol No. 21, March 29, 2019) approved the experiments for the study. Experiments on animals were designed based on the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ets no. 123), Strasbourg, 2006, and all the manipulations were made in order to decrease suffering and possible stress for animals. For animals breeding we used 20 mature Wistar rats (15 females and 5 males). Every four rats (3 females and 1 male) kept in cages (18.5 × 60 × 38 cm) in stable temperature conditions at 12:12 h light–dark cycle, in humidity frames between 55 and 65%; and had unlimited food and water consumption (“Chara”, JSC “Range-Agro”, Russia). The durations of pregnancy in females were 21–23 days. The offspring of one female was represented by 6–8 individuals. The male rats were randomly divided into 2 groups of three ages.
2.2. Determination of tolerance to hypobaric hypoxia
Hypoxic tolerance in the first group of Wistar rats of three age division (n = 18) was measured once in special decompression chamber via gasping time detection (the time before respiratory disturbances and signs of asphyxia appear). Based on literature data, such characteristics reflects the organism's hypoxia tolerance (Lukyanova et al., 2009; Jain et al., 2013; Kirova et al., 2013; Tregub et al., 2013). The conditions equivalent to the altitude of 11,500 m (equivalent to 180 mmHg) were modeled in the decompression chamber, combined with a mercury barometer, as was described previously (Lukyanova et al., 2009; Kirova et al., 2013; Tregub et al., 2013; Dzhalilova et al., 2018, 2019a,b; Dzhalilova, Makarova, 2020). The air from the decompression chamber was evacuated with a vacuum pump for 1 min. The level of atmospheric pressure was determined by the altimeter during the whole experiment. Decompressions and recompressions were made step by step with the 600 m (≈40 mmHg)/min rate to avoid organisms injury due to the unpredicted pressure fall or rise. The rate of airflow determined, was 2 L/min, at the same time the humidity was at about 45%. In the place, where the experiments were performed, was stable temperature 20–22°С. The time period determined for the first gasping sign, was checked via the electronic stopwatch. To restore the initial level of atmospheric pressure, the decompression chamber had an intake valve. Pressure recovery occurred within 1 min after the vacuum pump was switched off. Following the tests, all animals were alive and had normal activity without any markers of pathology.
2.3. Sample collection
The second group of rats (n = 18) was euthanized by an overdose (15 mg/kg) of anesthetic Zoletil («Virbac Sante Animale», France). For the research of genes and proteins expression, was chosen the liver, which is a sensitive to hypoxia organ. In adult animals, it was demonstrated that basal HIF-1α level in the neocortex and other oxygen-dependent organs, especially in the liver, varies depending the individual hypoxia tolerance of the organism and is higher in less resistant to hypoxia rats (Kirova et al., 2013; Dzhalilova et al., 2019a). Liver fragments were fixed for subsequent PCR in IntactRNA Reagent (Evrogen CJSC, Russia) and for Western Blot analysis in a liquid nitrogen. Jugular venous blood was obtained (Parasuraman et al., 2010), then it was centrifuged at 200 g during 20 min at room conditions. The serum collected was freezed at -70 °C and was kept no longer than two months.
2.4. Real-time PCR (qPCR)
Expression of Hif-1α in the liver of newborn (n = 6), prepubertal (n = 6) and adult (n = 6) Wistar rats was investigated by real-time PCR. Liver tissue in a volume of about 30 mm3, was put in IntactRNA Reagent (Evrogen CJSC, Russia) right after sampling. It was incubated during the night at 4 °C, and kept at -80°С before use. Total RNA was obtained from the tissue probes via RNeasy Plus Mini Kit (QIAGEN, Germany) according to the manufacturer protocol. The purified RNA concentration in the eluate was about 0.1 g/l; samples quality was checked by electrophoresis. Total RNA reverse transcription was done using MMLV RT Kit (Evrogen CJSC, Russia) for obtaining the single-stranded cDNA. Final dilution of the mixture in PCR constituted 1:250. Polymerase chain reactions were made twice based on the qPCRmix-HS SYBR (Evrogen CJSC, Russia) with oligonucleotide primers (SYNTOL, Russia). Primers for PCR were selected using the on-line Primer-BLAST program in accordance with generally accepted requirements (Hif-1α f: 5′-GAGCCTTAACCTATCTGTCA-3′, r: 5′-CACAATCGTAACTGGTCAGC-3′). Amplification with detection and digital analysis of fluorescence in real-time was made on DT-96 Real-Time PCR Cycler (DNA-Technology JSC, Russia) in standard mode of 95 °C for 5 min followed by (95 °C for 15 s, 62° for 10 s + detection, 72° for 20 s) x 45 cycles. Characteristic values (Cp) were generated automatically thanks to nonlinear regression analysis, and the relative expression values were measured by approach originally introduced by (Pfaffl, 2001) using b2m (f: 5′-CTCGCTCGGTGACCGTGAT-3′, r: 5′-CACAATCGTAACTGGTCAGC-3′) as reference target.
2.5. Western blot analysis
Liver fragments were lysed in Protein Solubilization Buffer (PSB, Bio-Rad, USA) with Complete Protease Inhibitor Cocktail (Roche, USA), homogenized with pestle and centrifuged 30 min at 14,000 g. Thereafter, 2x loading buffer was added to the supernatant, and the sample was incubated at 65 °C for 5 min. For the protein separation, 10%–12.5% SDS-PAGE was performed. Transfer from the gel to PVDF membranes by semi-wet approach was conducted using Trans-Blot® Turbo™ RTA Mini LF PVDF Transfer Kit (Bio-Rad, USA). The membranes were blocked with milk (5%) in Tris-buffered saline with Tween 0.1% (TTBS) for 1 h at room temperature, then incubated overnight with primary antibodies to HIF1α (ab179483, 1:1000, abcam) and GAPDH (sc-25778, 1:1000, Santa Cruz), PHD2 (ab244389, 1:500, abcam) overnight at 4 °C with gentle shaking. Thereafter samples were stained with horseradish peroxidase (HRP) conjugated secondary antibodies (Bio-Rad, USA) for 1 h at room temperature. Target proteins were visualized by Novex ECL Kit (Invitrogen™ Thermo Fisher Scientific, USA) in ChemiDoc (Bio-Rad, USA). For optical density measurements of the protein bands Image Lab Software tool was used with GAPDH as a reference protein.
2.6. Isolation and cultivation of splenic cells
Isolation and cultivation of splenic cells were carried out as describe in (Kosyreva et al., 2018). Splenic cells were aseptically isolated from each rat, then immediately put in Potter homogenizer with the medium Roswell Park Memorial Institute (RPMI) 1640 and single-cell suspensions were made. Erythrocytes were lysed by distilled water. In order to start synthesis and secretion of cytokines, the spleen cells were cultivated in 106/ml concentration in 1 ml of medium containing concanavalin A (5 μg/ml) during 20 h at 5% CO2 and 37 °C conditions in 24-well cultured plates. The content of culture medium included RPMI-1640 (PanEco, Russia), 5% inactivated foetal bovine serum (FBS), 50 μg/ml gentamicin, and 2 mM glutamine (Lin et al., 2015). The viability potential of cells was checked based on trypan blue exclusion (Lefèvre et al., 2017).
2.7. ELISA
The corticosterone (IBL, Germany) concentration was determined in the serum by ELISA. The levels of TNF-α, IFN-γ, and IL-10 were measured in the culture fluid of splenic cells by ELISA test systems (eBioscience, USA).
2.8. Oxidative stress assessment
Oxidative stress was evaluated in the serum using the «CR3000» analyser (Callegari, the Catellani group, Parma, Italy). The analyser uses two colorimetric assays to evaluate oxidative stress: the free oxygen radicals testing (FORT) and the free oxygen radicals defence (FORD). ROS were revealed in the FORT assay, which includes colorimetric method. It is based on the metals catalyzing properties. The FORD test supposes the use of preformed colored and stable radicals and defines the decrease in absorbance that is proportional to the antioxidant concentration in blood sample (Palmieri, Sblendorio, 2007; Garelnabi et al., 2008; Pavlatou et al., 2009; Găman et al., 2020). The FORT reflects the levels of ROS in the blood (Găman et al., 2020).
2.9. Statistics
Statistical analysis included the obtained data normality test with the Kolmogorov–Smirnov method in Statistica 8.0. We used the nonparametric multiple comparison procedures. Multiple comparison procedures were examined with Kruskal–Wallis test, in the situations when P was <0.05, Dunn's post-hoc test was used in order to reveal the differences in each pair of groups. For values of the measured parameters the median and IQR (Me, Low–High) were calculated. The differences were decided to be statistically significant when P was <0.05. In each group experiments included at least five observations. For the data graphical representation, we used box-and-whisker plots, reflecting the median, IQR, upper extreme (75%) and lower extreme (25%) of the data.
3. Results
3.1. Age-related differences of hypoxia tolerance
The first set of questions aimed to reveal which studied group (newborn, prepubertal or adult rats) was the most tolerant to hypoxic exposure. After the assessment of animals gasping time in the decompression chamber at 11,500 m, it was demonstrated that newborn and adult rats are more tolerant than prepubertal animals (Figure 1). Not less important is the high variability of the parameter in adult rats. This may be due to the fact that in this study we did not divide adult rats into tolerant and susceptible to hypoxia. It was demonstrated (Lukyanova et al., 2009; Kirova et al., 2013; Dzhalilova, Makarova, 2020) that subgroups of animals could be distinguished in rat population in puberty, according to the gasping time, differing by 3 or more times. Since in our work we aimed to study age-related changes in resistance to hypoxia, we estimated the gasping time in the decompression chamber in adult animals without dividing them into tolerant and susceptible groups.
Figure 1.
Evaluation of gasping time for newborn, prepubertal and adult Wistar rats in the decompression chamber (Me; 25%–75%). In all groups there were 6 observations. p – statistically significant differences, Kruskal–Wallis method with Dunn's post-hoc test. Newborn and adult rats are more tolerant to hypoxia than prepubertal animals. Not less important is the high variability of the parameter in adult rats.
3.2. Age-related differences of HIF-1α and PHD2 expression
The next section of the study was concerned with the determining of the expression and protein production levels of two hypoxia-associated markers – HIF-1α and PHD2 in the liver. Liver is an organ sensitive to hypoxia. Furthermore, during SIRS, we established age-related differences in pathological changes in hepatocytes (Kosyreva et al., 2019). Therefore, we chose this organ to assess the expression of proteins involved in adaptive response during hypoxia in accompanying inflammation.
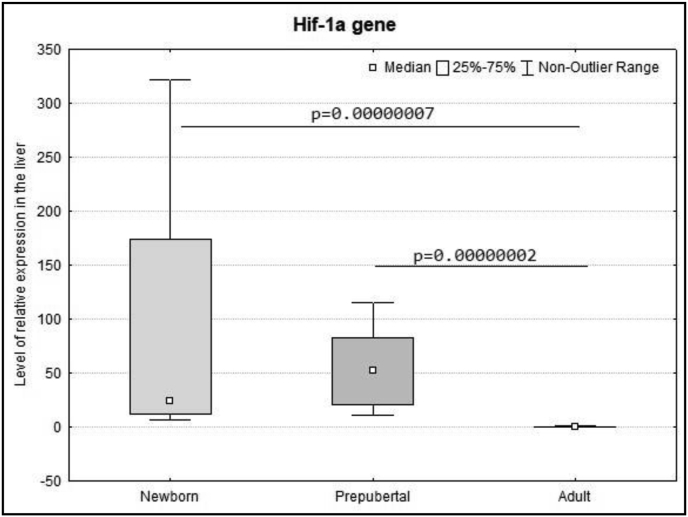
The Hif-1α expression level in the liver of newborn and prepubertal rats was significantly higher in comparison to adult animals, which means this indicator decreased throughout aging (Figure 2). Meanwhile, the HIF-1α protein level was minimal in newborn and prepubertal rats and increased towards puberty. It should be stated that there is a high variability in the HIF-1α protein level in adult rats. This corresponds to the spread in the parameters of the gasping time at altitude in adult animals and once again proves the fact that testing in the decompression chamber is an adequate in vivo method for dividing animals according to their resistance to hypoxia.
Figure 2.
The expression level of mRNA Hif-1α in the liver of newborn, prepubertal and adult Wistar rats (Me; 25%–75%). In all groups there were 6 observations. p – statistically significant differences, Kruskal–Wallis method with Dunn's post-hoc test. The Hif-1α expression level in the liver of newborn and prepubertal rats was significantly higher in comparison to adult animals, that means this indicator decreased throughout aging.
PHD2 is the key oxygen sensing enzyme involved in regulating HIF-1α levels under normoxic conditions. The PHD2 protein level in the liver of newborn animals was significantly higher than in prepubertal and adult rats (Figure 3). Therefore, we've demonstrated for the first time, that PHD2 is the age-related protein.
Figure 3.
(a) – Relative protein levels of HIF-1α and PHD2 in the liver of newborn, prepubertal and adult Wistar rats, normalized on GAPDH level, representative Western blot images are shown. Full-length blots are presented in Supplementary Figure S1 (a-c). (b, c) – The expression level of HIF-1α (b) and PHD2 (c) proteins in the liver of newborn, prepubertal and adult Wistar rats (Me; 25%–75%). In all groups there were 6 observations. p – statistically significant differences, Kruskal–Wallis method with Dunn's post-hoc test. HIF-1α protein level was minimal in newborn and prepubertal rats and increased towards puberty. It should be stated that there is a high variability in the HIF-1α protein level in adult rats. PHD2 protein level in the liver of newborn animals was significantly higher than in prepubertal and adult rats.
3.3. Age-related differences of free radical oxidation and antioxidant protection
HIF-1α and PHD2 protein levels could be determined by ROS levels. The hypoxic exposure is connected with the ROS production, and the resistance to the action of oxygen deficiency can be largely due to the effective work of the antioxidant system. Therefore, at the next stage, we measured the level of free radical oxidation and antioxidant protection using the FORT/FORD method. The level of free radical oxidation was higher in adult rats and was significantly different from prepubertal rats. At the same time, there were no age-related differences in the extent of antioxidant protection (Table 1).
Table 1.
The FORD and FORT values in the blood of newborn, prepubertal and adult Wistar rats (Me; 25%–75%). In all groups there were 6 observations. p - statistically significant differences, Kruskal–Wallis method with Dunn's post-hoc test. The level of free radical oxidation was higher in adult rats and was significantly different from prepubertal rats. At the same time, there were no age-related differences in the extent of antioxidant protection. 1-2 – the differences between newborn and prepubertal groups, 1-3 – the differences between newborn and adult groups, 2-3 – the differences between prepubertal and adult groups.
| Newborn1 | Prepubertal2 | Adult3 | p-value | |
|---|---|---|---|---|
| Free oxygen radical defence (mmol/L Trolox) | 1.72 (1.72–1.81) | 2.02 (2.0–2.02) | 1.66 (1.59–1.85) | 0.091−2 0.991−3 0.142−3 |
| Free oxygen radical testing (mmol/L H2O2) | 0.61 (0.58–0.64) | 0.53 (0.43–0.61) | 2.40 (2.20–3.11) | 0.991−2 0.121−3 0.007492−3 |
3.4. Age-related differences of cytokine production
A high level of free radical oxidation can lead to a predisposition to the development of inflammatory reactions. In addition, it was demonstrated that HIF regulates NF-κB dependent production of pro- and anti-inflammatory cytokines (Walmsley et al., 2005; Rius et al., 2008). Since resistance to hypoxia and HIF expression change with age, it is possible that the previously established differences in the severity of SIRS are associated with age-related changes in cytokine production. Therefore, we further investigated age-related differences in the production of pro- and anti-inflammatory cytokines by spleen cells in newborn, prepubertal, and adult male Wistar rats. It was revealed that the production of anti-inflammatory cytokine IL-10 by spleen cells was significantly higher in newborn and prepubertal rats in comparison to adult rats (Table 2). Simultaneously, the production of pro-inflammatory cytokines TNF-α and IFN-γ was minimal in newborn rats and increased by puberty. We demonstrated that the production of IFN-γ and TNF-α by spleen cells is increased in prepubertal rats in comparison with newborns.
Table 2.
Production of cytokines IL-10, TNF-α and IFN-γ by splenic cells in newborn, prepubertal and adult Wistar rats (Me; 25%–75%). In all groups there were 6 observations. p - statistically significant differences, Kruskal–Wallis method with Dunn's post-hoc test. Production of anti-inflammatory cytokine IL-10 by spleen cells was significantly higher in newborn and prepubertal rats in comparison to adult rats. Simultaneously, the production of pro-inflammatory cytokines TNF-α and IFN-γ was minimal in newborn rats and increased by puberty. 1-2 – the differences between newborn and prepubertal groups, 1-3 – the differences between newborn and adult groups, 2-3 – the differences between prepubertal and adult groups.
| Cytokines in the culture fluid of splenic cells, pg/mL | Newborn1 | Prepubertal2 | Adult3 | p-value (p < 0.05) |
|---|---|---|---|---|
| IL-10 | 110.0 (81.5–175.5) | 106.0 (100.5–127.7) | 39.9 (31.2–44.2) | 0.0031−3 0.0062−3 |
| TNF-α | 10.78 (1.35–13.48) | 149.2 (69.88–281.8) | 325.3 (254–456) | 0.0000041−2 0.000000021−3 0.0042−3 |
| IFN-γ | 0 (0–0) | 20.5 (5.86–50.37) | 1228 (205.4–2457) | 0.011−2 0.0000031−3 0.032−3 |
3.5. Age-related differences of corticosterone level in the blood serum
It is known that with the increase in pro-inflammatory mediators level, the level of corticosterone increases compensatory. For example, the release of certain inflammatory cytokines such as TNF-α, IL-10, and IL-6 activates the hypothalamic–pituitary–adrenal (HPA) axis and causes the release of cortisol (Turnbull, Rivier, 1999; Steensberg et al., 2003). The anti-inflammatory effects of cortisol then feedback and suppress further release of cytokines (Reichlin, 1993; Elenkov and Chrousos, 1999). Therefore, we estimated the concentration of one of the hypoxia tolerance and inflammation regulators – corticosterone in the serum of newborn, prepubertal and adult Wistar rats. The level of corticosterone is determined by the adrenocorticotropic hormone (ACTH) production of the hypothalamus and activation of the peripheral nervous system. The blood serum concentration of corticosterone was the highest in prepubertal and the lowest in newborn rats (Figure 4). In the prepubertal period there is an active development of the nervous system, including the pituitary gland and hypothalamus, that may determine the high level of corticosterone. Moreover, an increase in the corticosterone concentration is probably a compensatory reaction associated with the increase in the pro-inflammatory cytokines synthesis by the functionally maturing immune system in prepubertal rats.
Figure 4.
The corticosterone concentration in blood serum of newborn, prepubertal and adult Wistar rats (Me; 25%–75%). In all groups there were 6 observations. p – statistically significant differences, Kruskal–Wallis method with Dunn's post-hoc test. The blood serum corticosterone concentration was the highest in prepubertal and the lowest in newborn rats.
4. Discussion
Hypoxia tolerance depends on the age and is determined by a complex of factors, responsible for adaptive reactions to oxygen deficiency and immune processes. The study was aimed to age-related differences in hypoxia-associated genes and cytokine profile in newborn, prepubertal, and adult Wistar rats investigation. According to the results of gasping time in the decompression chamber determining at the 11,500 m, it was demonstrated that the most tolerant to hypoxia animals are newborns, and prepubertal animals are characterized by minimum gasping time values, which is in accordance with the data of previous studies (Volpe, 1995; Singer, 1999; Bickler, 2004; Kosyreva et al., 2019). In comparison with adult, newborn rats demonstrated the high level of liver Hif-1α mRNA expression, but the HIF-1 protein level, on the contrary, was low. It can be associated with a high PHD2 activity, which contributes to the HIF-1 protein degradation. Hydroxylation of proline residues of HIF-α is a key mechanism of negative regulation of its activity, since it contributes proteasome degradation of HIF-α via the von Hippel-Lindau (VHL) E3 ubiquitin ligase complex (Koyasu et al., 2018; Stothers et al., 2018). According to the earlier investigations, PHD2 is the key oxygen sensing enzyme involved in regulating HIF-1α levels under normoxic conditions (Berra et al., 2003; Appelhoff et al., 2004). Based on the literature, inhibition of PHD2 induces active synthesis of the HIF-1α protein (Berra et al., 2003). Probably, under physiological conditions in newborns, the mRNA Hif-1α expression level is constitutionally high in response to possible hypoxia. As data show, low tension of oxygen in utero is in connection with the much greater hypoxia resistance of neonatal or embryonic rats (Friedman, Haddad, 1993; Bickler, Hansen, 1998). Under normoxia, when the oxygen content is sufficient, prolyl hydroxylases contribute to the HIF-1 protein degradation in the proteasome, its level decreases, and its physiological and biochemical effects are not realized. Perhaps, newborn rats are characterized by the high induction level of HIF-1 protein synthesis based on the available mRNA, which, under hypoxic conditions in the decompression chamber, ensures the maximum gasping time at the altitude. Other studies also proved that HIF-1α protein was not found in the brain of newborn rats under normoxia (Lu et al., 2014).
In comparison to newborn and prepubertal rats, the minimum expression of Hif-1α mRNA was revealed in the liver of adult rats, while the HIF-1α protein level, vice versa, was high, which might be associated with low mRNA stability and high protein stability, as well as any miRNA functioning (Serocki et al., 2018). In accordance with the data of (Belaiba et al., 2007), in human pulmonary artery smooth muscle cells (PASMC) under hypoxic conditions, Hif-1α mRNA levels were increased in response to hypoxia within 0.5 h, peaking at 1 h after stimulation and returning to basal levels after 4 h of stimulation. Simultaneously, HIF-1α protein level were rapidly increased in response to hypoxia after 0.5 h, and it remained elevated for up to 8 h. In addition, we demonstrated that in adult period the level of PHD2 protein in liver was low, which contributes to the HIF-1α protein stabilization and its translocation into the nucleus.
The high HIF-1α protein level and low level of PHD2 in adult rats could also be explained by high ROS level, determined by the FORT. There're several research, reflecting that mitochondrial ROS exerts a negative regulation of PHD activity. High levels of ROS are able to affect the catalytic domain of PHD2, thus reducing its activity, or it can induce the specific post-translational modifications that in turn inhibit PHD2, which promotes HIF activation (Emerling et al., 2005; Salmeen, Barford, 2005). Pronounced relationship between FORT and other indices of inflammation and oxidative stress was found (Mantovani et al., 2004; Ridker et al., 2004; Abramson et al., 2005; Harris et al., 2007). Studies demonstrated that addition of exogenous H2O2 or glucose oxidase, which generates H2O2, is sufficient to stabilize HIF-1α protein in normoxia conditions (Chandel et al., 2000; Brunelle et al., 2005; Guzy et al., 2005). Many stress signals, including ROS can force accumulation of HIF even in conditions of normoxia, known as pseudohypoxia (Salminen et al., 2016). ROS was discovered to be the stimulating factor for HIF-1α stabilization, that eventually making possible for HIF pathway activation (Chandel et al., 1998, 2000; Bell et al., 2007). In addition, high levels of oxidative stress can lead to pro-inflammatory activation (Tafani et al., 2016). Aging and age-related degenerative diseases are associated with oxidative stress, probably partly linked to increased inflammation (Salminen et al., 2016).
The data obtained in this work indicate a high variability of both resistance to hypoxia and HIF-1α protein level in adult rats. This may be due to the fact that in this study we did not divide adult rats into tolerant and susceptible to hypoxia. However, it is known that in susceptible and tolerant to hypoxia animals the gasping time at altitude differs in at least three times, and HIF-1α expression level also varies (Kirova et al., 2013; Dzhalilova et al., 2019a; Dzhalilova, Makarova, 2020). Adult animals with different resistance to hypoxia differ in many parameters, which are discussed in details in (Dzhalilova, Makarova, 2020). The concept of the existence of different evolutionarily developed ‘functional and metabolic patterns’ corresponding to two types of animals with different tolerances to acute oxygen deficiency has been proposed (Mironova et al., 2010, 2019; Lukyanova, Kirova, 2015). These patterns are based on the characteristic features of the state of the central nervous system, the energy apparatus, and neurohumoral regulation, which determine the organism's response to hypoxia. The specific ultrastructure of the mitochondria in tolerant and susceptible rats, demonstrated by (Mironova et al., 2010, 2019; Pavlik et al., 2018; Belosludtsev et al., 2020), also supports the concept that animals with different tolerance to hypoxia have two different functional and metabolic profiles. They are associated with differences in the functional activity of energy system, the status of membranes and receptors. In comparison to newborn and prepubertal rats, the gasping time at critical altitude and HIF-1α protein expression level in adult animals had a pronounced variability, which may be due to the existence of different phenotypes in the population of adult animals, differing in hypoxia tolerance. According to literature, there is no data about the possibility of newborn and prepubertal experimental animals division into tolerant and susceptible subgroups.
In our work, the highest level of pro-inflammatory TNF-α and IFN-γ production by spleen cells was revealed in adult rats. Simultaneously, in comparison with newborn and prepubertal rats, the level of production of anti-inflammatory IL-10 in adult animals was low. It is known that normal term newborns cannot make IFN-γ in response to common mitogens or specific antigens (Bryson et al., 1980; Wakasugi, Virelizier, 1985). According to the existing data, in comparison with older children, newborns are characterized by higher values of the relative number of T-lymphocytes producing IL-2, and low rates of T-lymphocytes producing IFN-γ (Gasparoni et al., 2003). The ability to produce IFN-γ in humans is acquired normally during the first 3–6 months after birth (Frenkel, Bryson, 1987). It was also demonstrated (Wiegering et al., 2009) that the level of production of IFN-γ and TNF-α by T-helper cells increases with age. According to literature, hypoxia and HIF-1α activation induce NF-κB, which promotes the production of pro-inflammatory cytokines (Walmsley et al., 2005; Cummins et al., 2006). The increase with age in the HIF-1α protein level revealed is accompanied by the growth in the production of pro-inflammatory cytokines level, which confirms the key role of HIF in the development of immune responses in ontogenesis and functional maturation of the immune system (Corrado, Fontana, 2020). In addition, the increase in pro-inflammatory cytokines level in adult rats may be due to high levels of ROS. They activate NF-κB and promote the synthesis of pro-inflammatory cytokines by immune cells (Tafani et al., 2016).
The shortest gasping time in the decompression chamber at a critical altitude was revealed in prepubertal rats. At the same time, the Hif-1α mRNA expression level in them was significantly higher than in adults. In comparison with the neonatal period, the PHD2 protein level in prepubertal rats decreased, while the HIF-1α protein level did not change and remained lower than in adult rats. Probably, in prepubertal animals, additional mechanisms not related to the activity of prolyl hydroxylases, play a dominant role in the regulation of HIF-1α protein degradation (for example, Kruppel-like factor 2 (KLF2), receptor for activated C-kinase 1 (RACK1), etc.).
According to the literature, corticosterone is one of the regulators of hypoxia tolerance, and after the hypoxic exposure, the corticosterone content in plasma is appreciably higher in the susceptible to hypoxia animals (Jain et al., 2014). According to our data, the serum level of corticosterone in prepubertal rats was several times higher than in newborn and adult rats, which can determine the low tolerance of prepubertal animals to hypoxic exposure.
Low tolerance to hypoxia and the presence of additional regulation of HIF-1α degradation mechanisms in prepubertal rats may determine their high predisposition to the development of SIRS (Kosyreva et al., 2020). It is known that the development of LPS-induced pro-inflammatory reactions occurs through the activation of NF-κB. NF-κB is a transcription factor which, like HIF, regulates a diverse group of genes including immune receptors, cytokines, and stress-response genes (Pahl, 1999). In some contexts, NF-kB activity is linked to hypoxia signaling in a HIF-dependent manner (Walmsley et al., 2005; Rius et al., 2008). According to existing data, a low level of prolyl hydroxylases promotes the activation of NF-κB (Cummins et al., 2006; Fitzpatrick et al., 2016). This was indirectly confirmed by the increasing levels of TNF-α and IFN-γ associated with NF-κB throughout aging. NF-κB, in its turn, contributes the activation of HIF-1α mRNA expression (Rius et al., 2008; van Uden et al., 2008, 2011). In prepubertal animals an increased level of NF-κB expression can also be indicated by a compensatory high level of corticosterone.
Probably, prepubertal age with low tolerance to hypoxia is a risk factor for the development of inflammatory and other hypoxia-related diseases. During this period, the formation of the immune, endocrine and other systems of the organism occurs, hesitations in the levels of hormones are observed, which helps to reduce the general tolerance of the organism to stress factors, including hypoxia. Possibly, corticosterone plays a significant role in these processes as it is known that the excessive exposure to sustained elevated levels of stress hormones, including corticosterone, can be harmful and predispose to reproductive, immune, metabolic, and cardiovascular disorders (McEwen, 1998). In adult rats, despite the increased level of oxidative stress and production of pro-inflammatory cytokines, tolerance to hypoxia was higher, and SIRS severity was also less than in prepubertal animals (Kosyreva et al., 2019, 2020).
5. Conclusions
Thus, prepubertal animals are the least tolerant to hypoxia and are characterized by the shortest gasping time at the critical altitude (Figure 5). In this investigation we identified age-related differences in tolerance to hypoxia, characterized by different HIF-1α and PHD2 expression levels in the liver. The obtained data indicate that newborn rats are characterized by high mRNA Hif-1α expression level, accompanied by a low content of HIF-1 protein and high level of PHD2 prolyl hydroxylase. The relatively low Hif-1α mRNA expression level in adult rats in comparison to newborn and prepubertal rats during high levels of HIF-1α protein is in accordance with the low level of PHD2 activity. High HIF-1α protein level in adult rats is observed likely due to high ROS levels. The increase in HIF-1α protein content throughout age is accompanied by the growth in the production of pro-inflammatory cytokines level, which confirms the key role of HIF in the development of immune responses in ontogenesis and functional maturation of the immune system. In prepubertal animals, the level of Hif-1α mRNA expression was significantly higher than in adult animals. Simultaneously, the activity of PHD2 in prepubertal animals was significantly reduced in comparison with newborn rats, but the HIF-1α protein level was not changed. Further studies require the identification of additional mechanisms, determining the regulation of the HIF-1α protein level in prepubertal animals. Hopefully, this study will provide new insights into age-related differences in tolerance to hypoxia.
Figure 5.
Summary results of the study on the differences in the gasping time in the decompression chamber and the features of the expression of HIF-1α and PHD2 in newborn, prepubertal and adult Wistar rats. Newborn: high mRNA Hif-1α expression level, accompanied by a low content of HIF-1 protein and high PHD2 level. Prepubertal: the level of Hif-1α mRNA expression was higher than in adult animals. PHD2 activity in prepubertal animals was significantly reduced in comparison with newborn rats, but the HIF-1α protein level was not changed. Prepubertal animals are the least tolerant to hypoxia and are characterized by the shortest gasping time at the critical altitude. Adult: the relatively low level of Hif-1α mRNA expression in comparison to newborn and prepubertal rats during high levels of HIF-1α protein is in accordance with the low level of PHD2 activity. High HIF-1α protein level in adult rats is observed likely due to high ROS levels.
Declarations
Author contribution statement
Dzhuliia Dzhalilova: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Anna Kosyreva: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Polina Vishnyakova: Performed the experiments; Analyzed and interpreted the data.
Natalia Zolotova, Ivan Tsvetkov: Performed the experiments.
Vladimir Mkhitarov: Analyzed and interpreted the data.
Liliya Mikhailova: Conceived and designed the experiments.
Lev Kakturskiy: Contributed reagents, materials, analysis tools or data.
Olga Makarova: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (No. АААА-А19-119021490067-4). A part of the study concerning protein expression level was supported by the RUDN University Strategic Academic Leadership Program.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abramson J.L., Hooper W.C., Jones D.P., Ashfaq S., Rhodes S.D., Weintraub W.S., Harrison D.G., Quyyumi A.A., Vaccarino V. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178:115–121. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Adolph E.F. Regulations during survival without oxygen in infant mammals. Respir. Physiol. 1969;7:356–368. doi: 10.1016/0034-5687(69)90019-x. [DOI] [PubMed] [Google Scholar]
- Appelhoff R.J., Tian Y.M., Raval R.R., Turley H., Harris A.L., Pugh C.W., Ratcliffe P.J., Gleadle J.M. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Belaiba R.S., Bonello S., Zähringer C., Schmidt S., Hess J., Kietzmann T., Görlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E.L., Klimova T.A., Eisenbart J., Moraes C.T., Murphy M.P., Budinger G.R., Chandel N.S. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosludtsev K.N., Dubinin M.V., Talanov E.Y., Starinets V.S., Tenkov K.S., Zakharova N.M., Belosludtseva N.V. Transport of Ca2+ and Ca2+-dependent permeability transition in the liver and heart mitochondria of rats with different tolerance to acute hypoxia. Biomolecules. 2020;10:114. doi: 10.3390/biom10010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneke R., Hütler M., Jung M., Leithäuser R.M. Modeling the blood lactate kinetics at maximal short-term exercise conditions in children, adolescents, and adults. J. Appl. Physiol. 2005;99:499–504. doi: 10.1152/japplphysiol.00062.2005. (Bethesda, Md.: 1985) [DOI] [PubMed] [Google Scholar]
- Berra E., Benizri E., Ginouvès A., Volmat V., Roux D., Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert P. Baillie`re; Paris, France: 1870. Lec¸ons sur la physiologie compare´e de la respiration. [Google Scholar]
- Bickler P.E. Clinical perspectives: neuroprotection lessons from hypoxia-tolerant organisms. J. Exp. Biol. 2004;207:3243–3249. doi: 10.1242/jeb.00977. [DOI] [PubMed] [Google Scholar]
- Bickler P.E., Hansen B.M. Hypoxia-tolerant neonatal CA1 neurons: relationship of survival to evoked glutamate release and glutamate receptor-mediated calcium changes in hippocampal slices. Brain Res. Develop. Brain Res. 1998;106:57–69. doi: 10.1016/s0165-3806(97)00189-2. [DOI] [PubMed] [Google Scholar]
- Bonello S., Zähringer C., BelAiba R.S., Djordjevic T., Hess J., Michiels C., Kietzmann T., Görlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Britton S.W., Kline R.F. Age, sex, carbohydrate, adrenal cortex and other factors in anoxia. Am. J. Physiol. 1945;145:190–202. doi: 10.1152/ajplegacy.1945.145.2.190. [DOI] [PubMed] [Google Scholar]
- Brunelle J.K., Bell E.L., Quesada N.M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R.C., Chandel N.S. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metabol. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Bryson Y.J., Winter H.S., Gard S.E., Fischer T.J., Stiehm E.R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell. Immunol. 1980;55:191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Burtscher M., Mairer K., Wille M., Gatterer H., Ruedl G., Faulhaber M., Sumann G. Short-term exposure to hypoxia for work and leisure activities in health and disease: which level of hypoxia is safe? Sleep Breath. Schlaf Atmung. 2012;16:435–442. doi: 10.1007/s11325-011-0521-1. [DOI] [PubMed] [Google Scholar]
- Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel N.S., McClintock D.S., Feliciano C.E., Wood T.M., Melendez J.A., Rodriguez A.M., Schumacker P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chen J.J., Gendy E., Leu S., Zaldivar F., Gallassetti P., Nussbaum E., Cooper D., Random-Aizik S. Age, sex and weight effects on lactate and leukocyte response to exercise in children and adolescents. Eur. J. Sports Excer, Sci. 2018;6:1–10. [PMC free article] [PubMed] [Google Scholar]
- Corrado C., Fontana S. Hypoxia and HIF signaling: one Axis with divergent effects. Int. J. Mol. Sci. 2020;21:5611. doi: 10.3390/ijms21165611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins E.P., Berra E., Comerford K.M., Ginouves A., Fitzgerald K.T., Seeballuck F., Godson C., Nielsen J.E., Moynagh P., Pouyssegur J., Taylor C.T. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D., Yu S., Cui Y. Vol. 295. 2012. Morphological study of the sinus node and its artery in yak; pp. 2045–2056. (Anatomical Record (Hoboken, N.J.: 2007)). [DOI] [PubMed] [Google Scholar]
- Duffy T.E., Kohle S.J., Vannucci R.C. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J. Neurochem. 1975;24:271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Dzhalilova D., Makarova O. Differences in tolerance to hypoxia: physiological, biochemical, and molecular-biological characteristics. Biomedicines. 2020;8:428. doi: 10.3390/biomedicines8100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhalilova D.S., Diatroptov M.E., Tsvetkov I.S., Makarova O.V., Kuznetsov S.L. Expression of Hif-1α, Nf-κb, and Vegf genes in the liver and blood serum levels of HIF-1α, erythropoietin, VEGF, TGF-β, 8-Isoprostane, and corticosterone in Wistar rats with high and low resistance to hypoxia. Bull. Exp. Biol. Med. 2018;165:781–785. doi: 10.1007/s10517-018-4264-x. [DOI] [PubMed] [Google Scholar]
- Dzhalilova D.S., Kosyreva A.M., Diatroptov M.E., Ponomarenko E.A., Tsvetkov I.S., Zolotova N.A., Mkhitarov V.A., Khochanskiy D.N., Makarova O.V. Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats. J. Inflamm. Res. 2019;12:73–86. doi: 10.2147/JIR.S194581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhalilova D.S., Kosyreva A.M., Diatroptov M.E., Zolotova N.A., Tsvetkov I.S., Mkhitarov V.A., Makarova O.V., Khochanskiy D.N. Morphological Characteristics of the thymus and spleen and the subpopulation composition of lymphocytes in peripheral blood during systemic inflammatory response in male rats with different resistance to hypoxia. Int. J. Inflamm. 2019;2019:7584685. doi: 10.1155/2019/7584685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I.J., Chrousos G.P. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metabol. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Emerling B.M., Platanias L.C., Black E., Nebreda A.R., Davis R.J., Chandel N.S. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol. Cell Biol. 2005;25:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick S.F., Fábián Z., Schaible B., Lenihan C.R., Schwarzl T., Rodriguez J., Zheng X., Li Z., Tambuwala M.M., Higgins D.G., O'Meara Y., Slattery C., Manresa M.C., Fraisl P., Bruning U., Baes M., Carmeliet P., Doherty G., von Kriegsheim A., Cummins E.P., Taylor C.T. Prolyl hydroxylase-1 regulates hepatocyte apoptosis in an NF-κB-dependent manner. Biochem. Biophys. Res. Commun. 2016;474:579–586. doi: 10.1016/j.bbrc.2016.04.085. [DOI] [PubMed] [Google Scholar]
- Frenkel L., Bryson Y.J. Ontogeny of phytohemagglutinin-induced gamma interferon by leukocytes of healthy infants and children: evidence for decreased production in infants younger than 2 months of age. J. Pediatr. 1987;111:97–100. doi: 10.1016/s0022-3476(87)80353-0. [DOI] [PubMed] [Google Scholar]
- Friedman J.E., Haddad G.G. Major differences in Ca2+i response to anoxia between neonatal and adult rat CA1 neurons: role of Ca2+o and Na+o. J. Neurosci.: Off. J. Scoi. Nourosci. 1993;13:63–72. doi: 10.1523/JNEUROSCI.13-01-00063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Găman M.A., Epîngeac M.E., Diaconu C.C., Găman A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes. 2020;11:193–201. doi: 10.4239/wjd.v11.i5.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelnabi M.O., Brown W.V., Le N.A. Evaluation of a novel colorimetric assay for free oxygen radicals as marker of oxidative stress. Clin. Biochem. 2008;41:1250–1254. doi: 10.1016/j.clinbiochem.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Gasparoni A., Ciardelli L., Avanzini A., Castellazzi A.M., Carini R., Rondini G., Chirico G. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol. Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- Glass H.G., Snyder F.F., Webster E. The rate of decline in resistance to anoxia of rabbits, dogs and Guinea pigs from the onset of viability to adult life. Am. J. Physiol. 1944;140:609–615. [Google Scholar]
- Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metabol. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hansen A.J. Extracellular potassium concentration in juvenile and adult rat brain cortex during anoxia. Acta Physiol. Scand. 1977;99:412–420. doi: 10.1111/j.1748-1716.1977.tb10394.x. [DOI] [PubMed] [Google Scholar]
- Harris M.T., Davis W.W., Le N.A., Eggleston B., Austin G.E., Moussa M., Brown W.V. Free oxygen radicals in whole blood correlate strongly with high-sensitivity C-reactive protein. J. Clin. Lipidol. 2007;1:593–598. doi: 10.1016/j.jacl.2007.10.008. [DOI] [PubMed] [Google Scholar]
- He Y., Munday J.S., Perrott M., Wang G., Liu X. Association of age with the expression of hypoxia-inducible factors HIF-1α, HIF-2α, HIF-3α and VEGF in lung and heart of Tibetan Sheep. Animals: Open Aces. J. MDPI. 2019;9:673. doi: 10.3390/ani9090673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Yu S., Hu J., Cui Y., Liu P. Changes in the anatomic and microscopic structure and the expression of HIF-1α and VEGF of the yak heart with aging and hypoxia. PloS One. 2016;11 doi: 10.1371/journal.pone.0149947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K. Basic biology of hypoxic responses mediated by the transcription factor HIFs and its implication for medicine. Biomedicines. 2020;8:32. doi: 10.3390/biomedicines8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., von Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J., Maxwell P.H., Pugh C.W., Ratcliffe P.J. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Jain K., Suryakumar G., Ganju L., Singh S.B. Differential hypoxic tolerance is mediated by activation of heat shock response and nitric oxide pathway. Cell Stress & Chaperones. 2014;19:801–812. doi: 10.1007/s12192-014-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K., Suryakumar G., Prasad R., Ganju L. Upregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxia. High Alt. Med. Biol. 2013;14:65–77. doi: 10.1089/ham.2012.1064. [DOI] [PubMed] [Google Scholar]
- Jones N.M., Lee E.M., Brown T.G., Jarrott B., Beart P.M. Hypoxic preconditioning produces differential expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and its regulatory enzyme HIF prolyl hydroxylase 2 in neonatal rat brain. Neurosci. Lett. 2006;404:72–77. doi: 10.1016/j.neulet.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kaelin W.G. Proline hydroxylation and gene expression. Annu. Rev. Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- Kirova Y.I., Germanova E.L., Lukyanova L.D. Phenotypic features of the dynamics of HIF-1α levels in rat neocortex in different hypoxia regimens. Bull. Exp. Biol. Med. 2013;154:718–722. doi: 10.1007/s10517-013-2038-z. [DOI] [PubMed] [Google Scholar]
- Korneev A.A., Komissarova I.A., Nartsissov I. Ispol'zovanie glutationa v kachestve protektornogo sredstva pri gipoksicheskom vozdeĭstvii [The use of glutathione as a protector agent in hypoxic exposure] Biulleten Eksp. Biol. Meditsiny. 1993;116:261–263. [PubMed] [Google Scholar]
- Kosyreva A.M., Dzhalilova D.S., Makarova O.V., Tsvetkov I.S., Zolotova N.A., Diatroptova M.A., Ponomarenko E.A., Mkhitarov V.A., Khochanskiy D.N., Mikhailova L.P. Sex differences of inflammatory and immune response in pups of Wistar rats with SIRS. Sci. Rep. 2020;10:15884. doi: 10.1038/s41598-020-72537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosyreva A.M., Dzhalilova D.S., Tsvetkov I.S., Diatroptov M.E., Makarova O.V. Age-specific features of hypoxia tolerance and intensity of lipopolysaccharide-induced systemic inflammatory response in wistar rats. Bull. Exp. Biol. Med. 2019;166:699–703. doi: 10.1007/s10517-019-04421-3. [DOI] [PubMed] [Google Scholar]
- Kosyreva A.M., Makarova O.V., Kakturskiy L.V., Mikhailova L.P., Boltovskaya M.N., Rogov K.A. Sex differences of inflammation in target organs, induced by intraperitoneal injection of lipopolysaccharide, depend on its dose. J. Inflamm. Res. 2018;11:431–445. doi: 10.2147/JIR.S178288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Kobayashi M., Goto Y., Hiraoka M., Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: two decades of knowledge. Canc. Sci. 2018;109:560–571. doi: 10.1111/cas.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre N., Noyon B., Biarent D., Corazza F., Duchateau J., Casimir G. Sex differences in inflammatory response and acid-base balance in prepubertal children with severe sepsis. Shock. 2017;47:422–428. doi: 10.1097/SHK.0000000000000773. [DOI] [PubMed] [Google Scholar]
- Lieb M.E., Menzies K., Moschella M.C., Ni R., Taubman M.B. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochemistry and cell biology = Biochem. Cell Bilo. Biochimie et biologie cellulaire. 2002;80:421–426. doi: 10.1139/o02-115. [DOI] [PubMed] [Google Scholar]
- Lin K.H., Lin K.C., Lu W.J., Thomas P.A., Jayakumar T., Sheu J.R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2015;17:44. doi: 10.3390/ijms17010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Jiang L., Zhu H., Zhang L., Wang T. Hypoxia-inducible factor-1α and erythropoietin expression in the hippocampus of neonatal rats following hypoxia-ischemia. J. Nanosci. Nanotechnol. 2014;14:5614–5619. doi: 10.1166/jnn.2014.8728. [DOI] [PubMed] [Google Scholar]
- Lukyanova L.D., Germanova E.L., Kopaladze R.A. Development of resistance of an organism under various conditions of hypoxic preconditioning: role of the hypoxic period and reoxygenation. Bull. Exp. Biol. Med. 2009;147:400–404. doi: 10.1007/s10517-009-0529-8. [DOI] [PubMed] [Google Scholar]
- Lukyanova L.D., Kirova Y.I. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Front. Neurosci. 2015;9:320. doi: 10.3389/fnins.2015.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon P.C., Hirota K., Semenza G.L. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani G., Madeddu C., Macciò A., Gramignano G., Lusso M.R., Massa E., Astara G., Serpe R. Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Canc. Epidemiol. Biomarkers Prev.: Publicat. Am. Ass. Canc. Res. Cosponsor. Am Soci. Prevent. Oncol. 2004;13:1651–1659. [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mironova G.D., Pavlik L.L., Kirova Y.I., Belosludtseva N.V., Mosentsov A.A., Khmil N.V., Germanova E.L., Lukyanova L.D. Effect of hypoxia on mitochondrial enzymes and ultrastructure in the brain cortex of rats with different tolerance to oxygen shortage. J. Bioenerg. Biomembr. 2019;51:329–340. doi: 10.1007/s10863-019-09806-7. [DOI] [PubMed] [Google Scholar]
- Mironova G.D., Shigaeva M.I., Gritsenko E.N., Murzaeva S.V., Gorbacheva O.S., Germanova E.L., Lukyanova L.D. Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal's adaptation to hypoxia. J. Bioenerg. Biomembr. 2010;42:473–481. doi: 10.1007/s10863-010-9316-5. [DOI] [PubMed] [Google Scholar]
- Pahl H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Palmieri B., Sblendorio V. Oxidative stress tests: overview on reliability and use. Part II. Eur. Rev. Med. Pharmacol. Sci. 2007;11:383–399. [PubMed] [Google Scholar]
- Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlatou M.G., Papastamataki M., Apostolakou F., Papassotiriou I., Tentolouris N. FORT and FORD: two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2009;58:1657–1662. doi: 10.1016/j.metabol.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Pavlik L.L., Mikheeva I.B., Al'-Mugkhrabi Y.M., Berest V.P., Kirova Y.I., Germanova E.L., Luk'yanova L.D., Mironova G.D. Specific features of immediate ultrastructural changes in brain cortex mitochondria of rats with different tolerance to hypoxia under various modes of hypoxic exposures. Bull. Exp. Biol. Med. 2018;164:376–381. doi: 10.1007/s10517-018-3993-1. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M., Brown N.J., Vaughan D.E., Harrison D.G., Mehta J.L. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Neuroendocrine-immune interactions. N. Engl. J. Med. 1993;329:1246–1253. doi: 10.1056/NEJM199310213291708. [DOI] [PubMed] [Google Scholar]
- Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A.S., Nizet V., Johnson R.S., Haddad G.G., Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeen A., Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxidants Redox Signal. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- Salminen A., Kaarniranta K., Kauppinen A. Hypoxia-inducible histone lysine demethylases: impact on the aging process and age-related diseases. Aging and disease. 2016;7:180–200. doi: 10.14336/AD.2015.0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. Oxygen homeostasis. Wiley Interdisc. Rev. Sys. Biolo. Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serocki M., Bartoszewska S., Janaszak-Jasiecka A., Ochocka R.J., Collawn J.F., Bartoszewski R. miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis. 2018;21:183–202. doi: 10.1007/s10456-018-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp. Biochem. Physiol. Mol. Integr. Physiol. 1999;123:221–234. doi: 10.1016/s1095-6433(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Smolková K., Plecitá-Hlavatá L., Bellance N., Benard G., Rossignol R., Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int. J. Biochem. Cell Biol. 2011;43:950–968. doi: 10.1016/j.biocel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Steensberg A., Fischer C.P., Keller C., Møller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Stothers C.L., Luan L., Fensterheim B.A., Bohannon J.K. Hypoxia-inducible factor-1α regulation of myeloid cells. J. Mol. Med. 2018;96:1293–1306. doi: 10.1007/s00109-018-1710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafani M., Sansone L., Limana F., Arcangeli T., De Santis E., Polese M., Fini M., Russo M.A. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxidat. Med. Cell. Long. 2016:3907147. doi: 10.1155/2016/3907147. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston J.H., McDougal D.B., Jr. Effect of ischemia on metabolism of the brain of the newborn mouse. Am. J. Physiol. 1969;216:348–352. doi: 10.1152/ajplegacy.1969.216.2.348. [DOI] [PubMed] [Google Scholar]
- Tregub P., Kulikov V., Bespalov A. Tolerance to acute hypoxia maximally increases in case of joint effect of normobaric hypoxia and permissive hypercapnia in rats. Pathophysiology: Off. J. Int. Soci. Pathophysiol. 2013;20:165–170. doi: 10.1016/j.pathophys.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Trippenbach T., Richter D.W., Acker H. Hypoxia and ion activities within the brain stem of newborn rabbits. J. Appl. Physiol. 1990;68:2494–2503. doi: 10.1152/jappl.1990.68.6.2494. (Bethesda, Md.: 1985) [DOI] [PubMed] [Google Scholar]
- Turnbull A.V., Rivier C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- van Uden P., Kenneth N.S., Webster R., Müller H.A., Mudie S., Rocha S. Evolutionary conserved regulation of HIF-1β by NF-κB. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uden P., Kenneth N.S., Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Neurology of the Newborn. third ed. Saunders; Philadelphia, USA: 1995. Hypoxic-ischemic encephalopathy: biochemical and physiological aspects. [Google Scholar]
- Wakasugi N., Virelizier J.L. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J. Immunol. 1985;134:167–171. (Baltimore, Md.: 1950) [PubMed] [Google Scholar]
- Walmsley S.R., Print C., Farahi N., Peyssonnaux C., Johnson R.S., Cramer T., Sobolewski A., Condliffe A.M., Cowburn A.S., Johnson N., Chilvers E.R. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts E.R., Walmsley S.R. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol. Med. 2019;25:33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Wiegering V., Eyrich M., Wunder C., Günther H., Schlegel P.G., Winkler B. Age-related changes in intracellular cytokine expression in healthy children. Eur. Cytokine Netw. 2009;20:75–80. doi: 10.1684/ecn.2009.0149. [DOI] [PubMed] [Google Scholar]
- Wong B.W., Kuchnio A., Bruning U., Carmeliet P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem. Sci. 2013;38:3–11. doi: 10.1016/j.tibs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang C., Chen Y., Luo Y.J. Association between acute mountain sickness (AMS) and age: a meta-analysis. Militar. Med. Res. 2018;5:14. doi: 10.1186/s40779-018-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yu S., He J., Cui Y. Segmentation features and structural organization of the intrapulmonary artery of the yak. Anat. Rec. 2013;296:1775–1788. doi: 10.1002/ar.22790. (Hoboken, N.J.: 2007) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.