Abstract
Early diagnosis of neurodegenerative diseases, especially Alzheimer's disease (AD), is essential for implementing the appropriate treatment protocols and controlling disease progression. Early AD diagnosis helps patients achieve the best therapeutic outcomes, lessening irreversible neurodegenerative damage and severe cognitive decline. The measurement of brain waves and structural modifications, including gray/white matter and brain volume, have recently been considered a promising approach for brain biometrics because of the inherent specificity, degree of confidentiality, and reproducibility. Brain printing biometrics (BPB) is thus becoming more commonly considered as tool for early AD detection. This review proposes using BPB as a tool for the detection of AD prior to the appearance of persistent hallmark depositions, including Aβ and tau protein aggregations in different brain regions. It also describes BPB authentication, a method of implementation, as well as potential outcomes.
Keywords: AD, Biometrics, Brain printing, BPB, Early diagnosis, EEG, sMRI
Graphical abstract

Highlights
-
•
Biometrics is an authorized digital storage form for unique human characters.
-
•
BPB is a technique for early AD detection by monitoring brain structure and waves.
-
•
BPB will noninvasively distinguish AD among other neurodegenerative diseases.
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease that results in a loss of neuronal viability within the cortex and hippocampus as well as other brain regions important for cognition. A main goal for slowing disease progression lies in identifying individuals in the early asymptomatic stage, several years before the appearance of the neuropathological hallmarks of AD (Van Deerlin, 2012; Holzer et al., 2013). Such specific high-quality early detection techniques can provide patients with the time needed to make life plans regarding their careers or other personal issues, in addition to identifying plans to help manage their treatment. Even for familial forms of AD, especially the rare form known as early-onset familial Alzheimer disease (eFAD) that is associated with mutations in genes encoding the amyloid precursor protein (APP), presenilin-1, and/or presenilin-2, it would be valuable to have a diagnostic technique that predicts the onset of the disease (Haupt et al., 1992; Nochlin et al., 1993; Campion et al., 1999). Nowadays, novel biometric techniques have been widely explored as “Hidden Signature Biometrics.” The goal of these commonly used biometric devices such as sMRI (Aloui et al., 2011; Naït-ali 2020) or EEG (Palaniappan, 2008; Naït-ali 2020) is to extract a large number of features from inaccessible areas of the human body for the purpose of authorized personal identification.
Meanwhile examining changes in brain structure and electrical signals that accompany the onset of AD using the brain printing biometrics technique (BPB) would allow neurologists to determine whether BPB can serve as a reliable, noninvasive technique for early AD diagnosis. The onset of AD typically correlates with the overexpression of Aβ in brain tissue (Bae and Yi, 2020; Calabrò et al., 2021). At this early stage, the profound structural changes that occur can be correlated with the BPB technique which would be designed to generate personalized IDs using both computerized brain structural analysis as well as brain activity via sMRI (Chen et al., 2014) and EEG intra-connected devices, respectively (Jackson and Snyder, 2008). The current review highlights the idea that AD can be characterized and diagnosed by monitoring the early morphological and structural changes developed during the preclinical stage using the BPB technique. The use of this proposed approach would provide early and accurate AD diagnosis before the deposition or appearance of irreversible AD hallmarks in different brain areas. Additionally, the BPB technique would provide insight into the sequences of pathogenic events that occur in AD in addition to previously characterized preclinical inflammatory features and events associated with the sequences.
1.1. Proposed brain-printing biometrics technique
Biometrics were recently developed as an authorized digital form of storage for unique human measurable characteristics from various areas of the body with the goal of achieving nearly 100% accurate identification of individuals. The technique takes advantage of unique, inherited, characteristics as personal identifiers. Several types of biometrics are well-known, including physiological shape biometrics, behaviometrics, and, finally, hidden structure biometrics, the latter represented in the current review as brain-printing biometrics (Aloui, Naït-ali and Naceur, 2012; Barra et al., 2017). The idea of using the BPB approach in the early identification of significant cognitive disease modifications, including hallmark AD depositions, is mainly based on the fact that in addition to the early influence of neuroinflammation and genetic mutations on brain structural morphology, altered brain features have been linked to, and used in the dection of, long term brain disorders (Nochlin et al., 1993; Campion et al., 1999; Aloui et al., 2011). Diagnosing Alzheimer's disease was and still is mainly based on identifying the neuropathological hallmarks in the patient's brain, a positive family history, and/or genetic screening for familial forms of AD, all of which can be described as late-stage methods of AD diagnosis. The amyloid cascade hypothesis assumes that different inherited congenital defects or other unknown inflammatory and environmental factors may lead to direct or indirect over-accumulation of toxic Aβ, resulting in severe brain tissue damage many years before disease diagnosis. This highlights the importance of developing a reliable, noninvasive early method of detecting AD (Jackson and Snyder, 2008; Barra et al., 2017; Calabrò et al., 2021).
The BPB approach described here is proposed to be a precise, noninvasive technique for early diagnosis that utilizes two forms of computerized, interconnected mapping techniques: sMRI (Fujita and Hagiwara, 2019) and EEG (Tsolaki et al., 2014) for detecting unique structural and electrical alterations, respectively (Fig. 1). sMRI is used for deep brain image scanning to identify the onset of brain deformities and associated unique hidden brain structural changes before or as the disease precipitates, including the surface area of brain, white/gray matter volume, cortical-subcortical folds, and brain gyrification index (Aloui et al., 2012; Chen et al., 2014; Fujita and Hagiwara, 2019) (Fig. 1). EEG, on the other hand, measures brain function at different frequencies and can report the degree of deterioration (Jackson and Snyder, 2008; Tsolaki et al., 2014) (Fig. 1).
Fig. 1.
Illustrating outlines and phases of the brain biometrics computerized intervention system (BBCIS).
1.2. Alzheimer's disease and brain-printing biometrics technique
Despite the dramatic increase in the prevalence rate, AD has been severely underdiagnosed, misdiagnosed, or the diagnosis delayed until later stages when treatments are no longer beneficial and oligomeric aggregation hallmarks are irreversible (Relkin, 2000). Apart from misdiagnosis, the eFAD inherited form is mainly identified by a positive family history and the AD inflammatory form by hallmark Aβ aggregations that give rise to brain atrophy. Although the family history maybe 80% negative, the probability of having the eFAD gene mutation remains high, as the negative family history instead may be due to the early death or misdiagnosis of affected family members (Kaye, 1998; Relkin, 2000). Furthermore, AD inflammatory forms can also be misdiagnosed because of the dependence on the appearance of hallmarks of AD in different brain regions that usually appear at a late stage already associated with the symptoms of irreversible cognitive dysfunction. The inclusion of very late misdiagnosed patients in BPB studies may therefore lead to serious consequences, including the appearance of extreme mental, behavioral and functional disabilities associated with structural deformities that occur within a short time frame as well as vague predisposing factors that produce biased outcomes and incorrect feedback. For these reasons, careful consideration must be given to creation of the inclusion and exclusion criteria. On the other hand, raising awareness about identifying AD forms at an early asymptomatic stage might give neuroscientists the chance to find novel agents and palliative treatments that could delay or block severe disease progression (Braak and Braak, 1997; Kaye, 1998). The BPB technique allows us to hypothesize that AD can be accurately noninvasively distinguished from other neurodegenerative diseases, including inheritance and other high risk inflammatory forms of AD, by generating a characterized diagnostic feature to be named as “AD-BPB early hidden structural modifications."
1.3. Brain-printing biometrics technique implementation
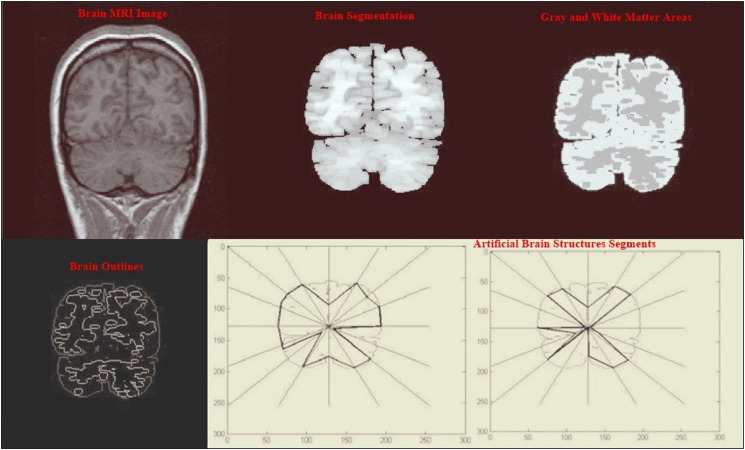
The new approach of measuring BPB for early AD detection is based on designing the brain biometrics computerized intervention system (BBCIS) (Fig. 1), which will be conducted on suspected AD patients with early cognitive signs. The construction of BBCIS includes eight phases as follows: the 1st and 2nd phases depend on creating a secret key and ID for the purpose of authentication/identification of each enrolled patient, followed by the acquisition of brain images performed by sMRI (phases 1 and 2) (Fig. 1, Fig. 2). The 3rd phase includes the acquisition of EEG brain wave signals and their integration with simultaneous sMRI imaging (Fig. 2, Fig. 3). The next two phases (4 and 5) include respective brain image slicing, segmentation, and analysis of extracted features (volume, surface and boundaries) (Fig. 4). While the 7th phase includes creating computerized artificial brain transformation and coordination analysis and then storing the data/geographical changes under the previously identified secret ID key assigned to each enrolled patient for the eight assessed phases (Fig. 2, Fig. 3, Fig. 4). Concerning the last phase, which mainly depends on raw data imaging and the analysis of brain waves, the degree of association depends on using preprocessing, extraction, and sequencing tools along with the appropriate use of ROC curve to avoid high image false-positive results (Fig. 4). In contrast, routine sMRI and EEG will be conducted weekly on the suspected AD subjects at certain fixed time intervals in order to detect morphological brain changes from one week to another.
Fig. 2.
Proposed BPB imaging modifications detection and verification technique.
Fig. 3.
Illustration of EEG signal main processing stages for detecting brain abnormalities. EEG is considered a practical detection method for detecting neuron abnormalities when being integrated with structural neuroimages or incorporated in the BPB technique.
Fig. 4.
Illustration of obtained BPB images and results that can be used in early AD detection.
1.4. Cortical thickness and brain-printing biometrics
It was previously reported that AD is highly associated with detected changes in the cortical thickness, especially in the frontal, temporal and occipital lobes (Lerch et al., 2005). The early decrease in cortical thickening as a drawback of AD is highly consistent with the BPB theory. Cortical thickness analysis via BPB allows searching for the direct link between cortical depth and clinical or psychological variable conditions across the entire brain surface (Lerch et al., 2005; Du et al., 2007). Brain-printing biometrics share many advantages over other global search algorithms, including voxel-based morphometry (VBM) and complicated deformation field analysis. Unlike VBM, a definite accurate description of the cortical thickness in millimeters allows promising quantitative descriptive results enabling successful early disease detection (Lerch et al., 2005; Du et al., 2007). Since significant disadvantages of using cortical thickness via MRI include failure to detect subcortical structures and white matter areas (Du et al., 2007; Querbes et al., 2009), measuring the cortical thickness deformities via BPB provides vast advantages for measuring WM and subcortical structures changes with high accuracy. Additionally, it directly links cortical thickness and the degree of cognitive impairment in AD (Lerch et al., 2005; Lerch and Evans, 2005).
1.5. Effect of psychological and physiological factors on BPB
BPB is suggested to be affected by different psychological and physiological factors such as vascular diseases, stress, anxiety, and sleep disorders (Marsland et al., 2015). Thus, these factors have to be excluded to avoid results bias (Curie et al., 2013; Marsland et al., 2015). Other factors such as medications may cause a power increase in both beta and theta bands resulting in results falsification (Blume, 2006). Emotional states may also induce different functional connectivity patterns in brain waves (Chan et al., 2015). It was also found that patients suffering from AD exhibit lower EEG, especially in the theta band, than normal controls (Lerch et al., 2005; Radić et al., 2019). Thus, developing a stable and efficient BPB system requires understanding its features in order to maintain high accuracy. AD-related inflammatory markers are associated with total brain volume changes (TBV) and primary brain structural morphology (Marsland et al., 2015). It was reported that increased neuroinflammation would result in increased white matter hyperintensities (WMH) and decreased TCB. These recognized changes can be used as an early diagnosis parameter for AD prior to the appearance of hallmark depositions (Jefferson et al., 2007). YKL-40, known as chitinase 3–like 1 protein, has been identified as the primary marker of glial inflammation and was found to be significantly higher in AD patients than in other types of neurodegenerative diseases. Some studies also reported variations in the YKL-40 level during predementia stages, where YKL-40 has also been reported to be higher in the cerebrospinal fluid (CSF) of AD patients indicating the progressed neurodegenerative pathophysiological process in brain tissues (Alcolea et al., 2015) (Table-1). Furthermore, patients with high sensitivity C-reactive protein (hsCRP) showed a decrease in cortical gray matter volume (Taki et al., 2013) (Table-1). Additionally, elevated levels of plasminogen activator inhibitor-1(PAI-1) can also be correlated with low speed and motor coordination, in addition to a significant loss in WM regions in cortico-subcortical regions. The cortical thickness was also found to be attenuated with elevated IL-6 level indicating general cortical atrophy (McCarrey et al., 2014) (Table-1). These observed changes can be considered a consequence of the onset of AD-associated progressive damage (Miralbell et al., 2012, Anwar et al., 2021b) (Table-1).
Table 1.
Effect of different and elevated inflammatory markers on brain imaging findings.
| Inflammatory Markers | Main cortical Findings |
|---|---|
| YKL-40 | YKL-40 levels are found to be significantly increased in preclinical and AD groups. Furthermore, high levels can be positively correlated with cortical thinning in both middle and inferior temporal areas (Alcolea et al., 2015). |
| CRP | The direct association of CRP level with cognitive performance can't be proven, although higher levels can be associated with impaired cognitive executive functions. CRP levels may be related to white matter integrity in cortical regions, association fibers in both frontal and temporal lobes, and gray matter volume in the cortex (Taki et al. 2013) |
| PAI-1 | An elevated level of PAI-1 can also be correlated with low speed and motor coordination's in addition to a significant loss in WM located in cortico-subcortical regions (Miralbell et al., 2012) |
| Il-6 | Il-6 can be negatively associated with spatial processing, memory, verbal proficiency, and executive functions. Higher levels can also be related to decreased cortical thickness in the inferior occipital gyrus as well as the inferior temporal gyrus (McCarrey et al., 2014). |
| TNF-α | The TNF-α level was found to be associated with alterations in the gray matter GM structural network volume (Taishi et al., 2007; Benson et al., 2020) |
Meanwhile, high serum TNF-α level was found to be associated with alterations in the gray matter GM structural volume as stemming from inflammatory cascades and the exaggerated release of inflammatory type microglia-M1 (Taishi et al., 2007; Benson et al., 2020; Anwar et al., 2021a, Anwar et al., 2021b) (Table-1). Additionally, exploratory examination of the volume of subcortical structures revealed inverse association of inflammation with volumes of the hippocampus, pallidum, and thalamus, where these findings also support the inverse associations of inflammatory markers with both global measures of gray matter volume/white matter integrity, and with regional gray matter volume of the hippocampus among healthy midlife adults (Marsland et al., 2015). Thus, the current findings suggest that inflammation relates to midlife cognitive function via its inverse association with brain structure modifications. This indicates that inflammatory factors cannot always be defined as a consequence of neurodegenerative diseases but, on the contrary, can be defined as major predisposing factors.
1.5.1. AD early detections arguments
The idea of early AD screening is still not wholly embraced largely because of the lack of an accurate, noninvasive diagnostic technique but also because of psychological issues that might result, including depression, stress, anxiety and social exclusion. Nevertheless, acknowledgment of its importance is increasing. A global endorsement for the idea of screening to achieve early accurate AD diagnosis would benefit patients by facilitating control over their condition, engaging safe modes of transportation to limit accidents or becoming lost, planning for the future, adopting a new lifestyle with regular mental exercise and having the full rights to receive the proper workplace benefits such as working hours, job type, job location, and required assistive devices. Along with the extensive efforts towards highlighting the need for early diagnosis, steps must be implemented to establish governmental post-AD diagnostic support to attain full rights of the patients in all aspects of life. The current review highlights that the under-recognition of AD is a severe problem in the population as early treatment and counseling would benefit both patients and caregivers. Additionally, early AD detection would allow physicians to recognize the AD preclinical symptoms to avoid severe cognitive consequences and provide cost-effective therapies.
1.5.2. Anticipated conclusion
Since every human being has its own BPB, it is suggested that structural manifestations related to cognitive disorders can be reflected as brain mapping changes. Thus, along with accurately distinguishing brain sectors, structures, and brain wave functions, alterations identified by the designed BBCIS can be considered as hidden structural modifications developed during the early AD asymptomatic period compared with healthy brain images. Thereby, BPB is suggested to be recognized as a potential early diagnostic technique for accurate noninvasive discrimination of different AD forms, including late-onset familial, non-familial, and environmentally caused AD forms without the need for late AD hallmark depositions or genetic screening. Furthermore, since the implementation of BPB is in progress, the current review anticipates the future benefits of BPB in neurodegenerative diseases, including improved disease detection and diagnostic accuracy leading to cost-effective observation and effective intervention studies. Therefore, it is essential that clinical studies are carried out to ensure the efficiency, accuracy, specificity, and verification necessary for it to be officially considered an early diagnostic technique.
Funding
This review article did not receive any grant, whether from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Mai M. Anwar: was responsible for designing, writing and revising the whole submitted manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to Turkiye Burslari Scholarships (YTB) for providing MMA a postdoctoral research scholarship at Koç University, Istanbul, Turkey.
References
- Alcolea D., Vilaplana E., Pegueroles J., Montal V., Sánchez-Juan P., González-Suárez A., Pozueta A., Rodríguez-Rodríguez E., Bartrés-Faz D., Vidal-Piñeiro D., González-Ortiz S., Medrano S., Carmona-Iragui M., Sánchez-Saudinós M., Sala I., Anton-Aguirre S., Sampedro F., Morenas-Rodríguez E., Clarimón J., Blesa R., Lleó A., Fortea J. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer's disease. Neurobiol. Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Aloui K., Naït-Ali A., Naceur M.S. 2011 IEEE Workshop on Computational Intelligence in Biometrics and Identity Management (CIBIM) 2011. A novel approach based brain biometrics: some preliminary results for individual identification; pp. 91–95. [Google Scholar]
- Aloui K., Naït-ali A., Naceur S. A new useful biometrics tool based on 3D brain human geometrical characterizations. J. Signal Inf. Process. 2012;3 [Google Scholar]
- Anwar M.M., Ali O.S.M., Rashed L.A., Badawi A.M., Eltablawy N.A. The therapeutic potential and efficiency of Intracerebroventricular transplantation and intravenous injection of Mesenchymal stem cells in relieving Aß hallmarks and improving cognitive dysfunction in AD induced model. Gene Reports. 2021;25:101323. [Google Scholar]
- Anwar M.M., Özkan E., Gürsoy-Özdemir Y. The role of Extracellular Matrix alterations in mediating Astrocytes damage, and Pericytes dysfunction in Alzheimer's disease: a comprehensive review. Eur. J. Neurosci. 2021 doi: 10.1111/ejn.15372. Epub ahead of print. PMID: 34182602. [DOI] [PubMed] [Google Scholar]
- Bae M., Yi H.G. 2020. Microphysiological Systems for Neurodegenerative Diseases in Central Nervous System; p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra S., Casanova A., Fraschini M., Nappi M. Multimedia Tools and Applications; 2017. Fusion of physiological measures for multimodal biometric systems; p. 76. [Google Scholar]
- Benson C.A., Powell H.R., Liput M., Dinham S., Freedman D.A., Ignatowski T.A., Stachowiak E.K., Stachowiak M.K. Immune factor, TNFα, disrupts human brain organoid development similar to schizophrenia-schizophrenia increases developmental vulnerability to TNFα. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.00233. 233-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume W.T. Drug effects on EEG. J. Clin. Neurophysiol. 2006;23:306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Calabrò M., Rinaldi C., Santoro G., Crisafulli C. The biological pathways of Alzheimer disease: a review. AIMS Neurosci. 2021;8:86–132. doi: 10.3934/Neuroscience.2021005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D., Dumanchin C., Hannequin D., Dubois B., Belliard S., Puel M., Thomas-Anterion C., Michon A., Martin C., Charbonnier F., Raux G., Camuzat A., Penet C., Mesnage V., Martinez M., Clerget-Darpoux F., Brice A., Frebourg T. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H.L., Chen L.F., Chen I.T., Chen Y.S. Beamformer-based spatiotemporal imaging of linearly-related source components using electromagnetic neural signals. Neuroimage. 2015;114:1–17. doi: 10.1016/j.neuroimage.2015.03.038. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhou Z., Shen H., Hu D. The potential of using brain images for authentication. The Scientific World Journal, 2014. 2014:749096. doi: 10.1155/2014/749096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie T., Mongrain V., Dorsaz S., Mang G.M., Emmenegger Y., Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–323. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A.T., Schuff N., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K., Miller B.L., Weiner M.W. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S., Hagiwara A. 3D quantitative synthetic MRI-derived cortical thickness and subcortical brain volumes. Scan-rescan repeatability and comparison with conventional T(1) -weighted images. 2019;50:1834–1842. doi: 10.1002/jmri.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt M., Kurz A., Pollmann S., Romero B. Alzheimer's disease: identical phenotype of familial and non-familial cases. J. Neurol. 1992;239:248–250. doi: 10.1007/BF00810345. [DOI] [PubMed] [Google Scholar]
- Holzer S., Warner J.P., Iliffe S. Diagnosis and management of the patient with suspected dementia in primary care. Drugs Aging. 2013;30:667–676. doi: 10.1007/s40266-013-0098-4. [DOI] [PubMed] [Google Scholar]
- Jackson C.E., Snyder P.J. Electroencephalography and event-related potentials as biomarkers of mild cognitive impairment and mild Alzheimer's disease. Alzheimers Dement. 2008;4:S137–S143. doi: 10.1016/j.jalz.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jefferson A.L., Massaro J.M., Wolf P.A., Seshadri S., Au R., Vasan R.S., Larson M.G., Meigs J.B., Keaney J.F., Jr., Lipinska I., Kathiresan S., Benjamin E.J., DeCarli C. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J.A. Diagnostic challenges in dementia. Neurology. 1998;51:S45–S52. doi: 10.1212/wnl.51.1_suppl_1.s45. discussion S65-S67. [DOI] [PubMed] [Google Scholar]
- Lerch J.P., Evans A.C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lerch J.P., Pruessner J.C., Zijdenbos A., Hampel H., Teipel S.J., Evans A.C. Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cerebr. Cortex. 2005;15:995–1001. doi: 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Gianaros P.J., Kuan D.C., Sheu L.K., Krajina K., Manuck S.B. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Immun. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey A.C., Pacheco J., Carlson O.D., Egan J.M., Thambisetty M., An Y., Ferrucci L., Resnick S.M. Interleukin-6 is linked to longitudinal rates of cortical thinning in aging. Transl. Neurosci. 2014;5:1–7. doi: 10.2478/s13380-014-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralbell J., Soriano J.J., Spulber G., López-Cancio E., Arenillas J.F., Bargalló N., Galán A., Barrios M.T., Cáceres C., Alzamora M.T., Pera G., Kivipelto M., Wahlund L.-O., Dávalos A., Mataró M. Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiol. Aging. 2012;33:1003. doi: 10.1016/j.neurobiolaging.2011.09.020. e9-1003.e17. [DOI] [PubMed] [Google Scholar]
- Naït-ali A. 2020. Hidden Biometrics When Biometric Security Meets Biomedical Engineering:When Biometric Security Meets Biomedical Engineering. [Google Scholar]
- Nochlin D., van Belle G., Bird T.D., Sumi S.M. Comparison of the severity of neuropathologic changes in familial and sporadic Alzheimer's disease. Alzheimer Dis. Assoc. Disord. 1993;7:212–222. [PubMed] [Google Scholar]
- Palaniappan R. Two-stage biometric authentication method using thought activity brain waves. Int. J. Neural Syst. 2008;18:59–66. doi: 10.1142/S0129065708001373. [DOI] [PubMed] [Google Scholar]
- Querbes O., Aubry F., Pariente J., Lotterie J.A., Démonet J.F., Duret V., Puel M., Berry I., Fort J.C., Celsis P. Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radić B., Petrović R., Golubić A., Bilić E., Borovečki F. EEG analysis and spect imaging in Alzheimer's disease, vascular dementia and mild cognitive impairment. Psychiatr. Danub. 2019;31:111–115. doi: 10.24869/psyd.2019.111. [DOI] [PubMed] [Google Scholar]
- Relkin N. Screening and early diagnosis of dementia. Am. J. Manag. Care. 2000;6:S1111–S1118. discussion S1119-24. [PubMed] [Google Scholar]
- Taishi P., Churchill L., Wang M., Kay D., Davis C.J., Guan X., De A., Yasuda T., Liao F., Krueger J.M. TNFalpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res. 2007;1156:125–132. doi: 10.1016/j.brainres.2007.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y., Thyreau B., Kinomura S., Sato K., Goto R., Wu K., Kakizaki M., Tsuji I., Kawashima R., Fukuda H. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum. Brain Mapp. 2013;34:2418–2424. doi: 10.1002/hbm.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki A., Kazis D., Kompatsiaris I., Kosmidou V., Tsolaki M. Electroencephalogram and alzheimer's disease: clinical and research approaches. International Journal of Alzheimer’s Disease, 2014. 2014:349249. doi: 10.1155/2014/349249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin V.M. The genetics and neuropathology of neurodegenerative disorders: perspectives and implications for research and clinical practice. Acta Neuropathol. 2012;124:297–303. doi: 10.1007/s00401-012-1032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]