Abstract
Physical activity (PA) and exercise are safe and beneficial for children and adolescents affected by cancer. Yet, this population is not active enough to receive benefits. PA guideline and recommendation statements can support individual behavior and practice change. The purpose of this project was to develop the international Pediatric Oncology Exercise Guidelines (iPOEG), comprised of guideline and recommendation statements, to promote PA among children and adolescents affected by cancer. Guideline development procedures, stakeholder engagement strategies, and the Delphi technique were used. Four online surveys were distributed to the iPOEG network (n = 9 core team members, n = 122 expert consensus committee members). Surveys included closed- and open-ended items informed by a literature synthesis and an in-person meeting. Responses were analyzed using descriptive statistics and content analysis. Consensus was defined as ≥ 80% agreement. Response rates to online surveys ranged from 82% to 91%. The iPOEG network agreed on four guideline and five recommendation statements, which highlight that movement is important for all children and adolescents affected by cancer. These statements are generic in nature as more research is still required to provide specific guidance on the frequency, intensity, time, and type of PA for this population. Nevertheless, the iPOEG statements represent available evidence and expert opinion, collectively suggesting that it is time for children and adolescents affected by cancer to move more.
Keywords: Childhood cancer, Physical activity, Delphi technique, Consensus
The evidence- and expert-informed international Pediatric Oncology Exercise Guidelines state it’s time for children and adolescents affected by cancer to move more.
Lay Summary
Physical activity is safe and beneficial for children and adolescents affected by cancer. Yet, most are not active enough to receive benefits. Guideline and recommendation statements can help change individual behavior and practice. To develop such statements, guideline development procedures, stakeholder engagement strategies, and the Delphi technique were used. Four online surveys were distributed to an international network (n = 131 experts). Surveys asked closed- and open-ended questions informed by a literature synthesis and an in-person meeting. Findings from the online surveys resulted in the international Pediatric Oncology Exercise Guidelines statements, which highlight that it is time for children and adolescents affected by cancer to move more.
Implications.
Practice: Movement is safe, beneficial, and recommended for all children and adolescents affected by cancer.
Policy: Policymakers who want to enhance movement among children and adolescents affected by cancer should explore sustainable physical activity or exercise programs, and include qualified exercise professionals as part of standard care to facilitate program implementation and uptake.
Research: Researchers should focus on conducting high-quality, multisite studies to continue providing evidence for the benefits of moving more during and after treatment for cancer.
Background
Physical activity (PA; i.e., any bodily movement produced by skeletal muscles that requires energy expenditure; [1]) and exercise (i.e., planned, structured, and repetitive PA for the purpose of conditioning any part of the body, improving health, and maintaining fitness; [1]) can confer positive outcomes for children and adolescents affected by cancer. Although there are gaps in the literature, researchers have reported that PA and exercise are associated with a range of benefits from helping manage symptoms (e.g., fatigue and pain), to enhancing physical and psychosocial well-being (e.g., improving body composition and reducing anxiety), to extending the length of survivorship [2–5]. This evidence has been presented in numerous cross-sectional and prospective studies [6–10], experimental articles [11–15], and systematic reviews [2–5]. Combined, findings suggest that PA, including exercise, is an important part of treatment and recovery for children and adolescents affected by cancer.
To promote PA and exercise in this cohort, resources (e.g., manuals and pamphlets) and models have been developed [16–19] and researchers have published manuscripts detailing best practice examples for integrating PA and exercise into standard pediatric oncology care [20]. Notwithstanding these contributions, an important gap remains: there are no widely agreed upon PA and exercise guideline statements for children and adolescents affected by cancer, nor strategies for tailoring PA and exercise (i.e., recommendations) for this cohort. Guideline and recommendation statements can support PA and exercise behavior change at multiple levels (e.g., the child/adolescent, parents/guardians, and health care providers; [21, 22]). In the absence of guideline and recommendation statements, children, adolescents, and their parents may be unsure about how much PA or exercise to engage in, and clinicians may be unsure about how much PA or exercise to recommend to their patients.
Consensus methods, such as the Delphi technique, are widely used and accepted for developing guidelines and recommendations in medical and health service research [23]. For example, the Delphi technique was recently used to develop supportive care clinical practice guidelines for children and adolescents affected by cancer [24] and recommendations for PA and exercise for adults with osteoporosis [25]. Furthermore, the Delphi technique can be used in circumstances when there are gaps in knowledge as it can consolidate available evidence and expert opinion [26]. Thus, the purpose of this project was to develop internationally agreed upon PA and exercise guideline and recommendation statements (i.e., the international Pediatric Oncology Exercise Guidelines; iPOEG).
METHODS
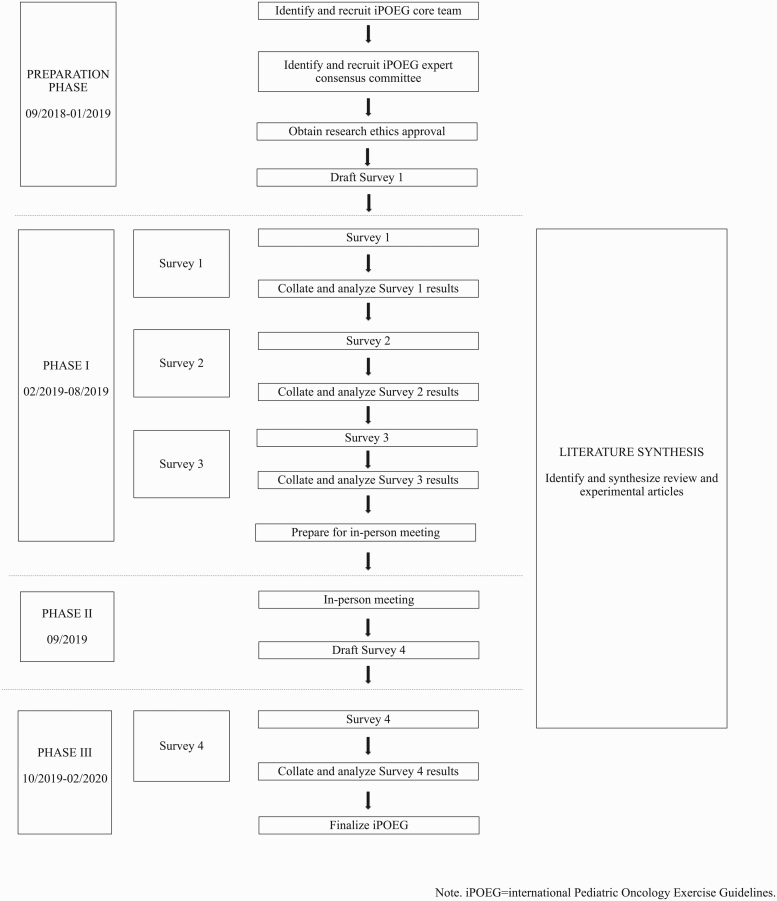
This project was guided by literature detailing clinical guideline development procedures [27], strategies to engage a range of stakeholders using online processes [28], and the Delphi technique [26]. Ethical approval was granted by the Health Research Ethics Board of Alberta—Cancer Care Committee and informed consent was obtained from all those who responded to the online surveys. No formal eligibility criteria were specified a priori for participants; rather, individuals who were experts in the field of pediatric exercise oncology—a field covering medicine, rehabilitation, physiology, kinesiology, and psychology—were invited or self-identified to participate in English. Figure 1 provides a visual depiction of the different phases comprising the development of the iPOEG.
Fig 1.
Overview of the international Pediatric Oncology Exercise Guidelines (iPOEG) project.
Preparation phase (September 2018–January 2019)
Identifying and recruiting international experts: core team and expert consensus committee
A core team of nine international experts from six countries were identified and recruited to participate via email by the first and last authors based on a recently completed international environmental scan [29] and the authors’ preexisting networks. Once established, a larger expert consensus committee was recruited. Similar to above, the expert consensus committee was identified using findings from the international environmental scan [29], common adopted criteria (i.e., actively practicing, publishing, and/or working in the field of pediatric exercise oncology; [30]), and snowball sampling. The decision was made post hoc to add new members with relevant expertise throughout this project. At the end of the preparation phase (January 2019), the expert consensus committee was comprised of 115 individuals from 18 countries. Throughout Phase I (February 2019–August 2019) and Phase II (September 2019), seven additional experts from six countries self-identified or were recruited to the expert consensus committee to total 122 individuals from 21 countries. The core team (n = 9) and iPOEG expert consensus committee (n = 122) together comprise the iPOEG network (n = 131 experts).
Phase I—information gathering (February 2019– August 2019)
Surveys
Emails were sent to the iPOEG network following guidance from Jones and Hunter [26]. These emails contained a link to the survey that collected informed consent and basic descriptive information (i.e., area of expertise, years working in field, and geographic location). Following this, a series of closed- and open-ended items were presented covering terminology and content areas for the iPOEG. Closed-ended items directed respondents to select the level to which they agreed or disagreed with statements, whereas open-ended items collected additional information and/or comments. Throughout, respondents could indicate “I do not feel I have the expertise to complete this section (skip to next section)” to skip a question or series of questions. Responses were anonymized [26] and data for open-ended items was analyzed via content analysis [31, 32] by two independent researchers (E.M. and D.C.). Findings were used to inform subsequent survey rounds. Consensus was defined a priori for closed-ended items as ≥80% agreement. Three surveys were administered in Phase I (February 2019–August 2019). Only those who completed Survey 1 were invited to participate in Survey 2, and only those who completed Survey 2 were invited to participate in Survey 3. Supplementary File 1 provides further details on these surveys as well as example items.
Literature synthesis
To provide an overview of the available evidence reporting on the effects of PA and exercise for children and adolescents affected by cancer, a literature synthesis was conducted following guidance for the design, conduct, and reporting of scoping reviews [33–35], systematic reviews [36, 37], and reviews of reviews [38]. Also, pragmatic constraints were considered. Review and experimental articles published in English, summarizing or reporting on the effects of PA (including exercise) interventions for children and adolescents affected by cancer, were included. Articles were identified by a team of researchers through a recently published environmental scan [29], systematic searching of Google and Google Scholar, reference list scanning, stakeholder engagement, and a database update, which was conducted in MEDLINE, PsycINFO, and SPORTDiscus from January 2017 to January 2020. Data were extracted, articles were assessed for quality (reviews; AMSTAR 2 [39]) or risk of bias (experimental articles; Cochrane Handbook for Systematic Reviews of Interventions [40] or Risk of Bias Assessment tool for Non-randomized Studies [41] as appropriate based on study design), and narrative summaries were prepared [42]. A total of 1,380 articles were identified. Twenty reviews and 69 experimental articles were included. Articles examined the effects of PA (or exercise) on PA behavior, physical, psychosocial, cognitive, and ‘other’ outcomes. Improvements, no change, and mixed results were found across the majority of outcomes. Two adverse events (e.g., a treatable injury and fatigue) were described. Article quality and risk of bias varied widely. Overall, findings suggest that the field of pediatric exercise oncology is rapidly advancing, and that PA, including exercise, is beneficial and safe. Nevertheless, more adequately powered research adhering to reporting standards is required. The full literature synthesis, including all methods and results, has been published elsewhere [43].
Phase II—international team meeting (September 2019)
The in-person, international team meeting took place in Banff, Alberta, September 2019. This meeting included the core team, local health care providers (i.e., an oncologist and nurse administrator), and trainees. A family affected by childhood cancer was also invited; however, they were unable to attend at the last minute due to personal circumstances. Just over 2 weeks prior to the meeting, attendees received a document consisting of findings from Surveys 1–3 and a summary of the findings from the in-progress literature synthesis. The objectives of this meeting were to discuss the results from the information gathered in Phase I (February 2019–August 2019) and to draft the iPOEG guideline and recommendation statements. Also, attendees reviewed and commented on the literature synthesis to identify research and innovation needs in the field (see [44] for a manuscript detailing these needs).
Phase III—Finalizing the iPOEG (October 2019–February 2020)
The statements drafted in Phase II (September 2019) were provided to members of the iPOEG network who completed at least Survey 1 or who had joined between February 2019 and October 2019. This survey explored agreement on the statements drafted during the in-person meeting. Supplementary File 1 provides the general details on the survey as well as example items, respectively. For each statement, respondents were asked to judge whether the statement should be included within the iPOEG (“yes” or “no”) and to provide their thoughts. As above, consensus was defined as ≥80%.
RESULTS
The combined response rate across the four surveys was 89%. On average, most respondents self-selected being a researcher (n = 52) and had >10 years in their field (n = 38). The majority of respondents were from Canada (n = 21), followed by Germany (n = 19), and the USA (n = 15; numbers represent averages across the four surveys; see Table 1).
Table 1.
Survey response rates and responder characteristics
| Survey 1 | Survey 2 | Survey 3 | Survey 4 | |
|---|---|---|---|---|
| Response rate details | ||||
| Invited (n) | 124 | 113 | 97 | 121 |
| Completed (n) | 113 | 97 | 93 | 99 |
| Response rate (%) | 91 | 86 | 96 | 82 |
| Area of expertise | ||||
| Exercise/sport specialist (n) | 31 | 28 | 32 | 30 |
| Health care or allied health care provider (e.g., nurse, oncologist, doctor, and physiotherapist) (n) | 60 | 46 | 42 | 50 |
| Movement instructor/provider (e.g., PA and yoga) (n) | 00 | 02 | 03 | 01 |
| Researcher (n) | 58 | 49 | 47 | 57 |
| Years in the field | ||||
| <1 year (n) | 02 | 02 | 00 | 02 |
| ≥1–2 years (n) | 07 | 05 | 04 | 05 |
| >2–5 years (n) | 24 | 23 | 23 | 16 |
| >5–10 years (n) | 33 | 31 | 30 | 39 |
| >10 years (n) | 46 | 36 | 36 | 37 |
| Respondent’s geographic location | ||||
| Australia (n) | 14 | 10 | 09 | 12 |
| Brazil (n) | 02 | 02 | 02 | 01 |
| Canada (n) | 23 | 21 | 21 | 22 |
| Colombia (n) | 01 | 01 | 01 | 01 |
| Denmark (n) | 03 | 02 | 02 | 02 |
| Finland (n) | 05 | 05 | 05 | 04 |
| France (n) | 02 | 01 | 01 | 01 |
| Germany (n) | 21 | 18 | 18 | 20 |
| Italy (n) | 08 | 08 | 08 | 08 |
| Netherlands (n) | 03 | 04 | 04 | 04 |
| New Zealand (n) | 00 | 00 | 00 | 01 |
| Norway (n) | 01 | 01 | 01 | 01 |
| Portugal (n) | 01 | 01 | 01 | 01 |
| Qatar (n) | 01 | 01 | 01 | 01 |
| Spain (n) | 04 | 03 | 03 | 03 |
| Switzerland (n) | 01 | 00 | 00 | 01 |
| Turkey (n) | 01 | 01 | 01 | 01 |
| UK (n) | 02 | 02 | 02 | 02 |
| USA (n) | 19 | 16 | 14 | 13 |
PA physical activity. For ‘area of expertise’ respondents were able to choose all categories that were applicable—the categories in this table represent those presented to the iPOEG network in the online surveys, prior to the network achieving consensus on the terminology for different groups of experts (e.g., exercise physiologist and physical therapist). For Survey 1, 112 (of 113) completed select demographic questions.
Survey 1
Respondents agreed on the definitions for the iPOEG (see Supplementary File 2). In addition, respondents reached consensus for the content of future surveys. There was no consensus on items covering specific criteria for PA/exercise prescription in pediatric oncology.
Survey 2
Respondents indicated that pediatric oncology-specific evidence should be used to inform the guideline and recommendation statements and that the core team’s expertise might be an important source of information. There was no consensus on how the recommendation statements could be written to address the need to tailor PA/exercise for children and adolescents affected by cancer.
Survey 3
Respondents agreed that only pediatric oncology-specific evidence should be used along with the core team expertise. Furthermore, a list of population-specific conditions that would require modifying or adapting PA/exercise were agreed upon (n = 21; e.g., anemia, cardiotoxicity, and veno-occlusive disease; see Supplementary File 3).
In-person meeting
Core team members (n = 9), local health care providers (i.e., pediatric oncologist and nurse administrator; n = 2), and trainees (n = 8) attended the in-person meeting. During this meeting, attendees discussed and modified previously agreed upon language and terminology. Meeting attendees then drafted four guideline statements that contained information to advise children and adolescents affected by cancer, their families, and health care providers, on how to engage in movement (i.e., bodily motion that requires energy expenditure; e.g., how often and how much) and five recommendation statements for tailoring exercise based on specific needs/circumstances.
Survey 4
Respondents agreed with modifying language and terminology and with each statement drafted during the in-person meeting (pending minor modifications to wording). Fig. 2 presents the final iPOEG guideline and recommendation statements, which are generic in nature.
Fig 2.
International Pediatric Oncology Exercise Guidelines (iPOEG) guideline and recommendation statements.
Discussion
The purpose of this project was to develop PA and exercise guideline and recommendation statements via iterative survey rounds using the Delphi technique to achieve consensus, a literature synthesis to build from existing evidence, and an in-person meeting to bring together clinical and research expertise. The guideline and recommendation statements are generic in nature as work remains to be done in this field. Nevertheless, moving more was described as safe—with no broad categories of contraindications for movement identified—and beneficial for all children and adolescents affected by cancer.
Notable strengths of this project include the international core team who provided varied perspectives, oversaw survey development, and offered input throughout the ancillary literature synthesis, ensuring that statements were based on available evidence and expert opinion. Also, the in-person meeting in Phase II (September 2019) enabled the discussion of available evidence, reflection on current practice, and review of findings from Surveys 1–3. Finally, the iterative and open-ended nature of Surveys 1–4 enabled consensus building and utilized the iPOEG networks’ feedback to refine subsequent surveys. Thus, the iPOEG statements presented herein are evidence-informed and reflect consensus from a large and diverse group of experts spanning disciplines and countries. Consensus throughout this project was high and comparable to that reported in the literature [45], which may be due (in part) to calls for guideline and recommendation statements by researchers in this field [2–5] and the relative rarity of pediatric cancer [46, 47], which has compelled small yet coordinated efforts locally and internationally.
When interpreting the iPOEG statements, there are important limitations that must be considered. First, the survey respondents were invited based on their self-identification as ‘experts’ in the field of pediatric exercise oncology. Although the intent was to develop an iPOEG network comprised of respondents with varied expertise, the full range of health care providers working with this population was not captured. It will be critical to continue to recruit and build a network that is inclusive of experts from different disciplines and who hold differing clinical and nonclinical positions. For example, including more psychologists, social workers, and child life specialists may ensure a greater emphasis on developmental perspectives and enjoyment during movement (i.e., the fun factor), which are paramount to promoting lasting behavior change in this population. Furthermore, distinguishing within and across groups of experts (e.g., health care providers, movement-based allied health care providers [e.g., physiotherapists and kinesiologists], other allied health care providers [e.g., psychologists, social workers, and child life specialists], and researchers [e.g., kinesiology and medicine]) is needed to ensure adequate representation from different perspectives when updating the iPOEG guideline and recommendation statements. Related to this, those who participated in this project may not be completely representative of all those with relevant experiences and/or training in this field internationally. Specifically, participation in this project was limited to those who could complete the surveys in English. Nonnative English speakers may have had difficulty understanding and responding and/or may have elected not to participate. Second, while the questions presented to the iPOEG network in Surveys 1–4 were devised by the core team, it is possible that additional or different questions could have been included that may have yielded different results. To minimize such biases, open-ended response options were included, iterative survey rounds were conducted to inform subsequent rounds, and a literature synthesis was performed. Finally, findings from this project and the literature synthesis (that occurred concurrently; [43]) indicate that more evidence is required to facilitate specific guideline and recommendations statements (e.g., following frequency, intensity, time, and type to provide a higher degree of specificity and guidance). As evidence continues to accumulate, further efforts will be required to refine these statements to ensure that they reflect the current evidence, practice, and a range of expert opinions. Notably, the iPOEG network continues to expand. At the time of manuscript revisions, the iPOEG network was comprised of 158 individuals from 26 countries. Interested individuals can join the iPOEG network by visiting: https://survey.ucalgary.ca/jfe/form/SV_2tUtHWgGmqUOPkx.
Although the publication of this manuscript is a necessary first step in knowledge translation, additional efforts are required to move these statements beyond academia and into practice. To ensure end users (e.g., health care providers, children, and adolescents affected by cancer and their families/caregivers, exercise professionals, educators, and cancer support organizations) have access to this information, a series of integrated knowledge translation projects are being undertaken to develop iPOEG Toolkits, which will consist of educational videos, infographics, brochures, and posters. These resources are being created with end users and will be hosted online to reduce barriers to access. To stay up-to-date or to learn more about this project, please see: https://kinesiology.ucalgary.ca/labs/health-and-wellness/research-projects/pediatric-oncology-research/international-pediatric-oncology. In addition, given the important role of exercise specialists, as detailed within the iPOEG recommendation statements, pediatric cancer and exercise education modules are being developed to help ensure that those wishing to work with this population have appropriate training Please see https://thrivehealthservices.doki.io/pediatric-cancer-and-exercise-module for more details.
CONCLUSION
The iPOEG guideline and recommendation statements are based on available evidence and consensus from a large team of international experts. The statements represent a first step to support end users engaging in and promoting movement and exercise among children and adolescents affected by cancer. Although further work is required, the experts agree, it is time for children and adolescents affected by cancer to move more.
Supplementary Material
Acknowledgments
The authors would like to thank the iPOEG expert consensus committee members who responded to at least one of the online surveys and who provided their input and guidance throughout this process. Also, they would like to thank the continually growing iPOEG network for their interest in and support of this work. Finally, the authors would like to acknowledge the work performed by Alyssa Froese, Vivien Lösse, and David Chiu in Phase I (February 2019–August 2019). This manuscript was prepared while the first author was supported by a Canadian Institutes of Health Research Fellowship.
Funding: This project was supported by funding to the corresponding author from the Daniel Family Leadership Chair in Psychosocial Oncology, Social Sciences and Humanities Research Council of Canada, the Faculty of Kinesiology at the University of Calgary, and the University Research Grants Committee.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Authors’ contributions: A.W. and S.N.R. are coleads on the iPOEG project. E.M. and C.L. are trainees engaged in iPOEG research. The core team consists of C.C.V., S.G., L.H., S.K., F.R., M.G., and P.v.d.T. Both G.M.T.G. and K.M. provided clinical input. All coauthors contributed meaningfully to this project and critically reviewed and approved of the manuscript.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval for the design and conduct of this study was provided by the Health Research Ethics Board of Alberta—Cancer Care Committee (HREBA-CC; Ref. HREBA.CC-18–0565).
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Study registration: This study was not formally registered.
Analytic plan preregistration: The analysis plan for this study was not formally preregistered.
Data availability
Deidentified data from this study are not available in a public archive. Summaries of the deidentified data from this study will be made available (as allowable according to institutional research ethics board standards) by emailing the corresponding author.
Analytic code availability: There is no analytic code associated with this study.
Materials availability: Materials used to conduct the study are not available in a public archive. Materials may be made available (upon reasonable request) by emailing the corresponding author.
References
- 1. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 2. Baumann FT, Bloch W, Beulertz J. Clinical exercise interventions in pediatric oncology: A systematic review. Pediatr Res. 2013;74(4):366–374. [DOI] [PubMed] [Google Scholar]
- 3. Grimshaw SL, Taylor NF, Shields N. The feasibility of physical activity interventions during the intense treatment phase for children and adolescents with cancer: A systematic review. Pediatr Blood Cancer. 2016;63(9):1586–1593. [DOI] [PubMed] [Google Scholar]
- 4. Rustler V, Hagerty M, Daeggelmann J, Marjerrison S, Bloch W, Baumann FT. Exercise interventions for patients with pediatric cancer during inpatient acute care: A systematic review of literature. Pediatr Blood Cancer. 2017;64(11):1–5. [DOI] [PubMed] [Google Scholar]
- 5. Morales JS, Valenzuela PL, Rincón-Castanedo C, et al. Exercise training in childhood cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev. 2018;70:154–167. [DOI] [PubMed] [Google Scholar]
- 6. Agüero G, Sanz C. Assessment of cardiometabolic risk factors among adolescent survivors of childhood cancer. Arch Argent Pediatr. 2015;113(2):119–125. [DOI] [PubMed] [Google Scholar]
- 7. Green DM, Zhu L, Wang M, et al. Pulmonary function after treatment for childhood cancer. A report from the St. Jude Lifetime Cohort Study (SJLIFE). Ann Am Thorac Soc. 2016;13(9):1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hooke MC, Rodgers C, Taylor O, et al. Physical activity, the childhood cancer symptom cluster-leukemia, and cognitive function: A longitudinal mediation analysis. Cancer Nurs. 2018;41(6):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: A report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang FF, Hudson MM, Huang IC, et al. Lifestyle factors and health-related quality of life in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer. 2018;124(19):3918–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braam KI, van Dijk-Lokkart EM, Kaspers GJL, et al. Effects of a combined physical and psychosocial training for children with cancer: A randomized controlled trial. BMC Cancer. 2018;18(1):1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chamorro Viña C, Valentín J, Fernández L, et al. Influence of a moderate-intensity exercise program on early NK cell immune recovery in pediatric patients after reduced-intensity hematopoietic stem cell transplantation. Integr Cancer Ther. 2017;16(4):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Götte M, Kesting SV, Gerss J, Rosenbaum D, Boos J. Feasibility and effects of a home-based intervention using activity trackers on achievement of individual goals, quality of life and motor performance in patients with paediatric cancer. BMJ Open Sport Exerc Med. 2018;4(1):e000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manchola-González JD, Bagur-Calafat C, Girabent-Farrés M, et al. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: results of a randomised clinical trial. Support Care Cancer. 2020;28(7):3171–3178. [DOI] [PubMed] [Google Scholar]
- 15. Riggs L, Piscione J, Laughlin S, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: A controlled clinical trial with crossover of training versus no training. Neuro Oncol. 2017;19(3):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chamorro-Viña C, Keats M, Culos-Reed SN. Pediatric oncology exercise manual. Available at https://www.ucalgary.ca/poem/.
- 17. Pediatric Integrated Cancer Service. A practical guide to keeping active during cancer treatment. Childhood cancer: Physical activity. Available at https://pics.org.au/wp-content/uploads/2018/04/PICS-Physical-Activity-booklet_Final.compressed.pdf.
- 18. Children’s Cancer and Leukaemia Group. Keeping active during and after treatment: A parent’s guide to physical activity, sport, and exercise for children and young people with cancer. Available at https://www.cclg.org.uk/write/MediaUploads/Publications/PDFs/Keeping_active_during_and_after_treatment_2017.pdf. 2017.
- 19. Kilka R, Tamburini A, Galanti G, Mascherini G, Stefani L. The role of exercise in pediatric and adolescent cancer: A review of assessments and suggestions for clinical implementation. J Funct Morphol Kinesiol. 2018;3(7):1–19.30221205 [Google Scholar]
- 20. Söntgerath R, Küpper L, Wulftange M, Schepper F, Christiansen H. Physical activity promotion and exercise in pediatric oncology—Structural requirements and financing options based on the Leipzig movement concept. Kinische Pädiatrie. 2019;231(3):150–156. [DOI] [PubMed] [Google Scholar]
- 21. Chauhan BF, Jeyaraman MM, Mann AS, et al. Behavior change interventions and policies influencing primary healthcare professionals’ practice-an overview of reviews. Implement Sci. 2017;12(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuso P. Strategies to increase physical activity. Permanente J. 2015;19(4):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falzarano M, Pinto Zipp G. Seeking consensus through the use of the Delphi technique in health sciences research. J Allied Health. 2013;42(2):99–105. [PubMed] [Google Scholar]
- 24. Loeffen EA, Mulder RL, Kremer LC, et al. Development of clinical practice guidelines for supportive care in childhood cancer—Prioritization of topics using a Delphi approach. Support Care Cancer. 2015;23(7):1987–1995. [DOI] [PubMed] [Google Scholar]
- 25. Giangregorio LM, McGill S, Wark JD, et al. Too fit to fracture: outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos Int. 2015;26(3):891–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: Developing guidelines. BMJ. 1999;318(7183):593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khodyakov D, Grant S, Denger B, et al. Practical considerations in using online modified-Delphi approaches to engage patients and other stakeholders in clinical practice guideline development. Patient. 2020;13(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wurz A, Daeggelmann J, Albinati N, Kronlund L, Chamorro-Viña C, Culos-Reed SN.. Physical activity programs for children diagnosed with cancer: an international environmental scan. Support Care Cancer. 2019;27(4):1153–1162. [DOI] [PubMed] [Google Scholar]
- 30. Hsu C-C, Sandford BA. The Delphi technique: Making sense of consensus. Pract Assess Res Eval. 2007;12(10):1–8. [Google Scholar]
- 31. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 32. Shepherd SK, Achterberg CL. Qualitative research methodology: data collection, analysis, interpretation, and verification. In: Monsen ER, ed. Research: Successful Approaches. American Dietetic Association; 1992:82–88. [Google Scholar]
- 33. Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2007;8(1):19–32. [Google Scholar]
- 34. Levac D, Colquhoun H, O’Brien KK. Scoping studies: Advancing the methodology. Implement Sci. 2010;5(69):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: A large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13(48):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [Updated March 2011]. Cochrane Collab. Available at ; www.handbook.cochrane.org.2011. [Google Scholar]
- 41. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–414. [DOI] [PubMed] [Google Scholar]
- 42. Snilstveit B, Oliver S, Vojtkova M. Narrative approaches to systematic review and synthesis of evidence for international development policy and practice. J Dev Effectiveness. 2012;4(3):409–429. [Google Scholar]
- 43. Wurz A, McLaughlin E, Lategan C, Ellis K, Culos-Reed SN. Synthesizing the literature on physical activity among children and adolescents affected by cancer: evidence for the international Pediatric Oncology Exercise Guidelines (iPOEG). Transl Behav Med. 2021; ibaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wurz A, McLaughlin E, Chamorro Viña C,et al. Advancing the field of pediatric exercise oncology: research and innovation needs. Curr Oncol. 2021;28(1):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elwyn G, O’Connor A, Stacey D, et al. ; International Patient Decision Aids Standards (IPDAS) Collaboration . Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process. BMJ. 2006;333(7565):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steliarova-Foucher E, Colombet M, Ries LAG, et al. ; IICC-3 contributors . International incidence of childhood cancer, 2001-10: A population-based registry study. Lancet Oncol. 2017;18(6):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun PR. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019;20(4):483–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from this study are not available in a public archive. Summaries of the deidentified data from this study will be made available (as allowable according to institutional research ethics board standards) by emailing the corresponding author.
Analytic code availability: There is no analytic code associated with this study.
Materials availability: Materials used to conduct the study are not available in a public archive. Materials may be made available (upon reasonable request) by emailing the corresponding author.