Abstract
Background
In the night of February 20, 2020, the first epidemic of the novel coronavirus disease (COVID-19) outside Asia was uncovered by the identification of its first patient in Lombardy region, Italy. In the following weeks, Lombardy experienced a sudden increase in the number of ascertained infections and strict measures were imposed to contain the epidemic spread.
Methods
We analyzed official records of cases occurred in Lombardy to characterize the epidemiology of SARS-CoV-2 during the early phase of the outbreak. A line list of laboratory-confirmed cases was set up and later retrospectively consolidated, using standardized interviews to ascertained cases and their close contacts. We provide estimates of the serial interval, of the basic reproduction number, and of the temporal variation of the net reproduction number of SARS-CoV-2.
Results
Epidemiological investigations detected over 500 cases (median age: 69, IQR: 57–78) before the first COVID-19 diagnosed patient (February 20, 2020), and suggested that SARS-CoV-2 was already circulating in at least 222 out of 1506 (14.7%) municipalities with sustained transmission across all the Lombardy provinces. We estimated the mean serial interval to be 6.6 days (95% CrI, 0.7–19). Our estimates of the basic reproduction number range from 2.6 in Pavia (95% CI, 2.1–3.2) to 3.3 in Milan (95% CI, 2.9–3.8). A decreasing trend in the net reproduction number was observed following the detection of the first case.
Conclusions
At the time of first case notification, COVID-19 was already widespread in the entire Lombardy region. This may explain the large number of critical cases experienced by this region in a very short timeframe. The slight decrease of the reproduction number observed in the early days after February 20, 2020 might be due to increased population awareness and early interventions implemented before the regional lockdown imposed on March 8, 2020.
Keywords: COVID-19, SARS-CoV-2, Epidemiology, Transmission dynamics, Reproduction number, Coronavirus, Lombardy outbreak
Highlights
-
•
Over 500 cases (median age: 69, IQR: 57–78) declaring symptom onset before the notification of the first case (20 February 2020) were retrospectively detected.
-
•
SARS-CoV-2 was already circulating in at least 222 out of 1506 (14.7%) municipalities of Lombardy.
-
•
The estimated mean serial interval was 6.6 days (95% CrI, 0.7–19).
-
•
The basic reproduction number in the 12 provinces of Lombardy ranged from 2.6 (95% CI, 2.1–3.2) to 3.3 (95% CI, 2.9–3.8).
-
•
A decreasing trend in the net reproduction number following the detection of the epidemic and the introduction of the first restrictive measures.
1. Introduction
In the late night of February 20, 2020, the first case of novel coronavirus infectious disease (COVID-19) was confirmed in the Lombardy Region, Italy, a district with 10 million inhabitants. The patient was a 38-year-old otherwise healthy man (patient 1), admitted to the Hospital of Codogno (province of Lodi), with a mild pneumonia resistant to therapy, no relevant travel history and no apparent exposure to diseased contacts. In the weeks that followed, the Codogno area, as well as several neighboring towns in southern Lombardy, experienced a very rapid increase in the number of detected cases (Riccardo et al., 2020). Given the rapid upsurge of positive cases in the area, regional and local health authorities in strong collaboration with the National Public Health Institute introduced coordinated actions such as intensive testing, contact tracing, isolation of confirmed cases and quarantine of case contacts to limit the spread of the infection. Following the increase in the number and in the spatial distribution of detected cases, all teaching activities were suspended on February 24, 2020 and a quarantined area was defined on the same day around 10 municipalities which were identified as the possible epicenter of the ongoing outbreak. In the following days, public health authorities implemented further restrictions such as the cancellation of all sport events in the whole region (February 25), restrictions on indoor sport and food services in the province of Bergamo, Cremona, and Lodi (March 2), the closure of indoor recreational and cultural venues in the whole region (March 4). Eventually, a regional lockdown was imposed on March 8, briefly followed by a national one on March 11, 2020 (Ministero della Salute, 2020a, Ministero della Salute, 2020b) (see Table S1 in Supplementary Materials).
Although the patient zero was never identified, evidence from environmental and observational studies suggests that SARS-CoV-2 was circulating in Lombardy as early as mid-January 2020 (Alteri et al., 2021, Giardina et al., 2021, Percivalle et al., 2020). Thanks to consolidation of case records, here we discuss the results of the epidemiology of COVID-19 in its early epidemic phase, which suggest a widespread circulation of the virus at the time of first case notification when no control measures were in place and SARS-CoV-2 circulation was not even suspected. Moreover, we present the epidemiological analyses arising from the retrospective investigation of the first 16,665 laboratory-confirmed cases in Lombardy as well as results concerning the transmission dynamics of the infection within Lombardy (up to March 9, 2020).
2. Method
2.1. Study population
Following the detection of the first patient, a strict proactive intervention for tracing and testing all patients’ contacts was set up on February 21, 2020. Following the WHO recommendations, nasal swabs (UTM viral transport ®, Copan Italia S.p.a) from all suspected cases were tested by applying a real-time RT PCR assay targeting two different genes (E and RdRp) of SARS-CoV-2. (Corman et al., 2020). When a positive result was obtained for one of these two genes only, the individual was retested to confirm her/his positivity. Positivity was confirmed only when a positive result was obtained for both genes. From February 21 to February 25, all suspected cases as well as their symptomatic and asymptomatic contacts were tested. From February 26 onward, testing was applied only to symptomatic patients. Epidemiological data were complemented by records collected through standardized interviews of confirmed cases and their close contacts. Operators of the local health protection agencies, called Agenzia di Tutela della Salute (ATS), performed the interviews. For each case and contact, operators collected data on their demographic characteristics (such as their name, gender, date of birth, home address, municipality of residence) and clinical symptoms (such the list of symptoms experienced and the date of symptom onset). Personal information such as name, home address, municipality of residence was used only within contact tracing activities, but it was removed before conducting the analysis. Interviews aimed at determining the history of exposure in the 2 weeks preceding the symptom onset of positive subjects (and/or their close contacts where necessary), including the time and type of events associated with individuals’ potential exposure to SARS-CoV-2 and the relationships between the case and its contacts. Individual records were used to build a central dataset managed by the Lombardy region and providing for each ascertained infection their age, sex, place of residence, respiratory tract specimen results, the full list of case contacts along with their clinical history including possible dates of symptom onset and hospitalization. The definition of a COVID-19 case and of a case contact used in the epidemiological field investigation is provided in the Supplementary Materials. In our analysis, we used a version of the database consolidated in July 2020. To reduce recall biases, we excluded symptomatic cases whose nasal swab test was carried out more than 30 days after the declared date of symptom onset. The statistical analysis was performed using the statistical software R version 4.0.1 and the programming language C. The C code used for the estimation of Rt and R0 is available at https://github.com/majelli/Rt. The code for the estimation of the serial interval and data used in the analysis can be found at doi: 10.6084/m9.figshare.16621888.
2.2. COVID-19 serial interval
Data collected through contact tracing, which allowed to investigate the transmission chains, were analyzed to estimate the distribution of the serial interval (i.e., the time period between the time of onset of symptoms in a primary case and the time of onset in her/his secondary cases) from confirmed cases with well-established epidemiological links. A gamma distribution was fitted to the set of anonymized serial intervals using maximum likelihood estimation (see Supplementary Materials).
2.3. SARS-CoV-2 transmission dynamics
The basic reproduction number R0 represents the average number of secondary cases generated by a primary infector in a fully susceptible population. The transmission potential of the disease at a given time t is better estimated in terms of a time dependent measure such as the net reproduction number Rt (Liu et al., 2018, WHO Response Team, 2014, Zhang et al., 2020). We provide estimates of both R0 and Rt for the initial phase of the epidemic, for each province in the Lombardy region, by using a Bayesian approach widely adopted in the literature (Liu et al., 2018, WHO Response Team, 2014, Zhang et al., 2020). Estimates of R0 and Rt were obtained by analyzing the daily number of symptomatic SARS-CoV-2 positive individuals in each province of Lombardy, as retrieved from records summarized in Table 1. As an approximation of the generation time, we used the estimated distribution of the serial interval. We then used MCMC Metropolis-Hastings sampling to estimate the posterior distribution of Rt (details reported in the Supplementary Materials). To estimate R0, we identified an initial phase of exponential growth by visual inspection of the curve of symptomatic cases (see Supplementary Materials), we assumed that during this phase Rt=R0 and estimated R0 by applying the procedure described above. We also run a sensitivity analysis by considering different estimates of the serial interval and different cut-off values for limiting the recall bias on symptom onset dates (see Supplementary Materials).
Table 1.
Characteristics of COVID-19 patients in Lombardy Region, Italy (January 30 – March 9, 2020).
| Jan 30 – Mar 9, 2020 (N = 16,665) | Jan 30 – Feb 19, 2020 (N = 527) | Feb 20 – Mar 9, 2020 (N = 16,138) | |
|---|---|---|---|
| Demographics | |||
| Median age - years (range) | 65 (0–100) | 69 (0–97) | 65 (0–100) |
| Male sex - no./total no. (%) | 10,369/16,645 (62.3%) | 337/527 (63.9%) | 10,032/16,118 (62.2%) |
| Health care workers - no./total no. (%) | 1934/16,665 (11.6%) | 39/527 (7.4%) | 1895/16,138 (11.7%) |
| Age group - no./total no. (%) | |||
| < 18 yr | 81/16,665 (0.5%) | 3/527 (0.6%) | 78/16,138 (0.5%) |
| 18–24 yr | 110/16,665 (0.7%) | 2/527 (0.4%) | 108/16,138 (0.7%) |
| 25–49 yr | 2977/16,665 (17.9%) | 79/527 (15%) | 2898/16,138 (18%) |
| 50–64 yr | 4805/16,665 (28.8%) | 132/527 (25%) | 4673/16,138 (29%) |
| 65–74 yr | 3712/16,665 (22.3%) | 136/527 (25.8%) | 3573/16,138 (22.2%) |
| 75 + yr | 4989/16,665 (29.9%) | 175/527 (33.2%) | 4805/16,138 (29.8%) |
| Deaths - no./total no. (%) | |||
| < 55 yr | 121/4592 (2.6%) | 2/107 (1.9%) | 119/4485 (2.7%) |
| 55–64 yr | 351/3381 (10.4%) | 6/109 (5.5%) | 345/3272 (10.5%) |
| 65–74 yr | 1181/3712 (31.8%) | 42/136 (30.9%) | 1139/3576 (31.9%) |
| 75 + yr | 2786/4980 (55.9%) | 95/175 (54.3%) | 2691/4805 (56%) |
| Total | 4439/16,665 (26.6%) | 145/527 (27.5%) | 4294/16,138 (26.6%) |
| Province of residence – Total (Incidence per 100,000) | |||
| Bergamo (BG) | 3928 (354.47) | 123 (11.10) | 3805 (343.37) |
| Brescia (BS) | 3482 (277.35) | 60 (4.78) | 3422 (272.57) |
| Milan (MI) | 2921 (89.46) | 55 (1.68) | 2866 (87.77) |
| Cremona (CR) | 1909 (536.37) | 72 (20.23) | 1837 (516.14) |
| Lodi (LO) | 1290 (567.25) | 156 (68.60) | 1134 (498.65) |
| Pavia (PV) | 836 (154.71) | 33 (6.11) | 803 (148.60) |
| Monza Brianza (MB) | 674 (77.45) | 6 (0.69) | 668 (76.76) |
| Mantua (MN) | 394 (96.83) | 12 (2.95) | 382 (93.88) |
| Lecco (LC) | 381 (113.74) | 1 (0.30) | 380 (113.45) |
| Como (CO) | 296 (49.53) | 3 (0.50) | 293 (49.03) |
| Varese (VA) | 268 (30.29) | 1 (0.11) | 267 (30.17) |
| Sondrio (SO) | 82 (45.45) | 3 (1.66) | 79 (43.79) |
| Outside Lombardy | 204 | 2 | 202 |
*Denominators deviating from the total indicate missing data. Percentages may not sum up to 100 because of rounding.
3. Results
We identified 527 cases with symptom onset before the detection of the first case, on February 20, 89.2% of which were hospitalized and 27.5% who died. Of these patients, 337 (63.9%) were males and 39 (7.4%) were healthcare workers and their median age was 69 years (interquantile range: 57–78). Similar population characteristics and clinical outcomes were observed for patients with symptoms onset date in the following weeks up to March 9, 2020, by which time a total of 16,138 additional symptomatic cases were identified in the whole region (Table 1).
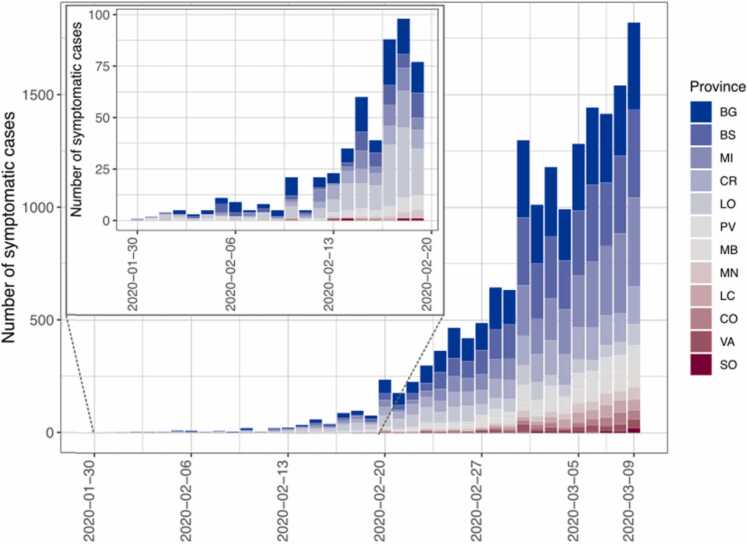
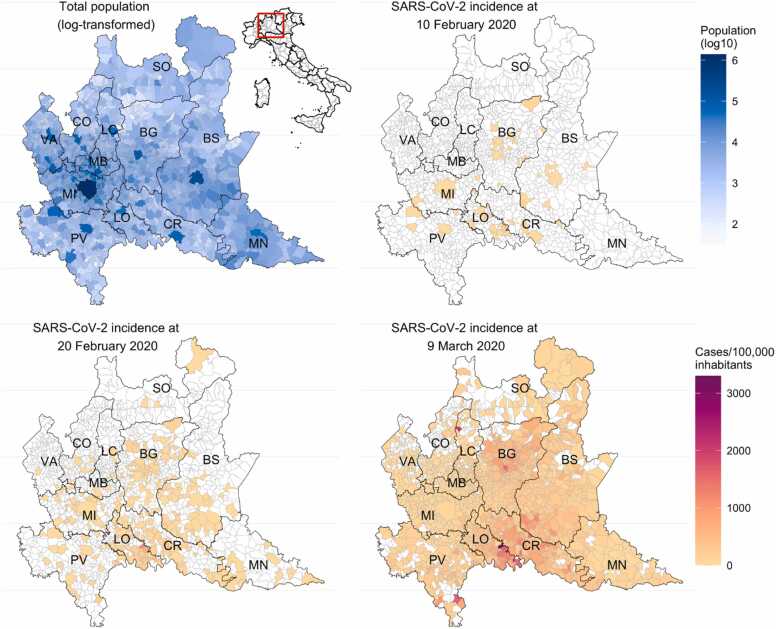
As of February 20, 2020, symptomatic cases were already present in all Lombardy provinces, with the provinces of Lodi and Bergamo accounting for 53% of the total and 3 more provinces (Brescia, Cremona, Milano) contributing for 35% (Table 1, Fig. 1, Fig. 2). In each of these 5 provinces, more than 50 cases had already developed symptoms before the discovery of the epidemic. As of March 9, 2020, 11 of 12 provinces counted at least 250 symptomatic cases (Table 1).
Fig. 1.
Daily number of reported cases in each province by date of symptom onset. The inset zooms in on the initial part of the epidemic curve.
Fig. 2.
Maps of Lombardy region, its provinces, and the total population by municipality as of December 2019. Location of Lombardy within Italy is highlighted by a red box (upper left panel). Cumulative number of cases/100,000 inhabitants as of February 10, 2020 (upper right panel), February 20, 2020 (lower left panel), and March 9, 2020 (lower right panel) in each municipality of Lombardy.
Using data from 55 clusters (i.e., groups of contacts identified by an index case) with an average number of contacts equal to 1.6 (range: 1–13), we extracted 90 observations of individual serial intervals. From these, we estimated the distribution of the serial interval to follow a gamma distribution with mean 6.6 days (shape: 1.28, rate=0.27; 2.5- and 97.5-percentiles of the distribution: 0.7–19.0) and we found that 95% of symptomatic secondary cases developed symptoms within 16.1 days after symptom onset in their infector (see Supplementary Materials for further details).
For each province, we identified an initial phase of exponential growth lasting between six and ten days. Using the epidemic curve of symptom onset, we estimated a basic reproduction number ranging from 2.3 in Sondrio (95%CI 1.8–2.9) to 3.3 in Milan and Brescia (95%CI 2.9–3.8 and 2.9–3.7, respectively) (Table 2). For some of these provinces (Monza-Brianza, Sondrio, Como, Lecco, Mantua, Varese), the time window of exponential growth includes interventions, therefore the value reported in Table 2 is expected to be an underestimate of the actual R0.
Table 2.
Model estimates of the basic reproductive number in each province of Lombardy; February 12 – March 9, 2020.
| Province | R0 (95% CI) | Exponential growth period | Symptomatic cases during the exponential growth period |
|---|---|---|---|
| Bergamo (BG) | 3 (2.5–3.5) | Feb 12 –20, 2020 | 155 |
| Cremona (CR) | 2.9 (2.3–3.4) | Feb 12 –20, 2020 | 102 |
| Lodi (LO) | 2.7 (2.4–3) | Feb 14 –23, 2020 | 338 |
| Pavia (PV) | 2.6 (2.1–3.2) | Feb 15 –23, 2020 | 75 |
| Brescia (BS) | 3.3 (2.9–3.7) | Feb 18 –24, 2020 | 211 |
| Milan (MI) | 3.3 (2.9–3.8) | Feb 19 –25, 2020 | 212 |
| Monza Brianza (MB)1 | 2.7 (2.1–3.4) | Feb 21 –27, 2020 | 66 |
| Varese (VA)1 | 2.9 (2.3–3.6) | Feb 23 – Mar 1, 2020 | 79 |
| Mantua (MN)1 | 2.8 (2.3–3.5) | Feb 25 – Mar 2, 2020 | 85 |
| Lecco (LC)1 | 2.7 (2.3–3.1) | Feb 26 – Mar 5, 2020 | 168 |
| Como (CO)1 | 2.4 (2.1–2.8) | Feb 27 – Mar 8, 2020 | 232 |
| Sondrio (SO)1 | 2.3 (1.8–2.9) | Mar 1–9, 2020 | 68 |
Provinces for which interventions were already in place at the time of exponential growth.
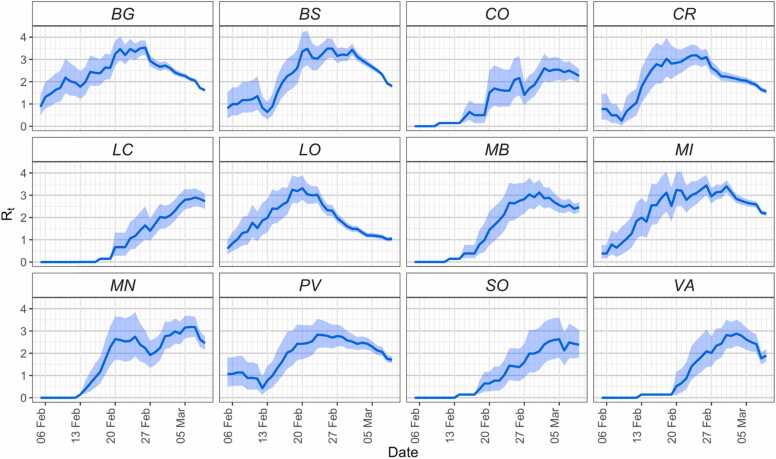
We found that the estimated net reproduction number Rt followed a common temporal pattern across different provinces, showing a steady and rapid increase until the end of February, followed by a decrease thereafter (Fig. 3). This decline starts in the days immediately after the detection of the first case and is more marked in the provinces of Lodi, Bergamo and Cremona for which early interventions were undertaken (e.g., the definition of quarantined areas in Lodi, the increase of smart working and restrictions on indoor sport and food services in Bergamo and Cremona; see Table S1). These results are robust to assuming different distributions of the serial interval for the estimation of Rt (see Supplementary Materials).
Fig. 3.
Estimated net reproduction number Rt (weekly moving average) from the curve of symptom onset across different provinces of Lombardy.
4. Discussion
We provided a detailed analysis of the COVID-19 epidemic in the month preceding its detection in Italy and in successive weeks until the national lockdown. Records of all laboratory-confirmed cases consolidated by public health authorities over the course of the epidemic allowed to shed some light on the early transmission dynamics that characterized the arrival of the pandemic in Europe. Thanks to the retrospective analysis of these records, we highlighted that the brisk increase in COVID-19 patients that caused high pressure on the public health system few weeks after the first detected case was rooted in the high transmissibility of the infection and the widespread silent transmission of the pathogen occurring between January and mid-February 2020. Undetected SARS-CoV-2 transmission could have raised from the small fraction of infected individuals developing recognizable symptoms (Poletti et al., 2020b) combined with the role played by asymptomatic carriers (He et al., 2021, Johansson et al., 2021, Lavezzo et al., 2020) and the lack of awareness of COVID-19 circulation in the population and the medical staff.
By analyzing contact tracing records, we estimated the mean serial interval to be 6.6 days, which lies in the same range as values obtained in the early days of the pandemic (Li et al., 2020, Zhang et al., 2020), when isolation of contacts was rare and often late.
We estimated the mean R0 to be rather homogenous across provinces of Lombardy, in the range 2.6–3.3. Retrospective epidemiological investigations confirmed that cases were scattered all around the region by the time of the first detection in Codogno (February 20). The threat posed by the emergence of COVID-19 was inflated by the limited knowledge on the new pathogen, while regional and national policy makers had to take rapid decisions on how to mitigate the spread of the epidemic, minimizing morbidity and mortality, delaying an epidemic peak that would have saturated and overwhelmed the entire healthcare system in a very short time (Grasselli et al., 2020a, Grasselli et al., 2020b, Guzzetta et al., 2020, Poletti et al., 2020a). Our findings suggest that the set-up of a quarantine area around the initial epicenter of the outbreak in the province of Lodi played a critical role in controlling the infection locally, with the reproduction number falling to approximately 1 before the initiation of the national lockdown. However, it was insufficient to suppress the transmission altogether because of the widespread circulation of SARS-CoV-2 prior to February 20, 2020. By mid- February, Rt was already above the epidemic threshold in at least seven provinces (Bergamo, Lodi, Milan, Cremona. Brescia, Pavia, Mantua). The increase of public concern about the ongoing epidemic and the progressive introduction of restrictive control measures may explain the decrease in Rt at the end of February. However, as Rt remained well above one, the initial set of interventions and the spontaneous behavioral response to the risk of infection were not sufficient to contain the ongoing epidemic. In fact, after the implementation of the initial set of intervention, the number of cases was still increasing exponentially, although at a lower rate.
This study is affected by limitations deriving from the collection of data in rapidly evolving infectious disease outbreaks. The identification of cases was made challenging by the lack of knowledge on the novel virus and by the availability of testing resources, producing large delays between the dates of symptom onset and reporting (see Supplementary Materials). These delays may introduce recall biases in the correct identification of the symptom onset dates. Our estimates of the net reproduction number from symptom onset might have been affected by the change in the testing protocol occurred on February 25, as the estimation of the net reproduction number is robust to an unknown level of underreporting but is affected by time-varying reporting rates (O’Driscoll et al., 2020). Furthermore, no data is available to disentangle the contribution on SARS-CoV-2 transmission of imported vs. locally generated infections. However, the travel bans from and to China, introduced on January 30, 2020, the restrictions on individual movements, and the lockdown imposed in early March 2020 could have limited the importation of SARS-CoV-2 positive individuals.
Despite these limitations, our analysis provides robust evidence about the widespread circulation of SARS-CoV-2 in Lombardy prior to its detection and shows that the stringent measures enforced in the entire region in March 2020 were necessary to mitigate the epidemic.
CRediT authorship contribution statement
Danilo Cereda: Conceptualization, Supervision, Investigation, Writing - review & editing, Mattia Manica: Investigation, Methodology, Writing - original draft, Marco Ajelli: Conceptualization, Investigation, Writing - review & editing, Piero Poletti: Methodology, Validation, Writing - original draft, Francesca Rovida: Data curation, Investigation, Vittorio Demicheli: Data curation, Investigation, Marco Ajelli: Investigation, Methodology, Supervision, Writing - review & editing, Piero Poletti: Investigation, Methodology, Writing - original draft, Filippo Trentini: Investigation, Methodology, Writing - review & editing., Giorgio Guzzetta: Investigation, Methodology, Writing - original draft, Valentina Marziano: Investigation,Methodology, Writing - review & editing, Raffaella Piccarreta: Investigation, Methodology, Writing - review & editing, Antonio Barone: Data curation, Investigation, Michele Magoni: Data curation, Investigation, Silvia Deandrea: Data curation, Investigation, Giulio Diurno: Data curation, Investigation, Massimo Lombardo: Data curation, Investigation, Marino Faccini: Data curation, Investigation, Angelo Pan: Data curation, Investigation, Raffaele Bruno: Data curation, Investigation, Elena Pariani: Data curation, Investigation, Giacomo Grasselli: Data curation, Investigation, Alessandra Piatti: Data curation, Investigation, Maria Gramegna: Data curation, Supervision, Investigation, Fausto Baldanti: Investigation, Supervision, Writing - review & editing, Alessia Melegaro: Investigation, Conceptualization, Supervision, Writing - review & editing, Stefano Merler: Conceptualization, Project administration, Methodology, Resources, Supervision, Writing - review & editing.
Declarations of interest
M.M., G.G., V.M., P.Po., F.T. and S.M. acknowledge funding from EU grant 874850 MOOD. A.M. and R.P. acknowledge support from Italian Ministry of Education Progetti di Rilevante Interesse Nazionale Grant 20177BRJXS. A.M. acknowledges the European Research Council Consolidator Grant 101003183 and Fondazione Romeo and Enrica Invernizzi. M.A. has received research funding from Seqirus. The funding is not related to COVID-19. The contents of this publication are the sole responsibility of the authors and don't necessarily reflect the views of the funders. All other authors declare no competing interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2021.100528.
Contributor Information
Marcello Tirani, Email: marcello_tirani@regione.lombardia.it.
Stefano Merler, Email: merler@fbk.eu.
Appendix A. Supplementary material
Supplementary material
.
References
- Alteri C., Cento V., Piralla A., Costabile V., Tallarita M., Colagrossi L., Renica S., Giardina F., Novazzi F., Gaiarsa S., Matarazzo E., Antonello M., Vismara C., Fumagalli R., Epis O.M., Puoti M., Perno C.F., Baldanti F. Genomic epidemiology of SARS-CoV-2 reveals multiple lineages and early spread of SARS-CoV-2 infections in Lombardy, Italy. Nat. Commun. 2021;12:434. doi: 10.1038/s41467-020-20688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina F., Galli C., Pellegrinelli L., Paolucci S., Tallarita M., Pariani E., Piralla A., Baldanti F. No evidence of SARS-CoV-2 circulation in the framework of influenza surveillance between October 2019 and February 2020 in Lombardy, Italy. Travel Med. Infect. Dis. 2021;40 doi: 10.1016/j.tmaid.2021.102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., Cattaneo S., Cereda D., Colombo S., Coluccello A., Crescini G., Forastieri Molinari A., Foti G., Fumagalli R., Iotti E., Langer T., Latronico N., Lorini F.L., Mojoli F., Natalini G., Pessina C.M., Ranieri V.M., Rech R., Scudeller L., Rosano A., Storti E., Thompson B.T., Tirani M., Villani P.G., Pesenti A., Cecconi M., COVID-19 Lombardy ICU Network Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 2020;180:1345. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. J. Am. Med. Assoc. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Guzzetta G., Poletti P., Ajelli M., Trentini F., Marziano V., Cereda D., Tirani M., Diurno G., Bodina A., Barone A., Crottogini L., Gramegna M., Melegaro A., Merler S. Potential short-term outcome of an uncontrolled COVID-19 epidemic in Lombardy, Italy, February to March 2020. Eurosurveillance. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.12.2000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Guo Y., Mao R., Zhang J. Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta‐analysis. J. Med. Virol. 2021;93:820–830. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.A., Quandelacy T.M., Kada S., Prasad P.V., Steele M., Brooks J.T., Slayton R.B., Biggerstaff M., Butler J.C. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., Rossi L., Manganelli R., Loregian A., Navarin N., Abate D., Sciro M., Merigliano S., De Canale E., Vanuzzo M.C., Besutti V., Saluzzo F., Onelia F., Pacenti M., Parisi S.G., Carretta G., Donato D., Flor L., Cocchio S., Masi G., Sperduti A., Cattarino L., Salvador R., Nicoletti M., Caldart F., Castelli G., Nieddu E., Labella B., Fava L., Drigo M., Gaythorpe K.A.M., Brazzale A.R., Toppo S., Trevisan M., Baldo V., Donnelly C.A., Ferguson N.M., Dorigatti I., Crisanti A., Ainslie K.E.C., Baguelin M., Bhatt S., Boonyasiri A., Boyd O., Cattarino L., Ciavarella C., Coupland H.L., Cucunubá Z., Cuomo-Dannenburg G., Djafaara B.A., Donnelly C.A., Dorigatti I., van Elsland S.L., FitzJohn R., Flaxman S., Gaythorpe K.A.M., Green W.D., Hallett T., Hamlet A., Haw D., Imai N., Jeffrey B., Knock E., Laydon D.J., Mellan T., Mishra S., Nedjati-Gilani G., Nouvellet P., Okell L.C., Parag K.V., Riley S., Thompson H.A., Unwin H.J.T., Verity R., Vollmer M.A.C., Walker P.G.T., Walters C.E., Wang H., Wang Y., Watson O.J., Whittaker C., Whittles L.K., Xi X., Ferguson N.M., Imperial College COVID-19 Response Team Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Tong S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.-H., Ajelli M., Aleta A., Merler S., Moreno Y., Vespignani A. Measurability of the epidemic reproduction number in data-driven contact networks. Proc. Natl. Acad. Sci. USA. 2018;115:12680. doi: 10.1073/pnas.1811115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministero della Salute, 2020a. Decree of the Prime Minister. Disposizioni attuative del decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia di contenimento e gestione dell’emergenza epidemiologica da COVID-19. (20A01228) (G.U. Serie Generale, n. 45 del 23 febbraio 2020). [Enactment of decree of 23 February no. 6 concerning urgent measures for the containment and management of the epidemiologic emergency caused by COVID-19].
- Ministero della Salute, 2020b. Decree of the Prime Minister. Ulteriori disposizioni attuative del decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia di contenimento e gestione dell’emergenza epidemiologica da COVID-19, applicabili sull’intero territorio nazionale. (20A01605) (G.U. Serie Generale, n. 64 del 11 marzo 2020). [Further enactment of decree of 23 February no. 6 concerning urgent measures for the containment and management of the epidemiologic emergency caused by COVID-19].
- O’Driscoll M., Harry C., Donnelly C.A., Cori A., Dorigatti I. A comparative analysis of statistical methods to estimate the reproduction number in emerging epidemics with implications for the current COVID-19 pandemic. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percivalle E., Cambiè G., Cassaniti I., Nepita E.V., Maserati R., Ferrari A., Di Martino R., Isernia P., Mojoli F., Bruno R., Tirani M., Cereda D., Nicora C., Lombardo M., Baldanti F. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Eurosurveillance. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti P., Tirani M., Cereda D., Trentini F., Guzzetta G., Marziano V., Buoro S., Riboli S., Crottogini L., Piccarreta R., Piatti A., Grasselli G., Melegaro A., Ajelli M., Merler S. Age-specific SARS-CoV-2 infection fatality ratio and associated risk factors, Italy, February to April 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.31.2001383. pii=2001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti P., Tirani M., Cereda D., Trentini F., Guzzetta G., Sabatino G., Marziano V., Castrofino A., Grosso F., Del Castillo G., Piccarreta R., Andreassi A., Melegaro A., Gramegna M., Ajelli M., Merler S., ATS Lombardy COVID-19 Task Force Association of Age With Likelihood of Developing Symptoms and Critical Disease Among Close Contacts Exposed to Patients With Confirmed SARS-CoV-2 Infection in Italy. Jama Network Open. 2020;14(3) doi: 10.1001/jamanetworkopen.2021.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardo F., Ajelli M., Andrianou X.D., Bella A., Del Manso M., Fabiani M., Bellino S., Boros S., Urdiales A.M., Marziano V., Rota M.C., Filia A., D’Ancona F., Siddu A., Punzo O., Trentini F., Guzzetta G., Poletti P., Stefanelli P., Castrucci M.R., Ciervo A., Di Benedetto C., Tallon M., Piccioli A., Brusaferro S., Rezza G., Merler S., Pezzotti P., the COVID-19 working group Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Eurosurveillance. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.49.2000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Response Team Ebola virus disease in West Africa — the first 9 months of the epidemic and forward projections. N. Engl. J. Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Litvinova M., Wang W., Wang Y., Deng X., Chen Xinghui, Li M., Zheng W., Yi L., Chen Xinhua, Wu Q., Liang Y., Wang X., Yang J., Sun K., Longini I.M., Halloran M.E., Jr., Wu P., Cowling B.J., Merler S., Viboud C., Vespignani A., Ajelli M., Yu H. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect. Dis. 2020;20:793–802. doi: 10.1016/S1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material