Abstract
(1) Background: Wait times to chemotherapy are associated with morbidity and mortality in breast cancer patients; however, it is unclear how primary care physician (PCP) continuity impacts these wait times, or whether this association is different in immigrants, who experience cancer care inequities. We assessed the association between PCP continuity and the contact-to-chemotherapy interval (wait time from when a patient first presents to healthcare to the first day of receiving breast cancer chemotherapy), with a specific look at the immigrant population. (2) Methods: Population-based, retrospective cohort study of women who were diagnosed with stage I–III breast cancer in Ontario who received surgery and adjuvant chemotherapy. We used quantile regression at the median and 90th percentile to quantify the effect of PCP continuity on the contact-to-chemotherapy interval, performing a separate analysis on the immigrant population. (3) Results: Among 12,781 breast cancer patients, including 1706 immigrants, the median contact-to-chemotherapy interval (126 days) was 3.21 days shorter (95% confidence interval (CI) 0.47–5.96) in symptom-detected patients with low PCP continuity, 10.68 days shorter (95% CI 5.36–16.00) in symptom-detected patients with no baseline PCP visits and 17.43 days longer (95% CI 0.90–34.76) in screen-detected immigrants with low PCP continuity compared to the same groups with high PCP continuity. (4) Conclusions: Higher PCP continuity was not associated with a change in the contact-to-chemotherapy interval for most of our study population, but was associated with a marginally longer interval in our symptom-detected population and a shorter contact-to-chemotherapy interval in screen-detected immigrants. This highlights the importance of PCP continuity among immigrants with positive screening results. Additionally, having no PCP visits at baseline was associated with a shorter contact-to-chemotherapy interval in symptom-detected patients.
Keywords: breast cancer, primary health care, population health, wait times
1. Introduction
Breast cancer is the second most common cause of cancer death in Canadian women [1]. Treating breast cancer often involves surgery and sometimes adjuvant chemotherapy to reduce the risk of recurrence. From 2010 to 2012, 88% of Canadian women with breast cancer received surgery [2], and from 2007 to 2012, 35–41% of Canadian women with stages I–III breast cancer received adjuvant chemotherapy [3]. Increased wait times to receive adjuvant breast cancer chemotherapy were linked with worse morbidity and mortality outcomes [4,5,6,7,8,9,10,11]. Internationally, longer wait times were associated with minority race, older age, comorbidity, rural residence, lower education, stage I breast cancer, mastectomy, gene expression profile testing and being covered through public insurance [12,13,14,15,16,17,18,19,20]. Meanwhile, within Canada and Ontario, shorter wait times were associated with assessment through dedicated breast assessment centres and treatment in South Central Ontario [21,22,23].
Immigrants make up a large proportion of the Ontario population (29.1% of the population according to the 2016 Census) [24], and the association between primary care use and wait times for cancer treatment may be different in this group. Canadian immigrants, despite similar primary care access, are less likely to have their breast cancer detected through screening and experience longer times to diagnosis than long-term residents [25]. In Ontario, immigrants are more likely to be diagnosed with advanced-stage breast cancers than Canadian-born women and are younger at diagnosis [26].
Breast cancer patients frequently visit their primary care physicians (PCPs) during the course of their cancer journey [27]. While PCP involvement in cancer care was shown to increase cancer screening rates, reduce late-stage diagnosis, decrease the use of avoidable hospital and emergency department (ED) visits and improve survival outcomes [28,29,30,31,32], the role of PCPs in affecting wait times to receiving breast cancer treatment is unclear. In qualitative studies and surveys, patients have reported that shorter wait times to cancer diagnosis and treatment are related to PCP responsiveness to patient symptoms, comprehensiveness or breadth of primary care services offered and accessibility of primary care [33,34]. However, a lack of continuity, or a “fresh pair of eyes”, may sometimes shorten the time to diagnosis [35]. The quantitative association between PCP continuity and wait times to receive breast cancer chemotherapy has not been established.
Our study objective was to determine whether PCP continuity was associated with time to chemotherapy. We hypothesised that high baseline PCP continuity is associated with a shorter contact-to-chemotherapy interval (i.e., a shorter wait time from when a patient first presents to healthcare to the first day of receiving adjuvant breast cancer chemotherapy) and that this association is different in the immigrant population.
2. Methods
2.1. Study Design
We conducted a population-based, retrospective cohort study using linked provincial-level administrative health databases that are housed at ICES, which is a not-for-profit research institute that stores an array of Ontario’s health-related data [36]. This study used data from the Ontario cohort of a larger, nationwide study (the Canadian Team to Improve Community-Based Cancer Care along the Continuum—CanIMPACT) [37].
2.2. Study Population
We included women aged 18+ years diagnosed with stage I–III breast cancer from 1 January 2007 to 31 December 2011 (allowing for 5 years of follow-up data for other CanIMPACT studies [38,39]) who received surgery and adjuvant chemotherapy. We excluded patients who had a prior cancer diagnosis or a new primary cancer that was diagnosed within 14 months of the breast cancer diagnosis, had received neoadjuvant chemotherapy or radiation therapy prior to adjuvant chemotherapy or were living in a long-term care facility at the time of diagnosis. Immigrants were defined based on inclusion in the Immigration Refugee and Citizenship Canada permanent resident (IRCC-PR) database, which includes data on immigrants who landed in Ontario from 1985 onward. The remainder of the study population, including Canadian-born citizens or immigrants who landed before 1985, were considered “long-term residents”. Individuals who were identified in the IRCC-PR database were linked deterministically and probabilistically to the registered population of Ontario with an 86% linkage rate [40].
2.3. Variables and Data Sources
Our main outcome variable was the contact-to-chemotherapy interval: the number of days from the index contact date (first presentation to any healthcare with a positive mammogram screening or symptoms warranting breast cancer investigation) to the start date of adjuvant chemotherapy. We also looked at two sub-intervals of the contact-to-chemotherapy interval: the primary care interval (from the index contact date to the date of first breast cancer specialist consultation, as defined by the Aarhus Statement used by the International Cancer Benchmarking Partnership (ICBP) [41,42]), and the surgery-to-chemotherapy interval (from the date of last breast surgery to the start of adjuvant chemotherapy).
Our main predictor variable was PCP continuity. Continuity of care is a patient’s experience of coherent and linked care over time. Relational continuity, one aspect of continuity of care, refers to the ongoing relationship between patients and providers [43]. Relational PCP continuity was determined in our study using the Usual Provider of Care (UPC) index, which is a validated measure that is commonly used to assess continuity of care [44,45]: the proportion of visits to the most-often-visited PCP (identified from billings data of ambulatory visits, excluding emergency room visits, to physicians with a ‘General Practitioner/Family Physician’ or ‘Family Physician/Emergency Medicine’ designation) during a 2-year baseline interval (6–30 months prior to the breast cancer diagnosis). We did not calculate the UPC index in patients with fewer than 3 visits to any PCP during the baseline interval since UPC values are less meaningful in this group, where UPC values are limited to 0.0, 0.5 and 1.0. As such, PCP continuity categories in our study were: 0 PCP visits, 1–2 PCP visits, low continuity (UPC ≤ 0.75) and high continuity (UPC > 0.75), as categorised in other studies [25,39,46,47].
We pre-specified potential confounders in our study based on the literature [12,13,14,15,16,17,18,19,20,23] and clinical insight. Potential confounders included the age at diagnosis, neighbourhood income quintile, rurality, physical comorbidity (determined using the Johns Hopkins ACG® System Aggregated Diagnosis Groups (ADGs [48]) and excluding psychosocial ADGs), mental health history (determined by having a mental health visit to a PCP during the baseline period [49]), health region (of which Ontario has fourteen) and primary care practice type (determined by patient enrolment in a particular funding model at diagnosis: ‘team-based capitation’, ‘enhanced fee-for-service (FFS)’, ‘capitation’, ‘straight FFS’ and ‘other’). Patients were considered to be screen-detected if their earliest test within 6 months prior to diagnosis was a documented mammogram screening, or a bilateral mammogram with additional mammogram and/or breast ultrasound ordered by a radiologist the same day or performed on a different day with no other tests that day. Otherwise, the patient was classified as symptom-detected. Databases used to obtain data elements are shown in Appendix A, Table A1. These datasets were linked using unique encoded identifiers and analysed at ICES.
2.4. Statistical Analysis
We used chi-squared tests to compare the nominal demographic characteristics across PCP continuity groups. We used Wilcoxon rank-sum tests and Kruskall–Wallis ANOVA to compare the median interval lengths across demographic characteristics and reported the 90th percentile intervals. We performed multivariable quantile regressions at the median and 90th percentile intervals by examining the association between baseline PCP continuity and interval length while adjusting for potential confounders. The contact-to-chemotherapy and primary care intervals were stratified via cancer detection method. We repeated our quantile regression analyses on the immigrant-only population. The few (n < 6) patients with implausible interval lengths or missing index contact dates were excluded from our multivariable analyses. All analyses were performed using SAS software, version 9.4 [50]. All p-values < 0.05 were considered statistically significant.
2.5. Ethics Approval
We obtained approval from the University of Toronto research ethics board.
3. Results
There were 12,781 women in our cohort (Table 1), including 1706 Canadian immigrants. Those with no baseline PCP visits (n = 800, 6.3%) were more likely to live in remote rural locations, be in the lowest two income quintiles and be diagnosed with stage II/III (versus stage I) disease. Those with low PCP continuity (n = 3914, 30.6%) were more likely to be <40 years old, live in urban areas, be immigrants, have more comorbidities and have symptom-detected breast cancer. High PCP continuity (n = 6531, 51.1%) was associated with age >60 years, being enrolled in a primary care model and screen-detected cancers.
Table 1.
Baseline characteristics according to continuity of care at baseline.
| Total n = 12,781 |
PCP Continuity | p-Value | ||||
|---|---|---|---|---|---|---|
| 0 Visits | 1–2 Visits | UPC ≤ 0.75 (Low) | UPC > 0.75 (High) | |||
| Total | 800 (100%) | 1536 (100%) | 3914 (100%) | 6531 (100%) | ||
| Age (Categorical) | ||||||
| <40 years | 1102 (8.6%) | 69 (8.6%) | 142 (9.2%) | 457 (11.7%) | 434 (6.6%) | <0.001 |
| 40–49 years | 3481 (27.2%) | 226 (28.3%) | 499 (32.5%) | 1237 (31.6%) | 1519 (23.3%) | |
| 50–59 years | 4225 (33.1%) | 302 (37.8%) | 533 (34.7%) | 1251 (32.0%) | 2139 (32.8%) | |
| 60–69 years | 3045 (23.8%) | 176 (22.0%) | 309 (20.1%) | 779 (19.9%) | 1781 (27.3%) | |
| 70–74 years | 607 (4.7%) | 15 (1.9%) | 37 (2.4%) | 126 (3.2%) | 429 (6.6%) | |
| >74 years | 321 (2.5%) | 12 (1.5%) | 16 (1.0%) | 64 (1.6%) | 229 (3.5%) | |
| Urban/Rural Residence | ||||||
| Urban | 11,189 (87.5%) | 664 (83.0%) | 1283 (83.5%) | 3549 (90.7%) | 5693 (87.2%) | <0.001 |
| Rural | 699 (5.5%) | 45 (5.6%) | 108 (7.0%) | 149 (3.8%) | 397 (6.1%) | |
| Rural—remote | 596 (4.7%) | 62 (7.8%) | 94 (6.1%) | 119 (3.0%) | 321 (4.9%) | |
| Rural—very remote | 292–297 (2.3%) | 25–30 (3.1–3.8%) | 50–55 (3.3–3.6%) | 93–98 (2.4–2.5%) | 115–120 (1.8%) | |
| Rural—unknown | * | * | * | * | * | |
| Unknown | * | * | * | * | * | |
| Immigration Status | ||||||
| Long-term residents | 11,075 (86.7%) | 681 (85.1%) | 1373 (89.4%) | 3281 (83.8%) | 5740 (87.9%) | <0.001 |
| Immigrants | 1706 (13.3%) | 119 (14.9%) | 163 (10.6%) | 633 (16.2%) | 791 (12.1%) | |
| Immigrant Region of Origin | ||||||
| East Asia and Pacific | 544 (4.3%) | 34 (4.3%) | 51 (3.3%) | 191 (4.9%) | 268 (4.1%) | <0.001 |
| Eastern Europe and Central Asia | 286 (2.2%) | 29 (3.6%) | 43 (2.8%) | 96 (2.5%) | 118 (1.8%) | |
| Latin America and Caribbean | 239 (1.9%) | 13 (1.6%) | 16 (1.0%) | 94 (2.4%) | 116 (1.8%) | |
| Middle East and North Africa | 145 (1.1%) | 16 (2.0%) | 6 (0.4%) | 55 (1.4%) | 68 (1.0%) | |
| South Asia | 270 (2.1%) | 12 (1.5%) | 16 (1.0%) | 111 (2.8%) | 131 (2.0%) | |
| Sub-Saharan Africa | 87 (0.7%) | 3–7 (0.4–0.9%) | 6–10 (0.4–0.7%) | 44 (1.1%) | 30 (0.5%) | |
| USA/New Zealand/Australia | 37 (0.3%) | * | 5–9 (0.3–0.6%) | 14 (0.4%) | 12 (0.2%) | |
| Western Europe | 98 (0.8%) | 6 (0.8%) | 16 (1.0%) | 28 (0.7%) | 48 (0.7%) | |
| Neighbourhood Income Quintile | ||||||
| 1 (lowest) | 2020 (15.8%) | 150 (18.8%) | 227 (14.8%) | 597 (15.3%) | 1046 (16.0%) | <0.001 |
| 2 | 2384 (18.7%) | 191 (23.9%) | 276 (18.0%) | 696 (17.8%) | 1221 (18.7%) | |
| 3 | 2523 (19.7%) | 140–144 (17.5–18.0%) | 274–278 (17.8–18.1%) | 807 (20.6%) | 1298 (19.9%) | |
| 4 | 2819 (22.1%) | 153 (19.1%) | 351 (22.9%) | 873 (22.3%) | 1442 (22.1%) | |
| 5 (highest) | 2994 (23.4%) | 160 (20.0%) | 401 (26.1%) | 928 (23.7%) | 1505 (23.0%) | |
| Unknown | 41 (0.3%) | * | * | 13 (0.3%) | 19 (0.3%) | |
| Comorbidity Burden | ||||||
| 0–5 ADGs | 7287 (57.0%) | 788 (98.5%) | 1472 (95.8%) | 1773 (45.3%) | 3254 (49.8%) | <0.001 |
| 6–9 ADGs | 4425 (34.6%) | 10–14 (1.3–1.8%) | 55–59 (3.6–3.8%) | 1661 (42.4%) | 2695 (41.3%) | |
| 10+ ADGs | 1069 (8.4%) | * | * | 480 (12.3%) | 582 (8.9%) | |
| History of Mental Health Visits | ||||||
| Yes | 4127 (32.3%) | 18 (2.3%) | 149 (9.7%) | 1486 (38.0%) | 2474 (37.9%) | <0.001 |
| Cancer Detection Method | ||||||
| Screening | 2916 (22.8%) | 164 (20.5%) | 328 (21.4%) | 776 (19.8%) | 1648 (25.2%) | <0.001 |
| Symptomatic | 9865 (77.2%) | 636 (79.5%) | 1208 (78.6%) | 3138 (80.2%) | 4883 (74.8%) | |
| Stage | ||||||
| Stage I | 2839 (22.2%) | 140 (17.5%) | 328 (21.4%) | 886 (22.6%) | 1485 (22.7%) | 0.017 |
| Stage II | 7311 (57.2%) | 470 (58.8%) | 889 (57.9%) | 2251 (57.5%) | 3701 (56.7%) | |
| Stage III | 2631 (20.6%) | 190 (23.8%) | 319 (20.8%) | 777 (19.9%) | 1345 (20.6%) | |
| Primary Care Practice Model | ||||||
| Straight FFS | 1887 (14.8%) | 301 (37.6%) | 277 (18.0%) | 542 (13.8%) | 767 (11.7%) | <0.001 |
| Enhanced FFS | 6281 (49.1%) | 228 (28.5%) | 553 (36.0%) | 2036 (52.0%) | 3464 (53.0%) | |
| Capitation | 2235 (17.5%) | 110 (13.8%) | 303 (19.7%) | 654 (16.7%) | 1168 (17.9%) | |
| Team-based capitation | 2206 (17.3%) | 123 (15.4%) | 369 (24.0%) | 642 (16.4%) | 1072 (16.4%) | |
| Other | 172 (1.3%) | 38 (4.8%) | 34 (2.2%) | 40 (1.0%) | 60 (0.9%) | |
| Health Region | ||||||
| 1 Erie St. Clair | 713 (5.6%) | 47 (5.9%) | 88 (5.7%) | 221 (5.6%) | 357 (5.5%) | <0.001 |
| 2 South West | 992 (7.8%) | 55 (6.9%) | 145 (9.4%) | 242 (6.2%) | 550 (8.4%) | |
| 3 Waterloo Wellington | 654 (5.1%) | 59 (7.4%) | 125 (8.1%) | 140 (3.6%) | 330 (5.1%) | |
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 101 (12.6%) | 198 (12.9%) | 413 (10.6%) | 756 (11.6%) | |
| 5 Central West | 543 (4.2%) | 25 (3.1%) | 30 (2.0%) | 197 (5.0%) | 291 (4.5%) | |
| 6 Mississauga Halton | 750 (5.9%) | 47 (5.9%) | 67 (4.4%) | 280 (7.2%) | 356 (5.5%) | |
| 7 Toronto Central | 1061 (8.3%) | 65 (8.1%) | 121 (7.9%) | 357 (9.1%) | 518 (7.9%) | |
| 8 Central | 1784 (14.0%) | 72 (9.0%) | 152 (9.9%) | 626 (16.0%) | 934 (14.3%) | |
| 9 Central East | 1710 (13.4%) | 90 (11.3%) | 177 (11.5%) | 495 (12.6%) | 948 (14.5%) | |
| 10 South East | 520 (4.1%) | 49 (6.1%) | 81 (5.3%) | 125 (3.2%) | 265 (4.1%) | |
| 11 Champlain | 1335 (10.4%) | 108 (13.5%) | 183 (11.9%) | 444 (11.3%) | 600 (9.2%) | |
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 12–16 (1.5–2.0%) | 70–74 (4.6–4.8%) | 165–169 (4.2–4.3%) | 266–270 (4.1%) | |
| 13 North East | 478 (3.7%) | 44 (5.5%) | 64 (4.2%) | 129 (3.3%) | 241 (3.7%) | |
| 14 North West | 252 (2.0%) | 24 (3.0%) | 34 (2.2%) | 78 (2.0%) | 116 (1.8%) | |
| Unknown | * | * | * | * | * | |
* denotes too few cases to report. Ranges are provided in associated rows or columns to prevent the reidentification of small cells as per the ICES policy. Note: PCP—primary care provider, UPC—usual provider of care index, ADG—Aggregated Diagnosis Groups, FFS—fee for service.
The median contact-to-chemotherapy interval was 126 days (Figure 1; Table 2). This median interval was 7–12 days longer in those >74 years old and 12–18 days shorter in those <40 years old. The contact-to-chemotherapy interval varied by health region. Women in the Champlain health region had 19–21-day-longer median intervals. Those in the Waterloo Wellington health region had 6–15-day-shorter intervals. Within the screen-detected group, longer intervals were seen in rural areas, with 30-day-longer median intervals in very remote rural neighbourhoods compared to urban neighbourhoods. Within the symptom-detected group, longer intervals were seen in immigrants by 7 days, high comorbidity groups by 12 days and those with a mental health history by 7 days. Among immigrants, the median contact-to-chemotherapy intervals varied by region of origin. Immigrants from the US/New Zealand/Australia or Western Europe had shorter median intervals compared to our full cohort, and immigrants from East Asia/Pacific, Latin America/Caribbean or Sub-Saharan Africa had longer median intervals such that, among symptom-detected immigrants, those from East Asia/Pacific, Latin America/Caribbean and Sub-Saharan Africa experienced 27–30-day-longer median contact-to-chemotherapy intervals than immigrants from Western Europe.
Figure 1.
Boxplots of all intervals in days separated by the method of breast cancer detection. Note: Surgery-to-chemotherapy interval not separated by detection method since breast cancer detection is not relevant during this interval.
Table 2.
Baseline characteristics according to median contact-to-adjuvant-chemotherapy interval (in days) stratified by screened versus symptomatic detection.
| Total n = 12,781 |
Contact-to-Adjuvant-Chemotherapy Interval in Days | ||||||
|---|---|---|---|---|---|---|---|
| Screened n = 2916 (22.8%) | Symptomatic n = 9865 (77.2%) | ||||||
| Median (IQR) | 90th Percentile | p-Value * | Median (IQR) | 90th Percentile | p-Value * | ||
| Total | 125 (103, 154) | 185 | 127 (99, 171) | 228 | |||
| Age (Categorical) | <0.0001 | <0.0001 | |||||
| <40 years | 1102 (8.6%) | 107 (85, 124) | 189 | 115 (90, 155) | 205 | ||
| 40–49 years | 3481 (27.2%) | 115 (93, 147) | 178 | 126 (99, 170) | 228 | ||
| 50–59 years | 4225 (33.1%) | 124 (103, 154) | 187 | 128 (101, 175) | 233 | ||
| 60–69 years | 3045 (23.8%) | 126 (105, 155) | 184 | 132 (103, 176) | 231 | ||
| 70–74 years | 607 (4.7%) | 125 (104, 158) | 185 | 138 (108, 179) | 224 | ||
| >74 years | 321 (2.5%) | 137 (118, 162) | 187 | 134 (104, 175) | 221 | ||
| Urban/Rural Residence | <0.0001 | 0.4999 | |||||
| Urban | 11,189 (87.5%) | 123 (102, 153) | 182 | 127 (99, 170) | 227 | ||
| Rural | 699 (5.5%) | 127 (110, 159) | 189 | 125 (102, 175) | 223 | ||
| Rural—remote | 596 (4.7%) | 134 (110, 164) | 194 | 127 (98, 173) | 225 | ||
| Rural—very remote | 292–297 (2.3%) | 153 (122, 184) | 231 | 132 (104, 182) | 259 | ||
| Rural—unknown | ≤5 | ** | ** | ** | ** | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
| Immigration Status | 0.1425 | 0.0008 | |||||
| Long-term residents | 11,075 (86.7%) | 125 (103, 154) | 184 | 126 (99, 170) | 227 | ||
| Immigrants | 1706 (13.3%) | 129 (104, 161) | 194 | 133 (104, 175) | 231 | ||
| Immigrant Region of Origin | 0.9288 | 0.0085 | |||||
| East Asia and Pacific | 544 (4.3%) | 135 (106, 161) | 191 | 138 (104, 175) | 231 | ||
| Eastern Europe and Central Asia | 286 (2.2%) | 135 (102, 167) | 191 | 127 (100, 173) | 230 | ||
| Latin America and Caribbean | 239 (1.9%) | 129 (116, 154) | 258 | 141 (108, 179) | 241 | ||
| Middle East and North Africa | 145 (1.1%) | 124 (104, 147) | 191 | 134 (108, 181) | 218 | ||
| South Asia | 270 (2.1%) | 126 (98, 160) | 194 | 134 (109, 169) | 217 | ||
| Sub-Saharan Africa | 87 (0.7%) | 137 (103, 155) | 163 | 139 (106, 180) | 225 | ||
| USA/New Zealand/Australia | 37 (0.3%) | 119 (103, 148) | 162 | 119 (100, 178) | 231 | ||
| Western Europe | 98 (0.8%) | 123 (105, 176) | 203 | 111 (94, 144) | 231 | ||
| Neighbourhood Income Quintile | 0.1196 | 0.1620 | |||||
| 1 (lowest) | 2020 (15.8%) | 128 (106, 160) | 188 | 130 (100, 175) | 226 | ||
| 2 | 2384 (18.7%) | 125 (104, 155) | 181 | 128 (100, 170) | 231 | ||
| 3 | 2523 (19.7%) | 125 (104, 155) | 183 | 127 (101, 174) | 225 | ||
| 4 | 2819 (22.1%) | 127 (103, 153) | 186 | 126 (99, 168) | 226 | ||
| 5 (highest) | 2994 (23.4%) | 122 (100, 151) | 184 | 125 (98, 170) | 231 | ||
| Unknown | 41 (0.3%) | 170 (119, 226) | 247 | 143 (102, 182) | 234 | ||
| Comorbidity Burden | 0.7763 | <0.0001 | |||||
| 0–5 ADGs | 7287 (57.0%) | 124 (104, 153) | 183 | 123 (98, 166) | 219 | ||
| 6–9 ADGs | 4425 (34.6%) | 126 (103, 155) | 189 | 133 (103, 178) | 238 | ||
| 10+ ADGs | 1069 (8.4%) | 126 (104, 158) | 182 | 135 (104, 183) | 245 | ||
| History of Mental Health Visits | 0.9609 | <0.0001 | |||||
| Yes | 4127 (32.3%) | 124 (102, 155) | 191 | 132 (103, 176) | 233 | ||
| No | 8654 (67.7%) | 126 (104, 154) | 183 | 125 (98, 169) | 225 | ||
| Stage | 0.0010 | <0.0001 | |||||
| Stage I | 2839 (22.2%) | 128 (105, 158) | 188 | 136 (105, 185) | 242 | ||
| Stage II | 7311 (57.2%) | 125 (103, 154) | 184 | 127 (100, 169) | 225 | ||
| Stage III | 2631 (20.6%) | 119 (100, 146) | 182 | 119 (93, 162) | 219 | ||
| Primary Care Model | 0.0373 | 0.0012 | |||||
| Straight FFS | 1887 (14.8%) | 127 (104, 152) | 182 | 126 (100, 169) | 221 | ||
| Enhanced FFS | 6281 (49.1%) | 127 (104, 159) | 190 | 128 (100, 172) | 230 | ||
| Capitation | 2235 (17.5%) | 121 (102, 153) | 180 | 127 (100, 175) | 233 | ||
| Team-based capitation | 2206 (17.3%) | 121 (101, 149) | 182 | 122 (97, 166) | 228 | ||
| Other | 172 (1.3%) | 126 (108, 157) | 190 | 117 (91, 155) | 203 | ||
| Primary Care Enrolment Status | 0.7247 | 0.6580 | |||||
| Rostered | 10,900 (85.3%) | 125 (103, 155) | 185 | 127 (99, 171) | 230 | ||
| Not rostered | 1881 (14.7%) | 127 (104, 152) | 183 | 127 (100, 169) | 221 | ||
| Health Region | <0.0001 | <0.0001 | |||||
| 1 Erie St. Clair | 713 (5.6%) | 118 (99, 142) | 179 | 120 (92, 157) | 208 | ||
| 2 South West | 992 (7.8%) | 138 (113, 167) | 200 | 133 (103, 172) | 227 | ||
| 3 Waterloo Wellington | 654 (5.1%) | 119 (98, 141) | 167 | 112 (91, 150) | 207 | ||
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 118 (100, 140) | 170 | 116 (96, 155) | 213 | ||
| 5 Central West | 543 (4.2%) | 120 (99, 150) | 182 | 126 (99, 171) | 223 | ||
| 6 Mississauga Halton | 750 (5.9%) | 120 (96, 154) | 196 | 124 (96, 173) | 234 | ||
| 7 Toronto Central | 1061 (8.3%) | 126 (106, 155) | 184 | 134 (105, 185) | 247 | ||
| 8 Central | 1784 (14.0%) | 124 (101, 154) | 188 | 128 (101, 174) | 231 | ||
| 9 Central East | 1710 (13.4%) | 114 (95, 146) | 179 | 127 (98, 171) | 220 | ||
| 10 South East | 520 (4.1%) | 126 (106, 159) | 183 | 120 (99, 157) | 217 | ||
| 11 Champlain | 1335 (10.4%) | 144 (121, 169) | 189 | 148 (120, 189) | 249 | ||
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 126 (103, 162) | 176 | 122 (102, 176) | 237 | ||
| 13 North East | 478 (3.7%) | 118 (98, 147) | 190 | 117 (88, 160) | 216 | ||
| 14 North West | 252 (2.0%) | 143 (108, 161) | 198 | 128 (92, 173) | 231 | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
* p-values calculated for median values. ** values suppressed due to small cells. Note: ADG—Aggregated Diagnosis Groups, FFS—fee for service.
The median primary care interval was 34 days (Figure 1; Appendix B, Table A2). This interval was longer in those with stage I disease by 3–5 days and in the Champlain health region by 10–12 days. In the screen-detected group, the median primary care interval was 13–14 days shorter for those aged <50 years, and 9 days longer for those in rural remote areas. In the symptom-detected group, the median primary care interval was 5–6 days shorter for those aged <40 years or >74 years. The median surgery-to-chemotherapy interval was 58 days (Figure 1; Appendix C, Table A3). This interval was longer in those aged >74 years old by 7 days, those living very remotely rural by 8 days and those in the Champlain health region by 7 days.
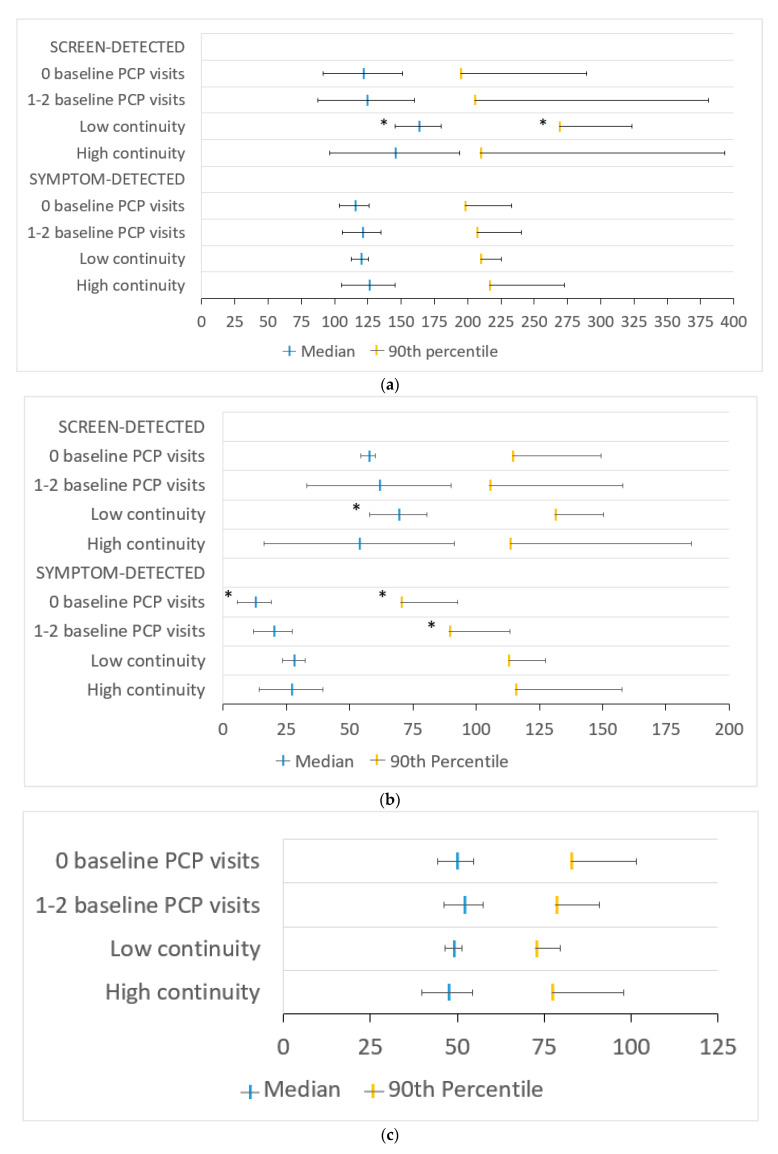
In our multivariable model, symptom-detected patients with low versus high PCP continuity had a shorter median contact-to-chemotherapy interval by 3.21 days (95% CI 0.47–5.96). Symptom-detected patients with no baseline PCP visits versus high continuity had shorter median and 90th percentile intervals by 10.68 (95% CI 5.36–16.00) and 25.38 days (95% CI 11.09–39.67), respectively. Neither PCP continuity nor having few baseline PCP visits was associated with a change in the contact-to-chemotherapy interval among screen-detected patients (Figure 2a). Symptom-detected patients with no baseline PCP visits versus high PCP continuity had shorter median and 90th percentile primary care intervals by 8.04 (95% CI 5.52–10.55) and 28.14 days (95% CI 16.60–39.68), respectively. PCP continuity was not associated with a change in the primary care interval (Figure 2b). Neither PCP continuity nor having few baseline PCP visits was associated with a change in the surgery-to-chemotherapy interval (Figure 2c).
Figure 2.
Adjusted median and 90th percentile (a) contact-to-chemotherapy, (b) primary care and (c) surgery-to-chemotherapy intervals in days by continuity of primary care at baseline separated by method of breast cancer detection, where applicable, with 95% confidence intervals in the entire cohort. PCP—primary care provider; low continuity—usual provider of care (UPC) index ≤ 0.75; high continuity—UPC index > 0.75. * indicates statistical significance.
In screen-detected immigrants, low versus high PCP continuity was associated with longer median and 90th percentile contact-to-chemotherapy intervals by 17.43 (95% CI 0.90–34.76) and 59.37 days (95% CI 4.06–114.67), respectively (Figure 3a). The longer median interval in screen-detected immigrants was mostly accounted for by the longer median primary care sub-interval by 15.45 days (95% CI 4.00–26.90), whereas the 90th percentile primary care interval was not significantly longer in this group (17.64 days, 95% CI −1.72–37.00). In symptom-detected immigrants, having no baseline PCP visits versus high continuity was associated with shorter median and 90th percentile primary care intervals by 14.52 (95% CI 7.79–21.25) and 45.25 days (95% CI 22.49–68.01), respectively (Figure 3b). Similar to the whole cohort, there was no association between PCP continuity or having a low number of baseline PCP visits and the surgery-to-chemotherapy interval among immigrants (Figure 3c).
Figure 3.
Adjusted median and 90th percentile (a) contact-to-chemotherapy, (b) primary care and (c) surgery-to–chemotherapy intervals in days by continuity of primary care at baseline separated by method of breast cancer detection, where applicable, with 95% confidence intervals (CIs) in the immigrant-only population. PCP—primary care provider; low continuity—usual provider of care (UPC) index ≤ 0.75; high continuity—UPC index > 0.75. * indicates statistical significance. Only the upper portion of the 95% CI is shown for 90th percentile intervals for clarity.
4. Discussion
In this population-based study of Ontario breast cancer patients that were diagnosed in 2007–2011, the median contact-to-chemotherapy, primary care and surgery-to-chemotherapy intervals were 126, 34 and 58 days, respectively. Other studies looked at different sub-intervals, making comparisons between studies difficult. The ICBP compared wait times for colorectal and lung cancer across several jurisdictions, including Ontario [51,52], but has not yet published findings for breast cancer wait times. Similar to other studies, we found in our unadjusted analyses that the time to breast cancer treatment was longer with increasing age [12,18], in certain Ontario health regions [23], in rural areas (for our screen-detected group) [15,18] and in those with higher comorbidity (for our symptom-detected group) [16]. Additionally, a history of mental health visits was associated with a longer time to treatment in symptom-detected patients. It may be that patients with higher age, comorbidity and/or mental health history require more preparation, counselling and/or stabilisation prior to breast cancer chemotherapy. Furthermore, in Ontario, women that were screened under 50 years of age are either considered high-risk according to the Ontario Breast Screening Program (OBSP) or are specifically referred for screening by their PCP [53], which may be associated with higher vigilance surrounding timely treatment in these younger women. Longer wait times in rural areas after positive screening may be due to the longer delays in organising follow-up tests (e.g., ultrasound) [54]. Within the immigrant population, inequities were noted based on the country of origin. Symptom-detected immigrant women from East Asia/Pacific, Latin America/Caribbean and Sub-Saharan Africa had 27–30-day-longer median contact-to-chemotherapy intervals than those from Western Europe. This is similar to another CanIMPACT study that looked at the time to diagnosis, where immigrant women in Ontario who were born in USA/New Zealand/Australia or Western Europe had the shortest adjusted time to breast cancer diagnosis and the longest adjusted time to diagnosis if they were born in East Asia/Pacific or Latin America/Caribbean [25]. Many women emigrating from East Asia/Pacific, Latin America/Caribbean and Sub-Saharan Africa would be considered racialised in Canada compared to those from Western Europe, and several international studies have found that minority race is associated with longer wait times to cancer treatment [12,13,14,19,55].
In our study, PCP continuity was not associated with the contact-to-chemotherapy interval, except in a few specific subsets of our population: in symptom-detected patients, low versus high continuity was associated with a 3-day-shorter median contact-to-chemotherapy interval, and in screen-detected immigrants, half of those with low PCP continuity waited at least 2.5 weeks longer, and 10% waited approximately 2 months longer to receive chemotherapy compared to immigrants with high PCP continuity. It is unclear why PCP continuity does not appear to play a huge role in the contact-to-chemotherapy interval length, acknowledging that a 3-day-longer median contact-to-chemotherapy interval in symptom-detected patients is not a large difference and may not be clinically meaningful. The median primary care sub-interval, i.e., the wait time from first contact with the healthcare system to the date of breast cancer consultation where primary care is thought to be mostly involved, made up only a quarter of the median contact-to-chemotherapy interval. As such, the contact-to-chemotherapy interval may be more influenced by factors outside of the realm or control of primary care. However, even the primary care interval was not associated with PCP continuity in our study. It may be that other elements of continuity of care, such as informational continuity between healthcare providers, are more important than relational continuity in determining wait times along the breast cancer care pathway. It is notable, therefore, that low relational PCP continuity was associated with such a large increase in the contact-to-chemotherapy interval among screen-detected immigrants specifically, with most of the longer interval being due to an increased primary care interval. This suggests that PCP–patient relational continuity plays an important role in influencing wait times to breast cancer consultation in immigrants with abnormal screen results. While work was done to identify wait time disparities [25,56] and barriers to cancer screening in immigrants [57,58], little has been done to explore how the handling of abnormal screening results might vary within the immigrant population. High relational PCP continuity might result in stronger patient–PCP relationships [59], allowing PCPs to provide more efficient care coordination and navigation through potentially unfamiliar healthcare institutions after abnormal screening results. Therefore, high relational PCP continuity may be particularly important for reducing wait times within this vulnerable population.
In symptom-detected patients, the contact-to-chemotherapy interval was more associated with the number of baseline PCP visits than with PCP continuity. Those with no baseline visits had shorter median and 90th percentile contact-to-chemotherapy intervals by 11 and 25 days, respectively, which was mostly due to a shorter primary care interval in this group. Patients with no baseline PCP visits may be more likely to present with later-stage disease and more alarming symptoms, which might prompt earlier referral and consultation with oncology. While this might lead to a shorter time to chemotherapy [60], this could also lead to worse outcomes [61]. This is supported by our data since those with no baseline PCP visits were more likely to be diagnosed at a later stage (stage II/III versus stage I) in our unadjusted analyses. It is also possible that PCPs are more prompted to initiate timely investigations and/or referrals for patients who they do not see often for the treatment of other conditions [35]. Those with no baseline PCP visits were more likely to live in remote rural locations or be in the lowest two income quintiles, which suggested that these groups may have a more difficult time accessing primary care.
These results lay the groundwork for future research and areas for practice and policy improvement. We showed that PCP continuity and the number of baseline PCP visits impact the contact-to-chemotherapy interval in certain populations. Future research from our team will look at the impact of these interval lengths on survival outcomes. Other future studies could examine the data record availability, completion of referral documents and/or follow-up of previously identified problems as a way to study the association between informational continuity and wait times to chemotherapy. Further work, including qualitative research with patients and providers, can explore why PCP continuity generally was not associated with the contact-to-chemotherapy interval, why immigrants with low continuity and abnormal screening experienced longer wait times to breast cancer consultation and why the longest intervals were seen for immigrants from Latin America/Caribbean, East Asia/Pacific and Sub-Saharan Africa. Other work can also look into why disparities in interval lengths were seen across the different health regions, and whether there are any specific interventions to address these disparities. It is possible that access to dedicated Breast Assessment Centres, which was shown to reduce wait times to diagnosis and sometimes treatment [21,22,62], may vary by region, and that expanding these programs to be more widely available may help to address the disparities seen across health regions. Having more structured, clear-cut referral criteria in these Breast Assessment Centres may also provide more equitable and timely care across other groups that were shown to have disparate interval lengths (e.g., age or comorbidity groups). Additionally, the median surgery-to-chemotherapy interval in our cohort was just over 8 weeks. Surgery-to-chemotherapy intervals >4 weeks were associated with higher mortality [4,5]. Therefore, shortening this interval may be an important target for breast cancer specialists and policymakers in Ontario. Policymakers should also make efforts to ensure that everyone in the population, particularly immigrants and other people who may experience challenges navigating the system, has a regular PCP.
Our results should be interpreted in light of certain limitations. Most notably, we were unable to examine the patient interval (from first symptoms to first healthcare presentation). PCP continuity may have a large part to play in decreasing the patient interval. Unfortunately, it is not possible to capture this interval using health administrative data. Second, since we used health administrative data in our study, the information on some variables, such as primary language, race and marital status, were not available. Third, we did not capture ED involvement in our study. While the number of breast cancer patients first presenting to the ED is small (~3–4%) [34,63], ED presentation may mediate the relationship between baseline PCP continuity/number of PCP visits and the contact-to-chemotherapy interval. Fourth, we did not explore the route to screening (through the OBSP versus through the PCP) or whether this impacted the contact-to-chemotherapy interval. Fifth, the CanIMPACT cohort that was used in this study included patients that were diagnosed in 2007–2011. While breast cancer treatment principles have not changed greatly since 2011 [64], and there has not been any major primary care reform in Ontario since that time [65], we need to consider that the effect of PCP continuity on the contact-to-chemotherapy interval may have changed from when these patients were treated.
5. Conclusions
We found that baseline PCP continuity was not associated with the contact-to-chemotherapy interval in the Ontario breast cancer population except in specific groups: we found that high baseline PCP continuity was associated with shorter wait times to breast cancer consultation and receiving adjuvant chemotherapy in screen-detected immigrants, and marginally increased wait times to chemotherapy in symptom-detected patients. Additionally, having no baseline PCP visits was associated with increased wait times to breast cancer consultation and receiving adjuvant chemotherapy. This highlights the importance of having access to PCPs and ensuring that immigrants and others who may have difficulty navigating the healthcare system have high PCP continuity.
Acknowledgments
Parts of this material are based on data and information provided by Ontario Health (Cancer Care Ontario (CCO)). The opinions, results, views and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred. Parts of this material are based on data and information that were compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the authors and not necessarily those of CIHI. All inferences, opinions and conclusions drawn in this paper are those of the authors and do not reflect the opinions or policies of the data stewards. The authors would like to acknowledge the work of Patti Groome and the quantitative team of CanIMPACT who created the initial cohort and whose work helped to shape the methods used in this study, as well as Marlo Whitehead, an ICES analyst, for her efficiency and expertise in managing the ICES datasets.
Appendix A
Table A1.
Data sources that were used to obtain the data elements for variable creation.
| Data Source | Data Elements |
|---|---|
| Ontario Cancer Registry (OCR) | Date of breast cancer diagnosis, age at diagnosis, sex, other cancer diagnoses, cancer stage |
| Registered Persons Database (RPDB) | Postal code at time of diagnosis, health region |
| 2006 Statistics Canada Census and Postal Code Conversion File Plus, version 5C | Rurality, neighborhood income quintile |
| Immigration Refugee and Citizenship Canada permanent resident (IRCC-PR) database | Immigration status |
| Ontario Health Insurance Plan (OHIP) | Number of PCP visits (billed encounters) total and per provider, reasons for visits, diagnostic codes, chemotherapy receipt, start of adjuvant chemotherapy |
| ICES Physician Database | Physician specialty |
| Client Agency Program Enrolment database (CAPE) and Corporate Provider Database | Primary care enrolment model |
| Canadian Institute for Health Information: Discharge Abstract Database (DAD) and Same-Day Surgery (SDS) database | Diagnosis codes, surgery receipt |
| Cancer Activity Level Reporting (ALR) database | Date of radiotherapy receipt |
Appendix B
Table A2.
Baseline characteristics according to the median and 90th percentile primary care intervals in days stratified by the method of detection.
| Total n = 12,781 |
Primary Care Interval in Days (from First Contact to First Oncology Visit) | ||||||
|---|---|---|---|---|---|---|---|
| Screened n = 2916 (22.8%) | Symptomatic n = 9865 (77.2%) | ||||||
| Median (IQR) | 90th Percentile | Kruskal–Wallis p-value * | Median (IQR) | 90th Percentile | Kruskal–Wallis p-value * | ||
| Total | 34 (21, 50) | 72 | 34 (17, 62) | 111 | |||
| Age (Categorical) | <0.0001 | <0.0001 | |||||
| <40 years | 1102 (8.6%) | 20 (18, 34) | 51 | 29 (14, 56) | 101 | ||
| 40–49 years | 3481 (27.2%) | 21 (9, 42) | 85 | 35 (19, 64) | 117 | ||
| 50–59 years | 4225 (33.1%) | 34 (21, 51) | 74 | 35 (16, 64) | 113 | ||
| 60–69 years | 3045 (23.8%) | 35 (22, 50) | 70 | 34 (16, 63) | 108 | ||
| 70–74 years | 607 (4.7%) | 35 (20, 50) | 67 | 34 (17, 59) | 99 | ||
| >74 years | 321 (2.5%) | 37 (21, 60) | 79 | 28 (14, 55) | 83 | ||
| Urban/Rural Residence | <0.0001 | 0.0078 | |||||
| Urban | 11,189 (87.5%) | 34 (20, 49) | 70 | 34 (17, 63) | 110 | ||
| Rural | 699 (5.5%) | 36 (21, 50) | 74 | 35 (17, 62) | 113 | ||
| Rural—remote | 596 (4.7%) | 41 (25, 60) | 84 | 32 (15, 56) | 106 | ||
| Rural—very remote | 292–297 (2.3%) | 43 (26, 70) | 91 | 28 (12, 58) | 113 | ||
| Rural—unknown | ≤5 | ** | ** | ** | ** | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
| Immigration Status | 0.4322 | 0.9899 | |||||
| Long-term residents | 11,075 (86.7%) | 34 (21, 50) | 71 | 34 (17, 62) | 111 | ||
| Immigrants | 1706 (13.3%) | 33 (17, 54) | 76 | 34 (17, 63) | 109 | ||
| Immigrant Region of Origin | 0.2853 | 0.0783 | |||||
| East Asia and Pacific | 544 (4.3%) | 33 (17, 62) | 94 | 33 (15, 63) | 112 | ||
| Eastern Europe and Central Asia | 286 (2.2%) | 27 (9, 51) | 56 | 33 (16, 62) | 112 | ||
| Latin America and Caribbean | 239 (1.9%) | 36 (21, 53) | 84 | 41 (19, 72) | 107 | ||
| Middle East and North Africa | 145 (1.1%) | 41 (16, 51) | 62 | 42 (19, 75) | 121 | ||
| South Asia | 270 (2.1%) | 30 (15, 44) | 78 | 28 (15, 53) | 95 | ||
| Sub-Saharan Africa | 87 (0.7%) | 41 (23, 55) | 69 | 37 (18, 69) | 90 | ||
| USA/New Zealand/Australia | 37 (0.3%) | 36 (28, 76) | 105 | 36 (15, 60) | 117 | ||
| Western Europe | 98 (0.8%) | 38 (24, 67) | 113 | 29 (14, 49) | 87 | ||
| Neighbourhood Income Quintile | 0.7635 | 0.7172 | |||||
| 1 (lowest) | 2020 (15.8%) | 35 (21, 54) | 75 | 34 (17, 62) | 105 | ||
| 2 | 2384 (18.7%) | 34 (21, 52) | 71 | 34 (17, 63) | 106 | ||
| 3 | 2523 (19.7%) | 35 (21, 51) | 72 | 34 (17, 63) | 108 | ||
| 4 | 2819 (22.1%) | 35 (21, 49) | 70 | 34 (16, 59) | 107 | ||
| 5 (highest) | 2994 (23.4%) | 34 (21, 49) | 70 | 34 (17, 64) | 122 | ||
| Unknown | 41 (0.3%) | 42 (25, 89) | 102 | 42 (25, 63) | 112 | ||
| Comorbidity Burden | 0.9419 | <0.0001 | |||||
| 0–5 ADGs | 7287 (57.0%) | 35 (21, 50) | 71 | 33 (16, 59) | 105 | ||
| 6–9 ADGs | 4425 (34.6%) | 34 (21, 51) | 73 | 36 (18, 66) | 115 | ||
| 10+ ADGs | 1069 (8.4%) | 35 (21, 53) | 71 | 34 (17, 63) | 120 | ||
| History of Mental Health Visits | 0.6662 | 0.0007 | |||||
| Yes | 4127 (32.3%) | 34 (21, 51) | 72 | 35 (18, 65) | 115 | ||
| No | 8654 (67.7%) | 35 (21, 50) | 72 | 33 (16, 61) | 108 | ||
| Stage | <0.0001 | <0.0001 | |||||
| Stage I | 2839 (22.2%) | 37 (23, 54) | 76 | 39 (21, 71) | 122 | ||
| Stage II | 7311 (57.2%) | 33 (20, 49) | 71 | 34 (17, 61) | 106 | ||
| Stage III | 2631 (20.6%) | 32 (19, 48) | 69 | 29 (14, 56) | 107 | ||
| Primary Care Practice Model | 0.5489 | 0.0078 | |||||
| Straight FFS | 1887 (14.8%) | 36 (20, 52) | 71 | 32 (15, 62) | 103 | ||
| Enhanced FFS | 6281 (49.1%) | 35 (21, 51) | 71 | 35 (17, 62) | 108 | ||
| Capitation | 2235 (17.5%) | 35 (21, 50) | 73 | 35 (17, 68) | 119 | ||
| Team-based capitation | 2206 (17.3%) | 33 (20, 50) | 72 | 33 (17, 61) | 115 | ||
| Other | 172 (1.3%) | 35 (25, 48) | 63 | 27 (12, 49) | 77 | ||
| Primary Care Enrolment Status | 0.4377 | 0.0256 | |||||
| Rostered | 10,900 (85.3%) | 34 (21, 50) | 72 | 34 (17, 62) | 112 | ||
| Not rostered | 1881 (14.7%) | 36 (20, 52) | 71 | 32 (15, 62) | 103 | ||
| Health Region | <0.0001 | <0.0001 | |||||
| 1 Erie St. Clair | 713 (5.6%) | 35 (21, 49) | 71 | 40 (21, 68) | 116 | ||
| 2 South West | 992 (7.8%) | 44 (27, 67) | 89 | 40 (20, 69) | 119 | ||
| 3 Waterloo Wellington | 654 (5.1%) | 33 (20, 44) | 57 | 27 (14, 51) | 105 | ||
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 29 (15, 43) | 57 | 32 (16, 55) | 99 | ||
| 5 Central West | 543 (4.2%) | 39 (25, 50) | 63 | 33 (17, 52) | 90 | ||
| 6 Mississauga Halton | 750 (5.9%) | 31 (17, 49) | 78 | 35 (16, 65) | 116 | ||
| 7 Toronto Central | 1061 (8.3%) | 34 (19, 55) | 76 | 34 (16, 67) | 120 | ||
| 8 Central | 1784 (14.0%) | 29 (17, 47) | 69 | 31 (15, 61) | 114 | ||
| 9 Central East | 1710 (13.4%) | 29 (18, 41) | 52 | 32 (15, 58) | 104 | ||
| 10 South East | 520 (4.1%) | 40 (25, 61) | 80 | 31 (17, 56) | 103 | ||
| 11 Champlain | 1335 (10.4%) | 44 (30, 57) | 70 | 46 (28, 74) | 127 | ||
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 27 (17, 43) | 68 | 27 (15, 58) | 110 | ||
| 13 North East | 478 (3.7%) | 32 (21, 43) | 63 | 27 (12, 53) | 91 | ||
| 14 North West | 252 (2.0%) | 56 (37, 77) | 95 | 35 (14, 63) | 107 | ||
| Unknown | ≤5 | ** | ** | ** | ** | ||
* p-values calculated for median values. ** values suppressed due to small cells. Note: ADG—Aggregated Diagnosis Groups, FFS—fee for service.
Appendix C
Table A3.
Baseline characteristics according to median surgery-to-chemotherapy interval in days.
| Total n = 12,781 |
Surgery-to-Adjuvant-Chemotherapy Interval in Days | |||
|---|---|---|---|---|
| Median (IQR) | 90th percentile | Kruskal–Wallis p-value * | ||
| Total | 58 (46, 74) | 93 | ||
| Age (Categorical) | <0.0001 | |||
| <40 years | 1102 (8.6%) | 52 (41, 68) | 87 | |
| 40–49 years | 3481 (27.2%) | 56 (44, 71) | 91 | |
| 50–59 years | 4225 (33.1%) | 58 (46, 74) | 93 | |
| 60–69 years | 3045 (23.8%) | 60 (48, 76) | 96 | |
| 70–74 years | 607 (4.7%) | 62 (49, 81) | 98 | |
| >74 years | 321 (2.5%) | 65 (49, 81) | 105 | |
| Urban/Rural Residence | <0.0001 | |||
| Urban | 11,189 (87.5%) | 57 (45, 73) | 92 | |
| Rural | 699 (5.5%) | 62 (48, 77) | 94 | |
| Rural—remote | 596 (4.7%) | 63 (50, 79) | 98 | |
| Rural—very remote | 292–297 (2.3%) | 66 (49, 86) | 110 | |
| Rural—unknown | ≤5 | ** | ** | |
| Unknown | ≤5 | ** | ** | |
| Immigration Status | 0.2876 | |||
| Long-term residents | 11,075 (86.7%) | 58 (46, 74) | 93 | |
| Immigrants | 1706 (13.3%) | 57 (44, 75) | 96 | |
| Immigrant Region of Origin | 0.1119 | |||
| East Asia and Pacific | 544 (4.3%) | 57 (43, 77) | 98 | |
| Eastern Europe and Central Asia | 286 (2.2%) | 56 (45, 70) | 88 | |
| Latin America and Caribbean | 239 (1.9%) | 59 (46, 77) | 107 | |
| Middle East and North Africa | 145 (1.1%) | 59 (43, 76) | 91 | |
| South Asia | 270 (2.1%) | 60 (46, 78) | 102 | |
| Sub-Saharan Africa | 87 (0.7%) | 53 (43, 70) | 99 | |
| USA/New Zealand/Australia | 37 (0.3%) | 52 (38, 72) | 91 | |
| Western Europe | 98 (0.8%) | 55 (42, 67) | 87 | |
| Neighbourhood Income Quintile | 0.0456 | |||
| 1 (lowest) | 2020 (15.8%) | 57 (45, 75) | 93 | |
| 2 | 2384 (18.7%) | 58 (46, 75) | 94 | |
| 3 | 2523 (19.7%) | 58 (46, 76) | 96 | |
| 4 | 2819 (22.1%) | 58 (45, 73) | 92 | |
| 5 (highest) | 2994 (23.4%) | 57 (45, 72) | 91 | |
| Unknown | 41 (0.3%) | 62 (48, 85) | 104 | |
| Comorbidity Burden | 0.0561 | |||
| 0–5 ADGs | 7287 (57.0%) | 57 (46, 73) | 92 | |
| 6–9 ADGs | 4425 (34.6%) | 58 (46, 74) | 94 | |
| 10+ ADGs | 1069 (8.4%) | 59 (45, 78) | 99 | |
| History of Mental Health Visits | 0.0595 | |||
| Yes | 4127 (32.3%) | 58 (46, 75) | 94 | |
| No | 8654 (67.7%) | 57 (45, 74) | 92 | |
| Stage | <0.0001 | |||
| Stage I | 2839 (22.2%) | 60 (48, 76) | 98 | |
| Stage II | 7311 (57.2%) | 58 (47, 75) | 93 | |
| Stage III | 2631 (20.6%) | 53 (41, 69) | 87 | |
| Primary Care Practice Model | 0.1546 | |||
| Straight FFS | 1887 (14.8%) | 57 (45, 74) | 96 | |
| Enhanced FFS | 6281 (49.1%) | 58 (45, 74) | 94 | |
| Capitation | 2235 (17.5%) | 57 (45, 73) | 92 | |
| Team-based capitation | 2206 (17.3%) | 59 (47, 73) | 91 | |
| Other | 172 (1.3%) | 56 (42, 75) | 97 | |
| Primary Care Enrolment Status | 0.5820 | |||
| Rostered | 10,900 (85.3%) | 58 (46, 74) | 93 | |
| Not rostered | 1881 (14.7%) | 57 (45, 74) | 96 | |
| Health Region | <0.0001 | |||
| 1 Erie St. Clair | 713 (5.6%) | 50 (40, 69) | 86 | |
| 2 South West | 992 (7.8%) | 63 (52, 79) | 98 | |
| 3 Waterloo Wellington | 654 (5.1%) | 52 (41, 68) | 83 | |
| 4 Hamilton Niagara Haldimand Brant | 1468 (11.5%) | 56 (45, 70) | 86 | |
| 5 Central West | 543 (4.2%) | 58 (44, 74) | 98 | |
| 6 Mississauga Halton | 750 (5.9%) | 55 (43, 71) | 92 | |
| 7 Toronto Central | 1061 (8.3%) | 57 (45, 71) | 96 | |
| 8 Central | 1784 (14.0%) | 56 (43, 73) | 93 | |
| 9 Central East | 1710 (13.4%) | 57 (45, 74) | 94 | |
| 10 South East | 520 (4.1%) | 59 (48, 73) | 91 | |
| 11 Champlain | 1335 (10.4%) | 65 (53, 79) | 96 | |
| 12 North Simcoe Muskoka | 518–522 (4.1%) | 63 (51, 78) | 93 | |
| 13 North East | 478 (3.7%) | 61 (45, 77) | 107 | |
| 14 North West | 252 (2.0%) | 52 (36, 72) | 97 | |
| Unknown | ≤5 | ** | ** | |
* p-values calculated for median values. ** values suppressed due to small cells. Note: ADG—Aggregated Diagnosis Groups, FFS—fee for service.
Author Contributions
Conceptualisation, R.L.W., A.L., R.M., M.K. and E.G.; methodology, R.L.W., A.L., R.M. and E.G.; software, R.L.W. and R.M.; validation, R.L.W.; formal analysis, R.L.W. and R.M.; investigation, R.L.W.; resources, E.G.; data curation, R.L.W.; writing—original draft preparation, R.L.W.; writing—review and editing, R.L.W., A.L., R.M., M.K. and E.G.; visualisation, R.L.W.; supervision, A.L., R.M., M.K. and E.G.; project administration, R.L.W., A.L. and E.G.; funding acquisition, E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Canadian Institutes of Health Research (CIHR; grant 128272). This study is supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The opinions, results and conclusions reported in this paper are those of the authors and are independent of those held by the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of The University of Toronto (protocol code 37074, approved on 26 March 2019).
Informed Consent Statement
Patient consent was waived since only secondary data sources that were housed at the ICES were used. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyse healthcare and demographic data without consent for health system evaluation and improvement.
Data Availability Statement
The dataset from this study is held securely in a coded form at ICES. While data-sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (accessed on 19 December 2019). The full dataset creation plan is available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. [(accessed on 24 June 2021)]. Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/canadian-cancer-statistics.html.
- 2.Statistics Canada Table 2: Count and Percentage of Surgically Treated Female Breast Tumours, by American Joint Committee on Cancer Stage at Diagnosis, by Province and Territory, Canada (except Quebec), 2010 to 2012 Combined. [(accessed on 27 July 2021)];2018 Available online: https://www150.statcan.gc.ca/n1/pub/82-003-x/2018008/article/00001/tbl/tbl02-eng.htm.
- 3.Powis M., Groome P., Biswanger N., Kendell C., Decker K.M., Grunfeld E., McBride M.L., Urquhart R., Winget M., Porter G.A., et al. Cross-Canada Differences in Early-Stage Breast Cancer Treatment and Acute-Care Use. Curr. Oncol. 2019;26:624–639. doi: 10.3747/co.26.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raphael M.J., Biagi J.J., Kong W., Mates M., Booth C.M., MacKillop W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2016;160:17–28. doi: 10.1007/s10549-016-3960-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhan Q.-H., Fu J.-Q., Fu F.-M., Zhang J., Wang C. Survival and time to initiation of adjuvant chemotherapy among breast cancer patients: A systematic review and meta-analysis. Oncotarget. 2017;9:2739–2751. doi: 10.18632/oncotarget.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tørring M.L., Falborg A.Z., Jensen H., Neal R.D., Weller D., Reguilon I., Menon U., Vedsted P., Almberg S.S., Anandan C., et al. Advanced-stage cancer and time to diagnosis: An International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur. J. Cancer Care. 2019;28:e13100. doi: 10.1111/ecc.13100. [DOI] [PubMed] [Google Scholar]
- 7.Tørring M.L., Frydenberg M., Hansen R.P., Olesen F., Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care. Eur. J. Cancer. 2013;49:2187–2198. doi: 10.1016/j.ejca.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Neal R.D., Tharmanathan P., France B., Din N., Cotton S., Fallon-Ferguson J., Hamilton W., Hendry A., Hendry M., Lewis R., et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer. 2015;112((Suppl. 1)):S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermiah E., Abdalla F., Buhmeida A., Larbesh E., Pyrhönen S., Collan Y. Diagnosis delay in Libyan female breast cancer. BMC Res. Notes. 2012;5:452. doi: 10.1186/1756-0500-5-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo Q., Cai C., Zhang Y., Kong X., Jiang L., Ma T., Zhang N., Yang Q. Delay in Diagnosis and Treatment of Symptomatic Breast Cancer in China. Ann. Surg. Oncol. 2015;22:883–888. doi: 10.1245/s10434-014-4076-9. [DOI] [PubMed] [Google Scholar]
- 11.Richards M.A., Westcombe A.M., Love S.B., Littlejohns P., Ramirez A.J. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/S0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 12.Chavez-MacGregor M., Clarke C.A., Lichtensztajn D.Y., Giordano S.H. Delayed Initiation of Adjuvant Chemotherapy Among Patients with Breast Cancer. JAMA Oncol. 2016;2:322–329. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujar M., Dahlui M., Yip C.H., Taib N.A. Delays in time to primary treatment after a diagnosis of breast cancer: Does it impact survival? Prev. Med. 2013;56:222–224. doi: 10.1016/j.ypmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Cabral A.L.L.V., Giatti L., Casale C., Cherchiglia M.L. Social vulnerability and breast cancer: Differentials in the interval between diagnosis and treatment of women with different sociodemographic profiles. Cienc. Saude Coletiva. 2019;24:613–622. doi: 10.1590/1413-81232018242.31672016. [DOI] [PubMed] [Google Scholar]
- 15.Saint-Jacques N., Younis T., Dewar R., Rayson D. Wait times for breast cancer care. Br. J. Cancer. 2007;96:162–168. doi: 10.1038/sj.bjc.6603523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khorana A.A., Tullio K., Elson P., Pennell N.A., Grobmyer S.R., Kalady M.F., Raymond D., Abraham J., Klein E.A., Walsh R.M., et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS ONE. 2019;14:e0213209. doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez A.J., Westcombe A.M., Burgess C.C., Sutton S., Littlejohns P., Richards M.A. Factors predicting delayed presentation of symptomatic breast cancer: A systematic review. Lancet. 1999;353:1127–1131. doi: 10.1016/S0140-6736(99)02142-X. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Zhou Y., Mao F., Guan J., Wang X., Zhang Y., Zhang X., Shen S., Sun Q. The influence on survival of delay in the treatment initiation of screening detected non-symptomatic breast cancer. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-019-46736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X., Ye F., Zhao B., Tang H., Wang J., Xiao X., Xie X. Risk factors for delay of adjuvant chemotherapy in non-metastatic breast cancer patients: A systematic review and meta-analysis involving 186982 patients. PLoS ONE. 2017;12:e0173862. doi: 10.1371/journal.pone.0173862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losk K., Freedman R.A., Lin N.U., Golshan M., Pochebit S.M., Lester S.C., Natsuhara K., Camuso K., King T.A., Bunnell C.A., et al. Implementation of Surgeon-Initiated Gene Expression Profile Testing (Onco type DX) Among Patients with Early-Stage Breast Cancer to Reduce Delays in Chemotherapy Initiation. J. Oncol. Pract. 2017;13:e815–e820. doi: 10.1200/JOP.2017.023788. [DOI] [PubMed] [Google Scholar]
- 21.Blackmore K.M., Weerasinghe A., Holloway C.M.B., Majpruz V., Mirea L., O’Malley F.P., Harris C.P., Hendry A., Hey A., Kornecki A., et al. Comparison of wait times across the breast cancer treatment pathway among screened women undergoing organized breast assessment versus usual care. Can. J. Public Health. 2019;110:595–605. doi: 10.17269/s41997-019-00210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKevitt E.C., Dingee C.K., Leung S.-P., Brown C.J., Van Laeken N.Y., Lee R., Kuusk U. Reduced Time to Breast Cancer Diagnosis with Coordination of Radiological and Clinical Care. Cureus. 2017;9:e1919. doi: 10.7759/cureus.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotogea A., Chiarelli A.M., Mirea L., Prummel M.V., Chong N., Shumak R.S., O’Malley F.P., Holloway C.M.B., the Breast Screening Study Group Factors associated with wait times across the breast cancer treatment pathway in Ontario. SpringerPlus. 2013;2:388. doi: 10.1186/2193-1801-2-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statistics Canada Immigration and Ethnocultural Diversity in Canada. [(accessed on 12 July 2019)];2018 Available online: https://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm.
- 25.Lofters A.K., CanIMPACT for the CanIMPACT Team. McBride M.L., Li D., Whitehead M., Moineddin R., Jiang L., Grunfeld E., Groome P.A. Disparities in breast cancer diagnosis for immigrant women in Ontario and BC: Results from the CanIMPACT study. BMC Cancer. 2019;19:42. doi: 10.1186/s12885-018-5201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal J., Ginsburg O., Fischer H.D., Austin P.C., Creatore M.I., Narod S.A., Rochon P.A. A Population-Based Cross-Sectional Study Comparing Breast Cancer Stage at Diagnosis between Immigrant and Canadian-Born Women in Ontario. Breast J. 2017;23:525–536. doi: 10.1111/tbj.12785. [DOI] [PubMed] [Google Scholar]
- 27.Del Giudice L., Bondy S.J., Chen Z., Maaten S. Physician Care of Cancer Patients. In: Jaakkimainen L., Upshur R.E.G., Klein-Geltink J.E., Leong A., Maaten S., Schultz S.E., editors. Primary Care in Ontario: ICES Atlas. The Institute for Clinical Evaluative Sciences (ICES); Toronto, ON, Canada: 2006. pp. 161–174. [Google Scholar]
- 28.Roetzheim R.G., Ferrante J.M., Lee J.-H., Chen R., Love-Jackson K.M., Gonzalez E.C., Fisher K.J., McCarthy E.P. Influence of Primary Care on Breast Cancer Outcomes Among Medicare Beneficiaries. Ann. Fam. Med. 2012;10:401–411. doi: 10.1370/afm.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrante J.M., Lee J.H., McCarthy E.P., Fisher K.J., Chen R., Gonzalez E.C., Love-Jackson K., Roetzheim R.G. Primary care utilization and colorectal cancer incidence and mortality among Medicare beneficiaries: A population-based, case-control study. Ann. Intern. Med. 2013;159:437–446. doi: 10.7326/0003-4819-159-7-201310010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones L.E., Doebbeling C.C. Beyond the Traditional Prognostic Indicators: The Impact of Primary Care Utilization on Cancer Survival. J. Clin. Oncol. 2007;25:5793–5799. doi: 10.1200/JCO.2007.13.6127. [DOI] [PubMed] [Google Scholar]
- 31.Arnold L.D., McGilvray M.M.O., Cooper J.K., James A.S. Inadequate Cancer Screening: Lack of Provider Continuity is a Greater Obstacle than Medical Mistrust. J. Health Care Poor Underserved. 2017;28:362–377. doi: 10.1353/hpu.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canadian Institute for Health Information Continuity of Care with Family Medicine Physicians: Why It Matters. [(accessed on 24 June 2021)]. Available online: https://secure.cihi.ca/estore/productFamily.htm?locale=en&pf=PFC2865.
- 33.Mathews M., Ryan D., Bulman D. What does satisfaction with wait times mean to cancer patients? BMC Cancer. 2015;15:1017. doi: 10.1186/s12885-015-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Provost S., Pineault R., Tousignant P., Roberge D., Tremblay D., Breton M., Benhadj L., Diop M., Fournier M., Brousselle A. Does the primary care experience influence the cancer diagnostic process? Int. J. Fam. Med. 2015;2015:176812. doi: 10.1155/2015/176812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsonage R.K., Hiscock J., Law R.-J., Neal R.D. Patient perspectives on delays in diagnosis and treatment of cancer: A qualitative analysis of free-text data. Br. J. Gen. Pract. 2017;67:e49–e56. doi: 10.3399/bjgp16X688357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ICES Working with ICES Data. 2019. [(accessed on 4 July 2019)]. Available online: https://www.ices.on.ca/Data-and-Privacy/ICES-data/Working-with-ICES-Data.
- 37.Grunfeld E. It takes a team: CanIMPACT: Canadian Team to Improve Community-Based Cancer Care along the Continuum. Can. Fam. Physician Med. Fam. Can. 2016;62:781–782. [PMC free article] [PubMed] [Google Scholar]
- 38.Kendell C., Decker K.M., Groome P.A., McBride M.L., Jiang L., Krzyzanowska M., Porter G., Turner D., Urquhart R., Winget M., et al. Use of Physician Services during the Survivorship Phase: A Multi-Province Study of Women Diagnosed with Breast Cancer. Curr. Oncol. 2017;24:81–89. doi: 10.3747/co.24.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McBride M.L., CanIMPACT for the CanIMPACT Team. Groome P.A., Decker K., Kendell C., Jiang L., Whitehead M., Li D., Grunfeld E. Adherence to quality breast cancer survivorship care in four Canadian provinces: A CanIMPACT retrospective cohort study. BMC Cancer. 2019;19:659. doi: 10.1186/s12885-019-5882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu M., Lebenbaum M., Lam K., Chong N., Azimaee M., Iron K., Manuel D., Guttmann A. Describing the linkages of the immigration, refugees and citizenship Canada permanent resident data and vital statistics death registry to Ontario’s administrative health database. BMC Med. Inform. Decis. Mak. 2016;16:135. doi: 10.1186/s12911-016-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weller D., Vedsted P., Rubin G., Walter F.M., Emery J., Scott S., Campbell C.E., Andersen R.S., Hamilton W., Olesen F., et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer. 2012;106:1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weller D., Vedsted P., Anandan C., Zalounina A., Fourkala E.O., Desai R., Liston W., Jensen H., Barisic A., Gavin A., et al. An investigation of routes to cancer diagnosis in 10 international jurisdictions, as part of the International Cancer Benchmarking Partnership: Survey development and implementation. BMJ Open. 2016;6:e009641. doi: 10.1136/bmjopen-2015-009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Defusing the Confusion: Concepts and Measures of Continuity of Healthcare. [(accessed on 20 October 2021)]. Available online: https://www.worldcat.org/title/defusing-the-confusion-concepts-and-measures-of-continuity-of-health-care/oclc/50248087.
- 44.Breslau N., Reeb K.G. Continuity of care in a university-based practice. J. Med. Educ. 1975;50:965–969. doi: 10.1097/00001888-197510000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez H.P., Marshall R.E., Rogers W.H., Safran D.G. Primary Care Physician Visit Continuity: A Comparison of Patient-reported and Administratively Derived Measures. J. Gen. Intern. Med. 2008;23:1499–1502. doi: 10.1007/s11606-008-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang L., Lofters A., Moineddin R., Decker K., Groome P., Kendell C., Krzyzanowska M., Li D., McBride M.L., Mittmann N., et al. Primary care physician use across the breast cancer care continuum: CanIMPACT study using Canadian administrative data. Can. Fam. Physician. 2016;62:e589–e598. [PMC free article] [PubMed] [Google Scholar]
- 47.Silver S.A., Bota S.E., McArthur E., Clemens K.K., Harel Z., Naylor K.L., Sood M.M., Garg A.X., Wald R. Association of Primary Care Involvement with Death or Hospitalizations for Patients Starting Dialysis. Clin. J. Am. Soc. Nephrol. 2020;15:521–529. doi: 10.2215/CJN.10890919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starfield B., Weiner J., Mumford L., Steinwachs D. Ambulatory care groups: A categorization of diagnoses for research and management. Health Serv. Res. 1991;26:53–74. [PMC free article] [PubMed] [Google Scholar]
- 49.Steele L.S., Glazier R.H., Lin E., Evans M. Using Administrative Data to Measure Ambulatory Mental Health Service Provision in Primary Care. Med. Care. 2004;42:960–965. doi: 10.1097/00005650-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 50.SAS Institute Inc. SAS 9.4. [(accessed on 24 June 2021)]. Available online: https://www.sas.com/content/dam/SAS/en_ca/User%20Group%20Presentations/Hamilton-User-Group/OrrLawrence-NewFeaturesSAS%209.4-Spring2014.pdf.
- 51.Weller D., Menon U., Falborg A.Z., Jensen H., Barisic A., Knudsen A.K., Bergin R., Brewster D., Cairnduff V., Gavin A.T., et al. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP) BMJ Open. 2018;8:e023870. doi: 10.1136/bmjopen-2018-023870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menon U., Vedsted P., Falborg A.Z., Jensen H., Harrison S., Reguilon I., Barisic A., Bergin R., Brewster D.H., Butler J., et al. Time intervals and routes to diagnosis for lung cancer in 10 jurisdictions: Cross-sectional study findings from the International Cancer Benchmarking Partnership (ICBP) BMJ Open. 2019;9:e025895. doi: 10.1136/bmjopen-2018-025895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ontario Breast Screening Program (OBSP) Guidelines Summary. [(accessed on 24 June 2021)]. Available online: https://better-program.ca/fr/evidence/resource/ontario-breast-screening-program-obsp-guidelines-summary/
- 54.Barisic A., Kish M., Gilbert J., Mittmann N., Moineddin R., Sisler J., Vedsted P., Grunfeld E. Family physician access to and wait times for cancer diagnostic investigations: Regional differences among 3 provinces. Can. Fam. Physician Med. Fam. Can. 2016;62:e599–e607. [PMC free article] [PubMed] [Google Scholar]
- 55.Velikova G., Booth L., Johnston C., Forman D., Selby P. Breast cancer outcomes in South Asian population of West Yorkshire. Br. J. Cancer. 2004;90:1926–1932. doi: 10.1038/sj.bjc.6601795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thøgersen H., Møller B., Åsli L.M., Bhargava S., Kvåle R., Fjellbirkeland L., Robsahm T.E., Aaserud S., Babigumira R., Larsen I.K. Waiting times and treatment following cancer diagnosis: Comparison between immigrants and the Norwegian host population. Acta Oncol. 2020;59:376–383. doi: 10.1080/0284186X.2019.1711167. [DOI] [PubMed] [Google Scholar]
- 57.Wang A.M.Q., Yung E.M., Nitti N., Shakya Y., Alamgir A.K.M., Lofters A.K. Breast and Colorectal Cancer Screening Barriers Among Immigrants and Refugees: A Mixed-Methods Study at Three Community Health Centres in Toronto, Canada. J. Immigr. Minor. Health. 2019;21:473–482. doi: 10.1007/s10903-018-0779-5. [DOI] [PubMed] [Google Scholar]
- 58.Vahabi M., Lofters A., Kumar M., Glazier R.H. Breast cancer screening disparities among immigrant women by world region of origin: A population-based study in Ontario, Canada. Cancer Med. 2016;5:1670–1686. doi: 10.1002/cam4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarrant C., Dixon-Woods M., Colman A.M., Stokes T. Continuity and Trust in Primary Care: A Qualitative Study Informed by Game Theory. Ann. Fam. Med. 2010;8:440–446. doi: 10.1370/afm.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Redaniel M.T., Martin R.M., Ridd M.J., Wade J., Jeffreys M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: Historical cohort study using the Clinical Practice Research Datalink. PLoS ONE. 2015;10:e0126608. doi: 10.1371/journal.pone.0126608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lukács G., Kovacs A., Csanádi M., Moizs M., Repa I., Kaló Z., Vokó Z., Pitter J.G. Benefits of Timely Care in Pancreatic Cancer: A Systematic Review to Navigate Through the Contradictory Evidence. Cancer Manag. Res. 2019;11:9849–9861. doi: 10.2147/CMAR.S221427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webber C., Whitehead M., Eisen A., Holloway C.M.B., Groome P.A. Breast cancer diagnosis and treatment wait times in specialized diagnostic units compared with usual care: A population-based study. Curr. Oncol. 2020;27:377–385. doi: 10.3747/co.27.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McPhail S., Elliss-Brookes L., Shelton J., Ives A., Greenslade M., Vernon S.W., Morris E.J.A., Richards M. Emergency presentation of cancer and short-term mortality. Br. J. Cancer. 2013;109:2027–2034. doi: 10.1038/bjc.2013.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waks A.G., Winer E.P. Breast Cancer Treatment: A Review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 65.Marchildon G.P., Hutchison B. Primary care in Ontario, Canada: New proposals after 15 years of reform. Health Policy. 2016;120:732–738. doi: 10.1016/j.healthpol.2016.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset from this study is held securely in a coded form at ICES. While data-sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (accessed on 19 December 2019). The full dataset creation plan is available from the authors upon request.