Abstract
Cells have the ability to respond to various types of environmental cues, and in many cases these cues induce directed cell migration towards or away from these signals. How cells sense these cues and how they transmit that information to the cytoskeletal machinery governing cell translocation is one of the oldest and most challenging problems in biology. Chemotaxis, or migration towards diffusible chemical cues, has been studied for more than a century, but information is just now beginning to emerge about how cells respond to other cues, such as substrate-associated cues during haptotaxis (chemical cues on the surface), durotaxis (mechanical substrate compliance) and topotaxis (geometric features of substrate). Here we propose four common principles, or pillars, that underlie all forms of directed migration. First, a signal must be generated, a process that in physiological environments is much more nuanced than early studies suggested. Second, the signal must be sensed, sometimes by cell surface receptors, but also in ways that are not entirely clear, such as in the case of mechanical cues. Third, the signal has to be transmitted from the sensing modules to the machinery that executes the actual movement, a step that often requires amplification. Fourth, the signal has to be converted into the application of asymmetric force relative to the substrate, which involves mostly the cytoskeleton, but perhaps other players as well. Use of these four pillars has allowed us to compare some of the similarities between different types of directed migration, but also to highlight the remarkable diversity in the mechanisms that cells use to respond to different cues provided by their environment.
Cell migration is critical in a wide array of physiological, developmental and disease-related processes, and basic tenets governing this process have been uncovered over the years. In vivo, cells must be able to perceive a variety of cues in their environment and migrate towards or away from these cues so as to execute morphogenetic programmes during development, mount an immune response and repair damaged tissues. When this process goes awry, devastating consequences often ensue. Failure of cells to migrate in the appropriate way can lead to defects during neuronal development linked to cognitive deficits1, chronic wounds that never heal2 and immune deficiencies3. Improperly initiated or misdirected cell migration can be equally detrimental, leading to invasive metastatic cancer4, autoimmune disease5 and fibrosis6. Biologists have studied the process of directed migration for more than a century, but many mysteries remain about how this process works at a mechanistic level.
To define how cells move directionally towards various cues, it is critical to understand the basics of cell migration. Perhaps the most influential paradigm for describing cell migration is the four-step cycle of cell crawling developed by Michael Abercrombie, an early pioneer of the field7. This paradigm arose from his observations of migrating primary and cultured fibroblasts by phase contrast and interference reflection microscopy8. In this scheme, the first event is the protrusion of a leading edge, which in fibroblasts is dominated by lamellipodia and filopodia. Next, the cell generates initial adhesions with the substrate. These adhesions connect to the contractile machinery of actomyosin stress fibres and, through a combination of pulling from the front and squeezing from the rear, the cell body moves forward. Finally, old adhesions are detached from the substrate or dissolved at the trailing edge. Although textbooks describe this as a stepwise cycle of sequential steps, in reality, all events of the cycle occur simultaneously and likely influence the other steps rather than proceeding as a series of independent events. In addition, many of the concepts of the Abercrombie cycle are specific to mesenchymal cells such as fibroblasts on 2D surfaces and may not apply to other cell types that use different modes of migration or during migration in different physiological environments (see Box 1 for a primer on different modes of migration).
Box 1 |. Modes of cell migration and plasticity.
Cells can migrate singly or collectively as groups227–229. Historically, single-cell migration has been divided into mesenchymal and amoeboid modes of migration, although these classifications are complicated by the plasticity of migration modes in different environments (see below). Fibroblasts, various stem cells and some cancer cells often use the mesenchymal migration mode, which is defined by several characteristics, such as a strong dependence on adhesion to the extracellular matrix (ECM), an elongated morphology in 3D environments, actin-based protrusions such as lamellipodia or filopodia at their leading edge, and the ability to generate strong traction forces on the substrate through contractile actin networks. These characteristics usually give rise to slower migration velocity93. The amoeboid migration mode is used by a wide range of cells, including primordial germ cells, single-cell social amoebas such as Dictyostelium discoideum and immune cells such as leukocytes230. In this mode, cells exhibit a more rounded morphology, undergo constant shape changes through rapid extension and retraction of membrane protrusions, and engage with the substrate through weak adhesions, which usually lead to higher migration velocity. Amoeboid protrusions vary from lamellipodia and filopodia driven by actin polymerization to actin-free transient spherical blebs that rely on myosin-based contraction and pressure-driven cytosolic flow217,230.
Cells can also move collectively as groups, which presents its own challenges and opportunities. Both epithelial and mesenchymal cells exhibit collective migration, which is important for tissue remodelling during morphogenesis, wound closure and cancer cell invasion211,229. During collective migration, signals from external cues are transmitted to the entire mass of cells through the integration of intracellular and intercellular signalling cascades as well as mechanotransduction at cell–cell junctions and cell–ECM interfaces212. This results in front–rear polarity at a supracellular level. A group of cells situated at the front of the supracellular unit generally becomes the leader cells in response to external cues and extend stable lamellipodia or filopodia towards the substrate, whereas the follower cells situated at the rear extend small transient cryptic lamellipodia. The stable protrusions possibly together with the transient ones promote the formation of focal adhesions with the ECM to exert traction forces towards the substrate. Application of these forces physically deforms the matrix, creating a path for the entire cohort211,229. Leader cells can also secrete matrix metalloproteinases that remodel the surrounding ECM, paving the way for collective migration during cancer invasion. In addition to this traction-based collective migration, cells can adopt a propulsion-based motility analogous to the amoeboid mode detected, for instance, in colon cancer cell clusters231.
Complicating any classification scheme of cell migration is the fact that cells can switch between modes of single-cell migration and between single-cell and collective migration, depending on a variety of factors, such as tissue topology, ECM composition and the degree of adhesion to it, as well as the presence of biochemical cues232,233. For example, physical confinement and low adhesion enable slow-moving fibroblasts and epithelial cells to transition into a faster migration mode, where large stable blebs are favoured by high cell contractility232. Similar stable bleb-based fast migration is observed in zebrafish progenitor cells induced by spatial confinement in vitro233 and in vivo at transplantation-induced wound sites of the embryo, where the cortical contractility is elevated. Confinement-associated plasticity in the migration mode is also detected in leukocytes. For example, neutrophils with a genetically disrupted branched actin network switch from multiple finger-like protrusions to smooth bleb-based leading-edge protrusions when exposed to confined microenvironments234. Cancer cells can also adopt a rounded, bleb-based migration mode in low-adhesion 3D environments or when their matrix metalloproteinase activities are disrupted. In addition, physicochemical parameters such as hypoxia, which is a prominent feature of solid tumours, have been shown to promote the transition of collectively invading cancer cells into individually moving amoeboid cells, and enhanced cancer dissemination235. The extreme plasticity in migration modes displayed by cancer cells may allow them to adapt to many tissue environments and contribute to disease progression.
In this Review, we present a four-part conceptual framework for understanding directed cell migration towards a variety of cues, including diffusible chemical cues (chemotaxis), chemical cues on a surface (haptotaxis), mechanical substrate compliance (durotaxis), geometric features of the substrate (topotaxis; also known as contact guidance) and electric fields (galvanotaxis; also known as electrotaxis) (TABLE 1 ). This framework is deliberately generic to facilitate comparisons and contrasts between different types of migration-inducing cues and migration modalities. By comparing different forms of directed migration, we highlight the progress made in the field and reveal gaps in our understanding of molecular underpinnings driving the directionality of cell migration. This Review cannot comprehensively cover all aspects of this large topic. Thus, we will avoid descriptions of new technologies for generating signal gradients or for quantifying migration or mathematical/theoretical models of this process, although we point to a few appropriate reviews of these topics (see REFS9,10). Instead, we will focus on how new findings provide insight into the underlying principles of directed migration and suggest questions to be addressed by future studies.
Table 1 |.
The four pillars of directed cell migration
| Migration mode | Cue | Signal generation | Signal sensing | Signal transmission | Signal execution |
|---|---|---|---|---|---|
| Chemotaxis | Diffusible chemical released from cells or deposited extracellular vesicles | Simple diffusion Regulated removal by degradation of the chemoattractant or decoy receptors Release of extracellular vesicles |
GPCRs RTKs Other receptors, such as axon guidance receptors |
Classical signalling pathways involving small and large G proteins PI3K TORC2 PLA2 MAPK/ERK |
Leading-edge protrusions (all types) Localized regulation of non-muscle myosin II and contractility |
| Haptotaxis | Substrate-bound chemical cues such as an immobilized chemokine or ECM | ECM secretion and deposition Binding of soluble factors to a substrate (mostly ECM) Exposing new sites on the substrate through enzymatic action |
For ECM, integrins, but different adhesion structure impacts signalling outcome For substrate-bound chemokines, regular receptors, but signalling kinetics may be different for different receptor–ligand pairs |
Classical integrin signalling pathways: Rho-family GTPases, FAK–Src, etc. Bound chemokine: probably same as diffusible cue |
Biased protrusion generation through a positive-feedback loop. Requires the Arp2/3 complex |
| Durotaxis | Differential substrate compliance | Passive: creating a stiff substrate by crosslinking of ECM components or ECM deposition Active: cells or tissues exerting a force on the substrate that is sensed by other cells |
Integrins Membrane tension and/or invagination Focal adhesion components Actomyosin filaments LINC complex |
Unclear, but two mechanisms have been proposed: role of pure mechanics using actomyosin system or the involvement of mechanically triggered signalling events | Similar to other forms of migration but biased relative to stiffness gradient |
| Topotaxis | Geometric properties of the migration substrate irrespective of mechanical or chemical properties | Preformed tunnels created by other cells Trails created by proteolytic ECM remodelling Topological features created by non-lytic ECM deformation 1D fibrils such as bundles of collagen Topology of natural tissue elements |
Cells adhere and conform to the topology and/or the geometry of the migration substrate with the help of focal adhesion components Membrane curvature-sensing proteins Nucleus deformation |
Cell and nuclear shape change may affect both signalling and the cytoskeleton but the mechanisms remain unclear | Topology/geometry biases force-generating mechanisms of actin polymerization and actomyosin contractility |
| Galvanotaxis | Electric fields | Ionic differences generated by transepithelial barriers such as in the skin, disrupted by wounding | Electrophoretic movement of charged surface proteins and lipids within the plane of the membrane | Clustering of membrane proteins/lipids must activate signalling, but the mechanisms remain unclear | Similar to chemotaxis but biased relative to charge |
Arp2/3 complex, actin-related protein 2/3 complex; ECM, extracellular matrix; FAK, focal adhesion kinase; GPCR, G protein-coupled receptor; LINC complex, linker of nucleoskeleton and cytoskeleton complex; PI3K, phosphoinositide 3-kinase; PLA2, phospholipase A2; RTK, receptor tyrosine kinase; TORC2, target of rapamycin complex 2.
Lamellipodia
Broad, sheet-like protrusions that contain branched and linear actin filaments. A variety of cell types, including fibroblasts, neural crest cells and macrophages, use lamellipodia to explore longer distances through the extracellular matrix.
Four pillars of directed migration
To organize the large amount of information necessary to understand directed migration in response to various cues, we developed a generic, conceptual framework of four events that must occur during all forms of directed migration, which we term the ‘four pillars of directed migration’ (TABLE 1 ). The pillars include generating the signal, sensing the signal, transmitting the signal and executing the signal. For each pillar, we separate the various forms of directed migration by cue except for the fourth pillar, where we consider how the various signalling mechanisms converge on a common set of cell translocation machineries.
Generating the signal
For directed migration to occur, a signal must first be generated. This signal may be a transient cue such as a diffusible chemical signal secreted into the environment meant to direct cells for a short burst of migration. Alternatively, the signal may involve a long-lasting change to the environment that guides cells for an extended time, such as the generation of physical paths.
Chemotaxis.
Chemotaxis is mediated by the generation of diffusible cues. When these cues are presented uniformly, cells undergo chemokinesis, where they migrate randomly with either higher speed and/or higher turning frequency relative to unstimulated cells11. However, if the promigratory signal is presented in the form of a gradient, directed migration occurs12 (FIG. 1a). The diffusible agents that induce directed migration include a large and diverse group of chemoattractants produced by different sources. These comprise formylated peptides13, products of the complement cascade14, phospholipid metabolites15 and a large family of chemokines and growth factors that are derived from endothelial, epithelial and stromal cells16. In addition, ATP and hydrogen peroxide have been reported to act as autocrine signals to amplify chemotactic signals17–19. Furthermore, specialized secreted proteins, such as Slits, netrins, semaphorins and ephrins, are well-known axon guidance cues20–23. The diverse biochemical nature of these chemotactic cues, with distinct diffusion coefficients and affinities for their cognate receptors, presents considerable challenges for the generation and maintenance of stable gradients during chemotaxis.
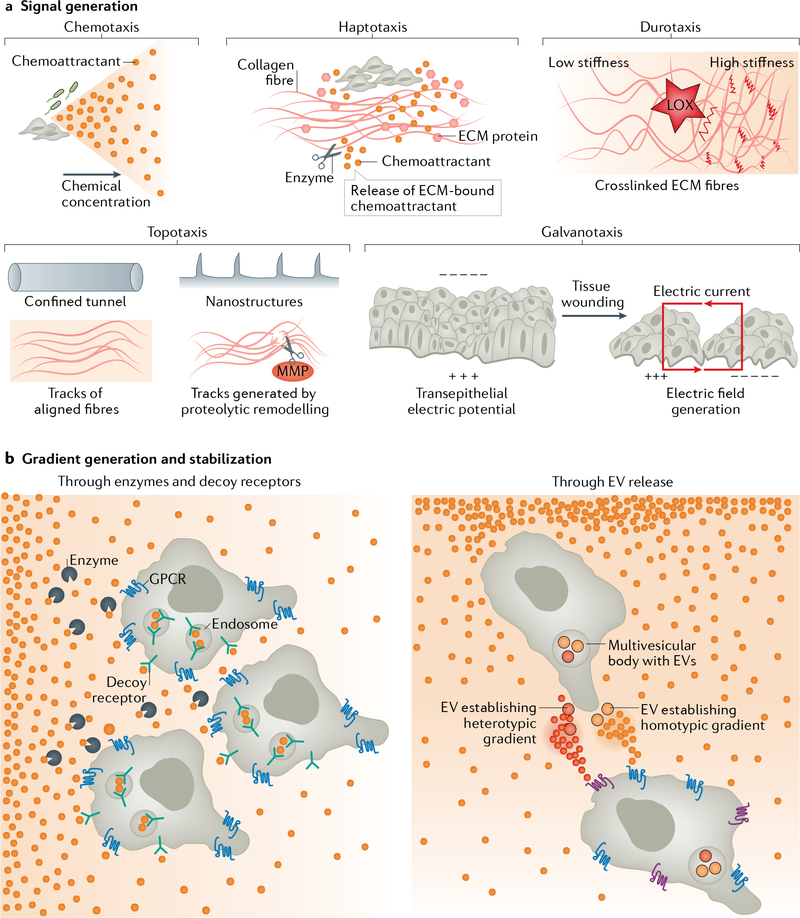
Fig. 1 |. Generating the signal.
a | The diverse ways by which cues for directional migration are generated. During chemotaxis, soluble chemoattractants released from bacteria or cellular sources diffuse to form chemical gradients. During haptotaxis, extracellular matrix (ECM) proteins and chemokines released from cellular sources are deposited onto the ECM and generate gradients of immobilized chemical cues. In some cases, ECM-bound chemokines are released from the matrix by cellular proteolytic activities (scissors) and provide soluble cues for chemotaxis. During durotaxis, gradients of stiffness can be generated by lysyl oxidase (LOX)-mediated ECM crosslinking. During topotaxis, the geometry of the existing tissue structures, aligned fibres or tracks generated by proteolytic remodelling (via matrix metalloproteinases (MMPs), scissors) or deformation provides directional signals. During galvanotaxis, electric fields generated at wounding sites as a result of the loss of transepithelial potential provide guidance cues for cells involved in damage repair. b | Cartoon explaining how stable gradients are generated and maintained during chemotaxis. Uniformly present soluble chemicals are either degraded by enzymes or scavenged (via endocytic internalization) by decoy receptors to establish a gradient (left). Cells release extracellular vesicles (EVs), such as exosomes carrying either identical (homotypic gradient) or distinct (heterotypic gradient) chemical cues, to generate a stable secondary gradient (right). GPCR, G protein-coupled receptor.
A gradient can be established by simple diffusion from a source or by regulating the removal of the attractant24. Examples of mechanisms cells use to regulate gradient formation and avoid receptor saturation (a situation when cells are no longer able to perceive concentration differences of the chemoattractant) include degradation of chemoattractants by enzymatic or proteolytic breakdown, and endocytosis of cell surface-bound chemoattractants via scavenger/decoy receptors that specifically remove their ligand without initiating cell polarity/migration signalling (reviewed in25) (FIG. 1b). For example, it was established that a negative-feedback loop between CXCL12 and its receptor CXCR7 is required to maintain optimal CXCL12 concentration in the zebrafish posterior lateral line primordium26. Using a clever synthetic approach, where GFP is used to generate diffusible gradients, it was recently shown that combining the expression of non-signalling decoy receptors with receptors engineered to respond to GFP allows the synthetic GFP gradient to generate normal growth and patterning of the Drosophila melanogaster wing pouch27. In addition, self-generating gradients have recently been proposed as an alternative mechanism to generate chemical gradients. In this case, migrating cells would secrete enzymes that break down chemoattractants initially distributed uniformly — as observed for Dictyostelium discoideum cells migrating towards folic acid28, and melanoma and pancreatic cells responding to lysophosphatidic acid29,30. Such a mechanism can theoretically give rise to steep gradients that work over long distances and convoluted migratory pathways. Accordingly, with use of artificial complex environments and mathematical modelling, it was recently shown that the breakdown of attractants allows D. discoideum and pancreatic metastatic cell lines to navigate long, complex paths in a manner that is dependent on attractant diffusibility, cell speed and path complexity31.
Filopodia
Finger-like protrusions that contain bundles of linear F-actin. filopodia serve to probe local environmental cues, provide directionality and maintain persistence of migrating cells.
Stress fibres
Contractile arrays of actin and non-muscle myosin II that are mechanically coupled to the substrate through integrin-based focal adhesions.
Substrate compliance
The mechanical resistance provided by non-rigid substrates (for example, collagen gels) to the contractile forces exerted by cells as they engage the substrate.
Complement
Complement proteins are products of the complement pathway generally activated as part of the innate immune response to infection. some complement proteins, such as C5a, act as chemoattractants that guide leukocytes to sites of infection.
Gradients can be propagated by means other than simple diffusion32. For instance, morphogens have long been known to be secreted in precursor forms that harbour motifs that bind to extracellular matrix (ECM) components and can be later released by cell-mediated proteolysis of the ECM component33. Similarly, it has been shown that neutrophils migrating in 3D collagen matrices activate discoidin domain receptor 2 (DDR2), which binds collagen I, and induces the secretion of matrix metalloproteinases and the release of collagen-derived chemotactic peptides that act in an autocrine manner to stabilize neutrophil directionality34. The concept, referred to as ‘autologous chemotaxis’, has also been reported to be involved during the CCR7-driven directed migration of tumour cells. In this case, chemoattractant gradients were generated from autocrine signals as a result of interstitial flow that produced advection fields35,36.
Posterior lateral line primordium
A group of cells that migrate together from the ear to the tip of the tail of zebrafish as they periodically deposit primary neuromasts.
During development, the transportation of signalling molecules along filopodium-like protrusions called ‘cytonemes’ or ‘tunnelling nanotubes’ (thin cellular protrusions involved in cell–cell communication) or through transcellular transport (transcytosis) has been reported to be involved in the generation of mitogen gradients37,38. There is also evidence that the packaging of chemoattractants in extracellular vesicles (in particular exosomes) importantly contributes to the generation of gradients during chemotaxis. In D. discoideum cells, extracellular vesicles have been shown to contain the machinery to synthesize and release the chemoattractant cAMP; these extracellular vesicles mediate the relay of chemotactic signals during chemotaxis and the alignment of cells in a head-to-tail fashion in a process referred to as ‘streaming’39,40. In macrophages and dendritic cells, the secondary chemoattractant leukotriene B4 (LTB4) has been reported to be present in exosomes and promote migration41. In neutrophils, LTB4 is released in response to primary chemoattractant stimulation and acts in an autocrine and paracrine fashion to stabilize neutrophil cell polarization and to relay signals to distant neutrophils42,43. Similarly, dendritic cell migration was recently reported to depend on exosomes released from lymphatic endothelial cells in a CX3CL1 (also known as fractalkine)-dependent fashion44. Moreover, chemokine-containing exosomes isolated from stressed tumour cells have been reported to activate and induce the migration of T cells45, and neutrophils have been shown to package and release CXCL12 via secretory vesicles, leaving behind CXCL12-containing trails that attract T cells to infection sites46. In these contexts, the packaging of chemotactic cues in extracellular vesicles/exosomes is poised to protect attractants from harsh extracellular environments, degradation and/or rapid diffusion. Furthermore, we envision that as the vesicles are deposited, they can persist after the cells have left the area and continue to deliver chemotactic cues to generate long-lasting secondary gradients to recruit distant cells to sites required for their action (such as sites of inflammation for leukocytes) (FIG. 1b). In addition, extracellular vesicles have also been reported to mediate directional migration by regulating cell–ECM adhesion assembly in tumour cells. In this case, autocrine secretion of fibronectin-coated exosomes at the leading edge of cells expressing fibronectin receptors allowed them to establish connections to the ECM, which became coated with these extracellular vesicles47,48.
Morphogens
Signal molecules that originate from a tissue and diffuse to generate a concentration gradient. Morphogens exert long-range signalling effects important for growth and tissue patterning during development.
Advection fields
Fluid flows such as interstitial flow in tissues that can create an advection field or directional transfer of molecules in the liquid phase around cells, which in turn can create asymmetries in secreted autocrine chemoattractants, leading to autologous chemotaxis. Advection fields can also form in the cytoplasm.
Extracellular vesicles
A group of heterogeneous vesicles (several nanometres to micrometres in size) that carry a variety of cargos, including proteins, lipids and nucleic acids, and are secreted by cells to the extracellular space to facilitate cell–cell communication.
Exosomes
The smallest subtype of extracellular vesicles, with a size ranging from 50 to 150 nm. Exosomes are generated as intraluminal vesicles which are secreted to the extracellular space when intraluminal vesicle-carrying multivesicular bodies fuse with the plasma membrane.
Caveolin
integral membrane protein family required for flask-shaped (caveola) membrane structure formation. Caveolins are also involved in membrane trafficking, exocytosis, endocytosis, extracellular vesicle formation and extracellular vesicle cargo selection.
Rho-family GTPases
A family of small proteins that bind GDP or GTP and regulate a wide array of downstream signalling events. CDC42, Rac, and RhoA are widely studied members of this family of proteins.
Haptotaxis.
Haptotaxis is the sensing of surface-bound chemical cues (FIG. 1a). A primary haptotactic cue is provided by the components of the ECM. A variety of cells can secrete ECM proteins, such as fibronectin, laminin and various collagens, into the environment to form insoluble arrays that become migration substrates or bind to existing substrates, thereby functioning as migration cues for the cells themselves or for other cells in the vicinity49,50. Unlike diffusible chemotactic cues, ECM haptotactic cues tend to be relatively stable and long-lasting. As many components of the ECM can bind to each other, their deposition can be iterative to create complex mixtures of haptotactic cues51. In addition, cells can locally degrade ECM components to further sculpt remarkably complex migration environments52.
The specific molecular mechanisms of ECM secretion remain poorly understood. Recent work suggests that caveolin-dependent regulation of exosome biogenesis is a key step in ECM secretion and deposition in fibroblasts53, but the universality of this mechanism will need to be explored in other cell types. Furthermore, initial cell engagement with the ECM can trigger the deposition of fibronectin at the leading edge of cells through a mechanism involving one of the Rho-family GTPases, CDC42 (REF.54). This creates a positive-feedback loop for cells to essentially lay down tracks and facilitate persistent migration on ECM compositional gradients. Cell engagement with the ECM can also modify the structure of the array, whereby integrin-dependent cell contacts induce the reorganization of fibronectin fibrils55. An interesting pathophysiological example of ECM deposition occurs in the retina of patients with diabetes or macular degeneration, where inappropriate deposition of fibronectin leads to thickening of Bruch’s membrane (the innermost layer of the retina) and inappropriate neovascularization56. Such increased deposition of ECM proteins could generate abnormal haptotactic cues. However, increased ECM deposition will also change the mechanical landscape of the microenvironment, which could trigger a pathological durotactic response as described later.
In addition to ECM haptotactic cues, cells secrete factors such as chemokines or other guidance factors that bind tightly to existing ECM arrays57, thus generating haptotactic cues that are sensed by direct cell engagement rather than acting at a distance through diffusion like during chemotaxis. Generation of these cues is regulated by mechanisms similar to the ones responsible for the generation of diffusible, chemotactic cues. Nevertheless, as noted already, such ECM-bound chemokines can also be released to switch a haptotactic cue into a locally functioning chemotactic cue58,59.
Durotaxis.
In addition to sensing molecular cues, cells have the ability to sense differences in substrate stiffness and respond by migrating towards or away from areas of higher stiffness (FIG. 1a). Such stiffness gradients have recently been demonstrated in vivo in the embryonic mouse limb bud60. The generation of these durotactic cues requires changes in the mechanical environments that cells encounter, which can persist for long periods and influence the migration of cells for days or much longer. For example, increased amounts of ECM deposition can often lead to changes in the mechanical properties of the local microenvironment, which can produce a hybrid haptotactic-durotactic-topotactic cue61. Another means by which mechanical cues can be generated is through mechanical modification of the existing ECM, either stiffening it or relaxing it. For example, the lysyl oxidase enzymes (LOXL1-LOLX4) can crosslink collagen fibrils and other ECM components to render a stiffer network62,63. Interestingly, this group of enzymes is frequently misregulated in cancer and other disease states such as during fibrosis, speaking to the importance of mechanical control of the cell’s environment62. These mechanical changes can influence the proliferation and migration of tumour cells64 as well as surrounding stromal cells such as endothelial cells in the vasculature65. Conversely, matrix-degrading enzymes such as matrix metalloproteinases can relax or soften the environment to further sculpt the mechanical landscape encountered by cells66,67.
Topotaxis.
Topotaxis is driven by biophysical cues, where cells sense the topographical features of the surrounding microenvironment. Natural tissue elements, including aligned collagen fibres, muscle strands, nerve fibres, vascular tracks and pores or tunnel-like confined trails within the ECM, often provide an anisotropic surface architecture at nanometre or micrometre scale68,69 (FIG. 1a). Migrating cells tend to adapt their shape to the available geometry of the surrounding substrate to migrate in a preferred direction. However, it is important to consider the cell types (taking into account their unique properties) as well as the size of the confining space when one is reflecting on how topological cues prescribe directional choices to migrating cells. For instance, it has been shown that migrating leukocytes, which exhibit amoeboid movement, follow pre-existing trails in 3D reconstituted collagen matrices70,71. Unlike tumour cells and fibroblasts, these leukocytes do not actively break down the ECM. Instead, leukocytes migrating in interstitial tissue undergo a robust shape change guided by the matrix that induces transient deformation of the collagen network, and squeeze through the preformed trails of larger pore size and least resistance. By contrast, cancer cells during tissue invasion frequently depend on proteolytic remodelling of the ECM to create their own trails, especially when encountering environments with limited space and more resistance 69,72. However, non-proteolytic strategies, including ECM deformation, have been reported in cancer cells with amoeboid features as they make their way through tissue73. Depending on the tumour type, a customized migratory approach may also exist, where tumour invasion relies on protease-dependent tunnel formation in the matrix, which guides the migration of leading tumour or non-tumour stromal cells (including fibroblasts, endothelial cells and smooth muscle cells), whereas the follower cells are simply carried along those tunnels without the need for active ECM remodelling74. Further, it has been reported that cancer cells during invasion can orient themselves parallel to different topological features of the surrounding tissue, and migrate directionally without requiring major ECM remodelling, suggesting that cells are capable of sensing complex topological features of the microenvironment and tune their migratory response accordingly75.
G protein-coupled receptors (GPCRs).
A family of plasma membrane receptors composed of seven transmembrane domains that couple to heterotrimeric G proteins to regulate responses mediated by a variety of external signals.
Axon growth cone
Motile structure at the tip of growing axons that guides directed extension of the axon and is important for patterning of the nervous system.
Galvanotaxis.
The existence of electric fields around cutaneous wound sites has been known since the 1840s76, and these fields can serve as cell migration cues in a process called ‘electrotaxis’ or ‘galvanotaxis’ (FIG. 1a). During the wounding process, the electric potential maintained by transepithelial resistance is short-circuited, and the resulting electric field can reach up to 10 μA cm−2, a value in the range that can be sensed by cells77. In addition to wounds, electric fields have been documented during embryogenesis through transepithelial ion transport78. These transient electric fields are sensed by cells in their vicinity, often provoking a directed migration response.
Sensing the signal
Once a signal has been generated, cells must be able to sense the signal. In the case of chemotaxis, this is a fairly straightforward process of receptor–ligand interactions. However, the sensing step for other cues, such a mechanical compliance of the substrate, is less straightforward and involves more complex mechanotransduction pathways engaging both surface proteins and mechanically coupled intracellular proteins.
Chemotaxis.
The mechanisms underlying how cells sense extracellular cues are, by far, best understood for chemical cues in the context of chemotaxis (FIG. 2a). Work using D. discoideum in the late 1980s first identified G protein-coupled receptors (GPCRs) as the surface receptor responsible for sensing cAMP — the main chemoattractant for D. discoideum chemotaxis79. This work was followed by a flurry of reports in the early 1990s showing that chemokines are also sensed by GPCRs80–83. More than 50 distinct chemokines have been identified in humans and are grouped into the CL, CCL, CXCL or CX3CL subfamily, depending on the sequential positioning of highly conserved cysteine residues84,85. These are recognized by ~20 known conventional chemokine receptors, referred to as ‘CCRs’ or ‘CXCRs’, that share 25–80% sequence identity and exhibit the ability to bind multiple chemokines within a given chemokine subfamily83,86,87. In addition, some chemokines can bind to atypical chemokine receptors, which are structurally related to conventional chemokine receptors but do not couple to signalling modules. Finally, formylated peptides88, products of the complement cascade16, phospholipid metabolites89 and the small molecules ATP and ADP17,90 are all known to bind to GPCRs to mediate their chemotactic activities, making GPCRs the main molecular chemotactic sensors.
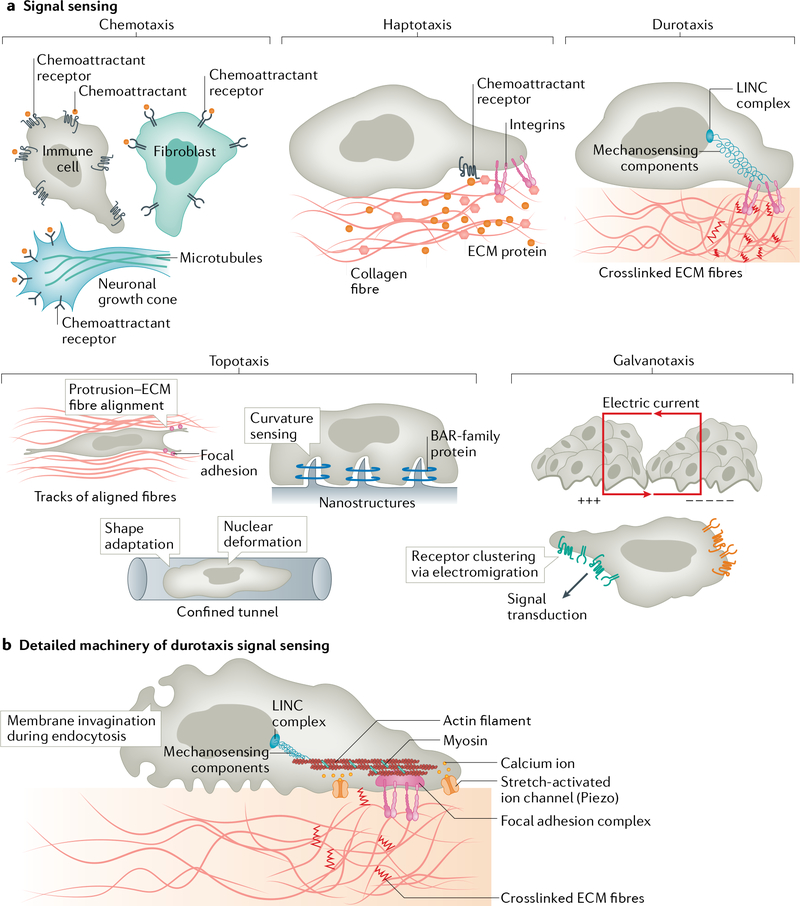
Fig. 2 |. Sensing the signal.
a | Various ways cells sense directional cues. During chemotaxis, cells sense the signal through surface receptors (G protein-coupled receptor (GPCRs), receptor tyrosine kinases or other transmembrane receptors), which bind the soluble chemical cues. During haptotaxis, cells detect surface-bound cues through integrin receptors and GPCRs. During durotaxis, substrate stiffness is sensed by an array of mechanically coupled components located on the cell surface, in the cytosol or at the nuclear envelope. During topotaxis, cells detect the geometry of available space and adapt their shape by changing the orientation of membrane protrusions in parallel to the aligned extracellular matrix (ECM) fibres, sensing topology-induced membrane curvature by BAR-family proteins, or gauging nuclear deformation resulting from compression and shape change. During galvanotaxis, the electric field is sensed by electromigration of membrane components (including signalling receptors) towards the cathode (+++) or the anode (− − − − −). b | Molecular machinery for durotactic sensing. Cells sense gradients of stiffness using mechanosensors at the cell surface (including integrin receptors in focal adhesions, invaginated membranes and stress-activated ion channels), inside the cytoplasm (including components of focal adhesions, actin filaments, microtubules and other mechanosensitive proteins) or at the nucleus (LINC complex).
Receptor tyrosine kinases (RTKs) that interact with growth factors, such as EGF and PDGF, are a second class of receptors that mediate chemotaxis91. Whereas GPCR-mediated chemotactic signalling prevails in the context of amoeboid chemotaxis, mesenchymal cells often use RTK-mediated signalling during directional migration, perhaps reflecting fundamental differences in physiological and environmental conditions encountered by each cell type92,93. In addition, axon growth cone guidance, which is required for patterning the nervous system, is mediated by several families of transmembrane receptors that bind to secreted guidance cues (such as Robo–Slit proteins or netrin-netrin receptors) and mediate either attractive or repulsive responses94–96. Neuronal guidance cues are also known to regulate immune responses97, where, again, they can inhibit or promote migration98–100.
Haptotaxis.
Haptotactic cues are also sensed by cognate cell surface receptors (FIG. 2a). Integrins are used to sense haptotactic gradients composed of ECM components, where the spatial organization or clustering of integrins is key to their signalling properties101. Numerous studies have shown that small, nascent adhesions at the leading edge of migrating cells recruit a different subset of signalling and mechanical effectors compared with larger, maturer adhesions further back under the cell body102. Moreover, these small, nascent adhesions are critical for ECM haptotaxis through a specific signalling pathway described in the next section. In the case of surface-bound chemokines such as CCL21, the same GPCR (CCR7) that senses the diffusible version of this cue is used to sense immobilized gradients103. Interestingly, dendritic cells following surface-immobilized CCL21 gradients require non-linear, exponential gradients for haptotaxis and do not undergo haptotaxis on linear surface gradients, suggesting differences in how the same cue is sensed when it is diffusible or surface-bound. In theory, cells could use other receptors such as cadherins at cell–cell junctions to perceive haptotactic cues presented by other cells, but this has yet to be reported.
Cell–cell junctions
Stable or dynamic sites where borders of two neighbouring cells contact each other. Cell–cell adhesion receptors and recruited adaptor proteins are mechanically coupled to the actin cytoskeleton.
Durotaxis.
The mechanism of durotactic sensing is a matter of intense recent interest (reviewed in104–106). Unlike the cell-impermeant chemical cues driving chemotaxis and haptotaxis, which must be sensed by cell surface receptors, mechanical force is not limited by membranes. Thus, the ‘receptors’ or ‘sensors’ for durotaxis could theoretically be on either side of the plasma membrane or even much deeper in the cytoplasm of the cell, as long as these components are mechanically coupled to the substrate. We envision that durotaxis results from the ensemble activation of multiple mechanically sensitive ‘receptors’ that act in concert to drive directed cell migration.
Focal adhesions
Multiprotein assemblies that physically connect extracellular matrix components to the intracellular actin cytoskeleton through integrin clusters. Integrin-mediated adhesion to extracellular matrix ligands recruits a plethora of signaling (Src and FA K) and structural (talin, paxillin and vinculin) molecules to focal adhesions. Large, mechanically engaged focal adhesions play a crucial role in sensing mechanical cues, while smaller, nascent adhesions are critical for sensing haptotactic cues.
Several candidate durotactic ‘receptors’ have been identified (FIG. 2b). Working from outside the cell inward, the first candidate receptor are integrins107,108. Several recent biophysical studies have demonstrated that these surface proteins are sensitive to mechanical load and are concentrated in structures relevant for cell migration, such as filopodia, lamellipodia and focal adhesions109,110. However, as some cells can migrate by integrin-independent mechanisms111, if they are sensing substrate compliance, they may be using an integrin-independent mechanism. Another potential source of mechanical sensing is the membrane itself, which seems to prominently involve invaginations of the membrane formed during either clathrin-based or caveolin-based endocytosis. Indeed, several studies have shown that these endocytosis pathways display different dynamics and internalization frequencies when cells are plated on uniform substrates of varying compliance112–114. However, differences in endocytic structures across single cells plated on stiffness gradients have not been shown. In addition, stretch-activated ion channels, such as PIEZO1/2, have been reported to sense substrate stiffness115. One possibility is that as cells apply traction force to the substrate on stiffness gradients, these channels will become differentially activated and produce local differences in migration-relevant signalling intermediates such as intracellular calcium. More work will be required to test these ideas.
Traction force
The stress vector at the interface between a migrating cell and its substrate.
LIM domains
Protein structural domains named after the proteins LIN-11, ISL1 and MEC-3. A subset of these domains, such as those found in the proteins zyxin, paxillin and testin, bind actin filaments in a mechanical stress-dependent manner.
LINC complex
Linker of nucleoskeleton and cytoskeleton (LINC) complex is a complex of nuclear envelope proteins that connects the cytoskeleton to the nuclear lamina and is thus involved in transferring signals from sensing mechanical cues at the cell surface or in the cytosol into nucleus.
Moving just inside the plasma membrane, many cytoplasmic components of integrin-containing focal adhesions have been shown to be mechanically sensitive, including talin116, vinculin117 and p130Cas118. When focal adhesions are under differential mechanical load, many of these proteins show altered conformation and/or altered interactions with other cytoplasmic components. However, many cells lack the large, stable focal adhesion structures of mesenchymal cells, where these phenomena have been mostly studied, and still display sensitivity to substrate rigidity119. This suggests that focal adhesions may not be a universal durotactic sensing structure across all cell types. Regardless of whether integrins are clustered in classic focal adhesion structures, they are still mechanically coupled to actin filaments, and several recent studies have shown that actin filaments themselves are sensitive to mechanical load by differentially binding proteins containing LIM domains depending on the mechanical conditions (varying load)120,121. This differential response to mechanical load could serve as a type of ‘durotactic receptor’, leading to altered cytoskeletal dynamics and structure, as well as alteration of signalling pathways. Recent work has also shown that a subset of microtubules originating from the Golgi apparatus are critical for durotaxis by regulating focal adhesion dynamics122.
Finally, actin filaments and microtubules are connected to the nucleus by the mechanically sensitive LiNC complex123,124, which could function as a sensor for differential mechanical load on either side of the nucleus. Indeed, differential positioning of the nucleus relative to the rest of the cell has been linked to regulation of cell polarization during scratch-induced migration of epithelial monolayers125 as well as cell locomotion through piston-like generation of pressure gradients in fibroblasts migrating in a lamellipodium-independent manner in certain 3D environments126 (see the section Executing the signal for details).
Topotaxis.
We are only beginning to understand the mechanisms for sensing topology (FIG. 2a). Similarly to durotaxis, sensors of topological cues are mechanically coupled elements that are located on either side of the cell surface or within internal compartments, such as the nucleus. Some of the sensing elements for topotaxis (for instance, focal adhesions) may overlap with those for both haptotaxis and durotaxis. Over the past decade, a large number of studies have monitored changes in cell shape, protrusion shape, actin cytoskeleton and motility in response to artificial matrix landscapes of diverse topology. These landscapes are generally engineered to house symmetric or asymmetric microstructures or nanostructures of the synthetic substrates that are arrayed on a flat surface in specific patterns127–129 or 3D channels of open or closed ratchets to mimic topological features of tissue architecture in vivo130. However, studies aimed at teasing apart the mechanisms of landscape sensing to the downstream intracellular events, which translate into motility, are sparse. In a few studies, cells were reported to sense topographical features at nanometre to micrometre scales by extending leading-edge protrusions. For instance, lamellipodia in fibroblasts were shown to orient themselves parallel to the micropatterned lines coated with ECM component, promoting elongated cell shape and directional migration131. In addition to parallel orientation, a smaller size of the protrusions has been reported to promote directed migration, possibly by limiting migration perpendicular to the direction of ECM patterns131. In contrast to lamellipodia used by fibroblasts, neurons use both transient, non-aligned and stabler, aligned filopodium populations to sense nanotopographical cues, and to coordinate the signals that promote neurite outgrowth along ECM-coated lined patterns132. In addition to the orientation of the structures, the availability of continuous adhesive surfaces on the ECM-coated aligned fibres assists individual protrusions in sensing the topology133. Such adhesion points give rise to the formation of sequential focal adhesions along the continuous stretch of fibres, which promote persistence of the protrusions in a parallel orientation with respect to the aligned fibres.
BAR-family proteins
Bin/amphiphysin/Rvs161 domain (BAR) proteins are membrane-binding proteins that aid in regulating membrane shape.
Arp2/3 complex
A seven-subunit protein complex that possesses actin nucleation and branching activities leading to the generation of branched actin networks.
N-WASP
Neuronal Wiskott–Aldrich syndrome protein activates the Arp2/3 complex and promotes branched actin filament formation.
Local deformation or curvature of the plasma membrane at the interface of the cell and the substrate was recently identified as the sensor of nanotopography134. Topography-induced membrane curvature serves as a bridge between surface topography and intracellular actin reorganization. Key pillars of the bridge include curvature-sensitive BAR-family proteins, particularly FBP17, which recognize and accumulate within high-curvature areas present on either end of nanobars fabricated to provide nanoscale topological cues. Localized FBP17 then facilitates nucleation of F-actin by activating key actin cytoskeleton modulators, including the Arp2/3 complex, N-WASP and cortactin134. The resulting branched F-actin network accumulates at both ends of the nanobars and undergoes rapid polymerization–depolymerization cycles. Of note, curvature sensing by FBP17 recruitment is restricted to a curvature diameter of less than 400 nm, indicating that topography sensing by curvature-sensitive proteins is size specific. Mechanistically, the identification of membrane curvature as the topology sensor fills the gap in our understanding of how topological cues from the surface translate into actin fibre reorganization inside the cells and, hence, is groundbreaking.
Cortactin
A nucleation promoting factor that activates the Arp2/3 complex and promotes branched actin filament formation.
Finally, a rather non-conventional sensor of topography is the cell nucleus. The nucleus is a large, bulky and relatively rigid cellular organelle, repositioning of which is rate limiting during migration in constrained environments. Recent studies have revealed that topological cues trigger changes in nuclear subcellular location and shape, which have a significant impact on path finding and cell migration71,135,136. In the case of immune cells using amoeboid migration, cytoskeletal forces position the nucleus in the front portion of the cells. This allows cells to sample densely packed tissue, gauge the available space and choose the path of least resistance71. Notably, two recent studies proposed that the nucleus acts as an internal ruler that interprets cell shape in confined spaces, and facilitates rapid movement136,137. Specifically, it was shown that when the level of confinement is increased above a certain point sensed via nuclear deformation, cells exert an active contractile force. This contractile response was connected to the progressive expansion and unfolding of the nuclear envelope with increased confinement. A completely unfolded nuclear envelope triggered a contractile response that allowed cells to resist the physical compression and squeeze out of the confined space. Such a specific adaptive response tailored by the nucleus may be of particular importance for immune cell patrolling through dense tissues, for progenitor cells migrating through a densely packed cell mass during embryonic development or even during cancer cell invasion.
Galvanotaxis.
How cells sense electric fields has been debated for several decades138. Unlike mechanical cues, which can be sensed either at the cell surface or inside the cell, electric fields must be sensed outside the plasma membrane due to its high electrical resistance. The two predominant models for this sensing are membrane depolarization and electromigration of surface proteins. Recent reports favour electromigration of surface proteins as a mechanism of galvanotactic sensing139,140. In these studies, the charge on the extracellular domains of model proteins, as well as some lipids and carbohydrates, resulting from the application of an electric field induces electrophoresis of these molecules within the plane of the plasma membrane, either towards or away from the cathode140,141 (FIG. 2a). Moreover, it has been shown that galvanotaxis itself is sensitive to extracellular pH, which likely changes the charge state of proteins through protonation139.
Transmitting the signal
In the third pillar of directed migration, the signal must be transmitted from the sensor to the machinery necessary to move the cell. In some cases, this occurs via polarized second messenger pathways, but in other cases the signal transduction machinery and the motility machinery may overlap, such as during durotaxis. During the transmission step, a weak signal (in the form of a shallow gradient of the cue) is often amplified to produce a robust cellular response.
Chemotaxis.
For cells to migrate, their cytoskeletal machinery must be polarized142. The important questions are how shallow gradients of extracellular chemical cues can be transformed into steep polarized cellular responses, and at what point in the signalling cascade are responses confined to a defined subcellular location. The introduction of GFP technology and live-cell imaging have been critical in addressing this question. It was established that chemotactic GPCRs remain uniformly distributed in D. discoideum cells and neutrophils undergoing chemotaxis143,144, suggesting that the signals leading to the segregation of the cytoskeletal machinery are downstream of receptor occupancy. However, in lymphocytes, chemokine receptors have been reported to be clustered at the front of cells145. In mesenchymal tumour cells undergoing chemotaxis, the EGF receptor, like GPCRs, is also distributed homogeneously on the plasma membrane, but accumulates in endocytic vesicles on the side of the cell exposed to the high concentration of the chemoattractant146. Because the EGF receptor signalling can continue from internalized endosomes, this suggests that polarized receptor signalling could occur for RTKs in mesenchymal chemotaxis147.
Nevertheless, from findings in amoeboid cells it is clear that the polarization of signalling molecules at the front and rear of cells undergoing chemotaxis occurs downstream of sensing receptors and upstream of the cytoskeletal machinery (FIG. 3a,b) (although the flow of actin may also contribute to the polarization of signalling molecules and hence cytoskeletal rearrangements may also fine-tune the distribution and thus the reception of the signal)148. Indeed, with use of probes that specifically label lipids downstream of phosphoinositide 3-kinase (PI3K), it was shown that phosphatidylinositol 3,4,5-trisphosphate lipids are spatially restricted to the leading edge of D. discoideum, neutrophils and fibroblasts undergoing chemotaxis149–151. These polarized phosphatidylinositol 3,4,5-trisphosphate sites, which are dependent on Ras signalling (reviewed in152), are then poised to spatially recruit a subset of pleckstrin homology (PH) domain-containing proteins that regulate actin assembly through the regulators of Rho-family GTPases, such as Rho guanine nucleotide exchange factors, and DOCK–ELMO153–155. However, pharmacological inhibition or genetic ablation of PI3K does not completely inhibit chemotaxis, particularly in steep chemotactic gradients152,156,157, and several parallel pathways have been reported to regulate D. discoideum and neutrophil chemotaxis, including TORC2 (REFS158–161), phospholipase A2 (REFS162–166) and MAPK/ERK167–170. In fibroblasts undergoing chemotaxis towards the RTK ligand PDGF, the phospholipase PLCγ appears to be crucial for this process through localized hydrolysis of phosphatidylinositol 4,5-bisphosphate to yield diacylglycerol at the leading edge facing the highest concentration of PDGF156. This stable enrichment of diacylglycerol triggers the localized activation of the kinase PKCα and the subsequent inactivation or inhibition of myosin II through non-canonical phosphorylation of Ser1/Ser2 on the regulatory light chain, thereby creating asymmetry in myosin II-mediated contractility. Furthermore, in carcinoma cells migrating towards the RTK ligand EGF, amplification of the signal has been reported to occur through the activation of the actin-severing protein cofilin by PLCγ thereby allowing asymmetric actin polymerization required for protrusion towards the EGF gradient171. Finally, positive-feedback and negative-feedback mechanisms centred at the regulation of signal transduction via the Ras–PI3K–ERK pathway have been reported to be involved in regulating the modes of migration in D. discoideum172 and the metastatic potential of epithelial cells173. Such self-organizing excitable signal transduction activities were proposed to underlie the control of the extension of actomyosin-based protrusions and were suggested to have a key role during development (reviewed in142).
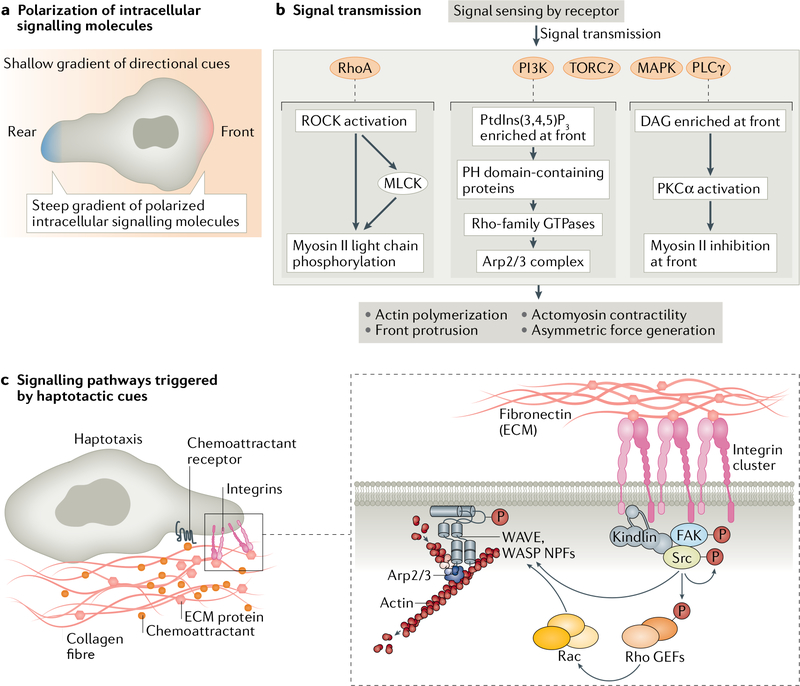
Fig. 3 |. Transmitting the signal.
a | Cartoon depicting how shallow gradients of extracellular directional cues are transmitted into steep gradients of intracellular signalling molecules at the front and rear of cells. b | Flow chart describing how various signalling pathways activated by sensing of directional cues lead to changes in the cytoskeletal machinery. c | Cartoon highlighting the molecular machinery that transmits haptotactic signals. Sensing gradients of fibronectin through integrin engagement activates non-receptor tyrosine kinases: focal adhesion kinase (FAK) and Src-family kinases. These kinases phosphorylate a variety of substrates, triggering the formation of new protein complexes, including guanine nucleotide exchange factors (GEFs), and the activation of Rac, which leads to branched actin network formation through Arp2/3 complex activation via the nucleation-promoting factors (NPFs) WASP and WAVE, ultimately generating lamellipodial structures. DAG, diacylglycerol; PH, pleckstrin homology; PI3K, phosphoinositide 3-kinase; PtdIns(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate.
Pleckstrin homology (PH) domain
Small protein domains of approximately 120 amino acids that are known to have phosphoinositide-binding specificity.
DOCK-ELMO
A protein complex consisting of an adaptor protein, ELMO, and a Rac-specific guanine nucleotide exchange factor, DOCK.
Importantly, although many aspects of chemotactic signalling are common in amoeboid and mesenchymal cells, the fundamental difference in migration behaviours between these two types of cells, with specific mechanisms, timescales and dynamics, requires distinct signalling pathways that are not fully understood92,93.
Haptotaxis.
The transmission of haptotactic signals and the transmission of chemotactic signals are similar, involving traditional Rho-family GTPase signalling pathways. The most well-studied form of haptotaxis is sensing gradients of ECM proteins through integrin engagement (FIG. 3c). In this case, integrin engagement leads to the activation of the non-receptor tyrosine kinases FAK and Src-family kinases174. These kinases phosphorylate a variety of substrates, triggering the formation of new protein complexes including those involving guanine nucleotide exchange factors, such as β-PIX and TIAM1, and the activation of Rac, which leads to the formation of lamellipodial structures. When these lamellipodia protrude up the gradient of fibronectin and encounter more ECM ligands for the integrins, a positive-feedback loop is established that is critical for haptotaxis of fibroblasts on gradients of fibronectin. However, it is not clear whether signal amplification akin to what happens during chemotaxis occurs or is required during ECM haptotactic signalling. In the case of haptotactic migration on gradients of surface-bound chemokines, the presumption is that the signalling pathways activated by the cognate GPCRs are the same as the signalling pathways activated by the diffusible version of the chemotactic cue, but this presumption needs further experimental validation. Interestingly, one study reported differential regulation of CCR7 towards haptotactic ECM-bound gradients of CCL21 versus chemotactic, diffusible CCL21 (REF.103). This suggests that a cell response to haptotactic versus chemotactic cues, even when involving the same receptor/pathway, may be specifically modulated to adjust cell behaviour to the migratory context.
TORC2
Target of rapamycin complex 2 is composed of seven conserved subunits and is involved in regulating proliferation, survival, cell migration and cytoskeletal reorganization.
Phospholipase A2
An enzyme that cleaves phospholipids to give rise to lipid products (arachidonic acid or lysophosphatidic acid) that either have the ability to regulate signalling events or are substrates in the generation of bioactive lipids.
MAPK/ERK
A group of protein kinases that transduce signals from cell surface receptors to the nucleus.
Durotaxis.
Delineating how durotactic signals are transmitted is challenging as the ‘receptor’ or ‘receptors’ responding to durotactic cues are not definitively known. Furthermore, the distinction between sensing and transmitting a mechanical signal could overlap considerably, and thus is not nearly as clear-cut as during other directed migration types, such as chemotaxis. However, the signal transduction mechanisms involved during durotaxis can be broadly grouped into two classes, depending on the cell type and environmental context.
Förster resonance energy transfer
A mechanism describing energy transfer between two light-sensitive molecules.
First, mechanically sensitive enzymes and proteins can generate traditional second messengers such as intracellular calcium changes. The best studied examples of these are the non-selective PIEZO1/2 stretch-activated ion channels175. These proteins have been linked to a wide variety of mechanosensation events but, to our knowledge, have not yet been specifically tested during durotaxis. Cytoplasmic enzyme kinetics can also be controlled by mechanical load176, which could theoretically lead to altered second messenger signalling, but this has not been documented during mechanically triggered cell migration.
Second, mechanical force can result in protein conformational changes, such as unfolding, or changes in biophysical properties, such as catch-bond behaviour, where mechanical load increases bond strength. One of the first proteins shown to display a mechanically sensitive conformational change and altered binding partners was p130Cas, an adaptor protein regulating tyrosine kinase-based signalling related to cell adhesion118. This paradigm of molecular stretching under mechanical load has strongly influenced how we think about mechanosensing177. This has been further reinforced by the generation of Förster resonance energy transfer-based molecular tension biosensors, including those of integrin adhesion-associated vinculin and talin, which enabled the visualization of local changes in tension that may constitute signals for directed migration117,178–181. Both vinculin and talin show F-actin binding that is increased under mechanical load182,183. This catch-bond binding for both proteins is asymmetric, with a bias towards binding the pointed (minus) end of the actin filament. In nascent adhesion structures at the leading edge, the proximal actin networks — which generally face the membrane with their barbed (plus) ends — are flowing backwards by retrograde flow from the leading edge. This polarized flowing population of filaments can be engaged by vinculin and talin associated with the nascent adhesions due to their asymmetric catch-bond properties, possibly contributing to localized adhesion maturation and hence directional migration. The next step will be to observe how these local, tension-dependent conformational changes translate to more global mechanotransduction networks across single cells migrating on gradients of substrate stiffness.
Topotaxis.
How guidance signals from tissue topography are transmitted within cells is not clearly defined. One can speculate that it will involve calcium, in light of the well-recognized role of calcium in converting external information into biological signals that subsequently lead to actin polymerization. Enhanced intracellular calcium activity has been detected in astrocytes seeded onto a micropatterned surface184. Astrocytes were found to be elongated and aligned along the direction of the grooves accompanied by frequent calcium peaks in the aligned cells relative to randomly oriented, rounded cells on flat surfaces184. Whether such calcium activity also tunes cell motility on such patterned surfaces is less clear. It has been partly addressed using surface topology with different degrees of confinement that mimic narrow channels and fibre-like tracks of natural ECM in vivo. In one of the recent studies, elevated intracellular calcium concentration was found to be important in mobilizing cells through confined microchannels, where the confinement-driven force was transmitted inside the cells as calcium influx via activation of PIEZO1 (REF.185). However, cell migration in the study was induced in the presence of a chemotactic source, indicating the need to further investigate whether topology or confined space-driven calcium influx controls cell motility independently of chemical cues.
Furthermore, PIEZO1 activation was recently shown to be regulated by the geometric features, such as roughness and stiffness, of the surrounding substrate when force was applied externally by substrate deflection at cell–substrate interfaces186. For instance, when individual pillars of a deformable micropillar array were deflected, the amplitude of the PIEZO1-mediated current was higher in cells surrounded by sparsely arrayed pillars, which led to less substrate roughness than for the densely arrayed pillars. Notably, the amplitude of the PIEZO1-mediated current induced by pillar deflection decreased when cells contacted substrates with greater stiffness but relatively low roughness186. Whether modulation of PIEZO1 channel activation by substrate mechanics affects calcium spatio-temporal dynamics and ultimately cell migration in response to various degrees of compressive, tensile and shear forces relevant in physiological contexts, such as dense tissues or blood capillaries, remains to be addressed.
There is also evidence that topotactic cues, and in particular cell compaction, are sensed via the nucleus, which will inevitably undergo deformation in this context, and that both calcium and the lipid-based second messenger arachidonic acid are essential to transmit this signal to the cytoskeleton71,136,137. In this mechanism, nuclear envelope stretching and unfolding resulting from the compressive force of compaction is associated with calcium release from internal stores, possibly through stretch-sensitive calcium channels. This then triggers the redistribution of the calcium-dependent phospholipase A2 (cPLA2) from the cytosol to the stretched nuclear envelope. Subsequent activation of cPLA2 leads to release of arachidonic acid from the nuclear envelope phospholipids. cPLA2-mediated release of arachidonic acid is critical for the biosynthesis of lipid mediators, including LTB4, which have well-established roles in activating myosin contractility and immune cell migration in an autocrine and paracrine manner42,187. Notably, LTB4-synthesizing enzymes also reside in the nucleus, which thereby identifies the nucleus as a dynamic hub for both sensing elements and second messenger production during cell motility in response to confined topology.
Pseudopodia
Protrusive structures in amoeboid cells generated by branched and linear actin filament arrays in the leading edge and aligned with the direction of movement.
Ena/VASP
Enabled/vasodilator-stimulated phosphoproteins are actin polymerases that drive actin filament elongation and antagonize filament capping, leading to the generation of linear actin filaments.
Galvanotaxis.
Cells in electric fields activate a wide variety of intracellular signalling pathways, including AKT, Src-family kinases, MEK–ERK and JAK1 (REF.77). Consistent with this finding, galvanotaxis is sensitive to many pharmacological inhibitors and genetic perturbation of intracellular signalling pathways77,188. In this way, galvanotaxis is most similar to chemotaxis in terms of signal transmission. Consistent with this idea, a large-scale genetic screen for D. discoideum mutants defective in galvanotaxis revealed an impressive overlap of identified genes with those previously implicated in chemotaxis188. The activation of a wide array of signalling pathways may arise due to the electromigration-based clustering of a range of cell surface receptors on either side of the cell (relative to the electric field) that somehow activates downstream, intracellular signalling pathways leading to polarized signal transduction139,140. Indeed, recent data indicate that localized, photo-induced clustering of GPCRs can induce ligand-independent signalling189. Interestingly, in keratocytes, inhibition of one of the kinases commonly associated with galvanotaxis, PI3K, can actually reverse the polarity of galvanotaxis, leading to migration of cells towards the anode rather than the cathode139. Although the mechanism of this reversal is not known, it may indicate that electric field-induced signalling is being activated on both the anode-facing side and the cathode-facing side of the cell, resulting in a ‘tug of war’ for cell polarization decisions that can be influenced by perturbation of intracellular signalling.
Formin
A group of actin polymerases that drives the formation of linear actin filaments.
Executing the signal
The translocation of cells, individually or in groups, relative to their environment requires the generation of asymmetric or polarized forces relative to the substrate. There are several sources of this force generation, but a key concept is that directed migration-promoting signalling biases these forces either towards or away from environmental cues. Directed cell migration cues do not activate special mechanisms of cell translocation but bias the migration and cell polarity machinery that operates during normal, random cell migration12. Depending on the mode of migration used by a particular cell type (BOX 1), multiple directed migration cues can converge on a common set of force generation mechanisms leading to cell translocation. Thus, with this fourth pillar of directed migration, we will not separate the directed migration forms by the environmental cue; rather, we will discuss the different forms of asymmetric force generation activated during directed migration (FIG. 4) and describe how the signal transmission step regulates this force generation.
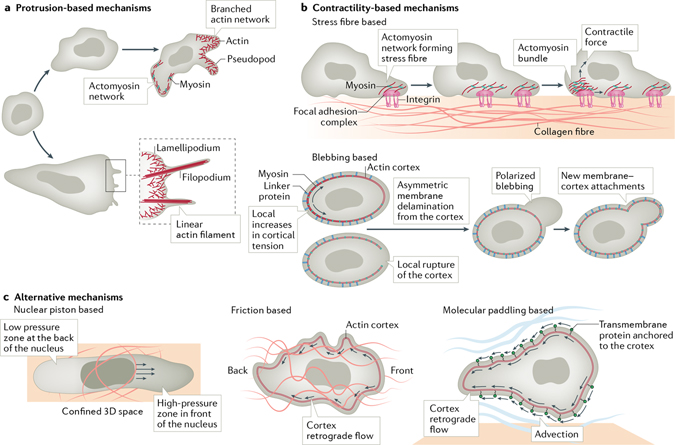
Fig. 4 |. Executing the signal.
Depiction of how asymmetric force is generated through protrusion-based, contractility-based or alternative mechanisms. a | Formation of protrusions such as pseudopodia, lamellipodia and/or filopodia is driven by branched and linearly polymerized actin networks. b | Contractility-based mechanisms of migration (characteristic of mesenchymal cells) depend on the establishment of stress fibres, where a contractile array of actomyosin networks is mechanically coupled to the substrate through integrin-based focal adhesions (top). Many cell types, in particular in the in vivo context, migrate via extension of blebs (bottom), which are generated through local increases in cortical tension on the non-blebbing side of the cell (marked with arrows) and asymmetric membrane tearing (delamination), or by local rupture of the cortex, or both. c | Cells moving through a confined space use the nuclei as a piston to generate zones of high cytosolic pressure at the leading edge. Cells moving in the absence of substrate adhesion depend on friction between cells and the environment, generated by retrograde flow of the cortical actin. Cells can also use molecular paddling to swim through the environment using horizontal rearward flow of transmembrane proteins anchored to the actin cortex (advection).
Protrusion-based mechanisms.
One of the characteristic features of most migrating cells is that they have some kind of protrusive structure at their leading edge, which is aligned with the direction of movement (FIG. 4a). With a few notable exceptions, such as nematode sperm cells190, most protrusions are driven by the polymerization of actin filament arrays191. In amoeboid cells, these are known as pseudopodia, and the actin is a mixture of branched actin networks produced by the activation of the Arp2/3 complex and linear actin arrays produced by Ena/VASP and formin proteins192. In fibroblasts and other more adherent cells, the protrusions often take on a flatter appearance and fan-like architecture (referred to as ‘lamellipodia’) that is more dominated by Arp2/3-branched actin, although they also contain linear actin microspikes that can extend beyond the edge as filopodia193. Some cells, such as neuronal growth cones, have protrusions that are almost entirely formed by filopodia, containing bundled linear arrays of actin with concentrated actin polymerases such as Ena/VASP and formins at their tip194. Of note, these different protrusions are not finite entities and are highly plastic and can dynamically interconvert over time, such as when filopodia direct where lamellipodia can form195 or when lamellipodia yield clusters of filopodia196. The proportion of actin network types within protrusions (branched versus linear) is probably more important than the historical naming convention for the protrusion type.
SCAR/WAVE
Suppressor of cAR/WAsP family verprolin-homologous protein is a nucleation-promoting factor that activates actin nucleation activity of the Arp2/3 complex.
Over the years, many lines of evidence have linked the formation of protrusions with various directed migration cues193,197. For example, one of the most satisfying connections between signalling pathways and protrusions is the activation of the Arp2/3 complex, via Rho-family GTPases and nucleation-promoting factors such as SCAR/WAVE, a topic that has been extensively reviewed elsewhere191,198. However, several groups have shown that cells lacking the Arp2/3 complex can still undergo chemotaxis in various contexts, albeit with reduced migration efficiency197,199–201. This indicates that the Arp2/3-branched actin pathway is not strictly required for chemotaxis in all circumstances and points towards other possible mechanisms of asymmetric force generation.
Other mechanisms responsible for triggering or tuning protrusions have been identified. In the case of haptotaxis, the activation of the Arp2/3 complex and the subsequent generation of branched actin networks at nascent integrin adhesions requires both FAK–Src and the RAC1 GTPase, integrated through the WAVE regulatory complex174,197 (FIG. 3c). In the case of neuronal growth cones responding to chemotactic guidance cues, emerging evidence suggests that monoubiquitinylation of VASP mediated by the ubiquitin ligase TRIM9 helps tune the activity of this actin polymerase at the tips of filopodia202. In the case of durotaxis, both Arp2/3-based lamellipodia203 and filopodia204 are required to sense and respond to varying substrate stiffness, although whether these protrusions are regulated through traditional, second messenger-type signalling pathways that are responsive to substrate stiffness remains to be determined. One consistent mechanism of protrusion control is the splitting of existing protrusions and the selective stabilization of the protrusion that is oriented more strongly towards the gradient of the signal. This has been demonstrated in the chemotactic migration of both D. discoideum and fibroblasts205,206, and may reflect a common strategy for many forms of directed migration. Finally, in cells undergoing topotaxis, including D. discoideum, neutrophils and breast cancer cells, it was observed that asymmetric microstructures of the substratum induce actin waves, which propagate unidirectionally, defining the direction of cell translocation. Curiously, the waves could propagate in different directions, depending on the cell type, and this was associated with the establishment of different focal adhesion patterns and differences in membrane dynamics and protrusion extension, which were linked to distinct local cell cortical plasticity in different cell types129,207.
Contractility-based mechanisms.
The other main generator of asymmetric force relative to the substrate for migrating cells is contractile arrays of actin and non-muscle myosin II (REF.208) (FIG. 4b). In the case of firmly adherent cells, such as mesenchymal cells, these arrays of contractile actin form stress fibres that are mechanically coupled to the substrate through integrin-based focal adhesions. Asymmetric strengthening or weakening of these adhesion structures provides a dynamic way to control the direction of cell migration. Indeed, many signalling pathways have been linked to adhesion turnover, including phosphorylation of specific adhesion proteins209 and the myosin II regulatory light chain156, selective proteolytic cleavage of adhesion proteins by calpain210 and relaxation of adhesions through contacts with microtubules68. Remodelling of focal adhesions almost certainly underpins durotactic migration in many cell types122,209 but may also be relevant for chemotactic migration of fibroblasts156.
Calpain
Calcium-activated cysteine protease that cleaves adhesion complex proteins.
Border cell
A specialized cell type that migrates as a group through the egg chambers in Drosophila melanogaster.
Blebs
Spherical membrane protrusions that rely on myosin-based contraction and pressure-driven cytosolic flow. Bleb-like protrusions are commonly used for motility by amoebas and embryonic cells. However, leukocytes and tumour cells can use blebbing motility especially in 3D environments under confined conditions.
In the case of cells with robust cell–cell adhesions, such as sheets of epithelial cells, myosin-based contractility can also be a potent mechanism to direct migration211,212. In this case, the contractile arrays are linked to the cell–cell adhesion sites and control the shape of cells relative to their neighbours, as well as coordinate the transmission of mechanical force across multiple cells. Cells with strong intercellular junctions often move collectively as groups, as in the case of border cell migration in D. melanogaster and neural crest migration in vertebrates. In recent work on neural crest migration in zebrafish and Xenopus laevis embryos, the migrating cluster of cells was shown to have a mechanically continuous band of actomyosin contractility around the periphery of the cluster that can be locally inhibited by chemotactic cues at the front to produce a form of ‘rear wheel’ drive motility213.
Reynolds number
A dimensionless number important in fluid mechanics. Cellular scales are inherently a low Reynolds number environment where inertia and momentum are negligible and thus movement requires strategies different from those for human-relevant length scales.
At the single-cell level, localized control of contractility (either positive or negative) has a profound effect on polarization and directed migration214. In fibroblasts migrating in a gradient of the chemoattractant PDGF, local inhibition of myosin II at the leading edge is critical for chemotaxis156. At the opposite end of the cell, recent studies in neutrophils and keratocytes have shown that control of rear contractility governs the direction of cell movement215,216. Even in cells that lack strong cell–substrate or cell–cell adhesions, such as amoeboid tumour cells, local contractility may have a key role in the generation of cellular blebs through local increases in cortical tension on the non-blebbing side of the cell, local relaxation of the cortex to permit blebbing or both217. However, the signals upstream of polarized blebbing are incompletely understood218.
Alternative mechanisms via pistons, ridges and molecular paddles.
Beyond actin-based protrusions and actomyosin contractility, cells have other ways to generate asymmetric traction force relative to their environment and move (FIG. 4c). However, most of these have not been definitively linked to specific directed migration cues. Cells moving through confined environments can use their nucleus as a piston to generate cytoplasmic pressure gradients at the leading edge to facilitate migration126. Possible cellular regulation of this ‘nuclear piston’ mode could involve the association of the nucleus with lateral membrane or localized osmotic control. Other mechanisms are observed in immune cells, which do not require integrins for their migration in 3D environments111. In one recent study it was shown that T cells can translocate using friction generated between the cell and its environment, which is coupled to cellular translocation through retrograde flow of the actin cortex219. In another recent study, non-adherent leukocytes were shown to ‘swim’ through their environment using a fascinating ‘molecular paddling’ mechanism, which is mediated by transmembrane proteins coupled to the actin cortex and moving by advection across the cell surface in a coherent manner relative to their environment220. This is a remarkably efficient migration strategy, with cell speeds similar to those of adhesion-based migration. Such mechanisms could be of great importance for migration through in vivo environments, given that cellular environments are characterized by a low Reynolds number221 — meaning that at cellular scales, inertia and momentum are negligible and thus movement requires strategies different from those for human-relevant length scales. However, it is unclear whether this paddling mechanism is used only for random migration or is also responsive to external environmental cues.
Conclusions and perspective
Directed migration has been studied for many decades, but our understanding of the mechanisms underpinning this process has increased dramatically only over the past 10 years. New approaches for producing stable and/or tunable gradients of various factors, controlled substrate geometries and new methods for imaging cells in 3D, physiological contexts have expanded our knowledge of the molecular and cellular processes required to execute directed migration. The unifying, conceptual framework presented herein has allowed us to highlight this progress. Going forwards, we hope that this framework will prompt the field to fill in gaps in our understanding (see BOX 2).
Box 2 |. Key open questions for specific modes of directed cell migration.
Chemotaxis
How are stable gradients of diffusible cues generated and maintained in tissues with interstitial fluid flows as in most multicellular organisms?
Haptotaxis
Is haptotaxis using extracellular matrix as a migratory cue used only by mesenchymal cells that have strong substrate adhesion? Do low-adhesion amoeboid cells still sense these cues?
Durotaxis
How do cells sense durotactic cues? Are there multiple/cell type-specific ‘receptors’ for durotaxis, and how do they connect to the cell polarity and translocation machinery?
Topotaxis
How are topotactic cues sensed by cells? Are there different mechanisms that are specific to particular 2D or 3D geometries? How does topology sensing by membrane curvature-sensitive proteins affect leading-edge protrusions and translate into guided migration?
Galvanotaxis
Electrophoretic migration of cell surface receptors within the plane of the plasma membrane and their activation seems to be critical for galvanotaxis, but how are those receptors activated by electric current-induced clustering? Is there polarized autocrine secretion of ligands that accompanies this clustering or is clustering of receptors sufficient to activate signalling in a ligand-independent manner?
One of the broader challenges in studying directed cell migration is understanding it in physiological contexts. Studying cell migration on the rigid, 2D surfaces commonly used for microscopy has been criticized as not reflecting these physiological contexts222. To be fair, the tools and imaging approaches needed to study migration in these more physiological contexts or in living organisms have only recently become widely available to researchers, and the field will need to continue to embrace this transition. However, studying directed cell migration using simplified 2D systems and model organisms has revealed underlying principles and insights that have become the building blocks to understand migration in more complex systems. By analogy, studying the biochemical properties of purified proteins, even though these systems do not replicate the complexity of the cytoplasm, continues to yield essential information. Multiple approaches to problems as complex as directed migration are required to achieve the level of understanding necessary to manipulate this process or develop therapeutic interventions.
A particularly difficult aspect of understanding directed migration in vivo is that cells are being exposed to multiple migration-inducing cues simultaneously. This ‘multicue’ problem is both fascinating and experimentally challenging. For example, if a cell is presented with a chemotactic cue orienting its migration in one direction but a topological or durotactic cue is orienting it in the opposite direction, how does the cell prioritize or integrate these conflicting signals? One compelling recent example of this problem comes from D. melanogaster border cell migration where the relative contributions of chemotactic and topological cues were dissected by elegant genetic approaches223. However, knowing the specific perturbations necessary to dissect multicue migration in vivo requires a specific understanding of the underlying processes, and more work in simplified, single-cue systems will be needed. In a related problem, how do cells switch their migration direction? For example, neutrophils initially home to injured sites but later reverse their migration and move away from the inflamed area as part of the resolution process224–226. Again, a more comprehensive understanding of the mechanisms of directed migration will be required to understand complex cases such as these.
A deeper understanding of directed cell migration will be necessary to effectively treat human diseases. Interventions that could block tumour cells from leaving the primary tumour or intravasating into blood or lymph vessels could be a potent way to block metastasis — the clinically most deadly aspect of cancer. Similarly, attenuating the recruitment of immune cells to inflamed areas in the context of chronic illnesses such as arthritis, asthma and atherosclerosis could improve disease outcome. Conversely, encouraging the migration of subsets of immune cells into tumours could improve anticancer immune therapies. The inappropriate migration of immune cells or fibroblasts can also lead to autoimmune disorders and fibrosis, respectively. Finally, finding ways for neuronal cells to ignore ‘stop’ cues such as in nerve injuries could lead to regenerative treatments for paralysis. Understanding how cells, using different migration modes (for example, amoeboid versus mesenchymal), migrate in response to the various chemical, mechanical, geometric and electrical cues that are present in these disease states has the potential to lead to the identification of novel therapeutic avenues.
Acknowledgements
The authors apologize to their colleagues whose work on this vast topic they were unable to cite due to length constraints. They thank M. Butler for designing some of the artwork in Fig. 3c, and J. Haugh for thoughtful discussions. J.E.B. acknowledges support from the NIH (R35GM130312 and U01EB018816). C.A.P. acknowledges support from the NIH (R01AI152517).
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Molecular Cell Biology thanks R. Insall, M. Sixt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Borrell V Recent advances in understanding neocortical development. F1000Res 10.12688/f1000research.20332.1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawar KB, Desai S, Bhonde RR, Bhole RP & Deshmukh AA Wound with diabetes: present scenario and future. Curr. Diabetes Rev. 10.2174/1573399816666200703180137 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Janssen E & Geha RS Primary immunodeficiencies caused by mutations in actin regulatory proteins. Immunol. Rev. 287, 121–134 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Novikov NM, Zolotaryova SY, Gautreau AM & Denisov EV Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 10.1038/s41416-020-01149-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Cuesta EM et al. The role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases. Front. Endocrinol. 10, 585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass DS et al. Idiopathic pulmonary fibrosis: molecular mechanisms and potential treatment approaches. Respir Investig. 58, 320–335 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Abercrombie M The Croonian Lecture, 1978 - the crawling movement of metazoan cells. Proc. Royal Soc. B Biol. Sci. 207, 129–147 (1980). [Google Scholar]
- 8.Abercrombie M, Dunn GA & Heath JP The shape and movement of fibroblasts in culture. Soc. Gen. Physiol. Ser. 32, 57–70 (1977). [PubMed] [Google Scholar]
- 9.Sardelli L et al. Engineering biological gradients. J. Appl. Biomater. Funct. Mater. 17, 2280800019829023 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Buttenschon A & Edelstein-Keshet L Bridging from single to collective cell migration: a review of models and links to experiments. PLoS Comput. Biol. 16, e1008411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson PC Random locomotion; chemotaxis and chemokinesis. a guide to terms defining cell locomotion. Immunol. Today 6, 273–278 (1985). [DOI] [PubMed] [Google Scholar]
- 12.Petrie RJ, Doyle AD & Yamada KM Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 10, 538–549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorward DA et al. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 185, 1172–1184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrengruber MU, Geiser T & Deranleau DA Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 346, 181–184 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Sadik CD & Luster AD Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 91, 207–215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald B & Kubes P Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. 89, 1079–1088 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Junger WG Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo SK, Starnes TW, Deng Q & Huttenlocher A Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109–112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katikaneni A et al. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat. Cell Biol. 22, 1049–1055 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blockus H & Chedotal A Slit-Robo signaling. Development 143, 3037–3044 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Boyer NP & Gupton SL Revisiting netrin-1: one who guides (axons). Front. Cell Neurosci. 12, 221 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S & Zhu L Semaphorins and their receptors: from axonal guidance to atherosclerosis. Front. Physiol. 9, 1236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kania A & Klein R Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 17, 240–256 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Crick F Diffusion in embryogenesis. Nature 225, 420–422 (1970). [DOI] [PubMed] [Google Scholar]
- 25.Majumdar R, Sixt M & Parent CA New paradigms in the establishment and maintenance of gradients during directed cell migration. Curr. Opin. Cell Biol. 30, 33–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau S et al. A negative-feedback loop maintains optimal chemokine concentrations for directional cell migration. Nat. Cell Biol. 22, 266–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stapornwongkul KS, de Gennes M, Cocconi L, Salbreux G & Vincent JP Patterning and growth control in vivo by an engineered GFP gradient. Science 370, 321–327 (2020). Using a synthetic morphogen in D. melanogaster, the authors show that the expression of non-signalling decoy receptors improves the ability of receptors engineered to respond to the synthetic morphogen to organize patterning and growth in vivo.
- 28.Tweedy L, Knecht DA, Mackay GM & Insall RH Self-generated chemoattractant gradients: attractant depletion extends the range and robustness of chemotaxis. PLoS Biol. 14, e1002404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muinonen-Martin AJ et al. Melanoma cells break down LPA to establish local gradients that drive chemotactic dispersal. PLoS Biol. 12, e1001966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juin A et al. N-WASP control of LPAR1 trafficking establishes response to self-generated LPA gradients to promote pancreatic cancer cell metastasis. Dev. Cell 10.1016/j.devcel.2019.09.018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tweedy L et al. Seeing around corners: cells solve mazes and respond at a distance using attractant breakdown. Science 10.1126/science.aay9792 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Pond KW, Doubrovinski K & Thorne CA Wnt/β-catenin signaling in tissue self-organization. Genes (Basel) 10.3390/genes11080939 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu P, Takai K, Weaver VM & Werb Z Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect Biol. 10.1101/cshperspect.a005058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afonso PV, McCann CP, Kapnick SM & Parent CA Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood 121, 1644–1650 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields JD et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Polacheck WJ, Charest JL & Kamm RD Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl Acad. Sci. USA 108, 11115–11120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller P, Rogers KW, Yu SR, Brand M & Schier AF Morphogen transport. Development 140, 1621–1638 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Restrepo S, Zartman JJ & Basler K Coordination of patterning and growth by the morphogen DPP. Curr. Biol. 24, R245–R255 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Kriebel PW, Barr VA, Rericha EC, Zhang G & Parent CA Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J. Cell Biol. 183, 949–961 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriebel PW et al. Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. J. Cell Biol. 217, 2891–2910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esser J et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 126, 1032–1040 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Afonso PV et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lämmermann T et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown M et al. Lymphatic exosomes promote dendritic cell migration along guidance cues. J. Cell. Biol. 217, 2205–2221 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T, Guo J, Yang M, Zhu X & Cao X Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 186, 2219–2228 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Lim K et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349, aaa4352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung BH, Ketova T, Hoshino D, Zijlstra A & Weaver AM Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 6, 7164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sung BH & Weaver AM Exosome secretion promotes chemotaxis of cancer cells. Cell Adh. Migr. 11, 187–195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hynes RO Stretching the boundaries of extracellular matrix research. Nat. Rev. Mol. Cell Biol. 15, 761–763 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Charras G & Sahai E Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 15, 813–824 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Mouw JK, Ou G & Weaver VM Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771–785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnans C, Chou J & Werb Z Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albacete-Albacete L et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. J. Cell Biol. 10.1083/jcb.202006178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerman SP, Asokan SB, Kuhlman B & Bear JE Cells lay their own tracks - optogenetic Cdc42 activation stimulates fibronectin deposition supporting directed migration. J. Cell Sci. 130, 2971–2983 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh P, Carraher C & Schwarzbauer JE Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 26, 397–419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller CG, Budoff G, Prenner JL & Schwarzbauer JE Minireview: fibronectin in retinal disease. Exp. Biol. Med. 242, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schultz GS & Wysocki A Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17, 153–162 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Li Q, Park PW, Wilson CL & Parks WC Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111, 635–646 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Schumann K et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010). [DOI] [PubMed] [Google Scholar]
- 60. Zhu M et al. Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. Proc. Natl Acad. Sci. USA 117, 4781–4791 (2020). This is a technical tour de force demonstrating the existence of stiffness gradients in mouse embryos.
- 61.Tran VD & Kumar S Transduction of cell and matrix geometric cues by the actin cytoskeleton. Curr. Opin. Cell Biol. 68, 64–71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai L, Xiong X, Kong X & Xie J The role of the lysyl oxidases in tissue repair and remodeling: a concise review. Tissue Eng. Regen. Med. 14, 15–30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Handorf AM, Zhou Y, Halanski MA & Li WJ Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11, 1–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye M et al. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 215, 107633 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Alcudia JF et al. Lysyl oxidase and endothelial dysfunction: mechanisms of lysyl oxidase down-regulation by pro-inflammatory cytokines. Front. Biosci. 13, 2721–2727 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Peng W, Raffetto JD & Khalil RA Matrix metalloproteinases in remodeling of lower extremity veins and chronic venous disease. Prog. Mol. Biol. Transl Sci. 147, 267–299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quintero-Fabián S et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 9, 1370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaverina I, Krylyshkina O & Small JV Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell. Biol. 146, 1033–1044 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul CD, Mistriotis P & Konstantopoulos K Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf K, Müller R, Borgmann S, Bröcker EB & Friedl P Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–3269 (2003). [DOI] [PubMed] [Google Scholar]
- 71. Renkawitz J et al. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature 568, 546–550 (2019). This seminal article shows that leukocytes position the nucleus frontwards to gauge the available space and choose their path.
- 72.Castro-Castro A et al. Cellular and molecular mechanisms of MT1-MMP-dependent cancer cell invasion. Annu. Rev. Cell Dev. Biol. 32, 555–576 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS & Sahai E ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Fisher KE et al. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J. Cell Sci. 122, 4558–4569 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weigelin B, Bakker GJ & Friedl P Intravital third harmonic generation microscopy of collective melanoma cell invasion: Principles of interface guidance and microvesicle dynamics. Intravital 1, 32–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du Bois-Reymond EH Vorläufiger Abriss einer Untersuchung über den sogenannten Froschstrom und über die elektromotorischen Fische. Ann. Phys. 10.1002/andp.18431340102 (1843). [DOI] [Google Scholar]
- 77.Zhao M et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442, 457–460 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Hotary KB & Robinson KR Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev. Biol. 166, 789–800 (1994). [DOI] [PubMed] [Google Scholar]
- 79.Klein P S. et al. A chemoattractant receptor controls development in Dictyostelium discoideum. Science 241, 1467–1472 (1988). [DOI] [PubMed] [Google Scholar]
- 80.Murphy PM & Tiffany HL Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 253, 1280–1283 (1991). [DOI] [PubMed] [Google Scholar]
- 81.Holmes WE, Lee J, Kuang WJ, Rice GC & Wood WI Structure and functional expression of a human interleukin-8 receptor. Science 253, 1278–1280 (1991). [DOI] [PubMed] [Google Scholar]
- 82.Griffith JW, Sokol CL & Luster AD Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Hughes CE & Nibbs RJB A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zlotnik A & Yoshie O Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Sallusto F & Baggiolini M Chemokines and leukocyte traffic. Nat. Immunol. 9, 949–952 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Locati M & Murphy PM Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu. Rev. Med. 50, 425–440 (1999). [DOI] [PubMed] [Google Scholar]
- 87.Proudfoot AE Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2, 106–115 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He HQ & Ye RD The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules 10.3390/molecules22030455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokomizo T Two distinct leukotriene B4 receptors, BLT1 and BLT2. J. Biochem. 157, 65–71 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Sadik CD, Kim ND & Luster AD Neutrophils cascading their way to inflammation. Trends Immunol. 32, 452–460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anand-Apte B & Zetter B Signaling mechanisms in growth factor-stimulated cell motility. Stem Cells 15, 259–267 (1997). [DOI] [PubMed] [Google Scholar]
- 92.Vorotnikov AV & Tyurin-Kuzmin PA Chemotactic signaling in mesenchymal cells compared to amoeboid cells. Genes Dis. 1, 162–173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bear JE & Haugh JM Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr. Opin. Cell Biol. 30, 74–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorla M & Bashaw GJ Molecular mechanisms regulating axon responsiveness at the midline. Dev. Biol. 466, 12–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bellon A & Mann F Keeping up with advances in axon guidance. Curr. Opin. Neurobiol. 53, 183–191 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Tessier-Lavigne M & Goodman CS The molecular biology of axon guidance. Science 274, 1123–1133 (1996). [DOI] [PubMed] [Google Scholar]
- 97.Mirakaj V & Rosenberger P Immunomodulatory functions of neuronal guidance proteins. Trends Immunol. 38, 444–456 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Wu JY et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410, 948–952 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tole S et al. The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. J. Leukoc. Biol. 86, 1403–1415 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Pilling D, Chinea LE, Consalvo KM & Gomer RH Different isoforms of the neuronal guidance molecule Slit2 directly cause chemoattraction or chemorepulsion of human neutrophils. J. Immunol. 202, 239–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huttenlocher A & Horwitz AR Integrins in cell migration. Cold Spring Harb. Perspect Biol. 3, a005074 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henning Stumpf B, Ambriović-Ristov A, Radenovic A & Smith AS Recent advances and prospects in the research of nascent adhesions. Front. Physiol. 11, 574371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schwarz J et al. Dendritic cells interpret haptotactic chemokine gradients in a manner governed by signal-to-noise ratio and dependent on GRK6. Curr Biol 27, 1314–1325 (2017). This study shows differential regulation of the same chemokine receptor in the context of a chemotactic and haptotactic cue.
- 104.Shellard A & Mayor R Durotaxis: the hard path from in vitro to in vivo. Dev. Cell 10.1016/j.devcel.2020.11.019 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Sunyer R & Trepat X Durotaxis. Curr. Biol. 30, R383–r387 (2020). [DOI] [PubMed] [Google Scholar]
- 106.Janmey PA, Fletcher DA & Reinhart-King CA Stiffness sensing by cells. Physiol. Rev. 100, 695–724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Astrof NS, Salas A, Shimaoka M, Chen J & Springer TA Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry 45, 15020–15028 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang G, Huang AH, Cai Y, Tanase M & Sheetz MP Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys. J. 90, 1804–1809 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nordenfelt P et al. Direction of actin flow dictates integrin LFA-1 orientation during leukocyte migration. Nat. Commun. 8, 2047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moore TI, Aaron J, Chew TL & Springer TA Measuring integrin conformational change on the cell surface with super-resolution microscopy. Cell Rep. 22, 1903–1912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lämmermann T et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008). [DOI] [PubMed] [Google Scholar]
- 112. Hetmanski JHR et al. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell 51, 460–475.e410 (2019). This recent study shows the importance of membrane tension in directed migration.
- 113.Sitarska E & Diz-Muñoz A Pay attention to membrane tension: mechanobiology of the cell surface. Curr. Opin. Cell Biol. 66, 11–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baschieri F et al. Frustrated endocytosis controls contractility-independent mechanotransduction at clathrin-coated structures. Nat. Commun. 9, 3825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Douguet D & Honoré E Mammalian mechanoelectrical transduction: structure and function of force-gated ion channels. Cell 179, 340–354 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Goult BT, Yan J & Schwartz MA Talin as a mechanosensitive signaling hub. J. Cell Biol. 217, 3776–3784 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Grashoff C et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010). This seminal article establishes a tension-based Förster resonance energy transfer biosensor using the focal adhesion protein vinculin.
- 118.Sawada Y et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacKay JL & Hammer DA Stiff substrates enhance monocytic cell capture through E-selectin but not P-selectin. Integr. Biol. 8, 62–72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun X et al. Mechanosensing through direct binding of tensed F-Actin by LIM domains. Dev. Cell 55, 468–482.e467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Winkelman JD, Anderson CA, Suarez C, Kovar DR & Gardel ML Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proc. Natl Acad. Sci. USA 117, 25532–25542 (2020). This article, along with Sun et al. (2020), shows that LIM domain-containing proteins bind differentially to actin filaments based on filament tension.
- 122.Rong Y et al. The Golgi microtubules regulate single cell durotaxis. EMBO Rep. 10.15252/embr.202051094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guilluy C et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16, 376–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arsenovic PT et al. Nesprin-2G, a component of the nuclear LINC complex, is subject to myosin-dependent tension. Biophys. J. 110, 34–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gomes ER, Jani S & Gundersen GG Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463 (2005). [DOI] [PubMed] [Google Scholar]
- 126.Petrie RJ, Koo H & Yamada KM Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lou HY, Zhao W, Zeng Y & Cui B The role of membrane curvature in nanoscale topography-induced intracellular signaling. Acc. Chem. Res. 51, 1046–1053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Driscoll MK, Sun X, Guven C, Fourkas JT & Losert W Cellular contact guidance through dynamic sensing of nanotopography. ACS Nano 8, 3546–3555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen S et al. Actin cytoskeleton and focal adhesions regulate the biased migration of breast cancer cells on nanoscale asymmetric sawteeth. ACS Nano 13, 1454–1468 (2019). This article shows that breast cancer cell lines exhibit different migration phenotypes on asymmetric sawtooth nanopatterns through the distinct regulation of actin polymerization waves and focal adhesion dynamics.
- 130. Le Maout E, Lo Vecchio S, Kumar Korla P, Jinn- Chyuan Sheu J & Riveline D Ratchetaxis in channels: entry point and local asymmetry set cell directions in confinement. Biophys. J. 119, 1301–1308 (2020). This article shows how cell direction through 3D ratchet channels is dictated by spatial asymmetry in two-sided confined channels and the entry point of the all-sided confined channels.
- 131.Ramirez-San Juan GR, Oakes PW & Gardel ML Contact guidance requires spatial control of leading-edge protrusion. Mol. Biol. Cell 28, 1043–1053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jang KJ et al. Two distinct filopodia populations at the growth cone allow to sense nanotopographical extracellular matrix cues to guide neurite outgrowth. PLoS ONE 5, e15966 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kubow KE, Shuklis VD, Sales DJ & Horwitz AR Contact guidance persists under myosin inhibition due to the local alignment of adhesions and individual protrusions. Sci. Rep. 7, 14380 (2017). This is a key article showing that cells can sense the topology of aligned fibres even in the absence of myosin activity.
- 134. Lou HY et al. Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl Acad. Sci. USA 116, 23143–23151 (2019). This is an important article identifying membrane curvature-sensing BAR-family protein FBP17 as the bridge between surface topology and topology-induced reorganization of the actin network.
- 135.McGregor AL, Hsia CR & Lammerding J Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 40, 32–40 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lomakin AJ et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 10.1126/science.aba2894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Venturini V et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 10.1126/science.aba2644 (2020). This article, along with Lomakin et al. (2020), shows that cells interpret cell shape and control movement in confined spaces by gauging nuclear deformation and initiating signalling events at the nucleus.
- 138.Zhao M Electrical fields in wound healing — an overriding signal that directs cell migration. Semin. Cell Dev. Biol. 20, 674–682 (2009). [DOI] [PubMed] [Google Scholar]
- 139. Allen GM, Mogilner A & Theriot JA Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr. Biol. 23, 560–568 (2013). This is a key article establishing the mechanism of galvanotaxis.
- 140.Sarkar A, Kobylkevich BM, Graham DM & Messerli MA Electromigration of cell surface macromolecules in DC electric fields during cell polarization and galvanotaxis. J. Theor. Biol. 478, 58–73 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Lin BJ et al. Lipid rafts sense and direct electric field-induced migration. Proc. Natl Acad. Sci. USA 114, 8568–8573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X, Miao Y, Pal DS & Devreotes PN Excitable networks controlling cell migration during development and disease. Semin. Cell. Dev. Biol. 100, 133–142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiao Z, Zhang N, Murphy DB & Devreotes PN Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J. Cell Biol. 139, 365–374 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Servant G, Weiner OD, Neptune ER, Sedat JW & Bourne HR Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol. Biol. Cell 10, 1163–1178 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nieto M et al. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J. Exp. Med. 186, 153–158 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailly M et al. Epidermal growth factor receptor distribution during chemotactic responses. Mol. Biol. Cell 11, 3873–3883 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bergeron JJ, Di Guglielmo GM, Dahan S, Dominguez M & Posner BI Spatial and temporal regulation of receptor tyrosine kinase activation and intracellular signal transduction. Annu. Rev. Biochem. 85, 573–597 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Callan-Jones AC & Voituriez R Actin flows in cell migration: from locomotion and polarity to trajectories. Curr. Opin. Cell Biol. 38, 12–17 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB & Devreotes PN G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91 (1998). [DOI] [PubMed] [Google Scholar]
- 150.Servant G et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haugh JM, Codazzi F, Teruel M & Meyer T Spatial sensing in fibroblasts mediated by 3’ phosphoinositides. J. Cell Biol. 151, 1269–1280 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Artemenko Y, Lampert TJ & Devreotes PN Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell Mol. Life Sci. 71, 3711–3747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kunisaki Y et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 174, 647–652 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao T et al. Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J. Leukoc. Biol. 81, 1127–1136 (2007). [DOI] [PubMed] [Google Scholar]
- 155.Yan J et al. A Gβγ effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev. Cell 22, 92–103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Asokan SB et al. Mesenchymal chemotaxis requires selective inactivation of myosin II at the leading edge via a noncanonical PLCgamma/PKCalpha pathway. Dev. Cell 31, 747–760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoeller O & Kay RR Chemotaxis in the absence of PIP3 gradients. CB 17, 813–817 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Liu L, Das S, Losert W & Parent CA mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell 19, 845–857 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen MY, Long Y & Devreotes PN A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev 11, 3218–3231 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee S et al. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol. Biol. Cell 16, 4572–4583 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.He Y et al. Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton. Mol. Biol. Cell 24, 3369–3380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen L et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev. Cell 12, 603–614 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van Haastert PJ, Keizer-Gunnink I & Kortholt A Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J. Cell Biol. 177, 809–816 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Heit B et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat. Immunol. 9, 743–752 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Meliton AY et al. Cytosolic group IVa phospholipase A2 mediates IL-8/CXCL8-induced transmigration of human polymorphonuclear leukocytes in vitro. J. Inflamm. 7, 14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yonker LM et al. Neutrophil-derived cytosolic PLA2α contributes to bacterial-induced neutrophil transepithelial migration. J. Immunol. 199, 2873–2884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma H, Gamper M, Parent C & Firtel RA The Dictyostelium MAP kinase kinase DdMEK1 regulates chemotaxis and is essential for chemoattractant-mediated activation of guanylyl cyclase. EMBO J. 16, 4317–4332 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mendoza MC, Booth EO, Shaulsky G & Firtel RA MEK1 and protein phosphatase 4 coordinate Dictyostelium development and chemotaxis. Mol. Cell Biol. 27, 3817–3827 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heit B, Tavener S, Raharjo E & Kubes P An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159, 91–102 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu X et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 13, 457–464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mouneimne G et al. Spatial and temporal control of cofilin activity is required for directional sensing during chemotaxis. Curr. Biol. 16, 2193–2205 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Miao Y et al. Altering the threshold of an excitable signal transduction network changes cell migratory modes. Nat. Cell Biol. 19, 329–340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhan H et al. An excitable Ras/PI3K/ERK signaling network controls migration and oncogenic transformation in epithelial cells. Dev. Cell 54, 608–623.e605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.King SJ et al. Lamellipodia are crucial for haptotactic sensing and response. J. Cell Sci. 129, 2329–2342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cox CD, Bavi N & Martinac B Biophysical principles of ion-channel-mediated mechanosensory transduction. Cell Rep. 29, 1–12 (2019). [DOI] [PubMed] [Google Scholar]
- 176.Pal N, Wu M & Lu HP Probing conformational dynamics of an enzymatic active site by an in situ single fluorogenic probe under piconewton force manipulation. Proc. Natl Acad. Sci. USA 113, 15006–15011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hu X, Margadant FM, Yao M & Sheetz MP Molecular stretching modulates mechanosensing pathways. Protein Sci. 26, 1337–1351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kumar A et al. Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J. Cell Biol. 213, 371–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Rothenberg KE, Scott DW, Christoforou N & Hoffman BD Vinculin force-sensitive dynamics at focal adhesions enable effective directed cell migration. Biophys. J. 114, 1680–1694 (2018). This recent work using the vinculin Förster resonance energy transfer-based tension sensor shows precise regulation of tension at different focal adhesions during directed migration.
- 180.Ringer P et al. Multiplexing molecular tension sensors reveals piconewton force gradient across talin-1. Nat Methods 14, 1090–1096 (2017). [DOI] [PubMed] [Google Scholar]
- 181.LaCroix AS, Lynch AD, Berginski ME & Hoffman BD Tunable molecular tension sensors reveal extension-based control of vinculin loading. eLife 10.7554/eLife.33927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang DL, Bax NA, Buckley CD, Weis WI & Dunn AR Vinculin forms a directionally asymmetric catch bond with F-actin. Science 357, 703–706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Owen LM, Bax NA, Weis WI & Dunn AR The C-terminal actin binding domain of talin forms an asymmetric catch bond with F-actin. Preprint at bioRxiv 10.1101/2020.09.01.276568 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singh AV et al. Astrocytes increase ATP exocytosis mediated calcium signaling in response to microgroove structures. Sci. Rep. 5, 7847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hung WC et al. Confinement sensing and signal optimization via Piezo1/PKA and myosin II pathways. Cell Rep. 15, 1430–1441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bavi N, Richardson J, Heu C, Martinac B & Poole K PIEZO1-mediated currents are modulated by substrate mechanics. ACS Nano 13, 13545–13559 (2019). This article shows that the surface geometry, including roughness and stiffness, modulates PIEZO1 channel activation in response to the external mechanical force applied at cell–substrate interfaces.
- 187. Subramanian BC et al. The LTB4-BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. J. Cell Biol. 10.1083/jcb.201910215 (2020). This article reports a critical role for neutrophil-derived LTB4 in the regulation of non-muscle myosin II and β2 integrin that depends on the release of extracellular vesicles during neutrophil arrest and extravasation in live mice.
- 188.Gao R et al. A large-scale screen reveals genes that mediate electrotaxis in Dictyostelium discoideum. Sci. Signal 8, ra50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sánchez MF, Els-Heindl S, Beck-Sickinger AG, Wieneke R & Tampé R Photo-induced receptor confinement drives ligand-independent GPCR signaling. Science 10.1126/science.abb7657 (2021). [DOI] [PubMed] [Google Scholar]
- 190.Smith HE Nematode sperm motility. WormBook; 10.1895/wormbook.1.68.2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pollard TD & Borisy GG Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003). [DOI] [PubMed] [Google Scholar]
- 192.Bodor DL, Ponisch W, Endres RG & Paluch EK Of cell shapes and motion: the physical basis of animal cell migration. Dev. Cell 52, 550–562 (2020). [DOI] [PubMed] [Google Scholar]
- 193.Krause M & Gautreau A Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 15, 577–590 (2014). [DOI] [PubMed] [Google Scholar]
- 194.McCormick LE & Gupton SL Mechanistic advances in axon pathfinding. Curr. Opin. Cell Biol. 63, 11–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Johnson HE et al. F-actin bundles direct the initiation and orientation of lamellipodia through adhesion-based signaling. J. Cell Biol. 208, 443–455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Svitkina TM et al. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160, 409–421 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wu C et al. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell 148, 973–987 (2012). This is the first article to establish that the Arp2/3 complex is required for ECM haptotaxis but is dispensable for RTK-dependent chemotaxis in fibroblasts.
- 198.Schaks M, Giannone G & Rottner K Actin dynamics in cell migration. Essays Biochem. 63, 483–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Collins SR et al. Using light to shape chemical gradients for parallel and automated analysis of chemotaxis. Mol. Syst. Biol. 11, 804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davidson AJ, Amato C, Thomason PA & Insall RH WASP family proteins and formins compete in pseudopod- and bleb-based migration. J. Cell Biol. 217, 701–714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vargas P et al. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 18, 43–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Menon S et al. The E3 ubiquitin ligase TRIM9 is a filopodia off switch required for netrin-dependent axon guidance. Dev. Cell 35, 698–712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oakes PW et al. Lamellipodium is a myosin-independent mechanosensor. Proc. Natl Acad. Sci. USA 115, 2646–2651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wong S, Guo WH & Wang YL Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. Proc. Natl Acad. Sci. USA 111, 17176–17181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Andrew N & Insall RH Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 9, 193–200 (2007). [DOI] [PubMed] [Google Scholar]
- 206.Welf ES, Ahmed S, Johnson HE, Melvin AT & Haugh JM Migrating fibroblasts reorient directionality by a metastable, PI3K-dependent mechanism. J. Cell Biol. 197, 105–114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sun X et al. Asymmetric nanotopography biases cytoskeletal dynamics and promotes unidirectional cell guidance. Proc. Natl Acad. Sci. USA 112, 12557–12562 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vicente-Manzanares M, Ma X, Adelstein RS & Horwitz AR Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Plotnikov SV, Pasapera AM, Sabass B & Waterman CM Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chan KT, Bennin DA & Huttenlocher A Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK). J. Biol. Chem. 285, 11418–11426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mayor R & Etienne-Manneville S The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 17, 97–109 (2016). [DOI] [PubMed] [Google Scholar]
- 212.Ladoux B & Mège RM Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 18, 743–757 (2017). [DOI] [PubMed] [Google Scholar]
- 213. Shellard A, Szabó A, Trepat X & Mayor R Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science 362, 339–343 (2018). This article shows a rear wheel drive-like motility during collective chemotaxis by actomyosin contractility in a cluster of rear cells.
- 214.Yam PT et al. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J. Cell Biol. 178, 1207–1221 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Tsai TY et al. Efficient front-rear coupling in neutrophil chemotaxis by dynamic myosin II localization. Dev. Cell 49, 189–205.e186 (2019). This article shows that the side-to-side redistribution of myosin II at the back of cells in response to leading-edge turning is critical for maintaining persistence and turning ability in neutrophils undergoing chemotaxis.
- 216.Allen GM et al. Cell mechanics at the rear act to steer the direction of cell migration. Cell Syst. 11, 286–299.e284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Charras G & Paluch E Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9, 730–736 (2008). [DOI] [PubMed] [Google Scholar]
- 218.Zatulovskiy E, Tyson R, Bretschneider T & Kay RR Bleb-driven chemotaxis of Dictyostelium cells. J. Cell Biol. 204, 1027–1044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Reversat A et al. Cellular locomotion using environmental topography. Nature 582, 582–585 (2020). This article identifies friction force generated by retrograde flow of actin propelling cells forward through a 3D environment in the absence of integrin engagement.
- 220. Aoun L et al. Amoeboid swimming is propelled by molecular paddling in lymphocytes. Biophys. J. 119, 1157–1177 (2020). This fascinating recent work demonstrates a new mechanism of cell swimming using ‘molecular paddling’ of cell surface proteins coupled to flow of the underlying cell cortex.
- 221.Purcell EM Life at low Reynolds number. Am. J. Phys. 45, 3–11 (1977). [Google Scholar]
- 222.Yamada KM & Sixt M Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 (2019). [DOI] [PubMed] [Google Scholar]
- 223. Dai W et al. Tissue topography steers migrating Drosophila border cells. Science 370, 987–990 (2020). This article shows that while chemical cues promote posterior movement, topographical cues provide orthogonal information and a path of least resistance during the migration of border cells in the egg chamber of D. melanogaster embryos.
- 224.Mathias JR et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288 (2006). [DOI] [PubMed] [Google Scholar]
- 225.Wang J et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Hind LE & Huttenlocher A Neutrophil reverse migration and a chemokinetic resolution. Dev. Cell 47, 404–405 (2018). [DOI] [PubMed] [Google Scholar]
- 227.Paluch EK, Aspalter IM & Sixt M Focal adhesion-independent cell migration. Annu. Rev. Cell Dev. Biol. 32, 469–490 (2016). [DOI] [PubMed] [Google Scholar]
- 228.Shellard A & Mayor R All roads lead to directional cell migration. Trends Cell Biol. 30, 852–868 (2020). [DOI] [PubMed] [Google Scholar]
- 229.Haeger A, Wolf K, Zegers MM & Friedl P Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 25, 556–566 (2015). [DOI] [PubMed] [Google Scholar]
- 230.Lämmermann T & Sixt M Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644 (2009). [DOI] [PubMed] [Google Scholar]
- 231.Pagès D-L et al. Cell clusters adopt a collective amoeboid mode of migration in confined non-adhesive environments. Preprint at bioRxiv 10.1101/2020.05.28.106203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu YJ et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015). [DOI] [PubMed] [Google Scholar]
- 233.Ruprecht V et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160, 673–685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Graziano BR et al. Cell confinement reveals a branched-actin independent circuit for neutrophil polarity. PLoS Biol. 17, e3000457 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lehmann S et al. Hypoxia induces a HIF-1-dependent transition from collective-to-amoeboid dissemination in epithelial cancer cells. Curr. Biol. 27, 392–400 (2017). [DOI] [PubMed] [Google Scholar]