Abstract
Introduction:
Parathyroid carcinoma (PC) is rare and often diagnosed incidentally after local resection (LR) for other indications. Although recommended treatment has traditionally been radical surgery (RS), more recent guidelines suggest that LR alone may be adequate. We sought to further investigate outcomes of RS versus LR for localized PC.
Materials and methods:
PC patients from 2004 to 2015 with localized disease were identified from the National Cancer Database, then stratified by surgical therapy: LR or RS. Demographic and clinicopathologic data were compared. Cox proportional hazard models were constructed to estimate associations of variables with overall survival (OS). OS was estimated from time of diagnosis using Kaplan-Meier curves.
Results:
A total of 555 patients were included (LR = 522, RS = 33). The groups were comparable aside from LR patients having higher rates of unknown nodal status (66.9% versus 39.4%; p = 0.003). By multivariable analysis, RS did not have a significant association with OS (hazard ratio (HR) = 0.43, 95% confidence interval (95%CI) = 0.10, 1.83; p = 0.255), nor did positive nodal status (HR = 0.66, 95% CI = 0.09, 5.03; p = 0.692) and unknown nodal status (HR = 1.30, 95%CI = 0.78, 2.17; p = 0.311). There was no difference in OS between the LR and RS groups, with median survival not reached by either group at 10 years (median follow-up = 60.4 months; p = 0.20).
Conclusions:
There was no difference in OS between LR and RS for localized PC. RS and nodal status may not impact survival as previously identified, and LR should remain a valid initial surgical approach. Future higher-powered studies are necessary to assess the effects of surgical approaches on morbidity and oncologic outcomes.
Keywords: Parathyroid carcinoma, Radical surgery, Local resection
Introduction
Parathyroid carcinoma (PC) is a rare malignancy, and its low incidence has resulted in a dearth of evidence-based guidelines for management [1–3]. In addition, it is difficult to diagnose preoperatively in the absence of overt symptoms [4–8]. This presents a unique clinical quagmire for surgeons when final histopathology reveals carcinoma after parathyroidectomy for a presumed benign indication.
Radical surgery (RS), in the form of en bloc resection of the tumor and ipsilateral thyroid, central neck dissection, and often lateral neck dissection, has long been considered the index operation of choice if PC is suspected, or as a second operation after final pathologic evaluation [8]. This recommendation was based on the aggressive nature of PC, lack of effective systemic chemotherapeutic agents, and poor response to radiation [1–3]. However, recent studies have suggested that RS, compared to local resection (LR), may not affect overall survival as much as previously postulated [9–12]. The American Association of Endocrine Surgeons guidelines for primary hyperparathyroidism management comment that if PC is encountered, en bloc resection should be performed if necessary to avoid capsular disruption, but prophylactic central or lateral neck dissection should be avoided [13]. Considering the lack of convincing data for or against RS, and the significant morbidity that may be associated with RS, especially in a re-operative setting, the role for RS warrants further evaluation [14,15]. We sought to compare outcomes of LR versus RS for localized PC using a large national database.
Materials and Methods
The National Cancer Database (NCDB) is a large national dataset maintained by the American College of Surgeons and the American Cancer Society. Due to the retrospective, deidentified nature of the data, this study was deemed exempt from review by our institutional review board.
NCDB data was queried for data between January 2004 and December 2015 for the primary site of the parathyroid glands (World Health Organization International Classification of Diseases 3.0.1 topography code 75.0). Patients with histologically confirmed carcinoma of the parathyroid glands (World Health Organization International Classification of Diseases 3.0.1 histology code 8010) treated with surgery were identified. The LR group included patients with surgical extent defined by the NCDB as “total surgical removal of primary site” (NCDB C75.0 site-specific surgery code 40), while RS was defined by the NCDB as “partial or total removal of the primary site WITH a resection in continuity (partial or total removal) with other organs” (NCDB C75.0 site-specific surgery code 60). Patients with distant metastatic disease at time of surgery were excluded (n = 42) as surgeries for these patients were likely palliative. Those with regional lymphatic metastatic disease were not excluded. Patients who received chemotherapy were excluded (n = 55) to avoid confounding due to a small sample size and lack of information regarding indications. Patients with missing follow-up information (n = 38) were excluded as well. The cohort was then stratified into two groups: those who underwent LR and those who underwent RS (Fig. 1).
Fig. 1.
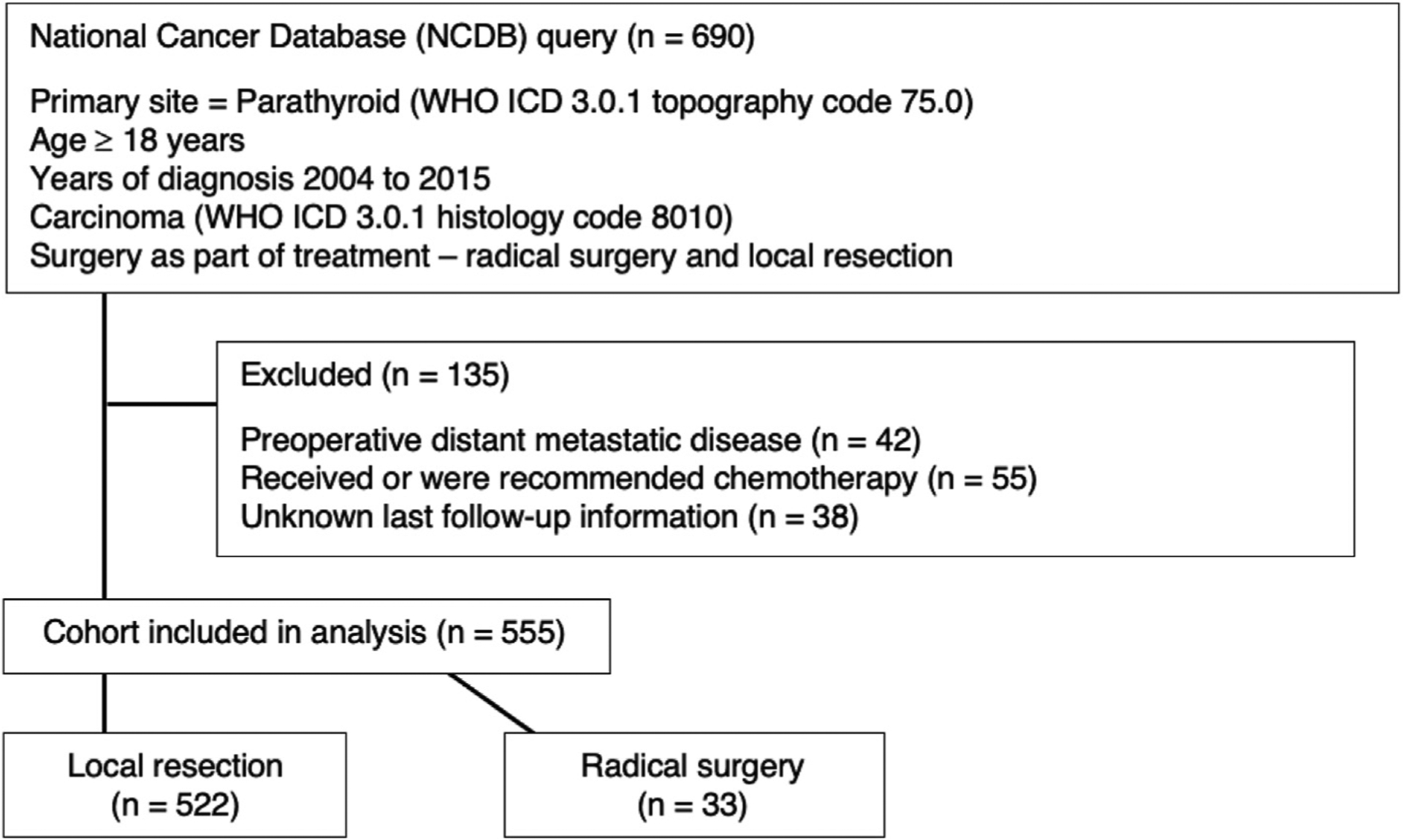
Data sorting process from National Cancer Database query to final cohort selection. Abbreviations: ICD, International Classification of Diseases; WHO, World Health Organization.
Demographic and clinicopathologic data were compared between RS and LR groups using two-sample t-test, Wilcoxon rank-sum test, chi-square test, or Fisher’s exact test where appropriate. Overall survival was estimated from time of diagnosis using Kaplan-Meier curves and compared using log-rank test. Associations of variables with overall survival were analyzed with univariate and multivariable cox proportional hazard models and reported as hazard ratios (HR) with 95% confidence intervals (95% CI). The multivariable model was constructed by purposeful selection using variables whose association with overall survival was measured to be p < 0.10 by univariate analysis. Significance was established at p < 0.05. Statistical analyses were performed using R version 3.5.1 (R Foundation, Vienna, Austria).
Results
A total of 555 patients were included in our analysis, with 522 in the LR cohort and 33 in the RS cohort. The LR and RS cohorts were comparable in terms of demographics, including age, sex, ethnicity, income, insurance, area of treatment, facility type, and Charlson/Deyo comorbidity index (Table 1). Patients were predominantly white (373 [67.2%]), male (286 [51.5%]), and younger (in years: 18–54 = 229 [41.3%], 55–64 = 156 [28.1%], 65–74 = 110 [19.8%], ≥75 = 60 [10.8%]). The RS cohort was more likely to have known lymph node status (LR = 173 [33.1%], RS = 20 (60.6%); p = 0.003). The proportion of patients receiving radiation therapy were not significantly different between the two groups (LR = 457 [87.5%], RS = 25 [75.8%]; p = 0.063).
Table 1.
Comparison of demographics and clinicopathologic characteristics between local resection and radical surgery groups.
| Characteristic | Variable | Local Resection Total (%) n = 522 | Radical Surgery Total (%) n = 33 | p-Value |
|---|---|---|---|---|
| Age | 18–54 | 211 (40.4) | 18 (54.5) | 0.999 |
| 55–64 | 148 (28.4) | 8 (24.2) | ||
| 65–74 | 107 (20.5) | 3 (9.1) | ||
| ≥75 | 56 (10.7) | 4 (12.1) | ||
| Sex | Female | 253 (48.5) | 16 (48.5) | 0.999 |
| Male | 269 (51.5) | 17 (51.5) | ||
| Ethnicity | White | 348 (66.7) | 25 (75.8) | 0.778 |
| Black | 84 (16.1) | 6 (18.2) | ||
| Hispanic | 44 (8.4) | 1 (3.0) | ||
| Other | 17 (3.3) | 0 (0.0) | ||
| Unknown | 29 (5.6) | 1 (3.0) | ||
| Year of diagnosis | 2004–2007 | 204 (39.1) | 10 (30.3) | 0.419 |
| 2008–2011 | 180 (34.5) | 15 (45.5) | ||
| 2012–2015 | 138 (26.4) | 8 (24.2) | ||
| Income | Below median | 211 (40.4) | 14 (42.4) | 0.873 |
| Above median | 309 (59.2) | 19 (57.6) | ||
| Unknown | 2 (0.4) | 0 (0.0) | ||
| Insurance | Private | 262 (50.2) | 22 (66.7) | 0.151 |
| Government | 209 (40.0) | 8 (24.2) | ||
| None | 33 (6.3) | 3 (9.1) | ||
| Unknown | 18 (3.4) | 0 (0.0) | ||
| Area | Metropolitan | 446 (85.4) | 26 (78.8) | 0.328 |
| Unknown | 8 (1.5) | 0 (0.0) | ||
| Urban/rural | 68 (13.0) | 7 (21.2) | ||
| Treatment facility | Academic | 234 (44.8) | 14 (42.4) | 0.720 |
| Community | 191 (36.6) | 11 (33.3) | ||
| Other/unknown | 97 (18.6) | 8 (24.2) | ||
| Charlson/Deyo score | 0 (none) | 432 (82.8) | 27 (81.8) | 0.417 |
| 1 (mild) | 68 (13) | 6 (18.2) | ||
| ≥2 (moderate/severe) | 22 (4.2) | 0 (0.0) | ||
| Surgical margins | Negative | 308 (59.0) | 21 (63.6) | 0.790 |
| Positive | 104 (19.9) | 5 (15.2) | ||
| Unknown | 110 (21.1) | 7 (21.2) | ||
| Pathologic nodal status | Negative | 163 (31.2) | 18 (54.5) | 0.003 |
| Positive | 10 (1.9) | 2 (6.1) | ||
| Unknown | 349 (66.9) | 13 (39.4) | ||
| Radiotherapy | No | 65 (12.5) | 8 (24.2) | 0.063 |
| Yes | 457 (87.5) | 25 (75.8) |
Age, ethnicity, income, insurance, Charlson/Deyo score, and pathologic nodal status were identified by the univariate model as variables to be included in the multivariable model (Table 2). By multivariable analysis, age over 75 years (HR 2.87, 95% CI 1.42–5.77; p = 0.003), unknown ethnicity (HR 2.38, 95% CI 1.19–4.74; p = 0.014), government insurance (HR 2.11, 95% CI 1.10–4.06; p = 0.024), and Charlson/Deyo score of 1 (HR 2.12, 95% CI 1.25–3.60; p = 0.005), and Charlson/Deyo score of ≥2 (HR 2.73, 95% CI 1.32–5.67; p = 0.007) were associated with worse survival. RS (HR 0.43, 95% CI 0.10–1.83; p = 0.255), positive nodal status (HR 0.66, 95% CI 0.09–5.03; p = 0.692), and unknown nodal status (HR 1.30, 95% CI 0.78–2.17; p = 0.311) did not have significant associations with overall survival (Table 2). By the Kaplan-Meier method, there was no difference in overall survival between the LR and RS groups, with median survival not reached by either group at 10 years (median follow-up 60.4 months; p = 0.20) (Fig. 2).
Table 2.
Univariate and multivariable Cox proportional hazards model analysis of selected patient variables and their associations with overall survival.
| Characteristic | Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% Cl) | p-Value | ||
| Treatment group | Local resection | – | – | – | – |
| Radical surgery | 0.38 (0.09, 1.54) | 0.174 | 0.43 (0.10, 1.83) | 0.255 | |
| Age | 18–54 | – | – | – | – |
| 55–64 | 1.13 (0.59, 2.13) | 0.721 | 1.15 (0.60, 2.20) | 0.682 | |
| 65–74 | 2.32 (1.33, 4.08) | 0.003 | 1.43 (0.73, 2.80) | 0.295 | |
| ≥75 | 4.85 (2.76, 8.60) | <0.001 | 2.87 (1.42, 5.77) | 0.003 | |
| Ethnicity | White | – | – | – | – |
| Black | 1.79 (1.07, 3.00) | 0.026 | 1.65 (0.94, 2.88) | 0.079 | |
| Hispanic | 0.87 (0.35, 2.17) | 0.762 | 0.80 (0.31, 2.07) | 0.639 | |
| Other | 0.93 (0.23, 3.85) | 0.927 | 1.01 (0.24, 4.24) | 0.989 | |
| Unknown | 2.44 (1.24, 4.78) | 0.010 | 2.38 (1.19, 4.74) | 0.014 | |
| Income | Below median | – | – | – | – |
| Above median | 0.66 (0.43, 0.99) | 0.046 | 0.71 (0.46, 1.09) | 0.119 | |
| Unknown | 0.00 (0.00, Inf) | 0.995 | 0.00 (0.00, Inf) | 0.994 | |
| Insurance | Private | – | – | – | – |
| Government | 3.67 (2.29, 5.91) | <0.001 | 2.11 (1.10, 4.06) | 0.024 | |
| None | 1.36 (0.41, 4.52) | 0.616 | 1.30 (0.37, 4.51) | 0.680 | |
| Unknown | 1.90 (0.57, 6.29) | 0.298 | 1.96 (0.56, 6.84) | 0.289 | |
| Charlson/Deyo score | 0 (none) | – | – | – | – |
| 1 (mild) | 2.18 (1.32, 3.62) | 0.002 | 2.12 (1.25, 3.60) | 0.005 | |
| ≥2 (moderate/severe) | 4.62 (2.29, 9.39) | <0.001 | 2.73 (1.32, 5.67) | 0.007 | |
| Pathologic nodal status | Negative | – | – | – | – |
| Positive | 0.66 (0.09, 4.95) | 0.691 | 0.66 (0.09, 5.03) | 0.692 | |
| Unknown | 1.79 (1.09, 2.91) | 0.020 | 1.30 (0.78, 2.17) | 0.311 | |
| Sex | Female | – | – | ||
| Male | 1.07 (0.71, 1.63) | 0.724 | |||
| Year of diagnosis | 2004–2007 | – | – | ||
| 2008–2011 | 0.99 (0.61, 1.61) | 0.964 | |||
| 2012–2015 | 0.61 (0.25, 1.51) | 0.290 | |||
| Area | Metropolitan | – | – | ||
| Unknown | 1.01 (0.14, 7.27) | 0.994 | |||
| Urban/rural | 0.95 (0.49, 1.84) | 0.884 | |||
| Treatment facility | Academic | – | – | ||
| Community | 0.90 (0.58, 1.41) | 0.657 | |||
| Other/unknown | 0.61 (0.32, 1.16) | 0.132 | |||
| Surgical margins | Negative | – | – | ||
| Positive | 0.79 (0.44, 1.42) | 0.424 | |||
| Unknown | 1.00 (0.60, 1.65) | 0.991 | |||
| Radiotherapy | No | – | – | ||
| Yes | 0.72 (0.36, 1.43) | 0.350 | |||
HR, hazard ratio; Inf, infinity.
Fig. 2.
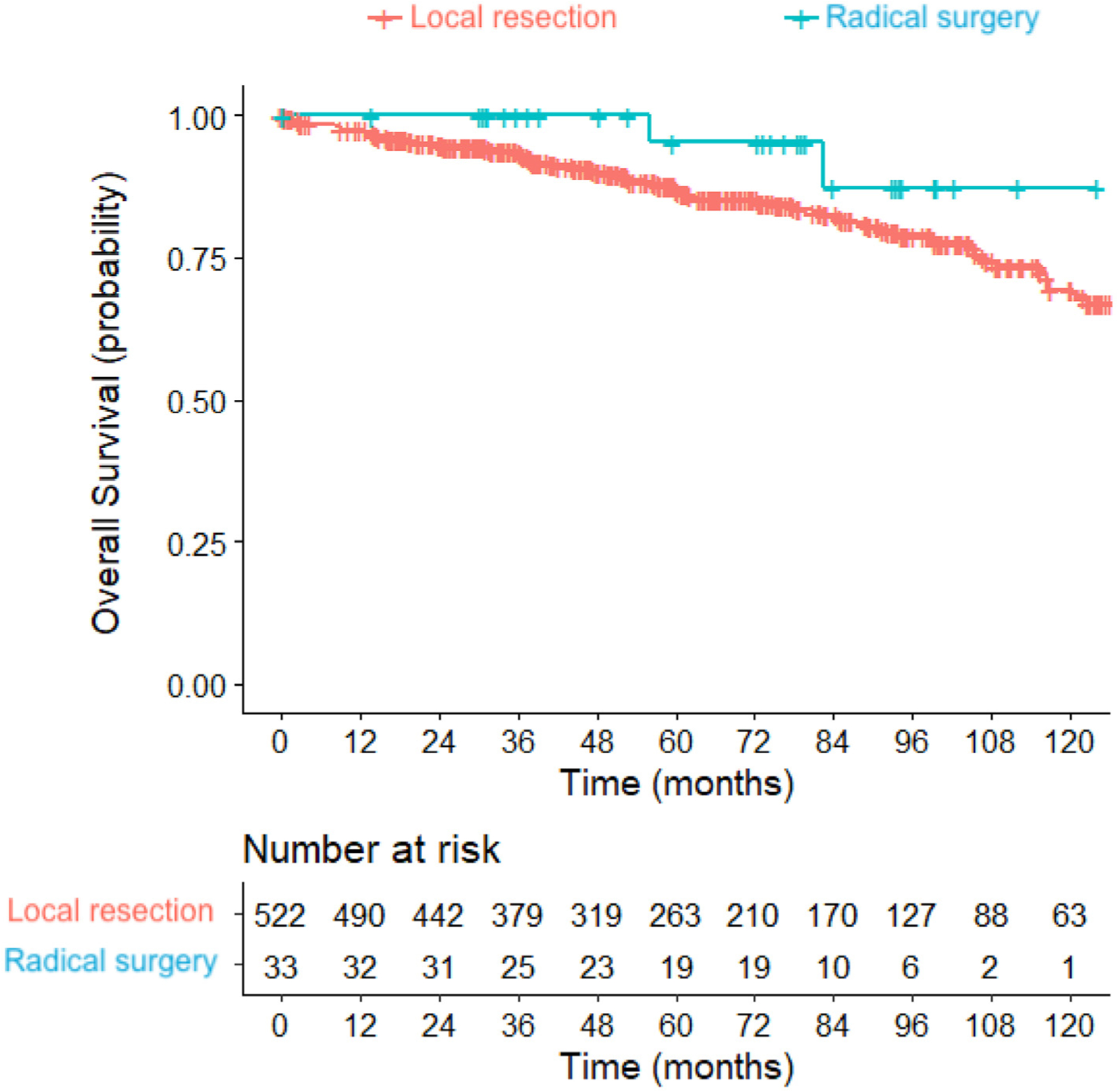
Kaplan-Meier curves of overall survival for local resection (LR) versus radical surgery (RS) groups extended to 10 years. Median survival not reached by either group at 10 years, with median follow-up time of 60.4 months. Logrank test: p = 0.20.
Discussion
In our study, we found no significant difference in overall survival between patients with parathyroid carcinoma treated with LR when compared to RS. To our knowledge, this is the largest study examining the impact of the extent of surgical resection on survival for parathyroid carcinoma.
Current treatment guidelines for the management of PC recommend complete parathyroidectomy with en bloc resection of adherent structure to avoid capsular disruption [1–3,13]. Surgery is considered the cornerstone of treatment as radiotherapy and chemotherapy remain unproven with regards to disease control or survival [1–3,13]. However, RS can cause significant morbidity in the form of recurrent laryngeal nerve palsy and muscular dysfunction of the neck and shoulder [14,15]. Furthermore, the risk of morbidity is increased in a re-operative setting [15]. This is often the case for RS, as many PC patients undergo RS as a second procedure after LR for a presumed benign indication [4–7,15]. PC is associated with its own set of morbidities, namely hypercalcemia and morbidity associated with local invasion, such as dysphagia. However, these conditions are much more likely in patients with advanced disease [16]. Additionally, there is conflicting evidence regarding the role for surgery for the control of hypercalcemia in patients with PC [17,18]. Considering the morbidity associated with RS, and the lack of proven oncologic or disease-specific morbidity benefits, this radical approach is being challenged [19].
Arguments in favor of RS cite locoregional control as the main advantage over a less aggressive surgical approach in terms of extending survival, as locoregional control is intended to control local disease and prevent distant metastases [2,8]. However, there is a growing body of evidence that a less radical approach may be able to achieve locoregional control in certain situations [4,9–13]. Pathologic evidence of locoregional control with LR was shown in one study, where 7 patients were diagnosed with PC on final pathology after LR for benign indications. Of those patients, 6 underwent RS to achieve locoregional control, and none were found to have any residual disease on final pathological evaluation [4]. With regards to preventing distant metastases, this was demonstrated in a retrospective review of 11 patients with PC who were treated with LR. They found that only one out of 11 patients (9.1%) developed metastatic disease during a median follow-up period of 99.6 months [9]. Another study of 75 patients with PC demonstrated no association between extent of resection and rates of distant metastases with a median follow-up period of 77.0 months [11].
More recent research has focused on evaluating surgical approaches by overall survival [2,11]. In a previous NCDB analysis, it was demonstrated that overall survival was improved in patients with PC by any surgical approach, and not specifically by RS, among 733 patients with a median follow-up period of 65.1 months [12]. Most notably, when compared to no surgery by multivariable analysis, RS had a higher HR for death than both complete tumor removal and incomplete tumor removal. However, they included patients with metastatic disease, which precluded making any conclusions about LR for localized PC. Finally, a study of patients in California with PC analyzed a total of 136 patients, 60 of whom were treated with LR, 58 with RS, and 18 with LR followed by RS. Overall survival did not differ between groups, nor did rates of disease-related complications, over a median follow-up period of 63.6 months [10]. All of these results, including our study, challenge the belief that RS is superior to LR in preventing metastatic disease and improving survival.
The NCDB captures more than 70% of incident cancer cases in the United States every year and is continuously audited for accuracy. Despite this, there are several limitations to this study inherent to the nature of the database. The retrospective nature of the study is subject to selection and misclassification bias. There is no information on recurrence or disease specific survival, nor is there information regarding surgeries prior to the procedure classified as each patient’s definitive surgery. Morbidity data is lacking in the dataset, including information regarding emergency department visits, hospital admissions, calcium levels, or complications related to local invasion. Data regarding whether surgeries involved debulking to prevent or address aerodigestive complications are also lacking in the dataset. However, these types of surgeries are most often palliative and performed in very advanced cases where metastatic disease is present – a subset of patients that we did not include in our study [16,20]. Coding of surgical procedures provided sufficient detail to distinguish local resection from radical surgery, but further information regarding extent of lymphadenectomy, specific adjacent organs removed, and dissection planes was not available. Calcium-related morbidity could not be evaluated due to limitations of the database, including hypercalcemic crisis events and development of chronic renal failure directly attributed to hypercalcemia.
Conclusions
Extent of surgery did not impact overall survival (OS) in patients with localized parathyroid carcinoma in our study. We propose that patients who are found to have parathyroid carcinoma after undergoing LR for a presumed benign indication may be safely and closely monitored for recurrence, rather than be committed to the morbidity of RS. This affirms current guidelines that recommend against routine ipsilateral thyroidectomy and lymphadenectomy. Studies addressing parathyroid carcinoma disease-specific morbidity are required to further clarify the role of LR compared to RS.
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB includes approximately 70% of incident cancer cases in the United States. The Commission on Cancer’s NCDB and the hospitals participating in the Commission on Cancer’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Acknowledgements
We would like to thank Stephanie Stebens, MLIS, for her assistance with editing and formatting.
Funding
The Mort and Brigitte Harris Pancreas Cancer Research Fund was used to fund research analysis. The source of funding had no role in study design, data collection, data analysis, data interpretation, writing of this report, or the decision to submit for publication.
Footnotes
CRediT authorship contribution statement
Shravan Leonard-Murali: Conceptualization, Methodology, Data acquisition, Quality control of data and algorithms, Formal analysis, and interpretation, Statistical analysis, Writing – original draft, Writing – review &; editing, Manuscript review. Tommy Ivanics: Conceptualization, Data acquisition, Formal analysis, and interpretation, Writing – review &; editing, Manuscript review. David S. Kwon: Conceptualization, Methodology, Data acquisition, Quality control of data and algorithms, Formal analysis, and interpretation, Writing – original draft, Writing – review &; editing, Manuscript review. Xiaoxia Han: Methodology, Quality control of data and algorithms, Formal analysis, and interpretation, Statistical analysis. Christopher P. Steffes: Data acquisition, Writing – review &; editing, Manuscript review. Rupen Shah: Writing – review &; editing, Manuscript review.
Declaration of competing interest
David S. Kwon is a paid consultant for Ethicon, Inc. This affiliation did not represent a conflict of interest. The authors have no other disclosures to report.
Previous Presentations: Presented at the American Association of Endocrine Surgeons Annual Meeting in Los Angeles, California, April 2019.
References
- [1].Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol 2012;13:11–23. [DOI] [PubMed] [Google Scholar]
- [2].Witteveen JE, Haak HR, Kievit J, Morreau H, Romijn JA, Hamdy NAT. Challenges and pitfalls in the management of parathyroid carcinoma: 17-year follow-up of a case and review of the literature. Horm Cancer 2010;1:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Betea D, Potorac I, Beckers A. Parathyroid carcinoma: challenges in diagnosis and treatment. Ann Endocrinol 2015;76:169–77. [DOI] [PubMed] [Google Scholar]
- [4].O’Neill CJ, Chan C, Symons J, et al. Parathyroid carcinoma encountered after minimally invasive focused parathyroidectomy may not require further radical surgery. World J Surg 2011;35:147–53. [DOI] [PubMed] [Google Scholar]
- [5].Libansky P, Adamek S, Broulik P, Fialov M, Kubinyi J, Lischke R. Parathyroid carcinoma in patients that have undergone surgery for primary hyperparathyroidism. In Vivo 2017;31:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Christakis I, Vu T, Chuang HH, et al. The diagnostic accuracy of neck ultrasound, 4D-computed tomography and sestamibi imaging in parathyroid carcinoma. Eur J Radiol 2017;95:82–8. [DOI] [PubMed] [Google Scholar]
- [7].Xue S, Chen H, Lv C, et al. Preoperative diagnosis and prognosis in 40 parathyroid carcinoma patients. Clin Endocrinol 2016;85:29–36. [DOI] [PubMed] [Google Scholar]
- [8].Dotzenrath C, Goretzki PE, Sarbia M, Cupisti K, Feldkamp J, Röher HD. Parathyroid carcinoma: problems in diagnosis and the need for radical surgery even in recurrent disease. Eur J Surg Oncol 2001;27:383–9. [DOI] [PubMed] [Google Scholar]
- [9].Basceken SI, Genc V, Ersoz S, Sevim Y, Celik SU, Bayram IK. Is local resection sufficient for parathyroid carcinoma? Clinics 2015;70:247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Young S, Wu JX, Li N, Yeh MW, Livhits MJ. More extensive surgery may not improve survival over parathyroidectomy alone in parathyroid carcinoma. Ann Surg Oncol 2016;23:2898–904. [DOI] [PubMed] [Google Scholar]
- [11].Asare EA, Silva-Figueroa A, Hess KR, et al. Risk of distant metastasis in parathyroid carcinoma and its effect on survival: a retrospective review from a high-volume center. Ann Surg Oncol 2019;26:3593–9. [DOI] [PubMed] [Google Scholar]
- [12].Asare EA, Sturgeon C, Winchester DJ, et al. Parathyroid carcinoma: an update on treatment outcomes and prognostic factors from the National Cancer Data Base (NCDB). Ann Surg Oncol 2015;22:3990–5. [DOI] [PubMed] [Google Scholar]
- [13].Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016;151:959–68. [DOI] [PubMed] [Google Scholar]
- [14].Gane EM, Michaleff ZA, Cottrell MA, et al. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: a systematic review. Eur J Surg Oncol 2017;43:1199–218. [DOI] [PubMed] [Google Scholar]
- [15].van Dijk D, van Dijk BAC, Weistra A, Links TP, Plukker JTM. Surgical complications and referral patterns in 567 patients with differentiated thyroid cancer in the Northern Region of The Netherlands: a population-based study towards clinical management implementation. Ann Surg Oncol 2020;27: 3872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Machado NN, Wilhelm SM. Parathyroid cancer: a review. Cancers 2019;11: 1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lo WM, Good ML, Nilubol N, Perrier ND, Patel DT. Tumor size and presence of metastatic disease at diagnosis are associated with disease-specific survival in parathyroid carcinoma. Ann Surg Oncol 2018;25:2535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Medas F, Erdas E, Loi G, et al. Controversies in the management of parathyroid carcinoma: a case series and review of the literature. Int J Surg 2016;28: S94–8. [DOI] [PubMed] [Google Scholar]
- [19].Christakis I, Silva AM, Kwatampora LJ, et al. Oncologic progress for the treatment of parathyroid carcinoma is needed. J Surg Oncol 2016;114: 708–13. [DOI] [PubMed] [Google Scholar]
- [20].Busaidy NL, Jimenez C, Habra MA, et al. Parathyroid carcinoma: a 22-year experience. Head Neck 2004;26:716–26. [DOI] [PubMed] [Google Scholar]


