Abstract
Background
Bone metastasis (BM) and skeletal-related events (SREs) happen to advanced lung cancer (LC) patients without warning. LC-BM patients are often passive to BM diagnosis and surgical treatment. It is necessary to guide the diagnosis and treatment paradigm for LC-BM patients from reactive medicine toward predictive, preventive, and personalized medicine (PPPM) step by step.
Methods
Two independent study cohorts including LC-BM patients were analyzed, including the Surveillance, Epidemiology, and End Results (SEER) cohort (n = 203942) and the prospective Fudan University Shanghai Cancer Center (FUSCC) cohort (n = 59). The epidemiological trends of BM in LC patients were depicted. Risk factors for BM were identified using a multivariable logistic regression model. An individualized nomogram was developed for BM risk stratification. Personalized surgical strategies and perioperative care were described for FUSCC cohort.
Results
The BM incidence rate in LC patients grew (from 17.53% in 2010 to 19.05% in 2016). Liver metastasis was a significant risk factor for BM (OR = 4.53, 95% CI = 4.38–4.69) and poor prognosis (HR = 1.29, 95% CI = 1.25–1.32). The individualized nomogram exhibited good predictive performance for BM risk stratification (AUC = 0.784, 95%CI = 0.781–0.786). Younger patients, males, patients with high invasive LC, and patients with other distant site metastases should be prioritized for BM prevention. Spine is the most common site of BM, causing back pain (91.5%), pathological vertebral fracture (27.1%), and difficult walking (25.4%). Spinal surgery with personalized spinal reconstruction significantly relieved pain and improved daily activities. Perioperative inflammation, immune, and nutrition abnormities warrant personalized managements. Radiotherapy needs to be recommended for specific postoperative individuals.
Conclusions
The presence of liver metastasis is a strong predictor of LC-BM. It is recommended to take proactive measures to prevent BM and its SREs, particularly in young patients, males, high invasive LC, and LC with liver metastasis. BM surgery and perioperative management are personalized and required. In addition, adjuvant radiation following separation surgery must also be included in PPPM-guided management.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13167-022-00270-9.
Keywords: Predictive preventive personalized medicine (PPPM), Cancer, Bone metastasis, Metastatic spinal tumors, Surgery, Lung cancer, Perioperative management, Predictive factors, Targeted prevention, Personalization of medical services, Risk assessment, Multidisciplinary team, Adjuvant radiation
Introduction
Bone metastasis (BM) is a potential complication of advanced lung cancer (LC) patients. When BM occurs, patients are frequently vulnerable to skeletal-related events (SREs), which can seriously impair their daily activities and even result in death [1–3]. LC has the highest BM risk among cancers and is the most common primary source of BM [4, 5]. As a result, it is necessary to identify high-risk LC-BM patients, actively prevent BM and its SREs, and develop personalized treatment strategies for LC-BM based on predictive, preventive, and personalized medicine (PPPM or 3PM) principles [6]. Major risk factors identification, predictive tools development, and treatment outcome research are critical approaches to implementing PPPM/3PM in cancer [6].
The LC-BM is osteolytic and causes many intractable SREs by drug therapy. BM surgery is a direct and effective way to treat SREs [7]. Nonetheless, given the poor prognosis of advanced LC, many LC-BM patients are negative to BM surgery. Over the last few decades, due to great leaps in LC chemotherapy and targeted therapy, advanced LC patients have exhibited a prolonged overall survival [8, 9]. Hence, BM surgery is increasingly necessary for SREs treatment [10, 11]. BM surgery, especially spinal metastasis surgery, is a highly complex and individualized technique [12]. It includes various procedures, such as open BM resection and minimally invasive percutaneous vertebroplasty (PVP) [13, 14]. Our institution Fudan University Shanghai Cancer Center (FUSCC) is one of the largest national comprehensive cancer centers in China and has extensive experience in LC-BM treatment based on our multidisciplinary team (MDT). This study seeks to (1) develop an individualized risk stratification model for BM, (2) identify key populations for BM prevention, and (3) provide personalized BM surgical strategies and perioperative management key points. We attempted to take the first step toward shifting the diagnosis and treatment paradigms of LC-BM patients away from reactive medicine and toward PPPM/3PM [15, 16].
Methods
Study population
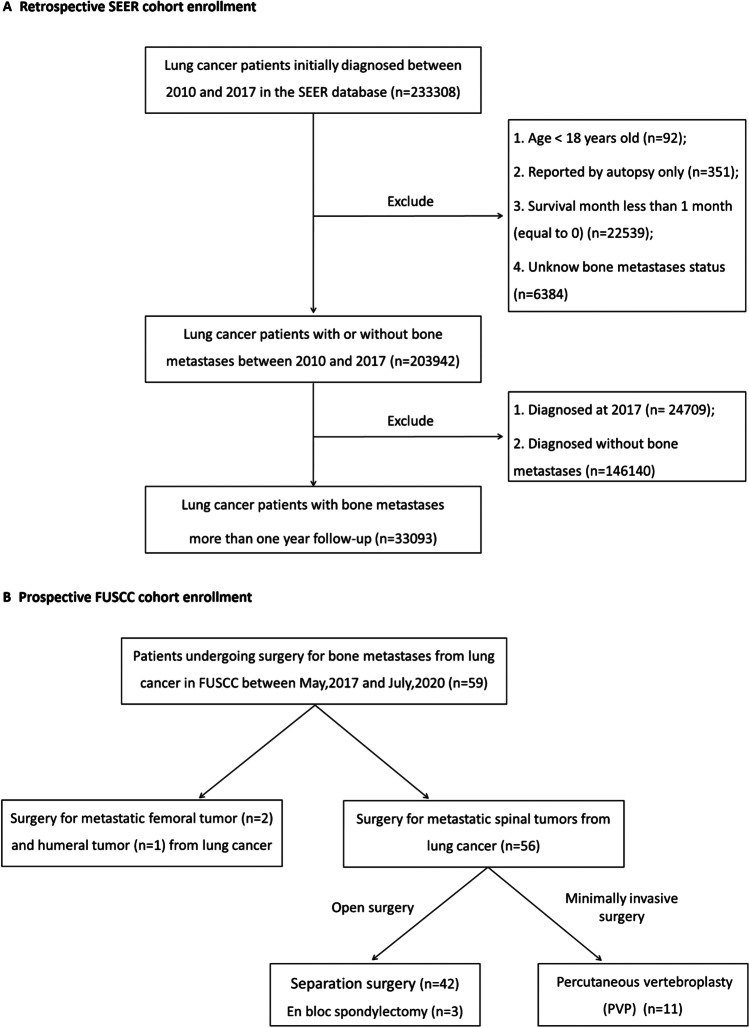
This study included LC-BM patients from the Surveillance, Epidemiology, and End Results (SEER) and FUSCC study cohorts. SEER cohort extracted data from the SEER-18 registries program (https://seer.cancer.gov/). LC patients were extracted using the site recode ICD-O-3/WHO 2008 of “Lung and bronchus.” The inclusion criteria were (1) LC as first primary cancer and (2) LC diagnosed with positive histology. A total of 233,308 LC patients were included from January 1, 2010, to December 31, 2017. LC patients less than 18 years old, patients reported by autopsy only, patients with survival less than 1 month, and patients with unknown BM status were excluded. Then 203,942 LC patients with and without BM were analyzed for BM risk factors. After excluding patients in 2017 and patients without BM, 33,093 LC-BM patients with at least 1-month follow-up were examined for survival (Fig. 1A).
Fig. 1.
Flowchart showing patient selection. A Retrospective SEER cohort between 2010 and 2017; B prospective Fudan University Shanghai Cancer Center (FUSCC) cohort between 2017 and 2020. Abbreviations: SEER, Surveillance, Epidemiology, and End Results
FUSCC cohort prospectively enrolled LC-BM patients who underwent BM surgery from May 1, 2017, to July 31, 2020 (Fig. 1B). The inclusion criteria were (1) patients diagnosed with LC; (2) clinical evidence supporting BM lesion from LC; (3) patients with an estimated survival of more than 3 months; and (4) patients with BM surgery indications. Patients who do not tolerate anesthesia, surgery, and those who lack medical information were excluded. We enrolled 59 representative patients, including 56 patients for spinal metastasis surgery, 2 patients for femoral metastasis surgery, and 1 patient for humeral metastasis surgery. Of 56 patients with spinal metastasis, 11 underwent minimally invasive PVP, 42 underwent separation surgery, and 3 underwent total en bloc spondylectomy (TES). Tomita’s score was used for surgery candidates with spinal metastasis, and those with a score > 8 were deemed unfit for surgery [17]. This study was approved by FUSCC review board and obtained informed consent.
Data collection
In SEER cohort, patients’ age, sex, LC site, laterality, histologic type, differentiation, and T and N stage were extracted. Specific distant metastasis sites were extracted, such as the bone, lung, brain, and liver metastasis. Surgery treatment information was obtained. Patients’ overall survival (OS) status and time were obtained.
In FUSCC cohort, patient’s age, gender, body mass index (BMI), smoking, and alcohol intake status were recorded. Patient’s symptoms and signs were questioned at hospital admissions, such as lower limbs pain and numbness status, difficultly walking, and paraplegia. Pain was scored by skilled nurses at hospital admission using the numerical rating scale (NRS) [18]. Walking ability on level ground and to climb stairs were assessed using the Barthel Index scale [19]. Briefly, walking ability on level ground was scored at 15 (complete independence), 10 (some assistance is required), 5 (much assistance is required), and 0 (full dependence). Ability to climb stairs was scored at 10 (complete independence), 5 (some assistance is required), and 0 (much assistance is required).
Imaging examinations (X-ray, computed tomography (CT), and magnetic resonance imaging (MRI)) and blood and biochemical routine tests were performed before and after BM surgery. Inflammatory and immune indexes (leucocytes, neutrophils percentage, lymphocytes percentage, monocytes percentage, neutrophil to lymphocyte ratio), hemoglobin, platelets, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), hepatic function indexes (alanine transaminase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL)), nutrition indexes (total protein, albumin, pre-albumin, and globulin), and serum Ca2+ level were all recorded.
Skilled anesthesiologists conducted the American Society of Anesthesiologists (ASA) grade before BM surgery [20]. Surgical approaches, duration, blood loss, and transfusion volume were recorded. Resected BM specimens were sent to pathological examination using hematoxylin–eosin (HE) and immunohistochemistry (IHC) staining. Radiation is an excellent adjuvant treatment option for LC-BM patients after separation surgery to kill spinal metastatic cancer cells, suppress osteoclasts, and promote osteolytic lesions calcification. Follow-up on survival was conducted via institutional outpatient records and telephone until November 22, 2021.
Statistical analysis
Continuous data were presented by mean ± standard deviation (SD) or median (interquartile range) and examined by Student’s t-test or Mann-Whiney U test, respectively. Categorical data were presented by number with percentage and examined by chi-square test. In SEER cohort, univariable and multivariable logistic regression analyses were conducted to identify BM risk factors. Age, sex, LC site, laterality, LC histology, LC differentiation, T and N stage, and lung, liver, and brain metastasis were entered into a multivariable logistic regression modeling in a method of stepwise forward LR. Odds ratio (OR) with 95% confidence interval (CI) was reported. An individualized nomogram for BM risk assessment was accordingly constructed based on logistic regression model using R software 4.1.2 (https://www.r-project.org/). Receiver operator characteristic (ROC) curve with calculated area under the curve (AUC) and calibration curve were employed to internally validate the nomogram predictive performance. Survival differences stratified by clinicopathologic characteristics were displayed using Kaplan–Meier curves and examined using log-rank test. Univariable and multivariable Cox proportional hazard regression analyses were conducted to examine independent prognostic factors for LC-BM patients. In FUSCC cohort, paired t-test and Wilcoxon matched-pairs signed-rank test was conducted for pre- and postoperative comparison of biochemical index, pain score, and activity ability score. Statistics and graphs were conducted using SPSS 24.0 and GraphPad Prism 7.0. A two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics of LC-BM patients in SEER
Baseline demographic and clinical characteristics of SEER cohort were presented in Supplementary Table 1. LC-BM patients from 2010 to 2016 were selected for epidemiological features analysis. LC-BM patient’s number increased over the years. BM incidence in LC was significantly increased from 17.53% in 2010 to 19.05% in 2016. LC-BM patient’s 3-month survival rates increased from 59.1% in 2010 to 62.3% in 2016. LC-BM patients’ 6-month survival rates increased from 40.3% in 2010 to 45.2% in 2016. More details were presented in Supplementary Fig. 1.
Risk factors for BM and individualized nomogram
As shown in Table 1, BM incidence was obviously high in young patients, males, main bronchus LC, bilateral LC, LC adenocarcinoma/small cell carcinoma, poorly/undifferentiated LC, LC with higher T and N stages, and LC with lung, liver, and brain metastases. Powerful BM independent risk factors mainly included liver metastases (OR = 4.53, 95% CI = 4.38–4.69), followed by poorly/undifferentiated LC (OR = 2.74, 95% CI = 2.52–2.99), N3 stage (OR = 2.28, 95% CI = 2.18–2.39), and adenocarcinoma (OR = 2.07, 95% CI = 2.00–2.14).
Table 1.
Univariable and multivariable logistic regression analysis for BM risk factors (n = 203942)
| Characteristics | Patient’s number (2010–2017) | Univariable logistic | Multivariable logistic | ||||
|---|---|---|---|---|---|---|---|
| BM | Total | Incidence (%) | OR (95% CI) | P | OR (95% CI) | P | |
| Age (years) | |||||||
| < 60 | 10352 | 46612 | 22.21 | 1.00 | 1.00 | ||
| 60–69 | 12835 | 67306 | 19.07 | 0.83 (0.80–0.85) | < 0.001* | 0.94 (0.91–0.97) | < 0.001* |
| 70–79 | 10216 | 62132 | 16.44 | 0.69 (0.67–0.71) | < 0.001* | 0.86 (0.83–0.89) | < 0.001* |
| ≥ 80 | 4322 | 27892 | 15.50 | 0.64 (0.62–0.67) | < 0.001* | 0.81 (0.78–0.85) | < 0.001* |
| Sex | |||||||
| Female | 16429 | 98773 | 16.6 | 1.00 | 1.00 | ||
| Male | 21296 | 105169 | 20.2 | 1.27 (1.24–1.30) | < 0.001* | 1.27 (1.24–1.30) | < 0.001* |
| LC site | |||||||
| Upper lobe | 18674 | 109015 | 17.1 | 1.00 | 1.00 | ||
| Lower lobe | 9520 | 55388 | 17.2 | 1.00 (0.98–1.03) | 0.768 | 1.08 (1.05–1.11) | < 0.001* |
| Middle lobe | 1528 | 9362 | 16.3 | 0.94 (0.89–1.00) | 0.05 | 1.02 (0.96–1.08) | 0.540 |
| Main bronchus | 1913 | 8448 | 22.6 | 1.42 (1.34–1.49) | < 0.001* | 1.06 (1.00–1.13) | 0.053 |
| Overlapping lesion | 379 | 2215 | 17.1 | 1.00 (0.89–1.12) | 0.981 | 0.88 (0.78–0.99) | 0.041* |
| NOS | 5711 | 19514 | 29.3 | 2.00 (1.93–2.07) | < 0.001* | 1.15 (1.09–1.20) | < 0.001* |
| LC laterality | |||||||
| Left | 15038 | 81832 | 18.4 | 1.00 | 1.00 | ||
| Right | 20312 | 115193 | 17.6 | 0.95 (0.93–0.97) | < 0.001* | 0.92 (0.90–0.95) | < 0.001* |
| Bilateral | 654 | 1840 | 35.5 | 2.45 (2.22–2.70) | < 0.001* | 0.89 (0.80–1.00) | 0.051 |
| NOS | 1721 | 5077 | 33.9 | 2.28 (2.14–2.42) | < 0.001* | 0.96 (0.89–1.04) | 0.299 |
| LC histology | |||||||
| Squamous cell carcinoma | 5231 | 48681 | 10.7 | 1.00 | 1.00 | ||
| Adenocarcinoma | 20444 | 99228 | 20.6 | 2.16 (2.09–2.23) | < 0.001* | 2.07 (2.00–2.14) | < 0.001* |
| Small cell carcinoma | 5943 | 24857 | 23.9 | 2.61 (2.51–2.72) | < 0.001* | 1.04 (1.00–1.09) | 0.074 |
| Other | 3430 | 20020 | 17.1 | 1.72 (1.64–1.80) | < 0.001* | 1.35 (1.29–1.43) | < 0.001* |
| NSCLC, NOS | 2677 | 11156 | 24.0 | 2.62 (2.49–2.76) | < 0.001* | 1.71 (1.62–1.81) | < 0.001* |
| LC differentiation | |||||||
| Well | 659 | 14155 | 4.7 | 1.00 | 1.00 | ||
| Moderately | 3519 | 40668 | 8.7 | 1.94 (1.78–2.11) | < 0.001* | 1.77 (1.62–1.93) | < 0.001* |
| Poorly | 9661 | 60680 | 15.9 | 3.88 (3.58–4.21) | < 0.001* | 2.74 (2.52–2.99) | < 0.001* |
| Unknown | 23886 | 88439 | 27.0 | 7.58 (7.00–8.21) | < 0.001* | 4.36 (4.01–4.74) | < 0.001* |
| T stage | |||||||
| T1 | 2788 | 33091 | 8.4 | 1.00 | 1.00 | ||
| T2 | 6390 | 43413 | 14.7 | 1.88 (1.79–1.97) | < 0.001* | 1.38 (1.31–1.45) | < 0.001* |
| T3 | 6366 | 29963 | 21.2 | 2.93 (2.80–3.08) | < 0.001* | 1.58 (1.49–1.66) | < 0.001* |
| T4 | 8720 | 33142 | 26.3 | 3.88 (3.71–4.06) | < 0.001* | 1.69 (1.61–1.78) | < 0.001* |
| Unknown | 13461 | 64333 | 20.9 | 2.88 (2.75–3.00) | < 0.001* | 1.64 (1.54–1.74) | < 0.001* |
| N stage | |||||||
| N0 | 5209 | 62045 | 8.4 | 1.00 | 1.00 | ||
| N1 | 2235 | 13449 | 16.6 | 2.18 (2.06–2.29) | < 0.001* | 1.64 (1.54–1.73) | < 0.001* |
| N2 | 12831 | 52041 | 24.7 | 3.57 (3.45–3.70) | < 0.001* | 2.05 (1.97–2.13) | < 0.001* |
| N3 | 6031 | 19663 | 30.7 | 4.83 (4.63–5.03) | < 0.001* | 2.28 (2.18–2.39) | < 0.001* |
| Unknown | 11419 | 56744 | 20.1 | 2.75 (2.65–2.85) | < 0.001* | 1.59 (1.50–1.68) | < 0.001* |
| Lung metastases | |||||||
| No | 26361 | 174887 | 15.1 | 1.00 | 1.00 | ||
| Yes | 9997 | 26569 | 37.6 | 3.40 (3.31–3.50) | < 0.001* | 1.98 (1.92–2.05) | < 0.001* |
| Unknown | 1367 | 2486 | 55.0 | 6.88 (6.35–7.46) | < 0.001* | 2.60 (2.36–2.86) | < 0.001* |
| Liver metastases | |||||||
| No | 25=818 | 181558 | 14.2 | 1.00 | 1.00 | ||
| Yes | 11019 | 21032 | 52.4 | 6.64 (6.44–6.84) | < 0.001* | 4.53 (4.38–4.69) | < 0.001* |
| Unknown | 888 | 1352 | 65.7 | 11.5 (10.3–12.9) | < 0.001* | 3.78 (3.31–4.31) | < 0.001* |
| Brain metastases | |||||||
| No | 27728 | 176150 | 15.7 | 1.00 | 1.00 | ||
| Yes | 9054 | 26513 | 34.1 | 2.78 (2.70–2.86) | < 0.001* | 1.59 (1.54–1.64) | < 0.001* |
| Unknown | 943 | 1279 | 73.7 | 15.0 (13.3–17.0) | < 0.001* | 4.54 (3.92–5.25) | < 0.001* |
*Statistically significant
Abbreviations: LC lung cancer, BM bone metastases, NOS not otherwise specified. NSCLC non-small cell lung cancer, OR odds ratio, CI confidence interval
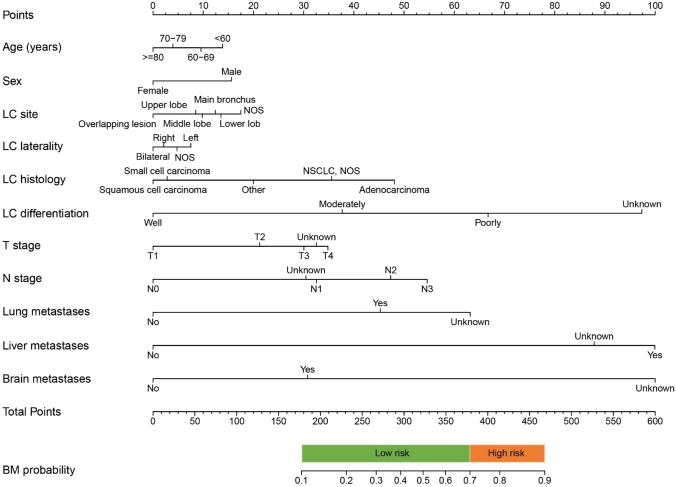
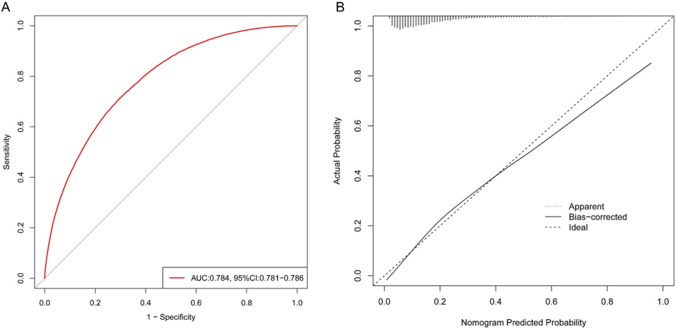
As shown in Fig. 2, an individualized nomogram was established for BM risk estimation and stratification. The nomogram incorporated a patient’ age, sex, LC site, laterality, histology, differentiation, T stage, N stage, lung metastasis, liver metastasis, and brain metastasis status. A patient with a BM probability more than 70% was considered as high-risk individual while that less than 70% was considered as low-risk individual. As shown in Fig. 3, internal validation by ROC and calibration curves demonstrated that the nomogram had a good predictive performance for BM risk estimation (AUC = 0.784, 95%CI = 0.781–0.786).
Fig. 2.
An individualized nomogram for predicting BM risk. A specific LC patient’s BM risk can be estimated by adding the points from each variable and vertically projecting the total points on the BM probability scale. A BM probability more than 70% is considered as high-risk while that less than 70% is considered low-risk. Abbreviations: LC, lung cancer; BM, bone metastasis
Fig. 3.
ROC curve with reported AUC and calibration curve to display the nomogram predictive performance for BM risk. Abbreviations: ROC, receiver operator characteristic curve; AUC, area under the curve
Survival and prognostic factors of LC-BM patients
Overall, the median OS time of LC-BM patients was 5.0 (4.91–5.09) months in SEER cohort. As shown in Supplementary Fig. 2, poor prognosis was more common in older patients, males, LC in overlapping lesions/main bronchus, bilateral LC, squamous and small cell carcinoma, poorly/undifferentiated LC, LC with higher T and N stages, LC with other organs metastases, patients without LC surgery, and patients without metastases surgery. OS did not differ between patients with BM with and without BM surgery.
Cox regression analyses revealed that powerfully independent prognostic factors included older age (HR = 1.66, 95% CI = 1.59–1.72) followed by poorly/undifferentiated LC (HR = 1.33, 95% CI = 1.22–1.46), liver metastases (HR = 1.29, 95% CI = 1.25–1.32), brain metastases (HR = 1.20, 95% CI = 1.16–1.23), and males (HR = 1.18, 95% CI = 1.15–1.21) (Table 2). To encourage the use of 3PM, an individualized nomogram was also accordingly constructed to predict a specific LC-BM patient’s survival rate (Supplementary Fig. 3).
Table 2.
Univariable and multivariable Cox regression analysis for LC-BM patient’s survival (n = 33093)
| Characteristics | Patient’s number (2010–2016) | Median OS time (95% CI), months | Univariable Cox | Multivariable Cox | ||||
|---|---|---|---|---|---|---|---|---|
| Total | All-cause deaths | Death rate (%) | HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | ||||||||
| < 60 | 9217 | 8351 | 90.6 | 7 (6.78–7.22) | 1.00 | 1.00 | - | |
| 60–69 | 11206 | 10354 | 92.4 | 6 (5.82–6.18) | 1.13 (1.10–1.16) | < 0.001* | 1.14 (1.11–1.18) | < 0.001* |
| 70–79 | 8899 | 8390 | 94.3 | 5 (4.84–5.16) | 1.30 (1.26–1.34) | < 0.001* | 1.33 (1.29–1.38) | < 0.001* |
| ≥ 80 | 3771 | 3635 | 96.4 | 3 (2.83–3.17) | 1.55 (1.49–1.61) | < 0.001* | 1.65 (1.58–1.72) | < 0.001* |
| Sex | ||||||||
| Female | 14359 | 13087 | 91.1 | 6 (5.83–6.17) | 1.00 | 1.00 | - | |
| Male | 18734 | 17643 | 94.2 | 5 (4.89–5.11) | 1.20 (1.17–1.23) | < 0.001* | 1.18 (1.15–1.21) | < 0.001* |
| LC site | ||||||||
| Upper lobe | 16340 | 15107 | 92.5 | 6 (5.85–6.15) | 1.00 | 1.00 | - | |
| Lower lobe | 8343 | 7700 | 92.3 | 5 (4.80–5.20) | 1.01 (0.98–1.04) | 0.578 | 1.00 (0.98–1.03) | 0.794 |
| Middle lobe | 1323 | 1215 | 91.8 | 6 (5.43–6.57) | 0.96 (0.91–1.02) | 0.179 | 0.96 (0.90–1.01) | 0.129 |
| Main bronchus | 1679 | 1589 | 94.6 | 5 (4.57–5.43) | 1.15 (1.09–1.21) | < 0.001* | 1.09 (1.04–1.15) | 0.001* |
| Overlapping lesion | 331 | 315 | 95.2 | 5 (3.98–6.03) | 1.18 (1.05–1.32) | < 0.001* | 1.16 (1.04–1.30) | 0.008* |
| NOS | 5077 | 4804 | 94.6 | 4 (3.79–4.21) | 1.17 (1.13–1.20) | < 0.001* | 1.14 (1.09–1.18) | < 0.001* |
| LC laterality | ||||||||
| Left | 13149 | 12183 | 92.7 | 6 (5.85–6.15) | 1.00 | 1.00 | - | |
| Right | 17836 | 16558 | 92.8 | 5 (4.88–5.12) | 1.03 (1.00–1.05) | 0.040* | 1.02 (1.00–1.05) | 0.05 |
| Bilateral | 597 | 559 | 93.6 | 4 (3.46–4.54) | 1.15 (1.06–1.25) | 0.001* | 1.01 (0.92–1.11) | 0.819 |
| NOS | 1511 | 1430 | 94.6 | 4 (3.61–4.39) | 1.15 (1.09–1.22) | 0.001* | 1.01 (0.94–1.07) | 0.842 |
| LC histology | ||||||||
| Squamous cell carcinoma | 4604 | 4410 | 95.8 | 4 (3.86–4.15) | 1.00 | 1.00 | ||
| Adenocarcinoma | 17826 | 16111 | 90.4 | 6 (5.84–6.16) | 0.68 (0.66–0.71) | < 0.001* | 0.72 (0.69–0.74) | < 0.001* |
| Small cell carcinoma | 5218 | 5074 | 97.2 | 7 (6.80–7.20) | 0.84 (0.81–0.87) | < 0.001* | 0.77 (0.74–0.81) | < 0.001* |
| Other | 3031 | 2848 | 94.0 | 4 (3.77–4.23) | 0.91 (0.87–0.95) | < 0.001* | 0.89 (0.85–0.94) | < 0.001* |
| NSCLC, NOS | 2414 | 2287 | 94.7 | 4 (3.76–4.24) | 0.91 (0.86–0.95) | < 0.001* | 0.91 (0.86–0.96) | < 0.001* |
| LC differentiation | ||||||||
| Well | 595 | 533 | 89.6 | 9 (7.71–10.3) | 1.00 | 1.00 | - | |
| Moderately | 3144 | 2849 | 90.6 | 7 (6.56–7.44) | 1.09 (0.99–1.19) | 0.081 | 1.07 (0.98–1.18) | 0.134 |
| Poorly | 8634 | 8083 | 93.6 | 5 (4.85–5.15) | 1.44 (1.32–1.57) | < 0.001* | 1.34 (1.22–1.46) | < 0.001* |
| Unknown | 20720 | 19265 | 93.0 | 5 (4.89–5.12) | 1.34 (1.23–1.47) | < 0.001* | 1.25 (1.15–1.37) | < 0.001* |
| T stage | ||||||||
| T1 | 2788 | 2592 | 93.0 | 7 (6.57–7.43) | 1.00 | 1.00 | - | |
| T2 | 6390 | 6069 | 95.0 | 6 (5.77–6.23) | 1.15 (1.10–1.20) | < 0.001* | 1.09 (1.04–1.14) | < 0.001* |
| T3 | 6366 | 6061 | 95.2 | 5 (4.80–5.20) | 1.23 (1.18–1.29) | < 0.001* | 1.14 (1.09–1.19) | < 0.001* |
| T4 | 8720 | 8365 | 95.9 | 5 (4.82–5.18) | 1.27 (1.21–1.32) | < 0.001* | 1.16 (1.11–1.21) | < 0.001* |
| Unknown | 8829 | 7643 | 86.6 | 5 (4.83–5.18) | 1.19 (1.14–1.24) | < 0.001* | 1.12 (1.07–1.18) | < 0.001* |
| N stage | ||||||||
| N0 | 5209 | 4848 | 93.1 | 6 (5.72–6.28) | 1.00 | 1.00 | - | |
| N1 | 2235 | 2117 | 94.7 | 5 (4.61–5.39) | 1.10 (1.04–1.15) | < 0.001* | 1.08 (1.03–1.14) | 0.003* |
| N2 | 12831 | 12311 | 95.9 | 5 (4.86–5.14) | 1.20 (1.16–1.24) | < 0.001* | 1.15 (1.11–1.19) | < 0.001* |
| N3 | 6031 | 5767 | 95.6 | 6 (5.76–6.24) | 1.15 (1.11–1.19) | < 0.001* | 1.10 (1.06–1.15) | < 0.001* |
| Unknown | 6787 | 5687 | 83.8 | 5 (4.79–5.21) | 1.10 (1.06–1.14) | < 0.001* | 1.04 (0.99–1.09) | 0.128 |
| Lung metastases | ||||||||
| No | 23054 | 21329 | 92.5 | 6 (5.88–6.12) | 1.00 | 1.00 | - | |
| Yes | 8756 | 8179 | 93.4 | 5 (4.81–5.19) | 1.08 (1.05–1.10) | < 0.001* | 1.03 (1.00–1.06) | 0.062 |
| Unknown | 1283 | 1222 | 95.2 | 5 (4.57–5.43) | 1.10 (1.04–1.16) | 0.002* | 0.98 (0.92–1.05) | 0.542 |
| Liver metastases | ||||||||
| No | 22616 | 20683 | 91.5 | 6 (5.87–6.13) | 1.00 | 1.00 | - | |
| Yes | 9668 | 9266 | 95.8 | 4 (3.84–4.16) | 1.33 (1.29–1.36) | < 0.001* | 1.29 (1.26–1.32) | < 0.001* |
| Unknown | 809 | 781 | 96.5 | 5 (4.47–5.53) | 1.21 (1.12–1.29) | < 0.001* | 1.13 (1.03–1.23) | 0.007* |
| Brain metastases | ||||||||
| No | 24406 | 22626 | 92.7 | 6 (5.88–6.12) | 1.00 | 1.00 | - | |
| Yes | 7821 | 7279 | 93.1 | 5 (4.83–5.17) | 1.09 (1.06–1.12) | < 0.001* | 1.17 (1.14–1.20) | < 0.001* |
| Unknown | 866 | 825 | 95.3 | 4 (3.55–4.45) | 1.16 (1.08–1.24) | < 0.001* | 1.09 (1.01–1.19) | 0.032* |
| Surgery of LC | ||||||||
| No | 32448 | 30194 | 93.1 | 5 (4.91–5.09) | 1.00 | - | 1.00 | - |
| Yes | 599 | 493 | 82.3 | 10 (8.49–11.5) | 0.59 (0.54–0.64) | < 0.001* | 0.66 (0.60–0.72) | < 0.001* |
| Unknown | 46 | 43 | 93.5 | 3 (1.10–4.90) | 1.23 (0.91–1.65) | 0.184 | 1.07 (0.64–1.78) | 0.795 |
| Surgery of metastases | ||||||||
| No | 31349 | 29133 | 92.9 | 5 (4.91–5.09) | 1.00 | 1.00 | ||
| Yes | 1699 | 1554 | 91.5 | 5 (4.57–5.43) | 0.92 (0.88–0.97) | 0.002* | 0.98 (0.93–1.03) | 0.450 |
| Unknown | 45 | 43 | 95.6 | 3 (1.03–4.97) | 1.24 (0.92–1.67) | 0.157 | 1.18 (0.71–1.96) | 0.535 |
Abbreviations: OS overall survival, LC lung cancer, BM bone metastases, NOS not otherwise specified, HR hazard ratio, CI confidence interval
*Statistically significant
LC-BM clinical and laboratory characteristics in FUSCC
Patient’s baseline characteristics in FUSCC cohort were presented in Table 3. Back pain (91.5%) was the most common symptom causing hospitalization, including lumbar back pain (40.7%), chest back pain (23.7%), and lumbar back pain with radiation to the lower limb (22.0%) and neck pain (5.1%). Other common symptoms and signs included lower limbs numbness (18.6%), vertebral body pathological fracture (27.1%), walking difficultly (25.4%), and lower limbs paraplegia (8.5%). Approximately 79.7% of BM was synchronously multiple lesions. The most common BM surgical location was the thoracic vertebra (52.5%), followed by the lumber (35.6%) and cervical vertebra (5.1%).
Table 3.
Clinical and laboratory characteristics of patients undergoing BM surgery in FUSCC (n = 59)
| Characteristics | Value |
|---|---|
| Age (years), mean ± SD | 61.1 ± 9.2 |
| ≤ 49 | 6 (10.2) |
| 50–59 | 20 (33.9) |
| 60–69 | 21 (35.6) |
| ≥ 70 | 12 (20.3) |
| Gender | |
| Female | 28 (47.5) |
| Male | 31 (52.5) |
| BMI (Kg/m2), mean ± SD | 23.2 ± 3.3 |
| Smoking | 12 (20.3) |
| excessive drinking | 8 (13.6) |
| Symptoms and signs at admission | |
| Back pain | 54 (91.5) |
| Location of the pain | |
| Lumbar back pain | 24 (40.7) |
| Lumbar back pain with radiation to lower limb | 13 (22.0) |
| Chest back pain | 14 (23.7) |
| Neck pain | 3 (5.1) |
| Numbness of the lower limbs | 11 (18.6) |
| Pathological fracture of vertebral body | 16 (27.1) |
| Difficultly walking | 15 (25.4) |
| Lower limb paraplegia | 5 (8.5) |
| Number of BM lesions | |
| Single | 12 (20.3) |
| Multiple | 47 (79.7) |
| Surgical location of BM lesions | |
| Cervical vertebra | 3 (5.1) |
| Thoracic vertebra | 31 (52.5) |
| Lumber vertebra | 21 (35.6) |
| Sacral vertebra | 1 (1.7) |
| Femur | 2 (3.4) |
| Humerus | 1 (1.7) |
| Histology of LC | |
| Adenocarcinoma | 38 (64.4) |
| Squamous cell carcinoma | 7 (11.9) |
| Small cell carcinoma | 3 (5.1) |
| Not otherwise specified | 11 (18.6) |
| Liver metastasis | 4 (6.8) |
| Brain metastasis | 9 (15.3) |
| Previous treatments | |
| Surgery of LC | 15 (25.4) |
| Chemo-radiotherapy | 20 (32.2) |
| Targeted therapy (EGFR-TKIs) | 16 (25.4) |
| Blood routine tests on admission, mean ± SD | |
| Leucocytes (× 109/L; normal range 3.5 ~ 9.5) | 7.4 ± 3.6 |
| Increased | 11 (18.6) |
| Neutrophils percentage (%; normal range 40 ~ 75) | 70.1 ± 10.6 |
| Increased | 16 (27.1) |
| Lymphocytes percentage (%; normal range 20 ~ 50) | 21.2 ± 8.9 |
| Decreased | 26 (44.1) |
| Monocytes percentage (%; normal range 3 ~ 10) | 6.3 ± 2.1 |
| Increased | 1 (1.7) |
| Decreased | 4 (6.8) |
| Platelets (× 109/L; normal range 125 ~ 350) | 232.2 ± 74.1 |
| Increased | 5 (8.5) |
| Decreased | 4 (6.8) |
| Neutrophil to lymphocyte ratio | 5.2 ± 6.7 |
| Hemoglobin (g/L; normal range 130.0–175.0) | 126.7 ± 16.4 |
| Anemia | 12 (20.3) |
| Biochemical tests on admission, mean ± SD | |
| ALP (U/L; normal range 45–125) | 127.1 ± 84.8 |
| Increased | 19 (32.2) |
| Decreased | 1 (1.7) |
| LDH (U/L; normal range 120–250) | 232.3 ± 147.6 |
| Increased | 14 (23.7) |
| Decreased | 3 (5.1) |
| ALT (U/L; normal range 9–50) | 31.1 ± 35.5 |
| Increased | 9 (15.3) |
| Decreased | 5 (8.5) |
| AST (U/L; normal range 15–40) | 27.8 ± 29.1 |
| Increased | 6 (10.2) |
| Decreased | 12 (20.3) |
| TBIL (umol/L; normal range 3.4–17.1) | 10.2 ± 5.0 |
| Increased | 4 (6.8) |
| Total protein (g/L; normal range 65–85) | 68.7 ± 4.5 |
| Decreased | 12 (20.3) |
| Albumin (g/L; normal range 40–55) | 40.8 ± 4.8 |
| Decreased | 28 (47.5) |
| Prealbumin (mg/L; normal range 250–400) | 253.6 ± 60.8 |
| Decreased | 25 (42.4) |
| Globulin (g/L; normal range 20–40) | 28.4 ± 3.3 |
| Ca2+ (mmol/L; normal range 2.11–2.52) | 2.23 ± 0.11 |
| Decreased | 8 (13.6) |
Abbreviations: BM bone metastases, LC lung cancer, BMI body mass index, EGFR-TKIs epidermal growth factor receptor-tyrosine kinase inhibitors, ALP alkaline phosphatase, LDH lactate dehydrogenase, ALT alanine transaminase, AST aspartate aminotransferase, TBIL total bilirubin, SD standard deviation
Adenocarcinoma was the main LC type (71.9%). Approximately 25.4% of patients underwent LC surgery, 32.2% underwent chemo-radiotherapy, and 25.4% underwent targeted therapy (EGFR-TKIs). Before BM surgery, 27.1% of patients had elevated neutrophil levels, 44.1% had decreased lymphocyte levels, and 20.3% had anemia. Low nutrition status was common in LC-BM patients, with 20.3% having decreased total protein, 47.5% having low albumin in, and 42.4% having reduced pre-albumin. ALP levels were elevated in 32.2% of patients. No hypercalcemia was found in the patients.
LC-BM limb surgery
A representative LC-BM limb surgery was presented in Fig. 4A–I. A female patient aged 62 had a pulmonary nodule (Fig. 4A) and presented left medial thigh pain for 1 week. An X-ray revealed a low-density mass in the middle of the left femur (Fig. 4B). After anesthesia, the incision (5 cm) was made above the tip of the left femur greater trochanter, passing through the lateral side of the thigh and the patella to the upper tibia (Fig. 4C). Then, the skin and subcutaneous tissues were incised to expose the proximal femur and hip joint. The separation was performed toward the gluteus medius fibers direction. Vastus lateralis muscle at the great trochanter basolateral margin was separated (Fig. 4D). The femoral neck and anterior articular capsule were sufficiently exposed, and the femoral head was removed using a head extractor. The right knee capsule was cut open, the cruciate ligament was cut off, and the meniscus was removed. Next, the vastus intermediates muscle and the whole femur involving the metastasis were removed (Fig. 4E). Then, the knee, femoral head, and femoral shaft prosthesis were installed (Fig. 4F). The strength line and flexion and extension function were confirmed to be satisfactory. The surgical lower extremity was as long as the contralateral normal lower extremity. Finally, the wound was washed and sutured layer by layer, with drainage tubes places (Fig. 4G). Postoperative X-ray showed a satisfactory prosthesis implantation surgery effect (Fig. 4H and I).
Fig. 4.
Personalized LC-BM limb surgery procedure. A X-ray shows that the primary pulmonary nodule (23 mm) is located in the right lower lung lobe (arrow indicated), with spicules of margin and pleural indentation; B X-ray shows a low-density mass in the medullary cavity of the middle left femur, with damage of cortical bone and rough thickening of local periosteum; C preoperative body surface marking; D exposure of femur lesions and soft tissues that need to be removed; E measurement of the resected specimen of femur tumor; F installation of knee joint prosthesis, femoral head prosthesis, and femoral shaft prosthesis; G drainage tubes with negative pressure are placed, and the wound is sutured layer by layer; H and I postoperative X-ray shows a satisfactory prosthesis implantation
PVP for LC spinal metastasis
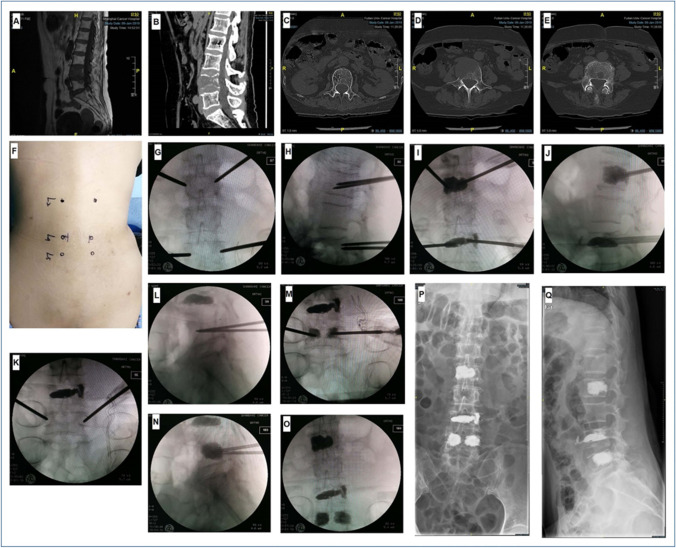
The clinical characteristics of patients undergoing PVP were presented in Table 4. PVP was recommended for (1) patients experiencing severe pain due to vertebral body deformation; (2) patients with no nerve root compression; and (3) patients with poor body conditions who cannot tolerate open surgery. A representative PVP in FUSCC was presented in Fig. 5. A 67-year-old female patient was diagnosed with multiple LC metastatic spinal tumors. Preoperative MRI and CT scans showed L2, L4, and L5 metastases (Fig. 5A–E). After anesthesia, the bilateral vertebral pedicle body surface projection points of L2 and L4 were located and punctured (Fig. 5F). Puncture needles were located in L2 and L4 vertebral bodies under C-arm fluoroscopy (Fig. 5G and H). A working channel was placed through the guide needle. Then, bone cement (4–5 mL) was pushed into the vertebral body (Fig. 5I and J). C-arm fluoroscopy revealed adequate bone cement diffusion. Finally, the puncture needles were pulled out after the bone cement had hardened. A similar PVP operation performed for L5 (Fig. 5K-O). Postoperative X-rays revealed a good surgical effect of bone cement dispersion in L2, L4, and L5 (Fig. 5P and Q).
Table 4.
Perioperative characteristics and surgical effects of patients receiving PVP and separation surgery/TES in FUSCC (n = 56)
| Characteristics | Minimally invasive PVP | Separation surgery/TES |
|---|---|---|
| Age | 54.5 ± 11.1 | 62.8 ± 8.3 |
| Male | 4 (36.4) | 25 (55.6) |
| BMI (kg/m2) | 25.1 ± 4.0 | 22.9 ± 3.0 |
| Tomita score | 6.1 ± 0.7 | 5.8 ± 0.7 |
| ASA grade | ||
| I | 7 (63.6) | 13 (28.9) |
| П/Ш | 4 (36.4) | 32 (71.1) |
| Duration of surgery (min) | 69 (60–118) | 157.5 (115.8–188.3) |
| Volume of blood loss (mL) | 10 (10–10) | 1000 (650–1550) |
| Blood transfusion | 0 (0) | 31 (68.9) |
| Length of hospital stay (days) | 4 (2–7) | 11 (7–15.5) |
| Surgical cost (¥) | 6871.0 ± 1658.0 | 11,686.0 ± 2324.0 |
| Pain (NRS scale) | ||
| Preoperative | 5.0 ± 0.9 | 4.8 ± 1.3 |
| Postoperative or follow-up | 0.5 ± 0.8 | 1.2 ± 1.0 |
| P | 0.001* | < 0.001* |
| Walk on level ground (Barthel scale) | ||
| Preoperative | 14.1 ± 2.0 | 8.9 ± 5.0 |
| Postoperative or follow-up | 14.6 ± 1.5 | 13.7 ± 3.1 |
| P | > 0.999 | < 0.001* |
| Climb stairs (Barthel scale) | ||
| Preoperative | 9.1 ± 2.0 | 5.1 ± 3.9 |
| Postoperative or follow-up | 9.5 ± 1.5 | 8.3 ± 3.0 |
| P | > 0.999 | < 0.001* |
Abbreviations: PVP percutaneous vertebroplasty, TES total en bloc spondylectomy, BMI body mass index, ASA American Society of Anesthesiologists, NRS numerical rating scale
*Statistically significant
Walk on level ground measured by Barthel scale: 15, full independence; 10, need of some help; 5, need of much help; 0, full dependence
Climb stairs measured by Barthel scale: 10, full independence; 5, need of some help; 0, need of much help
Fig. 5.
Personalized percutaneous vertebroplasty (PVP) procedure. A and B Preoperative sagittal MRI and CT scans show L4 and L5 involvement (low signal on T1WI and slightly high signal on T2WI); C–E preoperative transverse CT scans show L2, L4, and L5 involvements; F preoperative body surface location of L2, L4, and L5; G and H C-arm fluoroscopy shows that puncture needles are located in L2 and L4 vertebrae; I and J bone cement is injected into the vertebral bodies of L2 and L4; K and L C-arm fluoroscopy shows that puncture needles are located in L5 vertebra; M and N bone cement is injected into L5; O C-arm fluoroscopy shows good dispersion of bone cement in L2, L4, and L5; P and Q postoperative X-ray shows a good dispersion of bone cement in L2, L4, and L5
The median PVP time was 69 (60–118) min, with a median blood loss of 10 mL. No bone cement leakage-related symptoms were recorded. The pain was significantly relieved after PVP [NRS score: (0.5 ± 0.80) vs. (5.0 ± 0.9), P < 0.05]. The PVP preoperative and postoperative activities in the group (walk on level ground and climb stairs) were both nearly in full independence (Table 4).
Separation surgery and TES for LC metastatic spinal tumors
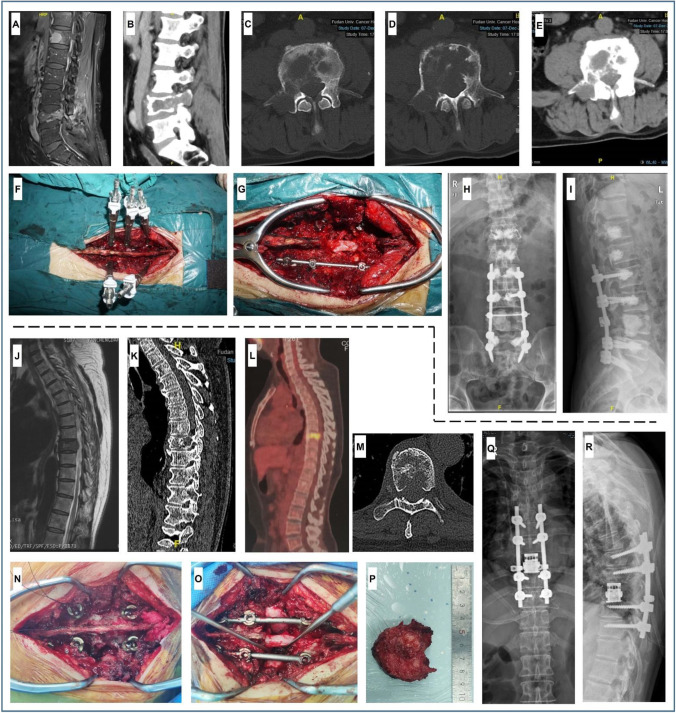
The clinical characteristics of patients receiving separation surgery/TES were presented in Table 4. A representative separation surgery in FUSCC was presented in Fig. 6A–I. A male patient aged 63 was admitted to hospital due to lumbar back pain for 1 month. His pain aggravated when he bent down, accompanied by right lower limb pain and numbness. He had a lung adenocarcinoma resection history 2 years ago. Preoperative MRI and CT scan suggested L4 metastasis with a compression fracture, with T12, L1, and L2 metastasis (Fig. 6A–E). After anesthesia, a posterior median longitudinal incision was performed from T12 to the 1st sacrum. The spinous process, bilateral lamina, facet joint, and transverse process from T12 to the 1st sacrum were well exposed. Five bone-cemented-pedicle screws were placed on L2 and L5 bilateral pedicles and L3 right pedicle (Fig. 6F). Bone cement was similarly injected into T12 and L1. Then the spinous process, bilateral lamina, and L3-4left facet joint were resected to expose the dural sac at the L3–4 level. The left connecting rod was installed with a temporary pull open (Fig. 6G). Next, with careful bilateral lumbar nerve roots protection, the metastatic LC and its eroded bone and soft tissues were piecemeal completely resected under a magnifying glass. The space between the dura and the tumor was sufficiently large to favor postoperative radiotherapy. The L4 body residual lumen was tamped with bone cement. The right connecting rod and the cross-connection were installed. Postoperative X-ray showed a good surgical effect of the internal fixation and bone cement dispersion (Fig. 6H and I).
Fig. 6.
Personalized separation surgery and TES procedures. A and B Preoperative sagittal MRI and CT scans show L4 metastasis with compression fracture, with T12, L1, and L2 involvements (high signal on T2WI and low signal on T1WI); C–E preoperative transverse CT scans show that the right appendage and vertebral body of L4 are eroded by tumor tissues. Bone destruction and multiple osteolytic lesions are visible; F installation of bone cement injection system; G the dural sac at the L3–4 level is well exposed to piecemeal resect the metastasis and its eroded bone and soft tissues; H and I postoperative X-ray shows a good internal fixation and bone cement dispersion in T12, L1, L2, and L4. J and K preoperative sagittal MRI and CT scans show T8 metastasis with pathological fracture. BM protruded into the spinal canal and compacted the dural sac; L preoperative PET-CT shows an increased SUVmax of the T8 vetebra; M preoperative transverse CT shows the right appendage and vertebral body of T8 involvement; N pedicle screws are placed bilaterally at T6–7 and T9–10; O the right intervertebral discs (T7–8 and T8–9) are dissected, and the right connecting rod is installed, so are the left intervertebral discs; P measurement of the resected T8 metastasis; Q and R postoperative X-ray shows a good surgical effect of the internal fixation and the artificial vertebral body. Abbreviation: TES, total en bloc spondylectomy; PET-CT, positron emission tomography-computed tomography; SUVmax, maximum standard uptake value
A representative TES in FUSCC was presented in Fig. 6J–R. A female patient aged 55 was admitted to hospital due to chest back pain for 3 months. Preoperative MRI, CT, and PET-CT scans showed T8 vertebral body pathological fracture, with an increased SUVmax (Fig. 6J–M) and no other vertebra metastasis. After anesthesia, a posterior median longitudinal incision was performed using T8 as the midpoint. The spinous process, bilateral lamina, and zygapophyses of T6–10 were well exposed. Pedicle screws were placed bilaterally at T6–7 and T9–10 (Fig. 6N), and then the ribs were exposed to both sides along the T8 transverse process (about 3 cm). The spinous process, bilateral lamina, and ligamentum flavum of T7 were removed to expose the dural sac and intervertebral foramen at T7–8 level. The T8 adnexa structure was resected to expose the dural sac at T7–9 level. After the upper and lower T8 intervertebral discs were determined, the annulus fibrosus and the posterior longitudinal ligament were cut. The right intervertebral discs (T7–8 and T8–9) were dissected, and the right connecting rod was installed, as well as the left intervertebral discs (Fig. 6O). T8 was completely removed under careful protection of spinal cord and nerve roots (Fig. 6P). An artificial vertebral body was installed between T7 and T9. Finally, the bilateral connecting rods with cross-connection were installed. Postoperative X-ray showed a good surgical effect of the internal fixation and the artificial vertebral body (Fig. 6Q and R).
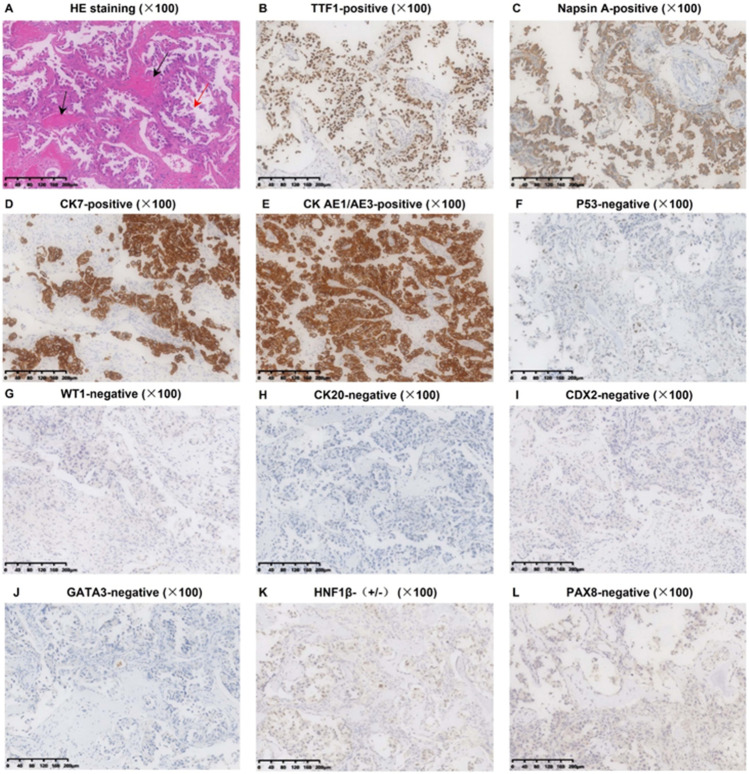
The resected T8 vertebra was sent for pathological examination. HE staining showed wide adenocarcinoma tissues around the bone tissues. IHC staining [TTF-1 ( +), Napsin A ( +), CK7 ( +), CK AE1/AE3 ( +), p53 ( −), WT-1 ( −), CK20 ( −), CDX2 ( −), GATA3 ( −), HNF1β ( ±), and PAX8 ( −)] verified the lung adenocarcinoma origin (Fig. 7A–L).
Fig. 7.
Pathological immunophenotype of the resected BM (T8 vertebra). A HE Staining showing the adenocarcinoma tissues (red arrow) and the bone tissues (black arrow); B-D the positive expressions of TTF-1, Napsin A, and CK7 are currently the best combination indicating an origin of lung adenocarcinoma (TTF-1 and Napsin A are generally positive in 80% of lung adenocarcinoma, and CK7 is nearly positive in all lung adenocarcinoma); E positive CK (AE1/AE3) expression indicated carcinoma instead of sarcoma; F negative p53 expression indicated an unlikely squamous carcinoma; G negative WT-1 expression indicated an unlikely origin of mesothelioma; H and I negative CK20 and CDX2 expression indicated an unlikely origin of colorectal cancer; J negative GATA-3 expression indicated an unlikely origin of breast cancer; K and L negative HNF-1β and PAX8 expression indicated an unlikely origin of ovary and thyroid
The median separation surgery/TES time in FUSCC was 223 (176–265) min. A median blood loss was 1000 mL, and 68.9% of the patients received intraoperative blood transfusion. Patients undergoing open surgery obtained ambulation improvement at hospital discharge, which generally takes 11 days. LC-specific mortality instead of SREs was the main causes of death, such as dyspnea, chest fluid, hemoptysis, and multiple organ failure. The median survival time of FUSCC cohort was 11 (6–14) months. During the survival, pain was significantly relieved [(1.2 ± 1.0) vs. (4.8 ± 1.3), P < 0.05], and walking ability on the level ground was significantly improved [(13.7 ± 3.1) vs. (8.9 ± 5.0), P < 0.05]. Ability to climb stairs was also significantly improved [(8.3 ± 3.0) vs. (5.1 ± 3.9), P < 0.05] (Table 4).
Comparisons between BM open surgery and minimally invasive PVP on inflammation, immune, and nutrition status were presented in Supplementary Fig. 4. For patients undergoing PVP, inflammatory markers such as leucocytes, neutrophils, and NLR were significantly increased after PVP. Lymphocytes percentage significantly decreased after PVP. Hemoglobin and total protein were not significantly changed. For patients undergoing separation surgery/TES, inflammatory markers such as leucocytes, neutrophils, and NLR were significantly increased after surgery. Lymphocytes percentage was significantly decreased after surgery, as well as the hemoglobin and total protein.
Discussion
PPPM from cancer systematic biology to imageology
PPPM can be applied to many cancer care fields, such as prostate cancer [21], colorectal cancer [22], and breast cancer [23]. For instance, Kucera and colleagues [21] suggested that PPPM-based liquid biopsy tests and multi-omics phenotyping and genotyping are instrumental for prostate cancer diagnosis and prevention. Colorectal cancer is characterized by various genetic alterations, such as KRAS and BRAF mutations. An integrative PPPM viewpoint by Hagan et al. considered that those molecular mutations found in colorectal cancer can guide targeted treatments such as anti-EGFR therapy and estimate the patient’s prognosis in the future [22]. Therefore, it is interesting to characterize LC-BM using PPPM-based principles. Cheng T and Zhan X [24] considered that PPPM could explain cancer pathophysiological processes from systematic biology to radiomics and metabolomics. In the case of cancer cells, the bone is a convenient metastasis destination. LC-BM is a multi-step process that includes the following steps: First, circulating LC cells settle in the bone microenvironment and become dormant to avoid being attacked by the body’s immune system; second, dormant LC cells re-activate and initiate proliferation, resulting in large metastatic lesions; Lastly, LC cells disrupt the dynamic balance between osteoblasts and osteoclasts, causing bone resorption and osteolytic lesions [25–27]. Besides the classical “seed and soil” theory, “pre-metastatic niches” should be noted in cancer metastasis [23], especially in BM. In our study, the LC-BM X-ray revealed a worm-eaten type of bony defect with an obscure and irregular boundary. CT and MRI images revealed BM size, range, and adjacent tissues which are crucial to preoperative assessment. PET-CT has high sensitivity and specificity (both > 90%) for LC-BM diagnosis [28]. In our study case, metastatic LC at T8 vertebrae highly took in fluorodeoxyglucose (FDG). The T8 malignant BM was easily identified as hyper-metabolic on PET-CT.
Predictive factors and target population for prevention
The timing of diagnosis impacts LC-BM treatment and prognosis. The most common clinical examinations for BM diagnosis, according to standard guidelines, are X-ray, radionuclide bone scanning (ECT), CT, and MRI. Regardless, BM is frequently discovered when bone-related symptoms and signs (SREs) are severe. In most cases, LC-BM patients have advanced metastasis, with multiple BM lesions. It is important to predict BM risk and thus early diagnosis of BM in LC patients. In this study, we identified that liver metastasis is a strong BM predictor in LC patients. This might be because liver metastasis is a key indicator of cancer cells entering veins, which are the primary pathways for LC cells to reach the bones. Additionally, we discovered that liver metastasis is linked to a poor prognosis. Young male patients, those with more invasive LC, and distant site metastases should be considered key populations for BM prevention. Few previous SEER studies included metastasis surgery in the survival analysis [29, 30]. Our study discovered that BM surgery did not improve LC-BM patients’ survival in SEER cohort. This might be due to the high malignant biological behavior of LC when compared to breast and prostate cancers. PPPM plays an important role in cancer patients’ targeted prevention. The time interval of medical examinations should be narrowed in those patients to detect BM at early stage and prevent extensive metastasis.
PPPM in personalized surgery
PPPM has its unique meaning in surgery, such as preoperative planning, intraoperative execution, and postoperative management [31]. Prediction is reflected in the preoperative planning, such as PVP or open surgery/TES options. Prevention is reflected in intraoperative execution, such as preventing complications (e.g., bone cement leakage). In our study, the spine was the most common BM site causing SREs, owing to the abundance of venous plexuses surrounding the vertebrae [32]. Spinal structure is complex and variable once tumor involvement. It motivates us to develop patient-specific surgical plan and use personalized internal fixation. Those SREs significantly impact patients’ quality of life and can only be effectively treated surgically [33, 34]. The Tomita score is a simple and effective tool for predicting the prognosis of metastatic spinal tumors [35]. Spinal metastasis surgery requires an expected survival longer than 3 and even 6 months [36–38]. Our study discovered that the 3- and 6-month LC-BM survival rates improved significantly over time, indicating that an increasing number of LC-BM patients are candidates for BM surgery.
We demonstrated that the personalized PVP significantly reduced patients’ pain, with less blood loss, hospital stay length, and cost. The possible reasons for pain reduction after PVP include as follows: The injected bone cement stabilizes the fracture; bone cement and its thermogenesis destroy sensory nerve endings; and bone cement’s anti-tumor effects [39–41]. Bone cement leakage is a severe complication after PVP [42–45]. As demonstrated in our procedures, personalized strategies to prevent bone cement leakage included precisely controlled viscosity of bone cement and close monitoring of its distribution under X-ray.
In our study, besides pain relief, personalized separation surgery/TES significantly improved movement ability. Separation surgery mainly applies to patients with spinal cord or nerve roots compression and spinal instability and fracture [46]. It enlarges the space between BM and the spinal dural mater via a 360°-circular spinal cord decompression [47, 48]. In our study, individualized artificial vertebral body was used to match with bone defect as closely as possible. It should be noted that radiation is necessary after separation surgery to prevent a local recurrence [49, 50].
PPPM in personalized perioperative critical care
In our study, LC-BM patients had increased inflammation and decreased immune, nutritional, and hemoglobin levels before surgery. Since our institution is a specialized cancer center, many of the advanced patients have received comprehensive therapy before surgery. It is well known that malignancy itself and its associated systemic therapies significantly impact inflammation, immunity, anemia, and nutrition [51, 52]. We showed that the increased inflammation and decreased immune, nutrition, and hemoglobin levels aggravated after BM surgery strike. Therefore, personalized perioperative managements such as anti-inflammation and blood transfusion must be prepared to prevent vital organs injury.
Limitations and prospects
There are inevitably some limitations in our study. This study takes the first step to incorporate 3PM into LC-BM’s interdisciplinary problem. We recognize that, at present, there remains a long way to go before fully elucidating and managing LC-BM via PPPM. Nonetheless, we are confident that our current work contributes to the progression of 3PM and can guide LC-BM diagnosis and treatment paradigm to 3PM in a step-by-step fashion. Secondly, external validation is required for our existing nomogram. Inclusion of LC-BM patients from various centers is required to test and improve the nomogram’s predictive performance. Furthermore, an online nomogram is suggested for ease of use. Thirdly, radiation as a key adjuvant therapy after separation surgery, it also needs to be characterized via PPPM-based approaches. In the future, it is critical to conduct an independent study to understand PPPM-oriented radiation use in LC-BM patients.
Conclusions and recommendations
LC patients have a longer survival time due to rapid advancements in systemic therapy, but they are also exposed to more BM risk. The PPPM concept should be actively incorporated into BM-LC management including diagnosis, prevention, treatment, and prognosis. The term “cancer prevention” refers to a broad concept that encompasses three dimensions: primary prevention entails avoiding carcinogenic factors and lowering cancer incidence; secondary prevention involves early detection, diagnosis, and treatment of cancer to reduce cancer mortality; and tertiary prevention entails reducing and treating cancer recurrence and metastasis, as well as preventing complications and sequelae. The current LC-BM study is primarily concerned with secondary and tertiary prevention. The present study can help us predict a LC individual’s BM risk, identify a more targeted population for BM prevention, and provide a more personalized BM surgery experience (Fig. 8). From passive to active, PPPM shifts cancer diagnostic and therapeutic patterns [53]. With the help of PPPM, LC patients can prevent from BM and its associated SREs, predict BM risks and prognosis, and receive a personalized surgical treatment. PPPM principles should be sufficiently developed and applied throughout the LC-BM process from screening to surgical therapy. Moreover, PPPM also apply to investigate cancer molecular biology and immunology. In the future, PPPM-based strategies are expected to further manage LC-BM targeted therapy (EGFR-TKIs) and immune checkpoint therapy.
Fig. 8.
A schematic diagram showing 3PM-oriented LC-BM care pattern. BM predictors, key population for BM prevention, and personalized surgery experience and effects are well depicted. 3PM-based radiation use is the next focus. Abbreviations: LC, lung cancer; BM, bone metastasis; 3PM, predictive, preventative, and personalized medicine
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BM
Bone metastasis
- LC
Lung cancer
- SREs
Skeletal-related events
- SEER
Surveillance, Epidemiology, and End Results
- FUSCC
Fudan University Shanghai Cancer Center
- MDT
Multidisciplinary team
- PVP
Percutaneous vertebroplasty
- TES
Total en bloc spondylectomy
- NOS
Not otherwise specified
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- BMI
Body mass index
- NRS
Numerical rating scale
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- ECT
Radionuclide bone scanning
- PET-CT
Positron emission tomography-computed tomography
- SUVmax
Maximum standard uptake value
- ALP
Alkaline phosphatase
- LDH
Lactate dehydrogenase
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- TBIL
Total bilirubin
- ASA
American Society of Anesthesiologists
- FDG
Fluorodeoxyglucose
- HE staining
Hematoxylin–eosin staining
- IHC
Immunohistochemistry
- OR
Odds ratio
- HR
Hazards ratio
- CI
Confidence interval
- ROC
Receiver operator characteristic curve
- AUC
Area under the curve
Author contribution
Wangjun Yan and Yangbai Sun contributed to the study conception and design. Xianglin Hu, Wending Huang, Zhengwang Sun, Hui Ye, Kwong Man, and Qifeng Wang retrieved, analyzed, and interpreted the data. Xianglin Hu drafted the manuscript. Wangjun Yan and Yangbai Sun revised the manuscript. All authors read and approved the submission.
Funding
This work was supported by the National Natural Science Foundation of China (Number: 81872179; Recipients: Wangjun Yan).
Data availability
Data for the SEER cohort were extracted from the Surveillance, Epidemiology, and End Results Program (https://seer.cancer.gov/). Data for the FUSCC cohort were available from the corresponding authors upon appropriate request.
Code availability
Not applicable
Declarations
Ethics approval
This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center, Shanghai, 200032, China. All procedures performed in the study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent for participation in the FUSCC cohort was obtained in this study.
Consent for publication
All authors read and approved the submission.
Conflict of interest
The authors declare no competing interests.
Footnotes
Highlights
The current study introduces a predictive, preventive, and personalized medicine (3PM) viewpoint throughout lung cancer (LC)-bone metastasis (BM) management.
1. Predictive diagnostics: Liver metastasis is a significant risk factor for BM. the individualized nomogram established in our study can well stratify a patient’s BM risk.
2. Targeted prevention: Young patients, males, patients with high invasive LC, and patients with other distant site metastases must be the key population for BM prevention.
3. Personalization of medical services: Personalized spinal metastasis surgery with spinal reconstruction significantly relieves pain and improves daily activities.
4. Personalization of medical services: Perioperative managements such as anti-inflammation and blood transfusion must be individualized to prevent vital organs injury. Radiotherapy needs to be recommended for specific postoperative individuals
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xianglin Hu, Wending Huang and Zhengwang Sun contributed equally to this work
Contributor Information
Yangbai Sun, Email: drsunyb@fudan.edu.cn.
Wangjun Yan, Email: yanwj@fudan.edu.cn.
References
- 1.Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res. 2018;33(12):2099–2113. doi: 10.1002/jbmr.3618. [DOI] [PubMed] [Google Scholar]
- 2.Choi SH, Koo JW, Choe D, et al. The incidence and management trends of metastatic spinal tumors in South Korea: a nationwide population-based study. Spine. 2020;45(14):E856–E863. doi: 10.1097/BRS.0000000000003445. [DOI] [PubMed] [Google Scholar]
- 3.Adogwa O, Rubio DR, Buchowski JM, et al. Spine-specific skeletal related events and mortality in non-small cell lung cancer patients: a single-institution analysis. J Neurosurg Spine. 2020;27:1–8. doi: 10.3171/2020.7.SPINE20829. [DOI] [PubMed] [Google Scholar]
- 4.Shih JT, Yeh TT, Wang SH, et al. Incidence of bone metastases in patients with organ-specific cancers: a nationwide population-based cohort study. Int J Clin Pract. 2021;5:e13997. doi: 10.1111/ijcp.13997. [DOI] [PubMed] [Google Scholar]
- 5.Ryan C, Stoltzfus KC, Horn S, et al. Epidemiology of bone metastases. Bone. 2020;1:115783. [DOI] [PubMed]
- 6.Grech G, Zhan X, Yoo BC, et al. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):9. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilai O, Fisher CG, Bilsky MH. State of the art treatment of spinal metastatic disease. Neurosurgery. 2018;82(6):757–769. doi: 10.1093/neuros/nyx567. [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 9.Patil V, Noronha V, Joshi A, et al. Phase III non-inferiority study evaluating efficacy and safety of low dose gemcitabine compared to standard dose gemcitabine with platinum in advanced squamous lung cancer. EClinicalMedicine. 2019;9:19–25. doi: 10.1016/j.eclinm.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi D, Fox Z, Albert T, et al. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br J Neurosurg. 2016;30(3):337–344. doi: 10.3109/02688697.2015.1133802. [DOI] [PubMed] [Google Scholar]
- 11.Spencer KL, van der Velden JM, Wong E, et al. Systematic review of the role of stereotactic radiotherapy for bone metastases. J Natl Cancer Inst. 2019;111(10):1023–1032. doi: 10.1093/jnci/djz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domenicucci M, Nigro L, Delfini R. Total en-bloc spondylectomy through a posterior approach: technique and surgical outcome in thoracic metastases. Acta Neurochir (Wien) 2018;160(7):1373–1376. doi: 10.1007/s00701-018-3572-2. [DOI] [PubMed] [Google Scholar]
- 13.Kushchayev SV, Wiener PC, Teytelboym OM, et al. Percutaneous vertebroplasty: a history of procedure, technology, culture, specialty, and economics. Neuroimaging Clin N Am. 2019;29(4):481–494. doi: 10.1016/j.nic.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Rothrock R, Pennington Z, Ehresman J, et al. Hybrid therapy for spinal metastases. Neurosurg Clin N Am. 2020;31(2):191–200. doi: 10.1016/j.nec.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Koklesova L, Liskova A, Samec M, et al. Protective effects of flavonoids against mitochondriopathies and associated pathologies: focus on the predictive approach and personalized prevention. Int J Mol Sci. 2021;22(16):8649. doi: 10.3390/ijms22168649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liskova A, Samec M, Koklesova L, et al. Liquid biopsy is instrumental for 3PM dimensional solutions in cancer management. J Clin Med. 2020;9(9):2749. doi: 10.3390/jcm9092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 18.Chiarotto A, Maxwell LJ, Ostelo RW, et al. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: a systematic review. J Pain. 2019;20(3):245–263. doi: 10.1016/j.jpain.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Knauf T, Buecking B, Hack J, et al. Development of the Barthel Index 5 years after hip fracture: results of a prospective study. Geriatr Gerontol Int. 2019;19(8):809–814. doi: 10.1111/ggi.13723. [DOI] [PubMed] [Google Scholar]
- 20.Mannion AF, Bianchi G, Mariaux F, et al. Can the Charlson Comorbidity Index be used to predict the ASA grade in patients undergoing spine surgery? Eur Spine J. 2020;29(12):2941–2952. doi: 10.1007/s00586-020-06595-1. [DOI] [PubMed] [Google Scholar]
- 21.Kucera R, Pecen L, Topolcan O, et al. Prostate cancer management: long-term beliefs, epidemic developments in the early twenty-first century and 3PM dimensional solutions. EPMA J. 2020;11(3):399–418. doi: 10.1007/s13167-020-00214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagan S, Orr MC, Doyle B. Targeted therapies in colorectal cancer-an integrative view by PPPM. EPMA J. 2013;4(1):3. doi: 10.1186/1878-5085-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bubnov R, Polivka J, Jr, Zubor P, et al. “Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer Syndrome” relevance to address the question. EPMA J. 2017;8(2):141–157. doi: 10.1007/s13167-017-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng T, Zhan X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 2017;8(1):51–60. doi: 10.1007/s13167-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shupp AB, Kolb AD, Bussard KM. Novel techniques to study the bone-tumor microenvironment. Adv Exp Med Biol. 2020;1225:1–18. doi: 10.1007/978-3-030-35727-6_1. [DOI] [PubMed] [Google Scholar]
- 26.Xiang L, Gilkes DM. The contribution of the immune system in bone metastasis pathogenesis. Int J Mol Sci. 2019;20(4):999. doi: 10.3390/ijms20040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F, Cao Y, Wu G, et al. BMP2 signalling activation enhances bone metastases of non-small cell lung cancer. J Cell Mol Med. 2020;24(18):10768–10784. doi: 10.1111/jcmm.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim CH, Ahn TR, Moon SH, et al. PET/CT features discriminate risk of metastasis among single-bone FDG lesions detected in newly diagnosed non-small-cell lung cancer patients. Eur Radiol. 2019;29(4):1903–1911. doi: 10.1007/s00330-018-5764-9. [DOI] [PubMed] [Google Scholar]
- 29.Song Q, Shang J, Zhang C, et al. Impact of the homogeneous and heterogeneous risk factors on the incidence and survival outcome of bone metastasis in NSCLC patients. J Cancer Res Clin Oncol. 2019;145(3):737–746. doi: 10.1007/s00432-018-02826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Chen L, Huang C, et al. The homogeneous and heterogeneous risk factors for occurrence and prognosis in lung cancer patients with bone metastasis. J Bone Oncol. 2019;17:100251. doi: 10.1016/j.jbo.2019.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joskowicz L. Computer-aided surgery meets predictive, preventive, and personalized medicine. EPMA J. 2017;8(1):1–4. doi: 10.1007/s13167-017-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa Y, Ito T, Mizuno Y, et al. Symptoms related to moderate skeletal-related events as clues for the diagnosis of bone metastasis. J Nippon Med Sch. 2019;86(3):159–164. doi: 10.1272/jnms.JNMS.2019_86-304. [DOI] [PubMed] [Google Scholar]
- 33.Laganà M, Gurizzan C, Roca E, et al. High prevalence and early occurrence of skeletal complications in EGFR mutated NSCLC patients with bone metastases. Front Oncol. 2020;10:588862. doi: 10.3389/fonc.2020.588862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Zhu W, Biskup E, et al. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal-related events (SREs) in breast cancer patients: a systematic review of the real world data. J Bone Oncol. 2018;11:38–50. doi: 10.1016/j.jbo.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH, Chung CK, Jahng TA, et al. Which one is a valuable surrogate for predicting survival between Tomita and Tokuhashi scores in patients with spinal metastases? A meta-analysis for diagnostic test accuracy and individual participant data analysis. J Neurooncol. 2015;123(2):267–275. doi: 10.1007/s11060-015-1794-1. [DOI] [PubMed] [Google Scholar]
- 36.Chinese Medical Association Society of Orthopedics Bone Oncology Group. Guidelines for surgical treatments of metastatic spinal tumors. Chin J Orthop. 2019; 39(12): 717–6. 10.3760/cma.j.issn.0253?2352.2019.12.001
- 37.Bollen L, Dijkstra SPD, Bartels RHMA, et al. Clinical management of spinal metastases-the Dutch national guideline. Eur J Cancer. 2018;104:81–90. doi: 10.1016/j.ejca.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Spratt DE, Beeler WH, de Moraes FY, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol. 2017;18(12):e720–e730. doi: 10.1016/S1470-2045(17)30612-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Yang S, Cai K, et al. Bioactive poly (methyl methacrylate) bone cement for the treatment of osteoporotic vertebral compression fractures. Theranostics. 2020;10(14):6544–6560. doi: 10.7150/thno.44428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan L, Wen B, Guo Z, et al. The effect of bone cement distribution on the outcome of percutaneous vertebroplasty: a case cohort study. BMC Musculoskelet Disord. 2020;21(1):541. doi: 10.1186/s12891-020-03568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wegener B, Zolyniak N, Gülecyüz MF, et al. Heat distribution of polymerisation temperature of bone cement on the spinal canal during vertebroplasty. Int Orthop. 2012;36(5):1025–1030. doi: 10.1007/s00264-011-1382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu SY, Zhong ZM, Wu Q, et al. Risk factors for bone cement leakage in percutaneous vertebroplasty: a retrospective study of four hundred and eighty five patients. Int Orthop. 2016;40(6):1205–1210. doi: 10.1007/s00264-015-3102-2. [DOI] [PubMed] [Google Scholar]
- 43.Tang S, Fu W, Zhang H, et al. Efficacy and safety of high-viscosity bone cement vertebroplasty in treatment of osteoporotic vertebral compression fractures with intravertebral cleft. World Neurosurg. 2019;132:e739–e745. doi: 10.1016/j.wneu.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Wang Q, Wang L, et al. Bone cement distribution in the vertebral body affects chances of recompression after percutaneous vertebroplasty treatment in elderly patients with osteoporotic vertebral compression fractures. Clin Interv Aging. 2017;12:431–436. doi: 10.2147/CIA.S113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuan TA, Luong TV, Cuong PM, et al. Cement leakage in percutaneous vertebroplasty for multiple osteoporotic vertebral compression fractures: a prospective cohort study. Orthop Res Rev. 2020;12:105–111. doi: 10.2147/ORR.S255517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quraishi NA, Arealis G, Salem KM, et al. The surgical management of metastatic spinal tumors based on an Epidural Spinal Cord Compression (ESCC) scale. Spine J. 2015;15(8):1738–1743. doi: 10.1016/j.spinee.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 47.Di Perna G, Cofano F, Mantovani C, et al. Separation surgery for metastatic epidural spinal cord compression: a qualitative review. J Bone Oncol. 2020;25:100320. doi: 10.1016/j.jbo.2020.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzilai O, Laufer I, Robin A, et al. Hybrid therapy for metastatic epidural spinal cord compression: technique for separation surgery and spine radiosurgery. Oper Neurosurg (Hagerstown) 2019;16(3):310–318. doi: 10.1093/ons/opy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiaozhou L, Xing Z, Xin S, et al. Efficacy analysis of separation surgery combined with SBRT for spinal metastases-a long-term follow-up study based on patients with spinal metastatic tumor in a single-center. Orthop Surg. 2020;12(2):404–420. doi: 10.1111/os.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redmond KJ, Lo SS, Soltys SG, et al. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine. 2017;26(3):299–306. doi: 10.3171/2016.8.SPINE16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorphe P, Bouhir S, Garcia GCTE, et al. Anemia and neutrophil-to-lymphocyte ratio in laryngeal cancer treated with induction chemotherapy. Laryngoscope. 2020;130(4):E144–E150. doi: 10.1002/lary.28021. [DOI] [PubMed] [Google Scholar]
- 52.Desai N, Schofield N, Richards T. Perioperative patient blood management to improve outcomes. Anesth Analg. 2018;127(5):1211–1220. doi: 10.1213/ANE.0000000000002549. [DOI] [PubMed] [Google Scholar]
- 53.Zubor P, Dankova Z, Kolkova Z, et al. Rho GTPases in Gynecologic cancers: in-depth analysis toward the paradigm change from reactive to predictive, preventive, and personalized medical approach benefiting the patient and healthcare. Cancers (Basel) 2020;12(5):1292. doi: 10.3390/cancers12051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the SEER cohort were extracted from the Surveillance, Epidemiology, and End Results Program (https://seer.cancer.gov/). Data for the FUSCC cohort were available from the corresponding authors upon appropriate request.
Not applicable