Abstract
The aim of the current study was to assess the influence of embryonic exposure to cadmium on basic and derived erythrocyte indices, the morphology and morphometric properties of erythrocytes, as well as erythrocyte spectrin distribution in newly hatched Gallus gallus domesticus chicks. The eggs were injected with cadmium (Cd) at a dose of 2 µg, 4 µg, 6 µg, or 8 µg per egg on the sixth day of incubation. Blood samples were collected on the first day after hatching. Exposure to cadmium resulted in higher levels of red blood cell count, hemoglobin concentration, and hematocrit value, while derived erythrocyte indices were lower (mean corpuscular volume) or higher (mean corpuscular hemoglobin concentration) in comparison to the control. These changes occurred in animals exposed to higher doses of this toxic agent. In cadmium-treated individuals (2 and 8 µg of Cd), the percentage of erythrocytes which exhibited changed shape increased. Increases in the length (6 and 8 µg) and width (2, 6, and 8 µg) of erythrocytes and the length and width of the nucleus (2–8 µg) of red blood cells were observed. Changes in spectrin distribution were also observed, which indicate alterations at structural and molecular levels.
Key words: hematology, erythrogram, spectrin, heavy metal, bird
INTRODUCTION
Hematological analysis is an important tool in the assessment of the physiological state of vertebrates. Hematological alterations may be a result of exposure to various toxic agents, including pesticides (e.g., Owagboriaye et al., 2019; Bojarski and Witeska, 2020), antibiotics (e.g., Al-Mayah and Al-Ahmed, 2005; Bojarski et al., 2020), and heavy metals (e.g., Mroczek-Sosnowska et al., 2013; Waghmare et al., 2015). Usually, standard red and white blood cell parameters are analyzed, but the influence of toxic substances on blood cell morphology is rarely taken into account. Nevertheless, in the case of fish, the morphology of red blood cells may change as a result of exposure to various chemicals, for example, insecticides (Sawhney and Johal, 2000; Babu et al., 2014), zinc (Tomova et al., 2008), or cadmium (Witeska et al., 2011). According to Witeska et al. (2010,2011), the morphology of fish erythrocytes is more sensitive than basic red blood cell parameters to various environmental agents. Thus, cellular anomalies of fish erythrocytes can be noticed even if the values of basic hematological indices such as hematocrit value, erythrocyte count or hemoglobin concentration do not change significantly (Witeska et al., 2010,2011). It is not known whether a similar phenomenon occurs in other vertebrates. Scientific data regarding the influence of xenobiotics on mammalian red blood cells is limited. However, according to Pagano and Faggio (2015), the erythrocytes of mammals can be used to study cellular and molecular treatment mechanisms, and they are a good model with which to assess the cytotoxicity of organic and inorganic chemicals. To our knowledge there is no available literature data concerning the effects of toxic substances on avian erythrocytes.
Another marker that is rarely studied in the context of xenobiotic exposure is the distribution of spectrin in red blood cells. Spectrin is a cytoskeletal protein that was first discovered in erythrocytes and is important for maintaining the stability, structure, and shape of the cell membrane (Zhang et al., 2013). In vitro exposure of human erythrocytes to cadmium resulted in changes in the biochemical structure of spectrin that were not observed after zinc treatment (Yang, 1993).
The avian embryo is an approved biological model which can be used to reflect environmental contamination under laboratory conditions (e.g., Liu et al., 2015; Dżugan et al., 2018; Bojarski et al., 2021). The aim of the current study was to assess the influence of embryonic exposure to cadmium (administrated as CdCl2 × 2.5 H2O) on basic (RBC – erythrocyte count, Ht – hematocrit value, and Hb – hemoglobin concentration) and derived (MCV – mean corpuscular volume, MCH – mean corpuscular hemoglobin, and MCHC – mean corpuscular hemoglobin concentration) erythrocyte indices, the morphology and morphometric properties of erythrocytes, as well as erythrocyte spectrin distribution in newly hatched Gallus gallus domesticus chicks. To our knowledge, this study is the first attempt to determine whether erythrocyte morphology may be considered an indicator of cadmium exposure in the case of birds.
MATERIAL AND METHODS
Experimental Design
According to Directive 2010/63/EU, the experimental and animal procedures used in this study did not need to be approved by the Local Animal Ethics Committee.
Incubation Procedure
A total of 450 embryonated eggs weighing 57.1 g ± 2.6 g (mean ± SD) from a 29-wk-old parental stock of broiler Cobb 500 line (Cobb Germany, Avimex GmbH; Sławomir Domagała's Poultry Farm, Gołaczewy, Poland) were used in the experiment. Before incubation, the eggs were stored for three days (T 18°C, RH 65%) and disinfected by ozonation (0.5% O3, 10 min).
Next, the hatching eggs were set on trays and incubated in the laboratory incubator (IgloTech, Poland). Between the first and the 18th incubation day (E1–E18) the incubation conditions were T 37.8 ± 0.1°C and 50 ± 1% RH, and the trays with the eggs were tilted 45° and turned 90° every hour. The eggs were transferred to baskets on E19 and incubation was continued at T 37.2 ± 0.1°C and RH 55 to 70% RH. Before in ovo injection (E6) and during transfer to hatch baskets (E19), candling was performed to eliminate eggs that were damaged or in which no embryo was developing.
In Ovo Procedure and Sampling
At E6, the embryonated eggs were randomly divided into 5 equal groups (n = 90). Next, the eggshell surface above the air cell was disinfected with 70% ethanol, and a hole was made using an 18G needle (1.20 mm × 40 mm). The experimental solution (100 µL) was in ovo injected into the albumen using a G20 needle (0.9 mm × 40 mm) with an insulin syringe (1 mL) (Lis et al., 2009; Dżugan et al., 2011; Lis et al., 2011; Dżugan et al., 2012; Tombarkiewicz et al., 2020).
The eggs were injected with cadmium (administrated as CdCl2 × 2.5 H2O (Merck KGaA, Darmstadt, Germany) in 0.7% saline solution) at a dose of 2 µg (Cd 2 group), 4 µg (Cd 4 group), 6 µg (Cd 6 group) or 8 µg (Cd 8 group) of cadmium per egg. The eggs in the control group (C group) were only injected with 0.7% saline solution. After the injections, the holes were sealed with hot paraffin, and incubation was continued.
Blood samples were collected from randomly selected (n = 20 per group) chicks on the first day after hatching (D1). After animal decapitation, the blood was collected from the jugular vessels into test tubes containing EDTA-K3 (ethylenediaminetetraacetic acid tripotassium salt) (FLmedical, Equimed, Poland).
Hematological and Morphometric Analyses
The analysis of basic red blood cell parameters (RBC, Ht and Hb) and derived erythrocyte indices (MCV, MCH, and MCHC) was performed using Procyte Dx Hematology analyzer (IDEXX Laboratories, Inc., Westbrook, ME). Blood smears were prepared, and after 48 h they were stained with the HemKolor kit (Stamar, Poland) according to the instruction provided by the manufacturer. Next, determination of erythrocyte alterations (according to Witeska et al. 2010,2011) and morphometric analysis were conducted, for which 300 or 30 red blood cells were included, respectively. The diameter of the erythrocyte was measured at × 100 magnification using ImageJ software; https://imagej.nih.gov/ij/docs/intro.html. The diameters of the cell and nucleus were measured. The mean was determined for each animal, and the data were expressed in μm.
Immunocytochemistry
The cell smears were dried in air and permeabilized with 0.1% Triton X-100 in Tris-buffered saline (TBS; 0.05M Tris–HCl containing 0.15M NaCl, pH = 7.6). Next, cells were blocked with 0.3% H2O2 in TBS for 15 min and with 10% normal goat serum for 30 min. Thereafter, the cells were incubated overnight at 4°C in a humidified chamber in the presence of a polyclonal antibody (anti-spectrin β II; ES7262; ELK Biotechnology, Wuhan, Hubei, China; dilution 1:20). A biotinylated secondary antibody, goat anti-rabbit IgG (dilution 1:400; Vector Labs., Burlingame, CA), was applied for 1h in RT. Finally, avidin-biotinylated horse radish peroxidase complex (Vectastain ABC Kit; dilution1:100; Vector Labs., Burlingame, CA) was used for a further 30 min. After each step, the cells were carefully rinsed with TBS. A bound antibody was visualized with TBS containing 0.05% 3,3′-diaminobenzidine (DAB) and 0.07% imidazole. Controls included incubating cells with 5% normal serum in place of the primary antibody. Cells were counterstained with hematoxylin. An optical microscope (Eclipse 80i, Nikon, Japan) was used to examine the stains.
Statistical Analysis
The normality of distribution was examined using the Shapiro-Wilk test. Due to the lack of compliance of the analyzed data with normal distribution, most of the results were analyzed statistically using a nonparametric Kruskal-Wallis test followed by Dunn's test (post hoc). Only one parameter (erythrocyte length) showed normal distribution; it was analyzed with a one-way ANOVA, followed by the Tukey test (post hoc). The R software was used for these analyses. The level of significance was set at α = 0.05.
RESULTS
Basic and Derived Red Blood Cell Parameters
The chicks that had been exposed to 2 µg of cadmium (Cd 2 group) exhibited statistically significant lower MCV value in comparison to the control (P = 0.020) (Table 1). The individuals from Cd 6 group showed significantly higher Hb concentration (P = 0.035), lower MCV (P = 0.003) and higher MCHC (P = 0.000) compared to the control (Table 1). The animals exposed to 8 µg of cadmium (Cd 8 group) had significantly higher RBC count (P = 0.000), Ht value (P = 0.001), Hb level (P = 0.000) and MCHC value (P = 0.000), while MCV was significantly lower compared to the control (P = 0.001) (Table 1).
Table 1.
Red blood cell parameters of newly hatched hen chicks after embryonic exposure to cadmium (mean ± SD; n = 20 in the case of the Control, Cd 2, Cd 4, and Cd 5 groups; n = 11 in the case of the Cd 8 group).
| Type of cell | Control | Cd 2 | Cd 4 | Cd 6 | Cd 8 |
|---|---|---|---|---|---|
| RBC [1012/l] | 2.26 ± 0.16 | 2.23 ± 0.21 | 2.40 ± 0.26 | 2.41 ± 0.35* | 2.69 ± 0.19⁎⁎⁎ |
| Ht [l/l] | 0.26 ± 0.02 | 0.25 ± 0.02 | 0.27 ± 0.03 | 0.27 ± 0.04 | 0.30 ± 0.02⁎⁎ |
| Hb [g/l] | 74.00 ± 8.28 | 70.70 ± 8.18 | 78.26 ± 10.35 | 80.05 ± 14.21⁎ | 90.91 ± 7.71⁎⁎⁎ |
| MCV [fl] | 116.91 ± 4.90 | 113.23 ± 3.21* | 114.64 ± 3.55 | 112.49 ± 3.55⁎⁎ | 112.12 ± 1.61⁎⁎ |
| MCH [pg] | 32.64 ± 2.14 | 31.70 ± 1.41 | 32.57 ± 1.94 | 33.01 ± 1.92 | 33.75 ± 0.78 |
| MCHC [g/l] | 279.06 ± 10.28 | 279.85 ± 8.93 | 283.84 ± 11.44 | 293.11 ± 11.59⁎⁎⁎ | 301.00 ± 7.00⁎⁎⁎ |
RBC – red blood cell count; Ht – hematocrit value, Hb – hemoglobin concentration, MCV – mean corpuscular volume; MCH – mean corpuscular hemoglobin; MCHC – mean corpuscular hemoglobin concentration.
Significant differences compared to the control values are marked with asterisks.
P < 0.05.
P < 0.01.
P < 0.01.
Erythrogram (Red Blood Cell Morphology Based on a Blood Smear)
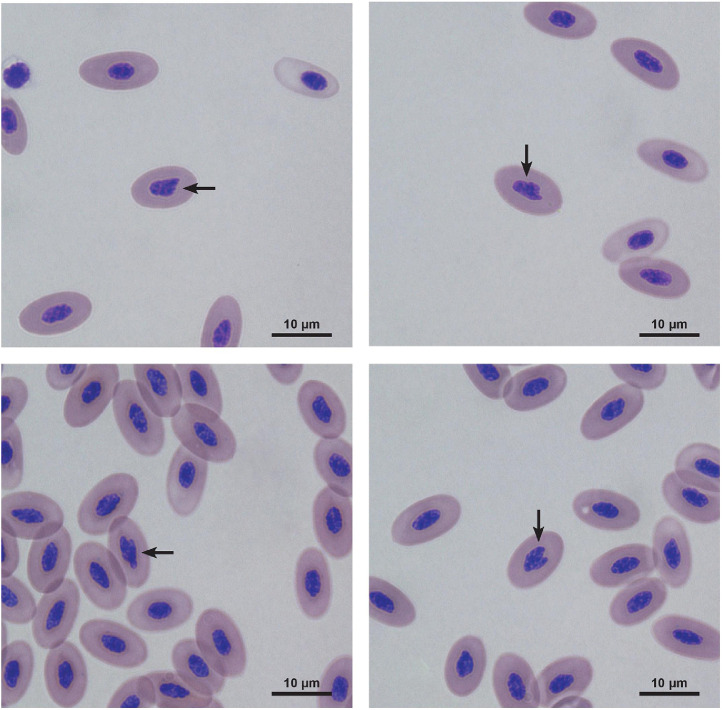
The obtained results showed that embryonic cadmium exposure resulted in a statistically significant higher count of erythrocytes exhibiting altered cell shape (Figure 1) in Cd 2 (P = 0.023) and Cd 8 (P = 0.002) groups compared to the control (Table 2). No other significant changes in erythrogram outcomes were observed: the number of red blood cells of altered nucleus shape (Figure 2), erythrocytes of vacuolated cytoplasm (Figure 3), and hemolyzed erythrocytes (Figure 4) were similar to the control values (Table 2).
Figure 1.
The abnormal cell shape of erythrocytes of newly hatched hen chicks after embryonic exposure to cadmium.
Table 2.
Erythrogram of newly hatched hen chicks after embryonic exposure to cadmium (mean ± SD; n = 20).
| Type of cell | Control | Cd 2 | Cd 4 | Cd 6 | Cd 8 |
|---|---|---|---|---|---|
| EAS | 0.85 ± 1.18 | 1.80 ± 1.24* | 1.65 ± 1.50 | 1.90 ± 2.20 | 2.25 ± 1.48** |
| EAN | 0.25 ± 0.55 | 0.35 ± 0.75 | 0.35 ± 0.49 | 0.35 ± 0.75 | 0.20 ± 0.41 |
| EVC | 1.70 ± 2.68 | 3.30 ± 6.03 | 2.45 ± 5.09 | 3.30 ± 4.45 | 2.45 ± 3.22 |
| HE | 1.20 ± 1.82 | 0.60 ± 1.14 | 1.55 ± 2.19 | 1.75 ± 2.55 | 3.25 ± 3.81 |
| CE | 296.00 ± 2.66 | 293.95 ± 5.44 | 294.00 ± 5.10 | 292.70 ± 4.88 | 291.85 ± 4.69 |
CE: correct (normal) erythrocytes; EAS: erythrocytes of altered cell shape; EAN: erythrocytes of altered nucleus shape; EVC: erythrocytes of vacuolated cytoplasm; HE: hemolyzed erythrocytes.
Significant differences compared to the control values are marked with asterisks.
P < 0.05.
P < 0.001.
Figure 2.
The abnormal nucleus shape of erythrocytes of newly hatched hen chicks after embryonic exposure to cadmium.
Figure 3.
The vacuolation of erythrocytes of newly hatched hen chicks after embryonic exposure to cadmium.
Figure 4.
The hemolysis of erythrocytes of newly hatched hen chicks after embryonic exposure to cadmium.
Morphometric Properties of Erythrocytes
Morphometric analysis revealed that Cd-exposed individuals (Cd 6 and Cd 8 groups) showed higher red blood cell length in comparison to the control value (P = 0.001 and P = 0.001, respectively) (Table 3). Animals in the Cd 2, Cd 6, and Cd 8 groups exhibited higher erythrocyte width compared to the control (P = 0.000 in each mentioned difference) (Table 3). Nucleus length and width were elevated in individuals exposed to cadmium at doses from 2 to 8 µg per egg compared to the control group (P = 0.000 in each mentioned difference) (Table 3).
Table 3.
Erythrocyte morphometry of newly hatched hen chicks after embryonic exposure to cadmium (mean ± SD; n = 20).
| Parameter | Control | Cd 2 | Cd 4 | Cd 6 | Cd 8 |
|---|---|---|---|---|---|
| erythrocyte length [µm] | 12.41 ± 0.44 | 12.44 ± 0.39 | 12.33 ± 0.54 | 12.82 ± 0.46⁎⁎ | 12.70 ± 0.36⁎⁎ |
| erythrocyte width [µm] | 6.73 ± 0.26 | 6.84 ± 0.26⁎⁎⁎ | 6.69 ± 0.31 | 6.90 ± 0.33⁎⁎⁎ | 6.85 ± 0.25⁎⁎⁎ |
| nucleus length [µm] | 5.00 ± 0.23 | 5.24 ± 0.46⁎⁎⁎ | 5.28 ± 0.42⁎⁎⁎ | 5.40 ± 0.35⁎⁎⁎ | 5.26 ± 0.25⁎⁎⁎ |
| nucleus width [µm] | 2.64 ± 0.12 | 2.97 ± 0.36⁎⁎⁎ | 2.84 ± 0.18⁎⁎⁎ | 2.87 ± 0.25⁎⁎⁎ | 2.79 ± 0.18⁎⁎⁎ |
Significant differences compared to the control values are marked with asterisks.
P < 0.01.
P < 0.001.
Immunocytochemical Localization of Spectrin in Erythrocytes
In the erythrocytes of the control chickens, a strong positive immunosignal for spectrin indicated that uniform distribution was visible in the cytoplasm of all cells (Figure 5A). Similarly, in animals from the Cd 2 group, the majority of erythrocytes expressed nondisturbed distribution of spectrin and a strong signal; however, in single cells the immunosignal was slightly blurred (Figure 5B). In the erythrocytes of chickens treated with 4 or 6 µg of cadmium, a marked decrease in the amount of positive spectrin immunosignal was revealed (Figure 5C, D). In addition, in erythrocytes from the Cd 6 group, single cells showed a lack of immunostaining (Figure 5D). In the erythrocytes of chickens exposed to 8 µg of cadmium, less than 50% of erythrocytes showed strong positive spectrin immunostaining; in the remaining cells, very weak or no spectrin immunostaining was observed (Figure 5E).
Figure 5.
Spectrin distribution in erythrocytes of control (A) and cadmium-exposed (B-E) newly hatched hen chicks.
DISCUSSION
The analysis showed that exposure to cadmium resulted in higher levels of basic red blood cell parameters (RBC count, Hb concentration, Ht value), while the derived erythrocyte indices were either increased (MCHC) or decreased (MCV) in comparison to the control. These changes were observed in the case of animals exposed to higher doses of the toxic agent. Therefore, it is reasonable to conclude that the red blood cell parameters that are commonly determined in hematological analyses are a useful marker of embryonic cadmium exposure. On the other hand, the study performed by Bojarski et al. (2021) showed that embryonic chromium (VI) exposure (1.56 or 15.6 mg per egg) did not cause major alterations in the hematological parameters of newly hatched chickens. The only significant change was a decrease in the RBC value in animals exposed to a higher dose of the tested substance. Mroczek-Sosnowska et al. (2013) studied the effect of copper (Cu) nanoparticles administered in ovo on the hematological markers of broiler chickens after 6 wk of rearing. The authors observed that this exposure caused an increase in RBC, Hb, and Ht values. Other studies also showed the influence of various elements on the hematological indices of birds. Sun et al. (2014) revealed that dietary tin (Sn) (720 mg/kg feed, 6 wk) treatment led to lower hemoglobin content, lower red blood cell count, and lower hematocrit level in broilers as compared to control individuals. It was also demonstrated that zinc-intoxicated mallards (Anas platyrhynchos) exhibited a lower Ht value, a higher reticulocyte count, as well as poikilocytosis (Levengood et al., 2000). On the other hand, Kucharska et al. (2019) revealed that mercury (Hg) does not affect the hematocrit value in mute swans (Cygnus olor).
The erythrogram (red blood cell morphology determined on the basis of a stained blood smear) turned out to be a less sensitive marker of cadmium exposure; nevertheless, the percentage of erythrocytes which exhibited changed shape increased in some experimental groups. In individuals exposed to cadmium, it is interesting that the morphometric analysis revealed an increase in the length and width of erythrocytes, as well as increased length and width of the nucleus of these cells. The changes were dose-dependent (most of the changes were observed in birds from groups exposed to higher doses of the tested metal). The results allow the conclusion that the morphometric properties of chicken erythrocytes are a sensitive marker of embryonic cadmium exposure, and morphometric analysis can be considered a significant supplement to analysis of erythrocyte morphology in hematological tests. Nevertheless, to the best of our knowledge, there is no data in the scientific literature on the effects of heavy metals on the erythrograms of birds or the morphometric characteristics of erythrocytes.
The observed changes in spectrin distribution indicate that alterations at structural and molecular levels occurred in chick erythrocytes due to cadmium exposure. Thus, determination of spectrin distribution appears to be useful marker of cadmium toxicity. However, to the best of our knowledge, there is no data in the literature on the influence of heavy metals on spectrin distribution in avian red blood cells.
Despite the physiological significance of red blood cells, studies focused on the influence of toxic substances on erythrocyte morphology are much less common than studies focused on standard hematological tests. As previously mentioned, in the case of fish, erythrocyte morphology is of great importance in assessing the toxicity of xenobiotics (especially heavy metals). The results of the present study do not allow a similar conclusion in the context of birds; however, it has been demonstrated that erythrocyte morphometric indices (length and width of the erythrocyte cell as well as length and width of erythrocyte nucleus) may be useful and reliable markers of toxicant in ovo exposure. Moreover, red blood cell spectrin distribution may be a new and interesting marker of cadmium embryonic exposure in birds. However, the analysis of routinely assessed red cell markers, such as RBC, Hb, Ht etc., should not be omitted in toxicological research.
ACKNOWLEDGMENTS
This research was supported by the Ministry of Science and Higher Education of the Republic of Poland (Subject No. 215-DZ06 and Subject No. 080100-D6, the University of Agriculture in Krakow).
Acknowledgments to Mr. Michael Timberlake for professional linguistic proofreading of the manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Al-Mayah A.S., Al-Ahmed J.A. Influence of antibiotics treatment on hematological aspect in chickens. Int. J. Poult. Sci. 2005;4:323–325. [Google Scholar]
- Babu V., Mariadoss S., Ipek C.E., Serbest B., Ali S. Surface structures of gill, scale and erythrocyte of Anabas testudineus exposed to sublethal concentration of cypermethrin. Environ. Toxicol. Pharmacol. 2014;37:1109–1115. doi: 10.1016/j.etap.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Bojarski B., Buchko O., Kondera E., Ługowska K., Osikowski A., Trela M., Witeska M., Lis M.W. Effects of embryonic exposure to chromium (VI) on blood parameters and liver microstructure of 1-day-old chickens. Poult. Sci. 2021;100:366–371. doi: 10.1016/j.psj.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarski B., Kot B., Witeska M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals. 2020;13:189. doi: 10.3390/ph13080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarski B., Witeska M. Blood biomarkers of herbicide, insecticide, and fungicide toxicity to fish—a review. Environ. Sci. Poll. Res. 2020;27:19236–19250. doi: 10.1007/s11356-020-08248-8. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Lis M., Droba M., Niedziółka J. Effect of cadmium injected in ovo on hatching results and the activity of plasma hydrolytic enzymes in newly hatched chicks. Acta Vet. Hung. 2011;59:337–347. doi: 10.1556/AVet.2011.020. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Lis M.W., Droba M., Niedziółka J.W. Protective effect of zinc on cadmium embryotoxicity and antioxidant status of blood plasma in newly hatched chicks. J. Environ. Sci. Health A. 2012;47:1288–1293. doi: 10.1080/10934529.2012.672133. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Trybus W., Lis M., Wesołowska M., Trybus E., Kopacz-Bednarska A., Król T. Cadmium-induced ultrastructural changes in primary target organs of developing chicken embryos (Gallus domesticus) J. Trace Elem. Med. Biol. 2018;50:167–174. doi: 10.1016/j.jtemb.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Kucharska K., Binkowski Ł.J., Batoryna M., Dudzik K., Zaguła G., Stawarz R. Blood mercury levels in mute swans (Cygnus olor) are not related to sex, but are related to age, with no blood parameter implications. Environ. Poll. 2019;252:21–30. doi: 10.1016/j.envpol.2019.05.075. [DOI] [PubMed] [Google Scholar]
- Levengood J.M., Sanderson G.C., Anderson W.L., Foley G.L., Brown P.W., Seets J.W. Influence of diet on the hematology and serum biochemistry of zinc-intoxicated mallards. J. Wildlife Dis. 2000;36:111–123. doi: 10.7589/0090-3558-36.1.111. [DOI] [PubMed] [Google Scholar]
- Lis M.W., Głodek K., Sechman A., Wątor D., Pawlak K., Niedziółka J.W. Effect of in ovo injection of Aroclor 1254 on embryonic development, time of hatching, and blood thyroid hormone levels in one-day-old chicken. Bull. Vet. Inst. Pulawy. 2011;55:293–298. [Google Scholar]
- Lis M.W., Sechman A., Pawlak K., Tombarkiewicz B., Niedziółka J., Rząsa J. Effects of in ovo exposure to acetylsalicylic acid and hyperthermia on the hatchability and thyroid hormone concentrations in newly-hatched chicks. Bull. Vet. Inst. Pulawy. 2009;53:527–534. [Google Scholar]
- Liu M., Liu Y., Cheng Z., Liu J., Chai T. Effects of chromic chloride on chick embryo fibroblast viability. Toxicol. Rep. 2015;2:555–562. doi: 10.1016/j.toxrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek-Sosnowska N., Batorska M., Lukasiewicz M., Wnuk A., Sawosz E., Jaworski S., Niemiec J. Effect of nanoparticles of copper and copper sulfate administered in ovo on hematological and biochemical blood markers of broiler chickens. Ann. Warsaw Univ. Life Sci. – SGGW, Anim. Sci. 2013;52:141–149. [Google Scholar]
- Owagboriaye F., Dedeke G., Ademolu K., Olujimi O., Aladesida A., Adeleke M. Comparative studies on endogenic stress hormones, antioxidant, biochemical and hematological status of metabolic disturbance in albino rat exposed to roundup herbicide and its active ingredient glyphosate. Environ. Sci. Poll. Res. 2019;26:14502–14512. doi: 10.1007/s11356-019-04759-1. [DOI] [PubMed] [Google Scholar]
- Pagano M., Faggio C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015;33:351–355. doi: 10.1002/cbf.3135. [DOI] [PubMed] [Google Scholar]
- Sawhney A.K., Johal M.S. Erythrocyte alterations induced by malathion in Channa punctatus (Bloch) Bull. Environ. Contam. Toxicol. 2000;64:398–405. doi: 10.1007/s001280000014. [DOI] [PubMed] [Google Scholar]
- Sun L.H., Zhang N.Y., Zhai Q.H., Gao X., Li C., Zheng Q., Krumm C.S., Qi D. Effects of dietary tin on growth performance, hematology, serum biochemistry, antioxidant status, and tin retention in broilers. Biol. Trace Elem. Res. 2014;162:302–308. doi: 10.1007/s12011-014-0129-y. [DOI] [PubMed] [Google Scholar]
- Tombarkiewicz B., Trzeciak K., Bojarski B., Lis W.M. The effect of methionine and folic acid administered in ovo on the hematological parameters of chickens (Gallus gallus domesticus) Poult. Sci. 2020;99:4578–4585. doi: 10.1016/j.psj.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova E., Arnaudov A., Velcheva I. Effects of zinc on morphology of erythrocytes and spleen in Carassius gibelio. J. Environ. Biol. 2008;29:897–902. [PubMed] [Google Scholar]
- Waghmare T.E., Nayaka H.B., Banklgi S.K.C., Tukappa A. Quantitative estimation of heavy metals in river water and their toxicity, hematology, gravimetric, serum and tissue biochemistry effects in albino rats. World J. Pharmaceut. Res. 2015;4:1415–1425. [Google Scholar]
- Witeska M., Kondera E., Szczygielska K. The effects of cadmium on common carp erythrocyte morphology. Pol. J. Environ. Stud. 2011;20:783–788. [Google Scholar]
- Witeska M., Kondera E., Szymańska M., Ostrysz M. Hematological changes in common carp (Cyprinus carpio L.) after short-term lead (Pb) exposure. Pol. J. Environ. Stud. 2010;19:825–831. [Google Scholar]
- Yang M. Effect of sulfhydryl reagents on spectrin states on the erythrocyte membrane. Biochem. Biophysic. Res. Comm. 1993;192:918–925. doi: 10.1006/bbrc.1993.1503. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhang C., Zhao Q., Li D. Spectrin: structure, function and disease. Sci. China Life Sci. 2013;56:1076–1085. doi: 10.1007/s11427-013-4575-0. [DOI] [PubMed] [Google Scholar]