Abstract
It is still an unsolved problem to achieve both immediate intraoperative feedback and satisfactory surgical experience in percutaneous endoscopic lumbar discectomy under local anesthesia for lumbar disk herniation (LDH) patients. Herein, we compared the analgesic and sedative effects of local anesthesia alone and local anesthesia with conscious sedation in LDH patients during percutaneous endoscopic lumbar discectomy. Ninety-two LDH patients were enrolled and divided into the following groups: control group (Con Group), dexmedetomidine group (Dex Group), oxycodone group (Oxy Group), and dexmedetomidine + oxycodone group (Dex + Oxy Group). Various signs, including mean arterial pressure (MAP), heart rate (HR), pulse oximeter oxygen saturation (SpO2) and Ramsay score, were compared before anesthesia (T1), working cannula establishment (T2), nucleus pulposus removal (T3), and immediately postoperation (T4). Clinical outcomes, including VAS score, operation time, hospitalization period, Macnab criteria, and SF-36 score, were also evaluated. The Dex + Oxy Group showed the most stable MAP and HR at T2 and T3 in all groups. The clinical outcomes, such as VAS, hospitalization period, Macnab criteria, and SF-36 score, have no significant differences among groups (p > 0.05). Local anesthesia combined with conscious sedation is a safe and effective method to improve the surgical experience and achieve satisfying clinical outcomes for LDH patients during percutaneous endoscopic lumbar discectomy.
Subject terms: Diseases, Medical research, Neurology
Introduction
Lumbar disk herniation (LDH) is a common disease in spinal surgery that often requires surgical treatment1,2. Lumbar discectomy can improve pain, function, and quality of life for LDH patients3. Conventional open lumbar surgery has been perceived as an effective intervention but carries several disadvantages, including postoperative back pain and a long recovery period. Subsequently, percutaneous endoscopic lumbar discectomy has been developed to facilitate lumbar discectomy4–6, with the following advantages such as paravertebral soft tissue protection, shorter hospital stays, less blood loss, and faster patient recovery6–8. Consequently, the percutaneous endoscopic technique received increasing attention from spinal surgeons around the worldwide9.
Local and general anesthesia are common analgesic methods used in percutaneous endoscopic lumbar discectomy10–13. General anesthesia can achieve an excellent surgical experience for patients11–13. However, it lacks intraoperative feedback during the operation, which indicates that the unconscious patients cannot communicate with the surgeon if their nerve root or spinal cord was damaged. Therefore, general anesthesia may increase the surgical risk of percutaneous endoscopic lumbar discectomy. Moreover, local anesthesia has the advantage of intraoperative feedback to improve safety during spinal surgery10. Nevertheless, it provides a poor surgical experience for pain-sensitive patients, which may cause anxiety and psychentonia, leading to an increase of blood pressure and heart rate, and even cardiovascular or cerebrovascular accidents10. Thus, achieving effective pain relief, immediate feedback during operation, and satisfactory surgical experience simultaneously in percutaneous endoscopic lumbar discectomy for patients with LDH remains to be a challenge.
Dexmedetomidine is a new alpha 2 adrenal receptor agonist with good sedative and analgesic effects, which has no inhibiting respiration and is easy to wake up after surgery14–18. Gadjradj et al.19 has reported that percutaneous transforaminal endoscopic discectomy performed under local anesthesia and conscious sedation using dexmedetomidine is safe and effective in treating sciatica and yields high satisfaction rates from surgeons, anesthesiologists, and patients. Moreover, oxycodone hydrochloride, as the only opioid and double receptor agonist, has an excellent inhibitory effect on mixed somatic and visceral pain20,21. We hypothesized that percutaneous endoscopic lumbar discectomy under local anesthesia and conscious sedation, which uses dexmedetomidine combined with oxycodone hydrochloride, could achieve a good analgesic effect and improve the surgical experience intraoperatively. Herein, we evaluated local anesthesia only versus local anesthesia combined with conscious sedation for LDH patients who underwent percutaneous endoscopic lumbar discectomy.
Results
Patient characteristics
The demographic characteristics of all 92 patients were compared by chi-square test (gender and the segment of LDH) and one-way ANOVA (age), and the results showed that there was no statistical difference in the demographic characteristics among the four groups.
Vital signs and sedation
The results of MAP, HR, SpO2, and Ramsay scores at T1, T2, T3, and T4 in four groups are shown in Fig. 1A–D. Firstly, a homogeneity test of variance was done to determine whether one-way ANOVA or Welch ANOVA would be used to compare the values of MAP, HR, SpO2, and Ramsay scores at different time points, and the results are shown in Table 1.
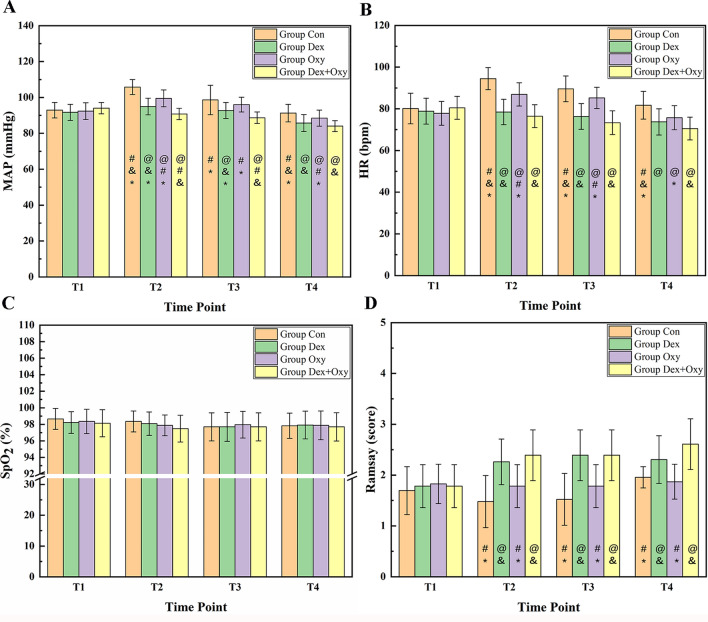
Figure 1.
The results of MAP (A), HR (B), SpO2 (C), and Ramsay score (D) at T1-T4 in Con Group, Dex Group, Oxy Group, and Dex + Oxy Group. "@", "#", "&", "*" represent the difference was statistical when compared with Con Group, Dex Group, Oxy Group, and Dex + Oxy Group, respectively.
Table 1.
P values of ANOVA for MAP, HR, SpO2, and Ramsay at four time points.
| Time point | Items | ||||
|---|---|---|---|---|---|
| MAP | HR | SpO2 | Ramsay | ||
| One-way ANOVA | Welch ANOVA | ||||
| T1 | 0.301 | 0.457 | 0.623 | 0.769 | – |
| T2 | < 0.001* | < 0.001* | 0.196 | – | < 0.001* |
| T3 | < 0.001* | < 0.001* | 0.937 | – | < 0.001* |
| T4 | < 0.001* | < 0.001* | 0.974 | – | < 0.001* |
MAP mean arterial pressure, HR heart rate, pulse SpO2 oximeter oxygen saturation, T1 before anesthesia, T2 working cannula establishment, T3 nucleus pulposus removal, T4 immediately postoperation;
*Difference was statistically significant.
At the time point of T1,there was no significant difference in MAP, HR, SpO2, and Ramsay scores among the four groups (p > 0.05). Results also showed that there was no statistical difference in SpO2 among groups at all the time points (p > 0.05). There were statistical differences in MAP, HR, and Ramsay in time points of T2, T3, and T4. Thus, LSD was used to compare MAP and HR among groups in time points of T2, T3, and T4 to clarify the differences between groups, and the Games-Howell test was used to compare Ramsay scores among T2, T3, and T4 time points. The results are shown in Table 2.
Table 2.
The P values of LSD and Games-Howell test for MAP, HR, and Ramsay at the time points of T2-T4.
| Items group | MAP | HR | Ramsay | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group Dex | Group Oxy | Group Dex + Oxy | Group Dex | Group Oxy | Group Dex + Oxy | Group Dex | Group Oxy | Group Dex + Oxy | |
| T2 | |||||||||
| Group Con | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | 0.139 | < 0.001* |
| Group Dex | – | < 0.001* | 0.001* | – | < 0.001* | 0.23 | – | 0.003* | 0.788 |
| Group Oxy | < 0.001* | – | < 0.001* | < 0.001* | – | < 0.001* | 0.003* | – | < 0.001* |
| Group Dex + Oxy | 0.001* | P < 0.001* | – | 0.23 | < 0.001* | – | 0.788 | < 0.001* | – |
| T3 | |||||||||
| Group Con | < 0.001* | 0.103 | < 0.001* | < 0.001* | 0.013* | < 0.001* | < 0.001* | 0.248 | < 0.001* |
| Group Dex | – | 0.042* | 0.012* | – | < 0.001* | 0.083 | – | < 0.001* | 1.000 |
| Group Oxy | 0.042* | – | < 0.001* | < 0.001* | – | < 0.001* | < 0.001* | – | < 0.001* |
| Group Dex + Oxy | 0.012* | < 0.001* | – | 0.083 | < 0.001* | – | 1.000 | < 0.001* | – |
| T4 | |||||||||
| Group Con | < 0.001* | 0.033* | < 0.001* | < 0.001* | 0.001* | < 0.001* | 0.015* | 0.730 | < 0.001* |
| Group Dex | – | 0.036* | 0.190 | – | 0.266 | 0.075 | – | 0.005* | 0.160 |
| Group Oxy | 0.036* | – | 0.001* | 0.266 | – | 0.004* | 0.005* | – | < 0.001* |
| Group Dex + Oxy | 0.190 | < 0.001* | – | 0.075 | 0.004* | – | 0.160 | < 0.001* | – |
MAP mean arterial pressure, HR heart rate, T2 working cannula establishment, T3 nucleus pulposus removal, T4 immediately postoperation.
*Difference was statistically significant; “–” The same group;
According to Fig. 1A,B and Table 2, the MAP and HR values at the time point of T2, T3, and T4 were decerased in Oxy Group, Dex Group, and Dex + Oxy Group than those in Con Group, especially in Dex + Oxy Group. According to Fig. 1D and Table 2, Ramsay scores in Dex Group and Dex + Oxy Group were significantly decreased than those in Con Group and Oxy Group at the time point of T2, T3, and T4 (p < 0.05).
Clinical outcomes
Operation time and hospitalization period
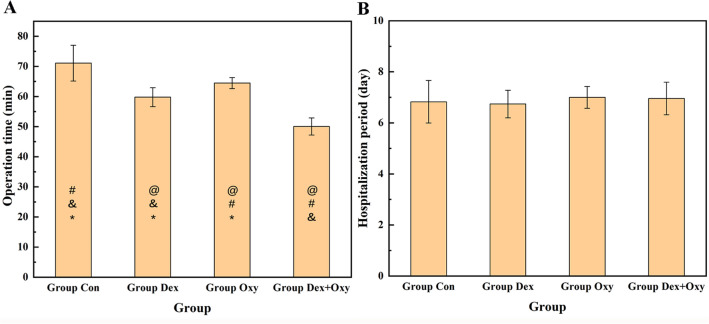
The results of the homogeneity test of variance in operation time and hospitalization period were p > 0.05, and the results of one-way ANOVA showed that there were differences in operation time (p < 0.001) and no statistical differences in hospitalization period among different groups (p > 0.05). LSD was used to compare the operation time between groups to clarify the difference in operation time between groups (Fig. 2A), and the results showed that the Con Group had the longest operation time, followed by Oxy Group, Dex Group, and Dex + Oxy Group, with significant statistically differences (p < 0.001). In the control group, one patient suffered from intense pain accompanied by nervousness, which led to a significant prolongation of the operation time. The results of the hospitalization period are shown in Fig. 2B.
Figure 2.
The results of operation time (A) and hospitalization period (B) in different groups. "@", "#", "&", "*" represent the difference was statistical when compared with Group Con, Group Dex, Group Oxy, and Group Dex + Oxy, respectively.
VAS score
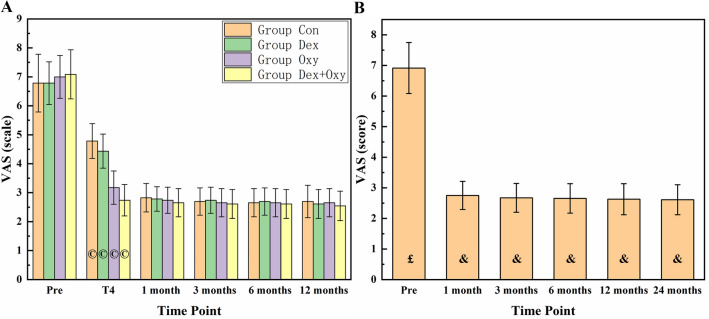
The VAS scores of the patients in the four groups were evaluated preoperatively and 1 day, 1 month, 3 months, 6 months, 12 months, and 24 months after surgery (Fig. 3A). There was no loss of follow-up at 6 months postoperatively; total 5 patients lost at 24 months follow-up. The results of the homogeneity test of variance were p > 0.05. The one-way ANOVA results showed that at the other time points, there was no statistical difference among the 4 groups (p > 0.05) (Fig. 3A). Therefore, we combined the data of each group at the same time point and compared the differences in VAS results between different time points to evaluate the therapeutic effect. The result of the homogeneity test of variance was p < 0.05, and the result of Welch ANOVA was also p < 0.05, so the Games-Howell test was used to compare the difference between every two different time points. The results were shown in Table 3 and Fig. 3B, and significant statistical differences existed between every two time points (p < 0.001), but no significant statistical differences were found between 12 and 24 months postoperatively (p > 0.05).
Figure 3.
The results of the VAS score at different times of the four groups (A). VAS results in follow-up duration (B). "©" represents the differences were all statistical when compared with the other groups. "£" represents differences were all statistical when compared with the other time points. "&" represents differences was statistical only when compared with the time point of preoperation.
Table 3.
The P values of VAS score between every two time points by LSD.
| Time point | preoperation | 1 mon- post-op | 3 mon- post-op | 6 mon- post-op | 12 mon- post-op | 24 mon- post-op |
|---|---|---|---|---|---|---|
| 1 mon- post-op | < 0.001* | – | < 0.001* | < 0.001* | < 0.001* | < 0.001* |
| 3 mon- post-op | < 0.001* | < 0.001* | – | < 0.001* | < 0.001* | < 0.001* |
| 6 mon- post-op | < 0.001* | < 0.001* | < 0.001* | – | < 0.014* | < 0.001* |
| 12 mon- post-op | < 0.001* | < 0.001* | < 0.001* | 0.014* | – | 0.579 |
| 24 mon- post-op | < 0.001* | < 0.001* | < 0.001* | < 0.001* | 0.579 | – |
VAS visual analogue scale, mon- month or months, post-op postoperation.
*Difference was statistically significant; “–” The same time point;
SF-36 (PCS + MCS) score, ODI score, and Macnab criteria
The SF-36 Physical Component Summary (PCS), the SF-36 Mental Component Summary (MCS), and the ODI score in the four groups were shown in Fig. 4A–C. The results of variance homogeneity and one-way ANOVA results showed no statistical difference among the 4 groups at each same time point (p > 0.05). Therefore, we combined the data of the four groups at each same time point as a whole, then compared SF-36 and ODI between different time points to evaluate the clinical outcomes. The results were showed in Fig. 4D.
Figure 4.
The clinical outcomes of PCS (A), MCS (B), and ODI (C) were shown in different groups. "£" represents differences were all statistical when compared with the other time points.
The clinical outcomes were rated according to modified Macnab criteria as "Excellent" (patient symptom-free, able to resume normal life and work), "Good" (slight symptoms remain, activity slightly limited, no effect on life and work), "Fair" (some symptom relief, activity significantly limited, life and work affected) or "Poor" (post-operation symptoms the same or worse as preoperation symptoms)22. The results of modified Macnab criteria at 24 months postoperation were shown in Table 4, Chi-square test was used for comparison among groups, and the p-value was more than 0.05. This result showed that local anesthesia with sedation and without sedation did not improve the clinical outcomes.
Table 4.
The results of modified Macnab criteria at 24 months postoperation.
| Group | Poor/total | Fair/total | Good/total | Excellent/total | χ2 | P |
|---|---|---|---|---|---|---|
| Group Con | 0/22 | 0/22 | 4/22 | 18/22 | 0.770 | 0.857 |
| Group Dex | 0/22 | 0/22 | 3/22 | 19/22 | ||
| Group Oxy | 0/21 | 0/21 | 3/21 | 18/21 | ||
| Group Dex + Oxy | 0/22 | 0/22 | 2/22 | 20/22 |
Discussion
The essential findings in the present study indicated that local anesthesia with conscious sedation is a safe, effective, and reliable method to achieve satisfying pain control during percutaneous endoscopic lumbar discectomy surgery. Another finding was that dexmedetomidine combined with oxycodone under local anesthesia could reduce anxiety, relieve pain, and improve the surgical experience during percutaneous endoscopic surgery for the treatment of LDH. Both local anesthesia and general anesthesia are effective methods for minimally invasive surgery in spinal surgery. Local anesthesia is inferior to general anesthesia in surgical experience for patients. However, local anesthesia may be superior to general anesthesia in information feedback. According to previous studies, simultaneous intraoperative feedback and good surgical experience remain challenging in LDH patients who underwent percutaneous endoscopic lumbar discectomy23. We, herein, applied local anesthesia combined with conscious sedation to improve the surgical experience for LDH patinets in percutaneous endoscopic lumbar discectomy.
Opioids24–27 and NSAIDs28–30 are two commonly used agents for pain management in clinical practice. Opioids are mainly utilized to relieve pain by binding to the opioid receptors in the central and peripheral nervous systems31. However, it has several side effects, including nausea, vomiting, dizziness, itching, sedation, respiratory depression, uroschesis, constipation, euphoria, nausea, vomiting, respiratory depression, excessive sedation, and liver dysfunction32–37. Meanwhile, NSAIDs are predominantly applied to inhibit the synthesis of prostaglandins and the release of bradykinin in the process of inflammation to relieve pain38,39. Nevertheless, NSAIDs could induce severe gastrointestinal reactions39. Therefore, choosing agents with low side effects and good analgesic effects durinng percutaneous endoscopic lumbar discectomy surgery is relatively crucial.
Dexmedetomidine is a highly selective alpha 2 receptor agonist characterized by sedation, analgesia, anti-anxiety, easy arousal, and mild respiratory inhibition14,16,18. Mantz et al.40 found that dexmedetomidine has a good analgesic effect for controlling acute and chronic inflammatory pain, postoperative pain, and chronic pain. In this study, we found that both Dex Group and Dex + Oxy Group have a lower MAP and HR than Con Group and Oxy Group at the time points of T2, T3, and T4 (p < 0.05), indicating that dexmedetomidine plays an essential role in controlling blood pressure and HR, which contribute to alleviating stress response and maintaining stability. Our results are consistent with the previous outcomes conducted by Greenberg et al.14.
Regarding the analgesic mechanism of dexmedetomidine, it activates alpha 2 receptors in the presynaptic membrane and inhibits neuronal excitation and norepinephrine release by negative feedback, thus stopping the transmission of pain signals14,16,18. Dexmedetomidine can also stimulate 2 receptors in the postsynaptic membrane, inhibit sympathetic activity, cause slow heart rate, decrease blood pressure, and produce sedative and anti-anxiety effects15,16,18. Moreover, it can directly bind to 2 receptors in the intramedullary system, exerting analgesic and sedative effects. In our opinion, the stimulation of paravertebral soft tissue, spinal cord, and nerve root during operation is the main cause of poor surgical experience. Thus, the pharmacological mechanism of dexmedetomidine might explain why Dex Group and Dex + Oxy Group have a better surgical experience than Con Group and Oxy Group at the time of T2, T3, and T4.
Oxycodone is a semisynthetic tibrazi derivative of opioid alkaloids14–18,40, characterized by the easy crossing of the blood–brain barrier, quick onset of action, long half-life, low affinity for receptors, mild respiratory inhibition, mild adverse reactions, little effect on hemodynamics, strong sedative and good analgesic effect20,21. In the current study, we found that the Oxy Group has a better analgesic effect than the Con Group, a similar analgesic effect compared to the Dex Group, and a worse analgesic effect than the Dex + Oxy Group. Our results were consistent with a previous study provided by Han et al.21, who reported that oxycodone had better analgesic effects, lower incidence of adverse complications, and less analgesic drug consumption than sufentanil in pain management postoperatively. Thus, we believe that oxycodone is an effective agent in suppressing pain but less effective in anti-anxiety. In the present study, we found that the values of MAP and HR in the Oxy Group were lower than those in the Con Group. In our opinion, oxycodone has a good analgesic effect, and this view was supported by authors20,21. Besides, the application of oxycodone combined with dexmedetomidine can play a synergistic analgesic effect durting minimal invasive spinal surgery.
We found that MAP and HR in the Con Group were significantly higher than that in other groups during the operation steps of catheter insertion and nucleus removal. We believe that this is due to the pain and anxiety caused by surgical manipulations. In addition, we detected that HR and MAP in the Dex Group and Dex + Oxy Group were significantly lower than that in the Con Group and Oxy Group during catheter insertion and nucleus removal. This result indicates that dexmedetomidine could reduce sympathetic activity, control heart rate and blood pressure, and reduce myocardial oxygen consumption, which is beneficial to alleviating anxiety and improving the surgical experience intraoperatively.
Pain is produced by the coordinated action of the central and peripheral nerve conduction systems25,41. Thus, the combined application of multiple analgesic strategies for pain management has been developed42–45. Nevertheless, there are numerous side effects of combined analgesic strategies, including nausea, vomiting, pruritus, and even excessive sedation. In our study, the Dex + Oxy Group showed lower side effects and provided a prolonged analgesia effect than the control group. This viewpoint was also consistent with previous studies46,47. Moreover, the blood oxygen saturation in the Dex + Oxy Group had no significant defference between intraoperative and preoperative (p > 0.05), demonstrating that the application of dexmedetomidine combined with oxycodone did not increase the risk of intraoperative respiratory depression and oxygen saturation reduction.
The Dex Group, Oxy Group, and Dex + Oxy Group had a shorter operative time than the Con Group (p < 0.05). We believe that the result is directly related to the patient's cooperation with the surgeon intraoperatively. However, the ODI score, Macnab score, and SF-36 score showed no significant differences among groups during the follow-up visits (p > 0.05). Thus, we believe that the local anesthetic combined with conscious sedative in percutaneous endoscopic lumbar discectomy for LDH cannot facilitate the surgical effect but might improve the patient's surgical experience.
In the control group, a patient was very nervous, anxious, and even shouting intraoperatively, which seriously affected the operation, resulting in a prolonged operation time. Unfortunately, bleeding causes blurred endoscopic vision and eventually leads to a dural tear in this patient. Bradycardia occurred in two patients after sedation with dexmedetomidine, and atropine (0.5 mg) was given for symptomatic treatment, then the HR returned to a normal level. No other complications, such as vascular injury, nerve injury, visceral injury, infection, nonunion of incisions, or relapsed LDH, were found.
Positive clinical results were achieved in this study. However, several limitations still deserve our attention. In the present study, there was no pharmacodynamics and pharmacokinetics evaluation of the combined therapy. Thus, pharmacodynamics and pharmacokinetics evaluation for the combination of oxycodone and dexmedetomidine in percutaneous endoscopic lumbar discectomy could be further studied.
Local anesthesia combined with conscious sedation is a safe and effective method to improve the surgical experience and achieve satisfying clinical outcomes for LDH patients who underwent percutaneous endoscopic lumbar discectomy.
Methods
Subjects and groups
This is a multicenter, retrospective study conducted at Zhengzhou orthopaedic hospital, Zhengzhou, Henan Province, China; and the second hospital of Jilin University, Changchun, Jilin Province, China. From January 2016 to June 2019, a total of 92 consecutive single-level LDH patients underwent percutaneous endoscopic lumbar discectomy under local anesthesia only or local anesthesia with conscious sedation. Patients were divided into four groups, including Con Group, Dex Group, Oxy Group, and Dex + Oxy Group.
All four groups were given local infiltration anesthesia with 1% lidocaine 30 ~ 40 mL layer by layer on the skin, subcutaneous fascia, muscle, and articular process. In the Dex + Oxy Group, oxycodone hydrochloride (1 mL/10 mg) was slowly injected intravenously (0.05 mg/kg) (Duration: 1 min) at 10 min before operation; Dexmetomidine hydrochloride (2 mL/200 μg) (0.5 μg/kg) was infused continuously for 10 min, and then maintained at a rate of 0.4 μg/ kg·h until the end of the operation. In the Dex Group, 0.3 mL of normal saline was slowly injected at 10 min before operation; Dexmetomidine hydrochloride (2 mL/200 μg) (0.5 μg/kg) was infused continuously for 10 min and then maintained at a rate of 0.4 μg/kg/h until the end of the operation. In the Oxy Group, oxycodone hydrochloride (1 mL/10 mg) was slowly injected intravenously (0.05 mg/kg) (Duration: 1 min) at 10 min before operation; 0.3 mL of normal saline was infused continuously for 10 min, and then maintained at a rate of 0.4 μg/kg·h until the end of the operation. In the Con Group, 0.3 mL of normal saline was slowly injected intravenously at 10 min before the operation; 0.3 mL of normal saline was infused continuously for 10 min and then maintained at a rate of 0.4 μg/kg h until the end of the operation. Subsequently, all patients underwent percutaneous endoscopic lumbar discectomy via the foraminal approach48. During the operation, if the heart rate was less than 50 beats/min, atropine (0.5 mg) would be given for symptomatic treatment.
Evaluation parameters
Vital signs, including MAP, HR, and SpO2, were compared at T1, T2, T3, and T4. The Ramsay score is a 6-level clinical score that scores the patients’ level of sedation on a scale from 1 (patient is anxious and agitated or restless or both) to 6 (patient exhibits no response to stimulus)49. Moreover, the clinical outcomes, including the hospitalization period, VAS score, the SF-36 score, ODI score, and Macnab criteria, were evaluated. Telephone follow-up was performed at 1 and 3 months after surgery, and patients were followed up at the hospital from 6 to 24 months after surgery. The X-rays, CT, and MRI of the lumbar were performed preoperatively. All of the LDH patients treated with percutaneous endoscopic lumbar discectomy underwent routine X-ray examination in the outpatient department during follow-up visits at 1 month and 6, 12, and 24 months postoperatively.
Statistical methods
SPSS (version 26.0) was used for statistical analysis. The chi-square test was used for counting data and analysis of variance (ANOVA) for measurement data. Before ANOVA, a homogeneity test of variance was performed. If p > 0.05, one-way ANOVA would be performed. If p < 0.05, Welch ANOVA would be used for statistical analysis. Least-Significant Difference (LSD) was used to test the differences between groups if the results of one-way ANOVA showed that p < 0.05, and the Games-Howell test was used to test the differences between groups if the results of Welch ANOVA showed that p < 0.05. p < 0.05 is considered to be a statistical difference. For the inter-group comparison of multiple groups, the corrected p* is used for comparison according to the number of pair comparisons (k), p* = p/k.
Ethical approval and consent to partcipate
This study was based on the principles outlined in the Helsinki Declaration, which the Ethics Committee approved of Zhengzhou Orthopaedic Hospital (No.2021014). All volunteers who participated in the study signed written informed consent.
Abbreviations
- LDH
Lumbar disk herniation
- Dex
Dexmedetomidine group
- Oxy
Oxycodone group
- VAS
Visual analogue scale
- MAP
Mean arterial pressure
- HR
Heart rate
- SpO2
Pulse oximeter oxygen saturation
- SF-36
Short form-36 health survey questionnaire
- ODI
Oswestry disability index
- CT
Computed tomography
- MRI
Magnetic resonance imaging
Author contributions
L.T.Y. and Y.L. participated in the study design and surgery; Y.L. and C.Z. participated in surgery and radiographic outcome assessment. D.X.G. collected all data. Data analysis was performed by D.X. under the supervision of X.Z. All authors contributed to the reviewal and interpretation of data. The manuscript was drafted by L.Y. and X.Z., reviewed by all authors, and revised with contributions from all authors under the supervision and final revision of X.Z. X.Z. was responsible for the integrity of the work from inception to the finished article. All authors read and approved the final manuscript.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harada GK, et al. Artificial intelligence predicts disk re-herniation following lumbar microdiscectomy: Development of the "RAD" risk profile. Eur. Spine J. 2021;30:2167–2175. doi: 10.1007/s00586-021-06866-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim ES, Kim CY. The association between continuity of care and surgery in lumbar disc herniation patients. Sci. Rep. 2021;11:5550. doi: 10.1038/s41598-021-85064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson AM, et al. Who should undergo surgery for degenerative spondylolisthesis? Treatment effect predictors in sport. Spine. 2013;38:1799–1811. doi: 10.1097/BRS.0b013e3182a314d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Zhang Z, Liu B, Liu S. Evaluation of percutaneous transforaminal endoscopic discectomy in the treatment of lumbar disc herniation: A retrospective study. Orthop. Surg. 2021;13:599–607. doi: 10.1111/os.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, et al. Adjuvant surgical decision-making system for lumbar intervertebral disc herniation after percutaneous endoscopic lumber discectomy: A retrospective nonlinear multiple logistic regression prediction model based on a large sample. Spine J. 2021 doi: 10.1016/j.spinee.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Gadjradj PS, et al. Full endoscopic versus open discectomy for sciatica: Randomised controlled non-inferiority trial. BMJ. 2022;376:e065846. doi: 10.1136/bmj-2021-065846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin. Neurol. Neurosurg. 2019;179:74–80. doi: 10.1016/j.clineuro.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Lewandrowski KU. Incidence, management, and cost of complications after transforaminal endoscopic decompression surgery for lumbar foraminal and lateral recess stenosis: A value proposition for outpatient ambulatory surgery. Int. J. Spine Surg. 2019;13:53–67. doi: 10.14444/6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li ZZ, Ma SY, Cao Z, Zhao HL. Percutaneous isthmus foraminoplasty and full-endoscopic lumbar discectomy for very highly upmigrated lumbar disc herniation: Technique notes and 2 years follow-up. World Neurosurg. 2020;141:e9–e17. doi: 10.1016/j.wneu.2020.03.141. [DOI] [PubMed] [Google Scholar]
- 10.Wan Q, et al. Posterior percutaneous full-endoscopic cervical discectomy under local anesthesia for cervical radiculopathy due to soft-disc herniation: A preliminary clinical study. J. Neurosurg. Spine. 2018;29:351–357. doi: 10.3171/2018.1.SPINE17795. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH, et al. Minimally invasive cervical foraminotomy and diskectomy for laterally located soft disk herniation. Eur. Spine J. 2015;24:3005–3012. doi: 10.1007/s00586-015-4198-1. [DOI] [PubMed] [Google Scholar]
- 12.Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: A prospective, randomized, controlled study. Spine. 2008;33:940–948. doi: 10.1097/BRS.0b013e31816c8b67. [DOI] [PubMed] [Google Scholar]
- 13.Yang JS, et al. Anterior or posterior approach of full-endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine. 2014;39:1743–1750. doi: 10.1097/BRS.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg RG, et al. Population pharmacokinetics of dexmedetomidine in infants. J. Clin. Pharmacol. 2017;57:1174–1182. doi: 10.1002/jcph.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye AD, et al. Dexmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain. Curr. Pain Headache Rep. 2020;24:21. doi: 10.1007/s11916-020-00853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, et al. Effects of dexmedetomidine on cognitive function in elderly patients after laparoscopic cholecystectomy: A protocol for systematic review and meta-analysis. Medicine. 2020;99:e20177. doi: 10.1097/MD.0000000000020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Ren Y, Feng B, Weng X. Pain relieving effect of dexmedetomidine in patients undergoing total knee or hip arthroplasty: A meta-analysis. Medicine. 2020;99:e18538. doi: 10.1097/MD.0000000000018538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, He J, Yu N, Jia C, Wang S. Mechanisms of dexmedetomidine in neuropathic pain. Front. Neurosci. 2020;14:330. doi: 10.3389/fnins.2020.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadjradj PS, Arjun Sharma JRJ, Harhangi BS. Quality of conscious sedation using dexmedetomidine during full-endoscopic transforaminal discectomy for sciatica: A prospective case series. Acta Neurochir. (Wien) 2022 doi: 10.1007/s00701-021-05100-x. [DOI] [PubMed] [Google Scholar]
- 20.Ruan X, Mancuso KF, Kaye AD. Revisiting oxycodone analgesia: A review and hypothesis. Anesthesiol. Clin. 2017;35:e163–e174. doi: 10.1016/j.anclin.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Han L, et al. Oxycodone versus sufentanil in adult patient-controlled intravenous analgesia after abdominal surgery: A prospective, randomized, double-blinded, multiple-center clinical trial. Medicine. 2018;97:e11552. doi: 10.1097/MD.0000000000011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu T, Wu JP, Zhang J, Yu HC, Liu QY. Comparative evaluation of posterior percutaneous endoscopy cervical discectomy using a 3.7 mm endoscope and a 6.9 mm endoscope for cervical disc herniation: A retrospective comparative cohort study. BMC Musculoskelet. Disord. 2021;22:131. doi: 10.1186/s12891-021-03980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu T, et al. Zina percutaneous screw fixation combined with endoscopic lumbar intervertebral fusion under intraoperative neuromonitoring: A case report. Medicine. 2021;100:e24220. doi: 10.1097/MD.0000000000024220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng XQ, Zhu LL, Zhou Q. Opioid analgesics-related pharmacokinetic drug interactions: From the perspectives of evidence based on randomized controlled trials and clinical risk management. J. Pain Res. 2017;10:1225–1239. doi: 10.2147/JPR.S138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers AH, Bakhshaie J, Zvolensky MJ, Vowles KE. Pain anxiety as a mechanism linking pain severity and opioid misuse and disability among individuals with chronic pain. J. Addict. Med. 2019 doi: 10.1097/ADM.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 26.Shah R, et al. Inhibition by divalent metal ions of human histidine triad nucleotide binding protein1 (hHint1), a regulator of opioid analgesia and neuropathic pain. Biochem. Biophys. Res. Commun. 2017;491:760–766. doi: 10.1016/j.bbrc.2017.07.111. [DOI] [PubMed] [Google Scholar]
- 27.Streicher JM, Bilsky EJ. Peripherally acting micro-opioid receptor antagonists for the treatment of opioid-related side effects: Mechanism of action and clinical implications. J. Pharm. Pract. 2017;31:658–669. doi: 10.1177/0897190017732263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhtar M, Nadeem RDA, Shah Gillani SF, Cheema OI, Nadeem MR. Comparison of intra articular NSAID (ketorolac) injection versus hyaluronic acid injection for the mean decrease of pain score (according to UCLA shoulder rating scale) in the management of adhesive capsulitis. Pak. J. Pharm. Sci. 2019;32:953–956. [PubMed] [Google Scholar]
- 29.Ravindhran B, Rajan S, Balachandran G, Mohan LN. Do ice packs reduce postoperative midline incision pain, NSAID or Narcotic use? World J. Surg. 2019;43:2651–2657. doi: 10.1007/s00268-019-05129-1. [DOI] [PubMed] [Google Scholar]
- 30.Stevens AM, Liu L, Bertovich D, Janjic JM, Pollock JA. Differential expression of neuroinflammatory mRNAs in the rat sciatic nerve following chronic constriction injury and pain-relieving nanoemulsion NSAID delivery to infiltrating macrophages. Int. J. Mol. Sci. 2019;20:5269. doi: 10.3390/ijms20215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barletta JF, Asgeirsson T, Senagore AJ. Influence of intravenous opioid dose on postoperative ileus. Ann. Pharmacother. 2011;45:916–923. doi: 10.1345/aph.1Q041. [DOI] [PubMed] [Google Scholar]
- 32.Goettsch WG, Sukel MP, van der Peet DL, van Riemsdijk MM, Herings RM. In-hospital use of opioids increases rate of coded postoperative paralytic ileus. Pharmacoepidemiol. Drug Saf. 2007;16:668–674. doi: 10.1002/pds.1338. [DOI] [PubMed] [Google Scholar]
- 33.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth. Analg. 2005;101:S5–22. doi: 10.1213/01.ane.0000177099.28914.a7. [DOI] [PubMed] [Google Scholar]
- 34.Gillis A, et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci. Signal. 2020;13:eaaz3140. doi: 10.1126/scisignal.aaz3140. [DOI] [PubMed] [Google Scholar]
- 35.Jung H, et al. Effect of fentanyl-based intravenous patient-controlled analgesia with and without basal infusion on postoperative opioid consumption and opioid-related side effects: A retrospective cohort study. J. Pain Res. 2020;13:3095–3106. doi: 10.2147/JPR.S281041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiyokawa M, Haning WF. Hydromorphone-induced tactile hallucinations: Rare opioid side effect. Cureus. 2021;13:e13622. doi: 10.7759/cureus.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahl EL, Bohn LM. Low intrinsic efficacy alone cannot explain the improved side effect profiles of new opioid agonists. Biochemistry. 2021 doi: 10.1021/acs.biochem.1c00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEvoy L, Carr DF, Pirmohamed M. Pharmacogenomics of NSAID-induced upper gastrointestinal toxicity. Front. Pharmacol. 2021;12:684162. doi: 10.3389/fphar.2021.684162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinrinde AS, Hameed HO. Glycine and L-Arginine supplementation ameliorates gastro-duodenal toxicity in a rat model of NSAID (Diclofenac)-gastroenteropathy via inhibition of oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2021 doi: 10.1515/jbcpp-2020-0307. [DOI] [PubMed] [Google Scholar]
- 40.Mantz J, Josserand J, Hamada S. Dexmedetomidine: New insights. Eur. J. Anaesthesiol. 2011;28:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 41.Schmelz M, et al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: Mechanism of action in the context of efficacy and safety. Pain. 2019;160:2210–2220. doi: 10.1097/j.pain.0000000000001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SI, Ha KY, Oh IS. Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: A randomized controlled trial. Eur. Spine J. 2016;25:1614–1619. doi: 10.1007/s00586-015-4216-3. [DOI] [PubMed] [Google Scholar]
- 43.Blancher M, et al. Intranasal sufentanil versus intravenous morphine for acute severe trauma pain: A double-blind randomized non-inferiority study. PLoS Med. 2019;16:e1002849. doi: 10.1371/journal.pmed.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, et al. Evaluation of intravenous parecoxib infusion pump of patient-controlled analgesia compared to fentanyl for postoperative pain management in laparoscopic liver resection. Med. Sci. Monit. 2018;24:8224–8231. doi: 10.12659/MSM.913182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekar C, et al. Preemptive analgesia for postoperative pain relief in lumbosacral spine surgeries: A randomized controlled trial. Spine J. 2004;4:261–264. doi: 10.1016/j.spinee.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Raja SD, Shetty AP, Subramanian B, Kanna RM, Rajasekaran S. A prospective randomized study to analyze the efficacy of balanced pre-emptive analgesia in spine surgery. Spine J. 2019;19:569–577. doi: 10.1016/j.spinee.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Lieblich S. Pre-emptive analgesia. J. Oral Maxillofac. Surg. 2017;75:245–246. doi: 10.1016/j.joms.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Yeung AT, Yeung CA. Advances in endoscopic disc and spine surgery: Foraminal approach. Surg. Technol. Int. 2003;11:255–263. [PubMed] [Google Scholar]
- 49.Mason KP, et al. Value of bispectral index monitor in differentiating between moderate and deep Ramsay Sedation Scores in children. Paediatr. Anaesth. 2006;16:1226–1231. doi: 10.1111/j.1460-9592.2006.01975.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.