Abstract
Background
COVID-19 is associated with inflammation and an increased risk of thromboembolic complications. Prophylactic doses of low-molecular-weight heparin have been used in hospitalised and non-critically ill patients with COVID-19. We aimed to evaluate the efficacy and safety of prophylactic low-molecular-weight heparin (enoxaparin) versus standard of care (no enoxaparin) in at-risk outpatients with COVID-19.
Methods
This open-label, multicentre, randomised, controlled, phase 3b trial (ETHIC) was done at 15 centres in six countries (Belgium, Brazil, India, South Africa, Spain, and the UK). We consecutively enrolled participants aged at least 30 years who had not received a COVID-19 vaccine and had symptomatic, confirmed COVID-19 in the outpatient setting plus at least one risk factor for severe disease. Within 9 days of symptom onset and by use of a web-based random block design (block size either 2 or 4), eligible participants were randomly assigned (1:1) to receive either subcutaneous enoxaparin for 21 days (40 mg once daily if they weighed <100 kg and 40 mg twice daily if they weighed ≥100 kg) or standard of care (without enoxaparin). The primary efficacy endpoint was the composite of all-cause hospitalisation and all-cause mortality at 21 days after randomisation and, in our main analysis, was analysed in the intention-to-treat population, which comprised all patients who were randomly assigned. Safety was also analysed in the intention-to-treat population for our main analysis. This trial is registered with ClinicalTrials.gov, NCT04492254, and is complete.
Findings
Following the advice of the Data and Safety Monitoring Board, this study was terminated early due to slow enrolment and a lower-than-expected event rate. Between Oct 27, 2020, and Nov 8, 2021, 230 patients with COVID-19 were assessed for eligibility, of whom 219 were enrolled and randomly assigned to receive standard of care (n=114) or enoxaparin (n=105). 96 (44%) patients were women, 122 (56%) were men, and one patient had missing sex data. 141 (65%) of 218 participants with data on race and ethnicity were White, 60 (28%) were Asian, and 16 (7%) were Black, mixed race, or Arab or Middle Eastern. Median follow-up in both groups was 21 days (IQR 21–21). There was no difference in the composite of all-cause mortality and hospitalisation at 21 days between the enoxaparin group (12 [11%] of 105 patients) and the standard-of-care group (12 [11%] of 114 patients; unadjusted hazard ratio 1·09 [95% CI 0·49–2·43]; log-rank p=0·83). At 21 days, two (2%) of 105 patients in the enoxaparin group (one minor bleed and one bleed of unknown severity) and one (1%) of 114 patients in the standard-of-care group (major abnormal uterine bleeding) had a bleeding event. 22 (21%) patients in the enoxaparin group and 13 (11%) patients in the standard-of-care group had adverse events. The most common adverse event in both groups was COVID-19-related pneumonia (six [6%] patients in the enoxaparin group and five [4%] patients in the standard-of-care group). One patient in the enoxaparin group died and their cause of death was unknown.
Interpretation
The ETHIC trial results suggest that prophylaxis with low-molecular-weight heparin had no benefit for at-risk outpatients with COVID-19. Although the trial was terminated early, our data, combined with data from similar studies, provide further insights to inform international guidelines and influence clinical practice.
Funding
The Thrombosis Research Institute and Sanofi UK.
Introduction
Venous thromboembolism is common in patients admitted to hospital with COVID-19. Additionally, microvascular thrombosis, which is associated with the inflammatory response to SARS-CoV-2 infection, contributes to organ dysfunction, including acute respiratory distress syndrome. Thrombosis is an important contributor to clinical deterioration and death from COVID-19.1 Compared with a prophylactic dose, a therapeutic dose of low-molecular-weight heparin might reduce the need for organ support and progression to intubation and death in non-critically ill patients hospitalised with COVID-19 but is of no benefit in those who are critically ill.2, 3 The question arises as to whether intervention with prophylactic-dose low-molecular-weight heparin during the early stages of COVID-19 in individuals in the community might prevent deterioration.
Research in context.
Evidence before this study
COVID-19 is associated with an increased risk of thromboembolic complications. As a result, debates have arisen regarding how anticoagulation might impact the natural history of disease. We searched PubMed without language restrictions for studies published between database inception and May 5, 2020, investigating the impact of thromboprophylaxis on outcomes in patients with COVID-19. Combinations of key search terms were applied, including “COVID”, “anticoagulation”, “low-molecular-weight heparin”, “thromboprophylaxis”, “venous thromboembolism”, “bleeding”, and “clinical guidelines”. We found 18 relevant studies, which together suggested that thromboprophylaxis might prevent thrombi in patients with COVID-19. Despite a paucity of robust clinical evidence, the administration of heparin was recommended as early as Aug 26, 2021, in several published guidance statements to prevent deterioration in patients with COVID-19.
Added value of this study
We investigated the potential impact of the early administration of prophylactic low-molecular-weight heparin on outcomes in outpatients with COVID-19. Although this trial was terminated early due to slow enrolment and a lower-than-expected event rate, and therefore is inconclusive, the data suggest that there was no difference in the composite of all-cause mortality and hospitalisation at 21 days between patients given low-molecular-weight heparin (enoxaparin) versus standard of care (no enoxaparin).
Implications of all the available evidence
Our results add to those of the ACTIV-4B trial, which reported no difference in outcomes between symptomatic patients with COVID-19 treated with aspirin or apixaban versus placebo. These results suggest that early anticoagulation for the prevention of thromboembolic complications in non-critical outpatients with COVID-19 might have no clinical benefit and should not be used routinely in this clinical setting. Combined with the data from other studies, our results will inform guidelines and guide clinical practice.
Low-molecular-weight heparin has been validated for its efficacy and safety in preventing venous thromboembolic disease in medical and surgical patients at high risk in both the acute hospitalised and post-discharge settings.4 However, its use has been advocated as part of routine care for patients with COVID-19 in the community, despite the absence of prospective clinical evidence in this setting.5, 6, 7 Therefore, we aimed to evaluate the efficacy and safety of prophylactic enoxaparin versus standard of care (without enoxaparin) in at-risk patients with COVID-19 in the community.
Methods
Study design and participants
The Early Thromboprophylaxis In COVID-19 (ETHIC) trial was an open-label, multicentre, randomised, controlled, phase 3b trial done at 15 centres in six countries (Belgium, Brazil, India, South Africa, Spain, and the UK). Investigator sites were identified and selected to include representative care settings (general practice, office-based, testing centres, and hospitals) in each participating country. Feasibility discussions and site selection visits were done by remote telephone or video conferences. Feasibility analysis used our site database to identify countries and sites that could deliver the protocol. Study sites in which standard of care included low-molecular-weight heparin were not feasible for the study. National coordinating investigators were assigned to each country and assisted with mapping the impact of COVID-19 in their respective countries, the treatment pathway for eligible patients, and appropriate sites and care settings. A confirmation letter was sent to selected sites to confirm site participation and to arrange a site initiation visit. During the site initiation visit, the sponsor was responsible for providing appropriate training materials to ensure that all personnel involved in the conduct of the trial were adequately qualified and trained. Regular monitoring calls were scheduled for each participating site. A close-out visit was done remotely for each site at the end of the study.
We consecutively enrolled participants aged at least 30 years who had not received a COVID-19 vaccine and had symptomatic (up to 9 days in duration; see appendix p 3 for the list of symptoms), confirmed (positive SARS-CoV-2 RT-quantitative PCR test) COVID-19 in the outpatient setting plus at least one risk factor for severe disease (appendix pp 38–39). In the initial protocol, patients were required to be aged at least 55 years and have at least two predefined risk factors: older age (≥70 years), a body-mass index of more than 25 kg/m2, chronic lung disease, diabetes, cardiovascular disease, or corticosteroid use. Applicable risk factors were identified via an extensive literature search of PubMed for literature published between database inception and May 5, 2020, before we finalised the protocol. Initial exclusion criteria comprised contraindication to unfractionated heparin or low-molecular-weight heparin; recent (<48 h) or planned spinal or epidural anaesthesia or puncture; percutaneous coronary intervention; thrombolytic therapy within the preceding 24 h; an increased risk of bleeding complications; pregnancy; severe renal impairment (glomerular filtration rate <30 mL/min); current anticoagulant or antiplatelet therapy (except low-dose aspirin); and current participation in another interventional study outside the purview of studies sponsored by the Thrombosis Research Institute. On Jan 13, 2021, the steering committee voted to amend the protocol to include patients who were aged at least 30 years, to allow current clopidogrel (≤75 mg) monotherapy, and to reduce the number of required risk factors to at least one. Further risk factors were added to the eligibility criteria: previous venous thromboembolism; liver disease; anaemia of chronic disease or sickle cell disease; and an immunocompromised state (in addition to that due to corticosteroid use; appendix p 38). These changes were made to address the slower-than-expected enrolment; after making these protocol amendments, the event rate was deemed satisfactory, with reference to usual event rates in the published literature of patients with only one risk factor for severe COVID-19.8, 9, 10, 11, 12 Additionally, we excluded patients who had received any COVID-19 vaccines due to the expected impact of vaccination status on the rate of study endpoints in our trial.
The study was evaluated and approved by local or central independent ethics committees or institutional review boards and regulatory authorities according to requirements for each participating country. The ETHIC trial was done in accordance with the Declaration of Helsinki and guidelines from the International Conference on Harmonisation on Good Clinical Practice and the Medicines for Human Use (Clinical Trials) Regulations 2004, and adheres to all applicable national laws and regulations. Clinical trial authorisation was obtained from each regulatory authority. All eligible participants were provided with a patient information sheet and consent form. Signed, written informed consent was obtained from all participating individuals, according to local requirements at each participating site. Enrolled patients could withdraw consent at any time by notifying the investigator. If lost to follow-up, the investigator attempted to obtain the cause of withdrawal. All events were managed and reported in accordance with the full requirements of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Clinical Safety Data Management: Definitions, and Standards for Expedited Reporting, Topic E2. The study protocol can be found in the appendix (pp 14–68).
Randomisation and masking
Within 9 days of COVID-19 symptom onset, eligible patients were consecutively enrolled and randomly assigned (1:1) to receive either enoxaparin or the current standard of care (no enoxaparin). Patients were enrolled by the clinicians involved in the study, who also were involved in data interpretation and manuscript review. The randomisation sequence was established before enrolment of the first patient and was done by use of a prespecified, secure, central, web-based randomisation system. Only key members of the data management team and the unmasked statistician had access to patient treatment allocations. Once a patient's data were entered as meeting criteria for the study, including informed consent, the electronic enrolment system provided the site with the appropriate random treatment assignment and the patient was assigned a randomisation number. Randomisation was generated with a random block design (block size either 2 or 4), blocking within each site to allow equal allocation of the two treatments within each site. The study was unblinded and therefore no allocation concealment was applied.
Procedures
Eligible patients received either enoxaparin for 21 days (40 mg once daily if they weighed <100 kg and 40 mg twice daily if they weighed ≥100 kg) or the current standard of care (without enoxaparin). Patients in the enoxaparin group received pre-loaded syringes from the site and instructions for subcutaneous self-administration. Sites recorded the batch number, expiry date, number of syringes dispensed, and dispensation date for each patient in the Investigational Product Patient Dispensing/Accountability Log. Participants randomly assigned to enoxaparin were required to notify the enrolling site if they had discontinued treatment. Information regarding the date and cause of discontinuation was collected by the study coordinators. In the event of enoxaparin intolerance, hospitalisation, or major bleeding, the decision to continue the randomised treatment, convert to a lower dose or higher dose, or discontinue treatment was at the discretion of the treating physician. Patients were required to return all used and partially used syringes to the enrolling site at the end of their participation in the study to allow drug reconciliation and destruction.
Patient data were collected at 21 days, 50 days, and 90 days following randomisation by the treating physician using an electronic case report form designed by the Thrombosis Research Institute. For a list of the assessments done at each follow-up visit, see the appendix (pp 40–46). Briefly, we collected information on death, hospital admission, and safety (eg, bleeding) at days 21, 50, and 90. The Thrombosis Research Institute ensured accurate data collection from the medical records of enrolled patients. As stated in the protocol (appendix p 61), source data verification was done by use of a risk-based approach to confirm that source data entered into the electronic case report forms by authorised site personnel were accurate, complete, and verifiable from source documents. Electronic audit trails are available for all data modifications and critical variables were subjected to additional audit. Participants received electronic or paper diaries to record compliance and the development, resolution, and severity of symptoms throughout the study.
Routine monitoring (every 2–4 weeks) of safety information for adverse events and serious adverse events and the electronic case report form was done by the Thrombosis Research Institute Safety Department. Adverse events were classified as per International Council for Harmonisation guidelines. Post-hoc, clinicians judged the relatedness of adverse events to the study drug. Serious adverse events must have met one of the following criteria: fatality; life-threatening; new or prolonged hospitalisation; disability or incapacity; congenital anomaly or birth defect in an infant born to a mother exposed to the drug; or a considerable medical event that might require medical or surgical intervention to prevent one of the aforementioned outcomes.
The severity of bleeding (major, clinically relevant non-major, or minor) was defined according to the International Society of Thrombosis and Haemostasis criteria.13 Major bleeding was defined as overt bleeding associated with death, within a critical area or organ, or caused by a decrease in haemoglobin concentration of 20 g/L (1·24 mmol/L) or more or leading to transfusion of two or more units of whole blood or red blood cells. Clinically relevant non-major bleeding was defined as bleeding requiring medical intervention by a health-care professional, leading to hospitalisation or an increase in the level of care, or promoting face-to-face evaluation. Minor bleeding was defined as any overt bleeding that did not meet the criteria for major or clinically relevant non-major bleeding. An independent safety review committee adjudicated Medical Dictionary for Regulatory Activities codes and the safety classification of all adverse events entered into the system.
Outcomes
The primary outcome was the composite of all-cause death and all-cause admission to hospital (hospitalisation) at 21 days after randomisation (with further assessments at days 50 and 90). Secondary outcomes were the diagnosis of venous thromboembolism at days 21, 50, and 90, and bleeding events at days 21 and 50. Bleeding events at day 90 was added as a post-hoc outcome.
The primary efficacy outcome and the bleeding events outcome were centrally adjudicated. Personal identifiers and treatment information were removed from all medical records before sharing the data with the Clinical Events Committee. The Clinical Events Committee were responsible for systematically adjudicating death, hospitalisation, and the classification of bleeding in a blinded way to the treatment assignment according to predefined clinical outcome definitions.
Statistical analysis
Sample size calculations were based on an α level of 0·05 and an event rate of 25% in the standard-of-care group, which was calculated using information on event rates reported by the Centers for Disease Control and Prevention at the beginning of the study (appendix p 1).8, 9, 10, 11, 12 Assuming a relative risk of 0·75 (ie, an event rate of 18·75% with enoxaparin) and 80% power, the study required a total of 1370 study participants. As enoxaparin use was well understood, a formal interim analysis by the Data and Safety Monitoring Board (DSMB) was not planned. Initially, the DSMB were to review safety after approximately a third and two-thirds of patients had been enrolled. However, as the COVID-19 landscape evolved, the DSMB were tasked to evaluate the possible need for a change in sample size after a third of patients had been enrolled.
In November, 2021, due to concerns that the overall event rate and rate of enrolment were lower than expected, the steering committee requested that the DSMB review unblinded data to provide guidance for trial continuation. The DSMB reviewed the evaluated data, calculating the probability of the observed control event rate (or less than the observed control event rate if the actual event rate was 25% assuming a binomial distribution) occurring. Conditional power calculations were done to determine either the number of patients required, assuming the event rate seen to date, or the change in relative risk required to detect a significant difference at the end of the study.14, 15
Continuous variables are expressed as median (IQR) and categorical variables are expressed as frequency and percentage. Differences in the primary outcome were tested for statistical significance with a log-rank test and analysed in a Kaplan–Meier curve; we estimated the unadjusted hazard ratio of enoxaparin versus standard of care by Cox regression. 95% CIs were based on the Wald test.
Three population sets were analysed during this study. We analysed all outcomes and safety in the intention-to-treat population, which comprised all patients who provided signed, written informed consent and were randomly assigned, for our main analysis. Supplementarily, we repeated our outcome and safety analyses in the per-protocol population, which was a subset of the intention-to-treat population, excluding patients who complied with less than 50% of the randomised treatment or had major protocol deviations, and in the as-treated population, in which the participants were assigned to the treatment they received, irrespective of assigned randomisation group. In the as-treated population, if a participant who was randomly assigned to enoxaparin did not receive the initial randomised drug, then the participant was assigned to the standard-of-care group. If a participant who was randomly assigned to standard of care initiated enoxaparin up to 2 days after randomisation, the participant was assigned to the enoxaparin group. We planned a sensitivity analysis to compare the primary and secondary outcomes before versus after the protocol amendment on Jan 13, 2021.
Data were extracted from the study database on Jan 18, 2022. All analyses were done by use of SAS Enterprise Guide 7.1. This study is registered with ClinicalTrials.gov, NCT04492254. The DSMB were responsible for reviewing trial data at prespecified timepoints and providing recommendations for the study protocol and progress. A full list of committee members is provided in the appendix (pp 12–14).
Role of the funding source
Sanofi funded this investigator-sponsored study and provided enoxaparin free of charge. The Thrombosis Research Institute had input in study design, data collection, data analysis, data interpretation, and writing of the report.
Results
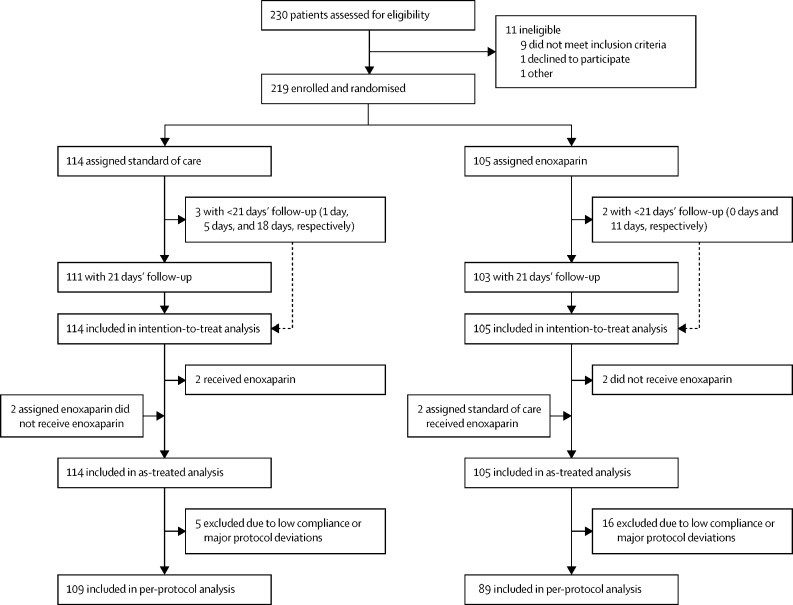
Enrolment into the ETHIC trial commenced on Oct 27, 2020. On Nov 8, 2021, due to the low overall event rate, slower-than-anticipated rate of enrolment, and the rapid adoption of COVID-19 vaccination, the steering committee recommended terminating the study once the DSMB had evaluated the data and concurred. The last patient was randomly assigned on Nov 8, 2021. Participating sites reported overwhelming workloads in caring for the large number of hospitalised patients with COVID-19; thus, incorporating the extra responsibility of clinical trial management was not possible in many cases, which contributed to slow enrolment. Of 230 patients with COVID-19 assessed for eligibility, 219 were randomly assigned to receive either standard of care (114 [52%]) or enoxaparin (105 [48%]; figure 1 ).
Figure 1.
Trial profile
The median age of patients in both groups was 59·0 years, more patients were male than female, and the majority were White or Asian (table 1 ). Data on the distribution of patients according to country of enrolment and care setting are provided in the appendix (p 2). For both groups, the median time from COVID-19 diagnosis to randomisation was 2·0 days and the median time from first symptom to randomisation was 5·0 days (table 1).
Table 1.
Demographics and clinical characteristics at baseline in the intention-to-treat population
| Enoxaparin group (n=105) | Standard-of-care group (n=114) | ||
|---|---|---|---|
| Age, years | 59·0 (51·0–66·0) | 59·0 (50·0–67·0) | |
| Sex | |||
| Female | 45 (43%) | 51/113 (45%) | |
| Male | 60 (57%) | 62/113 (55%) | |
| Missing data | 0 | 1 | |
| Race | |||
| Arab or Middle Eastern | 1 (1%) | 1/113 (1%) | |
| Asian | 29 (28%) | 31/113 (27%) | |
| Black | 4 (4%) | 1/113 (1%) | |
| Mixed race | 5 (5%) | 4/113 (4%) | |
| Not known | 0 | 1/113 (1%) | |
| White | 60 (57%) | 69/113 (61%) | |
| White (Hispanic) | 6 (6%) | 6/113 (5%) | |
| Missing data | 0 | 1 | |
| Body-mass index, kg/m2 | 30·1 (27·5–31·9) | 28·8 (26·3–32·2) | |
| Smoking status | |||
| Current smoker | 5/100 (5%) | 13/110 (12%) | |
| Previous smoker | 21/100 (21%) | 20/110 (18%) | |
| Never smoker | 74/100 (74%) | 77/110 (70%) | |
| Missing data | 5 | 4 | |
| Alcohol consumption from case report form | |||
| No or light consumption | 81/90 (90%) | 91/99 (92%) | |
| Moderate consumption | 9/90 (10%) | 7/99 (7%) | |
| Heavy consumption | 0/90 | 1/99 (1%) | |
| Missing data | 15 | 15 | |
| COVID-19 testing method | |||
| Nasal swab | 5/104 (5%) | 3/111 (3%) | |
| Nasopharyngeal swab | 81/104 (78%) | 85/111 (77%) | |
| Oropharyngeal swab | 3/104 (3%) | 2/111 (2%) | |
| Nasopharyngeal and oropharyngeal swab | 15/104 (14%) | 21/111 (19%) | |
| Missing data | 1 | 3 | |
| Time from COVID-19 diagnosis to randomisation, days | 2·0 (1·0–3·0) | 2·0 (1·0–3·0) | |
| Time from first symptom to randomisation, days | 5·0 (3·0–6·0) | 5·0 (4·0–6·0) | |
| Medical history | |||
| Chronic lung disease* | 6/76 (8%) | 14/86 (16%) | |
| Diabetes | 24/76 (32%) | 26/86 (30%) | |
| Active cancer† | 0/76 | 2/86 (2%) | |
| Vascular disease‡ | 12/76 (16%) | 14/86 (16%) | |
| Moderate or severe heart valve disease | 0/76 | 1/86 (1%) | |
| Treated arrhythmia | 1/76 (1%) | 2/86 (2%) | |
| Heart failure | 0/76 | 1/86 (1%) | |
| Hypertension | 56/76 (74%) | 58/86 (67%) | |
| Congenital heart disease | 1/76 (1%) | 0/86 | |
| Previous stroke or transient ischaemic attack | 2/76 (3%) | 1/86 (1%) | |
| Carotid artery disease | 2/76 (3%) | 0/86 | |
| Previous venous thromboembolism | 2/76 (3%) | 0/86 | |
| Chronic liver disease | 1/76 (1%) | 0/86 | |
| Immunocompromised condition§ | 1/76 (1%) | 3/86 (3%) | |
| Missing data | 29 | 28 | |
Data are median (IQR), n (%), or n/N (%).
Asthma, emphysema, chronic obstructive pulmonary disease, or pulmonary fibrosis.
Diagnosed within the past 6 months or receiving treatment for cancer.
Coronary artery disease or peripheral artery disease.
Receiving immunosuppressive therapy, including oral steroids, or presence of HIV infection.
Due to the very low number of events and the study's early termination, only the primary efficacy outcome was tested for statistical significance. Median follow-up for the primary efficacy outcome was 21 days (IQR 21–21) for both groups. At 21 days, in the intention-to-treat analysis, the composite of all-cause mortality and all-cause hospitalisation was observed in 12 (11%) of 105 patients in the enoxaparin group and in 12 (11%) of 114 patients in the standard-of-care group (unadjusted hazard ratio 1·09 [95% CI 0·49–2·43]; log-rank p=0·83; figure 2 ; table 2 ). When considering only 21 days of follow-up, all events of the primary composite outcome occurred within 14 days of commencing treatment. All-cause death was recorded for one patient in the enoxaparin group and their cause of death was unknown; this patient was hospitalised before death. 12 patients were hospitalised in each treatment group. Among the 12 patients in the enoxaparin group who were hospitalised, four were admitted to intensive care units or required acute medical care and three received mechanical ventilation or extracorporeal membrane oxygenation. No patients in the standard-of-care group were similarly admitted or treated. At 21 days, one patient in each group had a venous thromboembolism (deep vein thrombosis in the patient who received enoxaparin and pulmonary embolism in the patient who received standard of care). Neither of these two patients had a history of venous thromboembolism before randomisation. At 90 days, the composite outcome of cumulative all-cause death and all-cause hospitalisation remained at 12 patients in each group and there was a further venous thromboembolism event in the standard-of-care group (table 2).
Figure 2.
Cumulative survival probability for all-cause death or hospitalisation by treatment group
Table 2.
Cumulative incidence of efficacy endpoints during follow-up in the intention-to-treat population
|
21 days |
50 days |
90 days |
||||
|---|---|---|---|---|---|---|
| Enoxaparin group (n=105) | Standard-of care-group (n=114) | Enoxaparin group (n=105) | Standard-of-care group (n=114) | Enoxaparin group (n=105) | Standard-of-care group (n=114) | |
| All-cause death | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 1 (1%) | 1 (1%) |
| All-cause hospitalisation | 12 (11%) | 12 (11%) | 12 (11%) | 12 (11%) | 12 (11%) | 12 (11%) |
| Composite of all-cause death and all-cause hospitalisation | 12 (11%) | 12 (11%) | 12 (11%) | 12 (11%) | 12 (11%) | 12 (11%) |
| Venous thromboembolism | 1 (1%) | 1 (1%) | 1 (1%) | 2 (2%) | 1 (1%) | 2 (2%) |
Two patients who were randomly assigned to standard of care received enoxaparin and two patients who were randomly assigned to enoxaparin did not receive enoxaparin; thus, in our as-treated analysis, 105 patients were in the enoxaparin group and 114 patients were in the standard-of-care group. Five patients from the standard-of-care group and 16 patients from the enoxaparin group were excluded from the per-protocol analysis, totalling 109 patients in the standard-of-care group and 89 patients in the enoxaparin group. The incidences of the composite outcome of all-cause mortality and all-cause hospitalisation plus the incidences of venous thromboembolism remained similar between the two study groups in the as-treated and per-protocol analyses (appendix p 7). We planned a sensitivity analysis to compare patients before versus after the protocol amendment on Jan 13, 2021. Unfortunately, only 28 patients were enrolled before the date the protocol was changed, so conducting this sensitivity analysis was not possible.
The most common concomitant medications recorded at baseline in the enoxaparin group were statins (n=28), β blockers (n=25), oral antidiabetic agents (n=23), low-dose aspirin (n=21), and angiotensin receptor blockers (n=17; appendix p 6). The most common concomitant medications recorded at baseline in the standard-of-care group were β blockers (n=31), statins (n=30), low-dose aspirin (n=24), oral antidiabetic agents (n=19), and calcium channel blockers (n=18; appendix p 6). Concomitant use of a low-molecular-weight heparin other than enoxaparin was recorded in one (1%) patient in the enoxaparin group and in one (1%) patient in the standard-of-care group.
In the enoxaparin group, treatment was started on the day of randomisation in 95 (93%) of 102 patients; the remainder (n=7) started treatment within 5 days of randomisation. Two patients who were randomly assigned to the enoxaparin group did not start enoxaparin; thus, the start date was missing for these patients. Additionally, one patient had started enoxaparin 5 days before randomisation and was thus considered missing. Hypertension, diabetes, vascular disease, and chronic lung disease were the most common medical histories in both groups (table 1). Of the 105 patients who were randomly assigned to enoxaparin, 88 (84%) received a single daily dose of 40 mg and 17 (16%) received a twice-daily dose of 40 mg.
Before enrolment, there were two protocol violations regarding previous COVID-19 vaccination: one patient in the enoxaparin group had received their first dose of COVID-19 vaccine 119 days before enrolment and one patient in the standard-of-care group had received their first dose 18 days before enrolment (appendix p 4). After enrolment, 43 (41%) of 105 patients in the enoxaparin group and 50 (44%) of 114 patients in the standard-of-care group received at least a first-dose vaccination (appendix p 4). The median time from enrolment to first-dose vaccination was 40 days (IQR 31–61) in the enoxaparin group and 42 days (36–57) in the standard-of-care group.
Of the 105 patients in the enoxaparin group, five (5%) received their first vaccine dose within 21 days of randomisation, 21 (20%) received their first vaccine dose within 50 days of randomisation, and 17 (16%) received their first vaccine dose within 90 days of randomisation. The remaining 62 (59%) patients did not receive their first dose during follow-up. Of the 114 patients in the standard-of-care group, five (4%) received their first vaccine dose within 21 days of randomisation, 27 (24%) received their first vaccine dose within 50 days of randomisation, and 18 (16%) received their first vaccine dose within 90 days of randomisation. The remaining 64 (56%) patients did not receive a first dose during follow-up.
At 21 days, two (2%) of 105 patients in the enoxaparin group (one minor bleed and one bleed of unknown severity) and one (1%) of 114 patients in the standard-of-care group (major abnormal uterine bleeding) had any bleeding event (appendix p 8). Furthermore, 22 (21%) patients in the enoxaparin group and 13 (11%) patients in the standard-of-care group had adverse events (appendix p 8). The most common adverse event in both groups was COVID-19-related pneumonia (six [6%] patients in the enoxaparin group and five [4%] patients in the standard-of-care group; table 3 ). Adverse events were less likely to be serious in the enoxaparin group (13 [59%] of 22 patients) than in the standard-of-care group (12 [92%] of 13 patients). Most adverse events were related to the clinical course of COVID-19 and seemed unrelated to the administration of study drug according to post-hoc clinican judgement.
Table 3.
Adverse events in the intention-to-treat population at 21 days following randomisation
| Enoxaparin group (n=105) | Standard of care group (n=114) | ||
|---|---|---|---|
| Severe | 6 (6%) | 2 (2%) | |
| COVID-19 pneumonia | 3 (3%) | 0 | |
| Hypoxaemia | 1 (1%) | 2 (2%) | |
| Decreased oxygen saturation | 1 (1%) | 0 | |
| Respiratory failure | 1 (1%) | 0 | |
| Moderate | 10 (10%) | 8 (7%) | |
| COVID-19 pneumonia | 3 (3%) | 5 (4%) | |
| COVID-19 respiratory infection | 0 | 1 (1%) | |
| High fibrin D dimer | 0 | 1 (1%) | |
| Decreased oxygen saturation | 1 (1%) | 0 | |
| Pulmonary embolism | 0 | 1 (1%) | |
| Chills | 1 (1%) | 0 | |
| Injection site bruising | 1 (1%) | 0 | |
| Nausea | 1 (1%) | 0 | |
| Rash on legs and arms | 1 (1%) | 0 | |
| Shortness of breath | 1 (1%) | 0 | |
| Mild | 6 (6%) | 3 (3%) | |
| COVID-19 respiratory infection | 0 | 1 (1%) | |
| Contusion of multiple sites of trunk | 1 (1%) | 0 | |
| Fall | 1 (1%) | 0 | |
| Fever | 0 | 1 (1%) | |
| Decreased oxygen saturation | 0 | 1 (1%) | |
| Chest discomfort | 1 (1%) | 0 | |
| Hypermenorrhoea | 1 (1%) | 0 | |
| Hypotension | 1 (1%) | 0 | |
| Shingles | 1 (1%) | 0 | |
At 50 days, two (2%) of 105 patients in the enoxaparin group (both minor bleeds) and two (2%) of 114 patients in the standard-of-care group (one major and one minor bleed) had any bleeding events. 23 (22%) patients in the enoxaparin group and 17 (15%) patients in the standard-of-care group had adverse events (appendix p 8).
At 90 days, three (3%) patients in the enoxaparin group and three (3%) patients in the standard-of-care group had any bleeding (appendix p 8). 24 (23%) patients in the enoxaparin group and 19 (17%) patients in the standard-of-care group had adverse events (appendix p 8). Of note, there was a range of adverse event rates across countries and no adverse events were identified in India (appendix p 9). Most adverse events were reported in Belgium and Brazil (appendix p 9). To verify, sites and study monitors were asked to review all adverse event data, and patient diaries were compared with the electronic case report form records. No additional events were found after this added scrutiny. Results for the secondary outcome of bleeding and safety in the as-treated and per-protocol populations were similar to those in the intention-to-treat population (appendix p 10).
Enoxaparin treatment most often ceased due to reaching the end of planned treatment (n=76), adverse events (n=13), or patient choice (n=13; appendix p 5). Adverse events that led to the cessation of enoxaparin were COVID-19 pneumonia (n=6), chills (n=1), a fall (n=1), hypoxaemia (n=1), nausea (n=1), decreased oxygen saturation (n=1), respiratory failure (n=1), and shortness of breath (n=1). One patient had their dose changed during treatment (appendix p 5). Six patients had their treatment interrupted and restarted (appendix p 5).
Discussion
The ETHIC trial was unable to show a therapeutic advantage for the early administration of low-molecular-weight heparin (enoxaparin) compared with standard of care in patients with COVID-19 and at least one risk factor for severe disease in the outpatient setting—there was no difference in the primary efficacy outcome of all-cause hospitalisation and death. The use of a prophylactic dose of low-molecular-weight heparin was safe and did not cause an increase in major bleeding episodes.
The study was terminated early due to a lower-than-expected enrolment rate and a lower-than-expected event rate, so no firm conclusions can be drawn. We evaluate three explanations for the negative results. First, the prophylactic dose of low-molecular-weight heparin might have been insufficient. Retrospective studies initially showed that anticoagulation was associated with lower mortality in severely ill patients with COVID-19.16 Randomised trials2, 3, 17, 18 have compared lower versus higher heparin doses in hospitalised patients with COVID-19. Higher doses were mostly not associated with a mortality benefit compared with lower doses in critically ill patients, although some higher dose regimens have shown moderate benefit, in terms of an increased probability of survival to hospital discharge with reduced need for organ support, in non-critically ill patients.2 A meta-analysis of 42 studies of hospitalised patients with COVID-19 found that higher versus lower doses of anticoagulants did not significantly reduce in-hospital mortality and the incidence of thrombotic events, but significantly increased bleeding events.19 The optimal dose of heparin for outpatients with COVID-19 remains to be determined, but it seems unlikely that the use of higher doses would have provided better outcomes.
Second, there is debate as to whether thrombotic events in COVID-19 might be due to venous thromboembolism versus immunothrombosis.20 Indeed, immunothrombosis, which appears to be mechanistically distinct from venous thromboembolism, might be less amenable to traditional anticoagulants, resulting in an absence of treatment effect. Immunothrombosis is a mechanism of vascular immunity involving coagulation activation, immune cell recruitment, neutrophil extracellular trap formation, and platelet recruitment. If uncontrolled, this network of interconnected mechanisms can initiate the formation of microthrombi in small blood vessels.21 The associated increase in fibrinogen, factor VIII, and platelet count might confer resistance to heparin treatment.20 Certainly, patients with COVID-19 treated in intensive care units with unfractionated heparin or low-molecular-weight heparin have been shown to have substantial heparin resistance.22 Heparin resistance might also, in part, explain the high rates of thrombosis reported in critically ill patients.22
Finally, an important factor in explaining our negative results is the lower-than-expected event rate. The expected event rate was calculated on the basis of reported event rates available at the beginning of the study. In the early days of the pandemic, reported thrombotic event rates were much higher than during later stages of the pandemic.23, 24 This variation might be due to initial undertesting, changes in the pathogenicity of SARS-CoV-2 with the emergence of new variants, and improved disease management. Additionally, participating sites reported overwhelming workloads in caring for the large number of hospitalised patients with COVID-19; thus, incorporating the extra responsibility of clinical trial management was not possible in many cases, which contributed to slow enrolment. Furthermore, during the resulting delay in enrolment, population vaccination programmes progressed, therefore rendering large groups of patients ineligible. The ACTIV-4B trial25 dealt with similar difficulties. The ACTIV-4B study25 aimed to assess outcomes in clinically stable outpatients with symptomatic COVID-19 when treated with aspirin or apixaban compared with a placebo control. However, the study was also terminated early after achieving just 9% of the anticipated enrolment, as event rates were lower than expected. Notably, ACTIV-4B also reported no difference in a composite outcome (all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalisation for cardiovascular or pulmonary cause) between the treatment groups. Compared with the ACTIV-4B trial, the ETHIC trial required patients to have at least one risk factor for severe COVID-19 and patients started treatment much earlier after diagnosis. In ETHIC, the median time from COVID-19 diagnosis to randomisation was 2·0 days (vs 7 days [IQR 3–10] in ACTIV-4B) and 93% of patients received enoxaparin on the day of randomisation (vs a median time to receive treatment of 3 days [IQR 2–5] after randomisation in ACTIV-4B). Nevertheless, these dissimilarities were not reflected in the results of the two trials.
In our study, bleeding occurred infrequently. At 21 days, there had been two bleeding episodes in the enoxaparin group and one bleeding episode in the standard-of-care group. No major bleeding was seen with enoxaparin treatment. At 90 days' follow-up, the number of bleeding episodes was still similar between groups. The enoxaparin group had a higher rate of adverse events than the standard-of-care group and most adverse events were reported in Belgium and Brazil. Surprisingly, no adverse events were reported in India. Given that the other countries in the study reported an adverse event rate of approximately 25%, adverse events might have been under-reported in India. Adverse events were mostly mild or moderate in severity. Most adverse events were related to the clinical course of COVID-19 and seemed unrelated to the administration of study drug. When considering only 21 days of follow-up, all events of the primary composite outcome occurred within 14 days of commencing treatment. It is possible that a longer duration of treatment would therefore not be of value; however, due to the small sample size of this study, such cannot be definitively concluded and would require further confirmation.
On the basis of the available evidence, current society guidelines, such as those from the American Society of Hematology,26 the National Institutes of Health,27 and the National Institute for Health and Care Excellence,28 recommend a prophylactic dose of anticoagulation (preferably low-molecular-weight heparin) for the treatment of hospitalised patients with COVID-19, except for select non-critically ill patients. Earlier society guidelines, such as that of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis,7 promoted the use of increased doses of heparin for the treatment of critically ill patients with COVID-19; however, higher doses are no longer recommended by current guidance statements due to the lack of benefit and increased bleeding risk.26, 27 This recommendation does not preclude individual decision making for selected patients with a high thrombotic risk. For example, the MICHELLE trial29 showed a significant reduction in post-discharge thrombotic events with 10 mg of rivaroxaban versus no anticoagulation in a population with a high thrombotic risk but low bleeding risk after hospitalisation for COVID-19. Therefore, for these patients, post-discharge anticoagulation prophylaxis should be carefully selected.
This study had several limitations. First, the trial had an open-label design because treatment with a parenteral placebo would have been practically challenging in the control group. Second, adjustments to the inclusion criteria were made during the course of the study, with the age limit being adjusted from at least 55 years to at least 30 years. Third, we recruited participants who were not vaccinated, limiting generalisability in the current pandemic era, particularly in the context of breakthrough infections. However, because vaccination reduces the risk of serious COVID-19,30 the chances that outcomes in vaccinated patients will be further positively influenced by routine anticoagulation prophylaxis seem low. Finally, difficulty with patient enrolment during the pandemic led to low patient numbers and early termination of the study. As other related studies have reported early termination for similar causes, a collective, combined evaluation of the patients with COVID-19 recruited in these studies might provide valuable insight into the treatment effect.25
In conclusion, the ETHIC trial results suggest that prophylaxis with standard dose low-molecular-weight heparin administered within a few days of symptom onset does not improve the rate of all-cause hospitalisation and mortality compared with the standard of care among outpatients with symptomatic COVID-19 and at least one risk factor for severe disease. The study, however, was underpowered due to lower-than-expected enrolment and event rates. Our data, combined with data from similar studies, provide further information to inform guidelines and guide clinical practice. At this point in time, there is no evidence to support the routine, early use of low-molecular-weight heparin for prophylaxis in outpatients with COVID-19.
Data sharing
Requests for data will be reviewed by the Thrombosis Research Institute Leadership Committee. If accepted, a deidentified dataset containing the requested data elements and any necessary documentation will be provided for analytical purposes. The data will be shared via a secure portal. Data sharing will be available once combined analysis is complete, which will be on Oct 1, 2022. The study protocol can be found in the appendix (pp 14–68).
Declaration of interests
FC reports speaker fees from Boehringer Ingelheim Pharma, Bayer, Pfizer, and Daiichi-Sankyo Europe, and a modest research grant from Daiichi-Sankyo Europe. JS reports personal fees from Pfizer, AstraZeneca, Novartis, Sanofi, BMS, Dr Reddy's Laboratory, Lupin, and Abbot. RDL reports research grants from Bristol Myers Squibb, Pfizer, Amgen, GlaxoSmithKline, Medtronic, and Sanofi Aventis, and personal fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, and Bayer, outside the submitted work. BJ reports personal fees from Bayer HealthCare and Sanofi-Aventis. JIA reports speaker fees from Sanofi, Rovi, Bayer, and Aspen. FDRH acknowledges part support as Director of the National Institute for Health and Care Research (NIHR) Applied Research Collaboration Oxford Thames Valley and Theme Lead of the NIHR Oxford University Hospital Biomedical Research Centre, and has also received occasional fees or expenses for speaking or consultancy from AstraZeneca, Boehringer Ingelheim, Bayer, BMS/Pfizer, and Novartis. HG reports personal fees from Pfizer, Bayer, and Boehringer Ingelheim. PM reports honoraria from Bayer Pharma and Portolo. SS reports speaker fees from Bayer Pharma, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Sanofi Aventis, and Pfizer, and consultancy fees from Bayer Pharma, Boehringer Ingelheim, Daiichi Sankyo, Sanofi Aventis, Aspen, and Pfizer. SH reports personal fees from Bayer, Bristol Myers Squibb, Daiichi Sankyo, Portola, and Sanofi, outside the submitted work. AGGT reports grants from Bayer Healthcare and personal fees from Bayer Healthcare, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, and Boehringer Ingelheim, outside the submitted work. WA reports honoraria from Bayer Pharma, Bristol Myers Squibb, Pfizer, Daiichi Sankyo, Portola, Aspen, Sanofi, Leo Pharma, Norgine, and Werfen. ATR reports consultancy fees from Bayer Pharma, Daiichi Sankyo, Sanofi, Aspen, and Pfizer. KP reports a consultancy fee from Johnson & Johnson. AKK reports research grants from Anthos, Bayer, and Sanofi and personal fees from Anthos Therapeutics, Bayer, and Sanofi. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The ETHIC trial was funded by The Thrombosis Research Institute and Sanofi UK. Programming support was provided by Uma Maheshwari (Thrombosis Research Institute, London, UK). Rebecca Watkin (Thrombosis Research Institute, London, UK) provided drafts and editorial assistance during the preparation of this manuscript. We thank the physicians, nurses, and patients involved in the ETHIC trial.
Acknowledgments
Contributors
All authors contributed to study design, study execution, and data collection. SV analysed the data. KP supervised the data analysis and provided the interpretation of the results. SV and KP accessed and verified the underlying data. All authors contributed to drafting and critically reviewing the manuscript. AKK and GK were responsible for securing funding for the study. All authors approved the final draft of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
ETHIC investigators:
Ajit Avhad, Murillo Antunes, Ana Thereza Rocha, Jesus Gonzales Lama, Atul Abyankar, Adrian Paulo Morales Kormann, Louis Van Zyl, Upendra Kaul, Frances Adams, Ivan Aloysius, Matthew Capehorn, Pradeep Kumar, and Rajesh Mahajan
Supplementary Material
References
- 1.Bradbury CA, McQuilten Z. Anticoagulation in COVID-19. Lancet. 2022;399:5–7. doi: 10.1016/S0140-6736(21)02503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agnelli G. Prevention of venous thromboembolism in surgical patients. Circulation. 2004;110(suppl 1):IV4–I12. doi: 10.1161/01.CIR.0000150639.98514.6c. [DOI] [PubMed] [Google Scholar]
- 5.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oetjens MT, Luo JZ, Chang A, et al. Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID-19 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72:e695–e703. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrillo-Vega MF, Salinas-Escudero G, García-Peña C, Gutiérrez-Robledo LM, Parra-Rodríguez L. Early estimation of the risk factors for hospitalization and mortality by COVID-19 in Mexico. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares RCM, Mattos LR, Raposo LM. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo state, Brazil. Am J Trop Med Hyg. 2020;103:1184–1190. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosetto A, Castaman G, Rodeghiero F. Bleeders, bleeding rates, and bleeding score. J Thromb Haemost. 2013;11(suppl 1):142–150. doi: 10.1111/jth.12248. [DOI] [PubMed] [Google Scholar]
- 14.Proschan MA. Two-stage sample size re-estimation based on a nuisance parameter: a review. J Biopharm Stat. 2005;15:559–574. doi: 10.1081/BIP-200062852. [DOI] [PubMed] [Google Scholar]
- 15.Gould AL. Interim analyses for monitoring clinical trials that do not materially affect the type I error rate. Stat Med. 1992;11:55–66. doi: 10.1002/sim.4780110107. [DOI] [PubMed] [Google Scholar]
- 16.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes RD, de Barros e Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Li Y, Liu G, Su B. Intermediate-to-therapeutic versus prophylactic anticoagulation for coagulopathy in hospitalized COVID-19 patients: a systemic review and meta-analysis. Thromb J. 2021;19:91. doi: 10.1186/s12959-021-00343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 22.White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50:287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 24.Moll M, Zon RL, Sylvester KW, et al. VTE in ICU patients with COVID-19. Chest. 2020;158:2130–2135. doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA. 2021;326:1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Society of Hematology COVID-19 and VTE/anticoagulation: frequently asked questions. 2022. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
- 27.National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. 2022. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf [PubMed]
- 28.National Institute for Health and Care Excellence COVID-19 rapid guideline: managing COVID-19. 2022. https://www.nice.org.uk/guidance/ng191/resources/covid19-rapid-guideline-managing-covid19-pdf-51035553326 [PubMed]
- 29.Ramacciotti E, Barile Agati L, Calderaro D, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399:50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data will be reviewed by the Thrombosis Research Institute Leadership Committee. If accepted, a deidentified dataset containing the requested data elements and any necessary documentation will be provided for analytical purposes. The data will be shared via a secure portal. Data sharing will be available once combined analysis is complete, which will be on Oct 1, 2022. The study protocol can be found in the appendix (pp 14–68).