Abstract
Purpose
To evaluate the diagnostic performance of layer-specific cardiac MRI feature-tracking (FT) strain analysis in patients with acute myocarditis.
Materials and Methods
Seventy patients (mean age, 43 years ± 19 [SD]; 46 men) with clinically defined acute myocarditis and 42 healthy controls who underwent cardiac MRI from March 2014 to November 2018 were retrospectively analyzed. FT-based left ventricular peak systolic global longitudinal strain (GLS) and global circumferential strain (GCS) were assessed at subendocardial, midmyocardial, and subepicardial layers. The 2018 Lake Louise criteria (LLC) were assessed. Patients with myocarditis were dichotomized into two groups: those with preserved and those with reduced ejection fraction. For statistical analysis, unpaired t test, one-way analysis of variance, Pearson correlation, and receiver operating characteristic analysis were used.
Results
GLS and GCS values of all layers (eg, midmyocardial GCS: −21.3% ± 5.5 vs −28.0% ± 4.3; P < .001) were impaired in patients with myocarditis compared with controls. Only subepicardial GLS (−20.0% ± 3.3 vs −17.5% ± 3.3; P < .001) and midmyocardial GCS values (−28.0% ± 4.3 vs −23.1% ± 4.3; P < .001) could differentiate between controls and patients with preserved ejection fraction. Midmyocardial GCS correlated with inflammatory myocardial parameters (eg, late gadolinium enhancement percentage, r = 0.48, P < .001). Midmyocardial GCS (area under the receiver operating characteristic curve [AUC], 0.82) and subepicardial GLS (AUC, 0.77) had the highest diagnostic performance for acute myocarditis diagnosis (P < .05 against all other strain parameters). The diagnostic performance of the 2018 LLC was significantly improved by inclusion of these two strain parameters (AUC, 0.92 vs 0.97; P = .04).
Conclusion
Diagnostic performance of cardiac MRI FT strain was different between myocardial layers in acute myocarditis, with midmyocardial GCS and subepicardial GLS providing the highest diagnostic performance.
Keywords: MRI, Cardiac, Heart, Left Ventricle, Inflammation, Tissue Characterization, MR–Functional Imaging, Feature-Tracking Strain, Acute Myocarditis
Supplemental material is available for this article.
© RSNA, 2022
Keywords: MRI, Cardiac, Heart, Left Ventricle, Inflammation, Tissue Characterization, MR–Functional Imaging, Feature-Tracking Strain, Acute Myocarditis
Summary
The diagnostic performance of cardiac MRI feature-tracking strain analysis for acute myocarditis varied among myocardial layers, which might be explained by the pathophysiology of myocarditis.
Key Results
■ In multilayer strain analysis, midmyocardial circumferential strain (area under the receiver operating characteristic curve [AUC], 0.82) and subepicardial longitudinal strain (AUC, 0.77) had the highest diagnostic performance in patients with suspected acute myocarditis.
■ Diagnostic performance of 2018 Lake Louise criteria was significantly improved by inclusion of midmyocardial circumferential and subepicardial longitudinal strain (AUC, 0.92 vs 0.97; P = .04).
■ Only midmyocardial circumferential strain (−28.0% ± 4.3 vs −23.1% ± 4.3, P < .001) and subepicardial longitudinal strain (−20.0% ± 3.3 vs −17.5% ± 3.3, P < .001) could differentiate between controls and patients with myocarditis with preserved ejection fraction.
Introduction
Diagnosis of acute myocarditis can be challenging given its various causes and clinical manifestations, which range from asymptomatic courses to cardiogenic shock (1). Due to its ability to noninvasively characterize myocardial tissue, cardiac MRI has become the first-line imaging modality for the diagnostic workup of hemodynamically stable patients with clinically suspected acute myocarditis (2). Diagnosis of acute myocarditis at cardiac MRI can be established with the application of the Lake Louise criteria (LLC), which were updated in 2018 (3,4). Compared with the detection of nonischemic myocardial injury and edema, the detection of global or regional myocardial dysfunction can be more challenging in myocarditis. Most patients with uncomplicated acute myocarditis have a preserved left ventricular ejection fraction (LVEF) (5). Thus, systolic LV dysfunction is defined only as a supportive criterion in the current LLC (3). However, myocardial strain analysis using cardiac MRI feature tracking (FT) can improve the detection of myocardial dysfunction and provide additional prognostic information (6–8). Impaired strain parameters have also been described in acute myocarditis with preserved LVEF (6,8,9). In most of these studies, an endocardial layer approach was used for strain analysis (10). However, the LV wall consists of three distinct layers: the inner endocardium, the middle myocardium, and the outer epicardium (11,12). Recent cardiac MRI and echocardiographic studies described that multilayer strain analysis provides incremental diagnostic and prognostic value in patients with ischemic heart disease and heart failure (13–17). Some echocardiographic reports showed accentuated strain alterations at the subepicardial layer in myocarditis (18). However, a layer-specific approach using cardiac MRI FT has not yet been validated in acute myocarditis.
The objective of this study was to evaluate the diagnostic performance of cardiac MRI FT layer-specific strain analysis in patients with suspected acute myocarditis.
Materials and Methods
Study Sample
This study complies with the Declaration of Helsinki and was approved by the institutional ethics committee. The requirement for written informed consent was waived due to the retrospective study design. From March 2014 until November 2018, all patients with a clinically defined diagnosis of acute myocarditis according to the criteria of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases were included in this study (1). Main inclusion criteria were acute symptoms (acute chest pain or acute dyspnea) and clinical signs of acute myocardial injury (elevated troponin levels and/or abnormal electrocardiogram changes) with an accompanying clinically suspicious infectious constellation (laboratory parameters or medical history). Clinical evidence was the reference standard against which the diagnostic performance of traditional cardiac MRI and strain parameters was tested. This clinical validation approach was used as previously described (4,19–21). Healthy volunteers without known or clinically suspected cardiac disease who had undergone cardiac MRI for study control reasons were retrospectively identified. All controls had an unremarkable history of cardiovascular disease and showed normal cardiac MRI results without structural abnormalities.
Cardiac MRI Acquisition
All cardiac MRI investigations were performed with a clinical whole-body scanner (Ingenia 1.5 T; Philips Healthcare). A 16-channel torso coil with digital interface was used for signal reception. All patients underwent a routine cardiac MRI protocol for diagnostic workup of myocarditis. For functional myocardial assessment and strain analysis, electrocardiographically gated steady-state free precession cine images were acquired in short-axis, four-chamber, two-chamber, and three-chamber views (for technical parameters, see Table E1 [supplement]). To visualize myocardial edema and calculate T2 signal intensity ratio, T2-weighted short-tau inversion-recovery (STIR) sequences were performed. For late gadolinium enhancement (LGE) imaging, segmented inversion-recovery gradient-echo sequences were performed in short-axis, four-chamber, and two-chamber views. Native T1 and T2 mapping were acquired in end-diastole and short-axis view covering the apical, midventricular, and basal segments. Acquisition of T2 maps was applied by six-echo gradient spin-echo (ie, GraSE) sequence (22). For contrast enhancement, a single bolus of 0.2 mmol of gadobutrol (Gadovist; Bayer Healthcare) per kilogram of body weight was administered. T1 mapping was performed before and 10 minutes after contrast material administration using a standard 3(3)3(3)5 modified Look-Locker inversion recovery (ie, MOLLI) acquisition scheme (23).
Cardiac MRI Analysis
Image analysis was performed by a board-certified physician (J.A.L., 9 years of experience in cardiac MRI) using commercially available software (IntelliSpace Portal, version 10.1; Philips Medical System). All visual and quantitative analyses were performed according to recent recommendations of the Society for Cardiovascular Magnetic Resonance (24). Short-axis cine images were used to calculate LVEF and end-diastolic volume. Focal myocardial edema was evaluated by visual assessment of high signal intensities at T2 STIR sequences. Markers of diffuse myocardial edema were assessed semiquantitatively by calculating T2 signal intensity ratio and quantitatively by measuring T2 relaxation times (19). The presence and distribution of myocardial lesions was visually assessed on LGE images (25). LGE extent (LGE%) was calculated using the full width at half maximum technique in short-axis view. As a marker of myocardial injury, hyperemia, and inflammation, native T1 relaxation times were assessed as previously described (19). Hematocrit-corrected extracellular volume values were calculated as previously described (19,24).
LV layer-specific strain measurements were derived from cine images using dedicated software (QStrain, version 2.0, Medis Suite MR; Medis Medical Imaging Systems). Endocardial and epicardial contours were drawn in manually on cine images. Peak systolic global longitudinal strain (GLS) and strain rate values were obtained from cine images in four-chamber view; peak systolic global circumferential strain (GCS), peak systolic global radial strain, and strain rate values were derived from cine images in short-axis view (values were averaged using basal, midventricular, and apical short-axis sections). By default, GLS and GCS values were calculated by the software at three distinct layers (subendocardial, midmyocardial, and subepicardial). Additional analysis of 15 patients with myocarditis was performed by the first reader and a second reader (A.I., 5 years of experience in cardiac MRI) to assess intra- and interobserver reproducibility. The readers were blinded to patient history.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics (version 26; IBM), Prism (version 8.4.3; GraphPad Software), and MedCalc (version 18.11.3; MedCalc Software). Data were checked for normal distribution using Shapiro-Wilk test and visually using histograms. Patient characteristics are presented as means ± SDs or as absolute frequencies. Unpaired t test was used to compare continuous variables between patients with myocarditis and controls. Dichotomous variables were compared using the Fisher exact test. Patients with myocarditis were dichotomized into a group with preserved LVEF (>50%) and reduced LVEF (<50%) (26). One-way analysis of variance with Tukey post hoc test was used to compare strain values between dichotomized myocarditis groups and the control group. Correlation analysis was performed using Pearson correlation coefficient. Receiver operating characteristic (ROC) analysis was applied to calculate area under the ROC curve (AUC). Youden index was used to determine optimal cutoff values, and sensitivities and specificities were calculated. For the combination of the 2018 LLC with single predictive variables, new scores were derived on the basis of logistic regression analysis. Differences between ROC curves were tested using the DeLong method. Intraclass correlation coefficients (ICCs) were applied to assess intra- and interobserver reproducibility. P value less than .05 was considered indicative of statistically significant difference.
Results
Sample Characteristics
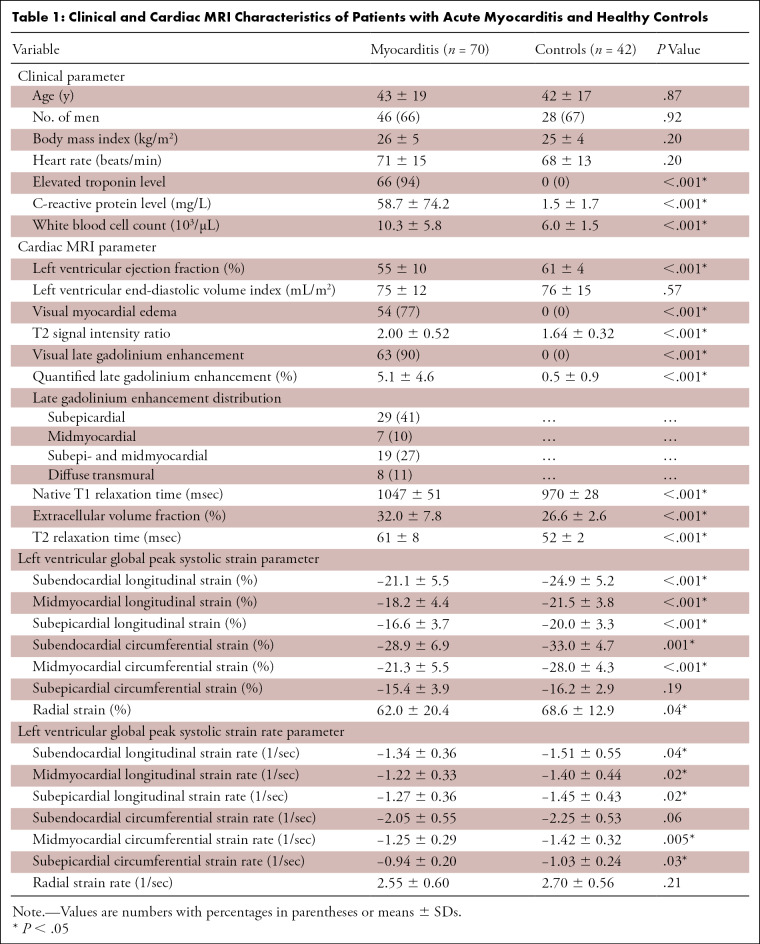
Seventy patients with suspected acute myocarditis (mean age, 43 years ± 19; 46 men) and 42 controls (mean age, 42 years ± 17; 28 men) were included. Patients underwent cardiac MRI 2.8 days ± 1.8 after admission. Detailed clinical characteristics are provided in Table 1.
Table 1:
Clinical and Cardiac MRI Characteristics of Patients with Acute Myocarditis and Healthy Controls
Cardiac MRI Results
There was a significant difference in LVEF between controls and patients with acute myocarditis (61% ± 4 vs 55% ± 10, P < .001). However, the vast majority of patients with myocarditis (55 of 70, 79%) had a preserved LVEF above 50%. Visual myocardial edema was present in 54 of 70 (77%) patients and in none of the controls (0%, P < .001). T2 signal intensity ratio was elevated in the myocarditis group compared with the control group (2.00 ± 0.52 vs 1.64 ± 0.32, P < .001). LGE lesions were present in 63 of 70 (90%) patients with myocarditis and in none of the controls (0%, P < .001). LGE was typically found in a nonischemic distribution (60 of 70 patients, 88%) and was primarily apparent at the subepicardium (29 of 70 patients, 41%) or combined at both the subepicardium and the midwall (19 of 70 patients, 27%). In seven of 70 (10%) patients, lesions were confined only to the midmyocardium. A diffuse pattern with transmural enhancement was observed in eight of 70 (11%) patients. Patients with myocarditis displayed higher T1 (1047 msec ± 51 vs 970 msec ± 28, P < .001) and T2 relaxation times (61 msec ± 8 vs 52 msec ± 2, P < .001), as well as extracellular volume values (32.0% ± 7.8 vs 26.6% ± 2.6, P < .001). Cardiac MRI parameters are given in Table 1.
Layer-specific Strain Results
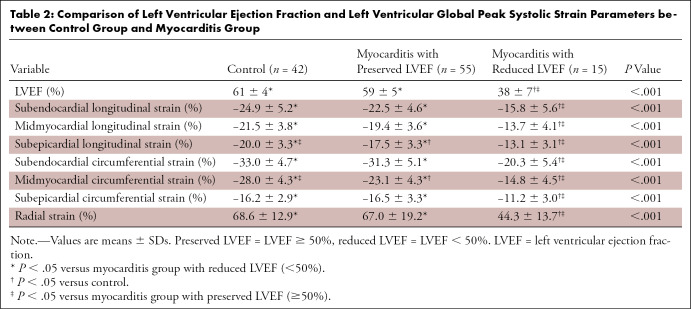
In both the myocarditis and control groups, GLS and GCS values showed a significant increase from the subepicardial layer to the midmyocardial and subendocardial layer (Table 1). Patients with myocarditis had impaired LV GLS values at the subepicardial (−16.6% ± 3.7 vs −20.0% ± 3.3, P < .001), midmyocardial (−18.2% ± 4.4 vs −21.5% ± 3.8, P < .001), and subendocardial layers (−21.1% ± 5.5 vs −24.9% ± 5.2, P = .001) compared with controls (Fig 1). Subendocardial and midmyocardial GCS values were reduced in the myocarditis group compared with controls (−28.9% ± 6.9 vs −33.0% ± 4.7, P = .001; and −21.3% ± 5.5 vs −28.0% ± 4.3, P < .001, respectively). A representative clinical example is shown in Figure 2. Subepicardial GLS (−20.0% ± 3.3 vs −17.5% ± 3.3, P < .001) and midmyocardial GCS (−28.0% ± 4.3 vs −23.1% ± 4.3, P < .001) were the only strain parameters that showed a significant difference between healthy controls and patients with preserved LVEF (Table 2).
Figure 1:
Box and whisker plots show peak systolic global longitudinal and circumferential strain values at different layers of the left ventricular myocardium in patients with myocarditis and controls.
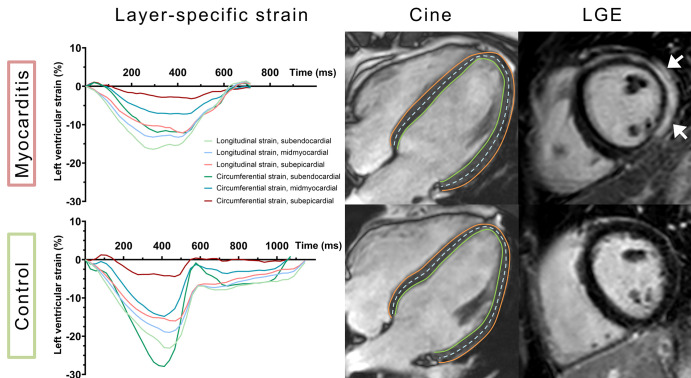
Figure 2:
Cardiac MR images show a clinical example of a 39-year-old man with acute myocarditis (upper row) and a healthy control (lower row). Coordinate axes display layer-specific curves of left ventricular peak systolic global longitudinal and circumferential strain, indicating reduced values in the patient with myocarditis. Non–contrast-enhanced cine images in four-chamber view demonstrate manually drawn contours at the subendocardial border (green line) and the subepicardial border (red line), as well as a representative line of the midmyocardium (dashed blue line). Late gadolinium enhancement (LGE) imaging in short-axis view shows distinct subepicardial and midmyocardial enhancement of the left ventricular lateral wall (white arrows).
Table 2:
Comparison of Left Ventricular Ejection Fraction and Left Ventricular Global Peak Systolic Strain Parameters between Control Group and Myocarditis Group
Good to excellent intra- and interobserver reproducibility was observed for multilayer strain assessment (eg, ICCs for subepicardial GLS: 0.90 [95% CI: 0.75, 0.97] and 0.88 [95% CI: 0.69, 0.96]; ICCs for midmyocardial GCS: 0.92 [95% CI: 0.79, 0.97] and 0.91 [95% CI: 0.80, 0.97], respectively) (Table E2 [supplement]).
Correlations between Strain and Other Cardiac MRI Parameters
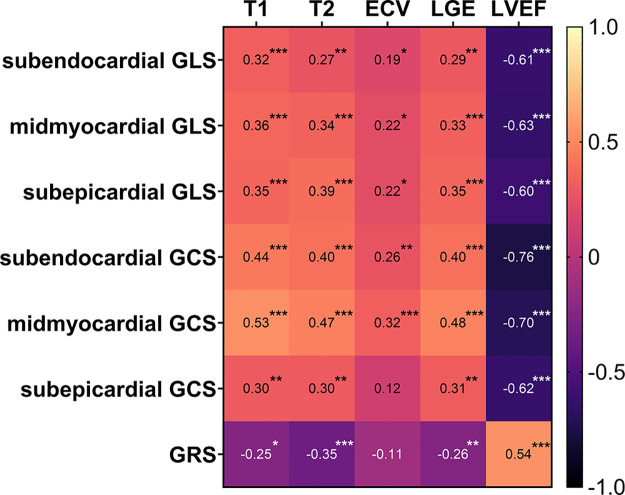
Correlation coefficients of layer-specific strain values and cardiac MRI parameters are provided in a correlation heatmap (Fig 3). Among all layer-specific strain parameters, midmyocardial GCS showed the strongest correlations with other cardiac MRI parameters (T1 relaxation time: r = 0.53, P < .001; T2 relaxation time: r = 0.47, P < .001; LGE%: r = 0.48, P < .001; LVEF: r = −0.70, P < .001). Correlations were also observed between subepicardial GLS (T1 relaxation time: r = 0.35, P < .001; T2 relaxation time: r = 0.39, P < .001; LGE%: r = 0.35, P < .001; LVEF: r = −0.60, P < .001), and midmyocardial GLS (T1 relaxation time: r = 0.36, P < .001; T2 relaxation time: r = 0.34, P < .001; LGE%: r = 0.33, P < .001, LVEF: r = −0.63, P < .001).
Figure 3:
Correlation heatmap shows Pearson correlation coefficient for layer-specific strain parameters and cardiac MRI parameters. Significance level of each correlation is indicated by asterisks: * = P < .05, ** = P < .01, *** = P < .001. ECV = extracellular volume fraction, GCS = global circumferential strain, GLS = global longitudinal strain, GRS = global radial strain, LGE = late gadolinium enhancement, LVEF = left ventricular ejection fraction, T1 = T1 relaxation time, T2 = T2 relaxation time.
Diagnostic Performance
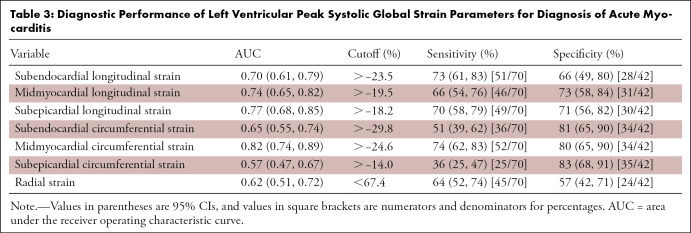
All sensitivity and specificity values, as well as calculated cutoff values, are provided in Table 3. In comparison to other layer-specific strain parameters, midmyocardial GCS (AUC, 0.82 [95% CI: 0.74, 0.89]), subepicardial GLS (AUC = 0.77 [95% CI: 0.68, 0.85]), and midmyocardial GLS (AUC = 0.74 [95% CI: 0.65, 0.82]) showed the highest diagnostic performance for diagnosis of acute myocarditis (Fig 4). Comparison of ROC curves showed a significant difference between midmyocardial GCS and midmyocardial GLS (∆AUC = 0.083 ± 0.039 [95% CI: 0.007, 0.159], P = .03) but not between subepicardial and midmyocardial GLS (ΔAUC = 0.033 ± 0.025 [95% CI: −0.015, 0.081], P = .18) or between midmyocardial GCS and subepicardial GLS (∆AUC = 0.050 ± 0.041 [95% CI: −0.030, 0.130], P = .22). Diagnostic performance of the 2018 LLC (AUC: 0.92 [95% CI: 0.87, 0.98]) was increased by the addition of midmyocardial GCS (AUC: 0.95 [95% CI: 0.91, 0.99], P = .18), subepicardial GLS (AUC: 0.97 [95% CI: 0.94, 1.00], P = .04), and by the addition of both strain parameters (AUC: 0.97 [95% CI: 0.94, 1.00], P = .04) (Fig 5).
Table 3:
Diagnostic Performance of Left Ventricular Peak Systolic Global Strain Parameters for Diagnosis of Acute Myocarditis
Figure 4:
![Graph shows receiver operating characteristic curves for different strain parameters: global longitudinal strain (GLS) at subendocardial (area under the receiver operating characteristic curve [AUC], 0.70), midmyocardial (AUC, 0.74), and subepicardial layer (AUC, 0.77); global circumferential strain (GCS) at subendocardial (AUC, 0.65), midmyocardial (AUC, 0.82), and subepicardial layer (AUC, 0.57); and global radial strain (GRS; AUC, 0.62).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3ab1/9274313/ad217cdabed0/ryct.210318.fig4.jpg)
Graph shows receiver operating characteristic curves for different strain parameters: global longitudinal strain (GLS) at subendocardial (area under the receiver operating characteristic curve [AUC], 0.70), midmyocardial (AUC, 0.74), and subepicardial layer (AUC, 0.77); global circumferential strain (GCS) at subendocardial (AUC, 0.65), midmyocardial (AUC, 0.82), and subepicardial layer (AUC, 0.57); and global radial strain (GRS; AUC, 0.62).
Figure 5:
![Graph shows receiver operating characteristic curves for 2018 Lake Louise criteria (LLC) (area under the receiver operating characteristic curve [AUC], 0.92) and for the respective combination of 2018 LLC with global midmyocardial circumferential strain (AUC, 0.95) and with global subepicardial longitudinal strain (AUC, 0.97), as well as with both strain parameters (AUC, 0.97). GCS = global circumferential strain, GLS = global longitudinal strain.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3ab1/9274313/01ab27a10d0f/ryct.210318.fig5.jpg)
Graph shows receiver operating characteristic curves for 2018 Lake Louise criteria (LLC) (area under the receiver operating characteristic curve [AUC], 0.92) and for the respective combination of 2018 LLC with global midmyocardial circumferential strain (AUC, 0.95) and with global subepicardial longitudinal strain (AUC, 0.97), as well as with both strain parameters (AUC, 0.97). GCS = global circumferential strain, GLS = global longitudinal strain.
Discussion
The present cardiac MRI FT-based study reveals the diagnostic benefits of a multilayer strain assessment in patients with acute myocarditis. Among all evaluated strain parameters, midmyocardial GCS (AUC, 0.82) and subepicardial GLS (AUC, 0.77) showed the highest diagnostic performance. Both strain parameters correlated with parameters of myocardial inflammation, especially T1 and T2 relaxation times and LGE%. Even in patients with preserved LVEF and in patients without positive LGE, midmyocardial GCS and subepicardial GLS were altered, indicating systolic dysfunction. Inclusion of midmyocardial GCS and subepicardial GLS significantly improved the diagnostic performance of 2018 LLC (AUC, 0.92 vs 0.97; P = .04).
According to cardiac physiology, we found a subepicardial-to-subendocardial strain gradient across the heart wall in both groups. Midmyocardial and subepicardial strain parameters were more affected than subendocardial layers in acute myocarditis. On the basis of common anatomic and pathophysiologic concepts, it can be assumed that myocardial dysfunction depends on the predominantly affected layer in different cardiac diseases (eg, predominantly subendocardial involvement in ischemic cardiac disease and subepicardial or midwall involvement in inflammatory disease). Although LV myocardial layers are coupled and the contraction process is complex, myocardial dysfunction can be principally classified according to the involved layer into subendocardial, transmural, or subepicardial myocardial dysfunction (27).
Our results are in general accordance with previous cardiac MRI FT-based studies that found incremental value of multilayer strain assessment for the identification of systolic dysfunction in patients with heart failure (13,14,17) or noncompaction cardiomyopathy (28). For the detection of different stages of heart failure, highest performance was achieved by GLS and GCS at endocardial level (13); however, heart failure with preserved EF was best diagnosed with epicardial GLS (17). Interestingly, a recent echocardiography-based multilayer strain study in patients after ST-segment myocardial infarction showed that reduced GLS at the epicardium (likely reflecting transmural ischemic scar) was associated with all-cause mortality.
Furthermore, a speckle-tracking echocardiography study reported that subepicardial GLS could improve detection of LV systolic dysfunction in patients with history of acute myocarditis (29). Another echocardiography study found comparable correlations between GLS and presence of myocardial edema at both the endocardial and epicardial borders, but no correlation between layer-specific strain parameters and the amount of myocardial edema (30). However, it must be noted that cardiac MRI FT and speckle-tracking echocardiography strain assessment are based on different tracking methods, so comparability of the techniques is limited (10).
In addition to the pathophysiologic explanation on the basis of inflammatory myocardial disease, our findings could also be explained by the physiologic arrangement of the LV myofibers. Common models of the LV depict a counterdirectional myofiber arrangement with a gradual change from a right-handed helical geometry in the subendocardium into a left-handed orientation in the subepicardium (31). Furthermore, the myofibers are predominantly arranged longitudinally in the subendocardium and subepicardium, while circumferentially in the midwall (11,12). These aspects might explain why the highest diagnostic performance was observed at the midmyocardial and subepicardial layers for circumferential and longitudinal strain, respectively. A proposed pathogenic hypothesis is provided in Figure 6.
Figure 6:
Illustration schematically shows the localization of cardiac involvement in acute myocarditis on the basis of a simplified representation of heart wall layer composition. Inflammatory myocardial necrosis (red areas) is typically located at the subepicardium and the midwall, sparing the subendocardium. Simplified, the left ventricular wall is composed of three distinct layers: the inner endocardium, the middle myocardium, and the outer epicardium. Myofibers are predominantly longitudinally oriented (dot pattern) in the endocardium and epicardium and predominantly circumferentially oriented (line pattern) in the middle myocardium. Double-sided arrows represent vectors of longitudinal strain (purple), circumferential strain (blue), and radial strain (green). The figure contains modified free medical images from Servier Medical Art (https://smart.servier.com) under a CC BY 3.0 license.
We found significant correlations between midmyocardial GCS and subepicardial GLS with quantitative markers of myocardial inflammation, which is in line with previous cardiac MRI FT studies that used a different software and endocardial-layer approach in patients with acute myocarditis (6,8). The strongest correlations were observed between midmyocardial GCS and T1 and T2 relaxation times, which indicate a link between systolic dysfunction and acute myocardial injury. Furthermore, significant correlations were observed between strain parameters and the extent of LGE, implicating an association between strain parameters and myocardial necrosis. Interestingly, T2 relaxation times—a highly sensitive marker of myocardial edema—and LGE extent were more strongly correlated with subepicardial GLS than subendocardial GLS. These findings consolidate the thesis that strain parameters of different layers might have different diagnostic value depending on the myocardial layer affected by the respective disease. In acute myocarditis, strain values of the midwall and subepicardium affected by inflammation might be more reduced compared with the unaffected subendocardial layer.
According to previous studies, not only patients with reduced LVEF but also patients with preserved LVEF had impaired strain parameters (6,32). Notably, midmyocardial GCS and subepicardial GLS were the only layer-specific strain parameters that showed group differences between patients with myocarditis with preserved LVEF and controls. These findings suggest that strain imaging may be more sensitive than LVEF to detect mild systolic functional abnormalities (eg, in patients with only subtle or focal myocarditis).
Of all evaluated layer-specific strain parameters, midmyocardial GCS and subepicardial GLS showed the highest diagnostic performance for the diagnosis of acute myocarditis. The addition of both strain parameters to the 2018 LLC significantly improved the diagnostic performance of multiparametric cardiac MRI. Interestingly, the addition of subepicardial GLS alone also enhanced diagnostic performance. As LVEF alone is often preserved in acute myocarditis, it is considered only as a supportive marker in the 2018 LLC. The improved detection of systolic dysfunction by layer-specific strain, however, might further improve future diagnostic criteria.
Cardiac MRI FT multilayer strain may be more sensitive for detecting systolic dysfunction than the commonly used endocardial approach and has the potential to improve current diagnostic criteria. Prospective studies are needed to evaluate the diagnostic and prognostic value of layer-specific strain parameters in myocarditis, including chronic and arrhythmogenic forms. The layer-specific approach might also enable and promote contrast-free investigations, such as in the setting of acute myocardial injury, as there are distinct differences in the pattern of myocardial dysfunction between possible differential diagnoses (myocardial infarction, myocarditis, takotsubo syndrome) (33). Although only patients with acute myocarditis were investigated in our study, layer-specific strain might also improve the detection of myocardial dysfunction in other cardiomyopathies. The technique might also be used for an early detection of incipient myocardial dysfunction associated with inflammatory myocardial processes in patients with systemic diseases or in patients receiving systemic therapies (eg, immunotherapy or chemotherapy) (34,35).
Our study had some limitations. Due to lack of endomyocardial biopsy, no histopathologic reference standard was available, and diagnosis of acute myocarditis was clinically defined, as was also done in other studies (4,19,21). Additionally, our cohort consisted mainly of patients with acute infarct-like myocarditis; therefore, generalizability to other types of myocarditis (eg, subacute or chronic myocarditis) is limited (2). Furthermore, the use of a healthy control group may have led to an overestimation of the diagnostic performance of MRI parameters in acute myocarditis. Finally, although good inter- and intrareader reproducibility was achieved, assessment of layer-specific strain can vary among different readers and centers depending on hardware and software.
In conclusion, in patients with acute myocarditis, a layer-specific cardiac MRI FT strain analysis may be more sensitive than the common endocardial approach, which might be pathophysiologically explained. Multilayer cardiac MRI FT-derived midmyocardial GCS and subepicardial GLS showed the highest diagnostic performance for acute myocarditis assessment, and diagnostic performance of the 2018 LLC was significantly improved by the addition of subepicardial GLS. Even in patients with preserved LVEF or without positive LGE, impairment of both strain parameters indicated systolic dysfunction. Multilayer cardiac MRI FT strain might further improve diagnostic use of cardiac MRI in the future.
A.I. supported by BONFOR-Forschungskommission der Medizinischen Fakultät Bonn and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy–EXC2151–390873048.
Disclosures of conflicts of interest: A.I. Funded by BONFOR-Forschungskommission der Medizinischen Fakultät Bonn and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy, EXC2151–390873048; funders had no influence on study conceptualization and design, collection and analysis of the data, manuscript preparation, or the decision to publish. D. Kravenchko No relevant relationships. N.M. No relevant relationships. C.E. No relevant relationships. L.M.B. No relevant relationships. T.V. No relevant relationships. D.T. No relevant relationships. D.D. No relevant relationships. S.Z. No relevant relationships. U.A. No relevant relationships. D. Kuetting No relevant relationships. J.A.L. Received payments for lectures from Philips Healthcare and for activities related to the scientific advisory board for Bayer HealthCare.
Abbreviations:
- AUC
- area under the ROC curve
- FT
- feature tracking
- GCS
- global circumferential strain
- GLS
- global longitudinal strain
- ICC
- intraclass correlation coefficient
- LGE
- late gadolinium enhancement
- LGE%
- LGE extent
- LLC
- Lake Louise criteria
- LVEF
- left ventricular ejection fraction
- ROC
- receiver operating characteristic
- STIR
- short-tau inversion recovery
References
- 1. Caforio ALP , Pankuweit S , Arbustini E , et al . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Eur Heart J 2013. ; 34 ( 33 ): 2636 – 2648 . 2648a–2648d . [DOI] [PubMed] [Google Scholar]
- 2. Ammirati E , Frigerio M , Adler ED , et al . Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document . Circ Heart Fail 2020. ; 13 ( 11 ): e007405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferreira VM , Schulz-Menger J , Holmvang G , et al . Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations . J Am Coll Cardiol 2018. ; 72 ( 24 ): 3158 – 3176 . [DOI] [PubMed] [Google Scholar]
- 4. Luetkens JA , Faron A , Isaak A , et al . Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort . Radiol Cardiothorac Imaging 2019. ; 1 ( 3 ): e190010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tschöpe C , Ammirati E , Bozkurt B , et al . Myocarditis and inflammatory cardiomyopathy: current evidence and future directions . Nat Rev Cardiol 2021. ; 18 ( 3 ): 169 – 193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luetkens JA , Schlesinger-Irsch U , Kuetting DL , et al . Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema . Eur Radiol 2017. ; 27 ( 11 ): 4661 – 4671 . [DOI] [PubMed] [Google Scholar]
- 7. Fischer K , Obrist SJ , Erne SA , et al . Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features . JACC Cardiovasc Imaging 2020. ; 13 ( 9 ): 1891 – 1901 . [DOI] [PubMed] [Google Scholar]
- 8. Luetkens JA , Petry P , Kuetting D , et al . Left and right ventricular strain in the course of acute myocarditis: a cardiovascular magnetic resonance study . Rofo 2018. ; 190 ( 8 ): 722 – 732 . [DOI] [PubMed] [Google Scholar]
- 9. Baeßler B , Schaarschmidt F , Dick A , Michels G , Maintz D , Bunck AC . Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis . Eur J Radiol 2016. ; 85 ( 1 ): 218 – 227 . [DOI] [PubMed] [Google Scholar]
- 10. Pedrizzetti G , Claus P , Kilner PJ , Nagel E . Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use . J Cardiovasc Magn Reson 2016. ; 18 ( 1 ): 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenbaum RA , Ho SY , Gibson DG , Becker AE , Anderson RH . Left ventricular fibre architecture in man . Br Heart J 1981. ; 45 ( 3 ): 248 – 263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez-Tendero A , Zhang C , Balicevic V , et al . Whole heart detailed and quantitative anatomy, myofibre structure and vasculature from X-ray phase-contrast synchrotron radiation-based micro computed tomography . Eur Heart J Cardiovasc Imaging 2017. ; 18 ( 7 ): 732 – 741 . [DOI] [PubMed] [Google Scholar]
- 13. Tanacli R , Hashemi D , Lapinskas T , et al . Range variability in CMR feature tracking multilayer strain across different stages of heart failure . Sci Rep 2019. ; 9 ( 1 ): 16478 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu L , Pagano JJ , Haykowksy MJ , et al . Layer-specific strain in patients with heart failure using cardiovascular magnetic resonance: not all layers are the same . J Cardiovasc Magn Reson 2020. ; 22 ( 1 ): 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarvari SI , Haugaa KH , Zahid W , et al . Layer-specific quantification of myocardial deformation by strain echocardiography may reveal significant CAD in patients with non-ST-segment elevation acute coronary syndrome . JACC Cardiovasc Imaging 2013. ; 6 ( 5 ): 535 – 544 . [DOI] [PubMed] [Google Scholar]
- 16. Skaarup KG , Iversen A , Jørgensen PG , et al . Association between layer-specific global longitudinal strain and adverse outcomes following acute coronary syndrome . Eur Heart J Cardiovasc Imaging 2018. ; 19 ( 12 ): 1334 – 1342 . [DOI] [PubMed] [Google Scholar]
- 17. Tanacli R , Hashemi D , Neye M , et al . Multilayer myocardial strain improves the diagnosis of heart failure with preserved ejection fraction . ESC Heart Fail 2020. ; 7 ( 5 ): 3240 – 3245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caspar T , Germain P , El Ghannudi S , et al . Acute myocarditis diagnosed by layer-specific 2d longitudinal speckle tracking analysis . Echocardiography 2016. ; 33 ( 1 ): 157 – 158 . [DOI] [PubMed] [Google Scholar]
- 19. Luetkens JA , Doerner J , Thomas DK , et al . Acute myocarditis: multiparametric cardiac MR imaging . Radiology 2014. ; 273 ( 2 ): 383 – 392 . [DOI] [PubMed] [Google Scholar]
- 20. Kotanidis CP , Bazmpani MA , Haidich AB , Karvounis C , Antoniades C , Karamitsos TD . Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta-analysis . JACC Cardiovasc Imaging 2018. ; 11 ( 11 ): 1583 – 1590 . [DOI] [PubMed] [Google Scholar]
- 21. Isaak A , Bischoff LM , Faron A , et al . Multiparametric cardiac magnetic resonance imaging in pediatric and adolescent patients with acute myocarditis . Pediatr Radiol 2021. ; 51 ( 13 ): 2470 – 2480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sprinkart AM , Luetkens JA , Träber F , et al . Gradient spin echo (GraSE) imaging for fast myocardial T2 mapping . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messroghli DR , Radjenovic A , Kozerke S , Higgins DM , Sivananthan MU , Ridgway JP . Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart . Magn Reson Med 2004. ; 52 ( 1 ): 141 – 146 . [DOI] [PubMed] [Google Scholar]
- 24. Schulz-Menger J , Bluemke DA , Bremerich J , et al . Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing . J Cardiovasc Magn Reson 2020. ; 22 ( 1 ): 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isaak A , Praktiknjo M , Jansen C , et al . Myocardial fibrosis and inflammation in liver cirrhosis: MRI study of the liver-heart axis . Radiology 2020. ; 297 ( 1 ): 51 – 61 . [DOI] [PubMed] [Google Scholar]
- 26. Aquaro GD , Perfetti M , Camastra G , et al . Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study . J Am Coll Cardiol 2017. ; 70 ( 16 ): 1977 – 1987 . [DOI] [PubMed] [Google Scholar]
- 27. Claus P , Omar AMS , Pedrizzetti G , Sengupta PP , Nagel E . Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications . JACC Cardiovasc Imaging 2015. ; 8 ( 12 ): 1444 – 1460 . [DOI] [PubMed] [Google Scholar]
- 28. Zhang J , Jiang M , Zheng C , et al . Evaluation of isolated left ventricular noncompaction using cardiac magnetic resonance tissue tracking in global, regional and layer-specific strains . Sci Rep 2021. ; 11 ( 1 ): 7183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caspar T , Fichot M , Ohana M , El Ghannudi S , Morel O , Ohlmann P . Late detection of left ventricular dysfunction using two-dimensional and three-dimensional speckle-tracking echocardiography in patients with history of nonsevere acute myocarditis . J Am Soc Echocardiogr 2017. ; 30 ( 8 ): 756 – 762 . [DOI] [PubMed] [Google Scholar]
- 30. Løgstrup BB , Nielsen JM , Kim WY , Poulsen SH . Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis . Eur Heart J Cardiovasc Imaging 2016. ; 17 ( 9 ): 1018 – 1026 . [DOI] [PubMed] [Google Scholar]
- 31. Sengupta PP , Korinek J , Belohlavek M , et al . Left ventricular structure and function: basic science for cardiac imaging . J Am Coll Cardiol 2006. ; 48 ( 10 ): 1988 – 2001 . [DOI] [PubMed] [Google Scholar]
- 32. Doerner J , Bunck AC , Michels G , Maintz D , Baeßler B . Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis . Eur J Radiol 2018. ; 104 : 120 – 128 . [DOI] [PubMed] [Google Scholar]
- 33. Pathik B , Raman B , Mohd Amin NH , et al . Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging . Eur Heart J Cardiovasc Imaging 2016. ; 17 ( 10 ): 1146 – 1152 . [DOI] [PubMed] [Google Scholar]
- 34. Oikonomou EK , Kokkinidis DG , Kampaktsis PN , et al . Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis . JAMA Cardiol 2019. ; 4 ( 10 ): 1007 – 1018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Faron A , Isaak A , Mesropyan N , et al . Cardiac MRI depicts immune checkpoint inhibitor-induced myocarditis: a prospective study . Radiology 2021. ; 301 ( 3 ): 602 – 609 . [DOI] [PubMed] [Google Scholar]