Abstract
Cisplatin is one of the most widely used chemotherapeutic anti-cancer drugs that is associated with multiple systemic toxicities limiting its use. The present study aimed to evaluate the hepato-protective effect of hesperidin against cisplatin-induced toxicity. Thirty-two adult male albino rats were equally split into four groups, the first group served as control received normal saline, the second group (CIS) received a single intraperitoneal dose of cisplatin (7.5 mg/kg bw) on the 22nd day of the experiment, the third group (HES) treated once daily with hesperidin (200 mg/kg bw, orally) for 21 days, and the last group (HES + CIS) pretreated once daily with hesperidin followed by a single intraperitoneal dose of cisplatin. Twenty-four hours later, samples were collected for further investigations. CIS-intoxication resulted in a significant decrease in the erythrogram along with thrombocytopenia leukopenia, and lymphopenia. Furthermore, CIS administration significantly elevated serum activity of liver enzymes, total, and indirect bilirubin as well serum glucose, total cholesterol, and triglycerides levels, meanwhile serum total protein, and globulin levels were significantly reduced. The hepatic MDA was markedly elevated with a concomitant decline in the hepatic antioxidant enzymes and severe alterations in the hepatic tissue architecture in CIS-intoxicated rats. Additionally, CIS-induced overexpression of hepatic Bax, caspase-3, and TNF-α, with no effect on hepatic expression of IL-10. Interestingly, HES pretreatment improved the CIS-induced hemato-biochemical, molecular and histopathological alterations. In conclusion, hesperidin hepato-protective effects against CIS might be mediated by its antioxidant, anti-inflammatory, and anti-apoptotic properties.
Keywords: Cisplatin, Hesperidin, Hepatic oxidative stress, Inflammation, Apoptosis
1. Introduction
Cisplatin (CIS) is one of the most common and potent anti-neoplastic agents used against a broad range of malignancies including testicular, ovarian, cervical, bladder, head, neck as well as the lung. Despite its beneficial application as an anti-neoplastic agent, CIS causes several toxicities that limit its clinical use including nephrotoxicity, hepatotoxicity, cardiotoxicity, neurotoxicity, and ototoxicity (Hassan et al., 2020, Yadav, 2019, Neamatallah et al., 2018). Many experimental studies documented the common toxic side effects associated with CIS, especially nephrotoxicity (Tahoon, 2017). Besides, hepatotoxicity has been identified as a significant dose-limiting side effect of CIS-based chemotherapy (Apaydin et al., 2018, Omar et al., 2016a, İşeri et al., 2007;) whereas CIS accumulates in the liver at a significant amount secondly to kidney (Liao et al., 2008). The CIS toxicity is developed by the generation of reactive oxygen species (ROS) such as superoxide anion and hydroxyl radicals leading to elevation in lipid peroxidation and damage of cells. Excess ROS induces cell arrest and apoptosis in both cancer cells and non-target normal cells (Galadari et al., 2017, Palipoch et al., 2014).
Recently a lot of attention has been focused on the protective role and mechanism of action of naturally occurring compounds that have antioxidants and anti-inflammatory properties as bioflavonoids that are widely available for dietary intake. Hesperidin (HES) (hesperetin- 7-rutinoside), one of the most biologically active compounds in the flavonoid family, is a flavanone glycoside found at high levels in citrus fruits such as lemons, limes, and oranges. It has been reported to exhibit a wide range of pharmacological effects, including: anti-carcinogenic, anti-inflammatory, and antioxidant activities via preventing oxidant injury and cell death by several mechanisms including; scavenging oxygen radicals, protecting against lipid peroxidation, and chelating metal ions (Omar et al., 2016b, Kamel et al., 2014, Bentli et al., 2013, Tanaka et al., 2012). There were few previous studies on the effect of hesperidin as a natural flavonoid against CIS-induced hepatotoxicity, at the time that many results proved the protective role of HES against CIS-induced nephrotoxicity (Mohamed et al., 2015, Adikay et al., 2012). Therefore, the focus of the current study was to address the ability of HES pretreatment to relive CIS induced hepatotoxicity by evaluating blood picture, liver function and hepatic oxidative damage biomarkers in combination with the gene expression of hepatic pro-inflammatory and anti-inflammatory biomarker. Also, histopathological changes and immunohistochemical expressions of apoptosis marker in rat liver were detected.
2. Material and methods
2.1. Chemicals
Hesperidin (>80%, Product No.: H5254) was purchased from Sigma Chemical Company (St. Louis, MO, USA) and cisplatin (Cis-Diamminedichloroplatinum (II), Pt 64.5 % min, Product No.: 10471) was purchased from Alfa Aesar, Germany. HES and CIS were dissolved immediately before use in physiological saline (0.9% sodium chloride).
2.2. Animals and experimental design
Thirty-two adult male healthy albino rats about 8 weeks of age, weighing about 200–230 g were obtained from the animal house, Faculty of Veterinary Medicine, Zagazig University (Zagazig, Egypt), housed 4 animals per cage and maintained at 25 ± 2 °C with free access to water and food, under a 12/12 h light–dark cycle. All procedures in this study were carried out according to the guidelines of the Animal Research Ethical Committee of the Faculty of Veterinary Medicine, Mansoura University, Egypt.
Rats were acclimatized for 2 weeks before the experiment and then randomly divided into equal four groups of 8 rats each as follows; first group (Control) served as a negative control group received saline orally (HES vehicle) once daily using gastric tube for 21 consecutive day followed by a single intraperitoneal injection with saline (CIS vehicle) on 22nd day, the second group (CIS) received saline orally once daily for 21 day followed by a single intraperitoneal dose of cisplatin (7.5 mg/kg) on the 22nd day of the experiment according to Neamatallah et al., (2018), the third group (HES) orally administered a daily dose of hesperidin (200 mg/kg) for 21 consecutive days according to Omar et al. (2016b) followed by a single intraperitoneal injection with saline, while the fourth group (HES + CIS) was pretreated orally once daily with hesperidin for 21 consecutive days followed by a single dose of cisplatin on the 22nd day of the experiment. All over the experiment, the animals were observed for any abnormal signs.
2.3. Sample collection and preparation
After 24 h from CIS injection, blood samples were collected from the medial canthus of the eye under anesthesia (mixture of ketamine and xylazine at dose 50 and 10 mg/kg respectively by IP Injection) and put into blood collection tubes either with anticoagulant (dipotassium salt of EDTA) for hematological parameters or without anticoagulant, that was allowed to stand for half an hour until blood clotted, left in the refrigerator for retraction of clot for 4 h, centrifuged to separate the serum and finally stored at −20 °C to be used for estimation of various biochemical parameters.
Rats were then euthanized by cervical dislocation, and the liver was dissected out, rinsed with ice-cold phosphate-buffered saline (PBS), and dried between two filter papers. To produce hepatic tissue homogenates,1 g of hepatic tissue was homogenized in an ice-cold PBS (pH 7.4), the homogenates were centrifuged at 825 × g for 15 min at 4 °C and the clear supernatants were removed and stored at −20 °C to be used later for the estimation of hepatic MDA and antioxidant parameters. Another portion of the liver was collected for gene expression and preserved at − 80 °C. Specimens from the liver were fixed in 10% neutral buffered formalin for histopathological and immunohistochemical studies.
2.4. Hematological studies
Whole blood samples were used for counting the erythrocytes (RBCs), leukocytes (TLC), platelets, and for estimating hemoglobin (Hb) concentration, and packed cell volume (PCV). Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated. Moreover, stained blood smears for manual differential leukocyte count were prepared as previously described (Feldman et al., 2000).
2.5. Assay of serum biochemical parameters
The collected frozen serum samples were analyzed for alanine transaminase (ALT, Cat No: 20764957), aspartate transaminase (AST, Cat No: 20764949), alkaline phosphatase (ALP, Cat No: 03333752), total bilirubin (Cat No: 03146022), direct bilirubin (Cat No: 05589061), total protein (Cat: 03183734), albumin (Cat No: 03183688), glucose (CAT No: 04404483), cholesterol (Cat: 03039773) and triglycerides (Cat No: 20767107) were estimated on Cobas Integra 400 plus analyzer (Roche Diagnostics, Germany); each with its specific reagent per manufacturer's instructions. All kits were obtained from Roche Diagnostics, Germany.
2.6. Assay of lipid peroxidation and antioxidant status in the hepatic homogenate
The levels of malondialdehyde (MDA, Cat No: MD2529), catalase (CAT, Cat No: 2517), reduced glutathione (GSH, Cat No: TA2511), and superoxide dismutase (SOD, Cat No: SD2521) were assessed in the liver homogenates using commercially available kits (Biodiagnostic, Egypt) following the manufacturer's instructions.
2.7. Gene expression analysis
According to the manufacturer's instructions, extraction of RNA was done using RNeasy Mini Kit (Qiagen company, Hilden, Germany). The purity of RNA samples was verified by measuring their absorbance using Nano Drop spectrophotometer, ND-1000 (Thermo Scientific, USA) at 260 and 280 nm. Later, the extracted RNA was used to synthesize cDNA using cDNA synthesis kit (TOP script™ RT DryMIX, Enzynomics Co. Ltd, Korea). Moreover, relative expression of IL-10 and TNF-α were performed by real-time PCR thermal cycler (Stratagene MX3005P) using SYBR Green PCR Master Mix (Quantitect SYBR green PCR kit, Qiagen company, Hilden, Germany). Primer sequences of rat IL-10 (Shynlova et al., 2014) and TNF- α (Khan et al., 2013) are shown in Table 1. β-actin (Banni et al., 2010) was used as a reference housekeeping gene to normalize the expression of target genes. The reaction mixture was carried out in a total volume of 25 μl consisted of 12.5 μl of QuantiTect SYBR Green PCR Master Mix, 0.5 μl of reverse transcriptase, 8.25 μl of RNase free water, 0.5 μl of forward primer, and the same for the reverse primer, and finally 3 μl of cDNA as template. The SYBR green real-time PCR cycling conditions were as follows: reverse transcription at 50 °C for 30 min. then primary denaturation at 94 °C for 15 min. followed by 40 cycles of 94 °C for 15 sec, annealing at 60 °C for 30 sec, and extension at 72 °C for 30 sec. At the end of the amplification phase, a dissociation curve (1 cycle of 94 °C for 1 min then 60 °C for 1 min, and finally 94 °C for 1 min) was performed. Amplification curves and CT values were determined by the Stratagene MX3005P software. Relative expression on the target genes was calculated by using the 2- ΔΔCt method (Yuan et al., 2006).
Table 1.
Primer sequences for RT-PCR.
| Gene | Primer sequence(5′-3′) |
|---|---|
| Rat β-actin | TCCTCCTGAGCGCAAGTACTCT |
| GCTCAGTAACAGTCCGCCTAGAA | |
| IL10 | GCGGCTGAGGCGCTGTCAT |
| CGCCTTGTAGACACCTTGGTCTTGG | |
| TNF-α | CACCAGCTCTGAACAGATCATGA |
| TCAGCCCATCTTCTTCCAGATGGT |
2.8. Histopathological examination
The formalin-fixed liver specimen was embedded in paraffin, sectioned in 5-µm thickness, stained by hematoxylin and eosin (H&E) stain, and examined under the microscope according to Bancroft and Gamble, (2008). Captured sections from all tested groups were subjected to careful histopathological examination and scoring (0, absent; 1, spotty necrosis; one or few necrotic hepatocytes; 2, confluent necrosis; 3, bridging necrosis) according to González‐Périz et al. (2006).
2.9. Immunohistochemistry of Bax and Caspase-3 expression
Immunohistochemistry of the formalin fixed, and paraffin embedded liver sections were carried out as described by (Elshopakey and Elazab, (2021). After deparaffinization the tissue sections, potassium citrate (pH, 6) was used for the antigen retrieval. the polyclonal mice anti-rat primary antibody (Biogenex, Fremont, CA) against BAX (1: 250) and monoclonal rabbit anti-mouse primary antibody (Biocare Medical, Pacheco, CA) against caspase-3 (1:100). Incubated overnight at 4 °C, followed by washing by TBS (tris buffer saline) and then incubated with HRP (Horse radish peroxidase) conjugated donkey anti-mouse (1:000) and goat anti-rabbit (1:1000) corresponding to their primary antibodies, for 1 h at room temperature, the developing was done for 1.5 min by 3, 3′-diaminobenzidine tetrahydrochloride (DAB) and counterstained by hematoxylline then coverslipping was done.The pattern of distribution for the immuno-positive cells in the hepatic lobule were semi-quantitative analyzed using light microscopy (Nikon Eclipse TE2000-U, NIKON, Japan) and the number of them per 1000 cell were counted and analyzed with Image-J analysis software (Image J, 1.46a, NIH, USA).
2.10. Statistical analysis
Statistical analysis was carried out using a statistical software program (SPSS for Windows, version 20, USA). All values were presented as a mean ± standard error of the mean (SEM). Mean differences were compared by one-way analysis of variance (ANOVA), using post-hoc comparison by least significant difference method (LSD) and by Duncan multiple comparison tests. P < 0.05 denoted the presence of a statistically significant difference.
3. Results
3.1. Hesperidin relives CIS-induced abnormal clinical signs
Cisplatin-treated rats showed bloody diarrhea, nose bleeding, hemoglobinuria, and poor appetite. While treatment with hesperidin ameliorated all above mentioned clinical signs but they did not return to normalcy.
3.2. Hesperidin ameliorates the CIS-induced hematological alterations
Alterations in blood picture of rats exposed to CIS was demonstrated by the significant reduction in the RBCs count, Hb concentration, PCV %, and blood counts of leukocytes, lymphocytes, and platelets compared to the control group (Table 2). HES pretreatment improved the previous hematological changes but without returning them to normal values. Moreover, HES treatment had no effect on all hematological parameters.
Table 2.
Effect of hesperidin on the hematological parameters in cisplatin-intoxicated rats.
| Parameters | Treatments |
|||
|---|---|---|---|---|
| Control | CIS | HES | HES + CIS | |
| RBCs (106/µL) | 7.34 ± 0.16 a | 5.61 ± 0.24c | 7.26 ± 0.30 a | 6.41 ± 0.13b |
| Hb (g/dl) | 13.95 ± 0.59 a | 10.17 ± 0.11c | 14.45 ± 0.25 a | 12.15 ± 0.25b |
| PCV (%) | 40.55 ± 0.49 a | 29.25 ± 0.47c | 42.92 ± 2.79 a | 35.00 ± 1.04b |
| MCV (fl) | 55.36 ± 1.74 a | 52.44 ± 2.46 a | 58.92 ± 1.65 a | 54.69 ± 2.55 a |
| MCH (pg) | 18.98 ± 0.49 a | 18.24 ± 0.81 a | 19.95 ± 0.59 a | 18.97 ± 0.65 a |
| MCHC (%) | 34.43 ± 1.65 a | 34.81 ± 0.64 a | 34.03 ± 2.01 a | 34.75 ± 0.74 a |
| Platelets (103/µL) | 686.00 ± 16.43a | 473.25 ± 20.80c | 637.50 ± 16.45a | 549.75 ± 21.41b |
| TLC (103/µL) | 13.60 ± 0.30 a | 6.59 ± 0.08c | 13.27 ± 0.41 a | 7.73 ± 0.16b |
| Neutrophil (103/µL) | 3.33 ± 0.04 a | 3.35 ± 0.13 a | 3.33 ± 0.06 a | 3.40 ± 0.07 a |
| Lymphocyte (103/µL) | 10.08 ± 0.27 a | 3.03 ± 0.17c | 9.75 ± 0.12 a | 4.13 ± 0.41b |
| Monocyte (103/µL) | 0.19 ± 0.002 a | 0.21 ± 0.013 a | 0.19 ± 0.010 a | 0.20 ± 0.005 a |
Data are expressed as mean ± standard error of the mean (n = 8). Means in the same row with different superscripts letters are significantly different (p < 0.05).
Control; normal, CIS; Cisplatin, HIS; Hesperidin, RBCs; Red blood cell count, Hb; Hemoglobin, PCV; Packed cell volume, MCV; Mean corpuscular volume, MCH; Mean corpuscular hemoglobin, MCHC; Mean corpuscular hemoglobin concentration, TLC; Total leukocytic count.
3.3. Hesperidin administration improves CIS-induced alterations of liver function biomarkers and lipids profile
In this study, CIS hepatotoxicity was reflected by elevation in the serum ALT, AST, ALP, total bilirubin, indirect bilirubin, glucose, cholesterol, and triglyceride values, with a significant reduction in the total protein and globulin levels compared to the control rats. HES pretreatment significantly ameliorated all biochemical alteration induced by CIS except total cholesterol level that remained elevated. It is important to note that the HES treatment had no effect on the measured biochemical parameters (Table 3).
Table 3.
Effect of hesperidin on liver function biomarkers and lipids profile in cisplatin-intoxicated rats.
| Parameters | Treatments |
|||
|---|---|---|---|---|
| Control | CIS | HES | HES + CIS | |
| ALT (U/L) | 40.40 ± 0.81c | 90.40 ± 1.91 a | 45.60 ± 1.36c | 59.00 ± 2.70b |
| AST (U/L) | 56.00 ± 2.19c | 105.20 ± 3.26 a | 56.80 ± 1.35c | 73.60 ± 3.96b |
| ALP (U/L) | 165.80 ± 7.79c | 435.40 ± 7.98 a | 162.80 ± 9.25c | 277.00 ± 16.41b |
| T. Bilirubin (mg/dl) | 0.47 ± 0.02b | 0.73 ± 0.06 a | 0.50 ± 0.002b | 0.57 ± 0.01b |
| D. Bilirubin (mg/dl) | 0.11 ± 0.005 a | 0.20 ± 0.056 a | 0.12 ± 0.004 a | 0.13 ± 0.007 a |
| Ind.Bilirubin(mg/dl) | 0.36 ± 0.02c | 0.53 ± 0.02 a | 0.38 ± 0.005 bc | 0.44 ± 0.01b |
| Total Protein (g/dl) | 7.06 ± 0.09 a | 5.88 ± 0.08c | 6.80 ± 0.09 ab | 6.48 ± 0.10b |
| Albumin (g/dl) | 4.06 ± 0.08 a | 3.87 ± 0.05 a | 3.98 ± 0.06 a | 3.86 ± 0.08 a |
| Globulin (g/dl) | 3.00 ± 0.17 a | 2.01 ± 0.06b | 2.82 ± 0.11 a | 2.62 ± 0.17 a |
| A/G ratio | 1.38 ± 0.11b | 1.93 ± 0.07 a | 1.42 ± 0.07b | 1.50 ± 0.12b |
| Glucose (mg/dl) | 96.50 ± 7.88c | 245.00 ± 10.40 a | 121.25 ± 6.42c | 208.25 ± 13.15b |
| Cholesterol (mg/dl) | 82.60 ± 2.85b | 143.60 ± 5.76 a | 70.60 ± 3.07b | 128.80 ± 8.44 a |
| Triglyceride (mg/dl) | 97.00 ± 4.23b | 134.60 ± 8.09 a | 102.40 ± 3.60b | 106.60 ± 2.99b |
Data are expressed as mean ± standard error of the mean (n = 8). Means in the same row with different superscripts letters are significantly different (p < 0.05).
Control; normal, CIS; Cisplatin, HIS; Hesperidin, ALT; Alanine aminotransferase, AST; Aspartate aminotransferase, ALP; Alkaline phosphastase, A/G ratio; Albumin/globulin ratio, D. Bilirubin; Direct bilirubin, Ind. Bilirubin; Indirect Bilirubin, T. Bilirubin; Total Bilirubin.
3.4. Hesperidin diminishes the hepatic oxidative stress and improves the hepatic antioxidant status
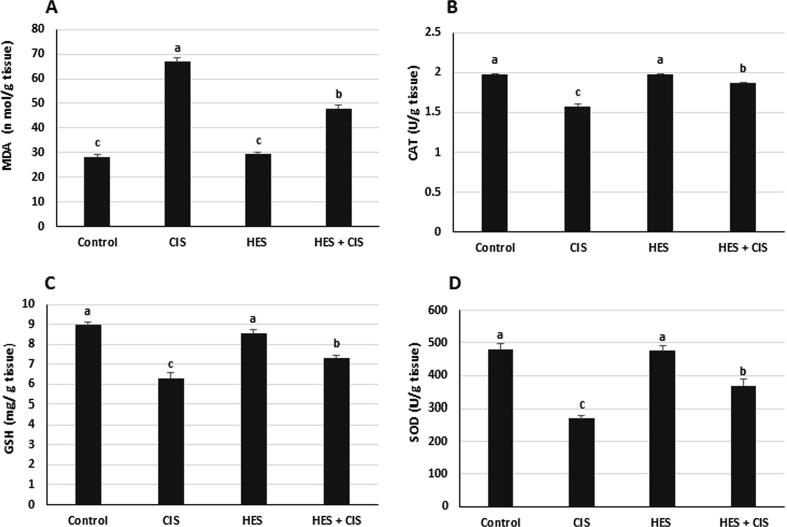
CIS significantly increased the hepatic MDA level, and significantly reduced the hepatic CAT and SOD activities as well hepatic GSH level compared to the control group (Fig. 1). On the other hand, HES pretreatment produced a significant protection against CIS-induced hepatotoxicity by decreasing the elevated MDA levels and activating the antioxidant biomarkers (CAT, GSH, and SOD) in the liver tissue.
Fig. 1.
Effect of hesperidin on the hepatic oxidative stress and antioxidant markers in cisplatin-intoxicated rats (A) MDA content, (B) CAT activity (C) GSH level (D) SOD activity. Control; normal, CIS; Cisplatin, HIS; Hesperidin, MDA; malondialdehyde, CAT; catalase, GSH; reduced glutathione, SOD; superoxide dismutase. Data are presented as mean ± standard error of the mean. Values with different letters within the same figure are significantly different (p < 0.05).
3.5. Hesperidin relieves CIS-induced hepatic inflammatory response in rats
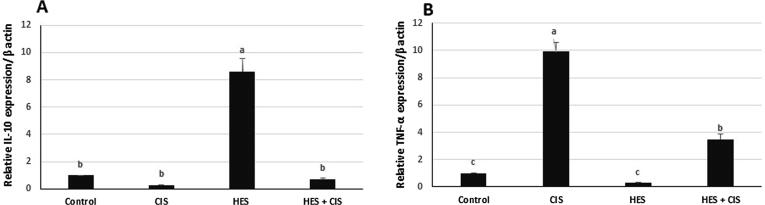
Hesperidin treatment in the HES group induced a potent upregulation in IL-10 expression (8.5-fold change) and an insignificant downregulation in the TNF-α expression (0.3-fold change) compared to the control rats. Remarkably, treatment with CIS significantly upregulated the TNF-α expression (9.9-fold change) with no effect on IL-10 expression in comparison to the control group. Besides, HES pretreatment significantly reduced the expression of TNF-α in comparison with the CIS-intoxicated rats (Fig. 2).
Fig. 2.
Effect of hesperidin on relative gene expression of hepatic IL-10 and TNF-α in cisplatin-intoxicated rats (A) Relative IL-10 expression normalized to β-actin (B) Relative TNF- α expression normalized to β-actin in all experimental groups. Each bar represents mean of fold change ± SEM (n = 8). Bars with different superscript letters within the same figure are significantly different (p < 0.05). Control; normal, CIS; Cisplatin, HIS; Hesperidin, IL-10; inerleukin-10, TNF- α; tumor necrosis factor-alpha.
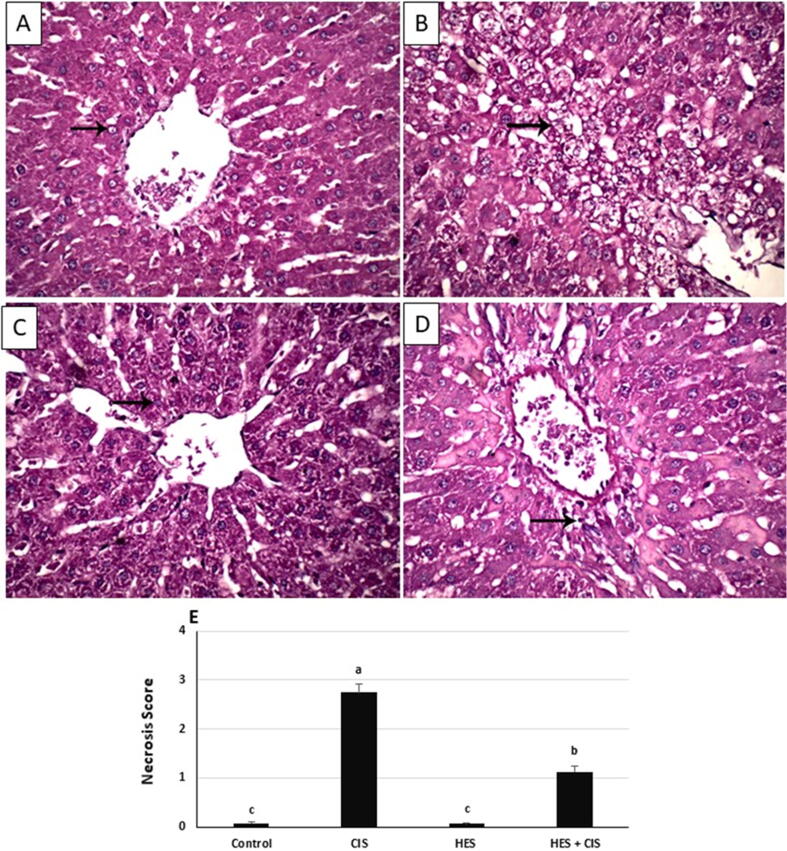
3.6. Hesperidin mitigated CIS-induced hepatic histopathological injury in rats
Histological analysis showed that CIS administration resulted in changes in liver architecture as indicated by local extensive necrosis of hepatocytes (Fig. 3B). The liver of control (Fig. 3A) and hesperidin (Fig. 3C) treated rats showed normal hepatic architecture. Treatment with HES mitigated the histopathological changes induced by CIS (Fig. 3D) and showed focal necrosis of hepatocytes.
Fig. 3.
Effect of hesperidin on the hepatic histopathological alterations in CIS-intoxicated rats. micrograph of hepatic tissues displays normal hepatocytes (arrow) and normal hepatic architecture (H&E staining) in (A) Normal control rats and (C) Hesperidin group. (B) The micrograph is taken from cisplatin group, displays local extensive necrosis of hepatocytes (arrow), (D) The micrograph shows focal necrosis of hepatocytes (arrow) in Hesperidin + Cisplatin group. (E) Hepatic necrosis scoring in the different experimental groups. Each bar represents mean ± SEM (n = 8). Bars with different superscript letters are significantly different (p < 0.05). (0, absent; 1, spotty necrosis; one or few necrotic hepatocytes; 2, confluent necrosis; 3, bridging necrosis). Control; normal, CIS; Cisplatin, HIS; Hesperidin.
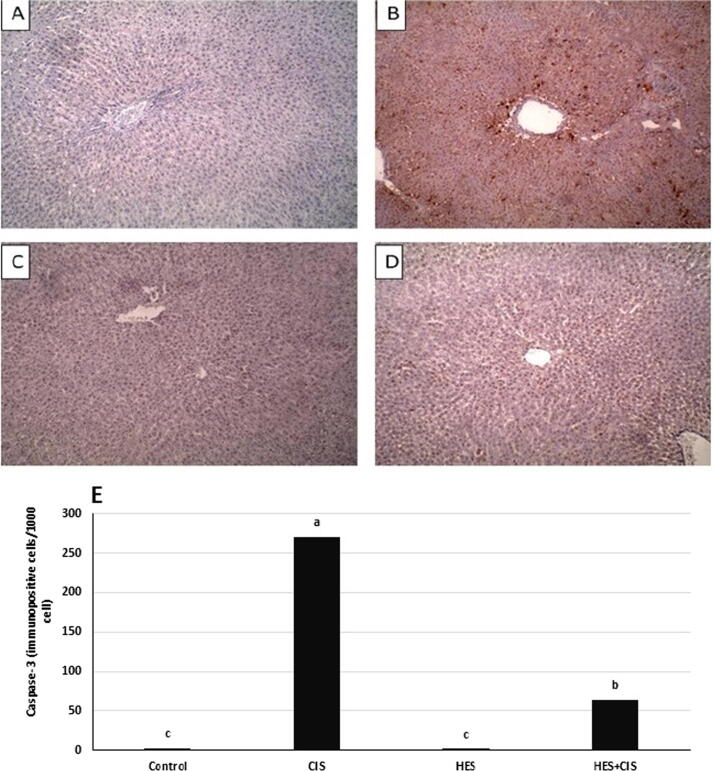
3.7. Hesperidin modulates CIS-induced apoptosis in rat liver
Immunohistochemical staining of Bax and caspase-3 proteins in liver tissue of all groups is demonstrated in Fig. 4, Fig. 5. CIS treatment revealed a prominent significant increase in Bax and Caspase-3 expression compared to the control group. Alternatively, HES counteracts CIS-induced apoptotic changes of Bax and Caspase-3 in liver tissue. Additionally, the HES control group did not show any difference from the normal control group.
Fig. 4.
Effect of hesperidin on the immunohistochemical expression of Bax in rat livers. Representative images for the immunohistochemical expression of Bax (A-D) (IHC, DAB immunostaining, hematoxylin as counter stain, 100x). (A): liver of control rats displays negative immunostaining against Bax, (B): liver of cisplatin group shows strong immunostaining in the cytoplasm of hepatocytes against Bax, (C): liver of HES displays mild immunostaining against Bax, (D): liver of CIS + HES displays moderate immunostaining in the hepatocytes against Bax. Graph (E) counting (number of immunopositive cells per 1000 cells) for Bax in liver tissue in all groups that was done by Image J analysis. Each bar represents mean ± SEM (n = 8). Bars with different superscript letters are significantly different (p < 0.05). Control; normal, CIS; Cisplatin, HIS; Hesperidin.
Fig. 5.
Effect of hesperidin on the immunohistochemical expression of Caspase-3 in rat livers. Representative images for the immunohistochemical expression of Caspase-3 (IHC, DAB immunostaining, hematoxylin as counter stain, 100x). (A): liver of Control group displays negative immunostaining against caspase-3 (B) liver of CIS group displays strong immunostaining in the cytoplasm and nucleus of hepatocytes against caspase-3, (C) liver of HES group displays mild immunostaining against caspase-3, (D) liver of HES + CIS group displays moderate immunostaining in the cytoplasm and nucleus of hepatocytes against caspase-3. (E) counting (number of immunopositive cells per 1000 cells) for Caspase-3 in liver tissue in all experimental groups that was done by Image J analysis. Each bar represents mean ± SEM (n = 8). Bars with different superscript letters are significantly different (p < 0.05). Control; normal, CIS; Cisplatin, HIS; Hesperidin.
4. Discussion
Cisplatin is one of the most active cytotoxic anticancer agents; however, hepatotoxicity is one of its side effects that has been attributed to excessive release of ROS and damage of cells (Dkhil et al., 2013). Moreover, inflammation and the production of cytokines as TNF-α and IL-6 occur as a complication of oxidative stress. Increased ROS and pro-inflammatory cytokines have been documented to produce hepatocyte apoptosis (Ingawale et al., 2014, Dkhil et al., 2013, Hoek and Pastorino, 2002).
In this context, it was observed that CIS treatment-induced normocytic normochromic anemia, which was demonstrated by decreased RBCs count, Hb, and PCV% with normal MCV and MCHC. These alterations are appertaining to the incidence of hemolytic anemia which may be attributed to RBCs destruction due to lipid peroxidation and membrane disruption (Trotta et al., 1983). This was confirmed in our study by the observed clinical signs which included bloody diarrhea, nose bleeding, hemoglobinuria, and poor appetite. Moreover, CIS-induced anemia could either results from drug-induced suppression of bone marrow that disturbs cell cycle causing anemia and leukopenia, or from CIS-induced renal dysfunction resulted in erythropoietin hormone deficiency (Mazur et al., 2002, Wood and Hrushesky, 1995).
Furthermore, leukopenia with prominent lymphopenia along with thrombocytopenia was obvious in the CIS-treated rats. This may result from oxidative stress in platelets and lymphocytes induced by CIS which disturbs life span and induces apoptosis, hence the reduction in the number of these blood cells (Olas et al., 2005).
Conjointly, a single dose of CIS (7.5 mg/kg) in our study resulted in a severe array of events of hepatotoxicity accompanied with the severe degenerative changes in the liver confirmed by the histopathological examination that exhibited local extensive necrosis of hepatocytes. The ability of CIS to induce an elevation in the serum activities of ALT, AST, and ALP is supposed to be a secondary event following CIS-induced liver damage with the consequent leakage of these enzymes from hepatocytes (Mansour et al., 2006), and may indicate hepatocytes necrosis (Gressner et al., 2007). Also, (Okoko and Ndoni, 2018) reported an elevation in the serum ALT, AST, and ALP activities in CIS-induced hepatotoxicity. However, the normal albumin level reported with CIS injection was the same as previously reported by Neamatallah et al. (2018), even though there was a significant decrease in the total protein and globulin levels.
Bilirubin is a well-established indicator of tissue damage by toxic substances, our results showed hyperbilirubinemia due to elevated levels of indirect and total bilirubin in CIS-treated rats. The increased levels of indirect bilirubin may be due to hemolysis that is confirmed by the hemolytic anemia in the hematological picture. Also, maybe resulted either from reduced hepatic uptake of bilirubin that happens as a result of some medications or from the reduced rate of bilirubin conjugation in the liver (VanWagner and Green, 2015). Additionally, the significant rise in levels of triglycerides and cholesterol in our work may be attributed to the deposition of small lipid droplets in the liver due to CIS treatment (Cho et al., 2012).
In the present work, CIS-treated rats showed hyperglycemia that may be secondary to the presence of marked glucose intolerance, in association with an impaired insulin response, and abnormal glucagon response to a glucose stimulus (Portilla et al., 2006). Also, Goldstein et al., 1983, Wang and Aggarwal, 1997 pointed the elevated blood glucose level to the impaired insulin secretion and possibly by induction of somatostatin and nitric oxide.
The excess production of ROS in CIS-treated rats leads to an imbalance between oxidant-antioxidant levels, which in turn reduces the scavenging power toward ROS and causes oxidative stress. This emphasized in our results by the elevation of hepatic MDA content, along with a diminution of the enzymatic antioxidants including hepatic CAT and SOD. Moreover, the depletion of GSH levels in CIS-induced rats makes the hepatic tissue more susceptible to oxidative stress. This is similar to the results obtained by Bentli et al., 2013, Omar et al., 2016a, Omar et al., 2016b, Yüce et al., 2007.
In the present study, hepatic oxidative stress triggered by a single dose of CIS (7.5 mg/kg) has been prospected to cause apoptosis that confirmed by increased hepatic Bax and caspase-3 expression and produces inflammation assessed by numerical downregulation of IL-10 and significant upregulation of TNF-α in the hepatic tissue. Since, oxidative stress induced by CIS in virtue of the activation of the NF-κB pathway, which, in sequence causes inflammation and apoptosis (Neamatallah et al., 2018).
Pro-inflammatory cytokines, such as TNF- α, IL-1β, and IL-6, are released into the bloodstream from the liver during hepatotoxic injury (Cho et al., 2012). TNF- α is a protein involved in inflammatory reactions and closely associated with apoptosis (Safhi et al., 2018). Apoptotic cells can also release IL-10 (Gao et al., 1998). Therefore, upregulation of TNF- α in our study is a relevant biomarker associated with inflammation pathways in CIS-induced hepatotoxicity, while IL-10 expression was decreased but statistically insignificant compared with the control group. The significant increase in hepatic TNF- α and decrease in hepatic IL-10 expression accompanied by CIS injection at doses 7.5 mg/kg and 12 mg/kg respectively was already detailed in previous studies (Hassan et al., 2020, Omar et al., 2016b).
Cisplatin intoxication shifts the balance between pro-and anti-apoptotic signals towards proapoptotic cascade (Indran et al., 2011), which is confirmed in our results by increased Bax and Caspase-3 expression in hepatic tissue of CIS-treated rats. Our results are compatible with Hassan et al., 2020, Neamatallah et al., 2018 which confirms that CIS administration is accompanied by apoptosis. Since, caspase activation is the essential step for the initiation of apoptosis induced by various stimuli. When cells are undergoing apoptosis, activation of the initiator caspases which include caspases 8 and 9, results in activation of executioner caspases such as caspases 3 and 7 (Salvesen and Dixit, 1997). Moreover, executioner caspase-3 triggers cellular proteins and DNA fragmentation factors that cause characteristic changes of apoptosis (Tong et al., 2004). Other studies have demonstrated that p53 activates caspase 3 by a variety of mechanisms, including the activation of the pro-apoptotic proteins as Bax; that trigger a sequence of events that leads to alterations in mitochondrial permeability transition and stimulation of cytochrome c release, all of which increase caspase 3, 8, and 9 activities in numerous cell types in response to chemical-induced apoptosis (Saad et al., 2009, Chen et al., 2001, Schuler et al., 2000).
In agreement with previous studies (Hamdy et al., 2016, Omar et al., 2016b), the HES alone in this study did not induce any adverse effects on all tested parameters, but interestingly provided significant protection against CIS-induced hematological, biochemical, and hepatic dysfunction. The hepatoprotective action of hesperidin may be attributed to stabilizing the hepatic cellular membrane damage and protecting the hepatocyte from CIS hepatotoxicity by its antioxidant effect and its free radical scavenging activity, consequently protecting membrane permeability (Omar et al., 2016b, Haková and Mišúrová, 1993, Pari et al., 2015).
Oral administration of HES (200 mg/kg) for 21 days was efficient in counteracting CIS toxicity whereas, it improved the anemic condition, leukopenia, and thrombocytopenia induced by CIS, which may be referred to the antioxidative potentials of hesperidin (Omar et al., 2016b). Also, hesperidin can stimulate the formation or secretion of the erythropoietin hormone, which stimulates stem cells in the bone marrow to produce RBCs (Aher and Ohlsson, 2020).
Interestingly, the serum biochemical changes were significantly abrogated by pretreatment with HES (200 mg/kg), which may be attributed to its free radical scavenging and antioxidant properties. This is in parallel with Omar et al. (2016a) who revealed that HES significantly improved the alterations in ALT, AST, and ALP activities. Besides, HES has a lipid-lowering ability (Chen et al., 2010) that may explain the improvement in the serum TG level in CIS-treated rats by inhibiting HMG-CoA reductase and acyl CoA: cholesterol acyltransferase in rats (Bok et al., 1999). Furthermore, HES pretreatment reduced the elevated blood glucose level in CIS-intoxicated group but remain comparable to the control rats. The hypoglycemic effect by of HES may be attributed to the changes in the activities of glucose-regulating enzymes and the ability of HES as flavonoids to normalize blood glucose by affect functions of GLUT-4 stimulating the glucose uptake in the skeletal muscle and adipocytes or through up-regulation of the mRNA expression of peroxisome proliferator-activated receptor (PPARs) that may be improved insulin–resistance (Akiyama et al., 2009).
Data obtained in this study indicated that pretreatment of rats with HES inhibited the elevation of lipid peroxidation induced by CIS and produced a significant increase in CAT and SOD activities and GSH level, resulting in values nearly close to those observed in the control rats. These findings are following other investigators (Mansour et al., 2006, Omar et al., 2016a). Since, the protective action of HES against CIS-induced oxidative stress is ascribed to its free radical scavenging as well as anti-lipid peroxidation properties in biological membranes (Suarez et al., 1998).
Hesperidin supplementation for successive 21 days inhibited the increase in TNF-α expression induced by CIS, while IL-10 was improved but also statistically indifferent. This protective role is due to the anti-inflammatory effect of hesperidin (Chen et al., 2010), which is demonstrated in our results by the significant elevation of IL-10 in rats supplemented with hesperidin. In parallel with previous reports, hesperidin treatment reduced inflammatory cytokine production (TNF-α) and suppressed apoptosis induced by CIS toxicity (Apaydin et al., 2018, Omar et al., 2016b). Our histopathological finding confirmed the reduction of hepatic damage following hesperidin administration in CIS-intoxicated rats; reflecting its antioxidant and antiapoptotic activity.
5. Conclusions
It could be concluded that oxidative stress, inflammation and apoptosis play an important role in CIS-induced hepatotoxicity. Interestingly, hesperidin pretreatment provides potent protective effects against CIS-induced liver injury may be mediated by its antioxidant, anti-inflammatory and anti-apoptotic properties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all members of the Clinical Pathology Department, for help and encouragement during the course of this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adikay S., Spandana U., Bharathi K. Effect of hesperdin isolated from orange peels on cisplatin-induced nephrotoxicity. Int. J. Pharmacogn. Phytochem. Res. 2012;4:49–53. [Google Scholar]
- Aher S.M., Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Systematic Rev. 2020 doi: 10.1002/14651858.CD004865.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama S., Katsumata S.-I., Suzuki K., Nakaya Y., Ishimi Y., Uehara M. Hypoglycemic and Hypolipidemic Effects of Hesperidin and Cyclodextrin-Clathrated Hesperetin in Goto-Kakizaki Rats with Type 2 Diabetes. Biosci. Biotechnol. Biochem. 2009;73(12):2779–2782. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- Apaydin F.G., KaltalioĞLu K., Balabanli B., Cevher Ş.C. Morin and hesperidin ameliorate cisplatin-induced hepatotoxicity and nephrotoxicity in rats: A histopathological study. Gazi University J. Sci. 2018;31:399–406. [Google Scholar]
- Bancroft J.D., Gamble M. Theory and Practice of Histological Techniques. sixth ed. Elsevier; 2008. Preface to the. [Google Scholar]
- Banni M., Messaoudi I., Said L., El Heni J., Kerkeni A., Said K. Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Arch. Environ. Contam. Toxicol. 2010;59(3):513–519. doi: 10.1007/s00244-010-9494-5. [DOI] [PubMed] [Google Scholar]
- Bentli R., Parlakpinar H., Polat A., Samdanci E., Sarihan M.E., Sagir M. Molsidomine Prevents Cisplatin-induced Hepatotoxicity. Arch. Med. Res. 2013;44(7):521–528. doi: 10.1016/j.arcmed.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Bok, S.-H., Lee, S.-H., Park, Y.-B., Bae, K.-H., Son, K.-H., Jeong, T.-S., Choi, M.-S., 1999. Plasma and Hepatic Cholesterol and Hepatic Activities of 3-Hydroxy-3-methyl-glutaryl-CoA Reductase and Acyl CoA: Cholesterol Transferase Are Lower in Rats Fed Citrus Peel Extract or a Mixture of Citrus Bioflavonoids. The Journal of Nutrition 129, 1182-1185. [DOI] [PubMed]
- Chen M., Gu H., Ye Y., Lin B., Sun L., Deng W., Zhang J., Liu J. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem. Toxicol. 2010;48(10):2980–2987. doi: 10.1016/j.fct.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cai J., Anders M.W., Stevens J.L., Jones D.P. Role of Mitochondrial Dysfunction in S-(1,2-Dichlorovinyl)-l-cysteine-Induced Apoptosis. Toxicol. Appl. Pharmacol. 2001;170(3):172–180. doi: 10.1006/taap.2000.9107. [DOI] [PubMed] [Google Scholar]
- Cho, Y.-E., Singh, T.S.K., Lee, H.-C., Moon, P.-G., Lee, J.-E., Lee, M.-H., Choi, E.-C., Chen, Y.-J., Kim, S.-H., Baek, M.-C., 2012. In-depth Identification of Pathways Related to Cisplatin-induced Hepatotoxicity through an Integrative Method Based on an Informatics-assisted Label-free Protein Quantitation and Microarray Gene Expression Approach. Molecular & Cellular Proteomics 11, M111.010884. [DOI] [PMC free article] [PubMed]
- Dkhil M.A., Al-Quraishy S., Aref A.M., Othman M.S., El-Deib K.M., Abdel Moneim A.E. The Potential Role ofAzadirachta indicaTreatment on Cisplatin-Induced Hepatotoxicity and Oxidative Stress in Female Rats. Oxid. Med. Cell. Longevity. 2013;2013:1–9. doi: 10.1155/2013/741817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshopakey G.E., Elazab S.T. Cinnamon Aqueous Extract Attenuates Diclofenac Sodium and Oxytetracycline Mediated Hepato-Renal Toxicity and Modulates Oxidative Stress. Cell Apoptosis Inflamm. Male Albino Rats. 2021;8(1):9. doi: 10.3390/vetsci8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B., Zinkl J., Jain V. 5th ed. Lippincott Williams and Wilkins; Canada: 2000. Schalm's Veterinary Hematology. [Google Scholar]
- Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radical Biol. Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Gao Y., Herndon J.M., Zhang H., Griffith T.S., Ferguson T.A. Antiinflammatory Effects of CD95 Ligand (FasL)-induced Apoptosis. J. Exp. Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.S., Mayor G.H., Gingerich R.L., Hook J.B., Rosenbaum R.W., Bond J.T. The effects of cisplatin and other divalent platinum compounds on glucose metabolism and pancreatic endocrine function. Toxicol. Appl. Pharmacol. 1983;69(3):432–441. doi: 10.1016/0041-008x(83)90266-1. [DOI] [PubMed] [Google Scholar]
- González‐Périz, A., Planaguma, A., Gronert, K., Miquel, R., López‐Parra, M., Titos, E., Horrillo, R., Ferré, N., Deulofeu, R., Arroyo, V.J.T.F.J., 2006. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S‐hydroxy‐DHA. 20, 2537-2539. [DOI] [PubMed]
- Gressner O.A., Weiskirchen R., Gressner A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta. 2007;381(2):107–113. doi: 10.1016/j.cca.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Haková, H., Mišúrová, E., 1993. The effect of silymarin and gamma radiation on nucleic acids in rat organs. Journal of Pharmacy and Pharmacology 45, 910-912. [DOI] [PubMed]
- Hamdy S.M., Sayed O.N., Abdel Latif A.K.M., Abd-Elazeez A.M., Amin A.M. Protective Effect Of Hesperidin And Tiger Nut Against DMBA Carcinogenicity In Female Rats. Biochem. Lett. 2016;11(1):150–167. [Google Scholar]
- Hassan H., Al-Wahaibi L., Elmorsy M., Mahran Y. Suppression of Cisplatin-Induced Hepatic Injury in Rats Through Alarmin High-Mobility Group Box-1 Pathway by Ganoderma lucidum: Theoretical and Experimental Study. Drug Des Development Therapy. 2020;14:2335–2353. doi: 10.2147/DDDT.S249093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J.B., Pastorino J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27(1):63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Indran I.R., Tufo G., Pervaiz S., Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. et Biophys. Acta (BBA) – Bioenerget. 2011;1807(6):735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Ingawale D.K., Mandlik S.K., Naik S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): A critical discussion. Environ. Toxicol. Pharmacol. 2014;37(1):118–133. doi: 10.1016/j.etap.2013.08.015. [DOI] [PubMed] [Google Scholar]
- İşeri S., Ercan F., Gedik N., Yüksel M., Alican İ. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230(2-3):256–264. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- Kamel K.M., Abd El-Raouf O.M., Metwally S.A., Abd El-Latif H.A., El-sayed M.E. Hesperidin and Rutin, Antioxidant Citrus Flavonoids, Attenuate Cisplatin-Induced Nephrotoxicity in Rats. J. Biochem. Mol. Toxicol. 2014;28(7):312–319. doi: 10.1002/jbt.21567. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Abdelhalim M.A., Alhomida A.S., Al Ayed M.S. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet. Mol. Res. 2013;12:5851–5857. doi: 10.4238/2013.November.22.12. [DOI] [PubMed] [Google Scholar]
- Liao Y., Lu X., Lu C., Li G., Jin Y., Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol. Res. 2008;57(2):125–131. doi: 10.1016/j.phrs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Mansour H.H., Hafez H.F., Fahmy N.M. Silymarin Modulates Cisplatin-Induced Oxidative Stress and Hepatotoxicity in Rats. BMB Rep. 2006;39(6):656–661. doi: 10.5483/bmbrep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- Mazur L., Czyzewska A., Bochenek M. Flow cytometric detection of apoptotic bone marrow cells with fractional DNA content after application of WR-2721, cyclophosphamide, cisplatin, and exposure of mice to gamma rays. Hum. Exp. Toxicol. 2002;21(6):335–341. doi: 10.1191/0960327102ht261oa. [DOI] [PubMed] [Google Scholar]
- Mohamed W.R., Arafa E.-S.-A., Shehata B.A., El Sherbiny G.A., Elgendy A.M. Beneficial Effects of Hesperidin against Cisplatin-Induced Nephrotoxicity and Oxidative Stress in Rats. British J. Pharmacol. Toxicol. 2015;6:56–63. [Google Scholar]
- Neamatallah T., El-Shitany N.A., Abbas A.T., Ali S.S., Eid B.G. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 2018;9(7):3743–3754. doi: 10.1039/c8fo00653a. [DOI] [PubMed] [Google Scholar]
- Okoko, T., Ndoni, S.A., 2018. Kolaviron protects against cisplatin-induced hepatic and renal oxidative damage in rats.
- Olas B., Wachowicz B., Majsterek I., Blasiak J. Resveratrol may reduce oxidative stress induced by platinum compounds in human plasma, blood platelets and lymphocytes. Anticancer Drugs. 2005;16(6):659–665. doi: 10.1097/00001813-200507000-00011. [DOI] [PubMed] [Google Scholar]
- Omar H.A., Mohamed W.R., Arab H.H., Arafa E.-S., Acharya K. Tangeretin Alleviates Cisplatin-Induced Acute Hepatic Injury in Rats: Targeting MAPKs and Apoptosis. PLoS ONE. 2016;11(3):e0151649. doi: 10.1371/journal.pone.0151649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar H.A., Mohamed W.R., Arafa E.-S., Shehata B.A., Sherbiny G.A.E., Arab H.H., Elgendy A.N.A.M. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol. Rep. 2016;68(2):349–356. doi: 10.1016/j.pharep.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Palipoch S., Punsawad C., Koomhin P., Suwannalert P. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Complement Altern. Med. 2014;14(1) doi: 10.1186/1472-6882-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari L., Karthikeyan A., Karthika P., Rathinam A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015;2:46–55. doi: 10.1016/j.toxrep.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portilla D., Li S., Nagothu K.K., Megyesi J., Kaissling B., Schnackenberg L., Safirstein R.L., Beger R.D. Metabolomic study of cisplatin-induced nephrotoxicity. Metabolomic Study Cisplatin-Induced Nephrotoxicity. 2006;69(12):2194–2204. doi: 10.1038/sj.ki.5000433. [DOI] [PubMed] [Google Scholar]
- Saad A.A., Youssef M.I., El-Shennawy L.K. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: The protective effect of grape seed proanthocyanidin extract. Food Chem. Toxicol. 2009;47(7):1499–1506. doi: 10.1016/j.fct.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Safhi M.M., Qumayri H.M., Masmali A.U.M., Siddiqui R., Alam M.F., Khan G., Anwer T. Thymoquinone and fluoxetine alleviate depression via attenuating oxidative damage and inflammatory markers in type-2 diabetic rats. Arch. Physiol. Biochem. 2019;125(2):150–155. doi: 10.1080/13813455.2018.1443141. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S., Dixit V.M. Caspases: Intracellular Signaling by Proteolysis. Cell. 1997;91(4):443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Schuler M., Bossy-Wetzel E., Goldstein J.C., Fitzgerald P., Green D.R. p53 Induces Apoptosis by Caspase Activation through Mitochondrial Cytochrome c Release. J. Biol. Chem. 2000;275(10):7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- Shynlova O., Dorogin A., Li Y., Lye S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J. Cell Mol. Med. 2014;18(9):1816–1829. doi: 10.1111/jcmm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J., Herrera M.D., Marhuenda E. In vitro scavenger and antioxidant properties of hesperidin and neohesperidin dihydrochalcone. Phytomedicine. 1998;5(6):469–473. doi: 10.1016/S0944-7113(98)80044-5. [DOI] [PubMed] [Google Scholar]
- Tahoon N. Biological effect of Parsley and honey on side effects of Cisplatin induced nephrotoxicity in experimental male rats. Bulletin National Nutrition Inst. 2017;48(1):1–23. [Google Scholar]
- Tanaka T., Tanaka T., Tanaka M., Kuno T. Cancer Chemoprevention by Citrus Pulp and Juices Containing High Amounts ofβ-Cryptoxanthin and Hesperidin. J. Biomed. Biotechnol. 2012;2012:1–10. doi: 10.1155/2012/516981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X., Lin S., Fujii M., Hou D.-X. Molecular mechanisms of echinocystic acid-induced apoptosis in HepG2 cells. Biochem. Biophys. Res. Commun. 2004;321(3):539–546. doi: 10.1016/j.bbrc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Trotta R.J., Sullivan S.G., Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem. J. 1983;212:759–772. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWagner L.B., Green R.M. Evaluating Elevated Bilirubin Levels in Asymptomatic Adults. JAMA. 2015;313(5):516. doi: 10.1001/jama.2014.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Aggarwal S.K. Effects of cisplatin and taxol on inducible nitric oxide synthase, gastrin and somatostatin in gastrointestinal toxicity. Anticancer Drugs. 1997;8(9):853–858. doi: 10.1097/00001813-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Wood P.A., Hrushesky W.J. Cisplatin-associated anemia: an erythropoietin deficiency syndrome. J. Clin. Invest. 1995;95(4):1650–1659. doi: 10.1172/JCI117840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav Y.C. Effect of cisplatin on pancreas and testes in Wistar rats: biochemical parameters and histology. Heliyon. 2019;5(8):e02247. doi: 10.1016/j.heliyon.2019.e02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.S., Reed A., Chen F., Stewart C.N. Statistical analysis of real-time PCR data. BMC Bioinf. 2006;7:1–12. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüce A., Ateşşahin A., Çeribaşı A.O., Aksakal M. Ellagic Acid Prevents Cisplatin-Induced Oxidative Stress in Liver and Heart Tissue of Rats. Basic Clin. Pharmacol. Toxicol. 2007;101(5):345–349. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]