Abstract
Wounds occur when skin integrity is broken and the skin is damaged. With progressive changes in the disease spectrum, the acute wounds caused by mechanical trauma have been become less common, while chronic wounds triggered with aging, diabetes and infection have become more frequent. Chronic wounds now affect more than 6 million people in the United States, amounting to 10 billion dollars in annual expenditure. However, the treatment of chronic wounds is associated with numerous challenges. Traditional remedies for chronic wounds include skin grafting, flap transplantation, negative-pressure wound therapy, and gauze dressing, all of which can cause tissue damage or activity limitations. Nanobiotechnology — which comprises a diverse array of technologies derived from engineering, chemistry, and biology — is now being applied in biomedical practice. Here, we review the design, application, and clinical trials for nanotechnology-based therapies for chronic wound healing, highlighting the clinical potential of nanobiotechnology in such treatments. By summarizing previous nanobiotechnology studies, we lay the foundation for future wound care via a nanotech-based multifunctional smart system.
Keywords: nanobiotechnology, chronic wound healing, scaffold systems, cell-carrying systems, stimuli-responsive systems
Introduction
The skin is the largest organ in the body, accounting for 15% of the total body weight. It is the first line of defense against physical, chemical, and biological factors.1,2 In some cases, the anatomical structure and biological function of the skin are impaired due to internal (local blood obstruction, inflammation, or underlying diseases) or external factors (mechanical injury, chemical corrosion, electric injury, or thermal injury).1,3
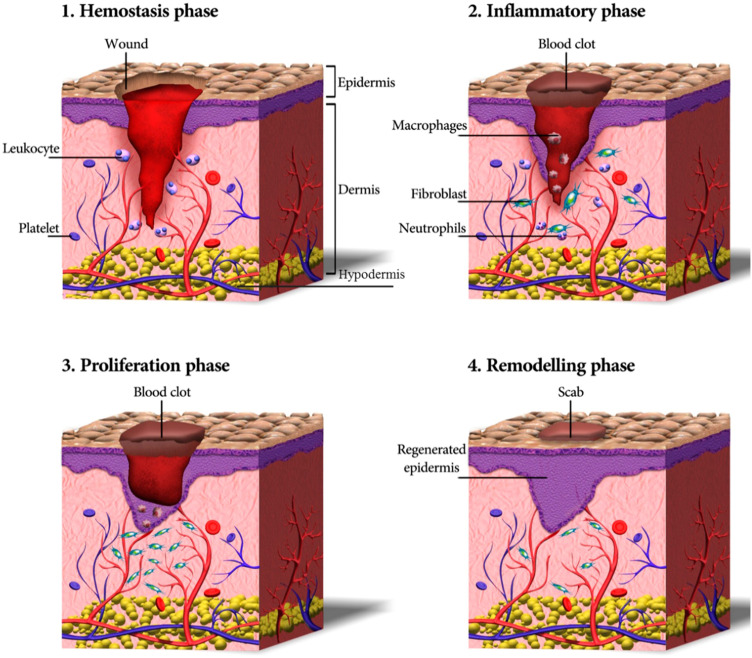
After damage, skin can self-heal, and this process involves four phases: hemostasis, inflammation, proliferation, and remodeling (Figure 1).4,5 In the first few minutes after skin damage, the platelets accumulate around the wound and get activated, forming a scab to preventing bleeding.6 After 2–3 days, the inflammatory phase starts around the wound, and the immune cells remove the dead and devitalized tissues and prevent microbial infections.4 The proliferation phase occurs after the inflammation phase, and it is characterized by the activation of keratinocytes, fibroblasts, endothelial cells, and macrophages, which contribute to wound closure, matrix formation and angiogenesis.7 In the 12 or more months after the primary repair is completed, the regenerated skin tissue is remodeled. During this phase, the processes activated after injury slow down, and the healed wound reaches it maximum mechanical strength.4,5
Figure 1.
Phases of wound healing, including the hemostasis, inflammatory, proliferation, and remodeling phase.
Notes: Reprinted from: Tavakoli S, Klar AS. Advanced Hydrogels as Wound Dressings. Biomolecules. 2020;10(8):1169. doi:10.3390/biom10081169.5 © 2020 by the authors. Licensee MDPI, Basel, Switzerland. Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
However, in some cases, the skin’s self-healing property is inadequate, leading to the formation of chronic wounds. Chronic wounds are defined as wounds that remain unhealed even after 12 weeks.8 The main factors delaying wound repair include diabetes, infections, and long-term inflammation. Diabetic mellitus damages the microenvironment of skin tissue, which is involved in wound regeneration. It causes increases in reactive oxygen species (ROS) levels and poor collagen deposition.9–11 The hyperglycemia weakens the functions of fibroblasts, keratinocytes, endothelial cells, and stem cells or progenitor cells involved in wound healing.12 Microbial infections deplete the energy and cells required for tissue regeneration, and the bacteria can form biofilms that display antibiotic resistance, immune evasion, and wound adherence.13,14 In unhealed skin, excess inflammation also contributes to wound chronicity owing to its cytotoxic effects and the induced tissue damage, both of which delay wound healing.15–17 Traditionally, the chronic wounds are treated with wound dressing made of gauze, skin grafting, or even flap transplantation. Moreover, targeted antibiotics are administered in case of infection. However, Surgery for chronic wounds can be challenging due to limited donor sites, donor damage, scar formation, and even severe functional and psycho-social disorders.18–20 Moreover, antibiotic overuse can lead to drug resistance, creating new problems for infectious chronic wounds.21,22 Moreover, chronic wounds become refractory due to infections, diabetes, ischemia, over-degradation of collagen, and other factors, leading to the failure of traditional treatment methods. Thus, novel methods for treating chronic wounds need to be explored.
Skin wounds are the most common type of tissue injury, and they can be caused by trauma, surgery, burns, chronic diseases, or cancers.4,23 Under adverse conditions, wounds often turn chronic. The acceleration of wound repair and improvement of the healing process are the primary objectives of chronic wound treatment. Nanobiotechnology, which involves the use of nano-sized particles in biological systems, represents the convergence of several scientific fields, including chemistry, biology, physics, optics, mechanics, and nanoscale Science and technology. Nanobiotechnology can provide tools and technologies for examining and modulating biological systems.24,25 By applying nanotechnology in the field of bioMedicine, several novel biomaterials, biosensors, and bio-therapies have been designed and studied. It is believed that the combination of nanotechnology and biology can aid in wound management, monitoring, and repair.26,27 Initially, the application of nanobiotechnology in chronic wound treatment was focused on the provision of scaffolds for cell migration and the replacement of traditional gauze dressing.28–30 However, with the development of nanotechnology and our understanding of wound healing mechanisms, various nanobiotechnology-based wound-treatments systems — including drug and gene delivery platforms, antimicrobial systems, and cell-carrying systems — have been developed and found to have prospective applications.31–36 Nevertheless, despite these advances, wound dressings remain largely primitive and lack functions that allow wound monitoring and dynamic wound responses. Therefore, smart hydrogels or bandage systems developed using nano-sized biomaterials, which can respond to stimuli or monitor the status of chronic wounds, have been examined.37,38
This review article provides a summary of nanobiotechnology-based scaffold, delivery, antimicrobial, cell-carrying, collagen modulating, stimuli-responsive, and wound monitoring systems for chronic wound healing. Further, the prospects of nanobiotechnology to achieve better treatment outcomes for chronic wounds are discussed.
Nanoplatforms Designed for Chronic Wound Healing
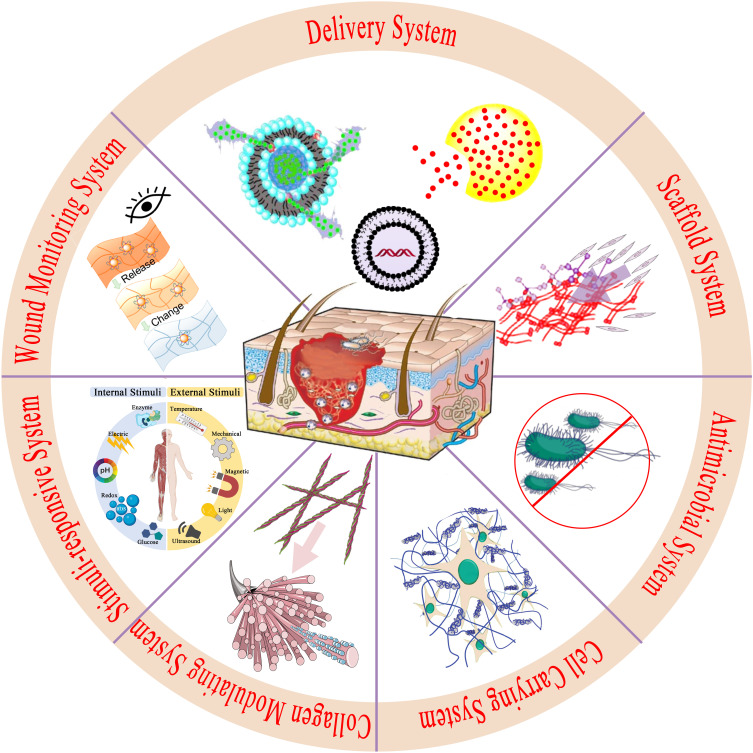
Physiologically, the wound healing process is affected by several factors, including gene expression; cell functions such as migration, proliferation, and differentiation; the skin microenvironment; infection; ischemia–hypoxia; inflammation; and collagen formation and arrangement.1,3,17,39–42 These factors are used as references for the design of nanobiotechnology systems that promote chronic wound repair (Figure 2) and need to be carefully considered before designing such systems.
Figure 2.
Nanoplatform for chronic wound healing.
To repair tissue defects in the wound area, a platform for cell adhesion, migration, and proliferation — ie, a scaffold for cells — needs to be established. Such a scaffold can also serve as a platform for multi-functional modification. Given their good biocompatibility, angiogenic capacity, and biomimetic behavior to natural human skin, nano-scaffold systems are widely used in tissue engineering.43–46
Tradition treatment methods for chronic wounds that show delayed Union involve local or systemic drug administration. However, the performance of these drugs is suboptimal owing to limitations such as low solubility and low bioactivity. Nanobiotechnology has thus been leveraged for the development of drug, gene, and exosome delivery systems that can help in overcoming these limitations.34,47,48
Infections, which impede tissue repair, should receive careful attention in chronic wound treatment. Silver nano-particles, a product of nanobiotechnology, have been used clinically in the treatment of microbial infection for decades. Moreover, several more recent studies have explored new nanoplatform-based anti-infection therapies, including potential anti-infection nanoparticles (NPs).49–52
Cell therapy, especially stem cell therapy, is currently a focus in regenerative medicine and diabetic wound repair. In some basic medical and preclinical studies, chronic wound treatment with stem cells has shown excellent outcomes.53–55 However, despite its great potential, the clinical translation of stem cell therapy for chronic wound healing is hindered by the lack of appropriate methods for cell encapsulation and transplantation. Thus, the development of nanobiotechnology-based cell-carrying systems can provide improved therapeutic effects.56,57
With the development of precision medicine, therapeutic systems that monitor wounds and respond to individual stimuli are expected to become popular. One such system is based on ferrihydrite NPs, which can respond to blue light and are effective for antimicrobial and wound healing treatments.58 More stimuli-responsive materials and monitoring systems for chronic wound healing can be generated through nanobiotechnology.
Nanoplatforms for Chronic Wound Healing
Scaffold Systems
The term scaffold system generally refers to materials that can integrate with living tissues and cells and can be implanted into different tissues where they supplement natural tissue function based on specific conditions. In order to enable seed cells to proliferate and differentiate, a scaffold composed of biological materials that acts as an artificial extracellular matrix (ECM) is required. Scaffolds are critical for tissue engineering systems, including those for bone, cartilage, blood vessels, nerves, skin, and artificial organs (eg, liver, spleen, kidney, and bladder).
Nano-scaffold systems aimed at chronic wound healing need to possess certain important features.
1. Safety and good biocompatibility: Scaffolds should be safe. Furthermore, their chemical components and degradation products should cause minimal immune or inflammatory responses in the body during a predetermined period.59
2. Appropriate size, dimensions, and mechanical strength: The chemical features of the scaffold should provide suitable microenvironments and maintain the biological activity of loaded cells or tissues for a long time.
3. Appropriate pore size and distribution: Scaffolds should have a highly and well-connected porous structure with an ideal pore size to allow cells, drugs, and bioactive molecules to get evenly distributed throughout the scaffold.60
4. Excellent biological behaviors: Scaffolds and the substances present in the scaffold should promote the proliferation and migration of fibroblasts, keratinocytes, and endothelial cells, thus promoting wound healing.61,62
5. Appropriate wound healing environment: The scaffold system should be able to absorb the wound exudate and prevent wound dehydration, reducing surface necrosis on the wound.63,64
Scaffold systems can be classified as follows based on the source and function of the materials.
Source of Materials
When designing scaffold systems for chronic wound, an appropriate matrix source needs to be selected. Table 1 lists a few sources of nanocomposites used in wound dressing. Natural nanomaterials and their derivates have good biocompatibility and can be degraded by enzymes or water. However, their characters and quality differ from batch to batch and cannot be standardized. In contrast, synthetic biomaterials, such as polyethylene glycol (PEG) nano-scaffolds, show more stable structural properties and can be chemically modified. However, the biosafety of synthetic materials needs to be strictly examined.
Table 1.
Sources of Nanocomposites
| Category | Examples |
|---|---|
| Natural biomaterials | Chitosan,65 gelatin, agar, glucan, hyaluronic acid,66 collagen,67 silk fibroin, alginate |
| Synthetic biomaterials | PEG-based nano-scaffold,68 alginate-polycaprolactone (PCL), electrospun membranes,69,70 polyurethane-based scaffold,71 microporous annealed particle scaffold72 |
Function of Materials
According to their functions, tissue engineering materials can be used for bones, nerves, blood vessels, skin, and other tissues (eg, tendon, ligament, cornea, liver, and kidneys).
Tissue engineering scaffolds for the skin can be of several types. These include natural polymers (chitosan, hyaluronic acid, and collagen), nanocomposite scaffolds (eg, nanobioactive glass and metal NPs), and conducting polymers (eg, polyaniline, polypyrrole, and polythiophene).73–75 Taghiabadi et al synthesized an intact amniotic membrane-based scaffold for cultivating adipose-derived stromal cells (ASCs). By ASCs on an acellular human amniotic membrane (HAM), they created a neoteric skin substitute.76 Zhang et al designed a conductive and antibacterial hydrogel based on polypyrrole and functionalized Zn–chitosan molecules for the management of infected chronic wounds. They demonstrated the promising potential of the hydrogel in promoting the healing of the infected chronic wound after electrical stimulation. Currently, other tissue engineering scaffolds such as calcium phosphates and composite materials (eg, hydroxyapatite, β-tricalcium phosphate, and whitlockite) for bone tissue engineering and amniotic membranes for corneal tissue engineering are under research.69,77
Skin tissue engineering scaffolds can be categorized as porous, fibrous, microsphere, hydrogel, composite, and acellular materials.73 Typically, natural biomaterials and their derivatives are biodegradable, absorbable, and harmless to the body, but their strength and processing performance are poor and their degradation speed cannot be controlled. Hence, in order to improve the mechanical and biological properties of scaffolds (eg, adhesion, strength, processing performance, and degradation speed) and accelerate wound healing, composite scaffolds have been developed by combining the characteristics and advantages of different materials. Depending on their constituents, these composite scaffolds can achieve specific functions. Currently, most novel scaffolds being developed use composite materials to obtain multifunctional characteristics.
Delivery Systems
Delivery systems are used to deliver drugs, cells, genes, and other neoteric bioactive molecules to the body or target area via transplantation or injection.78 Traditionally, delivery systems are broadly divided into two categories, drug delivery and cell delivery. With continuous Innovation in scientific research, new approaches, including gene delivery and the delivery of bioactive molecules such as growth factors, proteins, and peptides, are being developed.
Recently, there has been a significant increase in new biotechnology-based treatments, among which cell and gene therapies are quite sophisticated. Exosomes have shown superior therapeutic potential against various conditions, and delivery methods are being devised to maximize their therapeutic effectiveness. Moreover, exosomes are also emerging as a delivery system for other substances (eg, small molecules and miRNAs).79 NPs are essential for the delivery of these refined substances. In addition to serving as delivery vehicles, NPs can also act as diagnostic and therapeutic agents for some diseases.80 Research on nanoparticle-based drug delivery has mainly been focused on targeted drug delivery, and especially tumor-targeted drug delivery.81
Drug Delivery
A drug delivery system serves as a vehicle for therapeutic molecules. It allows drug delivery in the body, improves drug efficacy, and allows safe and controlled drug release.
The conventional routes for drug delivery80 are gastrointestinal drug delivery (eg, oral and rectal), parenteral administration (eg, subcutaneous, intramuscular, and intravenous injection) and topical administration (eg, percutaneous injection and wound dressings). Novel drug delivery systems for wound healing can be classified into the following categories: NPs, microcarriers, and tissue-engineered scaffolds.82 Skin tissue engineering scaffolds have been introduced earlier in this review, and NPs and microcarriers will be introduced in detail here (Table 2).
Table 2.
Drug Delivery Systems Developed Using Nanotechnology
| Category | Examples | |
|---|---|---|
| NPs | Inorganic NPs | Metal nanoparticles: CuNPs,87 AgNPs,88,89 and Cerium Oxide NPs90 |
| Carbon-based NPs91,92 | ||
| Quantum dots: self-assembled GQDs,93 Orange-emissive carbon quantum dots94 | ||
| Organic NPs | Dendrimers: act as antibacterial agents95,96 | |
| Hydrogels: chitosan,97 PVA,98 and PEG99 | ||
| Nano-emulsions can improve the solubility and reduce the enzymatic hydrolysis of drugs100 | ||
| Liposomal NPs: solid lipid NPs101,102 and nanostructured lipid carriers103,104 | ||
| Polymer NPs | PCL,69 PEG,68 and photothermal NPs105 | |
| Smart stimuli-responsive nanostructures | MMP9-responsive,106 pH-responsive,107 immune responsive,72 and thermosensitive nanostructures99 | |
| Nanofibers (NFs) | NFs can increase the transfer of various molecules and perform diverse functions108 | |
| Microspheres coated with nanocomposites | PCL microspheres,86 chitin microspheres,109 bacterial cellulose microspheres,110 and gelatin microspheres111 | |
| 3D-printed scaffolds | Typical three-dimensional porous matrix112 | |
| Engineered films | Agar-glycerol-sericin films113,114 | |
Abbreviations: GQD, graphene quantum dots; MMP, matrix metalloproteinase.
Drug-loaded nano-scaffolds that promote wound healing after topical administration have been developed. However, due to their poor solubility, short half-life, and other drawbacks, some drugs do not accumulate at an optimal concentration at the wound site for a long duration.83 Nano-scaffolds with varying porous structures can be used to load drugs or bioactive molecules, and the porous structure can provide a breathable environment for the wound.84 NPs carrying poorly soluble drugs are widely used to prepare controlled drug delivery systems. Nano-scaffolds typically show slow degradation, allowing long-term drug release and thereby maintaining an ideal concentration of the drug in the plasma.85 Shamloo et al developed polyvinyl alcohol (PVA)/chitosan/gelatin hydrogels to overcome the short half-life of basic fibroblast growth factor (bFGF). The biocompatibility of the hydrogel supported the continuous delivery of bFGF and significantly accelerated wound healing.86
During the treatment of chronic wounds, the drug is usually applied directly on affected region. Nanotechnology-based drug delivery systems could enable controlled drug release. Meanwhile, the degradability and stability of the drug could also be modified using nanosystems. Hence, these drug delivery systems could improve treatment compliance among patients with chronic wounds by reducing the application frequency and the cost of treatment.
It is widely acknowledged that metal ion-based biomaterials exhibit promising antimicrobial activity when applied to wounds, making them very suitable for the management of diabetic wounds, which are prone to infection. Given their reducing properties, under oxidative stress, cuprous ions provide a promising therapeutic option for diabetic wounds. Copper ions have also been reported to promote angiogenesis.115–117 Equipped with infrared absorption and efficient heat generation abilities, semiconductor cuprous sulfide (Cu2S) NPs are widely employed as photothermal agents. Wang et al utilized the photothermal effect of Cu2S and the angiogenic effect of Cu ions to prepare electrospun fibers containing Cu2S NPs, achieving a combination of advantages based on the components and successfully promoting diabetic wound healing. Moreover, their biomaterial could also effectively inhibit the growth of skin tumors both in vivo and in vitro.70 This system demonstrated the effectiveness of bifunctional tissue engineering biomaterials, providing a novel method for drug delivery for the treatment of biological conditions.
Gene Delivery
Classic gene therapy generally involves the expression of exogenous genes or the silencing of target genes via viral or non-viral delivery.118,119 In general, gene delivery via viral transfection may be carcinogenic.119 Most gene therapies for diabetic wounds are based on siRNAs. Gene therapy has become a promising strategy for the treatment of various diseases, and its effects are mediated via the regulation of RNA and protein expression.120 Many unmodified gene therapy agents, such as proteins, peptides, and nucleic acids, are rapidly degraded or eliminated from systemic circulation before they can accumulate at effective concentrations at the target site. Owing to poor pharmacokinetics, repeated administration is warranted. This, in addition to the narrow range of safe doses, often leads to adverse effects during treatment.121
Several studies on wound management and especially chronic diabetic wound management have focused on gene- or RNA-based (eg, mRNA, microRNA, circRNA, and lncRNA) therapies.122 Subcutaneous local injections can be used to directly deliver RNAs or proteins to the wound site.123 However, due to the short half-life of the therapeutic agent, repeated administration is required, often leading to pain and poor treatment compliance. Drug delivery systems not only solve these problems but also protect gene-related small molecules from degradation and eliminated from the body. The greatest challenge in gene therapy is ensuring the successful transduction or transfection of target genes into host cells by crossing extracellular and intracellular barriers. Therefore, the engineering of gene delivery vehicles is complex.118 Moreover, the materials used to encapsulate gene-related small molecules are required to have low toxicity and promote a high transfection efficiency.124 Currently, the NPs that deliver siRNAs to promote wound management are composed of lipids, polymers (eg, chitosan, PEG), hyperbranched cationic polysaccharides (HCP), and silicon.125–130
Shaabani et al developed layer-by-layer self-assembled siRNA-loaded gold NPs with two different outer layers — Chitosan (AuNP@CS) and Poly L-arginine (AuNP@PLA).126 They compared the two types of NPs, which had a similar core structure. They found that the two polymers had different escape mechanisms: the buffering capacity of chitosan resulted in endosome disruption,131 while PLA bound to the endosome lipid bilayer and promoted escaped through pore formation. Their results indicated that an outer layer of PLA allows the endosomal escape of siRNA, thus improving transfection efficiency and delivering target molecules to promote diabetic wound healing. Given that naked siRNAs are easily eliminated from the body, Li et al and Lan et al designed four HCP derivative-based vehicles128,129 for the delivery of siRNA against MMP9. This treatment led to the knockdown of MMP9, which prevents the healing of diabetic wounds, and thus promoted diabetic wound healing. Currently, nanocomposite-based gene delivery applications are focused on siRNA. However, efforts to deliver other products such as miRNA, lncRNA, or even DNA will be required in the future.
Exosome Delivery
Exosomes are endosome-derived vesicles (30 to 150 nm in size) secreted by a variety of cells, including adipose stem cells (ADSCs), bone marrow stem cells (BMSCs), and mesenchymal stem cells (MSCs).132,133 Different types of cells secrete exosomes with different specific markers, which account for their specific functions. Despite their different origins, exosomes have a similar appearance and size and often have a common composition. Once they are isolated from an extracellular medium or from biological fluids, the source of exosomes cannot be ascertained of.134 Exosomes can be employed as small molecules for wound treatment. The combination of exosomes with porous NPs can increase therapeutic effects while maintaining the advantages of a scaffold. Importantly, exosomes can also be used as nanocarriers for drug delivery and targeted therapy, and these are called engineered exosomes.133,135
Exosomes can effectively promote diabetic wound healing.136,137 Shiekh et al embedded ADSC-derived exosomes (ADSC-exo) into antioxidant polyurethane scaffolds to achieve sustained exosome release. Their nanosystem leveraged the advantages of the scaffold, including antioxidant and antibacterial effects, to accelerate diabetic wounds healing both in vivo and in vitro.71 To prolong the half-life and lower the clearance rate of exosomes, Lei et al designed an ultraviolet-shielding nano-dressing based on polysaccharides that allowed exosome delivery and had self-healing, anti-infection and thermo-sensitive properties.61 These findings indicate that exosomes can be stabilized and well-delivered to target cells by combining them with porous NPs or nanocarriers and can be applied for treating chronic wounds.
Antimicrobial System
It is widely accepted that infection is an important factor to monitor during the wound healing process as it can lead to progression of the chronic wound or even sepsis.138–140 Conventional prevention and treatment approaches for wound infection involve local or systemic antibiotic administration, which can lead to failed anti-infection treatment or even antibiotic resistance.141,142 Several nano-formulations that have antimicrobial ability have been developed and used in anti-infectious wound therapy, playing a critical role in infection management. Table 3 lists some antimicrobial nanobiotechnology-based systems used in wound healing.
Table 3.
Nanomaterials Used in Anti-Microbial Wound Dressing
| Components | Size (nm) | Target Pathogens | Wound Type | Ref |
|---|---|---|---|---|
| Ag/Fe3O4 NCs | 15–50 | S. aureus | Chronic wounds | [143] |
| AgNPs | 14–54 | S. aureus andP. aeruginosa | Acute and diabetic wounds | [144] |
| AgNPs | 3–5 | MRSA | Infectious wounds | [145] |
| AgNPs/ZnO | 50 | E. coli and S. aureus | Normal wounds | [146] |
| Au/Ag NRs | 50 | E. coli and MRSA | Normal wounds | [147] |
| Au/Ag/Cu2O NSs | 10–73 | E. coli and MRSA | Infectious and chronic wounds | [148] |
| AuNCs | 2 | E. coli and S. aureus | Normal wounds | [149] |
| AuNPs | 34 | S. aureus | Infectious and burn wounds | [150] |
| Carbon NTs | 150–250 | Mycobacterium tuberculosis | Secondary wounds | [151] |
| Ag nanofilms | 7–33 | S. aureus andP. aeruginosa | Thermal burn wounds | [152] |
| CuNCs | 30 | E. coli and S. aureus | Normal wounds | [153] |
| CuNPs | 110 | B. subtilis, P. aeruginosa, S. aureus, and E. coli | Normal wounds | [154] |
| Cu2WS4 NCs | 20 | E. coli and S. aureus | Normal wounds | [155] |
| Cu-TCPP(Fe) nanosheets | 3–5 | E. coli and S. aureus | Normal wounds | [156] |
| Fe3O4 | 20 | E. coli and S. aureus | Infectious wounds | [157] |
| Zn/SiO2 nanospheres | 80–120 | E. coli | Normal wounds | [158] |
| ZnO | 20 | E. coli and S. aureus | Normal wounds | [159] |
| ZnO/Au NPs | 20–50 | S. aureus and MRSH | Normal wounds | [160] |
| ZnO NPs | 20 | E. coli and S. aureus | Infectious and burn wounds | [161] |
Abbreviations: MRSA, multi-drug resistant S. aureus; MRSH, multi-drug resistant S. haemolyticus; NC, nanocomponent; NT, nanotube.
Inorganic Nano-Antimicrobial Materials
Metals have been used as inorganic antimicrobial agents for thousands of years and were even used as anti-infection agents in ancient Persia.162 Metal NPs, such as AgNPs, AuNPs, and CuNPs, have attracted great attention due to their anti-infection properties and low toxicity.163 Given that metal NPs do not cause antimicrobial resistance and release metal ions or produce ROS — which can kill microorganisms — they appear to be suitable alternatives to antibiotics as.164,165
AgNPs, which are the more well-known metal NPs, have been used widely in clinical practice and basic medical research. Wound treatment products containing AgNPs have been commercially available for decades.166 AgNPs can continuously generate Ag+, which reacts with proteins and nucleic acids, causing molecular defects and killing bacteria and viruses.167–170 Several studies have shown that AgNPs have good potential as antiseptics. Luna-Hernández et al found that a combination of functional chitosan and silver nanocomposites showed antibacterial effects against S. aureus and P. aeruginosa in burn wounds.152 Moreover, in mice treated with the composite dressing, silver accumulation was found to be far lower than that in mice treated with the clinically used AcasinTM nanosilver dressing. Zlatko et al demonstrated that the AgNPs hydrogel serves as a versatile platform, with features such as antibacterial efficacy, exudate absorbance, low cost, biocompatibility, hemocompatibility, and improved healing for chronic wounds.171 Huang et al constructed an organic framework-based microneedle patch containing AgNPs. The product showed transdermal delivery and could prevent S. aureus, E. coli, and P. aeruginosa infections in diabetic wounds.172 In addition, several commercialized products containing AgNPs have been developed for clinical treatment. These include Acticoat™, Allevyn® Ag, Aquacel® Ag Surgical, Atrauman Ag, Biatain® Silicone Ag, Flaminal®, Mepilex® Transfer Ag, SILVERCEL™, and Urgo Clean Ag.
Nano-sized gold is also useful as an anti-infection agent. It has been confirmed that AuNPs bind to bacterial DNA and show bactericidal and bacteriostatic properties.173,174 Some studies show that Au nanocomposites can kill MRSA and P. aeruginosa through photothermal effects and could promote wound closure.150,175
Compared with gold and silver, copper is less expensive and more easily available. CuNPs are considered the best candidates for developing future technologies for the management of infectious and communicable diseases.49 Cai et al developed a CuNP-embedded hydrogel that accelerated wound healing and showed effective antibacterial capacity against both gram-positive and gram-negative bacteria as well as great photothermal properties.176
Inorganic non-metal nano-materials have been also considered potential antimicrobial agents owing to their intrinsic anti-infection effects.177 Based on the unique structural and physio-chemical properties of carbon nanomaterials, a research team prepared a carbon nanofiber platform that inhibits the growth of E. coli and MRSA.178 In this study, CuNPs and ZnNPs were asymmetrically distributed in carbon NFs grown on an activated carbon fiber substrate using chemical vapor deposition (CVD). The carbon NFs platform inhibited the growth of gram-positive and gram-negative bacterial strains with superior efficiency than simple metal NPs. Another study showed that carbon nanotubes can be used to prepare wound-repairing bandages with infection-preventing properties.179
Organic Nano-Antimicrobial Materials
The natural organic biomaterial chitosan and its derivatives are popular in biomedicine. Chitosan possesses good biocompatibility, antimicrobial properties, and low immunogenicity.180 Using nanobiotechnology, Ganji et al fabricated a nanofiber with chitosan-encapsulated nanoparticles loaded with curcumin for wound dressing. The electrospun chitosan-based nanofiber inhibited the growth of E. coli and MRSA by 98.9% and 99.3% in infected wounds in mice.50 Another type of chitosan nanofiber also showed potential in wound care owing to its antibacterial and re-epithelialization-promoting effects.181 Antibiotic-loaded chitosan nanofibers have also been used for local drug delivery and wound treatment.182 Other metal–organic framework nanorods have also shown bacterial inhibition in infectious wounds.183 Dias et al developed a series of soluble potato starch nanofibers sized 70–264 nm. They incorporated carvacrol during the synthesis of the potato starch nanofibers, and the obtained nanocomposites showed great anti-pathogenic activity against S. aureus, E. coli, L. monocytogenes, and S. typhimurium, highlighting their potential as agents for wound dressing.184
With respect to organic nano-materials, anti-infection approaches focus on natural antibacterial compounds such as chitosan and its derivatives. Further, owing to the bactericidal effects of metals, metal-organic frameworks are also used. Given that metal NPs are associated with the potential risks of metal deposition, organic nano-antimicrobial materials, especially natural macromolecules with antibacterial properties, may become useful for wound dressing.
Anti-Biofilm Systems
Biofilm, which are made up of surface-attached groups of microbes, are considered to be the primary cause of chronic wounds owing to their role in antibiotic resistance.141,185–187 Most biofilms are formed on the surface of wounds. However, some special biofilms can get implanted into the deep layers of skin tissue, making traditional diagnose and treatment challenging.188 The clinical treatment of biofilms in wounds involves wound cleansing with polyvinylpyrrolidone or hydrogen peroxide, debridement, refashioning of wound edges, dressing, and the topical or general administration of antibiotics.189 With further insights into the mechanisms of biofilm formation and developments in nanobiotechnology, nanomaterials effective for biofilm therapy have been developed.
Nanomaterials based on metals or metal oxides are widely used against wound biofilms, including silver, copper, gold, titanium, zinc oxide, magnesium oxide, copper oxide, and iron oxide.190,191 Owing to the small size of these particles, metal or metal oxide NPs can move across bacterial membranes and rupture them. They can destroy enzyme activity and the respiratory chain in bacteria. It has been demonstrated that Ag NPs and silver oxide NPs are the most effective against microbial biofilms.192,193 Abdalla et al functionalized nano-silver with lactoferrin and incorporated them in a gelatin hydrogel, generating a dual-antimicrobial action dressing for infectious wounds and maximizing the anti-biofilm property of silver.194
Chitosan, bacterial cellulose (BC) and other natural antimicrobials have been modified using nanotechnology to treat wound biofilms. Owing to the positive charge on the polymeric chain of chitosan, chitosan NPs easily adhere to the negatively charged microbial membrane, triggering changes in permeability and preventing biofilm formation.195 Zemjkoski et al obtained chitosan NPs through gamma irradiation and encapsuled them into BC to form BC-nChiD hydrogels with excellent anti-biofilm potential. These hydrogels could provide a 90% reduction in viable biofilms and a 65% reduction in biofilm height.196 Mahtab reduced the amount of bacteria in a planktonic condition by treating bacterial biofilms with photodynamic therapy using curcumin encapsulated into silica NPs. After exposure to blue light, ROS was produced owing to the photodynamic properties of silica NPs. The ROS damaged biofilms, and the curcumin released prevented bacterial growth.197
The size of nanoparticles can be controlled, and they have a large specific area, can penetrate bacterial membranes, and show bactericidal properties. Hence, nanotechnology has great potential in destroying biofilms and treating infectious chronic wounds. In addition to providing nanoparticles with anti-infection properties, nanotechnology could also be used to provide a platform for antibiotics, enhance their solubility, prolong their half-life, and reduce the required treatment dose.
Cell-Carrying Systems
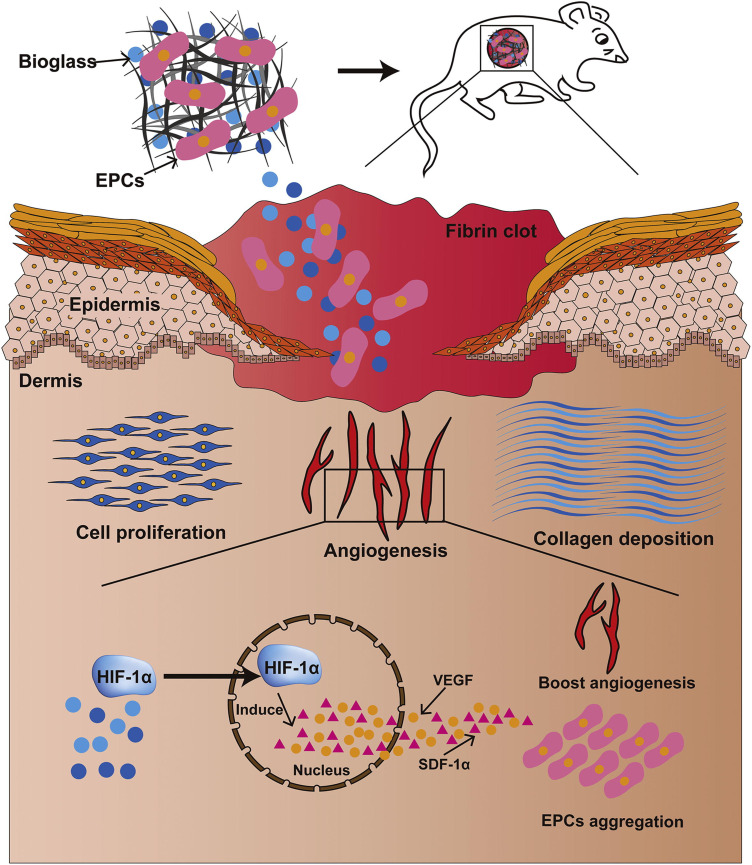
Due to its superiority with respect to tissue engineering, cell-based therapy is extensively used for chronic wound treatment.198–201 Stem cells derived from bone marrow, the umbilical cord, and adipose and cutaneous tissue can differentiate into various tissue types and modulate cell migration, collagen deposition, re-epithelialization, and tissue remodeling.198,202–205 Nanofibers prepared using electrostatic spinning are widely used for scaffolding. Mao et al prepared polycaprolactone nanofibrous scaffolds and combined collagen with bioactive glass NPs (CPB nanofibrous scaffold). The CPB nanofibrous scaffold exerted positive effects as a cell-carrying system containing epithelial progenitor cells (EPCs). The EPC-carrying CPB bioactive complex promoted wound healing by enhancing cell proliferation, granulation tissue formation, re-epithelialization, and cell adhesion (Figure 3).206 Khojasteh et al found that curcumin-carrying chitosan/poly(vinyl alcohol) nanofibers can carry pad-derived mesenchymal stem cells and show excellent curcumin release and improve cell adhesion and proliferation, indicating that they could be useful in wound dressings.207 Kaplan et al produced an injectable silk nanofiber hydrogel embedded with BMSCs. The nanofiber hydrogel maintained the stemness of the BMSCs, successfully carrying them to the target site and promoting wound healing through increased angiogenesis and collagen deposition.57
Figure 3.
Schematic of a CPB/EPC construct that promotes wound healing. CPB enhances cell proliferation, collagen deposition, and EPC differentiation via the Hif-1α/VEGF/SDF-1α pathway. This results in the rapid vascularization and healing of full-thickness wounds.
Notes: Reprinted from: Wang C, Wang Q, Gao W et al. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomaterialia. 2018;69:156–169. doi:10.1016/j.actbio.2018.01.019.206 © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved. With permission from Elsevier. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1742706118300308#f0060.
Usually, cell therapy in wound care is performed using micrometer-scale carriers as cell sizes fall in the range of microns. With the development of nanotechnology, an increasing number of nanofibers and NPs are being developed for cell therapy aimed at treating chronic wound given the excellent pro-differentiation, stemness-holding, and immunoregulation properties of the nanocomposites.
Collagen Modulating Systems
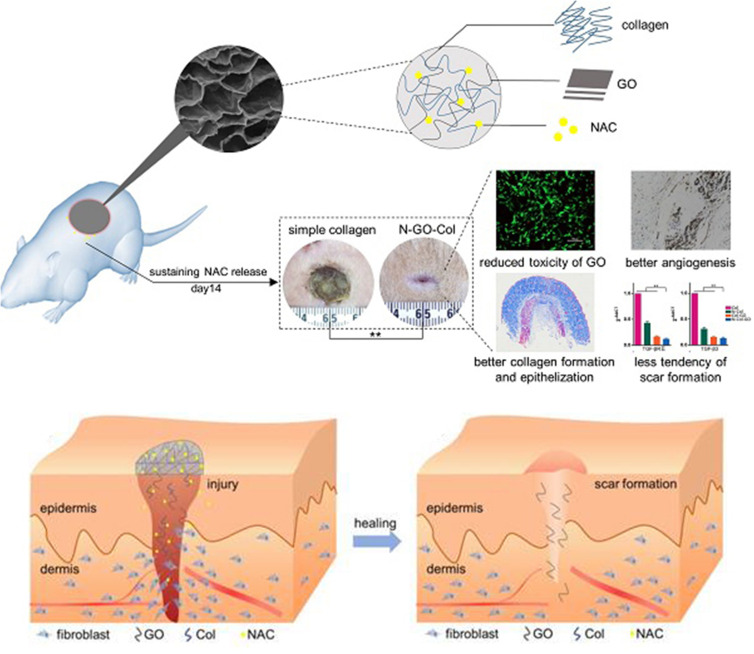
As an important component of the extracellular matrix, collagen mediates communication between cells, provides a scaffold for cell migration and adhesion, and plays a role in chronic wound healing.4 Some nanobiotechnology-based platforms have been used for collagen modulation. Sun et al loaded N-acetyl cysteine onto graphene oxide (GO) NPs to enable scarless wound healing (Figure 4).208 In their study, GO NPs decreased collagen metabolism and improved the balance between collagen formation and degradation, thus allowing the wound to heal without scarring. In another study by the same group, a polyamide nanofiber-based multi-layered scaffold was found to promote wound healing by encouraging the uniform arrangement of collagen.209 Krian et al synthesized a 3-D biomatrix with nanotized praseodymium that promotes collagen function via the stabilization of native collagen. Their rare-earth metal nanoparticles thus showed potential applications in wound care.210
Figure 4.
Wound healing effect of a scaffold based on GO NPs.
Notes: Adapted from: Li J, Zhou C, Luo C et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics. 2019;9(20):5839–5853. doi:10.7150/thno.34480.208 © The author(s). Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions.
In chronic wound treatment, deposited collagen acts as a natural scaffold for cells, and therefore, modulating collagens is synonymous with re-establishing tissue structure in the wound area. As a result, collagen-modulating nano-systems have mainly been used for accelerating tissue repair. However, the studies by Sun’s group are inspirational and demonstrate that this approach should also be utilized for developing chronic wound treatments that decrease scarring.
Stimuli-Responsive Systems
Despite the availability of dozens of commercial wound-care products, bionic systems have not yet been adopted for wound healing. There is an urgent need for smart wound-healing systems that can respond to the stimuli (temperature, pH, glucose, enzyme, etc.) at the site of the chronic wound area.211,212 Through developments in nanobiotechnology, NPs with stimuli-response characteristics have received great attention. Gong et al synthesized a nanozyme consisting of poly(acrylic acid)-coated Fe3O4 NPs (pFe3O4) and then combined them with GO to produce pFe3O4@GO NCs. The pFe3O4@GO NCs could react with glucose and function as a self-supplying H2O2 nanogenerator at the wound site, allowing the chemodynamic treatment of wound infections.157 Some researchers developed photoactive electrospun nanofibers using cellulose acetate, polyethylene oxide, methylene blue, and three-layered cellulose acetate/polyethylene oxide/silk fibroin/ciprofloxacin. The nanofibers could produce ROS after light irradiation at 635 nm, accelerating the healing of infectious wounds by inhibiting S. aureus, K. pneumoniae, and P. aeruginosa biofilms.213 Zhang et al developed a hybrid hydrogel with MnO2 nanosheets. The injectable MnO2 nanosheet hydrogel could perform thermogenesis under 808-nm laser irradiation, eliminating ROS and inflammation and promoting wound repair.214 Overall, nano-structures functionalized using stimuli-response properties could simulate the biological, chemical, and physical characteristics of natural skin, enabling tissue regeneration in refractory wounds.
Wound Monitoring System
Given the elucidation of mechanisms and physiological changes associated with wound healing, sensors that allow real-time monitoring of wound repair have been developed.215–217 A complex smart wound-monitoring wound dressing has also been invented.218 This dressing contains a nanofiber membrane made of chitosan/collagen, and promotes proliferation and regeneration by upregulating extracellular matrix secretion and promoting integrin/FAK signaling. Olivo et al added AgNPs to a fiber-based membrane monitor to increase the active surface area in the sensor, improving the detection sensitivity for biomarkers in the wound area.219 In order to avoid secondary wound damage caused by dressing changes, Jiang et al created bacterial cellulose-based membranes with aminobenzeneboronic acid-modified gold nanoclusters (A-GNCs), which could be used for treating wounds infected with multidrug-resistant bacteria.220 A-GNCs emit bright orange fluorescence under UV light, and the intensity of this fluorescence decreases with the release of A-GNCs. This allows healthcare professionals to determine when the dressing needs to be replaced. In the past few years, dressings that can monitor the status of chronic wounds in real-time have been tested. However, this field is relatively new, and current research on nanotech-based systems for monitoring chronic wounds is scarce.
Clinical Trials for Nanobiotechnology-Based Wound-Healing Treatments
Along with advances in nanobiotechnology research, several new nanosystems have advanced from the laboratory investigation stage to the clinical trial stage. Table 4 lists some clinical trials that have tested nano-therapies for wound healing. As early as 2014, Lopes et al investigated the cost-effectiveness of using nanocrystalline silver for treating burns. Their study showed that AgNPs provided faster wound healing than traditional silver sulfadiazine, requiring fewer dressing changes and reducing the human resource burden.221 Meanwhile, some clinical trials tested the use of nano-products for treating chronic wounds (Table 4). Although metal NPs were typically used for antimicrobial therapy, one clinical trial studied the efficacy and safety of autologous nano-fat combined with platelet-rich fibrin for treating refractory diabetic foot wounds. However, overall, there were few clinical trials examining the applications of nanoplatforms in chronic wound care, likely owing to inadequate previous research on biocompatibility. Moreover, few doctors participated in research on nanotechnology-based chronic-wound treatment, and hence, several clinical requirements were ignored or misunderstood.
Table 4.
List of Clinical Trials for Nanobiotechnology-Based Wound Treatment
| Registration Date | Title | Conditions | Treatment | Type of Platform | Trial Registration Number |
|---|---|---|---|---|---|
| 2014 | Comparative Analysis of Cost-effectiveness of Silver Dressing in Burns (ARGENTUM) | Second-degree burn | Nanocrystalline silver | Antimicrobial system | NCT02108535 |
| 2016 | Evaluation of the SPINNER Device for the Application of Wound Dressing: Treatment of Split Skin Graft Donor Sites | Skin wound | SPINNER (in situ nanofiber dressing) | Scaffold system | NCT02680106 |
| 2017 | A randomized, open label, parallel-controlled trial of the efficacy and safety of autologous nano-fat combined with platelet-rich fibrin in the treatment of refractory wounds of diabetic foot | Diabetic foot wound | Nano-fat combined with platelet-rich fibrin | Delivery system | ChiCTR-INR-17013540 |
| 2018 | Research on the Key Technology of Burn Wound Treatment | Burn | Nano-silver ion gel | Antimicrobial system | NCT03279549 |
| 2019 | A randomized, controlled, non-inferiority study of silver sulfate gauze self-adhesive dressings for non-chronic wounds | Non-chronic wound | Nano-silver trauma patch | Antimicrobial system | ChiCTR1900024140 |
| 2019 | Evaluation of Diabetic Foot Wound Healing Using Hydrogel/Nano Silver-based Dressing vs Traditional Dressing | Diabetic foot wound | Hydrogel/nano silver-based dressing | Antimicrobial system | NCT04834245 |
Conclusion and Future Prospects
As nanobiotechnology has developed, nano-sized biomaterials have been widely applied for treating chronic wounds. This review article highlights that the application of nanotechnology in chronic wound treatment has, so far, largely focused on scaffold construction, anti-infection treatment, and substance delivery.34,45,47,130,147
In scaffold systems, nanobiotechnology provides both materials and techniques for managing chronic wounds. Electrospinning, a nanotechnique, allows the production of biomimetic structures that mimic the natural skin and help in healing refractory wounds.50 Furthermore, some nano-scaffolds promote cell adhesion and migration by mimicking the construction of natural tissues, thus promoting chronic wound healing. Nevertheless, there is further scope to improve the quality of natural nano-biomaterials and the biocompatibility of synthetic nano-biomaterials to increase their application.
Dozens of metal NPs, and especially AgNPs, have been used in antimicrobial therapy for chronic wounds.163 However, metal deposition can cause DNA and cell damage. Hence, nanomaterials that prevent infection without causing toxicity are required. Further effort should be made to decrease the accumulation of heavy metals. Alternatively, nanocomposites without metal elements should be adopted more often in the future.
To overcome the ever-changing environment of the skin during chronic wound healing, several wound-monitoring and stimuli-responsive biomaterials have been developed.58,157,218 By leveraging specific characteristics, such as the photothermal effect, chemo-dynamic effect, fluorescence, and thermo-sensitivity, more nano-biomaterials that can be used in stimuli-responsive and dynamic monitoring systems for wound care should be developed. Most studies on wound healing have focused on migration-promoting effects, antimicrobial activity, and substance delivery. However, few nanotech-based multifunctional smart systems, such as smart dressings that show specific responses to stimuli, have been developed. Researchers in this field should work towards developing smart systems based on the mechanisms of disunion in chronic wounds, which could effectively demonstrate the potential of nanobiotechnology in promoting chronic wound repair.
Despite the decades-long history of nanotechnology research, few products and therapies based on nanobiotechnology have become available commercially or entered the clinical trial phase. One reason for this is that most basic nanotech research on chronic wound healing is performed in rodent models, such as C57BL/6 mice or Sprague–Dawley rats, even though the skin structure and chronic wound healing processes differ between rodents and humans.222 The wound healing effects observed in primates, such as humans, may not be as good as those in rats and mice. Meanwhile, the cost of nano-materials and processing platforms required for large-scale preparation also hinder the clinical translation of nanotechnologies.
During the past few years, numerous nano-materials and techniques have been used to repair chronic wounds. This review summarizes some nanobiotechnology-based systems and nanoplatform designs that can be used for treating chronic wounds. It highlights that a smart dressing for chronic wounds that allows real-time monitoring and has stimuli-responsive abilities is one possible direction for the future of nano-wound-repairing systems. We hope this review motivates the development of more sophisticated wound management systems based on nanobiotechnology in the future.
Acknowledgments
The authors acknowledge the support from the National Natural Science Foundation of China (81974289, 81772094), the Key Research and Development Program of Hubei Province (grant number 2020BCB031), the Guangdong Basic and Applied Basic Research Foundation (2019B1515120043), the international cooperation research project of Shenzhen, the international cooperation research project of Shenzhen (GJHZ20190822091601691), and the Key Project of Basic Research of Shenzhen (JCYJ20200109113603854).
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- 1.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci-Landmrk. 2004;9:283–289. doi: 10.2741/1184 [DOI] [PubMed] [Google Scholar]
- 2.Vig K, Chaudhari A, Tripathi S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. 2017;18(4):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stojadinovic A, Carlson JW, Schultz GS, Davis TA, Elster EA. Topical advances in wound care. Gynecol Oncol. 2008;111(2):S70–S80. [DOI] [PubMed] [Google Scholar]
- 4.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. [DOI] [PubMed] [Google Scholar]
- 5.Tavakoli S, Klar AS. Advanced hydrogels as wound dressings. Biomolecules. 2020;10(8):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson M, Jarbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114–125. [DOI] [PubMed] [Google Scholar]
- 9.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2ASuppl):26S–38S. [DOI] [PubMed] [Google Scholar]
- 10.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162(1):303–312. doi: 10.1016/S0002-9440(10)63821-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duscher D, Januszyk M, Maan ZN, et al. Comparison of the hydroxylase inhibitor dimethyloxalylglycine and the iron chelator deferoxamine in diabetic and aged wound healing. Plast Reconstr Surg. 2017;139(3):695e–706e. doi: 10.1097/PRS.0000000000003072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues M, Wong VW, Rennert RC, Davis CR, Longaker MT, Gurtner GC. Progenitor cell dysfunctions underlie some diabetic complications. Am J Pathol. 2015;185(10):2607–2618. doi: 10.1016/j.ajpath.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 14.Versey Z, da Cruz Nizer WS, Russell E, et al. Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol. 2021;12:648554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Coradin T, Helary C. Modulating inflammation in a cutaneous chronic wound model by IL-10 released from collagen-silica nanocomposites via gene delivery. Biomater Sci. 2018;6(2):398–406. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Liang B, Tian J, Wu J. Anti-inflammation biomaterial platforms for chronic wound healing. Biomater Sci. 2021;9(12):4388–4409. [DOI] [PubMed] [Google Scholar]
- 17.Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci-Us. 2008;97(8):2892–2923. [DOI] [PubMed] [Google Scholar]
- 19.Schiestl C, Stiefel D, Meuli M. Giant naevus, giant excision, eleg(i)ant closure? Reconstructive surgery with Integra Artificial Skin to treat giant congenital melanocytic naevi in children. J Plast Reconstr Aesthet Surg. 2010;63(4):610–615. [DOI] [PubMed] [Google Scholar]
- 20.Schiestl C, Neuhaus K, Biedermann T, Bottcher-Haberzeth S, Reichmann E, Meuli M. Novel treatment for massive lower extremity avulsion injuries in children: slow, but effective with good cosmesis. Eur J Pediatr Surg. 2011;21(2):106–110. [DOI] [PubMed] [Google Scholar]
- 21.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. [DOI] [PubMed] [Google Scholar]
- 22.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311–332. [DOI] [PubMed] [Google Scholar]
- 23.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradise J, Wolf SM, Kuzma J, RamacHandran G, Kokkoli E. Introduction: the challenge of developing oversight approaches to nanobiotechnology. J Law Med Ethics. 2009;37(4):543–545. [DOI] [PubMed] [Google Scholar]
- 25.Roco MC. Nanotechnology: convergence with modern biology and medicine. Curr Opin Biotechnol. 2003;14(3):337–346. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Kostarelos K, Nelson BJ, Zhang L. Trends in micro-/nanorobotics: materials development, actuation, localization, and system integration for biomedical applications. Adv Mater. 2021;33(4):2002047. [DOI] [PubMed] [Google Scholar]
- 27.Kargozar S, Baino F, Hamzehlou S, Hamblin MR, Mozafari M. Nanotechnology for angiogenesis: opportunities and challenges. Chem Soc Rev. 2020;49(14):5008–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenawy El R, Layman JM, Watkins JR, et al. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials. 2003;24(6):907–913. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Nagapudi K, Apkarian RP, Chaikof EL. Engineered collagen-PEO nanofibers and fabrics. J Biomater Sci Polym Ed. 2001;12(9):979–993. [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Lee MS, Song IB, et al. Preparation of sponge using porcine small intesinal submucosa and their applications as a scaffold and a wound dressing; Paper presented at: Tissue Engineering; 2007; Boston, MA. [DOI] [PubMed] [Google Scholar]
- 31.Henriques-Antunes H, Cardoso RMS, Zonari A, et al. The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano. 2019;13(8):8694–8707. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Ou-Yang W, Zhang C, et al. Synthetic polymeric antibacterial hydrogel for methicillin-resistant Staphylococcus aureus-infected wound healing: nanoantimicrobial self-assembly, drug- and cytokine-free strategy. ACS Nano. 2020;14(10):12905–12917. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Ma X, Hu H. Marine polysaccharides as a versatile biomass for the construction of nano drug delivery systems. Mar Drugs. 2021;19(6):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Lu KJ, Yu CH, Huang Q-L, Du Y-Z. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology. 2019;17(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng X, Ding Z, Cheng W, et al. Microskin-inspired injectable MSC-Laden hydrogels for scarless wound healing with hair follicles. Adv Healthcare Mater. 2020;9(10):2000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mester A, Opincariu D, Benedek I, Benedek I. Stem cell therapy in wound healing. J Interdiscip Med. 2017;2(s4):20–24. [Google Scholar]
- 37.Ma H, Zhou Q, Chang J, Wu C. Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano. 2019;13(4):4302–4311. [DOI] [PubMed] [Google Scholar]
- 38.Paneysar JS, Barton S, Ambre P, Coutinho E. Novel temperature responsive films impregnated with silver nano particles (Ag-NPs) as potential dressings for wounds. J Pharm Sci-US. 2022;111:810–817. [DOI] [PubMed] [Google Scholar]
- 39.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16(6):378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maranda LE, Rodriguez-Menocal L, Badiavas VE. Role of mesenchymal stem cells in dermal repair in burns and diabetic wounds. Curr Stem Cell Res Ther. 2017;12(1):61–70. [DOI] [PubMed] [Google Scholar]
- 41.Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003;55(12):1595–1611. [DOI] [PubMed] [Google Scholar]
- 42.Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24(6):737–764. [DOI] [PubMed] [Google Scholar]
- 43.Xi Y, Ge J, Guo Y, Lei B, Ma PX. Biomimetic elastomeric polypeptide-based nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano. 2018;12(11):10772–10784. [DOI] [PubMed] [Google Scholar]
- 44.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. [DOI] [PubMed] [Google Scholar]
- 45.Wang A, Liu Z, Hu M, et al. Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing. Nano Energy. 2018;43:63–71. [Google Scholar]
- 46.Xue C, Sutrisno L, Li M, et al. Implantable multifunctional black phosphorus nanoformulation-deposited biodegradable scaffold for combinational photothermal/ chemotherapy and wound healing. Biomaterials. 2021;269:120623. [DOI] [PubMed] [Google Scholar]
- 47.Maleki H, Khoshnevisan K, Sajjadi-Jazi SM, et al. Nanofiber-based systems intended for diabetes. J Nanobiotechnology. 2021;19(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cha H, Hong S, Park JH, Park HH. Stem cell-derived exosomes and nanovesicles: promotion of cell proliferation, migration, and anti-senescence for treatment of wound damage and skin ageing. Pharmaceutics. 2020;12(12):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ermini ML, Voliani V. Antimicrobial nano-agents: the copper age. ACS Nano. 2021;15(4):6008–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahimirad S, Abtahi H, Satei P, Ghaznavi-Rad E, Moslehi M, Ganji A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr Polym. 2021;259:117640. [DOI] [PubMed] [Google Scholar]
- 51.Stanescu P-O, Radu I-C, Leu Alexa R, et al. Novel chitosan and bacterial cellulose biocomposites tailored with polymeric nanoparticles for modern wound dressing development. Drug Deliv. 2021;28(1):1932–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes rocha Correa V, Assis martins J, Ribeiro de Souza T, et al. Melatonin loaded lecithin-chitosan nanoparticles improved the wound healing in diabetic rats. Int J Biol Macromol. 2020;162:1465–1475. [DOI] [PubMed] [Google Scholar]
- 53.Semon JA, Maness C, Zhang X, et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther. 2014;5(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. [DOI] [PubMed] [Google Scholar]
- 56.Jin G, Prabhakaran MP, Ramakrishna S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomaterialia. 2011;7(8):3113–3122. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Ding Z, Zheng X, Lu G, Lu Q, Kaplan DL. Injectable silk nanofiber hydrogels as stem cell carriers to accelerate wound healing. J Materi Chem B. 2021;9(37):7771–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian Q, Yang Y, Li A, et al. Ferrihydrite nanoparticles as the photosensitizer augment microbial infected wound healing with blue light. Nanoscale. 2021;13(45):19123–19132. [DOI] [PubMed] [Google Scholar]
- 59.Marler JJ, Upton J, Langer R, Vacanti JP. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev. 1998;33(1–2):165–182. [DOI] [PubMed] [Google Scholar]
- 60.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19(6):485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13(9):10279–10293. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. [DOI] [PubMed] [Google Scholar]
- 64.Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8(3):217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abd El-Hack ME, El-Saadony MT, Shafi ME, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int J Biol Macromol. 2020;164:2726–2744. [DOI] [PubMed] [Google Scholar]
- 66.Graca MFP, Miguel SP, Cabral CSD, Correia IJ. Hyaluronic acid-based wound dressings: a review. Carbohydr Polym. 2020;241:116364. [DOI] [PubMed] [Google Scholar]
- 67.Sorushanova A, Delgado LM, Wu Z, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater. 2019;31(1):e1801651. [DOI] [PubMed] [Google Scholar]
- 68.Liu S, Jiang T, Guo R, et al. Injectable and degradable PEG hydrogel with antibacterial performance for promoting wound healing. ACS Appl Bio Mater. 2021;4:2769–2780. [DOI] [PubMed] [Google Scholar]
- 69.Dodero A, Alloisio M, Castellano M, Vicini S. Multilayer alginate-polycaprolactone electrospun membranes as skin wound patches with drug delivery abilities. ACS Appl Mater Interfaces. 2020;12(28):31162–31171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Lv F, Li T, et al. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11(11):11337–11349. [DOI] [PubMed] [Google Scholar]
- 71.Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020. [DOI] [PubMed] [Google Scholar]
- 72.Griffin DR, Archang MM, Kuan CH, et al. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat Mater. 2021;20(4):560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17(12):1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui L, Liang J, Liu H, Zhang K, Li J. Nanomaterials for angiogenesis in skin tissue engineering. Tissue Eng Part B Rev. 2020;26(3):203–216. [DOI] [PubMed] [Google Scholar]
- 75.Talikowska M, Fu X, Lisak G. Application of conducting polymers to wound care and skin tissue engineering: a review. Biosens Bioelectron. 2019;135:50–63. [DOI] [PubMed] [Google Scholar]
- 76.Taghiabadi E, Beiki B, Aghdami N, Bajouri A. Cultivation of adipose-derived stromal cells on intact amniotic membrane-based scaffold for skin tissue engineering. Methods Mol Biol. 2019;1879:201–210. [DOI] [PubMed] [Google Scholar]
- 77.Palchesko RN, Carrasquilla SD, Feinberg AW. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Adv Healthc Mater. 2018;7(16):e1701434. [DOI] [PubMed] [Google Scholar]
- 78.Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 2012;29(1):1–63. [DOI] [PubMed] [Google Scholar]
- 79.Wan Z, Zhao L, Lu F, et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10(1):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jain KK. An overview of drug delivery systems. Methods Mol Biol. 2020;2059:1–54. [DOI] [PubMed] [Google Scholar]
- 81.Yun YH, Lee BK, Park K. Controlled drug delivery: historical perspective for the next generation. J Control Release. 2015;219:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–239. [DOI] [PubMed] [Google Scholar]
- 83.Zandi N, Dolatyar B, Lotfi R, et al. Biomimetic nanoengineered scaffold for enhanced full-thickness cutaneous wound healing. Acta Biomater. 2021;124:191–204. [DOI] [PubMed] [Google Scholar]
- 84.Son YJ, Tse JW, Zhou Y, Mao W, Yim EKF, Yoo HS. Biomaterials and controlled release strategy for epithelial wound healing. Biomater Sci. 2019;7(11):4444–4471. [DOI] [PubMed] [Google Scholar]
- 85.Shefa AA, Amirian J, Kang HJ, et al. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr Polym. 2017;177:284–296. [DOI] [PubMed] [Google Scholar]
- 86.Shamloo A, Sarmadi M, Aghababaie Z, Vossoughi M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int J Pharm. 2018;537(1–2):278–289. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Lv F, Li T, et al. Correction to electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2019;13(3):3740. [DOI] [PubMed] [Google Scholar]
- 88.Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shakya S, He Y, Ren X, et al. Ultrafine silver nanoparticles embedded in cyclodextrin metal-organic frameworks with GRGDS functionalization to promote antibacterial and wound healing application. Small. 2019;15(27):e1901065. [DOI] [PubMed] [Google Scholar]
- 90.Ma X, Cheng Y, Jian H, et al. Hollow, rough, and nitric oxide-releasing cerium oxide nanoparticles for promoting multiple stages of wound healing. Adv Healthc Mater. 2019;8(16):e1900256. [DOI] [PubMed] [Google Scholar]
- 91.Shahnawaz khan M, Abdelhamid HN, Wu HF. Near infrared (NIR) laser mediated surface activation of graphene oxide nanoflakes for efficient antibacterial, antifungal and wound healing treatment. Colloids Surf B Biointerfaces. 2015;127:281–291. [DOI] [PubMed] [Google Scholar]
- 92.Henna TK, Pramod K. Graphene quantum dots redefine nanobiomedicine. Mater Sci Eng C Mater Biol Appl. 2020;110:110651. [DOI] [PubMed] [Google Scholar]
- 93.Kumawat MK, Thakur M, Gurung RB, Srivastava R. Graphene quantum dots for cell proliferation, nucleus imaging, and photoluminescent sensing applications. Sci Rep. 2017;7(1):15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang P, Zhu Z, Zhang T, et al. Orange-emissive carbon quantum dots: toward application in wound ph monitoring based on colorimetric and fluorescent changing. Small. 2019;15(44):e1902823. [DOI] [PubMed] [Google Scholar]
- 95.Santos A, Veiga F, Figueiras A. Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity and biomedical applications. Materials. 2020;13(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Q, Liu K, Jiang T, et al. Injectable and self-healing chitosan-based hydrogel with MOF-loaded α-lipoic acid promotes diabetic wound healing. Mater Sci Eng C. 2021;131:112519. [DOI] [PubMed] [Google Scholar]
- 98.Zhao H, Huang J, Li Y, et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258:120286. [DOI] [PubMed] [Google Scholar]
- 99.Lee PY, Cobain E, Huard J, Huang L. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther. 2007;15(6):1189–1194. doi: 10.1038/sj.mt.6300156 [DOI] [PubMed] [Google Scholar]
- 100.Farahani H, Barati A, Arjomandzadegan M, Vatankhah E. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int J Biol Macromol. 2020;162:762–773. doi: 10.1016/j.ijbiomac.2020.06.175 [DOI] [PubMed] [Google Scholar]
- 101.Arantes VT, Faraco AAG, Ferreira FB, et al. Retinoic acid-loaded solid lipid nanoparticles surrounded by chitosan film support diabetic wound healing in in vivo study. Colloids Surf B Biointerfaces. 2020;188:110749. doi: 10.1016/j.colsurfb.2019.110749 [DOI] [PubMed] [Google Scholar]
- 102.Lopez-Iglesias C, Quilez C, Barros J, et al. Lidocaine-loaded solid lipid microparticles (SLMPs) produced from gas-saturated solutions for wound applications. Pharmaceutics. 2020;12(9):870. doi: 10.3390/pharmaceutics12090870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vairo C, Collantes M, Quincoces G, et al. Preclinical safety of topically administered nanostructured lipid carriers (NLC) for wound healing application: biodistribution and toxicity studies. Int J Pharm. 2019;569:118484. doi: 10.1016/j.ijpharm.2019.118484 [DOI] [PubMed] [Google Scholar]
- 104.Gainza G, Bonafonte DC, Moreno B, et al. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J Control Release. 2015;197:41–47. doi: 10.1016/j.jconrel.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 105.Hu C, Zhang F, Kong Q, et al. Synergistic chemical and photodynamic antimicrobial therapy for enhanced wound healing mediated by multifunctional light-responsive nanoparticles. Biomacromolecules. 2019;20(12):4581–4592. doi: 10.1021/acs.biomac.9b01401 [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Chen Z, Wang J, et al. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl Mater Interfaces. 2018;10(19):16315–16326. doi: 10.1021/acsami.8b03868 [DOI] [PubMed] [Google Scholar]
- 107.Rasool A, Ata S, Islam A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr Polym. 2019;203:423–429. doi: 10.1016/j.carbpol.2018.09.083 [DOI] [PubMed] [Google Scholar]
- 108.El-Aassar MR, Ibrahim OM, Fouda MMG, El-Beheri NG, Agwa MM. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohydr Polym. 2020;238:116175. doi: 10.1016/j.carbpol.2020.116175 [DOI] [PubMed] [Google Scholar]
- 109.Yu N, Cai T, Sun Y, et al. A novel antibacterial agent based on AgNPs and Fe3O4 loaded chitin microspheres with peroxidase-like activity for synergistic antibacterial activity and wound-healing. Int J Pharm. 2018;552(1–2):277–287. doi: 10.1016/j.ijpharm.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 110.Yu J, Huang TR, Lim ZH, et al. Production of hollow bacterial cellulose microspheres using microfluidics to form an injectable porous scaffold for wound healing. Adv Healthc Mater. 2016;5(23):2983–2992. doi: 10.1002/adhm.201600898 [DOI] [PubMed] [Google Scholar]
- 111.Chen H, Xing X, Tan H, et al. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):287–295. doi: 10.1016/j.msec.2016.08.086 [DOI] [PubMed] [Google Scholar]
- 112.Shafiee A, Cavalcanti AS, Saidy NT, et al. Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials. 2021;268:120558. doi: 10.1016/j.biomaterials.2020.120558 [DOI] [PubMed] [Google Scholar]
- 113.Tyeb S, Kumar N, Kumar A, Verma V. Flexible agar-sericin hydrogel film dressing for chronic wounds. Carbohydr Polym. 2018;200:572–582. doi: 10.1016/j.carbpol.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 114.Nun N, Cruz M, Jain T, et al. Thread size and polymer composition of 3D printed and electrospun wound dressings affect wound healing outcomes in an excisional wound rat model. Biomacromolecules. 2020;21(10):4030–4042. doi: 10.1021/acs.biomac.0c00801 [DOI] [PubMed] [Google Scholar]
- 115.Qiao Y, Ping Y, Zhang H, et al. Laser-activatable CuS nanodots to treat multidrug-resistant bacteria and release copper ion to accelerate healing of infected chronic nonhealing wounds. ACS Appl Mater Interfaces. 2019;11(4):3809–3822. doi: 10.1021/acsami.8b21766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dong C, Feng W, Xu W, et al. The coppery age: copper (Cu)-involved nanotheranostics. Adv Sci. 2020;7(21):2001549. doi: 10.1002/advs.202001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li S, Xie H, Li S, Kang YJ. Copper stimulates growth of human umbilical vein endothelial cells in a vascular endothelial growth factor-independent pathway. Exp Biol Med (Maywood). 2012;237(1):77–82. doi: 10.1258/ebm.2011.011267 [DOI] [PubMed] [Google Scholar]
- 118.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775 [DOI] [PubMed] [Google Scholar]
- 119.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763 [DOI] [PubMed] [Google Scholar]
- 120.Cutroneo KR. Gene therapy for tissue regeneration. J Cell Biochem. 2003;88(2):418–425. doi: 10.1002/jcb.10357 [DOI] [PubMed] [Google Scholar]
- 121.Kim J, Mirando AC, Popel AS, Green JJ. Gene delivery nanoparticles to modulate angiogenesis. Adv Drug Deliv Rev. 2017;119:20–43. doi: 10.1016/j.addr.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shende P, Patel C. siRNA: an alternative treatment for diabetes and associated conditions. J Drug Target. 2019;27(2):174–182. doi: 10.1080/1061186X.2018.1476518 [DOI] [PubMed] [Google Scholar]
- 123.Nguyen VT, Farman N, Palacios-Ramirez R, et al. Cutaneous wound healing in diabetic mice is improved by topical mineralocorticoid receptor blockade. J Invest Dermatol. 2020;140(1):223–234 e227. doi: 10.1016/j.jid.2019.04.030 [DOI] [PubMed] [Google Scholar]
- 124.Kim B, Park JH, Sailor MJ. Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Adv Mater. 2019;31(49):e1903637. doi: 10.1002/adma.201903637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor alpha to improve wound healing in diabetic mice. Bioeng Transl Med. 2019;4(1):75–82. doi: 10.1002/btm2.10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shaabani E, Sharifiaghdam M, Lammens J, et al. Increasing angiogenesis factors in hypoxic diabetic wound conditions by siRNA delivery: additive effect of LbL-Gold nanocarriers and desloratadine-induced lysosomal escape. Int J Mol Sci. 2021;22(17):9216. doi: 10.3390/ijms22179216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang S, Yan C, Zhang X, et al. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci. 2018;6(10):2757–2772. doi: 10.1039/C8BM00807H [DOI] [PubMed] [Google Scholar]
- 128.Li N, Yang L, Pan C, et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 2020;102:298–314. doi: 10.1016/j.actbio.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 129.Lan B, Wu J, Li N, et al. Hyperbranched cationic polysaccharide derivatives for efficient siRNA delivery and diabetic wound healing enhancement. Int J Biol Macromol. 2020;154:855–865. doi: 10.1016/j.ijbiomac.2020.03.164 [DOI] [PubMed] [Google Scholar]
- 130.Turner CT, Hasanzadeh Kafshgari M, Melville E, et al. Delivery of flightless I siRNA from porous silicon nanoparticles improves wound healing in mice. ACS Biomater Sci Eng. 2016;2(12):2339–2346. doi: 10.1021/acsbiomaterials.6b00550 [DOI] [PubMed] [Google Scholar]
- 131.Herce HD, Garcia AE, Litt J, et al. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys J. 2009;97(7):1917–1925. doi: 10.1016/j.bpj.2009.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bei HP, Hung PM, Yeung HL, Wang S, Zhao X. Bone-a-petite: engineering exosomes towards bone, osteochondral, and cartilage repair. Small. 2021;17(50):e2101741. doi: 10.1002/smll.202101741 [DOI] [PubMed] [Google Scholar]
- 133.Guo S, Perets N, Betzer O, et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13(9):10015–10028. doi: 10.1021/acsnano.9b01892 [DOI] [PubMed] [Google Scholar]
- 134.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 135.Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193–203. doi: 10.1038/s41582-018-0126-4 [DOI] [PubMed] [Google Scholar]
- 136.Liu W, Yu M, Xie D, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):259. doi: 10.1186/s13287-020-01756-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J, Wu H, Peng Y, et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology. 2021;19(1):202. doi: 10.1186/s12951-021-00942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59(Suppl 1):S4–16. [DOI] [PubMed] [Google Scholar]
- 139.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77(3):637–650. [DOI] [PubMed] [Google Scholar]
- 140.Mihai MM, Holban AM, Giurcaneanu C, et al. Microbial biofilms: impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr Top Med Chem. 2015;15(16):1552–1576. [DOI] [PubMed] [Google Scholar]
- 141.Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41(3):276–301. [DOI] [PubMed] [Google Scholar]
- 142.Simoes D, Miguel SP, Ribeiro MP, Coutinho P, Mendonca AG, Correia IJ. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130–141. [DOI] [PubMed] [Google Scholar]
- 143.Ghaseminezhad SM, Shojaosadati SA, Meyer RL. Ag/Fe3O4 nanocomposites penetrate and eradicate S. aureus biofilm in an in vitro chronic wound model. Colloids Surf B Biointerfaces. 2018;163:192–200. [DOI] [PubMed] [Google Scholar]
- 144.Singla R, Soni S, Patial V, et al. Cytocompatible anti-microbial dressings of Syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Sci Rep. 2017;7(1):10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yan X, Fang WW, Xue J, et al. Thermoresponsive in situ forming hydrogel with sol-gel irreversibility for effective methicillin-resistant Staphylococcus aureus infected wound healing. ACS Nano. 2019;13(9):10074–10084. [DOI] [PubMed] [Google Scholar]
- 146.Mao C, Xiang Y, Liu X, et al. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010–9021. [DOI] [PubMed] [Google Scholar]
- 147.Kim T, Zhang Q, Li J, Zhang L, Jokerst JV. A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano. 2018;12(6):5615–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qiao Y, He J, Chen W, et al. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14(3):3299–3315. [DOI] [PubMed] [Google Scholar]
- 149.Wang P, Yin B, Dong H, et al. Coupling biocompatible Au nanoclusters and cellulose nanofibrils to prepare the antibacterial nanocomposite films. Front Bioeng Biotechnol. 2020;8:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arafa MG, El-Kased RF, Elmazar MM. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci Rep. 2018;8(1):13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen G, Wu Y, Yu D, et al. Isoniazid-loaded chitosan/carbon nanotubes microspheres promote secondary wound healing of bone tuberculosis. J Biomater Appl. 2019;33(7):989–996. [DOI] [PubMed] [Google Scholar]
- 152.Luna-Hernández E, Cruz-Soto ME, Padilla-Vaca F, et al. Combined antibacterial/tissue regeneration response in thermal burns promoted by functional chitosan/silver nanocomposites. Int J Biol Macromol. 2017;105:1241–1249. [DOI] [PubMed] [Google Scholar]
- 153.Sun X, Dong M, Guo Z, et al. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int J Biol Macromol. 2021;167:10–22. [DOI] [PubMed] [Google Scholar]
- 154.Tiwari M, Narayanan K, Thakar MB, Jagani HV, Venkata Rao J. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014;8(4):230–237. [DOI] [PubMed] [Google Scholar]
- 155.Shan J, Li X, Yang K, et al. Efficient bacteria killing by Cu2WS4 nanocrystals with enzyme-like properties and bacteria-binding ability. ACS Nano. 2019;13(12):13797–13808. [DOI] [PubMed] [Google Scholar]
- 156.Liu X, Yan Z, Zhang Y, et al. Two-dimensional metal–organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano. 2019;13(5):5222–5230. [DOI] [PubMed] [Google Scholar]
- 157.Gong M, Xiao J, Li H, et al. Magnetically retained and glucose-fueled hydroxyl radical nanogenerators for H2O2-self-supplying chemodynamic therapy of wound infections. Mater Sci Eng C Mater Biol Appl. 2021;131:112522. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Y, Chang M, Bao F, et al. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale. 2019;11(13):6315–6333. [DOI] [PubMed] [Google Scholar]
- 159.Khorasani MT, Joorabloo A, Moghaddam A, Shamsi H, MansooriMoghadam Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int J Biol Macromol. 2018;114:1203–1215. [DOI] [PubMed] [Google Scholar]
- 160.Khan MI, Behera SK, Paul P, et al. Biogenic Au@ZnO core–shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency. Med Microbiol Immunol. 2019;208(5):609–629. [DOI] [PubMed] [Google Scholar]
- 161.Balaure PC, Holban AM, Grumezescu AM, et al. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int J Pharm. 2019;557:199–207. [DOI] [PubMed] [Google Scholar]
- 162.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. [DOI] [PubMed] [Google Scholar]
- 163.Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar M, Curtis A, Hoskins C. Application of nanoparticle technologies in the combat against anti-microbial resistance. Pharmaceutics. 2018;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang Y, Qin Z, Zeng W, et al. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol Rev. 2017;6(3):279–289. [Google Scholar]
- 166.Sibbald RG, Contreras-Ruiz J, Coutts P, Fierheller M, Rothman A, Woo K. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care. 2007;20(10):549–558. [DOI] [PubMed] [Google Scholar]
- 167.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52(6):1636–1653. [DOI] [PubMed] [Google Scholar]
- 168.Ramalingam B, Parandhaman T, Das SK. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl Mater Interfaces. 2016;8(7):4963–4976. [DOI] [PubMed] [Google Scholar]
- 169.Vonnemann J, Sieben C, Wolff C, et al. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale. 2014;6(4):2353–2360. [DOI] [PubMed] [Google Scholar]
- 170.Elbeshehy EKF, Elazzazy AM, Aggelis G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front Microbiol. 2015;6:453–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haidari H, Bright R, Garg S, Vasilev K, Cowin AJ, Kopecki Z. Eradication of mature bacterial biofilms with concurrent improvement in chronic wound healing using silver nanoparticle hydrogel treatment. Biomedicines. 2021;9(9):1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yin M, Wu J, Deng M, et al. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano. 2021;15(11):17842–17853. [DOI] [PubMed] [Google Scholar]
- 173.Rai A, Prabhune A, Perry CC. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem. 2010;20(32):6789–6798. [Google Scholar]
- 174.Li X, Robinson SM, Gupta A, et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8(10):10682–10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–148. [DOI] [PubMed] [Google Scholar]
- 176.Tao B, Lin C, Deng Y, et al. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J Materi Chem B. 2019;7(15):2534–2548. [DOI] [PubMed] [Google Scholar]
- 177.Zanin H, Margraf-Ferreira A, da Silva NS, Marciano FR, Corat EJ, Lobo AO. Graphene and carbon nanotube composite enabling a new prospective treatment for trichomoniasis disease. Mater Sci Eng C. 2014;41:65–69. [DOI] [PubMed] [Google Scholar]
- 178.Ashfaq M, Verma N, Khan S. Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: a novel potential antibiotic material. Mater Sci Eng C. 2016;59:938–947. [DOI] [PubMed] [Google Scholar]
- 179.Simmons TJ, Lee SH, Park TJ, Hashim DP, Ajayan PM, Linhardt RJ. Antiseptic single wall carbon nanotube bandages. Carbon. 2009;47(6):1561–1564. [Google Scholar]
- 180.Shariatinia Z. Pharmaceutical applications of chitosan. Adv Colloid Interface Sci. 2019;263:131–194. [DOI] [PubMed] [Google Scholar]
- 181.Zhou Z, Yan D, Cheng X, et al. Biomaterials based on N,N,N-trimethyl chitosan fibers in wound dressing applications. Int J Biol Macromol. 2016;89:471–476. [DOI] [PubMed] [Google Scholar]
- 182.Amiri N, Ajami S, Shahroodi A, et al. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int J Biol Macromol. 2020;162:645–656. [DOI] [PubMed] [Google Scholar]
- 183.Zhang L, Ouyang M, Zhang Y, et al. The fluorescence imaging and precise suppression of bacterial infections in chronic wounds by porphyrin-based metal–organic framework nanorods. J Materi Chem B. 2021;9(38):8048–8055. [DOI] [PubMed] [Google Scholar]
- 184.Fonseca LM, Cruxen C, Bruni GP, et al. Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int J Biol Macromol. 2019;139:1182–1190. [DOI] [PubMed] [Google Scholar]
- 185.Tolker-Nielsen T. Biofilm Development. Microbiol Spectr. 2015;3(2):MB-0001-2014. [DOI] [PubMed] [Google Scholar]
- 186.Haalboom M. Chronic wounds: innovations in diagnostics and therapeutics. Curr Med Chem. 2018;25(41):5772–5781. [DOI] [PubMed] [Google Scholar]
- 187.Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73(3):710–721. [DOI] [PubMed] [Google Scholar]
- 188.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13(1):34–40. [DOI] [PubMed] [Google Scholar]
- 189.Percival SL, Mayer D, Kirsner RS, et al. Surfactants: role in biofilm management and cellular behaviour. Int Wound J. 2019;16(3):753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim MH. Nanoparticle-based therapies for wound biofilm infection: opportunities and challenges. IEEE Trans Nanobioscience. 2016;15(3):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–284. [DOI] [PubMed] [Google Scholar]
- 192.Singh BN. Bactericidal, quorum quenching and anti-biofilm nanofactories: a new niche for nanotechnologists. Crit Rev Biotechnol. 2017;37(4):525–540. [DOI] [PubMed] [Google Scholar]
- 193.Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000;26(2):117–130. [DOI] [PubMed] [Google Scholar]
- 194.Suleman Ismail Abdalla S, Katas H, Chan JY, Ganasan P, Azmi F, Fauzi MB. Gelatin hydrogels loaded with lactoferrin-functionalized bio-nanosilver as a potential antibacterial and anti-biofilm dressing for infected wounds: synthesis, characterization, and deciphering of cytotoxicity. Mol Pharm. 2021;18(5):1956–1969. [DOI] [PubMed] [Google Scholar]
- 195.Sahariah P, Masson M. Antimicrobial chitosan and chitosan derivatives: a review of the structure-activity relationship. Biomacromolecules. 2017;18(11):3846–3868. [DOI] [PubMed] [Google Scholar]
- 196.Zmejkoski DZ, Zdravkovic NM, Trisic DD, et al. Chronic wound dressings - Pathogenic bacteria anti-biofilm treatment with bacterial cellulose-chitosan polymer or bacterial cellulose-chitosan dots composite hydrogels. Int J Biol Macromol. 2021;191:315–323. [DOI] [PubMed] [Google Scholar]
- 197.Mirzahosseinipour M, Khorsandi K, Hosseinzadeh R, Ghazaeian M, Shahidi FK. Antimicrobial photodynamic and wound healing activity of curcumin encapsulated in silica nanoparticles. Photodiagnosis Photodyn Ther. 2020;29:101639. [DOI] [PubMed] [Google Scholar]
- 198.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45(Suppl):A:A39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581. [DOI] [PubMed] [Google Scholar]
- 200.Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5(1):18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dabiri G, Heiner D, Falanga V. The emerging use of bone marrow-derived mesenchymal stem cells in the treatment of human chronic wounds. Expert Opin Emerg Drugs. 2013;18(4):405–419. [DOI] [PubMed] [Google Scholar]
- 202.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55(4):414–419. [DOI] [PubMed] [Google Scholar]
- 203.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. STEM CELLS. 2007;25(10):2648–2659. [DOI] [PubMed] [Google Scholar]
- 204.Kamolz LP, Kolbus A, Wick N, et al. Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under in vitro conditions. Burns. 2006;32(1):16–19. [DOI] [PubMed] [Google Scholar]
- 205.Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. [DOI] [PubMed] [Google Scholar]
- 206.Wang C, Wang Q, Gao W, et al. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomaterialia. 2018;69:156–169. [DOI] [PubMed] [Google Scholar]
- 207.Golchin A, Hosseinzadeh S, Staji M, Soleimani M, Ardeshirylajimi A, Khojasteh A. Biological behavior of the curcumin incorporated chitosan/poly(vinyl alcohol) nanofibers for biomedical applications. J Cell Biochem. 2019;120(9):15410–15421. [DOI] [PubMed] [Google Scholar]
- 208.Li J, Zhou C, Luo C, et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics. 2019;9(20):5839–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hou J, Chen L, Zhou M, et al. Multi-layered polyamide/collagen scaffolds with topical sustained release of N-Acetylcysteine for promoting wound healing. Int J Nanomedicine. 2020;15:1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vijayan V, Sreekumar S, Singh F, et al. Nanotized praseodymium oxide collagen 3-D pro-vasculogenic biomatrix for soft tissue engineering. Nanomedicine. 2021;33:102364. [DOI] [PubMed] [Google Scholar]
- 211.Lavrador P, Gaspar VM, Mano JF. Stimuli-responsive nanocarriers for delivery of bone therapeutics - Barriers and progresses. J Control Release. 2018;273:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhu Y, Yao Z, Liu Y, Zhang W, Geng L, Ni T. Incorporation of ROS-responsive substance P-loaded zeolite imidazolate framework-8 nanoparticles into a Ca(2+)-cross-linked alginate/pectin hydrogel for wound dressing applications. Int J Nanomedicine. 2020;15:333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Abdel Khalek MA, Abdel Gaber SA, El-Domany RA, El-Kemary MA. Photoactive electrospun cellulose acetate/polyethylene oxide/methylene blue and trilayered cellulose acetate/polyethylene oxide/silk fibroin/ciprofloxacin nanofibers for chronic wound healing. Int J Biol Macromol. 2021;193:1752–1766. [DOI] [PubMed] [Google Scholar]
- 214.Wang S, Zheng H, Zhou L, et al. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials. 2020;260:120314. [DOI] [PubMed] [Google Scholar]
- 215.Junker JPE, Caterson EJ, Eriksson E. The microenvironment of wound healing. J Craniofac Surg. 2013;24(1):12–16. [DOI] [PubMed] [Google Scholar]
- 216.Pang Q, Lou D, Li S, et al. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv Sci. 2020;7(6):1902673–1902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Matzeu G, Fay C, Vaillant A, Coyle S, Diamond D, Wearable A. Device for monitoring sweat rates via image analysis. IEEE Trans Biomed Eng. 2016;63(8):1672–1680. [DOI] [PubMed] [Google Scholar]
- 218.Zhang Y, Li T, Zhao C, et al. An integrated smart sensor dressing for real-time wound microenvironment monitoring and promoting angiogenesis and wound healing. Front Cell Dev Biol. 2021;9:701525–701525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Perumal J, Lim HQ, Attia ABE, et al. Novel cellulose fibre-based flexible plasmonic membrane for point-of-care SERS biomarker detection in chronic wound healing. Int J Nanomedicine. 2021;16:5869–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang L, Hou Q, Zheng W, Jiang X. Fluorescent and Antibacterial Aminobenzeneboronic Acid (ABA)-modified gold nanoclusters for self-monitoring residual dosage and smart wound care. ACS Nano. 2021;15(11):17885–17894. [DOI] [PubMed] [Google Scholar]
- 221.Moreira SS, Camargo MC, Caetano R, et al. Efficacy and costs of nanocrystalline silver dressings versus 1% silver sulfadiazine dressings to treat burns in adults in the outpatient setting: a randomized clinical trial. Burns. 2021;48:568–576. [DOI] [PubMed] [Google Scholar]
- 222.Abazari M, Ghaffari A, Rashidzadeh H, Momeni Badeleh S, Maleki Y. Current status and future outlook of nano-based systems for burn wound management. J Biomed Mater Res B Appl Biomater. 2020;108(5):1934–1952. [DOI] [PubMed] [Google Scholar]