Abstract
Introduction
Detailed evaluations of racial and ethnic trends and disparities in NSCLC outcomes are lacking, and it remains unclear whether recent advances in screening and targeted therapies for NSCLC have benefited all population groups equally.
Methods
Using the Surveillance, Epidemiology, and End Results 18-registry data, we evaluated trends in overall and stage-specific NSCLC incidence (2007–2018) among patients aged 55 to 79 years by sex and race and ethnicity. Overall and stage-specific 2-year cause-specific survival rates were calculated by sex and race and ethnicity. Health Disparities software calculated absolute (difference) and relative (ratio) disparity measures comparing racial and ethnic groups with the highest and lowest rates (range measures) and comparing white patients (reference group) with other groups (pairwise rate measures). Joinpoint software assessed changes in rates and disparities.
Results
Both men and women experienced substantial declines in NSCLC incidence from 2007 to 2018, largely due to significant declines in the incidence of distant-stage NSCLC over the study period (p < 0.05). During the same time period, the incidence of local-stage NSCLC significantly increased among black and Hispanic women (p < 0.05) and remained stable among all other groups. Overall, 2-year cause-specific survival rates improved across most racial and ethnic groups, especially among those diagnosed in regional and distant stages. For both sexes, absolute disparities in overall and stage-specific incidence of NSCLC significantly decreased over time (p < 0.05), whereas relative disparities remained unchanged. Pairwise comparison revealed persistent disparities in NSCLC burden between black and white men.
Conclusion
We found evidence of narrowing racial and ethnic disparities in NSCLC incidence over time; however, important disparities persist. More work is needed to ensure consistent and equitable access to high-quality screening, diagnosis, and treatment to reduce and eliminate cancer disparities.
Keywords: Non–small cell lung cancer, Screening, Stage at diagnosis, Disparities, Race, Ethnicity
Introduction
Mortality from the most common lung cancer, NSCLC, has fallen sharply in the United States in recent years.1,2 Despite nationwide declines in the past decade, certain population groups continue to suffer disproportionately from the disease.1,2 For example, black men experience higher lung cancer incidence and mortality rates than white counterparts.3 Furthermore, people of color have been found to develop cancer at earlier ages than white individuals and are more likely to present with advanced-stage disease at diagnosis.3,4
Because stage at diagnosis is an important determinant of survival, improvements in lung cancer screening and early detection have the potential to reduce disparities in lung cancer outcomes.
In 2011, the National Lung Screening Trial found a 20% reduction in lung cancer mortality with low-dose computed tomography (LDCT) screening in high-risk individuals.5 More recently, the Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON) confirmed the benefits of LDCT screening for lung cancer in high-risk individuals, revealing a 24% reduction in mortality in men and a 33% reduction in women in a 10-year period.6 In 2013, the U.S. Preventive Services Task Force (USPSTF) recommended annual screening for lung cancer with LDCT among eligible adults.7 Although LDCT screening has been covered by private insurers and Medicare since 2015, Medicaid coverage of LDCT is determined at the state level and Medicaid programs are one of the only insurers that are not required to cover lung cancer screening.8,9 This lack of uniform coverage for Medicaid recipients leaves a vulnerable segment of the population without equitable access to lung cancer screening opportunities. Treatment of NSCLC has also changed in the past decade with the identification of specific driver mutations and the development of molecularly targeted therapies and immunotherapies for NSCLC. Given that people of color are more likely to be uninsured and are more likely to be socioeconomically disadvantaged than white counterparts, there is concern that advances in screening and treatment of NSCLC could exacerbate existing disparities among vulnerable populations with limited access to care. Despite these concerns, detailed evaluations of racial and ethnic trends and disparities in NSCLC outcomes are lacking, and it remains unclear whether advances in screening and targeted therapies for NSCLC have benefited all population groups equally. Furthermore, existing studies of NSCLC trends do not include data past 20162,10 and may not fully capture the additional benefit of recent advances in screening, improvements in targeted treatment for NSCLC, and expanded insurance coverage of these services. We sought to evaluate racial and ethnic trends and disparities in NSCLC incidence and survival by stage at diagnosis during a time period in which new early detection screening and treatment options have become available.
Materials and Methods
Data Source
Patients aged 55 to 79 years diagnosed with having NSCLC from 2007 to 2018 were identified from the Surveillance, Epidemiology, and End Results (SEER) 18-registry database, which covers approximately 28% of the U.S. population.11 Cases were identified using International Classification of Diseases for Oncology, Third Edition morphology codes (Supplementary Table 1). The SEER data contain no identifiers and are publicly available for studies of cancer-based epidemiology and survival analysis. Therefore, the current study was deemed to be exempt from institutional review board approval, and the need for informed consent was waived.
We report trends in NSCLC incidence and 2-year cause-specific survival (CSS) rates by sex and race and ethnicity. We also report stage-specific incidence and 2-year CSS rates on the basis of SEER’s Summary Stage variable (localized, regional, distant, or unknown).12 Two-year CSS rates were derived from SEER’s cause-specific death classification.
Patient race and ethnicity were defined according to SEER’s Race and Origin variable and categorized into the following five mutually exclusive groups: American Indian or Alaskan Native (AIAN), Asian or Pacific Islander (API), black, Hispanic, and white. Patients with missing or unknown race and ethnicity information were excluded from the analysis (N = 725). We were unable to fully evaluate stage-specific incidence and survival trends among AIAN men and women owing to small numbers.
Statistical Analysis
We used SEER∗stat11 to calculate age-adjusted incidence and stage-specific incidence rates by sex, race and ethnicity, and calendar year (2007–2018). Overall and stage-specific 2-year CSS rates by sex, race and ethnicity, and year (2007–2016) were also calculated.
We used SEER Health Disparities (HD∗Calc) to calculate absolute (difference) and relative (ratio) disparity measures.13,14 Specifically, we used range difference as a summary measure of absolute disparity, defined as the difference between highest and lowest values for each outcome regardless of which specific groups are being compared. If no disparity exists, the range difference will be 0. Similarly, we used range ratio as a summary measure of relative disparity, derived from the highest divided by the lowest rate at each time point. If no disparity exists, the range ratio has a value of 1. Joinpoint analyses were used to evaluate trends in summary measures of absolute and relative disparities (i.e., range difference and range ratio).14 To identify which specific race and ethnic groups differ, we used rate difference and rate ratio to explore pairwise comparisons of incidence and 2-year CSS rates between the non-Hispanic whites (reference group) compared with the other racial and ethnic groups.
Joinpoint software was used to evaluate changes in trends over time.15 We calculated average annual percent change (AAPC) to summarize trends over time.16 A positive AAPC indicates an increasing trend, whereas a negative AAPC indicates a declining one. The AAPC is considered statistically significant when its value differs from 0 at α level 0.05.
Results
The SEER18 database identified 325,138 patients diagnosed with having NSCLC from 2007 to 2018 (Table 1). Of these, 75.2% were white, 12.1% black, 6.3% API, 5.8% Hispanic, and 0.5% AIAN. Most of the cases were of male sex (53.6%), aged more than or equal to 65 years (68.7%), with adenocarcinoma (54.0%), and with distant-stage tumors (50.3%). Black patients had the highest percentage of patients under age 65 years (40.4%) and the lowest percentage of local-stage diagnosis (18.6%). API patients had the highest percentage of distant-stage diagnosis (57.0%) and adenocarcinoma histology (69.5%) relative to the other four racial and ethnic groups.
Table 1.
Characteristics of Patients Aged 55 to 79 Years Diagnosed With NSCLC From 2007 to 2018
| Characteristic | Race and Ethnicity |
Total | ||||
|---|---|---|---|---|---|---|
| White | Black | American Indian/Alaskan Native | Asian/Pacific Islander | Hispanic | ||
| N (row %) | 244,552 (75.2) | 39,321 (12.1) | 1693 (0.5) | 20,623 (6.3) | 18,949 (5.8) | 325,138 |
| Sex | ||||||
| Male | 129,462 (52.9) | 22,510 (57.3) | 868 (51.3) | 11,495 (55.7) | 9991 (52.7) | 174,326 (53.6) |
| Female | 115,090 (47.1) | 16,811 (42.8) | 825 (48.7) | 9128 (44.3) | 8958 (47.3) | 150,812 (46.4) |
| Age | ||||||
| 55–59 | 29,733 (12.2) | 7224 (18.4) | 248 (14.7) | 2721 (13.2) | 2619 (13.8) | 42,545 (13.1) |
| 60–64 | 43,037 (17.6) | 8669 (22.1) | 374 (22.1) | 3872 (18.8) | 3381 (17.8) | 59,333 (18.3) |
| 65–69 | 57,251 (23.4) | 9211 (23.4) | 388 (22.9) | 4664 (22.6) | 4330 (22.9) | 75,844 (23.3) |
| 70–74 | 60,149 (24.6) | 7883 (20.1) | 382 (22.6) | 4758 (23.1) | 4492 (23.7) | 77,664 (23.9) |
| 75–79 | 54,382 (22.2) | 6334 (16.1) | 301 (17.8) | 4608 (22.3) | 4127 (21.8) | 69,752 (21.5) |
| Diagnosis stage | ||||||
| Localized | 59,846 (24.5) | 7320 (18.6) | 357 (21.1) | 3938 (19.1) | 3889 (20.5) | 75,350 (23.2) |
| Regional | 60,834 (24.9) | 9400 (23.9) | 426 (25.2) | 4386 (21.4) | 4189 (22.1) | 79,235 (24.4) |
| Distant | 118,826 (48.6) | 21,771 (55.4) | 861 (50.9) | 11,725 (57.0) | 10,247 (54.1) | 163,430 (50.3) |
| Unknown | 5046 (2.1) | 830 (2.1) | 49 (2.9) | 574 (2.8) | 623 (3.3) | 7119 (2.2) |
| Histology | ||||||
| Adenocarcinoma | 128,555 (52.6) | 20,886 (53.1) | 804 (47.5) | 14,339 (69.5) | 11,078 (58.5) | 175,662 (54.0) |
| Squamous | 71,404 (29.2) | 11,395 (29.0) | 570 (33.7) | 3553 (17.2) | 4347 (22.9) | 91,269 (28.1) |
| Large cell | 5354 (2.2) | 954 (2.4) | 29 (1.7) | 295 (1.4) | 308 (1.6) | 6940 (2.1) |
| Non–small cell, NOS | 24,533 (10.0) | 4302 (10.9) | 201 (11.9) | 1630 (7.9) | 1833 (9.7) | 32,499 (10.0) |
| Other specified | 14,706 (6.0) | 1784 (4.5) | 89 (5.3) | 806 (3.9) | 1383 (7.3) | 18,768 (5.8) |
| Diagnosis year | ||||||
| 2007 | 20,999 (8.6) | 2947 (7.5) | 105 (6.2) | 1364 (6.6) | 1356 (7.2) | 26,771 (8.2) |
| 2008 | 20,606 (8.4) | 3080 (7.8) | 135 (8.0) | 1472 (7.1) | 1439 (7.6) | 26,732 (8.2) |
| 2009 | 20,642 (8.4) | 3200 (8.1) | 113 (6.7) | 1529 (7.4) | 1426 (7.5) | 26,910 (8.3) |
| 2010 | 20,094 (8.2) | 3151 (8) | 126 (7.4) | 1602 (7.8) | 1446 (7.6) | 26,419 (8.1) |
| 2011 | 20,082 (8.2) | 3172 (8.1) | 138 (8.2) | 1620 (7.9) | 1499 (7.9) | 26,511 (8.2) |
| 2012 | 20,102 (8.2) | 3349 (8.5) | 130 (7.7) | 1698 (8.2) | 1570 (8.3) | 26,849 (8.3) |
| 2013 | 20,366 (8.3) | 3280 (8.3) | 137 (8.1) | 1791 (8.7) | 1617 (8.5) | 27,191 (8.4) |
| 2014 | 20,555 (8.4) | 3443 (8.8) | 150 (8.9) | 1762 (8.5) | 1639 (8.7) | 27,549 (8.5) |
| 2015 | 20,472 (8.4) | 3425 (8.7) | 189 (11.2) | 1925 (9.3) | 1671 (8.8) | 27,682 (8.5) |
| 2016 | 20,707 (8.5) | 3444 (8.8) | 150 (8.9) | 1952 (9.5) | 1830 (9.7) | 28,083 (8.6) |
| 2017 | 20,995 (8.6) | 3514 (8.9) | 168 (9.9) | 2071 (10.0) | 1793 (9.5) | 28,541 (8.8) |
| 2018 | 18,932 (7.7) | 3316 (8.4) | 152 (9.0) | 1837 (8.9) | 1663 (8.8) | 25,900 (8.0) |
Note: Numbers in parentheses are column percentages unless otherwise stated.
NOS, not otherwise specified.
Overall and Stage-Specific Incidence Rates
Among men, the incidence of NSCLC was highest among black men, and this was consistent across all stages (Fig. 1A–D). Among women, overall and local-stage incidence of NSCLC was highest among white women, whereas the incidence of regional- and distant-stage NSCLC was highest among black women (Fig. 1F–H).
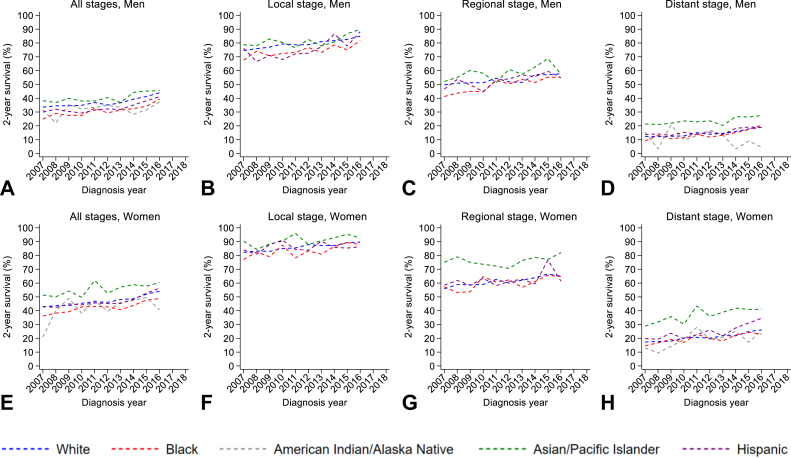
Figure 1.
Trends in NSCLC incidence. (A) All stages combined among men. (B) Local-stage incidence among men. (C) Regional-stage incidence among men. (D) Distant-stage incidence among men. (E) All stages combined among women. (F) Local-stage incidence among women. (G) Regional-stage incidence among women. (H) Distant-stage incidence among women.
Among men, the overall incidence of NSCLC significantly decreased over time across all racial and ethnic groups, with Hispanic men experiencing the steepest decline (AAPC = −3.45, p < 0.001) (Table 2). When stratified by stage, the incidence of local-stage disease in men remained stable over time, whereas the incidence of regional- and distant-stage NSCLC significantly decreased over time among men of all races and ethnicities (p < 0.05 for all). Declines in the regional-stage incidence were steepest among white men (AAPC = −3.93, p < 0.001), whereas black men experienced the steepest declines in incidence of distant-stage NSCLC (AAPC = −4.42, p < 0.001). The incidence of unknown-stage disease significantly decreased among white (AAPC = −4.79, p < 0.001) and black (AAPC = −4.25, p = 0.005) men and remained stable among Hispanic men (Supplementary Table 2). We could not fully evaluate trends in incidence of unknown-stage disease among AIAN and API men owing to sparse data.
Table 2.
Overall and Stage-Specific Trends in NSCLC Incidence Stratified by Sex and Race and Ethnicity From 2007 to 2018
| Sex/Race and Ethnicity | AAPC (95% CI) |
|||
|---|---|---|---|---|
| All Stages | Local Stage | Regional Stage | Distant Stage | |
| Men | ||||
| Total | −3.35 (−3.76 to −2.94)a | −0.91 (−2.04 to 0.23) | −3.93 (−4.99 to −2.88)a | −3.97 (−4.51 to −3.42)a |
| White | −3.30 (−3.72 to −2.88)a | −0.86 (−2.03 to 0.32) | −3.93 (−5.04 to −2.82)a | −3.95 (−4.44 to −3.46)a |
| Black | −3.18 (−3.88 to −2.48)a | −0.40 (−1.67 to 0.88) | −3.18 (−4.21 to −2.13)a | −4.45 (−6.02 to −2.87)a |
| American Indian/Alaskan Native | −3.08 (−5.32 to −0.78)a | b | b | −2.76 (−4.90 to −0.53)a |
| Asian/Pacific Islander | −2.13 (−2.98 to −1.27)a | +0.04 (−0.89 to 0.99) | −2.86 (−4.88 to −0.79)a | −2.49 (−3.74 to −1.23)a |
| Hispanic | −3.45 (−4.18 to −2.71)a | −0.08 (−2.89 to 2.82) | −3.64 (−5.03 to −2.23)a | −4.42 (−5.43 to −3.37)a |
| Women | ||||
| Total | −1.92 (−2.45 to −1.40)a | +0.63 (−0.50 to 1.78) | −2.96 (−4.22 to −1.69)a | −2.66 (−3.13 to −2.18)a |
| White | −1.87 (−2.40 to −1.33)a | +0.65 (−0.56 to 1.86) | −2.77 (−3.93 to −1.61)a | −2.67 (−3.17 to −2.19)a |
| Black | −1.49 (−1.99 to −0.98)a | +2.15 (1.07–3.26)a | −2.56 (−4.27 to −0.81)a | −2.34 (−3.13 to −1.53)a |
| American Indian/Alaskan Native | +1.40 (−1.42 to 4.31) | b | b | b |
| Asian/Pacific Islander | −0.89 (−1.88 to 0.10) | +0.86 (−1.49 to 3.26) | −2.37 (−5.06 to 0.40) | −0.94 (−1.60 to −0.27)a |
| Hispanic | −1.52 (−2.62 to −0.40)a | +1.86 (0.04 to 3.72)a | −2.76 (−5.47 to −0.01)a | −2.77 (−4.11 to −1.41)a |
AAPC, average annual percentage change; CI, confidence interval.
p < 0.05.
Statistic not reported owing to fewer than 20 cases in some years.
Among women, incidence of NSCLC from 2007 to 2018 significantly declined for white (AAPC −1.87, p < 0.001), Hispanic (AAPC = −1.52, p = 0.013), and black (AAPC = −1.49, p < 0.001) women but remained stable among API or AIAN women (Table 2 and Fig. 1E). When stratified by stage, the incidence of local-stage NSCLC significantly increased among black (AAPC = +2.15, p = 0.002) and Hispanic (AAPC = +1.86, p = 0.046) women but remained stable among white and API women. In contrast, the incidence of regional-stage NSCLC significantly decreased over time for white (AAPC = −2.77, p < 0.001), Hispanic (AAPC = −2.76, p = 0.049), and black (AAPC = −2.56, p = 0.009) women but remained stable among API women. The incidence of distant-stage NSCLC also decreased over time for all groups and was steepest among Hispanic (AAPC = −2.77, p < 0.001) women, followed by white (AAPC = −2.67, p < 0.001), black (AAPC = −2.34, p < 0.001), and API (AAPC = −0.94, p = 0.011) women. Incidence rates of unknown-stage NSCLC significantly decreased over time among white (AAPC = −4.00, p = 0.003) and black (AAPC = −3.18, p = 0.026) women; however, we were unable to evaluate trends in incidence of unknown-stage disease among AIAN, API, and Hispanic women owing to sparse data (Supplementary Table 2).
Overall and Stage-Specific 2-Year CSS Rates
Men had poorer 2-year CSS compared with women, regardless of race and ethnicity or stage (Fig. 2A and E). Specifically, 2-year CSS was generally highest among Hispanic men and women and poorest among black men and women, and this was consistent across all disease stages (Fig. 2A–H).
Figure 2.
Trends in 2-year cause-specific survival. (A) All stages combined among men. (B) Local-stage survival among men. (C) Regional-stage survival among men. (D) Distant-stage survival among men. (E) All stages combined among women. (F) Local-stage survival among women. (G) Regional-stage survival among women. (H) Distant-stage survival among women.
Among men, 2-year CSS rates increased across all race and ethnic groups except AIAN men, with black men (AAPC = +4.07, p < 0.001) experiencing the greatest improvement in 2-year CSS over time (Table 3 and Fig. 2A). When stratified by stage, 2-year CSS increased across all stages. Specifically, the largest increase in 2-year CSS for local-stage NSCLC occurred among Hispanic men (AAPC = +2.74, p < 0.001), whereas 2-year CSS for regional- and distant-stage NSCLC increased most among black men (AAPC = +3.19, p < 0.001 and AAPC = +7.31, p < 0.001, respectively). Changes in regional- and distant-stage 2-year CSS rates were not statistically significant for API and AIAN (respectively). Two-year CSS among men with unknown-stage disease remained generally stable over time; however, we were unable to evaluate trends by race and ethnicity owing to sparse data (Supplementary Table 3).
Table 3.
Trends in Overall and Stage-Specific 2-Year NSCLC Survival Stratified by Sex and Race and Ethnicity From 2007 to 2016
| Sex/Race and Ethnicity | AAPC (95% CI) |
|||
|---|---|---|---|---|
| All Stages | Local Stage | Regional Stage | Distant Stage | |
| Men | ||||
| Total | +2.97 (1.99–3.97)a | +1.41 (1.16–1.67)a | +1.86 (1.27–2.46)a | +5.21 (3.80–6.65)a |
| White | +2.85 (1.90–3.8)a | +1.31 (1.07–1.54)a | +1.65 (0.91–2.40)a | +5.13 (3.67–6.61)a |
| Black | +4.07 (2.43–5.73)a | +1.57 (0.76–2.40)a | +3.19 (2.00–4.40)a | +7.31 (4.13–10.58)a |
| American Indian/Alaskan Native | +1.15 (−2.14 to 4.55) | b | b | −7.29 (−19.34 to 6.55) |
| Asian/Pacific Islander | +2.34 (0.93–3.78)a | +1.42 (0.42–2.42)a | +1.80 (−0.18 to 3.82) | +2.90 (1.02–4.82)a |
| Hispanic | +3.39 (1.81–5.00)a | +2.74 (1.21–4.29)a | +2.00 (0.48–3.54)a | +4.20 (2.00–6.44)a |
| Women | ||||
| Total | +2.60 (2.04–3.16)a | +0.93 (0.74–1.12)a | +1.66 (1.12–2.21)a | +4.81 (3.95–5.67)a |
| White | +2.57 (2–3.13)a | +0.98 (0.77–1.19)a | +1.68 (1.13–2.23)a | +4.49 (3.55–5.65)a |
| Black | +2.87 (1.81–3.94)a | +1.17 (0.18–2.16)a | +1.60 (0.07–3.16)a | +4.59 (1.78–7.28)a |
| American Indian/Alaskan Native | +2.06 (−1.93 to 6.22) | b | b | b |
| Asian/Pacific Islander | +1.79 (0.23–3.38)a | +0.56 (−0.38 to 1.51) | +0.77 (−0.27 to 1.82) | +3.42 (0.92–5.99)a |
| Hispanic | +3.01 (1.81–4.23)a | +0.06 (−0.89 to 1.01) | +1.80 (−0.49 to 4.14) | +4.37 (1.71–7.09)a |
AAPC, average annual percentage change; CI, confidence interval.
p < 0.05.
Statistic not reported owing to fewer than 20 cases in some years.
Among women, 2-year CSS rates increased across all race and ethnic groups except AIAN women, with Hispanic women (AAPC = +3.00, p < 0.001) experiencing the greatest increase in 2-year CSS over time (Table 3, Fig. 2E). In stage-specific analyses, 2-year CSS rates for local- and regional-stage NSCLC significantly increased among white and black women (p < 0.05 for both) but remained stable over time for API and Hispanic women. Specifically, black women had the largest increase in local-stage 2-year CSS (AAPC = +1.17, p < 0.001) whereas white women experienced the largest improvement in regional-stage 2-year CSS rate (AAPC = +1.68, p = 0.005). Rates of 2-year CSS for distant-stage NSCLC increased among all women, with black (AAPC = +4.59, p < 0.001) women again experiencing the greatest improvement over time. Two-year CSS for unknown-stage disease increased slightly over time; however, we were unable to fully evaluate trends by race and ethnicity owing to sparse data (Supplementary Table 3).
Trends in Absolute and Relative Disparities
From 2007 to 2018, absolute disparities in incidence of NSCLC (measured as the range difference between the race or ethnic group with highest and lowest rates) have declined significantly among men (AAPC = −3.22, p < 0.001) and women (AAPC = 2.46, p < 0.001), driven largely by significant declines in absolute disparities in regional-stage (men, AAPC = −3.10, p < 0.001; women, AAPC = −3.62, p < 0.001) and distant-stage (men, AAPC = −3.79, p < 0.001; women, AAPC = −2.05, p < 0.001) diseases (Table 4). In contrast, relative disparities in overall and stage-specific incidence of NSCLC (derived by dividing the group with the highest rate by the group with the lowest rate) remained stable over time.
Table 4.
Trends in Racial and Ethnic Disparities in Overall and Stage-Specific NSCLC Incidence From 2007 to 2018
| Sex/Stage | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | AAPC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | |||||||||||||
| Range differencea | |||||||||||||
| All stages | 180.84 | 188.62 | 197.08 | 173.87 | 167.28 | 173.79 | 154.46 | 161.08 | 156.46 | 150.15 | 141.86 | 127.71 | −3.22 (−4.12 to −2.31)b |
| Localc | 33.82 | 29.43 | 30.16 | 29.65 | 32.73 | 24.78 | 26.04 | 27.27 | 27.12 | 25.99 | 28.71 | 28.63 | −1.67 (−2.88 to −0.44)b |
| Regionalc | 44.73 | 46.90 | 44.40 | 42.71 | 37.98 | 43.07 | 40.35 | 39.38 | 34.44 | 38.52 | 35.98 | 29.31 | −3.10 (−4.34 to −1.86)b |
| Distant | 101.94 | 109.37 | 117.15 | 98.77 | 95.72 | 102.71 | 85.88 | 91.78 | 96.09 | 82.37 | 74.93 | 66.80 | −3.79 (−5.33 to −2.23)b |
| Range ratiod | |||||||||||||
| All stages | 2.52 | 2.67 | 2.79 | 2.72 | 2.62 | 2.75 | 2.60 | 2.69 | 2.82 | 2.63 | 2.76 | 2.70 | +0.38 (−0.21 to 0.97) |
| Localc | 3.01 | 2.54 | 2.49 | 3.07 | 3.34 | 2.44 | 2.71 | 2.96 | 2.82 | 2.26 | 2.61 | 2.91 | −0.50 (−2.51 to 1.56) |
| Regionalc | 2.61 | 2.89 | 2.71 | 2.89 | 2.43 | 2.99 | 2.91 | 2.80 | 2.83 | 2.78 | 2.87 | 2.80 | +0.45 (−0.63 to 1.54) |
| Distant | 2.44 | 2.67 | 2.95 | 2.61 | 2.66 | 2.78 | 2.50 | 2.62 | 2.98 | 2.77 | 2.85 | 2.61 | +0.47 (−0.69 to 1.65) |
| Women | |||||||||||||
| Range differencea | |||||||||||||
| All stages | 103.93 | 93.46 | 96.61 | 86.44 | 91.68 | 83.52 | 85.23 | 88.76 | 80.52 | 80.25 | 78.16 | 75.08 | −2.46 (−3.14 to −1.78)b |
| Localc | 30.09 | 27.36 | 26.71 | 23.71 | 22.26 | 24.67 | 23.80 | 24.77 | 26.86 | 26.58 | 27.37 | 24.42 | −0.62 (−2.08 to 0.87) |
| Regionalc | 27.24 | 27.32 | 27.04 | 22.82 | 25.67 | 22.58 | 26.15 | 25.09 | 21.34 | 20.12 | 20.28 | 16.85 | −3.62 (−5.22 to −2.00)b |
| Distantc | 48.53 | 49.03 | 44.35 | 45.56 | 44.92 | 39.84 | 35.26 | 46.53 | 42.38 | 37.15 | 37.99 | 39.60 | −2.05 (−3.40 to −0.69)b |
| Range ratiod | |||||||||||||
| All stages | 2.46 | 2.24 | 2.35 | 2.18 | 2.37 | 2.16 | 2.23 | 2.39 | 2.20 | 2.18 | 2.15 | 2.36 | −0.46 (−1.26 to 0.35) |
| Localc | 2.99 | 2.68 | 2.50 | 2.47 | 2.27 | 2.62 | 2.47 | 2.52 | 2.79 | 2.40 | 2.35 | 2.43 | −0.96 (−2.21 to 0.32) |
| Regionalc | 2.67 | 2.85 | 2.87 | 2.26 | 2.79 | 2.36 | 3.01 | 3.04 | 2.44 | 2.26 | 2.35 | 2.71 | −0.80 (−2.88 to 1.33) |
| Distantc | 2.30 | 2.28 | 2.21 | 2.23 | 2.37 | 2.07 | 1.94 | 2.40 | 2.20 | 2.27 | 2.29 | 2.51 | +0.43 (−0.89 to 1.78) |
AAPC, average annual percentage change; CI, confidence interval.
Range difference (highest group rate – lowest group rate) represents a summary measure of disparities on the absolute scale.
p < 0.05.
American Indian/Alaskan Native excluded from stage-specific analyses owing to fewer than 20 cases in some years.
Range ratio (highest group rate ÷ lowest group rate) represents a summary measure of health disparity on the relative scale.
Pairwise comparison revealed marked variation in NSCLC incidence rates depending on sex, especially among white and black patients (Supplementary Tables 4 and 5). For example, incidence of NSCLC among black men was 26% higher (incidence rate ratio [IRR] = 1.26, 95% confidence interval [CI]: 1.19–1.32) than white men in 2007 and remained 33% higher (IRR = 1.33, 95% CI: 1.26–1.40) than white men by 2018. Stage-specific analyses again revealed consistently higher incidence of regional- and distant-stage NSCLC among black men compared with the other groups. For example, the incidence of regional-stage NSCLC among black men was 20% higher (IRR = 1.20, 95% CI: 1.08–1.33) compared with white men in 2007 and remained 39% (1.39, 95% CI: 1.24–1.55) higher by 2018. Incidence of distant-stage NSCLC among black men was 42% higher (IRR = 1.42, 95% CI: 1.33–1.53) than white counterparts in 2007 and remained 44% higher (IRR = 1.44, 95% CI: 1.34–1.54) by 2018. In contrast, we observed no such disparities in NSCLC incidence among black women, whose IRRs were similar to or lower than those of white women throughout the study period. For both sexes, API, AIAN, and Hispanic patients had consistently lower overall and stage-specific incidence compared with their white counterparts.
Absolute and relative disparities in overall and stage-specific 2-year CSS have generally decreased over time, although these trends did not reach statistical significance for either sex (Supplementary Table 6). In pairwise comparison, survival rate ratios (SRRs) for black and API men and women and Hispanic men moved closer to 1.00 (and the survival rate difference moved closer to 0) over time, indicating progress toward health equity for these groups (Supplementary Tables 7 and 8). Despite this improvement, black men and women continued to have worse 2-year CSS than their white counterparts in 2016 (men, SRR = 0.90, 95% CI: 0.84–0.95; women, SRR = 0.90, 95% CI: 0.86–0.96). When stratified by stage, black men had lower CSS rates for regional (SRR = 0.83, 95% CI: 0.72–0.96) and distant (SRR = 0.74, 95% CI: 0.58–0.94) stages of disease compared with white men in 2007, but by 2016, differences in stage-specific CSS rates between black and white men were no longer statistically significant. Alternatively, API women had consistently higher regional- and distant-stage CSS rates compared with white women over the study period (p < 0.05). Similar trends occurred among API men, who also had consistently higher distant-stage CSS rates compared with their white counterparts (p < 0.05).
Discussion
We provide a comprehensive evaluation of trends and patterns of racial and ethnic disparities in NSCLC incidence and survival by stage at diagnosis. Both men and women experienced declines in NSCLC incidence from 2007 to 2018, which was mainly the consequence of stark declines in the incidence of distant-stage NSCLC in the study period. During the same time period, the incidence of local-stage NSCLC significantly increased among black and Hispanic women and remained stable among all other groups. This improvement was further indicated by increases in 2-year CSS across all racial and ethnic groups.
Prior research has found an association between decreased NSCLC mortality and a corresponding diagnostic shift from later to earlier stages.10 In our analyses, incidence of local-stage NSCLC significantly increased among black and Hispanic women (p < 0.05) and remained stable over time for all other groups. Stable or increasing incidence of local-stage NSCLC along with substantial declines in incidence of regional- and distant-stage NSCLC may suggest a shift toward earlier detection of NSCLC when treatment is most effective.
Improvements in NSCLC outcomes have resulted in narrowing absolute disparities in NSCLC incidence for both men and women, and stage-specific analyses revealed that these trends were driven largely by reduced absolute disparities in incidence of regional- and distant-stage NSCLC. In contrast, relative disparities in overall and stage-specific NSCLC incidence remained constant over time, indicating more work is needed to not only reduce but eliminate disparities in NSCLC outcomes across groups.
Our findings also highlight a persistent gap in disease burden among black and white men. For example, NSCLC incidence among black men remained 33% higher than those of white men in 2018. Black men also had consistently higher incidence of regional- and distant-stage NSCLC and lower 2-year CSS rates compared with the other groups. Persistent disparities among black patients, during a time period of improved access to screening and advances in treatment, reflect inequity across the cancer continuum. Furthermore, findings of lower CSS among black patients underscore the need for additional research to identify and implement interventions to ensure consistent and equitable use of high-quality screening, diagnosis, and treatment to reduce and eliminate persistent racial and ethnic disparities.
Stark declines in NSCLC incidence and mortality in the past several years, as reported by us and others,2,12 correspond with recent advances in lung cancer screening and treatment of NSCLC. In the past decade, the identification of specific driver mutations, such as EGFR and ALK, has led to the development of new therapies and treatment targets for NSCLC.16,17 These advancements in targeted therapy for NSCLC have led to improvements in quality of life and overall survival among patients harboring the corresponding driver mutation.17,18 More recently, improved outcomes for patients with NSCLC have resulted from development of immune checkpoint inhibitor therapies, particularly those targeting the programmed cell death protein 1–programmed death-ligand 1 pathway.19, 20, 21 Studies have found EGFR mutation-positive NSCLC tumors to be more common in Asian patients than in non-Asian patients.22 This means that a greater proportion of Asian patients are likely to gain clinical benefit from EGFR-targeted treatments. As such, our findings of higher stage-specific CSS rates in API patients may be explained by the higher prevalence of EGFR mutation in the API population.
Although newly developed therapies undoubtedly play a role in population mortality, improvements in lung cancer screening and early detection can also contribute to mortality declines. LDCT scan is the only validated and reliable screening method for early detection of lung cancer and has been recommended by the USPSTF since 2013.23 Despite this, insurance coverage of LDCT scan did not become effective until 2015, which likely contributed to the initial low uptake of LDCT screening.24 Low screening rates led authors of prior studies to attribute the stark reductions in NSCLC mortality to advancements in targeted therapies for NSCLC rather than improved early detection through screening.2 Nevertheless, several recent studies have reported small, yet statistically significant increases in lung cancer screening utilization in recent years, likely as a result of improved insurance coverage and increased access to health care.25, 26, 27 As such, we believe that our findings of declines in NSCLC mortality, improvements in stage-specific survival, and the apparent shift toward earlier stage diagnosis reflect the combined benefit of advances in early detection of lung cancer by LDCT screening, improvements in targeted treatment and immunotherapies, and increased insurance coverage of these services. Despite this progress, substantial disparities in NSCLC burden remain.
Advances in lung cancer screening and targeted therapies have the potential to greatly improve racial and ethnic disparities in NSCLC outcomes, but only if implemented effectively and equitably. In 2021, the USPSTF released updated lung cancer screening guidelines that lowered the screening age from 55 to 50 years and smoking history from 30 to 20 pack-years.23 These changes are expected to expand screening access to racial and ethnic groups. Nevertheless, timely and equitable access to quality lung cancer treatment after diagnosis remains a critical concern. Future studies should monitor the impact of revised screening guidelines on disparities in access to lung cancer screening, treatment, stage-specific incidence, and survival among racial and ethnic populations.
Use of SEER data has several limitations. The SEER data do not include detailed information on patient insurance status, smoking history, diagnostic method, screening procedures, family history, and complete course of treatment.28 Therefore, our associations of improvements in NSCLC outcomes with increased access to LDCT screening and advancements treatment are speculative and should be interpreted with caution. In addition, we were unable to fully assess stage-specific trends for some racial and ethnic groups owing to sparse data. Furthermore, our use of broad racial and ethnic categories may mask important differences within and among subgroups. Although we used the most recent SEER data available at the time of the analysis, analyses should be repeated every few years owing to evolving health practices. In particular, results from the NELSON trial were presented at the World Lung Cancer Conference in 2018 and published in 2020, thus, practices in screening and consequent incidence data may be affected.6 As such, our efforts should be repeated as additional data become available.
In conclusion, declines in NSCLC incidence and improvements in stage-specific survival may reflect the population benefit of recent advancements in lung cancer screening and targeted treatments. Despite this progress, substantial racial and ethnic disparities in NSCLC burden persist. More work is needed to identify and implement interventions to ensure consistent and equitable use of high-quality screening, diagnosis, and treatment to reduce and eliminate persistent racial and ethnic disparities in NSCLC.
CRediT Authorship Contribution Statement
Kristin M. Primm: Conceptualization, methodology, software, validation, formal analysis, writing—original draft, visualization.
Hui Zhao: Resources, validation, writing—review and editing.
DaphneC.Hernandez: Supervision, writing—review and editing.
Shine Chang: Supervision, writing—review and editing, funding acquisition.
Acknowledgments
This research and Drs. Primm and Chang are supported by the CPRTP at MD Anderson Cancer Center and an award from the Cancer Prevention and Research Institute of Texas for the CPRTP Postdoctoral Fellowship in Cancer Prevention Program (RP 170259, Drs. Shine Chang and Sanjay Shete, Principal Investigators).
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Primm KM, Zhao H, Hernandez DC, Chang S. Racial and ethnic trends and disparities in NSCLC. JTO Clin Res Rep. 2022;3:100374.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100374.
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society Cancer facts & figures for African Americans 2019–2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf Accessed December 13, 2021.
- 4.Higgins R.S., Lewis C., Warren W.H. Lung cancer in African Americans. Ann Thorac Surg. 2003;76:S1363–S1366. doi: 10.1016/s0003-4975(03)01208-6. [DOI] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Koning H., van der Aalst C., de Jong P., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 7.Moyer V.A. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 8.Blandin Knight S., Crosbie P.A., Balata H., Chudziak J., Hussell T., Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7 doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 10.Flores R., Patel P., Alpert N., Pyenson B., Taioli E. Association of stage shift and population mortality among patients with non–small cell lung cancer. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.37508. e2137508-e2137508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute, DCCPS, Surveillance Research Program Surveillance, Epidemiology, and End Results (SEER) Program populations (1969–2018) www.seer.cancer.gov/popdata Accessed September 20, 2021.
- 12.Summary Staging Guide . National Cancer Institute - National Institutes of Health; 1977. Cancer Surveillance Epidemiology and End Results Reporting. [Google Scholar]
- 13.Breen N., Scott S., Percy-Laurry A., Lewis D., Glasgow R. Health disparities calculator: a methodologically rigorous tool for analyzing inequalities in population health. Am J Public Health. 2014;104:1589–1591. doi: 10.2105/AJPH.2014.301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn J., Harper S., Yu M., Feuer E.J., Liu B., Luta G. Variance estimation and confidence intervals for 11 commonly used health disparity measures. JCO Clin Cancer Inform. 2018;2:1–19. doi: 10.1200/CCI.18.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joinpoint Regression Program. Version 4.8.0.1; 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.
- 16.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Shaw A.T., Friboulet L., Leshchiner I., et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med. 2016;374:54–61. doi: 10.1056/NEJMoa1508887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soria J.-C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 19.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst R.S., Baas P., Kim D.-W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 21.Sacher A.G., Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non–small-cell lung cancer: a review. JAMA Oncol. 2016;2:1217–1222. doi: 10.1001/jamaoncol.2016.0639. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y., Au J.S.-K., Thongprasert S., et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Preventive Services Task Force (USPSTF) Lung cancer: screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
- 24.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedewa S.A., Kazerooni E.A., Studts J.L., et al. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. JNCI J Natl Cancer Inst. 2021;113:1044–1052. doi: 10.1093/jnci/djaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahnd W.E., Eberth J.M. Lung cancer screening utilization: a behavioral risk factor surveillance system analysis. Am J Prev Med. 2019;57:250–255. doi: 10.1016/j.amepre.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Okereke I.C., Nishi S., Zhou J., Goodwin J.S. Trends in lung cancer screening in the United States, 2016–2017. J Thorac Dis. 2019;11:873. doi: 10.21037/jtd.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James B.Y.M., Gross C.P., Wilson L.D., Smith B.D. NCI SEER public-use data: applications and limitations in oncology research. Oncology. 2009;23:288. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.