Abstract
Study Design:
Retrospective Study.
Objective:
To compare methods of assessing pre-operative bone density to predict risk for osteoporosis related complications (ORC), defined as proximal junctional kyphosis, pseudarthrosis, accelerated adjacent segment disease, reoperation, compression fracture, and instrument failure following spine fusions.
Methods:
Chart review of primary posterior thoracolumbar or lumbar fusion patients during a 7 year period. Inclusion criteria: preoperative dual-energy x-ray absorptiometry (DXA) test within 1 year and lumbar CT scan within 6 months prior to surgery with minimum of 1 year follow-up. Exclusion criteria: <18 years at time of index procedure, infection, trauma, malignancy, skeletal dysplasia, neuromuscular disorders, or anterior-posterior procedures.
Results:
140 patients were included. The average age was 67.9 years, 83 (59.3%) were female, and 45 (32%) had an ORC. There were no significant differences in patient characteristics between those with and without an ORC. Multilevel fusions were associated with ORCs (46.7% vs 26.3%, p = 0.02). Patients with ORCs had lower DXA t-scores (-1.62 vs -1.10, p = 0.003) and average Hounsfield units (HU) (112.1 vs 148.1, p ≤ 0.001). Multivariable binary logistic regression analysis showed lower average HU (Adj. OR 0.00 595% CI 0.0001-0.1713, p = 0.001) was an independent predictor of an ORC. The odds of an ORC increased by 1.7-fold for every 25 point decrease in average HU.
Conclusions:
The gold standard for assessing bone mineral density has been DXA t-scores, but the best predictor of ORC remains unclear. While both lower t-scores and average HU were associated with ORC, only HU was an independent predictor of ORC.
Keywords: hounsfield units, dual-energy x-ray absorptiometry, osteoporosis-related complications, lumbar fusion
Introduction
An aging population in the United States presents several challenges unique to an older demographic, including osteoporosis and osteopenia. It is estimated that 15% of the US population is at risk for disability or death as a result of osteoporotic complications.1,2 As the elderly population in the US continues to rise, the number of patients requiring instrumented spinal fusions in the setting of poor bone density will rise with it. 2
The medical and surgical algorithms for managing osteoporosis and osteopenia have evolved as our understanding of disease pathology and treatments have grown.3-5 The development of bisphosphonates, anabolic agents for bone synthesis, biologics and bone graft substitutes, and the recognition and treatment of vitamin D deficiency have enabled spinal fusion to become a valid and valuable treatment option for patients with diminished bone density, where it may have been inadvisable in the past. 5 However, even with advancements in the optimization of perioperative bone health, osteoporosis and osteopenia remain significant problems and have both been associated with increased complications following spinal fusion.5,6
One of the primary challenges osteoporosis and osteopenia pose on spine surgical outcomes is the lack of a gold standard prognostic preoperative radiographic measure to determine the risk of osteoporosis-related complications (ORC) following spinal fusion surgery. Several radiographic metrics such as Hounsfield units (HU), FRAX score, and t-scores on dual-energy x-ray absorptiometry (DXA) have been studied in the setting of osteoporosis/osteopenia and spine surgery.7-9 While DXA t-scores have been considered the gold standard for osteoporosis evaluation, the International Society for Clinical Densitometry recommends that in patients with degenerative spine disease including spinal deformity, lumbar spine DXA should not be used, as these focal structural changes may falsely elevate the reported BMD. 10 HU, as measured on routine CT of the lumbar spine, have shown promise in predicting bone density11,12 as well as various complications in patients with a degenerative lumbar spine.13-15 The purpose of this study was to compare patient characteristics and measures of bone density to determine which one or ones were associated with ORC following spinal fusion.
Methods
After obtaining approval from the Institutional Review Board, a retrospective cohort study was performed on an existing data set that has been previously analyzed for ORCs 5 composed of data from consecutive patients undergoing primary posterior thoracolumbar or lumbar fusion from 2 surgeons at an academic medical center from 2007 to 2014 were retrospectively reviewed. The inclusion criteria was patients with a DXA scan of the hips and/or spine performed within 1 year and CT scans within 6 months prior to the index procedure. Patients were excluded if they were younger than 18 years at the time of the index procedure or had infection, trauma, malignancy, skeletal dysplasia, neuromuscular disorders, or concomitant or staged anterior-posterior procedure.
Chart review was conducted of patients that met study criteria. Clinical notes and operative reports were reviewed to obtain clinical data regarding baseline characteristics, medical comorbidities, and surgical data. Preoperative CT scans obtained as a part of the patients’ routine clinical care were used to calculate the average Hounsfield units of the lumbar vertebrae. These were measured 3 times for all 5 lumbar vertebral bodies (superior, middle, and inferior portions) and averaged, as previously reported. The overall mean HU for the lumbar spine was then obtained from the average HU for each vertebral body. 16 HU measurements were excluded if there was prior pedicle screw instrumentation at those levels. All measurements were made utilizing our institution’s standard Picture Archiving and Communication Software (PACS). For complete details of how this was done please see the appendix. Preoperative DXA scans were used to obtain t-scores of the hips and lumbar spine. The lowest overall t-score from any region was used for grouping based on the World Health Organization (WHO) definitions. The WHO classifies bone health in adults age > 50 years using the lowest spine or total hip bone mineral density (BMD) compared to a reference standard taken from young white women. Osteoporosis is defined as a T-score ≤ -2.5, osteopenia defined with a T-score between -1.0 and -2.4, and normal bone density with a T-score > -1.0. 17 FRAX scores and ORCs were obtained from associated notes. ORCs were defined as one of the following categories: revision surgery, compression fracture, proximal junctional kyphosis, pseudarthrosis, accelerated adjacent segment disease, or instrumentation failure (including screw loosening).5,18,19 Determinations for ORCs were based on a review of imaging and the clinical record by a spine-fellowship trained surgeon.
Standard descriptive summary statistics (e.g. means and standard deviations for continuous variables such as age and percentage for categorical variables such as gender) were used to summarize demographic variables. Comparisons of categorical variables between subgroups were made using the Chi-square test or the Fisher’s exact test for cases when expected values were less than 5. Comparisons of continuous variables were completed using independent t-tests or the 1-way ANOVA for comparisons between 3 or more groups. A multivariable binary regression model was constructed to test associations between various clinical factors and the rate of ORCs. Only significant variables were entered into the model to prevent overfitting. Additionally, the multiplicative effects of the OR for continuous variables was used to estimate the increased odds of ORCs for greater than 1 unit change in the variable of interest. 20 Alpha was set at a significance level of p < 0.05. Statistical analysis was performed using JMP® software (JMP®, Version 14.1.0. SAS Institute Inc., Cary, NC, 1989-2019).
Results
Overall, 140 patients met appropriate criteria and all were included for analysis. Patient demographic information is reported in Table 1. Follow up period was an average of 2.1 years with a minimum of 1 year.
Table 1.
Comparison of Baseline Characteristics of Patients With and Without ORC.
| ORC | No ORC | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 70.2 (10.0) | 66.8 (9.9) | 0.0606 |
| Sex, n (% Female) | 23 (62.2) | 60 (63.2) | 0.9148 |
| Ethnicity, n (%) | 0.1545† | ||
| White | 70 (97.2) | 23 (92.0) | |
| Latino | 1 (1.4) | 0 (0) | |
| Native American | 0 (0) | 1 (1.4) | |
| Other/Unknown | 1 (1.4) | 1 (1.4) | |
| BMI, mean (SD) | 29.2 (5.8) | 29.8 (5.9) | 0.6634 |
| Diabetes, n (%) | 4 (8.9) | 13 (13.7) | 0.4058† |
| Active Smoker, n (%) | 1 (2.2) | 2 (2.1) | 1.000† |
| Bisphosphonate*, n (%) | 9 (20.0) | 10 (10.5) | 0.1846† |
| Teriparatide*, n (%) | 10 (22.2) | 7 (7.4) | 0.0236† |
ORC = Osteoporosis Related Complication, SD = standard deviation, BMI = Body Mass Index, * = treatment within 6 months of surgery. Significant results (p-value < 0.05) are bolded. P-values for continuous variables were obtained using independent t-tests. P-values for categorical variables were obtained using Chi-square tests. Fisher’s Exact test was used for small sample size, indicated with (†).
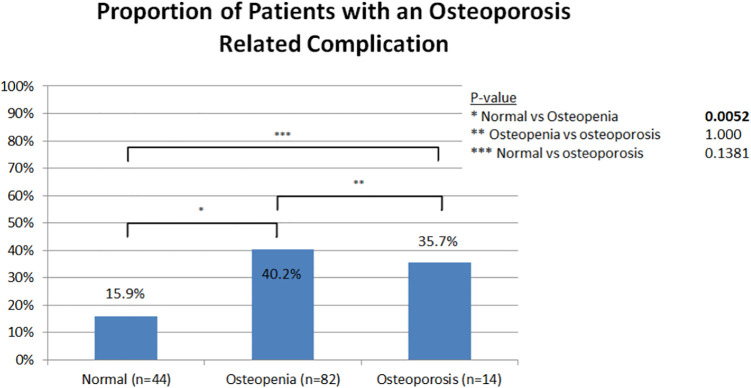
ORC was found in 45 (32%) patients. Patients were grouped according to the WHO definitions into patients with normal bone, osteopenia, and osteoporosis (Table 2). Among the 3 groups, there were 44 (31.4%) normal, 82 (58.6%) osteopenic, and 14 (10.0%) osteoporotic subjects. There were significant differences seen in the mean age, sex, and BMI of patients with normal bone density, osteopenia, and osteoporosis. The average age for the normal, osteopenic, and osteoporotic groups were 64.7 years, 68.6 years, and 73.3 years, respectively (p = 0.011). The normal bone density group was 47.7% female, the osteopenic group was 68.3% female, and the osteoporotic group was 78.6% female (p = 0.033). The average BMI among groups was 31.45 for the normal bone density group, 29.26 for the osteopenic group, and 25.83 for the osteoporotic group (p = 0.027). There were no significant differences in rates of multilevel fusion (3 or more levels) surgery among the 3 groups, with 25.0% in the normal bone density group, 35.4% in the osteopenia group, and 42.9% in the osteoporosis group (p = 0.350) (Table 2). The rate of ORC was 15.9% in patients with normal bone, 40.2% in patients with osteopenia, and 35.7% in patients with osteoporosis. There was a significant difference in rate of ORC between patients with normal bone density and osteopenia (p = 0.005). The difference in rates of ORC was not significant between the other categories (Figure 1). The most common ORCs were the following: 12 patients (8.6%) developed a pseudarthrosis, 10 patients (7.1%) required reoperation (2 for hardware failure, 1 vertebral body fracture, 3 pseudarthroses, 3 accelerated adjacent segment disease, 1 for PJK), and 5 patients (3.6%) had a compression fracture (Figure 2).
Table 2.
Comparison of Patient HU in Relation to Their Done Density on DXA Scan.
| DXA Classification | Normal | Osteopenia | Osteoporosis | P-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 64.7 (11.5) | 68.6 (8.8) | 73.3 (9.3) | 0.0106 |
| Sex, n (% Female) | 21 (47.7) | 56 (68.3) | 11 (78.6) | 0.0328 |
| BMI, mean (SD) | 31.45 (4.44) | 29.26 (6.48) | 25.83 (3.40) | 0.0272 |
| ≥ 3 levels fused, n (%) | 11 (25.0) | 29 (35.4) | 6 (42.9) | 0.3500 |
| Average Total HU, mean | 164.8 | 126.7 | 104.9 | <0.0001 |
| Average L1 HU, mean | 162.2 | 129.1 | 85.2 | <0.0001 |
| L1 > 110 HU, n (%) | 36 (90.0) | 41 (65.1) | 1 (10.0) | <0.0001 |
| L1 < 110 HU, n (%) | 4 (10.0) | 22 (34.9) | 9 (90.0) | <0.0001 |
HU = Hounsfield Units, DXA = Dual X-ray absorptiometry, SD = Standard Deviation. Significant results (p-value < 0.05) are bolded. P-values for continuous variables were obtained using 1-way ANOVA test. P-values for categorical variables were obtained using Fisher’s Exact test.
Figure 1.
Proportion of patients who developed an osteoporosis related complication by bone density group as determined from t-score impression. Pairwise Chi-squared tests were performed to evaluate for significant differences between groups.
Figure 2.
Overall number, relative frequency, and type of osteoporosis related complications among the entire patient sample.
There were no significant differences between patients with and without an ORC in terms of age, sex, ethnicity, body mass index (BMI), diabetes, and active smoking status. However, patients with an ORC were more likely to have been treated with teriparatide within 6 months of surgery (22% vs 7.4%, p = 0.024) (Table 1).
The region of the fusion (i.e. thoracic vs. lumbar…), instrumentation (90.7%) vs. uninstrumented (9.3%), decompression, and interbody fusion did not significantly differ between patients with and without an ORC (Table 3). Patients with 3 or more levels fused were more likely to have an ORC (46.7% vs 26.3%, p = 0.021). There was also a significant difference based on the treating surgeon. Comparing the patients with and without an ORC based on the surgeon, Surgeon 1 operated on 44.4% of the patients with an ORC and 23.2% of those without an ORC (p = 0.01) (Table 3).
Table 3.
Comparison of Surgical Data of Patients With and Without ORC.
| ORC | No ORC | P-value | |
|---|---|---|---|
| Numbers of levels fused, mean (SD) | 3.8 (4.1) | 2.6 (2.9) | 0.0790 |
| ≥ 3 levels fused, n (%) | 21 (46.7) | 25 (26.3) | 0.0211 |
| Fusion Location, n (%) | 0.0657 | ||
| Thoracic | 1 (2.2) | 0 (0) | |
| Thoracolumbar | 1 (2.2) | 3 (3.2) | |
| Lumbar | 16 (35.6) | 38 (40.0) | |
| Lumbosacral | 17 (37.8) | 47 (49.5) | |
| Thoracolumbosacral | 10 (22.2) | 7 (7.4) | |
| Instrumented Fusion, n (%) | 38 (84.4) | 89 (93.7) | 0.1161 |
| Interbody Fusion, n (%) | 20 (44.4) | 25 (55.6) | 1.0000 |
| Decompression, n (%) | 24 (96.0) | 68 (94.4) | 1.0000 |
| Treating Surgeon*, n (%) | 20 (44.4) | 22 (23.16) | 0.0103 |
ORC = Osteoporosis Related Complication, SD = standard deviation, * = proportion of patients treated by Surgeon 1 vs Surgeon 2. Significant results (p-value < 0.05) are bolded.
There were significant differences between patients with and without an ORC in terms of the various measures of bone density (Table 4). While the FRAX score was not significantly associated with an ORC, patients with ORCs had significantly lower DXA t-scores, (-1.62 vs -1.10, p = 0.003) and lower average HU (112.1 vs 148.1, p > 0.001) (Table 4). The rate of teriparatide use did not reach statistical significance based on bone density diagnosis for patients with normal BMD, osteopenia, or osteoporosis (13 (9%) vs 17 (12%) vs 30 (21%), respectively, p = 0.47).
Table 4.
Comparison of Measures of Bone Quality of Patients With and Without ORC.
| ORC | No ORC | P-value | |
|---|---|---|---|
| DXA—Lowest T-Score, mean (SD) | -1.62 (0.89) | -1.10 (1.09) | 0.0030 |
| Average HU of lumbar vertebral bodies, mean (SD) | 112.1 (42.3) | 148.1 (41.8) | 0.0001 |
| FRAX Score | |||
| Major Osteoporotic Fracture Score, mean (SD) | 14.3 (10.8) | 9.18 (5.9) | 0.0964 |
| Hip Fracture Score, mean (SD) | 2.3 (2.3) | 1.3 (1.4) | 0.1395 |
| *Major Osteoporotic Fracture Score ≥ 20%, n (%) | 3 (3.57) | 2 (2.11) | 0.3278 |
| *Hip Fracture Score ≥ 3%, n (%) | 4 (8.9) | 2 (2.11) | 0.0842 |
ORC = Osteoporosis Related Complication, SD = standard deviation, HU = Hounsfield Units, FRAX = Fracture Risk Assessment Tool, * = Treatment Threshold based on 10-year probability of a hip fracture ≥ 3% or a 10-year probability of a major osteoporosis-related fracture ≥ 20% based on the US-adapted WHO algorithm. Significant results (p-value < 0.05) are bolded.
When analyzed in a multivariable binary regression model (Table 5), the only factors that were independent predictors of an ORC were treatment with teriparatide (OR 5.20, 95% CI 1.48-18.32, p = 0.009) and lower average HU (OR 0.00 595% CI 0.0001-0.1713, p = 0.001). The odds of an ORC increased by 1.7-fold for every decrease in the average HU of 25 points. When analyzing the HU specifically there was a significant difference observed in the average HU between the 3 WHO bone density classifications (Table 2). Normal bone density had an average HU of 164.8, osteopenia had an average HU of 126.7, and osteoporosis had an average HU of 104.9 (p ≤ 0.001). A significant difference was also seen when analyzing L1 specifically. Normal bone density patients had an average L1 HU of 162.2, osteopenia had an average HU of 129.1, and osteoporosis had an average HU of 85.2 (p ≤ 0.001). When using 110 HU as the threshold for osteoporosis there was a significant different rate found among the 3 WHO groups. Based on an L1 HU of <110, 4 (10.0%) of normal WHO classified patients, 22 (34.9%) of osteopenic WHO classified patients, and 9 (90.0%) of osteoporotic WHO classified patients would be categorized as having osteoporosis based on an L1 HU of <110 (p ≤ 0.001) (Table 2).
Table 5.
Multivariable Binary Regression Model of Factors Associated With ORC.
| Odds Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| Average HU of lumbar vertebral bodies | 0.005* | 0.0001-0.1713 | 0.0010 |
| Teriparatide** | 5.20 | 1.48-18.32 | 0.0092 |
| Treating Surgeon*** | 2.65 | 0.80-8.7 | 0.1074 |
| ≥ 3 levels fused | 1.49 | 0.49-4.47 | 0.4783 |
| DXA—Lowest T-Score | 1.25 | 0.017-92.33 | 0.9169 |
ORC = Osteoporosis Related Complication, SD = standard deviation, HU = Hounsfield Unit, * = ORC increased by 1.73-fold for every decrease in the average HU of 25 points, ** = treatment within 6 months of surgery, ***= proportion of patients treated by Surgeon 1 vs Surgeon 2. Significant results (p-value < 0.05) are bolded.
Discussion
Establishing best practices for assessing and managing patients for diminished bone density prior to elective spinal fusion surgery is becoming an ever-greater challenge.5,21 Despite being commonly considered the method of choice for diagnosing osteoporosis, there are inherent limitations with using DXA as a measurement of bone density in the degenerative spine. The blastic effects of spondylosis can lead to a falsely elevated DXA t-score. 5 Furthermore, studies have shown limitations of bone densitometry, specifically pointing to discrepancies between t-scores and HU with medication use, as well as variation in HU measurements based on CT scanner settings and distance to the patient.16,22,23 As a result, our data showed that t-scores alone were not independently predictive of ORCs. Alternative methods such as HU measurements on CT are opportunistically available for many patients undergoing elective spinal fusion surgery and may provide more accurate assessments of bone density.24,25 Our data demonstrated that HUs were more predictive of ORCs than DXA t-scores or FRAX scores alone. While both the lowest t-score and HU were associated with ORCs in univariable analysis, only HU was an independent predictor of ORC in multivariable analysis. We estimate a 1.7-fold increase of ORC for every decrease in the average HU of 25 points, giving surgeons more predictable information for risk stratification in the pre-operative setting. Unfortunately, unlike DXA t-scores, which have universally recognized scoring stratification, accepted standard cut-off values for HU have not yet been determined. Some authors have suggested cut-offs between HU < 110-160 as predictive of clinically significant diminished bone density in the lumbar spine. Using 110 HU as the lower limit of normal provides 52-60% sensitivity for distinguishing osteoporosis from osteopenia and normal BMD in the lumbar region.26,27
Patients with osteoporosis have many treatments available including diet, vitamin D, calcium, and FDA-approved medications (bisphosphonates being the first-line agent).28,29 In severe cases or when first-line methods have failed, physicians and patients can use anabolic osteoporosis medications like teriparatide to help increase bone mass. 30 Abaloparatide is a similar parathyroid hormone analog but was not available for use during the time periods included in this study. However, in some patients, such as those with spinal fractures, anabolic agents have been recommended as first-line agents. 31 Teriparatide has been shown to improve bone density both clinically and radiographically, 16 as well as to increase fusion rates and decrease complications follow spinal fusion surgery.32,33 Oddly, in our study there appeared to be an association with increased rates of ORCs and the use of teriparatide.
We performed a separate sub-analysis (Table 6) to further investigate the association with the use of teriparatide and ORCs. We found no significant differences between the patients treated with teriparatide to those that were not in terms of the surgical data and baseline characteristics, except for patients treated with teriparatide were more likely to have been previously treated with a bisphosphonate (88% vs 3.3%, p < 0.0001), and to have a lower average HU (119.5 vs 140.8, p = 0.047); however, t-score did not reach statistical significance (-1.67 vs -1.21 p = 0.12). Given our sub-analysis and prior data demonstrating favorable effects of teriparatide on bone density, as well as the practice patterns of the 2 surgeons in this study, we expect that the use of teriparatide was a surrogate marker for patients with very poor bone density, who have failed other treatments and therefore are intrinsically at highest risk of an ORC, as opposed to establishing a causal relationship or lack of protective benefit between teriparatide and ORCs. The results of this study are not intended nor are they methodologically capable of determining the efficacy of teriparatide to reduce ORCs. Further, pointing out the effect of selection bias inherent to the 2 surgeon’s strict preoperative optimization requirements, while smoking 34 and poorly controlled diabetes 35 have been associated with surgical complications and non-union, we did not find an association between these 2 co-morbidities and ORC in this study. This is likely because there were few patients in our study with these medical co-morbidities (2.1% active smoker, 12.1% diabetic) as adequate glycemic control and smoking cessation are typically required prior to any elective spine surgery in these 2 surgeons’ practices.
Table 6.
Comparison of Patient Factors With and Without Teriparatide Treatment.
| Teriparatide | No Teriparatide | P-value | |
|---|---|---|---|
| Bisphosphonate*, n (%) | 15 (88.2) | 4 (3.3) | <.0001 |
| Average HU of lumbar vertebral bodies, mean (SD) | 119.5 (34.8) | 140.8 (45.6) | 0.0468 |
| DXA—Lowest T-Score, mean (SD) | -1.67 (1.03) | -1.21 (1.05) | 0.1200 |
* = treatment within 6 months of surgery, HU = Hounsfield Units, SD = Standard Deviation. Significant results (p-value < 0.05) are bolded.
This study does have limitations. As a retrospective review, we are not able to control for selection bias and confounders. This is particularly relevant to the use of teriparatide. We did attempt to account for confounders through the use of a multivariable regression model, though other factors, such as spine deformity, sagittal alignment, surgical technique, level selection, as well as other medical conditions may also play important roles. There was a significant difference between treating surgeons, though this result was unclear and potentially related to selection bias and subtle differences in the practice patterns. There may be additional confounding variables between these 2 samples, such as rates of deformity or surgical complexity, which we were not able to analyze. However, in multivariate analysis, the treating surgeon was not an independent predictor of complications. The heterogeneity of the patient population is another limitation, with different time periods of use of teriparatide and bisphosphonates, and unclear data regarding maintenance therapy following teriparatide treatment. Additionally, we were also not able to definitively determine that all the ORCs were due to poor bone quality and could have been due to a number of variables. Along with medications, there were demographic differences between groups. There were significant differences seen in the mean age, sex, and BMI of patients with normal bone density, osteopenia, and osteoporosis. However, this would be an expected result as studies have shown that poor bone density is much more prevalent in older adult females.36,37 There was a higher rate of teriparatide use in patients with ORCs, which seems somewhat counterintuitive. However, teriparatide was predominantly used in patients with osteoporosis, who were already at the highest risk for ORCs, so there may have been a selection bias in this result. Additionally, we only determined if patients were treated with teriparatide preoperatively, and did not compare the length of treatment preoperatively. Lastly, we did not have adequate data on whether or not the patients completed a full 2-year treatment course, and if they were appropriately transitioned to maintenance therapy following teriparatide treatment. Therefore, these limitations in the data may have played a role in the results we observed of higher teriparatide use in patients with ORCs.
Despite these limitations, we feel that data obtained from this large cohort is instructive on areas for future prospective and continued retrospective research aimed at identifying the best single or multi-modal method for assessing bone density prior to elective spine surgery. The demonstrated independent predictive value of HUs is one exemplary unique addition to the literature to come from our work.
Conclusions
DXA scans and associated t-scores have a suboptimal predictive value for ORCs following elective spine surgery. While both lower t-scores and average HU were associated with ORCs in our study, only HU was an independent predictor of ORC. The odds of an ORC increased by 1.7-fold for every decrease in the average HU of 25 points or 3-fold for every 50 point change. While this work suggests better methods for predicting ORCs is encouraging, further investigation is needed.
Appendix
Hounsfield units (HU) measurement consisted of 3 region of interest circles drawn at 3 different points in the vertebral body: just inferior to the superior endplate, mid-vertebral body, and just superior to the inferior end plate (Figure 3). These 3 values were then averaged together for each individual vertebral body from L1-L5 and then those 5 lumbar levels were averaged together to obtain a total value for the lumbar spine. Levels that contained instrumentation or vertebroplasty were not measured, as the artifact confounded the HU measurements.
Figure 3.
Three Hounsfield Unit (HU) measurements were taken at each vertebral body level, from L1-L5, by drawing region of interest circles just inferior to the superior endplate, mid-vertebral body, and just superior to the inferior endplate.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jeffery D. St. Jeor, BS  https://orcid.org/0000-0001-5609-282X
https://orcid.org/0000-0001-5609-282X
Brett A. Freedman, MD  https://orcid.org/0000-0002-3408-0163
https://orcid.org/0000-0002-3408-0163
Mohamad Bydon, MD  https://orcid.org/0000-0002-0543-396X
https://orcid.org/0000-0002-0543-396X
References
- 1.Forstein DA, Bernardini C, Cole RE, Harris ST, Singer A. Before the breaking point: reducing the risk of osteoporotic fracture. J Am Osteopath Assoc. 2013;113(2 Suppl 1):S5–24:quiz S25. [PubMed] [Google Scholar]
- 2.Iacono MV. Osteoporosis: a national public health priority. J Perianesth Nurs. 2007;22(3):175–180:quiz 181-172. [DOI] [PubMed] [Google Scholar]
- 3.Hassanzadeh H, Puvanesarajah V, Dalkin AC. Medical management of osteoporosis for elective spine surgery. Clin Spine Surg. 2016;29(4):134–140. [DOI] [PubMed] [Google Scholar]
- 4.Kirk D, Fish SA. Medical management of osteoporosis. Am J Manag Care. 2004;10(7 Pt 1):445–455. [PubMed] [Google Scholar]
- 5.Bjerke BT, Zarrabian M, Aleem IS, et al. Incidence of osteoporosis-related complications following posterior lumbar fusion. Global Spine J. 2018;8(6):563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson BC, Robinson WA, Wanderman NR, et al. A review and clinical perspective of the impact of osteoporosis on the spine. Geriatr Orthop Surg Rehabil. 2019;10:2151459319861591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254–262. [DOI] [PubMed] [Google Scholar]
- 8.Lim HK, Ha HI, Park S-Y, Lee K. Comparison of the diagnostic performance of CT Hounsfield unit histogram analysis and dual-energy X-ray absorptiometry in predicting osteoporosis of the femur. Eur Radiol. 2019;29(4):1831–1840. [DOI] [PubMed] [Google Scholar]
- 9.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(2):S3–S11. [DOI] [PubMed] [Google Scholar]
- 10.Densitometry ISfC.2015 ISCD official positions—adult. Official positions web site. Published 2015. Updated June 18, 2015. Accessed March 30, 2020, 2020.
- 11.Mikula AL, Puffer RC, Jeor JDS, et al. Teriparatide treatment increases Hounsfield units in the lumbar spine out of proportion to DEXA changes. J Neurosurg Spine. 2019:32(1):50–55. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93(11):1057–1063. [DOI] [PubMed] [Google Scholar]
- 13.Bokov A, Bulkin A, Aleynik A, Kutlaeva M, Mlyavykh S. Pedicle screws loosening in patients with degenerative diseases of the lumbar spine: potential risk factors and relative contribution. Global Spine J. 2019;9(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ullrich BW, Schenk P, Spiegl UJ, Mendel T, Hofmann GO. Hounsfield units as predictor for cage subsidence and loss of reduction: following posterior-anterior stabilization in thoracolumbar spine fractures. Eur Spine J. 2018;27(12):3034–3042. [DOI] [PubMed] [Google Scholar]
- 15.Zou D, Jiang S, Zhou S, et al. Prevalence of osteoporosis in patients undergoing lumbar fusion for lumbar degenerative diseases: a combination of DXA and Hounsfield units. Spine (Phila Pa 1976). 2020;45(7):E406–E410. [DOI] [PubMed] [Google Scholar]
- 16.Mikula AL, Naylor RM, St. Jeor JD, et al. P40. Teriparatide treatment improves bone quality in the vertebral body out of proportion to the pedicles and lamina of the lumbosacral spine as measured by Hounsfield units. Spine J. 2019;19(9):S176–S177. [Google Scholar]
- 17.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 18.Ehresman J, Ahmed AK, Lubelski D, et al. Vertebral bone quality score and postoperative lumbar lordosis associated with need for reoperation after lumbar fusion. World Neurosurg. 2020;140:e247–e252. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Xu W, Zhang X, Xi Z, Xie L. Biomechanical role of osteoporosis affects the incidence of adjacent segment disease after percutaneous transforaminal endoscopic discectomy. J Orthop Surg Res. 2019;14(1):131–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan P, Pramesh CS, Aggarwal R.Common pitfalls in statistical analysis: logistic regression. Perspect Clin Res. 2017;8(3):148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SB, Chung CK. Strategies of spinal fusion on osteoporotic spine. Journal of Korean Neurosurgical Society. 2011;49(6):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson GR, Luczak M, Włodarski KH. The limitation of DEXA analysis for bone mass determination in mice. Folia Biol (Krakow). 2004;52(1-2):125–129. [PubMed] [Google Scholar]
- 23.Lohman M, Tallroth K, Kettunen JA, Marttinen MT. Reproducibility of dual-energy x-ray absorptiometry total and regional body composition measurements using different scanning positions and definitions of regions. Metabolism. 2009;58(11):1663–1668. [DOI] [PubMed] [Google Scholar]
- 24.Treece GM, Gee AH. Independent measurement of femoral cortical thickness and cortical bone density using clinical CT. Med Image Anal. 2015;20(1):249–264. [DOI] [PubMed] [Google Scholar]
- 25.Klotz MCM, Beckmann NA, Bitsch RG, Seebach E, Reiner T, Jäger S. Bone quality assessment for total hip arthroplasty with intraoperative trabecular torque measurements. J Orthop Surg Res. 2014;9:109–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou D, Li W, Deng C, Du G, Xu N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur Spine J. 2019;28(8):1758–1766. [DOI] [PubMed] [Google Scholar]
- 28.Lehman RA, Jr, Kang DG, Wagner SC. Management of osteoporosis in spine surgery. J Am Acad Orthop Surg. 2015;23(4):253–263. [DOI] [PubMed] [Google Scholar]
- 29.Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. Guidelines for diagnosis and management of osteoporosis. Osteoporos Int. 1997;7(4):390–406. [DOI] [PubMed] [Google Scholar]
- 30.Bodenner D, Redman C, Riggs A. Teriparatide in the management of osteoporosis. Clin Interv Aging. 2007;2(4):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leder BZ.Anabolics and combination therapy. ASBMR. Published 2019. Accessed April 9, 2020.
- 32.Buerba RA, Sharma A, Ziino C, Arzeno A, Ajiboye RM. Bisphosphonate and teriparatide use in thoracolumbar spinal fusion: a systematic review and meta-analysis of comparative studies. Spine (Phila Pa 1976). 2018;43(17):E1014–E1023. [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, Park SW, Kim YB, Ko MJ. The effect of postoperative use of teriparatide reducing screw loosening in osteoporotic patients. J Korean Neurosurg Soc. 2018;61(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976). 2000;25(20):2608–2615. [DOI] [PubMed] [Google Scholar]
- 35.Deng H, Chan AK, Ammanuel S, et al. Risk factors for deep surgical site infection following thoracolumbar spinal surgery. J Neurosurg Spine. 2019;32(2):292–301. [PubMed] [Google Scholar]
- 36.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Mineral Res. 2014;29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. 2017;9(5):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]