Abstract
Objective
Although patients with significant coronary artery disease and aortic stenosis have traditionally undergone open valve replacement and bypass grafting, percutaneous coronary intervention (PCI) and transcatheter aortic valve replacement (TAVR) are increasingly considered. Because of the lack of data regarding timing of PCI/TAVR, in the present study we evaluated associations of staged and concomitant PCI/TAVR on outcomes in a nationally representative cohort.
Methods
Adults who underwent TAVR and PCI were identified using the 2016 to 2018 Nationwide Readmissions Database. If PCI/TAVR occurred on the same day, patients were considered Concomitant and otherwise considered Staged. Staged were further classified as Early-Staged if both occurred in the same hospitalization or Late-Staged if TAVR ensued PCI in a subsequent hospitalization. Multivariable regression models were developed to evaluate the association of TAVR timing on outcomes. The primary end point was in-hospital mortality whereas perioperative complications including acute kidney injury and hospitalization costs were secondarily considered.
Results
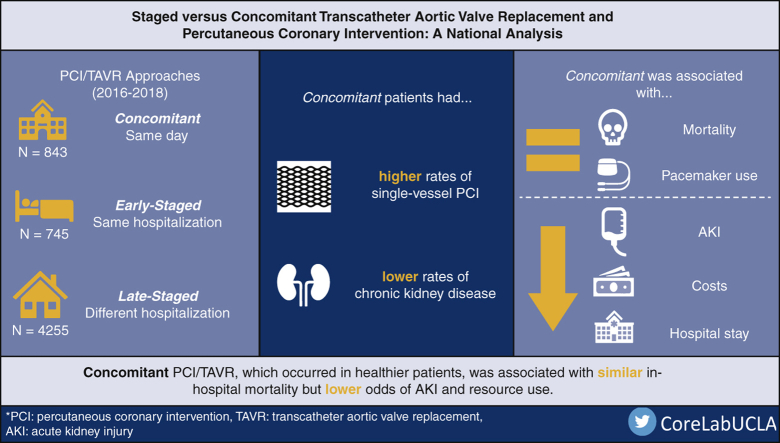
Of an estimated 5843 patients, 843 (14.4%) were Concomitant and 745 (12.7%) and 4255 (72.8%) were Early-Staged and Late-Staged, respectively. Although age and TAVR access were similar, Concomitant had a lower proportion of chronic kidney disease and more commonly underwent single-vessel PCI. Staged showed similar risk-adjusted mortality but greater odds of acute kidney injury (Early-Staged adjusted odds ratio: 2.68; 95% CI, 1.57-4.55 and Late-Staged: 1.97; 95% CI, 1.29-2.99) compared with Concomitant. Although post-TAVR hospitalization duration was similar, total length of stay and costs were increased in Staged.
Conclusions
Concomitant PCI/TAVR was associated with similar rates of in-hospital mortality but reduced rates of acute kidney injury and lower resource utilization. While evaluating patient-specific factors, concomitant PCI/TAVR might be reasonable in select individuals.
Key Words: transcatheter aortic valve replacement, percutaneous coronary intervention, nationwide readmissions database
Abbreviations and Acronyms: AKI, acute kidney injury; AOR, adjusted odds ratio; CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; ICD-10, International Classification of Disease, Tenth Revision; IQR, interquartile range; LOS, length of stay; NRD, Nationwide Readmissions Database; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement
Graphical abstract
Comparison of unadjusted procedural outcomes of treatment groups.
Central Message.
Concomitant PCI/TAVR was associated with similar rates of in-hospital mortality but reduced rates of acute kidney injury and lower resource utilization compared with staged PCI/TAVR.
Perspective.
Although patients with significant coronary artery disease and aortic stenosis have traditionally undergone open valve replacement and bypass grafting, PCI and TAVR are now more frequently considered. However, the timing of intervention for PCI and TAVR is unclear with large-scale studies lacking.
See Commentary on page 162.
With expanding indications for its use, transcatheter aortic valve replacement (TAVR) has been widely adopted for the management of aortic stenosis in patients in all risk categories for surgical aortic valve replacement (SAVR).1, 2, 3 Although low-risk surgical candidates with flow-limiting coronary artery disease and severe aortic stenosis frequently undergo SAVR with concomitant coronary artery bypass grafting (CABG), they are increasingly evaluated for percutaneous coronary intervention (PCI) and TAVR.4, 5, 6, 7 Notably, all PARTNER trials excluded patients with complex coronary artery disease or unprotected left main disease, leaving the role of percutaneous valve replacement and revascularization incompletely defined.1, 2, 3 In addition to these clinical trials, a limited number of reports have described the feasibility of combined PCI/TAVR with encouraging results.5,8 The timing of PCI then TAVR remains controversial with the staged approach shown to have greater bleeding-related complications potentially related to dual antiplatelet therapy (DAPT), as well as increased duration of hospitalization.6,7 In comparison, concomitant PCI/TAVR increases procedural complexity, might result in greater risk of acute kidney injury (AKI) because of larger volumes of intravenous contrast used, and have potential financial implications.5, 6, 7
In a meta-analysis of 209 patients, Yang and colleagues7 compared staged PCI then TAVR with concomitant PCI/TAVR and reported a similar risk of AKI for the 2 study groups. Among a cohort of 128 patients who underwent PCI then TAVR, the SURTAVI trial showed lower rates of AKI in the concomitant group.9 With no more than 10 patients experiencing AKI in each study, data remain limited and large-scale exploration of the optimal approach is lacking. In the present study we evaluated mortality, perioperative complications, and resource utilization associated with staged PCI then TAVR and concomitant PCI/TAVR in a nationally representative cohort. We hypothesized concomitant PCI/TAVR to be associated with lower hospitalization costs and shorter length of stay (LOS) while conferring similar risks of mortality and complications including AKI.
Methods
Study Design and Data Set
This was a retrospective cohort study of all adults (18 years of age and older) who underwent PCI then TAVR using the 2016 to 2018 Nationwide Readmissions Database (NRD). The NRD is an all-payer readmissions database that samples nearly 37 million annual hospitalization discharges and is part of the Healthcare Cost and Utilization Project. Validated algorithms using discharge weights provide accurate estimates for approximately 59% of all hospitalizations across the United States. The NRD contains linkage numbers for all sampled patients and allows capture of readmissions during the same calendar year across participating hospitals.
Study Groups and Outcomes
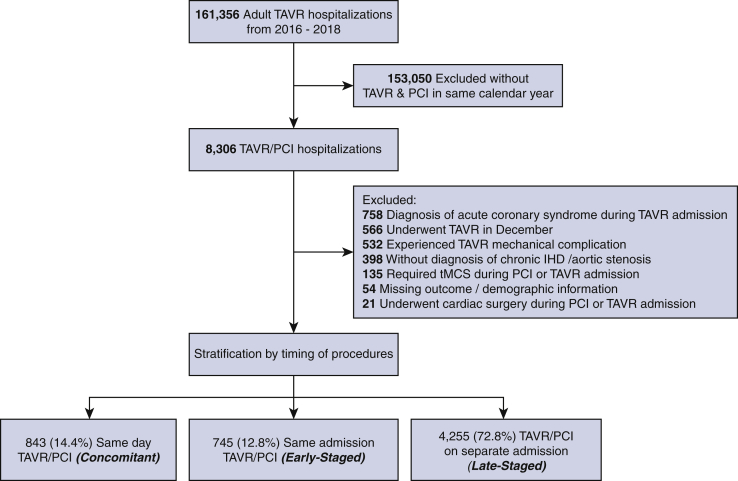
Patients who underwent PCI then TAVR with a history of chronic ischemic heart disease (I25) as well as aortic stenosis were identified using International Classification of Disease, Tenth Revision (ICD-10) codes. Timing from admission to intervention was determined using the “prday” variable reported by the NRD whereas those who underwent TAVR before PCI were not considered.10 Patients were considered as Concomitant if they underwent TAVR and PCI on the same procedure day and classified as Staged if PCI and TAVR were performed on separate days. Staged patients were further classified as Early-Staged if both procedures occurred in the same hospitalization and as Late-Staged if TAVR followed PCI in a different hospitalization during the calendar year. A Consolidated Standards of Reporting Trials diagram depicting inclusion and exclusion criteria is provided in Figure 1. To enhance homogeneity of the cohort, patients with a diagnosis of acute coronary syndrome during TAVR admission as well as those with a mechanical complication of TAVR, requiring temporary mechanical circulatory support, or undergoing cardiac surgery were omitted (Table E1). Additionally, those with missing key demographic information or mortality were omitted (0.9% of patients). Finally, because the NRD does not track hospitalizations across years, patients who underwent TAVR in December of each year were excluded to ensure adequate follow-up in the same calendar year.
Figure 1.
Consolidated Standards of Reporting Trials diagram of patient inclusion and exclusion criteria. TAVR, Transcatheter aortic valve replacement; PCI, percutaneous coronary intervention; IHD, ischemic heart disease; tMCS, temporary mechanical support.
Patient and hospital characteristics were defined using ICD-10 codes and in accordance with the NRD data dictionary.10 The Elixhauser Comorbidity Index, a previously validated composite score of 30 comorbidities, was used to quantify the burden of chronic conditions.11 For each year, hospitals were divided into low- (mean, 26.3 ± 23.1; range, 1.0-73.8), medium- (mean, 94.8 ± 31.0; range, 32.3-154.7), and high- (mean, 291.3 ± 180.9; range, 121.5-1692.7) volume tertiles on the basis of the annual institutional caseload of TAVR. The access approach for TAVR was identified as transapical versus other on the basis of ICD-10 coding. The primary outcome of the study was in-hospital mortality. Secondary outcomes included perioperative complications (stroke, postprocedural hemorrhage, blood transfusion, AKI, and pacemaker implantation within the calendar year), LOS, total hospitalization costs, non-home discharge, and 30-day readmissions after TAVR. Total LOS was defined as hospitalization days inclusive of PCI and TAVR whereas post-TAVR LOS was defined as hospitalization duration after TAVR. Total hospitalization costs were defined as costs associated with hospitalization for PCI and TAVR. Inpatient costs for the PCI/TAVR admission(s) were calculated by application of hospital-specific cost-to-charge ratios and inflation adjusted to the 2018 Personal Health Care Index.12
Statistical Methods
Temporal trends were assessed with a rank-based nonparametric test by Cuzick (nptrend). Continuous variables were compared using the adjusted Wald test and are reported as mean with standard deviation. Categorical variables were compared using the χ2 test and reported as proportions. Variables with non-normal distribution (costs and LOS) are reported as median with interquartile range (IQR) and were analyzed using the Mann–Whitney U test. Multivariable logistic and linear regression models were developed to evaluate the association of timing of PCI/TAVR on outcomes of interest. Elastic Net with retention of clinically relevant characteristics was used for variable selection. Aimed at reducing collinearity while applying penalties to decrease overfitting, Elastic Net uses a regressive least squares methodology to select explanatory variables.13 Optimization of the final model was on the basis of the receiver operating characteristic in addition to Akaike and Bayesian Information Criteria, when appropriate. Regression outcomes are reported as adjusted odds ratios (AOR) and β-coefficient (β) for dichotomous and continuous variables, respectively. To account for potential intergroup differences, entropy balancing was used as sensitivity analysis. Similar to propensity matching, entropy balancing addresses covariate balance but has the advantage of retaining the entire cohort for analysis.14 A multivariable logistic model predicting likelihood of receiving concomitant PCI/TAVR was used to apply treatment weighting. Standard mean differences reporting covariate balance before and after entropy balancing are shown in Figure E1.
Figure E1.
Pre- and post-covariate balance before and after entropy balancing. PCI, Percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement; AKI, acute kidney injury.
Prespecified comparisons between Concomitant and Staged groups were performed with a Bonferroni correction applied to account for 2 pairwise comparisons (α < .025). All statistical analyses were performed using Stata 16.0 (StataCorp LP). The study was deemed exempt from full review by the institutional review board at the University of California, Los Angeles.
Results
Of an estimated 161,356 hospitalizations for TAVR during the study period, 641 patients (0.40%) underwent TAVR then PCI whereas 5843 (3.62%) underwent PCI then TAVR. As a proportion of all TAVR performed, rates of PCI/TAVR remained low (2016: 4.76%; 2017: 3.18%; 2018: 3.18%). Of those who underwent PCI then TAVR, 843 comprised the Concomitant group whereas 745 and 4255 were Early-Staged and Late-Staged, respectively (Figure 1). The proportion of those in the Concomitant group increased slightly from 11.8% in 2016 to 16.8% in 2018 whereas Late-Staged decreased (2016: 75.2%; 2018: 69.5%) (nptrend = 0.005). The proportion of Early-Staged remained similar (2016: 13.0%; 2018: 13.7%). The median time from PCI to TAVR was 3 (IQR, 2-6) days for Early-Staged and 32 (IQR, 15-57) days for Late-Staged.
A comparison of demographic characteristics of the groups are reported in Table 1. Compared with Concomitant, patients in the Early-Staged and Late-Staged groups had a greater burden of comorbidities, measured according to the Elixhauser Comorbidity Index, and were less commonly female. Both Staged groups had a greater proportion of patients with pulmonary hypertension and chronic kidney disease than the Concomitant group. Late-Staged patients were more frequently treated at low-volume TAVR hospitals compared with those in the Concomitant group. Compared with Staged groups, the Concomitant group was admitted electively more frequently and underwent single-vessel PCI more commonly (Table 2). Age and TAVR access approach were not significantly different among groups.
Table 1.
Comparison of patient and hospital characteristics grouped according to timing of TAVR
| Concomitant (n = 843) | Early-Staged (n = 745) | Late-Staged (n = 4255) | P value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Mean age (SD), years | 80.4 (8.0) | 80.6 (8.6) | 80.3 (7.7) | .83 |
| Mean Elixhauser Comorbidity Index (SD) | 4.93 (1.63) | 5.71 (1.55)∗ | 5.15 (1.61)∗ | <.001 |
| Female sex | 49.9 | 40.9∗ | 40.9∗ | .003 |
| Income quartile | ∗ | .002 | ||
| 75th-100th | 35.1 | 25.6 | 23.1 | |
| 50th-75th | 27.2 | 28.1 | 27.5 | |
| 25th-50th | 22.7 | 23.6 | 27.8 | |
| 0-25th | 14.9 | 22.7 | 21.6 | |
| Primary payer | ∗ | .039 | ||
| Private insurance | 7.51 | 6.80 | 4.51 | |
| Medicare | 90.2 | 90.8 | 92.9 | |
| Medicaid | 0.18 | 1.38 | 0.93 | |
| Other | 2.09 | 1.00 | 1.67 | |
| Comorbidities | ||||
| Congestive heart failure | 69.4 | 81.9∗ | 67.9 | <.001 |
| Arrhythmia | 53.0 | 66.6∗ | 49.2∗ | <.001 |
| Pulmonary hypertension | 11.7 | 31.9∗ | 23.7∗ | <.001 |
| Peripheral vascular disease | 25.9 | 30.3 | 23.2 | .011 |
| Hypertension | 90.7 | 89.5 | 90.2 | .85 |
| Chronic lung disease | 22.8 | 31.9∗ | 26.4 | .006 |
| Diabetes | 35.3 | 43.1 | 42.5∗ | .02 |
| Hypothyroidism | 18.8 | 18.4 | 18.8 | .98 |
| Chronic kidney disease | ∗ | ∗ | <.001 | |
| Stage 1 | 0.48 | 0.00 | 0.05 | |
| Stage 2 | 2.41 | 2.25 | 2.27 | |
| Stage 3 | 14.6 | 22.4 | 20.0 | |
| Stage 4 | 2.38 | 6.11 | 5.46 | |
| Stage 5/end stage renal disease | 4.34 | 7.78 | 6.29 | |
| Liver disease | 2.19 | 4.94 | 2.63 | .043 |
| Coagulopathy | 9.7 | 15.2∗ | 6.91 | <.001 |
| Cancer | 3.04 | 2.45 | 3.72 | .38 |
| Rheumatologic diseases | 4.36 | 2.11 | 5.32 | .018 |
| Weight loss | 1.88 | 8.00∗ | 3.38 | <.001 |
| Electrolyte disorder | 9.48 | 30.9∗ | 23.5∗ | <.001 |
| Anemia | 3.19 | 9.19∗ | 6.26∗ | .002 |
| Psychiatric disorder | 4.77 | 11.1∗ | 10.0∗ | .0014 |
| Hospital characteristics | ||||
| Hospital type | .012 | |||
| Rural | 0.28 | 0.00 | 1.55 | |
| Metropolitan nonteaching | 15.6 | 9.07 | 15.4 | |
| Metropolitan teaching | 84.1 | 90.9 | 83.1 | |
| Hospital bed size | .059 | |||
| Large | 72.8 | 72.5 | 69.6 | |
| Medium | 24.2 | 25.2 | 24.1 | |
| Small | 2.95 | 2.95 | 6.31 | |
| Hospital volume tertile | ∗ | <.001 | ||
| Low | 2.58 | 2.37 | 7.05 | |
| Medium | 29.9 | 21.2 | 25.9 | |
| High | 67.5 | 76.4 | 67.1 | |
Values are reported as proportions unless otherwise noted.
Denotes P < .025 on pairwise comparison with Concomitant as reference.
Table 2.
Comparison of procedural characteristics grouped according to timing of TAVR
| Concomitant (n = 843) | Early-Staged (n = 745) | Late-Staged (n = 4255) | P value | |
|---|---|---|---|---|
| Elective admission for PCI | 89.4 | 34.9∗ | 27.8∗ | <.001 |
| Number of vessels stented | ∗ | ∗ | <.001 | |
| 1 | 96.4 | 88.4 | 82.8 | |
| 2 | 3.44 | 9.16 | 12.7 | |
| 3 | 0.00 | 2.29 | 3.14 | |
| ≥4 | 0.16 | 0.20 | 1.37 | |
| Elective admission for TAVR | 89.4 | 34.9∗ | 80.2∗ | <.001 |
| TAVR access approach | .45 | |||
| Transapical | 1.66 | 3.53 | 2.58 | |
| Other† | 98.3 | 96.5 | 97.4 |
Values are reported as proportions unless otherwise noted. PCI, Percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement.
Denotes P < .025 on pairwise comparison with Concomitant as reference.
Other TAVR approaches included but not limited to: transfemoral, transaxillary, and transaortic.
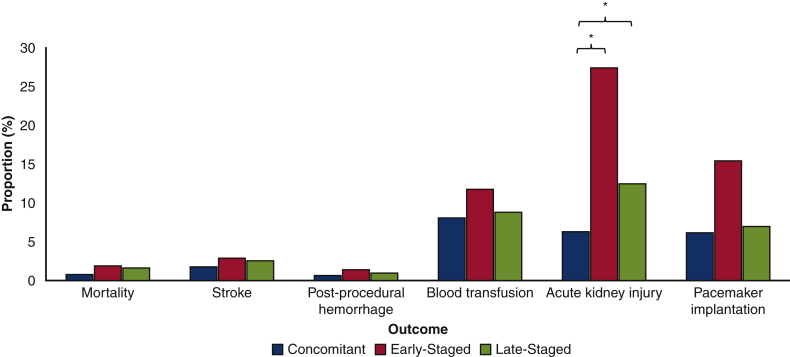
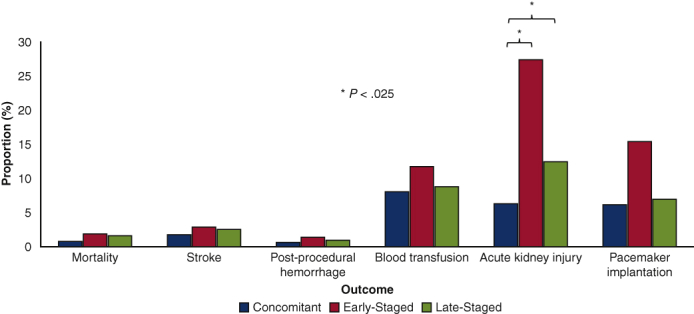
A bivariate comparison of outcomes is shown in Figure 2. Although mortality, stroke, postprocedural hemorrhage, and blood transfusion were similar between cohorts, AKI was more common in Early-Staged compared with Concomitant patients. Additionally, Concomitant patients had shorter post-TAVR and total LOS, lower total hospitalization costs, and higher rates of home discharge compared with others (Table E2). Readmissions within 30 days after the TAVR hospitalization were more common in Early-Staged and Late-Staged patients compared with Concomitant patients.
Figure 2.
Comparison of unadjusted procedural outcomes of treatment groups. ∗ Denotes P < .025 on pairwise comparison with Concomitant as reference.
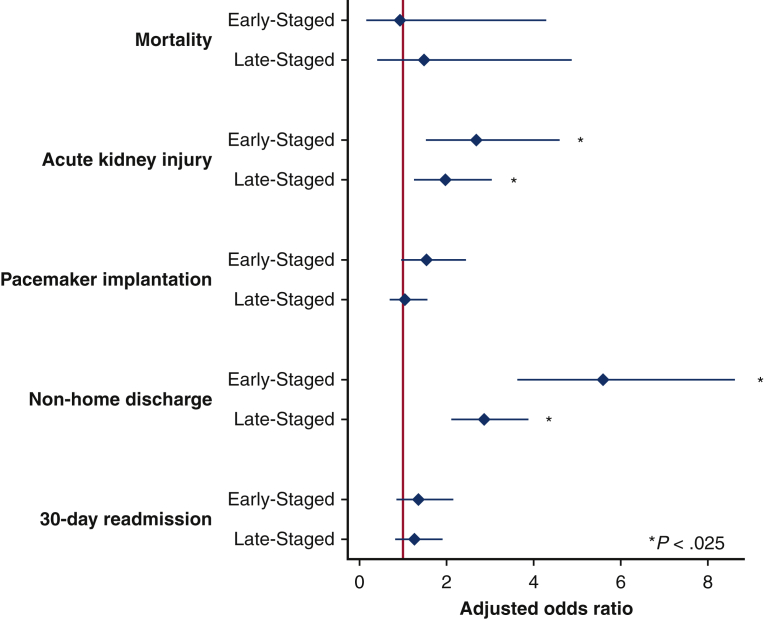
Multivariable linear and logistic models were developed using patient, hospital, and operative characteristics to assess the association of covariates on outcomes of interest. After adjustment, several factors remained associated with in-hospital mortality (Table 3). Rheumatologic diseases, electrolyte disorders, and 2-vessel PCI (compared with single-vessel PCI) were all associated with greater odds of mortality. Notably, center TAVR volume and timing of PCI/TAVR did not exhibit independent associations with the risk of death. As shown in Figure 3, adjusted odds of perioperative complications were generally similar between groups. However, both Staged approaches were associated with increased odds of AKI (Early-Staged AOR, 2.68; 95% CI, 1.57-4.55 and Late-Staged AOR, 1.97; 95% CI, 1.29-2.99) compared with Concomitant. Total hospitalization costs across PCI/TAVR admissions were greater for Early-Staged by $6600 (95% CI, 1400-11,900) and for Late-Staged by $13,300 (95% CI, 9200-17,400) compared with Concomitant (Table 4). Although post-TAVR LOS and 30-day readmission rates were similar between cohorts, Concomitant patients had greater odds of home discharge and shorter total LOS.
Table 3.
Risk-adjusted model for mortality
| Mortality, adjusted odds ratio (95% CI) | P value | |
|---|---|---|
| Patient characteristics | ||
| Age per year | 1.02 (0.97-1.07) | .48 |
| Elixhauser Comorbidity Index | 0.84 (0.64-1.10) | .20 |
| Female sex | 1.07 (0.55-2.11) | .84 |
| Income quartile | ||
| 75th-100th | 1 (Reference) | |
| 50th-75th | 1.19 (0.47-2.98) | .71 |
| 25th-50th | 1.24 (0.52-2.96) | .63 |
| 0-25th | 1.15 (0.49-2.69) | .76 |
| Primary payer | ||
| Private insurance | 1 (Reference) | |
| Medicare | 1.89 (0.20-18.1) | .58 |
| Medicaid | Omitted | |
| Other payer | Omitted | |
| Comorbidities | ||
| Congestive heart failure | 0.80 (0.37-1.73) | .57 |
| Pulmonary hypertension | 1.00 (0.44-2.27) | .99 |
| Peripheral vascular disease | 1.75 (0.78-3.93) | .18 |
| Chronic lung disease | 2.02 (0.91-4.50) | .09 |
| Diabetes | 0.70 (0.32-1.50) | .35 |
| Hypothyroidism | 0.61 (0.21-1.75) | .36 |
| Chronic kidney disease | ||
| No chronic disease | 1 (Reference) | |
| Stage 1 | Omitted | |
| Stage 2 | Omitted | |
| Stage 3 | 1.72 (0.82-3.61) | .15 |
| Stage 4 | 1.11 (0.29-4.19) | .88 |
| Stage 5/end stage renal disease | 0.72 (0.14-3.76) | .70 |
| Liver disease | 1.22 (0.18-8.25) | .84 |
| Coagulopathy | 1.48 (0.53-4.18) | .45 |
| Cancer | 1.39 (0.35-5.52) | .64 |
| Rheumatologic diseases | 2.97 (1.01-8.73) | .05 |
| Weight loss | 1.72 (0.47-6.28) | .41 |
| Electrolyte disorder | 2.74 (1.12-6.70) | .03 |
| Anemia | 1.71(0.64-4.54) | .28 |
| Procedural characteristics | ||
| Calendar year | 0.98 (0.66-4.54) | .91 |
| Timing approach | ||
| Concomitant | 1 (Reference) | |
| Early-Staged | 0.93 (0.20-4.25) | .93 |
| Late-Staged | 1.48 (0.45-4.86) | .52 |
| Elective admission for PCI | 1.41 (0.69-2.86) | .35 |
| Number of vessels stented | ||
| 1 | 1 (Reference) | |
| 2 | 2.47 (1.27-4.82) | .01 |
| 3 | 0.89 (0.14-5.51) | .90 |
| ≥4 | 3.28 (0.79-13.70) | .10 |
| Elective admission for TAVR | 0.31 (0.16-0.60) | .001 |
| TAVR access approach | ||
| Other∗ | 1 (Reference) | |
| Transapical | 2.53 (0.77-8.35) | .13 |
| Hospital characteristics | ||
| Hospital type | ||
| Rural | 1 (Reference) | |
| Metropolitan nonteaching | 1.60 (0.71-3.60) | .26 |
| Metropolitan teaching | Omitted | |
| Hospital bed size | ||
| Large | 1 (Reference) | |
| Medium | 1.53 (0.81-2.91) | .19 |
| Small | Omitted | |
| Hospital volume tertile | ||
| Low | 1 (Reference) | |
| Medium | 1.69 (0.40-7.17) | .47 |
| High | 1.18 (0.31-4.56) | .81 |
Covariates used were those retained in elastic net regression. Adjusted odds ratio reported with 95% confidence intervals. Variables with omitted values are because of low number of patients in respective group. CI, Confidence interval; PCI, percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement.
Other approach defined as transfemoral, transaxillary, and other approaches not otherwise specified.
Figure 3.
Selected risk-adjusted outcomes of treatment groups with Concomitant as reference. Reported as adjusted odds ratio. Area under curve for models were: mortality (0.76), stroke (0.74), procedural complication (0.82), acute kidney injury (0.80), pacemaker implantation (0.61), non-home discharge (0.67), and 30-day readmission.
Table 4.
Comparison of adjusted outcomes of treatment groups with Concomitant as reference
| Adjusted odds ratio or β-coefficient (95% CI) | P value | |
|---|---|---|
| Mortality | ||
| Early-Staged | 0.93 (0.20-4.25) | .93 |
| Late-Staged | 1.48 (0.45-4.86) | .52 |
| Stroke | ||
| Early-Staged | 1.38 (0.48-4.01) | .55 |
| Late-Staged | 1.65 (0.76-3.57) | .21 |
| Post-procedural hemorrhage | ||
| Early-Staged | 1.86 (0.43-8.09) | .41 |
| Late-Staged | 1.45 (0.41-5.08) | .56 |
| Blood transfusion | ||
| Early-Staged | 0.92 (0.53-1.57) | .75 |
| Late-Staged | 1.18 (0.78-1.79) | .43 |
| Acute kidney injury | ||
| Early-Staged | 2.68 (1.57-4.55) | <.001 |
| Late-Staged | 1.97 (1.29-2.99) | .002 |
| Pacemaker implantation | ||
| Early-Staged | 1.47 (0.94-2.28) | .088 |
| Late-Staged | 0.98 (0.68-1.41) | .91 |
| Non-home discharge | ||
| Early-Staged | 5.60 (3.65-8.59) | <.001 |
| Late-Staged | 2.87 (2.14-3.86) | <.001 |
| Post-TAVR length of stay, days | ||
| Early-Staged | 0.5 (−0.2 to 1.2) | .14 |
| Late-Staged | 0.3 (−0.1 to 0.7) | .21 |
| Total length of stay, days | ||
| Early-Staged | 3.0 (2.0-4.1) | <.001 |
| Late-Staged | 4.7 (4.0-5.3) | <.001 |
| Total hospitalization costs per $1000 | ||
| Early-Staged | 6.6 (1.4-11.9) | .014 |
| Late-Staged | 13.3 (9.2-17.4) | <.001 |
| 30-Day readmission | ||
| Early-Staged | 1.35 (0.86-2.12) | .19 |
| Late-Staged | 1.27 (0.86-1.87) | .24 |
Binary outcomes are reported as adjusted odds ratio whereas continuous outcomes are reported as β-coefficient. CI, Confidence interval; TAVR, transcatheter aortic valve replacement.
Sensitivity analyses were performed for those who underwent single-vessel PCI (Table E3) and after excluding patients with acute coronary syndrome during the PCI hospitalization (Table E4). Along with an additional analysis after entropy balancing (Table E5), similar findings were observed. A separate analysis was done on patients with chronic kidney disease stages 3, 4, and 5 and risk of AKI was similar among groups (Early-Staged AOR, 1.66; 95% CI, 0.69-3.97 and Late-Staged AOR, 1.17; 95% CI, 0.57-2.39) with Concomitant as reference.
Discussion
As an alternative to combined CABG and SAVR, PCI/TAVR has emerged as a potential alternate treatment option.5, 6, 7, 8 However, the optimal timing of PCI and TAVR remains unclear with no large-scale studies to date. In the present study we evaluated short-term clinical outcomes, resource utilization, and readmissions between staged versus concomitant PCI/TAVR using a nationally representative cohort. The concomitant approach, which generally had patients with fewer comorbidities, was associated with lower odds of AKI but similar risk of in-hospital mortality and other complications. Furthermore, the Concomitant group had lower total hospitalization costs and overall LOS but similar post-TAVR LOS. Our findings suggest that concomitant PCI/TAVR might be preferable to staged intervention in select patients.
Despite theoretical concerns regarding greater risk of contrast-induced nephropathy in concomitant PCI/TAVR, the present study showed paradoxically lower rates of AKI in this group compared with staged intervention. Notably, this finding did not persist in subanalysis of chronic kidney disease stage 3 to 5 patients, suggesting that the heart team might have been more conscientious regarding contrast administered. Several reasons might explain such a paradoxical observation in the full cohort. First, earlier intervention of the aortic stenosis might have improved cardiac output to prevent possible subsequent AKI. Furthermore, they had a greater proportion of single-vessel PCI, which might have led to lower contrast load. Patient selection might have also played a role. The Concomitant group, comprised of generally healthier patients with a lower frequency of chronic kidney disease, might have been more likely offered single-session treatment. Because they were predominantly electively admitted, they might have also undergone medical optimization before intervention. Similarly, Early-Staged might have had their TAVR delayed after experiencing an episode of AKI after PCI. Although we are unable to determine timing of diagnosis, our findings are similar to those found in the prospective SURTAVI trial in which staged patients experienced greater rates of AKI compared with concomitant patients.9 The SURTAVI trial might also have suffered selection bias because patients were not randomized into concomitant or staged cohorts and those with high SYNTAX scores (>22) were excluded. Regardless, causative factors leading to AKI after TAVR are likely multifactorial. Although previous studies on the effect of contrast load on AKI have been inconclusive, factors such as preexisting chronic kidney disease, degree of coronary disease, age, and hypotension from rapid ventricular pacing might appear to play a role.15,16 Focused investigation is needed to fully characterize the risk factors for AKI in this population.
Interestingly, Concomitant patients had a greater proportion of single-vessel PCI compared with Staged. A previous meta-analysis by Yang and colleagues7 using institutional data observed that patients who underwent concomitant PCI/TAVR had lower SYNTAX scores than their staged counterparts. This finding suggests the strong influence of appropriate patient selection for various strategies. With concerns regarding procedure time and contrast volume, the interventionalist might have opted to stage TAVR separately in those who required multi-vessel PCI. Furthermore, rates of postprocedural hemorrhage and blood transfusions across all groups were similarly low despite staged patients presumably receiving DAPT before TAVR. In an institutional series van Rosendael and colleagues17 compared early (<30 days) with late PCI (≥30 days) before TAVR and showed higher rates of minor vascular complications in early PCI potentially related to DAPT. Although our database does not have sufficient granularity to evaluate minor bleeding complications, the role of DAPT may affect vascular access planning for TAVR in this population. Although in the present study we evaluated the association between timing of TAVR and PCI, future work will help delineate objective criteria in patient risk stratification for either approach. A recently completed randomized controlled trial, ACTIVATION, might serve to address these questions.18
Regardless of timing, PCI/TAVR was observed to be safe with acceptable outcomes in patients who underwent the combined procedure. Our findings add to the growing body of literature supporting the short-term feasibility of this combined approach to severe aortic stenosis and coronary artery disease.8 Our studied in-hospital mortality, stroke, and pacemaker implantation rates were low, which are comparable with reported outcomes of SAVR/CABG and PCI/TAVR cohorts in the SURTAVI trial.9 Consequently, in patients with contraindications to surgical intervention, PCI/TAVR might be a reasonable treatment alternative. However, in instances in which complete revascularization is imperative, the role of SAVR/CABG should continue as the gold standard modality.19
From a value perspective, particular consideration must be given to the concomitant approach. With greater hospitalization costs in Staged groups compared with Concomitant ($6600 for Early-Staged and $13,300 for Late-Staged), there might be considerable cost savings in patients deemed appropriate for single-session intervention. Additionally, Concomitant patients were observed to have greater likelihood of home discharge. Patient quality of life might subsequently be greatly improved with more recovery time at home. Notably, utilization of the concomitant approach was low with only 14.4% of all PCI/TAVR patients and a mere 0.5% of all TAVRs performed. Greater awareness of its safety and patient-centered benefits might increase its application.
The present study has several important limitations. Clinical data regarding contrast load, procedural complexity, and device manufacturer could not be ascertained. Similarly, anatomical considerations including vessel stented and echocardiographic findings were not available in this data set. Information regarding European System for Cardiac Operative Risk Evaluation (EuroSCORE), SYNTAX, or Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), which might have played a role in patient selection, was not available in the present data set. Similarly, we were unable to assess the specific patient eligibility and decision-making made by the heart team, which might vary considerably across hospitals. The study groups were assigned to those who ultimately received treatment and not as an intention-to-treat analysis. Our study encompassed the period after the PARTNER 2 trial, which might not necessarily reflect the current proportion of low-risk patients. Furthermore, we cannot ascertain long-term outcomes, particularly out-of-hospital mortality. Staged patients might have experienced complications before or after TAVR. Despite these limitations, we used validated methodology to report generalizable outcomes after staged and concomitant PCI/TAVR.
Conclusions
The concomitant approach with PCI/TAVR was associated with similar risks of in-hospital mortality but paradoxically lower rates of AKI, potentially because of selection bias (Video 1). Furthermore, concomitant patients were associated with lower in-hospital costs and shorter overall LOS (Figure 4). With PCI/TAVR deemed safe regardless of timing of intervention, concomitant PCI/TAVR is worth consideration for patients who do not qualify for surgical intervention.
Figure 4.
Methods and results of the study with an implications statement. PCI, Percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement; AKI, acute kidney injury.
Conflict of Interest Statement
Richard J. Shemin serves as a consultant to the Edwards Lifesciences Advisory Board and as an institutional local Co-Principal Investigator on the PARTNER II Trial. The remaining authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Background, methods, results, and conclusion of this report. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00083-3/fulltext.
Appendix E1
Table E1.
ICD-10 diagnosis and procedure codes of interest
| Procedure name | ICD-10-PCS code |
|---|---|
| Transapical TAVR | 02RF37H, 02RF38H, 02RF3JH |
| Other TAVR | 02RF37Z, 02RF38Z, 02RF3JZ, 02RF3KH, 02RF3KZ, 02QF3ZJ, 02QF3ZZ |
| tMCS | 5A15223, 5A1522F, 5A1522G, 5A1522H, 02HA0QZ, 02HA3QZ, 02HA4QZ, 02HA0RJ, 02HA3RJ, 02HA4RJ, 5A02116, 5A0211D, 5A02216, 5A0221D, 5A02110, 5A02210 |
| Cardiac surgery | 02100, 02110, 02120, 02130, 02RF07, 02RF08, 02RF0K, 02RF0J, 02RF47, 02RF48, 02RF4J, 02RF4K, 02QF0Z, 02QF4Z, 02UF07, 02UF08, 02UF0J, 02UF0K, 02UF47, 02UF48, 02UF4J, 02UF4K, 02RG07, 02RG08, 02RG0J, 02RG0K, 02RG47, 02RG48, 02RG4J, 02RG4K, 02QG0Z, 02QG4Z, 02UG07, 02UG08, 02UG0J, 02UG0K, 02UG47, 02UG48, 02UG4J, 02UG4K, 02RJ07, 02RJ08, 02RJ0J, 02RJ0K, 02RJ47, 02RJ48, 02RJ4J, 02RJ4K, 02QJ0Z, 02QJ4Z, 02UJ07, 02UJ08, 02UJ0J, 02UJ0K, 02UJ47, 02UJ48, 02UJ4J, 02UJ4K, 02RH07, 02RH08, 02RH0J, 02RH0K, 02RH47, 02RH48, 02RH4J, 02RH4K, 02QH0Z, 02QH4Z, 02UH07, 02UH08, 02UH0J, 02UH0K, 02UH47, 02UH48, 02UH4J, 02UH4K |
| Diagnosis name | ICD-10-CM code |
|---|---|
| Chronic ischemic heart disease | I25 |
| Acute coronary syndrome | I22, I210, I211, I212, I213, I214, I219 |
| Mechanical complication of TAVR | T820, T823, T826, T828, T8222 |
ICD-10-PCS, International Classification of Diseases, 10th Revision, Procedure Coding System; TAVR, transcatheter aortic valve replacement; tMCS, temporary mechanical circulatory support; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification.
Table E2.
Comparison of unadjusted outcomes of treatment groups
| Concomitant | Early-Staged | Late-Staged | P value | |
|---|---|---|---|---|
| Mortality | 0.79 | 1.89 | 1.62 | .34 |
| Stroke | 1.76 | 2.88 | 2.54 | .50 |
| Post-procedural hemorrhage | 0.63 | 1.42 | 0.96 | .39 |
| Blood transfusion | 8.11 | 11.8 | 8.83 | .15 |
| Acute kidney injury | 6.32 | 27.5∗ | 12.5∗ | <.001 |
| Pacemaker implantation | 12.4 | 16.7 | 12.0 | .044 |
| Non-home discharge | 30.1 | 62.4∗ | 55.8∗ | <.001 |
| Median post-TAVR length of stay (IQR), d | 2 (1-3) | 3 (2-6) | 2 (2-4) | <.001 |
| Median total length of stay (IQR), d | 2 (1-3) | 11 (6-17)∗ | 8 (5-13)∗ | <.001 |
| Median total hospitalization costs per $1000 (IQR) | 55.6 (43.9-70.7) | 75.8 (60.8-95.4)∗ | 73.4 (58.0-93.7)∗ | <.001 |
| 30-Day readmission | 10.9 | 19.6∗ | 15.4∗ | .003 |
Data are reported as percentages unless otherwise noted. TAVR, Transcatheter aortic valve replacement; IQR, interquartile range.
Denotes P < .025 on pairwise comparison with Concomitant as reference.
Table E3.
Sensitivity analysis with only patients who underwent single-vessel stenting
| Adjusted odds ratio or β-coefficient (95% CI) | P value | |
|---|---|---|
| Mortality | ||
| Early-Staged | 1.09 (0.24-5.02) | .91 |
| Late-Staged | 1.57 (0.47-5.22) | .46 |
| Stroke | ||
| Early-Staged | 1.49 (0.48-4.60) | .49 |
| Late-Staged | 1.80 (0.82-3.94) | .14 |
| Post-procedural hemorrhage | ||
| Early-Staged | 2.68 (0.50-14.4) | .25 |
| Late-Staged | 1.66 (0.38-7.37) | .50 |
| Blood transfusion | ||
| Early-Staged | 0.81 (0.47-1.42) | .47 |
| Late-Staged | 1.14 (0.74-1.76) | .56 |
| Acute kidney injury | ||
| Early-Staged | 2.69 (1.55-4.67) | <.001 |
| Late-Staged | 2.14 (1.39-3.28) | .001 |
| Pacemaker implantation | ||
| Early-Staged | 1.37 (0.86-2.18) | .18 |
| Late-Staged | 0.99 (0.69-1.44) | .97 |
| Non-home discharge | ||
| Early-Staged | 5.05 (3.28-7.78) | <.001 |
| Late-Staged | 3.03 (2.25-4.08) | <.001 |
| Post-TAVR length of stay (d) | ||
| Early-Staged | 0.6 (−0.1 to 1.4) | .073 |
| Late-Staged | 0.2 (−0.2 to 0.6) | .27 |
| Total length of stay (d) | ||
| Early-Staged | 3.2 (2.2-4.3) | <.001 |
| Late-Staged | 4.6 (3.9-5.3) | <.001 |
| Total hospitalization costs per $1000 | ||
| Early-Staged | 7.5 (2.0-12.9) | .007 |
| Late-Staged | 12.8 (8.7-16.8) | <.001 |
| 30-Day readmission | ||
| Early-Staged | 1.43 (0.89-2.29) | .14 |
| Late-Staged | 1.19 (0.79-1.80) | .40 |
Binary outcomes are reported as adjusted odds ratio whereas continuous outcomes are reported as β-coefficient. CI, Confidence interval; TAVR, transcatheter aortic valve replacement.
Table E4.
Sensitivity analysis after excluding patients with acute coronary syndrome during the PCI hospitalization
| Adjusted odds ratio or β-coefficient (95% CI) | P value | |
|---|---|---|
| Mortality | ||
| Early-Staged | 1.63 (0.29-9.16) | .58 |
| Late-Staged | 1.78 (0.45-7.08) | .41 |
| Stroke | ||
| Early-Staged | 1.31 (0.41-4.20) | .65 |
| Late-Staged | 1.63 (0.75-3.57) | .22 |
| Post-procedural hemorrhage | ||
| Early-Staged | 2.14 (0.58-7.85) | .25 |
| Late-Staged | 1.22 (0.36-4.16) | .75 |
| Blood transfusion | ||
| Early-Staged | 0.73 (0.39-1.38) | .33 |
| Late-Staged | 1.10 (0.70-1.73) | .69 |
| Acute kidney injury | ||
| Early-Staged | 2.77 (1.52-5.09) | .001 |
| Late-Staged | 2.34 (1.46-3.75) | <.001 |
| Infectious complication | ||
| Early-Staged | 1.37 (0.72-2.61) | .33 |
| Late-Staged | 0.98 (0.59-1.64) | .94 |
| Pacemaker implantation | ||
| Early-Staged | 1.59 (0.98-2.56) | .059 |
| Late-Staged | 0.91 (0.60-1.39) | .68 |
| Non-home discharge | ||
| Early-Staged | 4.72 (3.10-7.17) | <.001 |
| Late-Staged | 2.88 (2.14-3.88) | <.001 |
| Post-TAVR length of stay, d | ||
| Early-Staged | 0.6 (−0.1 to 1.3) | .11 |
| Late-Staged | 0.3 (−0.1 to 0.7) | .18 |
| Total length of stay, d | ||
| Early-Staged | 3.0 92.0-4.0) | <.001 |
| Late-Staged | 4.6 (3.9-5.3) | <.001 |
| Total hospitalization costs per $1000 | ||
| Early-Staged | 6.9 (1.5-12.3) | .013 |
| Late-Staged | 13.5 (9.0-17.9) | <.001 |
| 30-Day readmission | ||
| Early-Staged | 1.29 (0.80-2.06) | .30 |
| Late-Staged | 1.27 (0.84-1.91) | 1.90 |
CI, Confidence interval; TAVR, transcatheter aortic valve replacement.
Table E5.
Sensitivity analysis with entropy balancing
| Adjusted odds ratio or β-coefficient (95% CI) | P value | |
|---|---|---|
| Mortality | ||
| Early-Staged | 1.16 (0.20-6.77) | .87 |
| Late-Staged | 1.83 (0.60-5.63) | .29 |
| Stroke | ||
| Early-Staged | 1.67 (0.47-5.92) | .43 |
| Late-Staged | 2.22 (0.84-5.85) | .11 |
| Post-procedural hemorrhage | ||
| Early-Staged | 1.53 (0.20-11.8) | .69 |
| Late-Staged | 1.18 (0.30-4.72) | .81 |
| Blood transfusion | ||
| Early-Staged | 0.60 (0.27-1.32) | .21 |
| Late-Staged | 1.27 (0.80-2.02) | .31 |
| Acute kidney injury | ||
| Early-Staged | 3.56 (1.77-7.16) | <.001 |
| Late-Staged | 2.53 (1.52-4.20) | <.001 |
| Infectious complication | ||
| Early-Staged | 1.48 (0.71-3.07) | .30 |
| Late-Staged | 0.88 (0.52-1.49) | .64 |
| Pacemaker implantation | ||
| Early-Staged | 1.57 (0.89-2.78) | .12 |
| Late-Staged | 1.03 (0.68-1.55) | .90 |
| Non-home discharge | ||
| Early-Staged | 1.98 (1.28-3.06) | .002 |
| Late-Staged | 4.25 (3.20-5.65) | <.001 |
| Post-TAVR length of stay, d | ||
| Early-Staged | 0.8 (−0.1-1.6) | .07 |
| Late-Staged | 0.1 (−0.3-0.4) | .62 |
| Total length of stay, d | ||
| Early-Staged | 4.7 (3.5-5.8) | <.001 |
| Late-Staged | 4.0 (3.5-4.4) | <.001 |
| Total hospitalization costs per $1000 | ||
| Early-Staged | 8.1 (2.6-13.7) | .004 |
| Late-Staged | 11.9 (8.9-14.9) | <.001 |
| 30-Day readmission | ||
| Early-Staged | 1.21 (0.65-2.23) | .55 |
| Late-Staged | 1.30 (0.85-2.01) | .23 |
CI, Confidence interval; TAVR, transcatheter aortic valve replacement.
References
- 1.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Leon M.B., Smith C.R., Mack M.J., Makkar R.R., Svensson L.G., Kodali S.K., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 3.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Griese D.P., Reents W., Tóth A., Kerber S., Diegeler A., Babin-Ebell J. Concomitant coronary intervention is associated with poorer early and late clinical outcomes in selected elderly patients receiving transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2014;46:e1–e7. doi: 10.1093/ejcts/ezu187. [DOI] [PubMed] [Google Scholar]
- 5.Wenaweser P., Pilgrim T., Guerios E., Stortecky S., Huber C., Khattab A.A., et al. Impact of coronary artery disease and percutaneous coronary intervention on outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Eurointervention. 2011;7:541–548. doi: 10.4244/EIJV7I5A89. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj A., Pancholy S., Sethi A., Rathor P. Safety and feasibility of PCI in patients undergoing TAVR: a systematic review and meta-analysis. Heart Lung. 2017;46:92–99. doi: 10.1016/j.hrtlng.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y., Huang F.Y., Huang B.T., Xiong T.Y., Pu X.B., Chen S.J., et al. The safety of concomitant transcatheter aortic valve replacement and percutaneous coronary intervention. Medicine (Baltimore) 2017;96:e8919. doi: 10.1097/MD.0000000000008919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conradi L., Seiffert M., Franzen O., Baldus S., Schirmer J., Meinertz T., et al. First experience with transcatheter aortic valve implantation and concomitant percutaneous coronary intervention. Clin Res Cardiol. 2011;100:311–316. doi: 10.1007/s00392-010-0243-6. [DOI] [PubMed] [Google Scholar]
- 9.Søndergaard L., Popma J.J., Reardon M.J., Van Mieghem N.M., Deeb G.M., Kodali S., et al. Comparison of a complete percutaneous versus surgical approach to aortic valve replacement and revascularization in patients at intermediate surgical risk results from the randomized SURTAVI trial. Circulation. 2019;140:1296–1305. doi: 10.1161/CIRCULATIONAHA.118.039564. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality NRD description of data elements. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddde.jsp
- 11.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality Using appropriate price indices for analyses of health care expenditures or income across multiple years. https://meps.ahrq.gov/about_meps/Price_Index.shtml
- 13.Zou H., Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Stat Methodol Ser B. 2005;67:301–320. [Google Scholar]
- 14.Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20:25–46. [Google Scholar]
- 15.Penkalla A., Pasic M., Drews T., Buz S., Dreysse S., Kukucka M., et al. Transcatheter aortic valve implantation combined with elective coronary artery stenting: a simultaneous approach. Eur J Cardiothorac Surg. 2015;47:1083–1089. doi: 10.1093/ejcts/ezu339. [DOI] [PubMed] [Google Scholar]
- 16.Cheungpasitporn W., Thongprayoon C., Kashani K. Transcatheter aortic valve replacement; a kidney's perspective. J Ren Inj Prev. 2016;5:1–7. doi: 10.15171/jrip.2016.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Rosendael P.J., Van Der Kley F., Kamperidis V., Katsanos S., Al Amri I., Regeer M., et al. Timing of staged percutaneous coronary intervention before transcatheter aortic valve implantation. Am J Cardiol. 2015;115:1726–1732. doi: 10.1016/j.amjcard.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Khawaja M.Z., Wang D., Pocock S., Redwood S.R., Thomas M.R. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: study protocol for a randomized controlled trial. Trials. 2014;15:1–8. doi: 10.1186/1745-6215-15-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huckaby L.V., Sultan I., Mulukutla S., Kliner D., Gleason T.G., Wang Y., et al. Revascularization following non-ST elevation myocardial infarction in multivessel coronary disease. J Card Surg. 2020;35:1195–1201. doi: 10.1111/jocs.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Background, methods, results, and conclusion of this report. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00083-3/fulltext.