Abstract
SMARCB1 (INI1)-deficient carcinoma of the sinonasal tract is a rare and distinct entity characterized by the loss of INI1 immunostain expression. These tumors are morphologically diverse, with isolated cases of yolk sac differentiation reported. We report the first case of SMARCB1-deficient sinonasal carcinoma that demonstrated co-loss of SMARCA4 immunostain, and reduced SMARCA2 and ARID1A staining, with the entire tumor showing histological and immunohistochemical evidence of yolk sac differentiation. The clinical, histological, immunohistochemical and molecular features were discussed and compared against SMARCB1-deficient sinonasal carcinomas with yolk sac differentiation and SMARCA4-deficeint sinonasal carcinomas reported in the literature. With a highly aggressive clinical course leading to mortality two months after presentation, the behavior of this tumor appears to be more comparable to that of SMARCA4-deficient sinonasal carcinomas. A comprehensive immunopanel including SMARCB1, SMARCA4, SMARCA2 and ARID1A may be advisable for assessment and prognostication of SWI/SNF-deficient tumors.
Keywords: SMARCB1-deficient sinonasal carcinoma, INI1, SMARCA4
Introduction
SMARCB1 (INI1) deficient carcinoma of the sinonasal tract was first reported by two independent groups in 2014 [1, 2]. The histological features of this tumor were initially described as basaloid cells without squamous or glandular differentiation, and the occasional presence of rhabdoid or plasmacytoid cells. The morphological spectrum of this entity has expanded to include pagetoid spread, glandular differentiation, sarcomatoid differentiation and yolk sac differentiation [1, 3–5], with more cases being described in the literature accompanied by increasing understanding of this entity. The loss of SMARCB1 (INI1) immunostain expression is a prerequisite for the diagnosis of SMARCB1-deficient sinonasal carcinoma. To date, none of the reported cases of SMARCB1-deficient sinonasal carcinoma, with or without yolk sac differentiation, have demonstrated co-loss of SMARCA4 immunostain. We report a case of SMARCB1-deficient sinonasal carcinoma with yolk sac differentiation, showing co-loss of SMARCA4 immunostaining, and reduced SMARCA2 and ARID1A staining. Clinical, histological, immunohistochemical and molecular features of this previously undescribed tumor were detailed and compared with a literature review of reported cases of SMARCB1-deficient sinonasal carcinoma with yolk sac differentiation.
Case Presentation
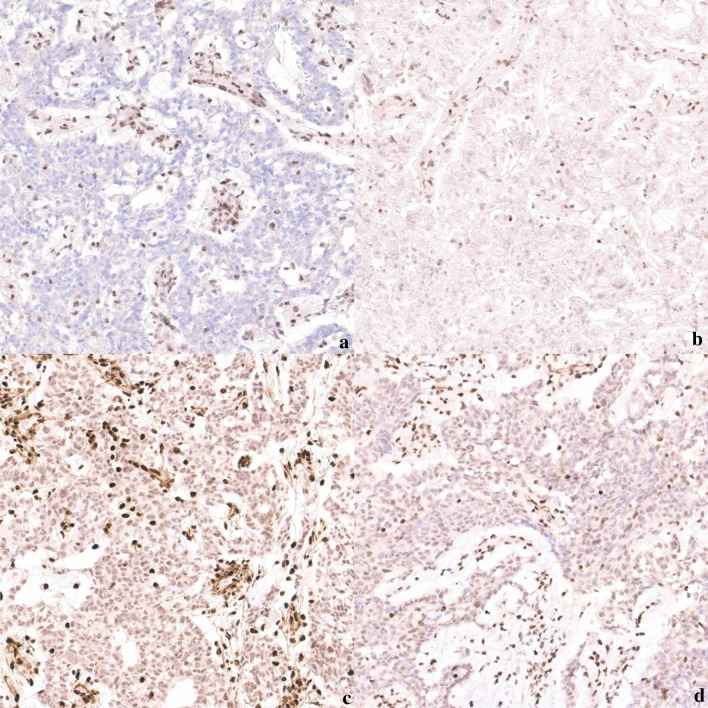
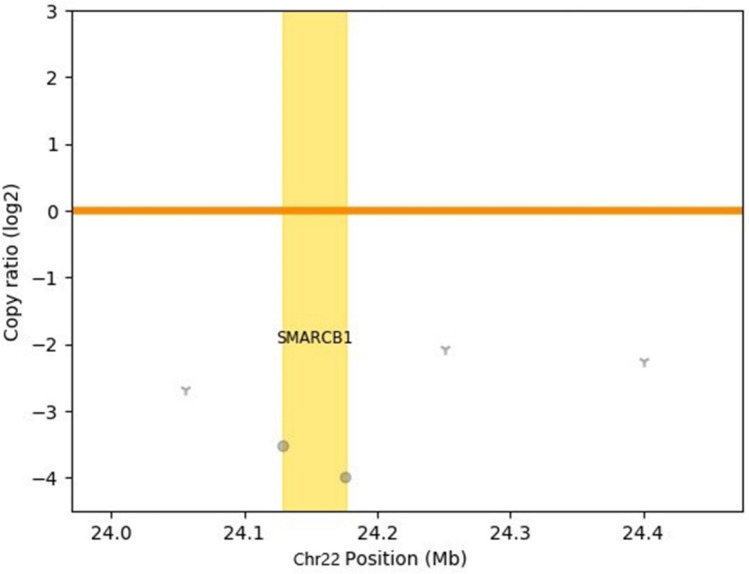
A man in his ninth decade, with a history of hypertension, ischemic heart disease, and obstructive sleep apnea presented with recurrent right epistaxis for two months. Flexible laryngoscopy revealed a right nasal cavity tumor extending to the right nasopharynx with contact bleeding. A biopsy was taken. Histology showed tumor cells forming complex cribriform, and irregular angulated glandular architecture in myxoedematous stromal background, recapitulating yolk sac differentiation (Fig. 1). No other histologically distinct tumor component was seen. Immunohistochemically, the tumor cells were positive for germ cell markers supportive of yolk sac differentiation, namely CDX2, HNF1β, SALL4, glypican3 (focal), c-KIT (focal) and AFP (focal) [6, 7] (Fig. 2). Immunostains for CK7, p16, p63, S100, SOX10, GATA3 and MYC were negative. There were loss of both SMARCB1 and SMARCA4 staining, and reduced SMARCA2 and ARID1A staining (Fig. 3). Subsequently, the tumor tissue was subjected to the Chinese University of Hong Kong somatic cancer panel v.3 which covered the single-nucleotide variation, indel and copy-number variation of 415 genes as well as tumor mutation burden. The result revealed homozygous deletion of SMARCB1 with a low tumor mutation burden of 3.64mut/Mb. While awaiting further work up and imaging, the patient was readmitted for fever and decreased conscious level. Computed tomography scan was performed and found an expansile soft tissue mass, measuring 4.4 cm in greatest dimension, in the right ethmoid extending to the right sphenoid with destruction of right lamina papyracea, crossing the midline and involving left posterior ethmoid sinus (Fig. 4). Opacification of right frontal and maxillary sinus was also noted. In view of the extensive skull base involvement and clinical presentation, empirical antibiotics were started with the presumptive diagnosis of central nervous system infection. Unfortunately, the patient’s condition rapidly deteriorated and passed away soon after admission, two months after the initial presentation (Fig. 5).
Fig.1.
Histological sections of the tumor. a Low-power magnification showing that the tumor is entirely composed of glandular structures with yolk-sac features, H&E, 4x magnification; b tumor cells forming irregular angulated glands in a myxoedematous stromal background, H&E, 10x magnification
Fig. 2.
Immunoreactivity to yolk sac markers. a CDX2, 20x magnification; b HNF1β, 20x magnification; c SALL4, 20x magnification; d glypican3, 20x magnification; e c-KIT, 20x magnification; f AFP, 20x magnification
Fig. 3.
Loss of a SMARCB1 and b SMARCA4 staining, and reduced c SMARCA2 and d ARID1A staining, 20x magnification
Fig. 4.
Copy number variation plot showed that the SMARCB1 locus was deleted
Fig. 5.
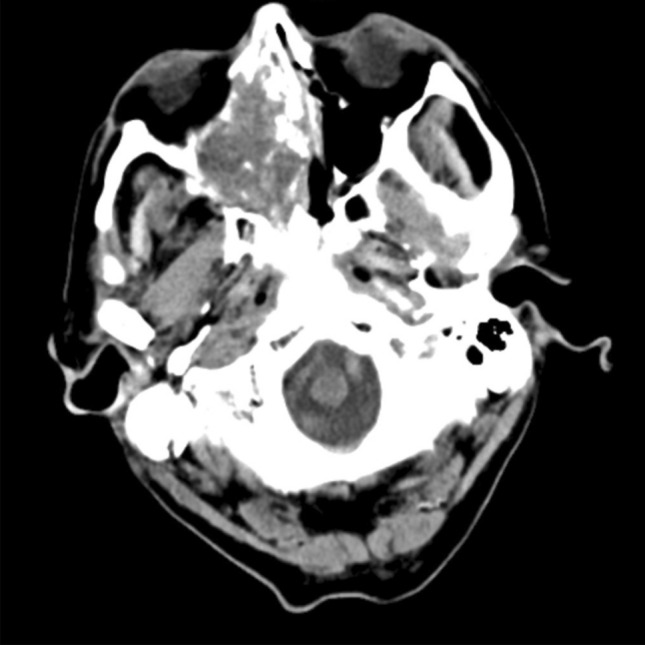
Computed tomography showing the tumor in the nasal cavity involving orbit
Discussion
SMARCB1, SMARCA4, SMARCA2 and ARID1A are subunits of the switch/sucrose non-fermentable (SWI/SNF) complexes, which are chromatin remodeling complexes promoting genomic DNA responsiveness by interacting with transcription factors and involving in tumorigenesis [8, 9]. The diagnosis of SWI/SNF-deficient tumors requires the use of immunohistochemical study, and loss of SMARCB1 and SMARCA4 is believed to be mutually exclusive [10]. Concurrent loss of SMARCB1 and SMARCA4 expression in human cancers, by immunostaining or molecular testing, has not been reported in literature.
SMARCB1-deficient carcinoma is included in the 4th edition of the WHO classification of the Head and Neck Tumors as a differential diagnosis under the sections of various carcinomas, in particular non-keratinizing squamous cell carcinoma, sinonasal undifferentiated carcinoma, and NUT carcinoma [11]. Histologically, these tumors encompass diverse morphological features. The tumor cells most commonly appear basaloid, squamoid and/or oncocytic [12]. Glandular, rhabdoid and plasmacytoid differentiation can occasionally be present [12]. Yolk sac differentiation is more recently reported and only present in a minority of cases [3]. A case series of 12 cases of SMARCB1-deficient sinonasal carcinomas reported histological evidence of yolk sac differentiation in three cases [3]. Three more cases of SMARCB1-deficient carcinomas with yolk sac differentiation were separately reported by different authors [5, 13, 14] [Table 1].
Table 1.
Clinical features of INI1-deficient sinonasal carcinoma with yolk sac differentiation
| Case | Age | Sex | Presentation | Site | Size | Treatment | Follow-up | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Current case | 84 | M | Epistaxis | Right nasal cavity | 4.4 cm, with sphenoid, orbital and maxillary sinus extension | None | 2 months | Died of disease | |
| 2 | Li et al. (13) | 55 | M |
Nasal obstruction Epistaxis |
Right nasal cavity | 3 cm | Lumpectomy | 6 months | Disease-free |
| 3 | Hazir et al. (14) | 55 | M | Cheek swelling | Left maxillary sinus | NR, but with orbital extension |
Neoadjuvant chemotherapy Orbital exenteration Subtotal maxillectomy Radical parotidectomy Selective neck dissection Adjuvant chemoradiation |
Local recurrence at 4 months Subsequent distant metastasis to lung and bone Died in the 20 at months |
Died of disease |
| 4 | Shah et al. (3) | 71 | F | NR | Maxillary sinus | NR | NR | NR | NR |
| 5 | 58 | M | NR | Frontal sinus | NR | NR | NR | NR | |
| 6 | 58 | M | NR | Ethmoid sinus | NR | NR | NR | NR | |
| 7 | Zamecnik et al. (5) | 44 | F | Pansinusitis | Left nasal cavity | NR, but with orbital and maxillary sinus extension | Chemotherapy | NR | NR |
NR – not recorded
In contrast, SMARCA4-deficient sinonasal carcinomas are less well delineated compared to SMARCB1-deficient sinonasal carcinomas. A case series in 2020 described 10 cases of SMARCA4-deficient sinonasal carcinoma defined by immunohistochemical study [4]. One of the cases showed co-loss of SMARCA4 and SMARCA2 staining. Although tumor DNA testing was performed, no pathogenic mutation was detected in any of the tumors in this series. The tumor cells were described as basaloid or epithelioid with extensive necrosis. No yolk sac differentiation was identified in any of the cases [4]. Until now, the presence of yolk sac differentiation has only been reported in SMARCB1-deficient sinonasal tumors, but not in SMARCA4-deficient sinonasal tumors.
Co-loss of multiple subunits of the SWI/SNF complexes were also documented in carcinomas of other organs. Concurrent loss of SMARCB1 and SMARCA2 was reported in a case series of gastrointestinal undifferentiated carcinoma [15], co-loss of ARID1A with SMARCA2/SMARCA4 in esophageal adenocarcinomas [9], and co-loss of SMARCA2 with SMARCB1/SMARCA4 in a case series of endometrial carcinoma with differentiation [10]. None of the reported cases of SMARCB1 or SMARCA4 deficient carcinoma demonstrated SMARCA4 and SMARCB1 co-loss.
Reviewing the six reported cases of SMARCB1-deficient sinonasal carcinoma with yolk sac differentiation, only one case reported by Li et al. [13] ran a relatively indolent disease course with the patient disease-free at the end of follow-up. In the current case and two cases reported by Hazir et al. and Zamecnik et al. [5, 14], the tumors behaved aggressively. There was extensive local tumor spread to the orbit and maxillary sinuses, and poor disease outcome. Except for the current case and the case from Li et al. [13], presence of other tumor components were noted, most commonly consisting of basaloid, squamoid and oncocytic tumor cells. The yolk sac component of all tumors was positive to germ cell markers with loss of SMARCB1/INI1 staining (Table 2). These findings confirm the observation by Zamecnik et al. [5] that SMARCB1/INI1 loss is distinctive and can be used to differentiate SMARCB1-deficient sinonasal carcinoma with yolk sac differentiation from primary gonadal yolk sac tumors.
Table 2.
Histological and molecular features of INI1-deficient sinonasal carcinoma with yolk sac differentiation
| Case | Non-yolk sac components | Immunomarkers | Molecular testing | |
|---|---|---|---|---|
| Current case | None | CDX2+, HNF1β+, SALL4+, glypican3+, c-KIT+, AFP+, INI1- |
Somatic cancer panel – homozygous deletion of SMARCB1 low tumor mutation burden (3.64mut/Mb) |
|
| 2 | Li et al. [13] | None | CK+, glypican3+, CDX2+, SALL4+, AFP+, CEA+, p16+, EMA+, CK20+, vimentin+, CD117(c-KIT)+, INI1- | FISH – homogenous deletion of SMARCB1 |
| 3 | Hazir et al. (14) | Basaloid and squamoid cell nests |
AFP+, SALL4+, glypican3+, MOC31+, CDX2+, vimentin+, INI1- (yolk sac areas) CK5/6+, CK7+, EMA+, INI1- (basaloid/squamoid areas) |
Not performed |
| 4 | Shah et al. (3) | Oncocytoid and basaloid cells | CK7+, glypican3+, PLAP+, SALL4+, INI1- | Not performed |
| 5 | Oncocytoid cells | AFP+, glypican3+, SALL4+, INI1- | Not performed | |
| 6 | Oncocytoid and clear cells | CDX2+, p40+, glypican3+, HepPar1+, INI1- | Not performed | |
| 7 | Zamecnik et al. (5) |
Basaloid, squamoid, oncocytic, rhabdoid and plasmacytoid cells Spindle, sarcomatoid areas |
AFP+, glypican3+, CDX2+, INI1- | Not performed |
In general, SMARCB1-deficient carcinomas were observed to progress rapidly with a reported median survival of 15 months [12]. Involvement of the sinuses and skull base was also not uncommon [12]. Although limited in number, the addition of a yolk-sac component does not seem to impact disease prognosis (Table 1). SMARCA4-deficient sinonasal carcinomas appears to be just as, if not more aggressive than SMARCB1 deficient carcinomas with a median survival of three months [4]. Progression was rapid in the current case with a disease course more similar to that of SMARCA4-deficient sinonasal carcinomas. The case of SMARCA4-deficient sinonasal carcinoma with co-loss of SMARCA2 reported by Agaimy et al. also died of disease at three months follow-up [4]. Even though only SMARCB1 deletion was confirmed by molecular techniques in our case, the co-loss of SMARCB1 and SMARCA4 staining, regardless of underlying molecular alterations, may indicate a worse disease outcome.
Conclusions
SMARCB1-deficient sinonasal carcinoma is a rare but aggressive tumor with variable histological features. Yolk sac differentiation is increasingly recognized and should be considered as a part of the spectrum in SMARCB1-deficient sinonasal carcinomas. SMARCA4-deficient sinonasal carcinomas are less understood but more rapidly progressive tumors, which are identified only by immunostaining without defining molecular features. We report a case of SMARCB1-deficient sinonasal carcinoma with yolk sac differentiation with co-loss of SMARCA4 staining, and reduced SMARCA2 and ARID1A staining. Its clinical course appears to be more comparable to that of SMARCA4-deficient sinonasal carcinomas, with the patient succumbing to disease two months after diagnosis. It may be advisable to perform a comprehensive immunopanel including SMARCB1, SMARCA4, SMARCA2 and ARID1A for SWI/SNF-deficient tumors for accurate prognostication and delineation of these recently identified entities.
Acknowledgements
The abstract of this article was accepted and presented at the Hong Kong College of Otorhinolaryngologists Annual Scientific Meeting 2021.
Authors’ contributions
JKMN and ABWC conceived the idea of the study. JKMN, JJXL and ABWC reviewed the slides and performed the literature review. JYKC, KT and DCMY collected and interpreted clinical data. JKMN drafted the manuscript. JYKC and ABWC edited and critically revised the manuscript. All authors have read and approved the manuscript.
Funding
The authors have no funding to declare
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethics approval
Attempt has been made in contacting family members of the deceased patient for the publication of this case report. Identifiers of the patient have been anonymized.
Consent to participate
Attempt has been made in contacting family members of the deceased patient for the publication of this case report. Identifiers of the patient have been anonymized.
Consent for publication
Attempt has been made in contacting family members of the deceased patient for the publication of this case report. Identifiers of the patient have been anonymized.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38(9):1282–9. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agaimy A, Koch M, Lell M, Semrau S, Dudek W, Wachter DL, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38(9):1274–81. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AA, Jain D, Ababneh E, Agaimy A, Hoschar AP, Griffith CC, et al. SMARCB1 (INI-1)-Deficient Adenocarcinoma of the Sinonasal Tract: A Potentially Under-Recognized form of Sinonasal Adenocarcinoma with Occasional Yolk Sac Tumor-Like Features. Head Neck Pathol. 2020;14(2):465–72. doi: 10.1007/s12105-019-01065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient Sinonasal Carcinoma: A Series of 10 Cases Expanding the Genetic Spectrum of SWI/SNF-driven Sinonasal Malignancies. Am J Surg Pathol. 2020;44(5):703–10. doi: 10.1097/PAS.0000000000001428. [DOI] [PubMed] [Google Scholar]
- 5.Zamecnik M, Rychnovsky J, Syrovatka J. Sinonasal SMARCB1 (INI1) Deficient Carcinoma With Yolk Sac Tumor Differentiation: Report of a Case and Comparison With INI1 Expression in Gonadal Germ Cell Tumors. Int J Surg Pathol. 2018;26(3):245–9. doi: 10.1177/1066896917741549. [DOI] [PubMed] [Google Scholar]
- 6.Gallo A, Fankhauser C, Hermanns T, Beyer J, Christiansen A, Moch H, et al. HNF1β is a sensitive and specific novel marker for yolk sac tumor: a tissue microarray analysis of 601 testicular germ cell tumors. Mod Pathol. 2020;33(11):2354–60. doi: 10.1038/s41379-020-0597-x. [DOI] [PubMed] [Google Scholar]
- 7.Bing Z, Pasha T, Tomaszewski JE, Zhang P. CDX2 expression in yolk sac component of testicular germ cell tumors. Int J Surg Pathol. 2009;17(5):373–7. doi: 10.1177/1066896909338598. [DOI] [PubMed] [Google Scholar]
- 8.Masliah-Planchon J, Bièche I, Guinebretière JM, Bourdeaut F, Delattre O. SWI/SNF chromatin remodeling and human malignancies. Annu Rev Pathol. 2015;10:145–71. doi: 10.1146/annurev-pathol-012414-040445. [DOI] [PubMed] [Google Scholar]
- 9.Schallenberg S, Bork J, Essakly A, Alakus H, Buettner R, Hillmer AM, et al. Loss of the SWI/SNF-ATPase subunit members SMARCF1 (ARID1A), SMARCA2 (BRM), SMARCA4 (BRG1) and SMARCB1 (INI1) in oesophageal adenocarcinoma. BMC Cancer. 2020;20(1):12. doi: 10.1186/s12885-019-6425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karnezis AN, Hoang LN, Coatham M, Ravn S, Almadani N, Tessier-Cloutier B, et al. Loss of switch/sucrose non-fermenting complex protein expression is associated with dedifferentiation in endometrial carcinomas. Mod Pathol. 2016;29(3):302–14. doi: 10.1038/modpathol.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Naggar, AKCJKCRGJTTSPJIAfRoC. WHO classification of head and neck tumours2017.
- 12.Agaimy A, Hartmann A, Antonescu CR, Chiosea SI, El-Mofty SK, Geddert H, et al. SMARCB1 (INI-1)-deficient Sinonasal Carcinoma: A Series of 39 Cases Expanding the Morphologic and Clinicopathologic Spectrum of a Recently Described Entity. Am J Surg Pathol. 2017;41(4):458–71. doi: 10.1097/PAS.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CY, Han YM, Xu K, Wu SY, Lin XY, Cao HY. Case Report: SMARCB1 (INI-1)-Deficient Carcinoma of the Nasal Cavity with Pure Yolk Sac Tumor Differentiation and Elevated Serum AFP Levels. Onco Targets Ther. 2021;14:2227–33. doi: 10.2147/OTT.S302613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazir B, Şímșek B, Erdemír A, Gürler F, Yazici O, Kizil Y, et al. Sinonasal SMARCB1 (INI1) Deficient Carcinoma with Yolk Sac Tumor Differentiation: A Case Report and Treatment Options. Head Neck Pathol; 2021. [DOI] [PMC free article] [PubMed]
- 15.Agaimy A, Daum O, Märkl B, Lichtmannegger I, Michal M, Hartmann A. SWI/SNF Complex-deficient Undifferentiated/Rhabdoid Carcinomas of the Gastrointestinal Tract: A Series of 13 Cases Highlighting Mutually Exclusive Loss of SMARCA4 and SMARCA2 and Frequent Co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016;40(4):544–53. doi: 10.1097/PAS.0000000000000554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Not applicable.