Abstract
Targeted Protein Degradation (TPD) has emerged as an exciting new era in chemical biology and drug discovery. PROteolysis TArgeting Chimera (PROTAC) technology targets cellular proteins for degradation by co-opting the Ubiquitin Proteasome System. Over the last five years, numerous studies have expanded our understanding of the unique mode of action and advantages of PROTACs, which has in turn spurred interest in both academia and industry to explore PROTACs as a novel therapeutic strategy. In this review, we first highlight the key advantages of PROTACs and then discuss the spatiotemporal regulation of protein degradation. Next, we explore current chemically tractable E3 ligases focusing on expanding the existing repertoire with novel E3 ligases to uncover the full potential of TPD. Collectively, these studies are guiding the development of the PROTAC technology as it emerges as a new modality in precision medicine.
Keywords: Targeted Protein Degradation, PROTACs, PhotoPROTACs, E3 ligase, Covalent ligands, Proteasome
eTOC blurb
Nalawansha et al. review the advantages and recent advances of the PROTAC technology focusing on the spatiotemporal regulation of protein degradation, the chemically tractable E3 ligases, as well as covalent and non-covalent PROTACs.
Graphical Abstract

1. Introduction
The Ubiquitin Proteasome System (UPS) is a key cellular machinery responsible for maintaining intracellular protein homeostasis (Finley, 2009; Hipp et al., 2019). Cellular protein homeostasis is maintained by a network of proteins including chaperones and the proteolytic system (Kim et al., 2013). While chaperones are responsible for correcting protein mis-folding, the proteolytic system, converging on the 26S proteasome, is responsible for the removal of unfolded or damaged proteins to maintain a healthy environment within the cell. The 26S proteasome consists of regulatory subunits (one or two 19S regulatory particles) and a 20S proteasome core (Kumar Deshmukh et al., 2019). Substrate specificity for proteasome-dependent cleavage is dictated by the 19S regulatory particle, which binds to ubiquitin-tagged proteins, deubiquitinates, unfolds and directs them into the catalytic 20S core particle for subsequent degradation.
Proteins that need to be degraded by the proteasome are first covalently tagged with ubiquitin (Ub) by a cascade of three enzymes known as the E1 ubiquitin activating enzyme, an E2 Ub conjugating enzyme and an E3 ubiquitin ligase (Kliza and Husnjak, 2020). Briefly, E1 activates Ub in an ATP-dependent mechanism to form an E1-Ub conjugate. Then, Ub is transferred from the E1 to the E2 enzyme via a trans-thioesterification reaction. In the final step, the E3 ubiquitin ligase simultaneously binds both E2-Ub and the substrate, and facilitates the ultimate transfer of Ub: directly from the E2 to the substrate or indirectly via the E3 ubiquitin ligase itself, depending on the E3 ligase family. Substrates tagged with Ub are destined for the 26S proteasome for degradation.
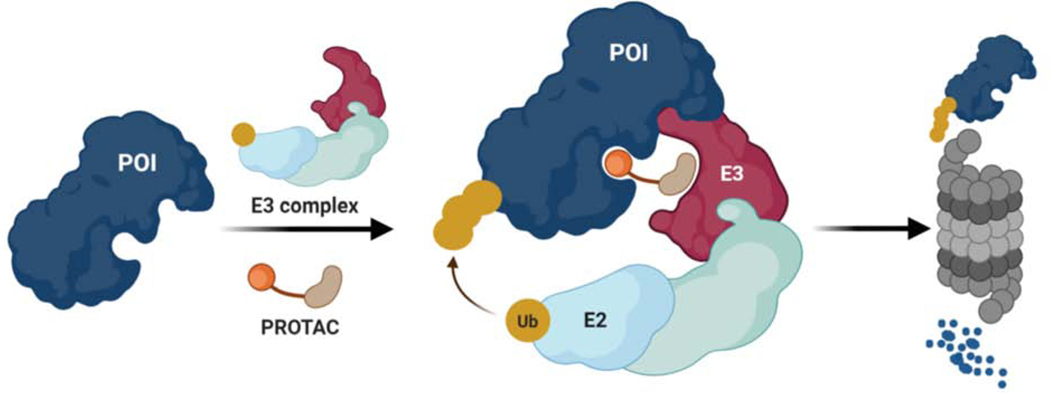
The PROteolysis TArgeting Chimera (PROTAC) technology was developed to artificially induce the degradation of a given protein of interest (POI) by hijacking the UPS (Sakamoto et al., 2001; Sakamoto et al., 2003; Schneekloth et al., 2004). PROTACs are heterobifunctional molecules comprising an E3 ligase ligand fused to a POI ligand via an intervening chemical linker. Upon forming the E3-PROTAC-POI ternary complex, the E3 ligase induces ubiquitination and subsequent proteasomal degradation of the target protein (Figure 1). Compared to traditional small molecules that chiefly function by blocking the catalytic activity of a druggable protein via an occupancy-driven pharmacology, PROTACs operate via an event-driven mode of action to eliminate the protein, thus removing all possible functions (i.e., enzymatic, scaffolding, regulatory, etc.) associated with it (Bondeson and Crews, 2017; Bondeson et al., 2015; Burslem and Crews, 2017; Lai and Crews, 2017; Lu et al., 2015; Salami and Crews, 2017; Zengerle et al., 2015). Moreover, PROTACs hold great promise as a versatile therapeutic modality since they require only transient interaction with any crevice on the target protein to promote its degradation (Crews, 2010). This feature of PROTACs offers the potential to target the undruggable proteome by overcoming the limitations of conventional small molecule inhibitors that can only target proteins with well-defined active sites.
Figure 1-. Targeted Protein Degradation by PROTACs.
PROTACs are heterobifunctional molecules that simultaneously bind to a Protein of Interest (POI) and an E3 ubiquitin ligase complex, leading to ubiquitination and degradation of the POI via the ubiquitin proteasome system. Ub: Ubiquitin. E2: Ub conjugating enzyme, E3: E3 ligase/ substrate adaptor protein.
PROTACs were first reported in 2001, demonstrating the proof of concept that target proteins can be ubiquitinated and degraded via the UPS by recruiting an E3 ligase in a PROTAC-dependent manner (Sakamoto et al., 2001). Since its discovery, the PROTAC technology has expanded and become widely used in both basic biological research and therapeutic development (An and Fu, 2018; BasuRay et al., 2019; Buckley et al., 2015; Konstantinidou et al., 2019; Moon and Lee, 2018; Neklesa et al., 2017; Raina and Crews, 2017; Salami et al., 2018). Over the years, the PROTAC technology has been successfully applied to many protein classes such as nuclear receptors, kinases, GPCRs, transmembrane proteins, epigenetic proteins, small GTPases, transcription factors, and protein aggregates (Bai et al., 2019; Bensimon et al., 2020; Bondeson et al., 2015; Buhimschi et al., 2018; Burslem et al., 2018a; Burslem et al., 2018b; Crew et al., 2018; Cromm et al., 2018; Han et al., 2019a; Hines et al., 2019; Hsu et al., 2020; Hu et al., 2019; Lai et al., 2016; Li et al., 2020a; Lu et al., 2015; Mares et al., 2020; Salami et al., 2018; Schiedel et al., 2018; Silva et al., 2019b; Smalley et al., 2020; Winter et al., 2015; Yang et al., 2018). Recent advances have broadened our understanding of the advantages of TPD, such as the event-driven mode of action, its catalytic nature, and the potential to target disease-causing proteins that are intractable via traditional small molecule-based inhibition (Bond et al., 2020; Bondeson et al., 2015; Bondeson et al., 2018; Burslem and Crews, 2020; Burslem et al., 2018a; Churcher, 2018; Jiang et al., 2019; Salami and Crews, 2017; Smith et al., 2019; Verma et al., 2020). A major accomplishment in the PROTAC technology is the development of orally-bioavailable PROTACs that have progressed to clinical trials (Mullard, 2019). Two PROTAC candidates, ARV-110 (https://clinicaltrials.gov/trialnumberNCT03888612) and ARV-471 (https://clinicaltrials.gov/trialnumberNCT04072952), targeting the androgen receptor and the estrogen receptor respectively, are currently in phase 1 clinical trials and have shown promising early data in terms of safety, tolerability, and efficacy.
This review will primarily focus on the advantages of PROTAC technology and recent advances, such as spatiotemporal regulation of light-induced protein degradation. Further, we discuss the current list of chemically tractable E3 ligases and covalent PROTACs based on novel E3 ligase ligands. Finally, we briefly compare conventional PROTACs to covalent PROTACs with their inherent features. We hope that these early studies will help expand the E3 ligase repertoire to fully uncover the potential of TPD. However, challenges and limitations of the PROTAC technology (e.g., hurdles in PROTAC development and optimization, hook effect, the lack of variety of E3 ligase ligands, and on/off target toxicity issues) will not be discussed here as they have been recently reviewed elsewhere (Burslem and Crews, 2020; Gao et al., 2020; Kostic and Jones, 2020).
2. Advantages of the PROTAC Technology
A. Induce Isoform-selective Degradation
Isoform-selective inhibitors are useful tools to decipher the biology of a given protein (Bieliauskas and Pflum, 2008; Edgar et al., 2010; Xiong et al., 2017). However, developing isoform-selective inhibitors is often stymied by high structural similarity and sequence identity among isoforms. PROTAC-mediated degradation has been shown to be effective in achieving isoform selectivity, given that stability of each isoform-PROTAC-E3 complex can dictate differential degradation outcomes (Figure 2A) (Bondeson et al., 2018). For instance, the p38 Mitogen-Activated Protein Kinase (MAPK) family has four different isoforms and has been implicated in cancer progression (Yang et al., 2016a). Even though p38-MAPK inhibitors have been developed to protect against chronic inflammation, they suffer from severe side effects due to their lack of isoform selectivity (Yang et al., 2016b). However, isoform-specific p38 degrading PROTACs based on the kinase inhibitor foretinib and a von Hippel-Lindau (VHL) ligand were recently developed by varying the linker length and linker attachment point to the VHL ligand to recruit the E3 ligase with a differential orientation relative to the targeted proteins (Smith et al., 2019). As a result, PROTACs “SJFα” and “SJFδ” demonstrated striking differential selectivity towards p38α and p38δ isoforms, respectively. In a separate study, isoform-selective degradation was achieved with PROTACs targeting the cyclin dependent kinase (CDK) family (Brand et al., 2019; Rana et al., 2019; Zhou et al., 2020). Palbociclib is a potent inhibitor of CDK4 and CDK6 (Fry et al., 2004). However, the PROTAC based on palbociclib induced selective degradation of CDK6 while sparing CDK4, further suggesting that PROTAC technology can be successfully applied to achieve isoform-selective degradation (Rana et al., 2019). Furthermore, CDK2 selective PROTACs have also been reported recently (Zhou et al., 2020). Tovell et al. developed isoform-specific PROTACs by selectively targeting serum/glucocorticoid-inducible protein kinase-3 (SGK3) for degradation, while leaving SGK1 and 2 isoforms unaffected (Tovell et al., 2019). These studies collectively underscore the capacity of the PROTAC technology to develop isoform-selective degraders, a feat that is challenging to achieve with conventional small molecule inhibitors. Moreover, these isoform-selective degraders can be used as valuable chemical biology tools to study the underlying biology associated with a given protein isoform. However, these pan-inhibitor-derived PROTACs could still inhibit all or most of the isoforms. Consequently, these PROTACs may display a mixture of activities such as selective degradation of a single isoform (event driven) and broader catalytic inhibition of all the isoforms (occupancy driven). Therefore, caution must be taken to differentiate inhibition- vs degradation-dependent effects to understand isoform-specific functions.
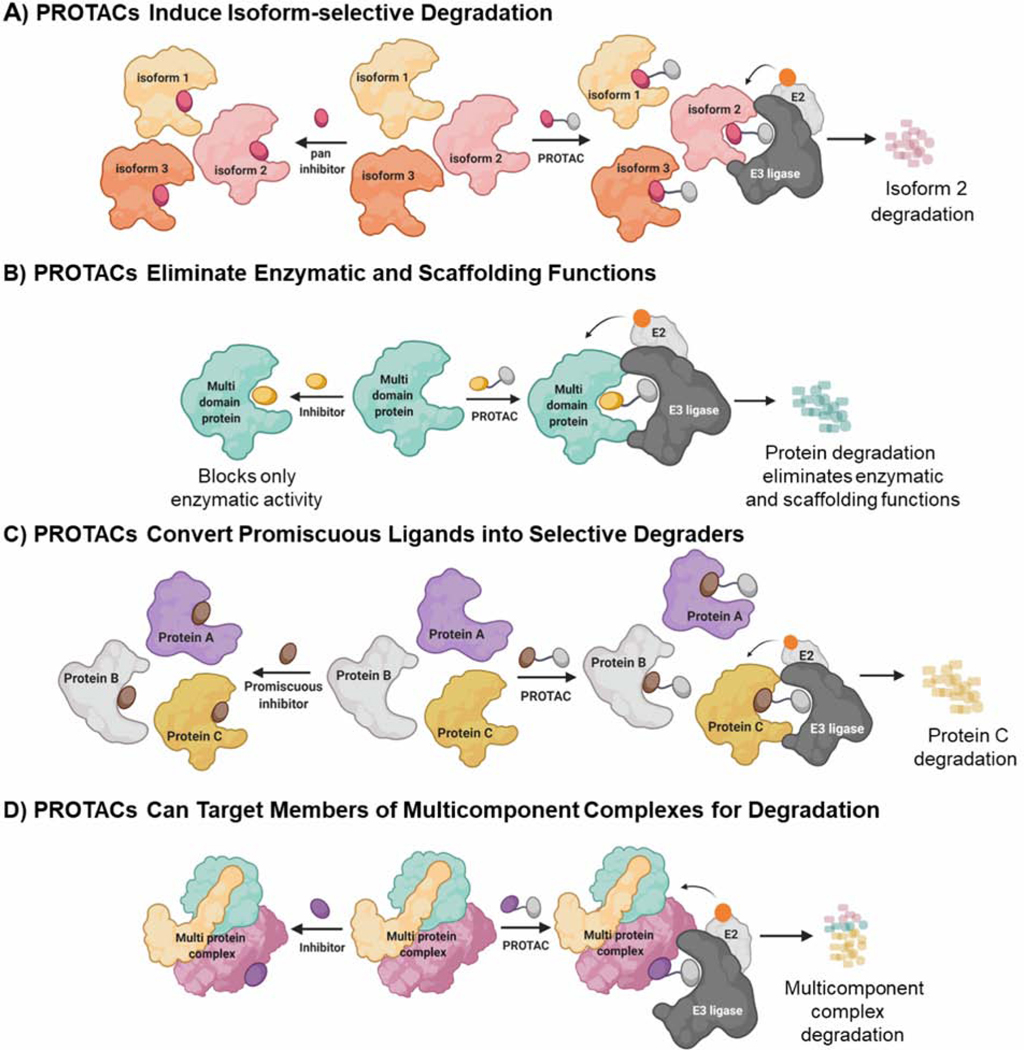
Figure 2-. Advantages of the PROTAC Technology.
A) PROTACs induce isoform-selective protein degradation. Pan inhibitors bind and inhibit multiple isoforms due to sequence and structural similarities among isoforms. In the depicted cartoon, the stable ternary complex between the E3 ligase and isoform 2 leads to ubiquitination and degradation, while sparing other isoforms. B) PROTACs induce degradation of multi domain proteins to eliminate both enzymatic and non-enzymatic/scaffolding roles. In contrast, small molecule inhibitors block only enzymatic functions. C) PROTACs convert promiscuous ligands into selective degraders. Promiscuous inhibitors bind to multiple proteins, however PROTACs derived from the promiscuous inhibitor do not induce degradation of bound proteins. As depicted in the carton, only protein C forms a stable ternary complex with E3 ligase, leading to effective ubiquitination and degradation. D) PROTACs can target multicomponent complexes for proteasomal degradation. Inhibitors that bind to a single protein in a complex are ineffective in blocking scaffolding functions of the protein complex. PROTACs bind to the protein complex via a single protein subunit and induce degradation of multiple proteins in the complex. E2- Ub conjugating enzyme. Orange circle on E2 represents ubiquitin.
B. Elimination of Enzymatic and Scaffolding Functions
When compared to small molecule inhibition, ligand-induced protein degradation has shown to be beneficial in targeting multi-functional proteins with both enzymatic and scaffolding roles (Figure 2B), e.g., receptor tyrosine kinases (RTKs), which harbor kinase activity, as well as kinase-independent scaffolding roles (Burslem et al., 2018a; Lemmon and Schlessinger, 2010). Even though RTK inhibitors can block only their kinase activity, the scaffolding roles of RTKs can often lead to signaling pathway reactivation (kinome rewiring effects) (Murtuza et al., 2019). In contrast, PROTACs targeting RTKs have the advantage of abrogating both enzymatic and scaffolding roles by eliminating the protein. In 2018, Burslem et al. showed that PROTAC-mediated degradation of RTKs inhibited both cell proliferation and downstream signaling (Burslem et al., 2018a). Moreover, this effect was durable compared to small molecule inhibition. This feature was also illustrated by targeting focal adhesion kinase (FAK) for degradation (Cromm et al., 2018; Popow et al., 2019). FAK plays a key role in tumor invasion and metastasis by acting as a kinase and a scaffold protein (Cance et al., 2013; Lee et al., 2015). A FAK PROTAC was shown to be effective in inhibiting invasion and migration, which was not affected by the FAK inhibitor defactinib (Cromm et al., 2018). Additionally, studies with FLT3-ITD, BCR-Abl, and PARP further confirm the advantages of PROTAC-mediated degradation of multi-functional proteins over small molecule inhibition of the enzymatic activity (Burslem et al., 2019; Burslem et al., 2018b; Wang et al., 2019a).
C. PROTACs Convert Promiscuous Ligands into Selective Degraders
As a consequence of the multiple mechanistic steps that need to be satisfied to achieve successful degradation of a POI, PROTACs display enhanced target selectivity compared to conventional small molecule inhibitors. Hence, promiscuous ligands can be converted to more selective degraders using the PROTAC technology (Bondeson et al., 2018; Huang et al., 2018). PROTAC-mediated protein degradation depends on the formation of protein-protein interactions (PPI) between the E3 ligase and POI to stabilize the ternary complex to effectively induce ubiquitination and proteasomal degradation of the latter (Figure 2C) (Gadd et al., 2017; Roy et al., 2019). Because the capacity to form favorable PPI with any target protein will vary among candidate E3 ligases, the nature of the E3 ligase recruited by PROTACs can impart selectivity towards a target protein relative to a small molecule. To this end, Bondeson et al. analyzed the degradation profile of PROTACs based on foretinib, a promiscuous kinase inhibitor, by unbiased mass spectrometry (Bondeson et al., 2018). Even though foretinib binds to more than 50 kinases, only 14 kinases were degraded by a VHL-recruiting PROTAC, and only 9 kinases were degraded by a cereblon (CRBN)-recruiting PROTAC, clearly demonstrating the enhanced selectivity of the PROTAC approach. Data further suggested that PPI between the target and E3 ligase are a key determinant in imparting selectivity and degradation. Of particular interest was the finding that binding affinity between PROTAC warhead and POI did not correlate with degradation outcome: a productive ternary complex that facilitates degradation is possible even in cases of PROTACs with weak binding affinity to their targets. This is exemplified by the aforementioned p38 PROTACs. A similar study by Huang et al. using a multi-kinase degrader (based on CRBN-binding ligand and a promiscuous kinase inhibitor) revealed a distinct degradation profile that guided the development of selective degraders for a subset of proteins, such as FLT3, BTK, and CDK proteins (Huang et al., 2018). This further demonstrates that PROTAC-mediated protein degradation can be highly selective compared to their cognate promiscuous inhibitors.
However, one limitation is that the PROTAC molecule can still bind and inhibit several proteins despite its ability to induce degradation. Therefore, we believe that future PROTAC studies will focus on overcoming these limitations by replacing the active site inhibitor moiety with ligands that bind the target at another, non-enzymatic site. In this manner, the target binding ligand will have no inhibitory effect on other proteins, hence deciphering the biological functions of the degraded protein more reliably.
D. PROTACs Can Target Members of Multicomponent Complexes for Degradation
In recent years, PROTAC technology has been applied to target epigenetic proteins for degradation (Vogelmann et al., 2020). Epigenetic proteins can be broadly classified into readers, writers, and erasers (Audia and Campbell, 2016), which play key roles in regulating gene expression via binding to histone proteins. Common reader proteins include acetyllysine- and methyllysine-binding proteins that contain bromodomains and chromodomains, respectively. Writers include histone acetyltransferases and methyltransferases, whereas erasers include histone deacetylases and demethylases. Given the aberrant expression of these proteins in many diseases, they have emerged as attractive drug targets (Helin and Dhanak, 2013).
In 2015, PROTACs were developed to target the epigenetic reader protein BRD4, a member of the bromodomain and extra terminal (BET) protein family, for degradation (Lu et al., 2015; Winter et al., 2015). ARV-825 and dBET1 PROTACs were developed by conjugating the BRD2–4 binding ligands OTX-015 and JQ1, respectively, to CRBN-recruiting ligands. ARV-771 and MZ1 PROTACs were developed by linking JQ1 to VHL ligand (Gadd et al., 2017; Raina et al., 2016). These PROTACs showed fast, efficient, and prolonged BRD degradation, resulting in c-Myc downregulation and inhibition of cell proliferation. In addition, PROTACs targeting epigenetic eraser proteins, such as sirtuins were also developed (Schiedel et al., 2018). Sirtuins belong to the NAD+-dependent deacetylase family and Sirtuin-2 (sirt-2) deregulation has been implicated in cancer and neurodegenerative diseases. In 2018, several PROTACs were developed by using a ligand selective for Sirt2 (SirReals) and thalidomide to target Sirt2 for degradation in Hela cells (Schiedel et al., 2018). Similarly, other epigenetic proteins including BRD7/9, TRIM24, HDAC1–3 and HDAC6 have been targeted for degradation using the PROTAC technology (An et al., 2019; Gechijian et al., 2018; Smalley et al., 2020; Wu et al., 2019; Zoppi et al., 2019).
Epigenetic proteins often exert their functions as multiprotein complexes when recruited to the desired site on chromatin (Cartron et al., 2020). These protein complexes harbor enzymatic and scaffolding roles to regulate gene expression. For instance, P300/CBP-associated factor (PCAF) and the general control nonderepressible 5 (GCN5) proteins harbor an acetyltransferase domain and a bromodomain (Nagy and Tora, 2007). Given the role of these proteins in cell growth, DNA damage repair, as well as, metabolic and immunological pathways, small molecule ligands have been developed to inhibit their functions (Humphreys et al., 2017). However, inhibition did not recapitulate the effects of PCAF/GCN5 knockdown in macrophage and dendritic cells, hampering the functional utility of these inhibitors in cells. The potential of PROTACs to degrade multiprotein complexes was illustrated by targeting PCAF/GCN5 acetyltransferase complex for degradation (Figure 2D) (Bassi et al., 2018). Bassi et al. developed a PROTAC by combining a potent and selective inhibitor of PCAF/GCN5 (GSK4027) with thalidomide. PCAF/GCN5 degradation by the PROTAC was more beneficial than small molecule inhibition and lowered inflammatory signaling. Another example of PROTAC-mediated degradation of epigenetic protein complex was demonstrated by targeting SMARCA2 and SMARCA4. SMARCA2 and SMARCA4 are acetyl-lysine reader proteins that exist as a part of the BAF chromatin remodeling complex to exert their biological function (St. Pierre and Kadoch, 2017). Using structure-based optimizations, Farnaby et al. developed PROTACs to target SMARCA2/4 proteins for degradation, further illustrating the advantages of degradation compared to inhibition in a multiprotein complex setting (Farnaby et al., 2019).
Recently, components of the polycomb repressive complex 2 (PRC2) have been targeted for degradation (Hsu et al., 2020; Potjewyd et al., 2020). The PRC2 is a multiprotein complex that regulates histone methylation at lysine 27 of histone H3 and is implicated in diseases such as cancer (Laugesen et al., 2016). EZH2 is the catalytic subunit with histone methyltransferase activity and EED is the regulatory subunit that assists in recruiting EZH2 to the chromatin as well as allosterically regulating the enzymatic activity of EZH2. Potjewyd et al. developed EED-targeting PROTACs using a highly potent EED binder, EED226, and the VHL ligand (Qi et al., 2017). In another study, Hsu et al. developed PROTACs comprised of an EED inhibitor and the VHL ligand (Dong et al., 2019). Both EED-targeting PROTACs induced varying levels of of EED, EZH2 and SUZ12 protein loss in the PRC2, affecting histone methylation and inhibiting cell proliferation. It is possible that each of these proteins is ubiquitinated in a direct, PROTAC-mediated manner or, alternatively, the overall PRC complex integrity is compromised upon PROTAC-mediated EED degradation, leading to destabilization and subsequent degradation of other complex components.
Collectively, these studies highlight the advantages of PROTAC-induced degradation of multiple proteins in a complex compared to inhibition of a single subunit by a small molecule ligand. Since acute and reversible post-translational protein knockdown by PROTACs offers superior advantages relative to existing genetic knockdown methods, these PROTACs could serve as chemical biology probes to decipher the function of epigenetic enzymes/complexes in pathological conditions.
In the following section, we will review the recent advances of TPD by first focusing on the light-controlled protein degradation, which is a significant advancement in the field. Next, the current state of the chemically tractable E3 ligases will be discussed thus emphasizing on expanding the E3 ligase toolbox to uncover the full potential of TPD with novel E3 ligases.
3. Recent Advances in Targeted Protein Degradation
Despite significant progress in advancing TPD as a modality for control of protein function and the many advantages inherent in the strategy, one limitation with the technology is the lack of conditional or spatiotemporal regulation of protein degradation, which can often lead to undesired systemic toxicity. Conditional protein degradation was first demonstrated in 2013 by the development of phosphor-dependent PROTACs (phosphoPROTACs) (Hines et al., 2013). PhosphoPROTACs use extracellular stimuli to induce protein degradation upon phosphorylation. PhosphoPROTACs consist of a VHL-recruiting peptide linked to peptide sequences that can bind known downstream TRK effector proteins, e.g., fibroblast growth factor receptor substrate 2α (FRS2α) or phosphatidylinositol-3-kinase (PI3K) (Kouhara et al., 1997; Li et al., 2001; Schneekloth et al., 2004). Upon growth factor stimulation, nerve growth factor receptor TrkA (tropomyosin receptor kinase A), or the neuregulin receptor ErbB2 (erythroblastosis oncogene B2) phosphorylate their cognate peptidic PROTAC, which then recruits either FRS2α or PI3K to VHL to induce effector protein ubiquitination and degradation (Cunningham et al., 1997; Kouhara et al., 1997). While this context-dependent and peptide-based approach worked, a more generalized approach was needed to control protein degradation in a spatial and temporal manner.
A. Spatiotemporal Control of Targeted Protein Degradation
Numerous studies have recently illustrated the feasibility of spatiotemporal regulation of protein degradation using light. This has opened a whole new avenue to explore TPD in a more controlled manner (Jin et al., 2020; Kounde et al., 2020; Liu et al., 2020; Naro et al., 2020; Pfaff et al., 2019; Reynders et al., 2020; Xue et al., 2019). Optical control has been used extensively in biological research given its non-invasive nature and tight spatiotemporal control. This has been accomplished by utilizing multiple strategies such as photolabile-caged molecules (photocaging), genetically engineered photoreceptors (optogenetics), or synthetic photoswitches (photopharmacology) (Albert and Vázquez, 2019; Ankenbruck et al., 2018; Renicke et al., 2013; Silva et al., 2019a). Light-induced protein degradation can be achieved by introducing a photoswitchable linker or by incorporating a caged molecule into the PROTAC design. These two approaches have been exploited with respect to different E3 ligases as well as POI by incorporating a light controlled element into the PROTAC structure.
Light-induced Protein Degradation by Photoswitchable PROTACs
Reversible optical control of TPD can be achieved by introducing a photoswitchable linker into the PROTAC design (Figure 3A) (Jin et al., 2020; Pfaff et al., 2019; Reynders et al., 2020). Upon light irradiation at the designated wavelength, the PROTAC interconverts between the cis and trans isomers, culminating in inactive or active PROTAC conformations. The inactive PROTAC is unable to form a stable ternary complex between the E3 ligase and POI, hence is defective for POI ubiquitination and degradation. Upon light irradiation, the inactive PROTAC switches to the active conformation, which forms a stable ternary complex between POI and E3, resulting in effective ubiquitination and proteasomal degradation of the POI. Moreover, degradation can be halted by restoring the inactive isomer with appropriate light irradiation.
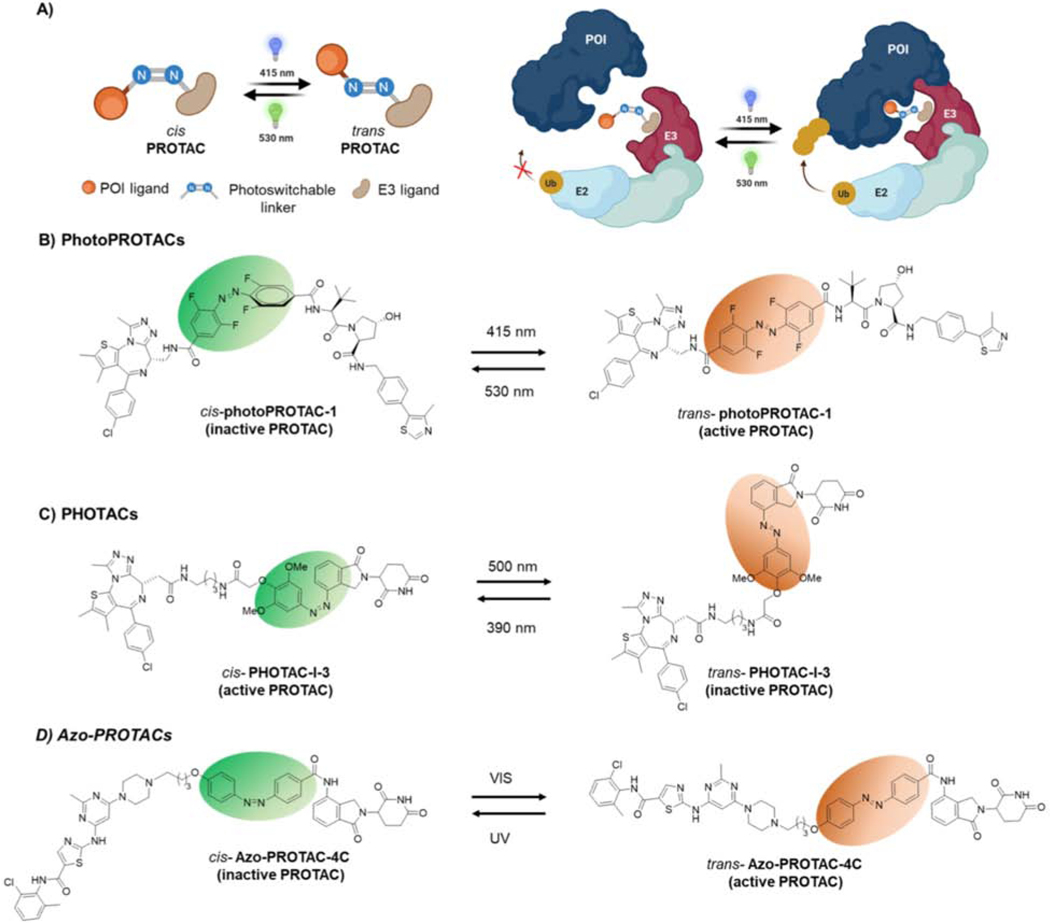
Figure 3-. Light-induced Protein Degradation by Photoswitchable PROTACs.
A) Schematic of photoswitchable PROTACs. Upon light irradiation, the PROTAC interconverts between its cis and trans conformers, leading to changes in PROTAC functionality. As depicted, the cis photoPROTAC is unable to form the stable ternary complex between E3 ligase and POI. Upon light irradiation, the cis isomer converts to the active trans isomer, leading to stable ternary complex formation, effective POI ubiquitination and degradation. B) PhotoPROTAC1 comprised of BRD-targeting JQ1 and a VHL ligand linked via a photoswitchable tetra fluoro azobenzene moiety. Light irradiation converts the inactive cis photoPROTAC1 into its active trans isomer, and vice versa. C) PHOTAC-I-3 consists of BRD targeting JQ1 and CRBN recruiting ligands. Light irradiation converts inactive trans PHOTAC-I-3 to active cis isomer and vice versa. D) Azo-PROTAC-4C consists of BCR-abl targeting ligand and CRBN recruiting ligands. Light irradiation converts active trans azo-PROTAC-4C to inactive cis isomer and vice versa. cis azobenzene and trans azobenzene are highlighted in green and orange ovals, respectively.
Pfaff and Samarasinghe et al. reported the application of a photoswitchable linker into a PROTAC molecule to achieve protein degradation upon light stimulation (Pfaff et al., 2019). These are termed “photoPROTACs”, where the VHL ligand is connected to JQ1, via a photoswitchable tetrafluoro azobenzene linker (Figure 3B). Light irradiation interconverts photoPROTACs between either cis or trans isomer, yielding photoswitchable PROTACs. The data indicate that the trans PROTAC is active in inducing ubiquitination and degradation of BRD2, while the cis PROTAC is inactive and unable to induce degradation. Further, the addition of a tetrafluoro moiety provides extra stability to the photostationary state of the photoPROTACs compared to azobenzene alone, averting the need for continuous irradiation of cells to retain the desired active conformation. Additionally, the different linker composition in the photoPROTAC enabled selective BRD2 degradation compared to its parent PROTAC, ARV-771 (Raina et al., 2016), which induces degradation of both BRD2 and 4, suggesting that a simple chemical upgrade to existing PROTACs may achieve more selective target degradation.
Reynders et al. developed a similar approach termed “PHOTACs”, where the POI ligand was connected to the E3 ligase recruiting ligand via an azobenzene-containing linker (Reynders et al., 2020). While the photoPROTAC utilized VHL as its E3 ligase, PHOTACs recruited CRBN to ubiquitinate target proteins such as BRD and FKBP12. The previously reported PROTACs dBET1 and dFKBP12 were modified to include an azobenzene moiety into the E3 ligase side of their linkers to achieve PHOTAC-I-3 and PHOTAC-II-5, respectively (Figure 3C) (Winter et al., 2015). In contrast to the photoPROTAC report, where the active isomer was trans, the data suggest that it is the cis isomer of the PHOTACs that actively promotes polyubiquitination and degradation of their targets. These differential behaviors could be attributed to the differences in linker length, composition, the positioning of the azobenzene moiety in the linker and/or the recruited E3 ligase. Jin et al. reported a similar strategy termed “Azo-PROTACs”, where they use the same photoswitchable azobenzene moiety (Figure 3D) (Jin et al., 2020). The data indicate that the trans-Azo-PROTAC-4C induced degradation of BCR-Abl by recruiting CRBN. Collectively, these studies demonstrate light-controlled degradation of target proteins such as BRD, FKBP12 and BCR-Abl using photoswitchable PROTACs by recruiting VHL or CRBN E3 ligases.
Light-induced Protein Degradation by Photocaged PROTACs
Irreversible light-induced protein degradation can be achieved by introducing a photocaging group into the PROTAC design (Kounde et al., 2020; Liu et al., 2020; Naro et al., 2020; Xue et al., 2019). Photocaging groups could be conjugated to either side of the PROTAC molecule (Figure 4A) to abrogate the binding to the E3 ligase or POI. However, upon light irradiation, the photocaging group will be released, allowing the formation of a stable ternary complex between POI-PROTAC-E3 ligase, leading to ubiquitination and degradation of the target protein.
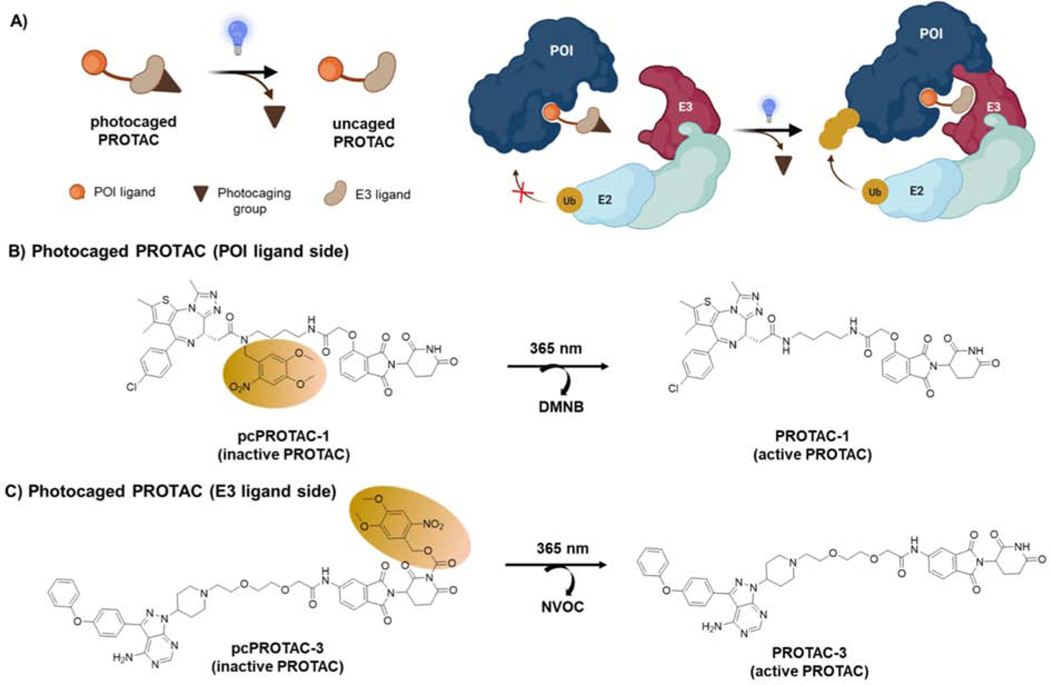
Figure 4-. Light-induced Protein Degradation by Photocaged PROTACs.
A) Schematic of photocaged PROTAC. Upon light irradiation, the photocaged group is removed to form the active PROTAC. As depicted in the cartoon, the inactive photocaged PROTACs (E3 ligand side) are unable to bind to the E3 ligase thereby blocking the formation of stable ternary complex between E3 ligase and POI. Upon light irradiation, the photocaging group will be removed allowing the formation of ternary complex between POI-PROTAC-E3 ligase, leading to ubiquitination and degradation of the target protein. B) Photocaged PROTAC-1 (pcPROTAC-1) comprised of BRD-targeting JQ1 and CRBN-recruiting ligand thalidomide. The inactive pcPROTAC-1 has a DMNB as the photocaging group on the POI ligand (JQ1) side. Upon light irradiation, the DMNB group is released, producing the active PROTAC-1. C) Photocaged PROTAC-3 (pcPROTAC-3) comprised of a BTK-targeting ligand and thalidomide. Inactive pcPROTAC-3 has a NVOC as the photocaging group on thalidomide. Upon light irradiation NVOC group is released, generating active PROTAC-3. Photocaging groups (DMNB: 4,5-Dimethoxy-2-nitrobenzyl, NVOC: 6-nitroveratryloxycarbonyl) are shown as brown ovals.
In 2019, a photocaging strategy was incorporated into PROTACs to achieve light-dependent protein degradation. A photocaging group was fused to mask JQ1 within dBET1, creating pcPROTAC-1 (Figure 4B) (Xue et al., 2019). Upon light irradiation (UV 365nm), active dBET1 was formed by photolysis of pcPROTAC-1, leading to BRD4 degradation and inhibition of cell growth. The activity of pcPROTAC-1 was also evaluated in zebrafish, further highlighting the utility of light-controlled protein degradation for phenotypic screening in model organisms. Additionally, the photocaging strategy was applied to a BTK-targeting PROTAC by masking the thalidomide side to abrogate CRBN recruitment, thereby creating pcPROTAC-3 (Figure 4C). Upon UV irradiation, the uncaged pcPROTAC-3 induced BTK degradation in a dose-dependent manner. This study demonstrated the suitability and potential for modifying either a POI ligand or a CRBN ligand using a photocaging group to achieve light-induced protein degradation. However, masking a POI ligand with a photocaging group would be advantageous over an E3 ligand, as it removes inhibitory effects on the POI in the absence of the light, thus reducing inhibition related on target effects.
Three independent studies by Naro et al. (Naro et al., 2020), Liu et al. (Liu et al., 2020), and Kounde et al. (Kounde et al., 2020) have further demonstrated the utility of photocaged PROTACs in TPD. In these three studies, they explored the potential of light-induced protein degradation by photocaging VHL or CRBN-based PROTACs targeting BRD, ERRα, and ALK proteins. Taken together, these studies demonstrate the flexibility and general applicability of photocaging to PROTAC-mediated degradation that recruits different E3 ligases to ubiquitinate a variety of targets. However, compared to reversible photoswitchable PROTACs, a limitation of the photocaged PROTACs is the irreversible release of active PROTACs into the cells. Protein degradation using photoswitchable PROTACs is, therefore, advantageous in terms of being mechanistically able to reduce the on-target toxicity associated with the prolonged presence of active trans isomer in cells.
Overall, these studies illustrate a novel approach to regulate protein degradation in a spatiotemporal manner. Given that these studies have explored commonly recruited E3 ligases (VHL and CRBN), this should be easily adaptable to either previously reported or the next generation of PROTACs to help uncover basic biological discoveries, such as downstream signaling pathways, kinetics, and spatiotemporal regulation of any given target protein through optically-controlled degradation. However, it is worth noting that these light-controlled PROTACs will need further optimizations to permit control using near IR irradiation (as opposed to ultraviolet) to be compatible in in vivo settings with the hope of using PROTACs as next generation medicines.
B. Expanding the Scope of Chemically-tractable E3 ligases
There are over 600 putative E3 ligases in the human proteome, yet only a handful of E3 ligases have so far been exploited for TPD due to the lack of small molecule ligands. The PROTAC concept was first introduced using a peptide-based PROTAC that recruits β-TRCP E3 ligase to induce degradation of methionine aminopeptidase-2 (MetAP2) (Sakamoto et al., 2001). The transition from peptide-based PROTACs to small molecule-based PROTACs was a key milestone in the TPD field (Bondeson et al., 2015; Itoh et al., 2010; Lu et al., 2015; Schneekloth et al., 2008; Winter et al., 2015). By 2015, all-small molecule PROTACs had been developed by recruiting VHL, CRBN, MDM2, or IAP E3 ligases given the discovery and availability of high affinity small molecule ligands. The classification, expression profile, and ligandability of E3 ligases have been extensively reviewed elsewhere (Schapira et al., 2019). In this section, we summarize the most common E3 ligases exploited for PROTAC design, novel E3 ligases with covalent ligands, and the development of covalent PROTACs. Finally, we foresee an interesting twist from conventional non-covalent PROTACs to covalent PROTACs in the TPD field.
VHL
Von Hippel-Lindau (VHL) is the substrate adaptor protein of the Cul2-Elongin BC and RBX2 complex and recognizes a core hydroxylated proline of the transcription factor HIF1α (Hon et al., 2002). In 2004, VHL was recruited to ubiquitinate the androgen receptor (AR) via a PROTAC composed of dihydroxytestosterone and a seven amino acid peptide derived from HIF1α (Schneekloth et al., 2004), the natural substrate of VHL. This PROTAC induced degradation of AR in HEK293 cells; however, due to the limitations of peptide-based strategies, high affinity small molecule ligands for VHL were developed by structure-based drug design (Buckley et al., 2012a; Buckley et al., 2012b). Subsequently, many PROTACs were developed using the small molecule VHL ligand to target variety of proteins for degradation such as RIPK2, ERRα, BCR/Abl, EGFR, p38, BRD2–4, FAK, BRD7/9, IRAK4, SMARCA2–4, and KRASG12C (Bond et al., 2020; Bondeson et al., 2015; Burslem et al., 2018a; Cromm et al., 2018; Farnaby et al., 2019; Smith et al., 2019; Zoppi et al., 2019). VHL is a ubiquitously expressed E3 ligase, thus VHL-based PROTACs are efficacious in many cell types. However, VHL-recruiting PROTACs are not active in some cancer types such as certain kidney cancers due to the inactivation or loss of VHL. A recent study showed that differential expression profile of VHL in platelets and tumor cells can be exploited to reduce on target drug toxicity (Khan et al., 2019; Zhang et al., 2020b). Given the low expression of VHL in platelets, the lead PROTAC, DT2216 derived from a BCL2/BCL-xL binding ligand and a VHL ligand, did not induce BCL-xL degradation in platelets, hence reducing platelet toxicity. Interestingly, this study demonstrates the advantage of PROTAC-induced selective protein degradation by exploiting differential expression of E3 ligases.
MDM2
Mouse double minute 2 homologue (MDM2) is an E3 ubiquitin ligase that negatively regulates the tumor suppressor p53 by inducing its ubiquitination and subsequent degradation by the proteasome (Wang et al., 2017). In 2008, the first all-small molecule based PROTAC was developed using a ligand, nutlin-3a, to recruit MDM2 to ubiquitinate and degrade the AR (Schneekloth et al., 2008). Because binding of nutlins to MDM2 disrupts the MDM2-p53 interaction, it leads to rapid stabilization of p53 (Tovar et al., 2006). Accordingly, a key advantage of using MDM2 as an E3 ligase in PROTAC design is this secondary effect of stabilizing tumor suppressor p53 that will not be accomplished by using other E3 ligases such as VHL or CRBN. In 2019, idasanutlin, a derivative of nutlin-3a, was successfully incorporated into a PROTAC targeting BRD4 for degradation (Ding et al., 2013; Hines et al., 2019). PROTAC A1874 demonstrated superior anti-proliferative activity compared to a VHL-recruiting BRD4 PROTAC, due to the dual ability of A1874 PROTAC to simultaneously induce BRD4 degradation and p53 stabilization.
CRBN
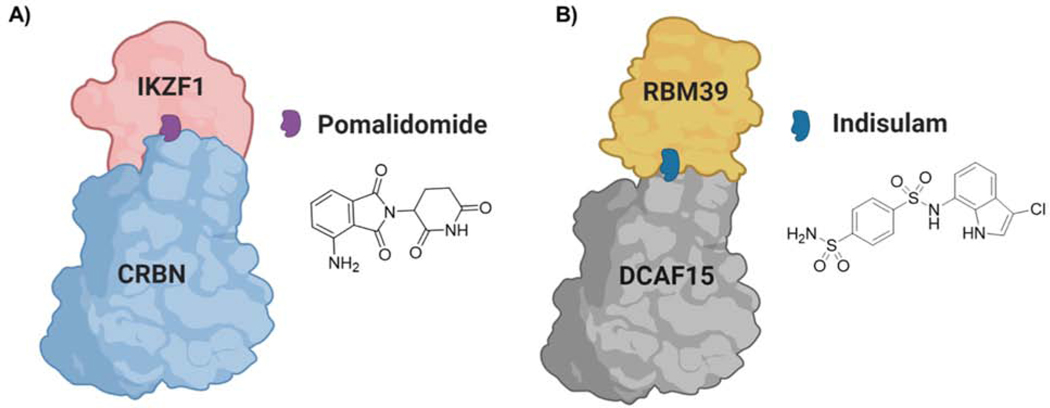
Cereblon (CRBN) is the substrate adaptor of Cul4-RBX1-DDB1- ubiquitin ligase complex that targets endogenous substrates such as glutamine synthetase, the homeo box protein MEIS2, 5’ AMP-activated protein kinase (AMPK), and the amyloid precursor protein (APP) for proteasomal degradation (Fischer et al., 2014; Kurihara et al., 2020; Kwon et al., 2019; Nguyen et al., 2016). In 2010, Ito et al. identified CRBN E3 ligase as the molecular target of the immunomodulatory drug (IMiD), thalidomide (Ito et al., 2010). Thalidomide and its analogs (IMiDs) represent a well characterized molecular glue mechanism where IMiDs alter the surface of CRBN to interact with neo-substrates such as IKZF1/3, CK1α, and GSPT1, resulting in their ubiquitination and degradation (Asatsuma-Okumura et al., 2019; Donovan et al., 2018; Kronke et al., 2015; Kronke et al., 2014; Matyskiela et al., 2016; Sievers et al., 2018) (Figure 5A). In 2015, IMiDs were incorporated into PROTAC development and successfully demonstrated that BRD4 can be degraded by recruitment of CRBN in a PROTAC-dependent manner (Lu et al., 2015; Winter et al., 2015). Subsequently, multiple proteins such as PCAF/GCN5, CDK4/6, BCL2, BCR-Abl, tau, MDM2, α1A-AR, BTK, BCL-xL, WEE1, and ALK have been targeted for degradation using CRBN-recruiting PROTACs (Bassi et al., 2018; Buhimschi et al., 2018; Fry et al., 2004; He et al., 2020; Lai et al., 2016; Li et al., 2019; Li et al., 2020a; Li et al., 2020b; Powell et al., 2018; Silva et al., 2019b; Wang et al., 2019b). Despite their wide use, one limitation of IMiD-based PROTACs is their ability to induce off-target degradation via neo-substrate recruitment to CRBN (Chamberlain and Hamann, 2019; Ishoey et al., 2018). However, the development of thalidomide analogs that are incapable of inducing neo-substrate degradation will be an interesting avenue to explore in refining CRBN-based PROTAC selectivity (Yang et al., 2020).
Figure 5-. Molecular Glue Degraders.
A) Pomalidomide binds to CRBN, stabilizes interactions with IKZF1, causing ubiquitination and degradation of latter. B) Indisulam binds to DCAF15 and induce protein-protein interactions with RBM39, causing ubiquitination and degradation of RBM39.
IAP
The E3 ligase cellular inhibitor of apoptosis proteins (cIAPs) were recruited to cellular retinoic acid binding proteins I (CRABPI) and CRABPII and induced their proteasomal degradation by a PROTAC composed of bestatin esters and all-trans retinoic acid (Itoh et al., 2010; Naito et al., 2019). However, bestatin esters show limited applicability in PROTAC development because they induce autoubiquitination and degradation of cIAP itself. Several other ligands for XIAPs have also been developed recently (Ohoka et al., 2017), and to date multiple proteins such as ER, AR, retinoic acid receptors, transforming acidic coiled coil-3-containing protein, BCL-xL, and BCR-Abl have been targeted for degradation by IAP-recruiting PROTACs (Demizu et al., 2012; Demizu et al., 2016; Itoh et al., 2011; Ohoka et al., 2014; Zhang et al., 2020a).
Exploring Novel E3 ligases in Targeted Protein Degradation
Only a handful of E3 ligases have been widely used in TPD for the last 20 years due to the scarcity of small molecule ligands with which to recruit them (Ottis and Crews, 2017). However, Ottis et al. explored the generalizability of other E3 ligase families in PROTAC design and demonstrated that five of six representative E3 ligases are amenable to TPD applications (Ottis et al., 2017). Therefore, a critical next step for the TPD field is to identify small molecule ligands for additional E3 ligases spanning a variety of structural and functional properties as well as unique expression profiles that can then be explored in PROTAC development to target intractable pathogenic proteins. This section will highlight recent studies that have identified ligands for new E3 ligases and explored their potential in TPD.
DCAF15
In 2017, the anti-cancer aryl sulfonamides indisulam, E7820 and chloroquinoxaline sulfonamide (CQS) were shown to bind to the E3 ligase DCAF15, thus targeting splicing protein RBM39 for degradation (Han et al., 2017; Uehara et al., 2017). DCAF15 is a substrate receptor of the Cul4/DDB1/RBX1 (CRL4) family of E3 ligases (Nguyen and Busino, 2020), however, the molecular details of aryl sulfonamide-induced DCAF15 and RBM39 interaction were initially unknown. Interestingly, recent crystallography and cryo-EM data revealed that indisulam and related analogs act as molecular glues. Indisulam stabilizes protein-protein interactions (PPI) between RBM39 and DCAF15 (Figure 5B) (Bussiere et al., 2020; Du et al., 2019; Faust et al., 2020), analogously to the manner in which IMiD binding to CRBN can induce PPI with neo-substrates such as IKZF1, leading to proteasomal degradation of the latter (Figure 5A) (Kronke et al., 2015; Kronke et al., 2014). In addition, levels of a related protein, RBM23, were decreased in indisulam-treated cells (Ting et al., 2019). However, the utility of DCAF15 ligands in TPD has yet to be determined (Coomar and Gillingham, 2019). Nonetheless, further studies will be needed to reveal endogenous substrates and indisulam analog-dependent neo-substrates of DCAF15 to fully understand its potential in TPD applications.
DCAF16
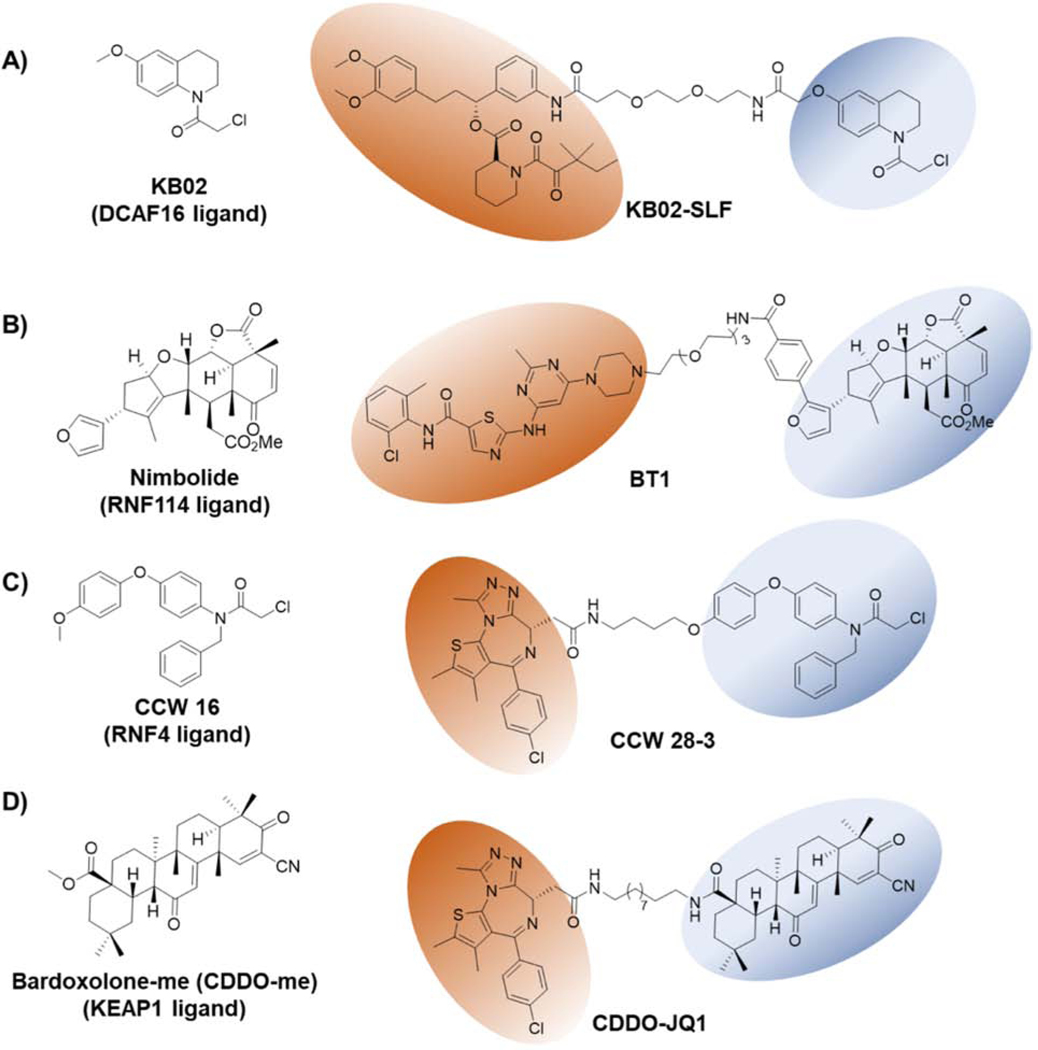
Zhang et al. used a chemoproteomic strategy to identify covalent E3 ligase binders using a cysteine targeting electrophilic library (Zhang et al., 2019c). In their study, they used heterobifunctional molecules consisting of covalent electrophilic fragments (scout fragments-KB02, KB03 and KB05) conjugated to known ligands for target proteins, such as BRD4 (JQ1) and FKBP12 (SLF). Using this chemoproteomic screen, they identified DCAF16, a poorly characterized, nuclear-localized substrate receptor of the Cul4/DDB1 E3 ligase complex (Lee and Zhou, 2007). KB02-SLF induced degradation of the nuclear FKBP12 selectively by recruiting nuclear-localized DCAF16 (Figure 6A). Further, data suggest that as little as 10–40% of DCAF16 engagement by PROTAC is sufficient to induce the degradation of the target protein: an advantage of using covalent E3 ligase ligands in PROTAC development. Although the endogenous substrates of DCAF16 are currently unknown, the demonstrated selectivity of this E3 ligase for nuclear-localized targets expands the potential utility of DCAF16 for selective degradation of nuclear-localized proteins.
Figure 6-. Structures of Covalent E3 ligase Ligands and Their Application in TPD.
A) KB02 is a covalent binder of the nuclear localized E3 ligase- DCAF16. KB02-SLF targets nuclear FKBP12 for degradation. B) Nimbolide covalently binds to the E3 ligase RNF114. BT1 is a PROTAC derived from a BCR-Abl ligand and nimbolide. C) CCW-16 covalently binds to RNF4 E3 ligase. CCW-28–3 is a PROTAC linking JQ1 to CCW-16 and targets BRD4 for degradation by recruiting RNF4. D) Bardoxolone-me (CDDO-me) is a reversible covalent ligand for KEAP1 E3 ligase. CDDO-JQ1 PROTAC links JQ1 to CDDO-me and targets BRD4 for proteasomal degradation by recruiting KEAP1. POI ligands and E3 ligase recruiting ligands are highlighted with orange and blue ovals, respectively.
RNF114
RNF114 belongs to the RING family E3 ligases and ubiquitinates several endogenous substrates including the tumor suppressors p21, p27, and p57 (Han et al., 2013). Activity-based proteomic profiling (ABPP) chemoproteomic studies are crucial to identify the unknown target of a novel compound of interest (Weerapana et al., 2010). In 2019, Spradlin et al. identified that the natural product nimbolide covalently binds to Cys8 in RNF114 E3 ligase (Figure 6B) (Spradlin et al., 2019). In doing so, nimbolide disrupts the interaction between p21 and RNF114, thereby causing p21 stabilization. To explore the potential of recruiting RNF114, a novel E3 ligase in TPD, the authors developed a PROTAC (XH2) based on nimbolide and JQ1 to degrade BRD4. Interestingly, XH2 induced the proteasomal degradation of BRD4 in breast cancer cells, thus validating that nimbolide can be used to recruit RNF114 in future TPD applications. Very recently, Tong et al. showed that a PROTAC (BT1) based on nimbolide and dasatinib, induces BCR-Abl degradation over c-Abl by recruiting RNF114 in leukemia cells (Figure 6B) (Tong et al., 2020b). In contrast to VHL- and CRBN-recruiting PROTACs, nimbolide binding to RNF114 has the additional advantage to stabilize the tumor suppressor p21. As a result, the BT1 PROTAC demonstrated an excellent anti-proliferative effect over VHL- or CRBN-recruiting PROTACs similar to how MDM2 recruiting PROTACs have demonstrated additional oncology benefits due to p53 stabilization (Hines et al., 2019). These studies substantiate the advantage of identifying ligands for new E3 ligases with additional anti-cancer properties such as stabilization of endogenous substrates with tumor suppressor functions (ex: p53 and p21).
RNF4
RNF4 belongs to the RING family of E3 ligases and targets polysumoylated proteins for proteasomal degradation (Tatham et al., 2008). Ward et al. identified cysteine reactive small molecules that target E3 ligase RNF4 by applying an ABPP-based covalent ligand screening method (Ward et al., 2019). Optimizing their initial hit, they developed CCW 16 as the lead RNF4-targeting ligand (Figure 6C). To evaluate the potential of using RNF4 in TPD, they developed a PROTAC by linking CCW16 to JQ1 (Figure 6C). The resulting PROTAC, CCW28–3, induced ubiquitination and degradation of BRD4 in a RNF4-dependent manner. While further optimization is needed to improve the potency, selectivity, and cell permeability of CCW 16, this study showcases the potential of ABPP-based covalent ligand screening methods to identify ligands for E3 ligases of interest.
KEAP1
KEAP1 is the substrate adaptor protein of the BTB-Cul3-RBX1 E3 ligase complex involved in redox-mediated protein quality control during oxidative stress (Zhang et al., 2004). KEAP1 is known to ubiquitinate and degrade NRF2, p62, BPTF, and PGAM5 (Jain et al., 2010; Kobayashi et al., 2004). In 2018, KEAP1 was recruited by a peptide-based PROTAC to degrade tau protein, which demonstrated that KEAP1 can be used as a potential E3 ligase in PROTAC-mediated TPD (Lu et al., 2018). Tong et al. recently reported that the first reversible covalent E3 ligase recruiting ligand in TPD applications, bardoxolone-me (CDDO-me), reversibly binds to cysteines on KEAP1 (Figure 6D) (Liby and Sporn, 2012; Sporn et al., 2011; Tong et al., 2020a). To explore the utility of bardoxolone to recruit KEAP1 to a POI, they developed a PROTAC (CDDO-JQ1) by linking JQ1 to bardoxolone. CDDO-JQ1 induced dose-dependent BRD4 degradation in a proteasome-dependent manner. However, at high PROTAC concentrations, KEAP1 degradation was also evident. Although this proof of concept study provides an interesting application of KEAP1 using novel ligands, a deeper understanding of the interaction between CDDO and KEAP1 will be needed, as well as further optimization to CDDO to minimize off-target effects. Nonetheless, this demonstrates the potential of using reversible covalent E3 ligase recruiters in PROTAC design in future TPD applications.
Outlook
For many years, the PROTAC technology utilized a limited number of E3 ligases, (i.e., VHL, CRBN, MDM2 and IAP) to induce targeted protein degradation (Bondeson and Crews, 2017; Lai and Crews, 2017) due to the lack of high affinity ligands for the majority of E3 ligases (Figure 7A). Interestingly, studies have demonstrated that even weak binders of E3 ligases can induce efficient degradation of the target protein, underscoring the advantages of the PROTAC technology (Buckley et al., 2015; Han et al., 2019b; Zhang et al., 2019c). Moreover, PROTACs composed of low affinity ligands for either the target protein or E3 ligase can still induce efficient target degradation via stabilizing PPIs. Because resistance to PROTACs has already arisen due to genomic alterations in the core components of the E3 ligase machinery, ligands for novel E3 ligases are desperately needed to overcome these resistance mechanisms (Ottis et al., 2019; Zhang et al., 2019a).
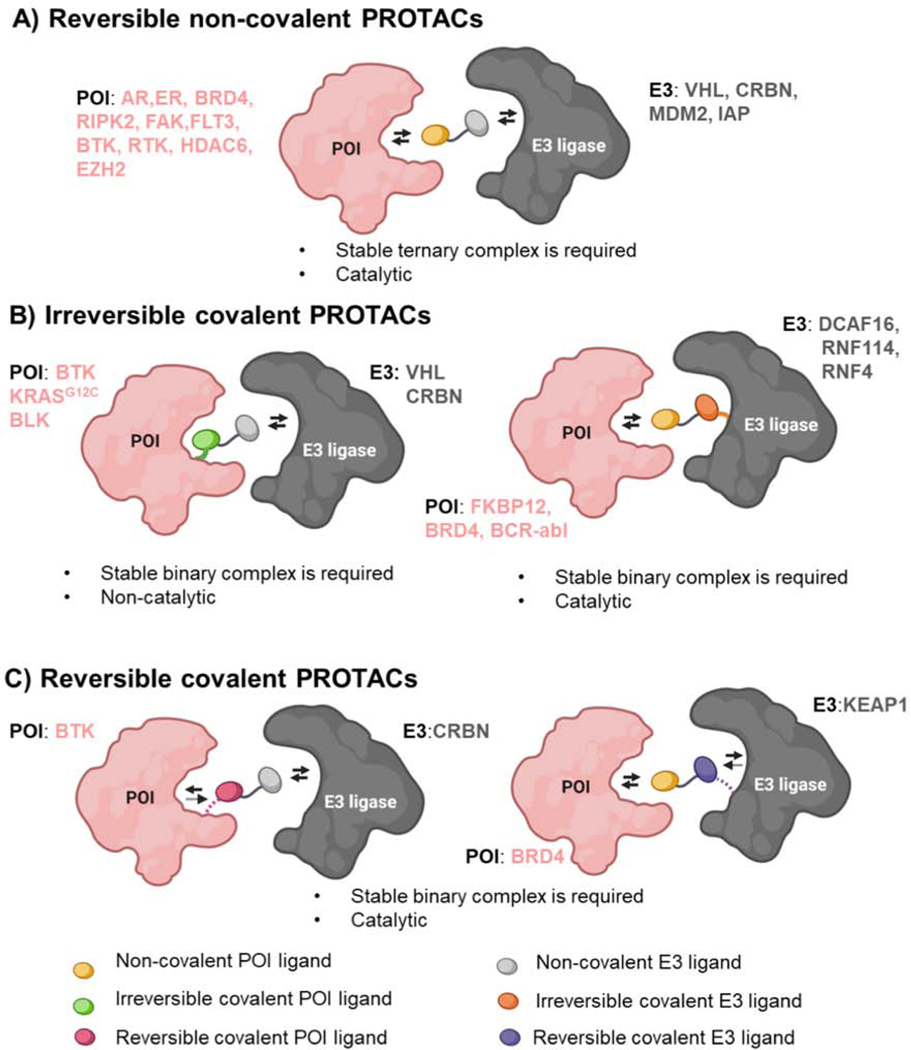
Figure 7-. Conventional Non-covalent PROTACs and Covalent PROTACs.
A) Conventional PROTACs derived from non-covalent ligands (POI-yellow oval and E3-gray oval) that reversibly engage both POI and E3 ligase, leading to a stable ternary complex formation to achieve successful degradation. These PROTACs maintain the catalytic nature, a unique feature of PROTAC technology compared to small molecule inhibitors. B) Irreversible covalent PROTACs can be based on covalent targeting of the POI (left) or the E3 ligase (right). These PROTACs form a stable binary complex given that one side of the PROTAC is irreversibly bound to the POI or E3 ligase. While covalent interaction of the PROTAC with the POI (left, green oval) can compromise the catalytic feature of the PROTAC, covalent targeting of the E3 ligase (right, orange oval) retains its catalytic nature. C) Reversible covalent PROTACs are derived from a reversible covalent ligand that binds to the POI (left, magenta oval) or E3 ligase (right, purple oval), therefore they retain the catalytic nature of non-covalent PROTACs. The representative examples of POI and E3 ligases exploited in each of these PROTAC categories are listed in pink and gray colors, respectively.
Covalent ligands are now increasingly appreciated in drug discovery due to their potentially enhanced selectivity and potency compared to reversible non-covalent ligands (Gehringer and Laufer, 2019; Zhang et al., 2019b). Covalent targeting of E3 ligases is emerging as an innovative approach to hijack novel E3 ligases. This is further underscored by the fact that covalent targeting of E3 does not affect the catalytic nature of the PROTAC (Figure 7B and 7C). Importantly, ligands have been discovered for novel E3 ligases (DCAF16, RNF114, and RNF4) due to the power of covalent ligand screening and chemoproteomics. Moreover, the PROTACs developed using these ligands induce degradation of their target proteins, demonstrating their potential applications in TPD.
Until recently, the TPD field deprioritized the use of covalent ligands for target proteins in PROTAC design, since covalent binding would abrogate the catalytic nature of PROTACs, which is a key advantage relative to small molecule inhibitors (Tinworth et al., 2019) (Figure 7A). Several studies have shown that PROTACs based on ligands that covalently bind to their target proteins such as BTK, BLK, and KRASG12C are successfully targeted for degradation (Figure 7B) (Bond et al., 2020; Gabizon et al., 2020; Xue et al., 2020). Very recently, using BTK as a clinically relevant model protein, PROTACs using reversible non-covalent, reversible covalent, and irreversible covalent BTK targeting ligands were compared for their selectivity and potency (Gabizon et al., 2020). These studies revealed that PROTACs based on a reversible covalent BTK ligand displayed enhanced selectivity and would be preferable over irreversible ligands as they maintain the catalytic nature of PROTACs (Figure 7C). One beneficial use of a covalent PROTAC would be to target intractable proteins for which there are currently only weak reversible ligands. Interestingly, irreversible covalent PROTACs can switch the requirement of a stable ternary complex to a more favorable binary interaction since one side of the PROTAC covalently modifies E3 ligase or POI. While, irreversible covalent targeting of POI could lose the catalytic mode of action of PROTACs, reversible or irreversible covalent ligands for E3 ligases are kinetically advantageous in the PROTAC design as it retains the catalytic ability to undergo multiple rounds of POI degradation (Figure 7B and 7C) (Zhang et al., 2019c). Nonetheless, future studies are needed to fully understand the advantages and limitations of covalent PROTACs relative to conventional non-covalent PROTACs.
Moving forward, chemoproteomic platforms, fragment-based ligand discovery, and DNA encoded library screening together will be useful in identifying ligands for intractable disease related targets, as well as for new E3 ligases (Goodnow et al., 2017; Jacquemard and Kellenberger, 2019). Most of the human E3 ligases are not well characterized; their roles in physiological and pathological settings as well as information on their endogenous substrates is lacking. Identification of endogenous substrates or even substrate degrons of novel E3 ligases could pave the way towards new ligand development. It would be intriguing to explore novel ligands for E3 ligases that are either tissue-specific, tumor-specific, or compartment-specific to achieve more precise control in TPD to treat disease. Given the lack of tumor-specific E3 ligase ligands, the use of tumor-specific POI ligands in PROTAC design is an intriguing approach in the context of precision medicine. This was illustrated by targeting oncogenic KRASG12C for degradation using MRTX-based VHL recruiting PROTAC (Bond et al., 2020). Alternatively, a tumor-specific PROTAC effect could be achieved by targeted delivery to cancer cells. For example, in a recent report, selective delivery of BRD4 PROTACs to Her2 positive breast cancer cells has been achieved using antibody-PROTAC conjugates, further highlighting advantages and the need to achieve tumor-specific degradation using PROTACs (Maneiro et al., 2020).
Currently, the majority of the PROTACs exploited in TPD are based on ubiquitously expressed E3 ligases using known target protein ligands. As TPD is rapidly emerging as a versatile therapeutic modality, the next generation of PROTACs will likely harness the power of disease specific E3 ligases using novel ligands to currently undruggable target proteins. It is tempting to speculate the profound level of targeting precision that could be engineered into a PROTAC by combination of these specificity-conferring measures (e.g. tumor-specific POI ligand linked to a tumor-specific E3 ligand and delivered by a tumor-specific antibody). These efforts will help further establish TPD as a new therapeutic modality in precision medicine.
SIGNIFICANCE.
TPD is emerging as an exciting modality in both the basic sciences as well as in therapeutic development. PROTACs offer the potential to target the undruggable proteome by overcoming the limitations of conventional small molecule inhibitors that can only target proteins with well-defined active sites. Here, we review the key advantages of PROTACs, i.e., the spatial and temporal regulation of protein degradation, and focus on expanding the E3 ligase toolbox to uncover the full potential of TPD. In the coming years, we anticipate tremendous potential in PROTAC technology to selectively target the undruggable, disease-causing proteins for degradation as it finds its way from the bench to bedside.
Acknowledgements
We would like to thank Dr. John Hines, Dr. George Burslem, Dr. Saul Jaime-Figueroa, Dr. Kusal T. G. Samarasinghe and Michael J. Bond for their insightful comments and critiques in the preparation of this manuscript. C.M.C. is funded by the NIH (R35CA197589) and is supported by an American Cancer Research Professorship.
Declaration of Competing Interests:
C.M.C is founder, shareholder, and consultant to Arvinas, Inc. and Halda, LLC, which support research in his laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert L, and Vázquez O.(2019). Photoswitchable peptides for spatiotemporal control of biological functions. Chemical Communications 55, 10192–10213. [DOI] [PubMed] [Google Scholar]
- An S, and Fu L.(2018). Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine 36, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z, Lv W, Su S, Wu W, and Rao Y.(2019). Developing potent PROTACs tools for selective degradation of HDAC6 protein. Protein & Cell 10, 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbruck N, Courtney T, Naro Y, and Deiters A.(2018). Optochemical Control of Biological Processes in Cells and Animals. Angewandte Chemie (International ed. in English) 57, 2768–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatsuma-Okumura T, Ando H, De Simone M, Yamamoto J, Sato T, Shimizu N, Asakawa K, Yamaguchi Y, Ito T, Guerrini L, et al. (2019). p63 is a cereblon substrate involved in thalidomide teratogenicity. Nature Chemical Biology 15, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Audia JE, and Campbell RM (2016). Histone Modifications and Cancer. Cold Spring Harbor perspectives in biology 8, a019521-a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Zhou H, Xu R, Zhao Y, Chinnaswamy K, McEachern D, Chen J, Yang CY, Liu Z, Wang M, et al. (2019). A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression In Vivo. Cancer Cell 36, 498–511.e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi ZI, Fillmore MC, Miah AH, Chapman TD, Maller C, Roberts EJ, Davis LC, Lewis DE, Galwey NW, Waddington KE, et al. (2018). Modulating PCAF/GCN5 Immune Cell Function through a PROTAC Approach. ACS Chem Biol 13, 2862–2867. [DOI] [PubMed] [Google Scholar]
- BasuRay S, Wang Y, Smagris E, Cohen JC, and Hobbs HH (2019). Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci U S A 116, 9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon A, Pizzagalli MD, Kartnig F, Dvorak V, Essletzbichler P, Winter GE, and Superti-Furga G.(2020). Targeted Degradation of SLC Transporters Reveals Amenability of Multi-Pass Transmembrane Proteins to Ligand-Induced Proteolysis. Cell Chem Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieliauskas AV, and Pflum MKH (2008). Isoform-selective histone deacetylase inhibitors. Chemical Society reviews 37, 1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MJ, Chu L, Nalawansha DA, Li K, and Crews CM (2020). Targeted Degradation of Oncogenic KRASG12C by VHL-Recruiting PROTACs. ACS Central Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson DP, and Crews CM (2017). Targeted Protein Degradation by Small Molecules. Annu Rev Pharmacol Toxicol 57, 107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson DP., Mares A., Smith IE., Ko E., Campos S., Miah AH., Mulholland KE., Routly N., Buckley DL., Gustafson JL., et al. (2015). Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol 11, 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime-Figueroa S, Wang J, Hamman BD, Ishchenko A, and Crews CM (2018). Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead. Cell Chem Biol 25, 78–87.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Jiang B, Bauer S, Donovan KA, Liang Y, Wang ES, Nowak RP, Yuan JC, Zhang T, Kwiatkowski N, et al. (2019). Homolog-Selective Degradation as a Strategy to Probe the Function of CDK6 in AML. Cell Chem Biol 26, 300–306.e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DL, Gustafson JL, Van Molle I, Roth AG, Tae HS, Gareiss PC, Jorgensen WL, Ciulli A, and Crews CM (2012a). Small-molecule inhibitors of the interaction between the E3 ligase VHL and HIF1alpha. Angew Chem Int Ed Engl 51, 11463–11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DL, Raina K, Darricarrere N, Hines J, Gustafson JL, Smith IE, Miah AH, Harling JD, and Crews CM (2015). HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins. ACS Chem Biol 10, 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, Jorgensen WL, Ciulli A, and Crews CM (2012b). Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J Am Chem Soc 134, 4465–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi AD, Armstrong HA, Toure M, Jaime-Figueroa S, Chen TL, Lehman AM, Woyach JA, Johnson AJ, Byrd JC, and Crews CM (2018). Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry 57, 3564–3575. [DOI] [PubMed] [Google Scholar]
- Burslem GM, and Crews CM (2017). Small-Molecule Modulation of Protein Homeostasis. Chem Rev 117, 11269–11301. [DOI] [PubMed] [Google Scholar]
- Burslem GM, and Crews CM (2020). Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 181, 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burslem GM, Schultz AR, Bondeson DP, Eide CA, Savage Stevens SL, Druker BJ, and Crews CM (2019). Targeting BCR-ABL1 in Chronic Myeloid Leukemia by PROTAC-Mediated Targeted Protein Degradation. Cancer Res 79, 4744–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burslem GM, Smith BE, Lai AC, Jaime-Figueroa S, McQuaid DC, Bondeson DP, Toure M, Dong H, Qian Y, Wang J, et al. (2018a). The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study. Cell Chem Biol 25, 67–77.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burslem GM, Song J, Chen X, Hines J, and Crews CM (2018b). Enhancing Antiproliferative Activity and Selectivity of a FLT-3 Inhibitor by Proteolysis Targeting Chimera Conversion. J Am Chem Soc 140, 16428–16432. [DOI] [PubMed] [Google Scholar]
- Bussiere DE, Xie L, Srinivas H, Shu W, Burke A, Be C, Zhao J, Godbole A, King D, Karki RG, et al. (2020). Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Nat Chem Biol 16, 15–23. [DOI] [PubMed] [Google Scholar]
- Cance WG, Kurenova E, Marlowe T, and Golubovskaya V.(2013). Disrupting the scaffold to improve focal adhesion kinase-targeted cancer therapeutics. Sci Signal 6, pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron P-F, Cheray M, and Bretaudeau L.(2020). Epigenetic protein complexes: the adequate candidates for the use of a new generation of epidrugs in personalized and precision medicine in cancer. Epigenomics 12, 171–177. [DOI] [PubMed] [Google Scholar]
- Chamberlain PP, and Hamann LG (2019). Development of targeted protein degradation therapeutics. Nature Chemical Biology 15, 937–944. [DOI] [PubMed] [Google Scholar]
- Churcher I.(2018). Protac-Induced Protein Degradation in Drug Discovery: Breaking the Rules or Just Making New Ones? J Med Chem 61, 444–452. [DOI] [PubMed] [Google Scholar]
- Coomar S, and Gillingham DG (2019). Exploring DCAF15 for reprogrammable targeted protein degradation. bioRxiv, 542506. [Google Scholar]
- Crew AP., Raina K., Dong H., Qian Y., Wang J., Vigil D., Serebrenik YV., Hamman BD., Morgan A., Ferraro C., et al. (2018). Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. J Med Chem 61, 583–598. [DOI] [PubMed] [Google Scholar]
- Crews CM (2010). Targeting the undruggable proteome: the small molecules of my dreams. Chem Biol 17, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromm PM, Samarasinghe KTG, Hines J, and Crews CM (2018). Addressing Kinase-Independent Functions of Fak via PROTAC-Mediated Degradation. J Am Chem Soc 140, 17019–17026. [DOI] [PubMed] [Google Scholar]
- Cunningham ME, Stephens RM, Kaplan DR, and Greene LA (1997). Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J Biol Chem 272, 10957–10967. [DOI] [PubMed] [Google Scholar]
- Demizu Y, Okuhira K, Motoi H, Ohno A, Shoda T, Fukuhara K, Okuda H, Naito M, and Kurihara M.(2012). Design and synthesis of estrogen receptor degradation inducer based on a protein knockdown strategy. Bioorganic & Medicinal Chemistry Letters 22, 1793–1796. [DOI] [PubMed] [Google Scholar]
- Demizu Y, Shibata N, Hattori T, Ohoka N, Motoi H, Misawa T, Shoda T, Naito M, and Kurihara M.(2016). Development of BCR-ABL degradation inducers via the conjugation of an imatinib derivative and a cIAP1 ligand. Bioorganic & Medicinal Chemistry Letters 26, 4865–4869. [DOI] [PubMed] [Google Scholar]
- Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, Chu XJ, Bartkovitz D, Podlaski F, Janson C, et al. (2013). Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem 56, 5979–5983. [DOI] [PubMed] [Google Scholar]
- Dong H, Liu S, Zhang X, Chen S, Kang L, Chen Y, Ma S, Fu X, Liu Y, Zhang H, et al. (2019). An Allosteric PRC2 Inhibitor Targeting EED Suppresses Tumor Progression by Modulating the Immune Response. Cancer Research 79, 5587. [DOI] [PubMed] [Google Scholar]
- Donovan KA, An J, Nowak RP, Yuan JC, Fink EC, Berry BC, Ebert BL, and Fischer ES (2018). Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Volkov OA, Czerwinski RM, Tan H, Huerta C, Morton ER, Rizzi JP, Wehn PM, Xu R, Nijhawan D, et al. (2019). Structural Basis and Kinetic Pathway of RBM39 Recruitment to DCAF15 by a Sulfonamide Molecular Glue E7820. Structure 27, 1625–1633.e1623. [DOI] [PubMed] [Google Scholar]
- Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, Friedman LS, and Belvin M.(2010). Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res 70, 1164–1172. [DOI] [PubMed] [Google Scholar]
- Farnaby W, Koegl M, Roy MJ, Whitworth C, Diers E, Trainor N, Zollman D, Steurer S, KarolyiOezguer J, Riedmueller C, et al. (2019). BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nature chemical biology 15, 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TB, Yoon H, Nowak RP, Donovan KA, Li Z, Cai Q, Eleuteri NA, Zhang T, Gray NS, and Fischer ES (2020). Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nat Chem Biol 16, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D.(2009). Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78, 477–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ES, Böhm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, et al. (2014). Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, et al. (2004). Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3, 1427–1438. [PubMed] [Google Scholar]
- Gabizon R, Shraga A, Gehrtz P, Livnah E, Shorer Y, Gurwicz N, Avram L, Unger T, Aharoni H, Albeck S, et al. (2020). Efficient targeted degradation via reversible and irreversible covalent PROTACs. J Am Chem Soc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd MS, Testa A, Lucas X, Chan K-H, Chen W, Lamont DJ, Zengerle M, and Ciulli A.(2017). Structural basis of PROTAC cooperative recognition for selective protein degradation. Nature chemical biology 13, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sun X, and Rao Y.(2020). PROTAC Technology: Opportunities and Challenges. ACS Med Chem Lett 11, 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechijian LN, Buckley DL, Lawlor MA, Reyes JM, Paulk J, Ott CJ, Winter GE, Erb MA, Scott TG, Xu M, et al. (2018). Functional TRIM24 degrader via conjugation of ineffectual bromodomain and VHL ligands. Nature chemical biology 14, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehringer M., and Laufer SA. (2019). Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J Med Chem 62, 5673–5724. [DOI] [PubMed] [Google Scholar]
- Goodnow RA, Dumelin CE, and Keefe AD (2017). DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nature Reviews Drug Discovery 16, 131–147. [DOI] [PubMed] [Google Scholar]
- Han J, Kim YL, Lee KW, Her NG, Ha TK, Yoon S, Jeong SI, Lee JH, Kang MJ, Lee MG, et al. (2013). ZNF313 is a novel cell cycle activator with an E3 ligase activity inhibiting cellular senescence by destabilizing p21(WAF1.). Cell Death Differ 20, 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T, Goralski M, Gaskill N, Capota E, Kim J, Ting TC, Xie Y, Williams NS, and Nijhawan D.(2017). Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 356. [DOI] [PubMed] [Google Scholar]
- Han X, Wang C, Qin C, Xiang W, Fernandez-Salas E, Yang CY, Wang M, Zhao L, Xu T, Chinnaswamy K, et al. (2019a). Discovery of ARD-69 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Androgen Receptor (AR) for the Treatment of Prostate Cancer. J Med Chem 62, 941–964. [DOI] [PubMed] [Google Scholar]
- Han X, Zhao L, Xiang W, Qin C, Miao B, Xu T, Wang M, Yang C-Y, Chinnaswamy K, Stuckey J, et al. (2019b). Discovery of Highly Potent and Efficient PROTAC Degraders of Androgen Receptor (AR) by Employing Weak Binding Affinity VHL E3 Ligase Ligands. Journal of Medicinal Chemistry 62, 11218–11231. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang X, Chang J, Kim HN, Zhang P, Wang Y, Khan S, Liu X, Zhang X, Lv D, et al. (2020). Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun 11, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin K, and Dhanak D.(2013). Chromatin proteins and modifications as drug targets. Nature 502, 480–488. [DOI] [PubMed] [Google Scholar]
- Hines J, Gough JD, Corson TW, and Crews CM (2013). Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs. Proc Natl Acad Sci U S A 110, 8942–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J, Lartigue S, Dong H, Qian Y, and Crews CM (2019). MDM2-Recruiting PROTAC Offers Superior, Synergistic Antiproliferative Activity via Simultaneous Degradation of BRD4 and Stabilization of p53. Cancer Res 79, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp MS, Kasturi P, and Hartl FU (2019). The proteostasis network and its decline in ageing. Nature Reviews Molecular Cell Biology 20, 421–435. [DOI] [PubMed] [Google Scholar]
- Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, and Jones EY (2002). Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 417, 975–978. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Rasmusson T, Robinson J, Pachl F, Read J, Kawatkar S, DH OD, Bagal S, Code E, Rawlins P, et al. (2020). EED-Targeted PROTACs Degrade EED, EZH2, and SUZ12 in the PRC2 Complex. Cell Chem Biol 27, 41–46.e17. [DOI] [PubMed] [Google Scholar]
- Hu J, Hu B, Wang M, Xu F, Miao B, Yang CY, Wang M, Liu Z, Hayes DF, Chinnaswamy K, et al. (2019). Discovery of ERD-308 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Estrogen Receptor (ER). J Med Chem 62, 1420–1442. [DOI] [PubMed] [Google Scholar]
- Huang HT, Dobrovolsky D, Paulk J, Yang G, Weisberg EL, Doctor ZM, Buckley DL, Cho JH, Ko E, Jang J, et al. (2018). A Chemoproteomic Approach to Query the Degradable Kinome Using a Multi-kinase Degrader. Cell Chem Biol 25, 88–99.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys PG., Bamborough P., Chung CW., Craggs PD., Gordon L., Grandi P., Hayhow TG., Hussain J., Jones KL., Lindon M., et al. (2017). Discovery of a Potent, Cell Penetrant, and Selective p300/CBP-Associated Factor (PCAF)/General Control Nonderepressible 5 (GCN5) Bromodomain Chemical Probe. J Med Chem 60, 695–709. [DOI] [PubMed] [Google Scholar]
- Ishoey M, Chorn S, Singh N, Jaeger MG, Brand M, Paulk J, Bauer S, Erb MA, Parapatics K, Muller AC, et al. (2018). Translation Termination Factor GSPT1 Is a Phenotypically Relevant Off-Target of Heterobifunctional Phthalimide Degraders. ACS Chem Biol 13, 553–560. [DOI] [PubMed] [Google Scholar]
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, and Handa H.(2010). Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ishikawa M, Naito M, and Hashimoto Y.(2010). Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J Am Chem Soc 132, 5820–5826. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kitaguchi R, Ishikawa M, Naito M, and Hashimoto Y.(2011). Design, synthesis and biological evaluation of nuclear receptor-degradation inducers. Bioorganic & Medicinal Chemistry 19, 6768–6778. [DOI] [PubMed] [Google Scholar]
- Jacquemard C, and Kellenberger E.(2019). A bright future for fragment-based drug discovery: what does it hold? Expert Opinion on Drug Discovery 14, 413–416. [DOI] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, and Johansen T.(2010). p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285, 22576–22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Wang ES, Donovan KA, Liang Y, Fischer ES, Zhang T, and Gray NS (2019). Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angew Chem Int Ed Engl 58, 6321–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Lu MC, Wang Y, Shan WX, Wang XY, You QD, and Jiang ZY (2020). Azo-PROTAC: Novel Light-Controlled Small-Molecule Tool for Protein Knockdown. J Med Chem. [DOI] [PubMed] [Google Scholar]
- Khan S, Zhang X, Lv D, Zhang Q, He Y, Zhang P, Liu X, Thummuri D, Yuan Y, Wiegand JS, et al. (2019). A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat Med 25, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, and Hartl FU (2013). Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82, 323–355. [DOI] [PubMed] [Google Scholar]
- Kliza K, and Husnjak K.(2020). Resolving the Complexity of Ubiquitin Networks. Front Mol Biosci 7, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, and Yamamoto M.(2004). Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24, 7130–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou M, Li J, Zhang B, Wang Z, Shaabani S, Ter Brake F, Essa K, and Domling A.(2019). PROTACs- a game-changing technology. Expert Opin Drug Discov 14, 1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic M, and Jones LH (2020). Critical Assessment of Targeted Protein Degradation as a Research Tool and Pharmacological Modality. Trends Pharmacol Sci 41, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, and Schlessinger J.(1997). A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89, 693–702. [DOI] [PubMed] [Google Scholar]
- Kounde CS, Shchepinova MM, Saunders CN, Muelbaier M, Rackham MD, Harling JD, and Tate EW (2020). A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chem Commun (Camb). [DOI] [PubMed] [Google Scholar]
- Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, Chamberlain PP, Mani DR, Man HW, Gandhi AK, et al. (2015). Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature 523, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronke J., Udeshi ND., Narla A., Grauman P., Hurst SN., McConkey M., Svinkina T., Heckl D., Comer E., Li X., et al. (2014). Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Deshmukh F, Yaffe D, Olshina MA, Ben-Nissan G, and Sharon M.(2019). The Contribution of the 20S Proteasome to Proteostasis. Biomolecules 9, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Asahi T, and Sawamura N.(2020). Cereblon-mediated degradation of the amyloid precursor protein via the ubiquitin-proteasome pathway. Biochemical and Biophysical Research Communications 524, 236–241. [DOI] [PubMed] [Google Scholar]
- Kwon E, Li X, Deng Y, Chang HW, and Kim DY (2019). AMPK is down-regulated by the CRL4ACRBN axis through the polyubiquitination of AMPKα isoforms. The FASEB Journal 33, 6539–6550. [DOI] [PubMed] [Google Scholar]
- Lai AC, and Crews CM (2017). Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov 16, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AC, Toure M, Hellerschmied D, Salami J, Jaime-Figueroa S, Ko E, Hines J, and Crews CM (2016). Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL. Angew Chem Int Ed Engl 55, 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen A, Højfeldt JW, and Helin K.(2016). Role of the Polycomb Repressive Complex 2 (PRC2) in Transcriptional Regulation and Cancer. Cold Spring Harbor perspectives in medicine 6, a026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Timpson P, Horvath LG, and Daly RJ (2015). FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther 146, 132–149. [DOI] [PubMed] [Google Scholar]
- Lee J, and Zhou P.(2007). DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell 26, 775–780. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, and Schlessinger J.(2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tennekoon GI, Birnbaum M, Marchionni MA, and Rutkowski JL (2001). Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci 17, 761–767. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Aguilar A, McEachern D, Przybranowski S, Liu L, Yang CY, Wang M, Han X, and Wang S.(2019). Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression. J Med Chem 62, 448–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lin Y, Song H, Qin X, Yu Z, Zhang Z, Dong G, Li X, Shi X, Du L, et al. (2020a). First small-molecule PROTACs for G protein-coupled receptors: Inducing α1A-adrenergic receptor degradation. Acta Pharmaceutica Sinica B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Pinch BJ, Olson CM, Donovan KA, Nowak RP, Mills CE, Scott DA, Doctor ZM, Eleuteri NA, Chung M, et al. (2020b). Development and Characterization of a Wee1 Kinase Degrader. Cell Chem Biol 27, 57–65.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby KT, and Sporn MB (2012). Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacological Reviews 64, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen H, Ma L, He Z, Wang D, Liu Y, Lin Q, Zhang T, Gray N, Kaniskan HU, et al. (2020). Light-induced control of protein destruction by opto-PROTAC. Sci Adv 6, eaay5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Qian Y., Altieri M., Dong H., Wang J., Raina K., Hines J., Winkler JD., Crew AP., Coleman K., et al. (2015). Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem Biol 22, 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y, You Q, and Jiang Z.(2018). Discovery of a Keap1dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur J Med Chem 146, 251–259. [DOI] [PubMed] [Google Scholar]
- Maneiro MA, Forte N, Shchepinova MM, Kounde CS, Chudasama V, Baker JR, and Tate EW (2020). Antibody-PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chem Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares A, Miah AH, Smith IED, Rackham M, Thawani AR, Cryan J, Haile PA, Votta BJ, Beal AM, Capriotti C, et al. (2020). Extended pharmacodynamic responses observed upon PROTAC-mediated degradation of RIPK2. Commun Biol 3, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyskiela ME, Lu G, Ito T, Pagarigan B, Lu CC, Miller K, Fang W, Wang NY, Nguyen D, Houston J, et al. (2016). A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 535, 252–257. [DOI] [PubMed] [Google Scholar]
- Moon S, and Lee BH (2018). Chemically Induced Cellular Proteolysis: An Emerging Therapeutic Strategy for Undruggable Targets. Mol Cells 41, 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A.(2019). First targeted protein degrader hits the clinic. Nat Rev Drug Discov. [DOI] [PubMed] [Google Scholar]
- Murtuza A, Bulbul A, Shen JP, Keshavarzian P, Woodward BD, Lopez-Diaz FJ, Lippman SM, and Husain H.(2019). Novel Third-Generation EGFR Tyrosine Kinase Inhibitors and Strategies to Overcome Therapeutic Resistance in Lung Cancer. Cancer Research 79, 689. [DOI] [PubMed] [Google Scholar]
- Nagy Z, and Tora L.(2007). Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26, 5341–5357. [DOI] [PubMed] [Google Scholar]
- Naito M, Ohoka N, and Shibata N.(2019). SNIPERs—Hijacking IAP activity to induce protein degradation. Drug Discovery Today: Technologies 31, 35–42. [DOI] [PubMed] [Google Scholar]
- Naro Y, Darrah K, and Deiters A.(2020). Optical Control of Small Molecule-Induced Protein Degradation. Journal of the American Chemical Society 142, 2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neklesa TK, Winkler JD, and Crews CM (2017). Targeted protein degradation by PROTACs. Pharmacol Ther 174, 138–144. [DOI] [PubMed] [Google Scholar]
- Nguyen KM, and Busino L.(2020). Targeting the E3 ubiquitin ligases DCAF15 and cereblon for cancer therapy. Seminars in Cancer Biology. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Lee JE, Sweredoski MJ, Yang S-J, Jeon S-J, Harrison JS, Yim J-H, Lee SG, Handa H, Kuhlman B, et al. (2016). Glutamine Triggers Acetylation-Dependent Degradation of Glutamine Synthetase via the Thalidomide Receptor Cereblon. Molecular cell 61, 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N, Nagai K, Hattori T, Okuhira K, Shibata N, Cho N, and Naito M.(2014). Cancer cell death induced by novel small molecules degrading the TACC3 protein via the ubiquitin–proteasome pathway. Cell Death & Disease 5, e1513–e1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N, Okuhira K, Ito M, Nagai K, Shibata N, Hattori T, Ujikawa O, Shimokawa K, Sano O, Koyama R, et al. (2017). In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs). J Biol Chem 292, 4556–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottis P, and Crews CM (2017). Proteolysis-Targeting Chimeras: Induced Protein Degradation as a Therapeutic Strategy. ACS Chem Biol 12, 892–898. [DOI] [PubMed] [Google Scholar]
- Ottis P, Palladino C, Thienger P, Britschgi A, Heichinger C, Berrera M, Julien-Laferriere A, Roudnicky F, Kam-Thong T, Bischoff JR, et al. (2019). Cellular Resistance Mechanisms to Targeted Protein Degradation Converge Toward Impairment of the Engaged Ubiquitin Transfer Pathway. ACS Chem Biol 14, 2215–2223. [DOI] [PubMed] [Google Scholar]
- Ottis P, Toure M, Cromm PM, Ko E, Gustafson JL, and Crews CM (2017). Assessing Different E3 Ligases for Small Molecule Induced Protein Ubiquitination and Degradation. ACS Chem Biol 12, 25702578. [DOI] [PubMed] [Google Scholar]
- Pfaff P, Samarasinghe KTG, Crews CM, and Carreira EM (2019). Reversible Spatiotemporal Control of Induced Protein Degradation by Bistable PhotoPROTACs. ACS Cent Sci 5, 1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popow J, Arnhof H, Bader G, Berger H, Ciulli A, Covini D, Dank C, Gmaschitz T, Greb P, Karolyi-Özguer J, et al. (2019). Highly Selective PTK2 Proteolysis Targeting Chimeras to Probe Focal Adhesion Kinase Scaffolding Functions. Journal of Medicinal Chemistry 62, 2508–2520. [DOI] [PubMed] [Google Scholar]
- Potjewyd F., Turner AW., Beri J., Rectenwald JM., Norris-Drouin JL., Cholensky SH., Margolis DM., Pearce KH., Herring LE., and James LI. (2020). Degradation of Polycomb Repressive Complex 2 with an EED-Targeted Bivalent Chemical Degrader. Cell Chem Biol 27, 47–56.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CE, Gao Y, Tan L, Donovan KA, Nowak RP, Loehr A, Bahcall M, Fischer ES, Jänne PA, George RE, et al. (2018). Chemically Induced Degradation of Anaplastic Lymphoma Kinase (ALK). Journal of Medicinal Chemistry 61, 4249–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Zhao K, Gu J, Huang Y, Wang Y, Zhang H, Zhang M, Zhang J, Yu Z, Li L, et al. (2017). An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat Chem Biol 13, 381–388. [DOI] [PubMed] [Google Scholar]
- Raina K, and Crews CM (2017). Targeted protein knockdown using small molecule degraders. Curr Opin Chem Biol 39, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AMK, Wang J, Chen X, Dong H, Siu K, et al. (2016). PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proceedings of the National Academy of Sciences, 201521738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Bendjennat M, Kour S, King HM, Kizhake S, Zahid M, and Natarajan A.(2019). Selective degradation of CDK6 by a palbociclib based PROTAC. Bioorganic & Medicinal Chemistry Letters 29, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renicke C, Schuster D, Usherenko S, Essen L-O, and Taxis C.(2013). A LOV2 Domain-Based Optogenetic Tool to Control Protein Degradation and Cellular Function. Chemistry & Biology 20, 619626. [DOI] [PubMed] [Google Scholar]
- Reynders M, Matsuura BS, Berouti M, Simoneschi D, Marzio A, Pagano M, and Trauner D.(2020). PHOTACs enable optical control of protein degradation. Sci Adv 6, eaay5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MJ, Winkler S, Hughes SJ, Whitworth C, Galant M, Farnaby W, Rumpel K, and Ciulli A.(2019). SPR-Measured Dissociation Kinetics of PROTAC Ternary Complexes Influence Target Degradation Rate. ACS Chemical Biology 14, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, and Deshaies RJ (2001). Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A 98, 8554–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM, and Deshaies RJ (2003). Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics 2, 1350–1358. [DOI] [PubMed] [Google Scholar]
- Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H, Jin M, McDonnell DP, Crew AP, Neklesa TK, et al. (2018). Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol 1, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami J, and Crews CM (2017). Waste disposal-An attractive strategy for cancer therapy. Science 355, 1163–1167. [DOI] [PubMed] [Google Scholar]
- Schapira M, Calabrese MF, Bullock AN, and Crews CM (2019). Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov 18, 949–963. [DOI] [PubMed] [Google Scholar]
- Schiedel M, Herp D, Hammelmann S, Swyter S, Lehotzky A, Robaa D, Olah J, Ovadi J, Sippl W, and Jung M.(2018). Chemically Induced Degradation of Sirtuin 2 (Sirt2) by a Proteolysis Targeting Chimera (PROTAC) Based on Sirtuin Rearranging Ligands (SirReals). J Med Chem 61, 482–491. [DOI] [PubMed] [Google Scholar]
- Schneekloth AR, Pucheault M, Tae HS, and Crews CM (2008). Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Lett 18, 5904–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneekloth JS Jr., Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, and Crews CM (2004). Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc 126, 3748–3754. [DOI] [PubMed] [Google Scholar]
- Sievers QL., Petzold G., Bunker RD., Renneville A., Slabicki M., Liddicoat BJ., Abdulrahman W., Mikkelsen T., Ebert BL., and Thoma NH. (2018). Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JM, Silva E, and Reis RL (2019a). Light-triggered release of photocaged therapeutics - Where are we now? Journal of controlled release : official journal of the Controlled Release Society 298, 154176. [DOI] [PubMed] [Google Scholar]
- Silva MC, Ferguson FM, Cai Q, Donovan KA, Nandi G, Patnaik D, Zhang T, Huang HT, Lucente DE, Dickerson BC, et al. (2019b). Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley JP, Adams GE, Millard CJ, Song Y, Norris JKS, Schwabe JWR, Cowley SM, and Hodgkinson JT (2020). PROTAC-mediated degradation of class I histone deacetylase enzymes in corepressor complexes. Chem Commun (Camb) 56, 4476–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BE, Wang SL, Jaime-Figueroa S, Harbin A, Wang J, Hamman BD, and Crews CM (2019). Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat Commun 10, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, and Gribble GW (2011). New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 74, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradlin JN, Hu X, Ward CC, Brittain SM, Jones MD, Ou L, To M, Proudfoot A, Ornelas E, Woldegiorgis M, et al. (2019). Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat Chem Biol 15, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Pierre R, and Kadoch C.(2017). Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Current Opinion in Genetics & Development 42, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, and Hay RT (2008). RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol 10, 538–546. [DOI] [PubMed] [Google Scholar]
- Ting TC, Goralski M, Klein K, Wang B, Kim J, Xie Y, and Nijhawan D.(2019). Aryl Sulfonamides Degrade RBM39 and RBM23 by Recruitment to CRL4-DCAF15. Cell Rep 29, 1499–1510.e1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinworth CP, Lithgow H, Dittus L, Bassi ZI, Hughes SE, Muelbaier M, Dai H, Smith IED, Kerr WJ, Burley GA, et al. (2019). PROTAC-Mediated Degradation of Bruton’s Tyrosine Kinase Is Inhibited by Covalent Binding. ACS Chem Biol 14, 342–347. [DOI] [PubMed] [Google Scholar]
- Tong B, Luo M, Xie Y, Spradlin J, Tallarico JA, McKenna JM, Schirle M, Maimone TJ, and Nomura D.(2020a). Targeted Protein Degradation via a Covalent Reversible Degrader Based on Bardoxolone (ChemRxiv) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong B, Spradlin JN, Novaes LFT, Zhang E, Hu X, Moeller M, Brittain SM, McGregor LM, McKenna JM, Tallarico JA, et al. (2020b). A Nimbolide-Based Kinase Degrader Preferentially Degrades Oncogenic BCR-ABL. ACS Chemical Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, et al. (2006). Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A 103, 1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovell H, Testa A, Zhou H, Shpiro N, Crafter C, Ciulli A, and Alessi DR (2019). Design and Characterization of SGK3-PROTAC1, an Isoform Specific SGK3 Kinase PROTAC Degrader. ACS Chem Biol 14, 2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T., Minoshima Y., Sagane K., Sugi NH., Mitsuhashi KO., Yamamoto N., Kamiyama H., Takahashi K., Kotake Y., Uesugi M., et al. (2017). Selective degradation of splicing factor CAPERalpha by anticancer sulfonamides. Nat Chem Biol 13, 675–680. [DOI] [PubMed] [Google Scholar]
- Verma R, Mohl D, and Deshaies RJ (2020). Harnessing the Power of Proteolysis for Targeted Protein Inactivation. Mol Cell 77, 446–460. [DOI] [PubMed] [Google Scholar]
- Vogelmann A, Robaa D, Sippl W, and Jung M.(2020). Proteolysis targeting chimeras (PROTACs) for epigenetics research. Curr Opin Chem Biol 57, 8–16. [DOI] [PubMed] [Google Scholar]
- Wang S, Han L, Han J, Li P, Ding Q, Zhang QJ, Liu ZP, Chen C, and Yu Y.(2019a). Uncoupling of PARP1 trapping and inhibition using selective PARP1 degradation. Nat Chem Biol 15, 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhao Y, Aguilar A, Bernard D, and Yang C-Y (2017). Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harbor perspectives in medicine 7, a026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, He N, Guo Z, Niu C, Song T, Guo Y, Cao K, Wang A, Zhu J, Zhang X, et al. (2019b). Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands. J Med Chem 62, 8152–8163. [DOI] [PubMed] [Google Scholar]
- Ward CC, Kleinman JI, Brittain SM, Lee PS, Chung CYS, Kim K, Petri Y, Thomas JR, Tallarico JA, McKenna JM, et al. (2019). Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. ACS Chemical Biology 14, 2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MBD, Bachovchin DA, Mowen K, Baker D, and Cravatt BF (2010). Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, and Bradner JE (2015). DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yang K, Zhang Z, Leisten ED, Li Z, Xie H, Liu J, Smith KA, Novakova Z, Barinka C, et al. (2019). Development of Multifunctional Histone Deacetylase 6 Degraders with Potent Antimyeloma Activity. J Med Chem 62, 7042–7057. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Li F, Babault N, Dong A, Zeng H, Wu H, Chen X, Arrowsmith CH, Brown PJ, Liu J, et al. (2017). Discovery of Potent and Selective Inhibitors for G9a-Like Protein (GLP) Lysine Methyltransferase. Journal of Medicinal Chemistry 60, 1876–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Chen J, Liu L, Zhou D, Zuo Y, Fu T, and Pan Z.(2020). Protein degradation through covalent inhibitor-based PROTACs. Chem Commun (Camb) 56, 1521–1524. [DOI] [PubMed] [Google Scholar]
- Xue G, Wang K, Zhou D, Zhong H, and Pan Z.(2019). Light-Induced Protein Degradation with Photocaged PROTACs. J Am Chem Soc 141, 18370–18374. [DOI] [PubMed] [Google Scholar]
- Yang C, Cao P, Gao Y, Wu M, Lin Y, Tian Y, and Yuan W.(2016a). Differential expression of p38 MAPK α, β, γ, δ isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Scientific reports 6, 22182–22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Cao P, Gao Y, Wu M, Lin Y, Tian Y, and Yuan W.(2016b). Differential expression of p38 MAPK α, β, γ, δ isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Scientific Reports 6, 22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Song Y, Xie H, Wu H, Wu YT, Leisten ED, and Tang W.(2018). Development of the first small molecule histone deacetylase 6 (HDAC6) degraders. Bioorg Med Chem Lett 28, 2493–2497. [DOI] [PubMed] [Google Scholar]
- Yang K, Zhao Y, Nie X, Wu H, Wang B, Almodovar-Rivera CM, Xie H, and Tang W.(2020). A CellBased Target Engagement Assay for the Identification of Cereblon E3 Ubiquitin Ligase Ligands and Their Application in HDAC6 Degraders. Cell Chem Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengerle M, Chan K-H, and Ciulli A.(2015). Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chemical Biology 10, 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, and Hannink M.(2004). Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24, 10941–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Riley-Gillis B, Vijay P, and Shen Y.(2019a). Acquired Resistance to BET-PROTACs(Proteolysis Targeting Chimeras) Caused by Genomic Alterations in Core Components of E3 ligase Complexes. Molecular Cancer Therapeutics, molcanther.1129.2018. [DOI] [PubMed] [Google Scholar]
- Zhang T., Hatcher JM., Teng M., Gray NS., and Kostic M. (2019b). Recent Advances in Selective and Irreversible Covalent Ligand Development and Validation. Cell Chem Biol 26, 1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Crowley VM, Wucherpfennig TG, Dix MM, and Cravatt BF (2019c). Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. Nat Chem Biol 15, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, He Y, Zhang P, Budamagunta V, Lv D, Thummuri D, Yang Y, Pei J, Yuan Y, Zhou D, et al. (2020a). Discovery of IAP-recruiting BCL-XL PROTACs as potent degraders across multiple cancer cell lines. Eur J Med Chem 199, 112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Thummuri D, Liu X, Hu W, Zhang P, Khan S, Yuan Y, Zhou D, and Zheng G.(2020b). Discovery of PROTAC BCL-XL degraders as potent anticancer agents with low on-target platelet toxicity. Eur J Med Chem 192, 112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Chen L, Cao C, Yu J, Luo X, Zhou P, Zhao L, Du W, Cheng J, Xie Y, et al. (2020). Development of selective mono or dual PROTAC degrader probe of CDK isoforms. Eur J Med Chem 187, 111952. [DOI] [PubMed] [Google Scholar]
- Zoppi V, Hughes SJ, Maniaci C, Testa A, Gmaschitz T, Wieshofer C, Koegl M, Riching KM, Daniels DL, Spallarossa A, et al. (2019). Iterative Design and Optimization of Initially Inactive Proteolysis Targeting Chimeras (PROTACs) Identify VZ185 as a Potent, Fast, and Selective von Hippel-Lindau (VHL) Based Dual Degrader Probe of BRD9 and BRD7. J Med Chem 62, 699–726. [DOI] [PMC free article] [PubMed] [Google Scholar]