Abstract
Objective
Although symptom relief is a critical aspect for successful drug development in Sjögren's disease, patient experiences with Sjögren's‐related symptoms are understudied. Our objective was to determine how pain, dryness, and fatigue, the cardinal symptoms of Sjögren's disease, drive cluster phenotypes.
Methods
We used data from the Sjögren's International Collaborative Clinical Alliance (SICCA) Registry and a Sjögren's Foundation survey. We performed hierarchical clustering of symptoms by levels of dryness, fatigue, and pain. Using international and US cohorts, we performed multiple logistic regression analysis to compare the clusters, which included comparisons of differences in symptoms, quality of life (QoL), medication use, and systemic manifestations.
Results
Four similar clusters were identified among 1,454 SICCA registrants and 2,920 Sjögren's Foundation survey participants: 1) low symptom burden in all categories (LSB); 2) dry with low pain and low fatigue (DLP); 3) dry with high pain and low to moderate fatigue (DHP); and 4) high symptom burden in all categories (HSB). Distribution of SICCA registrants matching the symptom profile for each cluster was 10% in the LSB cluster, 30% in the DLP cluster, 23% in the DHP cluster, and 37% in the HSB cluster. Distribution of survey participants matching the symptom profile for each cluster was 23% in the LSB cluster, 14% in the DLP cluster, 21% in the DHP cluster, and 42% in the HSB cluster. Individuals in the HSB cluster had more total symptoms and lower QoL but lower disease severity than those in the other clusters. Despite having milder disease as measured by laboratory tests and organ involvement, individuals in the HSB cluster received immunomodulatory treatment most often.
Conclusion
We identified 4 symptom‐based Sjögren's clusters and showed that symptom burden and immunomodulatory medication use do not correlate with Sjögren's end‐organ or laboratory abnormalities. Findings highlight a discordance between objective measures and treatments and offer updates to proposed symptom‐based clustering approaches.
INTRODUCTION
Sjögren's disease, a systemic autoimmune disease, is associated with increased health care costs, increased morbidity, and reduced quality of life (QoL) compared with these measures in people without the disease (1). Sjögren's disease has a heterogeneous phenotype ranging from isolated dryness to life‐threatening systemic organ involvement. The heterogeneity of Sjögren's disease creates unique experiences for each patient and complicates the choice of effective treatment. For example, depression and fatigue are common debilitating symptoms that reduce QoL, yet these symptoms do not respond to traditional immunosuppression (1, 2). Additionally, Sjögren's symptoms do not always parallel clinical signs. For example, symptoms of dryness do not necessarily correlate with objective tear or salivary flow measurements (3). In other autoimmune diseases, such as systemic lupus erythematosus, discordance between the severity of symptoms reported by the patient and physician assessment of disease severity has been posited to reduce patient satisfaction (1, 4, 5). These issues have made the identification of effective therapies in clinical trials challenging. Emphasis has therefore shifted toward Sjögren's treatments that are tailored to specific relevant subsets of patients (6). A critical first step of tailored therapy is to define symptom‐based patient clusters.
Recently, a UK‐based study used symptoms of pain, dryness, fatigue, anxiety, and depression to generate patient clusters. In their analyses of 608 patients with Sjögren's disease from the UK Primary Sjögren's Syndrome Registry, Tarn and colleagues defined 4 symptom‐based clusters with unique European League of Associations for Rheumatology (EULAR) Sjögren's Syndrome Disease Activity Index and laboratory profiles (7). Their findings were validated in 2 other European populations: the French Assessment of Systemic Signs and Evolution of Sjögren's Syndrome cohort and the Norwegian Stavanger cohort. Notably, however, 2 of the 5 symptoms included to generate clusters, anxiety and depression, are not cardinal symptoms in Sjögren's disease. In their retrospective analyses of outcomes of the JOQUER trial with hydroxychloroquine and the TRACTISS trial with rituximab, Tarn et al found considerably different responses to these therapies by cluster (8, 9). The cardinal symptoms caused by Sjögren's disease, however, are dryness, pain, and fatigue, regardless of anxiety and depression presence in a subgroup, which suggests the need for a more disease‐focused approach to clustering.
Our objective was to leverage a large international population to determine the clusters of Sjögren's disease based on the cardinal symptoms of dryness, pain, and fatigue. We compared differences in symptoms, QoL, medication use, and disease‐specific systemic manifestations between the symptom‐based clusters. We aimed to advance the understanding of unique Sjögren's disease phenotypes to 1) enhance mechanistic understanding of the pathogenesis driving distinct Sjögren's disease phenotypes, 2) improve symptom management through tailored therapy, 3) inform the identification of subgroups for clinical trial analyses, and 4) eventually, harmonize patient–provider expectations.
PATIENTS AND METHODS
We obtained data for this analysis from 2 sources: 1) the Sjögren's International Collaborative Clinical Alliance (SICCA) Registry, and 2) a Sjögren's Foundation survey.
SICCA Registry
The SICCA Registry is a National Institutes of Health–funded registry of individuals with suspected or known Sjögren's disease from 9 international research institutions from 2003 to 2012 (10). Participants who were age 21 years or older were enrolled in the registry if they had any of the following: repeated finding of tooth decay or cavities without other risk factors, a known diagnosis of Sjögren's disease, salivary gland enlargement, or abnormal findings on serology (anti‐SSA antibody or anti‐SSB antibody, antinuclear antibody, or rheumatoid factor [RF]). All registrants completed a standardized visit composed of an interview and questionnaires, physical examination, blood, tear and saliva collections, and labial salivary gland biopsy. Further registry details and enrollment procedures are described on the SICCA web page at https://sicca-online.ucsf.edu and in prior publications (11, 12, 13). Sjögren's disease was defined by the 2016 American College of Rheumatology (ACR)/EULAR criteria (14).
Data obtained from the SICCA Registry included depression severity, measured with the Patient Health Questionnaire 9 (PHQ‐9; scored 0–27, with higher scores indicating greater severity), and health‐related QoL, measured with the Short Form 12 (SF‐12; with lower scores indicating greater severity) (15). The SF‐12 is divided into mental and physical components. The mental component (scored 0–100) focuses on depression, anxiety, accomplishments, socialization, and carelessness. The physical component (scored 0–100) focuses on work limitations due to pain, work limitations due to physical issues, and limitations in climbing stairs. Of the 12 total questions in the SF‐12 health survey, 5 relate to mental health, 6 relate to physical health, and 1 relates to both.
Sjögren's Foundation survey
The content of the Sjögren's Foundation survey was developed in 2016 as a collaborative effort with the Harris Poll, a social science market research company, the Sjögren's Foundation, Sjögren's disease providers and experts, and patients with Sjögren's disease (16, 17). A total of 2,961 adults who self‐reported as having Sjögren's disease based on a physician's diagnosis completed the survey. The survey provided documentation of comprehensive details on the subjective experiences of patients with Sjögren's disease, which enriched our understanding of patient experiences within each cluster.
The survey contained 7 sections: 1) “patient profile” (Sjögren's diagnosis, general health, and past medical histories); 2) “severity” (frequency and impact of symptoms); 3) “emotional and physical well‐being” (effects of Sjögren's disease on daily emotional and physical experiences); 4) “effect on quality of life” (the effect of Sjögren's disease on QoL); 5) “treatment” (treatments or medications for Sjögren's disease); 6) “cost of disease” (costs and effects on career as a result of Sjögren's disease); and 7) “background information” (sociodemographic characteristics). Respondents recorded 40 symptoms by frequency of experience from never to daily. Bivariate comparisons considered each symptom present if the respondent indicated that it occurred at least weekly.
Symptom‐based cluster generation and statistical analysis
We generated separate symptom‐based clusters for each of the 2 samples. To generate hierarchical clusters in the SICCA Registry sample, we examined self‐reported 1) dryness using a weighted composite score of responses to 5 questions, 2) pain on a 5‐point Likert scale from “not at all” to “extremely,” and 3) fatigue on a 4‐point Likert scale from “not at all” to “nearly every day” (18).
Because we did not have a validated marker for dryness severity, we measured the burden of dryness with a dryness composite score based on 5 questions: 1) “do your eyes feel dry?” (yes or no), 2) “how often do you use artificial tears?” (≤3 times/day or >3 times/day), 3) “during the last week have you experienced any of the following symptoms with your eyes: gritty or scratchy sensation?” (5‐point Likert scale from none of the time to all the time), 4) “does your mouth feel dry?” (yes or no), and 5) “do you need to sip liquids to swallow dry foods?” (yes or no). Questions 2, 3, and 5 had been previously validated in a study that established the ability of these questions to correctly classify patients with Sjögren's disease versus controls and have been included in the 2002 (subjective components) and 2016 classification criteria for Sjögren's disease (entry criteria) (14, 18, 19). The other 2 questions (questions 1 and 4) are similar to other questions in the previously validated criteria but did not include the time elements (e.g., for >3 months). To ensure equal weight for all questions, we multiplied all the binary questions by 100 and the 5‐point Likert scale by 20. We then divided the sum by the number of completed questions to yield a final dryness composite score on a scale of 0–100.
Fatigue was evaluated with the following question: “Over the last two weeks how often have you felt bothered by the following problem: feeling tired or having little energy?” Pain was evaluated with the following question: “How much did pain interfere with your normal work?” We stratified clusters by levels of dryness, pain, and fatigue but not by anxiety and depression as previously reported (7). Dryness, pain, and fatigue are the main symptoms experienced by patients with Sjögren's disease as identified from patient interviews and the Profile of Fatigue and Discomfort–Sicca Symptoms Inventory (20, 21).
Among Sjögren's Foundation survey participants, we excluded participants for whom age and biologic sex were not reported. We performed unsupervised hierarchical clustering of symptoms with Ward's minimum variance method (22) to a priori identify 4 clusters to assess phenotypic similarity to the analogous 4 groups studied by Tarn et al based on self‐reported severity of pain (visual analog scale [VAS] 0–10), fatigue (VAS 0–10), and dryness (VAS 0–10).
We compared descriptive statistics for demographic features, symptom frequency, QoL, medication use, systemic manifestations, laboratory values, and histopathologic assessment of the labial salivary glands among the 4 symptom‐based clusters. We used one‐way analysis of variance or chi‐square tests to conduct hypothesis testing for differences between clusters. We used multiple logistic regression for analyses of categorical variables and linear regression for analyses of continuous variables, controlling for age, sex, race, ethnicity, education, and recruitment site for the SICCA Registry sample and age, sex, race, and disability for the Sjögren's Foundation survey sample. Statistical analyses were performed using JMP Pro statistical software, version 15.
RESULTS
SICCA cluster analysis
We identified 1,541 adults fulfilling the 2016 ACR/EULAR criteria for Sjögren's disease within the SICCA Registry. Three were excluded for missing data on age or sex, and 84 were excluded for missing data on clustering criteria. We thus included 1,454 adults from the SICCA Registry in the cluster analysis.
The 1,454 individuals in the SICCA Registry sample with complete data on the 3 cardinal symptoms had a mean age of 52 years, were predominantly women (94%), and were mostly White (50%), followed by Asian (35%) and other races (15%) (Table 1). The analysis yielded 4 clusters (Figure 1A). Clusters were characterized by low symptom frequency/severity burden of dryness and fatigue with rare pain (LSB; 10% prevalence), dry with low pain and low fatigue (dry low pain [DLP]; 30%), dry with high pain and low to moderate fatigue (dry high pain [DHP]; 23%), and high symptom frequency/severity burden in all categories (HSB; 37%).
Table 1.
Baseline demographic characteristics of patients with Sjögren's disease in the SICCA Registry, in total and according to symptom‐based clusters*
| All | LSB | DLP | DHP | HSB | Adjusted P † | |
|---|---|---|---|---|---|---|
| (n = 1,454) | (n = 146) | (n = 432) | (n = 336) | (n = 540) | ||
| Age, mean ± SD years | 52 (13) | 47 (15) | 54 (13) | 54 (14) | 52 (13) | <0.0001 |
| Female patient | 1,368 (94) | 133 (91) | 400 (93) | 314 (93) | 521 (96) | 0.02 |
| Race | <0.0001 | |||||
| White | 726 (50) | 38 (26) | 208 (48) | 163 (49) | 317 (59) | |
| Asian | 515 (35) | 90 (62) | 170 (39) | 131 (39) | 124 (23) | |
| Other‡ | 212 (15) | 18 (12) | 54 (13) | 42 (13) | 99 (18) | |
| Hispanic ethnicity | 170 (12) | 14 (10) | 50 (12) | 35 (10) | 71 (13) | 0.51 |
| Education | 0.03 | |||||
| Primary | 178 (12) | 14 (10) | 59 (14) | 49 (15) | 56 (10) | |
| High school | 409 (28) | 49 (34) | 116 (27) | 105 (31) | 139 (26) | |
| College/university | 857 (59) | 82 (56) | 256 (59) | 179 (53) | 340 (63) | |
| None | 10 (1) | 1 (1) | 1 (0) | 3 (1) | 5 (1) | |
| Employment | <0.0001 | |||||
| Full‐time | 533 (37) | 70 (48) | 186 (43) | 100 (30) | 177 (33) | |
| Part‐time | 200 (14) | 11 (8) | 62 (14) | 46 (14) | 81 (15) | |
| Homemaker | 193 (13) | 21 (14) | 50 (12) | 49 (15) | 73 (14) | |
| Retired | 318 (22) | 29 (20) | 107 (25) | 98 (29) | 84 (16) | |
| Student | 23 (2) | 4 (3) | 7 (2) | 3 (1) | 9 (2) | |
| Not working | 186 (13) | 11 (8) | 20 (5) | 40 (12) | 115 (21) | |
| Tobacco use | ||||||
| Current | 76 (5) | 10 (7) | 12 (3) | 21 (6) | 33 (6) | 0.04 |
| Ever | 435 (32) | 33 (23) | 127 (29) | 99 (29) | 176 (33) | 0.1 |
| Recruitment site | <0.0001 | |||||
| JHU | 119 (8) | 9 (6) | 26 (6) | 21 (6) | 63 (12) | |
| UPenn | 98 (7) | 6 (4) | 18 (4) | 18 (5) | 56 (10) | |
| UCSF | 283 (20) | 7 (5) | 77 (18) | 67 (20) | 132 (24) | |
| Argentina | 165 (11) | 15 (10) | 50 (12) | 34 (10) | 66 (12) | |
| China | 239 (16) | 60 (41) | 75 (17) | 68 (20) | 36 (7) | |
| Denmark | 202 (14) | 14 (10) | 67 (16) | 51 (15) | 70 (13) | |
| Japan | 205 (14) | 22 (15) | 77 (18) | 46 (14) | 60 (11) | |
| UK | 143 (10) | 13 (9) | 42 (10) | 31 (9) | 57 (11) |
Except where indicated otherwise, values are the number (%) of Sjögren's International Collaborative Clinical Alliance (SICCA) Registry patients. Missing data were as follows: race and ethnicity (n = 1 each), employment (n = 1), and tobacco use ever (n = 76). LSB = low symptom burden; DLP = dry, low pain; DHP = dry, high pain; HSB = high symptom burden; JHU = Johns Hopkins University; UPenn = University of Pennsylvania; UCSF = University of California, San Francisco.
Adjusted for age, sex, race, and disability.
Other race indicates all non‐White and non‐Asian races.
Figure 1.
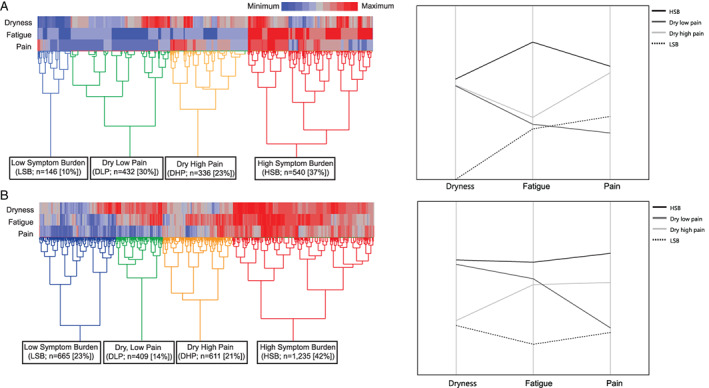
Heatmaps showing hierarchical clustering of Sjögren's disease symptoms according to severity level. A, Sjögren's International Collaborative Clinical Alliance (SICCA) Registry sample clusters generated by unsupervised hiararchical clustering based on evaluation of the following symptoms: oral and ocular dryness according to a weighted composite score of 5 items (presence of dry mouth, need sips of liquid to swallow food, presence of dry eye, presence of a gritty sensation in the eyes, and use of tear substitutes), fatigue (on a 4‐point Likert scale), and pain (on a 5‐point Likert scale). B, Sjögren's Foundation sample clusters generated by unsupervised hierarchical clustering based on evaluation of the following symptoms: oral and ocular dryness (on a 0–10‐mm visual analog scale [VAS]), fatigue (on a 0–10‐mm VAS), and pain (on a 0–10‐mm VAS).
Symptoms differed significantly among the symptom‐based clusters in the SICCA Registry. For example, dry mouth (“does your mouth feel dry?”) occurred in 96–99% of patients in the HSB, DLP, and DHP clusters but in only 35% of the patients in the LSB cluster (P < 0.0001). A similar pattern was shown for dry eye, which occurred in 87–94% of patients in the DLP, DHP, and HSB clusters but in only 21% of patients in the LSB cluster (P < 0.0001). There was an overarching pattern that non–sicca‐related symptoms predominated in the HSB cluster, followed by the DHP, DLP, and LSB clusters (Supplementary Table A, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42238).
The score for depression symptoms, as measured by the PHQ‐9, was higher (i.e., worse) in the HSB cluster (mean score 11.3) than in the DHP cluster (mean score 4.5), DLP cluster (mean score 2.9), and LSB cluster (mean score 2.2) (each P < 0.0001) (Figure 2A). Health‐related QoL, as measured by the SF‐12, also differed between the clusters (Figure 2A). The score for the physical components was lower (i.e., worse) in the HSB cluster (mean SF‐12 physical component summary score 36) than in the DHP, DLP, and LSB clusters (mean scores of 41, 53, and 51, respectively) (P < 0.0001). The score for the mental components was also lower (i.e., worse) in the HSB cluster (mean SF‐12 mental component summary score 39) than in the DHP, DLP, and LSB clusters (mean scores of 44, 44, and 46, respectively) (P < 0.0001).
Figure 2.
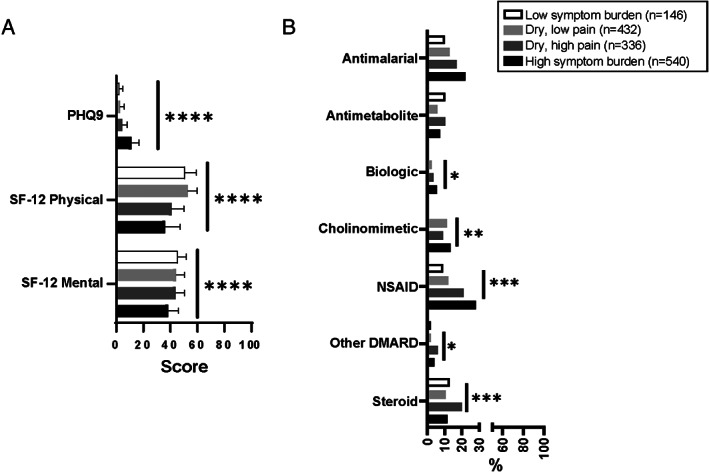
Depression, quality of life, and medication use in patients from the Sjögren's International Collaborative Clinical Alliance Registry (n = 1,454) categorized according to Sjögren's disease symptom–based clusters. A, Depression, measured by the Patient Health Questionnaire 9 (PHQ‐9), and health–related quality of life, measured by the physical and mental components of the Short Form 12 (SF‐12). Bars show the mean. B, Frequency of medication use. * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001, by one‐way analysis of variance or chi‐square test. Biologic = tumor necrosis factor inhibitor or anti‐CD20 antibody; NSAID = nonsteroidal antiinflammatory drug; DMARD = disease‐modifying antirheumatic drug.
Generally, patients in the HSB cluster more frequently took treatments such as cholinomimetics (14%), nonsteroidal antiinflammatory drugs (NSAIDs) (28%), and biologics (5%) than patients in the other clusters. When we compared the clusters, steroids (20%) and other disease‐modifying antirheumatic drugs (DMARDs) (6%) were the predominant treatment used by patients in the DHP cluster, and antimetabolites were the predominant treatment used by patients in the DHP and LSB clusters (each 10%) (Figure 2B).
Results from objective measurements of sicca symptoms in the mouth and eyes also differed between clusters in the SICCA Registry (Figure 3A). The unstimulated salivary flow was abnormal (≤5 ml/5 minutes) in 74% of the DLP cluster, in 70% of the HSB cluster, in 68% of the DHP cluster, and in 37% of the LSB cluster. The ocular surface staining score was abnormal (score ≥5) in 88% of the DLP cluster, whereas it was abnormal in only 74% of the HSB, 78% of the DHP, and 69% of the LSB clusters (P < 0.0001). Findings from the Schirmer's test (measured in mm/5 minutes) followed a similar pattern, in which patients in the DLP cluster had the greatest degree of sicca on objective ocular testing. Of 13 organ manifestations, 2 differed significantly between clusters, synovitis (which included metacarpophalangeal, wrist, or elbow synovitis) and primary biliary cholangitis (PBC), which were most common in the DHP cluster (11%) and DLP cluster (3%), respectively (Figure 3A).
Figure 3.
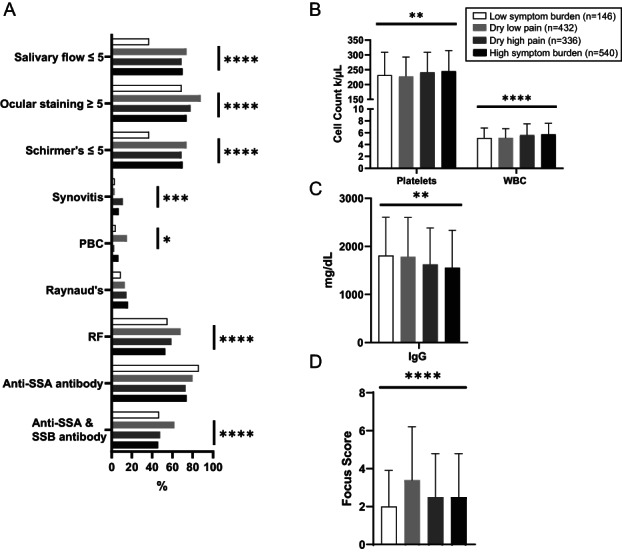
Oral and ocular dryness measurements, organ involvement, and abnormal laboratory and pathology results in patients from the Sjögren's International Collaborative Clinical Alliance Registry according to Sjögren's disease symptom–based clusters (n = 1,454). A, Frequency of patients with each laboratory or disease‐relevant feature according to dryness measurements, organ involvement, and abnormal laboratory results. B, Mean platelet and white blood cell (WBC) counts. C, Mean IgG levels. D, Mean focus score. Bars show the mean. * = P ≤ 0.05; ** = P ≤ 0.01, *** = P ≤ 0.001, by one‐way analysis of variance or chi‐square test. PBC = primary biliary cholangitis; RF = rheumatoid factor.
Despite the fact that ≥73% of patients were positive for SSA antibodies in all 4 clusters, laboratory evaluations notably showed significantly different frequencies of combined anti‐SSA and anti‐SSB antibody presence (Figure 3A) and levels of platelets and white blood cells (Figure 3B). The DLP cluster had the lowest level of platelets (mean 227.3 × 103 cells/microliter), and the LSB and DLP clusters had the lowest level of white blood cells (mean 5.1 × 103 cells/microliter in both). The LSB and DLP clusters had higher levels of IgG than the HSB and DHP clusters (Figure 3C). The DLP cluster had the highest predominance of RF positivity (68%) compared with the LSB cluster (55%), the DHP cluster (59%), and the HSB cluster (53%) (P < 0.0001) (Supplementary Table B, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42238). The DLP cluster had the highest focus score of mononuclear cell infiltrates in the labial salivary glands (mean score 3.4) (P < 0.0001) versus the other clusters (Figure 3D). Other laboratory findings, including levels of hemoglobin, lymphocytes, and IgM, did not differ among clusters (Supplementary Table B).
Sjögren's Foundation cluster analysis
Of the 3,072 respondents who completed the Sjögren's Foundation survey, 111 participants were excluded for being younger than age 18 years (n = 41), lack of a diagnosis of Sjögren's disease from a health care professional (n = 68), or incomplete survey demographics (n = 2). A further 41 participants were excluded from hierarchical cluster analysis due to missing item responses needed to generate the clusters (e.g., dryness, pain, or fatigue metrics). We thus included 2,920 participants in the Sjögren's Foundation cohort analysis.
Most of the 2,920 participants were White (93%) and women (96%), and the mean age at the time of the survey was 65 years. Distribution of Sjögren's Foundation survey participants in the 4 identified symptom‐based clusters was as follows: 23% in the LSB cluster, 14% in the DLP cluster, 21% in the DHP cluster, and 42% in the HSB cluster. Age at diagnosis, sex, and race were similar among the 4 clusters, but statistically these values differed (Table 2).
Table 2.
Baseline demographic and clinical characteristics of patients with Sjögren's disease who responded to the Sjögren's Foundation survey, in total and according to symptom‐based clusters*
| All | LSB | DLP | DHP | HSB | Adjusted P † | |
|---|---|---|---|---|---|---|
| (n = 2,920) | (n = 665) | (n = 409) | (n = 611) | (n = 1,235) | ||
| Age, mean ± SD years | ||||||
| Age at diagnosis | 52 (13) | 52 (12) | 54 (13) | 53 (12) | 52 (12) | <0.0001 |
| Age at time of survey | 65 (12) | 64 (12) | 67 (11) | 64 (13) | 65 (12) | 0.01 |
| Female sex | 2,791 (96) | 624 (94) | 392 (96) | 581 (95) | 1,194 (97) | 0.04 |
| Race | 0.03 | |||||
| White | 2,697 (93) | 612 (92) | 392 (96) | 573 (94) | 1,120 (91) | |
| Other‡ | 218 (7) | 52 (8) | 17 (4) | 37 (6) | 112 (9) | |
| Employment | <0.0001 | |||||
| Full‐time | 564 (20) | 151 (24) | 76 (20) | 123 (21) | 214 (19) | |
| Part‐time | 176 (6) | 50 (8) | 29 (7) | 36 (6) | 61 (5) | |
| Retired | 1,379 (50) | 321 (50) | 225 (58) | 284 (46) | 549 (48) | |
| Other§ | 648 (23) | 117 (18) | 59 (15) | 146 (25) | 326 (28) | |
| Medical comorbidity | ||||||
| GERD | 1,327 (48) | 237 (39) | 155 (41) | 288 (49) | 647 (54) | <0.0001 |
| Hypertension | 911 (33) | 143 (24) | 126 (33) | 200 (34) | 442 (37) | <0.0001 |
| Irritable bowel syndrome | 902 (32) | 122 (20) | 90 (24) | 179 (30) | 511 (42) | <0.0001 |
| Fibromyalgia | 861 (31) | 90 (15) | 52 (14) | 190 (32) | 529 (44) | <0.0001 |
| Autoimmune thyroid disease | 669 (24) | 126 (21) | 92 (24) | 147 (25) | 304 (25) | 0.48 |
| Stroke | 118 (4) | 17 (3) | 15 (4) | 20 (3) | 66 (5) | 0.14 |
| Myocardial infarction | 59 (2) | 7 (1) | 6 (2) | 14 (2) | 32 (3) | 0.15 |
| Other rheumatology disease | ||||||
| Rheumatoid arthritis | 597 (21) | 92 (15) | 52 (14) | 109 (19) | 344 (28) | <0.0001 |
| Mixed connective tissue disease | 374 (13) | 38 (6) | 43 (11) | 80 (14) | 213 (18) | <0.0001 |
| SLE | 287 (10) | 42 (7) | 28 (7) | 53 (9) | 164 (14) | <0.01 |
| Scleroderma | 81 (3) | 17 (3) | 11 (3) | 13 (2) | 40 (3) | 0.77 |
| Sarcoidosis | 31 (1) | 4 (1) | 3 (1) | 7 (1) | 17 (1) | 0.55 |
Except where indicated otherwise, values are the number (%) of respondents to the Sjögren's Foundation survey. Missing data were as follows: race and ethnicity (n = 1 each), employment (n = 1), and tobacco use ever (n = 76). GERD = gastroesophageal reflux disease; SLE = systemic lupus erythematosus (see Table 1 for other definitions).
Adjusted for age, sex, race, and disability.
Other race indicates all non‐White races.
Other employment indicates self‐employed, not employed but looking for work, not employed and not looking for work, not employed and unable to work due to disability or illness, student, or stay‐at‐home spouse or partner.
In the Sjögren's Foundation sample, members of each cluster experienced their Sjögren's disease differently. As expected, the LSB cluster experienced the lowest frequency of Sjögren's disease‐related symptoms and the HSB cluster experienced the highest frequency (Supplementary Table C, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42238). A fewer number of individuals in the LSB cluster experienced dry mouth and eye (86% and 87%, respectively) compared with the number of individuals in the other clusters. Additionally, only 51% of the individuals in the LSB cluster experienced fatigue compared with 94% in the HSB cluster (P < 0.0001). Although fibromyalgia occurred in 31% of the overall cohort, it was most prevalent among members of the HSB cluster (44%) (P < 0.0001 versus the other clusters) (Table 2).
Members of the HSB had the highest use of current opioid analgesics (34%) (P < 0.0001 versus the other clusters) (Figures 4A and B; Supplementary Table D, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42238). More members of the HSB cluster took nonprescription (93%) and prescription eye drops (53%) compared with the other clusters. DMARD use was highest in the DHP and HSB clusters (48% in each). Antidepressant use was high in the HSB cluster; however, current antidepressant use was lower than “ever” antidepressant use in the HSB cluster (56% compared with 34%). Among the 410 participants with depression in the HSB cluster, 321 participants (78%) had ever taken antidepressants and 241 participants (59%) were currently taking antidepressants.
Figure 4.

Medication use and cost of health care among participants of the Sjögren's Foundation survey according to Sjögren's disease symptom–based clusters (n = 2,920). In the survey, current medication use and exercise (A) and ever use of medications and exercise (B) were assessed, along with cost (in dollars) of specific aspects of health care for Sjögren's disease (C). Bars show the mean. Eye drops include artificial tears or eye ointments (nonprescription); prescription painkillers include, e.g., oxycodone, hydrocodone, tramadol; disease‐modifying antirheumatic drugs (DMARDs) include, e.g., hydroxychlroqouinme, methotrexate, azathioprine, mycophenolate, leflunomide, sulfasalazine; nerve pain medications include, e.g., gabapentin, pregabalin; injectable/infusible biologics include, e.g., rituximab, abatacept, tumor necrosis factor inhibitors. * = P ≤ 0.05; ** = P ≤ 0.01, *** = P ≤ 0.001, by one‐way analysis of variance or chi‐square test. OTC = over‐the‐counter; apt. = appointment.
Members of the HSB cluster had higher mean annual costs of over‐the‐counter medications ($785), prescription medications ($1,595), and health care appointment/copay costs ($1,052) than members of the other clusters (Figure 4C; Supplementary Table E, available at https://onlinelibrary.wiley.com/doi/10.1002/art.42238). Members of the DLP cluster had the lowest health care appointment/copay costs ($721), whereas members of the LSB cluster had the lowest prescription costs ($998). Mean dental care cost was lowest in the DHP cluster ($1,333) and highest in the DLP cluster ($2,636).
DISCUSSION
Sjögren's disease is a remarkably heterogeneous disease that lacks any US Food and Drug Administration–approved disease‐modifying therapy. This lack is partly because of gaps in our understanding of the pathogenesis of Sjögren's disease and because there may be different responses to therapy among the specific disease subgroups. Grouping patients with Sjögren's disease into symptom‐based categories has the potential to reduce heterogeneity, inform the understanding of processes driving these various subtypes, and promote tailored therapies to symptom clusters. In contrast to a prior approach that included anxiety and depression (7), we generated clusters that were derived from the cardinal Sjögren's disease symptoms of generalized dryness, pain, and fatigue. When we analyzed the 4 symptom‐based clusters that we generated and replicated across 2 large cohorts, we observed a discordance between the experience, disease severity, and treatment of Sjögren's disease, thus framing new opportunities for pathogenic insights, treatment, and approaches to clinical trials.
Our analyses of the SICCA Registry sample resulted in 4 clusters based on symptom severity: 1) a cluster of participants with low dryness and fatigue and rare pain (LSB cluster); 2) a cluster of participants with dryness and low pain and low fatigue (DLP cluster); 3) a cluster of participants with dryness and moderate to high pain and low to moderate fatigue (DHP cluster); and 4) a cluster of participants with high dryness, fatigue, and pain (HSB cluster). Notably, participants in the LSB cluster had infrequent dryness and extraglandular symptoms or organ involvement but had low white blood cell counts, higher levels of IgG, and low focus scores. Participants in the DLP cluster had dryness in the mouth and eyes based on objective measurements, had the highest frequency of PBC, and the most laboratory abnormalities, including anti‐SSA and anti‐SSB antibody positivity, RF, low blood cell counts, higher levels of IgG, and higher focus scores. However, participants in the DLP cluster took antimalarials, antimetabolites, biologics, and steroids less often than patients in the DHP and HSB cluster groups. Participants in the DHP cluster had a higher frequency of synovitis (11% frequency) and extraglandular symptoms than other clusters, but the frequency was still less than the frequency for patients in the HSB cluster. Participants in the HSB cluster had the highest overall symptom burden, level of depression, and impaired QoL, although they had less severe dryness, less frequent organ involvement, and fewer laboratory abnormalities. However, participants in the HSB cluster frequently received immune‐modulating medications.
We complemented the data generated from the SICCA Registry sample with data from the Sjögren's Foundation survey. We again focused on the same cardinal symptoms of pain, dryness, and fatigue, but data from the survey provided us more granular insight into patient experiences and costs. Although symptom‐based clusters between the SICCA Registry sample and the Sjögren's Foundation sample were similar overall, members of the DHP and DLP clusters from the Sjögren's Foundation sample appeared to have more fatigue. In addition, members of the DLP cluster from the Sjögren's Foundation sample reported greater burden of dryness than members of the similar cluster from the SICCA Registry sample. These differences might be attributed to the community‐based nature of the Sjögren's Foundation, where people with symptoms seek support for their disease. In contrast, the SICCA Registry may be enriched with patients referred by their physicians for a comprehensive evaluation, including biopsy of the labial salivary gland. Despite the different sources of members in the 2 cohorts, we identified similar clusters in both, strengthening our conclusions.
Symptom burden did not correlate well with traditional disease severity markers, such as abnormalities in laboratory results and extraglandular involvement, which are associated with outcomes like lymphoma and mortality. For example, patients in the DLP cluster had low symptom burden, yet patients in the cluster had the most significant glandular involvement and laboratory and pathology abnormalities. It is possible that the LSB cluster represents an earlier stage of the DLP cluster. This theory is supported by the higher prevalence of positivity for anti‐SSA and anti‐SSB antibodies in patients in the DLP cluster, potentially indicating epitope spreading. Furthermore, patients in the LSB cluster were younger (mean age 47 years) than patients in the DLP cluster (mean age 54 years). Interestingly, patients in the LSB cluster reported the lowest burden of dryness on objective measurements but had the highest frequency of anti‐SSA antibody positivity. This runs counter to prior studies that showed greater dryness in patients with Sjögren's disease who are anti‐SSA antibody positive (23). Accordingly, by separating the LSB and the DLP clusters, we revealed distinct subtypes of Sjögren's disease.
We found that treatment type paralleled symptom frequency and severity more than objective measurements of severity in patients with Sjögren's disease. For example, patients in the DHP and HSB clusters took antimalarial drugs, other DMARDs, NSAIDs, biologics, and steroids more frequently than patients in the DLP and LSB clusters despite an overall lower Sjögren's disease–specific activity metric. Similarly, although patients in the DLP cluster had the greatest level of dryness, patients in the HSB cluster more frequently took cholinomimetic therapy. It is possible that the higher use of cholinomimetics and immune‐modulating therapy among patients in the HSB cluster improved their respective measures of dryness and biologic activity. However, clinical practice and clinical trial experiences have demonstrated less response to therapy among those who have the HSB symptom subtype and who have low biologic disease activity (8, 24). Together with our results, these findings highlight the discordance between objective disease severity and treatment, with symptoms rather than disease severity measures driving therapies. Thus, the use of immunomodulatory therapy to address symptoms might unnecessarily increase risks for adverse outcomes. Our findings suggest that a more nuanced approach to therapy is needed in patients with Sjögren's disease.
Akin to systemic lupus erythematosus, cluster‐based treatment might improve communications between patients and providers as well as patient satisfaction and ultimately reduce costs and unnecessary exposure to high‐risk therapy, providing opportunities for improved care (5). Patients in the HSB cluster, characterized by heavy symptom burden, had lower overall end‐organ involvement and laboratory abnormalities, yet received more treatment. Patients in the HSB cluster had a high level of fatigue, which is a common and debilitating symptom of Sjögren's disease. Fatigue has been shown to be inversely related to the traditional proinflammatory cytokine profile in Sjögren's disease (25, 26, 27), and symptoms of fatique do not improve with immunomodulation. This discrepancy reveals an opportunity to focus on patient counseling and lifestyle interventions for individuals categorized in the HSB cluster (28).
Furthermore, patients in the HSB cluster had a high use of opioid analgesics (34%), indicating that they may be taking treatments that exacerbate their symptoms of dryness and pain. Opioid analgesics negatively impact individuals with fibromyalgia, which is frequently diagnosed in patients categorized in the HSB cluster (44%), and this treatment can lead to worsening pain, function, and depression (29, 30). Opioid analgesics also exacerbate dryness and, particularly in patients with Sjögren's disease, confound disease severity and patient response to therapies. We demonstrated that patients in the HSB cluster had higher medical care costs, which were up to twice the costs reported by the other clusters. By defining and counseling patients on therapies expected to benefit their particular subtype, providers might tailor treatment and control costs. Thus, we can potentially improve the symptom burden and QoL of patients with Sjögren's disease by targeting their particular phenotype with tailored therapy.
We observed interesting results in the DLP cluster because, although patients had lower overall symptom burden, they had high dryness levels, laboratory abnormalities, focus scores, and frequency of PBC. Akin to the DLP cluster, the LSB cluster also had low blood cell counts but was notable for having the highest levels of IgG. Accordingly, given these objective immunologic markers, members of these clusters might be more responsive to immunosuppressive therapies.
Tarn et al previously described distinct symptom‐based clusters generated on the basis of measures of pain, fatigue, and dryness plus anxiety and depression in European samples (7). The 4 main clusters described in their work included LSB, HSB, dryness and fatigue, and pain dominant with fatigue, and they observed different laboratory and transcriptomic profiles among the clusters. The investigators also retrospectively compared responses to treatment with hydroxychloroquine and rituximab from the JOUQER and TRACTISS trials, respectively, among the clusters. Patients in the HSB cluster improved with hydroxychloroquine treatment, and patients in the cluster with dryness dominant with fatigue improved with rituximab treatment (7, 24). Cluster membership might remain stable over time (31). Other studies have used latent class analysis to identify symptom‐based clusters in patients with Sjögren's disease but did not collect granular data on patient experiences, laboratory test results, or histopathology results (13). In contrast, we performed a simplified cluster analysis that focused on the cardinal symptoms of Sjögren's disease that have been used for validation of multiple patient‐reported outcome tools (20, 21). We expanded on the findings of Tarn et al by analyzing other clinically important metrics, such as organ involvement and focus score. We also reported whether categorization based on symptoms in patients with Sjögren's disease affected medical care and treatment costs.
Strengths of our study include the use of 2 large Sjögren's disease cohorts. To our knowledge, this is the largest study to report on symptom‐based clusters in patients with Sjögren's disease. In addition, the SICCA Registry sample included validated depression (PHQ‐9) and health care‐related QoL (SF‐12) metrics. Registrants were rigorously evaluated by rheumatologists and ophthalmologists with standardized examination, laboratory, and pathology protocols. However, we also acknowledge limitations.
First, registrants were referred to the SICCA Registry sample, so referral bias might have impacted our results. The Sjögren's Foundation survey was created by patients with Sjögren's disease and providers to describe the unique experience of each Sjögren's disease cluster but was not previously validated. The Sjögren's Foundation sample survey carries typical survey‐based limitations of response bias, recall bias, and misclassification bias. The Sjögren's Foundation cohort included self‐identified cases of Sjögren's disease, potentially allowing for inclusion of patients without a proven diagnosis. Furthermore, respondents to the Sjögren's Foundation survey did not have physical examinations or laboratory testing, so the severity and extent of their Sjögren's disease were unknown.
Another limitation of our analysis was that we did not statistically account for multiple testing. However, most of the P values were very small (<0.0001) and would be statistically significant even if we corrected for multiple testing, such as by using the Bonferroni correction method. Both data sources were of cross‐sectional design, and changes in clusters over time were not captured.
Extensive medication profiles and sleep habits were also not captured, and so we could not account for medications, such as antihypertensive drugs or sleep agents, that might confound analyses. Future studies should collect and analyze these data. Although our analysis and the other analyses summarized above emphasize the potential promise of targeted therapy for distinct subtypes of Sjögren's disease, further analyses are needed to define the biologic differences among symptom‐based clusters of Sjögren's disease for development of therapeutics. More research is also needed to determine the applicability of our findings to more diverse patient populations.
Our findings highlight a discordance in the experiences, disease severity, and treatment approaches among 4 relatively consistent symptom‐based clusters from 2 cohorts of patients with Sjögren's disease. We propose that further research into the pathogenesis underpinning these symptom‐based clusters could advance our understanding of this heterogeneous disease and move toward cluster‐targeted therapies and trials. We believe that clinical trials that account for the heterogeneous experiences of patients with Sjögren's disease might have a higher likelihood of success. In the short term, identification of a symptom‐based phenotype for Sjögren's disease could promote appropriate treatment regimens earlier in the disease, thereby improving patient QoL. A refined definition of treatments based on symptom clusters could have the added benefit of harmonizing the expectations and communication between patients and providers.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. McCoy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
McCoy, Woodham, Bunya, Maerz, Akpek.
Acquisition of data
McCoy, Woodham, Maerz, Makara.
Analysis and interpretation of data
McCoy, Woodham, Bartels, Saldanha, Maerz, Baer.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42238&file=art42238‐sup‐0001‐Disclosureform.pdf.
Supporting information
Disclosure Form
Appendix S1 Supplementary Information
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The data reported herein have been supplied by the Sjögren's International Collaborative Clinical Alliance (SICCA) Biorepository by the National Institute of Dental and Craniofacial Research (contract HHSN26S201300057C). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the SICCA investigators or the National Institute of Dental and Craniofacial Research. Dr. McCoy's work was supported by the Clinical and Translational Science Award program and by NIH grants UL1‐TR‐002373 and KL2‐TR‐002374 from the National Center for Advancing Translational Sciences and R03DE031340 from the National Institute of Dental and Craniofacial Research. Dr. Bunya's work was supported by NIH grant R01‐EY‐026972 from the National Eye Institute. Mr. Makara's work was supported by the Sjögren's Foundation. Dr. Baer's work was supported by NIH contract 75N92019P00427 from the National Institute of Dental and Craniofacial Research and the Jerome L. Greene Foundation.
REFERENCES
- 1. Miyamoto ST, Valim V, Fisher BA. Health‐related quality of life and costs in Sjögren's syndrome. Rheumatology (Oxford) 2019. doi: 10.1093/rheumatology/key370. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2. Meijer JM, Meiners PM, Huddleston Slater JJ, Spijkervet FK, Kallenberg CG, Vissink A, et al. Health‐related quality of life, employment and disability in patients with Sjogren's syndrome. Rheumatology (Oxford) 2009;489:1077–82. [DOI] [PubMed] [Google Scholar]
- 3. Fox RI, Fox CM. Sjögren syndrome: why do clinical trials fail? Rheum Dis Clin North Am 2016;423:519–30. [DOI] [PubMed] [Google Scholar]
- 4. Alarcón GS, McGwin G Jr, Brooks K, Roseman JM, Fessler BJ, Sanchez ML, et al. Systemic lupus erythematosus in three ethnic groups. XI. Sources of discrepancy in perception of disease activity: a comparison of physician and patient visual analog scale scores. Arthritis Rheum 2002;474:408–13. [DOI] [PubMed] [Google Scholar]
- 5. Pisetsky DS, Clowse ME, Criscione‐Schreiber LG, Rogers JL. A novel system to categorize the symptoms of systemic lupus erythematosus [review]. Arthritis Care Res (Hoboken) 2019;716:735–41. [DOI] [PubMed] [Google Scholar]
- 6. Fox RI, Fox CM, Gottenberg JE, Dörner T. Treatment of Sjögren's syndrome: current therapy and future directions [review]. Rheumatology (Oxford) 2021;60:2066–74. [DOI] [PubMed] [Google Scholar]
- 7. Tarn JR, Howard‐Tripp N, Lendrem DW, Mariette X, Saraux A, Devauchelle‐Pensec V, et al. Symptom‐based stratification of patients with primary Sjögren's syndrome: multi‐dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol 2019;12:PE85–E94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottenberg JE, Ravaud P, Puéchal X, Le Guern V, Sibilia J, Goeb V, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 2014;3123:249–58. [DOI] [PubMed] [Google Scholar]
- 9. Fisher BA, Everett CC, Rout J, O'Dwyer JL, Emery P, Pitzalis C, et al. Effect of rituximab on a salivary gland ultrasound score in primary Sjögren's syndrome: results of the TRACTISS randomised double‐blind multicentre substudy. Ann Rheum Dis 2018;773:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daniels TE, Cox D, Shiboski CH, Schiødt M, Wu A, Lanfranchi H, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjogren's syndrome among 1,726 registry participants. Arthritis Rheum 2011;637:2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels TE, Criswell LA, Shiboski C, Shiboski S, Lanfranchi H, Dong Y, et al. An early view of the international Sjogren's syndrome registry. Arthritis Rheum 2009;615:711–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data‐driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;644:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCoy SS, Sampene E, Baer AN. Association of Sjögren's syndrome with reduced lifetime sex hormone exposure: a case–control study. Arthritis Care Res (Hoboken) 2020;729:1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data‐driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;761:9–16. [DOI] [PubMed] [Google Scholar]
- 15. Ware J Jr, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;343:220–33. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez‐Najera C, Taylor S, Crawford S, Narasimhan P, Shao X, Li J. Characteristics and treatments of patients with Sjögren's syndrome in a real‐world setting. Paper presented at: EULAR Annual European Congress of Rheumatology; 2019. June 12–15; Madrid, Spain. [Google Scholar]
- 17. McCoy SS, Bartels CM, Saldanha IJ, Bunya VY, Akpek EK, Makara MA, et al. National Sjögren's foundation survey: burden of oral and systemic involvement on quality of life. J Rheumatol 2021;48:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002;616:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, et al. Preliminary criteria for the classification of Sjögren's syndrome: results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993;363:340–7. [DOI] [PubMed] [Google Scholar]
- 20. Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjogren's syndrome using a new questionnaire tool. Rheumatology (Oxford) 2004;436:758–64. [DOI] [PubMed] [Google Scholar]
- 21. Bowman SJ, Booth DA, Platts RG, Field A, Rostron J. Validation of the Sicca Symptoms Inventory for clinical studies of Sjögren's syndrome. J Rheumatol 2003;306:1259–66. [PubMed] [Google Scholar]
- 22. Milligan GW. An examination of the effect of six types of error perturbation on fifteen clustering algorithms. Psychometrika 1980;45:325–42. [Google Scholar]
- 23. Kontny E, Lewandowska‐Poluch A, Chmielińska M, Olesińska M. Subgroups of Sjögren's syndrome patients categorised by serological profiles: clinical and immunological characteristics. Reumatologia 2018;566:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collins A, Lendrem D, Wason J, Tarn J, Howard‐Tripp N, Bodewes I, et al. Revisiting the JOQUER trial: stratification of primary Sjögren's syndrome and the clinical and interferon response to hydroxychloroquine. Rheumatol Int 2021;419:1593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodewes ILA, Al‐Ali S, van Helden‐Meeuwsen CG, Maria NI, Tarn J, Lendrem DW, et al. Systemic interferon type I and type II signatures in primary Sjögren's syndrome reveal differences in biological disease activity. Rheumatology (Oxford) 2018;575:921–30. [DOI] [PubMed] [Google Scholar]
- 26. James K, Al‐Ali S, Tarn J, Cockell SJ, Gillespie CS, Hindmarsh V, et al. A transcriptional signature of fatigue derived from patients with primary Sjögren's syndrome. PloS One 2015;1012:e0143970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tripp NH, Tarn J, Natasari A, Gillespie C, Mitchell S, Hackett KL, et al. Fatigue in primary Sjögren's syndrome is associated with lower levels of proinflammatory cytokines. RMD Open 2016;22:e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azizoddin DR, Jolly M, Arora S, Yelin E, Katz P. Longitudinal study of fatigue, stress, and depression: role of reduction in stress toward improvement in fatigue. Arthritis Care Res (Hoboken) 2020;7210:1440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fitzcharles MA, Faregh N, Ste‐Marie PA, Shir Y. Opioid use in fibromyalgia is associated with negative health related measures in a prospective cohort study. Pain Res Treat 2013;2013:898493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng X, Robinson RL, Mease P, Kroenke K, Williams DA, Chen Y, et al. Long‐term evaluation of opioid treatment in fibromyalgia. Clin J Pain 2015;311:7–13. [DOI] [PubMed] [Google Scholar]
- 31. Lee JJ, Park YJ, Park M, Yim HW, Park SH, Kwok SK. Longitudinal analysis of symptom‐based clustering in patients with primary Sjogren's syndrome: a prospective cohort study with a 5‐year follow‐up period. J Transl Med 2021;191:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1 Supplementary Information


