Key Points
Question
How well does sentinel lymph node biopsy alone provide long-term disease control in the regional basin of patients with melanoma sentinel lymph node metastases?
Findings
In this randomized clinical trial including 823 patients, among patients with positive sentinel nodes randomized to nodal basin observation in the second Multicenter Selective Lymphadenectomy Trial, 80.2% (actuarial) of basins were free of recurrence at 10 years. Risk factors for in-basin recurrence included older age, thicker primary tumor, ulceration, and greater disease burden in the sentinel node.
Meaning
In this trial, sentinel lymph node biopsy, a standard staging procedure for melanoma, provided long-lasting regional nodal disease control in most patients with sentinel lymph node metastases.
This randomized clinical trial evaluates how frequently sentinel lymph node biopsy without completion lymph node dissection results in long-term regional nodal disease control in patients with sentinel lymph node metastases.
Abstract
Importance
Sentinel lymph node (SLN) biopsy is a standard staging procedure for cutaneous melanoma. Regional disease control is a clinically important therapeutic goal of surgical intervention, including nodal surgery.
Objective
To determine how frequently SLN biopsy without completion lymph node dissection (CLND) results in long-term regional nodal disease control in patients with SLN metastases.
Design, Setting, and Participants
The second Multicenter Selective Lymphadenectomy Trial (MSLT-II), a prospective multicenter randomized clinical trial, randomized participants with SLN metastases to either CLND or nodal observation. The current analysis examines observation patients with regard to regional nodal recurrence. Trial patients were aged 18 to 75 years with melanoma metastatic to SLN(s). Data were collected from December 2004 to April 2019, and data were analyzed from July 2020 to January 2022.
Interventions
Nodal observation with ultrasonography rather than CLND.
Main Outcomes and Measures
In-basin nodal recurrence.
Results
Of 823 included patients, 479 (58.2%) were male, and the mean (SD) age was 52.8 (13.8) years. Among 855 observed basins, at 10 years, 80.2% (actuarial; 95% CI, 77-83) of basins were free of nodal recurrence. By univariable analysis, freedom from regional nodal recurrence was associated with age younger than 50 years (hazard ratio [HR], 0.49; 95% CI, 0.34-0.70; P < .001), nonulcerated melanoma (HR, 0.36; 95% CI, 0.36-0.49; P < .001), thinner primary melanoma (less than 1.5 mm; HR, 0.46; 95% CI, 0.27-0.78; P = .004), axillary basin (HR, 0.61; 95% CI, 0.44-0.86; P = .005), fewer positive SLNs (1 vs 3 or more; HR, 0.32; 95% CI, 0.14-0.75; P = .008), and SLN tumor burden (measured by diameter less than 1 mm [HR, 0.39; 95% CI, 0.26-0.60; P = .001] or less than 5% area [HR, 0.36; 95% CI, 0.24-0.54; P < .001]). By multivariable analysis, younger age (HR, 0.57; 95% CI, 0.39-0.84; P = .004), thinner primary melanoma (HR, 0.40; 95% CI, 0.22-0.70; P = .002), axillary basin (HR, 0.55; 95% CI, 0.31-0.96; P = .03), SLN metastasis diameter less than 1 mm (HR, 0.52; 95% CI, 0.33-0.81; P = .007), and area less than 5% (HR, 0.58; 95% CI, 0.38-0.88; P = .01) were associated with basin control. When looking at the identified risk factors of age (50 years or older), ulceration, Breslow thickness greater than 3.5 mm, nonaxillary basin, and tumor burden of maximum diameter of 1 mm or greater and/or metastasis area of 5% or greater and excluding missing value cases, basin disease-free rates at 5 years were 96% (95% CI, 88-100) for patients with 0 risk factors, 89% (95% CI, 82-96) for 1 risk factor, 86% (95% CI, 80-93) for 2 risk factors, 80% (95% CI, 71-89) for 3 risk factors, 61% (95% CI, 48-74) for 4 risk factors, and 54% (95% CI, 36-72) for 5 or 6 risk factors.
Conclusions and Relevance
This randomized clinical trial was the largest prospective evaluation of long-term regional basin control in patients with melanoma who had nodal observation after removal of a positive SLN. SLN biopsy without CLND cleared disease in the affected nodal basin in most patients, even those with multiple risk factors for in-basin recurrence. In addition to its well-validated value in staging, SLN biopsy may also be regarded as therapeutic in some patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT00297895
Introduction
Achieving disease control in the regional node basin is an important therapeutic goal in the care of patients with clinically node-negative primary cutaneous melanoma. For many years, that goal was achieved by elective lymph node dissection, which was effective but subjected all patients to the risk of morbidity associated with full dissection. With the development of lymphatic mapping and sentinel lymph node (SLN) biopsy by Donald L. Morton, MD, one of us (A.J.C.), and colleagues, patients without nodal metastases (the great majority) were spared dissection, while those with SLN metastases were still afforded excellent in-basin disease control through the combination of SLN biopsy and immediate completion lymph node dissection (CLND).1,2 This benefit was observed in the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) and in the Sunbelt Melanoma trial.3,4
In these studies, most patients with SLN metastases did not have other nodal metastases detected in completion dissection specimens. Because of the potential morbidity of the additional surgery, the value of immediate CLND was questioned and subsequently evaluated in 2 large multicenter clinical trials: the second Multicenter Selective Lymphadenectomy Trial (MSLT-II)5 and the German Cooperative Oncology Group Selective Lymphadenectomy Trial (DeCOG-SLT).6 Those trials provided concordant data demonstrating no improvement in survival or decrease in distant metastases resulting from immediate CLND. Nodal observation has subsequently become a standard option in the management of patients with melanoma with SLN metastases.
The absence of a significant overall survival benefit from CLND has led to the suggestion that SLN biopsy is merely a staging procedure, without therapeutic value. Certainly, the question of whether there is any overall survival effect of early nodal surgery remains controversial, but there is little published information regarding the effect of SLN biopsy alone (ie, without CLND) on the other important therapeutic goal of such surgery: disease control in the regional node basin. We examined outcomes in patients with SLN metastases who were randomized to nodal observation in MSLT-II with regard to rates of in-basin nodal disease control and factors associated with such control.
Methods
MSLT-II is an international, multicenter, phase 3 randomized clinical trial that assessed the utility of CLND in patients with melanoma sentinel-node metastases. The protocol has been previously reported5 and can be found in Supplement 1. The protocol was approved by each institution’s institutional review board or ethics committee, and all participants provided written informed consent. Briefly, MSLT-II consisted of a screening phase in which patients enrolled prior to SLN biopsy, and a randomization phase, which enrolled patients who were found to have SLN metastases. Patients eligible for randomization were aged 18 to 75 years with Eastern Cooperative Oncology Group performance status of 0 to 1 and a non–melanoma-related life expectancy of at least 10 years. They were randomly assigned (1:1) to either immediate CLND or to nodal observation using clinical examination and ultrasonography. The primary end point of the trial has been previously reported.5 A secondary end point of MSLT-II was comparison of the frequency of same-basin recurrence among patients in the observation arm with the frequency of non-SLN metastases in the CLND arm. Another was determination of whether SLN tumor burden predicted non-SLN metastases or disease recurrence. In the current substudy, we examined patients receiving observation and the outcome of nodal recurrence in the lymph node basin that was previously found to be the site of SLN metastasis. Patients whose SLNs were positive only by a multimarker reverse transcriptase–polymerase chain reaction assay were included in the randomization phase of the trial but are not included in the current analysis. Patients in the observation arm were followed up with every 4 months for the first 2 years, every 6 months in years 3 to 5, and then annually. Ultrasonography assessment of the at-risk nodal basin was performed at each visit through 5 years.5,7 Adjuvant therapy was determined by each site’s standard practice, and only 6.5% of patients in the trial observation arm received adjuvant therapy, as previously reported.5 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients receiving observation were identified, and data regarding sex, age, sex, smoking status, primary tumor thickness, primary site, ulceration, number of positive SLNs, nodal basin site, and SLN tumor burden (measured by maximum diameter of largest metastasis or percentage area of node involved by tumor) were examined. The primary outcome was nodal recurrence in the basin from which a SLN containing metastatic melanoma had been removed. The nodal basin relapse-free survival (RFS) was calculated from the time of randomization. Data were summarized with means and SDs as well as medians and ranges. Cox proportional hazards regression modeling was used to evaluate the prognostic significance of clinical and pathological factors. Univariable and multivariable testing were performed to identify prognostic factors associated with nodal recurrence. Patients with incomplete data were not included in multivariable analyses, except for those with incomplete nodal tumor measurements (diameter or area). For tumor burden variables, a category of missing was included in the analysis. Stepwise method was used for variables selection with .10 significance level for entry and retention in the model. Differences in nodal RFS were analyzed using the Kaplan-Meier test and compared using the log-rank test. SPSS Statistic version 19.0 (IBM), SAS Enterprise Guide version 7 (SAS Institute), and Microsoft Excel 2010 (Microsoft) were used. All P values were 2-tailed, and significance was set at P < .05.
Results
From December 2004 through March 2014, 823 SLN-positive patients were randomized to and accepted nodal observation and had follow-up data for this analysis (eFigure in Supplement 2); 479 (58.2%) were male, and the mean (SD) age was 52.8 (13.8) years. A total of 791 patients had 1 SLN basin with metastasis and 32 had 2 SLN-positive basins, for a total of 855 node-positive basins examined. Demographic and pathologic features of the patient population are shown in Table 1. Most tumors were of intermediate thickness, with a median (IQR) Breslow depth of 2.20 (1.40-3.50) mm, and most were nonulcerated (495 of 823 [60.2%]). The trunk was the most common primary site (392 [47.6%]).
Table 1. Description of Patients.
| Characteristic | No. (%) |
|---|---|
| Total, No. | 823 |
| Sex | |
| Female | 344 (41.8) |
| Male | 479 (58.2) |
| Age, y | |
| Mean (SD) | 52.8 (13.8) |
| Median (IQR) | 54.6 (44-63) |
| Smoking status | |
| Never | 451 (56.4) |
| Quit | 201 (25.1) |
| Smoking | 148 (18.5) |
| Missing, No. | 23 |
| Breslow tumor thickness | |
| Mean (SD), mm | 2.81 (2.19) |
| Median (IQR), mm | 2.20 (1.40-3.50) |
| <1.50 mm | 212 (25.8) |
| 1.50-3.50 mm | 406 (49.3) |
| >3.50 mm | 205 (24.9) |
| Clark level | |
| II | 3 (0.4) |
| III | 128 (16.2) |
| IV | 618 (78.3) |
| V | 40 (5.1) |
| Missing, No. | 34 |
| Primary site | |
| Extremity | 350 (42.5) |
| Head or neck | 81 (9.8) |
| Trunk | 392 (47.6) |
| Ulceration | |
| Present | 328 (39.8) |
| Absent | 495 (60.2) |
Characteristics of the 855 SLN-positive basins examined are described in Table 2. The axilla was the most commonly involved site (433 [50.6%]), and 699 basins (81.8%) only had 1 positive SLN. Most patients (324 of 538 [60.2%]) had less than 5% nodal area involved with tumor, and the median (IQR) longest diameter of the largest focus of metastasis was 0.80 (0.20-2.00) mm. There were 148 nodal recurrences (17.3%) in the 855 basins examined over the 10-year period. Most nodal recurrences occurred by year 3, with a mean (SE) 3-year RFS rate of 83.2% (1.4%) and a mean (SE) 10-year RFS of 80.2% (1.5%). SLN basin disease-free survival rates were 90.5% and 80.2% at 5 and 10 years, respectively. To date, no regional nodal basin recurrences have been seen after year 7 (Figure, A).
Table 2. Nodal Pathology Characteristics.
| Characteristic | SLN basin, No. (%) |
|---|---|
| Total, No. | 855 |
| Basin site | |
| Axilla | 433 (50.6) |
| Epitrochlear | 3 (0.4) |
| Groin | 316 (37.0) |
| Popliteal | 9 (1.0) |
| Neck | 94 (11.0) |
| Positive SLNs, No. | |
| 1 | 699 (81.8) |
| 2 | 141 (16.5) |
| ≥3 | 15 (1.7) |
| SLN tumor burden | |
| Total, No. | 538 |
| SLN area involvement, % | |
| Mean (SD) | 7.4 (14.6) |
| Median (IQR) | 1.00 (1.00-5.00) |
| Missing, No. | 317 |
| <5% | 324 (60.2) |
| ≥5% | 214 (39.8) |
| Max diameter | |
| Total, No. | 571 |
| <0.1 mm | 76 (13.3) |
| 0.1-1.0 mm | 266 (46.6) |
| >1.0 mm | 229 (40.1) |
| Diameter, mm | |
| Mean (SD) | 1.68 (2.88) |
| Median (IQR) | 0.80 (0.20-2.00) |
| Missing, No. | 284 |
Abbreviation: SLN, sentinel lymph node.
Figure. Sentinel Lymph Node (SLN) Basin Disease-Free Survival.
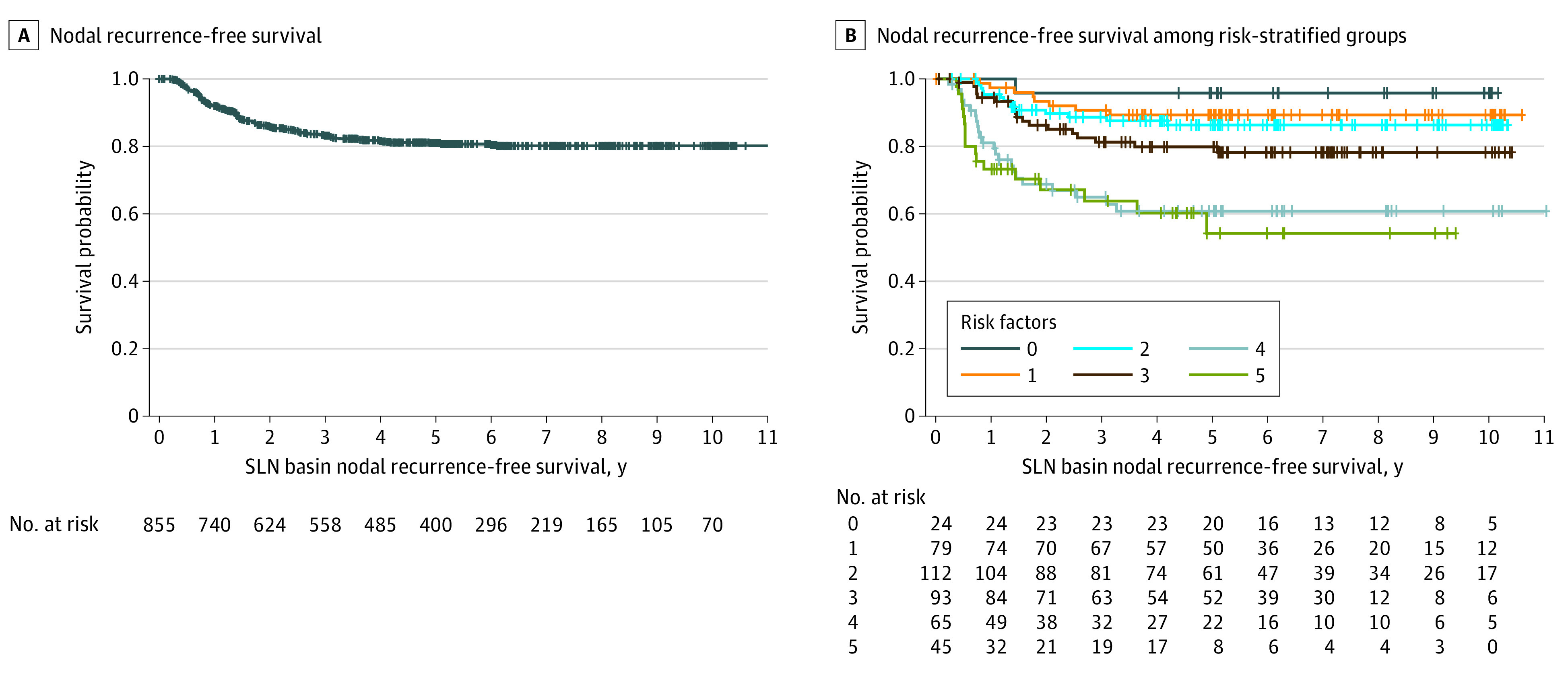
A, Kaplan-Meier plot of nodal recurrence–free survival. B, Nodal recurrence–free survival among risk-stratified groups. + indicates censored patients.
Univariable analysis showed that freedom from regional node basin recurrence was associated with age younger than 50 years (hazard ratio [HR], 0.49; 95% CI, 0.34-0.70; P < .001), a nonulcerated primary melanoma (HR, 0.36; 95% CI, 0.36-0.49; P < .001), lesser tumor thickness (less than 1.5 mm; HR, 0.46; 95% CI, 0.27-0.78; P = .004), axillary basin site (HR, 0.61; 95% CI, 0.44-0.86; P = .005), fewer positive SLNs (1 vs 3 or more; HR, 0.32; 95% CI, 0.14-0.75; P = .008), and lower SLN tumor burden (assessed by either diameter less than 1 mm [HR, 0.39; 95% CI, 0.26-0.60; P < .001] or area less than 5% [HR, 0.36; 95% CI, 0.24-0.54; P < .001]). Primary tumor site and smoking status were not significantly associated with nodal basin recurrence (Table 3). On multivariable analysis, younger age (HR, 0.57; 95% CI, 0.39-0.84; P = .004), a thinner primary melanoma (HR, 0.40; 95% CI, 0.22-0.70; P = .002), axillary basin (HR, 0.55; 95% CI, 0.31-0.96; P = .03), and smaller SLN metastasis diameter (less than 1 mm [HR, 0.52; 95% CI, 0.33-0.81; P = .007] or area less than 5% [HR, 0.58; 95% CI, 0.38-0.88; P = .01]) were independently associated with basin control (Table 4).
Table 3. Univariable Analysis Among 823 Patients With Sentinel Lymph Node (SLN) Metastases.
| Factor | HR (95% CI)a | P valuea | Factor effect, P value |
|---|---|---|---|
| Male vs female sex | 1.14 (0.82-1.58) | .43 | NA |
| Age | |||
| ≥50 y vs <50 y | 2.05 (1.42-2.96) | <.001 | NA |
| Continuous | 1.03 (1.01-1.04) | <.001 | |
| Primary tumor site | |||
| Extremity vs trunk | 1.25 (0.89-1.76) | .21 | .16 |
| Head or neck vs trunk | 1.61 (0.95-2.72) | .08 | |
| Extremity vs head or neck | 0.78 (0.46-1.31) | .34 | |
| Ulceration present vs absent | 2.80 (2.02-3.89) | <.001 | NA |
| Breslow tumor thickness, mm | |||
| <1.50 vs 1.50-3.50 | 0.46 (0.28-0.78) | .004 | <.001 |
| <1.50 vs >3.50 | 0.20 (0.12-0.34) | <.001 | |
| 1.50-3.50 vs >3.50 | 0.44 (0.31-0.62) | <.001 | |
| Nodal basin site | |||
| Axilla vs groin | 0.61 (0.44-0.86) | .005 | .01 |
| Axilla vs neck | 0.64 (0.38-1.08) | .10 | |
| Groin vs neck | 1.05 (0.62-1.76) | .86 | |
| Positive SLN, No. | |||
| 1 vs 2 | 0.71 (0.47-1.06) | .09 | .01 |
| 1 vs ≥3 | 0.32 (0.14-0.75) | .008 | |
| 2 vs ≥3 | 0.46 (0.19-1.12) | .09 | |
| Maximum SLN tumor diameter, mmb | |||
| <1 vs ≥1 | 0.36 (0.24-0.54) | <.001 | <.001 |
| <0.1 vs 0.1-1.0 | 0.66 (0.28-1.57) | .35 | |
| <0.1 vs >1 | 0.26 (0.11-0.60) | .002 | |
| 0.1-1.0 vs >1 | 0.39 (0.26-0.60) | <.001 | |
| SLN area involvement <5% vs ≥5%c | 0.36 (0.24-0.54) | <.001 | NA |
| Smoking statusd | |||
| Never vs quit | 0.84 (0.57-1.23) | .37 | .65 |
| Never vs smoking | 0.99 (0.64-1.53) | .97 | |
| Quit vs smoking | 1.18 (0.73-1.91) | .50 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Wald test.
A total of 284 missing.
A total of 317 missing.
A total of 24 missing.
Table 4. Multivariable Analysis Among 418 Patients With Sentinel Lymph Node (SLN) Tumor Burden Measurements.
| Factor | HR (95% CI)a | P value | Factor effect, P value |
|---|---|---|---|
| Age ≥50 y vs <50 y | 1.74 (1.19-2.54) | .004 | .004 |
| Ulceration present vs absent | 1.90 (1.32-2.74) | <.001 | <.001 |
| Breslow tumor thickness, mm | |||
| <1.5 | 0.40 (0.22-0.70) | .002 | <.001 |
| 1.5 – 3.5 | 0.53 (0.37-0.76) | <.001 | |
| >3.5 | 1 [Reference] | NA | |
| SLN basin site | |||
| Axilla | 0.55 (0.31-0.96) | .03 | .006 |
| Groin | 0.92 (0.53-1.59) | .77 | |
| Neck | 1 [Reference] | NA | |
| Maximum SLN tumor diameter, mm | |||
| ≥1 | 1.92 (1.24-2.98) | .007 | .01 |
| Missing | 1.61 (1.02-2.52) | .04 | |
| <1 | 1 [Reference] | NA | |
| SLN area involvement | |||
| ≥5% | 1.73 (1.13-2.63) | .01 | .002 |
| Missing | 0.85 (0.55-1.33) | .48 | |
| <5% | 1 [Reference] | NA |
Abbreviations: HR, hazard ratio; NA, not applicable.
Wald test.
When looking at the identified risk factors of age (50 years or older), ulceration, Breslow thickness greater than 3.5 mm, nonaxillary basin, and tumor burden of maximum diameter of 1 mm or greater and/or metastasis area of 5% or greater and excluding missing value cases, basin disease-free rates at 5 years were 96% (95% CI, 88-100) for patients with 0 risk factors, 89% (95% CI, 82-96) for 1 risk factor, 86% (95% CI, 80-93) for 2 risk factors, 80% (95% CI, 71-89) for 3 risk factors, 61% (95% CI, 48-74) for 4 risk factors, and 54% (95% CI, 36-72) for 5 or 6 risk factors (Figure, B).
Discussion
As a result of MSLT-II5 and DeCOG-SLT,6 treatment guidelines changed to allow nodal observation, which has become standard practice for patients with SLN metastases. This change has left gaps in our understanding of the clinical course of the disease, which will require additional study to clarify. Here, to our knowledge, we report findings from the largest prospective evaluation of long-term nodal basin control after SLN biopsy alone in patients with SLN metastases. Strikingly, although all of these patients had nodal metastases in their SLN, more than 80% of them never had an in-basin nodal recurrence, confirming that definitive basin disease clearance is achieved in the great majority of patients with positive SLNs through the minimally invasive SLN-biopsy procedure itself. The high rate of nodal control with removal of the SLN in the current study is consistent with the 16.3% regional lymph node recurrence rate over the 72-month median follow-up in the observation arm of the DeCOG-SLT.6 It is also consistent with the observation that, among patients who do undergo CLND, additional nodal metastases are not found in 67% to 90% of disease specimens.8,9,10,11 Of patients in the arm of MSLT-II who underwent CLND, 11.5% had positive non-SLNs.5 Although the percentage of patients with involved nodes is up to 10% higher with application of immunohistochemistry to non-SLNs, most patients still have only normal lymph nodes removed at dissection.12,13
In MSLT-I, patients were randomized to wide excision alone or wide excision with SLN biopsy and immediate CLND if metastasis was found. In that trial, the eventual rate of nodal recurrence in the observation arm was almost identical to the rate of SLN metastasis, and on recurrence, most patients underwent complete therapeutic lymph node dissection. It would appear reasonable to assume that this would have been the case for the patients in MSLT-II, meaning all would have developed clinical nodal disease and required full dissection had they not undergone SLN biopsy. However, as demonstrated by the results of this study, performance of the SLN biopsy itself eliminated all nodal disease in the great majority of cases and avoided the need for a significantly more morbid full dissection. Our data, therefore, support the proposition that SLN biopsy may be considered as therapeutic in this regard, rather than merely prognostic.
It has been pointed out that both in MSLT-II and DeCOG-SLT, a low tumor burden was present in the SLNs of most of the study patients. This burden was lower than that of MSLT-I and probably lower than the general population of patients with positive SLNs. However, both MSLT-II and DeCOG-SLT included a substantial absolute number of patients with SLN metastases more than 1 mm in greatest diameter (total, n = 462; MSLT-II, n = 403; DeCOG-SLT, n = 59), and in the current study, we have evaluated patients with high-risk features for nodal recurrence. Even in our highest risk category, with 5 or 6 high-risk features, most patients remained free of in-basin recurrences over an extended period of follow-up, and most of these recurrences happened within the first 3 years.
Our analysis found associations between freedom from recurrence and younger age, thinner primary tumors, axillary basin site, and small SLN tumor burden measured by either percentage nodal area occupied by tumor or by diameter of the largest metastasis. These findings are consistent with many but not all of the prognostic factors found in other studies to be associated with metastases in non-SLNs after SLN biopsy.14,15,16,17,18,19 Some studies have also shown that a combination of risk factors is associated with a higher probability of non-SLN metastasis.14,20 We did not include the number of SLNs removed as a variable, as we believe that number is determined by the lymphatic drainage anatomy of each patient, demonstrated by preoperative lymphoscintigraphy1 and consider that suggesting a minimum recommended number of SLNs may be misleading as a result. Of note, almost all prior studies predicting non-SLN metastasis used detection of disease in CLND specimens as the end point. It appears that the actual rate of non-SLN disease, manifested as nodal basin recurrence, is higher than that of histologically detected metastases in CLND specimens. In MSLT-II, cumulative non-SLN positivity rate was 8.4% higher in the observation arm, suggesting standard pathologic assessment of the completion dissection specimen, which does not include immunohistochemistry or step sectioning, misses up to 2 in every 5 non-SLN metastases.5 As a result, using CLND pathology to predict non-SLN metastases may not yield the most clinically useful models.
Our study helps continue the process of adapting clinical care to nodal observation after SLN metastasis and raises new questions for management. Are there patients (eg, those with 0 or 1 risk factor) who could safely be recommended for less frequent follow-up? Because nodal recurrence is uncommon after 3 years, could nodal follow-up after 3 years be curtailed? Are there patients whose risk of nodal recurrence is high enough to justify more aggressive follow-up or even advance consideration of immediate CLND? Further exploration and potential validation of these findings using other data sets will be important for validation. Because MSLT-II preceded the widespread availability of effective adjuvant systemic therapies, only 6.5% of observation patients in the study received adjuvant treatment.5 Since early 2014, when patient accrual to MSLT-II was completed, multiple effective and relatively well-tolerated adjuvant systemic regimens have become widely available. These therapies are expected to reduce the risk of nodal recurrence among observed basins even further than what we reported here. Specific reporting of regional node recurrence in adjuvant therapy studies in the post–MSLT-II and post–DeCOG-SLT era will be important to allow evaluation of this issue.
Limitations
This study has limitations. This report is limited as a single clinical trial, and confirmation of these findings in other data sets would be desirable. In addition, surgical practice in the context of a clinical trial at high-volume melanoma centers may not completely reflect practice for patients receiving surgery as part of standard of care outside of the trial. For example, trial patients may have had more complete removal of SLNs than might be found in general practice. This might also be investigated in future studies of real-world outcomes.
Conclusions
This study provides data on the largest prospectively monitored study group of patients managed with nodal observation after SLN metastasis. The findings strongly support a therapeutic effect of SLN biopsy in providing long-term regional node basin disease control in more than 80% of patients with SLN metastases. As clinicians consider alternative prognostic models through gene expression profiling of the primary melanoma, it is important to understand that the SLN excision provides excellent nodal control in addition to prognostic information. Age, primary tumor thickness, ulceration status, basin location, and SLN tumor burden were all independently associated with non-SLN status and, if validated, could be used to guide follow-up intensity and duration in the future.
Trial protocol.
eFigure. CONSORT flow diagram.
Data sharing statement.
References
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392-399. doi: 10.1001/archsurg.1992.01420040034005 [DOI] [PubMed] [Google Scholar]
- 2.Dessureault S, Soong SJ, Ross MI, et al. ; American Joint Committee on Cancer (AJCC) Melanoma Staging Committee . Improved staging of node-negative patients with intermediate to thick melanomas (>1 mm) with the use of lymphatic mapping and sentinel lymph node biopsy. Ann Surg Oncol. 2001;8(10):766-770. doi: 10.1007/s10434-001-0766-1 [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Thompson JF, Cochran AJ, et al. ; MSLT Group . Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599-609. doi: 10.1056/NEJMoa1310460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMasters KM, Egger ME, Edwards MJ, et al. Final results of the Sunbelt Melanoma Trial: a multi-institutional prospective randomized phase III study evaluating the role of adjuvant high-dose interferon alfa-2b and completion lymph node dissection for patients staged by sentinel lymph node biopsy. J Clin Oncol. 2016;34(10):1079-1086. doi: 10.1200/JCO.2015.63.3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211-2222. doi: 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiter U, Stadler R, Mauch C, et al. ; German Dermatologic Cooperative Oncology Group . Final analysis of DeCOG-SLT trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. 2019;37(32):3000-3008. doi: 10.1200/JCO.18.02306 [DOI] [PubMed] [Google Scholar]
- 7.Thompson JF, Haydu LE, Uren RF, et al. ; MSLT-II Trial Group . Preoperative ultrasound assessment of regional lymph nodes in melanoma patients does not provide reliable nodal staging: results from a large multicenter trial. Ann Surg. 2021;273(4):814-820. doi: 10.1097/SLA.0000000000003405 [DOI] [PubMed] [Google Scholar]
- 8.Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220(6):759-767. doi: 10.1097/00000658-199412000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson GW, Murray DR, Lyles RH, Staley CA, Hestley A, Cohen C. The amount of metastatic melanoma in a sentinel lymph node: does it have prognostic significance? Ann Surg Oncol. 2003;10(5):575-581. doi: 10.1245/ASO.2003.03.054 [DOI] [PubMed] [Google Scholar]
- 10.van Akkooi AC, de Wilt JH, Verhoef C, et al. Clinical relevance of melanoma micrometastases (<0.1 mm) in sentinel nodes: are these nodes to be considered negative? Ann Oncol. 2006;17(10):1578-1585. doi: 10.1093/annonc/mdl176 [DOI] [PubMed] [Google Scholar]
- 11.Guggenheim M, Dummer R, Jung FJ, et al. The influence of sentinel lymph node tumour burden on additional lymph node involvement and disease-free survival in cutaneous melanoma—a retrospective analysis of 392 cases. Br J Cancer. 2008;98(12):1922-1928. doi: 10.1038/sj.bjc.6604407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen DR, Huang RR, Binder S, Morton DL, Cochran AJ. Evaluation of melanoma in non-sentinel nodes: how much is enough? Mod Pathol. 2004;17(suppl 1):100. [Google Scholar]
- 13.Scolyer RA, Li LX, McCarthy SW, et al. Immunohistochemical stains fail to increase the detection rate of micrometastatic melanoma in completion regional lymph node dissection specimens. Melanoma Res. 2004;14(4):263-268. doi: 10.1097/01.cmr.0000136708.90534.71 [DOI] [PubMed] [Google Scholar]
- 14.Gershenwald JE, Andtbacka RH, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26(26):4296-4303. doi: 10.1200/JCO.2007.15.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger ME, Bower MR, Czyszczon IA, et al. Comparison of sentinel lymph node micrometastatic tumor burden measurements in melanoma. J Am Coll Surg. 2014;218(4):519-528. doi: 10.1016/j.jamcollsurg.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 16.van der Ploeg AP, van Akkooi AC, Haydu LE, et al. The prognostic significance of sentinel node tumour burden in melanoma patients: an international, multicenter study of 1539 sentinel node-positive melanoma patients. Eur J Cancer. 2014;50(1):111-120. doi: 10.1016/j.ejca.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 17.Kakavand H, Vilain RE, Wilmott JS, et al. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015;28(12):1535-1544. doi: 10.1038/modpathol.2015.110 [DOI] [PubMed] [Google Scholar]
- 18.Ryan M, Crow J, Kahmke R, Fisher SR, Su Z, Lee WT. FoxP3 and indoleamine 2,3-dioxygenase immunoreactivity in sentinel nodes from melanoma patients. Am J Otolaryngol. 2014;35(6):689-694. doi: 10.1016/j.amjoto.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 19.Speeckaert R, Vermaelen K, van Geel N, et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer. 2012;48(13):2004-2011. doi: 10.1016/j.ejca.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 20.Quaglino P, Ribero S, Osella-Abate S, et al. Clinico-pathologic features of primary melanoma and sentinel lymph node predictive for non-sentinel lymph node involvement and overall survival in melanoma patients: a single centre observational cohort study. Surg Oncol. 2011;20(4):259-264. doi: 10.1016/j.suronc.2010.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eFigure. CONSORT flow diagram.
Data sharing statement.


