Abstract
Purpose of Review
Complex environmental factors and human intervention influence the spread of arthropod vectors and the cycle of transmission of arboviruses. The spectrum of clinical manifestations is diverse, ranging from serious presentations like viral hemorrhagic fever (e.g., dengue, yellow fever, rift valley fever) or shock syndromes (e.g., dengue virus) to organ-specific illness like meningoencephalitis.
Recent Findings
A spectrum of clinical neurologic syndromes with potential acute devastating consequences or long-term sequelae may result from some arboviral infections.
Summary
In this review, we describe some of the most frequent and emerging neuro-invasive arboviral infections, spectrum of neurologic disorders including encephalitis, meningitis, myelitis or poliomyelitis, acute demyelinating encephalomyelitis, Guillain-Barré syndrome, and ocular syndromes.
Keywords: Encephalitis, Zika, Chikungunya, Yellow fever, Japanese encephalitis, Meningitis, Guillain-Barre syndrome, Transverse myelitis
Introduction
Arbovirus (arthropod-borne virus) is a broad term to include RNA viruses from different families including Flaviviridae, Phenuviridae, Togaviridae, and Reoviridae which require a life cycle in a host and vector (Table 1). Majority of the arboviral infections are seen in the tropical and sub-tropical regions. However, over the last century, increasing human influence on the environment with activities such as deforestation, industrialization, urbanization, international travel, and population growth has resulted in a change in the environment [1•]. One consequence of these changes is the unprecedented risk of exposure to previously unknown infectious agents or the reemergence of previously known microorganisms including some of the arboviruses. The geospatial distribution and epidemiology of the many members of the arboviral family occurs in complex interactions between hosts, reservoirs, and arthropod vectors [2]. Most arboviruses maintain dynamic enzoonotic or sylvatic transmission cycles between mosquitoes and non-human primates including birds, rodents, horses, or others. In the sylvatic cycle, humans are dead-end hosts. The human host acquires the infection during blood feeding of insects including mosquitos, ticks, or sandflies. Tick-borne encephalitis may also be transmitted by consumption of raw milk from infected animals like goats and cows [3]. Rare cases of transmission from transfusion and organ donation have been documented [4, 5]. Certain viruses like dengue, chikungunya, Zika, and yellow fever viruses can exist in urban epidemic cycle where the virus is fully adapted to the humans who act as the amplifying host without the needs for intermediary animal hosts. Arboviral transmission tends to be seasonal and could be variable depending on local climatic conditions, viral transmission, and vector activity.
Table 1.
Classification of arbovirus
| Arbovirus family | Common viruses |
|---|---|
| Flaviviridae | West Nile Virus, Japanese encephalitis virus, St. Louis encephalitis virus, dengue virus, Zika virus, yellow fever virus, tick-borne encephalitis virus, Usutu virus, Kyasanur forest disease |
| Togaviridae | Eastern equine encephalitis (EEE) virus, Venezuelan equine encephalitis (VEE) virus, Chikungunya virus, O’nyong nyong virus, Ross River virus, Sindbis virus |
| Peribunyaviridae | Jamestown Canyon, La Crosse, Cache valley |
| Phenuiviridae | Heartland, Rift Valley fever, Sandfly fever, Toscana |
| Reoviridae | Colorado tick fever |
Human infection may follow an asymptomatic clinical course or manifest as a spectrum of clinical manifestations ranging from undifferentiated febrile illness to serious presentations like hemorrhagic fever and shock syndromes. Many arboviruses have neurotropic avidity (Table 1) and may produce a spectrum of neurologic disorders due to direct invasion of either the central nervous system (leading to meningoencephalitis, myelitis, retinitis, and/or cerebral vasculitis), or the peripheral nervous system (which can lead to myositis or peripheral neuritis). Importantly, arboviruses are also associated with a variety of post-infectious immune-mediated conditions as well. These can impact the nervous system at any level and lead to a variety of syndromes such as autoimmune encephalitis, acute demyelinating encephalomyelitis (ADEM), acute inflammatory demyelinating polyradiculopathy (AIDP), and its variants [6]. Antibody cross-reactivity across different arboviruses, particularly flaviviruses, may pose challenges for serological diagnosis and serosurveys to assess prevalence or emergence of arboviruses [7–12]. Further advances in accurate point-of-care diagnostics will help not only with patient care but also surveillance of various co-circulating arboviruses in different regions. While new vaccines are being developed for prevention, treatment for most of the arboviral infections is supportive. The objective of this clinical review is to provide a comprehensive review of emerging neurotropic arboviral infections, clinical disorders associated with these infections, prevention, and management.
Epidemiology of Arboviruses and Emerging Neuroinvasive Arboviral Infections
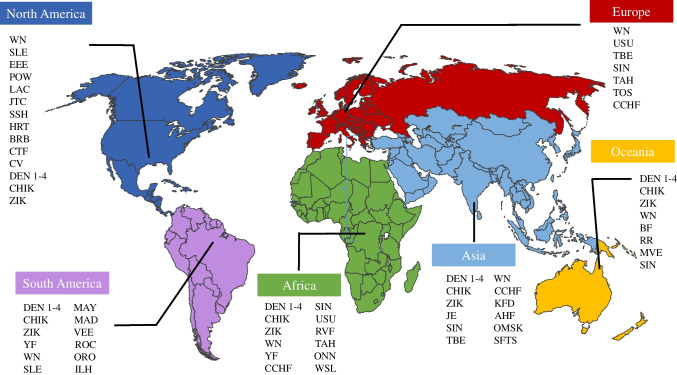
About 17% of all infectious diseases are attributed to vector-borne pathogens including parasites, viruses, and bacteria resulting in over 700,000 deaths annually [13]. In the last couple of decades, there have been emergence and re-emergence of a number of viral infections across the world (Fig. 1). Most of the emerging infections are considered to be of zoonotic origin. In addition to spread of endemic arboviruses in a wider geographic region resulting in larger, more frequent outbreaks, new viruses may be introduced in regions with vectors capable of disseminating the infection.
Fig. 1.
Map of arbovirus distribution around the world
West Nile virus, which was first reported in 1999 in New York, has spread all across North America, Europe, and also parts of South America. In the USA, over 52,000 cases of WNV including 25,849 cases of neuroinvasive WNV have been reported between 1999 and 2020 with a mortality of 8–12% [14]. Increased circulation of West Nile virus has been noted in central and Southern Europe with 300–1000 locally transmitted cases of West Nile virus documented annually [15]. Outbreaks of West Nile virus in Israel and West Asia have occurred since the introduction of the virus in that region in 2000 [16–18].
Zika virus re-emerged in 2007 in the Pacific Islands of Yap and caused pandemic spread in Brazil and the Americas resulting not only in febrile illness but over 3700 cases of congenital microcephaly [19••, 20•, 21]. The chikungunya and Zika virus outbreaks in the Americas in 2013 and 2015 demonstrated how rapidly these diseases could spread with the introduction of a virus in a region with vectors capable for transmission.
Many mosquito-borne viruses are endemic in Africa. Due to lack of disease tracking, the extent of disease caused by various arboviruses is unclear. Recently, there has been reporting of infections from new regions and also discovery of new mosquito-associated viruses [22]. Recent epidemics of yellow fever, Rift Valley fever, dengue, and West Nile viruses highlight the need for ongoing surveillance [23–25].
As in the African subcontinent, many arboviruses are endemic to Asia. There has been a 30-fold rise in incidence of dengue with 50–100 million infections annually, putting over 2.5 billion people at risk for serious illnesses [26]. The last couple of decades have also seen a rise in Japanese encephalitis and chikungunya virus disease cases with potential for widespread disease [27–33]. Along with these highly prevalent infections, outbreaks of other viruses like Rift Valley fever, Kyasanur Forest, Chandipura, and West Nile viruses have occurred and pose additional challenges [34–42].
Pathogenesis
Arboviruses are usually transmitted to human hosts when mosquitoes, ticks, or sandflies attach to the skin for a blood meal. Animal models have suggested that large amounts of virus may be inoculated extravascularly and the level of viral inoculation is proportional to feeding time [43]. The saliva of the arthropods which has anti-inflammatory and anti-hemostatic properties alter host immune response and plays a key role in disease pathogenesis [44]. Following viral inoculation, the initial viral replication occurs in the Langerhans dendritic cells followed by migration to regional lymph nodes. This is followed by a period of transient viremia which results in dissemination of the virus to the reticuloendothelial system [45••, 46•]. The degree and duration of viremia correlates with severity of the disease and neuroinvasion in case of neurotropic viruses [46•, 47–49].
Arboviruses may enter the central nervous system through direct infection of blood brain barrier (BBB), paracellular entry following disruption of BBB, Trojan horse mechanism (migration with infected leukocytes), retrograde transport along peripheral nerves, or by involvement of olfactory neurons with central nervous system entry through cribriform plate [50•, 51–53].
Spectrum of Neurological Clinical Disorders Associated with Arboviruses
Neurological manifestations of arboviral infections vary based on endemic area, virus serotype, and prior exposure to the virus (e.g., as seen with dengue) and host-viral interactions. Like other infectious pathogens, the classification of neurologic disorders caused by arboviral infections depends on the anatomic location (i.e., meningitis, encephalitis, myelitis, neuritis) and type of involvement-direct viral vs immune-mediated. Neuro-invasive arboviruses may concomitantly affect different anatomic locations and cause a multitude of clinical manifestations including central and peripheral nervous system involvement in the same episode of illness. Some of the arboviruses are thought to be neurotropic viruses like West Nile, Japanese encephalitis, and St. Louis encephalitis viruses. Other arboviruses like dengue, Zika, and chikungunya viruses predominantly cause a systemic syndrome but can also cause direct viral invasion of the brain with serious neurologic manifestations (Table 2). The dengue viruses, which are the most common arboviruses around the world, are associated with neurologic manifestations in 0.5–5% of patients [54, 55].
Table 2.
Specific clinical manifestations and diagnosis of major neurotropic arboviral infections
| Arbovirus | Specific clinical manifestations | Neurological complications | Diagnosis |
|---|---|---|---|
| Chikungunya | Chronic inflammatory rheumatism |
Guillain-Barre syndrome Encephalitis Myelopathy Myopathy ADEM Optic neuritis Retinitis Polyneuropathy |
Serum RT-PCR Serum IgM PRNT CSF RT-PCR, IgM Acute & convalescent serum IgG |
| Zika |
Non-purulent conjunctivitis Congenital microcephaly Arthrogryposis/fetal akinesia syndrome |
Guillain-Barre syndrome Transverse myelitis Meningoencephalitis Sensory neuropathy Cerebrovascular complications Retinitis Optic neuritis |
Serum, urine RT-PCR Serum IgM PRNT CSF RT-PCR, IgM Acute & convalescent serum IgG |
| Dengue |
Severe myalgias, arthralgias (“breakbone fever”) Hemorrhagic fever Shock syndrome |
Meningoencephalitis Stroke Transverse myelitis ADEM GBS Myositis Retinal vasculopathy Optic neuropathy Maculopathy Hypokalemic paralysis |
Serum RT-PCR Dengue nonstructural protein 1 Serum IgM PRNT Acute & convalescent serum IgG |
| West Nile |
Acute flaccid paralysis Autonomic instability Movement disorders |
Encephalitis Meningitis Poliomyelitis Seizures Parkinsonism Myoclonus Optic neuritis Retinal vasculitis |
Serum, CSF IgM Serum, CSF PCR in immunocompromised hosts Acute & convalescent serum IgG |
| Japanese encephalitis |
Seizures Acute flaccid paralysis Extrapyramidal features |
Encephalitis Brainstem encephalitis Poliomyelitis Parkinsonian syndrome Opisthotonus and rigidity spasms |
Serum, CSF IgM Serum, CSF RT-PCR Acute & convalescent IgG |
CSF, cerebrospinal fluid; RT-PCR, reverse transcriptase polymerase chain reaction; PRNT, plaque reduction neutralization test
Syndromes Associated with Direct Viral Invasion
Encephalitis
Encephalitis is characterized by inflammation of brain parenchyma and has various causes, infections (predominantly viral), and autoimmune disorders being the most commonly identified.
The etiology of encephalitis varies depending on the geographic region. In eastern Asia, a common cause of encephalitis is Japanese encephalitis virus infection. It is a member of the flavivirus family and causes an estimated 30,000 to 50,000 cases of encephalitis and 10,000 deaths in Asia yearly [56••, 57•]. The classic presentation of Japanese encephalitis includes flat, expressionless facies tremors and hypertonia. In the United States (US), encephalitis cases are associated with approximately 19,000 hospitalizations and more than 1000 deaths per year. Most sporadic viral encephalitis cases in the US are attributed to enteroviruses and herpesviruses (HSV, VZV). West Nile virus is the most common cause of epidemic arboviral encephalitis in the US.
Viral infections causing encephalitis produce two distinct clinico-pathological entities: acute viral encephalitis and post-infectious encephalitis which is described below. The pathogenesis of acute viral encephalitis involves direct infection of neurons with associated neuronal destruction, perivascular inflammation, neuronophagia, and tissue necrosis. The clinical features of arboviral encephalitis may vary by causal pathogen, degree of parenchymal involvement, its location (temporal lobe, limbic, brainstem, or other locations), and host factors. Elderly, neonates, and immunocompromised patients may have more severe presentations. Patients may present with fever, headache, nausea, vomiting, and features of neurological dysfunction including altered level of consciousness, gait instability, focal neurologic deficits, seizures, and extrapyramidal signs (hypertonia, tremors). Patients with encephalitis require hospitalization for supportive care, airway management, monitoring for raised ICP, and seizures. A significant proportion of patients can have residual neurological deficits. Approximately, two-thirds of deaths are caused by acute viral encephalitis and one-third attributed to post-infectious syndromes.
Meningitis
Most of the arboviruses which have been implicated in encephalitis may also cause an aseptic meningitis syndrome. Pathogens may cause involvement of both the meninges and the brain parenchyma leading to the term meningoencephalitis. Arboviral meningitis is characterized by fever, headache, and stiff neck and presentation may be indistinguishable from other aseptic meningitis. CSF studies may show lymphocytic pleocytosis with minimal change in CSF protein and glucose [58]. Patients with West Nile meningitis may have significant CSF pleocytosis with neutrophilia unlike typical aseptic meningitis. A strong suspicion for endemic arboviral infection should be maintained when patients present during seasonal periods of viral transmission.
Poliomyelitis/Acute Flaccid Myelitis
West Nile virus has been associated with poliomyelitis due to involvement of anterior horn cells in the spinal cord. In contrast to GBS, WNV poliomyelitis is usually associated with meningitis, and encephalitis, as part of an acute viral process. Pathologic features show motor neuron destruction in anterior horn cells with interstitial and perivascular lymphocytic infiltrate features [59, 60•]. In a population-based study of 32 patients with WNV associated with the clinical syndrome of acute flaccid paralysis, WNV poliomyelitis was the most common cause and occurred in 84% of the patients [61]. Similar anterior horn cell involvement with focal muscle wasting and lower motor neuron signs have also been noted with other flaviviruses including Japanese encephalitis and St. Louis Encephalitis viruses [62].
Neuro-Ophthalmologic Syndromes
Arboviruses may cause a multitude of ocular manifestations including conjunctivitis, uveitis, chorioretinitis, and optic neuropathy which may occur in the acute phase due to direct viral involvement or as post-infectious sequelae [63•]. Dengue, Zika, and chikungunya have all been noted to involve the posterior segment of the eye with vascular and neuro-ophthalmic manifestations. Maculopathy characterized by vasculitis and hemorrhages is the most common manifestation in dengue virus infection seen in up to 10% of the patients. Other manifestations include retinal hemorrhages, peripapillary hemorrhages, Roth’s spot, and diffuse retinal edema, choroidal neovascularization but optic neuritis has also been reported [64, 65]. Macular chorioretinal atrophy, optic nerve hypoplasia, and increased cup-to-disc ratio have been noted in infants with congenital Zika virus syndrome [65]. West Nile virus can cause a wide array of ocular manifestations like anterior uveitis, retinal vasculitis, optic neuritis, and sixth cranial nerve palsy [66, 67]. Asymptomatic multifocal chorioretinitis is the most common manifestation [66, 68–71]. Fundoscopic eye exam could be considered during clinical evaluation of neuroinvasive arboviral disease.
Post-Infectious Immune-Mediated Syndromes
Infectious agents are one of the common environmental factors which act as a trigger for autoimmune disorders. Proposed mechanisms include molecular mimicry resulting in altered recognition of self-antigens, epitope spreading which causes non-specific immune response to a wider range of antigens, and bystander activation whereby autoreactive immune cells are triggered following exaggerated inflammatory response to a pathogen [72, 73, 74•].
Acute Disseminated Encephalomyelitis
Acute disseminated encephalomyelitis is an immune-mediated disorder which occurs days to weeks after a viral infection and associated with white matter demyelination [75•]. The histopathologic findings of post-infectious ADEM include widespread perivenular inflammation and demyelination.
ADEM which is classically considered a monophasic illness may present with various neurological signs or symptoms including impaired consciousness, seizures, and/or motor weakness. Acute disseminated encephalomyelitis-like presentation has been described with Zika, dengue, and Japanese encephalitis viruses [76–79]. Patients usually present within 2 weeks after initial onset of infection with headache, vomiting, altered mental status, and seizures and could be mistakenly diagnosed as a meningoencephalitis syndrome.
MRI shows multifocal, asymmetric, white matter hyperintensities involving cerebral cortex, subcortical white matter, and basal ganglia. These lesions may show a pattern of ring-shaped contrast enhancement. As a consequence of the post-infectious inflammatory process, CSF studies may show pleocytosis and elevated protein. It is important to differentiate ADEM from other demyelinating disorders particularly multiple sclerosis. Monophasic, post-infectious illness with more acute clinical presentation, less pronounced periventricular distribution of lesions on MRI, and inflammatory CSF picture can be helpful. Most of these patients are treated with high dose steroids with a majority of patients recovering in 1–4 weeks. Residual neurological deficits are noted in about one-third of the patients [79].
Autoimmune Encephalitis
Antibodies to neuronal antibodies like N-methyl D-aspartate receptor (NMDA-R) may trigger autoimmune encephalitis. The association of herpes simplex encephalitis with post-infectious autoimmune encephalitis has been well documented. A recent report of three patients who presented with relapsing symptoms like choreoathetosis, sleep disorder, and agitation following proven Japanese encephalitis were found to have positive anti-NMDAR IgG in CSF and improved with immunotherapy [77, 80, 81]. Similar presentation of anti-NMDAR antibody-associated autoimmune encephalitis has been reported with Chikungunya virus as well [82]. Another report describes a patient with West Nile virus infection who developed autoimmune encephalitis with antibodies to anti-glycine receptor and improved with immunomodulatory therapy [83]. As the awareness of autoimmune encephalitis and access to testing improves, it is possible that more cases of arbovirus-associated autoimmune encephalitis may be reported in the future.
Post-Infectious Myelitis
Myelitis can occur as monophasic illness related to direct arboviral infection or as a post-infectious immune-mediated phenomenon. There have been case reports of transverse myelitis following dengue, Zika, chikungunya, and Japanese encephalitis viruses [84–92]. This may present as a monophasic post-infectious inflammatory event. However, acute infectious can also serve as a trigger for underlying autoimmune disorders such as myelin-oligodendrocyte glycoprotein-associated disease (MOGAD) [93•]. Thus, in patients with suspected post-infectious myelitis, a broad inflammatory work-up may be warranted.
Acute inflammatory Demyelinating Polyradiculopathy
AIDP is the most common cause of acute flaccid paralysis and is characterized by acute, progressive, bilateral, flaccid weakness, and sensory changes. It is commonly associated with albumin-cytologic dissociation in CSF studies. AIDP can be broadly classified into demyelinating and axonal subtypes, which have variable presentations. The main demyelinating forms include the classic Guillain-Barre syndrome (GBS), as well as Miller Fisher syndrome, characterized by ataxia, ophthalmoplegia, and areflexia [94••]. The main axonal variants include acute motor axonal neuropathy (AMAN), which may be clinically indistinguishable from acute flaccid myelitis (AFM) as they both typically present with flaccid weakness, areflexia, and preserved sensation, and acute motor and sensory axonal neuropathy (AMSAN) which presents similarly to GBS. Autoimmune processes (anti-ganglioside antibody) and molecular mimicry (cross-reacting antibodies to flavivirus antigens) have been thought to be the etiology for AIDP [94••]. Various AIDP variants have been described mostly following dengue infection but there are rare reports of AIDP presenting during acute febrile dengue illness [95–99]. Zika virus has emerged as an important cause of AIDP in regions with recent outbreaks particularly in South America [76, 100, 101, 102•, 103–105, 106•]. The prevalence of AIDP among Zika-infected individuals has been estimated to be about 1.2% but may be an underestimate due to limitations of data collection [100]. The acute motor axonal neuropathy (AMAN) variant of AIDP has been commonly reported following Zika infection [101, 106•]. Patients may have rapid progression of neurological symptoms with severe functional impairment with need for mechanical ventilation, and again can be clinically indistinguishable from AFM without further diagnostics such as spinal cord imaging and nerve conduction studies [101, 103]. Typical anti-ganglioside antibody has not been noted in arboviral infection-associated AIDP; however, in one study, antiglycolipid antibody was present in about 30% of patients with AIDP [101, 107]. In regions with high prevalence of multiple arboviral infections, AIDP has been being reported in arboviral co-infections [101, 103, 107, 108]. Similarly, AIDP has been reported with other arboviral infections including chikungunya, West Nile, and Japanese encephalitis viruses [109–117].
Differential Diagnosis of Arboviral Neurological Syndromes
A major consideration in the differential diagnosis of viral neuro-infections is to exclude other infectious agents that can mimic viral meningoencephalitis like bacterial, mycobacterial, fungal, rickettsial, or parasitic infections. Aseptic meningitis due to other viruses like herpes virus, varicella zoster, and enterovirus may be similar in presentation to arboviral meningitis. Acute flaccid weakness has a broad infectious differential and may be seen in non-arboviral poliomyelitis syndromes (as seen with enterovirus D68), infectious myeloradiculitis (which can be seen with various herpesviruses, or rarely parasites like schistosomiasis), or with other neuromuscular pathology that may be unrelated to other viral pathogens (AIDP due to other triggers such as Campylobacter jejuni, other infectious myositis).
Non-infectious Mimics of Arboviral Encephalitis
In addition to ruling out other infectious entities, non-infectious causes of these syndromes must be considered. Over the past decade, an increasing number of non-infectious, mainly autoimmune, encephalitis cases, have been identified and many do not meet the traditional existing criteria for encephalitis. Therefore, newly identified criteria, focusing on neurological and psychiatric manifestations without fever or CSF pleocytosis, have been proposed [118•]. The diagnosis of autoimmune encephalitis can be challenging due to the myriad manifestations. Autoimmune encephalitis is often associated with antibodies against neuronal cell surface or synaptic proteins; however, the absence of autoantibodies does not exclude the possibility that a disorder is immune-mediated [119, 120].
Acute flaccid weakness also has a broad non-infectious differential including some demyelinating syndromes such as MOGAD or neuromyelitis optica spectrum disorder, other inflammatory syndromes (sarcoidosis, antibody-mediated, or paraneoplastic syndromes), and malignancies such as lymphoma. Acute flaccid weakness may also be seen with non-infectious myopathies and neuropathies, motor neuron disease, neuromuscular junction disorders like myasthenia gravis, botulism, and tick paralysis which is a neurotoxin-mediated condition.
Long-Term Sequelae of Arboviral Neurological Syndromes
Though a majority of neurotropic arboviral infections are asymptomatic, residual long-term physical, mental, neurocognitive deficits have been noted in a significant proportion of recovered patients. West Nile, Japanese encephalitis, and eastern equine encephalitis viral disease have been associated with long-term sequelae in 50–75% of neuroinvasive cases, with symptoms lasting years after the onset of illness [121–127]. The common physical sequelae involve fatigue, myalgias, arthralgias, and generalized weakness and the cognitive/psychological sequelae involve memory loss, depression, difficulty concentrating, and anxiety. Other serious sequelae associated with West Nile virus also included paralysis, movement disorders, ataxia, and visual impairment [123, 124, 128]. Chikungunya infection has been associated with arthralgia, myalgia, and generalized weakness [129, 130, 131•]. Congenital Zika syndrome due to direct neuronal injury of the developing fetal brain during maternal Zika virus infection may cause severe microcephaly, brain atrophy, contractures, ocular abnormalities, and hypertonia [132, 133•, 134, 135].
Diagnosis
The diagnosis of neuroinvasive arboviral infection is established using serological or sometimes nucleic acid amplification tests (NAAT) in the serum and CSF. For dengue, chikungunya and Zika, NAAT, for detection of viral genome may be helpful when performed within 5–7 days of symptom onset, following which IgM should be considered [136••, 137••]. Immunoassay for nonstructural protein 1 can be used as an alternate test for dengue virus infection. IgM for the flaviviruses particularly dengue, Zika, and chikungunya may cross-react causing diagnostic uncertainties [7]. Plaque reduction neutralization tests (PRNTs) with > fourfold higher titers can sometimes be used for confirmation. However, PRNTs are labor- and time-intensive, limiting utility for high-volume and resource-limited settings. Also, in endemic areas with high prevalence of dengue and Zika, PRNTs may not be able to distinguish between the infecting virus and may not be able to confirm positive IgM results.
For most neurotropic arboviral infections, use of NAAT is limited by low viral loads and decreased sensitivity except in immunocompromised hosts who may have prolonged viral RT-PCR positivity in serum and CSF. In most clinical situations, serum and CSF IgM is used for diagnosis. IgM is detectable in the serum about 5–10 days from symptom onset. If initial IgM test is negative, repeat IgM testing should be performed around 10 days from symptom onset. Presence of IgM in the CSF is indicative of intrathecal synthesis and implies neuroinvasive infection. IgM antibodies may persist for 30–90 days but prolonged persistence has been documented [138, 139].
IgG detection in a single serum may not be helpful since it indicates prior infection but a fourfold rise in IgG titers between acute and convalescent serum can be used for diagnosis.
Prevention and Treatment
There are currently no human vaccines available for prevention for a majority of the arboviruses except for Japanese encephalitis virus, yellow fever virus, and tick borne encephalitis virus. The first licensed live attenuated dengue vaccine, Dengvaxia, has been approved by the FDA in the USA and being considered as part of vaccination strategy in various countries with high burden of dengue infection [140•, 141, 142]. This vaccine is recommended only for individuals who have been previously exposed to dengue virus and are seropositive due to the risk of severe infection in seronegative individuals who experience their first natural dengue infection.
Personal protection by decreasing exposed body surfaces while outdoors, minimizing outdoor time during dusk and dawn, and applying insect repellants like DEET and picardin in high transmission areas are recommended to decrease exposures to mosquitoes and ticks [143, 144]. One of the other major foci of prevention is vector control which requires a coordinated effort from health departments. Entomological surveys, early identification of virus in mosquitoes, ticks, elimination of standing water sources to decrease breeding of mosquitoes, and insecticide spraying are some of the strategies but could be expensive.
Treatment for neuroinvasive arboviral infections is largely supportive. Innovative therapies like monoclonal antibodies and antiviral compounds are being investigated [145–151].
Conclusions
Emerging and re-emerging arboviral infections will continue to pose a major challenge to human health in the coming decades. Most arboviral infections cause asymptomatic infections and undifferentiated acute febrile illness. Hence, the large majority may be undiagnosed and the burden of disease in the community may be difficult to assess. The “One Health approach” which promotes a collaborative, multidisciplinary framework linking human health to animals, plants, and the shared environment is crucial to understand and manage the emerging viral infections. Advances in accurate, rapid, point-of-care diagnostics, effective vaccine candidates, and new therapeutic strategies can herald a new era in the control and management of arboviral diseases.
Declarations
Conflicts of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on CNS Infections in Tropical Settings
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hotez PJ. Neglected tropical diseases in the anthropocene: the cases of Zika, Ebola, and other infections. PLoS Negl Trop Dis. 2016;10:e0004648. doi: 10.1371/journal.pntd.0004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould EA. West Nile virus: don’t underestimate its persistence. J Infect Dis. 2010;201:1. doi: 10.1086/648732. [DOI] [PubMed] [Google Scholar]
- 3.Encephalitis TB. https://www.cdc.gov/vhf/tbe/transmission/index.html [online].
- 4.Centers for Disease C, Prevention. West Nile virus transmission via organ transplantation and blood transfusion - Louisiana, 2008. MMWR Morb Mortal Wkly Rep 2009;58:1263-1267. [PubMed]
- 5.Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 6.Solbrig MV, Perng GC. Current neurological observations and complications of dengue virus infection. Curr Neurol Neurosci Rep. 2015;15:29. doi: 10.1007/s11910-015-0550-4. [DOI] [PubMed] [Google Scholar]
- 7.Morales I, Rosenberger KD, Magalhaes T, et al. Diagnostic performance of anti-Zika virus IgM, IgAM and IgG ELISAs during co-circulation of Zika, dengue, and chikungunya viruses in Brazil and Venezuela. PLoS Negl Trop Dis. 2021;15:e0009336. doi: 10.1371/journal.pntd.0009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koraka P, Zeller H, Niedrig M, Osterhaus AD, Groen J. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect. 2002;4:1209–1215. doi: 10.1016/S1286-4579(02)01647-7. [DOI] [PubMed] [Google Scholar]
- 9.van Meer MPA, Mogling R, Klaasse J, et al. Re-evaluation of routine dengue virus serology in travelers in the era of Zika virus emergence. J Clin Virol. 2017;92:25–31. doi: 10.1016/j.jcv.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Houghton-Trivino N, Montana D, Castellanos J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev Salud Publica (Bogota) 2008;10:299–307. doi: 10.1590/S0124-00642008000200010. [DOI] [PubMed] [Google Scholar]
- 11.Hoze N, Diarra I, Sangare AK, et al. Model-based assessment of Chikungunya and O’nyong-nyong virus circulation in Mali in a serological cross-reactivity context. Nat Commun. 2021;12:6735. doi: 10.1038/s41467-021-26707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endale A, Medhin G, Darfiro K, Kebede N, Legesse M. Magnitude of antibody cross-reactivity in medically important mosquito-borne flaviviruses: a systematic review. Infect Drug Resist. 2021;14:4291–4299. doi: 10.2147/IDR.S336351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Vector-borne disease. WHO fact sheet2020.
- 14.virus CWN. https://www.cdc.gov/westnile/index.html [online].
- 15.Control WNvsECfDPa. https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical [online].
- 16.Lustig Y, Kaufman Z, Mannasse B, et al. West Nile virus outbreak in Israel in 2015: phylogenetic and geographic characterization in humans and mosquitoes. Clin Microbiol Infect. 2017;23:986–993. doi: 10.1016/j.cmi.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Salama M, Amitai Z, Lustig Y, et al. Outbreak of West Nile Virus disease in Israel (2015): a retrospective analysis of notified cases. Travel Med Infect Dis. 2019;28:41–45. doi: 10.1016/j.tmaid.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Siegel-Itzkovich J. Twelve die of West Nile Virus in Israel. BMJ. 2000;321:724. [PMC free article] [PubMed] [Google Scholar]
- 19.•• Musso D, Ko AI, Baud D. Zika virus infection - after the pandemic. N Engl J Med 2019;381:1444-1457. This article provides an overview of the epidemiology of Zika virus infection and the risk of endemicity in many settings in Latin America. [DOI] [PubMed]
- 20.Weaver SC, Costa F, Garcia-Blanco MA, et al. Zika virus: history, emergence, biology, and prospects for control. Antivir Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas -Cc-DaoJ. https://www.paho.org/en/node/60231 [online].
- 22.Agboli E, Zahouli JBZ, Badolo A, Jost H. Mosquito-associated viruses and their related mosquitoes in West Africa. Viruses. 2021;13. [DOI] [PMC free article] [PubMed]
- 23.Ahmed A, Elduma A, Magboul B, Higazi T, Ali Y. The first outbreak of dengue fever in Greater Darfur, Western Sudan. Trop Med. Infect Dis Ther. 2019;4. [DOI] [PMC free article] [PubMed]
- 24.Shoemaker TR, Nyakarahuka L, Balinandi S, et al. First laboratory-confirmed outbreak of human and animal rift valley fever virus in Uganda in 48 years. Am J Trop Med Hyg. 2019;100:659–671. doi: 10.4269/ajtmh.18-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwachukwu WE, Yusuff H, Nwangwu U, et al. The response to re-emergence of yellow fever in Nigeria, 2017. Int J Infect Dis. 2020;92:189–196. doi: 10.1016/j.ijid.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Global Strategy for Dengue Prevention and Control.
- 27.Qiu S, Guo J, Li P, et al. Source-tracking of the Chinese Chikungunya viruses suggests that Indian subcontinent and Southeast Asia act as major hubs for the recent global spread of Chikungunya virus. Virol J. 2021;18:203. doi: 10.1186/s12985-021-01665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley J, Chongkolwatana V, Duong PT, et al. Detection of dengue, chikungunya, and Zika RNA in blood donors from Southeast Asia. Transfusion. 2021;61:134–143. doi: 10.1111/trf.16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wimalasiri-Yapa B, Stassen L, Huang X, et al. Chikungunya virus in Asia - Pacific: a systematic review. Emerg Microbes Infect. 2019;8:70–79. doi: 10.1080/22221751.2018.1559708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Japanese encephalitis: surveillance and immunization in Asia and the Western Pacific, 2016. Wkly Epidemiol Rec 2017;92:323–331. [PubMed]
- 31.Kuwata R, Torii S, Shimoda H, et al. Distribution of Japanese encephalitis virus, Japan and Southeast Asia, 2016-2018. Emerg Infect Dis. 2020;26:125–128. doi: 10.3201/eid2601.190235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakamoto R, Tanimoto T, Takahashi K, Hamaki T, Kusumi E, Crump A. Flourishing Japanese encephalitis, associated with global warming and urbanisation in Asia, demands widespread integrated vaccination programmes. Ann Glob Health. 2019;85. [DOI] [PMC free article] [PubMed]
- 33.Schuh AJ, Ward MJ, Leigh Brown AJ, Barrett AD. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J Virol. 2014;88:4522–4532. doi: 10.1128/JVI.02686-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.From the Centers for Disease Control. Update: outbreak of Rift Valley fever--Saudi Arabia, August-November 2000. JAMA 2000;284:2989-2990. [PubMed]
- 35.Al-Hazmi M, Ayoola EA, Abdurahman M, et al. Epidemic Rift Valley fever in Saudi Arabia: a clinical study of severe illness in humans. Clin Infect Dis. 2003;36:245–252. doi: 10.1086/345671. [DOI] [PubMed] [Google Scholar]
- 36.Jupp PG, Kemp A, Grobbelaar A, et al. The 2000 epidemic of Rift Valley fever in Saudi Arabia: mosquito vector studies. Med Vet Entomol. 2002;16:245–252. doi: 10.1046/j.1365-2915.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 37.Gladson V, Moosan H, Mathew S, P D. Clinical and laboratory diagnostic features of Kyasanur forest disease: a study from Wayanad, South India. Cureus 2021;13:e20194. [DOI] [PMC free article] [PubMed]
- 38.Gupta N, Chunduru K, Safeer KM, Saravu K. Clinical and laboratory profile of patients with Kyasanur forest disease: a single-centre study of 192 patients from Karnataka, India. J Clin Virol. 2021;135:104735. doi: 10.1016/j.jcv.2021.104735. [DOI] [PubMed] [Google Scholar]
- 39.Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566–570. doi: 10.4269/ajtmh.2005.73.566. [DOI] [PubMed] [Google Scholar]
- 40.Dwibedi B, Sabat J, Hazra RK, Kumar A, Dinesh DS, Kar SK. Chandipura virus infection causing encephalitis in a tribal population of Odisha in eastern India. Natl Med J India. 2015;28:185–187. [PubMed] [Google Scholar]
- 41.Gurav YK, Tandale BV, Jadi RS, et al. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J Med Res. 2010;132:395–399. [PubMed] [Google Scholar]
- 42.Tandale BV, Tikute SS, Arankalle VA, et al. Chandipura virus: a major cause of acute encephalitis in children in North Telangana, Andhra Pradesh, India. J Med Virol. 2008;80:118–124. doi: 10.1002/jmv.21041. [DOI] [PubMed] [Google Scholar]
- 43.Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262–1270. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.•• Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA 2013;310:308-315. This a state of the art article of West Nile Virus epidemiology with a focus of the outbreaks in the United States. [DOI] [PMC free article] [PubMed]
- 46.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagata N, Iwata-Yoshikawa N, Hayasaka D, et al. The pathogenesis of 3 neurotropic flaviviruses in a mouse model depends on the route of neuroinvasion after viremia. J Neuropathol Exp Neurol. 2015;74:250–260. doi: 10.1097/NEN.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Scarano F, Tyler KL. Molecular pathogenesis of neurotropic viral infections. Ann Neurol. 1987;22:565–574. doi: 10.1002/ana.410220502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- 50.Salimi H, Cain MD, Klein RS. Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics. 2016;13:514–534. doi: 10.1007/s13311-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charles PC, Walters E, Margolis F, Johnston RE. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 52.Li F, Wang Y, Yu L, et al. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol. 2015;89:5602–5614. doi: 10.1128/JVI.00143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suen WW, Prow NA, Hall RA, Bielefeldt-Ohmann H. Mechanism of West Nile virus neuroinvasion: a critical appraisal. Viruses. 2014;6:2796–2825. doi: 10.3390/v6072796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pancharoen C, Thisyakorn U. Neurological manifestations in dengue patients. Southeast Asian J Trop Med Public Health. 2001;32:341–345. [PubMed] [Google Scholar]
- 55.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848–851. doi: 10.4269/ajtmh.2001.65.848. [DOI] [PubMed] [Google Scholar]
- 56.•• Solomon T. Flavivirus encephalitis. N Engl J Med 2004;351:370-378. This article summarizes the epidemiology and clinical aspects of the major Flaviviruses that cause encephalitis [DOI] [PubMed]
- 57.Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arboviral meningitis/encephalitis CD. https://ndc.services.cdc.gov/case-definitions/arboviral-encephalitis-or-meningitis-2001/ [online].
- 59.Doron SI, Dashe JF, Adelman LS, Brown WF, Werner BG, Hadley S. Histopathologically proven poliomyelitis with quadriplegia and loss of brainstem function due to West Nile virus infection. Clin Infect Dis. 2003;37:e74–e77. doi: 10.1086/377177. [DOI] [PubMed] [Google Scholar]
- 60.Leis AA, Stokic DS. Neuromuscular manifestations of west nile virus infection. Front Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11:1021–1027. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misra UK, Kalita J. Anterior horn cells are also involved in Japanese encephalitis. Acta Neurol Scand. 1997;96:114–117. doi: 10.1111/j.1600-0404.1997.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 63.Venkatesh A, Patel R, Goyal S, Rajaratnam T, Sharma A, Hossain P. Ocular manifestations of emerging viral diseases. Eye (Lond) 2021;35:1117–1139. doi: 10.1038/s41433-020-01376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beral L, Merle H, David T. Ocular complications of dengue fever. Ophthalmology. 2008;115:1100–1101. doi: 10.1016/j.ophtha.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 65.de Andrade GC, Ventura CV, Mello Filho PA, Maia M, Vianello S, Rodrigues EB. Arboviruses and the eye. Int J Retina Vitreous. 2017;3:4. doi: 10.1186/s40942-016-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rousseau A, Haigh O, Ksiaa I, Khairallah M, Labetoulle M. Ocular manifestations of West Nile virus. Vaccines (Basel). 2020;8. [DOI] [PMC free article] [PubMed]
- 67.Kuchtey RW, Kosmorsky GS, Martin D, Lee MS. Uveitis associated with West Nile virus infection. Arch Ophthalmol. 2003;121:1648–1649. doi: 10.1001/archopht.121.11.1648. [DOI] [PubMed] [Google Scholar]
- 68.Adelman RA, Membreno JH, Afshari NA, Stoessel KM. West Nile virus chorioretinitis. Retina. 2003;23:100–101. doi: 10.1097/00006982-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 69.Anninger WV, Lomeo MD, Dingle J, Epstein AD, Lubow M. West Nile virus-associated optic neuritis and chorioretinitis. Am J Ophthalmol. 2003;136:1183–1185. doi: 10.1016/S0002-9394(03)00738-4. [DOI] [PubMed] [Google Scholar]
- 70.Bains HS, Jampol LM, Caughron MC, Parnell JR. Vitritis and chorioretinitis in a patient with West Nile virus infection. Arch Ophthalmol. 2003;121:205–207. doi: 10.1001/archopht.121.2.205. [DOI] [PubMed] [Google Scholar]
- 71.Khairallah M, Ben Yahia S, Attia S, Zaouali S, Ladjimi A, Messaoud R. Linear pattern of West Nile virus-associated chorioretinitis is related to retinal nerve fibres organization. Eye (Lond) 2007;21:952–955. doi: 10.1038/sj.eye.6702355. [DOI] [PubMed] [Google Scholar]
- 72.Horwitz MS, Sarvetnick N. Viruses, host responses, and autoimmunity. Immunol Rev. 1999;169:241–253. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim B, Kaistha SD, Rouse BT. Viruses and autoimmunity. Autoimmunity. 2006;39:71–77. doi: 10.1080/08916930500484708. [DOI] [PubMed] [Google Scholar]
- 74.• Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11. This article describes the various mechanisms described for viral-induced autoimmunity with clear illustrations. [DOI] [PMC free article] [PubMed]
- 75.Noorbakhsh F, Johnson RT, Emery D, Power C. Acute disseminated encephalomyelitis: clinical and pathogenesis features. Neurol Clin. 2008;26(759-780):ix. doi: 10.1016/j.ncl.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brito Ferreira ML, Antunes de Brito CA, Moreira AJP, et al. Guillain-Barre syndrome, acute disseminated encephalomyelitis and encephalitis associated with Zika virus infection in Brazil: detection of viral RNA and isolation of virus during late infection. Am J Trop Med Hyg. 2017;97:1405–1409. doi: 10.4269/ajtmh.17-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen WL, Liao MF, Chiang HL, Lin SK. A possible case of acute disseminated encephalomyelitis after Japanese encephalitis. Acta Neurol Taiwanica. 2013;22:169–173. [PubMed] [Google Scholar]
- 78.Gupta M, Nayak R, Khwaja GA, Chowdhury D. Acute disseminated encephalomyelitis associated with dengue infection: a case report with literature review. J Neurol Sci. 2013;335:216–218. doi: 10.1016/j.jns.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 79.Wan Sulaiman WA, Inche Mat LN, Hashim HZ, et al. Acute disseminated encephalomyelitis in dengue viral infection. J Clin Neurosci. 2017;43:25–31. doi: 10.1016/j.jocn.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 80.Ma J, Han W, Jiang L. Japanese encephalitis-induced anti-N-methyl-d-aspartate receptor encephalitis: a hospital-based prospective study. Brain and Development. 2020;42:179–184. doi: 10.1016/j.braindev.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Ma J, Zhang T, Jiang L. Japanese encephalitis can trigger anti-N-methyl-D-aspartate receptor encephalitis. J Neurol. 2017;264:1127–1131. doi: 10.1007/s00415-017-8501-4. [DOI] [PubMed] [Google Scholar]
- 82.Nobrega PR, Morais NMM, Braga-Neto P, et al. NMDAR encephalitis associated with acute Chikungunya virus infection: A New Trigger? Front Pediatr. 2020;8:176. doi: 10.3389/fped.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karagianni P, Alexopoulos H, Sourdi A, Papadimitriou D, Dimitrakopoulos AN, Moutsopoulos HM. West Nile Virus infection triggering autoimmune encephalitis: pathophysiological and therapeutic implications. Clin Immunol. 2019;207:97–99. doi: 10.1016/j.clim.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Seet RC, Lim EC, Wilder-Smith EP. Acute transverse myelitis following dengue virus infection. J Clin Virol. 2006;35:310–312. doi: 10.1016/j.jcv.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 85.Ankur Nandan V, Nilesh K, Dibyaranjan B, Ashutosh T, Ravi A, Arvind A. Acute transverse myelitis (ascending myelitis) as the initial manifestation of Japanese encephalitis: a rare presentation. Case Rep Infect Dis. 2013;2013:487659. doi: 10.1155/2013/487659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hameed S, Memon M, Imtiaz H, Kanwar D. Longitudinally extensive transverse myelitis with seropositive chikungunya. BMJ Case Rep. 2019;12. [DOI] [PMC free article] [PubMed]
- 87.Mecharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387:1481. doi: 10.1016/S0140-6736(16)00644-9. [DOI] [PubMed] [Google Scholar]
- 88.Neri VC, Xavier MF, Barros PO, Melo Bento C, Marignier R, Papais AR. Case report: acute transverse myelitis after Zika virus infection. Am J Trop Med Hyg. 2018;99:1419–1421. doi: 10.4269/ajtmh.17-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palacios E, Clavijo-Prado C, Ruiz A, Arias Antun A, Julian DE. Longitudinal extensive transverse myelitis and Zika virus: a diagnostic challenge in a hospital in Colombia. Neurologia (Engl Ed) 2019;34:204–206. doi: 10.1016/j.nrl.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Roman GC, Anaya JM, Mancera-Paez O, Pardo-Turriago R, Rodriguez Y. Concurrent Guillain-Barre syndrome, transverse myelitis and encephalitis post-Zika: a case report and review of the pathogenic role of multiple arboviral immunity. J Neurol Sci. 2019;396:84–85. doi: 10.1016/j.jns.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 91.Rueda-Lopes FC, da Cruz LCH, Fontes FL, et al. Clinical and magnetic resonance imaging patterns of extensive Chikungunya virus-associated myelitis. J Neuro-Oncol. 2021;27:616–625. doi: 10.1007/s13365-021-00962-4. [DOI] [PubMed] [Google Scholar]
- 92.Verma R, Praharaj HN, Patil TB, Giri P. Acute transverse myelitis following Japanese encephalitis viral infection: an uncommon complication of a common disease. BMJ Case Rep. 2012;2012. [DOI] [PMC free article] [PubMed]
- 93.Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. 2019;266:1280–1286. doi: 10.1007/s00415-018-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.•• Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med 2012;366:2294-2304. This neurologic syndrome is a major neurologic manifestation cause by many arboviral infections. [DOI] [PubMed]
- 95.Dalugama C, Shelton J, Ekanayake M, Gawarammana IB. Dengue fever complicated with Guillain-Barre syndrome: a case report and review of the literature. J Med Case Rep. 2018;12:137. doi: 10.1186/s13256-018-1626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohiuddin O, Khan AA, Waqar SHB, et al. Pharyngeal-cervical-brachial variant of Guillain-Barre syndrome: a case report of a rare complication following Dengue-Chikungunya co-infection. Pan Afr Med J. 2021;38:356. doi: 10.11604/pamj.2021.38.356.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prado MB, Jr, Narito KM, Adiao KJB. Anti-GM1 IgM antibody positive axonal variant of Guillain-Barre-syndrome in a pediatric patient with dengue fever. J Neuroimmunol. 2021;355:577572. doi: 10.1016/j.jneuroim.2021.577572. [DOI] [PubMed] [Google Scholar]
- 98.Sharma CM, Kumawat BL, Ralot T, Tripathi G, Dixit S. Guillain-Barre syndrome occurring during dengue fever. J Indian Med Assoc. 2011;109(675):682. [PubMed] [Google Scholar]
- 99.Umapathi T, Lim CS, Ooi EE, et al. Asymptomatic dengue infection may trigger Guillain-Barre syndrome. J Peripher Nerv Syst. 2016;21:375–377. doi: 10.1111/jns.12190. [DOI] [PubMed] [Google Scholar]
- 100.Barbi L, Coelho AVC, Alencar LCA, Crovella S. Prevalence of Guillain-Barre syndrome among Zika virus infected cases: a systematic review and meta-analysis. Braz J Infect Dis. 2018;22:137–141. doi: 10.1016/j.bjid.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dirlikov E, Major CG, Mayshack M, et al. Guillain-Barre syndrome during ongoing Zika virus transmission - Puerto Rico, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:910–914. doi: 10.15585/mmwr.mm6534e1. [DOI] [PubMed] [Google Scholar]
- 103.Grijalva I, Grajales-Muniz C, Gonzalez-Bonilla C, et al. Zika and dengue but not chikungunya are associated with Guillain-Barre syndrome in Mexico: a case-control study. PLoS Negl Trop Dis. 2020;14:e0008032. doi: 10.1371/journal.pntd.0008032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kassavetis P, Joseph JM, Francois R, Perloff MD, Berkowitz AL. Zika virus-associated Guillain-Barre syndrome variant in Haiti. Neurology. 2016;87:336–337. doi: 10.1212/WNL.0000000000002759. [DOI] [PubMed] [Google Scholar]
- 105.Leonhard SE, Halstead S, Lant SB, et al. Guillain-Barre syndrome during the Zika virus outbreak in Northeast Brazil: an observational cohort study. J Neurol Sci. 2021;420:117272. doi: 10.1016/j.jns.2020.117272. [DOI] [PubMed] [Google Scholar]
- 106.Parra B, Lizarazo J, Jimenez-Arango JA, et al. Guillain-Barre syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 107.Leonhard SE, Tan CY, van der Eijk AA, et al. Antecedent infections in Guillain-Barre syndrome in endemic areas of arbovirus transmission: a multinational case-control study. J Peripher Nerv Syst. 2021;26:449–460. doi: 10.1111/jns.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutta D, Debnath M, Nagappa M, et al. Antecedent infections in Guillain-Barre syndrome patients from south India. J Peripher Nerv Syst. 2021;26:298–306. doi: 10.1111/jns.12459. [DOI] [PubMed] [Google Scholar]
- 109.Agarwal A, Vibha D, Srivastava AK, Shukla G, Prasad K. Guillain-Barre syndrome complicating chikungunya virus infection. J Neuro-Oncol. 2017;23:504–507. doi: 10.1007/s13365-017-0516-1. [DOI] [PubMed] [Google Scholar]
- 110.Ahmed S, Libman R, Wesson K, Ahmed F, Einberg K. Guillain-Barre syndrome: an unusual presentation of West Nile virus infection. Neurology. 2000;55:144–146. doi: 10.1212/WNL.55.1.144. [DOI] [PubMed] [Google Scholar]
- 111.Beshai R, Bibawy D, Bibawy J. Guillain-Barre syndrome secondary to West Nile Virus in New York City. Case Rep Infect Dis. 2020;2020:6501658. doi: 10.1155/2020/6501658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brizzi K. Neurologic manifestation of Chikungunya virus. Curr Infect Dis Rep. 2017;19:6. doi: 10.1007/s11908-017-0561-1. [DOI] [PubMed] [Google Scholar]
- 113.Joseph N, Piccione EA. Guillain-Barre syndrome triggered by West Nile virus: a rare case scenario. J Clin Neuromuscul Dis. 2019;21:54–56. doi: 10.1097/CND.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 114.Oehler E, Fournier E, Leparc-Goffart I, et al. Increase in cases of Guillain-Barre syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Euro Surveill. 2015;20:30079. doi: 10.2807/1560-7917.ES.2015.20.48.30079. [DOI] [PubMed] [Google Scholar]
- 115.Ravi V, Taly AB, Shankar SK, et al. Association of Japanese encephalitis virus infection with Guillain-Barre syndrome in endemic areas of south India. Acta Neurol Scand. 1994;90:67–72. doi: 10.1111/j.1600-0404.1994.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 116.Stegmann-Planchard S, Gallian P, Tressieres B, et al. Chikungunya, a risk factor for Guillain-Barre syndrome. Clin Infect Dis. 2020;70:1233–1235. doi: 10.1093/cid/ciz625. [DOI] [PubMed] [Google Scholar]
- 117.Xiang JY, Zhang YH, Tan ZR, Huang J, Zhao YW. Guillain-Barre syndrome associated with Japanese encephalitis virus infection in China. Viral Immunol. 2014;27:418–420. doi: 10.1089/vim.2014.0049. [DOI] [PubMed] [Google Scholar]
- 118.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12:1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27:361–368. doi: 10.1097/WCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carson PJ, Konewko P, Wold KS, et al. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin Infect Dis. 2006;43:723–730. doi: 10.1086/506939. [DOI] [PubMed] [Google Scholar]
- 122.Klee AL, Maidin B, Edwin B, et al. Long-term prognosis for clinical West Nile virus infection. Emerg Infect Dis. 2004;10:1405–1411. doi: 10.3201/eid1008.030879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murray KO, Garcia MN, Rahbar MH, et al. Survival analysis, long-term outcomes, and percentage of recovery up to 8 years post-infection among the Houston West Nile virus cohort. PLoS One. 2014;9:e102953. doi: 10.1371/journal.pone.0102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patel H, Sander B, Nelder MP. Long-term sequelae of West Nile virus-related illness: a systematic review. Lancet Infect Dis. 2015;15:951–959. doi: 10.1016/S1473-3099(15)00134-6. [DOI] [PubMed] [Google Scholar]
- 125.Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–1624. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- 126.Sejvar JJ, Curns AT, Welburg L, et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J Neuropsychol. 2008;2:477–499. doi: 10.1348/174866407X218312. [DOI] [PubMed] [Google Scholar]
- 127.Ronca SE, Dineley KT, Paessler S. Neurological sequelae resulting from encephalitic alphavirus infection. Front Microbiol. 2016;7:959. doi: 10.3389/fmicb.2016.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kleinschmidt-DeMasters BK, Beckham JD. West Nile virus encephalitis 16 years later. Brain Pathol. 2015;25:625–633. doi: 10.1111/bpa.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elsinga J, Gerstenbluth I, van der Ploeg S, et al. Long-term Chikungunya Sequelae in Curacao: burden, determinants, and a novel classification tool. J Infect Dis. 2017;216:573–581. doi: 10.1093/infdis/jix312. [DOI] [PubMed] [Google Scholar]
- 130.Schilte C, Staikowsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Aalst M, Nelen CM, Goorhuis A, Stijnis C, Grobusch MP. Long-term sequelae of chikungunya virus disease: a systematic review. Travel Med Infect Dis. 2017;15:8–22. doi: 10.1016/j.tmaid.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Aragao M, Brainer-Lima AM, Holanda AC, et al. Spectrum of spinal cord, spinal root, and brain MRI abnormalities in congenital Zika syndrome with and without arthrogryposis. AJNR Am J Neuroradiol. 2017;38:1045–1053. doi: 10.3174/ajnr.A5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Franca GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 134.Krauer F, Riesen M, Reveiz L, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barre syndrome: systematic review. PLoS Med. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yepez JB, Murati FA, Pettito M, et al. Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017;135:440–445. doi: 10.1001/jamaophthalmol.2017.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharp TM, Fischer M, Munoz-Jordan JL, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep. 2019;68:1–10. doi: 10.15585/mmwr.rr6801a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of Chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis. 2016;214:S471–S474. doi: 10.1093/infdis/jiw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Edelman R, Schneider RJ, Vejjajiva A, Pornpibul R, Voodhikul P. Persistence of virus-specific IgM and clinical recovery after Japanese encephalitis. Am J Trop Med Hyg. 1976;25:733–738. doi: 10.4269/ajtmh.1976.25.733. [DOI] [PubMed] [Google Scholar]
- 139.Roehrig JT, Nash D, Maldin B, et al. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed west nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.• Dengue vaccine: WHO position paper, September 2018 - Recommendations. Vaccine 2019;37:4848-4849. Article presenting updated WHO recommendations on dengue vaccines. [DOI] [PubMed]
- 141.Paz-Bailey G, Adams L, Wong JM, et al. Dengue vaccine: recommendations of the advisory committee on immunization practices, United States, 2021. MMWR Recomm Rep. 2021;70:1–16. doi: 10.15585/mmwr.rr7006a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torres JR, Falleiros-Arlant LH, Gessner BD, et al. Updated recommendations of the International Dengue Initiative expert group for CYD-TDV vaccine implementation in Latin America. Vaccine. 2019;37:6291–6298. doi: 10.1016/j.vaccine.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 143.Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- 144.Diseases CDoV-B. https://www.cdc.gov/ncezid/dvbd/index.html [online].
- 145.Abdelnabi R, Kovacikova K, Moesslacher J, et al. Novel class of Chikungunya virus small molecule inhibitors that targets the viral capping machinery. Antimicrob Agents Chemother. 2020;64. [DOI] [PMC free article] [PubMed]
- 146.Feibelman KM, Fuller BP, Li L, LaBarbera DV, Geiss BJ. Identification of small molecule inhibitors of the Chikungunya virus nsP1 RNA capping enzyme. Antivir Res. 2018;154:124–131. doi: 10.1016/j.antiviral.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kovacikova K, van Hemert MJ. Small-molecule inhibitors of Chikungunya virus: mechanisms of action and antiviral drug resistance. Antimicrob Agents Chemother. 2020;64. [DOI] [PMC free article] [PubMed]
- 148.Goo L, Debbink K, Kose N, et al. A protective human monoclonal antibody targeting the West Nile virus E protein preferentially recognizes mature virions. Nat Microbiol. 2019;4:71–77. doi: 10.1038/s41564-018-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kotaki T, Kurosu T, Grinyo-Escuer A, et al. An affinity-matured human monoclonal antibody targeting fusion loop epitope of dengue virus with in vivo therapeutic potency. Sci Rep. 2021;11:12987. doi: 10.1038/s41598-021-92403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wan SW, Chen PW, Chen CY, et al. Therapeutic effects of monoclonal antibody against dengue virus NS1 in a STAT1 knockout mouse model of dengue infection. J Immunol. 2017;199:2834–2844. doi: 10.4049/jimmunol.1601523. [DOI] [PubMed] [Google Scholar]
- 151.Chikungunya. https://news.vumc.org/2019/02/21/chikungunya-antibody-set-to-enter-clinical-trial/ [online].