ABSTRACT
Background
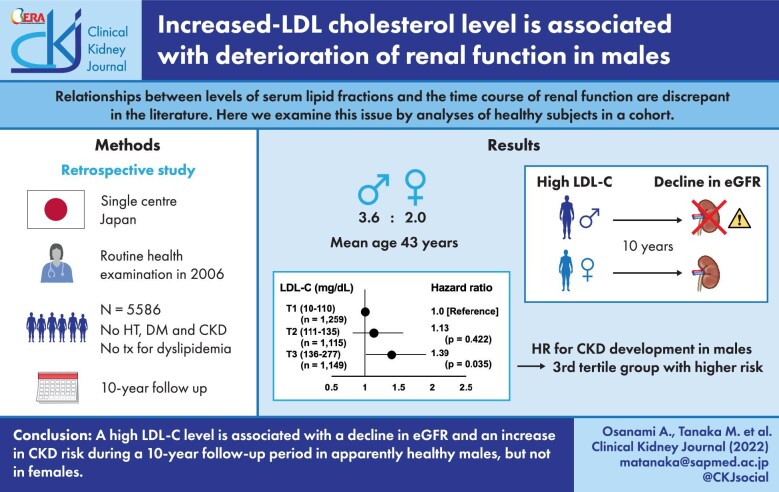
Relationships between levels of serum lipid fractions and the time course of renal function are discrepant in the literature. Here we examined this issue by analyses of healthy subjects in a cohort.
Methods
Of all subjects who received health examinations at Keijinkai Maruyama Clinic, Sapporo in 2006, subjects with hypertension, diabetes mellitus or chronic kidney disease (CKD) and those taking medication for dyslipidemia were excluded and a total of 5586 subjects (male/female: 3563/2023, mean age: 43 ± 8 years) were followed for 10 years.
Results
Linear mixed effect models showed that baseline low-density lipoprotein-cholesterol (LDL-C) level was negatively associated with estimated glomerular filtration rate (eGFR) during the 10-year follow-up period after adjustment for confounders. Interactions between the follow-up year and baseline level of LDL-C or high-density lipoprotein-cholesterol (HDL-C) for eGFR values during the follow-up period were significant in males but not in females. There were no significant interactions for eGFR between the follow-up year and baseline levels of total cholesterol, triglycerides, or HDL-C/triglycerides ratio. During the follow-up period, 346 males and 223 females developed CKD. When male subjects were divided into subgroups according to tertiles of baseline levels of LDL-C, the adjusted risk for CKD in the third tertial group was significantly higher than that in the first tertile group as a reference [hazard ratio (95% confidence interval): 1.39 (1.02–1.90), P = .035]. Such a difference was not observed for LDL-C tertiles in females or HDL-C tertiles in both sexes.
Conclusions
A high LDL-C level may be a risk factor for new-onset CKD in apparently healthy males.
Keywords: chronic kidney disease, dyslipidemia, high-density lipoprotein cholesterol, low-density lipoprotein-cholesterol
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Treatment of dyslipidemia is important for reducing major atherosclerotic events and its clinical benefits have also been shown in patients with chronic kidney disease (CKD), a risk factor of atherosclerosis [1, 2]. However, the impact of dyslipidemia on risk of CKD has not been fully characterized. To our knowledge, there have been five studies in which the relationships of disorders of lipid fractions with a decline in renal function and new onset of CKD were investigated, but the findings regarding a primary lipid fraction that is causally related to CKD are discrepant [3–7] (Supplementary data, Table S1). High levels of total cholesterol (TC), triglycerides (TG), TG-to-high-density-lipoprotein-cholesterol (HDL-C) ratio (TG/HDL-C), or low-density lipoprotein-cholesterol (LDL-C) and/or a low level of HDL-C have been reported to be risk factors for renal dysfunction in males or females depending on the study [3–7]. The reason for the discrepant results of earlier studies has not been clearly explained.
One possible explanation for the discrepant findings regarding serum lipids and CKD risk in earlier studies is different proportions of patients with comorbidities that increase CKD risk (such as hypertension and diabetes mellitus) in the study subjects [3–7]. Another possibility is a sex difference in renal sensitivity to detrimental effects of dyslipidemia. Sex differences in the risk of dyslipidemia for cardiovascular disease [8] and for the decline in eGFR [9] have been recognized, but there have been few studies in which differences between males and females in the relationships between levels of serum lipids and decline in eGFR were examined [3–7]. To avoid these potential problems, we enrolled subjects who did not have hypertension, diabetes mellitus or CKD in the present study. Longitudinal analyses using linear mixed effect models and Cox proportional hazard models were performed in male and female groups of the subjects to determine the associations between renal function, new onset of CKD and each of the lipid fractions including LDL-C, HDL-C, TC and TG.
MATERIALS AND METHODS
This study conformed to the principles outlined in the Declaration of Helsinki and was approved by the institutional ethical committee of Sapporo Medical University (Number: 29-2-64). Written informed consent was obtained from all subjects. This study was conducted as a project of the BOREAS (Broad-range Organization for REnal, Arterial and cardiac studies by Sapporo Medical University Affiliates) CKD investigators.
Study subjects and clinical endpoint
The present study was designed as a retrospective analysis of a cohort in which data have been prospectively collected. The cohort consists of individuals who received annual health examinations in Keijinkai Maruyama Clinic, a major health check-up institute in Sapporo, Japan. All individuals who received annual health examinations were initially enrolled (n = 28 990) for data retrieval in this study (Fig. 1). First, we excluded 7429 subjects with no data for blood pressure, no data for urinalysis, or no laboratory data for lipid variables, fasting plasma glucose, creatinine and eGFR in 2006. Next, we excluded 5484 subjects with eGFR <60 mL/min/1.73 m2, diabetes mellitus and/or hypertension or self-reported use of anti-dyslipidemic drugs at baseline. Subjects with TG level ≥400 mg/dL were also excluded in order to apply the Friedewald equation for the determination of the level of LDL-C. Finally, 10 491 subjects who did not receive health examinations in 2016 and at least once in the period from 2007 to 2015 were excluded. After exclusion, a total of 5586 subjects (male/female: 3523/2063) contributed to the present analyses. The characteristics of the enrolled and excluded subjects are shown in Supplementary data, Table S2. The clinical endpoint was new onset of CKD, defined as a decrease of eGFR to ˂60 mL/min/1.73 m2 [10] during a 10-year follow-up period. In a post hoc analysis, we repeated the analyses by using the chronicity criterion to define CKD: eGFR declined to ˂60 mL/min/1.73 m2 twice during the follow-up period.
FIGURE 1:

Selection of study participants. Among 28 990 individuals who received health examinations in 2006, a total of 5586 subjects (male/female: 3523/2063) were finally recruited for analyses in the present study. DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HT, hypertension.
Measurements
A self-administered questionnaire survey was performed to obtain information regarding smoking habits and medical history including treatment for hypertension, diabetes mellitus, dyslipidemia and ischemic heart disease. Medical examinations, blood pressure measurements and samplings of urine and blood were performed after an overnight fast. Proteinuria (0, 1+, 2+ or 3+) was evaluated by the dipstick method. Hematuria was defined by positivity (1+ or more) in the dipstick test. As an index of renal function, eGFR was calculated using the following equation: eGFR (mL/min/1.73 m2) = 194 × serum creatinine–1.094 × age–0.287 × 0.782 (if female) [11]. LDL-C was calculated by using the Friedewald equation. TG-to-HDL-C ratio (TG/HDL-C) was calculated as TG level (mg/dL) divided by HDL-C level (mg/dL).
Obesity was defined as body mass index (BMI) ≥25. Diabetes mellitus was diagnosed in accordance with the guidelines of the American Diabetes Association [12]: fasting plasma glucose ≥126 mg/dL or HbA1c ≥6.5% or self-reported use of anti-diabetic drugs. Hypertension was diagnosed as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or self-reported use of anti-hypertensive drugs. Ischemic heart disease was defined by self-reported treatment for ischemic heart disease.
Statistical analysis
Numeric variables are expressed as means ± SD for normal distributions or medians (interquartile ranges) for skewed distributions. The distribution of each parameter was tested for its normality using the Shapiro–Wilk test. Relationships between baseline levels of lipid fractions and absolute values of eGFR at the time of follow-up in each year and interactions between baseline lipid fractions and follow-up year for eGFR values during the follow-up period were examined by using linear mixed effect models. The models were adjusted for confounding factors including those used in earlier studies [5, 13, 14]: age, BMI, systolic blood pressure, smoking habit, hemoglobin, albumin, uric acid, fasting plasma glucose, hematuria and proteinuria at baseline. Cox proportional hazard model analysis was used to determine differences in risk for CKD among study subject groups divided by tertiles (T1∼T3) of baseline LDL-C or baseline HDL-C. The hazard ratio (HR) and 95% confidence interval (CI) of the T2 and T3 groups were calculated by use of the T1 group as the reference after adjustment for confounding factors. In the calculation of HR, we used confounding factors that were selected in earlier studies [5, 13, 14] (i.e., age, BMI, systolic blood pressure, smoking habit, hemoglobin, albumin, uric acid, fasting plasma glucose, hematuria and proteinuria at baseline) and new onsets of hypertension, diabetes mellitus and ischemic heart disease as time-dependent variables. A P-value of ˂.05 was considered statistically significant. All data were analyzed by using JMP 9.0.2 for Windows (SAS Institute, Cary, NC) and EZR [15].
RESULTS
Characteristics of the study subjects
Basal characteristics of recruited subjects are shown in Table 1. Female subjects had smaller BMI, smaller waist circumference, lower systolic and diastolic blood pressures, lower rate of smoking habit, lower levels of hemoglobin, albumin, blood urea nitrogen, creatinine, uric acid, fasting plasma glucose, TG, TG/HDL-C and LDL-C, higher rate of hematuria and higher levels of eGFR and HDL-C than did male subjects.
Table 1.
Characteristics of the subjects
| Total | Male | Female | |
|---|---|---|---|
| (n = 5586) | (n = 3523) | (n = 2063) | |
| Age (years) | 43 ± 8 | 44 ± 8 | 43 ± 8 |
| Body mass index | 22 ± 3 | 23 ± 3 | 21 ± 3 |
| Obesitya | 1690 (30.2) | 1386 (39.3) | 308 (14.9) |
| Waist circumference (cm) | 82 ± 9 | 84 ± 8 | 77 ± 8 |
| Systolic blood pressure (mmHg) | 111 ± 12 | 114 ± 11 | 106 ± 12 |
| Diastolic blood pressure (mmHg) | 71 ± 9 | 73 ± 8 | 67 ± 8 |
| Pulse rate (beats/min) | 62 ± 8 | 62 ± 8 | 63 ± 8 |
| Smoking habit | 2043 (36.5) | 1659 (47.0) | 384 (18.6) |
| Urinary data | |||
| Hematuria | 596 (10.6) | 174 (4.9) | 422 (19.3) |
| Proteinuria | 167 (2.9) | 93 (2.6) | 74 (3.5) |
| (1+) | 155 (2.7) | 86 (2.4) | 69 (3.3) |
| (2+) | 12 (0.2) | 7 (0.2) | 5 (0.2) |
| Biochemical data | |||
| Hemoglobin (g/dL) | 14.2 ± 1.5 | 15.1 ± 1.0 | 12.8 ± 1.1 |
| Albumin (g/dL) | 4.3 ± 0.2 | 4.4 ± 0.2 | 4.3 ± 0.2 |
| Blood urea nitrogen (mg/dL) | 13.8 ± 3.2 | 14.4 ± 3.2 | 12.8 ± 3.1 |
| Creatinine (mg/dL) | 0.71 ± 0.13 | 0.79 ± 0.09 | 0.58 ± 0.07 |
| eGFR (mL/min/1.73 m2) | 87.2 ± 13.5 | 86.1 ± 12.7 | 89.0 ± 14.5 |
| Uric acid (mg/dL) | 5.4 ± 1.4 | 6.0 ± 1.1 | 4.2 ± 0.8 |
| FPG (mg/dL) | 88 ± 8 | 89 ± 8 | 85 ± 7 |
| Hemoglobin A1c (%) | 5.1 ± 0.3 | 5.1 ± 0.3 | 5.1 ± 0.3 |
| C-reactive protein (mg/dL) | 0.05 (0.05–0.08) | 0.05 (0.05–0.09) | 0.05 (0.05–0.06) |
| LDL-C (mg/dL) | 119 ± 30 | 122 ± 30 | 113 ± 29 |
| HDL-C (mg/dL) | 61 ± 15 | 56 ± 13 | 70 ± 15 |
| TC (mg/dL) | 201 ± 32 | 202 ± 32 | 198 ± 32 |
| TG (mg/dL) | 85 (58–127) | 104 (74–149) | 60 (46–84) |
| TG/HDL-C | 1.43 (0.84–2.41) | 1.90 (1.21–3.02) | 0.85 (0.61–1.29) |
| Comorbidity | |||
| Ischemic heart disease | 25 (0.4) | 23 (0.6) | 2 (0.0) |
Variables are expressed as number (%), means ± SD or medians (interquartile ranges).
aObesity was defined as body mass index ≥25.
eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Of the 5586 subjects (male/female: 3523/2063), 10.2% of the subjects (n = 569, male/female: 346/223) developed new-onset of CKD during the follow-up period. The mean follow-up period was 9.5 years (range: 1–10 years) and follow-up summation was 53 764 (male/female: 34 075/19 689) persons-years. Of the 569 subjects with new-onset of CKD, the numbers of subjects who were categorized in G5 (eGFR <15), G4 (eGFR of 15–29), G3b (eGFR of 30–44) and G3a (eGFR of 45–59) stages of CKD [10] were 1, 2, 32 and 534, respectively. Characteristics of subjects who developed CKD and subjects who did not develop CKD during the follow-up period are shown in Supplementary data, Table S3. Subjects who developed CKD were older than subjects who did not develop CKD and had a lower level of eGFR and higher levels of LDL-C, TC, TG and TG/HDL-C than those in subjects who did not develop CKD. The level of HDL-C tended to be lower in subjects who developed CKD. During the follow-up period, 1414 subjects (male/female: 1100/314) and 258 subjects (male/female: 203/55) developed hypertension and diabetes mellitus, respectively. The number of subjects with ischemic heart disease was 25 (male/female: 23/2) at baseline (Table 1) and 176 subjects (male/female: 137/39) developed ischemic heart disease during the follow-up period.
Of the 569 subjects with new-onset of CKD during the follow-up period, 257 males and 172 females met the chronicity criterion in post hoc analysis. These numbers of CKD events are likely to have been underestimated since 10 491 subjects who could not be assessed for chronicity because they received the examination less than three times were excluded.
Associations between baseline levels of lipid fractions and eGFR during the follow-up period
Results of analyses of the linear mixed effect for eGFR during the follow-up period are summarized in Table 2. Follow-up year and LDL-C level at baseline were negatively associated with eGFR during the follow-up period in both male and female subjects (Model 1). There was a significant and negative interaction between follow-up year and level of LDL-C at baseline for eGFR during the follow-up period in male subjects but not in female subjects (Model 1). HDL-C level at baseline was not significantly associated with eGFR during the follow-up period in either male or female subjects (Model 2). There was a significant and positive interaction between the follow-up year and baseline HDL-C level for eGFR during the follow-up period only in male subjects. Among the lipid fractions including TC, TG and TG/HDL-C, only TC level at baseline was negatively associated with eGFR during the follow-up in male subjects but not in female subjects (Model 3). There was no significant interaction between the follow-up year and the baseline level of TC, TG or HDL-C/TG for eGFR during the follow-up period (Models 3–5). Visual depictions of linear mixed models for eGFR during the follow-up period in male and female subjects with low (50 mg/dL) and high (250 mg/dL) levels of baseline LDL-C are shown in Supplementary data, Fig. S1; the rate of eGFR decline during the follow-up period appears to be larger in male subjects with high baseline LDL-C than in those with low baseline LDL-C, while such a difference was not observed in female subjects.
Table 2.
Linear mixed effect models for eGFR during the follow-up period
| Male (n = 3563) | Female (n = 2023) | |||||
|---|---|---|---|---|---|---|
| Fixed-effect coefficient estimates (95% CI) | SE | P | Fixed-effect coefficient estimates (95% CI) | SE | P | |
| Model 1a | ||||||
| Follow-up year | −1.320 (−2.277, −0.826) | 0.366 | <.001 | −1.248 (−2.103, −0.307) | 0.437 | .004 |
| LDL-C at baseline | −0.022 (−0.036, −0.002) | 0.009 | .011 | −0.031 (−0.052, −0.002) | 0.013 | .015 |
| Interaction (follow-up year and LDL-C at baseline) | −0.001 (−0.002, −0.000) | 0.000 | .036 | 0.001 (−0.000, 0.002) | 0.001 | .238 |
| (AIC = 153 492) | (AIC = 93 982) | |||||
| Model 2a | ||||||
| Follow-up year | −1.444 (−2.489, −1.016) | 0.368 | <.001 | −1.320 (−2.218, −0.428) | 0.435 | .002 |
| HDL-C at baseline | 0.011 (−0.037, 0.039) | 0.020 | .549 | 0.006 (−0.046, 0.044) | 0.023 | .798 |
| Interaction (follow-up year and HDL-C at baseline) | 0.004 (0.001, 0.006) | 0.001 | <.001 | 0.000 (−0.002, 0.002) | 0.001 | .733 |
| (AIC = 155 186) | (AIC = 93 985) | |||||
| Model 3a | ||||||
| Follow-up year | −1.300 (−2.221, −0.800) | 0.362 | <.001 | −1.263 (−2.127, −0.371) | 0.436 | .003 |
| TC at baseline | −0.002 (−0.032, −0.000) | 0.001 | .031 | −0.021 (−0.041, 0.003) | 0.012 | .071 |
| Interaction (follow-up year and TC at baseline) | −0.000 (−0.001, 0.001) | 0.000 | .799 | 0.001 (−.0001, 0.002) | 0.001 | .267 |
| (AIC = 155 199) | (AIC = 93 985) | |||||
| Model 4a | ||||||
| Follow-up year | −1.328 (−2.235, −0.811) | 0.369 | <.001 | −1.368 (−2.245, −0.480) | 0.445 | .002 |
| Log TG at baseline | −0.235 (−1.071, 1.069) | 0.542 | .665 | 0.252 (−1.170, 2.061) | 0.814 | .756 |
| Interaction (follow-up year and LogTG at baseline) | 0.019 (−0.039, 0.075) | 0.029 | .506 | 0.027 (−0.060, 0.122) | 0.046 | .551 |
| (AIC = 155 187) | (AIC = 93 970) | |||||
| Model 5a | ||||||
| Follow-up year | −1.329 (−2.167, −0.387) | 0.369 | <.001 | −1.294 (−2.167, −0.387) | 0.437 | .003 |
| Log TG/HDL-C at baseline | −0.212 (−0.892, 1.665) | 0.426 | .619 | 0.141 (−0.892, 1.665) | 0.644 | .827 |
| Interaction (follow-up year and LogTG/HDL-C at baseline) | −0.017 (−0.057, 0.087) | 0.369 | .461 | 0.010 (−0.057, 0.871) | 0.036 | .788 |
| (AIC = 155 188) | (AIC = 93 972) | |||||
aThe models were adjusted for age, body mass index, systolic blood pressure, smoking habit, hemoglobin, albumin, uric acid, fasting plasma glucose, hematuria and proteinuria at baseline. Hematuria and proteinuria (1+ or more) were evaluated by the dipstick method.
eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides. AIC, Akaike's information criterion; CI, confidence interval; SE, standard error.
Relationships between baseline levels of lipid fractions and incidence of CKD during the follow-up period in males
Since significant associations of baseline levels of LDL-C and HDL-C with the subsequent decline of eGFR were found in males but not in females (Table 2), impacts of baseline LDL-C and HDL-C levels on incidence of new-onset of CKD were assessed in male subjects. Male subjects were divided into three subgroups according to tertiles in levels of LDL-C and HDL-C at baseline (T1∼T3). Cox proportional hazard model analysis showed that the HRs for new onset of CKD in the T2 and T3 groups of LDL-C were significantly higher than that in the T1 group of LDL-C in the unadjusted model (Fig. 2A, left panel). After adjustment for age, BMI, systolic blood pressure, smoking habit, hemoglobin, albumin, uric acid, fasting plasma glucose, hematuria and proteinuria at baseline and also new onsets of hypertension, diabetes mellitus and ischemic heart disease as time-dependent variables, HR in the T3 group of LDL-C was still significantly higher than that in the T1 group of LDL-C [HR: 1.39 (95% CI: 1.02–1.90), P = .035] as shown in the right panel of Fig. 2A. Although the HR for the new onset of CKD in the T3 group of HDL-C was significantly lower than that in the T1 group of HDL-C in the unadjusted model, there was no significant difference in the incidence of CKD between the three subgroups of HDL-C after adjustment for the above covariates (Fig. 2B). We conducted a similar analysis for the tertiles of non-HDL-C at baseline in males. HR in the T3 group of non-HDL-C was 1.81 (95% CI: 1.39–2.37, P <.001) compared with the T1 group as a reference in an unadjusted model and HR tended to be high [HR: 1.36 (95% CI: 0.98–1.87), P = .060] in the model with adjustment for the confounding variables stated above.
FIGURE 2:
Hazard ratio of chronic kidney disease (CKD) development by levels of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) at baseline in male subjects. (A, B) Cox proportional hazard models for the development of CKD defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 in first tertile (T1), second tertile (T2) and third tertile (T3) of levels of LDL-C (A) and HDL-C (B) at baseline. Adjusted model: adjustment for age, body mass index, systolic blood pressure, smoking habit, hemoglobin, albumin, uric acid, fasting plasma glucose, hematuria and proteinuria at baseline and new onset of hypertension, diabetes mellitus and ischemic heart disease during the follow-up period. CI, confidence interval; HR, hazard ratio.
As a post hoc analysis, Cox proportional hazard model analysis was repeated by using the chronicity criterion to define the new onset of CKD. As shown in Supplementary data, Fig. S2, results of the post hoc analysis also showed that HRs in the T3 group of LDL-C were significantly higher than those in the T1 group of LDL-C in both the unadjusted model [HR: 1.67 (95% CI: 1.32–2.11), P ≤ .001] and the adjusted model [HR: 1.304 (95% CI: 1.001–1.701), P = .049].
DISCUSSION
To our knowledge, this study is the first longitudinal study in which the relationships of comprehensive lipid fractions with the time course of renal function and new onset of CKD during a follow-up period of 10 years were investigated using linear mixed effect models in subjects with neither hypertension nor diabetes. In male subjects, there was a significant interaction between follow-up year and LDL-C at baseline for eGFR during the follow-up period, suggesting that a higher level of LDL-C at baseline is associated with a larger decline in eGFR during the follow-up period. Cox proportional hazard model analysis showed that a high level of LDL-C was a significant risk factor for the new onset of CKD. Interaction between follow-up year and HDL-C level at baseline was positively associated with the eGFR during the follow-up period in male subjects, but a low level of HDL-C was not a significant risk factor for the new onset of CKD. On the other hand, there was no significant interaction for eGFR during the follow-up period between the level of each of the lipid fractions and the follow-up year in overall female subjects. These findings suggest that a high level of LDL-C at baseline is associated with new onset of CKD in apparently healthy males, though such an impact of LDL-C level on the time course of renal function may not be significant in females.
The relationships between lipid fractions and the new onset of CKD in a general population have been reported by five groups of investigators [3–7] as summarized in Supplementary data, Table S1. The earlier studies differ in characteristics of study subjects, but HDL-C and/or TG/HDL-C were associated with a decline in renal function in four of the five studies [3–6]. Interestingly, such an impact of dyslipidemia on renal function has been observed in groups of subjects with comorbidities; an association of increased TG/HDL-C level with decline in renal function was reported for patients with type 2 diabetes mellitus [16] and patients with hypertension [17]. In contrast to the four earlier studies in general populations [3–6], a study by Kuma et al. [7] showed that an increased LDL-C level, but not an increased level of HDL-C or TG, was associated with a decline in eGFR in males without hypertension or diabetes mellitus, while such an association was not observed in males with hypertension and/or diabetes mellitus. Taken together, the results of the present study and the study by Kuma et al. [7] support the notion that elevation of LDL-C is significantly associated with a subsequent decline in eGFR in apparently healthy males and suggest that the discrepancy in the relationship of decline in eGFR with HDL-C or TG in earlier studies is due to different proportions of study subjects with pre-existing hypertension and diabetes mellitus, major risk factors of CKD [5, 18].
An association between baseline LDL-C level and subsequent decline of eGFR was observed in males but not in females (Table 2). The reason for this sex difference is unclear, but there are a few possibilities. First, renal sensitivity to detrimental effects of CKD risk factors might be higher in males than in females. The natural history of decline in eGFR with aging has been reported to be slower in females than in males [19] and multiple mechanisms have been proposed for the sex difference in decline in eGFR [9]. One of the mechanisms is the action of estrogen, which reduces renal damage through its antifibrotic and anti-apoptotic effects [20–22], its effect on nitric oxides [23] and its antioxidant effects [24–26]. In contrast, testosterone has pro-inflammatory, pro-apoptotic and pro-fibrotic effects [27, 28], possibly facilitating renal injury. Although estrogen decreases sharply after menopause, 1788 (86.6%) of the 2063 female subjects were under 52 years of age, the average menopause age in Japanese women [29], and thus were likely to be at pre-menopause. Second, the difference in qualities of LDL-C might contribute to the sex difference in effects of LDL-C on eGFR. Levels of oxidized LDL-C can be different between male and female populations with different levels of central obesity [30] and the levels of several enzymes influencing lipoprotein subclass distribution (such as lipoprotein lipase, cholesterol ester transfer protein and paraoxonase) are regulated by sex hormones [31]. Third, a lack of sufficient statistical power might be responsible for the apparent absence of an association between baseline LDL-C and GFR decline during the follow-up period in females. The number of enrolled male subjects was about 1.5-times larger than that of female subjects. These possible explanations need to be examined by a detailed analysis of serum lipid subclasses and qualities in a larger study population.
The lipid nephrotoxicity hypothesis has recently been proposed [32, 33], and possible mechanisms of the toxicity are inflammation, oxidative stress and endothelial dysfunction. Dyslipidemia increases reactive oxygen species and the increase in reactive oxygen species causes impairment of endothelium-dependent relaxation [32, 33] and is associated with elevation of oxidized LDL-C level [34]. Oxidized LDL-C induces inflammation at the microvascular level and promotes glomerular sclerosis via signaling of angiotensin II/receptor or transforming growth factor-β/receptors [35–37]. Whether such a mechanism underlies the increased risk of CKD by a high level of LDL-C in males (Fig. 2) remains to be elucidated.
Recently, the impact of dyslipidemia on the development of CKD was investigated by Mendelian randomization analysis [38, 39]. A study by Emanuelsson et al. indicated a significant relationship between LDL-C and a high risk of CKD [38], being consistent with the present observations. They used a weighted allele score, which reflects the combined effects of the included genetic variants on circulating LDL-C levels and increases study power. In contrast, Lanktree et al. did not find such an independent relationship between LDL-C and the risk of CKD. However, the study subjects had a higher prevalence of diabetes mellitus or hypertension and there was a more heterogenous comorbidity status in the study by Lanktree et al. [39] than those in the study by Emanuelsson et al. [38]. The differences in the background of study subjects are plausible explanations for the discrepancy.
The present study has some limitations. First, the possibility of selection bias in the samples cannot be excluded since the study subjects were not randomized and were urban residents who received annual health checkups in a single clinic. In the present study, a total of 10 491 subjects who did not receive health examinations in 2016 and at least once in the period from 2007 to 2015 were excluded. Annual medical checkups for employees in a company are obligatory in Japan, and most of the recruited subjects were employees of companies near the clinic. Some of the subjects would have moved to other cities during the 10-year period. Second, since proteinuria and hematuria were determined by the dipstick method, incipient kidney diseases might not have been detected in this study. Third, because subjects who had TG ≥400 mg/dL were excluded to calculate LDL-C by the Friedewald formula, the impact of hypertriglyceridemia on renal function could not be examined. Finally, since most of the subjects in the present study were in middle age, the present observations might not be directly extrapolated to elderly people.
In conclusion, the results of the present study using mostly middle-aged subjects suggest that a high level of LDL-C is associated with a decline in eGFR and an increase in CKD risk during the following 10 years in apparently healthy males. Such an association of LDL-C level with change in eGFR was not detected in apparently healthy females.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by Grants for Education and Research 2020 and 2021 from Sapporo Medical University. M.T. and M.F. were supported by grants from Japan Society for the Promotion of Science (19K08708, 20K08913).
Contributor Information
Arata Osanami, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Marenao Tanaka, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Masato Furuhashi, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Hirofumi Ohnishi, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan; Department of Public Health, Sapporo Medical University School of Medicine, Sapporo, Japan.
Nagisa Hanawa, Department of Health Checkup and Promotion, Keijinkai Maruyama Clinic, Sapporo, Japan.
Tomohisa Yamashita, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Norihito Moniwa, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Tetsuji Miura, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
CONFLICT OF INTEREST STATEMENT
The authors of this manuscript have no conflict of interest to disclose.
REFERENCES
- 1. Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014; 85: 1303–1309 [DOI] [PubMed] [Google Scholar]
- 2. Sandhu S, Natasha W, Linda Fet al. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17: 2006–2016 [DOI] [PubMed] [Google Scholar]
- 3. Schaeffner ES, Kurth T, Curhan GCet al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol 2003; 14: 2084–2091 [DOI] [PubMed] [Google Scholar]
- 4. Bae JC, Han JM, Kwon Set al. LDL-C/apoB and HDL-C/apoA-1 ratios predict incident chronic kidney disease in a large apparently healthy cohort. Atherosclerosis 2016; 251: 170–176 [DOI] [PubMed] [Google Scholar]
- 5. Yamagata K, Ishida K, Sairenchi Tet al. Risk factors for chronic kidney disease in a community-based population. A 10-year follow-up study. Kidney Int 2007; 71: 159–166 [DOI] [PubMed] [Google Scholar]
- 6. Tsuruya K, Yoshida H, Nagata Met al. Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: A longitudinal study in a large Japanese population. Am J Kidney Dis 2016; 66: 972–983 [DOI] [PubMed] [Google Scholar]
- 7. Kuma A, Uchino B, Ochiai Yet al. Impact of low-density lipoprotein cholesterol on decline in estimated glomerular filtration rate in apparently healthy young to middle-aged working men. Clin Exp Nephrol 2018; 22: 15–27 [DOI] [PubMed] [Google Scholar]
- 8. Mach F, Baigent C, Catapano ALet al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–188 [DOI] [PubMed] [Google Scholar]
- 9. Carrero JJ, Hecking M, Chesnaye NCet al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018; 14: 151–164 [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013). KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 11. Matsuo S, Imai E, Horio Met al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes Association . Classification and diagnosis of diabetes. Diabetes Care 2017; 40: S11–S24 [DOI] [PubMed] [Google Scholar]
- 13. Iseki K, Ikemiya Y, Inoue Tet al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004; 44: 642–650 [PubMed] [Google Scholar]
- 14. Kubo S, Kitamura A, Imano Het al. Serum albumin and high-sensitivity C-reactive protein are independent risk factors of chronic kidney disease in middle-aged Japanese individuals: the circulatory risk in communities study. J Atheroscler Thromb 2016; 23: 1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 2013; 48: 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zoppini G, Negri C, Stoico Vet al. Triglyceride-high-density lipoprotein cholesterol is associated with microvascular complications in type 2 diabetes mellitus. Metabolism 2012; 61: 22–29 [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Wang B, Yang Jet al. Serum lipids and risk of rapid renal function decline in treated hypertensive adults with normal renal function. Am J Hypertens 2019; 32: 393–401 [DOI] [PubMed] [Google Scholar]
- 18. Webster AC, Nagler E V, Morton RLet al. Chronic kidney disease. Lancet 2017; 389: 1238–1252 [DOI] [PubMed] [Google Scholar]
- 19. Valdivielso JM, Jacobs-Cachá C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens 2019; 28: 1–9 [DOI] [PubMed] [Google Scholar]
- 20. Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 2004; 15: 1546–1556 [DOI] [PubMed] [Google Scholar]
- 21. Catanuto P, Doublier S, Lupia Eet al. 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int 2009; 75: 1194–1201 [DOI] [PubMed] [Google Scholar]
- 22. Hutchens MP, Fujiyoshi T, Komers Ret al. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 2012; 303: F377–F385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nat Rev Nephrol 2009; 5: 384–396 [DOI] [PubMed] [Google Scholar]
- 24. Valdivielso JM, Jacobs-Cachá C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens 2019; 28: 1–9 [DOI] [PubMed] [Google Scholar]
- 25. Ruiz-Larrea MB, Leal AM, Martin Cet al. Antioxidant action of estrogens in rat hepatocytes. Rev Esp Fisiol 1997; 53: 225–229 [PubMed] [Google Scholar]
- 26. Borrás C, Gambini J, Gómez-Cabrera MCet al. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell 2005; 4: 113–118 [DOI] [PubMed] [Google Scholar]
- 27. Metcalfe PD, Leslie JA, Campbell MTet al. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab 2008; 294: E435–E443 [DOI] [PubMed] [Google Scholar]
- 28. Reckelhoff JF, Zhang H, Srivastava K.. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 2000; 35: 480–483 [DOI] [PubMed] [Google Scholar]
- 29. Yasui T, Hayashi K, Mizunuma Het al. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas 2012; 72: 249–255 [DOI] [PubMed] [Google Scholar]
- 30. Hermsdorff HH, Barbosa KB, Volp ACet al. Gender-specific relationships between plasma oxidized low-density lipoprotein cholesterol, total antioxidant capacity, and central adiposity indicators. Eur J Prev Cardiol 2014; 21: 884–891 [DOI] [PubMed] [Google Scholar]
- 31. Mascarenhas-Melo F, Marado D, Palavra Fet al. Diabetes abrogates sex differences and aggravates cardiometabolic risk in postmenopausal women. Cardiovasc Diabetol 2013; 12: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol 2009; 12: 713–721 [DOI] [PubMed] [Google Scholar]
- 33. Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol 2015; 310: F433–F445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vasconcelos EM, Degasperi GR, de Oliveira HCet al. Reactive oxygen species generation in peripheral blood monocytes and oxidized LDL are increased in hypercholesterolemic patients. Clin Biochem 2009; 42: 1222–1227 [DOI] [PubMed] [Google Scholar]
- 35. Lee HS, Song CY. Oxidized low-density lipoprotein and oxidative stress in the development of glomerulosclerosis. Am J Nephrol 2009; 29: 62–70 [DOI] [PubMed] [Google Scholar]
- 36. Peric-Golia L, Peric-Golia M. Aortic and renal lesions in hypercholesterolemic adult, male, virgin Sprague-Dawley rats. Atherosclerosis 1983; 46: 57–65 [DOI] [PubMed] [Google Scholar]
- 37. Kasiske BL, O'Donnell MP, Schmitz PGet al. Renal injury of diet-induced hypercholesterolemia in rats. Kidney Int 1990; 37: 880–891 [DOI] [PubMed] [Google Scholar]
- 38. Emanuelsson F, Nordestgaard BG, Tybjærg-Hansen Aet al. Impact of LDL cholesterol on microvascular versus macrovascular disease. J Am Coll Cardiol 2019; 74: 1465–1476 [DOI] [PubMed] [Google Scholar]
- 39. Lanktree MB, Thériault S, Walsh Met al. HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: a Mendelian Randomization Study. Am J Kidney Dis 2018; 71: 166–172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.