Abstract
Axons differ in their growth potential: whereas during development, axons rapidly grow to their targets, in the adult mammalian, CNS axons have lost their ability to grow and therefore fail to regenerate. Recent progress has enabled a better understanding of how developmental mechanisms direct axon regeneration. Focusing on neuronal polarization, where one neurite is singled out to become the axon, has uncovered the mechanisms initiating axon growth and growth restraint. This has helped to define the processes that need to be reactivated to induce axon regeneration: microtubule stabilization and actin dynamics. The molecular machinery underlying axon growth and axon regeneration is remarkably similar and includes the Rho-GTPases Cdc42, Rac-1, and RhoA, as well as the actin regulators cofilin and Myosin II. Importantly, neuron-intrinsic growth inhibitors in the adult nervous system, including the voltage-gated calcium channel subunit α2δ2 and the presynaptic active zone protein Munc13, restrain dynamics while the components driving axon growth remain largely present. The identified molecules suggest that synaptic transmission and axon growth may be processes that exclude each other. As a result, axon regeneration may be hampered by synaptic transmission and, thus, by the maturation of the CNS. This research has led to several translational avenues to induce axon regeneration and functional recovery after spinal cord injury and stroke; these include the drugs epothilones, gabapentinoids, and baclofen. Thus, the investigation of axon growth and regeneration side by side has been instrumental to coax the regenerative potential of the CNS.
Introduction
Most of us are fascinated by the complexity of the nervous system, which allows us to learn, generate, and control emotions, sense the environment and our own body, and direct muscles; occasionally, it is even used to think. A large part of the network relies on axons, propagating information in the form of action potentials. Axonal morphology is special: axons in humans reach lengths of up to 2 m, and they are even longer in larger animals. However, axons are also thin, with a diameter of around 1 µm. Thus, it almost comes as a surprise that these seemingly fragile structures remain intact for the whole lifespan of a mammal, where neurons, once differentiated, are not replenished, at least not to a large extent.
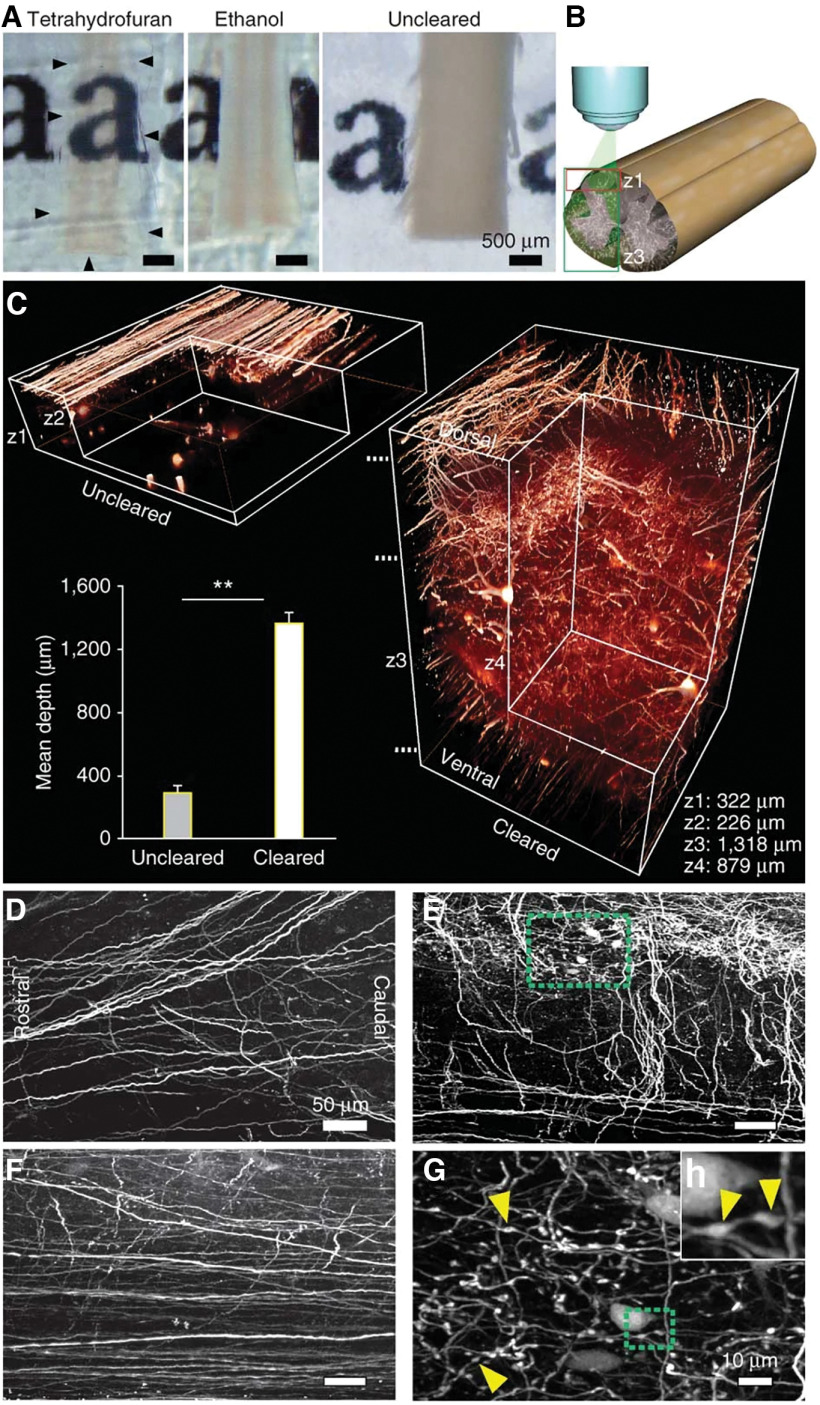
Since the times of Ramon y Cajal, it is well known that axons fail to regenerate in the CNS (Ramón y Cajal, 1928). This can lead to irreversible loss of functions associated with affected axons after insult or with age. Studying the degeneration and regeneration of the nervous system was through a tedious process, as our understanding of the wiring of the adult CNS was hampered by technical and methodological capacities. This changed with the development of 3D imaging of solvent-cleared organs (3-DISCO), which Ali Ertürk developed in our lab together with Hans-Ulrich Dodt (Fig. 1; Ertürk et al., 2011; Ertürk and Bradke, 2013). This pioneering work was followed by further developments from members of our laboratory and those of other research groups (Chung et al., 2013; Kuwajima et al., 2013; Ertürk et al., 2014; Susaki et al., 2014; Pan et al., 2016; Hilton et al., 2019). This provided unprecedented insights into the wiring of the nervous system, opening up a new view.
Figure 1.
Clearance allows high-resolution imaging of the unsectioned spinal cord. A, Clearance with THF renders the adult mouse spinal cord transparent (outlined by black arrowheads) while ethanol-treated tissue remains opaque (photographic images are shown). B, Schematic illustration of the two-photon–imaged regions and depths: only dorsal (z1) or entire dorsoventral (z3) spinal cord. C, Comparison between uncleared (left) and cleared (right) spinal cords of GFP-M mice imaged with two-photon microscopy. Values are mean ± SD. **p < 0.01. D–G, Horizontal projections from the cleared spinal cord at different depths marked in C: dorsal (∼100 μm; D), mid (∼500 μm; E), and ventral (∼1200 μm; F). G, Higher magnification of the area marked in green in E. H, Higher magnification of the area marked in green in G. Yellow arrowheads in G, H depict some of the axonal boutons in the gray matter. From Ertürk et al. (2011).
While those in the neuroscience community dealing with the diseased nervous system aim to find ways to elicit regeneration from frustratingly stubborn axons, developmental neurobiologists have a refreshingly different view of the axon. In their experience, neurons are experts in extending axons: only through their rapid growth and guidance can axons reach their targets to connect through synapses. Would it not be appealing to learn how neurons grow their axons during development? Once these mechanisms are better understood, one could reactivate them in the adult nervous system under pathologic conditions, such as after spinal cord injury, to induce axon regeneration. Obviously, in the adult nervous system, the regenerating axons would need to grow larger distances than in that of the embryo. Tuning the motor that drives axon growth will be essential for any regenerative growth. Moreover, beneficial integration of the regenerating axon does not necessarily need to occur at its original target, so that even axon growth of smaller distances could lead to functional improvements.
Neuronal Polarization: Growth versus Growth Restraint within a Single Neuron
During development, growth and growth restraint occur simultaneously. When hippocampal and cortical excitatory neuron polarize, one among multiple neurites starts to grow out rapidly, becoming the axon. The other neurites are restrained from growing at that stage and later become dendrites. The phenomenological description of this process by Carlos Dotti while he was in the laboratory of Gary Banker (Dotti et al., 1988) excited the community. Instantly, it raised the question as to how neurons polarize: how is it that they single out one neurite to grow, yet keep the other neurites restrained? This was the question that excited me, prompting me to join the lab of Carlos Dotti in the Cell Biology Programme at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany. His laboratory and the discussions with him during our daily morning coffee, ranging from science, policy, to football and literature, gave an irreversible and irresistible imprint. This, together with our PhD class of 1995 that also included my future wife Gaia Tavosanis, gave me an unforgettable start into research.
All neurites have the potential to become the axon, as axotomy experiments demonstrated early on in research (Dotti and Banker, 1987; Goslin and Banker, 1989). One prevalent idea in the polarization field was that a tug of war among the different neurites is what singles out the axon, such as by different pulling forces of the growth cones leading the neurites (Craig and Banker, 1994). This was largely fed by two lines of research, where first, Heidemann and Buxbaum showed in a number of elegant studies that pulling a neurite of various neurons with a calibrated glass pipette leads to its extension (Dennerll et al., 1989; Lamoureux et al., 1989, 1997; Zheng et al., 1993; Chada et al., 1997). Second, Mitchison and Kirschner proposed their clutch model of fibroblast migration, in which actin filaments move from the leading edge to the cell body, driven by an actin motor (Mitchison and Kirschner, 1988). Coupling the actin flow to the substrate, e.g., by adhesion sites, the clutch, and the traction, enables the fibroblast to pull forward along the substrate (Mitchison and Kirschner, 1988). From this perspective, axon growth could be driven by a similar mechanism: the growth cone might pull through actin-myosin adhesion along the substrate, pulling the axon behind.
While the idea that the growth cone might act as a fibroblast on a leash was appealing, and provided hope for a unifying migration model, it also raised questions. First, observations of growth cone movement along substrates argued that the forces may not be sufficient to extend the axon (Goldberg and Burmeister, 1986). Second, Mark Bretscher pointed out in a remarkable review that axons can extend without actin filaments (Bretscher, 1996). Forscher and Smith also provided a key piece in our understanding of growth cone actin in stationary Aplysia growth cones (Forscher and Smith, 1988). They showed that on pharmacological actin depolymerization, microtubules rapidly infiltrate the peripheral area of the growth cone, formerly occupied by the actin network. I therefore wondered whether the minor neurites are actively restrained from growing by a dense actin network, which prevents microtubules from protruding. Indeed, on actin depolymerization using cytochalasin or latrunculin, the typical restraint on development of polarity was lifted. Instead of one axon, multiple axons initiated their growth (Bradke and Dotti, 1999), even after one neurite was specified to become the axon (Bradke and Dotti, 2000). This, together with the enhanced traffic of membrane and organelles in the future axons (Bradke and Dotti, 1997), provided insights into the early events of neuronal polarization. It enabled our and other laboratories to further dissect, both molecularly and pharmacologically, the processes involved in a remarkable way (Jareb and Banker, 1997; Kunda et al., 2001; Schwamborn and Püschel, 2004; Garvalov et al., 2007; Montenegro-Venegas et al., 2010; Tahirovic et al., 2010; Flynn et al., 2012; Dupraz et al., 2019; Tedeschi et al., 2019). Only recently, Telma Santos and Barbara Schaffran demonstrated that developing CNS neurons indeed grow their axon in 3 dimensions (3D) very differently compared with fibroblasts: whereby CNS axons grow in an amoeboid movement without pulling on the substrate (Santos et al., 2020).
Axon Regeneration: Growth versus Growth Restraint
It became tempting to speculate that the concept of growth and growth restraint could be employed to induce axon regeneration in the adult nervous system. In the late 1980s, various proteins present at the injury site were identified to inhibit axon regeneration after injury. These proteins included several myelin factors, including Nogo-A and myelin-associated glycoprotein (MAG; Schwab and Caroni, 1988; Mukhopadhyay et al., 1994; Schwab and Strittmatter, 2014) as well as factors present in the scar formed after injury, including chondroitin sulfate proteoglycans (CSPGs; McKeon et al., 1995; Carulli et al., 2005). While the blockade of some these factors individually led to remarkable regrowth in preclinical models (Liebscher et al., 2005; Fawcett, 2006; Bradbury and Carter, 2011; Warren et al., 2018) I felt that in the long run it might be promising to identify the intracellular mechanisms that set the injured axon on hold, and onto which many of these extracellular factors likely converge. In other words, instead of taking some stop signs away, why not identify the brake acting in the axon? Loosening this putative brake could enable the axons to regenerate. Obviously, this plan needed a team effort; thus, the premise of our lab was laid out.
Reactivating the Mechanisms of Axon Growth to Induce Axon Regeneration: Microtubule Stabilization
In our lab, we first investigated the action of microtubules more closely. Are they just passively regulated by the actin filaments, or could they actively participate in axon growth? Harald Witte showed that the growing axon has more stable microtubules compared with the nongrowing neurites (Fig. 2). He also established causality between microtubule stability and axon growth in CNS neurons (Witte et al., 2008). To do so, he used the anticancer drug taxol at a low dose, enabling microtubule stabilization by enhancing the rate of microtubule rescue in their dynamic instability (Derry et al., 1995); the result being nongrowing neurites turning into growing axons. Hence, instead of a single axon, taxol-treated cells formed multiple axons. Interestingly, this process not only controls initial neuronal polarization but also maintains polarity in mature neurons. Susana Gomis-Rüth further showed that after the axon, containing the functional presynaptic apparatus, was cut close to the cell body (35 µm or less), a functional dendrite transformed to become the axon (Gomis-Rüth et al., 2008, 2014). Susana's work unraveled a remarkable degree of neuronal plasticity. Whereas previously, neuronal plasticity focused on a change in strengthening modalities of synaptic transmission, reflected by alterations in dendritic spine morphology, it seemed even the functional inputs and outputs are plastic and interchangeable. These findings were supported by the Rasband laboratory, with their demonstration that the axon initial segment maintains neuronal polarity (Hedstrom et al., 2008). It might be even possible that this polarity change could occur in vivo (Fenrich et al., 2007) but this needs to be investigated in the future.
Figure 2.

Differential distribution of acetylated and tyrosinated MTs in hippocampal neurons. A–H, Polarized stage 3 (A–D) and morphologically unpolarized stage 2 (E–H) rat hippocampal neurons stained for acetylated (B, F) and tyrosinated (C, G) tubulin (arrows, axons; arrowheads, minor neurites). Cells were permeabilized during fixation to remove unpolymerized tubulin subunits, therefore only tubulin incorporated in MTs was assessed. In stage 3 neurons, a high ratio of acetylated to tyrosinated tubulin is found in MTs in the axonal shaft (D, arrow) in comparison to MTs of minor neurites (D, arrowheads, I). In 35.0 ± 6.1% of morphologically unpolarized stage 2 neurons, the ratio of acetylated to tyrosinated tubulin is significantly increased in one of the minor neurites (H, white arrowhead with asterisk; p < 0.05 by Hampel outlier test). The areas boxed in H are shown in higher magnification in K, M. I, J, Ratio quantification of fluorescence intensities of acetylated and tyrosinated tubulin in MTs of stage 2 (J) and 3 (I) neurons (mean ± SEM; n > 105 neurons from three independent experiments for each stage 2 and 3). Values are normalized to the mean of nonaxonal or nonmaximal processes for stage 3 and 2, respectively; ***p < 0.001 by t test. K–N, Higher magnification views (K, M) and profiles of immunofluorescence intensity (in arbitrary units; L, N) of acetylated and tyrosinated tubulin of the neurites marked in H. Scale bars: 20 μm (A–H) and 10 μm (K, M). From Witte et al. (2008).
As Gomis-Rüth's axotomy studies showed that neuronal polarization can be reactivated in mature neurons, it raised the question of how this reactivation is disabled in vivo after an injury of the CNS. Using dorsal root ganglia (DRG) neurons, Ali Ertürk discovered that after an injury in the CNS, microtubules disassembled at the lesioned central tip (Ertürk et al., 2007). In contrast, after injury of their peripheral axon which courses in the peripheral nervous system, microtubules remained assembled. Additionally, pharmacological depolymerization of microtubules transformed the growing tips into nongrowing structures that resemble retraction bulbs: hallmarks of a degenerating axon tip found in the injured CNS (Tom et al., 2004). Live in vivo imaging further showed that on taxol treatment in the injured CNS, lesioned axons neither retract from the injury site nor form retraction bulbs (Fig. 3). This finding was of particular interest, and was followed up by other labs showing that in the PNS taxol could interfere with axon degeneration (Kleele et al., 2014), contrasting with the general view of taxol as a drug which causes peripheral neuropathy and axon degeneration at high doses (Scripture et al., 2006; Bhattacharya et al., 2012). Moreover, when treated with taxol, cerebellar granule neurons cultured on inhibitory CNS myelin and other inhibitory substrates grew longer axons (Ertürk et al., 2007). All of this pointed to the possibility that microtubules and their disassembly could be part of the brake that restrains the regeneration program. While this presented the possibility that microtubule stabilization could reactivate the axon growth program to induce axon regeneration, it also raised the question of where microtubules are assembled in the mature neuron.
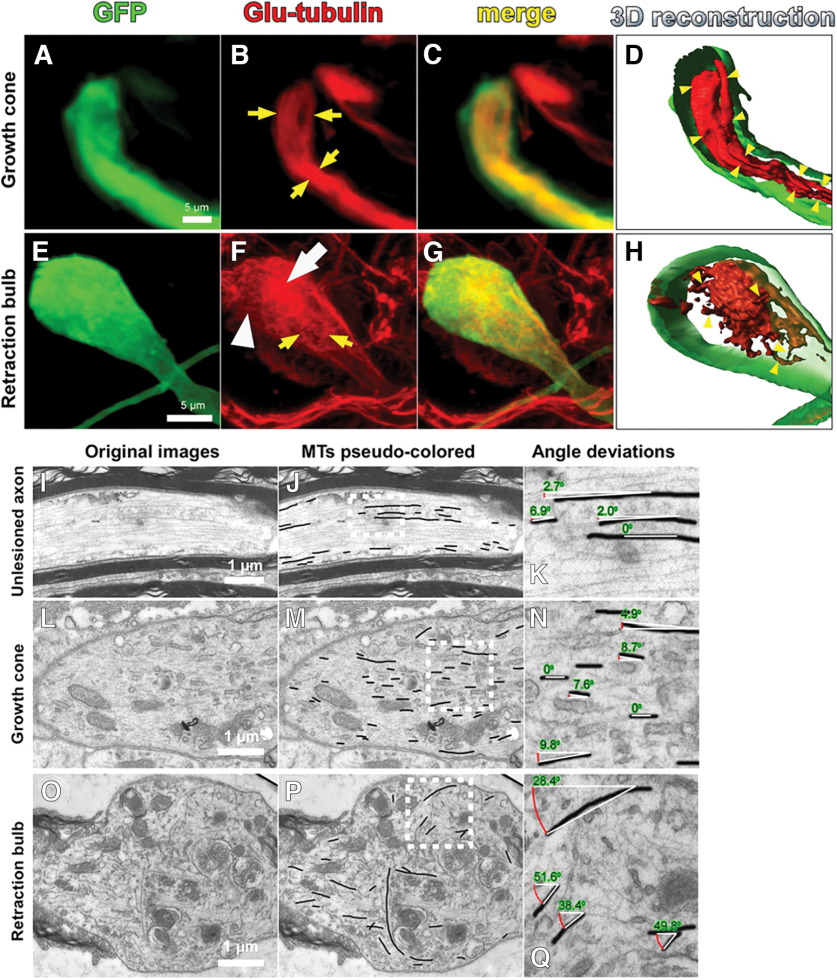
Figure 3.
Retraction bulbs have dispersed and disorganized microtubules. A–H, Immunostaining of axonal end structures with anti-Glu-tubulin antibody, recognizing the detyrosinated tubulin subunits, which are already assembled into microtubules, to visualize the organization of microtubules. A–D, Growth cones possess tightly bundled microtubules parallel to the axonal axis. GFP-positive growth cone (green; A), anti-Glu-tubulin staining (red; B), merge (C), and 3D reconstruction (D) are shown. B, D, The arrows (B) and arrowheads (D) point to some of the parallel microtubule bundles. E–H, Retraction bulbs have highly dispersed and disorganized microtubules. GFP-positive retraction bulb (green; E), anti-Glu-tubulin staining (red; F), merge (G), and 3D reconstruction (H) are shown. F, H, The yellow arrows (F) and arrowheads (H) indicate dispersed microtubules that are highly deviated. F, The white arrow indicates regions in which microtubules are densely accumulated, and the white arrowhead indicates regions without microtubules. I–Q, Electron micrographs of retraction bulbs and growth cones were analyzed to quantify the angle of deviation of microtubules. Unlesioned central axons were also quantified as a control. A representative unlesioned CNS axon (I), growth cone (L), and retraction bulb (O) are shown. J, M, P, The microtubules in I, L, and O were manually traced in black to visualize the overall microtubule organization in the axonal structures. K, N, Q, Higher magnifications of the marked areas in J, M, P, respectively. The angles between the traced microtubules and the axonal axis were quantified. From Ertürk et al. (2007).
Mitotic cells primarily generate microtubules from the centrosome. However, during differentiation, some cell types inactivate their centrosome as the microtubule organizing center (MTOC; Gonzalez et al., 1998). Michael Stiess, therefore, asked whether the function of the centrosome as an MTOC becomes inactivated during neuronal maturation. Indeed, he found that microtubules are generated acentrosomally to support axon extension (Stiess et al., 2010). Together with work in Aplysia neurons showing that microtubules could nucleate at the site of injury (Erez et al., 2007), this study changed the view of the neuronal centrosome and led to a novel branch of research focusing on the role of acentrosomal microtubule nucleation in neurons (Sánchez-Huertas et al., 2016; Cunha-Ferreira et al., 2018). The finding that taxol could induce microtubule formation and stabilization in injured CNS axons also led to additional questions: would this be sufficient to elicit axon regeneration? Furthermore, what is actually necessary for axon regeneration? It may be that the conditioning paradigm of regeneration could help to better pinpoint the minimal requirements for regeneration.
I had been exposed to this classical regeneration paradigm when I joined the lab of Marc Tessier-Lavigne, first at University of California San Francisco, and then later at Stanford University. At the same time, Simona Neumann started her postdoctoral work in the lab of Alan Basbaum. Simona was an expert in spinal cord injury research, effectively trained in the lab of Clifford Woolf. She taught me the conditioning effect: the classical paradigm of axon regeneration elicited by enhancing neuron-intrinsic growth competence. This effect was initially discovered by MacQuarrie and Grafstein in the peripheral axon of DRG neurons (McQuarrie and Grafstein, 1973). It was then transferred to the injured spinal cord by Richardson and Issa, when they showed that the central axon of DRG neurons also regenerates into a peripheral nerve graft after conditioning by prior lesioning of the peripheral axon (Richardson and Issa, 1984). The conditioning effect was mastered by Simona, who showed that it even enables injured axons to regenerate into the CNS in the absence of a graft (Neumann and Woolf, 1999). Simona and I investigated the role of the cAMP signaling pathway during axon regeneration and found in parallel with the lab of the late Marie Filbin that it partially mimics the conditioning effect. Intraganglionic membrane permeable dibutyryl (db) cAMP injection triggered enhanced axon growth of adult cultured neurons, even on inhibitory substrates, and enabled axon regeneration in the injured CNS (Neumann et al., 2002; Qiu et al., 2002). Yet still, this finding does not function within a clinically relevant time frame: if the conditioning is performed after the CNS injury, axons fail to regrow.
The failure of regeneration could be neuron-intrinsic; whereby the injured axon atrophies and cannot gain growth competence through a peripheral lesion. Alternatively, it could be neuron-extrinsic, where something has changed so drastically at the injury site that growth competent axons cannot execute their growth capacity. Bhavna Ylera uncovered that while postconditioned neurons still become growth competent, they cannot execute their growth, as the scar formation at the injury site prevents their regeneration (Ylera et al., 2009). To directly test this, together with Ali Ertürk, she injured CNS axons without creating a scar by performing a minimal two-photon laser lesion, followed by in vivo live imaging. They found that while these cut axons grew only little, the injured axons grew effectively on subsequent conditioning (Fig. 4). Through this study, it became clear that the minimal requirements for axons to regenerate are twofold: the axons need to be growth competent and the scar should be reduced.
Figure 4.
Two-photon lesioned axons show enhanced regeneration when followed by a conditioning peripheral lesion. Two-photon imaging and lesioning of GFP mice that received a central lesion only (unconditioned; A–E), a conditioning before CNS injury (F–J), and a conditioning after CNS injury (K–O). The mice were imaged before injury (A, F, K), at 5 min (B, G, L), 3 d (C, H, M), 5 d (D, I, N), and 9 d (E, J, O) postinjury. Lesion made with a two-photon laser is indicated with yellow arrowheads. White and red arrowheads indicate regenerating and stalled axons, respectively. Red line between M and N indicates the application of the peripheral lesion (conditioning) at 3 d after two-photon laser lesion. The purple dotted lines in the lesion sites align the images at the two-photon lesion. After two-photon imaging, the mice were perfused and spinal cords were stained with GFAP to reveal reactive astrocytes in central lesion only (P1–P3), conditioned before (Q1–Q3) or after CNS injury mice (R1–R3). Regenerating axonal sprouts in mice conditioned before (Q1) and after (R1) CNS injury. Extent of scar formation after two-photon laser is minimal as revealed by glial fibrillary acidic protein (GFAP) staining of reactive astrocytes indicated by purple arrows at the lesion site (P2, Q2, R2). Overlay showing the regenerating sprouts marked with white arrowheads and GFAP-stained reactive astrocytes indicated with purple arrows (P3, Q3, R3). In all spinal cord images, the rostral direction is to the left as indicated in A. Scale bars: 50 μm (E, J, O, P3–R3). Length quantification of sprouts in mice conditioned before (n = 8 animals; S) or after CNS injury (n = 7 animals; T) compared to unlesioned controls (n = 8 animals) at the indicated time points. Data are shown as the mean ± SEM. B.C.I., before central injury; A.C.I., after central injury; **p < 0.05 and ***p < 0.01. From Ylera et al. (2009).
In this context, it is noteworthy that taxol is used in the clinic not only as an anticancer drug, but is also used to reduce fibrotic scarring. For example, stents are coated with taxol to prevent scarring when introduced into the coronary system (Herdeg et al., 1998). Thus, taxol could, besides its action on loosening the intrinsic brake, also reduce fibrotic scarring after a spinal cord injury. Therefore, Farida Hellal tested the effects of Taxol delivered intrathecally through an osmotic pump after a spinal cord injury, and found that the fibrotic scar and the level of growth-inhibiting CSPGs were reduced. Indeed, she further demonstrated an increase of serotonergic axon density and an improvement in functional recovery (Hellal et al., 2011).
Other laboratories found similar aspects of the taxol effect, such as a reduction of fibrotic scarring and CSPGs in the injury site (Popovich et al., 2014), or enhancement of axon regrowth in the injured optic nerve (Sengottuvel et al., 2011). Yet if one starts to think of a therapeutic intervention, Taxol has the disadvantage in that it cannot cross the blood-brain barrier (BBB; Fellner et al., 2002), and needs to be delivered intrathecally for action in the CNS. Jörg Ruschel therefore tested the possibility of applying a microtubule stabilizing drug that can cross the BBB: epothilone B (Ruschel et al., 2015). He indeed found that systemic administration of epothilone B after spinal cord injury reduced fibrotic scarring and CSPGs, and increased regrowth of serotonergic axons (Fig. 5). This led to functional improvement of the animals, which depends on serotonergic axons. In this study, he also deciphered the mechanisms underlying reduction of fibrotic scarring. Whereas fibroblasts move in a directed fashion toward a lesion and contain stable microtubules in the direction of movement (Gundersen and Bulinski, 1988), this directionality of growth is abrogated with epothilone B, as this treatment causes microtubule stabilization all around the cell. The meningeal fibroblasts therefore cannot polarize; in contrast, axons can indeed regrow through epothilone B treatment. Imaging of the microtubule plus ends showed that microtubules polymerized and pushed the leading edge of neurites forward, even when exposed to growth inhibitory factors, such as Nogo-A. Intriguingly, these distinct actions on the different cell types can be attributed to the microtubule-associated protein (MAP) Tau, which is present in neurons but not in fibroblasts. This work opened a translational perspective that was followed by us and other laboratories in the spinal cord injury and stroke fields (Ruschel and Bradke, 2018; Sandner et al., 2018; Yang et al., 2018; Kugler et al., 2020; Duan et al., 2021; Xue et al., 2021). The next crucial step was to decipher the molecular machinery underlying axon regeneration.
Figure 5.

EpoB promotes regrowth of raphespinal axons and improves walking after spinal cord contusion injury. A, Serotonin (5HT) immunolabeling (dashed line, lesion border) and (B) number (#) of 5HT-labeled (+) fibers caudal to a spinal dorsal hemisection; n = 7–8 rats per group. i.p., intraperitoneal injection. C, Coronal sections of the lumbar spinal cord after contusion injury. Left panel, Coimmunostaining of 5HT, synaptophysin (Syn), and choline acetyltransferase (ChAT). Right panels, Magnification of each marker in boxed area (left panel) visualizing serotonergic innervation of motor neurons (arrowheads). D, Total length of 5HT-immunopositive fibers in the ventral horn (5,7-DHT, 5,7-dihydroxytryptamine); n = 4 (uninjured), 6 (7 dpi), 11–12 rats (56 and 70 dpi) per group. E, Number of footfalls on the horizontal ladder; n = 10–11 rats per group. dpi, days postinjury. Scale bars: 50 µm. Schemes in A, C indicate lesion and displayed region (red box). Values are plotted as mean ± SEM; *p < 0.05; n.s., not significant. From Ruschel et al. (2015).
Deciphering the Cytoskeletal Machinery Underlying Axon Growth and Regeneration
To uncover the molecular machinery that could drive axon regeneration, we used a similar conceptual strategy that led us to the idea of microtubule stabilization in the first place. We studied the physiological mechanisms underlying axon growth and growth restraint during neuronal polarization. Subsequently, we tested how lesioned axons are restrained from growing on a molecular level in mouse models of spinal cord injury. This, in turn, enabled us to manipulate these mechanisms to enable axon regeneration.
Boyan Garvalov demonstrated that the Rho-GTPase Cdc42 regulates neuronal polarization and axon formation in the developing brain (Garvalov et al., 2007). Conditional knock-out (KO) analysis showed that Cdc42 has fewer downstream effectors than previously anticipated, a deduction based on studies of overexpression of dominant-negative and constitutively active mutants. Through actions involving cofilin, Cdc42 dynamized the actin cytoskeleton to enable microtubules protrusion. Conversely, Sabina Tahirovic observed that Rac1 dynamizes the actin cytoskeleton through the WAVE complex (Tahirovic et al., 2010). Moving further downstream, Kevin Flynn revealed that the cofilin family of proteins directed the transformation of a round neuroblast to a neuron bearing neurites (Flynn et al., 2012). By binding to their minus ends, cofilin severs actin filaments, which in turn creates space for microtubules to protrude. This raises the possibility that the actin retrograde flow found in growth cones can, at least in CNS neurons, be regarded as a by-product of the actin severing at the minus end, and of the actin polymerization at the plus end in the leading edge. Thus, actin depolymerization itself restores neurite and axon formation in KO neurons deficient in members of the cofilin family. In fact, the interaction between microtubules and actin filaments remains to be investigated in far more detail (Coles and Bradke, 2015). The role of cytoplasmic linker proteins (CLIPs), which interact with both microtubules and actin filaments, provides just the first glimpse toward a better understanding of the tight interaction between these cytoskeletal networks during axon growth (Neukirchen and Bradke, 2011). But does dynamizing the actin cytoskeleton play a role in axon regeneration?
In a team headed by Andrea Tedeschi, Sebastian Dupraz, Michele Curcio, Claudia Laskowski, and Barbara Schaffran, we found that conditioning dynamizes the actin cytoskeleton through cofilin, which then enables axon growth (Tedeschi et al., 2019). In fact, overexpressing cofilin is sufficient to elicit regeneration of DRG axons in the injured spinal cord. Work from our lab, along with work on the actin regulator Profilin by the Sousa lab (Pinto-Costa et al., 2020), identified molecular regulators of the cytoskeleton as intrinsic regenerative modulators; we therefore felt that we were now in a position to decipher a whole signaling pathway controlling axon regeneration, from the extrinsic site down to its effectors on the cytoskeleton. As many growth inhibitors mediate their action through receptors that converge on RhoA signaling (Geoffroy and Zheng, 2014; Sami et al., 2020), Sina Stern investigated which physiological effectors downstream of RhoA may mediate growth restraint. Conditional KO analysis combined with pharmacological and molecular manipulation, alongside in vivo and in vitro imaging, pinpointed the cascade: RhoA activates the actin motor Myosin II. Myosin II, in turn, forms antiparallel actin bundles in the growth cone, which prevents microtubules from protruding forward to enable axon regrowth and axon regeneration (Stern et al., 2021). These data show that inactivation of RhoA, such as with the C3 exoenzyme (Lehmann et al., 1999), which has been used in clinical trials (Fehlings et al., 2011), acts ultimately by enabling microtubule protrusion to induce axon regeneration. However, Sina Stern's and Brett Hilton's work also uncovered that RhoA keeps astrocytes from becoming over-reactive after a spinal cord injury (Stern et al., 2021). As a result, only inactivation of RhoA in neurons leads to axon regeneration, whereas simultaneous inactivation of RhoA in astrocytes abrogates this regenerative effect and induces CSPG production (Fig. 6).
Figure 6.
Neuronal deletion of RhoA promotes regeneration, whereas astrocytic deletion of RhoA abrogates regeneration. A, Overview of the transgenic mice used in the experiment. B, Immunoblot for glial fibrillary acidic protein (GFAP) and CSPGs in spinal cord extracts from WT and RhoAGFAPKO mice with or without SCI. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown as a loading control. C, D, Quantification of B. For GFAP, the upper band at 48 kDa, for GAPDH the band at 35 kDa, and for CSPG the band at 63 kDa were used for quantification. Values are plotted as mean and SEM and normalized to the WT control; *p < 0.05, ***p < 0.001 by Student's t test. n = 3 individual animals. E, Immunoblot for GFAP and CSPGs in spinal cord extracts from WT and RhoASynKO mice with or without spinal cord injury (SCI). GAPDH is shown as a loading control. F, G, Quantification of E; *p < 0.05, **p < 0.01, ***p < 0.001 by Student's t test. n = 3 individual animals. H, 3D rendering of tile scan imaged dorsal column sensory axons labeled with GFP in whole-mount spinal cord immunostained with GFAP antibody four weeks after SCI. Asterisks indicate lesion centers. The GFAP signal was set to saturating levels to outline the lesion. Scale bar: 200 µm. I, Quantification of axon density caudal to the lesion site; **p < 0.01 by one-way ANOVA, followed by permutation test. WT, n = 7; RhoANesKO, n = 4; RhoAGFAPKO, n = 7; RhoASynKO, n = 5. J, Quantification of axon density rostral to the lesion site and at the lesion site (LS); scatterplot with mean; *p < 0.05, **p < 0.01, ***p < 0.001 by permutation test. WT, n = 7; RhoANesKO, n = 4; RhoAGFAPKO; n = 7; RhoASynKO, n = 5. AU, arbitrary unit. From Stern et al. (2021).
This finding may also explain why clinical trials using C3 exoenzyme were unsuccessful (Fehlings et al., 2021). Neuron-specific inactivation of RhoA or manipulating a neuron-specific downstream effector may be a more promising approach to try in the future. In this regard, it is interesting to note that neither taxol nor epothilones induce an increased glial reactivity after spinal cord injury (Hellal et al., 2011; Popovich et al., 2014; Ruschel et al., 2015; Stern et al., 2021). This has also helped to provide new insights for the current debate about the role of the scar in spinal cord injury (Sofroniew, 2009; Cregg et al., 2014; Anderson et al., 2016; Silver, 2016; Bradbury and Burnside, 2019) by showing that RhoA restrains glial reactivity, and its ablation in astrocytes leads to inhibition of axon regeneration. Finally, it also demonstrates that the signaling cascades employed to prevent axon regeneration are already active during development. Accordingly, Sebastian Dupraz showed that RhoA also signals to Myosin II to execute the neuronal polarization program, specifically to enable growth pauses for the axon during circuit formation (Dupraz et al., 2019). Thus, the mechanisms controlling axon growth are still present in the adult nervous system. But why are they inactive in the adult CNS, preventing axon regeneration?
Axon Growth and Regeneration versus Synaptic Transmission: The Rise of Intrinsic Growth Inhibitors
One question often asked is why neurons lose their regenerative competence when they mature. While science fails to address why questions overall, some interesting hints in this regard can be extracted. For example, axons probably need to downregulate their growth program once they have reached their target; otherwise, a chance remains that they cross their target area without stopping at the correct site. From this perspective, one could envision that axon growth and synaptic maturation and transmission may be processes that inhibit each other to a certain extent (Hilton and Bradke, 2017; Tedeschi and Bradke, 2017). Could it be then that electrical activity and synapse formation suppress axon formation?
Cohan and Kater showed decades ago that snail sensory neurons stop growing on electrical stimulation (Cohan and Kater, 1986). Joana Enes therefore tested the possibility that the beneficial effects of the conditioning paradigm stem partly from electrical silencing (Enes et al., 2010). Indeed, electrical activity ceases a few hours after peripheral nerve lesioning, while electrical activation of cultured adult DRG neurons causes them to stop (Enes et al., 2010). Consistent with this, Brett Hilton demonstrated that electrically inactivated DRG neurons, silenced by pharmacogenetics, regenerate their axon after a spinal cord injury (Hilton et al., 2022). Importantly, while electrical activity stopped growing axons, this effect was abrogated when transcription was inhibited: axons then continued to grow (Enes et al., 2010). This raised an exciting possibility: maybe the growth machinery is preserved in the adult axon, and an expressed growth inhibitor might simply restrain regenerative growth.
Andrea Tedeschi embarked on the quest for such a growth inhibitor that might also be involved in synapse formation. By performing RNA sequencing of growing and growth- incompetent DRG neurons during development in adult cell culture and after conditioning, and then performing bioinformatic analysis, he identified Cacna2d2, the gene encoding the voltage gated calcium channel auxiliary subunit α2δ2, as a growth inhibitor. α2δ2 can be blocked by gabapentinoids; and indeed, this pharmacological blockade caused axon regeneration in spinal cord-injured mice (Tedeschi et al., 2016). We early on shared our findings with the European Multicenter Study about Spinal Cord Injury (EMSCI) network, a consortium of >20 spinal cord injury clinics. Remarkably, when they looked at their patients' data, they found that those who had received gabapentinoids, such as gabapentin and pregabalin, which are used in the clinics as anticonvulsants, within one month after spinal cord injury performed better on a locomotor score (Warner and Figgitt, 2005; Warner et al., 2017; Cragg et al., 2020). This is by no means a clinical trial, but this meta-analysis combined with our experimental data raises hope that gabapentinoids could be an important part of a future therapy for patients. In fact, the effect of gabapentinoids in CNS injury was followed up by Andrea's own laboratory, as well as by other research groups, determining that gabapentinoids elicit functional recovery after spinal cord injury and stroke (Sun et al., 2020; Kugler et al., 2022; Tedeschi et al., 2022).
While this presents promising avenues for future translational work, many of the underlying mechanisms of axon regeneration are still unclear. For example, could the synaptic transmission apparatus be part of restraining axon regeneration? Brett Hilton addressed this question and found that proteins of the vesicle priming machinery, including Munc13, suppress axon regeneration (Fig. 7; Hilton et al., 2022). Importantly, he showed that the drug baclofen, which dampens synaptic transmission, induces axon regeneration. Excitingly, a meta-analysis showed that there are positive effects associated when baclofen is given to patients (Cragg et al., 2019).
Figure 7.

Munc13 deletion promotes axon regeneration following adult CNS injury. A, Multiphoton tile scan of GFP+ sensory axons (yellow) and glial fibrillary acidic protein (GFAP)+ astrocytes (blue) in the unsectioned spinal cord after complete dorsal column spinal cord injury (SCI) in the given conditions. R, rostral; C, caudal. Asterisks indicate lesion centers. Scale bar: 200 µm. B, Quantification of A. Scatterplot with means; ***p < 0.001, **p < 0.01 by permutation test. n = 11 animals per group. C, Scheme of voltage-gated calcium channel (VGCC) activation, presynaptic Ca2+ influx, and vesicle release. D, Representative fluorescence images of Tuj1 (red) and GFP/Cre-GFP (cyan) immunolabeled DRG neurons from Munc13-1fl/flMunc13-2KO/KOMunc13-3KO/KO mice administered AAV-GFP or AAV-Cre-GFP and cultured for 24 h in the presence of DMSO, KCl (40 mM), Roscovotine (20 µM), or GV-58 (20 µM). Scale bar: 200 µm. E, F, Length of the longest axon (E) and branching frequency (F) of AAV-GFP+ neurons in D. Values are plotted as mean ± SEM; ***p < 0.001 GFP DMSO versus GFP KCl, GFP Roscovotine, GFP GV-58 in E and **p < 0.01 GFP DMSO versus GFP KCl, *p < 0.05 GFP DMSO versus GFP Roscovotine, GFP DMSO versus GFP GV-58 in F by one-way ANOVA followed by Tukey's post hoc test; n = 72 GFP DMSO, 86 GFP GV-58, 37 GFP KCl, 53 GFP Roscovotine-treated neurons from three independent experiments. G, H, Length of the longest axon (G) and branching frequency (H) of AAV-Cre-GFP+ neurons in D. Values are plotted as mean ± SEM n = 82 Cre-GFP DMSO, 92 Cre-GFP GV-58, 48 Cre-GFP KCl, 23 Cre-GFP Roscovotine-treated neurons from three independent experiments. From Hilton et al. (2022).
Conclusion
Taken together, the recent years have seen the views of developmental biologists and medically oriented scientists on axon growth and regeneration start to merge. Axon regeneration in the adult CNS is possible by reactivating processes that induce axon growth during development. Indeed, there is a remarkable conservation between the growth machinery that exists during development and the components that are present in the adult. It emerges that this machinery is robustly restrained by growth inhibitors in the adult CNS, and that unlocking this growth restraint appears to be a way to elicit axon regeneration. I would like to stress that this topic is not a completed one. Instead, it is a field that is waiting for significant discoveries to be made, some of them translational, and some of them truly fundamental. For example, while we understand the signaling regulating axon growth relatively well, our basic understanding of how an axon grows is still fragmentary. Do axons grow by an amoeboid movement in general? Does it depend on whether an axon grows in the CNS or PNS, which themselves differ in type and stiffness of the substrate? Accordingly, our field focuses a lot of attention on the growth cone as the machinery of axon growth (Bradke et al., 2012). But how do the different parts of a neuron regulate their concerted growth? Are we too “growth-cone-centric”? How are different dynamic processes effectively regulated in a single cell, such as migration and axon growth, which builds a truly complex choreography? It needs to be stressed that the classical field of neuronal polarization is up for stunning discoveries. Even as this review goes to the press, Max Schelski has just discovered that the nongrowing minor neurites contain microtubules that move retrogradely; this retrograde flow is drastically reduced in the growing axon (Schelski and Bradke, 2021). In other words, the focus on the axon has hampered our view on the exciting intracellular dynamics in nongrowing neurites for decades. Moreover, much of our knowledge of the mechanisms underlying axon growth and regeneration relies on 2D substrates. Instead, maybe it is time to move forward to 3D and in vivo models to fuse cell biology and neuroscience effectively (Alfadil and Bradke, 2022). Considering how spinal cord injury shows the typical hallmarks of axon degeneration found also in neurodegenerative diseases, could this not help us to better define causalities in neurodegenerative diseases that lack a defined time 0? These are some of the basic questions we as a field need to ask in the future. There are exciting times ahead.
Footnotes
I am truly grateful to all past and present members of our lab. Without their dedication, we would not have been able to establish our research. It is a privilege to work together with such a group of fine people. And it is exciting to see how well the alumni are doing in leadership positions both in academy and in industry. I am indebted to Juliane Schiweck, Brett Hilton, and Emily Handley for carefully reading and editing the manuscript. I sincerely thank all of our collaborators throughout the years including Armin Blesch, John Bixby, Cord Brakebusch, Nils Brose, Oliver Brüstle, Armin Curt, Hans-Ulrich Dodt, Casper Hoogenraad, Cordelia Imig, Lukas Kapitein, John Kramer, Vance Lemmon, Gabor Petzold, Stefan Remy, Susanne Schoch, Joachim Schultze, Norbert Weidner, Corette Wierenga, Walter Witke, and Marcy Zenobi-Wong. Their input really made the huge difference to develop our projects together. Their commitments enabled the flourishment of our studies. I am indebted to our funding sources, including the Deutsche Forschungsgemeinschaft, International Foundation for Research in Paraplegia, Wings for Life, Human Frontier Science Program, European Molecular Biology Organization, European Community funding, and the Roger de Spoelberch Prize, which provided us generously with funding for our studies. And I sincerely thank my mentors. Last, I would like to thank my wife Gaia Tavosanis and wish our children Fausto, Samuele, and Emilia to find on their ways the excitement we have found for science.
References
- Alfadil E, Bradke F (2022) Moving through the crowd. Where are we at understanding physiological axon growth? Semin Cell Dev Biol. Advance online publication. Retrieved July 8, 2022. 10.1016/j.semcdb.2022.07.001. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya MR, Gerdts J, Naylor SA, Royse EX, Ebstein SY, Sasaki Y, Milbrandt J, DiAntonio A (2012) A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J Neurosci 32:5054–5061. 10.1523/JNEUROSCI.4951-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM (2011) Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull 84:306–316. 10.1016/j.brainresbull.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnside ER (2019) Moving beyond the glial scar for spinal cord repair. Nat Commun 10:3879. 10.1038/s41467-019-11707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG (1997) Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19:1175–1186. 10.1016/S0896-6273(00)80410-9 [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG (1999) The role of local actin instability in axon formation. Science 283:1931–1934. 10.1126/science.283.5409.1931 [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG (2000) Differentiated neurons retain the capacity to generate axons from dendrites. Curr Biol 10:1467–1470. 10.1016/s0960-9822(00)00807-1 [DOI] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME (2012) Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13:183–193. 10.1038/nrn3176 [DOI] [PubMed] [Google Scholar]
- Bretscher MS (1996) Moving membrane up to the front of migrating cells. Cell 85:465–467. 10.1016/S0092-8674(00)81246-5 [DOI] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW (2005) Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol 15:116–120. 10.1016/j.conb.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Chada S, Lamoureux P, Buxbaum RE, Heidemann SR (1997) Cytomechanics of neurite outgrowth from chick brain neurons. J Cell Sci 110:1179–1186. 10.1242/jcs.110.10.1179 [DOI] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K (2013) Structural and molecular interrogation of intact biological systems. Nature 497:332–337. 10.1038/nature12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan CS, Kater SB (1986) Suppression of neurite elongation and growth cone motility by electrical activity. Science 232:1638–1640. 10.1126/science.3715470 [DOI] [PubMed] [Google Scholar]
- Coles CH, Bradke F (2015) Coordinating neuronal actin-microtubule dynamics. Curr Biol 25:R677–691. 10.1016/j.cub.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Cragg JJ, Tong B, Jutzeler CR, Warner FM, Cashman N, Geisler F, Kramer JLK (2019) A longitudinal study of the neurologic safety of acute baclofen use after spinal cord injury. Neurotherapeutics 16:858–867. 10.1007/s13311-019-00713-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg JJ, Jutzeler CR, Grassner L, Ramer M, Bradke F, Kramer JLK (2020) Beneficial “pharmaceutical pleiotropy” of gabapentinoids in spinal cord injury: a case for refining standard-of-care. Neurorehabil Neural Repair 34:686–689. 10.1177/1545968320931516 [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G (1994) Neuronal polarity. Annu Rev Neurosci 17:267–310. 10.1146/annurev.ne.17.030194.001411 [DOI] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197–207. 10.1016/j.expneurol.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Ferreira I, Chazeau A, Buijs RR, Stucchi R, Will L, Pan X, Adolfs Y, van der Meer C, Wolthuis JC, Kahn OI, Schätzle P, Altelaar M, Pasterkamp RJ, Kapitein LC, Hoogenraad CC (2018) The HAUS complex is a key regulator of non-centrosomal microtubule organization during neuronal development. Cell Rep 24:791–800. 10.1016/j.celrep.2018.06.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennerll TJ, Lamoureux P, Buxbaum RE, Heidemann SR (1989) The cytomechanics of axonal elongation and retraction. J Cell Biol 109:3073–3083. 10.1083/jcb.109.6.3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry WB, Wilson L, Jordan MA (1995) Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry 34:2203–2211. 10.1021/bi00007a014 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Banker GA (1987) Experimentally induced alteration in the polarity of developing neurons. Nature 330:254–256. 10.1038/330254a0 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8:1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan YY, Chai Y, Zhang NL, Zhao DM, Yang C (2021) Microtubule stabilization promotes microcirculation reconstruction after spinal cord injury. J Mol Neurosci 71:583–595. 10.1007/s12031-020-01679-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz S, Hilton BJ, Husch A, Santos TE, Coles CH, Stern S, Brakebusch C, Bradke F (2019) RhoA controls axon extension independent of specification in the developing brain. Curr Biol 29:3874–3886.e9. 10.1016/j.cub.2019.09.040 [DOI] [PubMed] [Google Scholar]
- Enes J, Langwieser N, Ruschel J, Carballosa-Gonzalez MM, Klug A, Traut MH, Ylera B, Tahirovic S, Hofmann F, Stein V, Moosmang S, Hentall ID, Bradke F (2010) Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol 20:1154–1164. 10.1016/j.cub.2010.05.055 [DOI] [PubMed] [Google Scholar]
- Erez H, Malkinson G, Prager-Khoutorsky M, De Zeeuw CI, Hoogenraad CC, Spira ME (2007) Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol 176:497–507. 10.1083/jcb.200607098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertürk A, Bradke F (2013) High-resolution imaging of entire organs by 3-dimensional imaging of solvent cleared organs (3DISCO). Exp Neurol 242:57–64. 10.1016/j.expneurol.2012.10.018 [DOI] [PubMed] [Google Scholar]
- Ertürk A, Hellal F, Enes J, Bradke F (2007) Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci 27:9169–9180. 10.1523/JNEUROSCI.0612-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertürk A, Mauch CP, Hellal F, Förstner F, Keck T, Becker K, Jährling N, Steffens H, Richter M, Hübener M, Kramer E, Kirchhoff F, Dodt HU, Bradke F (2011) Three-dimensional imaging of the unsectioned adult spinal cord to assess axon regeneration and glial responses after injury. Nat Med 18:166–171. 10.1038/nm.2600 [DOI] [PubMed] [Google Scholar]
- Ertürk A, Lafkas D, Chalouni C (2014) Imaging cleared intact biological systems at a cellular level by 3DISCO. J Vis Exp (89):51382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW (2006) Overcoming inhibition in the damaged spinal cord. J Neurotrauma 23:371–383. 10.1089/neu.2006.23.371 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L (2011) A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 28:787–796. 10.1089/neu.2011.1765 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Chen Y, Aarabi B, Ahmad F, Anderson KD, Dumont T, Fourney DR, Harrop JS, Kim KD, Kwon BK, Lingam HK, Rizzo M, Shih LC, Tsai EC, Vaccaro A, McKerracher L (2021) A randomized controlled trial of local delivery of a rho inhibitor (VX-210) in patients with acute traumatic cervical spinal cord injury. J Neurotrauma 38:2065–2072. 10.1089/neu.2020.7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, Bernhardt G, Graeff C, Färber L, Gschaidmeier H, Buschauer A, Fricker G (2002) Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 110:1309–1318. 10.1172/JCI15451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich KK, Skelton N, MacDermid VE, Meehan CF, Armstrong S, Neuber-Hess MS, Rose PK (2007) Axonal regeneration and development of de novo axons from distal dendrites of adult feline commissural interneurons after a proximal axotomy. J Comp Neurol 502:1079–1097. 10.1002/cne.21362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KC, Hellal F, Neukirchen D, Jacob S, Tahirovic S, Dupraz S, Stern S, Garvalov BK, Gurniak C, Shaw AE, Meyn L, Wedlich-Söldner R, Bamburg JR, Small JV, Witke W, Bradke F (2012) ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron 76:1091–1107. 10.1016/j.neuron.2012.09.038 [DOI] [PubMed] [Google Scholar]
- Forscher P, Smith SJ (1988) Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol 107:1505–1516. 10.1083/jcb.107.4.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Wu X, Brakebusch C, Bamburg JR, Bradke F (2007) Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci 27:13117–13129. 10.1523/JNEUROSCI.3322-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy CG, Zheng B (2014) Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol 27:31–38. 10.1016/j.conb.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DJ, Burmeister DW (1986) Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol 103:1921–1931. 10.1083/jcb.103.5.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth S, Wierenga CJ, Bradke F (2008) Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr Biol 18:992–1000. 10.1016/j.cub.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth S, Stiess M, Wierenga CJ, Meyn L, Bradke F (2014) Single-cell axotomy of cultured hippocampal neurons integrated in neuronal circuits. Nat Protoc 9:1028–1037. 10.1038/nprot.2014.069 [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Tavosanis G, Mollinari C (1998) Centrosomes and microtubule organisation during Drosophila development. J Cell Sci 111:2697–2706. 10.1242/jcs.111.18.2697 [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G (1989) Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol 108:1507–1516. 10.1083/jcb.108.4.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC (1988) Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A 85:5946–5950. 10.1073/pnas.85.16.5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN (2008) AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183:635–640. 10.1083/jcb.200806112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F (2011) Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331:928–931. 10.1126/science.1201148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdeg C, Oberhoff M, Karsch KR (1998) Antiproliferative stent coatings: taxol and related compounds. Semin Interv Cardiol 3:197–199. [PubMed] [Google Scholar]
- Hilton BJ, Bradke F (2017) Can injured adult CNS axons regenerate by recapitulating development? Development 144:3417–3429. 10.1242/dev.148312 [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Blanquie O, Tedeschi A, Bradke F (2019) High-resolution 3D imaging and analysis of axon regeneration in unsectioned spinal cord with or without tissue clearing. Nat Protoc 14:1235–1260. 10.1038/s41596-019-0140-z [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Husch A, Schaffran B, Lin TC, Burnside ER, Dupraz S, Schelski M, Kim J, Müller JA, Schoch S, Imig C, Brose N, Bradke F (2022) An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron 110:51–69.e7. 10.1016/j.neuron.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jareb M, Banker G (1997) Inhibition of axonal growth by brefeldin A in hippocampal neurons in culture. J Neurosci 17:8955–8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleele T, Marinković P, Williams PR, Stern S, Weigand EE, Engerer P, Naumann R, Hartmann J, Karl RM, Bradke F, Bishop D, Herms J, Konnerth A, Kerschensteiner M, Godinho L, Misgeld T (2014) An assay to image neuronal microtubule dynamics in mice. Nat Commun 5:4827. 10.1038/ncomms5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler C, Thielscher C, Tambe BA, Schwarz MK, Halle A, Bradke F, Petzold GC (2020) Epothilones improve axonal growth and motor outcomes after stroke in the adult mammalian CNS. Cell Rep Med 1:100159. 10.1016/j.xcrm.2020.100159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler C, Blank N, Matuskova H, Thielscher C, Reichenbach N, Lin TC, Bradke F, Petzold GC (2022) Pregabalin improves axon regeneration and motor outcome in a rodent stroke model. Brain Commun 4:fcac170. 10.1093/braincomms/fcac170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda P, Paglini G, Quiroga S, Kosik K, Caceres A (2001) Evidence for the involvement of Tiam1 in axon formation. J Neurosci 21:2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C (2013) ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development 140:1364–1368. 10.1242/dev.091844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux P, Buxbaum RE, Heidemann SR (1989) Direct evidence that growth cones pull. Nature 340:159–162. 10.1038/340159a0 [DOI] [PubMed] [Google Scholar]
- Lamoureux P, Altun-Gultekin ZF, Lin C, Wagner JA, Heidemann SR (1997) Rac is required for growth cone function but not neurite assembly. J Cell Sci 110:635–641. 10.1242/jcs.110.5.635 [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L (1999) Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci 19:7537–7547. 10.1523/JNEUROSCI.19-17-07537.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME (2005) Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol 58:706–719. 10.1002/ana.20627 [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Höke A, Silver J (1995) Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol 136:32–43. 10.1006/exnr.1995.1081 [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B (1973) Axon outgrowth enhanced by a previous nerve injury. Arch Neurol 29:53–55. 10.1001/archneur.1973.00490250071008 [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M (1988) Cytoskeletal dynamics and nerve growth. Neuron 1:761–772. 10.1016/0896-6273(88)90124-9 [DOI] [PubMed] [Google Scholar]
- Montenegro-Venegas C, Tortosa E, Rosso S, Peretti D, Bollati F, Bisbal M, Jausoro I, Avila J, Cáceres A, Gonzalez-Billault C (2010) MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol Biol Cell 21:3518–3528. 10.1091/mbc.E09-08-0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13:757–767. 10.1016/0896-6273(94)90042-6 [DOI] [PubMed] [Google Scholar]
- Neukirchen D, Bradke F (2011) Cytoplasmic linker proteins regulate neuronal polarization through microtubule and growth cone dynamics. J Neurosci 31:1528–1538. 10.1523/JNEUROSCI.3983-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23:83–91. 10.1016/s0896-6273(00)80755-2 [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI (2002) Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34:885–893. 10.1016/s0896-6273(02)00702-x [DOI] [PubMed] [Google Scholar]
- Pan C, Cai R, Quacquarelli FP, Ghasemigharagoz A, Lourbopoulos A, Matryba P, Plesnila N, Dichgans M, Hellal F, Ertürk A (2016) Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13:859–867. 10.1038/nmeth.3964 [DOI] [PubMed] [Google Scholar]
- Pinto-Costa R, Sousa SC, Leite SC, Nogueira-Rodrigues J, Ferreira da Silva T, Machado D, Marques J, Costa AC, Liz MA, Bartolini F, Brites P, Costell M, Fässler R, Sousa MM (2020) Profilin 1 delivery tunes cytoskeletal dynamics toward CNS axon regeneration. J Clin Invest 130:2024–2040. 10.1172/JCI125771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Tovar CA, Lemeshow S, Yin Q, Jakeman LB (2014) Independent evaluation of the anatomical and behavioral effects of Taxol in rat models of spinal cord injury. Exp Neurol 261:97–108. 10.1016/j.expneurol.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT (2002) Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34:895–903. 10.1016/s0896-6273(02)00730-4 [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S (1928) Degeneration and regeneration of the nervous system. Oxford: Oxford University Press. [Google Scholar]
- Richardson PM, Issa VM (1984) Peripheral injury enhances central regeneration of primary sensory neurones. Nature 309:791–793. 10.1038/309791a0 [DOI] [PubMed] [Google Scholar]
- Ruschel J, Bradke F (2018) Systemic administration of epothilone D improves functional recovery of walking after rat spinal cord contusion injury. Exp Neurol 306:243–249. 10.1016/j.expneurol.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brüstle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F (2015) Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348:347–352. 10.1126/science.aaa2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami A, Selzer ME, Li S (2020) Advances in the signaling pathways downstream of glial-scar axon growth inhibitors. Front Cell Neurosci 14:174. 10.3389/fncel.2020.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Huertas C, Freixo F, Viais R, Lacasa C, Soriano E, Lüders J (2016) Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat Commun 7:12187. 10.1038/ncomms12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandner B, Puttagunta R, Motsch M, Bradke F, Ruschel J, Blesch A, Weidner N (2018) Systemic epothilone D improves hindlimb function after spinal cord contusion injury in rats. Exp Neurol 306:250–259. 10.1016/j.expneurol.2018.01.018 [DOI] [PubMed] [Google Scholar]
- Santos TE, Schaffran B, Broguière N, Meyn L, Zenobi-Wong M, Bradke F (2020) Axon growth of CNS neurons in three dimensions is amoeboid and independent of adhesions. Cell Rep 32:107907. 10.1016/j.celrep.2020.107907 [DOI] [PubMed] [Google Scholar]
- Schelski M, Bradke F (2021) Microtubule retrograde flow retains neuronal polarization in a fluctuating state. bioRxiv 458567. 10.1101/2021.09.01.458567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Caroni P (1988) Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci 8:2381–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Strittmatter SM (2014) Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol 27:53–60. 10.1016/j.conb.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn JC, Püschel AW (2004) The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 7:923–929. 10.1038/nn1295 [DOI] [PubMed] [Google Scholar]
- Scripture CD, Figg WD, Sparreboom A (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol 4:165–172. 10.2174/157015906776359568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D (2011) Taxol facilitates axon regeneration in the mature CNS. J Neurosci 31:2688–2699. 10.1523/JNEUROSCI.4885-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J (2016) The glial scar is more than just astrocytes. Exp Neurol 286:147–149. 10.1016/j.expneurol.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. 10.1016/j.tins.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Hilton BJ, Burnside ER, Dupraz S, Handley EE, Gonyer JM, Brakebusch C, Bradke F (2021) RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron 109:3436–3455.e9. 10.1016/j.neuron.2021.08.014 [DOI] [PubMed] [Google Scholar]
- Stiess M, Maghelli N, Kapitein LC, Gomis-Rüth S, Wilsch-Bräuninger M, Hoogenraad CC, Tolić-Nørrelykke IM, Bradke F (2010) Axon extension occurs independently of centrosomal microtubule nucleation. Science 327:704–707. 10.1126/science.1182179 [DOI] [PubMed] [Google Scholar]
- Sun W, Larson MJ, Kiyoshi CM, Annett AJ, Stalker WA, Peng J, Tedeschi A (2020) Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury. J Clin Invest 130:345–358. 10.1172/JCI130391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, Abe T, Kiyonari H, Shimizu Y, Miyawaki A, Yokota H, Ueda HR (2014) Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157:726–739. 10.1016/j.cell.2014.03.042 [DOI] [PubMed] [Google Scholar]
- Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, Flynn KC, Stradal TE, Chrostek-Grashoff A, Brakebusch C, Bradke F (2010) Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci 30:6930–6943. 10.1523/JNEUROSCI.5395-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Bradke F (2017) Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol 42:118–127. 10.1016/j.conb.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F (2016) The calcium channel subunit alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92:419–434. 10.1016/j.neuron.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Dupraz S, Curcio M, Laskowski CJ, Schaffran B, Flynn KC, Santos TE, Stern S, Hilton BJ, Larson MJE, Gurniak CB, Witke W, Bradke F (2019) ADF/cofilin-mediated actin turnover promotes axon regeneration in the adult CNS. Neuron 103:1073–1085.e6. 10.1016/j.neuron.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Larson MJE, Zouridakis A, Mo L, Bordbar A, Myers JM, Qin HY, Rodocker HI, Fan F, Lannutti JJ, McElroy CA, Nimjee SM, Peng J, Arnold WD, Moon LDF, Sun W (2022) Harnessing cortical plasticity via gabapentinoid administration promotes recovery after stroke. Brain 145:2378–2393. 10.1093/brain/awac103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J (2004) Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci 24:6531–6539. 10.1523/JNEUROSCI.0994-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FM, Cragg JJ, Jutzeler CR, Röhrich F, Weidner N, Saur M, Maier DD, Schuld C; EMSCI Sites, Curt A, Kramer JK (2017) Early administration of gabapentinoids improves motor recovery after human spinal cord injury. Cell Rep 18:1614–1618. 10.1016/j.celrep.2017.01.048 [DOI] [PubMed] [Google Scholar]
- Warner G, Figgitt DP (2005) Pregabalin: as adjunctive treatment of partial seizures. CNS Drugs 19:265–272, discussion 273–264. 10.2165/00023210-200519030-00007 [DOI] [PubMed] [Google Scholar]
- Warren PM, Steiger SC, Dick TE, MacFarlane PM, Alilain WJ, Silver J (2018) Rapid and robust restoration of breathing long after spinal cord injury. Nat Commun 9:4843. 10.1038/s41467-018-06937-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F (2008) Microtubule stabilization specifies initial neuronal polarization. J Cell Biol 180:619–632. 10.1083/jcb.200707042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zhang H, Fan Y, Xiao Z, Zhao Y, Liu W, Xu B, Yin Y, Chen B, Li J, Cui Y, Shi Y, Dai J (2021) Upregulation of Apol8 by epothilone D facilitates the neuronal relay of transplanted NSCs in spinal cord injury. Stem Cell Res Ther 12:300. 10.1186/s13287-021-02375-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang X, Ge H, Liu W, Sun E, Ma Y, Zhao H, Li R, Chen W, Yuan J, Chen Q, Chen Y, Liu X, Zhang JH, Hu R, Fan X, Feng H (2018) Epothilone B benefits nigrostriatal pathway recovery by promoting microtubule stabilization after intracerebral hemorrhage. J Am Heart Assoc 7:e007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylera B, Ertürk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, Oudega M, Kirchhoff F, Bradke F (2009) Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol 19:930–936. 10.1016/j.cub.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Zheng J, Buxbaum RE, Heidemann SR (1993) Investigation of microtubule assembly and organization accompanying tension-induced neurite initiation. J Cell Sci 104:1239–1250. 10.1242/jcs.104.4.1239 [DOI] [PubMed] [Google Scholar]