Abstract

In recent times, nutrition and diet have become prominent health paradigms due to sedentary lifestyle disorders. Preventive health care strategies are becoming increasingly popular instead of treating and managing diseases. A nutraceutical is an innovative concept that offers additional health benefits beyond its fundamental nutritional value. These nutraceuticals have the potential to reduce the exorbitant use of synthetic drugs because the modern medicine approach of treating diseases with high-tech, expensive supplements, and long-term consequences aggravates consumers. However, most nutraceuticals are plant-derived, making them susceptible to degradation and prone to chemical instability, poor solubility, unpleasant taste, and bioactivity loss before absorption to the targeted site. To counteract this problem, the bioavailability of these labile compounds can be maximized by encapsulating them in protective nanocarriers. It is crucial that nanoencapsulation technologies convert bioactive compounds into forms that can be easily combined with functional foods and beverages without adversely affecting their organoleptic properties. In recent years, nanoformulations using food-grade materials, such as polysaccharides, proteins, lipids, etc., have received considerable attention. Among them, microbial polysaccharides are biocompatible, nontoxic, and nonimmunogenic, and most of them are US-FDA approved and can undergo tailored modifications. The nanoformulation of microbial polysaccharide is a relatively new frontier which has several advantages over existing systems. The present article, for the first time, comprehensively reviews microbial polysaccharides-based nanodelivery systems for nutraceuticals and discusses various techno-commercial aspects of these nanotechnological preparations. Moreover, this has also attempted to draw a future research perspective in this area.
1. Introduction
The positive aspects of diet have been prominent recently due to the emergence of a new health paradigm. People have adopted a more contemporary lifestyle and have also changed their food habits. The prevalence of obesity has been recognized as a global issue, while in many developing countries, heart disease, cancer, osteoporosis, arthritis, and many other diseases continue to be the leading causes of death worldwide.1 Since modern medicines are expensive and high-tech, consumers seek complementary or alternative products, and nutraceuticals are a particularly appealing substitute because they are primarily obtained from natural resources. A nutraceutical is an amalgamation of pharmaceutical and nutrition, a food product or component that nourishes the body to prevent diseases.2,3 The relationship between nutrition and immunity is well-known, with undernourished individuals at a greater risk of bacterial, viral, or other infections. In order to prevent infections effectively, our body needs a healthy, balanced diet with adequate minerals and vitamins.4 Additionally, prebiotics, probiotics, herbs, amino acids, enzymes, and other botanicals may be beneficial.
The roots of nutraceuticals can be traced back to Ayurveda and science, making them a healthy lifestyle choice for consumers.5 Earlier, this sector and the products were viewed as a cure for heavily exercising and dieting individuals; however, people have been facing many critical health issues globally, resulting in a shift in perception. However, they are now used for preventative therapies.6 The pandemic ravaging the world has prompted people to seek ways to boost their immunity to fight against multiple diseases. Nutraceuticals have experienced tremendous growth that has significantly contributed to the food, biomedical, and pharmaceutical markets due to the emergence of awareness and the need to stay fit among people. According to a report by the Deccan Chronicle (July 2021) on “Nutraceutical paradigm shift-from curative to preventive,” nutraceuticals are projected to generate USD 722.5 billion revenue by 2027 globally.
Most nutraceuticals are plant-derived, making them susceptible to degradation and prone to chemical instability, poor solubility, unpleasant taste, and bioactivity loss before absorption to the targeted site.2,4 To counteract this problem, the bioavailability of these labile compounds can be maximized by encapsulating them in protective carriers. A delivery system should also use affordable, safe, reliable, and label-friendly ingredients. The encapsulation technologies using food-grade materials, such as polysaccharides, lipids, proteins, liposomes, etc., have received much attention. In particular, polysaccharides have gained prominence as the body easily recognizes them due to their similar biochemical properties to human extracellular matrixes.7 They are also involved in many biological processes, including immune recognition and cell signaling, which activate antimicrobial and anti-inflammatory responses in the body. Further, in vivo degradation of these biopolymers occurs through enzymatic or hydrolytic reactions, resulting in innocuous degradation products that can be reused by any biological system or easily cleared by the immune system.8 In view of such features, polysaccharides are regarded as promising delivery systems for the future. Polysaccharides are the long chain of branched or unbranched sugar monomeric units joined through glycosidic linkages and are obtained from plants, animals, fungi, bacteria, and algae.9 Among these, microbially obtained polysaccharides have become popular due to various advantages such as rapid fermentation process, large-scale economic production, biocompatibility, nonimmunogenicity, biodegradability, and most of them are US-FDA approved and, hence, regarded as safe for human consumption.10,11 The production of microbial polysaccharides is based on sustainable processes, utilizing renewable resources, as well as controlled cultivation conditions to ensure a high yield of products devoid of changes in environmental factors.12 Polysaccharides are high molecular weight compounds with varying compositions, polarity, functionality, and fermentability.13 It has been found that many microbial polysaccharides can undergo modification and form films that make them suitable for preparing encapsulation coatings with various properties.12
In light of nutraceuticals’ potential efficacy and limiting characteristics, nanotechnology may prove to be a revolutionary innovation for enhancing their effectiveness in several diseases by leveraging their beneficial properties.14 Nanomaterials, as defined by the European Commission, are materials with small particles that possess specific properties.15 Since nanomaterials have size-dependent properties, they are used widely in diverse applications, offering numerous advantages along with posing few inherent menaces. Typically, nanoencapsulation systems use particles with an average dimension under 100 nm. It is believed that these kinds of systems can enhance the performance of bioactive substances in a number of ways, such as improving water dispersibility, enhancing chemical stability, avoiding undesirable flavor, reducing volatilization, and enabling site-specific or controlled release of the substances.16 Nanoencapsulation technologies are known to convert bioactive molecules into forms that can be easily combined with functional foods and beverages without adversely affecting the properties of the product.13 The use of nanobiotechnology may allow nutraceuticals to be delivered directly to targeted organs in the human body, for instance, by crossing the blood–brain barrier to the brain.4 Besides human well being, nanomaterials have also proven helpful in food quality and safety. Nanoencapsulation systems can be constructed using polysaccharides because they have diverse functional groups possessing surface activity, gelation, and structure-forming capabilities.17 In recent years, a trend toward multifunctional and more complex controlled release systems has been apparent in nanomaterials made of polysaccharides, improving the therapeutic efficacy, mechanical properties, and safety profiles of theranostics and regenerative medicine.
This review, one of its kind, will provide a comprehensive outlook on the present status of microbial polysaccharides and their nanoformulations for nutraceutical delivery (Figure 1). We have focused on the diverse microbial sources of polysaccharides. Moreover, we have discussed different types of nutraceuticals and their global market. Further, utilization of these polymers for the preparation of nutraceutical-loaded nanoformulations like nanogels, nanoparticles, nanofibers, nanoemulsions, and nanocapsules is mentioned. As a result of the information provided in this article, more efficient nanotechnology-enabled delivery systems may be developed in the future.
Figure 1.
Overview of microbial polysaccharide-based nanoformulations for nutraceutical delivery (created with BioRender.com).
2. Microbial Polysaccharides
In nature, polysaccharides are extensively distributed and produced by all types of organisms. Depending on the type of polysaccharide, it may be a homopolymer or heteropolymer, contain anionic or neutral sugars (pentoses and hexoses), and may have linked nonsugar compounds.18 Polysaccharides derived from microorganisms have recently emerged as essential biopolymers despite the fact that the majority of polysaccharides are derived from plants. In the present day, polysaccharides from microbes are emerging as economic competition for other natural and synthetic polymers. Several advantages are associated with the production and utilization of microbial polysaccharides for diverse applications. Regional or climatic conditions do not influence the production of microbial polysaccharides. Unlike plants, microbes exhibit rapid growth, and their production is comparatively easy.18 Moreover, the genetic modification of microorganisms can enhance the polysaccharides production along with desired properties. In addition to the ingredients required for microbial growth media, industrial food wastes may also be employed as a growth medium, an inexpensive and sustainable method that promotes a circular bioeconomy.19 Here, we have mentioned a few microbially obtained polysaccharides utilized for nanoformulations and delivery of nutraceuticals.
2.1. Alginate
The polymer alginate is abundant in cell walls of brown algae, including Phyophyceae. They are also derived from several bacterial sources such as Azotobacter and Pseudomonas, Laminaria, Ascophyllum nodosum, Macrocystis pyrifera, and a few others.20,21 It is an anionic heteropolymer composed of alternating unbranched units of d-mannuronate (M) and l-guluronate (G) connected by β-1,4 glycosidic linkages.22,23 In the past few years, alginate has gained attention in several industries due to its biocompatibility, biodegradability, low immunogenicity, adaptability, water-solubility, comparatively low production cost, thickening, and gelling abilities. Moreover, they have excellent mucoadhesive properties due to their carboxyl and hydroxyl groups, making them suitable for topical delivery systems of nutraceuticals to increase their efficiency and bioavailability.23 However, materials that contain simple alginates are prone to rapid leakage of encapsulated compounds since their pores are relatively large.16 The upregulation of alginate performance can be attributed to the fact that it can be combined with other polymers, organic and inorganic fillers, and bioactive substances. Therefore, several nanoformulations of alginate and its modified forms are reported in combination with different proteins and polysaccharides for nutraceutical delivery, as represented in Table 1.
Table 1. Different Nanoformulations of Microbial Polysaccharides for Encapsulation and Delivery of Nutraceuticals.
| Polysaccharide | Nutraceutical | Nanoformulation | Ref |
|---|---|---|---|
| Alginate/Cashew Gum | Lippia sidoides essential oil | Nanoparticle | (83) |
| Alginate Aldehyde-Gelatin | Curcumin | Nanogel | (99) |
| Alginate and β-Lactoglobulin | β-Carotene, folic acid, curcumin and ergocalciferol | Nanocomplex | (111) |
| Alginate-Chitosan | Essential oils such as turmeric oil and lemongrass oil | Nanocapsule | (106) |
| Alginate-Chitosan-Casein | Curcumin | Nanoparticle | (85) |
| Alginate/Sodium caseinate/ γ-cyclodextrin | Curcumin | Nanoemulsion | (97) |
| Alginate-Whey Protein | Omega-3 fatty acid | Nanoemulsion | (113) |
| Alginate/Zein-Pectin | Curcumin | Nanoparticle | (114) |
| Oleoyl Alginate Ester | Vitamin D3 | Nanoparticle | (115) |
| Propylene Glycol Alginate-Zein | β-Carotene | Nanoparticle | (116) |
| Propylene Glycol Alginate-Zein-Tea Saponin | Resveratrol | Nanoparticle | (117) |
| Sodium Alginate | Corn oil, oleic acid, olive oil, and unrefined and refined coconut oil | Nanoemulsion | (118) |
| Sodium alginate-Chitosan | Vitamin B2 | Nanoparticle | (119) |
| Sodium Alginate/Zein/Caseinate | Curcumin | Nanoparticle | (91) |
| Carboxylic Curdlan-coated Zein | Curcumin | Nanoparticle | (87) |
| Casein-Graft-Dextran | β-carotene | Nanoparticle | (120) |
| Dextran | Isoflavone genistein | Nanoparticle | (121) |
| Dextran | Vitamin B12 | Nanoparticle | (122) |
| Dextran-Casein | Curcumin | Nanoparticle | (86) |
| Dextran-Gelatin | Tea polyphenol | Nanoparticle | (123) |
| Dextran and Glycosylated α-Lactalbumin | Curcumin | Nanoparticle | (124) |
| Dextran-Ovalbumin | Curcumin | Nanogel | (100) |
| Dextran Sulfate-Amphiphilic Chitosan Derivative | Epigallocatechin-3-gallate | Nanoliposome | (109) |
| Dextran-Whey Protein | β-carotene | Nanoemulsion | (96) |
| Phthalyl Dextran | Probiotics | Nanoparticles | (90) |
| Gellan-Chitosan | Resveratrol | Nanofibers | (104) |
| Gellan-Pectin | Resveratrol | Nanoparticle | (125) |
| Levan | Resveratrol | Nanoparticle | (37) |
| Levan | O-acetyl-α-tocopherol | Nanoparticle | (126) |
| Levan and Alginate | Curcumin | Nanoemulsion | (94) |
| Levan and Poly lactide-co-glycolide | Curcumin | Nanoparticle | (127) |
| Neorhizobium urealyticum K1T sp. nov. exopolysaccharide | Astaxanthin | Nanoemulsion | (95) |
| Pullulan | Probiotics | Nanoparticle | (128) |
| Pullulan-Ovalbumin | Curcumin | Nanogel | (101) |
| Xanthan Gum and Bovine Serum Albumin | Curcumin | Nanoparticle | (129) |
| Xanthan Gum-Chitosan | Curcumin | Nanofibers | (102) |
| Xanthan Gum-Zein | Curcumin | Nanoparticle | (130) |
2.2. Curdlan
A polysaccharide produced by a microbe and secreted outside its cells is known as an exopolysaccharide (EPS).24 Curdlan is one such EPS isolated from Alcaligenes faecalis var. myxogenes 10C3 in 1962 by Harada and colleagues for the first time.25 Moreover, bacteria from the genus Rhizobium, Alcaligenes, Bacillus, Agrobacterium, and Cellulomonas are also known to produce curdlan.26 It is a linear homopolysaccharide composed of glucose connected by β-1,3 glycosidic linkages. Recently, curdlan has acquired popularity in food and nonfood industries because of its unique physicochemical and rheological properties. In addition to thickening, stabilizing, and gelling agents, curdlan can mimic meat, fat, and seafood. Further, it is used for encapsulating drugs in the pharmaceutical industry. There is excellent potential in phosphorylated and carboxymethylated derivatives of curdlan.27 Curdlan also possesses antitumor, anti-inflammatory, and immunomodulating properties, which can be advantageous when using it as a delivery vehicle in therapeutics.
2.3. Dextran
Louis Pasteur discovered dextran in 1861, and its first known source was a slime-producing bacteria called Leuconostoc mesenteroides.28 The production of dextran by Gram-positive, facultatively anaerobes spherical-shaped bacteria such as Streptococcus and Leuconostoc strains has been demonstrated in subsequent studies. Dextran is a neutral polysaccharide composed of repeating units of d-glucose with α-1,6 glycosidic linkages between the monomers and α-1,2, α-1,3, and α-1,4 linkages in the branching.29 It is highly soluble, cytocompatible, biodegradable, and nonimmunogenic, making it the perfect biopolymer for generating nanomaterials for delivery. Moreover, Dextran 70 has been listed as an essential medicine by the World Health Organization (WHO) since April 2015 as a blood plasma substitute.30 Drug dissolution and permeation through gastrointestinal membranes can be facilitated by nanocarriers composed of dextran with high water solubility.28 Digestive enzymes metabolize dextran to be physiologically harmless, whereas artificial polymers may accumulate as toxins and cause side effects. For nanomaterials to uphold the integrity of delivery systems, prolonged retention time, improved bioavailability of cargo molecules, and colloidal stability against degradation by the enzyme in gastrointestinal tract are crucial.28 Amylases such as salivary amylase and malt amylase barely break down dextran, unlike other polysaccharides. Shingel reported that dextranase depolymerizes dextran only in the lumen of large intestine, kidney, liver, and spleen.30 Therefore, dextran-based delivery systems can protect compounds against chemical and enzymatic degradation throughout the stomach and small intestine, resulting in improved bioavailability. Another factor that facilitates dextran’s efficacy is its neutral charge. Due to electric repulsion, delivery systems with anionic nature have difficulty getting through the mucus layer. According to studies, mucus is best permeated through an electrically neutral and polar surface of a delivery system.31,32 Due to several reactive hydroxyl groups, various derivatives have been prepared through tailored modification of the dextran backbone. It is also necessary to investigate eco-friendly fabrication methods for dextran derivatives, as they are mainly synthesized using organic solvents and sometimes hazardous chemicals, which raise possible safety concerns.
2.4. Gellan
Sphingomonas elodea (Pseudomonas elodea) produces gellan, a natural anionic polysaccharide.33 It is a linear tetrasaccharide composed of repeating units of α-l-rhamnose, β-d-glucose, and β-d-glucuronate in a ratio of 1:2:1. These units are linked in the form of (1,3)-β-d-glucose-(1,4)-β-d-glucuronate-(1,4)-β-d-glucose-(1,4)-α-l-rhamnose-(1→).34 Further, gellan can be classified into high acyl (HA) and low acyl (LA) based on the degree of acyl substitution. It possesses rapid gelation, thermoresponsiveness, mucoadhesiveness, biocompatibility, nontoxicity, elasticity, easy derivatization, and the ability to tolerate acid and heat stress.35,36 As an environmentally friendly substitute to synthetic polymers in drug delivery systems, this biopolymer has gained considerable interest in pharmaceutical and biomedical research in the last few decades. A wide range of dosage forms is being explored for gellan. Tablet binder, granulating agent, and coating materials have been investigated for conventional dosage forms; however, their applications are less attractive.35 The development of relatively recent nanoformulations has assisted in altering drug pharmacokinetics and making gellan more bioavailable by delivering it in a sustained manner as well as by releasing it at a specific site or by targeting the receptor.
2.5. Levan
Levan is a natural fructan-type polysaccharide composed mainly of β-(2→6) linked β-d-fructofuranose residues that can be linear or branched with β-(2→1) linkages.37 In nonsterile, highly saline conditions, Halomonas smyrnensis cultures were the first extremophilic levan producers that provided high yields and were cost-effective. Moreover, bacteria from the genera Bacillus, Acinetobacter, Acetobacter, Pseudomonas, and Zymomonas are also known to produce levan.38 It is an amphiphilic polymeric material able to form films with high adhesion properties and is compatible with salts and surfactants. In addition, it exhibits biocompatibility, nontoxicity, stability in heat, acidic, and alkaline media, and high water holding capacity. Levan can self-assemble into nanoparticles in water, which is one of its most attractive properties. At the Critical Aggregation Concentration (CAC), when macromolecules reach a critical concentration, their chain reorganizes to reduce their hydrophobic sections’ exposure to water.39 However, levan is comparatively less utilized in pharmaceutical and biomedical formulations; hence, its effectiveness needs to be proven on a larger scale. The limited production strategies of levan prevent its widespread production and use despite its many industrial applications. It will be necessary to introduce novel techniques to both produce and purify this biopolymer so that production costs can be reduced and stability can be increased.
2.6. Pullulan
Several strains of Aureobasidium pullulans produce pullulan, an extracellular polysaccharide.40 This polysaccharide is neutral, composed of repeating maltotriose units attached by α-1,4 glycosidic bonds, and trimers are linked together via α-1,6 glycosidic bonds. In fact, the generally regarded as safe (GRAS) status of pullulan indicates it is nontoxic and nonimmunogenic. Furthermore, pullulan chains contain a high percentage of hydroxyl groups, making the polymer highly soluble and chemically flexible.41 Pullulan is extensively used in food, biomedical, and pharmacological industries due to its numerous properties, such as high solubility, structural flexibility, thickening, and encapsulation ability. With the incidence of several reactive hydroxyl groups, pullulan is a versatile polymer that can be altered with various conjugation techniques to introduce additional functional groups or to conjugate with different bioactive compounds.40 For efficient bioactive compound delivery, pullulan can be transformed into multiple forms, including nanospheres, nanogels, and nanoparticles. Integrin-mediated cell adhesion can lead to cell internalization of different pullulan forms.40
2.7. Xanthan
Xanthan is isolated from Gram-negative bacteria Xanthomonas campestris.42 It is an anionic polysaccharide composed of a linear backbone of glucose units connected by β-1,4 glycosidic bonds and pendant trisaccharide side-chains consisting of d-glucuronic acid (β-1,2) and d-mannose (β-1,4) along with d-mannose residues at the terminal.16,42 The intramolecular and intermolecular hydrogen bonding between the xanthan molecules occurs due to hydroxy and carboxyl groups.43 Interestingly, aqueous xanthan solutions exhibit high intrinsic viscosity at low concentrations and behave like pseudoplastic fluids because of their high molecular weight and hydrogen bonding.42 For food, pharmaceuticals, and cosmetics industries, xanthan’s high viscosity, low pH, and salt-resistance properties make it an ideal thickening, suspending, and stabilizing agent.44,45 The ability to undergo electrostatic interaction with cationic groups makes it helpful in developing polysaccharide-based delivery systems. Moreover, the human stomach or small intestine cannot digest xanthan, however, colon enzymes can degrade it. It is, therefore, feasible to deliver the nutraceuticals to the colon through xanthan matrices, which shield compounds from the stomach and small intestine environments.42 While native xanthan boasts many potential benefits, it also has some drawbacks, including microbial contamination, poor shear resistance, unstable viscosity, uncontrolled hydration rates, inadequate mechanical and thermal properties.42 Consequently, it has limited applications in the pharmaceutical and biomedical fields.
3. Nutraceuticals
Nutraceuticals focuses on prevention, as the Greek physician Hippocrates, the father of medicine, said, “let your food be your medicine and your medicine be your food”.46 A nutraceutical is a food product that may be made up of several ingredients, such as whole foods or even isolated ingredients. It may also consist of herbal products, processed foods (e.g., cereals, beverages), or genetically modified items.14 In terms of “nutraceutical products”, there has been controversy to date regarding their specific definition. The concept of “nutraceuticals” does not have a clear definition globally; however, the function of these products in promoting health and preventing disease is included as part of their nutritional value.47 The definition of nutraceutical varies according to different regulatory agencies. As per the Food Safety and Standards Authority of India (FSSAI), “nutraceutical” refers to a food (or part of a food) that has the ability to prevent and treat health problems.48 A nutraceutical product is regulated in the US as a drug, an ingredient in food, or a dietary supplement.49 Santini et al. have reviewed different definitions of nutraceuticals, accordingly, the Food and Drug Administration, FDA, Dietary Supplement Health and Education Act of 1994 (DSHEA), United States define nutraceuticals as the health-enhancing dietary supplements that deliver biologically active food components in a nonfood matrix.50 A significant interest in nutraceuticals has opened up innovative product development opportunities to meet consumer needs for healthier foods.51 There is growing evidence regarding the clinical significance of nutraceuticals since they can effectively prevent and treat several diseases, including allergies, inflammation, cardiovascular, Alzheimer’s, Parkinson’s disease, eye disorders, obesity, diabetes, and regulating immune system function.49 As a result of the merger between pharmaceutical, agribusiness, and nutrition conglomerates, nutraceuticals are found in a mosaic of products related to food, herbal supplements, and pharmaceutical manufacturers. Nutraceuticals can help humans maintain a healthy lifestyle and prevent disease as long as they are consumed within their recommended dietary intakes. There are multiple bases to classify nutraceuticals into different categories, among them, the most recent one is based on diverse sources, which is also illustrated in Figure 2.
Figure 2.
Classification of nutraceuticals based on diverse sources (created with BioRender.com).
3.1. Natural or Traditional
Traditional nutraceuticals are procured directly from natural sources and utilized without changing their components.52 Examples include lycopene in tomatoes, reservetol in grapes, soybeans rich in saponins, omega-3 fatty acids in salmon, naringin from oranges, and so on. They are further divided into nutrients, herbals, phytochemicals, probiotics, and enzymes based on their chemical constituents.53 Vitamins, minerals, fatty acids, and amino acids are all nutrients that are recognized for their nourishments.54 Vitamins are dietary supplements that can cure diseases like stroke, cataracts, and cardiovascular. Plants, animals, and dairy products contain minerals beneficial for health, including osteoporosis, teeth strength, anemia, bone growth, muscle strength, nerve impulses, and heart rhythms. A diet enriched with omega-3 polyunsaturated fatty acids (PUFAs) maintains brain function, reduces cholesterol deposition, and regulates inflammatory processes.53
Nutraceuticals made from herbal ingredients help prevent chronic diseases and improve health. They are analgesic, anti-inflammatory, antipyretic, astringent, and antiarthritic.53 Moreover, flavonoids in herbs such as apiol and psoralen act as diuretics, carminatives, and antipyretics. A component of peppermint is menthol, which has anti-inflammatory properties and helps cure colds. Proanthocyanidin found in some herbals helps treat or prevent cancer, ulcers, and urinary tract infections. In contrast, tannins found in some plants are believed to alleviate depression, colds, cough, hypertension, and asthma.49,55
A phytochemical is a plant compound that has active components for a variety of biological and metabolic reactions in humans.56 Neuroprotective effects of phytochemicals are attributed to their ability to maintain a chemical balance in the brain.57 Furthermore, phytochemical-rich vegetables and fruits can reduce cancer, cardiac and neurodegenerative disease risks. One of the most common and highly demanded phytochemicals is curcumin obtained from Curcuma longa L’s dried rhizomes.58 Traditional Indian and Chinese medicine agents have utilized curcumin for centuries as an anti-inflammatory. There are various biological and pharmacological benefits of curcumin, including anticarcinogenic properties, antioxidant properties, immunomodulatory properties, and antiangiogenic properties. Among other functions, phytochemicals act as substrates, cofactors, and inhibitors of biochemical reactions that bind to and eliminate unwanted constituents in the intestine and enhance vital nutrients’ absorption and stability.53
Probiotic means “for life” and refers to live microorganisms that confer health benefits on the human body when consumed in a tolerable amount.53,56,59 As a new concept, gerobiotics refers to probiotics or postbiotics (metabolites of microorganisms) that play a role in preventing or treating different ailments.59−61 Microorganisms such as bacteria aid digestion and absorb nutrients. As a result, they act as a symbiotic community to reduce the number of pathogens that cause human disease. The most common probiotic that survives in the human gut is Lactobacillus, which has different species. Several probiotic strains are currently being used as probiotics, including Bifidobacterium spp., Streptococcus, and Bacillus.1,62−64 Their antimicrobial properties are derived from altering microflora that prevents pathogen adhesion to the intestinal epithelium, competing for nutrients needed for pathogen survival, producing antitoxin effects, and reversing some intestinal epithelium’s effects caused by infection, such as secretory changes and neutrophil migration.49
Enzymes function as nutraceuticals because biological processes cannot take place without enzymes. The use of enzyme supplements in the diet can benefit those suffering from medical conditions such as diabetes, digestion problems, hypertension, obesity, etc.53 A variety of enzymes can be found in microbial, animal, and plant sources. In order to augment the nutritional value of several foods, enzymes are used for fortification. Carbohydrates, proteins, and fats in foods are broken down into simpler molecules by these enzymes, supporting digestion and metabolism.65 A study in spontaneously hypertensive rats found that alcalase and pepsin hydrolyzed Australian canola protein lowered blood pressure.66 Nutraceuticals and functional foods that contain these hydrolysates may be useful antihypertensive ingredients.
3.2. Unnatural or Nontraditional
In contrast to traditional ones, nontraditional nutraceuticals are foods or products that are artificially synthesized.56 Agriculture breeding or biotechnology is applied to enhance food properties and human health. Examples include several nutraceutical products containing provitamin A, responsible for boosting antioxidant activity, such as rice supplemented with carotene and cereals infused with vitamins as well as minerals.1,67 Nutraceuticals that are not traditional can be classified as fortified or recombinant, depending on how they are processed. “Designer food” or fortified foods are food products that are fortified with nutrients to enhance their effectiveness.52 The value of fortified nutraceuticals can be improved by adding micronutrients or vitamins, such as calcium to orange juice and cholecalciferol to milk.56 These foods contribute to the prevention of anemia and the improvement of health by providing the body with essential nutrients. As an example, infant milk fortified with prebiotic and probiotic Bifidobacterium lactis HN019 can help children with diarrhea, respiratory infections, and severe illnesses.53 According to reports, fortified foods are highly effective against micronutrient malnutrition for quite a few reasons. First, by choosing a form of fortified food that is attractive to many individuals, fortified food can be accessible to more people.68 A second reason is that people prefer not to change their food habits or characteristics. The third benefit of fortified food is that its introduction and benefits can be achieved quickly. In addition, they are generally considered safe and inexpensive by most people.52
On the other hand, biotechnology and genetic recombination are both involved in producing recombinant nutraceuticals.56 Genetically modified foods and crops can be used to create products containing recombinant compounds and proteins, thereby improving their health benefits. These nutraceuticals include golden rice, iron rice, golden mustard, maize, and gold kiwifruit.1,47,52,53 Malnourished people often consume meals that revolve around a staple crop, so they lack access to the wide variety of fruits and vegetables necessary for a healthy diet. Due to undernutrition, one-third of all childhood deaths under five occur worldwide, and one in four children is stunted. Genetically engineered food crops that have improved traits, such as an increase in iron storage, proteins, or a higher level of folate, can provide sufficient levels of these micronutrients and others that are regularly lacking in developing world diets.69 Nevertheless, genetically modified foods can potentially have detrimental effects, such as antibiotic-resistant diseases.52 A more thorough evaluation of the unknown, long-term effects on humans needs to be conducted.
4. Global Market of Nutraceuticals
Author: Over the past few years, the nutraceutical industry has been rapidly flourishing and expanding. Preventive health care strategies are becoming increasingly popular instead of treating and managing diseases.70 Healthcare costs are expected to rise in both developed and developing countries due to this trend. With chronic diseases being more prevalent, healthcare interventions are becoming more expensive, which is increasing the demand for nutraceuticals. Moreover, consumers are increasingly choosing natural and organic nutraceuticals over synthetic pharmaceuticals. Nutraceuticals are “unstoppable” in the global market despite the brevity of experts’ definitions of what they are (Figure 3).71 The global nutraceutical market evokes continuous controversy, so it is essential to evaluate it. The nutraceuticals industry is divided into functional foods, natural or herbal products, and dietary supplements.53 Functional foods dominated the nutraceuticals market share in 2020; however, functional beverages grew faster in the forecast period.
Figure 3.
Global market of nutraceuticals and its chief consumers. Reproduced with permission from ref (70). Copyright Elsevier 2022.
The nutraceuticals market was expected to grow by nearly USD 250 billion by 2018, as per the report by Klynveld Peat Marwick Goerdeler (KPMG).70 Furthermore, the market value of nutraceuticals was estimated to be over $275 billion by 2021, with an exponential growth rate of 7.3% CAGR. However, according to a market analysis report by Grand View Research, nutraceutical businesses rose rapidly and were worth USD 454.55 billion in 2021, which is projected to grow at a CAGR of 9.0% between 2021 and 2030.72 In the current scenario, a key driving force for market is demand for dietary supplements and functional foods in the wake of COVID-19 pandemic. As a result, nutraceuticals have gained significance in the global market during and after the COVID-19 pandemic. Moreover, the largest revenue share of around 30% in 2021 was contributed by the Asia Pacific. At the same time, a high product adoption rate leads the United States, Japan, and Germany to be the key market drivers.70 In the Asia Pacific region, India is another rapidly growing nutraceutical market. It was assessed to be around USD 2.2–2.8 billion in 2015, which grew to USD 6.1 billion by the year 2019–2020 and was likely to reach USD 8.5 billion by 2022.70,73 The Indian population has changed its lifestyle significantly over the past decade, resulting in the growth of this industry. Fast foods, packaged foods, and sedentary lifestyles have increased the incidence of lifestyle diseases. Consequently, nutraceuticals are preferred by Indian consumers, mainly those of higher socio-economic status, as an alternative to pharmaceuticals for improving health and preventing illness.72,73
5. Nanoformulations to Encapsulate Nutraceuticals
When using large-sized materials, there are several obstacles to overcome, including in vivo instability, poor solubility, poor absorption in the body, and the possibility of adverse reactions.74 In this light, nanotechnology plays a significant role in the design of advanced nanoformulations of polymers, their targeting arenas, controlled release, and delivery of bioactive compounds. Recent decades have seen nanotechnology as one of the most promising research fields. This new technology studies nanometer-sized materials and their exceptional properties compared to micro/macroscale counterparts.75 Foods and nutrients that are hydrophobic or poorly soluble are vital to health, including water-insoluble vitamins, essential oils, phenolic compounds, flavors, and aromatic compounds, to name a few.76 In addition to being prone to oxygen sensitivity, light sensitivity, and temperature changes, these ingredients have poor solubility and bioavailability, thus limiting their application.77 Moreover, the sensory quality of foods is affected by essential oils, fish oil, and other nutraceuticals with unpleasant flavors that limit their direct inclusion in food formulations.78,79 Thus, the nanoencapsulation technique is beneficial for masking undesirable flavors in food formulations. In the areas of food, pharmaceuticals, and cosmeceuticals, nanoencapsulation has received recognition owing to its ability to control the release of nutrients in a defined zone and time.75 It offers numerous advantages in terms of targeted delivery and bioavailability. Through encapsulation, nutraceuticals can be protected from damage and undesirable variations owing to processing stages and digestion conditions. This necessitates the development of a suitable carrier system.80 In fact, nanocarriers prolong the nutraceuticals’ presence in blood and cellular uptake. Some of the obstacles that limit the effectiveness of low-molecular-weight chemotherapeutics and functional biological macromolecules (such as proteins and oligonucleotides) include poor solubility, loss of bioactivity before reaching targeted sites, inadequate cellular uptake, enzymatic degradation, rapid renal clearance, drug resistance caused by overexpression of the efflux transporter, short plasma half-lives, as well as unwanted side effects.81 Compared to low-molecular-weight molecules and small nanoparticles, macromolecules such as polysaccharides and large nanoparticles are more effective at trapping cells due to their enhanced permeability and retention properties.7 Polysaccharides have a higher number of hydroxyl groups and other hydrophilic groups, such as carboxyls and amino groups, enhancing aqueous solubility along with bioadhesion and biorecognition characteristics through noncovalent interactions. Thus, making polysaccharides as most promising delivery systems. Previously, Yaxu et al. have reviewed polysaccharide-based nanodelivery systems for encapsulation, delivery, and pH-responsive release of bioactive ingredients. This review, however, focuses on the use of microbial polysaccharides nanoformulation for nutraceutical entrapment and delivery, which will lead to new outcomes and broaden the scope of applications. We have discussed a few microbial polysaccharide-based nanoformulations to encapsulate nutraceuticals for targeted delivery. Moreover, Table 1 summarizes the microbial polysaccharides utilized
5.1. Nanoparticles
Material with a nanoscale dimension, i.e., smaller than 100 nm, is called a nanoparticle.82 The use of these materials in modern medicine has expanded dramatically over the past few decades, from contrast agents in medical imaging to delivery vehicles (Figure 4). The interiors of polysaccharide-based nanoparticles are primarily composed of polysaccharide molecules instead of water.16 It is likely to alter the physicochemical and functional properties of this type of nanoparticle by changing its size, shape, composition, and charge. Oliveira et al. synthesized alginate/cashew gum nanoparticles via the spray-drying method for Lippia sidoides essential oil encapsulation and delivery.83 Conventional spray dryers use high shear mixers, homogenizers, or ultrasonicators to emulsify active ingredients in a solution of food-quality wall materials and then dry the emulsification.84 The nanoparticle encapsulated essential oil exhibited encapsulation efficiency of around 55% and in vitro release studies suggested that within 30 to 50 h, 45 to 95% of the oil was released. As a result of adding cashew gum to alginate, it is possible to maximize hydrophilicity of polymer matrixes, permitting a faster release of oil at an acceptable load level. The oil utilized in this study was reported to possess activity against dengue vector A. aegypti larvae; hence, this study paves the way for the development of larvicide to fight dengue. In another study, polysaccharide alginate and chitosan were conjugated with casein protein to form nanoparticles and encapsulate curcumin to enhance its bioavailability and therapeutic index.85In vitro and in vivo evaluations of the developed nanoformulation against Ehrlich carcinoma were assessed by delivering curcumin orally. Similarly, Meng et al. reported developing casein-dextran nanoparticles through heating in a dry/wet state and regulating pH to investigate their physiochemical properties.86 This nanoparticle was synthesized to encapsulate curcumin and its targeted delivery to the intestine. It was capable of exhibiting higher stability under simulated gastrointestinal conditions. Apart from this, a study conducted by Yu et al. attempted to encapsulate curcumin using carboxylic curdlans.87 As hydrophilic coatings for hydrophobic zein nanoparticles, carboxylic curdlans with different carboxylate contents, molecular weights, and chain conformations were prepared. The freshly prepared curcumin-loaded nanoparticles were spherical in shape, had a particle size of 18.3 nm, and had a zeta potential of +17.7 mV, showing 66.7% encapsulation efficiency. In this study, encapsulated curcumin’s particulate and physicochemical characteristics were affected by anionic carboxylic Curdlan coatings on zinc surfaces. As a result of this coating, the curcumin is more water dispersible and photo- and thermostable and exhibits sustained release behavior in aqueous environments. The carboxylic curdlans’ chain conformation, molecular weight, and carboxylate content were correlated with these properties. Moreover, hydrogen bonding, hydrophobic interactions, and electrostatic interactions among curcumin, zinc nanoparticles, and carboxylic curdlans were the key factors contributing to the observed improvements. Therefore, this nanomaterial formulation paves the way for enhancing the delivery of hydrophobic nutraceuticals.
Figure 4.
Sodium alginate/caseinate/zein nanoparticle formation with encapsulation and release of curcumin. Reproduced with permission from ref (91). Copyright Elsevier 2019.
Another interesting study on nanoformulation of levan nanoparticles utilized electrohydrodynamic atomization (EHDA) for entrapping resveratrol, a polyphenol from stilbene found in grape seed and skin displaying antioxidant, anticancer, anti-inflammatory, and cardioprotective activities.37 Atomization of conductive solutions under an electric field is the principle behind EHDA.88 When electrical forces overcome surface tension stress in a charged solution jet, it decomposes into tiny particles by Coulomb repulsion.89 Ultimately, this leads to nanoparticle formation after solvent evaporation. This system has potential applications in drug delivery, wound healing, and tissue engineering.
On the other hand, due to widespread bacterial resistance caused by the overuse of antibiotics, probiotics have attracted much attention as an alternative to antibiotics. In order to determine whether prebiotics enhance the cellular and antimicrobial properties of probiotics, Kim et al. prepared phthalyl dextran nanoparticles (PDNs) by conjugating phthalic anhydride with dextran to form a prebiotic.90 By internalizing PDNs, probiotics produced higher antimicrobial peptide levels, which resulted in more significant antimicrobial activity against Gram-positive and -negative pathogens. Additionally, pediocin produced by PDN-internalized probiotics suppressed pathogenic gut infections and altered the gut microbiome population in vivo. Pediococcus acidilactidi internalized with PDNs may have enhanced antimicrobial properties in mice, reducing pathogens and increasing beneficial bacteria species. Based on these results, PDNs might offer a new avenue for probiotic modulation and could help address the challenge of bacterial resistance as a new type of prebiotic. Nanoparticles of other microbial polysaccharides, such as gellan, xanthan, pullulan, etc., exhibit tremendous potential as a nutraceutical delivery vehicle.
5.2. Nanoemulsion
Nanoemulsions typically consist of two or more immiscible liquids dispersed with a surfactant, and droplets range from 50 to 500 nm.92 At least three ingredients make up nanoemulsions, i.e., oil, water, and an emulsifier. Here, polysaccharide functions as an emulsifier due to presence of diverse functional groups. High optical transparency, better physical stability, and improved bioavailability of nanoemulsions make them ideal delivery vehicles for bioactive components.16 Further, nanoemulsions are categorized as water-in-oil (W/O), oil-in-water (O/W), oil-in-water-in-oil (O/W/O), and water-in-oil-in-water (W/O/W) according to the spatial arrangement of hydrophobic and hydrophilic phases (Figure 5).93 Moreover, W/O nanoemulsions help deliver hydrophilic nutraceuticals such as vitamin C, whereas O/W nanoemulsions are responsible for delivering lipophilic compounds such as curcumin, carotenoids, essential oils, etc.16 Nanoemulsions have a few disadvantages, including their thermodynamic instability, aggregation, and flocculation. In order to prevent the separation of oil and water phases, stabilizers should be added. Interestingly, the polysaccharide may function as a stabilizer in O/W nanoemulsions. As a result of polysaccharides interacting with oil droplets, they form protective coatings to prevent aggregate formation. Richa et al. utilized a similar concept for O/W nanoemulsion formation using polysaccharides, i.e., levan and alginate, along with olive oil and castor oil to encapsulate curcumin.94 Another study by Rupsa et al. utilized marine exopolysaccharide isolated from Neorhizobium urealyticum K1T sp. nov. and clove oil for nanoemulsion formation via ultrasonication to entrap astaxanthin which is a carotenoid.95 A similar study by Fan et al. focused on modulating the bioaccessibility and physicochemical stability of β-carotene loaded oil-in-water (O/W) nanoemulsions in the gastrointestinal tract by utilizing protein-carbohydrate Maillard conjugates (WPI-dextran (DT) conjugates).96 Compared to other medium chain length triglycerides and saturated triglycerides, corn oil had higher β-carotene bioaccessibility which was utilized in this study. According to previous studies, whey protein isolate (WPI) reacts slowly with gastric pepsin due to its folded β-sheet conformation, and it can control the release of β-carotene in the stomach. According to this study, the bioavailability of nutraceuticals can be regulated by high molecular weight polymer. Researchers may use the findings from this study to enhance the encapsulation, stabilization, and application of nutraceuticals and pharmaceuticals using nanoemulsions. Additionally, Wang et al. prepared alginate/sodium caseinate/γ-cyclodextrin nanoemulsion to deliver curcumin. In this system, alginate was responsible for enhancing the acid stability.97
Figure 5.
Schematic representation of nanoemulsion formation and types of emulsions, i.e., oil-in-water (O/W), water-in-oil (W/O), water-in-oil-in-water (W/O/W), and oil-in-water-in-oil (O/W/O) (created with BioRender.com).
5.3. Nanogel
Nanogels are three-dimensional submicron-sized particles formed by physically or chemically cross-linking monomers or polymers (Figure 6).76 Nanogels possess unique swelling properties owing to the degree and type of cross-linking that may also impart stimuli-responsiveness to the system. They are porous in nature, having tunable and different pore sizes, allowing high loading capacity.98 Nanogels can easily encapsulate hydrophilic compounds, however, for entrapping hydrophobic molecules, one may require a lipophilic nanocarrier first.16 Sarika et al. prepared curcumin-loaded alginate aldehyde–gelatin nanogels through the inverse miniemulsion technique.99 These nanogels induce anticancer activity toward MCF-7 cells and can be utilized for targeted delivery to cancer cells. Another report on dextran-ovalbumin nanogels also mentioned encapsulation and delivery of curcumin.100 Here, nanogels were prepared as a result of the Maillard reaction. In this reaction, an amino group from protein is converted into a Schiff base by condensing it with a carbonyl group from dextran, which then cyclizes into an N-substituted glycosylamine.10 The results suggest that designing nanogels could improve the oral bioavailability of lipophilic nutraceutical curcumin that can be incorporated into functional foods. A similar method was reported for preparing pullulan-ovalbumin nanogels for encapsulating and delivering curcumin.101 The average particle diameter of nanogels and curcumin-loaded nanogels was around 190 and 160 nm, respectively, along with a polydispersity index of 0.227 and 0.146, respectively. This nanogel possessed an encapsulation efficiency of 88.38% with a loading capacity of 8.78% curcumin. Curcumin-loaded pullulan-ovalbumin nanogels were found to facilitate the controlled release of curcumin and underwent structural damage during in vitro digestion, as observed under transmission electron microscopes. Additionally, the 30-day storage stability of both nanogels and curcumin-loaded nanogels is noteworthy as that facilitates the effective delivery of drugs and nutrients.
Figure 6.

Polysaccharide-based nanogel formation. Reproduced with permission from ref (76) Copyright Elsevier 2019.
5.4. Nanofibers
Nanofibers are gaining researchers’ attention owing to their encapsulating properties and delivering hydrophobic and hydrophilic nutraceuticals.76 They are nanosized fibers ranging in diameter from 1 to 100 nm.16 For the fabrication of nanofibers, the electrohydrodynamic method has been extensively researched due to its high encapsulation efficiency, low processing temperature, and ability to incorporate sensitive and volatile bioactive compounds.102 Electrospinning is the newly developed approach for encapsulation that utilizes high voltage to produce nanofibers.75 By applying an appropriate electrical field, electrospinning uses a solution of polysaccharides to draw a thin stream of fluid through a nozzle, which causes the solvent to evaporate, depositing fibers on a collector plate (Figure 7).16 As polysaccharides differ in their molecular weights, entanglement concentrations, morphologies, and shear properties, they have different spinning properties. As a result of their high surface area to volume ratio, electrospun nanofibers are highly sensitive to environmental changes, proving them advantageous for controlled release of encapsulated bioactive compounds.103 Shekarforoush et al. reported encapsulation and release of curcumin that were successfully achieved via electrospinning technology using viscoelastic gels of xanthan gum and chitosan in formic acid.102 This study showed a low release of curcumin (∼20%) at acidic pH compared to neutral pH (∼50%), thereby suggesting the efficiency of nanofibers for encapsulating hydrophobic compounds. Likewise, another research group also reported a similar method for fabricating gellan-chitosan composite nanofibers to enhance resveratrol’s encapsulation efficiency.104 It was able to deliver 43–51% of total encapsulated resveratrol in the intestine region with significantly higher antioxidant activity, thus enhancing the stability and bioavailability of resveratrol.
Figure 7.
Fabrication of nanofibers using different electrospinning techniques. Reproduced with permission from ref (76). Copyright Elsevier 2019.
5.5. Nanocapsules
The vesicular structure of nanocapsules is enclosed by a polymeric membrane surrounding a central core where substances may either dissolve or adsorb on the surface.105 Nanocapsules have an advantage over nanoparticles in terms of polymer quantity required for the delivery system formulation. There are several methods to fabricate nanocapsules, such as interfacial polymerization, self-assembly, ionogel, and nanoprecipitation (Figure 8).16 However, there is only a single report on utilizing microbial polysaccharides to form nanocapsules for nutraceutical delivery. In this regard, Natrajan et al. have synthesized a alginate-chitosan nanocapsule to encapsulate essential oil through the ionogel method.106 The interaction between cationic chitosan and anionic alginate leads to forming a polyelectrolyte complex that remains stable in simulated gastrointestinal conditions and protects the bioactive compounds. Moreover, lemongrass oil and curcumin encapsulation efficiencies were approximately 87% and 71%, respectively. The authors also studied the pH-responsive delivery of nutraceuticals which suggested the release of 42% lemongrass oil and 90% curcumin at pH 7.4 in 48 h, whereas 38% and 70% were released at acidic pH.
Figure 8.
Schematic representation of polysaccharide nanocapsules formation through nanoprecipitation steps. Reproduced from ref (107). Copyright American Chemical Society 2019.
5.6. Nanoliposomes
Liposomes are spherical molecules with walls composed of an amphiphilic lipid bilayer of hydrophilic polar head and hydrophobic nonpolar tail.108 Such uniqueness in structure allows the encapsulation of hydrophilic compounds interiorly, hydrophobic compounds within their bilayers, and amphiphilic molecules in the lipid/water interface, thereby promoting its utilization as a carrier vehicle in different food, biomedical and therapeutic applications.79 However, they have specific limitations, such as physiochemical instability, that causes its degradation and phospholipids aggregation.76 The nanoformulation of liposomes can improve stability of volatile compounds and increase their solubility, that ultimately enhances their bioavailability. Therefore, by coating dextran sulfate on the surface of amphiphilic chitosan nanoliposome via electrostatic interaction, a novel delivery system for Epigallocatechin-3-gallate (EGCG) was reported.109 In this work, dextran sulfate helps in enhancing the stability of nanoliposome under simulated intestinal conditions. Dynamic high-pressure microfluidization (DHPM) combined with the film evaporation method was utilized for nanoliposome formation (Figure 9). This system was reported to possess high EGCH encapsulation efficiency with sustainable release behavior.
Figure 9.
Dynamic high-pressure microfluidization (DHPM) combined with the film evaporation method was utilized for nanoliposome formation of amphiphilic chitosan derivatives (DCMC) and dextran-sulfate (DS). Reproduced with permission from ref (109). Copyright Elsevier 2015.
5.7. Nanocomplex
Currently, aqueous delivery systems are being investigated for their ability to physically or chemically ’complicate’ or ’bind’ bioactive ingredients, especially hydrophobic ones, to molecules or supramolecular structures to prevent chemical or physical degradation of the active ingredients.110 Lipophilic and hydrophilic molecules can be bound by many types of biopolymers, thereby forming molecular complexes. In addition to binding to the individual biopolymer, the bioactive molecules can be incorporated into clusters created by a single type or a mixture of biopolymers at a specific or nonspecific binding site.111 In contrast to the two biopolymers considered individually, protein–polysaccharide complexes formed through noncovalent interactions can potentially have different functional properties owing to synergistic effects.112 Two types of phase separation can occur when proteins and polysaccharides are mixed in an aqueous medium, i.e., thermodynamic incompatibility (repulsive) and compatibility (attractive), depending on their charges and therefore on the factors that influence them, like pH and ionic strength. It is mainly electrostatic forces that hold proteins and polysaccharides together during interactions in biopolymer-rich phases, resulting in coacervates, complexes, and gels.112 These complexes can be further utilized for entrapment and delivery of bioactive compounds. A coacervate interacts with one another, forming transient multivesicular structures that coalesce further and eventually separate into a dense coacervated phase, making it ineligible for a delivery system in clear liquid foods where uniformity of structure is a concern.111 Therefore, nanocomplexes of protein–polysaccharides are prepared to enhance the encapsulation and delivery of nutraceuticals. Hosseini et al. reported a similar protein–polysaccharide nanocomplex of sodium alginate and β-lactoglobulin (BLG) through electrostatic interaction (Figure 10).111 As a result of chemical binding analysis using fluorescence spectroscopy, they showed that BLG complexed with four nutraceutical model compounds under all conditions that varied as a function of pH and nutraceutical type. Consequently, nanoscopic delivery systems can be designed to encapsulate hydrophobic and hydrophilic bioactive in clear liquid foods with acidic pH. As a result of BLG-sodium alginate interactions, stable nanocomplexes containing nutraceuticals with low water solubility were successfully encapsulated by electrostatically stable nanocomplexes. An electrophoretic mobility analysis indicated that soluble nanocomplexes were stable against aggregation.
Figure 10.
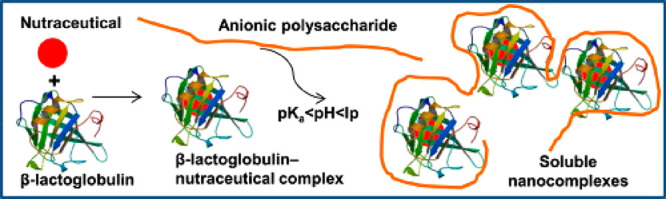
Schematic representation of nutraceuticals entrapped in sodium alginate-lactoglobulin-induced nanocomplexes. Reproduced from ref (111). Copyright Elsevier 2015.
6. Conclusion and Future Outlook
As healthcare costs rise, people focus on healthier living, preventive care, and secondary sources of medications. There is a global revolution in nutraceuticals due to adverse effects of drugs and toxicity risks. Nutraceutical usage can enhance health, longevity, and quality of life. However, these nutraceuticals require nanoencapsulation to improve their bioactivity and stabilize them for targeted delivery. In the food and biomedical sciences, polysaccharides have been extensively used for decades, providing significant benefits such as their biocompatibility, nontoxicity, consumer friendliness, cost-effectiveness, and the ability to interact efficiently with other biomaterials. In light of the increasing interest in polysaccharide-based nanomaterials in the last couple of years, it is clear that these materials can be used in various industries for incorporation in food products and nutraceuticals as stabilizers and delivery agents. Different types of microbial polysaccharides can be used for encapsulation and nutraceutical applications, allowing formulators to tailor properties and prepare delivery systems for specific objectives. It is also possible to chemically modify polysaccharides owing to the presence of different functional groups, which can enhance their delivery and encapsulation performance. Composite nanomaterials with improved properties can be created by combining polysaccharides with other edible substances such as polyphenols, proteins, or phospholipids. The combination of functional groups that induce multistimuli responsiveness for controllable release requires further consideration. In addition to delivering several bioactive substances in a single delivery system, polysaccharide-based nanoformulations may also be helpful in personalized nutrition. Despite this, polysaccharide-based nanocarriers still need to be thoroughly tested under more realistic conditions in vitro and in vivo for their safety and efficacy. Further intensive research must be done into the specific interactions of nanomaterials with human organs, tissues, cells, or biomolecules, their effect on human metabolism, and their application in drug delivery. Research into the exact mechanisms of polysaccharide bioactivities and their future applications requires extensive exploration of the structural activity relationship between polysaccharides. This review summarizes several microbial polysaccharides utilized for nanoformulations, such as nanogel, nanoparticle, nanofiber, etc. Additionally, we have discussed the role and importance of nutraceuticals along with the increasing global market with rising demand. Finally, we have described nanoformulation techniques involved in the encapsulation and delivery of nutraceuticals. Since there are a handful of reports on microbial polysaccharide nanoformulations, it paves the way for developing novel nanomaterials with exclusive properties, thus broadening the horizon of nutraceutical encapsulation and delivery applications. The development of nanoformulated delivery systems of essential nutraceuticals over the next few years is widely anticipated to continue, with a variety of novel food products expected to be used with a significant impact on addressing malnutrition among children in the future.
Acknowledgments
The authors are thankful for the fellowship and project grants from the Council of Scientific and Industrial Research (CSIR), Government of India. The Table of Contents graphic was created with BioRender.com.
Author Contributions
A.R.C. contributed to the conceptualization, supervision, and manuscript drafting. N.S. contributed to the data analysis, data compilation, and manuscript drafting.
The authors declare no competing financial interest.
References
- Das L.; Bhaumik E.; Raychaudhuri U.; Chakraborty R. Role of Nutraceuticals in Human Health. J. Food Sci. Technol. 2012, 49 (2), 173–183. 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende P.; Mallick C. Nanonutraceuticals: A Way towards Modern Therapeutics in Healthcare. J. Drug Delivery Sci. Technol. 2020, 58, 101838 10.1016/j.jddst.2020.101838. [DOI] [Google Scholar]
- Durazzo A.; Nazhand A.; Lucarini M.; Atanasov A. G.; Souto E. B.; Novellino E.; Capasso R.; Santini A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21 (7), 2285. 10.3390/ijms21072285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad R.; Gulshad L.; Haq I. U.; Farooq M. A.; Al-Farga A.; Siddique R.; Manzoor M. F.; Karrar E. Nanotechnology: A Novel Tool to Enhance the Bioavailability of Micronutrients. Food Sci. Nutr. 2021, 9 (6), 3354–3361. 10.1002/fsn3.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S.; Gokhale J. Immunity Boosting Nutraceuticals: Current Trends and Challenges. J. Food Biochem. 2022, 46 (3), 13902 10.1111/jfbc.13902. [DOI] [PubMed] [Google Scholar]
- Barve K. H.; Kulkarni Y. A.; Gaikwad A. B. Nutraceuticals as Therapeutic Agents for Inflammation. Fruits, Veg. Herbs Bioact. Foods Heal. Promot. 2016, 121–147. 10.1016/B978-0-12-802972-5.00007-X. [DOI] [Google Scholar]
- Miao T.; Wang J.; Zeng Y.; Liu G.; Chen X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5 (4), 1700513 10.1002/advs.201700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelke N. B.; James R.; Laurencin C. T.; Kumbar S. G. Polysaccharide Biomaterials for Drug Delivery and Regenerative Engineering. Polym. Adv. Technol. 2014, 25 (5), 448–460. 10.1002/pat.3266. [DOI] [Google Scholar]
- Casillo A.; Lanzetta R.; Parrilli M.; Corsaro M. M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Marine Drugs 2018, 16, 69. 10.3390/md16020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N.; Richa; Roy Choudhury A. Recent Advances in Composite Hydrogels Prepared Solely from Polysaccharides. Colloids Surfaces B Biointerfaces 2021, 205, 111891 10.1016/j.colsurfb.2021.111891. [DOI] [PubMed] [Google Scholar]
- Moscovici M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front. Microbiol. 2015, 6 (SEP), 1–11. 10.3389/fmicb.2015.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas F.; Alves V. D.; Reis M. A.; Crespo J. G.; Coelhoso I. M. Microbial Polysaccharide-Based Membranes: Current and Future Applications. J. Appl. Polym. Sci. 2014, 131 (6), 40047 10.1002/app.40047. [DOI] [Google Scholar]
- Singh H. Nanotechnology Applications in Functional Foods; Opportunities and Challenges. Prev. Nutr. food Sci. 2016, 21 (1), 1–8. 10.3746/pnf.2016.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino D.; Mancuso A.; Cristiano M. C.; Froiio F.; Lammari N.; Celia C.; Fresta M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomater. (Basel, Switzerland) 2021, 11 (3), 1–20. 10.3390/nano11030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. W. K.; Souto E. B.; Durazzo A.; Lucarini M.; Novellino E.; Tewari D.; Wang D.; Atanasov A. G.; Santini A. Big Impact of Nanoparticles: Analysis of the Most Cited Nanopharmaceuticals and Nanonutraceuticals Research. Curr. Res. Biotechnol. 2020, 2, 53–63. 10.1016/j.crbiot.2020.04.002. [DOI] [Google Scholar]
- Meng Y.; Qiu C.; Li X.; McClements D. J.; Sang S.; Jiao A.; Jin Z. Polysaccharide-Based Nano-Delivery Systems for Encapsulation, Delivery, and PH-Responsive Release of Bioactive Ingredients. Crit. Rev. Food Sci. Nutr. 2022, 1–15. 10.1080/10408398.2022.2105800. [DOI] [PubMed] [Google Scholar]
- Yang X.; Li A.; Li X.; Sun L.; Guo Y. An Overview of Classifications, Properties of Food Polysaccharides and Their Links to Applications in Improving Food Textures. Trends Food Sci. Technol. 2020, 102, 1–15. 10.1016/j.tifs.2020.05.020. [DOI] [Google Scholar]
- Jindal N.; Singh Khattar J.. Microbial Polysaccharides in Food Industry. In Biopolymers for Food Design; Elsevier, Inc., 2018; pp 95–123. 10.1016/B978-0-12-811449-0.00004-9. [DOI] [Google Scholar]
- Espro C.; Paone E.; Mauriello F.; Gotti R.; Uliassi E.; Bolognesi M. L.; Rodríguez-Padrón D.; Luque R. Sustainable Production of Pharmaceutical, Nutraceutical and Bioactive Compounds from Biomass and Waste. Chem. Soc. Rev. 2021, 50 (20), 11191–11207. 10.1039/D1CS00524C. [DOI] [PubMed] [Google Scholar]
- Ahmad A.; Gulraiz Y.; Ilyas S.; Bashir S. Polysaccharide Based Nano Materials: Health Implications. Food Hydrocoll. Heal. 2022, 2, 100075 10.1016/j.fhfh.2022.100075. [DOI] [Google Scholar]
- Ahmad N. H.; Mustafa S.; Man Y. B. C. Microbial Polysaccharides and Their Modification Approaches: A Review. Int. J. Food Prop. 2015, 18 (2), 332–347. 10.1080/10942912.2012.693561. [DOI] [Google Scholar]
- Choukaife H.; Doolaanea A. A.; Alfatama M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13 (11), 335–368. 10.3390/ph13110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodero A.; Alberti S.; Gaggero G.; Ferretti M.; Botter R.; Vicini S.; Castellano M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8 (22), 2100809 10.1002/admi.202100809. [DOI] [Google Scholar]
- Srivastava N.; Kumari S.; Kurmi S.; Pinnaka A. K.; Choudhury A. R. Isolation, Purification, and Characterization of a Novel Exopolysaccharide Isolated from Marine Bacteria Brevibacillus Borstelensis M42. Arch. Microbiol. 2022, 204 (7), 399. 10.1007/s00203-022-02993-9. [DOI] [PubMed] [Google Scholar]
- Aquinas N.; Bhat M R.; Selvaraj S. A Review Presenting Production, Characterization, and Applications of Biopolymer Curdlan in Food and Pharmaceutical Sectors. Polym. Bull. 2022, 79 (9), 6905–6927. 10.1007/s00289-021-03860-1. [DOI] [Google Scholar]
- Martinez C. O.; Ruiz S. P.; Nogueira M. T.; Bona E.; Portilho M.; Matioli G. Effective Immobilization of Agrobacterium Sp. IFO 13140 Cells in Loofa Sponge for Curdlan Biosynthesis. Molecules 2015, 20 (5), 7957–7973. 10.3390/molecules20057957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T. P. Production of the Polysaccharide Curdlan by Agrobacterium Species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation 2020, 6 (1), 16. 10.3390/fermentation6010016. [DOI] [Google Scholar]
- Hu Q.; Lu Y.; Luo Y. Recent Advances in Dextran-Based Drug Delivery Systems: From Fabrication Strategies to Applications. Carbohydr. Polym. 2021, 264, 117999 10.1016/j.carbpol.2021.117999. [DOI] [PubMed] [Google Scholar]
- Heinze T.; Liebert T.; Heublein B.; Hornig S.. Functional Polymers Based on Dextran. In Adv. Polym. Sci.; Springer: Berlin, Heidelberg, 2006; Vol. 205, pp 199–291. 10.1007/12_100. [DOI] [Google Scholar]
- Banerjee A.; Bandopadhyay R. Use of Dextran Nanoparticle: A Paradigm Shift in Bacterial Exopolysaccharide Based Biomedical Applications. Int. J. Biol. Macromol. 2016, 87, 295–301. 10.1016/j.ijbiomac.2016.02.059. [DOI] [PubMed] [Google Scholar]
- Shan W.; Zhu X.; Liu M.; Li L.; Zhong J.; Sun W.; Zhang Z.; Huang Y. Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS Nano 2015, 9 (3), 2345–2356. 10.1021/acsnano.5b00028. [DOI] [PubMed] [Google Scholar]
- Ensign L. M.; Schneider C.; Suk J. S.; Cone R.; Hanes J. Mucus Penetrating Nanoparticles: Biophysical Tool and Method of Drug and Gene Delivery. Adv. Mater. 2012, 24 (28), 3887–3894. 10.1002/adma.201201800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A. K.; Bera H.; Hasnain M. S.; De A.; Pal D.; Samanta A.. Gellan Gum-Based Nanomaterials in Drug Delivery Applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Academic Press, 2021; pp 313–336. 10.1016/B978-0-12-820874-8.00004-X. [DOI] [Google Scholar]
- Cui S. W.; Wu Y.; Ding H. The Range of Dietary Fibre Ingredients and a Comparison of Their Technical Functionality. Fibre-Rich Wholegrain Foods Improv. Qual. 2013, 96–119. 10.1533/9780857095787.1.96. [DOI] [Google Scholar]
- Costa A. L. R.; de la Torre L. G.. Gellan Gum Nanoparticles in Drug Delivery. In Micro- and Nanoengineered Gum-Based Biomaterials for Drug Delivery and Biomedical Applications; Elsevier, 2022; pp 127–156. 10.1016/B978-0-323-90986-0.00009-1. [DOI] [Google Scholar]
- Muthukumar T.; Song J. E.; Khang G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24 (24), 4514. 10.3390/molecules24244514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinan E.; Cesur S.; Haskoylu M. E.; Gunduz O.; Oner E. T. Resveratrol-Loaded Levan Nanoparticles Produced by Electrohydrodynamic Atomization Technique. Nanomater. 2021, 11 (10), 2582. 10.3390/NANO11102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Siqueira E. C.; Rebouças J. de S.; Pinheiro I. O.; Formiga F. R. Levan-Based Nanostructured Systems: An Overview. Int. J. Pharm. 2020, 580, 119242 10.1016/j.ijpharm.2020.119242. [DOI] [PubMed] [Google Scholar]
- González-Garcinuño Á.; Tabernero A.; Marcelo G.; Martín del Valle E. A Comprehensive Study on Levan Nanoparticles Formation: Kinetics and Self-Assembly Modeling. Int. J. Biol. Macromol. 2020, 147, 1089–1098. 10.1016/j.ijbiomac.2019.10.076. [DOI] [PubMed] [Google Scholar]
- Rajalekshmy G. P.; Annie Mariya R.; Rekha M. R. Pullulan-Based Nanomaterials in Drug Delivery Applications. Biopolym. Nanomater. Drug Delivery Biomed. Appl. 2021, 383–404. 10.1016/B978-0-12-820874-8.00010-5. [DOI] [Google Scholar]
- Grenha A.; Rodrigues S. Pullulan-Based Nanoparticles: Future Therapeutic Applications in Transmucosal Protein Delivery. Ther. Delivery 2013, 4 (11), 1339–1341. 10.4155/tde.13.99. [DOI] [PubMed] [Google Scholar]
- Patel J.; Maji B.; Moorthy N. S. H. N.; Maiti S. Xanthan Gum Derivatives: Review of Synthesis, Properties and Diverse Applications. RSC Adv. 2020, 10 (45), 27103. 10.1039/D0RA04366D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camesano T. A.; Wilkinson K. J. Single Molecule Study of Xanthan Conformation Using Atomic Force Microscopy. Biomacromolecules 2001, 2 (4), 1184–1191. 10.1021/bm015555g. [DOI] [PubMed] [Google Scholar]
- Palaniraj A.; Jayaraman V. Production, Recovery and Applications of Xanthan Gum by Xanthomonas Campestris. J. Food Eng. 2011, 106 (1), 1–12. 10.1016/j.jfoodeng.2011.03.035. [DOI] [Google Scholar]
- Katzbauer B. Properties and Applications of Xanthan Gum. Polym. Degrad. Stab. 1998, 59 (1–3), 81–84. 10.1016/S0141-3910(97)00180-8. [DOI] [Google Scholar]
- Witkamp R. F.; van Norren K. Let Thy Food Be Thy Medicine···When Possible. Eur. J. Pharmacol. 2018, 836, 102–114. 10.1016/j.ejphar.2018.06.026. [DOI] [PubMed] [Google Scholar]
- Aronson J. K.; Aronson J. K. Defining ‘Nutraceuticals’: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83 (1), 8–19. 10.1111/bcp.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putta S.FSSAI guidance and notification on nutraceuticals – An insight. Fnbnews.Com, https://www.fssai.gov.in/upload/media/FSSAI_News_Guidance_FNB_09_06_2020.pdf.
- Nasri H.; Baradaran A.; Shirzad H.; Kopaei M. R. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5 (12), 1487. [PMC free article] [PubMed] [Google Scholar]
- Santini A.; Cammarata S. M.; Capone G.; Ianaro A.; Tenore G. C.; Pani L.; Novellino E. Nutraceuticals: Opening the Debate for a Regulatory Framework. Br. J. Clin. Pharmacol. 2018, 84 (4), 659. 10.1111/bcp.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiaka T.; Kritsi E.; Tsiantas K.; Christodoulou P.; Sinanoglou V. J.; Zoumpoulakis P. Design and Development of Novel Nutraceuticals: Current Trends and Methodologies. Nutraceuticals 2022, 2 (2), 71–90. 10.3390/nutraceuticals2020006. [DOI] [Google Scholar]
- Helal N. A.; Eassa H. A.; Amer A. M.; Eltokhy M. A.; Edafiogho I.; Nounou M. I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Delivery Formul. 2019, 13 (2), 105–156. 10.2174/1872211313666190503112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwosu O. K.; Ubaoji K. I.. Nutraceuticals: History, Classification and Market Demand. In Functional Foods and Nutraceuticals; 2020; pp 13–22. 10.1007/978-3-030-42319-3_2. [DOI] [Google Scholar]
- Chauhan B.; Kumar G.; Kalam N.; Ansari S. H. Current Concepts and Prospects of Herbal Nutraceutical: A Review. J. Adv. Pharm. Technol. Res. 2013, 4 (1), 4–8. 10.4103/2231-4040.107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar V.; Singh T.; Katiyar S. K. Multi-Targeted Prevention and Therapy of Cancer by Proanthocyanidins. Cancer Lett. 2008, 269 (2), 378. 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alali M.; Alqubaisy M.; Aljaafari M. N.; Alali A. O.; Baqais L.; Molouki A.; Abushelaibi A.; Lai K. S.; Lim S. H. E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26 (9), 2540. 10.3390/molecules26092540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.; Khanum F.; Khanum F.; Khanum F. Neuroprotective Potential of Phytochemicals. Pharmacogn. Rev. 2012, 6 (12), 81–90. 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggers H. J.; Zaioncz S.; Cheleski J.; Mainardes R. M.; Khalil N. M. Curcumin, a Multitarget Phytochemical: Challenges and Perspectives. Stud. Nat. Prod. Chem. 2017, 53, 243–276. 10.1016/B978-0-444-63930-1.00007-7. [DOI] [Google Scholar]
- Yeşilyurt N.; Yılmaz B.; Ağagündüz D.; Capasso R. Microbiome-Based Personalized Nutrition as a Result of the 4.0 Technological Revolution: A Mini Literature Review. Process Biochem. 2022, 121, 257–262. 10.1016/j.procbio.2022.07.012. [DOI] [Google Scholar]
- Yesilyurt N.; Yılmaz B.; Agagunduz D.; Capasso R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biol. 2021, 1 (2), 89–110. 10.3390/biologics1020006. [DOI] [Google Scholar]
- Ağagündüz D.; Kocaadam-Bozkurt B.; Bozkurt O.; Sharma H.; Esposito R.; Özoğul F.; Capasso R. Microbiota Alteration and Modulation in Alzheimer’s Disease by Gerobiotics: The Gut-Health Axis for a Good Mind. Biomed. Pharmacother. 2022, 153, 113430 10.1016/j.biopha.2022.113430. [DOI] [PubMed] [Google Scholar]
- Konuray G.; Erginkaya Z. Potential Use of Bacillus Coagulans in the Food Industry. Foods 2018, 7 (6), 92–101. 10.3390/foods7060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q.; Li P.. Biosynthesis of Vitamins by Probiotic Bacteria. In Probiotics and Prebiotics in Human Nutrition and Health; IntechOpen, 2016; pp 135–148. 10.5772/63117. [DOI] [Google Scholar]
- Karakan T.; Ozkul C.; Akkol E. K.; Bilici S.; Sobarzo-Sánchez E.; Capasso R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13 (2), 1–18. 10.3390/nu13020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapal A.; Tiku P. K. Nutritional and Nutraceutical Improvement by Enzymatic Modification of Food Proteins. Enzym. Food Biotechnol. Prod. Appl. Futur. Prospect. 2019, 471–481. 10.1016/B978-0-12-813280-7.00027-X. [DOI] [Google Scholar]
- Alashi A. M.; Blanchard C. L.; Mailer R. J.; Agboola S. O.; Mawson A. J.; He R.; Malomo S. A.; Girgih A. T.; Aluko R. E. Blood Pressure Lowering Effects of Australian Canola Protein Hydrolysates in Spontaneously Hypertensive Rats. Food Res. Int. 2014, 55, 281–287. 10.1016/j.foodres.2013.11.015. [DOI] [Google Scholar]
- Malisorn C.; Suntornsuk W. Optimization of β-Carotene Production by Rhodotorula Glutinis DM28 in Fermented Radish Brine. Bioresour. Technol. 2008, 99 (7), 2281–2287. 10.1016/j.biortech.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Allen L.; De Benoist B.; Dary O.; Hurrell R.. Guidelines on Food Fortification with Micronutrients; JSTOR, 2006. [Google Scholar]
- Hefferon K. L. Nutritionally Enhanced Food Crops; Progress and Perspectives. Int. J. Mol. Sci. 2015, 16 (2), 3895. 10.3390/ijms16023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A. S.; Lordan R.; Horbańczuk O. K.; Atanasov A. G.; Chopra I.; Horbańczuk J. O.; Jóźwik A.; Huang L.; Pirgozliev V.; Banach M.; Battino M.; Arkells N. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 106001 10.1016/j.phrs.2021.106001. [DOI] [PubMed] [Google Scholar]
- Hoti G.; Matencio A.; Pedrazzo A. R.; Cecone C.; Appleton S. L.; Monfared Y. K.; Caldera F.; Trotta F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23 (8), 4102. 10.3390/ijms23084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutraceuticals Market Size & Share Report, 2021–2030, https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market (accessed 2022-09-04).
- Indian Nutraceuticals industry to touch US$ 8.5 billion by 2022: study | Business Standard News, https://www.business-standard.com/article/news-cm/indian-nutraceuticals-industry-to-touch-us-8-5-billion-by-2022-study-117041900191_1.html (accessed 2022-09-04).
- Patra J. K.; Das G.; Fraceto L. F.; Campos E. V. R.; Rodriguez-Torres M. D. P.; Acosta-Torres L. S.; Diaz-Torres L. A.; Grillo R.; Swamy M. K.; Sharma S.; Habtemariam S.; Shin H. S.. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018 1612018, 16 ( (1), ), 1–33. 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S. M. An Overview of Nanoencapsulation Techniques and Their Classification. Nanoencapsulation Technol. Food Nutraceutical Ind. 2017, 1–34. 10.1016/B978-0-12-809436-5.00001-X. [DOI] [Google Scholar]
- Rezaei A.; Fathi M.; Jafari S. M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds within Different Nanocarriers. Food Hydrocoll. 2019, 88 (October 2018), 146–162. 10.1016/j.foodhyd.2018.10.003. [DOI] [Google Scholar]
- Faridi Esfanjani A.; Assadpour E.; Jafari S. M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. 10.1016/j.tifs.2018.04.002. [DOI] [Google Scholar]
- Wen P.; Zhu D. H.; Wu H.; Zong M. H.; Jing Y. R.; Han S. Y. Encapsulation of Cinnamon Essential Oil in Electrospun Nanofibrous Film for Active Food Packaging. Food Control 2016, 59, 366–376. 10.1016/j.foodcont.2015.06.005. [DOI] [Google Scholar]
- Ghorbanzade T.; Jafari S. M.; Akhavan S.; Hadavi R. Nano-Encapsulation of Fish Oil in Nano-Liposomes and Its Application in Fortification of Yogurt. Food Chem. 2017, 216, 146–152. 10.1016/j.foodchem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Misra S.; Pandey P.; Mishra H. N. Novel Approaches for Co-Encapsulation of Probiotic Bacteria with Bioactive Compounds, Their Health Benefits and Functional Food Product Development: A Review. Trends Food Sci. Technol. 2021, 109, 340–351. 10.1016/j.tifs.2021.01.039. [DOI] [Google Scholar]
- Kirtane A. R.; Kalscheuer S. M.; Panyam J. Exploiting Nanotechnology to Overcome Tumor Drug Resistance: Challenges and Opportunities. Adv. Drug Delivery Rev. 2013, 65 (13–14), 1731–1747. 10.1016/j.addr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomedicine 2007, 2 (2), 129. [PMC free article] [PubMed] [Google Scholar]
- de Oliveira E. F.; Paula H. C. B.; Paula R. C. M. d. Alginate/Cashew Gum Nanoparticles for Essential Oil Encapsulation. Colloids Surfaces B Biointerfaces 2014, 113, 146–151. 10.1016/j.colsurfb.2013.08.038. [DOI] [PubMed] [Google Scholar]
- Chopde S.; Datir R.; Deshmukh G.; Dhotre A.; Patil M. Nanoparticle Formation by Nanospray Drying & Its Application in Nanoencapsulation of Food Bioactive Ingredients. J. Agric. Food Res. 2020, 2, 100085 10.1016/j.jafr.2020.100085. [DOI] [Google Scholar]
- Elbialy N. S.; Mohamed N. Fabrication of the Quaternary Nanocomplex Curcumin-Casein-Alginate-Chitosan as a Potential Oral Delivery System for Cancer Nutraceutical Therapy. J. Drug Delivery Sci. Technol. 2022, 70 (May 2021), 103226 10.1016/j.jddst.2022.103226. [DOI] [Google Scholar]
- Meng J.; Kang T.-T.; Wang H.-F.; Zhao B.-B.; Lu R.-R. Physicochemical Properties of Casein-Dextran Nanoparticles Prepared by Controlled Dry and Wet Heating. Int. J. Biol. Macromol. 2018, 107, 2604–2610. 10.1016/j.ijbiomac.2017.10.140. [DOI] [PubMed] [Google Scholar]
- Yu Y. B.; Wu M. Y.; Wang C.; Wang Z. W.; Chen T. T.; Yan J. K. Constructing Biocompatible Carboxylic Curdlan-Coated Zein Nanoparticles for Curcumin Encapsulation. Food Hydrocoll. 2020, 108, 106028 10.1016/j.foodhyd.2020.106028. [DOI] [Google Scholar]
- Xie J.; Jiang J.; Davoodi P.; Srinivasan M. P.; Wang C. H. Electrohydrodynamic Atomization: A Two-Decade Effort to Produce and Process Micro-/Nanoparticulate Materials. Chem. Eng. Sci. 2015, 125, 32–57. 10.1016/j.ces.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Huang J.; Si T.; Xu R. X. Coaxial Electrospray of Microparticles and Nanoparticles for Biomedical Applications. Expert Rev. Med. Devices 2012, 9 (6), 595–612. 10.1586/erd.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. S.; Han G. G.; Hong L.; Kang S. K.; Shokouhimehr M.; Choi Y. J.; Cho C. S. Novel Production of Natural Bacteriocin via Internalization of Dextran Nanoparticles into Probiotics. Biomaterials 2019, 218, 119360 10.1016/j.biomaterials.2019.119360. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Jing Y.; Han C.; Zhang H.; Tian Y. Encapsulation of Curcumin in Zein/ Caseinate/Sodium Alginate Nanoparticles with Improved Physicochemical and Controlled Release Properties. Food Hydrocoll. 2019, 93, 432–442. 10.1016/j.foodhyd.2019.02.003. [DOI] [Google Scholar]
- Assadpour E.; Maghsoudlou Y.; Jafari S. M.; Ghorbani M.; Aalami M. Optimization of Folic Acid Nano-Emulsification and Encapsulation by Maltodextrin-Whey Protein Double Emulsions. Int. J. Biol. Macromol. 2016, 86, 197–207. 10.1016/j.ijbiomac.2016.01.064. [DOI] [PubMed] [Google Scholar]
- Bhushani A.; Anandharamakrishnan C.. Food-Grade Nanoemulsions for Protection and Delivery of Nutrients; Springer, Cham, 2017; pp 99–139. 10.1007/978-3-319-53112-0_3. [DOI] [Google Scholar]
- Richa R.; Roy Choudhury A. Exploration of Polysaccharide Based Nanoemulsions for Stabilization and Entrapment of Curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. 10.1016/j.ijbiomac.2019.11.167. [DOI] [PubMed] [Google Scholar]
- Roychowdhury R.; Srivastava N.; Kumari S.; Pinnaka A. K.; Roy Choudhury A. Isolation of an Exopolysaccharide from a Novel Marine Bacterium Neorhizobium Urealyticum Sp. Nov. and Its Utilization in Nanoemulsion Formation for Encapsulation and Stabilization of Astaxanthin. LWT 2021, 151, 112105 10.1016/j.lwt.2021.112105. [DOI] [Google Scholar]
- Fan Y.; Yi J.; Zhang Y.; Wen Z.; Zhao L. Physicochemical Stability and in Vitro Bioaccessibility of β-Carotene Nanoemulsions Stabilized with Whey Protein-Dextran Conjugates. Food Hydrocoll. 2017, 63, 256–264. 10.1016/j.foodhyd.2016.09.008. [DOI] [Google Scholar]
- Wang C.; Li X.; Sang S.; Julian McClements D.; Chen L.; Long J.; Jiao A.; Wang J.; Jin Z.; Qiu C. Preparation, Characterization and in Vitro Digestive Behaviors of Emulsions Synergistically Stabilized by γ-Cyclodextrin/Sodium Caseinate/Alginate. Food Res. Int. 2022, 160, 111634 10.1016/j.foodres.2022.111634. [DOI] [PubMed] [Google Scholar]
- Gokmen M. T.; Du Prez F. E. Porous Polymer Particles - A Comprehensive Guide to Synthesis, Characterization, Functionalization and Applications. Prog. Polym. Sci. 2012, 37 (3), 365–405. 10.1016/j.progpolymsci.2011.07.006. [DOI] [Google Scholar]
- P. R S.; James N. R.; P. R A. k.; Raj D. K. Preparation, Characterization and Biological Evaluation of Curcumin Loaded Alginate Aldehyde–Gelatin Nanogels. Mater. Sci. Eng., C 2016, 68, 251–257. 10.1016/j.msec.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Feng J.; Wu S.; Wang H.; Liu S. Improved Bioavailability of Curcumin in Ovalbumin-Dextran Nanogels Prepared by Maillard Reaction. J. Funct. Foods 2016, 27, 55–68. 10.1016/j.jff.2016.09.002. [DOI] [Google Scholar]
- Zeng Q.; Zeng W.; Jin Y.; Sheng L. Construction and Evaluation of Ovalbumin-Pullulan Nanogels as a Potential Delivery Carrier for Curcumin. Food Chem. 2022, 367, 130716 10.1016/j.foodchem.2021.130716. [DOI] [PubMed] [Google Scholar]
- Shekarforoush E.; Ajalloueian F.; Zeng G.; Mendes A. C.; Chronakis I. S. Electrospun Xanthan Gum-Chitosan Nanofibers as Delivery Carrier of Hydrophobic Bioactives. Mater. Lett. 2018, 228, 322–326. 10.1016/j.matlet.2018.06.033. [DOI] [Google Scholar]
- Rezaei A.; Nasirpour A.; Fathi M. Application of Cellulosic Nanofibers in Food Science Using Electrospinning and Its Potential Risk. Compr. Rev. Food Sci. Food Saf. 2015, 14 (3), 269–284. 10.1111/1541-4337.12128. [DOI] [PubMed] [Google Scholar]
- Rostami M.; Ghorbani M.; Aman mohammadi M.; Delavar M.; Tabibiazar M.; Ramezani S. Development of Resveratrol Loaded Chitosan-Gellan Nanofiber as a Novel Gastrointestinal Delivery System. Int. J. Biol. Macromol. 2019, 135, 698–705. 10.1016/j.ijbiomac.2019.05.187. [DOI] [PubMed] [Google Scholar]
- Pinto Reis C.; Neufeld R. J.; Ribeiro A. J.; Veiga F.; Nanoencapsulation I. Methods for Preparation of Drug-Loaded Polymeric Nanoparticles. Nanomedicine 2006, 2 (1), 8–21. 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Natrajan D.; Srinivasan S.; Sundar K.; Ravindran A. Formulation of Essential Oil-Loaded Chitosan–Alginate Nanocapsules. J. Food Drug Anal. 2015, 23 (3), 560–568. 10.1016/j.jfda.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.; Ramos R. A. N. S.; Alcouffe P.; Munoz L. E.; Bilyy R. O.; Ganachaud F.; Bernard J. Programmable Hierarchical Construction of Mixed/Multilayered Polysaccharide Nanocapsules through Simultaneous/Sequential Nanoprecipitation Steps. Biomacromolecules 2019, 20 (10), 3915–3923. 10.1021/acs.biomac.9b00990. [DOI] [PubMed] [Google Scholar]
- Safinya C. R.; Ewert K. K. Liposomes Derived from Molecular Vases. Nat. 2012, 489 (7416), 372–374. 10.1038/489372b. [DOI] [PubMed] [Google Scholar]
- Zou L.; Peng S.; Liu W.; Chen X.; Liu C. A Novel Delivery System Dextran Sulfate Coated Amphiphilic Chitosan Derivatives-Based Nanoliposome: Capacity to Improve in Vitro Digestion Stability of (−)-Epigallocatechin Gallate. Food Res. Int. 2015, 69 (1), 114–120. 10.1016/j.foodres.2014.12.015. [DOI] [Google Scholar]
- Sagalowicz L.; Leser M. E. Delivery Systems for Liquid Food Products. Curr. Opin. Colloid Interface Sci. 2010, 15 (1–2), 61–72. 10.1016/j.cocis.2009.12.003. [DOI] [Google Scholar]
- Hosseini S. M. H.; Emam-Djomeh Z.; Sabatino P.; Van der Meeren P. Nanocomplexes Arising from Protein-Polysaccharide Electrostatic Interaction as a Promising Carrier for Nutraceutical Compounds. Food Hydrocolloids. 2015, 50, 16–26. 10.1016/j.foodhyd.2015.04.006. [DOI] [Google Scholar]
- McClements D. J. Non-Covalent Interactions between Proteins and Polysaccharides. Biotechnol. Adv. 2006, 24 (6), 621–625. 10.1016/j.biotechadv.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Abbasi F.; Samadi F.; Jafari S. M.; Ramezanpour S.; Shams Shargh M. Ultrasound-Assisted Preparation of Flaxseed Oil Nanoemulsions Coated with Alginate-Whey Protein for Targeted Delivery of Omega-3 Fatty Acids into the Lower Sections of Gastrointestinal Tract to Enrich Broiler Meat. Ultrason. Sonochem. 2019, 50, 208–217. 10.1016/j.ultsonch.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Huang X.; Huang X.; Gong Y.; Xiao H.; McClements D. J.; Hu K. Enhancement of Curcumin Water Dispersibility and Antioxidant Activity Using Core–Shell Protein–Polysaccharide Nanoparticles. Food Res. Int. 2016, 87, 1–9. 10.1016/j.foodres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Li Q.; Liu C. G.; Huang Z. H.; Xue F. F. Preparation and Characterization of Nanoparticles Based on Hydrophobic Alginate Derivative as Carriers for Sustained Release of Vitamin D3. J. Agric. Food Chem. 2011, 59 (5), 1962–1967. 10.1021/jf1020347. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Sun C.; Dai L.; Zhan X.; Gao Y. Structure, Physicochemical Stability and in Vitro Simulated Gastrointestinal Digestion Properties of β-Carotene Loaded Zein-Propylene Glycol Alginate Composite Nanoparticles Fabricated by Emulsification-Evaporation Method. Food Hydrocoll. 2018, 81, 149–158. 10.1016/j.foodhyd.2018.02.042. [DOI] [Google Scholar]
- Wei Y.; Li C.; Dai L.; Zhang L.; Liu J.; Mao L.; Yuan F.; Gao Y. The Construction of Resveratrol-Loaded Protein-Polysaccharide-Tea Saponin Complex Nanoparticles for Controlling Physicochemical Stability and: In Vitro Digestion. Food Funct. 2020, 11 (11), 9973–9983. 10.1039/D0FO01741H. [DOI] [PubMed] [Google Scholar]
- Branco I. G.; Sen K.; Rinaldi C. Effect of Sodium Alginate and Different Types of Oil on the Physical Properties of Ultrasound-Assisted Nanoemulsions. Chem. Eng. Process. - Process Intensif. 2020, 153, 107942. 10.1016/j.cep.2020.107942. [DOI] [Google Scholar]
- Azevedo M. A.; Bourbon A. I.; Vicente A. A.; Cerqueira M. A. Alginate/Chitosan Nanoparticles for Encapsulation and Controlled Release of Vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. 10.1016/j.ijbiomac.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Pan X.; Yao P.; Jiang M. Simultaneous Nanoparticle Formation and Encapsulation Driven by Hydrophobic Interaction of Casein-Graft-Dextran and Beta-Carotene. J. Colloid Interface Sci. 2007, 315 (2), 456–463. 10.1016/j.jcis.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Semyonov D.; Ramon O.; Shoham Y.; Shimoni E. Enzymatically Synthesized Dextran Nanoparticles and Their Use as Carriers for Nutraceuticals. Food Funct. 2014, 5 (10), 2463–2474. 10.1039/C4FO00103F. [DOI] [PubMed] [Google Scholar]
- Chalasani K. B.; Russell-Jones G. J.; Jain A. K.; Diwan P. V.; Jain S. K. Effective Oral Delivery of Insulin in Animal Models Using Vitamin B12-Coated Dextran Nanoparticles. J. Controlled Release 2007, 122 (2), 141–150. 10.1016/j.jconrel.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Sun X.; Zhang L.; Zhang P.; Li J.; Liu Y. N. Fabrication of Biopolymeric Complex Coacervation Core Micelles for Efficient Tea Polyphenol Delivery via a Green Process. Langmuir 2012, 28 (41), 14553–14561. 10.1021/la303062j. [DOI] [PubMed] [Google Scholar]
- Yi J.; Fan Y.; Zhang Y.; Wen Z.; Zhao L.; Lu Y. Glycosylated α-Lactalbumin-Based Nanocomplex for Curcumin: Physicochemical Stability and DPPH-Scavenging Activity. Food Hydrocoll. 2016, 61, 369–377. 10.1016/j.foodhyd.2016.05.036. [DOI] [Google Scholar]
- Prezotti F. G.; Boni F. I.; Ferreira N. N.; Silva D. S.; Almeida A.; Vasconcelos T.; Sarmento B.; Gremião M. P. D.; Cury B. S. F. Oral Nanoparticles Based on Gellan Gum/Pectin for Colon-Targeted Delivery of Resveratrol. Drug Dev. Ind. Pharm. 2020, 46 (2), 236–245. 10.1080/03639045.2020.1716374. [DOI] [PubMed] [Google Scholar]
- Nakapong S.; Pichyangkura R.; Ito K.; Iizuka M.; Pongsawasdi P. High Expression Level of Levansucrase from Bacillus Licheniformis RN-01 and Synthesis of Levan Nanoparticles. Int. J. Biol. Macromol. 2013, 54 (1), 30–36. 10.1016/j.ijbiomac.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Bahadori F.; Eskandari Z.; Ebrahimi N.; Bostan M. S.; Eroğlu M. S.; Oner E. T. Development and Optimization of a Novel PLGA-Levan Based Drug Delivery System for Curcumin, Using a Quality-by-Design Approach. Eur. J. Pharm. Sci. 2019, 138, 105037 10.1016/j.ejps.2019.105037. [DOI] [PubMed] [Google Scholar]
- Hong L.; Kim W. S.; Lee S. M.; Kang S. K.; Choi Y. J.; Cho C. S. Pullulan Nanoparticles as Prebiotics Enhance the Antibacterial Properties of Lactobacillus Plantarum through the Induction of Mild Stress in Probiotics. Front. Microbiol. 2019, 10 (FEB), 142 10.3389/fmicb.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannopoulos A.; Sklapani A. Xanthan-Based Polysaccharide/Protein Nanoparticles: Preparation, Characterization, Encapsulation and Stabilization of Curcumin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100075 10.1016/j.carpta.2021.100075. [DOI] [Google Scholar]
- Zhang D.; Jiang F.; Ling J.; Ouyang X.-k.; Wang Y.-G. Delivery of Curcumin Using a Zein-Xanthan Gum Nanocomplex: Fabrication, Characterization, and in Vitro Release Properties. Colloids Surfaces B Biointerfaces 2021, 204, 111827 10.1016/j.colsurfb.2021.111827. [DOI] [PubMed] [Google Scholar]










