Abstract
Background
Systemic lupus erythematosus (SLE) represents a principal prototype of a multisystemic autoimmune disease with the participation of both cell- and antibody-mediated mechanisms causing significant renal impairment. A renal biopsy diagnosis is the gold standard for clinical renal disease in SLE, which includes a broad range of indications.
Summary
Renal disease in SLE can involve glomerular, tubulointerstitial, and/or vascular compartments, none of which are mutually exclusive. In most instances, the basic pathogenetic mechanism involves tissue deposition of immune complexes and/or cell-mediated mechanisms, identified by light microscopy, immunohistochemical methods, and electron microscopy (EM), evoking intraglomerular proliferative, inflammatory, and other tissue responses. These produce a spectrum of histologic lesions, depending on the participation of a wide range of clinical triggers, namely, genetic, serological, and immunological factors, correlating with their underlying pathogenetic potential. In addition to light and immunofluorescence microscopy, EM in this setting facilitates an accurate diagnosis, assesses disease activity, delineates subclasses, differentiates from primary forms of non-lupus renal lesions, identifies organized deposits, and rarely, identifies other forms of nonimmune complex lesions such as podocytopathies, amyloidosis, and thrombotic microangiopathy.
Key Messages
EM findings that are distinctive for most of the renal lesions in SLE include immune complex and nonimmune complex diseases as well as overlapping entities. Routine ultrastructural examination not only provides significant diagnostic and prognostic information from both initial and repeat renal biopsies from lupus patients but also contributes toward the understanding of the underlying pathophysiology of the disease process.
Keywords: Systemic lupus erythematosus, Lupus nephritis, Lupus vasculopathy, Organized deposits, Thrombotic microangiopathy, Lupus podocytopathy, Electron microscopy
Introduction
Systemic lupus erythematosus (SLE) is the prototype of a multisystemic autoimmune disease involving the lung, heart, skin, joint, central nervous system, and kidney. Renal lesions in SLE are varied and are defined by the underlying pathogenetic mechanisms, mediated primarily by immune complex deposition causing lupus glomerulonephritis (GN) with associated inflammatory responses. Any of the compartments in the renal cortex (glomeruli, tubulointerstitium, and vasculature) can be affected alone or in combination, as they are not mutually exclusive (Table 1). Glomerular involvement (commonly termed “lupus nephritis” [LN], due to its relative ease of use rather than the longer and more accurate “lupus glomerulonephritis”) is most frequently encountered, which may or may not be accompanied by tubulointerstitial or less commonly vascular lesions. Isolated tubulointerstitial or vascular involvement may occur rarely [1, 2, 3].
Table 1.
Renal lesions in SLE
| Lupus GN (revised ISN/RPS classification 2018) [2] |
| Minimal mesangial LN (class 1) |
| Mesangial proliferative LN (class 2) |
| Focal LN (class 3) |
| Diffuse LN (class 4) |
| Membranous LN (class 5) |
| Advanced sclerosing LN (class 6) |
| TIN |
| Associated with lupus GN |
| Primary lupus TIN (not associated with significant lupus GN or lupus vascular lesions) |
| Vascular changes (isolated or with lupus GN) |
| Uncomplicated vascular immune deposits |
| Noninflammatory lupus vasculopathy with immune deposits |
| TMA |
| Necrotizing vasculitis (medium and/or small vessel vasculitis), rare |
| Lupus podocytopathy |
| Minimal change disease, FSGS, collapsing glomerulopathy |
| Renal lesions unrelated to lupus disease |
| Diabetic nephropathy, IgA nephropathy, amyloidosis, etc. |
ISN/RPS, International Society of Nephrology/Renal Pathology Society; FSGS, focal segmental glomerulosclerosis; SLE, systemic lupus erythematosus; GN, glomerulonephritis; LN, lupus nephritis; TIN, tubulointerstitial nephritis; TMA, thrombotic microangiopathy.
Clinical Manifestations
The diagnosis for SLE is well delineated by the 1997 modified American Society of Rheumatology criteria and the recently modified criteria of the Systemic Lupus International Collaborating Clinics [4, 5]. There is a high incidence of renal involvement in SLE, that is, LN, with 50–70% of patients developing subclinical or mild to severe clinical renal manifestations during the course of disease. SLE usually affects young women at a peak age between the 2nd and 4th decades with a female to male ratio of 8–9:1, while children as well as older adults can also be affected at a lower frequency. A higher predilection for certain racial and ethnic groups, particularly in individuals of African descent and Hispanic origin, is observed for the development of both SLE renal disease and the severity of LN. A proportion of cases of SLE have a tendency to occur in family members, suggesting an underlying genetic predisposition. The systemic nature of clinical SLE spans many years with periods of exacerbation/flare and remission and is manifested as skin rashes, often in those areas exposed to sunlight including the characteristic “butterfly” rash on the face, joint symptoms ranging from arthralgias to inflammatory arthritis, inflammatory lung disease, autoimmune hepatitis, polyserositis, hematological abnormalities of anemia, and thrombocytopenia. However, it is the involvement of the kidneys and the central nervous system that is more predictive of the more severe form of SLE and poor prognosis. The spectrum of clinical renal disease and urinary abnormalities generally reflects the type and severity of the renal/glomerular lesions and can vary from asymptomatic proteinuria/hematuria, nephritic or nephrotic syndrome, and acute or chronic renal failure. Active urine sediment with red blood cell (RBC) and protein casts is often correlated with active or proliferative forms of GN, while nephrotic range proteinuria is commonly associated with membranous lupus GN or a podocytopathy. The onset of hypertension is not an early event, unless it is also complicated by the development of lupus vasculopathy or thrombotic microangiopathy (TMA). Although the serological profile of patients with SLE or LN is heterogeneous that confirms the diagnosis, it is also indicative of the disease activity, based on low complement levels (C3 and C4), anti-C1q antibodies, the antibody specificities, and titers. These autoantibodies produce several patterns of nuclear staining for detection, occurring in varying frequencies in SLE and other autoimmune diseases. They include antibodies to nuclear antigens (ANA), single- or double-stranded DNA, RNA, nucleosomal proteins, and histones. Nonnuclear antigens may include matrix proteins, for example, laminin, vimentin, or heparan sulfate; complement components, particularly C1q; blood cell antigens, for example, RBCs, leukocytes, platelets, and endothelial cells; and membrane phospholipids. Apart from a transplant renal biopsy, perhaps renal disease in lupus may have the broadest range of indications for a renal biopsy that includes repeat biopsies for subsequent flares of disease activity, changes in therapy, and even discontinuation of therapy.
Pathogenesis
The underlying pathogenetic mechanisms of SLE include T-lymphocyte activation, B-lymphocyte stimulation leading to the development of a number of auto-ANA, other cellular/matrix antigens, and plasma proteins, triggered by varied genetic and environmental factors. The pathogenetic factors and eventual morphology of renal LN are complex and involve multiple signaling pathways [3, 6, 7, 8]. However, most pertinent to the pathological and electron microscopy (EM) findings is the renal tissue injury with or without immune-complex deposition. Accordingly, autoantibodies of diverse specificities bind to the target antigens, which are either in circulation (forming circulating immune complexes) or within the kidney (forming in situ complexes with native/tissue or planted antigens), followed by complement activation, often involving both the alternate and classical pathways of the complement cascade, deposited in the various locations of the kidney tissue. The size, avidity, nephritogenicity, and location of the immune complexes may dictate the type and severity of glomerular, tubulointerstitial, and vascular lesions, which in turn translate into expression of the clinical renal disease.
Light Microscopy
The histologic spectrum of LN ranges from mild to severe forms of GN patterns of injury mainly based on light microscopy (LM). The ISN/RPS, International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification continues to evolve focusing toward refined terminology and clinical relevance [1, 2, 9]. Several morphologic features overlap between the LN classes: for example, varying degrees of mesangial proliferation seen in all classes; the extent of endocapillary hypercellularity, necrosis, and crescent formation (involving <50% or >50%) qualifies classes 3 and 4; and class 5 membranous LN can occur in combination with classes 3 and 4 [2]. The currently used revised ISN/RPS classification with the introduction of the modified NIH activity and chronicity indices [2, 10] is listed in Tables 1 and 2. Standard processing of the renal biopsy specimen for LM, immunofluorescence (IF), and EM is recommended for a comprehensive examination [3, 9, 11].
Table 2.
| Activity index | |
| Endocapillary hypercellularity | 0–3 |
| Neutrophil infiltration and/or karyorrhexis | 0–3 |
| Fibrinoid necrosis | 0–3×2 |
| Cellular/FCs | 0–3×2 |
| Hyaline (immune) deposits | 0–3 |
| Interstitial inflammation | 0–3 |
|
| |
| Total range of score | 0–24 |
|
| |
| Chronicity index | |
| Total glomerulosclerosis (segmental and global) | 0–3 |
| Fibrous crescents | 0–3 |
| Tubular atrophy | 0–3 |
| Interstitial fibrosis | 0–3 |
|
| |
| Total range of score | 0–12 |
LN, lupus nephritis; FCs, fibrocellular crescents.
Class 1: Mild Mesangial LN
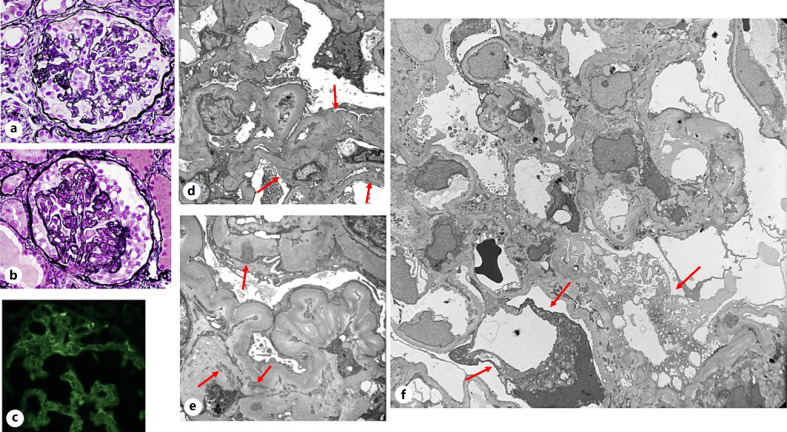
The renal tissue findings are generally normal by LM, and such cases are seldom seen in biopsy. This often depends on the biopsy practices of the treating nephrologist and the presenting symptoms of the patient, which may be microhematuria or low proteinuria and normal renal function. The glomerular architecture is well preserved with normal cellularity or only minor abnormalities of focal mesangial prominence or thickening (Fig. 1a). However, focal minimal or small but detectable immune deposits mainly limited to the mesangial areas are identified by IF (Fig. 1b) and EM (Fig. 1c-f).
Fig. 1.
Minimal mesangial LN (ISN/RPS class 1). a Normal glomerular appearance without mesangial hypercellularity (periodic acid-Schiff, ×400). b Variable/scant, granular IgG deposits in the mesangial areas (IF, ×400). c–f Finely granular, scant to small mesangial electron dense deposits (arrows), with relatively preserved foot processes, no significant changes of the mesangial cells, matrix, or glomerular basement membranes (c–f ×6,000). LN, lupus nephritis; IF, immunofluorescence; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
Class 2: Mesangial Proliferative LN
This class is defined as primarily mesangial disease with mesangial hypercellularity and variably increased mesangial matrix, of mild to severe degree (at least a minimum of 4 nuclei in the peripheral mesangial locations) (not including the hilar region) [2] (Fig. 2a, 3a), containing significant immune deposits restricted to the mesangial areas. Such lesions are best examined by PAS staining of a routine renal biopsy section of standard thickness having an adequate number of glomeruli. No evidence of endocapillary hypercellularity is noted in any of the glomeruli, and the peripheral capillary walls are relatively of normal thickness with patent capillary lumina. The tubulointerstitium appears largely preserved, with occasional RBCs, RBC casts, or protein casts in the tubular lumina. The clinical presentation again is commonly microhematuria and subnephrotic proteinuria with normal renal function. Nephrotic syndrome may occur in a few cases due to a superimposed podocytopathy with widespread glomerular foot process effacement.
Fig. 2.
Mesangial proliferative LN (ISN/RPS class 2). a Global moderate mesangial expansion due to hypercellularity and increased matrix (periodic acid-Schiff, ×400). b Global granular mesangial IgG deposits (IF, ×400). c Conspicuous granular mesangial electron dense deposits, mesangial hypercellularity, normal glomerular basement membranes, with intact foot processes (×6,000). d Rare subepithelial electron dense deposits (arrows) within the capillary basement membranes with localized foot process effacement, but preserved in areas with mesangial hypercellularity and deposits (×6,000). e Small areas of subendothelial expansion by electron dense deposits adjacent to the mesangium (arrows) may be seen, but still categorized as class 2 (×6,000). LN, lupus nephritis; IF, immunofluorescence; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
Fig. 3.
Severe mesangial proliferative LN (ISN/RPS class 2). a Glomerulus showing global severe mesangial cell proliferation with mild narrowing of capillary lumina and no endocapillary hypercellularity (in all of the glomeruli examined) (periodic acid-Schiff, ×400). b–e In addition to granular mesangial electron dense deposits, there are small, well-defined subendothelial electron dense deposits in the capillary basement membranes (b, e, arrows) and inflammatory cells in glomerular capillary lumina (d, e), suggesting the consideration of LN of higher activity (class 3 or class 4), which may be unsampled or in progression (often clinical presentation and serology may also be helpful to determine the activity). Scattered rare small subepithelial and intramembranous deposits (c, d, arrows) may also be noted in this setting without indicating a change in the class of LN (×6,000 for b, ×10,000 for c, ×8,000 for d, e). LN, lupus nephritis; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
IF and EM
In class 2, IF and EM play an important role in confirming isolated mesangial LN or may identify subtle changes that could qualify for an early form of a higher class of LN. Substantial immune deposits are recognized by IF with a “full house” composition (see below) predominantly in the mesangial areas, regardless of cellularity in a granular, global, and diffuse pattern, sparing the capillary basement membranes (Fig. 2b). On occasion, there may be focal/rare, small peripheral capillary wall deposits. Electron microscopic appearance can vary from case to case or glomerulus to glomerulus in the same biopsy and even within the mesangial areas of a single glomerulus, with regard to the amount of immune-type electron dense deposits. They are fine to coarsely granular and, when abundant, partly replace the matrix and displace the nuclei (Fig. 2c, 3c). Some cases may disclose minute scattered subepithelial (Fig. 2d, 3c, d) or subendothelial deposits (Fig. 3b, e), or minimal subendothelial extension of the mesangial deposits (Fig. 2e), which is not unusual in this class. But when significant stretches of capillary wall deposits are found, the potential for a higher class of LN should be suspected and reported (Fig. 2a-e).
The common differential diagnoses in this setting with microhematuria and subnephrotic proteinuria containing mainly mesangial deposits are IgA nephropathy, C1q nephropathy, some milder forms of infection, or autoimmune diseases. The clinical and serological data and the composition of immune deposits by IF will be helpful in resolving the differences.
Class 3 and Class 4: Focal and Diffuse Proliferative LN
Definition and Background
These classes are defined by the presence of segmental (<50% of capillary tuft) or global (≥50% of capillary tuft) glomerular endocapillary hypercellularity with or without tuft necrosis and crescent formation in <50% of total glomeruli as class 3 and ≥50% of total glomeruli as class 4 LN, in a given relatively adequate kidney biopsy for examination for a lupus patient (1). Often, more segmental lesions are encountered in class 3, while segmental and/or global lesions may predominate in class 4, with immune deposits. In addition, the glomerular lesions can be active or chronic sclerosing in nature to be included in a specific class. A majority of these patients present with a nephritic syndrome or a combination of nephrotic-nephritic features with hematuria, proteinuria, and varying degrees of renal insufficiency with or without hypertension.
Light Microscopy
Class 3: This category is also termed as “focal lupus nephritis” due to <50% involvement of the total glomeruli by active proliferative or necrotizing lesions with crescents (focal or global), which can be observed in PAS and H&E stains. Segmental lesions are more common in this class (Fig. 4a). The other features include influx of inflammatory cells, such a polymorphonuclear leukocytes, lymphocytes, and macrophages, and development of localized “wireloop” lesions and “hyaline thrombi” indicating subendothelial eosinophilic immune deposits as well as intracapillary aggregates of immune deposits, best seen in PAS and trichrome stains. This is accompanied by localized endothelial swelling and almost often mild to moderate mesangial proliferation. A small proportion of cases present with focal segmental, pauci-immune type of necrotizing lesions, resembling ANCA-associated crescentic GN, some with concomitant positive serology (Fig. 5a, b). These lesions typically tend to recur, thus showing active, subacute, and chronic healed lesions, all in 1 biopsy tissue. The tubulointerstitium can be relatively unaffected in the early stages or show patchy active or subacute interstitial inflammation or mild focal tubular atrophy and interstitial fibrosis.
Fig. 4.
Focal proliferative LN (ISN/RPS class 3). a Focal segmental glomerular endocapillary hypercellularity, overlaid by a small fibrocellular crescent with adhesion to the Bowman capsule and the rest of the glomerulus, shows focal mesangial prominence and patent capillary lumina (periodic acid-Schiff, ×200). b Segmental lesional IgG localization within the capillary walls and mainly mesangial deposits in the rest of the glomerulus (×200). c Focal, small subendothelial and mesangial deposits are noted in some capillary basement membranes (arrows), in 1 capillary loop, in a case of class 3 LN. Fairly widespread subendothelial deposits should lead to a suspicion of an active class of lupus (class 3 or class 4) (×8,000). d Endocapillary hypercellularity is characterized mainly by inflammatory cells occluding the glomerular capillary lumen, which may also be associated with subendothelial deposits (arrows) and overlaid by FC (×6,000). LN, lupus nephritis; FC, fibrocellular crescent; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
Fig. 5.
Pauci-immune focal proliferative nephritis in systemic lupus. a, b Segmental fibrinoid, necrotizing lesion without significant mesangial or endocapillary hypercellularity that usually shows scant mesangial deposits by IF staining for immunoglobulins and complement components (not shown here) (PAS, PASM ×400). A small proportion of these patients may have positive ANCA serology. c Glomeruli often contain some baseline minimal to mild glomerular mesangial electron dense deposits (arrows), confirming the underlying lupus disease (×2,500). d, e Extracapillary fibrin aggregate with a characteristic bundled, fibrillary substructure and tactoids in low and high magnification, within this lesion (×2,500, ×8,000). IF, immunofluorescence.
Class 4: This class is termed “diffuse lupus nephritis” by virtue of involvement of the >50% of total glomeruli in a biopsy with segmental or global endocapillary hypercellularity along with varying degrees of mesangial proliferation. In the more severe cases, segmental fibrinoid necrotizing changes with or without crescents are evident, some of which may have positive ANCA serology that may be driving the crescent formation. Significant inflammatory cell infiltration also contributes to the endocapillary hypercellularity. A higher number of polymorphonuclear leukocytes with or without accompanying karyorrhexis are scored as an activity index (Table 3). Glomerular endocapillary hypercellularity, capillary subendothelial, eosinophilic, PAS+ deposits as “wireloop” lesions, intracapillary “hyalin” thrombi representing immune deposits (Fig. 6a, 7a), necrosis, and crescent formation all are also scored as activity indices.
Table 3.
Diagnostic utility of EM in LN
| 1. | Confirmation of the diagnosis of lupus GN |
| 2. | Accurate assignment of lupus GN class |
| 3. | Identification of other concomitant classes of lupus GN |
| 4. | Differentiation between lupus and non-lupus membranous GN |
| 5. | Assessment of the extent and status of glomerular deposits and extracellular matrix for prognosis |
| 6. | Confirmation of specific interclass transformation of lupus GN |
| 7. | Documentation of TRIs and organized deposits |
| 8. | Diagnosis of non-lupus renal lesions in SLE patients including podocytopathies, TMA, amyloidosis, and infectious complications |
| 9. | Limited utility in the diagnosis and prognostic significance of tubulointerstitial or vascular lesions |
GN, glomerulonephritis; EM, electron microscopy; LN, lupus nephritis; TRIs, tubuloreticular inclusions; SLE, systemic lupus erythematosus; TMA, thrombotic microangiopathy.
Fig. 6.
Diffuse proliferative LN (ISN/RPS class 4). a Two glomeruli showing relatively global glomerular abundant eosinophilic capillary wall deposits, “wireloops” or “hyaline thrombi” in the lumina, with only focal endocapillary hypercellularity (periodic acid-Schiff, ×200). b Strong granular IgG deposition along the capillary walls, mesangial areas, and focal luminal globular aggregates, corresponding to the “hyaline thrombi,” having a “full house pattern” of composition (×400). c A single glomerular capillary loop distended with endocapillary hypercellularity containing lymphocytes and monocytes, trapping scattered and aggregates of electron dense deposits and focal subendothelial deposits (arrows). d A portion of a glomerulus demonstrating 2 intraluminal granular aggregates termed “hyaline thrombi” composed of immune deposits (arrows) (×6,000). e Same glomerulus as in (c) with abundant mesangial deposits (arrows) admixed with matrix, cells with adjacent glomerular endocapillary hypercellularity (×6,000). f Two glomerular capillary loops with circumferential subendothelial electron dense deposits (arrows) and mesangial deposits that appear in continuity with the subendothelial deposits, representing the ultrastructural correlate of “wireloop” lesions, with preserved lamina densa. The foot processes are generally preserved and no significant subepithelial deposits are identified (×7,000). g A “wireloop” lesion with massive finely granular subendothelial electron dense deposits on 1 side of the glomerular capillary basement membrane has infiltrating monocytes within the lumen (×6,000). h A higher magnification image of a mesangial area containing nodular aggregates of electron dense deposits, displacing cells and admixed with matrix at the periphery (×10,000). LN, lupus nephritis; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
Fig. 7.
Diffuse LN (ISN/RPS class 4). a This is another variant of diffuse lupus GN where the glomerular capillary walls are globally thickened by massive eosinophilic/PAS+, subendothelial deposits, with focal obstructive intracapillary “hyalin thrombi” having a smooth outer contour, suggesting the absence of subepithelial deposits. A small proportion of cases show this unique feature involving all of the glomeruli in a given biopsy without significant hypercellularity (PAS, ×400). b Strong IgG deposition is localized in the capillary walls in the same distribution as seen by LM (×400). c, d EM findings show corresponding large, confluent subendothelial markedly electron dense deposits involving most capillary subendothelial zones displacing the endothelial cells and partly filling capillary lumina, as well as expansive mesangial deposits replacing the matrix and cells (×2,000, ×3,000). GN, glomerulonephritis; LN, lupus nephritis; EM, electron microscopy; LM, light microscopy; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
IF Microscopy
Classes 3 and 4: A characteristic feature of IF in LN cases is the composition of the immune deposits as “full house” that stains for all 3 immunoglobulin deposits (IgG, IgM, and IgA) and both complement components C3 and C1q representing the alternate and classical pathways of complement activation, respectively. This can have a segmental/global glomerular pattern or focal/diffuse distribution in the given biopsy tissue, having a granular texture in both class 3 (Fig. 4b) and class 4 cases. All parameters of IF assessment are important while reviewing the slides, which include the composition, the intensity of individual immune reactants (0–3+), pattern of staining, and location with the glomeruli and renal tissue. This information can help determine the specific class of LN in some cases and exclude non-lupus lesions. Among the immunoglobulins, IgG is the most intense, with lesser staining for IgM and IgA, commonly found along the glomerular capillary walls, lumina, and mesangium (Fig. 6c, 7b), while IgM is seen more strongly in the subendothelial and mesangial areas. The deposits can be granular, irregular, segmental, or continuous in distribution. The terminal complement complex (C5b–9) and C4d (when used) are also localized within the deposits. Fibrin stain is positive in the sites of necrosis and crescent formation.
Electron Microscopy
Routine EM can contribute significantly toward the diagnosis of LN (Table 3). Since it is well known that the glomerular lesions in the different classes of LN are heterogeneous, varying from one case to another of the same class and among glomeruli within the same biopsy, 1 or 2 glomeruli examined for EM may display a range of segmental to global cellularity, varied quantity, and location of the deposits. While mesangial deposits are found in all classes of LN in a relatively global manner and in greater quantities in the higher classes, the precise localization and frequency of capillary basement membrane deposits are useful in deciding the milder forms, the active proliferative classes (3 and 4), and class 5 [3, 6, 10].
Class 3: The endocapillary hypercellularity, endothelial swelling, and luminal inflammatory cells are all visualized in greater detail (Fig. 4d), when available by EM, as these lesions are focal and segmental. In addition to mesangial deposits, focal stretches of small- or medium-sized, granular subendothelial deposits are noted (Fig. 4c). Small scattered subepithelial deposits may occur, but when they are found in significant numbers correlating with more extensive capillary wall IF staining, it raises the possibility of a concomitant membranous LN. Foot process effacement is variable depending on the number of subepithelial deposits and occasionally a superimposed podocytopathy. In cases of pauci-immune necrotizing segmental LN, except for small mesangial deposits, no conspicuous capillary wall deposits are visible (Fig. 5c), except for intra- or extracapillary fibrin deposits (Fig. 5d, e).
Class 4: The active glomerular features of class 4 are similar to those found in class 3, where the presence of segmental or global involvement of the glomeruli demonstrates varied hypercellularity, more widespread and abundant mesangial (Fig. 6c, e, h), intracapillary (Fig. 6d), and subendothelial electron dense deposits in a segmental or circumferential distribution. The latter finding represents the ultrastructural counterpart of the “wireloop” lesion by LM and the continuous capillary wall deposits by IF (Fig. 6f, g). Again, a few scattered subepithelial deposits may be part of this class. Sometimes massive mesangial, subendothelial, and intracapillary deposits are present without obvious hypercellularity (Fig. 7a-d).
A focal or diffuse membranoproliferative glomerular pattern associated with class 4 is occasionally seen by LM, containing substantial subendothelial deposits and hypercellularity, representing active lesions. But these do not necessarily signify a subacute or chronic nature of the lesion, as definitive new basement membrane formation and mesangial interposition are not always identified by EM (Fig. 8a-d). However, variable subendothelial expansion with rarefaction or resorption of deposits, with or without basement membrane remodeling by EM, may suggest a transition to a more subacute or chronic stage of the glomerular lesion (Fig. 8e). If numerous concomitant subepithelial deposits are also present in this setting, this may give the appearance of a previously described type 3 membranoproliferative pattern of glomerular injury.
Fig. 8.
Diffuse proliferative LN (ISN/RPS class 4). a Two glomeruli display lobular architecture with global endocapillary hypercellularity including an influx of inflammatory cells almost occluding the capillary lumina, irregular thickening of the peripheral capillary walls displaying focal double contours indicative of a membranoproliferative (MPGN) pattern of glomerular injury in lupus, one of which is overlaid by a small FC. This pattern is not seen very often (PAS ×200). b Glomerulus showing mainly stretches of subendothelial IgG deposits which vary from 1 capillary loop to another having a smooth outer contour, suggesting no obvious subepithelial deposits and focal mesangial deposits (×400). c A portion of a glomerulus, where at least 2 capillary loops are markedly thickened by double contours, and extensive cellular interposition (arrows), which is in continuity with the adjacent mesangial area, while a separate loop is uninvolved (×1,800). d Higher magnification of the previous image showing subendothelial cellular interposition, where the original basement membrane is above (arrows) infiltrated by coarsely granular electron dense deposits and trapping some cellular debris with totally effaced foot processes. Another layer of basement membrane material is noted below the interposed cells, which is lined in the inner aspect by endothelial cells, giving rise to the double contours by LM (×600). e EM of a glomerulus from a different lupus patient with diffuse LN and focal MPGN, demonstrating a circumferential cellular interposition of a capillary loop (arrows) with small deposits and new basement membrane formation in the inner aspect, in continuity with the mesangial area containing significant deposits. Note the adjacent 2 capillary loops do not show the cellular interposition or thickening (×3,000). LN, lupus nephritis; EM, electron microscopy; FC, fibrocellular crescent; LM, light microscopy; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
The usual renal parenchymal lesions of chronicity of LN are assessment of glomerulosclerosis, fibrous crescents, tubular atrophy, and interstitial fibrosis. The causes of segmental or global glomerulosclerosis in LN may be as a result of 1 or more specific and nonimmunologic injuries, such as proliferative or necrotizing GN with or without immune deposits, and hypertensive or progressive ischemic injury, which may not be resolved by morphological means (LM, IF, or EM), in all cases.
Class 5 Membranous LN
Definition
Pure membranous LN is defined by dominant glomerular capillary wall thickening by LM. This is mainly due to subepithelial immune electron dense deposits, involving >50% of the tuft and also >50% of total glomeruli in the biopsy, resembling primary membranous GN. These are often indolent and clinically manifest subnephrotic proteinuria or frequently full nephrotic syndrome with >3 g of proteinuria per 24 h (50–70%). Serology almost always includes positive ANA and other active or inactive serology, such as anti-dsDNA and low or near-normal levels of serum complements C3 and C4. The renal function is essentially normal, when occurring initially in a pure form and without significant tubulointerstitial scarring and vascular sclerosis. Membranous LN may develop as a new lesion during a subsequent lupus flare and can superimpose on pre-existing active or healing chronic lupus glomerular lesions.
Light Microscopy
The glomeruli disclose diffuse, mild to severe irregular thickening of the capillary walls involving all the glomeruli, depending on the stage of the diagnosis. The glomeruli may appear relatively normal in a new onset, early stage of membranous LN, where the biopsy was done for proteinuria (Fig. 9a). As the disease progresses, the capillary walls start to show developing basement membrane spikes by PAS and silver staining on the outer aspect of the basement membranes (Fig. 9b), while the inner lining appears smooth with generally patent capillary lumina. In much later stages, the basement membrane spikes fuse over the deposits, forming an irregular new membrane, visible by silver staining, enclosing lucent spaces, representing resorbed deposits. Mesangial expansion or proliferation may be imperceptible in some cases, but in most cases, it can range from mild to moderate with an increase in matrix and cellularity (Fig. 9c).
Fig. 9.
Membranous LN (ISN/RPS class 5). a–c Glomerular capillary wall irregular thickening, mild to severe by PAS+ deposits showing suggestion of spikes in some capillary loops, with varying amount of mesangial hypercellularity, but without endocapillary hypercellularity, in all the glomeruli within a given biopsy (periodic acid-Schiff, ×400). d Global granular IgG in the glomerular capillary walls with a “full house” pattern is observed (×400). e, f Numerous small or confluent, fine to coarsely granular subepithelial or intramembranous electron dense deposits are seen frequently interspersed by basement membrane spikes. Some of these deposits may penetrate the thickness of the glomerular basement membrane with attenuation of lamina densa. The presence of such “penetrating” deposits, as well as mesangial deposits, favors lupus membranous GN over primary membranous GN. Rare subendothelial deposits may occur (arrows), the extent of which will determine whether there is a more active component and a combined class 4 and class 5 should be considered. The foot processes are totally effaced but may also show some degenerative changes and denudation, due to the extensive basement membrane remodeling (×6,000). g An earlier stage of glomerular subepithelial deposits, which appear finely granular, discrete, and frequently interspersed with basement membrane spikes, showing an intact or even thickening of the lamina densa (×6,000). h, i Higher magnification of the glomerular capillary basement membranes showing numerous subepithelial and intramembranous deposits penetrating deep into the matrix with marked attenuation of the lamina densa. Small subendothelial deposits (arrows) may be found, without affecting the class designation (×15,000). j This image shows mainly intramembranous and focal subepithelial electron dense deposits in varying stages of resorption (lucencies or rarefaction, partially dense, clear, granular) (arrows) with persistent foot process effacement. These findings reflect resolving deposits and chronic evolution of the disease process, as seen in the various stages of primary membranous GN (×8,000). GN, glomerulonephritis; LN, lupus nephritis; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
IF Microscopy
The glomerular capillary deposits are qualified by diffuse, global, fine to coarsely granular staining (Fig. 9d) in a “full house” fashion for immunoglobulins, IgG being universally dominant, with variable IgM and IgA, along with similar intensity of C3 and variable C1q, depending on the stage and activity of disease. Focal mesangial staining is also evident in almost all cases.
Electron Microscopy
EM is helpful when studying the early lesions where the glomeruli appear close to normal by LM, where subepithelial electron dense deposits may be extremely small, embedded in the outer aspect of the basement membranes, just underneath the epithelial foot processes. Some of these deposits may be less dense and may escape detection in a few areas. As the stage of membranous LN progresses, the granular deposits become larger and more visible with developing basement membrane spikes between them, while the lamina densa is largely preserved (Fig. 9e, f). Eventually, the spikes fuse over the deposits, making them intramembranous (Fig. 9g), which may undergo resorption changes (Fig. 9j). A particular characteristic of the active and abundant subepithelial deposits in membranous LN is that they have a tendency to penetrate the lamina densa (Fig. 9h, i). These are often termed “transmembranous” and thus raise suspicion of lupus or lupus-like disease, despite the lack of “full house” immune complex deposits and rarely with negative lupus serology. As a rule, the epithelial foot processes are extensively effaced with numerous microvilli in the urinary space. A constant finding, as in all other LN classes, is the presence of a variable amount of mesangial deposits. A few, small subendothelial deposits found in the LN setting are acceptable and will not change the class designation, unless they are substantial and widespread involving most loops, then a combination of class 5 with class 3 or 4 (Fig. 11a–e) should be considered, based also on the clinical and laboratory data. If there is suspicion of this type, examination of more than 1 glomerulus may be indicated to confirm the diffuse nature of the glomerular lesion, as this could have therapeutic and prognostic implications.
Fig. 11.
Proliferative and membranous LN (combined ISN/RPS classes 4 and 5). a Two glomeruli with segmental endocapillary hypercellularity and marked irregularly thickened capillary walls, infiltrating inflammatory cells and mild to moderate mesangial expansion (periodic acid-Schiff, ×400). b Strong granular IgG deposits along the glomerular capillary walls and mesangial areas (×400). This pattern of staining can also be observed in isolated class 4 or class 5 LN. EM is the best way to identify this combined lesion when associated with global diffuse LN. c–e Glomerular capillaries show abundant granular dense deposits by EM in both subepithelial and subendothelial (arrows) locations of the glomerular basement membranes, some being superficial, others with basement membrane spikes, and a few fused over making them intramembranous and focal in the mesangial areas. The lamina densa may be largely intact or show focal “penetrating” deposits (×4,000, ×7,000 for c, d, ×8,000 for e). d This image also demonstrates the massive subendothelial and intraluminal accumulation of immune deposits. LN, lupus nephritis; EM, electron microscopy; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
Differential Diagnosis
When membranous LN is clinically silent or indolent, this needs to be differentiated from primary membranous GN in adults [12]. The former is most often observed in younger females, many of whom have a higher number of parameters of ARA SLE criteria for diagnosis, with positive ANA serology in almost all of them, while lesser cases may have low complement levels. In contrast to primary membranous GN, LM may show more intraglomerular cellularity, while IF exhibits a “full house” pattern of staining of immunoglobulins and both complement components. These deposits also localize mainly IgG1 and IgG3 subsets with fainter IgG2 [13] and are negative for phospholipase A2 receptor antibodies (PLA2R) staining, whereas positive staining for dominant IgG4 subset and the presence of positive serology for PLA2R antibodies with corresponding serology confirm the diagnosis in primary membranous GN. It was recently reported that exostosin can be detected by immunostaining in 80% of membranous GN associated with autoimmune diseases and 35% of membranous LN but virtually not seen in other types of membranous GN. This marker thus may be helpful in the differential diagnosis [1315]. Some of the more predictable differentiating points are also observed by EM in membranous LN, such as the presence of mesangial deposits, scattered small subendothelial deposits, focal glomerular “transmembranous” deposits, tubular basement membrane deposits, and occasional tubuloreticular inclusions (TRIs) within the glomerular endothelial cells. Membranous LN may be an initial manifestation of lupus, and rarely in the setting of seronegative lupus, which may convert to seropositivity nearly 5–10 years after the onset of the membranous LN. In this setting, there rarely is“full house” IF.
Class 6: Advanced Sclerosing LN
Definition
The advanced stage of sclerosing LN is defined by the ISN/RPS classification as global glomerulosclerosis in about or >90% of the glomeruli, with no evidence of any residual activity of the lesions (Fig. 10a). This is accompanied by extensive and severe tubular atrophy, chronic interstitial inflammation, fibrosis, and marked arteriosclerosis. These findings are almost always correlated by the clinical presentation of a high stage of chronic kidney disease with subnephrotic or minimal proteinuria. Such cases are seldom subject to a biopsy, unless a clear indication is presented for examination of the tissue. IF and EM when performed on relatively preserved glomeruli may show residual deposits (Fig. 10b, c) amid mostly sclerosing glomerular change without significant cellularity or activity in the tissue.
Fig. 10.
Sclerosing LN (ISN/RPS class 6). a Most of the glomeruli are sclerotic or almost completely sclerosed in this advanced class of LN, but mild focal residual cellularity may be observed (periodic acid-Schiff, ×400). b Immunoglobulins (IgG in this illustration) or complement components are either lost (arrows) or segmentally preserved (arrowheads). c EM of a portion of a sclerotic glomerulus showing matrix expansion, wrinkled/collapsed capillary basement membranes, and residual scattered, ill-defined, granular electron dense deposits (arrows) (×8,000). LN, lupus nephritis; EM, electron microscopy; ISN/RPS, International Society of Nephrology/Renal Pathology Society.
Mixed Class 3 or 4 with Class 5
Although the proliferative classes 3 and 4 (focal and diffuse) are clinically and histopathologically distinct in the setting of SLE renal disease, about 20–25% of the cases present with a combination of lesions of classes 3 and 5 or classes 4 and 5 in the same glomeruli, manifesting LM, IF, and EM features of both (Fig. 11a-e). Sometimes, in the presence or total absence of segmental or global hypercellularity, EM is the best modality to determine the location and extent of subepithelial and subendothelial immune electron deposits and resolve the diagnostic issue. This has significant clinical therapeutic and prognostic utility.
Lupus Podocytopathy
Definition
Lupus podocytopathy in patients with SLE manifests mainly with proteinuria, often of nephrotic range, secondary to podocyte injury demonstrating fairly widespread foot process effacement without significant glomerular endocapillary hypercellularity or capillary wall immune deposits, and has emerged as a distinct entity in a subset of SLE patients [16].
Clinical Features
Lupus podocytopathy is a rather uncommon renal manifestation of all cases undergoing renal biopsies in SLE (1–2%). The main clinical presentation is nephrotic range proteinuria or frank nephrotic syndrome with occasional mild nephritic urinary sediment. While ANA is positive in all cases confirming the diagnosis of SLE, about 25–50% are also positive for other parameters such as anti-dsDNA antibodies, anticardiolipin antibody, anti-Sm antibody, and low complement levels, suggesting active disease and with varied duration of SLE. The creatinine levels may be normal, or they may present with features of acute kidney injury in the absence of proliferative GN.
LM and IF Microscopy
The usual forms of glomerular lesions associated with lupus podocytopathy are minimal change disease, mesangial hypercellularity, and focal segmental glomerulosclerosis (Fig. 12a-c). However, segmental scarring as a result of healed proliferative lesions of lupus GN should be excluded. Severe renal failure with marked nephrotic syndrome is associated with segmental or global collapsing glomerulopathy [17], a severe form of podocytopathy, characterized by marked wrinkling and collapse of the capillary walls leading to occlusion of the capillary lumina, overlaid by hyperplastic and vacuolated epithelial cells in the Bowman space (Fig. 13a, b). The underlying pathogenetic mechanisms appear to be related to an immune-mediated podocyte injury in lupus patients, and in some populations expressing high-risk APOL1 genotypes, APOL1 may have a higher predisposition for the collapsing features [18].
Fig. 12.
Lupus podocytopathy. Minimal change disease. A young female SLE patient of 2 years duration, with no specific treatment, presented with a sudden onset of nephrotic syndrome. a Glomerulus with preserved capillary architecture and patent lumina (PAS ×200). b Occasionally, there may be mild to moderate glomerular mesangial hypercellularity and mild increased matrix in this setting (×400). FSGS. This may develop in a few SLE patients presenting with active lupus serology and nephrotic syndrome. c Glomerulus with minimal mesangial thickening and an area of segmental capillary tuft collapse with sclerosis and capsular adhesion at the periphery, resembling the NOS form of primary FSGS with minimal to mild mesangial immune deposits by IF (PAS ×200). d, e The EM findings are similar in both minimal change disease and FSGS. Portions of 2 glomeruli showing normal thickness of basement membranes, total effacement of foot processes (arrows) with epithelial swelling, and microvillous transformation in the urinary space. The endothelial fenestrations are largely preserved. However, the mesangial areas appear prominent with finely granular small electron dense deposits (arrows) (×1,800). EM, electron microscopy; FSGS, focal segmental glomerulosclerosis; IF, immunofluorescence.
Fig. 13.
Lupus podocytopathy. Collapsing glomerulopathy. This is a 35-year-old female patient who presented with severe nephrotic syndrome (10 g/24 h) and renal insufficiency (creatinine 2.5 mg/dL) with a recent diagnosis of SLE and high titers of ANA and anti-dsDNA. a, b Nearly 50% of the glomeruli showed segmental (a) or global (b) marked wrinkling and collapse with loss of patency of the lumina, but without an increase in intraglomerular cellularity or matrix, overlaid by hyperplastic and variably vacuolated epithelial cells in the areas of collapse (×400). There were mild tubulointerstitial changes initially, which progressed in a few months to significant tubular epithelial injury, vacuolization, and focal microcystic tubular changes. c Only focal fine IgG staining in the mesangial areas is found (×400). d, e On EM, the collapsed portions of the glomeruli show marked thickening and wrinkling of the capillary basement membranes, leading to mostly occlusion of the lumina with total foot process effacement and rare subepithelial, intramembranous, and mesangial densities, suggesting evidence of occasional immune deposits in the setting of lupus (d ×6,000, e ×12,000). f Glomerular findings adjacent to the areas of collapse, in addition to diffuse foot process effacement, demonstrate the severe visceral epithelial/podocyte injury changes, such as small and large confluent intracytoplasmic vacuolization with varying degenerative cytoplasmic changes (arrows) and condensation of the nuclear chromatin, and focal separation from the basement membranes. There is also total loss of endothelial fenestrations in the capillaries (×3,000). EM, electron microscopy; SLE, systemic lupus erythematosus.
IF may reveal variable but mainly glomerular mesangial deposits (Fig. 13c) in most mild cases or focal sparse capillary wall deposits in a few. Some studies have shown development of collapsing glomerulopathy with underlying active or previous lupus GN, where immune deposits may be detected by IF. The distinction of a concomitant or superimposed podocytopathy lesion should be made with caution [17, 18].
Electron Microscopy
To make a definitive diagnosis of a podocytopathy in the setting of lupus renal disease, EM is required. While at least 50–80% process effacement is recognized (Fig. 12d, e), this also allows for the assessment of other underlying immune complex-mediated lupus lesions that may or may not contribute to the extent of foot process effacement and the magnitude of proteinuria. Mesangial electron dense deposits could be present (Fig. 12d, e). Cases with substantial capillary wall deposits in the subepithelial and subendothelial areas may exclude the diagnosis of lupus podocytopathy. Although collapsing features in some of these instances may represent a superimposed lesion, features of ischemic collapse or globally sclerosing glomeruli as a result of healing LN should be considered and excluded. TRIs may be seen in the glomerular endothelial cells in patients with active systemic disease. In collapsing glomerulopathy, severe wrinkling and collapse of the capillaries are confirmed by EM with total foot process effacement and degenerative changes in the glomerular endothelial and mesangial areas (Fig. 13d, e). In addition, striking glomerular epithelial cell alterations are observed such as marked vacuolization, many of which are coalesced, as a result of severe injury, leading to focal separation from the underlying basement membranes, degenerative cytoplasmic change, and nuclear condensation (Fig. 13f).
Unusual EM Findings
In addition to the usual ultrastructural features observed in the various classes of lupus glomerular lesions and sometimes in the tubulointerstitium, some unique EM findings may be noted in LN, regardless of the histological findings, preferably in more active cases: organized deposits, hematoxylin body, and TRIs [19, 20, 21, 22].
Organized deposits in LN are not unusual and are visualized primarily by EM and not detected by LM and IF within the immune complex deposits. They are characterized by focal or rarely diffuse substructural changes, including microtubular, finger print-like, lattice-like, or having randomly arranged parallel bundles of fibrillary appearance, which are often seen within otherwise usual granular textured deposits [19, 20]. Sometimes, more than 1 pattern of organization can occur within the immune deposits in the same case, and occasionally, extensive or total tubulofibrillar transformation of the deposits may also be seen (Fig. 14a-e). The formation of organized deposits may depend on the composition and physicochemical characteristics of the immune complexes, local tissue conditions, and distribution of the deposits. These may occur more frequently in class 4 LN, where abundant deposits are present, and less frequently in class 5. Almost all these cases show a “full house” pattern of composition by IF. While fingerprint deposits (fragmented, partial, or fully formed), as evidenced by the concentric curved dark and light lines with a diameter of 10–15 nm (Fig. 15a-e), are more commonly observed, they do not all represent concomitant cryoglobulinemia in LN [20]. Those patients with both SLE and mixed cryoglobulinemia, with organized deposits, may show microtubular substructure ranging from 20 to 100 nm in diameter (Fig. 16b), admixed with a background of granular deposits. Additionally, Congo red-negative fibrillar deposits measuring 10–18 nm in diameter, with a solid cross section, may occur in rare cases (Fig. 16a), and a few others just have a loose microaggregate appearance (Fig. 16c, d). However, the clinical significance or impact of such organization within immune complex deposits is not entirely clear, except maybe in the presence of high titer mixed or polyclonal cryoglobulinemia.
Fig. 14.
Glomerular organized deposits in LN. a–c These are from 1 case where the glomerular capillary basement membranes show extensive subendothelial and mesangial deposits (arrows) that are totally transformed as tubulofibrillar appearance, occurring in bundles that are irregularly arranged, ranging from 20 to 60 nm in diameter, without any evidence of a background of granular deposits. The overlying basement membrane is mild to moderately thickened with denudation or effacement of foot processes. Such deposits have also been seen exclusively in the subepithelial areas in other rare LN cases in our files (a ×10,000, b ×20,000, c ×60,000). d Another case with similar randomly arranged tubulofibrillar deposits, now admixed with a background of finely granular deposits (arrows) found in the capillary basement membranes and the mesangial area (×10,000). e High magnification EM image of glomerular deposits in different cases showing randomly arranged tubulofibrillar deposits along with a 2nd form of organized partial fingerprint deposit (arrows) in the case field (×60,000). EM, electron microscopy.
Fig. 15.
Spectrum of organized fingerprint glomerular deposits in LN. a, b, e A case of diffuse LN with some granular subendothelial and mesangial deposits, where significant portions of them are transformed into mostly fragmented or partial “fingerprint-like” patterns (arrows), composed of concentric curved dark and light lines with a diameter of 10–15 nm (a ×25,000, b ×40,000, c, e ×60,000). c Deposits on both sides of the glomerular basement membrane with a fingerprint appearance (×40,000). d High magnification of an aggregate of fully formed “fingerprint-like” deposits in a glomerulus with LN (×40,000). LN, lupus nephritis.
Fig. 16.
Cryoglobulin and other organized glomerular deposits in LN. a Extensive subendothelial randomly arranged fibrillar deposits showing short rigid fibrils with a solid cross section measuring 10–18 nm (×40,000). b Subendothelial deposits composed of haphazard aggregates of large hollow tubular deposits (arrows) in a patient where circulating cryoglobulins were identified (×40,000). c, d A case of diffuse LN with deposits having an unusual loose somewhat mottled appearance (arrows), throughout the glomerulus. Occasionally, such an appearance may be seen in patients who were treated with immunosuppressive therapy for a short duration (×10,000). LN, lupus nephritis.
TRI [20], a cytomembranous structure, appears as intracellular reticular aggregates of tubular structures of uniform diameter, but of variable sizes, localized within dilated cisternae of the endoplasmic reticulum (Fig. 17a-c). It is shown to be induced by elevated cytokine levels, particularly alpha interferon, elaborated by activated T lymphocytes. The inclusions, single or several per glomerulus, are seen in endothelial cells of glomeruli and peritubular capillaries, rarely in glomerular epithelial or tubular epithelial cells, but not in other renal parenchymal cells. TRIs are also observed in glomerular lesions with other immune-mediated diseases such a mixed connective tissue disease and viral infections, for example, HIV, HCV, and rarely HBV. Although after a diligent search TRIs may be noted in most biopsies with LN of any class, even in lupus class 5, sometimes they may antedate the onset of SLE. A higher frequency and increased numbers of TRIs are readily identified in active class 3 and 4 LN. Additionally, another form of cytomembranous structure associated with TRIs termed “cylindric confronting cisternae,” also induced by alpha-interferon and previously reported in HIV nephropathy, is detected by EM in active diffuse LN cases, particularly in the glomerular and tubulointerstitial infiltrating macrophages [21].
Fig. 17.
Tubuloreticular inclusion. a–c A TRI body characterized by reticular aggregates of cytomembranous structures of uniform diameter (arrows) found within the glomerular and sometimes peritubular capillary endothelial cell smooth endoplasmic reticulum, readily seen in active LN cases. The glomerular capillary basement membrane in (a) shows both subepithelial and subendothelial immune complex deposits (a ×20,000, b ×15,000, ×15,000). d Hematoxylin body: This is rarely seen as an “in vitro” phenomenon, where the bare neutrophil or endothelial nuclei are exposed to circulating ANA, leading to swelling and homogeneous condensation of the chromatin, assuming an unusual morphology of enlargement and hyperchromatism with extremely dark osmiophilic staining. By EM, it represents an acellular mass of amorphous material, which is identified in the glomerular capillary lumen (×8,000). e Concentric laminated myelin-like structures (“zebra bodies”) within podocytes, overlying a capillary loop with subepithelial electron dense deposits. This patient having systemic lupus was treated with chloroquine, presenting with proteinuria. The kidney biopsy shows class 5 membranous LN. These abnormal podocyte lipid inclusions are reminiscent of Fabry disease, due to the interference of chloroquine with the cellular lysosomal processing of phospholipids, a known complication of chloroquine therapy in some lupus patients (×3,000). LN, lupus nephritis; EM, electron microscopy; TRI, tubuloreticular inclusion.
Hematoxylin body by LM is a darkly stained body composed of a central dense amorphous nuclear material, with a vague lobulated granular appearance and cytoplasmic fragments, partially surrounded by a damaged cell membrane. They reside within a large phagocytic vacuole of mesangial cells or monocytes, sometimes encountered in diffuse LN cases (Fig. 17d). These have been explained to be antibody-mediated apoptotic cells, with subsequent phagocytosis, or often represent an in vitro LE phenomenon following biopsy within the tissue [22]. On occasion, it can be recognized by LM as having a high affinity for hematoxylin stain.
Lupus Tubulointerstitial Nephritis
Definition and Background
This is defined as acute or chronic interstitial inflammation of any degree with associated tubular injury or tubulitis, also termed as tubulointerstitial nephritis (TIN), which may accompany lupus GN (secondary) in a renal biopsy. A few instances of isolated active interstitial inflammation that is devoid of glomerular lesions (primary) may occur in the setting of SLE [23, 24]. The TIN may be due to (a) factors leading to TIN injury common to any significant glomerular disease regardless of etiology, e.g., ischemia, cytokines, or inflammation; (b) lupus-specific cell-mediated injury; (c) concomitant immune complex deposits in tubular basement membranes or peritubular capillary wall; and (d) lupus-specific antitubular antibody-mediated injury. The clinical correlate of TIN is acute or chronic renal failure, with minimal or no proteinuria, unless there is concomitant glomerular lesion [23, 24]. The extent and severity of the tubulointerstitial disease in LN have an impact on the activity and renal function, as well as chronic changes serve as an important marker of prognosis and long-term renal survival.
Light Microscopy
The frequency of occurrence, and extent and severity of the secondary TIN can be generally correlated with the higher proliferative lupus class (e.g., lupus GN class 4). The infiltrating cells are composed of mainly activated T lymphocytes and macrophages with a smaller population of B lymphocytes and plasma cells. This can be patchy or diffuse with mild, moderate, or severe intensity with focal tubular infiltration (Fig. 18a, d).
Fig. 18.
Active and chronic TIN, associated with lupus GN. a Active and chronic TIN, associated with class 4 lupus GN noted within the glomeruli included, composed of mainly large aggregates of mononuclear inflammatory cell infiltrate lymphocytes, a small proportion of plasma cells and macrophages, and focal tubulitis (PAS, ×200). b Positive Ig in glomerulus (left), as well as granular staining along the tubular basement membranes and interstitial space. c EM image showing nonspecific chronic tubulointerstitial changes, including tubular epithelial injury, numerous granular electron dense deposits of pale density within the tubular basement membranes with irregular thickening, and infiltrating inflammatory cells (×4,000). d Mild chronic TIN, associated with lupus GN. d Active and chronic TIN, associated with class 4 lupus GN (left) with focal tubulitis (PAS, ×200). e Positive deposits of Ig in the glomeruli but not in the tubulointerstitial compartment (×200). f Ultrastructurally, the tubules show nonspecific chronic tubulointerstitial changes, including tubular cell injury, thickened and focal laminated tubular basement membrane without immune deposits, interstitial fibrosis, and interstitial inflammation (×4,000). GN, glomerulonephritis; EM, electron microscopy; TIN, tubulointerstitial nephritis.
IF and EM
Routine IF performed on the kidney biopsy can uncover the presence of granular tubular basement membrane, peritubular capillary, or interstitial deposits, in a focal/segmental or diffuse manner when present (Fig. 18b). A sizable number of cases may not localize tubular or interstitial deposits by IF despite the presence of an inflammatory reaction (Fig. 18e). These findings are correlated by EM depending on the sample available for examination. There are finely to coarsely granular, small or larger confluent intramembranous deposits or on the outer aspect of the basement membranes, with or without evidence of tubulitis and epithelial injury (Fig. 18c). Electron dense deposits may be seen in peritubular capillary wall (Fig. 19g). In contrast, no deposits by IF or EM are identified in other cases, despite the existence of immune complex glomerular lesions in the same biopsy (Fig. 18e, f). The interstitial deposits become visible when they occur in abundance or aggregates in active cases.
Fig. 19.
Lupus vascular lesion: lupus vasculopathy. a A preglomerular arteriole discloses intimal PAS+ immune complex deposits with a flocculent appearance (arrows), without associated inflammation, accompanied by proliferative LN seen in the adjacent glomerulus (PAS ×400). b Intimal IgG deposits in a small arteriole (arrows) corresponding to the light microscopic finding, often showing a “full house” pattern by IF. c, d Segmental granular electron dense deposits having varied density (arrows) seen mainly restricted to the intimal layer and focally involving the medial layer (c ×6,000, d ×8,000). e An arteriole displaying exclusively intimal immune deposits (arrows), surrounded by 1 or 2 layers of smooth muscle medial layer (×1,800). f High magnification of a small arterial wall with small deposits (arrows) infiltrating in between the smooth muscle cells in the media. These may represent the usual uncomplicated vascular immune deposits detected by IF in many active LN cases (×6,000). g A peritubular capillary with abundant granular deposits in the basal lamina (×12,000). IF, immunofluorescence; LN, lupus nephritis.
Primary lupus TIN, without glomerular lesions, is rarely observed, due to tubular deposition of circulating immune complexes or in situ localization of circulating anti-tubular basement antibodies, with similar LM, IF, and EM features as those seen with glomerular lesions [23].
Lupus Vascular Disease
Background
Renal vascular lesions in patients with SLE can be recognized in 8–25% of renal biopsies. They are most commonly associated with active class 3 or class 4 LN but can occur independently of glomerular or immune complex-mediated lesions. The vascular lesions show varied histopathological features, reflecting diverse pathogenesis and clinical significance [3, 25, 26]. They include uncomplicated vascular immune deposits, noninflammatory microvascular immune deposits also termed “lupus vasculopathy,” TMA of various causes, necrotizing vasculitis (microscopic or macroscopic polyangiitis type), and accelerated or chronic arteriosclerosis. The clinical renal manifestations depend on the type and severity of the vascular lesions, sometimes coinciding with glomerular and/or tubulointerstitial disease. A higher incidence of hypertension and initial elevated creatinine levels is observed in the presence of lupus-associated vascular lesions, with worse renal survival [25, 26].
Light Microscopy
The lupus vascular lesions in the kidney are readily recognized by LM because of their specific appearances, except for those with “uncomplicated vascular immune deposits” showing normal morphology or mild eosinophilic thickening. The latter lesions are often incidentally identified during IF staining with focal, minimal to mild intensity and are often considered as part of active LN (Fig. 19f). The “noninflammatory lupus vasculopathy” involves mainly preglomerular arterioles and rarely some small arteries showing mostly circumferential or focal segmental intimal accumulation with occasional medial extension of eosinophilic, PAS+, often massive immune complex deposits having a flocculent appearance and separating the endothelial cells. This appearance is different from the more homogeneous, pale, hyaline deposits in hypertensive vascular disease, leading to luminal narrowing or occlusion (Fig. 19a). These deposits localize immunoglobulins and complement components within the vascular wall (Fig. 19b). This may be focal or involve a number of small arterial vessels, often in cases of diffuse LN, with elevated creatinine and hypertension.
The most severe form of vascular lesion in SLE is TMA involving glomeruli and/or small arterial vessels with or without simultaneous glomerular lesions of LN. A number of causative factors are implicated in this setting, namely, secondary to immune complexes, presence of antiphospholipid antibodies, and underlying abnormalities of complement regulatory proteins, initiating endothelial injury and leading to progressive renal failure. The LM findings disclose glomerular capillary microthrombi, endothelial swelling, and subendothelial widening, which are somewhat discernible, and in some instances of concomitant proliferative lesion (Fig. 20a). Occlusive microvascular thrombosis with fibrin deposits and trapping of fragmented RBCs may also be seen in severe cases (Fig. 20b).
Fig. 20.
TMA in lupus: a, b This is a pediatric case of diffuse LN presenting with acute renal failure and severe clinical TMA features. The glomeruli show global endocapillary hypercellularity and focal capillary microthrombi (arrows) (a) and an occluded small artery and arteriolar thrombus trapping fragmented RBCs (arrows), with moderate ischemic collapse in an adjacent glomerulus (b) (a PAS ×200, b H&E ×200). c Same case as above, a glomerular capillary loop showing variable subendothelial widening with a lucent space and focal subendothelial deposits (arrows). The rest of the basement membrane is generally intact with relatively preserved foot processes (×12,000). d This is a lupus patient with detectable antiphospholipid antibodies presenting with a mild rise in creatinine and subnephrotic proteinuria without any evidence of immune complex-mediated glomerular lesions. Healing glomerular TMA lesion with organized subendothelial space containing increased matrix material trapping focal cellular debris (×6,000). e Another lupus patient with antiphospholipid antibodies, proteinuria, and mesangial LN, showing incidental intracapillary fibrin tactoids in 1 loop (arrows), while the adjacent capillary shows a foamy macrophage trapped in the lumen (×6,000). f, g Intrarenal arterioles from 2 different lupus patients with kidney biopsies, showing the loose edematous intimal expansion (arrows) with trapped dark fragments of RBCs and occluded by a thrombus with disintegrating cellular elements in (f). The other is completely occluded by the intimal mucoid swelling limited by the intact internal elastic lamina, with total separation of the endothelial cells, containing remnants of myointimal cellular elements in g (arrows) (f ×2,000, g ×1,800). LN, lupus nephritis; RBCs, red blood cells; TMA, thrombotic microangiopathy.
While leukocytoclastic vasculitis is commonly seen in the skin or rarely as alveolar capillaritis in the lungs causing hemorrhage, visceral involvement by small vessel lupus vasculitis is seldom encountered in SLE, leading to significant morbidity and mortality [25]. Mild to moderate arteriosclerosis or accelerated arterial thickening can ensue in relatively young patients, following these renal vascular lesions, often manifesting as new-onset hypertension.
Electron Microscopy
EM is not routinely required for the diagnosis of “noninflammatory lupus vasculopathy,” and the alterations usually depict the ultrastructural correlates of those identified by LM, for example, endothelial injury and varying amounts of intimal or focal medial accumulation of electron dense deposits (Fig. 19c-e). EM glomerular findings may help in the diagnosis of TMA which range from subendothelial widening with lucent space, intracapillary fibrin tactoids, to, in various stages of healing, basement membrane remodeling and replacement by matrix (Fig. 20c-e). The microvascular changes show the composition of the luminal thrombi and severe fibromyxoid intimal expansion, as a result of endothelial injury (Fig. 20f, g).
Conclusions
SLE is a multisystemic autoimmune disease with a high incidence of renal involvement during the course of the disease, affecting glomeruli, tubulointerstitial compartment, and vasculature. The morphological spectrum of the renal parenchymal disease in LN is broad with diverse patterns of injury, a majority of them being glomerular lesions that include immune complex-mediated mesangial GN, focal or diffuse proliferative GN with or without crescents and membranous GN, and rarely a podocytopathy associated with SLE. Varying degrees of active, subacute, and chronic tubulointerstitial and a variety of vascular lesions may accompany the glomerular lesions, sharing similar or different pathogenetic mechanisms. They may be antibody-driven immune complex-mediated, cell-mediated, complement-mediated, or hemodynamically mediated tissue injury. The role of EM in characterizing and defining these lesions cannot be overemphasized, based on its contributions toward proper diagnosis, improved specificity, and classification of the glomerular lesions; localization and status of immune deposits; and differentiation from primary glomerular lesions. The latter is particularly important: when serologic data are equivocal, “full house” immune deposits are not observed, and there is the concomitant presence of other non-lupus entities [27]. Thus, the practice of routine triaging and performance of EM on initial and repeat renal biopsies from SLE patients not only benefits the diagnosis but also provides valuable prognostic information, having important therapeutic implications.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
The authors did not receive any funding.
Author Contributions
Luan D. Truong and Surya V. Seshan participated in the preparation of the manuscript, assembling images, and references.
Acknowledgments
The authors would like to express their gratitude to the EM technical staff (Mr. Michael Ganger, Mrs. Lilian Antonio, and Ms. Melanie Wilson) for providing EM images and Mr. Mikhail Snigirev for secretarial and digital assistance in the preparation of the manuscript.
Funding Statement
The authors did not receive any funding.
References
- 1.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GE, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65((2)):521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018 Apr;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 3.D'Agati VD, Stokes MB. Renal disease in systemic lupus erythematosus, mixed connective tissue disease, Sjogren's syndrome and Rheumatoid arthiritis. In: Jennette JC, D'Agati VD, Olson JL, editors. Silva FG heptinstall's pathology of the kidney. 7th ed. Philadelphia: LWW; 2015. pp. p. 559–655. [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725–1736. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 5.Tedeschi SK, Johnson SR, Boumpas D, Daikh D, Dörner T, Jayne D, et al. Developing and refining new candidate criteria for systemic lupus erythematosus classification: an international collaboration. Arthritis Care Res (Hoboken) 2018;70((4)):571–581. doi: 10.1002/acr.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol. 2015;11:329–341. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
- 7.Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13((8)):483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 8.Anders H-J, Saxena R, Zhao M-H, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020 Jan 23;6((1)):7. doi: 10.1038/s41572-019-0141-9. [DOI] [PubMed] [Google Scholar]
- 9.Seshan SV, Jennette JC. Renal disease in Systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med. 2009;133:233–248. doi: 10.5858/133.2.233. [DOI] [PubMed] [Google Scholar]
- 10.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 11.Herrera GA. The value of electron microscopy in the diagnosis and clinical management of lupus nephritis. Ultrastruct Pathol. 1999;23:63–77. [PubMed] [Google Scholar]
- 12.Jennette JC, Iskandar SS, Dalldorf FG. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int. 1983;24:377–385. doi: 10.1038/ki.1983.170. [DOI] [PubMed] [Google Scholar]
- 13.Hemminger J, Nadasdy G, Satoskar A, Brodsky SV, Nadasdy T. IgG subclass staining in routine renal biopsy material. Am J Surg Pathol. 2016;40((5)):617–626. doi: 10.1097/PAS.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi S. New ‘Antigens’ in membranous nephropathy. J Am Soc Nephrol. 2021;32((2)):268–278. doi: 10.1681/ASN.2020071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu W, Chen Y, Wang S, Chen H, Liu Z, Zeng C, et al. Clinical-morphological features and outcomes of lupus podocytopathy. Clin J Am Soc Nephrol. 2016;11((4)):585–592. doi: 10.2215/CJN.06720615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvatore SP, Barisoni LM, Herzenberg AM, Chander PN, Nickeleit V, Seshan SV. Collapsing glomerulopathy in 19 patients with systemic lupus erythematosus or lupus-like disease. Clin J Am Soc Nephrol. 2012;7((6)):914–925. doi: 10.2215/CJN.11751111. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CP, Beggs ML, Saeed M, Walker PD. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol. 2013;24((5)):722–725. doi: 10.1681/ASN.2012121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grishman E, Porush JC, Rosen SM, Churg J. Lupus nephritis with organized deposits in the kidneys. Lab Invest. 1967;16((5)):717–725. [PubMed] [Google Scholar]
- 20.Hvala A, Kobenter T, Ferluga D. Fingerprint and other organised deposits in lupus nephritis. Wien Klin Wochenschr. 2000;112((15–16)):711–715. [PubMed] [Google Scholar]
- 21.Venkataseshan VS, Marquet E, Grishman E. Significance of cytoplasmic inclusions in lupus nephritis. Ultrastruct Pathol. 1991;15:1–14. doi: 10.3109/01913129109021300. [DOI] [PubMed] [Google Scholar]
- 22.Morita M, Sakaguchi H. Ultrastructure of renal glomerular hematoxylin bodies. Ultrastruct Pathol. 1984;7:13–9. doi: 10.3109/01913128409141849. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Xu J, Zhang X, Ren YL, Cheng M, Guo ZL, et al. Tubular basement membrane immune complex deposition is associated with activity and progression of lupus nephritis: a large multicenter Chinese study. Lupus. 2018;27:545–555. doi: 10.1177/0961203317732407. [DOI] [PubMed] [Google Scholar]
- 24.Dhingra S, Qureshi R, Abdellatif A, Gaber LW, Truong LD. Tubulointerstitial nephritis in systemic lupus erythematosus: innocent bystander or ominous presage. Histol Histopathol. 2014;29:553–565. doi: 10.14670/HH-29.10.553. [DOI] [PubMed] [Google Scholar]
- 25.Seshan SV. Lupus vasculopathy and vasculitis: What is the difference and when do they occur? Pathol Case Rev. 2007;129((5)):214–221. [Google Scholar]
- 26.Wu LH, Yu F, Tan Y, Qu Z, Chen MH, Wang SX, et al. Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. Kidney Int. 2013;83((4)):715–723. doi: 10.1038/ki.2012.409. [DOI] [PubMed] [Google Scholar]
- 27.Baranowska-Daca E, Choi YJ, Barrios R, Nassar G, Suki WN, Truong LD. Nonlupus nephritides in patients with systemic lupus erythematosus: a comprehensive clinicopathologic study and review of the literature. Hum Pathol. 2001 Oct;32((10)):1125–1128. doi: 10.1053/hupa.2001.28227. [DOI] [PubMed] [Google Scholar]