Abstract
Background.
The association between cortisol secretion and mortality in patients with adrenal incidentalomas is controversial. This study aimed to assess all-cause mortality, prevalence of comorbidities, and occurrence of cardiovascular (CV) events in uniformly stratified patients with cortisol autonomy.
Methods.
The Non-Aldosterone-Producing AdrenoCortical Adenoma (NAPACA) Outcome study is an international retrospective multi-centre cohort study investigating the effects of cortisol autonomy (defined as non-suppressible serum cortisol on dexamethasone-suppression testing) on mortality and CV morbidity in patients with adrenal incidentalomas. Patients with clinically apparent hormone excess, active malignancy, or follow-up <36 months were excluded. Patients were stratified according to the 0800-0900h serum cortisol values after a 1 mg dexamethasone-suppression test (<50nmol/L, non-functioning adenoma (NFA); 50-138nmol/L, possible Autonomous Cortisol Secretion (PACS); >138nmol/L, ACS). The primary study endpoint was all-cause mortality. Secondary endpoints were prevalence of cardiometabolic comorbidities, CV events, and cause-specific mortality.
Findings.
3656 patients (57% NFA, 36% PACS, 7% ACS) were included (64% women; median age 61 years; median follow-up 7·0 years). During follow-up, 352 patients (9·6%) died. All-cause mortality (adjusted for age, sex, comorbidities, and former CV events) was significantly increased in PACS (HR 1·52; 95%CI 1·19-1·94) and ACS (1·77; 1·20-2·62). In women <65 years, ACS was associated with higher mortality compared to NFA (HR 4·37; 95%CI 1·93-9·91), while in men this was not observed. Cardiometabolic comorbidities were significantly less frequent in NFA than in PACS and ACS (hypertension: n=1186 (59%), n=944 (74%), n=179 (75%); dyslipidaemia: n=724 (36%), n=547 (44%), n=123 (52%); diabetes: n=365 (18%), n=288 (23%), n=62 (27%); always p<0.001).
Interpretation.
Cortisol autonomy is associated with increased all-cause mortality, especially in women <65 years. However, until results from randomised interventional trials will be available, a conservative therapeutic approach seems to be justified in most patients with adrenal incidentaloma.
Funding.
Deutsche Forschungsgemeinschaft, Associazione Italiana per la Ricerca sul Cancro, Università di Torino.
Introduction
Over the last decades, wider availability and use of cross-sectional imaging have resulted in an increased incidental detection of clinically inapparent adrenal masses. Such adrenal ‘incidentalomas’ have an increasing age-dependent prevalence, ranging from 3% in adults of 50 years of age to 10% in those over 70 years.1–3
The majority of these tumours are benign non-functioning adrenal adenomas (NFA).3,4 However, endocrine workup may find biochemical evidence of hypercortisolism in 30-50% of patients without clinically overt glucocorticoid excess, a condition historically described as ‘subclinical Cushing syndrome’. As only very few of these cases progress to overt Cushing syndrome,5,6 it is currently recommended that patients be categorised by the serum cortisol value after the 1 mg overnight dexamethasone-suppression test (DST) as having ‘autonomous cortisol secretion’ (ACS: >138 nmol/L), ‘possible ACS’ (PACS: 50-138 nmol/L), and NFA (<50 nmol/L).7
Recently, a cohort study reported a slightly elevated mortality in 969 patients with adrenal incidentalomas compared to 2907 patients without.8 Furthermore, several studies have focused on the association between ACTH-independent cortisol autonomy (defined as non-suppressible serum cortisol after DST) and mortality in these patients, but results are conflicting. Three single centre studies that included 198 to 365 patients9–11 and one population-based study from Sweden (with 1048 patients)12 reported an increased mortality in persons with elevated cortisol after the 1 mg DST. In contrast, a systematic review (with 32 studies and 4121 patients) found cardiovascular (CV) and metabolic risk factors (i.e., hypertension, diabetes mellitus, dyslipidaemia, and obesity) to be more prevalent in the presence of what the authors termed ‘mild autonomous cortisol excess’.6 However, mortality was only studied in a subgroup of 1356 patients from nine studies and remained comparable to patients with NFA. In line with this, a population-based study from Minnesota (USA) compared 1004 patients with adrenal incidentalomas to sex- and age-matched subjects without adrenal tumours and found no difference in mortality.13 These discrepancies may be explained in part by the heterogeneity of the criteria used for the definition of cortisol autonomy in these studies.
Taken together, although it is plausible that there is an association between low-grade cortisol excess (as disclosed by DST), comorbidities (including CV events) and mortality, previously reported cohorts were limited by low numbers and potential single-centre bias. Accordingly, we have performed a large international multicentre cohort study to assess all-cause mortality, prevalence of comorbidities, and occurrence of CV events in patients with adrenal incidentalomas, applying unified diagnostic criteria to define cortisol autonomy.
Methods
Study design and setting
The Non-Aldosterone-Producing AdrenoCortical Adenoma (NAPACA) Outcome study was approved by the European Network for the Study of Adrenal Tumours (ENSAT) (www.ensat.org) in December 2014. Subsequently, a total of 30 centres from 16 countries agreed to participate. Each had local ethical approval for pseudonymised, standardised phenotype recording. All patients provided written informed consent (except for nine centres, where the Ethics Committees waived this requirement). Centres were asked to report patients in a consecutive manner to minimize selection bias. Retrospective data acquisition was carried out over a 56-month period (from January 2015 to August 2019).
Criteria for patient selection
Patients fulfilling the following inclusion criteria were considered eligible: age ≥18 years; adrenal incidentaloma (uni- or bilateral with a diameter ≥1cm) detected by cross-sectional imaging between January 1, 1996 and December 31, 2015; diagnosis of an adrenal adenoma based on typical imaging characteristics7 or follow-up imaging excluding malignancy; availability of a 1 mg DST result at the time of the initial diagnosis; follow-up data on living status and occurrence of CV events; follow-up duration ≥36 months. Exclusion criteria included a confirmed diagnosis of clinically overt Cushing syndrome (defined according to an established clinical practice guideline14 as presence of hypercortisolism along with specific clinical signs of cortisol excess (such as easy bruising, facial plethora, and proximal myopathy), ACTH-dependent hypercortisolism, phaeochromocytoma, primary aldosteronism, surgery within 36 months after initial diagnosis, or any active malignancy (including adrenocortical carcinoma) at the time of primary diagnosis of the adrenal mass. The considerable variation in use of other diagnostic tests at different centres, including plasma ACTH and urinary free cortisol, precluded formal analysis of other tests. Patients undergoing surgery after ≥36 months of follow-up were censored, setting the date of surgery as the date of last follow-up. For sub-analyses, patients were categorized according to their age at diagnosis (<65 vs. ≥65 years, based on age-dependent thresholds established to assess CV risk in patients with diabetes or hypertension15,16), with separate analyses based on sex.
Variables
Following the European guideline on the management of adrenal incidentalomas,7 patients were categorised according to their first serum cortisol 1 mg DST result after initial diagnosis of the adrenal incidentaloma: serum cortisol <50 nmol/L, NFA; 50-138 nmol/L, PACS; >138 nmol/L, ACS). The conversion factor for serum cortisol is: nmol/L divided by 27·59 = μg/dL (hence, important cutoffs for the 1 mg DST are 50 nmol/L = 1·8 μg/dL, and 138 nmol/L = 5·0 μg/dL).
The following clinical annotations were collected: age, sex, and body mass index (BMI) at the time of the initial diagnosis of adrenal incidentaloma; tumour characteristics (i.e., size and side); medical history (e.g., cardiometabolic risk factors and CV events) both at primary diagnosis and during follow-up. Diagnosis of comorbidities was done according to the existing guidelines available at the time of adrenal tumour diagnosis.
Outcomes
The primary endpoint of the NAPACA Outcome study was all-cause mortality. Pre-specified secondary endpoints were: prevalence of cardiometabolic comorbidities (hypertension, diabetes mellitus, and dyslipidaemia), occurrence of CV events, and cause-specific mortality. For CV morbidity, we defined a composite endpoint of the following Major Adverse Cardiovascular Events (MACE): myocardial infarction or coronary revascularization (either bypass surgery or percutaneous intervention), stroke, or CV-related death. In addition, we collected data on venous thrombosis and pulmonary embolism.
Statistical analysis
Absolute numbers and percentages were calculated for categorical data. Missing values were discounted when calculating proportions. The results for continuous variables are expressed as medians and quartiles. The intergroup differences between the different DST categories were analysed via χ2-test. All-cause mortality was calculated as the time between the initial diagnosis of the adrenal incidentaloma and death or last follow-up. A power analysis was performed based on the assumption of a clinical meaningful hazard ratio (HR) of at least 1.5 for a two-group comparison and a mortality rate of about 10%. Using a type 1 error alpha of 0.05 and a power of 80%, about 2000 patients with 191 deaths would have to be included. Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used for subgroup analysis. Data were censored either at the date of last follow up, adrenalectomy, or death. Relevant prognostic variables were identified by univariable and multivariable analyses, using the Cox proportional hazards model. HR were provided along with the corresponding 95% confidence intervals (CI). Multivariable Cox analyses included three different post-DST groups (NFA, PACS, ACS) and the following known prognostic factors for all-cause mortality and CV events as covariables: age, sex, diabetes mellitus, hypertension, dyslipidaemia, and any former CV event. To study the functional forms of a relationship between cortisol after the 1 mg DST as a continuous variable and all-cause mortality, we applied restricted cubic splines. In addition, we categorised the cohort based on age and sex. For this analysis we used a formal 3-way interaction test, using a Cox regression for age (<65, ≥65 years), sex (male, female), and DST category (NFA, PACS, ACS). Time to first MACE was defined as the time between the initial diagnosis of the adrenal incidentaloma and first documentation of any MACE thereafter. As a quality check for data integrity, a completeness index was calculated for each centre: patients with available follow-up data within the last 12 months on December 31st, 2018 were counted as complete (i.e., centres with an index of ≥90% qualified for a sub-analysis, and the results were then compared to those derived from the whole study group). Two-tailed p values of <0·05 were judged as significant. Statistical analysis was performed using SPSS (version28·0, New York, USA) and R (version4.0.2) software using the packages ‘survival’ (version3.2-13) and ‘smoothHR’ (version1.0.3).
Role of the funding sources
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Out of the entire cohort of 4374 reported cases, 3656 patients from 28 centres and 15 countries were eligible for the mortality analysis. As suggested (http://www.strobe-statement.org/), Supplementary Figure 1 provides the reasons for excluding patients. Supplementary Table 1 depicts details on the patients per centres. In 131 patients, adrenalectomy was performed later than 36 months after initial diagnosis (details in Supplementary Table 2). These patients were censored at the time of surgery.
According to the result of the first DST, subjects were categorised as NFA (n=2089, 57·1%), PACS (n=1320, 36·1%), and ACS (n=247, 6·8%). Median age at initial diagnosis was 61 years, and almost two-third of patients were women. Bilateral tumours were most frequent in ACS, and this group also had the largest median tumour diameter. Patient characteristics at initial diagnosis of the adrenal incidentaloma are summarized in Table 1.
Table 1.
Patient characteristics at initial diagnosis of the adrenal incidentaloma.
| Characteristics | All patients (n=3656) |
NFA (n=2089) |
PACS (n=1320) |
ACS (n=247) |
|---|---|---|---|---|
| Demographics A | ||||
| Women (n, %) | 2350 (64·3%) |
1321 (63·2%) |
860 (65·2%) |
169 (68·4%) |
| Men (n, %) | 1306 (35·7%) |
768 (36·8%) |
460 (34·8%) |
78 (31·6%) |
| Age, years | 61 (53-68) |
60 (52-67) |
63 (56-70) |
63 (55-70) |
| Age < 65 years (n, %) | 2264 (61·9%) |
1404 (67·2%) |
726 (55·0%) |
134 (54·3%) |
| Follow-up, years | 7·0 (4·7-10·2) |
7·2 (4·8-10·5) |
6·9 (4·7-10·0) |
6·9 (4·5-10·0) |
| Clinical characteristics B | ||||
| Body mass index, kg/m2 | 28·1 (25·0-32·3) |
28·6 (25·4-32·6) |
27·8 (24·6-31·9) |
27·7 (24·3-31·9) |
| Tumour characteristics C | ||||
| - Left (n, %) | 1497 (44·6%) |
946 (49·8%) |
468 (38·1%) |
83 (36·2%) |
| - Right (n, %) | 1093 (32·6%) |
646 (34·0%) |
385 (31·4%) |
62 (27·1%) |
| - Bilateral (n, %) | 764 (22·8%) |
306 (16·1%) |
374 (30·5%) |
84 (36·7%) |
| Maximum tumour diameter, mm | 22 (15-30) |
20 (15-25) |
26 (19-33) |
29 (20-37) |
| Biochemistry D | ||||
| 1-mg DST serum cortisol, nmol/L | 47 (30-72) |
33 (28-50) |
72 (61-94) |
190 (157-253) |
| Comorbidities | ||||
| Hypertension (n, %) E | 2309 (65·3%) |
1186 (58·6%) |
944 (74·0%) |
179 (75·2%) |
| Dyslipidaemia (n, %) F | 1394 (40·0%) |
724 (36·2%) |
547 (43·8%) |
123 (51·9%) |
| Diabetes mellitus (n, %) G | 715 (20·5%) |
365 (18·2%) |
288 (23·0%) |
62 (26·7%) |
| CV events before initial diagnosis of the adrenal incidentaloma | ||||
| Myocardial infarction and/or coronary intervention (n, %) H | 199 (6·0%) |
87 (4·6%) |
96 (8·0%) |
16 (7·1%) |
| Stroke (n, %) I | 70 (2·1%) |
31 (1·6%) |
27 (2·3%) |
12 (5·3%) |
| Deep vein thrombosis and/or pulmonary embolism (n, %) J | 62 (1·9%) |
31 (1·7%) |
26 (2·2%) |
5 (2·2%) |
| At least one CV event (n, %) K | 319 (9·3%) |
150 (7·6%) |
139 (11·4%) |
30 (13·2%) |
If not otherwise specified, numbers are given as median (quartiles). Number of patients for whom the reported variable was available:
n=3565,
n=3219,
n=3354,
n=3656,
n=3537,
n=3486,
n=3484,
n=3305,
n=3299,
n=3293,
n=3415.
Centre-specific data on ethnicity can be found in Supplementary Table 1. Abbreviations: ACS, autonomous cortisol secretion; CV, cardiovascular; DST, dexamethasone suppression test; NFA, non-functioning adenoma; PACS, possible autonomous cortisol secretion.
As shown in a scatter plot provided in Supplementary Figure 2, serum cortisol after the 1 mg DST increased with age. None of the patients developed overt Cushing syndrome during follow-up.
During a median follow-up of 7·0 (4·7-10·2) years, 352 of 3656 patients (9·6%) died. Figure 1A depicts the crude overall survival of the three study subgroups. Compared to the NFA group, the proportion of deaths observed in PACS and ACS was higher: 143/2089 (6·8%) vs. 168/1320 (12·7%) and 41/247 (16·6%). The hazard ratios for PACS and ACS remained significantly higher than the NFA group after multivariable Cox analysis adjusting for age, sex, hypertension, diabetes mellitus, dyslipidaemia, and former CV events (HR for death in PACS, 1·52 (95% CI 1·19-1·94; p=0·001) and ACS, 1·77 (1·20-2·62; p=0·004; Figure 1B). Bilateral adenomas had a greater association with PACS and ACS, but presence of bilateral adenomas was itself not an independent risk factor for death.
Figure 1.
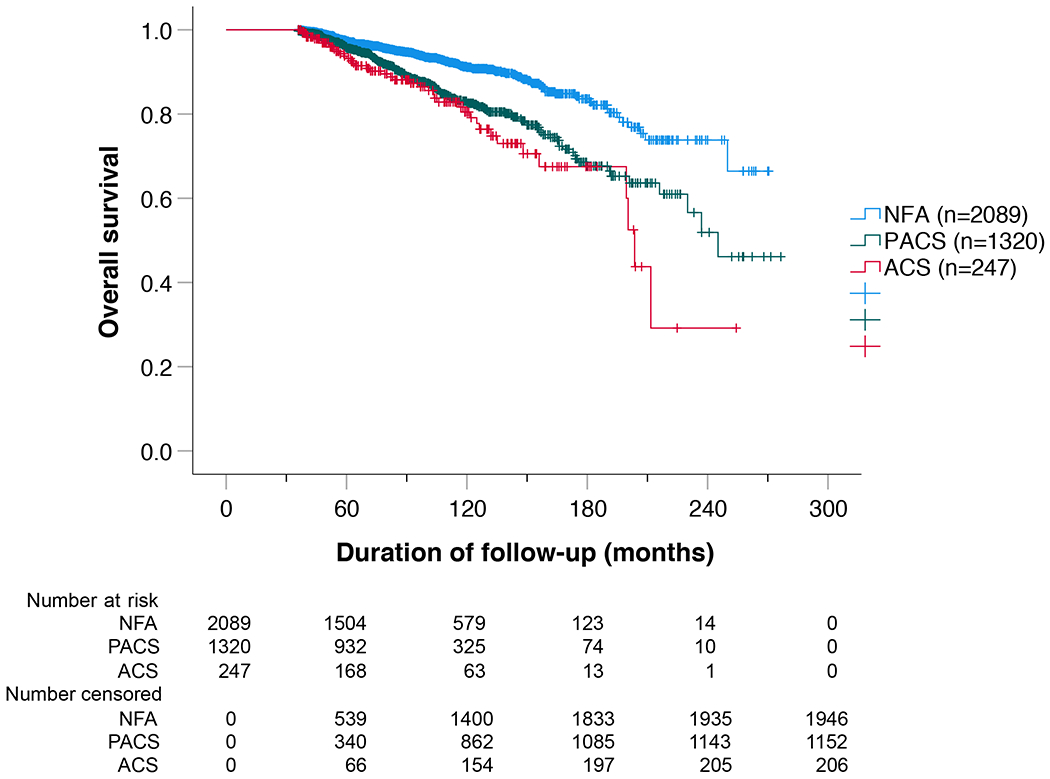
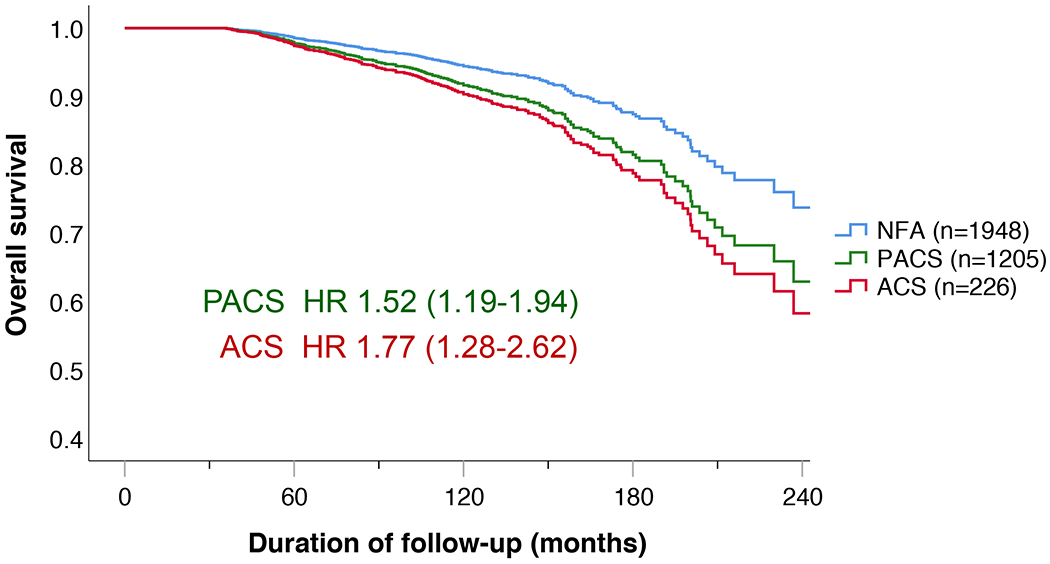
Overall survival of the entire cohort.
Results are presented as (A) Kaplan-Meier curve and (B) multivariable Cox regression analysis. (A) The Kaplan-Meier analysis included all 3656 patients. Median survival was not reached in NFA, was 246 months in PACS, and 206 months (95% CI 187-209) in ACS. Overall log-rank was p<0.001 (NFA vs. PACS, p<0.001; NFA vs. ACS, p<0.001; PACS vs. ACS, p=0.102). (B) Multivariable Cox regression analysis (including n=3379 cases; adjusted for sex, age, hypertension, dyslipidaemia, diabetes mellitus, and former CV events). Patients with missing variables were excluded from the analysis.
Abbreviations: ACS, autonomous cortisol secretion; HR, hazard ratio; NFA, non-functioning adenoma; PACS, possible autonomous cortisol secretion.
Following the cutoff criteria of a very recently published study,12 we performed a post-hoc analysis of our study. Here we divided our cohort in four subgroups (i.e., serum cortisol post-DST <50 nmol/L, 51-80 nmol/L, 81-138 nmol/L, and >138 nmol/L) and found that the mortality of the 766 patients with a serum cortisol after the 1 mg DST between 51 and 80 nmol/L was not significantly higher than the NFA group (HR 1·29, 95% CI 0·97-1·71; p=0·085); see also Supplementary Table 3. Furthermore, we studied serum cortisol after the 1 mg DST as a continuous variable in relation to all-cause mortality (Supplementary Figure 3). Whilst there was no significant linear relationship in the entire cohort, we found a linear increase in the HR for death for serum cortisol after the 1 mg DST ≤138 nmol/L.
Sensitivity analyses led to the following observations: (I) 10-year overall survival was heterogeneous among centres (ranging from 69% to 100%). To reduce the risk that overall survival was overestimated due to insufficient follow-up (leading to a lack of reported deaths), we performed an additional analysis restricted to the 21 centres with more reliable follow-up (as illustrated by a completeness index score ≥90%). However, overall survival of this cohort of 2730 patients was not changed in a relevant manner compared to the entire cohort (Supplementary Table 4). Accordingly, we decided not to exclude any centre from the analysis. (II) The association between mortality and the degree of cortisol autonomy was age-dependent: in patients <65 years, mortality was significantly higher in ACS than in NFA (adjusted HR for death: 3·16, 95% CI 1·65-6·05), whereas this was not the case for patients ≥65 years (adjusted HR for death: 1·43, 95% CI 0·87-2·33). (III) The association between mortality and serum cortisol after the 1 mg DST was much stronger in women than in men (adjusted HR for death, ACS vs. NFA: 2·50 [95% CI 1·45-4·31] in women vs. 1·19 [95% CI 0·67-2·10] in men). Consequently, we undertook a combined analysis of age- and sex- specific mortality, which is presented in Table 2 and Figure 3. This analysis revealed a significant interaction of age, sex, and the DST category (p<0.01). It is important to note, however, that the number of patients in each of these groups meant that a separate formal analysis group by group was underpowered.
Table 2.
Multivariable Cox regression analysis of sex- and age-specific all-cause mortality.
| Sex | Age (years) | All Subjects (n) | All Events (n) | PACS |
ACS |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | p | n | HR | 95% CI | p | |||||
| Women | < 65 | 1424 | 51 | 472 | 1·82 | 0·99-3·31 | 0·052 | 96 | 4·39 | 1·93-9·96 | <0·001 | |
| ≥ 65 | 723 | 108 | 302 | 1·99 | 1·31-3·01 | 0·001 | 57 | 1·80 | 0·86-3·76 | 0·118 | ||
| Men | < 65 | 734 | 43 | 222 | 1·35 | 0·70-2·59 | 0·370 | 34 | 1·77 | 0·59-5·33 | 0·307 | |
| ≥ 65 | 479 | 94 | 200 | 1·26 | 0·81-1·97 | 0·310 | 36 | 1·09 | 0·55-2·16 | 0·813 | ||
The analysis was adjusted for hypertension, diabetes mellitus, dyslipidaemia, and former CV events. Patients with missing variables were excluded from the analysis. Abbreviations: ACS, autonomous cortisol secretion; CI, confidence interval; HR, hazard ratio; PACS, possible autonomous cortisol secretion.
Figure 3.
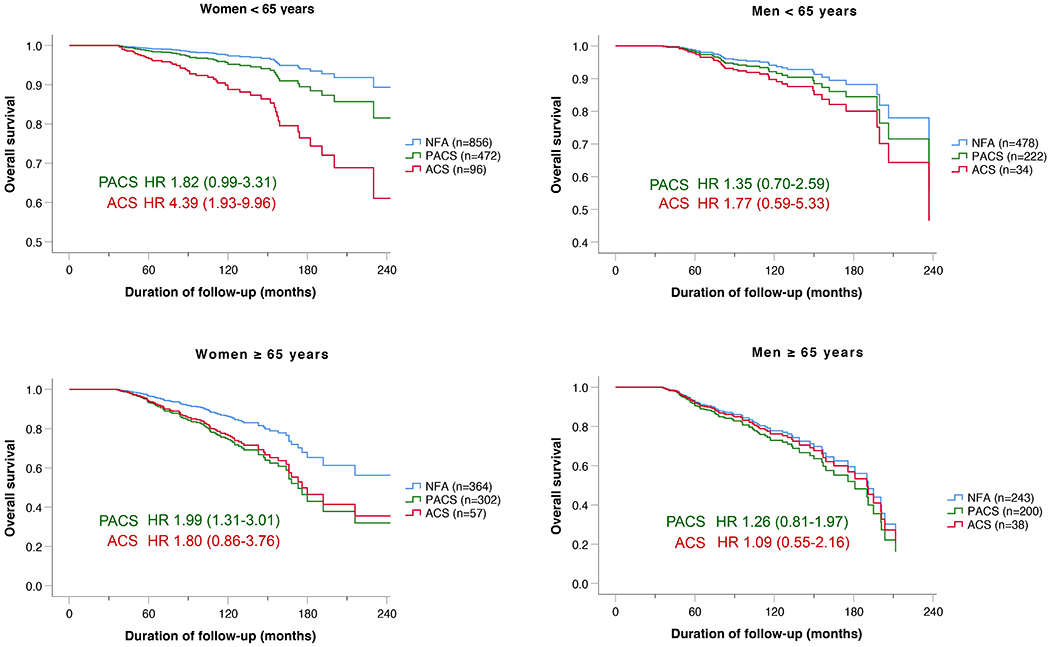
Overall survival according to sex and age.
Multivariable Cox regression analysis adjusted for hypertension, dyslipidaemia, diabetes mellitus, and former CV event. Patients with missing variables were excluded from the analysis. Abbreviations: ACS, autonomous cortisol secretion; HR, hazard ratio; NFA, non-functioning adenoma; PACS, possible autonomous cortisol secretion.
Information on the individual causes of death was available in 306 of 352 deceased patients (87·4%) (Figure 2). The two most frequent causes of death were cancer and CV-related events in 98 and 95 patients, respectively. Supplementary Table 5 depicts the cause of death according to age and sex.
Figure 2.
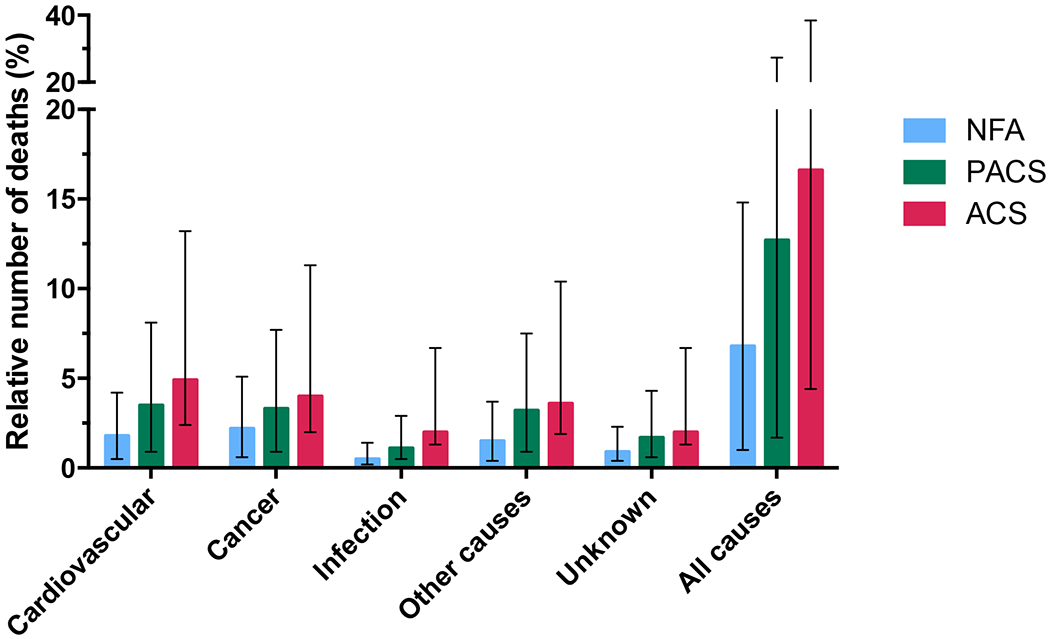
Mortality in patients with adrenal incidentalomas
Abbreviations: ACS, autonomous cortisol secretion; HR, hazard ratio; NFA, non-functioning adenoma; PACS, possible autonomous cortisol secretion.
Data on cardiometabolic morbidity and CV events were available in 3484 of 3656 patients (95·3%; 2002 NFA, 1250 PACS, 232 ACS). Overall, hypertension was the most frequent comorbidity at initial diagnosis (65·3%), followed by dyslipidaemia (40·0%), and diabetes mellitus (20·5%). As outlined in Table 1, the prevalence increased as a continuum from NFA to PACS and ACS patients, and this was true for each of these comorbid conditions.
For CV endpoints, 319 patients (9·3%) had experienced at least one CV event by the time of the initial diagnosis of the adrenal incidentaloma (Table 1). During follow-up, a total of 476 non-fatal CV events occurred in 375 patients with more CV events being found in patients with PACS and ACS: overall, 297 of 3484 patients with available data (8·5%) experienced a MACE (NFA, 7·3%; PACS, 10·3%; ACS, 9·4%). A detailed overview of the reported CV events in the three subgroups is provided in Supplementary Table 6. However, when adjusting for cardiometabolic comorbidities, time to the first MACE was only significantly shorter in the women ≥65 years with ACS (Table 3).
Table 3.
Multivariate Cox regression analysis for sex- and age-specific major cardiovascular events (MACE).
| Sex | Age (years) | All Subjects (n) | All Events (n) | PACS | ACS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| n | HR | 95% CI | p | n | HR | 95% CI | p | ||||
| Women | < 65 | 1377 | 75 | 466 | 1·20 | 0·74-1·95 | 0·463 | 94 | 1·61 | 0·71-3·61 | 0·252 |
| ≥ 65 | 705 | 92 | 296 | 1·33 | 0·84-2·06 | 0·224 | 56 | 2·09 | 1·08-4·05 | 0·028 | |
| Men | < 65 | 694 | 91 | 218 | 1·05 | 0·67-1·63 | 0·831 | 33 | 0·73 | 0·29-1·85 | 0·506 |
| ≥ 65 | 466 | 89 | 193 | 1·10 | 0·70-1·72 | 0·685 | 36 | 1·04 | 0·48-2·24 | 0·917 | |
The analysis was adjusted for hypertension, diabetes mellitus, dyslipidaemia, and former CV events. Patients with missing variables were excluded from the analysis. Time to first MACE was defined as the time lag between the initial diagnosis of the adrenal incidentaloma and first documentation of any MACE thereafter. Abbreviations: ACS, autonomous cortisol secretion; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event; PACS, possible autonomous cortisol secretion.
Discussion
The NAPACA Outcome study is by far the largest retrospective analysis on mortality and CV morbidity in patients with adrenal incidentalomas performed to date. In contrast to a meta-analysis from 2019,6 but similar to a very recent study from Sweden,12 we found overall an increased mortality in patients with PACS and ACS. Due to our large sample size (>3600 patients) we were able to reliably analyse effects of age and sex on mortality. Our data show that ACS in women <65 years of age was associated with a 4-fold increase in adjusted mortality, whereas mortality in older women and men <65 years was only moderately increased and not affected in older men.
We found that PACS and ACS were associated with an increased frequency of cardiometabolic comorbidities. In particular, hypertension had a higher prevalence in both PACS and ACS compared to NFA, while diabetes mellitus and dyslipidaemia showed a progressively increased frequency from NFA to PACS and ACS, reflecting a continuum in metabolic disturbance, as shown previously.17,18 Furthermore, CV events occurring either before or after the initial diagnosis of the adrenal tumour were more frequently observed in patients with PACS and ACS than in NFA. However, when adjusting for cardiometabolic comorbidities, a significant increase in MACE was only found in women with ACS ≥65 years, suggesting that glucocorticoid-related CV events may not be the main drivers of overall mortality in this cohort, as it has been suggested by others.9,10,12 This is in line with the reported causes of death, which indicated only few CV-related deaths in women <65 years with cortisol autonomy. In our study, we found a relative increase in CV-related mortality that paralleled that for other causes of death in patients with ACS. Another study pointed to cancer as the leading cause of death in presence of ACS;11 we could only partly confirm this observation in our large cohort in which CV and cancer-related deaths were almost equal in patients with cortisol autonomy (n=58 vs. n=56). In line with others, however, our study suggests that cortisol autonomy might have systemic detrimental effects.18–20 Nevertheless, we are well aware that a retrospective study can - by definition - never prove any causal relationship.
The fact that the association between ACS and mortality appeared to be clinically relevant mostly in younger women has not yet been described by others and may suggest that ACS is a prognostic factor that has greater influence at younger ages when other age-related comorbidities are less prominent. Although a different clinical presentation was observed for men and women with overt Cushing disease,21 less is known on sex-specific organ effects by hypercortisolism. Recent studies on stress associated with the COVID-19 pandemic showed that younger and middle-aged women were more susceptible to stress than men, displaying an increased vascular reactivity to glucocorticoids.22 Besides, it has been shown that women with diabetes or coronary heart disease were likely to receive less aggressive medical management of their CV risk factors and this may have contributed to sex differences in CV mortality.23,24 In the present study, however, we adjusted our survival analysis for comorbidities to mitigate the risk of such a confounder. Interestingly, a very recently published large prospective multi-centre study in 1305 patients with adrenal adenomas demonstrated an increased risk and severity of hypertension and type 2 diabetes in patients with cortisol autonomy and, like us, showed an increasing proportion of affected women with increasing cortisol after 1 mg DST.25 Whereas it would be important to screen for (and treat) ACS in young, and presumably otherwise more healthy patients, it is probably less relevant to do so in frail and elderly patients. However, only a large randomised intervention trial would provide a definitive answer, and such a trial is not available. Thus, for the time being, our study suggests that any decision on initiating cortisol-lowering treatment or surgery has to be taken with care, and on an individual basis.
We also observed that serum cortisol after the 1 mg DST increases with age. A retrospective study, however, cannot establish whether this association may also reflect chronic stress associated with age-related illnesses. Future studies will have to confirm this finding and to clarify if this is a hallmark of the brain aging process affecting the hypothalamic-pituitary-adrenal axis,26 reduced cortisol inactivation due to a reduced activity of 11β-hydroxysteroid dehydrogenase type 2 consequent to a lower nephron mass in ageing,27 a matter of increasing adrenal tumour mass with age,18 or potentially accelerated metabolism of dexamethasone (e.g., CYP3A4 induction due to polypharmacy in elderly patients)28. Overall, these data raise questions as to the significance of elevated serum cortisol after the 1 mg DST in the more elderly population. Besides, as recently reported,12 we could not find any clear relationship between cortisol after DST and all-cause mortality in the entire cohort. However, there was a near linear relationship when serum cortisol was ≤138 nmol/L. For higher values, the accuracy of the results are likely be limited by the low number of patients.
Our study has several limitations. First, a retrospective design is always prone to bias, including heterogeneous or possibly inaccurate capture of relevant clinical information. Nevertheless, we tried to minimize such an impact by requesting consecutively recruited patients, a minimum number of included patients per centre, and a sensitivity analysis focusing on centres with a follow-up rate of more than 90%. Second, the number of patients with the highest serum cortisol after the 1 mg DST (i.e., the ACS group) was small compared to the other two subgroups PACS and NFA; this may have weakened the statistical power of some analyses. However, the 247 ACS exceeded the total number of patients included in all previous studies on this topic (n=154).9–12 Third, we relied on a single 1 mg DST only, with variability in the performance of the cortisol assays used between centres over time, and without availability of dexamethasone serum concentrations29. However, the biochemical tests used to assess if there is cortisol autonomy have not been changed over the last 25 years.Fourth, it is possible that the inclusion criteria ‘1 mg DST result’ by itself leads to some bias, because some patients with adrenal incidentaloma may not have undergone testing. However, this bias is not resolvable, as shown by a recent population-based study in which only few patients with adrenal incidentalomas underwent some type of endocrine screening.13 In addition, we acknowledge that all participating institutions are tertiary care centres and our series might not be representative of cases seen in the community. Finally, the diagnostic criteria of the comorbidities were not uniform across centres and have obviously changed over the study period of 23 years.
In conclusion, our large retrospective international cohort study provides additional strong evidence for an overall association between PACS and ACS with increased mortality (of note, causality cannot be proven due to its retrospective nature). However, this risk is not equally distributed. Women <65 years with ACS bear the highest relative risk, whereas men ≥65 years do not appear to be at adverse risk (irrespective of the degree of cortisol autonomy). Although several studies have claimed benefits of adrenalectomy in patients with ACS, all of them were prone to bias and limited in numbers.30 Randomised interventional trials are needed to determine whether intervention (either medical treatment or surgery) is able to mitigate the cardiometabolic morbidity and mortality in patients with adrenal adenomas. Based on our findings, and until results from such trials will be available, we suggest that a conservative approach may be prudent, in particular in men with cortisol autonomy ≥65 years.
Supplementary Material
Research in context.
Evidence before this study
Adrenal incidentalomas are found in at least 3% of adults. In up to 50% of these individuals, endocrine investigation identifies evidence of biochemical hypercortisolism without clinically overt glucocorticoid excess, a condition historically described as ‘subclinical Cushing syndrome’. During preparation of the European Society of Endocrinology / European Network for the Study of Adrenal Tumours (ENSAT) Clinical Guidelines on Management of Adrenal Incidentalomas (2016), a comprehensive literature search was performed, using three well established databases (i.e., Pubmed, NHS Economic Evaluation Database (NHSEED), and Cochrane Database of Systematic Reviews and Database of Abstracts of Reviews of Effects), from January 1, 2000, to November 30, 2014, to identify all systematic reviews and studies that had assessed any association between autonomy of cortisol secretion (defined as non-suppressible serum cortisol on dexamethasone-suppression testing) with morbidity and mortality. This search revealed only two small studies, that together summarized 404 patients (including only 39 deaths), showing an increased mortality in patients with unsuppressed cortisol after dexamethasone. To confirm or refute this association, we initiated the present study under the auspices of ENSAT. Due to the lack of available multi-centre data for a sound power calculation, we aimed initially at the collection of data from at least 2000 patients. In 2021, we updated our previous literature search (now covering the period from December 1, 2014, to July 31, 2021), and identified a systematic review and a Swedish cohort study, published in 2020 and 2021, respectively. The review based on 1356 patients from nine studies and could not confirm the claimed association between cortisol autonomy and mortality, whereas the new cohort study with 1048 patients found increased mortality in patients in whom serum cortisol after dexamethasone was >83 nmol/L. In our current study, our pre-determined diagnostic criteria were those used in the above-mentioned guideline. We stratified, therefore, the patients according to the serum cortisol value after the 1 mg overnight dexamethasone-suppression test as having ‘autonomous cortisol secretion’ (ACS: >138 nmol/L), ‘possible ACS’ (PACS: 50-138 nmol/L), and ‘non-functioning adenoma’ (NFA: <50 nmol/L).
Added value of this study
Our large retrospective international cohort study with more than 3600 patients with adrenal adenomas and a follow-up of at least three years (median 7 years) provides additional strong evidence for an overall association between PACS and ACS with all-cause mortality. For the first time our study indicates that this risk varies by age and sex. Women below the age of 65 years with ACS bear the highest relative risk of death with an adjusted hazard ratio of 4.37 (95% CI 1.93-9.91), whereas men older than 65 years do not appear to be at increased risk (hazard ratio of 1.09 (95% CI 0.55-2.16)). We have also confirmed that the prevalence of cardiometabolic morbidity increases progressively with the degree of cortisol autonomy, itself more frequently detected in women and in the presence of bilateral tumours.
Implications of all the available evidence
Although our study confirms the association between cortisol autonomy, mortality and cardiometabolic morbidity, it calls for caution regarding therapeutic interventions. Our data suggest that women younger than 65 years of age could benefit most from normalizing cortisol secretion. However, only randomised interventional trials will determine whether any intervention (either medical treatment or surgery) is able to mitigate both cardiometabolic morbidity and mortality in patients with adrenal adenomas. Our study clearly provides the rationale and the statistical basis for such an outcome trial. Until these data are available, however, a conservative approach seems reasonable, especially in men older than 65 years.
Acknowledgements
This project has been supported by the European Network for the Study of Adrenal Tumours, the Deutsche Forschungsgemeinschaft (DFG) project number 314061271 (CRC/Transregio 205/1 ‘The Adrenal: Central relay of health and disease’, grant to Martin Fassnacht), the Associazione Italiana per la Ricerca sul Cancro (AIRC, grant number IG2019-23069 to Massimo Terzolo), and the Ricerca Locale Università di Torino 2020 (RILO 2020, grant to Giuseppe Reimondo). Irina Bancos is the recipient of a NIDDK/NIH K23 Award (grant number K23DK121888). Alessandro Prete is the recipient of a Diabetes UK Sir George Alberti Research Training Fellowship (grant number 18/0005782). We are grateful to Yvonne Möhres (University Hospital Würzburg) for her help in data management. We also thank the staff of the participating centres for their commitment to the NAPACA Outcome study. Mari Suzuki works in the meantime for the U.S. Federal government, however, the presented views are not necessarily those of the U.S. Federal government.
Disclosure Summary
Irina Bancos served as consultant for Corcept Therapeutics, Sparrow Pharmaceutics, and Spruce Biosciences, and was as member of advisory or data safety monitoring boards for Adrenas Therapeutics, Recordati and Strongbridge Biopharma (in all cases, institution fees were provided); in addition, personal honoraria were received from Elsevier ClinicalKey. Iacopo Chiodini received consulting fees and honoraria from HRA Pharma Rare Diseases and Recordati, was a member of advisory or data safety monitoring boards for HRA Pharma Rare Diseases and Recordati, and participated in clinical studies from Corcept Therapeutics. Alexandra Chrisoulidou received personal support for attending meetings and/or travel from Sanofi, and was a member of advisory or data safety monitoring boards for Ipsen; in addition, personal honoraria were received from Ipsen. Timo Deutschbein received personal consulting fees (for being a member of advisory or data safety monitoring boards for HRA Pharma Rare Diseases and Recordati), and personal honoraria from Novartis; in addition, he participated in clinical studies from Corcept Therapeutics and HRA Pharma Rare Diseases (for these, institution fees were provided). Martin Fassnacht participated in clinical studies from Corcept Therapeutics and HRA Pharma Rare Diseases (for these, institution fees were provided). Ljiljana Marina was a member of the expert panel ‘Focus Area Adrenal and Cardiovascular Endocrinology’ from the European Society of Endocrinology, and led the working group 5 of the project ‘CA20122 - Harmonizing clinical care and research on adrenal tumours in European countries’ from the European Cooperation in Science in Technology. John Newell-Price served as consultant for and received honoraria from HRA Pharma Rare Diseases and Recordati (in all cases, institution fees were provided). Carla Scaroni received consulting fees and honoraria from HRA Pharma Rare Diseases and Recordati, was a member of advisory or data safety monitoring boards for HRA Pharma Rare Diseases and Recordati, and served as coordinator of the Pituitary Club of the Italian Society of Endocrinology. Massimo Terzolo received personal consulting fees (for being a member of advisory or data safety monitoring boards for Corcept Therapeutics and HRA Pharma Rare Diseases), and participated in clinical studies from HRA Pharma Rare Diseases (for the latter, institution fees were provided). Stylianos Tsagarakis received personal support for attending meetings and/or travel from Ipsen, Pfizer, and Recordati, and participated in clinical studies from Crinetics Pharmaceuticals, Novartis, and Strongbridge Biopharma; in addition, personal honoraria were received from Recordati. The other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Data sharing
We will consider sharing de-identified, individual participant-level data that underlie the results reported in this article on receipt of a request detailing the study hypothesis and statistical analysis plan. All requests should be sent to the corresponding author. The corresponding author and lead investigators of this study will discuss all requests and make decisions about whether data sharing is appropriate based on the scientific rigour of the proposal. All applicants will be asked to sign a data access agreement.
References
- 1.Reimondo G, Castellano E, Grosso M, Priotto R, Puglisi S, Pia A, et al. Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. The Journal of clinical endocrinology and metabolism. 2020; 105(4). [DOI] [PubMed] [Google Scholar]
- 2.Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. The lancet Diabetes & endocrinology. 2020; 8(11): 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherlock M, Scarsbrook A, Abbas A, Fraser S, Limumpornpetch P, Dineen R, et al. Adrenal Incidentaloma. Endocrine reviews. 2020; 41(6): 775–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. The Journal of clinical endocrinology and metabolism. 2000; 85(2): 637–44. [DOI] [PubMed] [Google Scholar]
- 5.Cawood TJ, Hunt PJ, O’Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? European journal of endocrinology / European Federation of Endocrine Societies. 2009; 161(4): 513–27. [DOI] [PubMed] [Google Scholar]
- 6.Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L, et al. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Annals of internal medicine. 2019; 171(2): 107–16. [DOI] [PubMed] [Google Scholar]
- 7.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European journal of endocrinology / European Federation of Endocrine Societies. 2016; 175(2): G1–G34. [DOI] [PubMed] [Google Scholar]
- 8.Taya M, Paroder V, Bellin E, Haramati LB. The relationship between adrenal incidentalomas and mortality risk. European radiology. 2019; 29(11): 6245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014; 99(12): 4462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. The lancet Diabetes & endocrinology. 2014; 2(5): 396–405. [DOI] [PubMed] [Google Scholar]
- 11.Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine. 2017; 58(2): 267–75. [DOI] [PubMed] [Google Scholar]
- 12.Kjellbom A, Lindgren O, Puvaneswaralingam S, Londahl M, Olsen H. Association Between Mortality and Levels of Autonomous Cortisol Secretion by Adrenal Incidentalomas : A Cohort Study. Annals of internal medicine. 2021; 174(8): 1041–9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang CD, Li D, Kaur RJ, Ebbehoj A, Singh S, Atkinson EJ, et al. Cardiometabolic Outcomes and Mortality in Patients with Adrenal Adenomas in a Population-based Setting. The Journal of clinical endocrinology and metabolism. 2021; 106(11): 3320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2008; 93(5): 1526–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the u.s. Diabetes care. 2006; 29(11): 2415–9. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European heart journal. 2018; 39(33): 3021–104. [DOI] [PubMed] [Google Scholar]
- 17.Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” Adrenal Tumors and the Risk for Incident Diabetes and Cardiovascular Outcomes: A Cohort Study. Annals of internal medicine. 2016; 165(8): 533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prete A, Subramanian A, Bancos I, Chortis V, Tsagarakis S, Lang K, et al. Cardiometabolic Disease Burden and Steroid Excretion in Benign Adrenal Tumors : A Cross-Sectional Multicenter Study. Annals of internal medicine. 2022: epub 2022/01/04. [DOI] [PubMed] [Google Scholar]
- 19.Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vascular health and risk management. 2005; 1(4): 291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohd Azmi NAS, Juliana N, Azmani S, Mohd Effendy N, Abu IF, Mohd Fahmi Teng NI, et al. Cortisol on Circadian Rhythm and Its Effect on Cardiovascular System. International journal of environmental research and public health. 2021; 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pecori Giraldi F, Moro M, Cavagnini F. Gender-related differences in the presentation and course of Cushing’s disease. The Journal of clinical endocrinology and metabolism. 2003; 88(4): 1554–8. [DOI] [PubMed] [Google Scholar]
- 22.Dhaibar HA, Cruz-Topete D. Predisposition of Women to Cardiovascular Diseases: A Side-Effect of Increased Glucocorticoid Signaling During the COVID-19 Pandemic? Frontiers in global women’s health. 2021; 2: 606833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Ba Y, Cai RC, Xing Q. Association between diabetes mellitus and the risk for major cardiovascular outcomes and all-cause mortality in women compared with men: a meta-analysis of prospective cohort studies. BMJ open. 2019; 9(7): e024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansour O, Golden SH, Yeh HC. Disparities in mortality among adults with and without diabetes by sex and race. Journal of diabetes and its complications. 2020; 34(3): 107496. [DOI] [PubMed] [Google Scholar]
- 25.Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. The lancet Diabetes & endocrinology. 2016; 4(7): 611–29. [DOI] [PubMed] [Google Scholar]
- 26.Yiallouris A, Tsioutis C, Agapidaki E, Zafeiri M, Agouridis AP, Ntourakis D, et al. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front Endocrinol (Lausanne). 2019; 10: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinkler M, Zehnder D, Lepenies J, Petrelli MD, Moore JS, Hughes SV, et al. Expression of renal 11beta-hydroxysteroid dehydrogenase type 2 is decreased in patients with impaired renal function. European journal of endocrinology / European Federation of Endocrine Societies. 2005; 153(2): 291–9. [DOI] [PubMed] [Google Scholar]
- 28.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013; 138(1): 103–41. [DOI] [PubMed] [Google Scholar]
- 29.Vogg N, Kurlbaum M, Deutschbein T, Grasl B, Fassnacht M, Kroiss M. Method-Specific Cortisol and Dexamethasone Thresholds Increase Clinical Specificity of the Dexamethasone Suppression Test for Cushing Syndrome. Clin Chem. 2021; 67(7): 998–1007. [DOI] [PubMed] [Google Scholar]
- 30.Terzolo M, Reimondo G. Insights on the Natural History of Adrenal Incidentalomas. Annals of internal medicine. 2019; 171(2): 135–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We will consider sharing de-identified, individual participant-level data that underlie the results reported in this article on receipt of a request detailing the study hypothesis and statistical analysis plan. All requests should be sent to the corresponding author. The corresponding author and lead investigators of this study will discuss all requests and make decisions about whether data sharing is appropriate based on the scientific rigour of the proposal. All applicants will be asked to sign a data access agreement.


