Abstract
In consideration of the problems related to food safety, environmental pollution, and the spread of infected diseases nowadays, we urgently need testing methods that can be easily performed by common people. Smartphone-based detections are promising for general applications. However, some of these analytical strategies require a combination of accessories and instruments, such as portable electrochemical workstations, mini multi-mode microplate readers, and complex temperature control devices, etc., which are small but still expensive. Herein, we comprehensively introduce a free app (Spotxel® Reader) that can provide accurate data analysis for microplate or parallel-format test sensors without an instrument. By simulating the optical signal of the test samples through a smartphone, the sensing results can be obtained for free. We discuss the detection strategies involved in the reported smartphone-based analyses using Spotxel® Reader. Prospects for the development of this free app for future detection applications are presented. This review aims to popularize free analysis software, so that ordinary people may realize convenient tests.
Graphical abstract
Keywords: Spotxel® Reader, Instrument-free, Wide available, Smartphone
Introduction
Many hazards may threaten human health these days. For instance, the recently emerged SARS-CoV-2 that causes COVID-19, seriously influence people’s life [1]; The food contaminants such as aflatoxin are often found in cereals and has a high risk to cause cancer [2, 3]; Some microbial such as Helicobacter pylori may be infected by sharing food and seriously damage people’s stomach [4]. These toxic, harmful, or over-expressed substances may threaten people’s health, but people cannot know their safety without detection. If the detections required professional and expensive testing methods, enough tests may not be performed, since people are tired of complicated analyses. On the other hand, the development of inexpensive and convenient methods that are preferred by the public will make an important contribution to the choices of more detections, which in turn prevent harm to attack people’s health [5].
Recently, smartphone-based detection methods have been developed, which have a great opportunity to be adopted by normal people due to their convenience [6–8]. For instance, an analyte may cause a color or fluorescence change in a sensor/probe [9–11]. These sensors can be developed by test chips, which may show a visible color change in the presence of a certain amount of the analyte [12–14]. The naked eye alone can only roughly judge color change, but cannot accurately evaluate the concentration of the analyte [15–17]. On the other hand, the smartphone can photograph this optical signal change, and then use appropriate software to transmit the specific value of the signal, and calculate the concentration of the target [18, 19]. To achieve low-cost analysis, optical signal analysis software including Color picker [20–22], ImageJ [23–25], Matlab [21, 26–28], etc., have been used for the determination of different analytes in combination with a smartphone. For instance, the Color picker can convert an image obtained from a smartphone to RGB information. This information has to be converted into an optical signal by the researchers using a defined formula. Then, the data can be further correlated with the concentration of the analyte. In addition, this method can only analyze one image at a time, so it needs further simplification and optimization for sample analysis. ImageJ supports image processing, such as logical and arithmetical operations, convolution, contrast manipulation, Fourier analysis, and edge detection. This program supports the analysis of several images simultaneously. However, to establish the connection between the analyte and the image from the smartphone, the researcher needs to establish a formula to obtain the concentration information of the analyte, which is still difficult for ordinary users. Matlab can be used to analyze sensing data, which allows numeric computing, matrix manipulations, plotting of functions and data, and implementation of algorithms with a specific written program. Thus, it is relatively complicated and requires some professional training [26, 29]. Some other smartphone-based detections can be performed at the point of care and obtain the data directly using mature programs, but expensive instruments such as Sensit smart are involved [30–32].
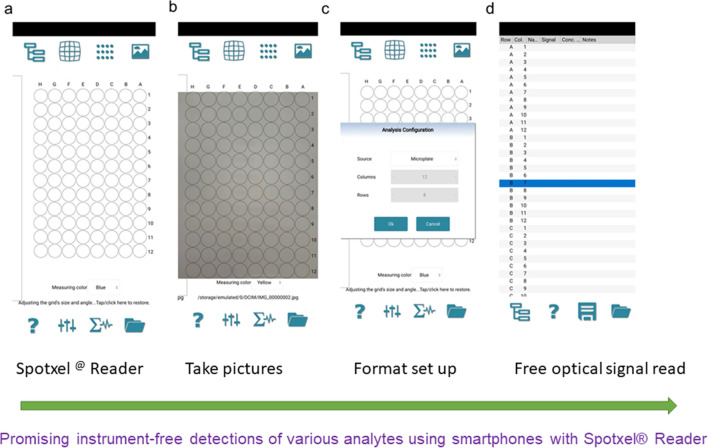
Spotxel® Reader is a free smartphone app that can read the color or fluorescence intensity of multiple samples in microplate wells and other samplers with plate formats (Fig. 1). It can also analyze images in rows, columns, or microarray formats. When people use Spotxel® Reader software to analyze the test objects, they no longer need to create formulas or further analysis. The premium version of this app enables the simulation of the assay’s standard curves and shows the unknown samples’ concentrations directly. This software mainly analyzes the intensity of optical signals through four channels including blue, yellow, purple, and brightness. Since its development in 2017, the Spotxel® Reader has been used by many researchers for the rapid and inexpensive detection of several analytes including environmental pollutants [33, 34], microbial [35], disease-related biomarkers [36–38], etc. The detained mechanism, detection limit, and analysis accuracy are shown in Table 1. However, there is still limited research on using this app to enable wide-available tests for common people. Herein, we introduce in detail the reported smartphone-based detection strategy using Spotxel® Reader to analyze different analytes. The related mechanisms of different strategies are systematically discussed. A prospect on the new applications of using a free app for future detections is also proposed. This review is aiming to enable common people to benefit from the free apps for cost-effective tests, which may guard people’s health against the attack of various hazards at early stages.
Fig. 1.
Spotxel® Reader app: bare interface (a), camera interface (b), analysis array design interface (c), and its optical signal intensity result display (d)
Table 1.
Simple analysis of various analytes based on Smartphones with Spotxel® Reader
| Probe | Mechanisms | Detection limit; accuracy | Analytes | Equipment | Reader | Ref. |
|---|---|---|---|---|---|---|
| m-CDs@SiO2 | The probe exhibited fluorescence changes on interaction with pyrethroids under UV irradiation | 0.048 μg/L; 87.93–101.4% | λ-Cyhalothrin | A portable UV-integrated box with a 365 nm light source |
Smartphone with Spotxel® Reader, Version 1.5.5 |
[33] |
| RCDs | The fluorescence of RCDs-based nanomaterials was quenched by interactions with λ-Cyhalothrin | 6.66 μg/L; 88–104% | λ-Cyhalothrin | A portable ultraviolet light box |
Smartphone with Spotxel® Reader, Version 1.5.5 |
[34] |
| RT-LAMP Assay | Primer recognition of the target genome of SARS-CoV-2 leads to a colorimetric reaction | Sensitivity: 81%; Specificity: 83%; Accuracy: 75–86% | SARS-CoV-2 | 65 °C incubator | Spotxel® Reader: Germersheim, Germany, 2017 | [35] |
| DTA-functionalized Tp | DTA-functionalized Tp showed chiral recognition with fluorescence change | 13 ng mL−1; 86.00–118.33% | Chiral amino acids | None | Spotxel® Reader (version: not mentioned) | [36] |
| TPTA-assembled GQDs | Chiral TPTA-modified GQDs showed fluorescence change with the recognition of D‑phenylalanine | 0.050 μM; 86.20–110.0% | D-Phe | A dark UV analyzer with 365 nm irradiation light | Spotxel® Reader (version: not mentioned) | [37] |
| Humanized organ-on-a-chip model | The model showed synergistic paracrine signaling from sympathetic neurons | Not mentioned | Neuro-breast cancer crosstalk |
G-Series Human Bone Metabolism Array 1000 (RayBiotech) |
Spotxel® software (Version 2.2.2, SICASYS Software GmbH) | [38] |
m-CDs@SiO2 silica nanoparticles embedded with carbon dots (CDs) based on m-phenylenediaminem, RCDs red-emission carbon dots; ( +)-diacetyl-L-tartaric anhydride-functionalized 1,3,5-triformylphloroglucinol, TPTA triformylphloroglucinol-functionalized chiral ( +)-diacetyl-L-tartaric anhydride, GQDs graphene quantum dots, D-Phe D-phenylalanine
Evaluation of food safety
Aflatoxin B1 (AFB1) is a carcinogen that naturally exists in food, especially spoiled grains [39–41]. There are many test devices for detecting ABF1 on the market based on the observation of color or fluorescence change [42]. However, the sensitivity of most naked eye-based tests can not accurately detect AFB1 in food at relatively low concentrations, though the amount may already be toxic. Some bioreceptors such as antibodies and aptamers can recognize AFB1, but the price is mostly considerable [43–45]. Molecularly Imprinted (MIP) membranes have synthetic binding sites, which can mimic natural bioreceptors to recognize ABF1 [46]. Meanwhile, these synthesized membranes are cost-effective to meet large-scale productions. Sergeyeva et al. developed a smartphone-based fluorescent sensor using MIP membranes (Fig. 2a) [47]. Two functional monomers [2-acrylamido-2-methyl-1-propansulfonic acid (AMPSA) and acrylamide (AA)] have binding sites to interact with AFB1 selectively. Although the authors did not mention the detailed binding sites, b2 and b3 of AMPSA and AA from the selected molecules containing amide-conjugated alkene groups might recognize b1 of ABF1 based on Michael reaction (Fig. 2b). With UV irradiation and the interaction of MIP membranes with the samples, the fluorescence changes as a function of the concentration of the target. This signal can be read by a smartphone with Spotxel® Reader, which calculates the concentration of AFB1 subsequently. The detection limit of ABF1 was 20 ng mL−1. By analysis of different extracts of the aflatoxin B1-free wheat and maize flour spiked with AFB1 of 10 ng mL−1, 50 ng mL−1, 80 ng mL−1, and 100 ng mL−1, 80–94%, 83–109%, 78–114%, and 88–104% recoveries were obtained. This method facilitates a cost-effective and simple approach to food safety evaluation.
Fig. 2.
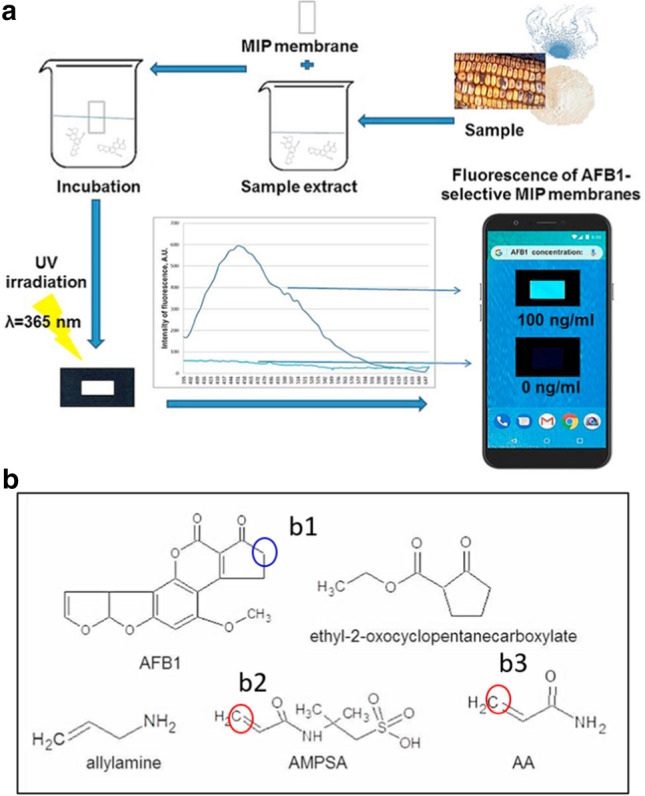
Evaluation of food safety using smartphone with Spotxel® Reader based on MIP sensing: a Scheme for the detection of AFB1 using an MIP-membrane-based smartphone sensor. Reproduced with permission from [47] by Elsevier. b the structure of AFB1 and several molecule candidates for recognizing ABFB1; the possible binding sites were marked based on Michael reaction
Fumonisin B1 (FB1) is a carcinogenic mycotoxin that is present in food and can cause health threats to animals or humans [48–50]. Yu et al. developed a colorimetric assay for the detection of FB1 (Fig. 3) [51]. Initially, AuNP@MnO2 nanoparticles were synthesized from KMnO4 and 13 nm AuNPs (Fig. 3A). Alkaline phosphatase (ALP) labeled goat anti-mouse IgG interacted with the target through the link of FB1 monoclonal antibody (McAb) (Fig. 3B). FB1-BSA was coated on the microplate and competes with FB1 to bind the McAb [Fig. 3B(a, b)], which further captured ALP-IgG [Fig. 3B(c)]. This conjugate hydrolyzed ascorbic acid 2-phosphate (AAP) and generated ascorbic acid (AA), which reduced the MnO2 shell to Mn2+ [Fig. 3B(d, e)]. The released Mn2+ induced the aggregation of gold nanoparticles (Au NPs), resulting in a color change of the colloid that can be read by the smartphone with Spotxel® reader [Fig. 3B(f)]. The method has a detection limit of 0.15 ng/mL and a recovery rate of 86.4–110.9% for FB1 in the maize flour samples.
Fig. 3.
Evaluation of food safety using smartphone with Spotxel® Reader based on an antibody-modified nanosensor: A schematic of synthesis of AuNP@MnO2. B Mn2+-mediated aggregation of the colloid. a coating of FB1-BSA on the well, b Competitive reaction, c ALP-IgG interacted with McAb, d the hydrolysis of AAP by ALP, e ascorbic acid induced the generation of Mn2+ causing aggregation of AuNPs. f Spotxel® Reader analysis. Reproduced with permission from [51] by Elsevier
Evaluation of food nutrition
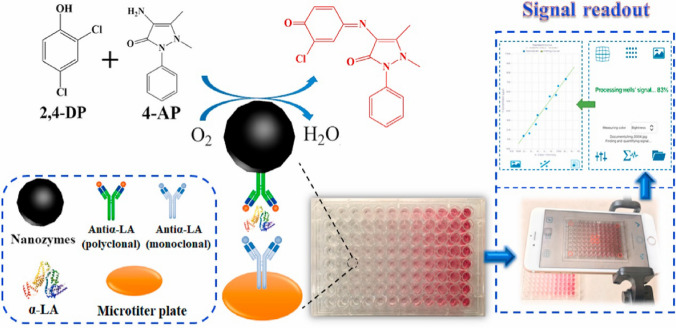
α-Lactalbumin (α-LA) is a component of the milk proteins and may possess some important functions of immunologic defense, which may induce apoptosis in lymphoid cell lines [52]. Zhang et al. employed a facile hydrothermal method and synthesized laccase mimics (named LM nanozymes) using glutathione (GSH) and copper (II) chloride as precursors [53]. LM nanozymes catalyze the oxidative coupling reaction between 4-aminoantipyrine (4-AP) and 2,4-dichlorophenol (2,4-DP) resulting in the production of a red product. This phenomenon was used for the ELISA analysis of alpha-lactalbumin (α-LA) (Fig. 4). LM nanozymes were modified by target antibodies that recognize α-LA and show color change by catalyzing the substrate in the presence of 4-AP and 2,4-DP. A smartphone with Spotxel® Reader detected the signal change and showed a detection limit of 0.056 ng/mL with high specificity for α-LA in food samples. The method was accurate as the recovery of α-LA from spiked raw milk, UHT milk, yogurt, candy, EHF, and PHF were 100.04%, 101.17%, 99.24%, 97.64%, 96.51%, and 99.48%, respectively. These strategies may enable the development of an inexpensive method for food monitoring for public use.
Fig. 4.
Evaluation of food nutrition using smartphone with Spotxel® Reader based on antibody-modified nanozymes: schematic illustration of the integration system of nanozyme-based immunoassay with a smartphone for the detection of α-LA. Reproduced with permission from [53] by Elsevier
Detection of pesticides
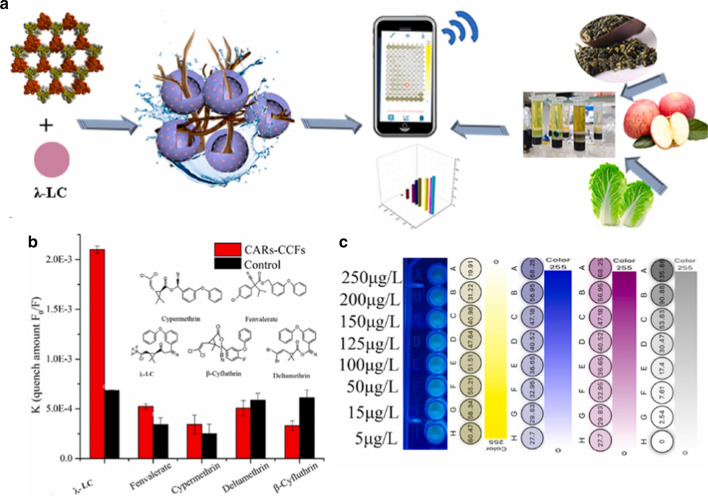
Pyrethroids are commercial pesticides that are toxic to insects, such as dragonflies, bees, gadflies, mayflies, and some other invertebrates [54–56]. The presence of pyrethroids in the aquatic environment may be toxic to fish and other organisms [57–59]. They also threaten people’s health indirectly. Jiang et al. developed a smartphone-based fluorescence detection method for the analysis of pyrethroids (Fig. 5). The artificial receptors inside a covalent Carbazole (CAR)-conjugated framework (CCFs) with strong fluorescence were used to recognize pyrethroids, such as λ-cyhalothrin (LC). The fluorescence changes as a function of the target under UV excitation at 365 nm. The smartphone with Spotxel® Reader detected the change and calculated the concentration of LC with a detection limit of 4.067 μg L−1. A recovery ratio of 88–103% was found for LC in food samples, which indicated the current method could accurately determine this pesticide.
Fig. 5.
Evaluation of pesticides using smartphone with Spotxel® Reader based on artificial receptors inside a CCFs sensor: scheme for the fabrication of the CARs-CCFs and fluorescence sensing of pyrethroids using artificial receptors bound inside a covalent organic framework by smartphone-based analysis; b the quenching constant of the CARs-CCFs and control group for LC and its analogs; c Spotxel® Reader software processing results. Reproduced with permission from [60] by Elsevier
Zhu et al. synthesized red fluorescent carbon dots (RCDs) and developed molecular imprinting technology to detect pyrethroids, i.e., Lambda-cyhalothrin (LC) [33]. The –NH2 groups on the surface of the RCDs interacted with LC, allowing the detection of pyrethroids in the concentration range of 1–120 μg/L) with a detection limit of 0.89 μg/L using a Synergy H1 microplate reader. Furthermore, a portable UV light box was used to induce fluorescence of the RCDs-based sensor, the smartphone with Spotxel® Reader facilitate the sensitive detection of LC without an instrument and the detection limit was 6.66 μg/L. 90–99% recovery rates were obtained for analysis of pyrethroids from tea samples.
Detection of SARS-CoV-2
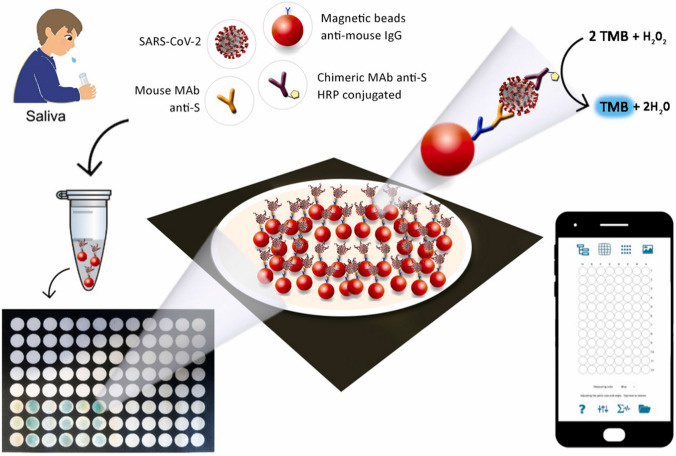
Due to the continued spread of COVID-19, there is a growing need for effective testing of the disease-causing virus, i.e., SARS-CoV-2 [61–63]. Various analysis strategies such as the detection of nucleic acid [64], antibodies [65], and antigens of SARS-CoV-2 have been used to evaluate the infection of COVID-19 patients [66]. However, most methods are relatively expensive and complicated. The applications of these tests are still limited. Fabiani et al. applied magnetic beads to support the immunological chain for developing a paper-based immunoassay for analysis of SARS-CoV-2 antigen (spiked protein). The color change was observed on a wax-printed 96-well paper plate (Fig. 6) based on the catalysis of TMB to ox-TMB [67]. A smartphone with Spotxel® Reader can read the signal can calculate the concentration of SARS-CoV-2 antigen with a detection limit of 0.1 μg/mL. Compared to the gold standard PCR tests for analysis of the nasopharyngeal swab specimens samples, 100% agreement was found for 12 saliva samples. This approach brings new hope for the popularity of virus self-testing.
Fig. 6.
Evaluation of SARS-CoV-2 using smartphone with Spotxel® Reader based on an Immuno nanosensor: scheme of the analysis of SARS-CoV-2 antigen using smartphone with Spotxel® Reader. Reproduced with permission from [67] by Elsevier
Prospect and conclusions
The detection strategies based on smartphones combined with Spotxel® Reader show prospects for instrument-free analysis in the fields of food safety evaluation, environmental pollutant detection, microbial infections, and disease diagnosis. Further development of strategies associated with it is likely to bring new hope for mass detections by common people. To facilitate the development of portable, inexpensive, and simple detection methods, we propose the following outlook.
At present, Spotxel® Reader mainly obtains the concentration of an analyte based on the analysis of color and fluorescence change of the reacted samples, and both of these signals are prone to have background interferences. The reported works have not studied in depth how to avoid the influence of background. Some simple devices with the necessary setup are expected to reduce the interferences and obtain more actuate results.
Spotxel® Reader currently cannot detect some signals with low interferences, such as near-infrared fluorescence. In this case, the near-infrared fluorescence is expected to transfer to the visible optical signal that can be directly analyzed. For instance, combined with nano-upconversion technology, near-infrared light may induce the generation of visible light for the analysis of some targets, so that the tests can be performed. However, to maintain the sensitivity of a test after a series of signal transformations, the high energy-transfer efficiency of the system is expected to be fabricated.
Finally, we also expect more free software to analyze different signals of the sensors in combination with smartphones, bringing more feasibility for ordinary people to detect analytes by themselves.
Acknowledgements
We acknowledge Jinzhou Medical University for its financial support.
Funding
This work is supported by University Student Innovation Project (grant number 10160101).
Data Availability Statement
Data sharing is not applicable to this review article as no new data were created in this study.
Declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Ningyi Qin and Zirui Liu have contributed equally to this work.
Contributor Information
Xifan Mei, Email: meixifan@jzmu.edu.cn.
Dan Li, Email: danli@jzmu.edu.cn.
References
- 1.Ye Q, Shao W, Meng H. Performance and application evaluation of SARS-CoV-2 antigen assay. J Med Virol. 2022;94:3548–3553. doi: 10.1002/jmv.27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharafi K, Matin BK, Omer AK, Mansouri B, Soleimani H, Fattahi N, Sharafi H, Kiani A. A worldwide systematic literature review for aflatoxin M1 in infant formula milk: human health risk assessment by Monte Carlo simulation. Food Control. 2022 doi: 10.1016/j.foodcont.2021.108681. [DOI] [Google Scholar]
- 3.Li B, Zhang Y, Ren X, Ma H, Wu D, Wei Q. No-wash point-of-care biosensing assay for rapid and sensitive detection of aflatoxin B1. Talanta. 2021;235:122772. doi: 10.1016/j.talanta.2021.122772. [DOI] [PubMed] [Google Scholar]
- 4.Yuan C, Adeloye D, Luk TT, Huang L, He Y, Xu Y, Ye X, Yi Q, Song P, Rudan I. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolescent Health. 2022;6:185–194. doi: 10.1016/S2352-4642(21)00400-4. [DOI] [PubMed] [Google Scholar]
- 5.Jean S, Burnham CD, Chapin K, Garner OB, Pant Pai N, Turabelidze G, Butler-Wu S. At-home testing for infectious diseases: the laboratory where you Live. Clin Chem. 2021;68:19–26. doi: 10.1093/clinchem/hvab198. [DOI] [PubMed] [Google Scholar]
- 6.Yang T, Luo Z, Bewal T, Li L, Xu Y, Mahdi Jafari S, Lin X. When smartphone enters food safety: a review in on-site analysis for foodborne pathogens using smartphone-assisted biosensors. Food Chem. 2022;394:133534. doi: 10.1016/j.foodchem.2022.133534. [DOI] [PubMed] [Google Scholar]
- 7.Hao Y, Yang Z, Dong W, Liu Y, Song S, Hu Q, Shuang S, Dong C, Gong X. Intelligently design primary aromatic amines derived carbon dots for optical dual-mode and smartphone imaging detection of nitrite based on specific diazo coupling. J Hazard Mater. 2022;430:128393. doi: 10.1016/j.jhazmat.2022.128393. [DOI] [PubMed] [Google Scholar]
- 8.Placer L, Lavilla I, Pena-Pereira F, Bendicho C. Bromine speciation by a paper-based sensor integrated with a citric acid/cysteamine fluorescent probe and smartphone detection. Sens Actuators B Chem. 2022 doi: 10.1016/j.snb.2022.131499. [DOI] [Google Scholar]
- 9.Lu Z, Chen M, Li M, Liu T, Sun M, Wu C, Su G, Yin J, Wu M, Zou P, Lin L, Wang X, Huang Q, Yin H, Rao H, Zhou X, Ye J, Wang Y. Smartphone-integrated multi-color ratiometric fluorescence portable optical device based on deep learning for visual monitoring of Cu2+ and thiram. Chem Eng J. 2022 doi: 10.1016/j.cej.2022.135686. [DOI] [Google Scholar]
- 10.Çifteci A, Çelik SE, Apak R. Gold-nanoparticle based turn–on fluorometric sensor for quantification of sulfhydryl and disulfide forms of biothiols: measurement of thiol/disulfide homeostasis. Analyt Lett. 2021;55:648–664. doi: 10.1080/00032719.2021.1958830. [DOI] [Google Scholar]
- 11.Jiang R, Lin D, Zhang Q, Li L, Yang L. Multiplex chroma-response based fluorescent smartphone sensing platform for rapid and visual quantitative determination of antibiotic residues. Sens Actuators B Chem. 2022;350:130902. doi: 10.1016/j.snb.2021.130902. [DOI] [Google Scholar]
- 12.Hunt JP, Zhao EL, Free TJ, Soltani M, Warr CA, Benedict AB, Takahashi MK, Griffitts JS, Pitt WG, Bundy BC. Towards detection of SARS-CoV-2 RNA in human saliva: a paper-based cell-free toehold switch biosensor with a visual bioluminescent output. N Biotechnol. 2022;66:53–60. doi: 10.1016/j.nbt.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Cui M, Zhang Y, Jiang L, Liu H, Liu Z. A colorimetric aptasensing assay with adjustable color mutation points for threshold-readout detection of carcinoembryonic antigen. Sens Actuators B Chem. 2022;350:130857. doi: 10.1016/j.snb.2021.130857. [DOI] [Google Scholar]
- 14.Hou J, Jia P, Yang K, Bu T, Zhao S, Li L, Wang L. Fluorescence and colorimetric dual-mode ratiometric sensor based on Zr-tetraphenylporphyrin tetrasulfonic acid hydrate metal-organic frameworks for visual detection of copper ions. ACS Appl Mater Interfaces. 2022;14:13848–13857. doi: 10.1021/acsami.1c23199. [DOI] [PubMed] [Google Scholar]
- 15.Sun BR, Zhou AG, Li X, Yu HZ. Development and application of mobile apps for molecular sensing: a review. ACS Sens. 2021;6:1731–1744. doi: 10.1021/acssensors.1c00512. [DOI] [PubMed] [Google Scholar]
- 16.Franca AS, Oliveira LS. Smartphone-based detection devices. Elsevier; 2021. pp. 249–268. [Google Scholar]
- 17.Junaid HM, Solangi AR, Batool M. Carbon dots as naked eye sensors. Analyst. 2021;146:2463–2474. doi: 10.1039/D0AN02399J. [DOI] [PubMed] [Google Scholar]
- 18.Sivakumar R, Lee NY. Recent progress in smartphone-based techniques for food safety and the detection of heavy metal ions in environmental water. Chemosphere. 2021;275:130096. doi: 10.1016/j.chemosphere.2021.130096. [DOI] [PubMed] [Google Scholar]
- 19.Chellasamy G, Ankireddy SR, Lee KN, Govindaraju S, Yun K. Smartphone-integrated colorimetric sensor array-based reader system and fluorometric detection of dopamine in male and female geriatric plasma by bluish-green fluorescent carbon quantum dots. Mater Today Bio. 2021;12:100168. doi: 10.1016/j.mtbio.2021.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Jian J, Sun B, Wei Y, Pan D, Cao J, Shen Y. Engineering of onsite point-of-care testing of Fe3+ with visual ratiometric fluorescent signals of copper nanoclusters-driven portable smartphone. Sens Actuators B Chem. 2022;370:132413. doi: 10.1016/j.snb.2022.132413. [DOI] [Google Scholar]
- 21.Tarasi S, Ramazani A, Morsali A, Hu ML. Highly sensitive colorimetric naked-eye detection of Hg(II) using a sacrificial metal-organic framework. Inorg Chem. 2021;60:13588–13595. doi: 10.1021/acs.inorgchem.1c01894. [DOI] [PubMed] [Google Scholar]
- 22.Yadav P, Laddha H, Agarwal M, Gupta R. Fun with smartphones: handy solution for quantification of debilitating fluoride ions in drinking water. J Cheml Educ. 2022;99:2677–2683. doi: 10.1021/acs.jchemed.2c00216. [DOI] [Google Scholar]
- 23.de Carvalho Oliveira G, Machado CCS, Inacio DK, Silveira Petruci JFD, Silva SG. RGB color sensor for colorimetric determinations: evaluation and quantitative analysis of colored liquid samples. Talanta. 2022;241:123244. doi: 10.1016/j.talanta.2022.123244. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen LH, Oveissi F, Chandrawati R, Dehghani F, Naficy S. Naked-eye detection of ethylene using thiol-functionalized polydiacetylene-based flexible sensors. ACS Sens. 2020;5:1921–1928. doi: 10.1021/acssensors.0c00117. [DOI] [PubMed] [Google Scholar]
- 25.Pinyorospathum C, Rattanarat P, Chaiyo S, Siangproh W, Chailapakul O. Colorimetric sensor for determination of phosphate ions using anti-aggregation of 2-mercaptoethanesulfonate-modified silver nanoplates and europium ions. Sens Actuators B Chem. 2019;290:226–232. doi: 10.1016/j.snb.2019.03.059. [DOI] [Google Scholar]
- 26.Fan K, Zeng J, Yang C, Wang G, Lian K, Zhou X, Deng Y, Liu G. Digital quantification method for sensitive point-of-care detection of salivary uric acid using smartphone-assisted muPADs. ACS Sens. 2022;7:2049–2057. doi: 10.1021/acssensors.2c00854. [DOI] [PubMed] [Google Scholar]
- 27.Caroleo F, Magna G, Damiano C, Cavalleri M, Gallo E, Di Natale C, Paolesse R. Colour Catcher® sheet beyond the laundry: a low-cost support for realizing porphyrin-based mercury ion sensors. Sens Actuators B Chem. 2022 doi: 10.1016/j.snb.2022.131900. [DOI] [Google Scholar]
- 28.Adeniyi O, Mashazi P. Kirigami paper-based colorimetric immunosensor integrating smartphone readout for determination of humoral autoantibody immune response. Microchem J. 2022;178:107427. doi: 10.1016/j.microc.2022.107427. [DOI] [Google Scholar]
- 29.Heinonen R, Mattila TJ. Smartphone-based estimation of green cover depends on the camera used. Agronomy J. 2021;113:5597–5601. doi: 10.1002/agj2.20752. [DOI] [Google Scholar]
- 30.Nelis JLD, Migliorelli D, Muhlebach L, Generelli S, Stewart L, Elliott CT, Campbell K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta. 2021;228:122215. doi: 10.1016/j.talanta.2021.122215. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Lillehoj PB. Microfluidic magneto immunosensor for rapid, high sensitivity measurements of SARS-CoV-2 nucleocapsid protein in serum. ACS Sens. 2021;6:1270–1278. doi: 10.1021/acssensors.0c02561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasquez V, Navas MC, Jaimes JA, Orozco J. SARS-CoV-2 electrochemical immunosensor based on the spike-ACE2 complex. Anal Chim Acta. 2022;1205:339718. doi: 10.1016/j.aca.2022.339718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Han L, Liu H, Sun B. A smartphone-based ratiometric fluorescent sensing system for on-site detection of pyrethroids by using blue-green dual-emission carbon dots. Food Chem. 2022;379:132154. doi: 10.1016/j.foodchem.2022.132154. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X, Yuan X, Han L, Liu H, Sun B. A smartphone-integrated optosensing platform based on red-emission carbon dots for real-time detection of pyrethroids. Biosens Bioelectron. 2021;191:113460. doi: 10.1016/j.bios.2021.113460. [DOI] [PubMed] [Google Scholar]
- 35.Londono-Avendano MA, Libreros G, Osorio L, Parra B. A rapid RT-LAMP assay for SARS-CoV-2 with colorimetric detection assisted by a mobile application. Diagnostics. 2022;12:848. doi: 10.3390/diagnostics12040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Yuan X, Jiang W, Liu H, Sun B. Chiroptical-responsive nanoprobe for the optosensing of chiral amino acids. Microchim Acta. 2022;189:184. doi: 10.1007/s00604-022-05282-w. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Zhang Y, Liu H, Sun B. A visual chiroptical system with chiral assembly graphene quantum dots for D-phenylalanine detection. Anal Bioanal Chem. 2022;414:4885–4896. doi: 10.1007/s00216-022-04113-4. [DOI] [PubMed] [Google Scholar]
- 38.Conceicao F, Sousa DM, Loessberg-Zahl J, Vollertsen AR, Neto E, Soe K, Paredes J, Leferink A, Lamghari M. A metastasis-on-a-chip approach to explore the sympathetic modulation of breast cancer bone metastasis. Mater Today Bio. 2022;13:100219. doi: 10.1016/j.mtbio.2022.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao L, Liu H, Yao L, Wen W, Chen M-M, Zhang X, Wang S. Construction of a dual-functional CuO/BiOCl heterojunction for high-efficiently photoelectrochemical biosensing and photoelectrocatalytic degradation of aflatoxin B1. Chem Eng J. 2022 doi: 10.1016/j.cej.2021.132297. [DOI] [Google Scholar]
- 40.Li Y, Sun L, Yu H, Zhao Q. Sensitive competitive aptamer surface plasmon resonance sensor for aflatoxin b1 using streptavidin as a signal enhancer. ACS Food Sci Technol. 2022;2:936–940. doi: 10.1021/acsfoodscitech.2c00079. [DOI] [Google Scholar]
- 41.Li J, Wang Q, Xiong C, Deng Q, Zhang X, Wang S, Chen MM. An ultrasensitive CH3NH3PbBr 3 quantum dots@SiO2-based electrochemiluminescence sensing platform using an organic electrolyte for aflatoxin B1 detection in corn oil. Food Chem. 2022;390:133200. doi: 10.1016/j.foodchem.2022.133200. [DOI] [PubMed] [Google Scholar]
- 42.Singh AK, Sri S, Garimella L, Dhiman TK, Sen S, Solanki PR. Graphene quantum dot-based optical sensing platform for aflatoxin B1 detection via the resonance energy transfer phenomenon. ACS Appl Bio Mater. 2022;5:1179–1186. doi: 10.1021/acsabm.1c01224. [DOI] [PubMed] [Google Scholar]
- 43.Mao X, Yu B, Li Z, Li Z, Shi G. Comparison of lateral flow immunoassays based on oriented and nonoriented immobilization of antibodies for the detection of aflatoxin B1. Anal Chim Acta. 2022;1221:340135. doi: 10.1016/j.aca.2022.340135. [DOI] [PubMed] [Google Scholar]
- 44.Charernchai S, Chikae M, Phan TT, Wonsawat W, Hirose D, Takamura Y. Automated paper-based femtogram sensing device for competitive enzyme-linked immunosorbent assay of aflatoxin B1 using submicroliter samples. Anal Chem. 2022;94:5099–5105. doi: 10.1021/acs.analchem.1c05401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang C, Wang Y, Sun Y, Duan J, Wang M, Ai S, Hou J. A novel immunocolorimetric probe for aflatoxin B1 based on multifunctional metal−organic frameworks. Sens Actuators B Chem. 2022;369:132362. doi: 10.1016/j.snb.2022.132362. [DOI] [Google Scholar]
- 46.Sergeyeva T, Yarynka D, Dubey L, Dubey I, Piletska E, Linnik R, Antonyuk M, Ternovska T, Brovko O, Piletsky S, El'skaya A. Sensor based on molecularly imprinted polymer membranes and smartphone for detection of fusarium contamination in cereals. Sensors. 2020;20:4304. doi: 10.3390/s20154304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sergeyeva T, Yarynka D, Piletska E, Linnik R, Zaporozhets O, Brovko O, Piletsky S, El'skaya A. Development of a smartphone-based biomimetic sensor for aflatoxin B1 detection using molecularly imprinted polymer membranes. Talanta. 2019;201:204–210. doi: 10.1016/j.talanta.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Zhang Q, Ma F, Li P. Simultaneous determination of aflatoxins, fumonisin B1, T-2 and cyclopiazonic acid in agri-products by immunomagnetic solid-phase extraction coupled with UHPLC-MS/MS. Food Chem. 2022;378:132020. doi: 10.1016/j.foodchem.2021.132020. [DOI] [PubMed] [Google Scholar]
- 49.Feng J, Xue Y, Wang X, Song Q, Wang B, Ren X, Zhang L, Liu Z. Sensitive, simultaneous and quantitative detection of deoxynivalenol and fumonisin B1 in the water environment using lateral flow immunoassay integrated with smartphone. Sci Total Environ. 2022;834:155354. doi: 10.1016/j.scitotenv.2022.155354. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Yu Q, Ouyang H, Zhang R, Li J, Xian R, Wang K, Li X, Cao C. Antagonistic effect of selenium on fumonisin b1 promotes neutrophil extracellular traps formation in chicken neutrophils. J Agric Food Chem. 2022;70:5911–5920. doi: 10.1021/acs.jafc.2c01329. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Li Y, Zhang Q, Zha Y, Lu S, Yang Y, Li P, Zhou Y. Colorimetric immunoassay via smartphone based on Mn2+—mediated aggregation of AuNPs for convenient detection of fumonisin B1. Food Control. 2022 doi: 10.1016/j.foodcont.2021.108481. [DOI] [Google Scholar]
- 52.Shi R, Chen W, Pan F, Zhao P, He Y, Yu R, Fu R, Munkh-Amgalan G, Jiang Z. Characterization of the binding behavior, structure and foaming properties of bovine α-lactalbumin combined with saponin by the multi-spectroscopic and silico approaches. Food Hydrocolloids. 2022;124:107259. doi: 10.1016/j.foodhyd.2021.107259. [DOI] [Google Scholar]
- 53.Zhang X, Wu D, Wu Y, Li G. Bioinspired nanozyme for portable immunoassay of allergenic proteins based on a smartphone. Biosens Bioelectron. 2021;172:112776. doi: 10.1016/j.bios.2020.112776. [DOI] [PubMed] [Google Scholar]
- 54.Andersen HR, David A, Freire C, Fernandez MF, D'Cruz SC, Reina-Perez I, Fini JB, Blaha L. Pyrethroids and developmental neurotoxicity—a critical review of epidemiological studies and supporting mechanistic evidence. Environ Res. 2022;214:113935. doi: 10.1016/j.envres.2022.113935. [DOI] [PubMed] [Google Scholar]
- 55.Singh S, Mukherjee A, Jaiswal DK, de Araujo Pereira AP, Prasad R, Sharma M, Kuhad RC, Shukla AC, Verma JP. Advances and future prospects of pyrethroids: toxicity and microbial degradation. Sci Total Environ. 2022;829:154561. doi: 10.1016/j.scitotenv.2022.154561. [DOI] [PubMed] [Google Scholar]
- 56.Li M, Lv M, Liu T, Du G, Wang Q. Lipid metabolic disorder induced by pyrethroids in nonalcoholic fatty liver disease of Xenopus laevis. Environ Sci Technol. 2022;54:8463–8474. doi: 10.1021/acs.est.2c00516. [DOI] [PubMed] [Google Scholar]
- 57.Xie W, Zhao J, Zhu X, Chen S, Yang X. Pyrethroid bioaccumulation in wild fish linked to geographic distribution and feeding habit. J Hazard Mater. 2022;430:128470. doi: 10.1016/j.jhazmat.2022.128470. [DOI] [PubMed] [Google Scholar]
- 58.El Ayari T, Mhadhbi L, Trigui El Menif N, El Cafsi M. Acute toxicity and teratogenicity of carbaryl (carbamates), tebufenpyrad (pyrazoles), cypermethrin and permethrin (pyrethroids) on the European sea bass (Dicentrarchus labrax L, 1758) early life stages. Environ Sci Pollut Res Int. 2022 doi: 10.1007/s11356-11022-20421-11359. [DOI] [PubMed] [Google Scholar]
- 59.Wongmaneepratip W, Gao X, Yang H. Effect of food processing on reduction and degradation pathway of pyrethroid pesticides in mackerel fillet (Scomberomorus commerson) Food Chem. 2022;384:132523. doi: 10.1016/j.foodchem.2022.132523. [DOI] [PubMed] [Google Scholar]
- 60.Jiang W, Zhao Y, Zhang D, Zhu X, Liu H, Sun B. Efficient and robust dual modes of fluorescence sensing and smartphone readout for the detection of pyrethroids using artificial receptors bound inside a covalent organic framework. Biosens Bioelectron. 2021;194:113582. doi: 10.1016/j.bios.2021.113582. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Duran SSF, Lim WYS, Tan CKI, Cheong WCD, Suwardi A, Loh XJ. SARS-CoV-2 in wastewater: from detection to evaluation. Mater Today Adv. 2022;13:100211. doi: 10.1016/j.mtadv.2022.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravi N, Cortade DL, Ng E, Wang SX. Diagnostics for SARS-CoV-2 detection: a comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens Bioelectron. 2020;165:112454. doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alygizakis N, Markou AN, Rousis NI, Galani A, Avgeris M, Adamopoulos PG, Scorilas A, Lianidou ES, Paraskevis D, Tsiodras S, Tsakris A, Dimopoulos MA, Thomaidis NS. Analytical methodologies for the detection of SARS-CoV-2 in wastewater: protocols and future perspectives. Trends Analyt Chem. 2021;134:116125. doi: 10.1016/j.trac.2020.116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Au WY, Cheung PPH. Diagnostic performances of common nucleic acid tests for SARS-CoV-2 in hospitals and clinics: a systematic review and meta-analysis. The Lancet Microbe. 2021;2:e704–e714. doi: 10.1016/S2666-5247(21)00214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werbel WA, Segev DL. SARS-CoV-2 antibody testing for transplant recipients: a tool to personalize protection versus COVID-19. Am J Transplant. 2022;22:1316–1320. doi: 10.1111/ajt.16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon-Loriere E, Schwartz O. Towards SARS-CoV-2 serotypes? Nat Rev Microbiol. 2022;20:187–188. doi: 10.1038/s41579-022-00708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabiani L, Mazzaracchio V, Moscone D, Fillo S, De Santis R, Monte A, Amatore D, Lista F, Arduini F. Paper-based immunoassay based on 96-well wax-printed paper plate combined with magnetic beads and colorimetric smartphone-assisted measure for reliable detection of SARS-CoV-2 in saliva. Biosens Bioelectron. 2022;200:113909. doi: 10.1016/j.bios.2021.113909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review article as no new data were created in this study.