Abstract
Oral cancer, with an around 50% mortality rate, is one of the most common malignancies world-wide. It is often detected in advanced or terminal stage and has a poor prognosis, although substantial progress in cancer management. Microbiome has become an increasingly recognized factor that may contribute to the cancerous development. Oral microbiological population comprising more than 700 bacterial species, varies since saliva and different habitats of oral cavity. A shift of composition of oral microbiome from usual condition to functional inflammation to pathological state has been discovered amongst patients with premalignant disorders and oral carcinoma, with evidence suggesting the tumor microenvironment (TME) could strongly exacerbate the influence of oral microorganisms. The complex interactions taking place in either cancer formation or progression have been evaluated in several publications, however given their results’ heterogeneity, a review is needed to correctly untangle the potential correlation in this group of pro-carcinogenesis. In this review, we briefly summarize our current knowledge of the role of oral microbiome, focusing on its potential crosstalk with TME in oral squamous cell carcinomas (OSCC) more precisely, and pave the way for manipulating oral microbiome to deal with OSCC in the future.
Key Words: Oral Squamous Cell Carcinoma, Microbiome, Tumor Microenvironment
Introduction
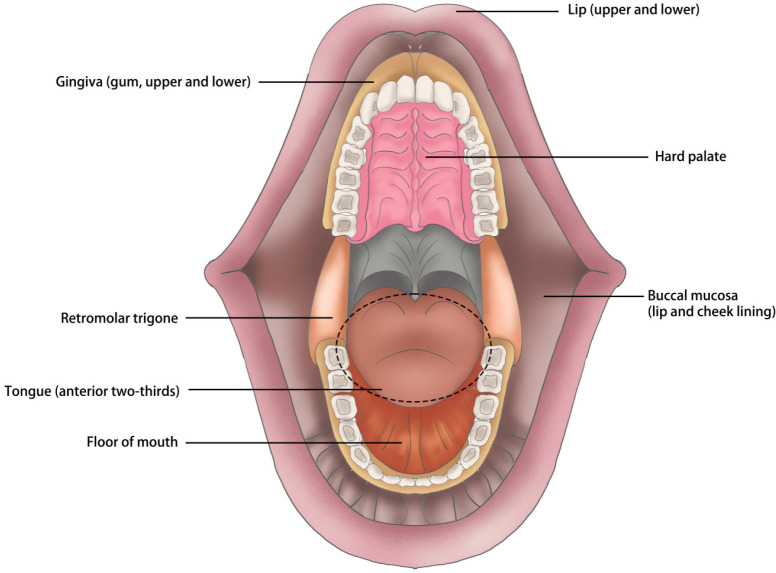
Head and neck cancers are a group of malignancies affecting the mouth, nose, pharynx, larynx, sinuses, and salivary glands (Chow, 2020), with an annual incidence of comprising approximately 600,000 new cases all over the world (Johnson et al., 2020). As the predominant histopathological subtype of epithelial-cell-derived malignant tumors occurred in head and neck, oral squamous cell carcinomas (OSCCs) account for around 90% of all head-neck malignancies (Vigneswaran and Williams, 2014; Jou and Hess, 2017). The major anatomical sites of OSCC embrace anterior tongue, buccal mucosa, lower and upper gum, floor of the mouth, hard palate, retromolar trigone as well as lips (Huang and O’Sullivan, 2017; Almangush et al., 2020) (Figure 1). Due to its high morbidity and mortality (Sung et al., 2021) (377,713 new cases and 177,757 deaths annually) estimated by GLOBOCAN (http://globocan.iarc.fr/), settling the tumorigenesis of OSCC has important clinical value whereby it will help for improving the poor prognosis.
Figure 1.
There is Marked Diversity of Tumors’ Primary Location Arising in the Oral Cavity
Tumor progression is governed by various pro-tumorigenic cytokines and growth factors in the tumor microenvironment (TME) (Hanahan and Weinberg, 2011). For OSCC, TME that mainly functions in its aggravation is created not only through some kinds of stromal cells, but also through malignant epithelial cells. It produces a marked effect in tumor growth and invasiveness coupled with early metastasis, increased recurrences and acquired therapeutic resistance in oral cancer (Naik et al., 2017). In recent decades great headways have made in the field of OSCC research, however, there seems to be a void regarding the role that the oral microbiome may play in OSCC by contrast with the traditional risk factors (e.g. tobacco smoking, alcohol consumption, human papillomavirus (HPV) infection, betel quid intake (Kumar et al., 2016; Alsahafi et al., 2019)). Bad habits of oral hygiene are established to be another risk that acts synergistically to enhance the oral cancer (Hashim et al., 2016; Perera et al., 2018; Chang et al., 2019). It is noteworthy that poor oral hygiene can also significantly determine the microbial habitat and its ecology in oral cavity (Kilian, 2018). Hence, the inner-relationship between oral microbiota and TME could hypothetically trigger the occurrence of OSCC.
Part 1 – Oral microbial distribution in human mouth
Human oral microbiome is defined as all microorganisms along with their genomes in human beings’ oral cavity (Dewhirst et al., 2010). According to the expanded Human Oral Microbiome Database (eHOMD, http://www.homd.org/), more than 770 identified bacterial strains are hosted in the mouth, but herein only 70% species are cultivable in the laboratory, and the remains of 30% can be detected with the existence of whole genome sequences of over 480 taxa (Verma et al., 2018). Oro-maxillofacial region is composed of many spaces, such as frontal sinus, sphenoid sinus, ethmoidal sinus, maxillary sinus, oral cavity, nasal cavity and so on. These structures and constitutes make an ideal niche, wherein the stable habitat of constant temperature (37 Celsius degree) or saliva pH value (6.5~7.5) provided, for the growth whether they are aerobic bacteria or anaerobes. And both aerobes and anaerobic bacteria can prevent themselves from external-environment through the oral biofilms shaping, as well as supply nutrients and keep hydrated by saliva (Takahashi, 2005; Arweiler and Netuschil, 2016). With regard to microflora, the most densely populated habitats can be arisen from the environmental diversity of oral cavity boosting the establishment of distinct microbial communities of physiological/pathological areas as follows, surfaces of teeth, gingival sulcus, supragingival and subgingival plaque, buccal mucosa, hard and soft palates, tonsils, and tongue coating (Aas et al., 2005). In addition, most of them may be found in the saliva attaching to exfoliated human epithelial cells (Dawes, 2003). Host-derived nutrition (e.g. proteins or glycoproteins from gingival crevicular fluid or saliva), moist and warm conditions are responsible for the maintenance of ecological equilibrium within microbial flora (van ‘t Hof et al., 2014).
Oral microorganisms are classified into five categories in line with their oxygen requirements (Rosan and Lamont, 2000; Avila et al., 2009): a. obligate aerobes are isolated only under pathogenic circumstances considering as pathogens, such as progressive periodontitis (Mitchell, 1984); b. microaerophiles (e.g. Actinomyces) which grow best at low O2 concentrations (2%~5%, >10% growth inhibition); c. facultative anaerobes (e.g. Streptococci); d. obligate anaerobe (e.g. Prevotella intermedia, Fusobacteriumnucleatum, Tannerella forsythia, Porphyromonas gingivalis, Treponema denticola) can be detected abnormal increase in patients with dentoalveolar abscesses (Sklavounos et al., 1986), stomatitis (Koopmans et al., 1988), advanced periodontitis (Kobayashi et al., 1990), pericoronitis (Sixou et al., 2003), etc; e. capnophiles (e.g. Aggregatibacter actinomycetemcomitans) which grow best at high concentrations of CO2 (5%~10%), can be detected in juvenile/localized aggressive periodontitis acting as the aetiology of this oral disease (Gholizadeh et al., 2017).
The distribution of bacterial species depends on their specific responses toward different biological zones in the oral cavity (Zarco et al., 2012). The colonization of bacterial species is ensured by a so-called “lock and key” or “ligand-receptor” mechanism holding assorted adhesins (adhesion molecules) (Piatti et al., 1997). Predominant microbial communities in oral cavity and oropharyngeal region are summarized in Tab. 1 (Lim et al., 2017).
Part 2 – An overview of the oral microbiome in OSCC
As the most frequent malignancies in the mouth, OSCCs represent 4.3% of all cancers globally (see the web-based platform of Global Cancer Observatory (GCO)). The radical resection in oral and maxillofacial region is very challenging in front of the subunits of complex anatomical structure and vital physiological functions. Correspondingly, the requirements of both aesthetic recovery and functional reconstruction make a heavy financial burden for the treatment (Huber and Tantiwongkosi, 2014). Additionally, an essential factor in OSCC deaths is the high level of recurrence after treatment (Szturz and Vermorken, 2020). Several studies, which contain practically 1,500 cases, have shown with approximately 30% as the overall recurrence rate (Mücke et al., 2009; Rogers et al., 2009; Wang et al., 2013). It would be of paramount importance that, the recurrence rate could be as high as ranging from 70% to 92% in the first 36 months (Boysen et al., 1992; Schwartz et al., 2000; Braakhuis et al., 2002; Mücke et al., 2009). Besides, the moment in which OSCC recurrence happens usually decides the subsequent 5-year survival status. Concretely, the survival rate drops to between 20.5% and 27.55% if relapse happens within the 18 months after treatment, whereas it might relatively improve to 38.1% to 42.3% if relapse happens afterwards (Liu et al., 2007; Mücke et al., 2009). All in all, given the overall survival rate associated with OSCC has still no substantial changes in the last forty years, despite multitudinous research advances in our knowledge of its causes and risk factors, a brand-new perspective is urgently needed to offer for early diagnostic and therapeutic work. Viruses have long been related with the risk of developing oral carcinomas. First and foremost to be mentioned is human papillomavirus (HPV), with an almost thirty years’ history being widely researched, in which is treated as the aetiological factor in OSCCs (Wittekindt et al., 2018). The oncogenic potential of HPV is well demonstrated in the context of OSCC via several comprehensive meta-analyses founded by about 150 studies addressing HPV and OSCC in human cases, and that has indicated that by the increment of HPV infection the risk of oral cancer adds up to three-fold (Liyanage et al., 2013; Hardefeldt et al., 2014). The prevalence of HPV infection among OSCC patients, estimating via HPV DNA detection in OSCC tumors, is close to 25% (Syrjänen K and Syrjänen S, 2013; Hardefeldt et al., 2014; Petrick et al., 2014). HPV-negative OSCCs occupy the vast majority of oral carcinoma cases nevertheless. Accordingly, the next goal gradually comes into our view.
In recent years, there has been increasing focus on the oral microbiome with the respect to its contribution to tumor development. There are evidences that bacteria have a direct causal association with oncogenesis in some types of cancers, including Salmonella typhi in gallbladder cancer (Di Domenico et al., 2017), Helicobacter pylori in gastric cancer (Amieva and Peek, 2016), though, no exactly distinguishable linkage between the oral microbiome and OSCC has been yet tapped. Tab. 2 presents a couple of studies concerning the effect of bacteria’s presence to cancers of the oral cavity and pharynx (Nagy et al., 1998; Sakamoto et al., 1999; Tateda et al., 2000; Mager et al., 2005; Sasaki et al., 2005; Hooper et al., 2006; Katz et al., 2011; Pushalkar et al., 2012; Guerrero-Preston et al., 2016; Amer et al., 2017; Lee et al., 2017; Wang et al., 2017; Zhao et al., 2017; Yang et al., 2018; Zhang et al., 2019; Bronzato et al., 2020; Guo et al., 2021).
Part 3 – The main immune-related cells of tumor microenvironment (TME)
In pace with a growing recognition that tumor could be a ‘unique organ’ whose complex property approaches to or might even exceed what normal healthy tissues are like, a concept of TME is proposed for the first time (Hanahan and Weinberg, 2011). From this path-breaking perspective, the tumors’ biological phenomena and outbreak regularity can only be understood by studying the individual specialized cell types within TME as well as the behaviors that they manifest during the course of multistep tumorigenesis. Further, since Stephen Paget firstly proposed the hypothesis of ‘seeds (pro-metastatic tumor cells) and soil (the supportive microenvironment in specific organ sites)’ that is essential prerequisite for tumor development and progression (Paget, 1989), this theory of biological importance of TME in fostering cancer recommitted has come to be widely accepted. TME is highly heterogeneous characterized with a principal compartment of host stromal cells as well as its inner extracellular matrix (ECM) which is a collection of fibrous proteins, fibronectins and collagens for example, that is secreted by mesenchymal cells and provides structural and biochemical support to the surrounding cells.
Cancer-associated fibroblasts (CAFs)
CAFs, a type of perpetually activated fibroblasts, are recognized as a prime source of collagen-producing cells. They can directly communicate with the cancer cells and other types of stromal cells (e.g. inflammatory cells and endotheliocytes) (Ishii et al., 2016). Extensive evidences have been implicated to support the premise of the biological importance of CAFs in cancerous progression (Zhang et al., 2017; Li et al., 2018; Qin et al., 2018; Qin et al., 2019; Matos et al., 2020). Nevertheless, CAF different subtypes with heterogeneous biological properties make distinct functional contributions (Tab. 3) (Chang et al., 2002; Orimo and Weinberg, 2007; Radisky et al., 2007; Zeisberg et al., 2007; Potenta et al., 2008; Council and Hameed, 2009; Kalluri and Weinberg, 2009; Spaeth et al., 2009; Wikström et al., 2009; Toullec et al., 2010; Bochet et al., 2013; Jia et al., 2013; Kim et al., 2015; Shiga et al., 2015; Kalluri, 2016; Öhlund et al., 2017; Bartoschek et al., 2018; Gunaydin, 2021; Helms et al., 2021). CAFs secrete a considerable variety of autocrine and paracrine cytokines and other tumor-promoting factors so as to modulate the TME primarily embodied in the aspects of cancer-elicited inflammation, angiogenesis, cancer cell proliferation, invasion, migration, metastasis, therapeutic resistance, etc. CAF-secreted products, including various growth factors, cytokines and chemokines, that impact cancer cells are summarized in Tab. 4 (Custódio et al., 2020).
Tumor-associated macrophages (TAMs)
TAMs indeed are among the most abundant and important tumor-infiltrating immune cell types in TME. Monocytes from blood differentiate into either of two functionally contrasting subtypes, namely classical activated M1 macrophages and alternatively activated M2 macrophages, depending on their interactions with other cells present in tissues (Noy and Pollard, 2014). M1 macrophages are induced by various stimuli, for instance, interferon gamma (IFN-γ), lipopolysaccharide (LPS). They demonstrate pro-inflammatory and microbicidal effects, as well as exert anti-tumor functions of directly regulate cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) to kill tumor cells (Pan et al., 2020; Yunna et al., 2020). M2 macrophages are induced by diverse stimuli such as interleukin (IL)-4, IL-13, prostaglandin E2 (PGE2), transforming growth factor-beta (TGF-β) and they show anti-inflammatory effects. They can promote the occurrence and metastasis of tumor cells, enhance tumor cell invasion, motility and intravasation, stimulate tumor angiogenesis, inhibit T / natural killer (NK) cell-mediated anti-tumor immune response, and lead to tumor progression and prevent tumor cells from chemo- or immuno-therapy (Funes et al., 2018; Pan et al., 2020). M2 macrophages may be further divided into subpopulations such as M2a, M2b, M2c and M2d (Ji et al., 2016; Wang et al., 2019; Pan et al., 2020). The role of TAMs in tumor progress, as well as inducible factors for M1/M2 polarization, and M1/M2 macrophages’ secretory proteins associating with the biological function, are displayed in Tab. 5.
Tumor-associated neutrophils (TANs)
Neutrophils (i.e. also known like polymorphonuclear leukocytes, PMNs) are the most preponderant leukocyte population existing in blood circulation, and are crucial effector cells originated from the innate immune system (Welch et al., 1989). Beside TAMS, another pivotal constitution as infiltrating immune cells engaged into the TME through cytokines and chemokines, is TANs, which can be distinguished in accordance with their activation status and influences on tumor cells growing in N1 (antitumor) or N2 (protumor) TANs (Masucci et al., 2019; Ohms et al., 2020). N2 subtype is characterized by increased expression of angiogenesis enhancing and invasion promoting factors CXCR4, VEGF and MMP-9 with absent IFN-β (Jablonska et al., 2010) and is acquired by neutrophils following the TGF-β treatment (Fridlender et al., 2009). However, neutrophils can revert back to the cytotoxic N1 subtype upon the TGF-β blockade or in the presence of the IFN-β (Andzinski et al., 2016), while expressing high levels of intercellular adhesion molecule 1 (ICAM-1) and tumor necrosis factor-alpha (TNF-α) as well as increasing neutrophil extracellular traps (NETs) formation. Comparative markers and biologic functions of TAN subtypes are listed in Tab. 6.
Natural killer cells (NK cells)
Natural killer cells (NK cells) play a critical role in the innate immune system (known to induce necrotic as well as apoptotic cell death in susceptible targets), since their main function in the organism is the ability to quickly detect and eliminate virally-infected or malignant cells, through releasing granzyme B and perforin regulated by cathepsins and cystatins (Lanier, 2005; Magister et al., 2015). They are characterized as cytotoxic effectors which are capable to recognize and lyse a number of poorly differentiated or even undifferentiated tumors (particularly oral cancer) by exhibiting higher CD44 expression whereas lower levels of CD54, PD-L1, and major histocompatibility complex (MHC) class I (Kozlowska et al., 2017; López-Soto et al., 2017; Jewett et al., 2018; Kaur et al., 2018). NK cells are treated as large granular CD3- lymphocytes that can be classified into two distinct subpopulations, depending on their expression levels of surface markers CD16 and CD56. The most prominent cytokines secreted via NK cells are IFN-γ and TNF-α. However, NK cells have been reported to produce a variety of other important factors, including IL-5, IL-8, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), CCL2, CCL3, CCL4, CCL5 and CXCL10 (Jewett et al., 2019; Zhu et al., 2020; Marofi et al., 2021).
Extracellular matrix (ECM)
The extracellular matrix (ECM) is a non-cellular network of macromolecules, including glycoproteins, proteoglycans, fibrous structural proteins, and growth factors that form a frame providing other surrounding cells with biochemical and physical support (Ziober et al., 2006; Lyons and Jones, 2007; Mughees et al., 2021). The progression of cancer has been found directly linked to the dysregulation, disorganization and degradation of the ECM (Walker et al., 2018) with recent studies showing that matrix metalloproteinases (MMPs) have been reported to activate growth factors or release them from the matrix, along with ECM degradation, eventually leading to the formation of primary tumours which can further metastasize (Johnson et al., 2014). Fascinatingly, the ECM components as a potential target for therapeutics will be a great ordeal as they are linked to various signalling pathways and may pave way for blocking the life-threatening metastasis of oral cancer.
Part 4 – Pathogenic impact of the microbiome on TME
Recent work has indicated that a certain association between oral microbial dysbiosis and cancer (Su et al., 2021); more to the point, tumor microenvironment (TME) could be decisive therein. Both pro-tumorigenic and anti-tumorigenic roles functioned through TME have been associated with the microbiome. Within TME, the communication between homogenous cell types is driven by an extremely complex network of chemokines, cytokines, growth factors, other inflammatory mediators, and matrix remodeling enzymes (Balkwill et al., 2012). Now, the microbiome can be served as a potent modulator among diverse non-malignant cells of the TME to impact the host immune responses. Considering that the metabolism of cancerous cells is strictly regulated by the TME, the microbiome may be a new component in the TME that impairs tumor cell metabolism maintaining a healthy barrier, inducing inflammation, and producing genotoxins and bacterial metabolites with different features. Below, the possible modalities of how dysbiosis interferes with carcinogenesis, and the potential mechanisms by which microbial dysbiosis modulates carcinogenesis in OSCC are reviewed in Fig. 2 and 3. Rationale of how microbiome and cancer microenvironment change each other, is forging a metagenomics cross-talk that the regulatory interplay between immunogenomics and the microbiome. The human microbiome encompasses up to 100-fold more genes than the host genome, is frequently referred to as the ‘second genome’. Importantly, the microbial genome is flexible and amendable to alter during the host’s lifetime, in contrast to human genome. The bi-directional interaction between microbial and host genome has been revealed in some diseases’ development, such as obesity, inflammatory bowel disease (e.g. Crohn’s disease, and ulcerative colitis) (Levy et al., 2015). Although the identification of key host genes and microbe-derived signals involved in TME immunity is still in its infancy, it could be hypothesized that, the influence of the microbiota on genetic and epigenetic regulation of gene expression in the immunocytes of TME is especially apparent via the host immune system.
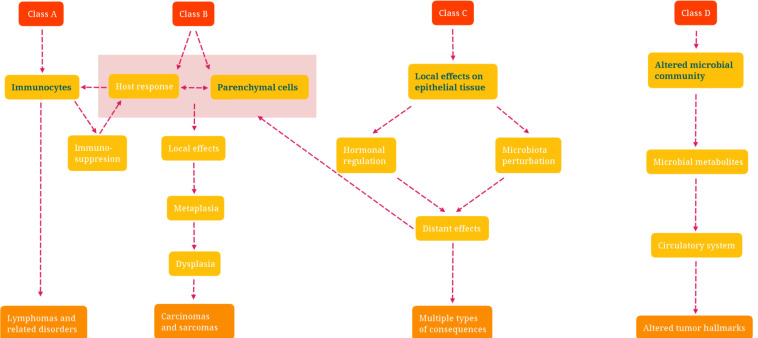
Figure 2.
Schematic Picture of the Classification of Microbiota-Associated Human Malignancies. Class A is defined by the involvement of the immune response; Class B requires direct microbial interactions with parenchymal cells; Class C covers distant effects from local interactions; and Class D shows the consequences of altered microbiome composition
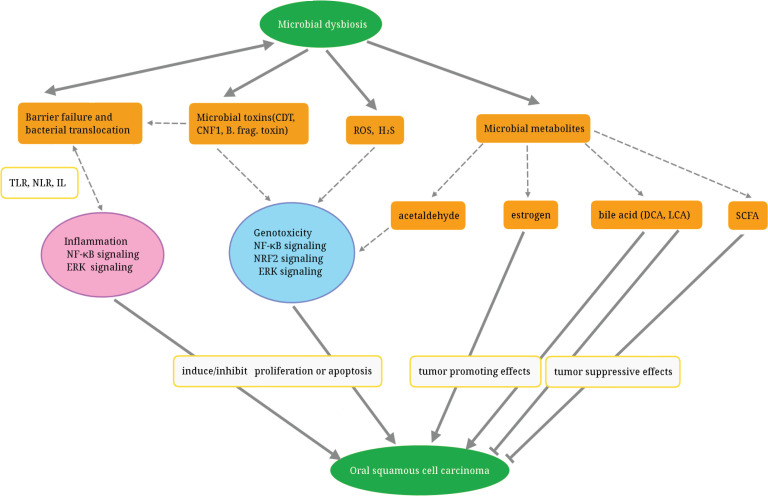
Figure 3.
Actions of Microorganism Contribute to the Pathogenesis of Oral Inflammation and Increase the Risk of OSCC
Part 5 – Effect of the microbiome in the outcomes of oral cancer therapy
The effect of human microorganism on the treatment of oral malignancy is merely starting to be investigated, in spite of its clinical importance. There is now proof indicating that the oral microbiome can impact patient reactions to the cancer treatment. Specifically, embracing chemotherapy and radiotherapy, it has been implicated in modulating the efficacy and cytotoxicity during cancer therapy (Dunnack et al., 2021). Moreover, preclinical data suggest that the microbiome modulation could become a novel strategy for improving the efficacy of immune-based therapies for cancer (Fessler et al., 2019). The oral microbiota has the potential to affect the capacity of cancer therapy. The microbiota, when affected by dysbiosis, may profoundly influence both cancer pathogenesis and its therapeutic outcome. In particular, the regulation of such a therapeutic outcome is related to the oral microbiota’s competence to process anti-cancer compounds and modify the host’s immune response. These two effects combined could clarify the substantive participation of the patient’s microbiome composition in affecting the efficiency of anti-cancer therapy (Irfan et al., 2020). We anticipate that the human microbiome will bit by bit play an undeniably conspicuous role in oral cancer treatment. As of now, the system of the microbiome’s impacts in cancer treatment is not surely known; be that as it may, some studies in respect of clinical pilot should be designed to assist with uncovering the ability of the microbiome in malignant tumor-relevant treatment. We reckon that the advancement of these clinical preliminaries will expel impediments for utilizing the microbiome to improve and help treatment utilizing immune check point inhibitors (Irfan et al., 2020).
Table 1.
Predominant Microbial Communities within Different Sites of Human Oral and Oropharyngeal Region
| Colonia location* | Bacterial species** |
|---|---|
| Tooth surface | Streptococcus mutans, |
| Actinomyces, | |
| Eubacterium, | |
| Peptostreptococcus | |
| Gingival crevice | Fusobacterium, |
| Prevotella, | |
| Porphyromonas | |
| Dental plaque | Actinomyces, |
| Rothia, | |
| Kocuria, | |
| Arsenicicoccus, | |
| Microbacterium, | |
| Propionibacterium, | |
| Mycobacterium, | |
| Dietzia, | |
| Turicella, | |
| Corynebacterium, | |
| Bifidobacterium, | |
| Scardovia, | |
| Parascardovia | |
| Tongue | Veillonella atypica, |
| Porphyronas gingivalis, | |
| Selenomonas species, | |
| Actinobacillus actinomycetemcomitans | |
| Prevotella intermedia, | |
| Capnocytophaga species, | |
| Streptococcus faecalis, | |
| Eikenella corrodens | |
| Tonsil | Streptococcus viridans, |
| Neisseria species, | |
| Haemophilus influenzae, | |
| coagulase-negative Staphylococci | |
| Oropharynx | Streptococcus salivarius, |
| Streptococcus mutans, | |
| Streptococcus anginosus, | |
| Streptococcus pyogenes, | |
| Streptococcus pneumoniae, | |
| Haemophilus influenzae, | |
| Haemophilus parainfluenzae |
*Bacteria bind with complementary receptors of the host, colonizing on non-shedding surfaces of the teeth and continually shedding surfaces of the mucosal epithelia; **Streptococci are commonly found genera in human oral cavity, but this group is highly genetically heterogeneous (Itzek et al., 2010).
Table 2.
Oral Microbiota in Craniomaxillofacial-Cervical Squamous Cell Carcinomas
| Cancer category | Microorganisms | Sample source | Bibliography |
|---|---|---|---|
| HNSCC | phyla Fusobacteria | Meta-analysis | (Bronzato et al., 2020) |
| HNSCC | Streptococcus anginosus | Tumor tissue | (Tateda et al., 2000) |
| HNSCC | Firmicutes, Proteobacteria, Bacteroidetes (Streptococcus, Prevotella, Haemophilus, Lactobacillus, Veillonella) | Saliva | (Guerrero-Preston et al., 2016) |
| OSCC | Streptococcus anginosus | Tumor tissue | (Sasaki et al., 2005) |
| OSCC | Capnocytophaga gingivalis, Prevotella melaninogenica, Streptococcus mitis | Saliva | (Mager et al., 2005) |
| OSCC | Micrococcus luteus, Prevotella melaninogenica, Exiguobacterium oxidotolerans, Fusobacterium naviforme, Staphylococcus aureus, Veillonella parvula, Prevotella sp. (oral clone BE073 phylotype), Rothia mucilaginosa, Streptococcus salivarius, Actinomyces odontolyticus, Moraxella osloensis, Prevotella veroralis, Propionibacterium acnes, Atopobium parvulum, Streptococcus parasanguinis, Veillonella dispar, Streptococcus mitis/oralis | Tumor tissue | (Hooper et al., 2006) |
| OSCC | Fusobacterium periodonticum, Parvimonas micra, Streptococcus constellatus, Haemophilus influenza, Filifactor alocis | Oral rinse | (Yang et al., 2018) |
| OSCC | Streptococcus sp. (oral taxon 058), Streptococcus salivarius, Streptococcus gordonii, Streptococcus parasanguinis, Peptostreptococcus stomatis, Gemella haemolysans, Gemella morbillorum, Johnsonella ignava | Tumor tissue | (Pushalkar et al., 2012) |
| HNSCC | Actinomyces depleted in HNSCC, Parvimonas increased in HNSCC | Tumor tissue | (Wang et al., 2017) |
| OSCC | Fusobacterium, Dialister, Peptostreptococcus, Filifactor, Peptococcus, Catonella, Parvimonas | Swabs of oral lesions | (Zhao et al., 2017) |
| Primary OECs | Porphyromonas gingivalis promoted cell migration which was slightly enhanced by co-infection with Fusobacterium nucleatum | Primary OECs | (Lee et al., 2017) |
| OSCC | Acinetobacter, Fusobacterium, Streptococcus, Prevotella | Tumor tissue, saliva, mouthwash | (Zhang et al., 2019) |
| Gingival squamous cell carcinoma | Porphyromonas gingivalis | Paraffin embedded cancer samples | (Katz et al., 2011) |
| Oral mucosal cancer | Oral streptococci (Streptococcus intermedius, Strep. constellatus, Strep. oralis, Strep. mitis, Strep. sanguis, Strep. salivarius), aerobic enteric bacteria (Enterococcus, Escherichia, Klebsiella etc.), anaerobic bacteria, Peptostreptococcus spp. | The regional lymph nodes in the neck | (Sakamoto et al., 1999) |
| OSCC | Veillonella, Fusobacterium, Prevotella, Porphyromonas, Actinomyces and Clostridium (anaerobes), and Haemophilus, Enterobacteriaceae and Streptococcus spp. (aerobes), Candida albicans | Biofilm from the central surface of the lesions | (Nagy et al., 1998) |
| Malignant oral leukoplakia | Fusobacterium, Leptotrichia, Campylobacter species, Rothia mucilaginosa, Leptotrichia spp. Campylobacter concisus | Swabs | (Amer et al., 2017) |
| OSCC | Porphyromonas gingivalis | Paraffin embedded cancer samples | (Guo et al., 2021) |
Table 3.
CAFs' Morphological, Phenotypical, and Functional Variability
| Characteristics of CAFs | Descriptions | Bibliography |
|---|---|---|
| Cellular origins of CAFs | CAFs can originate from various cell types such as MSCs, fibrocytes, adipocytes, endothelial cells via EndMT, smooth muscle cells, stellate cells, epithelial cells via EMT, pericytes as well as normal fibroblasts. | (Radisky et al., 2007; Potenta et al., 2008; Spaeth et al., 2009; Wikström et al., 2009; Bochet et al., 2013; Shiga et al., 2015; Kalluri, 2016; Bartoschek et al., 2018; Helms et al., 2021) |
| Markers of activated CAFs |
Several molecules, such as α-SMA, FAP, FSP-1, PDGFR α/β, vimentin, TGF-β1, FGF, SDF-1, and EGF are considered some of the markers of activated CAFs. | (Zeisberg et al., 2007; Council and Hameed, 2009; Kalluri and Weinberg, 2009; Toullec et al., 2010; Jia et al., 2013; Kim et al., 2015; Öhlund et al., 2017) |
| Heterogeneity of CAFs | CAFs as well as activated fibroblasts are known to be very heterogeneous, displaying different expression patterns.Resting fibroblast cells are morphologically spindle shaped in contrast to activated fibroblast cells which are stellate shaped. Activated fibroblast cells express the above molecular markers (mainly α-SMA, FAP and PDGFRβ). Resting fibroblast cells express FSP-1 (rarely), and α1β1 integrin. | (Chang et al., 2002; Kalluri, 2016) |
α-SMA, alpha-smooth muscle actin; CAFs, cancer-associated fibroblasts; EGF, epidermal growth factor; EMT, epithelial-mesenchymal transition; EndMT, endothelial to mesenchymal transition; FAP, fibroblast activation protein; FGF, fibroblast growth factor; FSP-1, fibroblast-specific protein-1; MSCs, mesenchymal stem cells; PDGFR α/β, platelet-derived growth factor receptor α/β; SDF-1, stromal derived factor 1; TGF-β1, transforming growth factor-beta1
Table 4.
CAF-Derived Proteins, Enzymes, Cytokines and Factors that have a Functional Impact on HPV-Negative HNSCC, OSCC
| CAF-derived products | Functional impact on cancer cells | Proposed mechanism | Bibliography |
|---|---|---|---|
| CCL2* | Proliferation, migration in oral cancer cells SCC9 and CAL27 | Over-expression of miR-124 in CAFs-OSCCs co-culture abrogated CAFs-promoted OSCCs cell growth and migration, and this inhibitory effect can be rescued by addition of CCL2. | (Li et al., 2017) |
| Migration, invasion in oral cancer cells SCC9 and CAL27; in vivo tumor growth using SCC9 | Induces ROS and subsequent activation of PI3k/Akt-mTOR in cancer cells and CAFs. In cancer, CCL2 further increases Cyclin D/E and CDK4. | (Li et al., 2014) | |
| IL-8* | Proliferation, migration in oral cancer cells SCC9 and CAL27 | Over-expression of miR-124 in CAFs-OSCCs co-culture abrogated CAFs-promoted OSCCs cell growth and migration, and this inhibitory effect can be rescued by addition of IL-8. | (Li et al., 2017) |
| IL-6** | Proliferation, migration, invasion in oral cancer cells SCC25 and CAL27 | Regulates osteopontin expression in a STAT3 dependent-way. Osteopontin binds to an integrin receptor and activates NF-κB in cancer. | (Qin et al., 2018) |
| BDNF*** | Migration, invasion in oral and pharyngeal cancer cells; in vivo tumor growth using OSC19 | Interacts with TrkB and upregulates MMP9 in cancer cells. | (Jiffar et al., 2017) |
| HGF** | Glycolysis in head and neck cancer cells HN5 and SCC1 | Induces the expression of key glycolytic enzymes and lactate efflux. Regulates bGFG expression in cancer cells. | (Kumar et al., 2018) |
| Proliferation in head and neck cancer cells HN5 and UM-SCC-1; migration and invasion in HN5, UM-SCC-1 and OSC-19 | Interacts with c-Met and phosphorylates STAT3 via a JAK dependent route in cancer cells. Synergism with IL-6 enhances the effect. | (Kumar et al., 2015) | |
| HGF*** | Migration in oropharyngeal cancer cells SCC072 and SCC089 | A synergistic relationship between HGF and IL-6 in the support of migration that relates JAK activation to HGF responsiveness in HPV-negative lines. In vitro evidence to support the clinical application of c-Met inhibitors in the control of early HPV-negative oropharyngeal carcinomas. | (Bolt et al., 2018) |
| MMPs** | Cetuximab resistance in laryngeal cancer cell line UT-SCC9 (40%) and tongue cancer cell line UT-SCC-24A (60%) | CAF-dependent modulation of cetuximab sensitivity and suggest that inhibiting MMPs may improve the effects of EGFR-targeted therapy. | (Johansson et al., 2012) |
| MMP-2* | Invasion in oral cancer cell H357 | Senescent CAFs from genetically unstable OSCC promote a more aggressive oral cancer phenotype by production of active MMP-2, disruption of epithelial adhesion and induction of keratinocyte invasion. | (Hassona et al., 2014) |
| Periostin** | Stemness in head and neck cancer cells SCC-25 and HN6 | Activates PTK7 and Wnt/β-Catenin in cancer cells. | (Yu et al., 2018) |
| Proliferation, migration, invasion in head and neck cancer cells HN13 and rat oral cancer cells Rca-T | Binds integrin receptors. | (Qin et al., 2016) | |
| MFAP5* | Proliferation, migration in tongue cancer cell SCC25 | Increases phosphorylation of PDK1, Akt and decreases cRAF, PTEN. | (Principe et al., 2018) |
| CXCL12* | EMT, invasion in oral cancer cells SCC9 and CAL27 | EMT and angiogenesis formation in tongue cancer through myofibroblast differentiation via SDF1 secretion. | (Zhou et al., 2015) |
| CXCL1* | Invasion in oral cancer cells YD10B and YD38 | CXCL1 can transform NFs into senescent CAFs via an autocrine mechanism. | (Kim et al., 2018) |
| Invasion and migration in oral cancer cell SAS | Increases MMP1 in both cancer cells and CAFs. | (Wei et al., 2019) |
"Normal fibroblasts (NFs) activated in co-culture used as a surrogate for CAFs; *Derived from a primary culture of oral cancer; **Derived from a primary culture of head and neck cancer, without specifying the subsite; ***Derived from a primary culture of the oral cavity and oropharyngeal area. Abbreviations: Akt, protein kinase B; BDNF, brain derived neutrophic factor; bFGF, basic fibroblast growth factor; CCL2, chemokine (C-C motif) ligand-2; c-Met, c-mesenchymal-epithelial transition factor; CXCL, C-X-C motif chemokine ligand; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; HGF, hepatocyte growth factor; IL, interleukin; JAK, Janus tyrosine kinase; MFAP5, microfibrillar-associated protein 5; MMP9, matrix metalloproteinase-9; mTOR, mammalian target of the rapamycin; NF-κB, nuclear factor-kappaB; PDK1, pyruvate dehydrogenase kinase isoenzyme-1; PI3K, phosphatidylinositol-3-kinase; PTEN, gene of phosphate and tension homology deleted on chromsome ten; PTK7, protein tyrosine kinase 7; ROS, reactive oxygen species; SDF1, stromal cell-derived factor-1; STAT3, signal transducer and activator of transcription-3; TrkB, tyrosine kinase receptor B; Wnt (Wingless / Integrated).
Table 5.
Inducible Factors for M1/M2 polarization, and M1/M2 macrophages’ Secretory Proteins Associating with the Biological Function
| Phenotypes | Stimuli | Markers | Potential signal axis | Functions | Bibliography | |
|---|---|---|---|---|---|---|
| Human monocytes/macrophages | M1 | IFN-α, TNF-α, LPS, GM-CSF | IL-12 high/IL-10 low, IL-6, TNF-α, CXCL9, CD80, CD86, iNOS | Expression of M1-associated transcripts was increased in THP-1 cells transfected with mimics of miR-29b, miR-125a-5p, or miR-155. The apparent inflammatory property of miR-29b and miR-125a-5p can be at least partially explained by repression of TNFAIP3, a negative regulator of NF-κB signaling. | Pro-inflammation, microbicidal effect, tumor resistance | (Martinez et al., 2008; Mosser and Edwards, 2008; Graff et al., 2012; Colin et al., 2014; Eigsti et al., 2014; Gensel and Zhang, 2015; Ito et al., 2016; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) |
| M2a | IL-4, IL-13 | "CCL17, IL-1R, CD206, Dectin-1, IL-10,DC-SIGN (CD209)" | miRNAs that are uniquely regulated in human macrophages polarized toward M2a (miR-193b) phenotype. |
Anti-inflammatory, wound healing | (Martinez et al., 2008; Mosser and Edwards, 2008; Graff et al., 2012; Eigsti et al., 2014; Gensel and Zhang, 2015; Nakamura et al., 2015; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) | |
| M2b | LPS+IC, IL-1α+IC | IL-10 high/IL-12 low, CD86, TNF-α, CCL1, IL-6 | miRNAs that are uniquely regulated in human macrophages polarized toward M2b (miR-27a*, miR-29b-1*, miR-132*, and miR-222*) phenotypes. | Immunoregulation, promoting infection, tumor progression | (Sironi et al., 2006; Martinez et al., 2008; Mosser and Edwards, 2008; Kobayashi et al., 2011; Graff et al., 2012; Orme and Mohan, 2012; Colin et al., 2014; Ohlsson et al., 2014; Gensel and Zhang, 2015; Chen et al., 2016; Fujiwara et al., 2016; Ito et al., 2016; Schulert et al., 2016; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) | |
| M2c | IL-10, glucocorticoids | "CD206, CD163, IL-10, TGF-β, CXCL13,MerTK" | Increased expression of MerTK, which binds indirectly to phosphatidyl serine through the bridging molecules GAS-6 and protein S, promoting the uptake of apoptotic cells, in active microscopic polyangiitis and in the M2c subtype of macrophages. | Immunosuppression, phagocytosis, tissue remodeling | (Martinez et al., 2008; Graff et al., 2012; Gensel and Zhang, 2015; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) | |
| M2d | LPS+A2R ligands, IL-6 | VEGF, IL-10, TGF-β | Jumonji domain containing-3-IRF4 pathway has been implicated in M2d activation. | Tumor progression, angiogenesis | (Duluc et al., 2007; Martinez et al., 2008; Ferrante and Leibovich, 2012; Shapouri-Moghaddam et al., 2018) | |
| Mouse monocytes/macrophages | M1 | IFN-α, TNF-α, LPS, GM-CSF" | IL-12 high/IL-10 low, IL-6, TNF-α, CXCL9, CD80, CD86, iNOS | Not applicable | Pro-inflammation, microbicidal effect, tumor resistance | (Martinez et al., 2008; Mosser and Edwards, 2008; Colin et al., 2014; Gensel and Zhang, 2015; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) |
| M2a | IL-4, IL-13 | CCL17, IL-1R, Dectin-1, IL-10, Arg-1, Chil3, FIZZ1 | Anti-inflammatory, wound healing | (Martinez et al., 2008; Mosser and Edwards, 2008; Lefèvre et al., 2010; Gensel and Zhang, 2015; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) | ||
| M2c | IL-10, glucocorticoids | "CD206, CD163, IL-10, TGF-β, CXCL13,MerTK, Arg-1" | Immunosuppression, phagocytosis, tissue remodeling | (Martinez et al., 2008; Gensel and Zhang, 2015; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) | ||
| M2d | LPS+A2R ligands, IL-6 | VEGF, IL-10, TGF-β, iNOS | Tumor progression, angiogenesis | (Martinez et al., 2008; Wang et al., 2010; Ferrante and Leibovich, 2012; De Paoli et al., 2014; Gensel and Zhang, 2015; Liberale et al., 2017; Shapouri-Moghaddam et al., 2018) |
Abbreviations: Arg-1, arginase 1; CCL, C-C motif chemokine ligand; CD, cluster of differentiation; CXCL, C-X-C motif chemokine ligand; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; GM-CSF, granulocyte macrophage colony stimulating factor; IFN-, interferon gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; IRF, interferon regulatory factor; LPS, lipopolysaccharide; miRNA, micro ribonucleic acid; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Table 6.
Tumor-Associated Neutrophils (TANs) can Either Promote or Inhibit Tumor Growth Depending on Their Polarization States
| Phenotype | Identificationmarkers/axis | Function | Bibliography | |
|---|---|---|---|---|
| N 1 | Arginase-1, ROS, STAT3, STAT/IRF-8, LOX-1, mPR3, MPO/hydrogen peroxide | Protumor | T/NK cell suppression | (el-Hag and Clark, 1987; Cemerski et al., 2002; Rotondo et al., 2009; Chalmin et al., 2010; Waight et al., 2013; Condamine et al., 2010; Waight et al., 2016; Yang et al., 2018) |
| VEGF, MMP-9, TNF-α, IL-8, NAMPT/STAT3, Oncostatin M, NETs, NE | Angiogenesis/metastasis | (McCourt et al., 1999; Kerfoot et al., 2001; Huang et al., 2002; Queen et al., 2005; Ardi et al., 2007; Houghton et al., 2010; Bald et al., 2014; Park et al., 2016; Chen et al., 2018; Pylaeva et al., 2019) | ||
| CCL2, CCL17 | Immune cell recruitment | (Mishalian et al., 2014; Zhou et al., 2016) | ||
| N 2 | ROS, Granzyme B, MET signaling, Trogoptosis,TNF-α | Antitumor | Tumor cytotoxicity | (Finisguerra et al., 2015; Comen et al., 2016; Gershkovitz et al., 2018; Martin et al., 2018; Matlung et al., 2018) |
| Mechanism unknown,NETs | T-cell activation/priming | (Beauvillain et al., 2007; Tillack et al., 2012; Eruslanov et al., 2014) |
Abbreviations: CCL, chemokine (C-C motif) ligand; LOX-1, low-density lipoprotein receptor-1; MMP-9, matrix metalloproteinase-9; MPO, myeloperoxidase; mPR3, membrane-associated proteinase 3; NAMPT, nicotinamide phosphoribosyltransferase; NE, neutrophil elastase; NET, neutrophil extracellular trap; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Future prospects
The field of microbiome-immunity-cancer has made huge strides over the past several years, nevertheless, few aim at microbiome-mediated regulation in the TME system of OSCC. Additional research is required to expand on correlative or functional observations toward mechanistic understanding and translation of these findings to the clinics.
Author Contribution Statement
LC, GZ and LH conceptualized, and designed the outline of the study; contributed to paper interpretation. LC and LH reviewed all the relevant articles. LC was in charge of findings’ acquisition, original draft writing, tables and figures preparing; and manuscript revision. GZ supervised the study. GZ and LH critically revised the main text, and obtained the financial body. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. The requirements for authorship as stated earlier in this document have been met, and each author believes that the manuscript represents honest work.
Acknowledgements
The authors would like to thank Dr. Ning-bo Zheng (Comprehensive Cancer Center, Wake Forest Baptist Health; Department of Microbiology & Immunology, Wake Forest School of Medicine. Winston-Salem, NC 27101, USA.), for the professional assistance to our work.
This review is a part of a PhD candidate’s thesis approved by the School / Hospital of Stomatology, Xinjiang Medical University.
Funding statement
All phases of this study were supported by the National Natural Science Foundation of China (grant number: 82160189); Tianshan Innovation Team of Xinjiang Uygur Autonomous Region (grant number: 2021D14001)
Ethics approval
Ethical approval will not be required because this article is a kind of secondary research that retrieved and synthesized data from already published studies.
Availability of data and materials
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almangush A, Mäkitie AA, Triantafyllou A, et al. Staging and grading of oral squamous cell carcinoma: An update. Oral Oncol. 2020;107:104799. doi: 10.1016/j.oraloncology.2020.104799. [DOI] [PubMed] [Google Scholar]
- Alsahafi E, Begg K, Amelio I, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A, Galvin S, Healy CM, Moran GP. The Microbiome of Potentially Malignant Oral Leukoplakia Exhibits Enrichment for Fusobacterium, Leptotrichia, Campylobacter, and Rothia Species. Front Microbiol. 2017;8:2391. doi: 10.3389/fmicb.2017.02391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andzinski L, Kasnitz N, Stahnke S, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982–93. doi: 10.1002/ijc.29945. [DOI] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–7. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arweiler NB, Netuschil L. The Oral Microbiota. Adv Exp Med Biol. 2016;902:45–60. doi: 10.1007/978-3-319-31248-4_4. [DOI] [PubMed] [Google Scholar]
- Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–11. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–13. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–6. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- Bartoschek M, Oskolkov N, Bocci M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvillain C, Delneste Y, Scotet M, et al. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–73. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- Bochet L, Lehuédé C, Dauvillier S, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–68. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- Bolt R, Foran B, Murdoch C, et al. HPV-negative, but not HPV-positive, oropharyngeal carcinomas induce fibroblasts to support tumour invasion through micro-environmental release of HGF and IL-6. Carcinogenesis. 2018;39:170–9. doi: 10.1093/carcin/bgx130. [DOI] [PubMed] [Google Scholar]
- Boysen M, Lövdal O, Tausjö J, Winther F. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur J Cancer. 1992;28:426–30. doi: 10.1016/s0959-8049(05)80068-1. [DOI] [PubMed] [Google Scholar]
- Braakhuis BJ, Tabor MP, Leemans CR, et al. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck. 2002;24:198–206. doi: 10.1002/hed.10042. [DOI] [PubMed] [Google Scholar]
- Bronzato JD, Bomfim RA, Edwards DH, et al. Detection of Fusobacterium in oral and head and neck cancer samples: A systematic review and meta-analysis. Arch Oral Biol. 2020;112:104669. doi: 10.1016/j.archoralbio.2020.104669. [DOI] [PubMed] [Google Scholar]
- Cemerski S, Cantagrel A, Van Meerwijk JP, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. 2002;277:19585–93. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Lee WT, Hsiao JR, et al. Oral hygiene and the overall survival of head and neck cancer patients. Cancer Med. 2019;8:1854–64. doi: 10.1002/cam4.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MB, Hajal C, Benjamin DC, et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc Natl Acad Sci U S A. 2018;115:7022–7. doi: 10.1073/pnas.1715932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Xiao X, Lao XM, et al. Polarization of Tissue-Resident TFH-Like Cells in Human Hepatoma Bridges Innate Monocyte Inflammation and M2b Macrophage Polarization. Cancer Discov. 2016;6:1182–95. doi: 10.1158/2159-8290.CD-16-0329. [DOI] [PubMed] [Google Scholar]
- Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–66. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- Comen E, Wojnarowicz P, Seshan VE, et al. TNF is a key cytokine mediating neutrophil cytotoxic activity in breast cancer patients. NPJ Breast Cancer. 2016;2:16009. doi: 10.1038/npjbcancer.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Dominguez GA, Youn JI, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council L, Hameed O. Differential expression of immunohistochemical markers in bladder smooth muscle and myofibroblasts, and the potential utility of desmin, smoothelin, and vimentin in staging of bladder carcinoma. Mod Pathol. 2009;22:639–50. doi: 10.1038/modpathol.2009.9. [DOI] [PubMed] [Google Scholar]
- Custódio M, Biddle A, Tavassoli M. Portrait of a CAF: The story of cancer-associated fibroblasts in head and neck cancer. Oral Oncol. 2020;110:104972. doi: 10.1016/j.oraloncology.2020.104972. [DOI] [PubMed] [Google Scholar]
- Dawes C. Estimates, from salivary analyses, of the turnover time of the oral mucosal epithelium in humans and the number of bacteria in an edentulous mouth. Arch Oral Biol. 2003;48:329–36. doi: 10.1016/s0003-9969(03)00014-1. [DOI] [PubMed] [Google Scholar]
- De Paoli F, Staels B, Chinetti-Gbaguidi G. Macrophage phenotypes and their modulation in atherosclerosis. Circ J. 2014;78:1775–81. doi: 10.1253/circj.cj-14-0621. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico EG, Cavallo I, Pontone M, Toma L, Ensoli F. Biofilm Producing Salmonella Typhi: Chronic Colonization and Development of Gallbladder Cancer. Int J Mol Sci. 2017;18:1887. doi: 10.3390/ijms18091887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- Dunnack HJ, Judge MP, Cong X, et al. An Integrative Review of the Role of the Oral and Gut Microbiome in Oral Health Symptomatology During Cancer Therapy. Oncol Nurs Forum. 2021;48:317–31. doi: 10.1188/21.ONF.317-331. [DOI] [PubMed] [Google Scholar]
- Eigsti RL, Sudan B, Wilson ME, Graff JW. Regulation of activation-associated microRNA accumulation rates during monocyte-to-macrophage differentiation. J Biol Chem. 2014;289:28433–47. doi: 10.1074/jbc.M114.599316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Hag A, Clark RA. Immunosuppression by activated human neutrophils Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–13. [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–80. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante CJ, Leibovich SJ. Regulation of Macrophage Polarization and Wound Healing. Adv Wound Care (New Rochelle) 2012;1:10–6. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7:108. doi: 10.1186/s40425-019-0574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finisguerra V, Di Conza G, Di Matteo M, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–53. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Hizukuri Y, Yamashiro K, et al. Guanylate-binding protein 5 is a marker of interferon-γ-induced classically activated macrophages. Clin Transl Immunol. 2016;5:e111. doi: 10.1038/cti.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–95. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Gershkovitz M, Caspi Y, Fainsod-Levi T, et al. TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells. Cancer Res. 2018;78:2680–90. doi: 10.1158/0008-5472.CAN-17-3614. [DOI] [PubMed] [Google Scholar]
- Gholizadeh P, Pormohammad A, Eslami H, et al. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb Pathog. 2017;113:303–11. doi: 10.1016/j.micpath.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287:21816–25. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget. 2016;7:51320–34. doi: 10.18632/oncotarget.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front Oncol. 2021;11:668349. doi: 10.3389/fonc.2021.668349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZC, Jumatai S, Jing SL, et al. Bioinformatics and immunohistochemistry analyses of expression levels and clinical significance of CXCL2 and TANs in an oral squamous cell carcinoma tumor microenvironment of Prophyromonas gingivalis infection. Oncol Lett. 2021;21 doi: 10.3892/ol.2021.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect. 2014;142:1119–37. doi: 10.1017/S0950268814000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann Oncol. 2016;27:1619–25. doi: 10.1093/annonc/mdw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br J Cancer. 2014;111:1230–7. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms EJ, Berry MW, Chaw RC, et al. Mesenchymal Lineage Heterogeneity Underlies Non-Redundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2021;21 doi: 10.1158/2159-8290.CD-21-0601. candisc.0601.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SJ, Crean SJ, Lewis MA, et al. Viable bacteria present within oral squamous cell carcinoma tissue. J Clin Microbiol. 2006;44:1719–25. doi: 10.1128/JCM.44.5.1719-1725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–42. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- Huang SH, O’Sullivan B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr Treat Options Oncol. 2017;18 doi: 10.1007/s11864-017-0484-y. [DOI] [PubMed] [Google Scholar]
- Huber MA, Tantiwongkosi B. Oral and oropharyngeal cancer. Med Clin North Am. 2014;98:1299–321. doi: 10.1016/j.mcna.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Irfan M, Delgado RZR, Frias-Lopez J. The Oral Microbiome and Cancer. Front Immunol. 2020;11:591088. doi: 10.3389/fimmu.2020.591088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–96. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Ito I, Bhopale KK, Nishiguchi T, et al. The Polarization of M2b Monocytes in Cultures of Burn Patient Peripheral CD14+ Cells Treated with a Selected Human CCL1 Antisense Oligodeoxynucleotide. Nucleic Acid Ther. 2016;26:269–76. doi: 10.1089/nat.2016.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzek A, Gillen CM, Fulde M, et al. Contribution of plasminogen activation towards the pathogenic potential of oral streptococci. PLoS One. 2010;5:e13826. doi: 10.1371/journal.pone.0013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett A, Kos J, Fong Y, et al. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin Cancer Biol. 2018;53:178–88. doi: 10.1016/j.semcancer.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Jewett A, Kos J, Kaur K, et al. Natural Killer Cells: Diverse Functions in Tumor Immunity and Defects in Pre-neoplastic and Neoplastic Stages of Tumorigenesis. Mol Ther Oncolytics. 2019;16:41–52. doi: 10.1016/j.omto.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Shu D, Zheng M, et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep. 2016;6:24838. doi: 10.1038/srep24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia CC, Wang TT, Liu W, et al. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One. 2013;8:e63243. doi: 10.1371/journal.pone.0063243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiffar T, Yilmaz T, Lee J, et al. Brain derived neutrophic factor (BDNF) coordinates lympho-vascular metastasis through a fibroblast-governed paracrine axis in the tumor microenvironment. Cancer Cell Microenviron. 2017;4:e1566. doi: 10.14800/ccm.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AC, Ansell A, Jerhammar F, et al. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol Cancer Res. 2012;10:1158–68. doi: 10.1158/1541-7786.MCR-12-0030. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Burtness B, Leemans CR, et al. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, De Costa AM, Young MR. Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer. Cancers (Basel) 2014;6:756–70. doi: 10.3390/cancers6020756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou A, Hess J. Epidemiology and Molecular Biology of Head and Neck Cancer. Oncol Res Treat. 2017;40:328–32. doi: 10.1159/000477127. [DOI] [PubMed] [Google Scholar]
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–15. doi: 10.4248/IJOS11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Nanut MP, Ko MW, et al. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: strategies to optimize their growth and expansion for effective cancer immunotherapy. Curr Opin Immunol. 2018;51:170–80. doi: 10.1016/j.coi.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, Raharjo E, Ho M, et al. Exclusive neutrophil recruitment with oncostatin M in a human system. Am J Pathol. 2001;159:1531–9. doi: 10.1016/S0002-9440(10)62538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. The oral microbiome - friend or foe? Eur J Oral Sci. 2018;126:5–12. doi: 10.1111/eos.12527. [DOI] [PubMed] [Google Scholar]
- Kim EK, Moon S, Kim DK, Zhang X, Kim J. CXCL1 induces senescence of cancer-associated fibroblasts via autocrine loops in oral squamous cell carcinoma. PLoS One. 2018;13:e0188847. doi: 10.1371/journal.pone.0188847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Jung WH, Koo JS. Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: an immunohistochemical analysis. J Transl Med. 2015;13:222. doi: 10.1186/s12967-015-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Jeschke MG, Asai A, et al. Propranolol as a modulator of M2b monocytes in severely burned patients. J Leukoc Biol. 2011;89:797–803. doi: 10.1189/jlb.1010553. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi A, Yoshikawa R, Okuda K, Hara K. The microbial flora from root canals and periodontal pockets of non-vital teeth associated with advanced periodontitis. Int Endod J. 1990;23:100–6. doi: 10.1111/j.1365-2591.1990.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Koopmans AS, Kippuw N, de Graaff J. Bacterial involvement in denture-induced stomatitis. J Dent Res. 1988;67:1246–50. doi: 10.1177/00220345880670091901. [DOI] [PubMed] [Google Scholar]
- Kozlowska AK, Topchyan P, Kaur K, et al. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J Cancer. 2017;8:537–54. doi: 10.7150/jca.15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Kandl C, Hamilton CD, et al. Mitigation of Tumor-Associated Fibroblast-Facilitated Head and Neck Cancer Progression With Anti-Hepatocyte Growth Factor Antibody Ficlatuzumab. JAMA Otolaryngol Head Neck Surg. 2015;141:1133–9. doi: 10.1001/jamaoto.2015.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, New J, Vishwakarma V, et al. Cancer-Associated Fibroblasts Drive Glycolysis in a Targetable Signaling Loop Implicated in Head and Neck Squamous Cell Carcinoma Progression. Cancer Res. 2018;78:3769–82. doi: 10.1158/0008-5472.CAN-17-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12:458–63. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lee J, Roberts JS, Atanasova KR, et al. Human Primary Epithelial Cells Acquire an Epithelial-Mesenchymal-Transition Phenotype during Long-Term Infection by the Oral Opportunistic Pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. doi: 10.3389/fcimb.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre L, Galès A, Olagnier D, et al. PPARγ ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS One. 2010;5:e12828. doi: 10.1371/journal.pone.0012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Thaiss CA, Elinav E. Metagenomic cross-talk: the regulatory interplay between immunogenomics and the microbiome. Genome Med. 2015;7:120. doi: 10.1186/s13073-015-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Fan Q, Li J, Song J, Gu Y. MiR-124 down-regulation is critical for cancer associated fibroblasts-enhanced tumor growth of oral carcinoma. Exp Cell Res. 2017;351:100–8. doi: 10.1016/j.yexcr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Li X, Xu Q, Wu Y, et al. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. 2014;35:1362–70. doi: 10.1093/carcin/bgu046. [DOI] [PubMed] [Google Scholar]
- Li YY, Tao YW, Gao S, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBio Med. 2018;36:209–20. doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberale L, Dallegri F, Montecucco F, Carbone F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb Haemost. 2017;117:7–18. doi: 10.1160/TH16-08-0593. [DOI] [PubMed] [Google Scholar]
- Lim Y, Totsika M, Morrison M, Punyadeera C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics. 2017;7:4313–21. doi: 10.7150/thno.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SA, Wong YK, Lin JC, et al. Impact of recurrence interval on survival of oral cavity squamous cell carcinoma patients after local relapse. Otolaryngol Head Neck Surg. 2007;136:112–8. doi: 10.1016/j.otohns.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Liyanage SS, Rahman B, Ridda I, et al. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis. PLoS One. 2013;8:e69238. doi: 10.1371/journal.pone.0069238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of Metastasis by NK Cells. Cancer Cell. 2017;32:135–54. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Lyons AJ, Jones J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int J Oral Maxillofac Surg. 2007;36:671–9. doi: 10.1016/j.ijom.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Mager DL, Haffajee AD, Devlin PM, et al. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magister Š, Tseng HC, Bui VT, Kos J, Jewett A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget. 2015;6:22310–27. doi: 10.18632/oncotarget.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marofi F, Abdul-Rasheed OF, Rahman HS, et al. CAR-NK cell in cancer immunotherapy; A promising frontier. Cancer Sci. 2021;112:3427–36. doi: 10.1111/cas.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Seignez C, Racoeur C, et al. Tumor-derived granzyme B-expressing neutrophils acquire antitumor potential after lipid A treatment. Oncotarget. 2018;9:28364–78. doi: 10.18632/oncotarget.25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Masucci MT, Minopoli M, Carriero MV. Tumor Associated Neutrophils Their Role in Tumorigenesis Metastasis, Prognosis and Therapy. Front Oncol. 2019;9:1146. doi: 10.3389/fonc.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlung HL, Babes L, Zhao XW, et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018;23:3946–59. doi: 10.1016/j.celrep.2018.05.082. [DOI] [PubMed] [Google Scholar]
- Matos LL, Menderico Junior GM, Theodoro TR, et al. Cancer-associated fibroblast regulation by microRNAs promotes invasion of oral squamous cell carcinoma. Oral Oncol. 2020;110:104909. doi: 10.1016/j.oraloncology.2020.104909. [DOI] [PubMed] [Google Scholar]
- McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–31; discussion 1331-2. doi: 10.1001/archsurg.134.12.1325. [DOI] [PubMed] [Google Scholar]
- Mishalian I, Bayuh R, Eruslanov E, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17--a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135:1178–86. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- Mitchell DA. Metronidazole: its use in clinical dentistry. J Clin Periodontol. 1984;11:145–58. doi: 10.1111/j.1600-051x.1984.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mücke T, Wagenpfeil S, Kesting MR, Hölzle F, Wolff KD. Recurrence interval affects survival after local relapse of oral cancer. Oral Oncol. 2009;45:687–91. doi: 10.1016/j.oraloncology.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Mughees M, Sengupta A, Khowal S, Wajid S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol Biol Rep. 2021;48:1773–86. doi: 10.1007/s11033-020-06054-6. [DOI] [PubMed] [Google Scholar]
- Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–8. [PubMed] [Google Scholar]
- Naik PP, Panda PK, Bhutia SK. Oral Cancer Stem Cells Microenvironment. Adv Exp Med Biol. 2017;1041:207–33. doi: 10.1007/978-3-319-69194-7_11. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito I, Kobayashi M, Herndon DN, Suzuki F. Orosomucoid 1 drives opportunistic infections through the polarization of monocytes to the M2b phenotype. Cytokine. 2015;73:8–15. doi: 10.1016/j.cyto.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi T, Ito I, Lee JO, et al. Macrophage polarization and MRSA infection in burned mice. Immunol Cell Biol. 2017;95:198–206. doi: 10.1038/icb.2016.84. [DOI] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson SM, Linge CP, Gullstrand B, et al. Serum from patients with systemic vasculitis induces alternatively activated macrophage M2c polarization. Clin Immunol. 2014;152:10–9. doi: 10.1016/j.clim.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Ohms M, Möller S, Laskay T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front Immunol. 2020;11:532. doi: 10.3389/fimmu.2020.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Heterogeneity of stromal fibroblasts in tumors. Cancer Biol Ther. 2007;6:618–9. doi: 10.4161/cbt.6.4.4255. [DOI] [PubMed] [Google Scholar]
- Orme J, Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discov Med. 2012;13:151–8. [PubMed] [Google Scholar]
- Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–96. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IR, Attygalla M, Jayasuriya N, Dias DK, Perera ML. Oral hygiene and periodontal disease in male patients with oral cancer. Br J Oral Maxillofac Surg. 2018;56:901–3. doi: 10.1016/j.bjoms.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Petrick JL, Wyss AB, Butler AM, et al. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. 2014;110:2369–77. doi: 10.1038/bjc.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti G, Gazzola T, Allegra L. Bacterial adherence in smokers and non-smokers. Pharmacol Res. 1997;36:481–4. doi: 10.1006/phrs.1997.0255. [DOI] [PubMed] [Google Scholar]
- Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–9. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principe S, Mejia-Guerrero S, Ignatchenko V, et al. Proteomic Analysis of Cancer-Associated Fibroblasts Reveals a Paracrine Role for MFAP5 in Human Oral Tongue Squamous Cell Carcinoma. J Proteome Res. 2018;17:2045–59. doi: 10.1021/acs.jproteome.7b00925. [DOI] [PubMed] [Google Scholar]
- Pushalkar S, Ji X, Li Y, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylaeva E, Harati MD, Spyra I, et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. Int J Cancer. 2019;144:136–49. doi: 10.1002/ijc.31808. [DOI] [PubMed] [Google Scholar]
- Qin X, Guo H, Wang X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20:12. doi: 10.1186/s13059-018-1604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yan M, Wang X, et al. Cancer-associated Fibroblast-derived IL-6 Promotes Head and Neck Cancer Progression via the Osteopontin-NF-kappa B Signaling Pathway. Theranostics. 2018;8:921–40. doi: 10.7150/thno.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yan M, Zhang J, et al. TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep. 2016;6:20587. doi: 10.1038/srep20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–9. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SN, Brown JS, Woolgar JA, et al. Survival following primary surgery for oral cancer. Oral Oncol. 2009;45:201–11. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- Rotondo R, Barisione G, Mastracci L, et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2009;125:887–93. doi: 10.1002/ijc.24448. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Naito H, Ohta Y, et al. Isolation of bacteria from cervical lymph nodes in patients with oral cancer. Arch Oral Biol. 1999;44:789–93. doi: 10.1016/s0003-9969(99)00079-5. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Yamaura C, Ohara-Nemoto Y, et al. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005;11:151–6. doi: 10.1111/j.1601-0825.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- Schulert GS, Fall N, Harley JB, et al. Monocyte MicroRNA Expression in Active Systemic Juvenile Idiopathic Arthritis Implicates MicroRNA-125a-5p in Polarized Monocyte Phenotypes. Arthritis Rheumatol. 2016;68:2300–13. doi: 10.1002/art.39694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Mehta RH, Wenig BL, Shaligram C, Portugal LG. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity. Head Neck. 2000;22:34–41. doi: 10.1002/(sici)1097-0347(200001)22:1<34::aid-hed6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- Shiga K, Hara M, Nagasaki T, et al. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel) 2015;7:2443–58. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M, Martinez FO, D’Ambrosio D, et al. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J Leukoc Biol. 2006;80:342–9. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- Sixou JL, Magaud C, Jolivet-Gougeon A, et al. Evaluation of the mandibular third molar pericoronitis flora and its susceptibility to different antibiotics prescribed in france. J Clin Microbiol. 2003;41:5794–7. doi: 10.1128/JCM.41.12.5794-5797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklavounos A, Legakis NJ, Ioannidou H, Patrikiou A. Anaerobic bacteria in dentoalveolar abscesses. Int J Oral Maxillofac Surg. 1986;15:288–91. doi: 10.1016/s0300-9785(86)80087-4. [DOI] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Chang LC, Huang HD, et al. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis. 2021;42:127–35. doi: 10.1093/carcin/bgaa062. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Syrjänen K, Syrjänen S. Detection of human papillomavirus in esophageal papillomas: systematic review and meta-analysis. APMIS. 2013;121:363–74. doi: 10.1111/apm.12003. [DOI] [PubMed] [Google Scholar]
- Szturz P, Vermorken JB. Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher. Oral Oncol. 2020;101:104492. doi: 10.1016/j.oraloncology.2019.104492. [DOI] [PubMed] [Google Scholar]
- Takahashi N. Microbial ecosystem in the oral cavity: metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congr Ser. 2005;1284:103–12. [Google Scholar]
- Tateda M, Shiga K, Saijo S, et al. Streptococcus anginosus in head and neck squamous cell carcinoma: implication in carcinogenesis. Int J Mol Med. 2000;6:699–703. doi: 10.3892/ijmm.6.6.699. [DOI] [PubMed] [Google Scholar]
- Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188:3150–9. doi: 10.4049/jimmunol.1103414. [DOI] [PubMed] [Google Scholar]
- Toullec A, Gerald D, Despouy G, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–30. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ‘t Hof W, Veerman EC, Nieuw Amerongen AV, Ligtenberg AJ. Antimicrobial defense systems in saliva. Monogr Oral Sci. 2014;24:40–51. doi: 10.1159/000358783. [DOI] [PubMed] [Google Scholar]
- Verma D, Garg PK, Dubey AK. Insights into the human oral microbiome. Arch Microbiol. 2018;200:525–40. doi: 10.1007/s00203-018-1505-3. [DOI] [PubMed] [Google Scholar]
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–41. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waight JD, Netherby C, Hensen ML, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–78. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C, Mojares E, Del Río Hernández A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang S, Yue K, Wang XD. The recurrence and survival of oral squamous cell carcinoma: a report of 275 cases. Chin J Cancer. 2013;32:614–8. doi: 10.5732/cjc.012.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Funchain P, Bebek G, et al. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017;9:14. doi: 10.1186/s13073-017-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345–58. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Ni H, Lan L, et al. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010;20:701–12. doi: 10.1038/cr.2010.52. [DOI] [PubMed] [Google Scholar]
- Wei LY, Lee JJ, Yeh CY, et al. Reciprocal activation of cancer-associated fibroblasts and oral squamous carcinoma cells through CXCL1. Oral Oncol. 2019;88:115–23. doi: 10.1016/j.oraloncology.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Welch DR, Schissel DJ, Howrey RP, Aeed PA. Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci U S A. 1989;86:5859–63. doi: 10.1073/pnas.86.15.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69:799–809. doi: 10.1002/pros.20927. [DOI] [PubMed] [Google Scholar]
- Wittekindt C, Wagner S, Sharma SJ, et al. HPV - A different view on Head and Neck Cancer. Laryngorhinootologie. 2018;97:S48–113. doi: 10.1055/s-0043-121596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Yeh YM, Yu HY, et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front Microbiol. 2018;9:862. doi: 10.3389/fmicb.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TH, St John LS, Garber HR, et al. Membrane-Associated Proteinase 3 on Granulocytes and Acute Myeloid Leukemia Inhibits T Cell Proliferation. J Immunol. 2018;201:1389–99. doi: 10.4049/jimmunol.1800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Wu K, Wang X, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9:1082. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- Zarco MF, Vess TJ, Ginsburg GS. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109–20. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li X, Sun W, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang J, Feng Q, et al. Compositional and Functional Analysis of the Microbiome in Tissue and Saliva of Oral Squamous Cell Carcinoma. Front Microbiol. 2019;10:1439. doi: 10.3389/fmicb.2019.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chu M, Huang Z, et al. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7:11773. doi: 10.1038/s41598-017-11779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhuang XM, Wang YY, et al. Tumor necrosis factor α induces myofibroblast differentiation in human tongue cancer and promotes invasiveness and angiogenesis via secretion of stromal cell-derived factor-1. Oral Oncol. 2015;51:1095–102. doi: 10.1016/j.oraloncology.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646–58. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- Zhu X, Qin X, Wang X, et al. Oral cancer cellderived exosomes modulate natural killer cell activity by regulating the receptors on these cells. Int J Mol Med. 2020;46:2115–25. doi: 10.3892/ijmm.2020.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober AF, Falls EM, Ziober BL. The extracellular matrix in oral squamous cell carcinoma: friend or foe? Head Neck. 2006;28:740–9. doi: 10.1002/hed.20382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.