Abstract
Gliomas are primary central nervous system tumors that arise from glial progenitor cells. Gliomas have been classically classified morphologically based on their histopathological characteristics. However, with recent advances in cancer genomics, molecular profiles have now been integrated into the classification and diagnosis of gliomas. In this review article, we discuss the clinical features, imaging findings, and molecular profiles of adult-type diffuse gliomas based on the new 2021 World Health Organization Classifications of Tumors of the central nervous system.
Keywords: Glioma, Brain Neoplasms, Diagnosis, Classification
Graphical Abstract
Highlights
• Isocitrate dehydrogenase (IDH) mutation status is key to classify the adult-type diffuse gliomas.
• Molecular and genetic profiles have been integrated into the diagnosis of gliomas.
• Next-generation sequencing became essential for the diagnosis of gliomas.
INTRODUCTION
Gliomas are a heterogeneous group of primary central nervous system (CNS) tumors that originate from glial progenitor cells [1]. These tumors constitute the most common type of malignant primary CNS tumors in adults. The annual incidence of gliomas is reported to be about 6 cases per 100,000 worldwide, and their incidence is significantly lower in non-Caucasian populations, especially for glioblastoma [2,3]. Classically, these tumors have been classified morphologically based on their histopathological characteristics, as with other tumors. However, with the vast advances in cancer genomics, there has been a paradigm shift in which molecular profiles have begun to be integrated into the diagnosis of gliomas [4,5]. This fundamental change is largely attributed to the acknowledgement that different genomic signatures lead to different clinical outcomes, thus necessitating different expectations and treatment methods; this reflects the basic concept of “precision medicine” [6]. This trend is clearly manifested in the new 2021 World Health Organization (WHO) Classification of Tumors of the CNS [7] which fully embraced the integrated diagnosis. Under this new classification, gliomas, glioneuronal tumors, and neuronal tumors are subcategorized into six groups: adult-type diffuse gliomas, pediatric-type diffuse low-grade gliomas, pediatric-type diffuse high-grade gliomas, circumscribed astrocytic gliomas, glioneuronal and neuronal tumors, and ependymal tumors. This review article will focus on the diagnosis and classification of adult-type diffuse gliomas, which account for the majority of gliomas. This study was approved by the Institutional Review Board of Seoul National University Hospital (No. 2206-067-1332) regarding the use of patients’ data.
CLASSIFICATIONS OF ADULT-TYPE DIFFUSE GLIOMAS
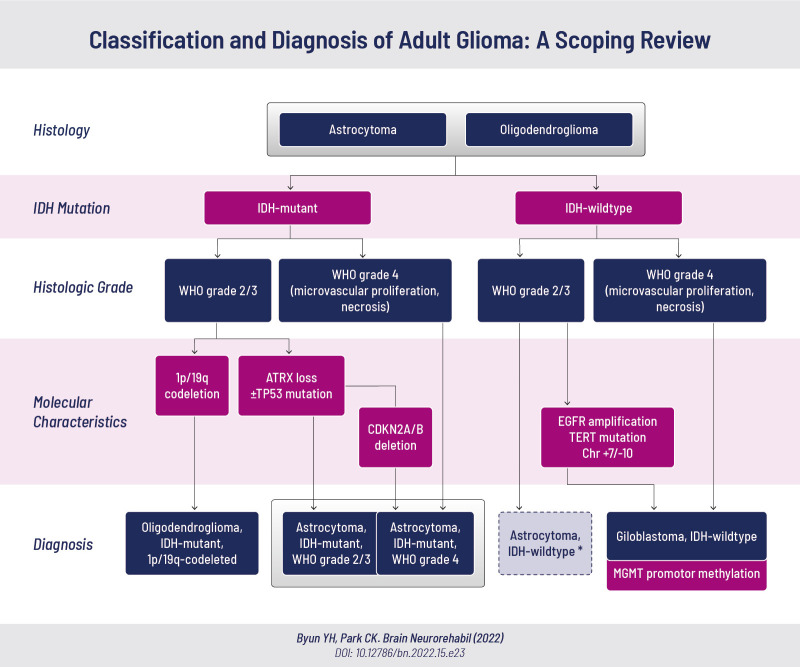
The 2021 WHO CNS classification separated adult-type diffuse gliomas from the pediatric type, and integrated the histopathological features of the tumors with the molecular profiles to provide a more concise diagnosis [8]. Adult-type diffuse gliomas are classified into: 1) astrocytoma, isocitrate dehydrogenase (IDH)-mutant (grade 2/3/4), 2) oligodendroglioma, IDH-mutant (grade 2/3) and 1p/19q-codeleted, and 3) glioblastoma, IDH-wildtype (grade 4).
Diffuse gliomas are first broadly divided into IDH-mutant and IDH-wildtype gliomas (Fig. 1). IDH-mutant glioma with histopathological high-grade features (WHO grade 4), including necrosis and/or microvascular proliferation, is diagnosed as astrocytoma, IDH-mutant, WHO grade 4. IDH-mutant gliomas without these features are graded as either WHO grade 2 or 3 and are subdivided according to their molecular profiles. IDH-mutant glioma with concurrent 1p/19q-codeletion is diagnosed as oligodendroglioma, IDH-mutant, 1p/19q-codeleted. IDH-mutant glioma without 1p/19q-codeletion is diagnosed as astrocytoma, IDH-mutant, grade 2 or 3. As alpha-thalassemia/mental retardation, X-linked (ATRX) mutations are mutually exclusive with 1p/19q-codeletion, the diagnosis of astrocytoma, IDH-mutant could be made if ATRX and/or TP53 mutations are present without further inspection for 1p/19q-codeletion. A point to note is that astrocytoma, IDH-mutant with cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) deletion is diagnosed as astrocytoma, IDH-mutant, WHO grade 4, even in the absence of histopathological high-grade features. This is based on studies that have shown CDKN2A/B deletion to be an independent negative prognostic factor in IDH-mutant astrocytoma [9].
Fig. 1. The 2021 WHO classification of adult-type diffuse gliomas.
Adult-type diffuse gliomas are classified as: 1) astrocytoma, IDH-mutant, 2) oligodendroglioma, IDH-mutant and 1p/19q-codeleted, and 3) glioblastoma, IDH-wildtype.
WHO, World Health Organization; IDH, isocitrate dehydrogenase; ATRX, alpha-thalassemia/mental retardation, X-linked; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; EGFR, epidermal growth factor receptor; TERT, telomerase reverse transcriptase; MGMT, O-5-methylguanine-DNA methyltransferase.
*IDH-wildtype glioma without high-grade features (WHO grade 2/3) should be investigated further and classified into other categories.
IDH-wildtype glioma with high-grade features is diagnosed as glioblastoma, IDH-wildtype. IDH-wildtype glioma without high-grade features should be investigated further to determine its further classification into other categories. Of note, IDH-wildtype glioma with epidermal growth factor receptor (EGFR) amplification, telomerase reverse transcriptase (TERT) promoter mutation, or chromosome 7 gain/chromosome 10 loss is diagnosed as glioblastoma, IDH-wildtype even in the absence of histopathological high-grade features. This is due to studies showing that astrocytoma, IDH-wildtype, WHO grade 2/3 with these mutations carries the same prognosis as histologically diagnosed glioblastoma [10]. When glioblastoma, IDH-wildtype is diagnosed, the presence of O-5-methylguanine-DNA methyltransferase (MGMT) promotor methylation should additionally be assessed, as it is associated with a better response to temozolomide and longer survival [11].
CLINICAL FEATURES
Gliomas present with various symptoms, which are often related to the location and grade of the tumor. Generally, low-grade, slow-growing tumors present with new-onset seizure or a more subtle progressive neurologic deficit [12]. In contrast, higher-grade, faster-growing tumors often present with more acute neurologic symptoms combined with other symptoms [13]. Common presenting symptoms include headache, seizure, cognitive dysfunction, and focal neurologic deficits [14]. Headache is one of the most common non-specific symptoms in patients with brain tumors; however, it has a very low positive predictive value [15]. Therefore, high clinical suspicion is required in patients with an acute onset of a new type of headache or with significant changes in the pattern of headaches. The incidence of cognitive dysfunction increase with the patient’s age and tumor grade, and it seems to be worse when the dominant hemisphere is involved [16]. However, these symptoms may not be confined to the affected lobe, as the tumor could interrupt the integrity of the neural network [17].
IMAGING FINDINGS (RADIOGENOMICS)
In recent years, there has been significant growth in the field of radiogenomics, where vast numbers of quantitative imaging features have been correlated with the molecular/genetic profiles of tumors [18]. There have been ongoing efforts to utilize radiogenomics for diagnosing glioma based on the new genetic classification, ultimately aiming to stratify patients to optimize individual treatment. Magnetic resonance imaging (MRI) is the most common imaging modality used to diagnose gliomas, and many attempts have been made to uncover the specific MRI features of genetic mutations related to gliomas [19].
IDH-mutated gliomas tend to be more frequently confined to a single lobe with larger areas of non-enhancing lesions and sharper borders than IDH-wildtype gliomas [20,21]. The imaging features of oligodendroglioma, IDH-mutant, 1p/19q-codeleted are well established, with the tumor traditionally localized to the frontal lobe, commonly showing calcification, cortical involvement, heterogeneous signal intensity on T1- and T2-weighted images, and indistinct tumor margins [22]. Additionally, the T2-fluid-attenuated inversion recovery (FLAIR) mismatch sign has been fairly recently introduced to identify IDH-mutant, 1p/19q intact tumors [23]. The T2-FLAIR mismatch sign is considered positive when a tumor shows homogeneously hyperintense T2 signal with relatively low signal intensity on FLAIR, except for a hyperintense peripheral rim. The sign is highly specific and helps to rule in IDH-mutant, 1p/19q intact tumors when positive [24].
HISTOPATHOLOGIC CHARACTERISTICS AND RELEVANT MOLECULAR PROFILES
Astrocytoma, IDH-mutant
In the new 2021 WHO classification, WHO grade 2, 3, and 4 astrocytomas with IDH mutations are all included in the diagnosis of astrocytoma, IDH-mutant. Astrocytic tumors typically show diffusely infiltrating fibrillary glial cells with a microcytic background and regional heterogeneity. Astrocytomas are classified as grade 3 when nuclear atypia and increased mitotic activity are present (Fig. 2), and grade 4 when microvascular proliferation or necrosis is present. An IDH mutation (IDH1 or IDH2) is one of the most important mutations in adult diffuse glioma, having both diagnostic and prognostic significance. IDH mutations lead to the overproduction and accumulation of the 2-hydroxyglutarate metabolite [25]. This leads to alteration in global DNA methylation and ultimately results in changes in the epigenetic status and blocking of cellular differentiation [26,27]. Ironically, IDH mutations, which seem to instigate tumorigenesis, are associated with positive prognostic factors. Gliomas with IDH mutations have much better prognosis than their IDH-wildtype counterparts [28].
Fig. 2. Diffuse astrocytoma, IDH-mutant, grade 3.
(A) A T2-FLAIR image and (B) a T1 contrast-enhanced image show an ill-defined T2 high SI lesion without significant contrast enhancement in the right frontal cortex. The tumor shows (C) increased cellularity and nuclear polymorphism, (D) an intermediate mitotic count (3/10 HPF) on PHH3 immunohistochemistry (arrowhead), (E) IDH1 mutation positivity, and (F) ATRX mutation positivity (ATRX loss).
FLAIR, fluid-attenuated inversion recovery; SI, signal intensity; PHH3, phosphohistone H3; HPF, high-power field; IDH, isocitrate dehydrogenase, ATRX, alpha-thalassemia/mental retardation, X-linked.
The majority of IDH-mutant gliomas are also associated with loss-of-function mutations in ATRX and TP53 [29]. ATRX is an X-linked gene associated with alpha thalassemia and intellectual disability located in Xq21.1. Its loss of function is most importantly associated with lengthening of telomeres, which essentially leads to a more robust oncogenic cellular proliferation [30]. ATRX mutation is mutually exclusive with 1p/19q-codeletion. Therefore, oligodendroglioma (1p/19q-codeleted) can be ruled out in tumors with ATRX mutation without additional 1p/19q testing. ATRX mutant gliomas also commonly harbor a mutation of TP53, which is a well-known tumor suppressor gene located in 17p13.1. TP53 mutation leads to unregulated oncogenic cellular proliferation. CDKN2A/B is located in 9p21.3 and is associated with regulating Rb and p53-dependent signaling pathways [31]. CDKN2A/B mutations in astrocytic gliomas have shown strong associations with poorer survival [32]. Therefore, when a CDKN2A/B mutation is present, astrocytoma, IDH-mutant, WHO grade 4 can be diagnosed even in the absence of high-grade histopathologic features.
Oligodendroglioma, IDH-mutant and 1p/19q-codeleted
Classically, oligodendroglioma (WHO grade 2) is a relatively slow-growing tumor with a predilection for the frontal lobe and is often associated with seizures [33]. Common histopathologic findings include a “fried egg” appearance with uniformly rounded nuclei and clear halos, “chicken-wire” patterned branching capillaries, and extensive calcifications [34]. Oligodendroglioma with features of microvascular proliferation, necrosis, and/or significantly increased mitotic activity is classified as WHO grade 3 and shows more aggressive features than WHO grade 2 tumors (Fig. 3). 1p/19q-codeletion is a disease-defining chromosomal alteration resulting from failed translocation of the corresponding chromosomes. The molecular manifestations that result from this alteration are currently unknown. However, it is generally associated with a favorable prognosis and a good response to chemotherapy [35].
Fig. 3. Oligodendroglioma, IDH-mutant, 1p/19q-codeleted.
(A) A T2-FLAIR image and (B) a T1 contrast-enhanced image show an ill-defined T2 high-SI lesion without significant contrast enhancement in the left frontoparietal lobe. (C) Gross photograph of the resected tumor. The tumor shows (D) a “fried egg” appearance with uniformly rounded nuclei and clear halos, (E) IDH1 mutation positivity, (F) ATRX mutation negativity, and (G) 1p/19q co-deletion.
IDH, isocitrate dehydrogenase; FLAIR, fluid-attenuated inversion recovery; SI, signal intensity; ATRX, alpha-thalassemia/mental retardation, X-linked; CNV, copy number variation.
Glioblastoma, IDH-wildtype
Glioblastoma is a highly malignant tumor that occurs most commonly in elderly patients, accounting for around 49% of primary malignant brain tumors [36]. It is characterized by rapid progression and has a median survival of 14–16 months after diagnosis [37]. The histologic hallmark of glioblastoma is necrosis and vascular proliferation, commonly seen together with marked pleomorphism and increased mitotic activity (Fig. 4) [38]. Molecular alterations including EGFR amplification, TERT promoter mutation, and gain of chromosome 7/loss of chromosome 10 have shown to be strongly associated with glioblastoma. If one of these three molecular alterations is present, glioblastoma can be diagnosed even in the absence of high-grade histopathologic features.
Fig. 4. Glioblastoma, IDH-wild type.
(A) A T2-FLAIR image and (B) a T1 contrast-enhanced image show an enhancing mass in the left parietal lobe with multiple necrosis and perilesional T2 high-SI infiltrations. The tumor shows (C) necrosis and microvascular proliferation, (D) IDH1 mutation negativity, and (E) positive results for MGMT methylation.
IDH, isocitrate dehydrogenase; FLAIR, fluid-attenuated inversion recovery; SI, signal intensity; MGMT, O-5-methylguanine-DNA methyltransferase.
EGFR (also known as HER1 or ERBB1) is located on chromosome 7q and its protein product serves as a type of transmembrane receptor tyrosine kinase. The EGFRvIII mutation is defined as deletion of exons 2–7 of the EGFR gene and is the most commonly seen EGFR mutation in glioblastoma. The EGFRvIII mutation is closely associated with EGFR amplification and is known to promote cell proliferation, angiogenesis, and invasion in various models [39]. TERT promoter mutations are associated with abnormal maintenance of telomeres, which ultimately leads to cellular longevity and cellular proliferation [40]. TERT mutations are mutually exclusive with ATRX mutations and are commonly seen in glioblastoma. The most significant aneuploidy harbored by glioblastoma involves chromosomes 7 and 10, typically showing trisomy of chromosome 7 and monosomy of chromosome 10 [41]. MGMT encodes a DNA repair enzyme that functions to remove alkyl adducts from DNA, preventing double-strand breaks and base mispairing [42]. Methylation of the MGMT promoter silences this gene and leads to inefficient repair of DNA alkylation, thereby enhancing the response to alkylating chemotherapeutic agents such as temozolomide [43]. Therefore, an evaluation for MGMT promoter methylation is advised when glioblastoma is diagnosed as a supportive measure to predict the response to temozolomide treatment and overall survival.
MOLECULAR TESTING METHODS
Various molecular testing methods are used to detect genetic mutations in gliomas, which have now become a prerequisite information for the diagnosis of these tumors. Classically, immunohistochemistry studies have been universally used to supplement the conventional hematoxylin and eosin histology [44]. The IDH1 mutation, which is a gain-of-function mutation, can be detected using mutation-specific monoclonal antibodies [45]. The ATRX mutation is detected by identifying the loss of nuclear ATRX, since it is a loss-of-function mutation. Fluorescence in situ hybridization has been most frequently used to identify EGFR amplification, 1p/19q-codeletion, and chromosome 7 and 10 alterations. The test requires a minimal tissue sample and can even detect deletions smaller than whole-arm deletions [46]. TERT promoter mutations could be detected by direct sequencing, whereas MGMT promoter methylation status is verified using methylation-specific polymerase chain reaction.
In recent years, next-generation sequencing (NGS) has been actively embraced by a growing number of institutions as the standard diagnostic tool for detecting gene mutations when diagnosing gliomas [47,48]. NGS enables an efficient analysis of a large number of genetic variations in a single session by sequencing millions of small fragments of DNA in parallel [49]. NGS technology continues to be developed with the goal of reducing its costs and improving the accuracy of DNA sequencing. Although NGS is not yet used in all medical institutions, many efforts are being made to utilize NGS in clinical practice.
SUMMARY
Advances in cancer genomics and molecular testing methods have led to a new era of glioma diagnosis. The integration of molecular profiles into the diagnosis of gliomas has resulted in a clearer classification of these tumors. The new classification based on an integrated diagnosis is expected to help better understand and treat gliomas, as different genomic signatures lead to different clinical outcomes.
Footnotes
Funding: None.
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 2.Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJ, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang H, Song SW, Ha J, Won YJ, Park CK, Yoo H, Jung KW. A nationwide, population-based epidemiology study of primary central nervous system tumors in Korea, 2007-2016: a comparison with United States data. Cancer Res Treat. 2021;53:355–366. doi: 10.4143/crt.2020.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez A, Huse JT. The evolving classification of diffuse gliomas: World Health Organization updates for 2021. Curr Neurol Neurosci Rep. 2021;21:67. doi: 10.1007/s11910-021-01153-8. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. World Health Organization classification of tumours of the central nervous system. 5th ed. Lyon: International Agency for Research on Cancer; 2021. [Google Scholar]
- 8.Komori T. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: the 10 basic principles. Brain Tumor Pathol. 2022;39:47–50. doi: 10.1007/s10014-022-00428-3. [DOI] [PubMed] [Google Scholar]
- 9.Appay R, Dehais C, Maurage CA, Alentorn A, Carpentier C, Colin C, Ducray F, Escande F, Idbaih A, Kamoun A, Marie Y, Mokhtari K, Tabouret E, Trabelsi N, Uro-Coste E, Delattre JY, Figarella-Branger D POLA Network. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-oncol. 2019;21:1519–1528. doi: 10.1093/neuonc/noz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesileanu CMS, Dirven L, Wijnenga MMJ, Koekkoek JAF, Vincent AJPE, Dubbink HJ, Atmodimedjo PN, Kros JM, van Duinen SG, Smits M, Taphoorn MJB, French PJ, van den Bent MJ. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro-oncol. 2020;22:515–523. doi: 10.1093/neuonc/noz200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128:47–58. doi: 10.1002/cncr.33918. [DOI] [PubMed] [Google Scholar]
- 12.Colman H. Adult gliomas. Continuum (Minneap Minn) 2020;26:1452–1475. doi: 10.1212/CON.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen BK, Hansen S, Laursen RJ, Kosteljanetz M, Schultz H, Nørgård BM, Guldberg R, Gradel KO. Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I-IV in the the Danish Neuro-Oncology Registry. J Neurooncol. 2017;135:571–579. doi: 10.1007/s11060-017-2607-5. [DOI] [PubMed] [Google Scholar]
- 14.Peeters MC, Dirven L, Koekkoek JA, Gortmaker EG, Fritz L, Vos MJ, Taphoorn MJ. Prediagnostic symptoms and signs of adult glioma: the patients’ view. J Neurooncol. 2020;146:293–301. doi: 10.1007/s11060-019-03373-y. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Hansen M, Berendse S, Hamilton W. Symptomatic diagnosis of cancer of the brain and central nervous system in primary care: a systematic review. Fam Pract. 2015;32:618–623. doi: 10.1093/fampra/cmv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 17.Posti JP, Bori M, Kauko T, Sankinen M, Nordberg J, Rahi M, Frantzén J, Vuorinen V, Sipilä JO. Presenting symptoms of glioma in adults. Acta Neurol Scand. 2015;131:88–93. doi: 10.1111/ane.12285. [DOI] [PubMed] [Google Scholar]
- 18.Badve C, Kanekar S. Radiogenomics of gliomas. Radiol Clin North Am. 2021;59:441–455. doi: 10.1016/j.rcl.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Smits M, van den Bent MJ. Imaging correlates of adult glioma genotypes. Radiology. 2017;284:316–331. doi: 10.1148/radiol.2017151930. [DOI] [PubMed] [Google Scholar]
- 20.Carrillo JA, Lai A, Nghiemphu PL, Kim HJ, Phillips HS, Kharbanda S, Moftakhar P, Lalaezari S, Yong W, Ellingson BM, Cloughesy TF, Pope WB. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol. 2012;33:1349–1355. doi: 10.3174/ajnr.A2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi S, Yu L, Li H, Ou Y, Qiu X, Ding Y, Han H, Zhang X. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014;7:1895–1902. doi: 10.3892/ol.2014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smits M. Update on neuroimaging in brain tumours. Curr Opin Neurol. 2021;34:497–504. doi: 10.1097/WCO.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 23.Patel SH, Poisson LM, Brat DJ, Zhou Y, Cooper L, Snuderl M, Thomas C, Franceschi AM, Griffith B, Flanders AE, Golfinos JG, Chi AS, Jain R. T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res. 2017;23:6078–6085. doi: 10.1158/1078-0432.CCR-17-0560. [DOI] [PubMed] [Google Scholar]
- 24.Corell A, Ferreyra Vega S, Hoefling N, Carstam L, Smits A, Olsson Bontell T, Björkman-Burtscher IM, Carén H, Jakola AS. The clinical significance of the T2-FLAIR mismatch sign in grade II and III gliomas: a population-based study. BMC Cancer. 2020;20:450. doi: 10.1186/s12885-020-06951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM, Dang L, Fantin VR, Mak TW. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeWeerdt S. The genomics of brain cancer. Nature. 2018;561:S54–S55. doi: 10.1038/d41586-018-06711-8. [DOI] [PubMed] [Google Scholar]
- 28.Śledzińska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. 2021;22:10373. doi: 10.3390/ijms221910373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XY, Gerges N, Korshunov A, Sabha N, Khuong-Quang DA, Fontebasso AM, Fleming A, Hadjadj D, Schwartzentruber J, Majewski J, Dong Z, Siegel P, Albrecht S, Croul S, Jones DT, Kool M, Tonjes M, Reifenberger G, Faury D, Zadeh G, Pfister S, Jabado N. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124:615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 30.Bai J, Varghese J, Jain R. Adult glioma WHO classification update, genomics, and imaging: what the radiologists need to know. Top Magn Reson Imaging. 2020;29:71–82. doi: 10.1097/RMR.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 31.Pacifico A, Leone G. Role of p53 and CDKN2A inactivation in human squamous cell carcinomas. J Biomed Biotechnol. 2007;2007:43418. doi: 10.1155/2007/43418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, Koelsche C, Wefers A, Reinhardt A, Huang K, Sievers P, Shimizu H, Nanjo H, Kobayashi Y, Miyake Y, Suzuki T, Adachi JI, Mishima K, Sasaki A, Nishikawa R, Bewerunge-Hudler M, Ryzhova M, Absalyamova O, Golanov A, Sinn P, Platten M, Jungk C, Winkler F, Wick A, Hänggi D, Unterberg A, Pfister SM, Jones DTW, van den Bent M, Hegi M, French P, Baumert BG, Stupp R, Gorlia T, Weller M, Capper D, Korshunov A, Herold-Mende C, Wick W, Louis DN, von Deimling A. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. doi: 10.1007/s00401-018-1849-4. [DOI] [PubMed] [Google Scholar]
- 33.Van den Bent MJ, Reni M, Gatta G, Vecht C. Oligodendroglioma. Crit Rev Oncol Hematol. 2008;66:262–272. doi: 10.1016/j.critrevonc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Wesseling P, van den Bent M, Perry A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:809–827. doi: 10.1007/s00401-015-1424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 36.Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro-oncol. 2021;23:iii1–iiiiii105. doi: 10.1093/neuonc/noab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: clinical presentation, diagnosis, and management. BMJ. 2021;374:n1560. doi: 10.1136/bmj.n1560. [DOI] [PubMed] [Google Scholar]
- 38.Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35:2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 39.An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37:1561–1575. doi: 10.1038/s41388-017-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell RJ, Rube HT, Xavier-Magalhães A, Costa BM, Mancini A, Song JS, Costello JF. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. 2016;14:315–323. doi: 10.1158/1541-7786.MCR-16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stichel D, Ebrahimi A, Reuss D, Schrimpf D, Ono T, Shirahata M, Reifenberger G, Weller M, Hänggi D, Wick W, Herold-Mende C, Westphal M, Brandner S, Pfister SM, Capper D, Sahm F, von Deimling A. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136:793–803. doi: 10.1007/s00401-018-1905-0. [DOI] [PubMed] [Google Scholar]
- 42.Thon N, Kreth S, Kreth FW. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. Onco Targets Ther. 2013;6:1363–1372. doi: 10.2147/OTT.S50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, Xia D, Lindeman NI, Wen PY, Chakravarti A, Mehta MP, Hegi ME, Stupp R, Aldape KD, Zadeh G. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro-oncol. 2019;21:167–178. doi: 10.1093/neuonc/noy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunbar E, Yachnis AT. Glioma diagnosis: immunohistochemistry and beyond. Adv Anat Pathol. 2010;17:187–201. doi: 10.1097/PAP.0b013e3181d98cd9. [DOI] [PubMed] [Google Scholar]
- 45.Tanboon J, Williams EA, Louis DN. The diagnostic use of immunohistochemical surrogates for signature molecular genetic alterations in gliomas. J Neuropathol Exp Neurol. 2016;75:4–18. doi: 10.1093/jnen/nlv009. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez FJ, Vizcaino MA, Lin MT. Recent advances on the molecular pathology of glial neoplasms in children and adults. J Mol Diagn. 2016;18:620–634. doi: 10.1016/j.jmoldx.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Synhaeve NE, van den Bent MJ, French PJ, Dinjens WNM, Atmodimedjo PN, Kros JM, Verdijk R, Dirven CMF, Dubbink HJ. Clinical evaluation of a dedicated next generation sequencing panel for routine glioma diagnostics. Acta Neuropathol Commun. 2018;6:126. doi: 10.1186/s40478-018-0633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahm F, Schrimpf D, Jones DT, Meyer J, Kratz A, Reuss D, Capper D, Koelsche C, Korshunov A, Wiestler B, Buchhalter I, Milde T, Selt F, Sturm D, Kool M, Hummel M, Bewerunge-Hudler M, Mawrin C, Schüller U, Jungk C, Wick A, Witt O, Platten M, Herold-Mende C, Unterberg A, Pfister SM, Wick W, von Deimling A. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131:903–910. doi: 10.1007/s00401-015-1519-8. [DOI] [PubMed] [Google Scholar]
- 49.Behjati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pract Ed. 2013;98:236–238. doi: 10.1136/archdischild-2013-304340. [DOI] [PMC free article] [PubMed] [Google Scholar]