Abstract
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has posed a serious threat to public health and has quickly become a global concern. The infection of SARS‐CoV‐2 begins with the binding of its spike protein to the receptor‐angiotensin‐converting enzyme 2 (ACE2), which, after a series of conformation changes, results in the fusion of viral‐cell membranes and the release of the viral RNA genome into the cytoplasm. In addition, infected host cells can express spike protein on their cell surface, which will interact with ACE2 on neighboring cells, leading to cell membrane fusion and the formation of multinucleated cells or syncytia. Both viral entry and syncytia formation are mediated by spike‐ACE2 interaction and share some common mechanisms of membrane fusion. Here in this review, we will summarize our current understanding of spike‐mediated membrane fusion, which may shed light on future broad‐spectrum antiviral development.
Keywords: antiviral agents, cell fusion, cellular effect, coronavirus, entry inhibitors, virus classification
1. INTRODUCTION
First identified in late December 2019, the coronavirus disease 2019 (COVID‐19) pandemic which had caused 581 348 223 infections including 6 412 121 deaths worldwide (https://coronavirus.jhu.edu/map.html, accessed on August 4, 2022), has quickly become a tremendous concern. The newly emerged human pathogen that has caused the pandemic, named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was rapidly characterized as a member of the subgenus Sarbecovirus of the genus Betacoronavirus and was shown to be closely related to two bat‐derived SARS‐like coronaviruses, bat‐SL‐CoVZC45 and bat‐SL‐CoVZXC21. 1 Notably, despite amino acid variations at some key residues, SARS‐CoV‐2 has a similar receptor‐binding domain (RBD) structure to that of SARS‐CoV, which caused the SARS outbreaks in China during 2002–2003. 2 , 3
Coronaviruses (CoVs) are enveloped viruses that can infect many species of animals including humans. They usually cause respiratory, enteric, hepatic, and neurologic diseases. 4 RNA viruses exhibit high rates of mutation and CoVs are estimated to have moderate to high mutation rates compared to other single‐stranded RNA viruses. 5 After more than 2 years of circulating in the human population, SARS‐CoV‐2 has accumulated mutations that lead to genetic drift and escape from immune recognition. 6 As of May 2022, World Health Organization has designated five variants of concern (VOCs), 7 and the current global epidemiology of SARS‐CoV‐2 is characterized by the continuous rapid global spread of the Omicron variant. To develop effective treatments and a better suppression strategy against this virus, more efforts need to be put in elucidating the virus life cycle and pathogenesis of this disease.
2. SARS‐COV‐2 AND ITS RECEPTORS
Coronavirus contains a single‐stranded positive‐sense RNA genome ranging from 26.4 to 31.7 kilobases, 8 which is one of the largest genomes among RNA viruses. The SARS‐CoV‐2 genome has a 5′‐cap structure and 3′‐poly A tail, as well as the typical genome structure of coronaviruses 5 , 9 : the 5′‐untranslated region (UTR) is followed by ORF1ab, encoding 16 nonstructural proteins (nsp1 to nsp16) that are involved in genome transcription and replication. The 3′ terminal encodes four structural proteins: the spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins followed by the 3′‐UTR. Among these structural proteins, the S protein participates in recognition of the host cell receptor before invading target cells. 10
2.1. Spike protein
Structural analyses of S from SARS‐CoV‐2 as well as other members of coronavirus family showed that S forms homotrimer on the viral surface and protrudes from the viral membrane to form a distinctive crown‐like appearance. 3 , 11 , 12 These structures also revealed that the RBD in the S1 subunit is located at the outermost part of the trimer (Figure 1A), allowing S to easily access the receptor.
Figure 1.
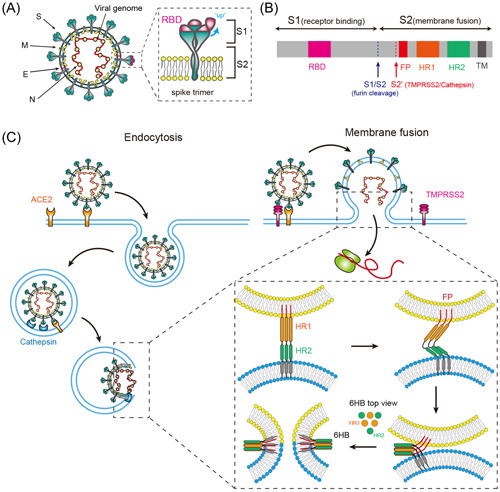
SARS‐CoV‐2 enters cells via endocytosis or cell surface membrane fusion. (A). Diagram of SARS‐CoV‐2 and spike trimer. S, spike; M, membrane; E, envelope; N, nucleocapsid protein; RBD, receptor‐binding domain. (B). The full‐length SARS‐CoV‐2 S protein. S could be cleaved by furin at S1/S2 and be processed by protease at S2′. FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat 2; TM, transmembrane domain. (C). Virus entry by endocytosis or membrane fusion. In the endocytic pathway, the entire viral particle enters the cell and fuses its membrane with the luminal side of the endosomal membrane. In the nonendocytosis pathway, a fusion pore is generated by the direct fusion of the viral membrane and cell membrane. Both pathways share similar mechanisms of membrane fusion. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
To bind to a host cell receptor, the RBDs of S1 undergo hinge‐like conformational movements that hide or expose the determinants of receptor binding, which are referred to as two distinct conformations, respectively: the “down” represents a receptor‐binding inactive state, and the “up” represents a receptor‐binding active state. 3 , 10 Interactions between S and the receptor destabilize the prefusion trimer, causing shedding of the S1 subunit and conformational changes in the S2 subunit, resulting in a stable postfusion conformation. 3 , 10
Unlike SARS‐CoV, the S of SARS‐CoV‐2 has been shown to have a polybasic cleavage site (PRRAR) at the S1/S2 site that can be cleaved by furin during biosynthesis 12 , 13 , 14 , 15 (Figure 1B). This cleavage site is required for efficient proteolytic cleavage of S, and well‐regulated cleavage at this site is essential for cell‐cell fusion and viral entry into human lung cells. 14 The SARS‐CoV‐2 Alpha variant (B.1.1.7) and Delta variant (B.1.617.2), which are both highly transmissible, exhibit mutations in position 681 that is adjacent to the furin cleavage site (HRRAR in Alpha, RRRAR in Delta). These mutations are predicted to alter furin cleavage and viral infection.
In addition, S is extensively decorated with host‐derived glycans. these glycosylations create sugary barriers called glycan shielding that is likely to be important for immune evasion. On the other hand, some glycans could be recognized as epitopes and elicit neutralizing antibodies. 16 Detailed information on S glycosylation and its role in virus infection has been discussed in a review. 17
2.2. ACE2 as SARS‐CoV‐2 receptors
Several molecules have been reported to be involved in the entry process of SARS‐CoV‐2 and act as either attachment factors or entry receptors, among which ACE2 is believed to be the most important functional receptor. ACE2 is a type I membrane protein with a single zinc‐binding catalytic motif, whose N‐terminal domain shares significant sequence homology with angiotensin‐converting enzyme (ACE). ACE2 is an important regulator in the renin‐angiotensin system, which is cardinal in renal and cardiovascular physiology and pathophysiology. 18
ACE2 also acts as a functional receptor for three coronaviruses: human CoV‐NL63, SARS‐CoV, and SARS‐CoV‐2. Among them, human CoV‐NL63 infection only causes mild symptoms, whereas SARS‐CoV and SARS‐CoV‐2 cause severe lower respiratory tract diseases. However, all three viruses can recognize and interact with common “virus‐binding hotspot” regions in ACE2. 19 , 20 , 21 , 22 It has been reported that SARS‐CoV‐2 RBD has higher ACE2 binding affinity than SARS‐CoV RBD, however, the full‐length S of SARS‐CoV‐2 has comparable ACE2 binding affinity to that of SARS‐CoV, 23 suggesting the SARS‐CoV‐2 RBD might be less exposed than SARS‐CoV RBD.
While the catalytic site and intracellular domain of ACE2 are not required for the infection, the interaction between peptidase domain (PD) of host ACE2 and RBD of S is shown to mediate the viral entry of SARS‐CoV‐2. 21 , 22 , 24 The structures of SARS‐CoV‐2 RBD/ACE2 complex have been determined by X‐ray crystallography and cryo‐electron microscopy. 3 , 21
ACE2 distribution in human tissues may indicate their susceptibility to SARS‐CoV‐2 infection. 25 Several studies have analyzed the expression of ACE2 in different tissues and reported that ACE2 protein is mainly expressed in the small intestine, kidney, gallbladder, testis, and to a lesser extent in the lung. 25 , 26 , 27 Although the respiratory route does not express the highest ACE2, it is the dominant route of infection, suggesting some in‐correlation between ACE2 expression and tissue susceptibility to SARS‐CoV‐2 infection.
The high expression of ACE2 in intestine and the successful detection of viral RNA in the stool and gastrointestinal tracts of patients with COVID‐19, indicate that fecal‐oral transmission is a potential transmission pathway. 28 However, one major obstacle to this transmission route is that SARS‐CoV‐2 could not tolerate gastric acid and survive passage into the gut. 28 , 29 Studies have shown that SARS‐CoV‐2 lost infectivity at low pH. 30 Consistent with this, patients taking proton pump inhibitors are more likely to be associated with higher infection rates or more severe clinical outcomes. 31
In the respiratory tract, higher ACE2 expression was detected in the nose, while lower expression throughout the lower respiratory tract was revealed by high‐sensitivity RNA in situ technology, 32 and this distribution is in parallel with the susceptibility to SARS‐CoV‐2 infection. In addition, although lung tissue has relatively low ACE2 expression, circulating soluble ACE2 (sACE2) could contribute to its high susceptibility to viral infection. 33 sACE2 is a form of protease‐cleaved ACE2 that is released into the plasma, which retains its ability to bind to SARS‐CoV‐2 and mediates viral endocytosis entry via AT1 or ACPR1B receptors. 33
2.3. Other co‐receptors and receptor‐independent virus infection
Besides ACE2, several other molecules have been reported to be involved in the SARS‐CoV‐2 entry process, which include neuropilin 1 (NRP1), phosphatidylserine receptors, the C‐type lectins, ASGR1, KREMEN1, and CD147. Detailed functions of these co‐receptors or cofactors have been summarized in reviews. 10 , 34
In addition, SARS‐CoV‐2 could also infect cells via receptor‐independent pathways. Viral spreads through cell‐cell contact have been proved in cell cultures, which is reported to be mediated by S. 35 Cell‐to‐cell transmission has been well documented for other viruses, like HIV and HCV, which could help virus to evade antibody neutralization, resulting in an efficient way of virus transferring to neighboring cells. ACE2 could enhance SARS‐CoV‐2 cell‐to‐cell transmission, but it is not absolutely required. 35
Another form of receptor‐independent transmission is mediated by exosomes or extracellular vesicles (EVs). EVs could transfer viruses or viral components from infected cells to healthy cells and thus facilitate infection. SARS‐CoV‐2 assembles in single membrane vesicles near ER‐Golgi intermediate compartment and egresses through exocytosis. 36 Large viral‐containing vesicles have been documented in SARS‐CoV‐2 infected cells and these multivesicular bodies are responsible for the generation of EVs. 36 , 37 EVs could transfer viruses or viral components to target cells by internalization, direct‐fusion or receptor‐ligand interaction. 37 Recent studies have indicated that EVs were involved in SARS‐CoV‐2 viral spreading and pathogenesis. 38 , 39 On the other hand, EVs could also transfer receptors, like ACE2 to receipt cells, 40 thus further contributing to the in‐correlation of ACE2 expression and SARS‐CoV‐2 tropism.
3. SPIKE MEDIATED VIRUS‐CELL MEMBRANE FUSION
Enveloped viruses exploit two basic routes to enter cells: the endocytic and non‐endocytic pathways. Both entry mechanisms have been reported for SARS‐CoV‐2, and the specific entry pathway to infect cells is mainly determined by the expression of host proteases (Figure 1C). 10 , 41 Cell entry of coronaviruses begins with the binding of S to the cellular receptors, followed by the activation of S before membrane fusion. S activation, the prerequisite event for membrane fusion, involves protease cleavage at two different locations, S1/S2 and S2′. 42
3.1. S1/S2 cleavage by furin
As described above, a polybasic cleavage site (PRRAR) at the S1/S2 boundary of SARS‐CoV‐2 S can be cleaved by furin in the infected cells, resulting in the formation of two noncovalently linked subunits with different functions‐S1 for receptor binding and S2 for membrane fusion. The S1 subunit in this non‐covalently linked structure is prone to premature dissociation, while the D614G mutation features new interactions that prevent premature loss of S1 subunit and enhances infectivity. 43 , 44 The cleavage of S1/S2 is essential for entry of SARS‐CoV‐2 into human lung cells as well as cell‐cell fusion. 14 In addition, this cleavage site has also been indicated to affect viral fitness and viral transmission, 45 , 46 and thus plays an important role in the infectivity of this virus. Notably, NRP1 has been shown to interact with the carboxyl‐terminal of cleaved S1 and enhanced viral entry into host cells. 47 , 48 As NRP1 is abundantly expressed in the olfactory epithelium of the nasal cavity, it has been suggested to be involved in the spread and infection of the virus in the central nervous system. 49
Furthermore, S1/S2 cleavage is prerequisite for the cleavage at the S2′ site. 50 Although cleavage at S1/S2 is essential for SARS‐CoV‐2 infection, this cleavage does not produce conformational changes necessary to induce membrane fusion. A further cleavage at the S2′ site mediated either by TMPRSS2 on cell surface or by cathepsins in endosome is required to expose the fusion peptide (FP) and initiate the fusion of membranes. 10 , 51
3.2. Direct membrane fusion and TMPRSS2
TMPRSS2 was first identified as a virus‐activating protease that cleaves the HA glycoprotein of influenza A virus. 52 TMPRSS2 belongs to type II transmembrane serine protease (TTSP) that is characterized by an N‐terminal transmembrane domain, a stem region, and a C‐terminal extracellular serine protease domain that contains a catalytic triad essential for proteolytic activity, comprising histidine, aspartic acid and serine. 53 TMPRSS2 is widely expressed in epithelial cells of respiratory tract, heart, prostate and gastrointestinal tracts. 42 , 54 , 55 It has been associated with a wide range of physiological and pathological processes, such as digestion, tissue remodeling, blood coagulation, fertility, inflammatory responses, and tumor cell invasion. 56
TMPRSS2 has been reported to be involved in cell entry of influenza virus H1N1, H7N9, and human coronaviruses HCoV‐229E, MERS‐CoV, and SARS‐CoV. 56 , 57 In vivo studies demonstrated that TMPRSS2‐deficient mice showed less severe lung pathology when infected with certain influenza A virus strains, SARS‐CoV or MERS‐CoV, 58 , 59 , 60 suggesting TMPRSS2 is an essential host factor for these viruses. Therefore, inhibition of TMPRSS2 has been proposed as a promising approach for antiviral development. 57 It was then shown that TMPRSS2 inhibitor, camostat mesylate reduced the entry of SARS‐CoV‐2 into TMPRSS2‐expressing cells, such as the human airway cells, suggesting TMPRSS2 was also crucial for SARS‐CoV‐2 infection of respiratory tract. 61 In addition to TMPRSS2, other TTSPs or metalloproteases have also been reported to activate S. 62 , 63 In vitro studies confirmed that furin cleaved at the S1/S2 boundary while TMPRSS2 cleaved at the S2′ site, TMPRSS2 and furin could not compensate for each other for S activation. 42 Therefore, S activation involves a cleavage at S1/S2 during synthesis and then a second cleavage at S2′ during viral entry into target cells. In particular, TMPRSS2 cleaves S at single arginine or lysine residues located immediately upstream of FP, 42 allowing FP to insert into the host cell membrane, and thus mediate viral entry at cell surface. 10 , 56
3.3. Endocytosis and cathepsin
On the other hand, many in vitro studies using various cell lines have demonstrated that lysosomotropic agents could block viral entry, 13 , 24 , 61 suggesting an endocytic entry pathway for SARS‐CoV‐2. Further studies revealed that if cell surface proteases are absent or insufficient, SARS‐CoV‐2 prefers to enter cells via endocytosis. 10 , 64 Endocytosis is the process through which cells take in external substances by engulfing them with the cell membrane. Clathrin‐mediated endocytosis (CME) is the best‐characterized pathway, during which clathrin‐coated vesicles are formed and extracellular substances are engulfed within. 65 SARS‐CoV has been reported to enter cells via CME as well as other pathways. 66 , 67 Similarly, SARS‐CoV‐2 could also enter cells via CME, 24 , 68 during which the cytoplasmic tail and enzymatic activity of ACE2 are not required. 24 , 69
During endocytosis, the S protein conformation changes in an acidic environment. It is generally believed that the cleavage of S at the S2′ site during endocytosis is mediated by the host protease cathepsin, followed by FP exposure and membrane fusion. 10 , 70 However, some studies have proposed that the cleavage sites for cathepsins are upstream of the S2′ site, 71 , 72 therefore, the exact cleavage sites for cathepsin or whether there are other proteases involved need further investigation. Cathepsins are nonspecific proteases with endopeptidase and exopeptidase activity, contributing to protein degradation in late endosomes and lysosomes. 73 Cathepsins are highly expressed in most human tissues and there are three catalytic classes of cathepsins: aspartic (D and E), serine (G), and cysteine (B, C, K, L, S, and V) proteases, of which cathepsin B is required for Ebola virus entry, while cathepsin L is more important for SARS‐CoV and SARS‐CoV‐2 virus entry. 74 , 75
As SARS‐CoV‐2 could exploit two entry pathways for viral entry, combinational use of inhibitors targeting TMPRSS2 and Cathepsin displayed strong synergy in blocking virus entry. 76
3.4. TMPRSS2 dictates the entry route used by SARS‐CoV‐2 to infect host cells
SARS‐CoV‐2 could infect cells via membrane fusion at cell surface or endocytosis, and the specific pathway used in a given cell type mainly depends on the expression of proteases, in particular TMPRSS2. The route of membrane fusion is preferred when TMPRSS2 is expressed, while in the absence of this protease, the virus will employ endocytosis for viral entry. 77 A study showed that cell surface membrane fusion occurs within 10 min after infection in a pH‐independent manner, while the pH‐dependent endocytosis takes about 40–60 min postinfection. 41 Since both ACE2 and TMPRSS2 are expressed in the respiratory tract, especially the upper respiratory tract, 78 , 79 TMPRSS2‐mediated membrane fusion is believed to play an important role in the infection of respiratory epithelium, while endocytosis might act as an alternative pathway during the amplification of SARS‐CoV‐2 in extrapulmonary organs. 50 In addition, overexpression of TMPRSS2 in non‐TMPRSS2 expressing cells eliminated the dependence of infection on the cathepsin, 41 highlighting the importance of TMPRSS2 expression in determining whether the viruses employ either membrane fusion or endocytosis pathways.
3.5. Membrane fusion
Enveloped viruses have evolved to employ a largely conserved mechanism to promote the fusion of viral and cellular membranes. 80 , 81 For most viruses with class I fusion proteins, glycoproteins are all homotrimers that contain coiled‐coil structures that must be primed to become fusion‐competent. 80 After the FP inserts into the target membrane, conformation changes cause the α‐helical heptad repeats to fold back to form a six‐helix bundle (6HB), which will pull membranes to form fusion pores. 81
In the case of SARS‐CoV‐2, the S2 subunit responsible for membrane fusion forms a central helical bundle with the heptanucleotide repeat 1 (HR1) and HR2 that are essential for membrane fusion. 10 After S binding to ACE2, the S2′ cleavage site is exposed and later processed by cathepsin or TMPRSS2. S2′ cleavage causes conformational changes in S2 and probably the dissociation of S1, followed by FP inserting into cell membrane. This fusion intermediate features a long central three‐stranded coiled coil and the 6HB is formed by the folding back of HR2 to HR1. 10 6HB pulls the viral and cell membranes to apposition (two membranes brought close together) and facilitates the formation of the fusion pore, through which the viral genome is released into the cytosol (Figure 1C). 10
Membrane fusion is not a spontaneous process and several energy barriers must be overcome before fusion occurs. For example, energies are required for membrane apposition, membrane curvature deformation and fusion‐pore formation and expansion. 82 Like other viral protein‐mediated membrane fusion, free energy generated during S conformation changes from a metastable state to a thermodynamically more favorable state will be used to overcome the energy barrier for lipid bilayer fusion. 83 , 84 , 85
4. SPIKE MEDIATED CELL–CELL MEMBRANE FUSION
A highly similar yet distinct membrane fusion event during SARS‐CoV‐2 infection is the viral protein mediated cell‐cell fusion or syncytia formation. Syncytia are multinucleated cells generated by infected cells fusing with neighboring ones. Enveloped viruses belonging to different viral families can induce syncytia, including viruses from herpeviridae, paramyxoviridae, coronaviridae, retroviridae family, hepatitis C virus and Ebola virus et al. 86 Syncytia facilitate rapid viral spread, and it might also promote immune evasion and contribute to pathogenesis. 86
4.1. Methods for syncytia detection
The traditional method of syncytia assessment is to count multinucleated giant cells under a light microscope. While this method is straightforward, quantification is time‐consuming and difficult: small syncytia may be easily overlooked while some unfused cell clusters may be counted by mistake. To overcome these issues, different methods have been developed to quantify syncytia (Figure 2). Slack et al. 87 established a fusion‐dependent promoter activation and green fluorescent protein (GFP) expression system to quantify syncytia, in which a LacR‐IE1 transcriptional activator is expressed in one group of cells, while a second group of cells is transfected with a plasmid containing the GFP gene under the control of a LacR‐IE1 regulated promoter. Fusion of these two cells leads to promoter activation and GFP expression. 87 On the other hand, Buchrieser et al. 88 measured SARS‐CoV‐2 induced syncytia with split GFP system. Fusion of two groups of cells expressing GFP1‐10 and GFP11 respectively, leads to the complementation of GFP, which can be detected with a fluorescent microscope or the high‐content imaging system for screening purposes. Sanders et al. 89 employed dual‐fluorescence labeling to quantify SARS‐CoV‐2 induced syncytia, in which they used RFP to label ACE2‐expressing cells and GFP to label spike‐expressing cells. Cell fusion of these two types of cells leads to multinucleated cells with both colors.
Figure 2.
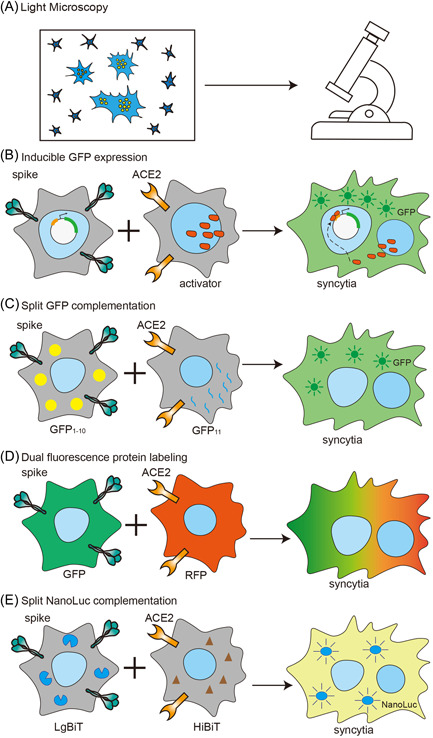
Diagrams showing different methods for syncytia detection. Syncytia could be observed with a light microscope for fused multinucleated cells (A); to detect the expression of fluorescence protein (B–D) or luciferase (E) in fused cells
In addition, syncytia could also be quantified with luminescence. Our group established a coculture system to measure spike‐induced syncytia based on split NanoLuc. 90 Fusion of cells expressing NanoLuc large subunit (LgBiT) and cells expressing small subunit (HiBiT) leads to complementation of NanoLuc luciferase, which can then be measured with live‐cell NanoLuc substrate. Compared to the split GFP system, this system is more rapid and sensitive and is more suitable for high‐throughput screening. With this system, we also showed that syncytia formation is much more dependent on ACE2 expression while moderate spike expression is able to induce syncytia, 90 partially explaining why syncytia is a commonly observed feature in SARS‐CoV‐2 infected tissue.
4.2. Syncytia formation as a pathological feature of severe COVID‐19
The presence of multinucleated pneumocytes has been well documented in severe COVID‐19 patients, 91 , 92 and patients who died as a direct consequence of SARS‐CoV‐2 infection had a higher rate of syncytia formation than those who died of other causes, 89 suggesting that syncytia contributed to the pathology of SARS‐CoV‐2 infections. Several COVID‐19 autopsies also confirmed the presence of multinucleated giant cells in lung tissues. 93 , 94 , 95
Syncytia could facilitate viral transmission as these large multinucleated syncytia could be released from the primary infection sites and then enable multiple viral genomes to be collectively transferred into single cells. Syncytia‐mediated viral spread has been proved during SARS‐CoV‐2 infection. 35 , 96 By using a reconstituted primary bronchial epithelia model, Beucher et al. 96 showed that syncytia released from the epithelium could transmit SARS‐CoV‐2 infection more efficiently than cell‐free supernatant, suggesting syncytia enable en bloc viral transmission. Another advantage of this syncytia‐mediated viral transmission is that it also enables viruses to spread without the need to assemble and/or enter the extracellular environment, thus protecting the virus from neutralizing antibodies as well as restriction factors that target viral assembly and release. 97
Although syncytia facilitate viral transmission, they might cause direct cytopathic effects to host cells. Syncytia‐related cell death has been well documented in viruses such as HIV and RSV, 98 , 99 and the syncytial death via apoptosis or pyroptosis could release viruses into surrounding cells and thus further spread viral infection. 97 Syncytia induced by SARS‐CoV‐2 S also led to cell death, which has been confirmed with in vitro cell culture models. 88 , 90 Ma et al. 100 further proved that the death of syncytia induced by SARS‐CoV‐2 infection was mediated by GSDME‐dependent pyroptosis.
In addition, syncytia have also been shown to target lymphocytes for cell death. Zhang et al. 94 reported that lymphocytes were detected within syncytia as a cell‐in‐cell structure among COVID‐19 patients. This phenomenon was further confirmed with a coculture cell model showing that syncytia could internalize different lymphocytes to form cell‐in‐cell structure and internalized cells readily underwent quick cell death. 94 These results suggested that syncytia might contribute to peripheral blood lymphocyte loss in patients.
4.3. Spike protein induces syncytia formation
Pathological studies of COVID‐19 patients or nonhuman primate models with syncytia suggest that most syncytia are of lung epithelial origin, but were generally negative for SARS‐CoV‐2 nucleocapsid protein. 89 , 101 Although many of the detailed mechanism of syncytia formation is unclear, it is generally believed that S protein expressed on infected cell surface interacts with the ACE2 receptor on neighboring cells and causes the syncytia formation, a process resembling the S‐mediated viral‐cell membrane fusion. Several studies have confirmed that syncytia could be recapitulated with in vitro cell culture systems by coculturing S‐expressing and ACE2‐expressing cells, 88 , 89 , 92 , 102 suggesting that S protein is the major fusogen protein involved. However, Buchrieser et al. 88 reported that no clear syncytia were formed in SARS‐CoV‐2 infected Caco2 cells, although these cells are permissive to infection, suggesting that syncytia formation is dependent on both viral and host factors (Figure 3).
Figure 3.
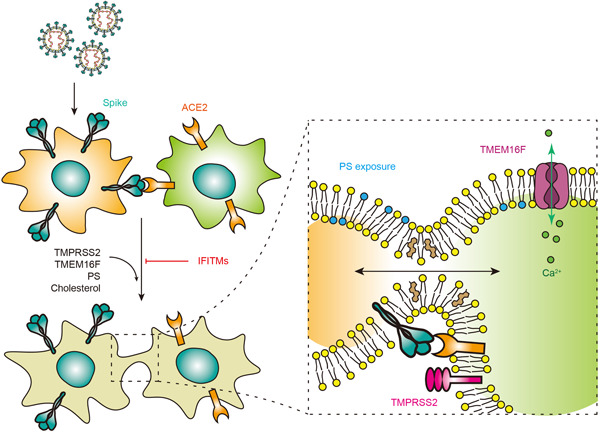
Spike mediated cell‐cell fusion. Syncytia are formed by S on infected cells interacting with ACE2 on neighboring cells. IFITMs could inhibit S‐mediated syncytia that can be reversed by TMPRSS2. In addition, TMEM16F, PS and cholesterol are host factors to enhance syncytia. PS, phosphatidylserine. ACE2, angiotensin‐converting enzyme 2
4.4. Viral factors affecting syncytia formation
The three highly pathogenic coronaviruses, MERS‐CoV, SARS‐CoV, and SARS‐CoV‐2 all could cause serious lung injury and fatal infections. However, their abilities to induce syncytia differ. First, S protein from SARS‐CoV has poor ability to induce syncytia, 14 , 94 while S from both MERS‐CoV and SARS‐CoV‐2 has been proven to cause syncytia. 14 Coincidentally, spikes from the latter two coronaviruses have the furin cleavage site, which is missing in SARS‐CoV S, suggesting that the furin cleavage site might play a role during syncytia formation. Consistently, inhibition of furin activity or mutations destroying the furin cleavage site of SARS‐CoV‐2 S have been proven to abolish syncytia, 14 , 102 and Zhang et al. 94 further proved that a bi‐arginine motif adjacent to the furin cleavage site containing R682 and R685 dictates syncytium formation. Second, compared to MERS‐CoV spike, a crucial third arginine at the furin cleavage site of SARS‐CoV‐2 conferred complete S1/S2 cleavage, while MERS‐CoV S was processed by furin at very low efficiency. 103 Consistently, nonhuman primates infected with MERS‐CoV showed fewer syncytia than those infected with SARS‐CoV‐2, 101 further arguing the role of furin cleavage in syncytia formation. In addition to the furin cleavage site, cellular proteases and ACE2 expression are also important factors in the formation of syncytia. TMPRSS2 moderately increased SARS‐CoV2 S‐driven fusion, while ACE2 strongly increased it. In contrast, TMPRSS2 strongly increased SARS‐CoV S‐driven fusion, while ACE2 moderately increased it. 104
As mentioned, S alone is sufficient to trigger syncytia in cells expressing ACE2 receptor. 88 , 94 , 102 Sequence analysis showed that the ER‐Golgi retrieval motif is not required for syncytia, however, a membrane‐proximal region of high cysteine content that can be palmitoylated is essential for membrane fusion. 89 Palmitoylation of this region has been reported to be required for murine coronavirus (mouse hepatitis virus, MHV), and SARS‐CoV spike‐mediated syncytia formation, 105 , 106 , 107 suggesting a conserved role of cysteine palmitoylation during coronavirus infection. In addition, other post‐translation modifications including phosphorylation could also affect cell‐cell fusion. For example, two serines at the edges of the furin cleavage site have been reported to be efficiently phosphorylated by proline‐directed and basophilic protein kinases, 103 which blocked furin cleavage and thus would compromise cell‐cell fusion.
4.5. S of VOC and syncytia formation
As an RNA virus, coronaviruses would accumulate mutations due to selection pressures during circulation. SARS‐CoV‐2 has evolved into different variants, among which, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529) have been listed as VOCs by the WHO. 7 Interestingly, some of the variants’ S proteins contain mutations that alter their ability to induce syncytia. Notably, the Delta S has been shown to promote syncytia formation with relatively larger and faster membrane fusion than the other variants. 24 , 108 , 109 , 110 In contrast, although the Omicron variant has increased transmissibility, its S protein has been shown to have a reduced ability to induce the formation of syncytia. 111 , 112 On the other hand, contradictory results have been reported for Alpha and Beta. Rajah et al. 108 reported S proteins of these variants are more syncytiogenic compared to Wuhan ancestral while Hoffmann et al. 113 showed that Alpha and Beta S did not increase syncytia formation. The discrepancy is possibly due to different assay systems employed by different groups.
Of all the mutations associated with the S protein, mutation at P681 draws a lot of attention due to its location at the S1/S2 cleavage site. Two kinds of naturally‐occurred mutations have been reported to be associated with variants, which are P681H (Alpha, Omicron) and P681R (Delta). 114 Both mutations have been suggested to enhance S cleavage efficiency and alter the ability of syncytia induction. 24 , 97 , 110 , 113 , 114 However, whether there is a direct correlation between S cleavage and syncytia formation still needs further investigation.
In addition to the S1/S2 cleavage efficiency, S‐ACE2 affinity has also been suggested to affect syncytia formation and high S‐ACE2 affinity correlates to high fusogenicity. 108 Mutations within the RBD, such as N501Y, K417N, and E484K, which are found in VOC, have been reported to increase the affinity of S protein to ACE2 and were associated with higher syncytia formation. 108
4.6. Host factors affecting syncytia formation
In addition to the fusogenic ability of S protein, many host factors can also modulate syncytia formation. Interferon‐induced transmembrane (IFITM) proteins are small transmembrane proteins that are products of interferon‐stimulated genes (ISGs). 115 IFITM can inhibit a broad spectrum of viral infections by blocking viral membrane fusion. 115 It has been reported that IFITMs could inhibit SARS‐CoV‐2 infection, although opposing results have also been reported. 116 , 117 , 118 SARS‐CoV‐2‐induced syncytia could be effectively inhibited by the expression of IFITMs, with IFITM1 being the most potent molecule. 88 , 108 But this inhibitory effect could be reversed by the expression of the serine protease, TMPRSS2, 88 which increased and accelerated S‐induced syncytia with or without IFITMs expression. In vitro cell culture model indicated that both IFITMs and TMPRSS2 modulate the efficiency of fusion when they are present in the same cell with ACE2, rather than in the S‐expressing cell. 88 Another ISG product that inhibits syncytia formation is lymphocyte antigen 6 complex, locus E (LY6E), 119 but the specific mechanism is not well understood.
An antisyncytial screening study with clinically approved drugs revealed that drugs regulating intracellular Ca2+ levels are top candidates. 92 Mechanism analysis revealed that the Ca2+‐activated TMEM16 family is essential for syncytia formation. In particular, overexpression of TMEM16F significantly stimulated S‐induced syncytia, while TMEM16F inhibition blunted syncytia. 92 TMEM16F, an ion channel protein, also functions as a lipid scramblase responsible for phosphatidylserine externalization onto the outer leaflet of the plasma membrane. Phosphatidylserine externalization is believed to serve as a conserved “fuse me” signal 120 and thus an important event for plasma membrane fusion.
In addition to phosphatidylserine, other lipid molecules are also essential for cell membrane fusion. Sanders et al. 89 identified several drugs that could perturb membrane lipid composition and inhibit syncytia through high‐throughput screening. In particular, cholesterol is required for syncytia formation but via a raft‐independent mechanism. Drugs such as 25‐hydroxycholesterol or methyl‐beta‐cyclodextrin that interfere with cell membrane cholesterol could inhibit syncytia in a dose‐dependent manner. 89 , 121 , 122
To summarize, successful infection of SARS‐CoV‐2 in human airway epithelial cells leads to viral replication and the expression of viral‐encoded proteins, including S. While most of the newly synthesized S accumulates in ER‐Golgi intermediate compartment for virion assembly, some of the S travels past the Golgi and expresses on cell surface. 123 , 124 , 125 The cell surface‐expressed S could then interact with ACE2 on neighboring cells and initiate cell–cell fusion. The expression of S on infected cell surface as well as ACE2 expression on target cells are both required for syncytia formation. 90 Similar to the process of viral‐cell membrane fusion, S1/S2 cleavage is also involved in syncytia formation. 14 , 46 , 90 Additionally, an efficient cell‐cell membrane fusion also requires cleavage at S2′ site, 126 which is further supported by the fact that TMPRSS2 enhances syncytia formation. 88 , 90 The exposure of the FP will then initiate the membrane fusion process, during which lipid bilayers rearrange with lots of host protein factors and lipid molecules mentioned above. SARS‐CoV‐2 induced multinucleated pneumocyte is a feature of severe COVID‐19 patients. Most of the syncytial cells end up dying together with internalized lymphocytes, with possible concomitant dissemination of viruses to initiate new infections.
5. PERSPECTIVES
Viral entry represents the first step of the virus life cycle and therefore an ideal target for antiviral research. For SARS‐CoV‐2 infection, inhibitors targeting Spike/ACE2 or their interaction, inhibitors targeting host proteases and endosome acidification, and inhibitors targeting membrane fusion have all been proposed and are in urgent development. 127 , 128 As enveloped viruses employ a highly conserved mechanism of membrane fusion for entry, antivirals targeting this process have emerged as a promising broad‐spectrum antiviral strategy. This strategy is superior to the traditional “one bug‐one drug” strategy in the era of emerging and re‐emerging infectious diseases. Some of the drugs target the physicochemical properties of membrane fusion process, which potentially broaden the antiviral spectrum, while others target the lipid components of membranes, presenting a potentially high barrier to resistance. 129 For fusogenic viruses like SARS‐CoV‐2, antimembrane fusion drugs will not only block viral infection, but also decrease damages caused by syncytia. With the advancement of our understanding towards membrane fusion, this kind of broad‐spectrum antiviral represents an encouraging antiviral paradigm.
AUTHORS CONTRIBUTIONS
Conceptualization: Hongliang Wang. Writing: Xinyu Li, Huijun Yuan, Xiaozhen Li, and Hongliang Wang. Visualization: Xinyu Li, and Hongliang Wang. Supervision: Hongliang Wang. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (81871662, 82150201, 82272309).
Li X, Yuan H, Li X, Wang H. Spike protein mediated membrane fusion during SARS‐CoV‐2 infection. J Med Virol. 2022;95:e28212. 10.1002/jmv.28212
Xinyu Li and Huijun Yuan contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were generated or analyzed in this study.
REFERENCES
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367:1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koyama T, Weeraratne D, Snowdon JL, Parida L. Emergence of drift variants that May affect COVID‐19 vaccine development and antibody treatment. Pathogens. 2020;9(5):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Tracking SARS‐CoV‐2 variants. https://wwwwhoint/en/activities/tracking-SARS-CoV-2-variants/. 2022.
- 8. Brant AC, Tian W, Majerciak V, Yang W, Zheng ZM. SARS‐CoV‐2: from its discovery to genome structure, transcription, and replication. Cell Biosci. 2021;11(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS‐CoV‐2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gui M, Song W, Zhou H, et al. Cryo‐electron microscopy structures of the SARS‐CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27(1):119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(2):281‐292 e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat Commun. 2020;11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann M, Kleine‐Weber H, Pohlmann S. A multibasic cleavage site in the spike protein of SARS‐CoV‐2 is essential for infection of human lung cells. Mol Cell. 2020;78(4):779‐784 e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xia S, Lan Q, Su S, et al. The role of furin cleavage site in SARS‐CoV‐2 spike protein‐mediated membrane fusion in the presence or absence of trypsin. Signal Transduct Target Ther. 2020;5(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinto D, Park YJ, Beltramello M, et al. Cross‐neutralization of SARS‐CoV‐2 by a human monoclonal SARS‐CoV antibody. Nature. 2020;583(7815):290‐295. [DOI] [PubMed] [Google Scholar]
- 17. Zhao X, Chen H, Wang H. Glycans of SARS‐CoV‐2 spike protein in virus infection and antibody production. Front Mol Biosci. 2021;8:629873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamming I, Cooper ME, Haagmans BL, et al. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu K, Li W, Peng G, Li F. Crystal structure of NL63 respiratory coronavirus receptor‐binding domain complexed with its human receptor. Proc Natl Acad Sci USA. 2009;106(47):19970‐19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science. 2005;309(5742):1864‐1868. [DOI] [PubMed] [Google Scholar]
- 21. Lan J, Ge J, Yu J, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215‐220. [DOI] [PubMed] [Google Scholar]
- 22. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367(6485):1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS‐CoV‐2. Proc Natl Acad Sci USA. 2020;117(21):11727‐11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang H, Yuan H, Zhao X, et al. Cytoplasmic domain and enzymatic activity of ACE2 are not required for PI4KB dependent endocytosis entry of SARS‐CoV‐2 into host cells. Virol Sin. 2022;37:380‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Wang Y, Luo W, et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS‐CoV‐2, in human tissues and blood cells. Int J Med Sci. 2020;17(11):1522‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal‐oral transmission of SARS‐CoV‐2. Nat Rev Gastroenterol Hepatol. 2021;18(4):269‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price E. Could the severity of COVID‐19 be increased by low gastric acidity? Crit Care. 2020;24(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan KH, Sridhar S, Zhang RR, et al. Factors affecting stability and infectivity of SARS‐CoV‐2. J Hosp Infect. 2020;106(2):226‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee SW, Ha EK, Yeniova AÖ, et al. Severe clinical outcomes of COVID‐19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70(1):76‐84. [DOI] [PubMed] [Google Scholar]
- 32. Hou YJ, Okuda K, Edwards CE, et al. SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429‐446 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeung ML, Teng JLL, Jia L, et al. Soluble ACE2‐mediated cell entry of SARS‐CoV‐2 via interaction with proteins related to the renin‐angiotensin system. Cell. 2021;184(8):2212‐2228 e2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans JP, Liu SL. Role of host factors in SARS‐CoV‐2 entry. J Biol Chem. 2021;297(1):100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeng C, Evans JP, King T, et al. SARS‐CoV‐2 spreads through cell‐to‐cell transmission. Proc Natl Acad Sci USA. 2022;119(1):e2111400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mendonça L, Howe A, Gilchrist JB, et al. Correlative multi‐scale cryo‐imaging unveils SARS‐CoV‐2 assembly and egress. Nat Commun. 2021;12(1):4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hassanpour M, Rezaie J, Nouri M, Panahi Y. The role of extracellular vesicles in COVID‐19 virus infection. Infect Genet Evol. 2020;85:104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon Y, Nukala SB, Srivastava S, et al. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS‐CoV‐2 coding sequence overexpressing lung epithelial cells. Stem Cell Res Ther. 2020;11(1):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zaid Y, Puhm F, Allaeys I, et al. Platelets can associate with SARS‐Cov‐2 RNA and are hyperactivated in COVID‐19. Circ Res. 2020;127(11):1404‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J, Chen S, Bihl J. Exosome‐mediated transfer of ACE2 (angiotensin‐converting enzyme 2) from endothelial progenitor cells promotes survival and function of endothelial cell. Oxid Med Cell Longev. 2020;2020:4213541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koch J, Uckeley ZM, Doldan P, Stanifer M, Boulant S, Lozach PY. TMPRSS2 expression dictates the entry route used by SARS‐CoV‐2 to infect host cells. EMBO J. 2021;40(16):e107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS‐CoV‐2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang J, Cai Y, Xiao T, et al. Structural impact on SARS‐CoV‐2 spike protein by D614G substitution. Science. 2021;372(6541):525‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang L, Jackson CB, Mou H, et al. SARS‐CoV‐2 spike‐protein D614G mutation increases virion spike density and infectivity. Nat Commun. 2020;11(1):6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lau SY, Wang P, Mok BW, et al. Attenuated SARS‐CoV‐2 variants with deletions at the S1/S2 junction. Emerg Microbes Infect. 2020;9(1):837‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peacock TP, Goldhill DH, Zhou J, et al. The furin cleavage site in the SARS‐CoV‐2 spike protein is required for transmission in ferrets. Nat Microbiol. 2021;6(7):899‐909. [DOI] [PubMed] [Google Scholar]
- 47. Cantuti‐Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science. 2020;370(6518):856‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daly JL, Simonetti B, Klein K, et al. Neuropilin‐1 is a host factor for SARS‐CoV‐2 infection. Science. 2020;370(6518):861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gudowska‐Sawczuk M, Mroczko B. The role of neuropilin‐1 (NRP‐1) in SARS‐CoV‐2 infection: review. J Clin Med. 2021;10(13):2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoffmann M, Pohlmann S. How SARS‐CoV‐2 makes the cut. Nat Microbiol. 2021;6(7):828‐829. [DOI] [PubMed] [Google Scholar]
- 51. Haque SM, Ashwaq O, Sarief A, Azad John Mohamed AK. A comprehensive review about SARS‐CoV‐2. Future Virol. 2020;15(9):625‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bottcher E, Matrosovich T, Beyerle M, Klenk HD, Garten W, Matrosovich M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol. 2006;80(19):9896‐9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284(35):23177‐23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paoloni‐Giacobino A, Chen H, Peitsch MC, Rossier C, Antonarakis SE. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics. 1997;44(3):309‐320. [DOI] [PubMed] [Google Scholar]
- 55. Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID‐19: serendipity or opportunity for intervention? Cancer Discov. 2020;10(6):779‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thunders M, Delahunt B. Gene of the month: TMPRSS2 (transmembrane serine protease 2). J Clin Pathol. 2020;73(12):773‐776. [DOI] [PubMed] [Google Scholar]
- 57. Shen LW, Mao HJ, Wu YL, Tanaka Y, Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hatesuer B, Bertram S, Mehnert N, et al. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog. 2013;9(12):e1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tarnow C, Engels G, Arendt A, et al. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J Virol. 2014;88(9):4744‐4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iwata‐Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6):e01815‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS‐CoV‐2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47):e01815‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jocher G, Grass V, Tschirner SK, et al. ADAM10 and ADAM17 promote SARS‐CoV‐2 cell entry and spike protein‐mediated lung cell fusion. EMBO Rep. 2022;23(6):e54305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tang T, Bidon M, Jaimes JA, Whittaker GR, Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178:104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kaksonen M, Roux A. Mechanisms of clathrin‐mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313‐326. [DOI] [PubMed] [Google Scholar]
- 66. Inoue Y, Tanaka N, Tanaka Y, et al. Clathrin‐dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81(16):8722‐8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin‐ and caveolae‐independent endocytic pathway. Cell Res. 2008;18(2):290‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bayati A, Kumar R, Francis V, McPherson PS. SARS‐CoV‐2 infects cells following viral entry via clathrin‐mediated endocytosis. J Biol Chem. 2021;296:100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Karthika T, Joseph J, Das VRA, et al. SARS‐CoV‐2 cellular entry is independent of the ACE2 cytoplasmic domain signaling. Cells. 2021;10(7):1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Q, Xiang R, Huo S, et al. Molecular mechanism of interaction between SARS‐CoV‐2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhao MM, Zhu Y, Zhang L, et al. Novel cleavage sites identified in SARS‐CoV‐2 spike protein reveal mechanism for cathepsin L‐facilitated viral infection and treatment strategies. Cell Discov. 2022;8(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bosch BJ, Bartelink W, Rottier PJ. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol. 2008;82(17):8887‐8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA. 2005;102(33):11876‐11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao MM, Yang WL, Yang FY, et al. Cathepsin L plays a key role in SARS‐CoV‐2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct Target Ther. 2021;6(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Padmanabhan P, Desikan R, Dixit NM. Targeting TMPRSS2 and cathepsin B/L together may be synergistic against SARS‐CoV‐2 infection. PLoS Comput Biol. 2020;16(12):e1008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murgolo N, Therien AG, Howell B, et al. SARS‐CoV‐2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLoS Pathog. 2021;17(2):e1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu Y, Qu HQ, Qu J, Tian L, Hakonarson H. Expression pattern of the SARS‐CoV‐2 entry genes ACE2 and TMPRSS2 in the respiratory tract. Viruses. 2020;12(10):1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hoffmann M, Hofmann‐Winkler H, Smith JC, et al. Camostat mesylate inhibits SARS‐CoV‐2 activation by TMPRSS2‐related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021;65:103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mas V, Melero JA. Entry of enveloped viruses into host cells: membrane fusion. SubCell Biochem. 2013;68:467‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. White JM, Whittaker GR. Fusion of enveloped viruses in endosomes. Traffic. 2016;17(6):593‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9(7):543‐556. [DOI] [PubMed] [Google Scholar]
- 83. Basanez G. Membrane fusion: the process and its energy suppliers. Cell Mol Life Sci. 2002;59(9):1478‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang J, Maschietto F, Guberman‐Pfeffer MJ, et al. Computational insights into the membrane fusion mechanism of SARS‐CoV‐2 at the cellular level. Comput Struct Biotechnol J. 2021;19:5019‐5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu Y, Qian R, Yang Y, Sheng Y, Li W, Wang W. Activation pathways and free energy landscapes of the SARS‐CoV‐2 spike protein. ACS Omega. 2021;6(36):23432‐23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leroy H, Han M, Woottum M, et al. Virus‐mediated cell‐cell fusion. Int J Mol Sci. 2020;21(24):9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Slack JM, Blissard GW. Measurement of membrane fusion activity from viral membrane fusion proteins based on a fusion‐dependent promoter induction system in insect cells. J Gen Virol. 2001;82(Pt 10):2519‐2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Buchrieser J, Dufloo J, Hubert M, et al. Syncytia formation by SARS‐CoV‐2‐infected cells. EMBO J. 2021;39(23):e106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sanders DW, Jumper CC, Ackerman PJ, et al. SARS‐CoV‐2 requires cholesterol for viral entry and pathological syncytia formation. eLife. 2021;10:e65962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang H, Guo S, Yang H. Rapid quantitative monitoring of SARS‐CoV‐2 spike protein‐mediated syncytia formation using split NanoLuc. J Med Virol. 2022;94:6073‐6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bussani R, Schneider E, Zentilin L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID‐19 pathology. EBioMedicine. 2020;61:103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Braga L, Ali H, Secco I, et al. Drugs that inhibit TMEM16 proteins block SARS‐CoV‐2 spike‐induced syncytia. Nature. 2021;594(7861):88‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Z, Zheng Y, Niu Z, et al. SARS‐CoV‐2 spike protein dictates syncytium‐mediated lymphocyte elimination. Cell Death Differ. 2021;28(9):2765‐2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS‐CoV‐2: the Mount Sinai COVID‐19 autopsy experience. Mod Pathol. 2021;34(8):1456‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Beucher G, Blondot ML, Celle A, et al. Bronchial epithelia from adults and children: SARS‐CoV‐2 spread via syncytia formation and type III interferon infectivity restriction. Proc Natl Acad Sci USA. 2022;119(28):e2202370119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rajah MM, Bernier A, Buchrieser J, Schwartz O. The mechanism and consequences of SARS‐CoV‐2 spike‐mediated fusion and syncytia formation. J Mol Biol. 2022;434:167280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ferri KF, Jacotot E, Geuskens M, Kroemer G. Apoptosis and karyogamy in syncytia induced by the HIV‐1‐envelope glycoprotein complex. Cell Death Differ. 2000;7(11):1137‐1139. [DOI] [PubMed] [Google Scholar]
- 99. Kotelkin A, Prikhod'ko EA, Cohen JI, Collins PL, Bukreyev A. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor‐related apoptosis‐inducing ligand. J Virol. 2003;77(17):9156‐9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ma H, Zhu Z, Lin H, et al. Pyroptosis of syncytia formed by fusion of SARS‐CoV‐2 spike and ACE2‐expressing cells. Cell Discov. 2021;7(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID‐19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cheng YW, Chao TL, Li CL, et al. Furin inhibitors block SARS‐CoV‐2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33(2):108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ord M, Faustova I, Loog M. The sequence at spike S1/S2 site enables cleavage by furin and phospho‐regulation in SARS‐CoV2 but not in SARS‐CoV1 or MERS‐CoV. Sci Rep. 2020;10(1):16944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hörnich BF, Großkopf AK, Schlagowski S, et al. SARS‐CoV‐2 and SARS‐CoV spike‐mediated cell‐cell fusion differ in their requirements for receptor expression and proteolytic activation. J Virol. 2021;95(9):e00002‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McBride CE, Machamer CE. Palmitoylation of SARS‐CoV S protein is necessary for partitioning into detergent‐resistant membranes and cell‐cell fusion but not interaction with M protein. Virology. 2010;405(1):139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Petit CM, Chouljenko VN, Iyer A, et al. Palmitoylation of the cysteine‐rich endodomain of the SARS‐coronavirus spike glycoprotein is important for spike‐mediated cell fusion. Virology. 2007;360(2):264‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yang J, Lv J, Wang Y, et al. Replication of murine coronavirus requires multiple cysteines in the endodomain of spike protein. Virology. 2012;427(2):98‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rajah MM, Hubert M, Bishop E, et al. SARS‐CoV‐2 alpha, beta, and delta variants display enhanced spike‐mediated syncytia formation. EMBO J. 2021;40(24):e108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mlcochova P, Kemp SA, Dhar MS, et al. SARS‐CoV‐2 B.1.617.2 delta variant replication and immune evasion. Nature. 2021;599(7883):114‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Saito A, Irie T, Suzuki R, et al. Enhanced fusogenicity and pathogenicity of SARS‐CoV‐2 delta P681R mutation. Nature. 2022;602(7896):300‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pia L, Rowland‐Jones S. Omicron entry route. Nat Rev Immunol. 2022;22(3):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao H, Lu L, Peng Z, et al. SARS‐CoV‐2 omicron variant shows less efficient replication and fusion activity when compared with delta variant in TMPRSS2‐expressed cells. Emerg Microbes Infect. 2022;11(1):277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hoffmann M, Arora P, Groß R, et al. SARS‐CoV‐2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384‐2393 e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mittal A, Khattri A, Verma V. Structural and antigenic variations in the spike protein of emerging SARS‐CoV‐2 variants. PLoS Pathog. 2022;18(2):e1010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liao Y, Goraya MU, Yuan X, Zhang B, Chiu SH, Chen JL. Functional involvement of Interferon‐Inducible transmembrane proteins in antiviral immunity. Front Microbiol. 2019;10:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shi G, Kenney AD, Kudryashova E, et al. Opposing activities of IFITM proteins in SARS‐CoV‐2 infection. EMBO J. 2021;40(3):e106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Winstone H, Lista MJ, Reid AC, et al. The polybasic cleavage site in SARS‐CoV‐2 spike modulates viral sensitivity to type I interferon and IFITM2. J Virol. 2021;95(9):e02422‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Prelli Bozzo C, Nchioua R, Volcic M, et al. IFITM proteins promote SARS‐CoV‐2 infection and are targets for virus inhibition in vitro. Nat Commun. 2021;12(1):4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pfaender S, Mar KB, Michailidis E, et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat Microbiol. 2020;5(11):1330‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Whitlock JM, Chernomordik LV. Flagging fusion: phosphatidylserine signaling in cell‐cell fusion. J Biol Chem. 2021;296:100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zang R, Case JB, Yutuc E, et al. Cholesterol 25‐hydroxylase suppresses SARS‐CoV‐2 replication by blocking membrane fusion. Proc Natl Acad Sci USA. 2020;117(50):32105‐32113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang S, Li W, Hui H, et al. Cholesterol 25‐Hydroxylase inhibits SARS‐CoV‐2 and other coronaviruses by depleting membrane cholesterol. EMBO J. 2020;39(21):e106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cattin‐Ortola J, Welch LG, Maslen SL, Papa G, James LC, Munro S. Sequences in the cytoplasmic tail of SARS‐CoV‐2 spike facilitate expression at the cell surface and syncytia formation. Nat Commun. 2021;12(1):5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Scherer KM, Mascheroni L, Carnell GW, et al. SARS‐CoV‐2 nucleocapsid protein adheres to replication organelles before viral assembly at the Golgi/ERGIC and lysosome‐mediated egress. Sci Adv. 2022;8(1):eabl4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ding S, Adam D, Beaudoin‐Bussières G, et al. SARS‐CoV‐2 spike expression at the surface of infected primary human airway epithelial cells. Viruses. 2021;14(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yu S, Zheng X, Zhou B, et al. SARS‐CoV‐2 spike engagement of ACE2 primes S2′ site cleavage and fusion initiation. Proc Natl Acad Sci USA. 2022;119(1):e2111199119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xiu S, Dick A, Ju H, et al. Inhibitors of SARS‐CoV‐2 entry: current and future opportunities. J Med Chem. 2020;63(21):12256‐12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chitsike L, Duerksen‐Hughes P. Keep out! SARS‐CoV‐2 entry inhibitors: their role and utility as COVID‐19 therapeutics. Virol J. 2021;18(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vigant F, Santos NC, Lee B. Broad‐spectrum antivirals against viral fusion. Nat Rev Microbiol. 2015;13(7):426‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were generated or analyzed in this study.


