Abstract
The present study developed a functional yoghurt supplemented with Lantiplantibacillus plantarum 200655 and evaluated its physicochemical properties and antioxidant activities. Yoghurt samples were prepared using commercial starter cultures and probiotics and grouped as follows: control sample without probiotics (C), GG (supplemented with Lacticaseibacillus rhamnosus GG), R (supplemented with L. plantarum KCTC 3108), and S (supplemented with L. plantarum 200655). The GG, R, and S samples had shorter fermentation time compared with the C sample. Lactic acid bacteria count, pH, and titratable acidity were similar in all samples during refrigerated storage. However, the GG, R, and S samples showed increased viscosity and water holding capacity (WHC), and decreased syneresis. The S sample had no adverse effect on organoleptic properties. Furthermore, the S sample had the highest antioxidant activity and significantly inhibited LPS-induced oxidative stress in intestinal cells. These findings suggest the potential use of L. plantarum 200655 in dairy products with therapeutic benefits.
Keywords: Yoghurt, Probiotics, ROS activity, Antioxidant activity, Functional food
Introduction
Oxidative stress occurs due to an imbalance between the free radicals production and the elimination capacity of them, which can damage biological components such as lipids, lipoproteins, proteins, membranes, and deoxyribonucleic acid (Pizzino et al., 2017). Oxidative stress is correlated with the natural aging process and pathogenesis of numerous diseases, including ulcers, arthritis, inflammatory bowel disease (IBD), cardiovascular disease, and cancer (Li et al., 2019). The human body has evolved a number of non-enzymatic defense mechanisms to combat the damaging effects of radical oxygen species (ROS). However, oxidative stress in the gastrointestinal tract is directly linked to diet and variations in the gut microbiota. Therefore, the beneficial effects and roles of gut microbiota on oxidative stress and pathogenesis of relevant diseases have been gaining interest (Kong et al., 2020). Furthermore, evidence showed that probiotics can reduce oxidative stress by modifying antioxidative enzymes (Yang et al., 2019). Lactobacillus and Bifidobacterium strains are considered the most important probiotics in this field. Recently, the amelioration of IBD by probiotics has been studied (Kawahara et al., 2015).
Probiotics are described as live microorganisms that present a helpful effect on the host when used in adequate amounts (FAO/WHO, 2002). Lactobacilli are beneficial microorganisms in fermented dairy products, have a long history of safety in foods, and are commonly accepted as safe by the Food and Drug Administration. These microorganisms also exhibit probiotic properties, which provide consumers with various health benefits (Widyastuti et al., 2021). Several Lactobacillus strains isolated from fermented foods, such as Korean kimchi, have been shown to improve cell-mediated immune responses, ameliorate hypocholesterolemia, and exhibit anti-allergy, antimutagenic, anticancer, and ROS scavenging activities (Lee et al., 2011; Ryu et al., 2014; Son et al., 2017; Yang et al., 2019). Furthermore, some reports have recommended the use of novel probiotics isolated from conventionally fermented foods in developing healthy fermented dairy products (Kariyawasam et al., 2021a). This is due to the ability of probiotics and other starter organisms to generate bioactive peptides, which are inactive in the primary structure of proteins and can be released during dairy fermentation via proteolytic activity of microbial enzymes. Bioactive peptides can be generated both from whey proteins such serum albumin, lactoferrin, α-lactalbumin, β-lactoglobulin, immunoglobulins, and protease-peptone fractions and from caseins of α-casein, β-casein, and κ-casein (Tonolo et al., 2020). These bioactive peptides are associated with therapeutic properties, such as antimicrobial, opioid, antithrombotic, immunomodulating, cyto-modulating properties, and antioxidant (Choi et al., 2012). Furthermore, the antioxidant activity of bioactive peptides can be affected by the amino acids sequence in the structure as well as the size of the peptides (Mushtaq et al., 2016). A novel approach to reducing oxidative stress is to consume probiotic yoghurt, which has antioxidant activity and counteracts oxidative stress. There have been several reports on the effect of probiotic yoghurt on oxidative stress alleviation using Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 (Rezazadeh et al., 2021), Levilactobacillus brevis 200019 (Kariyawasam et al., 2021b), and Lactobacillus delbrueckii subsp. bulgaricus DSM 20080 and L. acidophilus ATCC 4356 (El-Khadragy et al., 2019).
When selecting probiotic strains for application in foods, viability in fermentation and storage and sensory properties must be considered (Daniel et al., 2010). Lantiplantibacillus plantarum 200655, isolated from Korean kimchi, exhibits radical scavenging activity, lipid peroxidation inhibition activity, and immunomodulatory activity in vitro (Yang et al., 2019). The aim of this study was to define the potential use of L. plantarum 200655 as a probiotic organism in yoghurt and evaluate whether the developed yoghurt can inhibit LPS-induced oxidative stress in intestinal cells.
Materials and methods
Bacterial strains and culture methods
Lacticaseibacillus rhamnosus GG (LGG) is a clinical probiotic strain that has been extensively researched and is widely used as a standard probiotic strain. L. plantarum KCTC 3108 was originally isolated from pickled cabbage and was recommended for use as a reference organism in academic studies. Therefore, LGG and L. plantarum KCTC 3108 were used as the reference probiotics in this study. Probiotic cultures were propagated and retained in lactobacilli MRS broth (BD Difco™, Franklin Lakes, NJ, USA) at 37 °C. The yoghurt starter cultures included Streptococcus thermophilus and Lactobacillus bulgaricus were provided by Culture Systems Inc. (Mishawaka, IN, USA) in freeze-dried form.
Preparation of yoghurt samples
Yoghurt samples were prepared as described by Kariyawasam et al. (2021b). Reconstituted skim milk (12%) was heated (85 °C, 30 min) and subsequently cooled (to 37 °C) before adding 0.01% (w/v) commercial starter culture or probiotics (initial inoculum level, 7 Log CFU/mL). Yoghurt mixtures added into 50 mL containers and incubated at 42 °C until the pH reached 4.5 ± 0.1. The yogurt samples were labeled C (starter culture), GG (starter culture and L. rhamnosus GG), R (starter culture and L. plantarum KCTC 3108), and S (starter culture and L. plantarum 200655) as fermentation starter. The samples were stored under refrigerated conditions (4 °C) until further analysis.
Lactic acid bacteria (LAB) counts, pH, and titratable acidity
LAB count of the yoghurt samples was determined over the storage period at weekly intervals (0, 7, 14, and 21 days) by plating on MRS agar at the dilutions followed by incubation at 37 °C for 48 h (Kariyawasam et al., 2020). The pH of the samples was determined using a pH meter (WTW, Weilheim, Germany) throughout fermentation and over the storage period at weekly intervals. Titratable acidity was determined at weekly intervals during the storage period by titrating 10 g of sample with NaOH until the pH reached 8.2. The titratable acidity was measured using the following equation:
Physicochemical and organoleptic properties of the yoghurt samples
Yoghurt samples were separated by centrifugation at 600×g for 6 min. The clear supernatant was poured out and weighed to define the water holding capacity (WHC) and syneresis values (Zhang et al., 2019). The viscosity values of the yoghurt samples were determined using a DV-E viscometer (Brookfield, Toronto, Canada). The color values were measured using a colorimeter (Konica Minolta, Inc., Tokyo, Japan) as L* [darkness (0) − lightness (100)], a* [greenness (− 60) – redness (60)], and b* [blueness (− 60) − yellowness (60)].
The organoleptic properties of the developed yoghurt samples were valued using a 5-point hedonic scale: 5- highly acceptable, 1- not acceptable. A semi-trained panel of 30 members conducted an organoleptic evaluation, including the color and appearance, body and texture, flavor, aroma, and overall acceptability (ISO, 2017). This study was certified for organoleptic evaluation by the Institutional Review Board (IRB) of Konkuk University (7001355-202001-E-107).
Preparation of water-soluble extract (WSE)
WSEs of the yoghurt samples were made as depicted by Sah et al. (2016). The yoghurt samples were separated by centrifugation at 14,000×g at 4 °C for 30 min. The supernatant was filtered through a 0.45 μm filter, and the filtrate was freeze-dried. The protein content of the freeze-dried samples was determined using Bradford assay with a bovine serum albumin (BSA, 0.1–1.4 mg/mL) standard. The samples were remained at − 80 °C and used for further analysis.
Radical scavenging activity of the yoghurt samples
The radical scavenging activity of yoghurt samples was determined using 2,2-diphenyl-2-picrylhydrazyl radical (DPPH) and 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging assays after 7 days of storage. Briefly, 200 µL of WSE (0.5 mg protein/mL) was mixed 1 mL of 100 µM DPPH and reacted for 15 min at 25 °C in the dark. The absorbance of the samples was determined at 517 nm, and the DPPH radical scavenging activity of the samples was calculated using the following formula:
A control and A sample represent the absorbance of the control (distilled water) and WSE of the yoghurt samples, respectively.
Meanwhile, the ABTS radical scavenging activity was determined as described by Kariyawasam et al. (2021b). Twenty microliter of WSE (0.5 mg protein/mL) was blended with the ABTS radical cation solution (14 mM ABTS and 5 mM potassium persulfate) and reacted at 37 °C for 5 min in the dark. The absorbance of the samples was measured at 734 nm. The ABTS radical scavenging activity was calculated using the following formula:
A control and A samples represent the absorbance of the control (distilled water) and WSE yoghurt samples, respectively.
ROS scavenging activity of the yoghurt samples
The ROS-scavenging activity of yoghurt samples was defined using a human colon adenocarcinoma cell line (HT-29, KCLB 30038) after 7 days of storage (Kwon et al., 2019). HT-29 cells were cultured in 6-well polystyrene microtiter plates with RPMI 1640 medium, 10% fetal bovine serum, and 1% penicillin-streptomycin and grown to approximately 80% confluency. The cells were pretreated with WSE (0.5 mg protein/mL) for 15 h before being treated with LPS (0.1 µg/mL) for 24 h to induce ROS production. The cells were then treated with 20 µM 2′,7′-dichlorofluorescin diacetate (DCFH-DA) (Sigma-Aldrich, St. Louis, MO, USA) for 30 min, and washed with phosphate-buffered saline (Invitrogen, Carlsbad, CA, USA). Washed samples were observed using the Nikon Eclipse Ti2–U fluorescence microscope (Nikon Co. Ltd., Tokyo, Japan), and the fluorescent area was quantified using the ImageJ software.
Statistical analysis
Data were analyzed with one-way analysis of variance (ANOVA). Differences were regard as significant at p ≤ 0.05 by Duncan’s multiple range test.
Results and discussion
LAB viability, pH, and titratable acidity
The initial LAB counts (0 day) of the C, GG, R, and S samples were 8.46 ± 0.03, 8.58 ± 0.05, 8.51 ± 0.03, and 8.53 ± 0.06 log CFU/mL, respectively. The viable LAB counts increased slightly until 14 days of storage under refrigerated conditions (p > 0.05, data not shown). However, LAB viability declined slightly after 14 days in all yoghurt samples, and counts were comparable (p > 0.05, data not shown). The consistent decrease in pH during storage did not occur to have a significant effect on LAB survival. The LAB count remained above 6 log CFU/mL after 21 days of refrigerated storage for all yoghurt samples. Despite the different termination pH values, probiotic organisms persisted viable above the therapeutic level of 6 log CFU/mL (Donkor et al., 2007).
The pH of the yoghurt samples continued to decrease throughout the fermentation process (Fig. 1A). Yoghurt fermentation was accelerated by co-culture with probiotics. The total fermentation time for all probiotic-supplemented yoghurt samples was approximately 340 min, compared to that for the control sample (400 min). These outcomes have also been noted in a probiotic yoghurt with combination of a commercial starter with probiotics (Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium lactis) (Akan, 2022). These findings demonstrate the potential use of L. plantarum 200655 as a probiotics in dairy food products, particularly in accelerating the fermentation process.
Fig. 1.
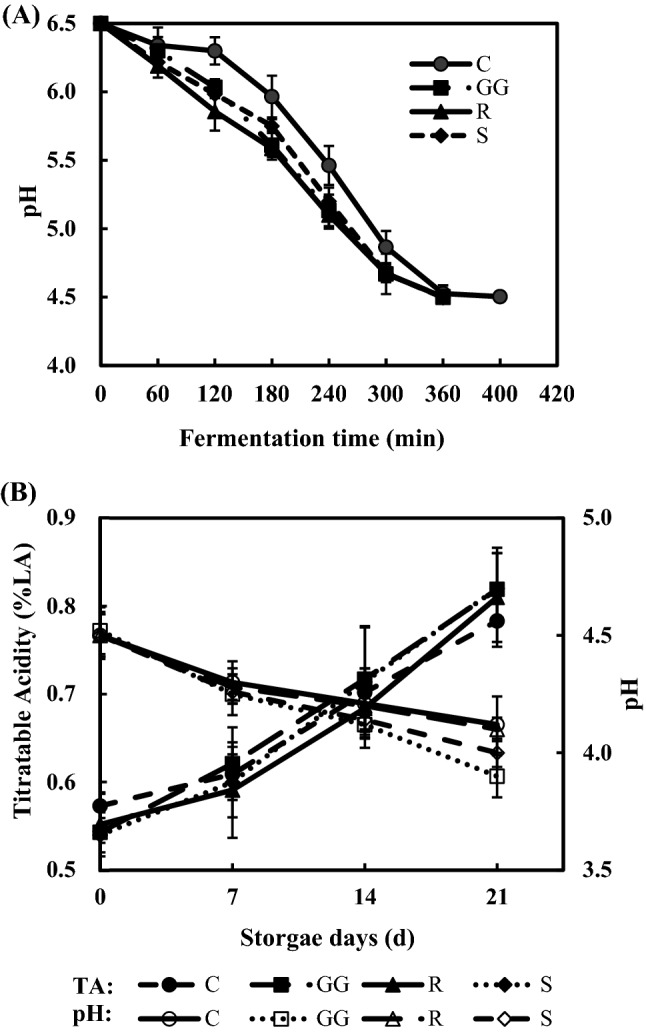
Changes in (A) pH during fermentation and (B) titratable acidity and pH during storage at 4 °C for 21 days. Values are expressed as mean ± SD. C, control yoghurt fermented with starter culture; GG, yoghurt fermented with starter culture and L. rhamnosus GG; R, yoghurt fermented with starter culture and L. plantarum KCTC 3108; S, yoghurt fermented with starter culture and L. plantarum 200655
The titratable acidity of the yoghurt samples showed a continuous increase during storage (Fig. 1B). The titratable acidity of the C, GG, R, and S samples at the end of the storage period were 0.78 ± 0.02%, 0.82 ± 0.04%, 0.81 ± 0.06%, and 0.82 ± 0.04%, respectively. Increased acidity occur by the continued production of organic acids and residual LAB activity (Habibi Najafi et al., 2019). Similarly, a consistent decrease in pH was observed during storage (Fig. 1B).
Physicochemical and organoleptic properties of the yoghurt samples
The addition of probiotics did not influence the L*, a*, and b* values (p > 0.05) (Table 1). However, an increase in WHC and viscosity and decrease in syneresis values (p < 0.05) were observed in the GG and S samples. The viscosity and WHC values of the S sample increased by 16.34% and 5.79%, respectively, whereas the syneresis value decreased by 5.43% compared to the C sample. These results indicate that probiotic supplementation increased viscosity. The exopolysaccharide produced by probiotics may be responsible for improved textural properties (Lee et al., 2021). Some studies have reported interactions concerning milk proteins and their ability to increase the firmness and viscosity of fermented milk, while decreasing syneresis and graininess (Buldo et al., 2016). The C sample had the highest overall acceptability, followed by the S, GG, and R samples (Table 2). However, the organoleptic properties of all samples did not show any differences (p > 0.05).
Table 1.
Physicochemical results of yoghurt samples after 7 days of refrigerated storage
| Parameter | C | GG | R | S |
|---|---|---|---|---|
| Color value | ||||
| L* | 87.92 ± 0.88a | 87.28 ± 0.46a | 87.69 ± 0.85a | 87.98 ± 0.78a |
| a* | − 2.49 ± 0.35a | − 2.54 ± 0.08a | − 2.46 ± 0.12a | − 2.55 ± 0.18a |
| b* | 2.30 ± 0.58a | 2.72 ± 0.34a | 2.86 ± 0.32a | 2.87 ± 0.34a |
| Viscosity (cP) | 1735.33 ± 28.57a | 1959.00 ± 31.58c | 1836.00 ± 24.25b | 1951.67 ± 27.57c |
| WHC (%) | 61.16 ± 0.44a | 66.87 ± 1.18c | 63.54 ± 1.61b | 66.59 ± 0.39c |
| Syneresis (%) | 38.84 ± 0.44c | 33.13 ± 1.18a | 36.46 ± 1.61b | 33.41 ± 0.39a |
All values are mean of three replicates (mean ± SD)
C, control yoghurt fermented with starter culture; GG, yoghurt fermented with starter culture and L. rhamnosus GG; R, yoghurt fermented with starter culture and L. plantarum KCTC 3108; S, yoghurt fermented with starter culture and L. plantarum 200655
a–cMeans within a row with different superscripts differ significantly (p < 0.05)
Table 2.
Organoleptic results of yoghurt samples after 7 days of refrigerated storage
| Sample | Color | Body/Texture | Flavor | Odor | Overall acceptability |
|---|---|---|---|---|---|
| C | 3.30 ± 1.26a | 3.27 ± 0.98a | 3.57 ± 1.07a | 3.10 ± 0.80a | 3.53 ± 1.14a |
| GG | 3.43 ± 1.14a | 3.60 ± 0.86a | 3.30 ± 0.88a | 3.03 ± 1.07a | 3.43 ± 1.14a |
| R | 3.37 ± 0.93a | 3.37 ±0.81a | 3.23 ± 1.00a | 3.07 ± 0.74a | 3.40 ± 0.81a |
| S | 3.47 ± 0.68a | 3.57 ± 0.86a | 3.23 ± 1.04a | 2.97 ± 0.96a | 3.47 ± 1.07a |
All values represent the mean from three replicates (mean ± SD)
C, control yoghurt fermented with starter culture; GG, yoghurt fermented with starter culture and L. rhamnosus GG; R, yoghurt fermented with starter culture and L. plantarum KCTC 3108; S, yoghurt fermented with starter culture and L. plantarum 200655
a–c Means within a column with different superscripts differ significantly (p < 0.05)
Radical scavenging activity of the yoghurt samples
The antioxidant activities of the probiotic samples after 7 days of storage under refrigerated conditions are shown in Fig. 2A and B. The radical scavenging activity values for C, GG, R, and S samples were 13.78 ± 4.68%, 30.70 ± 2.00%, 22.43 ± 1.13%, and 35.17 ± 3.05% in the DPPH assay, respectively; and 46.86 ± 2.28%, 57.02 ± 4.95%, 55.44 ± 0.69%, and 62.51 ± 1.23% in the ABTS assay, respectively. These results show that the antioxidant activity of the yoghurt samples increased when they were co-cultured with probiotics consistent with the reports of Mushtaq et al. (2016) and Kim et al. (2017). The highest antioxidant activity was defined in the S sample in both assays (p < 0.05); therefore, the addition of probiotics could increase the antioxidant activity of yoghurt. Previous studies have suggested that the antioxidant activity of yoghurt samples may be due to protein hydrolysis by microflora peptidases and release of bioactive peptides having antioxidant activity (Donkor et al., 2007; Mushtaq et al., 2016; Kariyawasam et al., 2021b). It has been described that milk proteins can release a number of strong antioxidant peptides, including Phe-Gly-Gly-Met-Ala-His, Phe-Pro-Tyr-Cys-Ala-Pro, Tyr-Val-Pro-Glu-Pro-Phe, Tyr- Pro-Pro-Tyr-Glu-Thr-Tyr, and Val-Tyr-Pro-Phe from goat milk using alkaline and neutral proteases and Phe-Ser-Asp-Ile-Pro-Asn-Pro-Ile-Gly-Ser-Glu-Asn-Ser-Glu-Lys-Thr-Thr-Met-Pro-Leu-Trp from bovine skim milk manufactured by Lactococcus lactis SL6 (Sah et al., 2015; Kim et al., 2017). However, the antioxidant activities of probiotic yoghurt samples differ because each LAB harbors unique proteases that are responsible for peptide bond cleavage. This cleavage produces peptides with variable antioxidant capacities, as peptide bioactivity is determined by a variety of factors, such as amino acids sequence, structure, and size. In addition, Rajapakse et al. (2005) reported that hydrolysates containing Cys, Gln, His, Lys, Met, Pro, Tyr, and Val had potent antiradical properties.
Fig. 2.
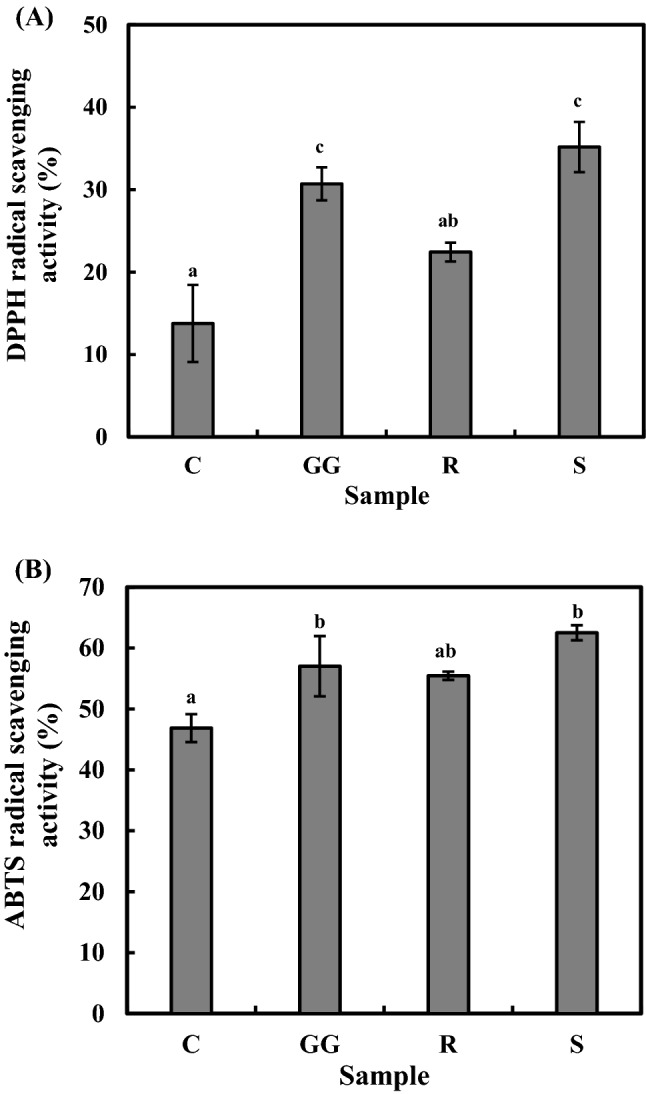
(A) DPPH and (B) ABTS radical scavenging activity of WSE of yoghurt samples at 7 days of refrigerated storage (4 °C). Values are expressed as mean ± SD. Means from different yoghurt samples denoted by lowercase letters (a, b, c) are significantly different (p < 0.05). C, control yoghurt fermented with starter culture; GG, yoghurt fermented with starter culture and L. rhamnosus GG; R, yoghurt fermented with starter culture and L. plantarum KCTC 3108; S, yoghurt fermented with starter culture and L. plantarum 200655
ROS scavenging activity of the yoghurt samples
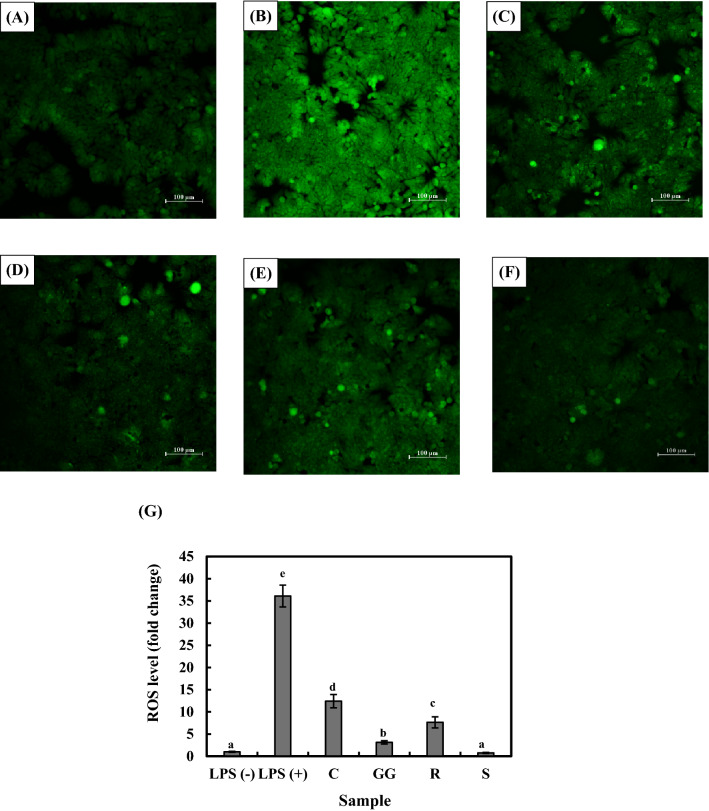
The yoghurt samples were investigated for their inhibitory ability to ROS production in HT-29 cells. DCFH-DA is a cell-permeable and non-fluorescent dye. DCFH-DA has been widely used as a probe for ROS due to the intense fluorescence seen when ROS are present. Therefore, it can be applied to measure the amount of intracellular ROS (Li et al., 2019). Figure 3B shows the green fluorescence is enhanced in the LPS treated sample compared to LPS untreated negative control sample (Fig. 3A). LPS treatment increased oxidative stress in HT-29 cells by 36.11-fold compared with the negative control. However, pretreatment with WSE decreased the green fluorescence, representing the excellent ROS scavenging ability (Fig. 3C–F). The ROS-scavenging activities of yoghurt samples were 12.42-, 3.13-, 7.65-, and 0.75-fold for the C, GG, R, and S samples, respectively. The ROS scavenging activity was high in the S sample (p < 0.05), similar with that observed in antioxidant activity. Thus, these results confirm that yoghurt samples produce bioactive peptides with varying antioxidant activities and that these antioxidant peptides can prevent free radical formation in HT-29 cells. Bioactive peptides produced during milk fermentation can reduce LPS-induced oxidative stress (Kariyawasam et al., 2021b; Khan et al., 2019; Tonolo et al., 2020). Furthermore, these findings suggest that L. plantarum 200655 supplementation in probiotic yoghurt increases antioxidative activity and modulates oxidative stress-induced damage in HT-29 cells, thereby improving colon health.
Fig. 3.
Fluorescence images of HT-29 cells in the presence of (A) negative control without LPS, (B) 0.1 µg/mL LPS, (C) 0.1 µg/mL LPS and 100 µL of the C sample, (D) 0.1 µg/mL LPS and 100 µL of the GG sample, (E) 0.1 µg/mL LPS and 100 µL of the R sample, (F) 0.1 µg/mL LPS and 100 µL of the S sample, and (G) ROS level (fold change). Values are expressed as mean ± SD. Means from different yoghurt samples denoted by lowercase letters (a-e) are significantly different (p < 0.05). C, control yoghurt fermented with starter culture; GG, yoghurt fermented with starter culture and L. rhamnosus GG; R, yoghurt fermented with starter culture and L. plantarum KCTC 3108; S, yoghurt fermented with starter culture and L. plantarum 200655
In conclusion, this study demonstrated the potential use of L. plantarum 200655 isolated from Korean kimchi as a probiotics in dairy products to improve the physicochemical properties of yoghurt and consumer health benefits. The addition of L. plantarum 200655 improved the textural properties of yoghurt, such as WHC, viscosity, and syneresis value, while having no adverse effect on its organoleptic properties. Furthermore, the results showed that yoghurt with L. plantarum 200655 has increased antioxidant activity and may control oxidative stress in the human colon adenocarcinoma cell line, with results comparable to those of the commercial probiotic strain L. rhamnosus GG. Therefore, this study highlights that consuming yoghurt supplemented with L. plantarum 200655 may protect against intestinal diseases, such as IBD, by reducing oxidative stress and has the potential to be utilized as a commercial probiotic strain in the food industry.
Declarations
Conflict of interest
The authors affirm that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kariyawasam Majuwana Gamage Menaka Menike Kariyawasam, Email: menaka.k@uwu.ac.lk.
Na-Kyoung Lee, Email: lnk11@konkuk.ac.kr.
Hyun-Dong Paik, Email: hdpaik@konkuk.ac.kr.
References
- Akan E. The effect of fermentation time and yogurt bacteria on the physicochemical, microbiological and antioxidant properties of probiotic goat yogurts. Anais da Academia Brasileira de Ciencias. 2022;94:1–16. doi: 10.1590/0001-3765202220210875. [DOI] [PubMed] [Google Scholar]
- Buldo P, Benfeldt C, Folkenberg DM, Jensen HB, Amigo JM, Sieuwerts S, Thygesen K, van den Berg F, Ipsen R. The role of exopolysaccharide-producing cultures and whey protein ingredients in yoghurt. LWT - Food Science and Technology. 2016;72:189–198. doi: 10.1016/j.lwt.2016.04.050. [DOI] [Google Scholar]
- Choi J, Sabikhi L, Hassan A, Anand S. Bioactive peptides in dairy products. International Journal of Dairy Technology. 2012;65:1–12. doi: 10.1111/j.1471-0307.2011.00725.x. [DOI] [Google Scholar]
- Daniel G, Gabriel F, Branco, Filomena N, Adriano GC, Jose AFF. Functional foods and non-dairy probiotic food development: Trends, concepts, and products. Comprehensive Reviews in Food Science and Food Safety. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- Donkor ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. International Dairy Journal. 2007;17:657–665. doi: 10.1016/j.idairyj.2006.08.006. [DOI] [Google Scholar]
- El-Khadragy MF, Al-Olayan EM, Elmallah MIY, Alharbi AM, Yehia HM, Moneim AEA. Probiotics and yogurt modulate oxidative stress and fibrosis in livers of Schistosoma mansoni-infected mice. BMC Complementary Medicine and Therapies. 2019;19:3. doi: 10.1186/s12906-018-2406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO. Guideline for the Evaluation of Probiotics in Food. London, Ontario, Canada (2002)
- Habibi Najafi MB, Fatemizadeh SS, Tavakoli M. Release of proteolysis products with ACE-inhibitory and antioxidant activities in probiotic yogurt containing different levels of fat and prebiotics. International Journal of Peptide Research and Therapeutics. 2019;25:367–377. doi: 10.1007/s10989-018-9679-8. [DOI] [Google Scholar]
- ISO. Sensory analysis–Methodology: General guidance for establishing a sensory profile. Geneva, Switzerland: International Organization for Standardization. ISO, 6658: 2017 (2017)
- Kariyawasam KMGMM, Yang SJ, Lee NK, Paik HD. Probiotic Properties of Lactobacillus brevis KU200019 and synergistic activity with fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Science of Animal Resources. 2020;40:297–310. doi: 10.5851/kosfa.2020.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam KMGMM, Lee NK, Paik HD. Fermented dairy products as delivery vehicles of novel probiotic strains isolated from traditional fermented Asian foods. Journal of Food Science and Technology. 2021;58:2467–2478. doi: 10.1007/s13197-020-04857-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam KMGMM, Lee NK, Paik HD. Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Bioscience. 2021;39:100835. doi: 10.1016/j.fbio.2020.100835. [DOI] [Google Scholar]
- Kawahara M, Nemoto M, Nakata T, Kondo S, Takahashi H, Kimura B, Kuda T. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. International Immunopharmacology. 2015;26:295–303. doi: 10.1016/j.intimp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Khan IT, Nadeem M, Imran M, Ullah R, Ajmal, Jaspal MH. Antioxidant properties of milk and dairy products: A comprehensive review of the current knowledge. Lipids in Health and Disease. 2019;18:1–13. doi: 10.1186/s12944-019-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee JY, Balolong MP, Kim JE, Paik HD, Kang DK. Identification and characterization of a novel antioxidant peptide from bovine skim milk fermented by Lactococcus lactis SL6. Food Science of Animal Resources. 2017;37:402–409. doi: 10.5851/kosfa.2017.37.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Olejar KJ, On SLW, Chelikani V. The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants. 2020;9:610. doi: 10.3390/antiox9070610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HC, Bae H, Seo HG, Han SG. Short communication: Chia seed extract enhances physiochemical and antioxidant properties of yogurt. Journal of Dairy Science. 2019;102:4870–4876. doi: 10.3168/jds.2018-16129. [DOI] [PubMed] [Google Scholar]
- Lee H, Yoon H, Ji Y, Kim H, Park H, Lee J, Shin H, Holzapfel W. Functional properties of Lactobacillus strains isolated from kimchi. International Journal of Food Microbiology. 2011;145:155–161. doi: 10.1016/j.ijfoodmicro.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Lee NK, Lim SM, Cheon MJ, Paik HD. Physicochemical analysis of yogurt produced by Leuconostoc mesenteroides H40 and its effects on oxidative stress in neuronal cells. Food Science of Animal Resources. 2021;41:261–273. doi: 10.5851/kosfa.2020.e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cao X, Fei X, Zhang S, Xian Y. Nanoscaled luminescent terbium metal-organic frameworks for measuring and scavenging reactive oxygen species in living cells. Journal of Material Chemistry B. 2019;7:3027–3033. doi: 10.1039/C9TB00361D. [DOI] [Google Scholar]
- Mushtaq M, Gani A, Masoodi FA, Ahmad M. Himalayan cheese (Kalari/Kradi) – Effect of different probiotic strains on oxidative stability, microbiological, sensory and nutraceutical properties during storage. LWT - Food Science and Technology. 2016;67:74–81. doi: 10.1016/j.lwt.2015.11.039. [DOI] [Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: Harms and benefits for human health. Oxidative Medicine and Cellular Longevity. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Research International. 2005;8:175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- Rezazadeh L, Alipour B, Jafarabadi MA, Behrooz M, Gargari BP. Daily consumption effects of probiotic yogurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 on oxidative stress in metabolic syndrome patients. Clinical Nutrition ESPEN. 2021;41:136–142. doi: 10.1016/j.clnesp.2020.12.003. [DOI] [PubMed] [Google Scholar]
- Ryu EH, Yang EJ, Woo ER, Chang HC. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiology. 2014;41:19–26. doi: 10.1016/j.fm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. Journal of Dairy Science. 2015;98:5905–5916. doi: 10.3168/jds.2015-9450. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Antibacterial and antiproliferative peptides in synbiotic yogurt—Release and stability during refrigerated storage. Journal of Dairy Science. 2016;99:4233–4242. doi: 10.3168/jds.2015-10499. [DOI] [PubMed] [Google Scholar]
- Son SH, Jeon HL, Jeon EB, Lee NK, Park YS, Kang DK, Paik HD. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT - Food Science and Technology. 2017;85:181–186. doi: 10.1016/j.lwt.2017.07.018. [DOI] [Google Scholar]
- Tonolo F, Fiorese F, Moretto L, Folda A, Scalcon V, Grinzato A, Ferro S, Arrigoni G, Bindoli A, Feller E, Bellamio M, Marin O, Rigobello MP. Identification of new peptides from fermented milk showing antioxidant properties: Mechanism of action. Antioxidants. 2020;9:1–24. doi: 10.3390/antiox9020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyastuti Y, Febrisiantosa A, Tidona F. Health-promoting properties of Lactobacilli in fermented dairy products. Frontiers in Microbiology. 2021;12:673890. doi: 10.3389/fmicb.2021.673890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Lee JE, Lim SM, Kim YJ, Lee NK, Paik HD. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Science and Biotechnology. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Jeong CH, Cheng WN, Bae H, Seo HG, Petriellob MC, Han SG. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT - Food Science and Technology. 2019;101:276–284. doi: 10.1016/j.lwt.2018.11.010. [DOI] [Google Scholar]