Abstract
Lymphomas are among the most common cancers in people with HIV (PWH). The lymphoma subtypes and pathogenesis of lymphoma in PWH are different from the immunocompetent population. It is well-known that HIV causes severe CD4+ T cell lymphopenia in the absence of antiretroviral therapy (ART); however, the risk of developing certain subtypes of lymphoma remains elevated even in people receiving ART with preserved CD4+ T cells. HIV contributes to lymphomagenesis and causes decreased immune surveillance via T cell depletion and dysregulation, B cell dysregulation, and the potential contribution of HIV-encoded proteins. The oncogenic gammaherpesviruses, Epstein-Barr virus (EBV) and Kaposi sarcoma herpesvirus (KSHV, also known as human herpesvirus 8), are the causative agents in the majority of HIV-associated lymphomas. HIV-associated T cell depletion and dysregulation allows EBV and KSHV to proliferate in infected B cells. Specific EBV- and KSHV-encoded proteins participate in B cell activation, and proliferation leading to B cell transformation. Understanding the distinct pathogenesis of HIV-associated lymphomas affords opportunities to develop therapies that specifically target these unique aspects and improve lymphoma outcomes in PWH. Agents being studied that target the specific roles of HIV, EBV, and KSHV in lymphomagenesis include immunotherapies, targeted agents, and cellular therapies.
Keywords: HIV, AIDS, lymphoma, Epstein-Barr virus, Kaposi sarcoma herpesvirus, human herpesvirus 8
Introduction
Early in the AIDS epidemic, before the discovery of the virus now known as HIV, it was recognized that individuals with this new syndrome had an increased risk of developing aggressive B cell lymphomas and these were included in the definition of AIDS.[1] Despite increased worldwide availability of modern antiretroviral therapy (ART), lymphomas are the most common cancers among people with HIV (PWH) in the United States and are among the most common globally.[2-5] The use of ART and control of HIV drastically decreases the risk of many lymphoma subtypes, but the overall risk of developing lymphoma among PWH is still 11-17 fold higher than the general population in the modern era of ART.[6, 7] Unfortunately, even with significant improvements in lymphoma survival over the past several decades, PWH have lower overall survival as compared to the general population.[8] While there are significant systemic barriers to healthcare for PWH that play a part in lower survival, immunosuppression from HIV and oncogenic viruses play large roles in lymphomagenesis and drive differences in disease presentation. For instance, PWH often present with lymphoma at more advanced stages with more rapid progression, have more frequent B symptoms, and have more frequent involvement of extranodal sites and the central nervous system (CNS) than the general population.[9, 10] Uncontrolled HIV viremia and the depth of the CD4+ nadir are both associated with an increased risk of lymphoma.[11, 12]
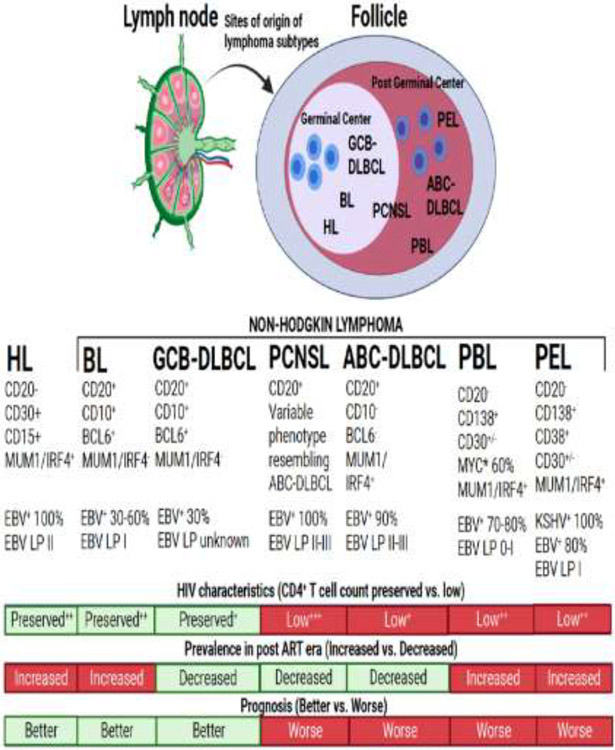
The frequent subtypes of lymphoma that occur in PWH, which differ from the distribution of subtypes in the general population, provide evidence of the unique drivers of lymphoma among PWH. These are generally aggressive B cell subtypes, some of which occur almost exclusively in PWH (Table 1, Figure 1). The most frequent subtypes are diffuse large B cell lymphoma (DLBCL), both germinal center B cell-like (GCB-DLBCL) and activated B cell-like (ABC-DLBCL); Burkitt lymphoma (BL); primary CNS lymphoma (PCNSL); plasmablastic lymphoma (PBL); primary effusion lymphoma (PEL); and KSHV-associated large cell lymphoma (KSHV-LCL). In addition, PWH have an increased incidence of Hodgkin lymphoma (HL). PCNSL is associated with the greatest level of immunosuppression and typically presents with the lowest CD4+ counts.[13] ABC-DLBCL, PBL, and PEL are also associated with substantial immunosuppression and low CD4+ T cell counts <200 cells/μL, while BL, GCB-DLBCL, as well as HL tend to present at lower levels of immunosuppression with relatively well-preserved CD4+ T cell counts.[14]
Table 1.
Characteristics of HIV-associated lymphomas and treatments
| Lymphoma Subtype |
EBV+ | KSHV+ | EBV Latency Pattern |
CD4+ T Cell Count |
Preferred Front-line Treatments per NCCN Guidelines[143, 144] |
|---|---|---|---|---|---|
| ABC-DLBCL | 90% | - | II/III | Low | DA-EPOCH-R |
| GCB-DLBL | 30% | - | - | Preserved | DA-EPOCH-R |
| BL | 30-60% | - | I | Preserved | DA-EPOCH-R Modified R-CODOX-M-IVAC |
| PBL | 70-80% | - | 0/I | Low | DA-EPOCH |
| PEL | 80-90% | 100% | I | Low | DA-EPOCH (add R if concurrent MCD) |
| KSHV-LCL | - | 100% | - | Low | DA-EPOCH (add R if concurrent MCD) |
| PCNSL | 100% | - | II/III | Very low | HD-MTX +/− R |
| HL | 100% | - | II | Preserved | A-AVD ABVD |
A-AVD indicates brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine; ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; CODOX-M-IVAC, cyclophosphamide, vincristine, doxorubicin, methotrexate alternating with ifosfamide, etoposide, and high-dose cytarabine; DA-EPOCH, dose-adjusted infusional etoposide, vincristine, and doxorubicin with cyclophosphamide and prednisone; HD-MTX, high-dose methotrexate; LCL, large cell lymphoma; MCD, multicentric Castleman disease; NCCN, National Comprehensive Cancer Network; R, rituximab.
Figure 1. Subtypes and characteristics of HIV-associated lymphomas.
HIV-associated lymphomas are comprised of a heterogeneous group of non-Hodgkin lymphomas (Burkitt lymphoma [BL], germinal center-like diffuse large B cell lymphoma [GcB-DLBCL], activated B cell-like DLBCL [ABC-DLBCL], primary central nervous system lymphoma [PCNSL], plasmablastic lymphoma [PBL], and primary effusion lymphoma [PEL]) and classical Hodgkin lymphoma (HL). The level of HIV-associated immune suppression plays a role in the development of various subtypes and the advent of antiretroviral therapy (ART) has had varying effects on the incidence of the various subtypes. Over time survival has improved for all subtypes, but those rare subtypes associated with significant immune suppression, such as PBL and PEL, still have poorer outcomes comparatively. Epstein-Barr virus (EBV) and Kaposi sarcoma herpesvirus (KSHV) play large roles in the pathogenesis of HIV-associated lymphomas. The EBV latency pattern (LP) determines the oncogenic pathways dysregulated in EBV+ lymphomas. This figure was created on biorender.com.
The majority of lymphomas among PWH are associated with the oncogenic viruses, Epstein-Barr virus (EBV) and Kaposi sarcoma herpesvirus (KSHV, also known as human herpes virus 8) (Table 1, Figure 1).[14] DLBCL and BL are the most common HIV-associated subtypes and are much more frequently associated with EBV than are these subtypes in the general population. PBL, a rare CD20 negative B cell lymphoma also associated with EBV, is diagnosed most frequently in PWH.[15] In PWH, 100% of PCNSL, 30-90% of DLBCL depending on the subtype, 30-60% of BL, and 70-80% of plasmablastic lymphoma are associated with EBV.[16] KSHV is the cause of PEL, both its classic and extracavitary variants, and also KSHV-LCL. These rare CD20-negative B cell lymphomas are seen almost exclusively in PWH, and their incidence may be increasing in the era of widespread ART use.[17] Unique among lymphomas, PEL is usually dually infected with EBV in addition to its causative agent, KSHV.[18] KSHV-LCL is always EBV-negative. HIV infection is also associated with increased risk of developing the polyclonal lymphoproliferative disorder, KSHV-associated multicentric Castleman disease (MCD), which increases the risk of developing aggressive lymphoma by up to 15 times.[19, 20] It is important to note that because they are caused by the same virus, KSHV-associated MCD and the endothelial tumor, Kaposi sarcoma, can be diagnosed concurrently in patients with PEL or KSHV-LCL.[21] Although there are fewer data, T cell and NK cell lymphomas also appear to be increased in PWH and may be associated with EBV.[6] There is a well characterized report of HIV-associated T cell lymphomas developing through integration of HIV proviruses into the signal transducer and activator of transcription 3 (STAT3) or lymphocyte-specific protein tyrosine.[22] HIV, EBV, and KSHV have distinct mechanisms that may interact with each other to drive lymphomagenesis, even in those with well-controlled HIV on ART (Figure 2). Understanding this complex relationship may lead to better therapeutic strategies to treat HIV-associated lymphomas.
Figure 2. Proposed effects of HIV, Epstein-Barr virus, and Kaposi sarcoma herpesvirus on lymphomagenesis.
HIV, Epstein-Barr virus (EBV), and Kaposi sarcoma herpesvirus (KSHV) have significant effects on lymphomagenesis through depletion of T cells but also through activation and transformation of B cells via chronic antigen stimulation, cytokine dysfunction, and through possible actions of specific viral oncoproteins. KSHV-encoded proteins are marked with an asterisk. This figure was created on biorender.com
HIV and Lymphomagenesis
HIV-related T cell loss and dysfunction, B cell dysfunction, and potentially HIV-encoded proteins themselves play roles in the decreased immunosurveillance and control of cancer in PWH. While ART reverses many of these effects and drastically decreases the risk of lymphoma, several of HIV’s effects on the immune system cannot be completely reversed with ART, and though undetectable on standard assays, low-level HIV replication continues in PWH on ART. Thus, lymphoma risk remains elevated even in those on long-term ART with preserved CD4+ T cell counts.
Uncontrolled HIV leads to profound CD4+ T cell loss over time, including virus-specific T cells required for controlling EBV and KSHV, which allows infected cells to proliferate.[23-25] In addition, the remaining CD8+ T cells are dysfunctional in part because they lack the support of helper CD4+ T cells.[26] Restoration of CD4+ T cell numbers with ART leads to significant declines in the risk of lymphoma, especially those subtypes most associated with advanced immunosuppression. However, even in people receiving ART with normal or near-normal CD4+ counts, there is an increased risk of lymphoma. This is partly due to a small subset of T cells that remain persistently infected with HIV. These HIV-infected cells (the HIV reservoir) cause chronic viral antigenemia leading to chronic T cell activation, decreased cytokine production, upregulation of inhibitory immune checkpoint proteins, and loss of cytotoxic T cell function.[27] T cells in this state of “exhaustion” have impaired ability to kill virus-infected and cancer cells.[28, 29] Expression of inhibitory immune checkpoint proteins, such program death 1 (PD-1), on T cells correlates with HIV viral load, CD4+ T cell count, and CD8+ T cell cytotoxic functions.[27, 30, 31] Also, there are a number of biologically important immune inhibitory receptors expressed in excess on the T cells of PWH, such as cytotoxic T lymphocyte antigen-4 (CTLA-4), lymphocyte activation gene protein (LAG-3), T cell immunoglobulin domain and mucin domain-containing protein (TIM-3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT).[32] It is possible to partially reverse upregulation of these inhibitory receptors on T cells with the introduction of ART, but not to the levels seen prior to HIV infection, which is an important reason the risk of lymphoma remains even in PWH with preserved CD4+ T cell counts.[33]
With regard to B cell dysfunction and lymphomagenesis, chronic antigenemia from HIV-encoded proteins and T cell dysfunction causes polyclonal B cell hyperactivity, including hypergammaglobulinemia, lymphoid hyperplasia, and an increased proportion of terminally differentiated circulating B cells.[34] The risk of non-Hodgkin lymphoma is increased eight-fold in PWH who have elevated serum free light chains showing the risk of chronically activated B cells.[35-37] HIV also stimulates abnormal elevations in interleukins (IL)-10 and -6, which promote B cell proliferation and are associated with the development of lymphoma in PWH.[38, 39] KSHV and EBV can also upregulate these cytokines or encode for viral homologues. Despite increased activation, these B cells exhibit poor immune responsiveness to neoantigens and recall antigens.[40] The normal interaction between CD4+ T cells and B cells is dysregulated in HIV infection due at least in part to impaired interactions between CD40L on T cells and CD40 on B cells.[41] HIV envelope has also been noted to carry CD40L when the virus buds from HIV-infected T cells and has the ability to strongly activate B cells leading to increased somatic hypermutation through expression of activation-induced cytidine deaminase (AID).[42-44] There is evidence that HIV glycoprotein gp120 can interact directly with B cells via membrane immunoglobulins and C-type lectin receptors, which upregulates AID triggering immunoglobulin class switching and somatic hypermutation.[45-47] Many of these changes in B cell function can be reversed with initiation of ART; however, HIV treatment does not appear to be able to reverse the HIV-related decrease in antigen-specific memory B cells, which may have an effect on control of EBV- and KSHV-specific proliferation and surveillance of tumor antigens even in those with well-controlled HIV on long-term ART.[48, 49]
Several HIV-encoded proteins may play important roles in lymphomagenesis. Tat protein may be particularly important in the development of lymphoma given its down-stream effects lead to DNA damage as well as increased reactive oxygen species, IL-6 and IL-10 production, expression of DNA repair β-polymerase, and angiogenesis.[50, 51] Tat protein’s effects also result in DNA damage that promotes translocation of the MYC oncogene to the immunoglobulin heavy chain locus, which may be important specifically in the development of BL.[52] Despite ART and undetectable HIV replication, HIV-encoded gp120 and matrix protein p17 can persist in lymph nodes contributing to the ongoing elevated risk of B cell lymphomas in PWH on ART.[53] Some p17 protein variants have been found to be potent activators of the Akt signaling pathway, which promotes B cell transformation and growth.[54] Interplay between EBV and p17 proteins may also increase lymphomagenesis. Binding of p17 protein variants to the chemokine receptor, CXCR2, upregulates EBV latent membrane protein 1 (LMP-1), which is a major oncogene as will be discussed below.[55] Despite the persistent contribution of HIV infection to lymphomagenesis even during viral suppression, ART significantly decreases the overall risk of lymphoma as randomized trials have shown that even short interruptions in ART with rebound in HIV viremia increase the risk of non-Hodgkin lymphoma by almost 4-fold.[56]
The Role of EBV and KSHV in Lymphomagenesis
EBV and KSHV are closely related gammaherpesviruses that play several important roles in lymphomagenesis that may have effects on lymphoma-related survival (Table 2). For instance, patients with EBV-positive DLBCL have worse overall survival than EBV-negative DLBCL.[57] KSHV-associated lymphomas overall have worse outcomes compared with other HIV-associated lymphomas, with medial survival being only about 10-22 months.[58, 59] Although PEL is driven by KSHV and its positivity in the tumor is a requirement for its pathologic diagnosis, a large percentage of PEL tumors are also positive for EBV. Unlike EBV-positive DLBCL, EBV-positive PEL has improved survival compared with EBV-negative PEL. Gene expression profiling of EBV-positive and EBV-negative PEL has shown that genes involved in the cell cycle and regulation of signal transduction are differentially expressed depending on EBV positivity, indicating that differences in survival by EBV status may be explained by differences in pathogenesis.[60]
Table 2.
EBV and KSHV latent gene products and their contributions to lymphomagenesis or immune escape
| Virus | Gene Product |
Role in lymphomagenesis |
|---|---|---|
| EBV | LMP-1 | Increases expression of anti-apoptotic proteins, BCL-2 and A20 Activates NF-κB and JAK-STAT pathways |
| LMP-2A | Mimics B cell receptor signaling; activates PI3K-AKT-mTOR pathways Induce downregulation of MHC-1 |
|
| EBNA-1 | Interacts with c-myc Promotes latent infection and development of cellular mutations |
|
| EBNA-2 | Increases PD-L1 expression Activates cyclin D2 in conjunction with EBNA-LP |
|
| EBNA-3 | Regulates host gene transcription Alters p53-induced apoptosis Causes cell cycle dysregulation via pRb, c-myc, and Cyclin D1 |
|
| EBNA-LP | Activates cyclin D2 in conjunction with EBNA-2 | |
| BART miRNAs | Suppresses innate and adaptive host immunity Modulate tumor-associated macrophages Suppresses IL-12 and differentiation of CD4+ T cells |
|
| BHRF1 miRNAs |
Suppresses innate and adaptive host immunity Suppresses IL-12 and differentiation of CD4+ T cells |
|
| KSHV | LANA | Inactivates TP53 and retinoblastoma |
| vFLIP | Upregulates NF-κB Interacts with c-myc |
|
| vIL-6 | Activates JAK-STAT and PI3K-AKT pathways Promotes B cell proliferation via autocrine and paracrine signaling |
|
| v-cyclin | Activates CDK6 to promote cell growth |
Like all herpesviruses, EBV and KSHV have both latent and lytic cycles. When EBV and KSHV infect B cells, their genetic material is eventually transported to the host nucleus where the genome circularizes and remains as an episome tethered to the host chromosomes via EBV Nuclear Antigen 1 (EBNA-1), in the case of EBV, or latency-associated nuclear antigen (LANA) in the case of KSHV.[61] For both viruses, the default pathway is generally to enter latency where only a few viral genes required for viral genome maintenance and host-cell survival are expressed. These viruses may undergo lytic reactivation, either sporadically or in response to certain signals, in which almost all viral proteins are expressed, new virions are produced, and the host cells subsequently die.[62, 63] EBV has three latency programs determined by the pattern of expression of its latency genes: EBV nuclear antigens (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, EBNA-LP), latent membrane proteins (LMP-1, LMP-2A, LMP-2B), non-coding RNAs (EBER-1, EBER-2), and microRNAs (BHRF1s and BARTs).[63] The genes expressed in each EBV latency pattern and the associated lymphoma subtypes are shown in Table 3 and Figure 1. Unlike EBV, KSHV has only one latency program, which is characterized by expression of viral FLICE inhibitory protein (vFLIP), v-cyclin, latency-associated nuclear protein (LANA), kaposin signaling proteins, and microRNAs. A viral homologue of interleukin-6 (vIL-6) and some other KSHV genes (e.g. K5) are also expressed at low levels during latency.[62] In general, EBER and LANA are used to establish EBV and KSHV positivity in tumor cells, respectively.
Table 3.
Epstein-Barr virus latency patterns
| Latency Pattern |
EBERs | LMP-1 | LMP-2A/B | EBNA-1 | EBNA- 2/3/LP |
BHRF1 miRNAs |
BART miRNAs |
|---|---|---|---|---|---|---|---|
| 0 | + | − | − | − | − | − | + |
| I | + | − | − | + | − | − | + |
| IIa | + | + | + | + | − | − | + |
| IIb | + | − | − | + | + | + | + |
| III | + | + | + | + | + | + | + |
One strategy used by both EBV and KSHV to maintain infection in B cells and evade host immune surveillance is through downregulation of surface immune proteins. Major histocompatibility complex 1 (MHC-1), which is required for T cell killing, is downregulated by EBV lytic proteins, BILF1 and BDLF3, and KSHV lytic proteins, K3 and K5.[64-66] The EBV latent protein LMP-2A can also induce downregulation of MHC-1 through the sonic hedgehog pathway.[67] Moreover, certain EBV- and KSHV-encoded miRNAs can target MHC-1 polypeptide-related sequence B to escape recognition by NK cells.[68] EBV and KSHV can also downregulate the immune surface markers intercellular adhesion molecule 1 (ICAM-1) and CD86 (B7-2), which are involved in both T cell and NK cell killing.[69, 70] Blocking the ability of EBV and KSHV to downregulate these immune surface markers is a potential way to improve T cell recognition of EBV- and KSHV-infected lymphoma cells.
Similar to HIV, chronic viral stimulation of T cells by EBV and KSHV leads to expression of PD-L1 on tumor cells and infiltrating immune cells, which has been documented in a variety of EBV- and KSHV-associated lymphomas and contributes to lymphomagenesis.[71] In EBV-negative HL, amplification of the chromosome 9p24.1 is associated with increased expression of PD-L1 on malignant Reed-Sternberg cells and thought to be the reason for a very high response rate to anti-PD-1 therapy in HL. However, studies have shown that 9p24.1 amplification and EBV-positivity are generally exclusive, and it is EBV that drives overexpression of PD-L1 in EBV+ HL.[72] In EBV+ DLBCL, EBNA-2 increases PD-L1 expression via downregulation of certain microRNAs and is associated with worse survival.[73, 74]
Because of the oncogenic role of EBV, EBV+ lymphomas are generally not as reliant on genetic mutations to drive activation of tumor-promoting pathways as compared to virus-negative tumors. EBV-encoded LMP-1 is one of the most important viral proteins for B cell transformation in vitro. It functions like a constitutively activated TNF family receptor; increases expression of anti-apoptotic proteins, BCL-2 and A20; and activates signaling pathways that promote B cell growth and survival, including the NF-κ B and JAK/STAT pathways.[63] LMP-1 is generally not expressed in B cells expressing BCL-6, a marker of germinal center differentiation. Instead, LMP-1 is most important as a driver of lymphomagenesis in ABC-DLBCL, which has a post-germinal center plasmacytic differentiation. LMP-2A is also expressed in ABC-DLBCL as well as HL; it mimics B cell receptor signaling by associated Syk and Src and is able to activate multiple oncogenic pathways, such as the PI3K, Akt, and mTOR pathways.[16, 75]
In contrast to their expression in other EBV-associated lymphomas, LMP-1 and EBNA-2 are not expressed in BL, and the main EBV-encoded protein expressed is EBNA-1. Unlike LMP-1, EBNA-1 does not drive B cell transformation in vitro but contributes to the survival of BL cells and allows for the development of other mutations in the setting of HIV infection that may eventually lead to BL.[63, 76] Comparing whole genome sequencing of EBV+ and EBV− BL, tumors showed that EBV+ cases had a higher mutational burden suggesting EBV plays a role in driving downstream genetic events as B cells are transformed.[77] In addition, c-myc translocations are seen in 100% of BL and EBNA-1 may also interact with c-myc to promote the development of BL.[78]
The EBNA-3 proteins are expressed in post-transplant lymphoproliferative disorders and are essential for EBV-induced B cell transformation.[79] Importantly, EBNA-3 proteins can regulate host gene transcription by binding to transcriptional regulators, including histone deacetylases, and modulate cellular genes via recruitment of chromatin remodeling proteins.[80, 81] EBNA-3 proteins also alter p53-induced apoptosis and cause cell cycle-dysregulation via interactions with a variety of host proteins, including pRb, c-myc, and Cyclin D1, which have known roles in lymphomagenesis.[82] EBNA-2 and EBNA-LP also play an essential role in B cell transformation.[83] These two proteins cooperate to activate cyclin D2 leading to progression of resting B cells in to the G1 phase of the cell cycle.[84]
EBV expresses regulatory microRNAs (miRNAs) that can modulate cell signaling and manipulate viral and cellular gene expression. BART miRNAs are expressed in all latency programs and play a role in the development of all HIV-associated lymphomas through suppression of innate and adaptive immunity.[85] These miRNAs regulate EBV gene expression through multiple mechanisms, including desensitization to B cell receptor signaling and therefore, help maintain latent infection of B cells.[86-88] BARTs may also modulate tumor-associated macrophages in the tumor microenvironment that lead to a pro-tumor state reducing host responses to EBV and are predictors of poor lymphoma survival.[89] BARTs as well as EBV-encoded BHRF1 miRNAs suppress expression of IL-12 in EBV-infected cells impairing differentiation of CD4+ T cells into T helper 1 cells. This ultimately reduces the number of EBV-specific cytotoxic T cells allowing for EBV-infected B cell proliferation.[90, 91]
KSHV-encoded LANA is expressed in all KSHV-infected cells and can inactivate tumor suppressor proteins TP53 and retinoblastoma leading to increased cellular survival and cellular proliferation via overexpression of MYC.[92-94] In contrast to EBV, KSHV does not transform B cells in cell culture despite the fact that it encodes many proteins that are homologs of human proteins known to play important roles in lymphomagenesis. For instance, vFLIP appears to be very important in the development of PEL via upregulation of the NF-κB pathway leading to expression of prosurvival proteins and cytokines, such as human IL-6.[95, 96] vFLIP may also interact with c-myc to promote lymphomagenesis.[97] vIL-6 is the viral homolog of human-IL6 and is able to activate downstream signaling pathways via binding to the membrane glycoprotein 130 (gp130), the chain of the IL-6 receptor that is shared with other receptors. Unlike human IL-6, its activation of gp130 does not require binding to the gp80 chain that confers specificity to the IL-6 receptor. Binding of vIL-6 to gp130 leads to activation of the JAK-STAT and PI3K-AKT pathways, both of which are known to be important in lymphomagenesis.[98] While it can be secreted, vIL-6 can activate cells by binding intracellularly to gp130 within the endoplasmic reticulum, and in fact, this appears to be its main mode of action, v-IL6 can also promote B cell proliferation via autocrine and paracrine signaling of nearby B cells.[99] v-cyclin is a homolog of cyclin D and can hijack control of the cell cycle. v-cyclin binds to and activates cyclin-dependent kinase 6 (CDK6), and this complex phosphorylates retinoblastoma in addition to other cellular proteins causing entry into the growth and synthesis phases of the cell cycle.[100, 101] In addition, v-cyclin is resistant to cellular CDK inhibitors further promoting cell growth and survival.[102]
Treatment
The unique pathogenesis of HIV-associated lymphomas provides the opportunity to improve treatment outcomes; however, current treatment strategies do not address the contribution of viruses with the exception of the concurrent use of ART for HIV suppression. There are no U.S. Food and Drug Administration-approved therapies specific to HIV-associated lymphomas, and for subtypes that occur almost exclusively in PWH, recommended therapies are based on small, retrospective studies and expert opinion. In general, front-line therapy of all HIV-associated lymphoma subtypes combines several cytotoxic chemotherapy drugs with the addition of rituximab for CD20+ lymphomas (Table 1). A pooled analysis of over 1500 patients with HIV-associated non-Hodgkin lymphomas treated on prospective clinical trials showed that survival was significantly improved with infusional etoposide, vincristine, and doxorubicin with cyclophosphamide and prednisone (EPOCH) compared with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), although there was no difference when rituximab was added to the regimens.[103] Prior to the development of effective, combination ART, HIV-associated lymphomas were generally treated with reduced-dose chemotherapy as infectious complications were frequent and therapy was poorly tolerated.[104] However, with the use of ART and great experience, HIV-associated lymphomas should now generally be treated with the full-dose recommended therapy for a given subtype and line of therapy.[105] This is particularly important to consider as treatment is potentially curative. There is evidence that anti-cancer therapies can be administered even to critically ill patients with HIV-associated lymphomas in the intensive care unit with successful long-term outcomes.[106] In addition, ART is viewed by practitioners with expertise in the field as an essential part of lymphoma therapy, and with the variety of modern regimens available, can be safely administered with chemoimmunotherapy.[107] Every log increase in the HIV viral load in the 6 months following lymphoma therapy increases the risk of mortality and underscores the importance of ART during and after treatment.[108] In addition, therapies that spare T cells and allow for immune reconstitution, particularly in the relapsed/refractory setting, are optimal in PWH. Lower CD4+ T cell counts following chemotherapy and/or radiation have been associated with increased mortality.[109] Because there is increased risk for infectious complications and T cells are expected to decrease with cytotoxic chemotherapy, appropriate antimicrobial prophylaxis against opportunistic infections is essential during and after lymphoma treatment.[105]
Anti-PD(L)-1 therapies have been successfully incorporated into the treatment of variety of cancers over the last decade and have been shown to be safe in PWH.[110-112] Anti-PD-1 therapies have substantially increased the survival in relapsed/refractory HL and may soon be incorporated into frontline therapy.[113-115] Unfortunately, like many immunotherapy trials, PWH were excluded from the trials in HL, and it is unknown whether the response rates in PWH are the same as the general population. However, there is reason to think that anti-PD-1 agents may work just as well if not better in PWH as PD-1 expression is increased in both HIV-specific CD4+ and CD8+ T cells and in T cells specific for viral antigens or tumor neoantigens in PWH.[116] Tumors with high mutation burden tend to have higher response rates to anti-PD(L)-1 therapy because mutations may lead to production of novel antigens.[117] Virus-related tumors tend to have fewer genetic mutations driving oncogenesis than those unassociated with viruses; however, since the viral-encoded proteins themselves are foreign antigens, it has been hypothesized that they might be susceptible to anti-PD(L)-1 therapy on this basis.[72, 118] Single-agent anti-PD-1 agents have not been used successfully to treat non-Hodgkin lymphomas in the general population, but our group has seen exceptional tumor responses with these agents in PWH and EBV- and KSHV-associated non-Hodgkin lymphomas.[119, 120] As noted, this greater success may reflect the fact that the gammaherpesviruses in HIV-associated lymphomas provide foreign antigens, and thus render tumors more susceptible to anti-PD(L)-1 therapy. Further exploration of anti-PD-1 agents specifically in HIV-associated lymphomas is of significant interest with respect to HIV infection, as these agents are T cell-sparing and have been shown to induce HIV latency reversal and in combination with other inventions have the potential to reduce the HIV reservoir.[121]
Targeted agents that can inhibit important pathways activated or dysregulated by HIV, EBV, and/or KSHV may be beneficial in HIV-associated lymphomas either as additions to front-line chemotherapy regimens or in subsequent lines of therapy for relapsed/refractory disease. Bruton tyrosine kinase (BTK) inhibitors, such as ibrutinib, are able to inhibit B cell receptor signaling and downstream activation of the NF-κB pathway, and have been used successfully to treat ABC-DLBCL in the immunocompetent population.[122] Given the activation of B cell receptor signaling by LMP-2A and the importance of the NF-κB pathway in HIV-associated ABC-DLBCL, ibrutinib is being tested in combination with cytotoxic chemotherapy by the AIDS Malignancy Consortium (AMC) (NCT03220022). The effects of KSHV-encoded v-cyclin on lymphomagenesis may be targeted by the FDA-approved CDK4/6 inhibitors which sensitize EBV- and KSHV-infected cells to T cell killing.[123] There is also rationale for testing agents that target the PI3K, Akt, and mTOR pathways, particularly since there are FDA-approved therapies targeting several of these pathways. The immunomodulatory drugs, lenalidomide and pomalidomide, inhibit cereblon, an E3 ubiquitin ligase, whose downstream effects lead to inhibition of NF-κB as well as activation of T and NK cells.[124] Immunomodulatory drugs are also able to reverse the EBV- and KSHV-mediated downregulation of immune surface markers improving antigen presentation and immune surveillance.[125, 126] These drugs are also directly toxic to PEL cells in the lab through targeting of IRF4.[70, 127] We are investigating the combination of an immunomodulatory drug with rituximab and cytotoxic chemotherapy in a phase 1/2 trial focused on PEL using lenalidomide and a phase 1 trial for all subtypes of HIV-associated lymphomas using pomalidomide at the National Cancer Institute (NCT02911142 and NCT05389423). Because immunomodulatory agents are able to activate T cells and improve antigen presentation to T cells, there is rationale to combine these agents with anti-PD-1 therapies. There is the added benefit that both of these therapies are T cell-sparing. To test this rationale, we are combining pomalidomide with the anti-PD-1 agent, nivolumab, for relapsed/refractory virus-associated cancers, including a specific cohort for patients with HIV-associated lymphomas, in a phase 1 clinical trial at the National Cancer Institute (NCT04902443).
Cellular therapies are also promising for the treatment of HIV-associated lymphomas. Donor-derived EBV-specific T cells have been used successfully for several decades to prevent or treat EBV+ post-hematopoietic stem cell transplant lymphoproliferative disorders.[128] KSHV-specific T cells have not yet been developed; however, research into understanding the repertoire of KSHV-infected T cells in patients with KSHV-associated diseases that may lead to the development of this type of therapy is ongoing.[129] The ability of virus-specific T cells to target and kill EBV-infected lymphomas depends on the immunogenicity of the virus-encoded proteins expressed by the lymphoma cells. The success of EBV-specific T cells in post-transplant lymphoproliferative disorders is due to the fact that the tumor cells express type III latency proteins, which includes the broadest array of proteins among the latency programs. EBNA-1-specific adoptive T cells have been successfully used to treat EBV reactivation and lymphoproliferative disorders after post-allogeneic stem cell transplant.[130] To treat lymphomas expressing the less immunogenic type II latency proteins, such as DLBCL or HL, additional modifications to the cell manufacturing process to enrich the EBV-specific T cells for LMP-1 and LMP-2 antigens may be required.[131] Other strategies to sensitize the tumors to EBV-specific T cells may include epigenetic modulation to increase expression of viral antigens or inducing EBV lytic replication to promote recognition by virus-specific T cells.[132, 133] This treatment strategy could apply not only to donor-derived virus-specific T cells but also the patient’s own T cells. This strategy was studied by the AMC using the histone deacetylase inhibitor, vorinostat, along with cytotoxic chemotherapy to treat a variety of HIV-associated non-Hodgkin lymphomas. Vorinostat was used as a way to disrupt EBV and KSHV latency and hopefully enhance chemotherapy-induced cell death.[134] While the combination was shown to be safe among this heterogeneous group of HIV-associated lymphomas, the addition of vorinostat to combination chemotherapy did not improve survival.
The first CAR T-cells were developed to target HIV, but when early efforts were unsuccessful, CAR T-cell technologies were redirected to cancer research.[135] CAR T-cells have now changed the treatment landscape of aggressive B cell lymphomas, and CAR T-cells directed against the B cell antigen CD19 are now standard-of-care in the second-line setting for refractory or early-relapsed DLBCL.[136] Unfortunately, PWH have been excluded from all cancer-directed CAR T-cell therapy trials to date due to concerns about safety and efficacy. There have been a few case reports of the successful manufacturing of CAR T-cells in PWH with lymphoma on ART with durable remissions, but significant questions about optimal cell manufacturing processes, safety, and efficacy remain.[137-139] The AMC is planning a phase 1 trial of the commercial anti-CD19 CAR T-cell product, axicabtagene ciloleucel, in HIV-associated non-Hodgkin lymphomas (NCT05077527). Learning from the success of cancer-directed CAR T-cells, the development of HIV-targeted CAR T-cells for HIV cure is ongoing.[140, 141] We look forward to upcoming advances in CAR T-cell technology that allow for the targeting of both HIV and lymphoma in one CAR T-cell product to treat patients with HIV-associated lymphomas.
Conclusions
PWH are at higher risk of a variety of aggressive lymphomas with worse overall survival than the general population. In this review, we have discussed the role of HIV, EBV, and KSHV in lymphomagenesis and potential therapeutic strategies to target these unique aspects. Further scientific progress and new developments in treatment will require that systemic barriers to healthcare experienced by PWH be removed in tandem to meaningfully impact the survival of PWH and lymphoma.[142] Improving access to clinical trials and novel therapies will be important keys to addressing the work still to be done in HIV-associated lymphomas.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
KL, RY, and RR report receiving research support from Bristol Myers Squibb through CRADAs with the NCI and receiving drugs for clinical trials from Merck, EMD-Serono, Eli Lilly, and CTI BioPharma through CRADAs with the NCI. RY reports receiving drug supply for laboratory research from Janssen Pharmaceuticals. RY is a co-inventor on US Patent 10,001,483 entitled "Methods for the treatment of Kaposi's sarcoma or KSHV-induced lymphoma using immunomodulatory compounds and uses of biomarkers." An immediate family member of RY is a co-inventor on patents or patent applications related to internalization of target receptors, epigenetic analysis, and ephrin tyrosine kinase inhibitors. All rights, title, and interest to these patents have been assigned to the U.S. Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502).
References
- 1.Pluda JM, Venzon DJ, Tosato G, Lietzau J, Wyvill K, Nelson DL, et al. Parameters affecting the development of non-Hodgkin's lymphoma in patients with severe human immunodeficiency virus infection receiving antiretroviral therapy. J Clin Oncol. 1993;11(6):1099–107. [DOI] [PubMed] [Google Scholar]
- 2.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;107(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin DO, Metzger M, Poeth K, Deng K, Dharsee A, Rico JC, et al. Malignancies, Particularly B-Cell Lymphomas, Are a Frequent Cause of Mortality in Human Immunodeficiency Virus-1 Patients Despite Highly Active Antiretroviral Therapy. Open Forum Infect Dis. 2015;2(4):ofv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarchoan R, Uldrick TS. HIV-Associated Cancers and Related Diseases. N Engl J Med. 2018;378(22):2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. Aids. 2014;28(15):2313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borges AH, Silverberg MJ, Wentworth D, Grulich AE, Fatkenheuer G, Mitsuyasu R, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. 2013;27(9):1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol. 2015;33(21):2376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noy A Optimizing treatment of HIV-associated lymphoma. Blood. 2019;134(17):1385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Ramirez RU, Qin L, Lin H, Leyden W, Neugebauer RS, Althoff KN, et al. Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV. 2019;6(4):e240–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaboration of Observational HIVERESG, Bohlius J, Schmidlin K, Costagliola D, Fatkenheuer G, May M, et al. Incidence and risk factors of HIV-related non-Hodgkin's lymphoma in the era of combination antiretroviral therapy: a European multicohort study. Antivir Ther. 2009;14(8):1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, Napravnik S, et al. Temporal Trends in Presentation and Survival for HIV-Associated Lymphoma in the Antiretroviral Therapy Era. JNCI: Journal of the National Cancer Institute. 2013;105(16):1221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai SY, Lurain K, Yarchoan R. How immunodeficiency can lead to malignancy. Hematology Am Soc Hematol Educ Program. 2021;2021(1):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323–30. [DOI] [PubMed] [Google Scholar]
- 16.Shindiapina P, Ahmed EH, Mozhenkova A, Abebe T, Baiocchi RA. Immunology of EBV-Related Lymphoproliferative Disease in HIV-Positive Individuals. Front Oncol. 2020;10:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramaswami R, Chia G, Dalla Pria A, Pinato DJ, Parker K, Nelson M, et al. Evolution of HIV-Associated Lymphoma Over 3 Decades. J Acquir Immune Defic Syndr. 2016;72(2):177–83. [DOI] [PubMed] [Google Scholar]
- 18.Cesarman E, Chadburn A, Rubinstein PG. KSHV/HHV8-mediated hematologic diseases. Blood. 2022;139(7):1013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oksenhendler E, Boulanger E, Galicier L, Du MQ, Dupin N, Diss TC, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331–6. [DOI] [PubMed] [Google Scholar]
- 20.Ramaswami R, Lurain K, Polizzotto MN, Ekwede I, Waldon K, Steinberg SM, et al. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Adv. 2021;5(6):1660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramaswami R, Lurain K, Yarchoan R. Oncologic Treatment of HIV-Associated Kaposi Sarcoma 40 Years on. J Clin Oncol. 2022;40(3):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellors JW, Guo S, Naqvi A, Brandt LD, Su L, Sun Z, et al. Insertional activation of STAT3 and LCK by HIV-1 proviruses in T cell lymphomas. Sci Adv. 2021;7(42):eabi8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Maza O, Breen EC. B-cell activation and lymphoma in patients with HIV. Curr Opin Oncol. 2002;14(5):528–32. [DOI] [PubMed] [Google Scholar]
- 24.de Martel C, Shiels MS, Franceschi S, Simard EP, Vignat J, Hall HI, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29(16):2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piriou E, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106(9):3166–74. [DOI] [PubMed] [Google Scholar]
- 26.Heather JM, Best K, Oakes T, Gray ER, Roe JK, Thomas N, et al. Dynamic Perturbations of the T-Cell Receptor Repertoire in Chronic HIV Infection and following Antiretroviral Therapy. Front Immunol. 2015;6:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–202. [DOI] [PubMed] [Google Scholar]
- 28.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. [DOI] [PubMed] [Google Scholar]
- 31.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M, et al. T-cell exhaustion in HIV infection. Immunol Rev. 2019;292(1):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122(1):12–9; quiz 20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiels MS, Landgren O, Costello R, Zingone A, Goedert JJ, Engels EA. Free light chains and the risk of AIDS-defining opportunistic infections in HIV-infected individuals. Clin Infect Dis. 2012;55(10):e103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landgren O, Goedert JJ, Rabkin CS, Wilson WH, Dunleavy K, Kyle RA, et al. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010;28(5):773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd L, Borges AH, Harvey R, Bower M, Grulich A, Silverberg M, et al. The extent of B-cell activation and dysfunction preceding lymphoma development in HIV-positive people. HIV Med. 2018;19(2):90–101. [DOI] [PubMed] [Google Scholar]
- 38.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong HL, Breen EC, Pfeiffer RM, Aissani B, Martinson JJ, Margolick JB, et al. Cytokine signaling pathway polymorphisms and AIDS-related non-Hodgkin lymphoma risk in the multicenter AIDS cohort study. Aids. 2010;24(7):1025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309(8):453–8. [DOI] [PubMed] [Google Scholar]
- 41.Moir S, Ogwaro KM, Malaspina A, Vasquez J, Donoghue ET, Hallahan CW, et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci U S A. 2003;100(10):6057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin G, Roy J, Barat C, Ouellet M, Gilbert C, Tremblay MJ. Human immunodeficiency virus type 1-associated CD40 ligand transactivates B lymphocytes and promotes infection of CD4+ T cells. J Virol. 2007;81(11):5872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol Rev. 2010;237(1):226–48. [DOI] [PubMed] [Google Scholar]
- 44.Epeldegui M, Thapa DR, De la Cruz J, Kitchen S, Zack JA, Martinez-Maza O. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS One. 2010;5(7):e11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berberian L, Goodglick L, Kipps TJ, Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 1993;261(5128):1588–91. [DOI] [PubMed] [Google Scholar]
- 46.Cagigi A, Du L, Dang LV, Grutzmeier S, Atlas A, Chiodi F, et al. CD27(−) B-cells produce class switched and somatically hyper-mutated antibodies during chronic HIV-1 infection. PLoS One. 2009;4(5):e5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176(7):3931–41. [DOI] [PubMed] [Google Scholar]
- 48.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108(5):1580–7. [DOI] [PubMed] [Google Scholar]
- 49.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. The Journal of infectious diseases. 2005;191(9):1442–50. [DOI] [PubMed] [Google Scholar]
- 50.Dolcetti R, Gloghini A, Caruso A, Carbone A. A lymphomagenic role for HIV beyond immune suppression? Blood. 2016;127(11):1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kundu RK, Sangiorgi F, Wu LY, Pattengale PK, Hinton DR, Gill PS, et al. Expression of the human immunodeficiency virus-Tat gene in lymphoid tissues of transgenic mice is associated with B-cell lymphoma. Blood. 1999;94(1):275–82. [PubMed] [Google Scholar]
- 52.Atallah-Yunes SA, Murphy DJ, Noy A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020;7(8):e594–e600. [DOI] [PubMed] [Google Scholar]
- 53.Popovic M, Tenner-Racz K, Pelser C, Stellbrink HJ, van Lunzen J, Lewis G, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2005;102(41):14807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolcetti R, Giagulli C, He W, Selleri M, Caccuri F, Eyzaguirre LM, et al. Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis. Proc Natl Acad Sci U S A. 2015;112(46):14331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martorelli D, Muraro E, Mastorci K, Dal Col J, Fae DA, Furlan C, et al. A natural HIV p17 protein variant up-regulates the LMP-1 EBV oncoprotein and promotes the growth of EBV-infected B-lymphocytes: implications for EBV-driven lymphomagenesis in the HIV setting. Int J Cancer. 2015;137(6):1374–85. [DOI] [PubMed] [Google Scholar]
- 56.Silverberg MJ, Neuhaus J, Bower M, Gey D, Hatzakis A, Henry K, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. Aids. 2007;21(14):1957–63. [DOI] [PubMed] [Google Scholar]
- 57.Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lurain K, Polizzotto MN, Aleman K, Bhutani M, Wyvill KM, Goncalves PH, et al. Viral, immunologic, and clinical features of primary effusion lymphoma. Blood. 2019;133(16):1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillet S, Gerard L, Meignin V, Agbalika F, Cuccini W, Denis B, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91(2):233–7. [DOI] [PubMed] [Google Scholar]
- 60.Fan W, Bubman D, Chadburn A, Harrington WJ Jr., Cesarman E, Knowles DM. Distinct subsets of primary effusion lymphoma can be identified based on their cellular gene expression profile and viral association. J Virol. 2005;79(2):1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juillard F, de Miranda MP, Li S, Franco A, Seixas AF, Liu B, et al. KSHV LANA acetylation-selective acidic domain reader sequence mediates virus persistence. Proc Natl Acad Sci U S A. 2020;117(36):22443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–68. [DOI] [PubMed] [Google Scholar]
- 64.Quinn LL, Williams LR, White C, Forrest C, Zuo J, Rowe M. The Missing Link in Epstein-Barr Virus Immune Evasion: the BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II. J Virol. 2016;90(1):356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuo J, Currin A, Griffin BD, Shannon-Lowe C, Thomas WA, Ressing ME, et al. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS pathogens. 2009;5(1):e1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HR, Brulois K, Wong L, Jung JU. Modulation of Immune System by Kaposi's Sarcoma-Associated Herpesvirus: Lessons from Viral Evasion Strategies. Frontiers in microbiology. 2012;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deb Pal A, Banerjee S. Epstein-Barr virus latent membrane protein 2A mediated activation of Sonic Hedgehog pathway induces HLA class Ia downregulation in gastric cancer cells. Virology. 2015;484:22–32. [DOI] [PubMed] [Google Scholar]
- 68.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5(4):376–85. [DOI] [PubMed] [Google Scholar]
- 69.Davis DA, Mishra S, Anagho HA, Aisabor AI, Shrestha P, Wang V, et al. Restoration of immune surface molecules in Kaposi sarcoma-associated herpesvirus infected cells by lenalidomide and pomalidomide. Oncotarget. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis DA, Mishra S, Anagho HA, Aisabor AI, Shrestha P, Wang V, et al. Restoration of immune surface molecules in Kaposi sarcoma-associated herpes virus infected cells by lenalidomide and pomalidomide. Oncotarget. 2017;8(31):50342–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wienand K, Chapuy B, Stewart C, Dunford AJ, Wu D, Kim J, et al. Genomic analyses of flow-sorted Hodgkin Reed-Sternberg cells reveal complementary mechanisms of immune evasion. Blood Adv. 2019;3(23):4065–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anastasiadou E, Stroopinsky D, Alimperti S, Jiao AL, Pyzer AR, Cippitelli C, et al. Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia. 2019;33(1):132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bultema R, Longnecker R, Swanson-Mungerson M. Epstein-Barr virus LMP2A accelerates MYC-induced lymphomagenesis. Oncogene. 2009;28(11):1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt's lymphomas. Proc Natl Acad Sci U S A. 2003;100(24):14269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panea RI, Love CL, Shingleton JR, Reddy A, Bailey JA, Moormann AM, et al. The whole-genome landscape of Burkitt lymphoma subtypes. Blood. 2019;134(19):1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drotar ME, Silva S, Barone E, Campbell D, Tsimbouri P, Jurvansu J, et al. Epstein-Barr virus nuclear antigen-1 and Myc cooperate in lymphomagenesis. Int J Cancer. 2003;106(3):388–95. [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharjee S, Ghosh Roy S, Bose P, Saha A. Role of EBNA-3 Family Proteins in EBV Associated B-cell Lymphomagenesis. Frontiers in microbiology. 2016;7:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knight JS, Lan K, Subramanian C, Robertson ES. Epstein-Barr virus nuclear antigen 3C recruits histone deacetylase activity and associates with the corepressors mSin3A and NCoR in human B-cell lines. J Virol. 2003;77(7):4261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allday MJ, Bazot Q, White RE. The EBNA3 Family: Two Oncoproteins and a Tumour Suppressor that Are Central to the Biology of EBV in B Cells. Curr Top Microbiol Immunol. 2015;391:61–117. [DOI] [PubMed] [Google Scholar]
- 82.Saha A, Robertson ES. Impact of EBV essential nuclear protein EBNA-3C on B-cell proliferation and apoptosis. Future Microbiol. 2013;8(3):323–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mannick JB, Cohen JI, Birkenbach M, Marchini A, Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991;65(12):6826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sinclair AJ, Palmero I, Peters G, Farrell PJ. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13(14):3321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iizasa H, Kim H, Kartika AV, Kanehiro Y, Yoshiyama H. Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases. Front Immunol. 2020;11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim H, Iizasa H, Kanehiro Y, Fekadu S, Yoshiyama H. Herpesviral microRNAs in Cellular Metabolism and Immune Responses. Frontiers in microbiology. 2017;8:1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin X, Tsai MH, Shumilov A, Poirey R, Bannert H, Middeldorp JM, et al. The Epstein-Barr Virus BART miRNA Cluster of the M81 Strain Modulates Multiple Functions in Primary B Cells. PLoS pathogens. 2015;11(12):e1005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y, Fachko D, Ivanov NS, Skinner CM, Skalsky RL. Epstein-Barr virus microRNAs regulate B cell receptor signal transduction and lytic reactivation. PLoS pathogens. 2019;15(1):e1007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Higuchi H, Yamakawa N, Imadome KI, Yahata T, Kotaki R, Ogata J, et al. Role of exosomes as a proinflammatory mediator in the development of EBV-associated lymphoma. Blood. 2018;131(23):2552–67. [DOI] [PubMed] [Google Scholar]
- 90.Tagawa T, Albanese M, Bouvet M, Moosmann A, Mautner J, Heissmeyer V, et al. Epstein-Barr viral miRNAs inhibit antiviral CD4+ T cell responses targeting IL-12 and peptide processing. J Exp Med. 2016;213(10):2065–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murer A, Ruhl J, Zbinden A, Capaul R, Hammerschmidt W, Chijioke O, et al. MicroRNAs of Epstein-Barr Virus Attenuate T-Cell-Mediated Immune Control In Vivo. mBio. 2019;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Friborg J Jr., Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402(6764):889–94. [DOI] [PubMed] [Google Scholar]
- 93.Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med. 2000;6(10):1121–7. [DOI] [PubMed] [Google Scholar]
- 94.Bubman D, Guasparri I, Cesarman E. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene. 2007;26(34):4979–86. [DOI] [PubMed] [Google Scholar]
- 95.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18(42):5738–46. [DOI] [PubMed] [Google Scholar]
- 96.Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J Biol Chem. 2002;277(16):13745–51. [DOI] [PubMed] [Google Scholar]
- 97.Ahmad A, Groshong JS, Matta H, Schamus S, Punj V, Robinson LJ, et al. Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 cooperates with Myc to promote lymphoma in mice. Cancer Biol Ther. 2010;10(10):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272(31):19625–31. [DOI] [PubMed] [Google Scholar]
- 99.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94(8):2871–9. [PubMed] [Google Scholar]
- 100.Jarviluoma A, Ojala PM. Cell signaling pathways engaged by KSHV. Biochim Biophys Acta. 2006;1766(1):140–58. [DOI] [PubMed] [Google Scholar]
- 101.Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, Godden K, et al. Cyclin encoded by KS herpesvirus. Nature. 1996;382(6590):410. [DOI] [PubMed] [Google Scholar]
- 102.Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390(6656):184–7. [DOI] [PubMed] [Google Scholar]
- 103.Barta SK, Xue X, Wang D, Tamari R, Lee JY, Mounier N, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122(19):3251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaplan LD, Straus DJ, Testa MA, Von Roenn J, Dezube BJ, Cooley TP, et al. Low-dose compared with standard-dose m-BACOD chemotherapy for non-Hodgkin's lymphoma associated with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med. 1997;336(23):1641–8. [DOI] [PubMed] [Google Scholar]
- 105.Reid E, Suneja G, Ambinder RF, Ard K, Baiocchi R, Barta SK, et al. Cancer in People Living With HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2018;16(8):986–1017. [DOI] [PubMed] [Google Scholar]
- 106.Hansen ME, Mangusan R, Lurain K, Odeny T, George J, Lu C, et al. Characteristics of patients admitted to the intensive care unit with Kaposi sarcoma herpesvirus-associated diseases. AIDS. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carbone A, Vaccher E, Gloghini A. Hematologic cancers in individuals infected by HIV. Blood. 2022;139(7):995–1012. [DOI] [PubMed] [Google Scholar]
- 108.Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, Napravnik S, et al. Association of early HIV viremia with mortality after HIV-associated lymphoma. Aids. 2013;27(15):2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calkins KL, Chander G, Joshu CE, Visvanathan K, Fojo AT, Lesko CR, et al. Immune Status and Associated Mortality After Cancer Treatment Among Individuals With HIV in the Antiretroviral Therapy Era. JAMA Oncol. 2020;6(2):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uldrick TS, Goncalves PH, Abdul-Hay M, Claeys AJ, Emu B, Ernstoff MS, et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol. 2019;5(9):1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gonzalez-Cao M, Moran T, Dalmau J, Garcia-Corbacho J, Bracht JWP, Bernabe R, et al. Assessment of the Feasibility and Safety of Durvalumab for Treatment of Solid Tumors in Patients With HIV-1 Infection: The Phase 2 DURVAST Study. JAMA Oncol. 2020;6(7):1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Odeny TA, Lurain K, Strauss J, Fling SP, Sharon E, Wright A, et al. Effect of CD4+ T cell count on treatment-emergent adverse events among patients with and without HIV receiving immunotherapy for advanced cancer. Journal for ImmunoTherapy of Cancer. 2022;10(9):e005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017;35(19):2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen PB, Savas H, Evens AM, Advani RH, Palmer B, Pro B, et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood. 2021;137(10):1318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lurain K, Ramaswami R, Yarchoan R, Uldrick TS. Anti-PD-1 and Anti-PD-L1 Monoclonal Antibodies in People Living with HIV and Cancer. Curr HIV/AIDS Rep. 2020;17(5):547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gandhi MK, Hoang T, Law SC, Brosda S, O'Rourke K, Tobin JWD, et al. EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity. Blood. 2021;137(11):1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34(23):2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lurain K, Ramaswami R, Mangusan R, Widell A, Ekwede I, George J, et al. Use of pembrolizumab with or without pomalidomide in HIV-associated non-Hodgkin's lymphoma. J Immunother Cancer. 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uldrick TS, Adams SV, Fromentin R, Roche M, Fling SP, Goncalves PH, et al. Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy. Sci Transl Med. 2022;14(629):eabl3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Y, Shrestha P, Heape NM, Yarchoan R. CDK4/6 inhibitors sensitize gammaherpesvirus-infected tumor cells to T-cell killing by enhancing expression of immune surface molecules. J Transl Med. 2022;20(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lacy MQ, McCurdy AR. Pomalidomide. Blood. 2013;122(14):2305–9. [DOI] [PubMed] [Google Scholar]
- 125.Davis DA, Shrestha P, Aisabor AI, Stream A, Galli V, Pise-Masison CA, et al. Pomalidomide increases immune surface marker expression and immune recognition of oncovirus-infected cells. Oncoimmunology. 2019;8(2):e1546544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shrestha P, Davis DA, Jaeger HK, Stream A, Aisabor AI, Yarchoan R. Pomalidomide restores immune recognition of primary effusion lymphoma through upregulation of ICAM-1 and B7-2. PLoS pathogens. 2021;17(1):e1009091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gopalakrishnan R, Matta H, Tolani B, Triche T Jr., Chaudhary PM. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene. 2016;35(14):1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heslop HE, Sharma S, Rooney CM. Adoptive T-Cell Therapy for Epstein- Barr Virus-Related Lymphomas. J Clin Oncol. 2021;39(5):514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roshan R, Labo N, Trivett M, Miley W, Marshall V, Coren L, et al. T-cell responses to KSHV infection: a systematic approach. Oncotarget. 2017;8(65):109402–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31(1):39–48. [DOI] [PubMed] [Google Scholar]
- 131.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dalton T, Doubrovina E, Pankov D, Reynolds R, Scholze H, Selvakumar A, et al. Epigenetic reprogramming sensitizes immunologically silent EBV+ lymphomas to virus-directed immunotherapy. Blood. 2020;135(21):1870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ghosh SK, Perrine SP, Williams RM, Faller DV. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood. 2012;119(4):1008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramos JC, Sparano JA, Chadburn A, Reid EG, Ambinder RF, Siegel ER, et al. Impact of Myc in HIV-associated Non-Hodgkin Lymphomas Treated with EPOCH, and Outcomes with Vorinostat (AMC075 Trial). Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rust BJ, Kiem HP, Uldrick TS. CAR T-cell therapy for cancer and HIV through novel approaches to HIV-associated haematological malignancies. Lancet Haematol. 2020;7(9):e690–e6. [DOI] [PubMed] [Google Scholar]
- 136.Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022;386(7):640–54. [DOI] [PubMed] [Google Scholar]
- 137.Abramson JS, Irwin KE, Frigault MJ, Dietrich J, McGree B, Jordan JT, et al. Successful anti-CD19 CAR T-cell therapy in HIV-infected patients with refractory high-grade B-cell lymphoma. Cancer. 2019;125(21):3692–8. [DOI] [PubMed] [Google Scholar]
- 138.Allred J, Bharucha K, Ozutemiz C, He F, Janakiram M, Maakaron J, et al. Chimeric antigen receptor T-cell therapy for HIV-associated diffuse large B-cell lymphoma: case report and management recommendations. Bone Marrow Transplant. 2021;56(3):679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abbasi A, Peeke S, Shah N, Mustafa J, Khatun F, Lombardo A, et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J Hematol Oncol. 2020;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anthony-Gonda K, Bardhi A, Ray A, Flerin N, Li M, Chen W, et al. Multispecific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci Transl Med. 2019;11(504). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maldini CR, Gayout K, Leibman RS, Dopkin DL, Mills JP, Shan X, et al. HIV-Resistant and HIV-Specific CAR-Modified CD4(+) T Cells Mitigate HIV Disease Progression and Confer CD4(+) T Cell Help In Vivo. Mol Ther. 2020;28(7):1585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lurain K, Yarchoan R, Ramaswami R. The Changing Face of HIV-Associated Malignancies: Advances, Opportunities, and Future Directions. Am Soc Clin Oncol Educ Book. 2019;39:36–40. [DOI] [PubMed] [Google Scholar]
- 143.Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw. 2019;17(6):650–61. [DOI] [PubMed] [Google Scholar]
- 144.Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Armand P, Bello CM, et al. NCCN Guidelines(R) Insights: Hodgkin Lymphoma, Version 2.2022. J Natl Compr Canc Netw. 2022;20(4):322–34. [DOI] [PubMed] [Google Scholar]