Abstract
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) which was identified in Wuhan, China in December 2019 and jeopardized human lives. It spreads at an unprecedented rate worldwide, with serious and still-unfolding health conditions and economic ramifications. Based on the clinical investigations, the severity of COVID-19 appears to be highly variable, ranging from mild to severe infections including the death of an infected individual. To add to this, patients with comorbid conditions such as age or concomitant illnesses are significant predictors of the disease’s severity and progression. SARS-CoV-2 enters inside the host cells through ACE2 (angiotensin converting enzyme2) receptor expression; therefore, comorbidities associated with higher ACE2 expression may enhance the virus entry and the severity of COVID-19 infection. It has already been recognized that age-related comorbidities such as Parkinson’s disease, cancer, diabetes, and cardiovascular diseases may lead to life-threatening illnesses in COVID-19-infected patients. COVID-19 infection results in the excessive release of cytokines, called “cytokine storm”, which causes the worsening of comorbid disease conditions. Different mechanisms of COVID-19 infections leading to intensive care unit (ICU) admissions or deaths have been hypothesized. This review provides insights into the relationship between various comorbidities and COVID-19 infection. We further discuss the potential pathophysiological correlation between COVID-19 disease and comorbidities with the medical interventions for comorbid patients. Toward the end, different therapeutic options have been discussed for COVID-19-infected comorbid patients.
Keywords: COVID-19, comorbidity, diabetes, cancer, Parkinson’s disease, cardiovascular disease
1. Introduction
Coronavirus disease (COVID-19) is a communicable disease associated with the dysfunction of the upper respiratory tract caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). The city of Wuhan, China was the first to document pneumonia cases of unknown etiology at the end of December 2019.1 It was spread to around 188 nations, resulting in many confirmed cases and severe health and socioeconomic consequences. The total number of confirmed cases worldwide reached 574,818,625 with 6,395,451 fatalities by the fourth week of July 2022; this data was presented by the Coronavirus Resource Center at Johns Hopkins University.2
Ejaz and colleagues reported that SARS-CoV-2 infections could cause various symptoms, from mild diseases that go away independently to dangerous ones that affect many organs. There are mainly four types of coronavirus (CoV), classified as α-CoV, β-CoV, γ-CoV, and δ-CoV. Of these, only α-CoV and β-CoV have been shown to cause animal sickness.3 Further, β-CoV was responsible for SARS in 2003 and the Middle East respiratory syndrome (MERS) in 2012.4 According to several genomic studies, SARS-CoV-2 is an encapsulated virus with a positive sense-RNA genome. The genome of SARS-CoV-2 is approximately 96% similar to that of bat CoV RaTG13. Furthermore, the genomic sequence and evolution of the analysis of SARS-CoV-2 have a 79.5% genomic similarity to the severe acute respiratory syndrome-coronavirus (SARS-CoV).5
SARS-CoV-2 enters human cells by attaching to the angiotensin convertase enzyme2 (ACE2) receptor of the upper respiratory tract,6 which acts as an entry point for this virus.6,7 Although the virus travels by intranasal and oral pathways, it affects olfactory sensory neurons. Eventually, it infects the central nervous system (CNS), causing hyposmia (loss of sensation of smell) and hypogeusia8 (loss of taste) as well as other sensory symptoms.8,9 Although SARS-CoV-2 infects people of all ages and genders, research shows that individuals with comorbidities are more susceptible to COVID-19 infection. Further evidence suggests that male patients 50 years of age with or without comorbidities show a significantly increased risk of death.10 According to the Centers for Disease Control and Prevention (CDC), USA, individuals aged 65 and above accounted for around 30% of COVID-19 infections, 45% of hospitalizations, 53% of intensive care unit (ICU) admissions, and 80% of deaths. In addition, after COVID-19 infection, those with a compromised immune system due to cancer treatments or steroids requiring hospitalization are prone to mortality.11 Given those the events of ICU admissions or mortality following COVID-19 infection increase. It is vital to comprehend the mechanisms and treatment alternatives that are most suited for marginalized populations.12 Based on clinical data on COVID-19, comorbidities like cardiovascular disease (CVD) including hypertension and diabetes have been the most prevalent.13,14 In this review, we focused on the link between COVID-19 and comorbidities such as Parkinson’s disease (PD), cancer, diabetes, and CVD. We also looked at epidemiological data, pathological relationships, and potential treatment options for COVID-19-infected people with comorbidities.
2. Methodology
As was highlighted, this review illustrates the relationship between several comorbidities and COVID-19. A literature search was conducted online using several databases and search engines like PubMed to exploit this. The keywords such as COVID-19, SARS-CoV-2, and comorbidity in COVID-19 were used to get the most relevant articles that support this study. On the other hand, the relationship between PD, cancer, diabetes mellitus, CVD, and hypertension with COVID-19 articles were also used to accomplish this study. Besides, the rest of the articles with mismatched or irrelevant keywords were not considered for this study. All the publications were examined and referenced based on their relevancy and compatibility with the current topic of discussion. We used PubMed’s “Boolean Operators” (AND, NOT, and OR) search criterion to acquire relevant search results for this. Figure 1 depicts the strategy for obtaining the data and subsequently filtering the articles, and Table 1 provides the keywords used for searching through Pubmed. The significant comorbidities of COVID-19, such as PD, cancer, diabetes, and CVD, have been included after a literature search and screening of published research articles, meta-analyses, and systemic review studies. In this study, 198 articles were discussed in depth to show how these significant comorbidities were to blame for hospitalization, ICU admission, and mortality in most cases. In addition, we looked at the molecular and cellular mechanisms of COVID-19 and how they relate to its pathophysiology, linked with substantial comorbidities. For instance, cytokine storm is typical of all the main comorbidities described above. We looked for several inflammatory cytokines connected to COVID-19-related comorbidities in this context. Then, we checked the further information on clinical trials web site15 to learn more about the clinical studies that used the repurposed drugs for the appropriate comorbidities. Furthermore, we have also included future projections as well as several treatment strategies for each comorbidity. Patients with COVID-19 have received treatment using a wide range of therapeutic modalities globally. In the absence of a vaccine or a SARS-CoV neutralizing antibody, convalescent plasma therapy and pharmaceutical repurposing lead the charge.
Figure 1.
Scheme depicting the process of screening of documented articles to prepare this manuscript. All relevant papers are listed according to the keywords searched in the NCBI search engine (PUBMED) using Boolean operators such as AND, OR, and NOT. The articles were fetched for COVID-19, coronavirus, COVID-19, and comorbidity, along with their possible treatment strategy.
Table 1. Keywords Used for the Literature Review and the Number of Articles Retrieved.
| Keywords | PUBMED |
|---|---|
| COVID-19 OR coronavirus | 3,844 |
| SARS-CoV-2 AND comorbidity | 10,591 |
| COVID-19 AND Parkinson’s disease | 684 |
| COVID-19 AND cancer | 21,020 |
| COVID-19 AND diabetes | 11,444 |
| COVID-19 AND CVD | 19,186 |
3. Variants of SARS-CoV-2
During genomic replication, a virus’s genetic code changes (gene mutation), a phenomenon also prevalent with the SARS-CoV-2 virus, wherein constant gene mutations have led to many variants (lineage) of the same virus over time. A lineage is a set of genetically related viral variants that share a common ancestor. SARS-CoV-2 has several lineages, all of which produce COVID-19 infection. Some lineage changes propagate more rapidly and easily than others, perhaps contributing to make COVID-19 cases more common. A rise in the number of cases have imposed a higher burden on healthcare resources, resulting in additional hospitalizations and, perhaps, fatalities. In the USA, epidemiological investigations into viral genetic sequence-based monitoring and laboratory research are routinely conducted to track SARS-CoV-2 genetic lineages. The SARS-CoV-2 Interagency Group (SIG) of the US government categorized Omicron as a Variant of Concern on November 30, 2021 (Control and Prevention, 2021). According to SIG, there are four types of SARS-CoV-2 variants: 1. Variant Being Monitored (VBM): Alpha (B.1.1.7 and Q lineages), Beta (B.1.351 and descendent lineages), Gamma (P.1 and descendent lineages), Epsilon (B.1.427 and B.1.429), Eta (B.1.525), Iota (B.1.526), Kappa (B.1.617.1), 1.617.3, Mu (B.1.621, B.1.621.1), Zeta (P.2); 2. Variant of Interest (VOI): None of the variant(s) yet identified; 3. A variant of Concern (VOC): Delta (B.1.617.2 and AY lineages), Omicron (B.1.1.529 and BA lineages); 4. A variants of High Consequence (VOHC): This sort of variation is yet to be detected internationally.16
Omicron is constantly evolving mutations even after 3 years of the pandemic and still giving rise to several new subvariants, such as BA.2.75 and BA.4.6. Importantly, several of these new variations, including BA.2.3.20, BA.2.75.2, CA.1, BR.2, BN.1, BM.1.1.1, BU.1, BQ.1.1, and XBB,17 exhibit notable growth advantages over BA.5.18 Recently reported in the second week of October 2022 is the name of the latest lineages variant of Omicron is XBB and sublineages XBB.1 (S:252 V) found in major regions such as China, United Kindom (UK), Europe, and North America, resulting in all these countries announcing that they are now going on nationwide lockdown-like restriction once again due to the sudden surge of the new COVID variant. XBB and XBB.1 (S:252 V) are mainly found in Bangladesh, Singapore, and India.
The prevalence of Delta and Omicron (BA.1) coinfections and Omicron lineages BA.1 and BA.2 coinfections were estimated at 0.18% and 0.26%, respectively. Among 6,242 hospitalized patients, ICU admission rates were 1.64%, 4.81, and 15.38% in Omicron, Delta, and Delta/Omicron patients, respectively. Among patients admitted to the ICU, there were no reports of BA.1/BA.2 coinfections. A total of 21 patients (39.6%) of the 53 coinfected patients missed vaccinations. Even though SARS-CoV-2 coinfections were rare in their clinical study, it is still essential to accurately identify them so that they can figure out how they affect patients and how likely they will make recombinants.19
One clinical study has been conducted in France to detect the prevalence of SARS-CoV-2 coinfection during spread of the Delta, Omicron, Delta/Omicron variant. This study was held from December 2021 to February 2022. They tested the effectiveness of four sets of whole-genome sequencing primers using 11 blends of Delta/Omicron isolates at multiple ratios, and they developed a bioinformatics technique that is impartial for identifying coinfections involving various genetic SARS-CoV-2 lineages. Applied to 21,387 samples collected from 6 December 2021 to 27 February 2022, random genomic surveillance in France, they detected 53 coinfections between different lineages.
The Delta variation was the most susceptible and transmissible of all of these variants, that resulted in an increase in the percentage of fatalities as well as comorbidities such as hospitalization of older persons. The Omicron variant may spread more quickly than other variants, such as Delta; however, Omicron was less lethal compared to the Delta variant. These differences in response could be attributed to many factors such as the less efficient cleaving of the S protein fraction of omicron and more α helix stabilization than the delta variant.20
4. COVID-19 Risk and Epidemiology in Relation to Comorbidity
4.1. Aging and Comorbidities
COVID-19 infection is associated with aging, which is a major risk factor for severe illness and mortality, especially for those who are in long-term care facilities. In addition, people at any age with serious underlying medical conditions are more at risk of getting COVID-19 infection. The elderly, SARS-CoV-2 infected persons with comorbidities, including PD, diabetes, cancer, and hypertension (HTN) and CVD, are at higher risk of death. Early evidence from several epidemiological data sets shows that the COVID-19 case fatality ratio (CFR) increases with age. Table 2 reflects the number of CFRs in various countries; overall, the CFR of China and USA is 2.3% and 2.7%, respectively, while the global CFR was at 2.8%.21
Table 2. Percentage Case Fatality Ratio of aged persons with COVID-19.
Italy was the first nation to be affected by the pandemic after China. The total CFR of Italy was higher (7.2%) compared to China (2.3%). This is attributed to a more significant proportion of older adults (22.8% and 11.9%, respectively) in Italy. In addition, an 82-year-old man in Brooklyn was the first COVID-19 fatality reported in New York City. A significant case series of 5,700 COVID-19 patients admitted to hospitals in New York City revealed a similar pattern of COVID-19 fatalities with age.21,24 However, the mechanism of SARS-CoV-2 infection is still unknown. However, the primary mechanism of SARS-CoV-2 underlies the ACE2 enzyme’s expression and utilization of the ACE2 receptor, which helps to enter the SARS-CoV-2 inside the cell. Lymphopenia, abnormal respiration, and a high level of pro-inflammatory cytokines in plasma are the main manifestations ascribed to individuals with COVID-19 infection along with very high body temperature and respiratory issues.30 COVID-19 is caused by several metabolic and viral disorders, all of which have a part in the developing of the more complicated symptoms.
The CFR for India seems to be lower than in several European nations. This might attributed to low percentage of population (6.38%) to be above the age of 65 as per 2019 statistics. According to reports, COVID-19 puts elderly persons (those over 60) at a greater risk of mortality. The relationship between CFR and several other health and socioeconomic factors, variations in the virulence of SARS-CoV-2 across geographical areas, and COVID-19 response indicators unique to certain countries has to be further studied.
The projected CFRs (July 2020) based on the random- and fixed-effect models were 1.42% (95% Cl 1.19–1.70%) and 2.97% (95% CI 2.94–3.00%), respectively. Estimates made using the random-effects model were more likely to accurately reflect the real CFR for India because of the high level of variability. In earlier research, the COVID-19 CFR was estimated using random-effect models, or the CFR was provided using both random- and fixed-effect models. We made sure that states with a lot of cases and fatalities got more weightage than those with fewer cases and deaths by using a random effects model.31Table 3 shows that COVID-19-infected patients with comorbidities had a higher death risk.
Table 3. Mortality Rates of COVID-19-Infected Patients with Comorbidities in Several Countries.
As a consequence of aging, the body experiences progressive biological alterations in immune function, concurrently causing an increased susceptibility to age-related inflammation (inflammaging) and other associated inflammatory conditions, which makes the elderly population vulnerable to enhanced risk of infection following exposure to the virus.32 Inflammaging is a chronic low-stage inflammation mediated by dysfunction in the basal responses of the pattern recognition receptors (PRRs) and pathogen-associated molecular patterns (PAMPs). A progressive impairment in autophagic signals affect the PRR signals in the aging population and consequently cause the exorbitant release of reactive oxygen species (ROS).33 This condition may further be worsened with the binding of the virus to immune cells as they also work in synchronization with PAMPs and PRRs, thereby causing oxidative damage to cells in older individuals. Another hallmark of inflammaging is increased production of interleukins (ILs), especially IL-1β and IL-18, due to activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome, in COVID-19 infections. NLRP3 activation is strongly correlated to the aging population. This consequently causes increased pyroptosis, which is central to cell death post-infection; increased release of IL-1β, IL-18 as well as damage-associated molecular patterns (DAMPs) further cumulates inflammatory responses in the elderly.38 This mechanism has also been reported to be the root cause of inflammatory conditions such as cancer, diabetes, PD, and acute myocardial injury.39 Furthermore, with age, there is a progressive hardening of the endothelial cells, causing the increased formation of plaque, and further leading to a hypercoagulative state. Following COVID-19 infection, reports have demonstrated that a hypercoagulative state is associated with an increased risk of comorbid conditions such as ischemic stroke and myocardial infarction.40
Therefore, it can be concluded that aging and comorbid conditions are crucial factors in eliciting the response mediated by COVID-19 infection alone. So, in the subsequent sections, we discussed the latest reports of age-related comorbidities, such as PD, cancer, diabetes, and CVD, and how they relate to the severity and pathology of COVID-19.
4.2. A Case-Control and Cohort Investigation to Ascertain the Association between Severe COVID-19 and Comorbidities
The population of patients needing hospital admission is disproportionately composed of older adults, men, and people with comorbid conditions, including diabetes and CVDs.21,24,41,42 After COVID-19 expanded to multiethnic communities in Western Europe and North America, multiple studies claimed that Black, Asian, or other minority ethnic groups43 were more likely to be affected by the illness.43,44 According to the statistical report from USA, in several cities or cumulative analyses across large states, Black and Hispanic people had higher per capita mortality rates than White people, but the underlying reasons were unknown.43,45 A large cohort study has been conducted in the United Kingdom (UK),46 wherein they reported higher overall death rates for Black and South Asian people compared to White people. However, the study ignores the wide variances in the ethnic makeup of local populations across various geographic locations.
In this regard, a case-control and cohort study was conducted at King’s College Hospital Foundation Trust, UK to investigate if ethnic origin influences the probability of hospital admission with severe COVID-19 and/or in-hospital mortality. Inner city adult patients with confirmed COVID-19 admitted to the hospital (n = 872 cases) were compared with 3,488 matched controls randomly drawn from a primary healthcare database consisting of 344,083 people dwelling in the same area. For the cohort study, the authors examined 1,827 people continuously hospitalized with COVID-19. Self-defined ethnicity served as the primary exposure factor and analyses were adjusted for socio-demographic and clinical characteristics. Based on the results obtained by conditional logistic regression analysis, it was demonstrated that the Black and Mixed/Other ethnicity were linked with greater admission risk than the White.47 Further, the Black and Mixed ethinicity could be linked to disease severity but not to in-hospital mortality. This was majorly attributed to ethinicity and partly to comorbities and socioeconomic factors. In addition, the study elucidated the association of increased in-hospital mortality with ICU admission for Asian ethinicity. Therefore,it could be concluded that COVID-19 disease outcome is influenced by the ethnic background.47
4.3. Sex Differences
Although aging is a prominent factor for comorbidities, it may not be as labeled as the only confounding factor for the disease severity. Reports have suggested that hospitalized males had the highest mortality rate as compared to females and this association was more prominent with patients with predisposing conditions such as hypertension, diabetes and obesity in an age-dependent manner.48 One study has shown male patients’ as a predictor of ICU admissions.49 Contrastingly, in context of the long term COVID-19 manifestations, women were more likely to report uneasiness, breathlessness, and fatigue following recovery.50 Outcomes in severity also resulted from biochemical differences in males and females; compared to male patients, females had higher lymphocyte counts, higher levels of high-density lipoprotein, as well as lower levels of highly sensitive C-reactive protein.51 Another hypothesis for gender disparity in protection is that the females have biallelic Toll-like receptor (TLR)7 expression, thereby leading to a better interferon (IFN)-mediated response after early infection.52 Interestingly, studies have reported that estrogen confers some protection against the severity of COVID-19.53 This has been validated in a preclinical setting, where mice infected with the SARS-CoV virus had a higher mortality rate following ovariectomy or estrogen receptor antagonist administration.54
Further, pregnant women with mild infection demonstrate same outcome as uninfected pregnant women. However, those with severe infection demonstrate a higher risk of perinatal infection as well as mortality, usually having a tendency to feel unwell and this further exacerbates during COVID-19 infection and may result in worsening of conditions of the patients. Children are disportionately infected with COVID-19 compared to older population but with low infection severity and could be attributed to lower concentration of ACE2 receptors in children as well as trained/acquired immunity as a result of vaccination, indigenous virus competition, as well as maternal immunity.55
Thus, it can be concluded that sex differences play a major role in the outcomes and severity of COVID-19 infection. Due to the pivotal role of female hormones, they may be less susceptible to long-term manifestations which are prevalent in male patients.
5. COVID-19 and Comorbidities
5.1. Parkinson’s Disease and COVID-19
SARS-CoV-2 is a neurotropic virus, that can enter the CNS either by hematogenous or neuronal retrograde dissemination.56 In an autopsy study, viral RNA in the brains of several COVID-19 patients was detected.57 Patients having neurological diseases are generally more vulnerable to the respiratory system infections.and could be attributed to the involvement of central respiratory centers in the case of COVID-19 infections.58 There are shreds of evidence that suggest the potential of SARS-CoV-2 to enter the brain through the olfactory epithelium and cause neuronal death in mice. Furthermore, there have been cases of COVID-19 individuals developing symptomatic parkinsonism after 2–5 weeks of viral infection. SARS-CoV was discovered in the cerebral fluid of individuals suffering from acute SARS-CoV disease, as was also reported in the COVID-19 cases.59 Recent data from a study performed in three designated COVID-19 care hospitals of the Union Hospital of Huazhong University of Science and Technology in Wuhan, China suggests that 78 patients out of 214 COVID-19 patients demonstrated neurologic manifestations. This involved the CNS, peripheral nervous system, and skeletal muscles, subsequently indicating the neurotropic potential of this virus.60
Hitherto, there is not enough evidence regarding the susceptibility of PD patients to COVID-19. According to a study from the Parkinson’s and Movement Disorders Unit in Padua, Italy and the Parkinson’s Foundation Centre of Excellence at King’s College Hospital in London, UK, PD patients with an average age of more than 78.3 years and with a disease period of greater than 12.7 years are more prone to COVID-19. They also have a significantly high mortality rate of 40%.61 The patients in extreme conditions of PD with respiratory muscle rigidity, dyspnoea, and on deep brain stimulation or levodopa infusion therapy showed high vulnerability and 50% mortality.61 Moreover, SARS-CoV and H1N1 viruses (structurally and functionally similar to COVID-19) can aggravate the mechanisms involved in PD pathophysiology as supported by previous studies.62 A few studies also suggest the role of the CNS in COVID-19 infection indicating that PD patients might be more prone to COVID-19 infection.
5.1.1. Possible Pathophysiological Association between COVID-19 and PD Patients
There are various theories about how SARS-CoV-2 enters the CNS. Peripheral SARS-CoV-2 infection can lead to a cytokine storm, which might disrupt the blood–brain barrier integrity and might be a mechanism for SARS-CoV-2 to infiltrate into the CNS. Besides this, ACE2 receptors are highly expressed in the substantia nigra and striatum (the regions which are potentially affected in PD) making dopaminergic neurons present in these brain regions more susceptible to SARS-CoV-2 infection.63
Furthermore, the accumulation of alpha-synuclein (α-Syn) is the major hallmark of PD.64,65 According to previous findings, the entrance of SARS-CoV-2 into the CNS may upregulate this protein, causing aggregation. On the contrary, few studies have indicated α-Syn’s protective effect in blocking viral entry and propagation into the CNS.66 Moreover, its expression in the neurons can act as a barrier to viral RNA replication. In a retrospective cohort study conducted in Japan, PD patients suffering from pneumonia showed a lower mortality rate.67 Furthermore, the main pathophysiological pathway involved in PD development,68 includes autophagy disruption,69 ER stress,70 and mitochondrial dysfunction.71 As a result, COVID-19 may trigger frequent modulations in these pathways, as seen in SARS-CoV and influenza A virus.
Proteostasis plays a vital role in protein translation, folding, and subsequent clearance with the help of heat shock proteins (HSPs). Viral infection hijacks the host cellular machinery for its replication and disrupts the proteostasis pathways by interacting with Hsp40.72 The viral-Hsp40 interaction results in the binding of Hsp40 with two subunits of viral RNA polymerase, which further assists the viral genome to get translocated into the nucleus via interaction with the viral nucleoprotein and inhibition of protein kinase R (PKR) activation, thereby restricting the host from producing an antiviral response. Hsp90 also modulates the activity of viral RNA polymerase after it enters the nucleus.73 Under normal conditions, Hsp9074 and Hsp70 restrict apoptosis initiation pathways thereby reducing apoptosis.74,75 However, infection with SARS-CoV-2 suppresses Hsp90 and Hsp70 function, leading to activation of caspase cascade followed by apoptosis and subsequent propogation of infection.
Autophagy lysosomal pathways and ubiquitin-proteasome pathways are two important components of proteostasis and are responsible for the degradation of impaired proteins.68 It has been reported that H1N1 infection obstructs autophagic flux at the initial stages resulting in the reduced number of autophagosomes and hindering autophagosome-lysosome fusion at later stages of autophagy. Both these activities result in autophagy disruption in human dopaminergic neurons and mouse brain and subsequently lead to α-Syn aggregation.76 Furthermore, when H1N1 was instilled intranasally in Rag knockout mice, α-Syn aggregates were found in the cells near olfactory bulbs, which may further spread in a prion-like manner to other regions of the brain and originate downstream events of PD pathogenesis.77 Furthermore, the ubiquitin-proteasome system can destroy viral proteins by ubiquitination; however, the H1N1 virus hijacks this mechanism and inhibits the host cell opponents of viral reproduction. This disrupts proteostasis and toxic protein aggregation.77,78 Further, SARS-CoV-2 has also been shown to act similarly to H1N1 virus and might be involved in α-Syn accumulation.77
Besides this, ER has also been reported as a target of various viruses. SARS-CoV-2 utilizes ER for the synthesis and processing of viral proteins. It has been shown previously that the Spike (S) protein gets collected in the ER and induces unfolded protein response (UPR) by transcriptional activation of several UPR effectors, including glucose-regulated protein 78 (GRP78), GRP94, and CCAAT/Enhancer-binding protein (C/EBP) homologous protein to aid viral replication.79 UPR may result in ER stress, which further activates cellular signals triggering neuronal death, are implicated in PD.63 Another study discovered that SARS-CoV open reading frames (ORF) 6 and 7a produce ER stress via GRP94 activation.80
Mitochondrial dysfunction is another pathway that connects PD with COVID-19. ORF-9b of SARS-CoV-2 degrades dynamin-like protein 1 (Drp1), involved in mitochondrial fission, thus causing mitochondrial elongation. Moreover, it suppresses antiviral cellular signaling by targeting the mitochondrial-associated adaptor molecule signalosome.81 ORF3b is located partially in mitochondria and is involved in apoptosis along with other accessory proteins (ORF3a, ORF6, and ORF 7a of SARS-CoV). The virus utilizes the mitochondria for caspase activation for apoptosis, thereby causing viral dissemination to other cells. The aforementioned cellular malfunctions result in increased ROS, redox imbalance, and mitochondrial and lysosomal dysfunction making the cells more susceptible to infection.82 According to current research, neuroinflammation is a defining factor in COVID-19 infection. Proinflammatory cytokine levels are higher in the periphery and cerebrospinal fluid in PD patients.83 Strikingly, studies have reported that viral infection can also induce neuroinflammation.84 Due to the compromised anti-inflammatory mechanisms in old age, the older population is more susceptible to develop neurodegenerative diseases as well as severe COVID-19 infection.
TLRs may play a role in the immunological response to coronavirus infections indicated by the presence of PAMPs (lipopolysaccharides, dsDNA/RNA, ssRNA) in the host cells recognized by specific TLRs derived from viruses following infection.85 TLR 3 is known to be activated in the case of HSV-I and influenza A infection.86 Whenever TLRs are triggered, pro-inflammatory cytokines are released (IL-1, IL-6, and tumor necrosis factor-alpha (TNF-alpha)) and type I IFN-α/β via MyD88-dependent and MyD88-independent pathways, which further translocate nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB), interferon regulatory factor (IRF), IRF-3 and IRF-7, inside the nucleus.87 Moreover, NF-κB has been reported to contribute to the pathogenesis of PD by triggering the release of pro-inflammatory mediators and subsequent neuroinflammation.88 Therefore, it can be concluded that NF-κB plays a common role in inflammation in both PD and COVID-19 pathogenesis. Neuroinflammation can also trigger misfolding and aggregation of α-Syn.89 Aggregated α-Syn leads to the activation of microglia which further favors the production of pro-inflammatory cytokines, ultimately causing neurodegeneration (Figure 2).90
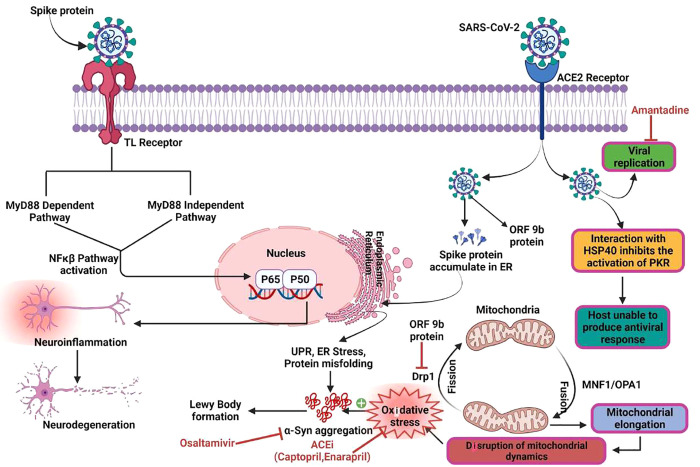
Figure 2.
Possible connection between the pathophysiology of Parkinson’s disease and SARS-CoV-2 infection. The virus enters the neurons by binding to the ACE-2 receptors or the TLRs and activates the NF-κB pathway triggering neuroinflammation. The spike protein in the SARS-CoV-2 virus accumulates in the ER and produces a UPR, potentially leading to α-Syn accumulation. The ORF-9b protein of the virus inhibits the Drp1 responsible for mitochondrial fission, thereby disrupting the mitochondrial dynamics. SARS-CoV-2 interacts with Hsp40 and inhibits protein kinase R activation, restricting the host from producing an antiviral response. ACEi, like Captopril and Enalapril, work as antioxidants by reducing oxidative stress and halting the accumulation of α-Syn in Lewy bodies and Lewy neurites. Amantadine inhibits viral replication by blocking the influenza M2 ion channel, thereby preventing the delivery of viral ribonucleoprotein into the cytoplasm of the host and might have a disruptive effect on the lysosomal pathway. As a result, amantadine might be used as potential treatment approach in COVID-19-positive PD patients to lower viral load in these individuals. Oseltamivir, an anti-influenza medication, has also been shown to be helpful in PD because it prevents H1N1-induced α-Syn aggregation.
5.1.2. Promising Therapeutic Strategies for PD Patients during COVID-19 Infection
None of the anti-Parkinsonian drugs render PD patients at risk for COVID-19; therefore, PD patients should not alter or stop any medicine without a clinician’s consultation. However, in order to avoid possible interactions, PD patients with COVID-19 infection should not use cough suppressants containing dextromethorphan and pseudoephedrine with Selegiline.91 Previous data suggest that the therapies used for COVID-19 such as angiotensin converting enzyme inhibitors (ACEi), might be safe for PD patients as well. Neuroprotective effects of ACEi like captopril and perindopril have been observed in PD animal models, which act by preventing dopaminergic cell loss and increasing striatal dopamine content, respectively.92 ACEi also work as antioxidants by reducing oxidative stress, which has been linked to PD93 and has been found to decrease the number of falls in a cross-sectional study involving 91 PD patients.94 Furthermore, hydroxychloroquine exhibits anti-Parkinsonian effects by raising Nurr1 expression, inhibiting glycogen synthase kinase-3 beta (GSK-3β)95 and functioning as an anti-inflammatory drug, making it a viable treatment option for PD patients infected with COVID-19.96 In another study, a COVID-19 patient with PD was cured with remdesivir in a clinical trial. In addition, the antiviral drug amantadine used in PD treatment has also been used for years in the treatment of influenza. Amantadine inhibits viral replication by blocking the influenza M2 ion channel, thereby preventing the delivery of viral ribonucleoprotein into the cytoplasm of the host and might have a disruptive effect on the lysosomal pathway. As a result, amantadine might be advantageous for treatment in COVID-19-positive PD patients as a potential treatment approach to lower viral load in these individuals.97
Oseltamivir phosphate, an anti-influenza drug, was found to be useful in PD as it inhibits H1N1-induced α-Syn aggregation.77 Many studies have highlighted that older people are typically deficient in vitamin D; vitamin D might have antiviral properties.73,98 As a result, vitamin D3 supplementation (2000–5000 IU/day) has been recommended in older PD patients, which might protect them against COVID-19. Moreover, it has been suggested that vitamin D3 can slow down the progression of PD.99 Above all, Fenoldopam, a dopamine D1 receptor agonist, was shown to be protective against inflammation as well as lung permeability and pulmonary edema in an endotoxin-induced acute lung injury mouse model.100 Taken together, it can be concluded that therapies used in PD and COVID-19 infection do not have any detrimental drug interaction. However, diagnosis presents a challenge in PD patients especially in the older population as they might neglect the COVID-19 symptoms because of other chronic diseases. Furthermore, early symptoms of COVID-19, for example dyssomnia, might be neglected by PD patients as they commonly suffer from olfactory dysfunction. Still, people with COVID-19 pneumonia who take medications for PD should have their doses changed because motor symptoms can impair breathing thereby worsening the condition.
5.1.3. Future Perspectives
There is a scarcity of data linking PD and COVID-19 outcomes. However, evidence from molecular processes of SARS coronaviruses changing proteostasis, mitochondrial and ER malfunction, and α-Syn aggregation suggests that it is necessary to identify medications acting on these pathways as a treatment for PD patients suffering from COVID-19. Detailed investigations on these mechanisms are required to determine PD patients’ sensitivity to COVID-19 as well as the safety of antiviral medicines, vaccinations, ACEi, and other antiviral drugs used for COVID-19. The indirect effect of this pandemic on PD patients including no direct patient–doctor visits, depression due to social distancing, reduced physical activity, and battery failure in patients on deep brain stimulation therapy also need attention and should be taken care of with the help of video conferencing and the availability of sufficient stock of medications.
5.2. Cancer and COVID-19
Reports suggest patients with lung cancer, hematological cancer, or any metastatic cancer are potentially at high risk of COVID-19 infection either due to treatment or disease susceptibility. Toward this, COVID-19-infected cancer patients were studied retrospectively; analysis showed a higher incidence of severe events following infection of COVID-19 infection, especially in the patients who received the anticancer treatment for 14 days. In one study, a total of 15 (53.6%) patients developed severe clinical event, and 28.6% were found to be morbid. Similar studies suggested being vigilant toward cancer patients who are on anticancer treatments as they are prone to COVID-19 infection as a result of reduced immunity.101
As per a nationwide study in China, roughly 39% (7 out of 18) of cancer patients infected with COVID-19 experienced severe symptoms, compared to only 8% (124 out of 1572) of patients not suffering with cancer.102 Another collective report has stated that there were 52 different studies worldwide involving 18,650 cancer patients infected with COVID-19 with 4,243 deaths. The data further implied that the risk of mortality of cancer patients is about 25.6 % COVID-19 as compared to 2.3% in the normal population.103 COVID-19 puts lung cancer patients at significant risk of developing severe episodes.104,105 In this regard, a study reported that out of 102 lung cancer patients infected with COVID-19 infection, 62% of lung cancer patients were hospitalized, and the mortality rate was approximately 25%.105 In another study, 55% (6 out of 11) mortality was observed in lung cancer patients infected with COVID-19 which was very high compared to other cancers.106
5.2.1. Possible Pathophysiological Association between COVID-19 and Cancer Patients
SARS-CoV-2 infects the host cell via the ACE2, which is a cell surface receptor. It is highly expressed on the lung epithelial cells and subsequently gets cleaved with the help of the host transmembrane serine protease2 (TMPRSS2). The viral internalization evokes the host immune response through the activation of alveolar macrophages and the complement cascade. This activation leads to a massive release of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and IFN-γ.107 This phenomenon, termed the cytokine storm, further causes alveolar endothelial tissue damage. Among these cytokines, IL-6 is involved in the pathophysiology of cancer, especially, lung cancer and other chronic diseases.108 It has been reported that IL-6 promotes tumorigenesis and anti-apoptotic signaling and is an important biomarker for cancer diagnosis and prognosis.106 The involvement of IL-6 in abnormally immune-activated conditions like cancer106,109 inflammation and immunosuppression have also been reported. Apart from IL-6, the activation of serine/threonine p21 activated kinase1 (PAK1), an essential component of malaria and some viral infections, is also a critical mediator of cytokine storm and gets overexpressed in SARS-CoV-2 infected lungs,109,110 resulting in mortality of COVID-19 patients. One study has reported that the human PAK, is an important component of host–pathogen interactions. PAK paralogues (Group I PAKs, include PAK1, PAK2, and PAK3; Group II PAKs, including PAK4, PAK5, and PAK6) are found in nearly all mammalian tissues, wherein they play important roles in a variety of processes including cell survival and proliferation, cell cycle progression, and cytoskeletal organization111 and are involved in different types of cancer. In drug development, the role of PAKs in cell survival along with proliferation, as well as participation in a variety of malignancies, is of significant interest. PAK1 activation can lead to the development of lung fibrosis112 by stimulation of the chemokine (C–C motif) ligand 2 (CCL2) production, thereby aggravating the patient’s condition. PAK1 blockers can help in restoring the immune response thereby combating virus-induced lung fibrosis. In this regard, the PAK1 inhibitor (propolis) was tested as a therapeutic approach for treating COVID-19 patients. Its extract components were shown to have inhibitory effects against other targets like ACE2 and TMPRSS2 (Figure 3).109
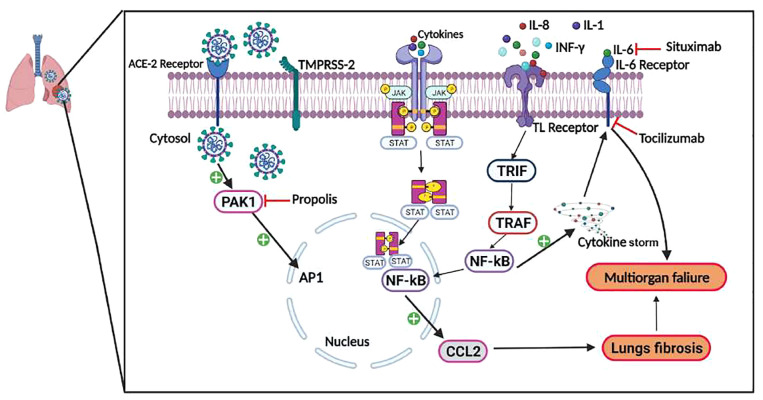
Figure 3.
Possible connection between the pathophysiology of cancer and SARS-CoV-2 infection. SARS-CoV-2 virus entry into the host cell is facilitated by spike protein binding to ACE and activation by TMPRSS2. After binding, viral particles undergo endocytosis and active PAK1. Moreover, due to the upregulation of cytokine levels (IL-6, IL-1, and IL-8), JAK/STAT pathway, and TLRs, gets activated, which will further activate the TIR-domain-containing adapter-inducing interferon-β family (TRIF). Following this, TRIF recruits TNF receptor-associated factor (TRAF) which is followed by activation of the inflammatory marker, NF-κB, present in the cytosol. This is followed by nuclear translocation of NF-κB and binding with DNA, and ultimately the formation of CCL2 protein, results in fibrosis. The phenomenon is termed as cytokine storm and is responsible for multiorgan failure and death in cancer patients with COVID-19. Toclizumab, a monoclonal antibody, and situximab, a chimeric mouse–human monoclonal antibody, were both used to block the IL-6 receptor and already exhibited antitumor efficacy under diverse randomized trials control trials for further assessment of its effectiveness for COVID-19 patients. Another drug, propolis, was used to treat lung fibrosis during COVID-19 infection of cancer patients. Propolis inhibits the PAK1 activation in lung fibrosis by stimulating CCL2 production. PAK1 blockers may help in reviving the immune system, preventing lung fibrosis caused by viruses. In this context, propolis, a PAK1 inhibitor, was explored as a therapeutic strategy for COVID-19 patients.
Besides, underlying conditions and altered immune responses increase the risk of developing venous thromboembolism, microvascular COVID-19 lung and vessels obstructive thrombo-inflammatory syndrome in cancer patients.113 The progressive endothelial thrombo-inflammatory syndrome may also cover the brain’s microvascular bed and other vital organs, resulting in multiple organ failures and death.113 Additionally, Bhotla et al. and colleagues hypothesized that platelets are getting infected due to COVID-19 infection which exacerbate the SARS-CoV-2 infection and ultimately lead to the bronchopneumonia or death.114
In conclusion, the biochemical and immunological characteristics outlined above demonstrate that cancer patients, are more susceptible to COVID-19 infections than the general population. However, non-biological variables such as increased contact with the healthcare system for cancer treatment may potentially contribute to the increase in COVID-19 prevalence in cancer patients.
5.2.2. Promising Therapeutic Strategies for Cancer Patients during COVID-19 Infection
Because of the current pandemic situation, as well as the associated risk factors, cancer patients must take additional precautions. As a result, in order to minimize the adverse consequences of the COVID-19 pandemic on highly susceptible cancer patients, hospitals should have, more robust management procedures in place. To this end, chemotherapy or surgery should be postponed, intense treatment should be provided, greater personal protection should be provided, telemedicine should be used, and a separate treatment approach for COVID-19 cancer patients should be implemented.115 As discussed in the previous section, IL-6 has been recognized as a crucial component of the immune response to SARS-CoV-2. Many clinical trials are currently underway to investigate treatment strategies targeting IL-6 by repurposing anti-IL-6 therapeutics for COVID-19 in cancer patients.116 Tocilizumab, a monoclonal antibody against the IL-6 receptor, has shown promising results in a double-blind, placebo-controlled phase-III study called EMPACTA (NCT04372186).116 Similarly, siltuximab, an IL-6 receptor chimeric mouse–human monoclonal antibody, has already exhibited its antitumor efficacy and is under diverse randomized control trials for further assessing its efficacy for COVID-19 patients (NCT04486521, NCT04330638, and NCT04329650).116
In addition, tumor reversion therapy might be the near future therapy for the treatment of cancer. The molecular biology behind the tumor reversal process is not only fascinating but alluring. Some chemical compounds used for tumor reversion include LY294002, metformin, sertraline, and ellipticine.117
Apart from this, T cell therapy such as immune checkpoint inhibitors (ICIs) and chimeric antigen receptor T cell (CAR-T) therapy may increase the risk of cytokine release resulting in increased severity of COVID-19 infection. Cytokine storm is directly linked to COVID-19-associated diseases such as acute respiratory distress syndrome (ARDS) and multiorgan failure. Moreover, an immunocompromised cancer patient with immunocompromised therapy and also those at risk for immune-related side effects in response to immuno-oncology treatments should be monitored closely.118
Another treatment approach is mesenchymal stem cell therapy; this is approved and is now used for cancer treatment alone or in combination with other drugs.119 It was even applied on 7 patients with COVID-19 infection, resulting in a negative effect on the expression of ACE2 along with TMPRSS2. On the sixth day, cytokine-secreting cells (CXCR3+ CD4+ T, CXCR3+ CD8+ T, and NK CXCR3+ cells) disappeared as peripheral lymphocyte counts increased. While the TNF-alpha levels were reduced, dendritic cell populations and IL10 levels were increased.119,120
Along with IL-6, other cytokines associated with the pathogenesis of COVID-19 infection in cancer patients include type I IFN, IL-1, IL-7, IL-17, and TNF-alpha. Even a clinical study named Bee-COVID was conducted with the Brazilian Green Propolis Extract for the treatment of the COVID-19 condition (NCT04480593). In conclusion, targeting these inflammatory markers may serve as a good approach to treating COVID-19 infection in cancer patients.121
5.2.3. Future Perspectives
The COVID-19 outbreak is a global threat to the health system. Despite the substantial study, there is currently no recognized treatment for COVID-19. Still, it is unclear why some people respond abruptly to SARS-CoV-2 infection and others are asymptomatic. Further, why people with coexisting comorbidities are more susceptible to severe clinical events of COVID-19 is unclear. Being at high risk of infection and deteriorating outcomes, cancer patients are suggested to be more cautious and follow the guidelines issued by World Health Organization and the European Society for Medical Oncology.
5.3. Diabetes and COVID-19
Diabetes mellitus (DM) is a common metabolic disorder with multiple etiologies primarily associated with a deficiency of insulin secretion and/or its action. Besides the clinical complication of the disease, an individual with diabetes is more susceptible to a broad range of infections (such as foot infection, rhinocerebral mucormycosis, malignant external otitis, and gangrenous cholecystitis) as well as predisposed to certain conditions that primarily affect lungs122 like influenza, tuberculosis, and legionella pneumonia.122,123 Moreover, diabetes and its complications such as disrupted glycemic control and ketoacidosis were found to be a potential risk factor for mortality in the influenza A (H1N1) pandemic in 2009, SARS-COV, and MERS coronavirus infection.124
Several studies have reported high mortality in COVID-19 patients with diabetes. More specifically, diabetes was the most common underlying comorbidity in approximately 22% of the 32 nonsurvivors from a cohort of 52 COVID-19 patients in intensive care.125 Detailed clinical research on 140 hospitalized COVID-19 patients in Wuhan indicated the second highest prevalence was diabetes (12.1%) after hypertension.125 Another study on a subset of 1099 patients discovered that out of 177 severe cases, 16.2% of patients with seriously infected conditions had diabetes. Eighteen of these patients had composite outcomes, including death, use of mechanical ventilation, and admission to an ICU.126 Recent epidemiological research of 72,314 COVID-19 patients at the Chinese Center for Disease Control and Prevention found that diabetics mortality in diabetic patients was three times greater than non-diabetic patients (7.3% mortality rate as compared to the overall 2.3% mortality rate).21,126 Besides, a recent retrospective multicenter cohort study on 191 laboratory-confirmed COVID-19 cases observed a statistically significant association between diabetes and increased mortality.42 In yet another study, Fadini and his colleagues at the University Hospital of Padova discovered that the mortality rate of diabetic SARS-CoV-2 patients were 1.75 times greater than the general population. This research, coupled with earlier research, suggested that diabetes may exacerbate the outcome of a new coronavirus illness.127 The CDC data further suggested that COVID-19-infected diabetic patients have a higher risk of the developing severe symptoms.128 Toward this, in a retrospective research conducted in Wuhan, China, 32% of cases patients had comorbidities, 20% of which were diabetics.129 In addition, hyperglycemia or dysregulated glycemic management is associated with a high risk of complications. Diabetes as a major risk factor for the course and prognosis of COVID-19 was further mentioned according to a study of 174 COVID-19 patients admitted to Wuhan Union Hospital.130
5.3.1. Possible Pathophysiological Association between COVID-19 and Diabetic Patients
COVID-19 infection in diabetic patients might increase stress hormone levels such as glucocorticoids and catecholamines, leading to high glucose levels. Hyperglycemia in diabetes induces glucose allowing non-enzymatic glycosylation of lung collagen and elastin by advanced glycation end products thereby resulting in reduced elasticity of the lungs COVID-19 infection. This causes thickening of the alveolar epithelial basal lamina and microvascular alterations in the pulmonary capillary beds, further leading to a reduction in pulmonary capillary blood volume and diffusing capacity, which influence the patient’s overall survival.131 Even a brief period of hyperglycemia has the potential to alter immune cell function.132 Diabetes increases pro-inflammatory cytokines, including IL-1, IL-6, and TNF-alpha. IL-6, C-reactive protein, serum ferritin, and coagulation index, D-dimer are significantly higher in diabetic individuals than in those without diabetes. Current research suggests that diabetic patients are more prone to cytokine storms.42 This may be further exaggerated in response to a stimulus as seen in patients with COVID-19 infection. In a study, patients infected with COVID-19 have developed a fatal hyperinflammatory syndrome characterized by a fulminant and fatal hypertyrosinemia, and was thought to be associated with disease severity as demonstrated increased IL-2, IL-7, IL-12, TNF-alpha, and IFN-γ inducible protein 10, macrophage inflammatory protein-1α (MIP-1α/CCL3).133 A prolonged hyperglycemic state causes an elevated immune response, which further leads to inflammation.131 Interestingly, increased glucose levels were discovered to directly promote SARS-CoV-2 replication in human monocytes and to sustain SARS-CoV-2 replication through the formation of ROS and activation of hypoxia-inducible factor-1α.134 This massive influx of inflammatory cells has the potential to disrupt the activities of the primary insulin-responsive organs, i.e., the skeletal muscles and liver, which are primarily responsible for insulin-mediated glucose absorption.135 High glucose concentration in the plasma leads to cytokine production, glucotoxicity, and viral-induced oxidative stress; these factors promoted a greater risk of thromboembolic problems as well as damage to important organs in diabetic patients (Figure 4).136 Moreover, one enzyme, dipeptidyl peptidase-4 (CD26 or adenosine deaminase complexing protein 2), tends to bind with the virus and promote the ACE2 expression which is involved to initiate the infectious disease.137
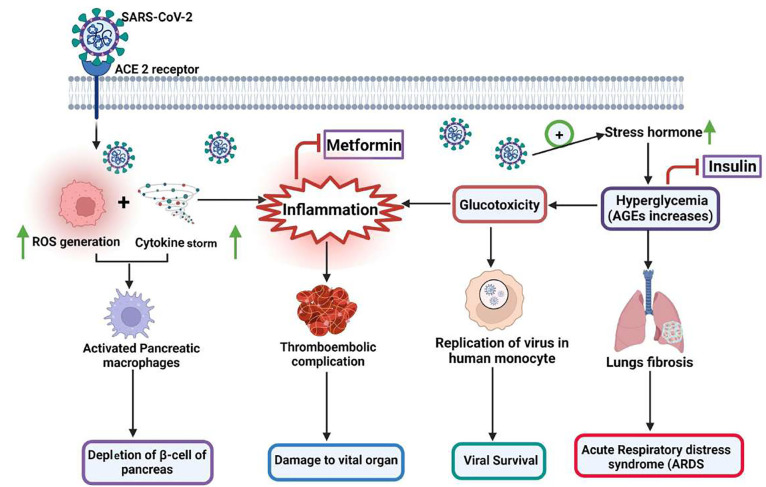
Figure 4.
Possible connection between the pathophysiology of diabetes and SARS-CoV-2 infection. Infection with SARS-CoV-2 leads to increased levels of stress hormones like glucocorticoids that cause lung fibrosis, acute lung damage, and acute respiratory distress syndrome (ARDS). Elevated levels of inflammatory cytokines lead to the development of hyperinflammation, further causing the activation of pancreatic macrophages and subsequent depletion of β islets cells. Glucotoxicity resulting from elevated stress levels favors viral survival and eventually causes elevation of inflammation. Insulin resistance, hyperglycemia, and inflammation-induced vascular endothelial damage contribute to cardiovascular events, and thromboembolism leads to multiple organ failures. Due to its anti-inflammatory property, Metformin has demonstrated potential for treatment of the SARS-CoV-2 virus-mediated infection. Metformin inhibits the interaction between the virus and the host cell, and suppresses the production of ACE2 via activation of adenosine monophosphate-activated protein kinase (AMPK).
Furthermore, studies have shown severe lung pathology with dysregulated immune response in mice (with existing Type2 DM (T2DM)) infected with MERS-CoV.138 In addition, COVID-19 patients with diabetes have an easier progression to acute respiratory distress syndrome and septic shock resulting in multiple organ failures.139
Another direct metabolic link that exists between coronavirus infection and diabetes is based on the expression of ACE2 present in specific tissue. ACE2 is a transmembrane glycoprotein that converts angiotensin II (Ang II) to angiotensin (Ang).140 ACE2 is also expressed in blood vessels, macrophages, and monocytes.141 The expression of ACE2 is of prime importance in the etiology of SARS-CoV-2. It has been observed that inflammatory signals produced by macrophages, such as type I IFN, increase ACE2 receptor expression.142 The infection of pancreatic macrophages may have triggered these inflammatory signals. As a result, more immune cells particularly pro-inflammatory monocyte/macrophages are recruited, causing further damage to islets of Langerhans and β-cells in the pancreas. Subsequently, it reduces insulin release, subsequently resulting in acute hyperglycemia and transitory diabetes in healthy people.143
Additionally, macrophages and monocytes are mobile, and once infected with the SARS-CoV-2 virus, they can infiltrate the cells of the pancreatic islets and spread the virus throughout the pancreas.139 Furthermore, SARS-CoV-2 infection of the β-cells can directly damage them, causing apoptosis, and further worsening the glycemic control of diabetic patients.144
Furthermore, anti-diabetic medicines, such as glucagon-like peptide 1 agonists, antihypertensive drugs, and statins, also increase ACE2 expression.145 COVID-19-infected patients were treated with different medications, such as systemic corticosteroids and antiviral medicines, which may potentially cause hyperglycemia. Glucocorticoid-induced DM (GIDM) is a frequent and potentially critical issue to address in clinical practice, although it may contribute to worsening hyperglycemia and ultimately aggravate the diabetic condition associated with COVID-19 infection. The detailed mechanism causing glucocorticoid-induced hyperglycemia is the promotion of weight gain, decrease in peripheral insulin sensitivity, increase in the production of glucose with the promotion of gluconeogenesis, β cell injury due to the destruction of pancreatic cells, increase in the levels of fatty acids, and impairment of insulin release.146
However, in the case of SARS-CoV-2 infection, the effect of diabetes on ACE2 expression still needs to be studied in detail. In a study, an increased expression of ACE2 was observed in the kidney of diabetic patients147,148 in the early stage followed by a decreased expression in later stages that relatively overlaps with the occurrence of diabetic nephropathy.147−149 Each of these pathways working synergistically may worsen the situation for diabetic patients making them frailer and further increasing the severity of COVID-19 disease.148
5.3.2. Promising Therapeutic Strategies for Diabetic Patients during COVID-19 Infection
The glucose level in diabetic patients is mainly maintained with the administration of insulin and is mainly recommended for critically ill patients infected with SARS-CoV-2. It has also been observed that insulin infusion significantly reduces the inflammatory cytokines and helps in lowering the severity of COVID-19.150 Metformin has been proven to have anti-inflammatory properties in preclinical investigations, and it has also been demonstrated to lower circulating levels of inflammatory biomarkers in persons with T2DM.151
Besides the anti-inflammatory action of metformin, it is potentially used against the SARS-CoV-2 virus.117,137 Metformin acts through inhibiting the virus–host-cell association as well as prevents the expression of ACE2 through the activation of adenosine monophosphate-activated protein kinase. Another promising molecule, DPP-4 antagonist (Linagliptin), an anti-diabetic agent with potential anti-aging properties, was repurposed to combat COVID-19 infection118 also demonstrates antiaging properties. Further, plant-based natural compounds such as resveratrol, catechin, curcumin, procyanidin, and theaflavin have been tested for the treatment of COVID-19 disease through in silico, in vitro, and in vivo studies.137 More recently, convalescent plasma therapy has also been applied for COVID-19 associated comorbidities wherein it was used to downregulate the inflammatory cytokines and the viral load in COVID-19 patients.120 Sulfonylurea must be avoided in COVID-19 patients comorbid with T2DM, as they can cause hypoglycemia. Thiazolidinediones can be used in mild diseases with caution as they have protective effects on the cardiovascular system.152 However, due to weight gain, edema, and heart failure, thiazolidinediones were not used in moderate and severe conditions.153
5.3.3. Future Perspectives
COVID-19 pathology majorly involves inflammation that eventually leads to multiple organ failure and even death. Comorbid diabetic patients are highly susceptible to hospitalization-based COVID-19 infection. Diabetes is characterized by a persistent state of hyperglycemia that can aggravate SARS-CoV-2-induced inflammation. Therefore, diabetic patients’ medication should be monitored as some drugs can increase the expression of viral entry receptors leading to a poor prognosis of COVID-19 in these patients. Further, insulin resistance has also been reported in comorbid, especially, T2DM patients. Hence, management of diabetes is of utmost importance in infected patients. Drugs that do not interact with ACE2 receptors could be given, though insulin treatment is unanimously given for COVID-19 patients with comorbid status of diabetes.
5.4. COVID-19 and Cardiovascular Diseases
CVD is a broad classification for a range of conditions that involve dysregulation of the heart and vascular systems. Since COVID-19 infection is a multiplexed pathophysiological condition, it is obvious that the cardiovascular systems are at the crux of exposure. Toward this, in a multicenter study conducted in Wuhan involving 191 patients, 24% of the patients that died had coronary heart disease.42 In addition, in a systemic review of 199 patients with COVID-19, 40% of them were diagnosed with myocarditis.154 Further, a meta-analysis study reported that 25% of the patients developed acute cardiac injury and the mortality rate was 20 times higher than those pre-existing comorbidities in comparison to those with no pre-existing comorbidities.155
As discussed in the previous section, systemic inflammation due to viral infection is prevalent and is a consequence of the cytokine storm. This inflammation is of particular concern for cardiac tissues and has been shown to cause myocarditis. The mechanism proposed for this pathology demonstrates the involvement of hepatocyte growth factor release,156 followed by priming of the immune cells such as T cells and subsequent release of IL-6156,157 As a result, COVID-19 patients have been found to have pericardial effusion, which can lead to inflammation of the membranes around the heart and pericarditis.158 Conversely, the safety of mRNA vaccines is of particular concern as they have been reported to cause myocarditis.159
In the earlier section we discussed PD, its association with COVID-19 and the importance of olfactory nerves and the brain. Although nerves express ACE2 receptors, which are of utmost importance for infectivity, one cannot rule out the importance of the possible involvement of the heart–brain axis. This bidirectional communication is important as it is mediated by the control of endothelial cells and the regulation of cerebral blood flow by somatosensory signaling mechanisms. Thus, infection with COVID-19 disrupts this normal physiological function and, as a consequence, vascular–neuronal communication is disturbed thereby causing headaches and increased incidences of stroke in infected patients. Along with this, serious implications such as the neural spread of viruses and consequent, central disturbances such as anxiety are predisposed due to neuroinflammation and lower brain-derived neurotropic factor levels.160 Elevated levels of inflammatory biomarkers, CRP and D-dimer are the most common markers for COVID-19-associated coagulopathy. Particularly, elevated levels of D-dimer in hypertensive patients indicate the severity of the disease since COVID-19 patients’ death rates are more likely to rise with D-dimers upon admission or throughout time.118 Alternatively, in patients with diabetic complications, depletion of the ACE2 receptor causes activation of the renin-angiotensin-aldosterone system (RAAS), leading to β-cell destruction and an increased risk of cardiomyopathy in an age-dependent manner.161 However, these concepts are still premature and need a discussion on hypertension, which has been studied to a great extent with COVID-19 and is a predisposing factor for other cardiovascular complications as well.
HTN is the most common disorder in people aged 50 years or more.162 Epidemiological studies have reported a high risk of hospitalization for patients with CVD such as HTN after COVID-19 infection.163 One study has shown hypertension (30%) and coronary heart disease (20%) as the most prevalent comorbidities (8%) in COVID-19-infected patients. Another investigation has shown that HTN (27%) and cardiovascular complications were the most prevalent comorbidities among COVID-19 patients with acute respiratory distress syndrome (6%).164,165 The high prevalence of hypertension in COVID-19 patients is not surprising, nor does it necessarily imply a causal relationship between hypertension and COVID-19 or its severity. Further, hypertension is extremely common in the elderly166 and older people appear to be at a higher risk of contracting SARS-CoV-2 and developing severe forms and complications of COVID-19.
Hypertensive patients are frequently treated with ACEi or angiotensin receptor blockers (ARBs) to lower the volume of blood and ultimately blood pressure. However, the application of ACEi or ARBs in hypertensive patients is questionable as ACEi or ARBs are reported to increase the levels of ACE2 in hypertensive patients, and importantly, SARS-CoV-2, is reported to enter inside the lung cells through ACE2 receptors.164 Therefore, it is crucial to understand how RAAS reacts to COVID-19 infection and whether it is feasible to use ACEi or ARBs.
5.4.1. Possible Pathophysiological Association between COVID-19 and Cardiovascular Diseases Patients
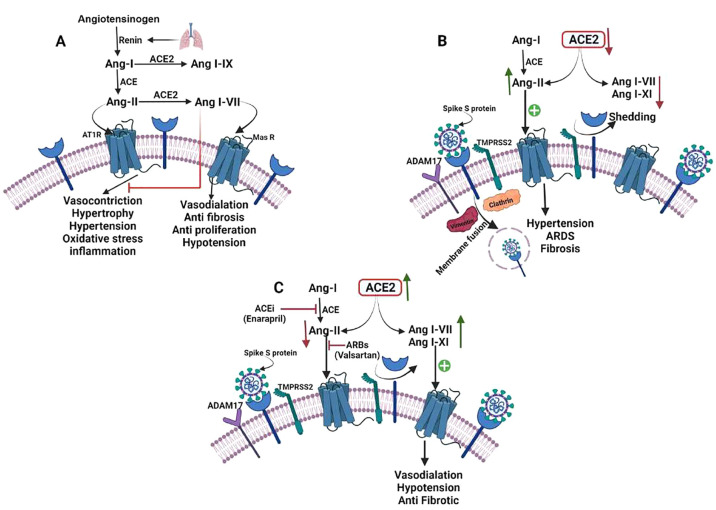
ACE2 is a monocarboxypeptidase that is homologous to ACE and has an an extracellular active site.167 Angiotensin I (Ang I) is cleaved by ACE to produce Ang II, which constricts blood vessels and increases salt and fluid retention by binding to and activating the angiotensin receptor 1 (AT1 receptor), causing HTN. However, the membrane-bound ACE2 inactivates Ang II to Angiotensin I–VII (Ang I–VII) which then binds to the Mas1 oncogene (Mas receptor), which has further shown to possess a vasodilator effect. Furthermore, ACE2 converts Ang II into Angiotensin I–IX (Ang I–IX) which is then transformed into Ang I–VII by ACE. In addition, ACE2 converts Ang I with less binding affinity as compared to Ang II. ACE2 acts as a negative regulator of the RAAS, modulating vasoconstriction, fibrosis, and hypertrophy.168,169 Hypertensive individuals have lower gene expression and/or ACE2 activity than normotensive patients. Ang II, on the other hand, inhibits ACE2. It has been reported in preclinical studies that ACE2 deficiency induces HTN in rats when Ang II exceeds.168,170
In several studies, SARS-CoV infection lowered ACE2 expression in cells, causing severe organ damage by disturbing the physiological homeostasis between ACE/ACE2171 and Ang172 II/Ang I–VII.171−173 The ACE2 transmembrane domain is internalized along with the virus during SARS-CoV-2 infection, which reduces ACE2 expression. For some transmembrane proteinases and proteins, disintegrin is one of the proteins that may be involved in the binding and membrane fusion processes and ADAM metallopeptidases domain 17 (ADAM17), TMPRSS2, and TNF-converting enzyme.174,175 TMPRSS2 cleaves ACE2 to increase viral uptake, and ADAM17 can cleave ACE2 to produce ectodomain shedding.174
Importantly, lower levels of ACE2 in COVID-19-infected hypertensive patients resulted in a lower degradation rate of Ang II, overexpression of the AT1 receptor with reduced activity of Ang I–IX and I–VII and promotion of hypertension, ARDS, hypertrophy, and myocardial injury.12,176
The ACE2/Ang I–VII/Mas axis has been shown to play a beneficial role in the heart.177 It can enhance post-ischemic heart178 functions by inducing coronary vessel vasorelaxation, inhibiting oxidative stress, attenuating abnormal cardiac remodeling, and inhibiting oxidative stress. ACE2 expression rises early in the course of a heart attack but declines as the disease progresses.177 ACE2 knockout mice develop myocardial hypertrophy and interstitial fibrosis, which accelerates heart failure.179,180 Furthermore, ACE2 deletion in mice exacerbates diabetes-related heart failure.181 ACE2 expression in cardiac cells has been shown to be significantly reduced in both SARS-CoV-infected humans180 and mice.182
Due to the significant downregulation of ACE2 and overexpression of Ang II in COVID-19 infection, the lack of protective actions of Ang I–VII may exacerbate and perpetuate cardiac damage. According to current research and several clinical studies,129 HTN is a comorbidity in a significant proportion of individuals with severe illness. RAAS overactivation may have already occurred in these people before infection. The absence of the protective effects of Ang I–VII may accelerate and perpetuate cardiac damage due to considerable downregulation of ACE2 and overexpression of Ang II in COVID-19 infection. ACE2/Ang I–VII/Mas receptor axis counteracts excessively activated ACE/Ang-II/AT1 receptor axis as seen in HTN (Figure 5).
Figure 5.
Possible connection between the pathophysiology of Hypertension and SARS-CoV-2 infection. (A) ACE2/Ang I–VII/Mas axis and the renin-angiotensin system (RAS). Angiotensinogen is transformed to Ang-I by the protease renin, which is then converted to Ang-II by the ACE. Vasoconstriction, hypertrophy, fibrosis, proliferation, inflammation, and oxidative stress can be caused by Ang-II following binding to the AT1 receptor. Ang-I and Ang-II can be converted to Ang I–IX and Ang I–VII respectively by ACE2. Vasodilatation, vascular protection, anti-fibrosis, anti-proliferation, and anti-inflammation are effects of Ang I–VII binding to the Mas receptor. (B) When SARS-CoV-2 binds to ACE2, the virus is internalized with the receptor, and ACE2 is removed via ADAM17. Reduced ACE2 availability causes a decrease in the levels of Ang I–VII, I–IX, and Ang-II degradation, as well as increased AT1 receptor activation, facilitating HTN, ARDS, and fibrosis. (C) Infection with SARS-CoV-2 and therapy with ACEi/ARB. After SARS-CoV-2 binding, ACE2 is upregulated by ACEi and ARB, and free ACE2 persists. Ang I–VII, a favorable metabolite of Ang II, is still destroyed by ACE2, although the AT1 receptor is less activated than Mas receptor-activated through increased levels of Ang I–VII and I–IX resulting in vasodilatation, hypotension, and antifibrotic activity. ARB prevents Ang II binding on the AT1 receptor, while ACE decreases Ang II production, resulting in decreased AT1 receptor activation and sustained interaction with ACE2, preventing ACE2 internalization.
Notably, soluble ACE2 molecules have been demonstrated to limit SARS-CoV infection183 and suggested that a soluble recombinant form of ACE2 molecules can act as competitive interceptor of SARS-CoV-2 virus and prevent it from latching onto cellular membrane-bound ACE2.184 In ARDS, recombinant human soluble ACE2 was planned to be tested clinically for its efficacy against COVID-19 infection so that a larger phase IIB trial can be performed which may potentially benefit the hypertensive COVID-19-infected patients (NCT04287686).185
Cardiac damage induced by SARS-CoV and SARS-CoV-2 is a major cause of mortality186 and morbidity,187 affecting up to a third of those who have the disease in its most severe form.182,186−188 SARS-CoV was found in one-third of human autopsy hearts, accompanied by a substantial drop in cellular ACE2.182 Also, the involvement of ACE2 has been studied in critically ill patients, wherein continued treatment with ACE2i (ACE2 inhibitor) was deemed to demonstrate less load on the heart as the alveolar spaces are critically dismantled in these patients.189 Even though the powerful immune response seen in these people might affect cardiac dysfunction similar to the lungs, Ang II is expected to contribute to the negative effects of SARS-CoV on the heart and SARS-associated cardiomyopathy.168 Inflammatory signals are thought to lower ACE2 cell-surface expression and transcription.168 Some contrasting studies report that ACE2 may also be involved in vasodilation, anti-inflammatory and antioxidant roles due to the generation of fragment Ang (I–VII) from Ang II.190 However, this is scantly reported and as a result, the classical hypothesis of disease worsening is most prevalent.
In conclusion, a decrease in cellular ACE2 may render the cells less sensitive to SARS-CoV-2, whereas overexpression of the AT1 receptor causes more severe tissue damage. On the other hand, as AT1 receptor activity is decreased, the cell membrane becomes more sensitive to viral particles with increase in ACE2 levels.
5.4.2. Promising Therapeutic Strategies for Cardiovascular Diseases Patients during COVID-19
Both ACEi and ARB have been demonstrated to upregulate ACE2, and some researchers have speculated that ARB and ACEi treatments may have a deleterious effect on SARS-CoV-2 infection.191 Given how commonly these compounds are used to treat HTN and heart failure, this might be a major source of concern.
In animal studies, ACEi treatment increased plasma Ang I–VII levels, decreased plasma Ang II levels, and increased ACE2 expression in the heart. In contrast, Ang II receptor blockers (ARBs) increase plasma levels of both Ang II and Ang I–VII, as well as ACE2 expression and activity in the heart. ACEi/ARBs, renin inhibitors, and Ang I–VII analogs may minimize organ damage by blocking the RAAS pathway and/or increasing Ang I–VII levels. In population-based research, the use of ACEi and ARBs significantly lowered the 30-day mortality in pneumonia patients requiring hospitalization. Treatment with ACEi/ARBs has also prompted concerns that increasing the expression of ACE2 in target organs might facilitate the infection-induced development in severe COVID-19 infection.12 According to two large cohort studies, administration of ACEi/ARBs were connected to hospitalized patients’ having a lower risk of all-cause mortality rather than an increased chance of SARS-CoV-2 infection. However, further research is warranted to examine the protective effects of ACEi/ARBs in COVID-19.191,192
Despite the various probable confounders, a decrease in membrane ACE2 expression might explain many of the anomalies seen in SARS-CoV-2 infection. There is not enough clinical evidence to suggest that there is a higher chance of acquiring a severe COVID-19 infection; further, it is unclear if continuation or discontinuation of ARB/ACEi is a wise decision. In addition, we do not know if switching to another treatment approach could worsen the patient’s situation, notably in individuals with heart failure and a poor ejection fraction. Further, whether RAAS inhibitor medication is useful or detrimental for virally induced lesions and switching to another medicine could make the patient’s situation even worse. Clinical trials to detect the effect of losartan as a potential treatment approach for COVID-19, are currently starting (NCT04311177 and NCT04312009). Further, trial to detect if stopping or continuing ACEi/ARB treatment has any consequences are underway (NCT04338009). ACE inhibitors and angiotensin-converting enzyme inhibitors (ARBs) are not only used to treat HTN and heart failure, but they also have a minor impact on ACE2. Although β-blockers are unlikely to interact with ACE or ACE2, they do lower plasma Ang II levels by preventing the conversion of pro-renin to renin.194 Calcium channel blockers appear to reduce Ang II-induced ACE2 downregulation. However, the data is limited to a single research article that studies nifedipine’s effect on fractionated cell extracts.195 Thiazides and mineralocorticoid receptor antagonists did not improve the hypertensive rats’ naturally low ACE2 activity,196 although mineralocorticoid receptor antagonists did reduce ACE expression.197 In heart failure patients, mineralocorticoid receptor antagonists, on the other hand, increase membrane ACE2 activity.198 Newer therapies such as DNA aptamers, short oligonucleotide sequences that bind to specific proteins, are being investigated for masking the ACE2 binding domain. In this regard, a group reported the synthesis of novel DNA aptamers that were shown to specifically bind to the ACE2-K353 domain and blocked the entry of the virus through ACE2 receptors.199 It is still controversial that non-ACEi/BRA drugs (β-blockers, calcium channel blockers, diuretics) are more likely to increase the risk of adverse outcomes than ACEi/BRA drugs that increase ACE2 and provide theoretical protection if the reduction in membranous ACE2 seen in HTN and obesity is important in the pathophysiology of severe COVID-19.
5.4.3. Future Perspectives
It has been confirmed that SARS-CoV-2 enters the lung through the ACE2 receptor followed by other tissues like the liver, bile duct, gastrointestinal system (small intestine, duodenum), esophagus, and kidney. SARS-CoV-2 can damage these organs associated with the heart and transmit it from human to human rapidly, resulting in serious illness and life-threatening diseases such as heart attack or cancer. Although higher levels of ACE2 enzyme may be expressed in hypertensive patients, this can be a marker for the severity of COVID-19. Effective treatment and prevention of coronavirus infection should begin immediately. However, developing vaccines or drugs for humans in a shorter period is difficult. Nevertheless, only Covaxin and Covishield are currently available in India. In the current scenario, if any person is infected with SARS-CoV-2, then he/she should be isolated and should be controlling the origin of the infection. Scientists are trying to develop vaccines or repurposing drugs with the help of different in vivo or in vitro studies resulting in some positive evidence for the treatment of COVID-19, but these pieces of evidence are not sufficient to cure the infection.
As COVID-19 treatment options are evaluated, it will be important to understand the possible side effects of CVD, with a focus on drug–drug interactions. Further, in order to learn more about how COVID-19 affects the heart, we need comprehensive molecular tests that may look at how things work and prospective and retrospective studies with good clinical methodology.
6. Conclusion
The COVID-19 pandemic is still globally stressful. SARS-CoV-2 has infected the entire globe and has caused pneumonia-like symptoms in severely infected populations. Conversely, some new variants have been reported which have less serious effects with some patients being asymptomatic post-infection. However, the population afflicted with comorbid conditions is found to be more vulnerable to death or ICU admissions comparable to people without comorbid conditions. Age is found to be the highest risk factor in COVID-19 infection, as aged people show enhanced sensitivity to immune responses. Similarly, SARS-CoV-2 causes the cytokine storm, which makes aged people more sensitive to severe infection. There was a significant increase in mortality observed with COVID-19-infected people having age-related disease conditions such as PD, cancer, diabetes, and CVD. COVID-19 infection in comorbid patients causes mitochondrial and ER malfunction that induces α-Syn aggregation, activates TLRs, and ultimately causes nuclear translocation of NFκB through JAK/STAT3 pathway in cancer patients. SARS-CoV-2 infection in diabetic individuals leads to depletion of beta cells of islets of Langerhans creating imbalance in glucose levels. Hypertensive patients infected with COVID-19 demonstrate enhanced Ang-II levels which are responsible for vasoconstriction ultimately worsening hypertensive condition. Therefore, the population infected with SARS-CoV-2 and with comorbid conditions needs to be monitored carefully at the onset of the infection, as the symptoms may worsen with time which may lead to severe life-threatening conditions. Toward this, more clinical studies are warranted for an improved mechanistic understanding of the susceptibility of comorbid patients to COVID-19 infection. Further, the comorbid population should be vaccinated on a priority basis as compared to people without comorbid conditions. Though various studies indicate the importance of comorbid disorders as the possible determinants for COVID-19 infected individuals, a comprehensive evaluation of an clarification regarding the vulnerability in COVID-19 patients is required. This article has limits on clinical-epidemiological conclusions and lacks critical and statistical analysis of COVID-19 and comorbidities and the reader should be aware of the aforementioned restrictions in our review.
Acknowledgments
The authors acknowledge National Institute of Pharmaceutical Education and Research (NIPER)-Ahmedabad administration for providing the facility and support for this review article. N.A. would like to acknowledge Science and Engineering Research Board (SERB), Government of India for SERB-Power Grant (Project No SPG/2021/004209) and All India Institute of Medical Sciences (AIIMS), Bhopal, Madhya Pradesh, India. A.M.K. would like to acknowledge project no. LX22NPO5107 (MEYS): Financed by European Union – Next Generation EU for support.
Glossary
Abbreviations
- ACEi
angiotensin converting enzyme inhibitor
- ADAM 17
ADAM metallopeptidase domain 17
- α-Syn
alpha-synuclein
- COVID-19
Coronavirus disease
- CVD
cardiovascular diseases
- PAMPs
pathogen-associated molecular patterns
- PAK1
serine/threonine p21 activated kinase1
- SARS-CoV-2
severe acute respiratory syndrome-coronavirus-2
- TRAF
tumor necrosis factor receptor-associated factor
- TMPRSS2
transmembrane serine protease2
Author Present Address
∇ International Clinical Research Center, St. Anne’s University Hospital Brno, Brno, Czech Republic
Author Contributions
We declare that all authors made fundamental contributions to the manuscript. S.C., A.M.K., and N.A. conceptualized the review and made table of contents. S.C. made the illustrations, prepared the draft and compiled the manuscript. L.V.N. and A.P. contributed in cancer and COVID-19 section. N.S. and M.M. contributed in diabetes and COVID-19 section. M.S. and F.M.M. contributed in PD and COVID-19 section. N.P. and M.G. assisted in compilation and illustrations. AAS revised the manuscript and wrote CVD and COVID-19. S.C., M.S., L.V.N., N.S., A.A.S., A.M.K., and N.A. proofread the manuscript. All authors read and approved the final version of the manuscript.
This supplement was supported by the National Institute of Pharmaceutical Education and Research (NIPER) seed fund-Ahmedabad, Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India. N.A. acknowledges the support from SERB-POWER Grant (Project No SPG/2021/004209), Science and Engineering Research Board, Government of India. A.M.K. gratefully acknowledges the support of the Ramaligaswami Fellowship (No. BT/RLF/Re-entry/24/2017) from the Department of Biotechnology, Ministry of Science and Technology, Government of India.
The authors declare no competing financial interest.
This article is made available via the ACS COVID-19 subset for unrestricted RESEARCH re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for the duration of the World Health Organization (WHO) declaration of COVID-19 as a global pandemic.
References
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus resources center.https://coronavirus.jhu.edu/ (acccessed 05–12–2022).
- Ejaz H.; Alsrhani A.; Zafar A.; Javed H.; Junaid K.; Abdalla A. E.; Abosalif K. O.; Ahmed Z.; Younas S. COVID-19 and comorbidities: Deleterious impact on infected patients. Journal of infection public health 2020, 13 (12), 1833–1839. 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.; Wunderink R. G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018, 23 (2), 130–137. 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtani A. A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): emergence, history, basic and clinical aspects. Saudi journal of biological sciences 2020, 27 (10), 2531–2538. 10.1016/j.sjbs.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Petitjean S. J.; Koehler M.; Zhang Q.; Dumitru A. C.; Chen W.; Derclaye S.; Vincent S. P.; Soumillion P.; Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11 (1), 1–10. 10.1038/s41467-020-18319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.-R.; Cao Q.-D.; Hong Z.-S.; Tan Y.-Y.; Chen S.-D.; Jin H.-J.; Tan K.-S.; Wang D.-Y.; Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-an update on the status. Military medical research 2020, 7 (1), 1–10. 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi A.; Chastang J.; Miyara M.; Lejeune J.; Soares A.; Ibanez G.; Saadoun D.; Morélot-Panzini C.; Similowski T.; Amoura Z.; et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40 (4), 691–697. 10.1007/s10096-020-04056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R.; von Bartheld C. S. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. neuroscientist 2021, 27 (6), 582–603. 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas M.; Rahaman S.; Biswas T. K.; Haque Z.; Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology 2021, 64 (1), 36–47. 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajamarca-Baron J.; Guavita-Navarro D.; Buitrago-Bohorquez J.; Gallego-Cardona L.; Navas A.; Cubides H.; Arredondo A. M.; Escobar A.; Rojas-Villarraga A. SARS-CoV-2 (COVID-19) en pacientes con algún grado de inmunosupresión. Reumatologia clinica 2021, 17 (7), 408–419. 10.1016/j.reumae.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. S.; Sehayek D.; Gabrielli S.; Zhang X.; McCusker C.; Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgraduate medicine 2020, 132 (8), 749–755. 10.1080/00325481.2020.1786964. [DOI] [PubMed] [Google Scholar]
- Adab P.; Haroon S.; O’Hara M.; Jordan R. Comorbidities and covid-19 Better understanding is essential for health system planning. Bmj 2022, 377, o1431. 10.1136/bmj.o1431. [DOI] [PubMed] [Google Scholar]
- Sanyaolu A.; Okorie C.; Marinkovic A.; Patidar R.; Younis K.; Desai P.; Hosein Z.; Padda I.; Mangat J.; Altaf M. J. S. c. c. m. Comorbidity and its impact on patients with COVID-19. SN Comprehensive clinical medicine 2020, 2 (8), 1069–1076. 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Library of Medicine . ClinicalTrials.gov, https://clinicaltrials.gov/ (acccessed 05–12–2022).
- SARS-CoV-2 Variant Classifications and Definitions; Centers for Disease Control and Prevention, https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html/ (accessed 07-02-2023). [Google Scholar]
- Jian F.; Yu Y.; Song W.; Yisimayi A.; Yu L.; Gao Y.; Zhang N.; Wang Y.; Shao F.; Hao X.; et al. Further humoral immunity evasion of emerging SARS-CoV-2 BA. 4 and BA. 5 subvariants. Lancet Infect. Dis. 2022, 22 (11), 1535–1537. 10.1016/S1473-3099(22)00642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Nadeau S.; Yared M.; Voinov P.; Xie N.; Roemer C.; Stadler T. CoV-Spectrum: analysis of globally shared SARS-CoV-2 data to identify and characterize new variants. Bioinformatics 2022, 38 (6), 1735–1737. 10.1093/bioinformatics/btab856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal A.; Simon B.; Destras G.; Chalvignac R.; Semanas Q.; Oblette A.; Queromes G.; Fanget R.; Regue H.; Morfin F.; et al. Detection and prevalence of SARS-CoV-2 co-infections during the Omicron variant circulation, France, December 2021-February 2022. Nat. Commun. 2022, 13 (1), 6316. 10.1038/s41467-022-33910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Thambiraja T. S.; Karuppanan K.; Subramaniam G. J. J. o. m. v. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein 2022, 94 (4), 1641–1649. 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]; Kumar S.; Thambiraja T. S.; Karuppanan K.; Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. Journal of medical virology 2022, 94 (4), 1641–1649. 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- Wu Z.; McGoogan J. M. J. j. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. jama 2020, 323 (13), 1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Klein S. L.; Garibaldi B. T.; Li H.; Wu C.; Osevala N. M.; Li T.; Margolick J. B.; Pawelec G.; Leng S. X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing research reviews 2021, 65, 101205. 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G.; Rezza G.; Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. jama 2020, 323 (18), 1775–1776. 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Richardson S.; Hirsch J. S.; Narasimhan M.; Crawford J. M.; McGinn T.; Davidson K. W.; Barnaby D. P.; Becker L. B.; Chelico J. D.; Cohen S. L. J. P. C.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323 (20), 2052–2059. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa H.; Rello J.; Tejada S.; Martín A.; Balziskueta G.; Vinuesa C.; Fernández-Miret B.; Villagra A.; Vallejo A.; San Sebastián A.; et al. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesthesia critical care & pain medicine 2020, 39 (5), 553–561. 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohamadi Y.; Tola H. H.; Abbasi-Ghahramanloo A.; Janani M.; Sepandi M. Case fatality rate of COVID-19: a systematic review and meta-analysis. J. Prev. Med. Hyg. 2021, 62 (2), E311–E320. 10.15167/2421-4248/jpmh2021.62.2.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J.; Ohmagari N.; Shin D.; Diaz G.; Asperges E.; Castagna A.; Feldt T.; Green G.; Green M. L.; Lescure F.-X.; et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020, 382 (24), 2327–2336. 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikpouraghdam M.; Jalali Farahani A.; Alishiri G.; Heydari S.; Ebrahimnia M.; Samadinia H.; Sepandi M.; Jafari N. J.; Izadi M.; Qazvini A.; et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J. Clin. Virol. 2020, 127, 104378. 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati H.; Ramezani M.; Najafi F.; Sayad B.; Nazeri M.; Sadeghi M. Evaluation of Angiotensin-converting Enzyme 2 (ACE2) in COVID-19: A Systematic Review on All Types of Studies for Epidemiologic, Diagnostic, and Therapeutic Purposes: Angiotensin-converting enzyme 2 (ACE2) in COVID-19. Open Access Macedonian J. Med. Sci. 2020, 8 (T1), 84–91. 10.3889/oamjms.2020.4763. [DOI] [Google Scholar]
- Singh B. B.; Ward M. P.; Lowerison M.; Lewinson R. T.; Vallerand I. A.; Deardon R.; Gill J. P.; Singh B.; Barkema H. W. Meta-analysis and adjusted estimation of COVID-19 case fatality risk in India and its association with the underlying comorbidities. One health 2021, 13, 100283. 10.1016/j.onehlt.2021.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V.; Gadi N.; Spihlman A. P.; Wu S. C.; Choi C. H.; Moulton V. R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections?. Frontiers in physiology 2021, 11, 571416. 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. C.; Goldstein D. R.; Montgomery R. R. Age-dependent dysregulation of innate immunity. Nature reviews immunology 2013, 13 (12), 875–887. 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H.; Lv Y.; Xu Y.; Wu Y.; Zeng W.; Wang T.; Cao X.; Xu Y. Characteristic of Parkinson’s disease with severe COVID-19: a study of 10 cases from Wuhan. Journal of Neural Transmission 2021, 128 (1), 37–48. 10.1007/s00702-020-02283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G.; Zangrillo A.; Zanella A.; Antonelli M.; Cabrini L.; Castelli A.; Cereda D.; Coluccello A.; Foti G.; Fumagalli R. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323 (16), 1574–1581. 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P. K.; Ghassemieh B. J.; Nichols M.; Kim R.; Jerome K. R.; Nalla A. K.; Greninger A. L.; Pipavath S.; Wurfel M. M.; Evans L.; et al. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020, 382 (21), 2012–2022. 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wu M.; Yao J.; Guo J.; Liao X.; Song S.; Li J.; Duan G.; Zhou Y.; Wu X.. Caution on kidney dysfunctions of COVID-19 patients. MedRxiv (Infections Disease (except HIV/AIDS)), March 27, 2020,2020.02.08.20021212, ver. 2. DOI: 10.1101/2020.02.08.20021212.
- Bartleson J. M.; Radenkovic D.; Covarrubias A. J.; Furman D.; Winer D. A.; Verdin E. SARS-CoV-2, COVID-19 and the aging immune system. Nature aging 2021, 1 (9), 769–782. 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia F.; Gerbino A.; Lionetti V.; Miragoli M.; Munaron L.; Pagliaro P.; Pasqua T.; Penna C.; Rocca C.; Samaja M.; et al. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. Geroscience 2020, 42 (4), 1021–1049. 10.1007/s11357-020-00198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanaroff A. C.; Garcia S.; Giri J. Myocardial infarction during the COVID-19 pandemic. Jama 2021, 326 (19), 1916–1918. 10.1001/jama.2021.19608. [DOI] [PubMed] [Google Scholar]
- Li Q.; Guan X.; Wu P.; Wang X.; Zhou L.; Tong Y.; Ren R.; Leung K. S.; Lau E. H.; Wong J. Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382 (13), 1199–1207. 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.; Yu T.; Du R.; Fan G.; Liu Y.; Liu Z.; Xiang J.; Wang Y.; Song B.; Gu X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395 (10229), 1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Hooper M.; Napoles A. M.; Perez-Stable E. J. COVID-19 and racial/ethnic disparities. JAMA 2020, 323 (24), 2466–2467. 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D.; Sze S.; Minhas J. S.; Bangash M. N.; Pareek N.; Divall P.; Williams C. M.; Oggioni M. R.; Squire I. B.; Nellums L. B.; et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine 2020, 23, 100404. 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca-Mandic P.; Georgiou A.; Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. Jama internal medicine 2021, 181 (1), 131–134. 10.1001/jamainternmed.2020.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E. J.; McDonald H. I.; Bhaskaran K.; Walker A. J.; Bacon S.; Davy S.; Schultze A.; Tomlinson L.; Bates C.; Ramsay M. J. b. Risks of covid-19 hospital admission and death for people with learning disability: population based cohort study using the OpenSAFELY platform. BMJ 2021, 374, n1592. 10.1136/bmj.n1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri R.; Bendayan R.; Ashworth M.; Bean D. M.; Dodhia H.; Durbaba S.; O’Gallagher K.; Palmer C.; Curcin V.; Aitken E.; et al. A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19. EClinicalMedicine 2020, 28, 100574. 10.1016/j.eclinm.2020.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman K. E.; Magder L. S.; Baghdadi J. D.; Pineles L.; Levine A. R.; Perencevich E. N.; Harris A. D. Impact of sex and metabolic comorbidities on coronavirus disease 2019 (COVID-19) mortality risk across age groups: 66 646 inpatients across 613 US hospitals. Clinical Infectious diseases 2021, 73 (11), e4113–e4123. 10.1093/cid/ciaa1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino G.; Grassi G.; Borghi C.; Carugo S.; Fallo F.; Ferri C.; Giannattasio C.; Grassi D.; Letizia C.; Mancusi C.; et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: The results of the SARS-RAS study of the Italian Society of Hypertension. PLoS One 2020, 15 (10), e0237297 10.1371/journal.pone.0237297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ya’qoub L.; Elgendy I. Y.; Pepine C. J. Sex and gender differences in COVID-19: More to be learned!. Am. Heart J. Plus 2021, 3, 100011. 10.1016/j.ahjo.2021.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha J.; Qie G.; Yao Q.; Sun W.; Wang C.; Zhang Z.; Wang X.; Wang P.; Jiang J.; Bai X.; et al. Sex differences on clinical characteristics, severity, and mortality in adult patients with COVID-19: A multicentre retrospective study. Frontiers in medicine 2021, 8, 607059. 10.3389/fmed.2021.607059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiering A. E.; De Vries T. J. Why females do better: the X chromosomal TLR7 gene-dose effect in COVID-19. Front. Immunol. 2021, 12, 756262. 10.3389/fimmu.2021.756262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwani M.; Yassin A.; Al-Zoubi R. M.; Aboumarzouk O. M.; Nettleship J.; Kelly D.; AL-Qudimat A. R.; Shabsigh R. Sex-based differences in severity and mortality in COVID-19. Rev. Med. Virol. 2021, 31 (6), e2223 10.1002/rmv.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R.; Fett C.; Mack M.; Ten Eyck P. P.; Meyerholz D. K.; Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. journal of immunology 2017, 198 (10), 4046–4053. 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyyazhagan A.; Pushparaj K.; Balasubramanian B.; Kuchi Bhotla H.; Pappusamy M.; Arumugam V. A.; Easwaran M.; Pottail L.; Mani P.; Tsibizova V.; et al. COVID-19 in pregnant women and children: Insights on clinical manifestations, complexities, and pathogenesis. Int. J. Gynecol. Obstet. 2022, 156 (2), 216–224. 10.1002/ijgo.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Kang H.; Li S.; Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. Journal of neurology 2020, 267 (8), 2179–2184. 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I. H.; Normandin E.; Bhattacharyya S.; Mukerji S. S.; Keller K.; Ali A. S.; Adams G.; Hornick J. L.; Padera R. F. Jr; Sabeti P. Neuropathological features of Covid-19. New england journal of medicine 2020, 383 (10), 989–992. 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl A. J.; Bhatia K. P.; Merello M. Movement Disorders in the World of COVID-19. Mov. Disord. Clin. Pract. 2020, 7 (4), 355–356. 10.1002/mdc3.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C.; Bai W. Z.; Hashikawa T. Response to Commentary on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. Journal of medical virology 2020, 92 (7), 707–709. 10.1002/jmv.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L.; Jin H.; Wang M.; Hu Y.; Chen S.; He Q.; Chang J.; Hong C.; Zhou Y.; Wang D.; et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020, 77, 683. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A.; Leta V.; Teo J.; Chaudhuri K. R. Outcome of Parkinson’s disease patients affected by COVID-19. Movement disorders 2020, 35, 905. 10.1002/mds.28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi A.; Domingues R.; Setz C.; Outeiro T. F.; Krisko A. SARS-CoV-2: at the crossroad between aging and neurodegeneration. Movement disorders 2020, 35 (5), 716. 10.1002/mds.28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquisiras-Burgos I.; Peralta-Arrieta I.; Alonso-Palomares L. A.; Zacapala-Gómez A. E.; Salmerón-Bárcenas E. G.; Aguilera P. Neurological complications associated with the blood-brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Molecular neurobiology 2021, 58 (2), 520–535. 10.1007/s12035-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.; Malim F. M.; Goswami A.; Sharma N.; Juvvalapalli S. S.; Chatterjee S.; Kate A. S.; Khairnar A. Neuroprotective Effect of Swertiamarin in a Rotenone Model of Parkinson’s Disease: Role of Neuroinflammation and Alpha-Synuclein Accumulation. ACS pharmacology translational science 2023, 6, 40. 10.1021/acsptsci.2c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Benito M.; Granado N.; García-Sanz P.; Michel A.; Dumoulin M.; Moratalla R. Modeling Parkinson’s disease with the alpha-synuclein protein. Front. Pharmacol. 2020, 11, 356. 10.3389/fphar.2020.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Wahmane S.; Achbani A.; Ouhaz Z.; Elatiqi M.; Belmouden A.; Nejmeddine M. The possible protective role of α-synuclein against severe acute respiratory syndrome coronavirus 2 infections in patients with Parkinson’s disease. Mov. Disord. 2020, 35 (8), 1293–1294. 10.1002/mds.28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo T.; Yasunaga H.; Michihata N.; Sasabuchi Y.; Hasegawa W.; Takeshima H.; Sakamoto Y.; Matsui H.; Fushimi K.; Nagase T.; et al. Influence of Parkinsonism on outcomes of elderly pneumonia patients. Parkinsonism 2018, 54, 25–29. 10.1016/j.parkreldis.2018.03.028. [DOI] [PubMed] [Google Scholar]
- Lehtonen Š.; Sonninen T.-M.; Wojciechowski S.; Goldsteins G.; Koistinaho J. Dysfunction of cellular proteostasis in Parkinson’s disease. Frontiers in neuroscience 2019, 13, 457. 10.3389/fnins.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K.; Matsuda N. Proteostasis and neurodegeneration: the roles of proteasomal degradation and autophagy. Biochimica et biophysica acta -molecular cell research 2014, 1843 (1), 197–204. 10.1016/j.bbamcr.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Wang H. Q.; Takahashi R. J. A. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxid. Redox Signaling 2007, 9 (5), 553–561. 10.1089/ars.2006.1524. [DOI] [PubMed] [Google Scholar]
- Park J.-S.; Davis R. L.; Sue C. M. Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Current neurology neuroscience reports 2018, 18 (5), 1–11. 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarenko A.The Host Heat Shock Response, Viral Immune Escape and Viral Replication; Massachusetts Institute of Technology, 2020. [Google Scholar]
- Momose F.; Naito T.; Yano K.; Sugimoto S.; Morikawa Y.; Nagata K. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J. Biol. Chem. 2002, 277 (47), 45306–45314. 10.1074/jbc.M206822200. [DOI] [PubMed] [Google Scholar]
- Pandey P.; Saleh A.; Nakazawa A.; Kumar S.; Srinivasula S. M.; Kumar V.; Weichselbaum R.; Nalin C.; Alnemri E. S.; Kufe D. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO journal 2000, 19 (16), 4310–4322. 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere H. M.; Wolf B. B.; Cain K.; Mosser D. D.; Mahboubi A.; Kuwana T.; Tailor P.; Morimoto R. I.; Cohen G. M.; Green D. R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature cell biology 2000, 2 (8), 469–475. 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Parekh P.; Sharma N.; Sharma M.; Gadepalli A.; Sayyed A. A.; Chatterjee S.; Kate A.; Khairnar A. AMPK-dependent autophagy activation and alpha-Synuclein clearance: a putative mechanism behind alpha-mangostin’s neuroprotection in a rotenone-induced mouse model of Parkinson’s disease. Metab. Brain Dis. 2022, 37, 2853–2870. 10.1007/s11011-022-01087-1. [DOI] [PubMed] [Google Scholar]
- Marreiros R.; Müller-Schiffmann A.; Trossbach S. V.; Prikulis I.; Hänsch S.; Weidtkamp-Peters S.; Moreira A. R.; Sahu S.; Soloviev I.; Selvarajah S.; et al. Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (12), 6741–6751. 10.1073/pnas.1906466117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack M. U.; Albrecht R. A.; Urano T.; Inn K.-S.; Huang I.-C.; Carnero E.; Farzan M.; Inoue S.; Jung J. U.; García-Sastre A. J. C. h.; et al. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell host microbiome 2009, 5 (5), 439–449. 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja I.; de Vries E.; Tscherne D. M.; García-Sastre A.; Rottier P. J.; de Haan C. A. Inhibition of the ubiquitin-proteasome system affects influenza A virus infection at a postfusion step. Journal of virology 2010, 84 (18), 9625–9631. 10.1128/JVI.01048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.-P.; Siu K.-L.; Chin K.-T.; Yuen K.-Y.; Zheng B.; Jin D.-Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. Journal of virology 2006, 80 (18), 9279–9287. 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-S.; Qi H.-Y.; Boularan C.; Huang N.-N.; Abu-Asab M.; Shelhamer J. H.; Kehrl J. H. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. journal of immunology 2014, 193 (6), 3080–3089. 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.; Wong C. K.; Li P.; Xie Y. A SARS-CoV protein, ORF-6, induces caspase-3 mediated, ER stress and JNK-dependent apoptosis. Biochimica et biophysica acta -general subjects 2008, 1780 (12), 1383–1387. 10.1016/j.bbagen.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costela-Ruiz V. J.; Illescas-Montes R.; Puerta-Puerta J. M.; Ruiz C.; Melguizo-Rodríguez L. J. C. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Zhao Y.; Zhang F.; Wang Q.; Li T.; Liu Z.; Wang J.; Qin Y.; Zhang X.; Yan X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z. Important aspects of Toll-like receptors, ligands and their signaling pathways. inflammation research 2010, 59 (10), 791–808. 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L.; Holt A. C.; Medzhitov R.; Flavell R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413 (6857), 732–738. 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Onofrio L.; Caraglia M.; Facchini G.; Margherita V.; Placido S. D.; Buonerba C. J. F. S. O. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future science OA 2020, 6, FSO605. 10.2144/fsoa-2020-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani T. B.; Vital M. A.; Rauh L. K. Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arq. Neuro-Psiquiatr. 2015, 73, 616–623. 10.1590/0004-282X20150057. [DOI] [PubMed] [Google Scholar]
- Lim S.; Chun Y.; Lee J. S.; Lee S. J. Neuroinflammation in synucleinopathies. Brain pathology 2016, 26 (3), 404–409. 10.1111/bpa.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira B. D. M.; Radford R. A.; Chung R. S.; Guillemin G. J.; Pountney D. L. Neuroinflammation in multiple system atrophy: response to and cause of α-synuclein aggregation. Frontiers in cellular neuroscience 2015, 9, 437. 10.3389/fncel.2015.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J. E.; Wagner M. L.; Sage J. I. Safety of selegiline with cold medications. Annals of pharmacotherapy 2003, 37 (3), 438–441. 10.1345/aph.1C175. [DOI] [PubMed] [Google Scholar]
- Reardon K.; Mendelsohn F.; Chai S. Y.; Horne M. K. The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson’s disease. Australian New Zealand journal of medicine 2000, 30 (1), 48–53. 10.1111/j.1445-5994.2000.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Sonsalla P. K.; Coleman C.; Wong L.-Y.; Harris S. L.; Richardson J. R.; Gadad B. S.; Li W.; German D. C. The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Experimental neurology 2013, 250, 376–383. 10.1016/j.expneurol.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudisio A.; Lo Monaco M. R.; Silveri M. C.; Bentivoglio A. R.; Vetrano D. L.; Pisciotta M. S.; Brandi V.; Bernabei R.; Zuccala G. Use of ACE-inhibitors and falls in patients with Parkinson’s disease. Gait Posture 2017, 54, 39–44. 10.1016/j.gaitpost.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Sharma N.; Soni R.; Sharma M.; Chatterjee S.; Parihar N.; Mukarram M.; kale R.; Sayyed A. A.; Behera S. K.; Khairnar A. Chlorogenic Acid: A Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022, 59 (11), 6834–6856. 10.1007/s12035-022-03005-z. [DOI] [PubMed] [Google Scholar]
- Hedya S. A.; Safar M. M.; Bahgat A. K. Hydroxychloroquine antiparkinsonian potential: Nurr1 modulation versus autophagy inhibition. Behavioural brain research 2019, 365, 82–88. 10.1016/j.bbr.2019.02.033. [DOI] [PubMed] [Google Scholar]
- Cady S. D.; Hong M. Amantadine-induced conformational and dynamical changes of the influenza M2 transmembrane proton channel. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (5), 1483–1488. 10.1073/pnas.0711500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telcian A. G.; Zdrenghea M. T.; Edwards M. R.; Laza-Stanca V.; Mallia P.; Johnston S. L.; Stanciu L. A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral research 2017, 137, 93–101. 10.1016/j.antiviral.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Hribar C. A.; Cobbold P. H.; Church F. C. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain sciences 2020, 10 (5), 284. 10.3390/brainsci10050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone N. B.; Liu Z.; Pittet J. F.; Zmijewski J. W. Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. Journal of leukocyte biology 2017, 101 (2), 357–365. 10.1189/jlb.3HI0216-068RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Zhu F.; Xie L.; Wang C.; Wang J.; Chen R.; Jia P.; Guan H.; Peng L.; Chen Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31 (7), 894–901. 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W.; Guan W.; Chen R.; Wang W.; Li J.; Xu K.; Li C.; Ai Q.; Lu W.; Liang H.; et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020, 21 (3), 335–337. 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini K. S.; Tagliamento M.; Lambertini M.; McNally R.; Romano M.; Leone M.; Curigliano G.; de Azambuja E. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. European journal of cancer 2020, 139, 43–50. 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A.; Weiskopf D.; Ramirez S. I.; Mateus J.; Dan J. M.; Moderbacher C. R.; Rawlings S. A.; Sutherland A.; Premkumar L.; Jadi R. S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181 (7), 1489–1501. 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Rizvi H.; Preeshagul I. R.; Egger J. V.; Hoyos D.; Bandlamudi C.; McCarthy C. G.; Falcon C. J.; Schoenfeld A. J.; Arbour K. C.; et al. COVID-19 in patients with lung cancer. Ann. Oncol. 2020, 31 (10), 1386–1396. 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V.; Goel S.; Kabarriti R.; Cole D.; Goldfinger M.; Acuna-Villaorduna A.; Pradhan K.; Thota R.; Reissman S.; Sparano J. A.; et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discovery 2020, 10 (7), 935–941. 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas A. J.; Harris C. C. Biomarker development in the precision medicine era: lung cancer as a case study. Nature reviews cancer 2016, 16 (8), 525–537. 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa L.; Melenotte C.; Griscelli F.; Gachot B.; Marabelle A.; Kroemer G.; Zitvogel L. The immuno-oncological challenge of COVID-19. Nature cancer 2020, 1 (10), 946–964. 10.1038/s43018-020-00122-3. [DOI] [PubMed] [Google Scholar]
- Berretta A. A.; Silveira M. A. D.; Cóndor Capcha J. M.; De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Wang H.; Xia L.; Oyang L.; Zhou Y.; Zhang B.; Chen X.; Luo X.; Liao Q.; Liang J. Overexpression of PAK1 correlates with aberrant expression of EMT markers and poor prognosis in non-small cell lung cancer. Journal of cancer 2017, 8 (8), 1484. 10.7150/jca.18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Von Freyend S.; Kwok-Schuelein T.; Netter H. J.; Haqshenas G.; Semblat J.-P.; Doerig C. Subverting host cell P21-activated kinase: a case of convergent evolution across pathogens. Pathogens 2017, 6 (2), 17. 10.3390/pathogens6020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta H.; He H. PAK1-blockers: Potential Therapeutics against COVID-19. Medicine in drug discovery 2020, 6, 100039. 10.1016/j.medidd.2020.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curigliano G. Cancer patients and risk of mortality for COVID-19. Cancer cell 2020, 38 (2), 161–163. 10.1016/j.ccell.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchi Bhotla H.; Kaul T.; Balasubramanian B.; Easwaran M.; Arumugam V. A.; Pappusamy M.; Muthupandian S.; Meyyazhagan A. Platelets to surrogate lung inflammation in COVID-19 patients. Med. Hypotheses 2020, 143, 110098. 10.1016/j.mehy.2020.110098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania A. S.; Prathipati P.; Abdul B. A.; Chava S.; Katta S. S.; Gupta S. C.; Gangula P. R.; Pandey M. K.; Durden D. L.; Byrareddy S. N.; et al. COVID-19 and cancer comorbidity: therapeutic opportunities and challenges. Theranostics 2021, 11 (2), 731. 10.7150/thno.51471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnquist C.; Ryan B. M.; Horikawa I.; Harris B. T.; Harris C. C. Cytokine storms in cancer and COVID-19. Cancer Cell 2020, 38 (5), 598–601. 10.1016/j.ccell.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A.; Kashyap A.; Tripathi G.; Yadav J.; Bibban R.; Aggarwal N.; Thakur K.; Chhokar A.; Jadli M.; Sah A. K.; et al. Tumor reversion: a dream or a reality. Biomarker Res. 2021, 9 (1), 1–27. 10.1186/s40364-021-00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender L. A.; Curran M.; Bates S. M.; Mairesse M.; Weigandt J.; Betts C. J. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Frontiers in immunology 2020, 11, 1991. 10.3389/fimmu.2020.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Z.; Zhu R.; Hou W.; Feng Y.; Yang Y.; Han Q.; Shan G.; Meng F.; Du D.; Wang S.; et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020, 11 (2), 216–228. 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.; Sah A. K.; Tripathi G.; Kashyap A.; Tripathi A.; Rao R.; Mishra P. C.; Mallick K.; Husain A.; Kashyap M. K. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Molecular cellular biochemistry 2021, 476 (2), 553–574. 10.1007/s11010-020-03924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Zhao Y.; Okwan-Duodu D.; Basho R.; Cui X. Medicine. COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol. Med. 2020, 17 (3), 519. 10.20892/j.issn.2095-3941.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara C.; Kusaka Y.; Kimura S.; Yamaguchi T.; Nanjo Y.; Ishii Y.; Udono H.; Standiford T. J.; Tateda K. Metformin mediates protection against Legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species. journal of immunology 2018, 200 (2), 623–631. 10.4049/jimmunol.1700474. [DOI] [PubMed] [Google Scholar]
- Martinez N.; Kornfeld H. Diabetes and immunity to tuberculosis. European journal of immunology 2014, 44 (3), 617–626. 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Feng Y.; Yuan M.; Yuan S.; Fu H.; Wu B.; Sun G.; Yang G.; Zhang X.; Wang L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabetic Med. 2006, 23 (6), 623–628. 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Zhang J.-j.; Dong X.; Cao Y.-y.; Yuan Y.-d.; Yang Y.-b.; Yan Y.-q.; Akdis C. A.; Gao Y.-d. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75 (7), 1730–1741. 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Guan W.-j.; Ni Z.-y.; Hu Y.; Liang W.-h.; Ou C.-q.; He J.-x.; Liu L.; Shan H.; Lei C.-l.; Hui D. S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382 (18), 1708–1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini G.; Morieri M.; Longato E.; Avogaro d. A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. Journal of endocrinological 2020, 43 (6), 867–869. 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebekozien O. A.; Noor N.; Gallagher M. P.; Alonso G. T. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care 2020, 43 (8), e83–e85. 10.2337/dc20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Hu B.; Hu C.; Zhu F.; Liu X.; Zhang J.; Wang B.; Xiang H.; Cheng Z.; Xiong Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323 (11), 1061–1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.; Li M.; Dong Y.; Zhou H.; Zhang Z.; Tian C.; Qin R.; Wang H.; Shen Y.; Du K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/metabolism research 2020, 36 (7), e3319 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler M. Is the lung a’target organ’in diabetes mellitus?. Archives of internal medicine 1990, 150 (7), 1385–1388. 10.1001/archinte.150.7.1385. [DOI] [PubMed] [Google Scholar]
- Jafar N.; Edriss H.; Nugent K. The effect of short-term hyperglycemia on the innate immune system. American journal of the medical sciences 2016, 351 (2), 201–211. 10.1016/j.amjms.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Mehta P.; McAuley D.; Brown M.; Sanchez E.; Tattersall R.; Manson J.; et al. COVID-19: Consider cytokine storm syndromes immunosuppression. Lancet 2020, 395 (10229), 1033. 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A. C.; Davanzo G. G.; Monteiro L. d. B.; de Souza G. F.; Muraro S. P.; Virgilio-da-Silva J. V.; Prodonoff J. S.; Carregari V. C.; de Biagi Junior C. A. O.; Crunfli F.; et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020, 32 (3), 437–446.e5. 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C.; Bonadonna R. C.; DelPrato S.; Ratheiser K.; Zyck K.; Ferrannini E.; DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 1989, 84 (1), 205–213. 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N.; Bai H.; Chen X.; Gong J.; Li D.; Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis 2020, 18 (5), 1094–1099. 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla U.; Kashyap M. K.; Husain A. Aging and diabetes drive the COVID-19 forwards; unveiling nature and existing therapies for the treatment. Molecular cellular biochemistry 2021, 476 (11), 3911–3922. 10.1007/s11010-021-04200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsar K.; Coleman C.; Beck S.; Frieman M. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019, 4 (20), e131774 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M.; Ghobrial R. M.; Lewicki S.; Kubiak J. Z. Macrophages in diabetes mellitus (DM) and COVID-19: do they trigger DM?. Journal of Diabetes Metabolic Disorders 2020, 19 (2), 2045–2048. 10.1007/s40200-020-00665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C.; Bernardi S.; Burns W. C. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr. Opin. Nephrol. Hypertens. 2011, 20 (1), 62–68. 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- Rutkowska-Zapała M.; Suski M.; Szatanek R.; Lenart M.; Węglarczyk K.; Olszanecki R.; Grodzicki T.; Strach M.; Gąsowski J.; Siedlar M. Human monocyte subsets exhibit divergent angiotensin I-converting activity. Clinical experimental immunology 2015, 181 (1), 126–132. 10.1111/cei.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C. G.; Allon S. J.; Nyquist S. K.; Mbano I. M.; Miao V. N.; Tzouanas C. N.; Cao Y.; Yousif A. S.; Bals J.; Hauser B. M.; et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020, 181 (5), 1016–1035. 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-K.; Lin S.-S.; Ji X.-J.; Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta diabetologica 2010, 47 (3), 193–199. 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.; Bae J. H.; Kwon H.-S.; Nauck M. A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17 (1), 11–30. 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocrine reviews 2020, 41 (3), bnaa011. 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. L.; Weiss R. E. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes/metabolism research reviews 2014, 30 (2), 96–102. 10.1002/dmrr.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindom S. M.; Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Molecular cellular endocrinology 2009, 302 (2), 193–202. 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Arora A.; Sharma P.; Anikhindi S. A.; Bansal N.; Singla V.; Khare S.; Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes metabolic syndrome: Clinical research Reviews 2020, 14 (4), 535–545. 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M.; Wysocki J.; Gonzalez-Pacheco F. R.; Salem M.; Evora K.; Garcia-Halpin L.; Poglitsch M.; Schuster M.; Batlle D. Murine Recombinant Angiotensin-Converting Enzyme 2: Effect on Angiotensin II-Dependent Hypertension and Distinctive Angiotensin-Converting Enzyme 2 Inhibitor Characteristics on Rodent and Human Angiotensin-Converting Enzyme 2. Hypertension 2012, 60 (3), 730–740. 10.1161/HYPERTENSIONAHA.112.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C.; D’Onofrio N.; Balestrieri M. L.; Barbieri M.; Rizzo M. R.; Messina V.; Maggi P.; Coppola N.; Paolisso G.; Marfella R. J. D. c. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control?. Diabetes care 2020, 43 (7), 1408–1415. 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. R.; Morrison V. L.; Levin D.; Mohan M.; Forteath C.; Beall C.; McNeilly A. D.; Balfour D. J.; Savinko T.; Wong A. K.; et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016, 119 (5), 652–665. 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan W. N.; Viscoli C. M.; Furie K. L.; Young L. H.; Inzucchi S. E.; Gorman M.; Guarino P. D.; Lovejoy A. M.; Peduzzi P. N.; Conwit R.; et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 2016, 374, 1321–1331. 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesto R. W.; Bell D.; Bonow R. O.; Fonseca V.; Grundy S. M.; Horton E. S.; Le Winter M.; Porte D.; Semenkovich C. F.; Smith S. J. C.; et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004, 27, 256–263. 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- Chung M. K.; Zidar D. A.; Bristow M. R.; Cameron S. J.; Chan T.; Harding III C. V.; Kwon D. H.; Singh T.; Tilton J. C.; Tsai E. J. COVID-19 and cardiovascular disease: from bench to bedside. Circ. Res. 2021, 128 (8), 1214–1236. 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtazmanesh S.; Shobeiri P.; Hanaei S.; Mahmoud-Elsayed H.; Dalvi B.; Malakan Rad E. Cardiovascular disease in COVID-19: a systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egyptian heart journal 2020, 72 (1), 1–17. 10.1186/s43044-020-00075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszak F.; Maryniak A.; Botti E.; Abrahim C.; Salifu M. O.; Youssef M.; Henglein V. L.; McFarlane S. I. Myocarditis associated with COVID-19. Am. J. Med. Case Rep. 2020, 8 (12), 498. 10.12691/ajmcr-8-12-19.33088905 [DOI] [Google Scholar]
- Siripanthong B.; Nazarian S.; Muser D.; Deo R.; Santangeli P.; Khanji M. Y.; Cooper L. T. Jr; Chahal C. A. A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart rhythm 2020, 17 (9), 1463–1471. 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arocutipa C.; Saucedo-Chinchay J.; Imazio M. Pericarditis in patients with COVID-19: a systematic review. Journal of cardiovascular medicine 2021, 22 (9), 693–700. 10.2459/JCM.0000000000001202. [DOI] [PubMed] [Google Scholar]
- Bozkurt B.; Kamat I.; Hotez P. J. Myocarditis with COVID-19 mRNA vaccines. Circulation research 2021, 144 (6), 471–484. 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V.; Bollini S.; Coppini R.; Gerbino A.; Ghigo A.; Iaccarino G.; Madonna R.; Mangiacapra F.; Miragoli M.; Moccia F.; et al. Understanding the heart-brain axis response in COVID-19 patients: A suggestive perspective for therapeutic development. Pharmacological Res. 2021, 168, 105581. 10.1016/j.phrs.2021.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A.; Rockman-Greenberg C.; Sareen N.; Lionetti V.; Dhingra S. An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning β-cell survival and cardiovascular conditions in diabetic patients. Molecular Cell. Biochem. 2022, 477, 1681–1695. 10.1007/s11010-022-04396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto E. Blood pressure and ageing. Postgraduate medical journal 2007, 83 (976), 109–114. 10.1136/pgmj.2006.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A. V.; Bakris G. L.; Black H. R.; Cushman W. C.; Green L. A.; Izzo J. L. Jr; Jones D. W.; Materson B. J.; Oparil S.; Wright J. T. J. J. Jr; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L.; Flack J. M.; Ito S.; Muntner P.; Webb R. C. Hypertension and COVID-19. Am. J. Hypertens. 2020, 33, 373–374. 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.; Chen X.; Cai Y.; Xia J.; Zhou X.; Xu S.; Huang H.; Zhang L.; Zhou X.; Du C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern.l Med. 2020, 180 (7), 934–943. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar M. H.; Afshin A.; Alexander L. T.; Anderson H. R.; Bhutta Z. A.; Biryukov S.; Brauer M.; Burnett R.; Cercy K.; Charlson F. J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388 (10053), 1659–1724. 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zores F.; Rebeaud M. E. COVID and the renin-angiotensin system: are hypertension or its treatments deleterious?. Frontiers in cardiovascular medicine 2020, 7, 71. 10.3389/fcvm.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K.; Imai Y.; Ohto-Nakanishi T.; Penninger J. M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacology therapeutics 2010, 128 (1), 119–128. 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshotels M. R.; Xia H.; Sriramula S.; Lazartigues E.; Filipeanu C. M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin ii type i receptor-dependent mechanism. Hypertension 2014, 64 (6), 1368–1375. 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M. J.; Wysocki J.; Batlle D. ACE2 alterations in kidney disease. Nephrology dialysis transplantation 2013, 28 (11), 2687–2697. 10.1093/ndt/gft320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S.; Yamamoto N.; Nakai-Murakami C.; Osawa Y.; Tokunaga K.; Sata T.; Yamamoto N.; Sasazuki T.; Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (22), 7809–7814. 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K.; Imai Y.; Rao S.; Gao H.; Guo F.; Guan B.; Huan Y.; Yang P.; Zhang Y.; Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Med. 2005, 11 (8), 875–879. 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I.; Bertram S.; Herzog P.; Pfefferle S.; Steffen I.; Muench M. O.; Simmons G.; Hofmann H.; Kuri T.; Weber F. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010, 84 (2), 1198–1205. 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H. P.; Look D. C.; Tan P.; Shi L.; Hickey M.; Gakhar L.; Chappell M. C.; Wohlford-Lenane C.; McCray P. B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2009, 297 (1), L84–L96. 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.; Kleine-Weber H.; Schroeder S.; Krüger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N.-H.; Nitsche A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181 (2), 271–280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. B.; Zhong J.-C.; Grant M. B.; Oudit G. Y. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016, 118 (8), 1313–1326. 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R. A. S.; Sampaio W. O.; Alzamora A. C.; Motta-Santos D.; Alenina N.; Bader M.; Campagnole-Santos M. J. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol. Rev. 2018, 98, 505. 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F.; Yang J.; Zhang Y.; Dong M.; Wang S.; Zhang Q.; Liu F. F.; Zhang K.; Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nature reviews cardiology 2014, 11 (7), 413–426. 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.; Basu R.; Guo D.; Chow F. L.; Byrns S.; Schuster M.; Loibner H.; Wang X.-h.; Penninger J. M.; Kassiri Z.; et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 2010, 122 (7), 717–728. 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- Oudit G. Y.; Kassiri Z.; Patel M. P.; Chappell M.; Butany J.; Backx P. H.; Tsushima R. G.; Scholey J. W.; Khokha R.; Penninger J. M. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007, 75 (1), 29–39. 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Patel V. B.; Bodiga S.; Basu R.; Das S. K.; Wang W.; Wang Z.; Lo J.; Grant M. B.; Zhong J.; Kassiri Z.; et al. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ. Res. 2012, 110 (10), 1322–1335. 10.1161/CIRCRESAHA.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.; Kassiri Z.; Jiang C.; Liu P.; Poutanen S.; Penninger J.; Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European journal of clinical investigation 2009, 39 (7), 618–625. 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Moore M. J.; Vasilieva N.; Sui J.; Wong S. K.; Berne M. A.; Somasundaran M.; Sullivan J. L.; Luzuriaga K.; Greenough T. C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426 (6965), 450–454. 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D.; Wysocki J.; Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy?. Clinical science 2020, 134 (5), 543–545. 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- Khan A.; Benthin C.; Zeno B.; Albertson T. E.; Boyd J.; Christie J. D.; Hall R.; Poirier G.; Ronco J. J.; Tidswell M.; et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 2017, 21 (1), 234. 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz M.; Yim E.; Klaff L.; Lokhandwala S.; Riedo F. X.; Chong M.; Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama 2020, 323 (16), 1612–1614. 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.; Qin M.; Shen B.; Cai Y.; Liu T.; Yang F.; Gong W.; Liu X.; Liang J.; Zhao Q.; et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5 (7), 802–810. 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Yang Y.; Zhang C.; Huang F.; Wang F.; Yuan J.; Wang Z.; Li J.; Li J.; Feng C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63 (3), 364–374. 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razeghian-Jahromi I.; Zibaeenezhad M. J.; Lu Z.; Zahra E.; Mahboobeh R.; Lionetti V. Angiotensin-converting enzyme 2: a double-edged sword in COVID-19 patients with an increased risk of heart failure. Heart Fail. Rev. 2021, 26 (2), 371–380. 10.1007/s10741-020-10016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso M.; Thanaraj T. A.; Abu-Farha M.; Alanbaei M.; Abubaker J.; Al-Mulla F. The two faces of ACE2: the role of ACE2 receptor and its polymorphisms in hypertension and COVID-19. Mol. Ther.--Methods Clin. Dev. 2020, 18, 321–327. 10.1016/j.omtm.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Zhu L.; Cai J.; Lei F.; Qin J.-J.; Xie J.; Liu Y.-M.; Zhao Y.-C.; Huang X.; Lin L.; et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020, 126 (12), 1671–1681. 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N.; Kalra A.; Nowacki A. S.; Anjewierden S.; Han Z.; Bhat P.; Carmona-Rubio A. E.; Jacob M.; Procop G. W.; Harrington S.; et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5 (9), 1020–1026. 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas-Boas W. W.; Ribeiro-Oliveira A. Jr.; da Cunha Ribeiro R.; Vieira R. L. P.; Almeida J.; Nadu A. P.; e Silva A. C. S.; Santos R. A. S. Effect of propranolol on the splanchnic and peripheral renin angiotensin system in cirrhotic patients. World J. Gastroenterol. 2008, 14 (44), 6824. 10.3748/wjg.14.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K.; Kusunoki A.; Machida T.; Hirafuji M. Angiotensin II reduces membranous angiotensin-converting enzyme 2 in pressurized human aortic endothelial cells. Journal of the renin-angiotensin-aldosterone system 2009, 10 (4), 210–215. 10.1177/1470320309343710. [DOI] [PubMed] [Google Scholar]
- Jessup J. A.; Brosnihan K. B.; Gallagher P. E.; Chappell M. C.; Ferrario C. M. Differential effect of low-dose thiazides on the renin angiotensin system in genetically hypertensive and normotensive rats. Journal of the American society of hypertension 2008, 2 (2), 106–115. 10.1016/j.jash.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y.; Zhu A.; Yoneda T.; Usukura M.; Takata H.; Yamagishi M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. American journal of hypertension 2007, 20 (10), 1119–1124. 10.1016/j.amjhyper.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Keidar S.; Gamliel-Lazarovich A.; Kaplan M.; Pavlotzky E.; Hamoud S.; Hayek T.; Karry R.; Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circulation research 2005, 97 (9), 946–953. 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- Villa A.; Brunialti E.; Dellavedova J.; Meda C.; Rebecchi M.; Conti M.; Donnici L.; De Francesco R.; Reggiani A.; Lionetti V.; et al. DNA aptamers masking angiotensin converting enzyme 2 as an innovative way to treat SARS-CoV-2 pandemic. Pharmacol. Res. 2022, 175, 105982. 10.1016/j.phrs.2021.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]