Abstract
Fas/CD95 is a key regulator of apoptotic signaling, which is crucial for the maintenance of homeostasis in peripheral lymphoid organs. TDAG51 has been shown to play critical roles in the up-regulation of Fas gene expression and T-cell apoptosis in vitro. In order to identify the role of TDAG51 in vivo, we generated TDAG51-deficient (TDAG51−/−) mice. Northern blotting revealed no expression of TDAG51 in TDAG51−/− mice, indicating that the TDAG51 gene was successfully targeted. TDAG51−/− mice were healthy and showed no gross developmental abnormalities. While Fas-deficient mice display marked lymphadenopathy, splenomegaly, and lymphocytosis, TDAG51−/− mice had no apparent defects in secondary lymphoid organs. Although TDAG51 is required for up-regulation of Fas expression in T-cell hybridomas, TDAG51−/− mice expressed normal levels of Fas and had normal T-cell apoptosis. Therefore, we conclude that TDAG51 is not essential for Fas up-regulation and T-cell apoptosis in vivo. There are several known homologs of TDAG51, and these homologs may substitute for TDAG51 in TDAG51−/− mice.
Apoptosis, or programmed cell death, is an important regulatory process for many cell types. In particular, apoptosis plays a major role in the clonal deletion of developing T cells and the elimination of activated T cells in the periphery (8). T-cell apoptosis is characterized by the expression of death cytokines, such as FasL and tumor necrosis factor, and their cognate receptors, such as Fas (CD95, Apo-1) and tumor necrosis factor receptor 1 (16).
Activation of the Fas receptor on cells of the immune system and various other tissues results in cell death (13). Fas is a type I membrane protein containing a death domain (DD) sequence in its cytoplasmic tail. The Fas DD tail binds to the cytoplasmic DD-containing adapter protein FADD (2). FADD then activates the caspase cascade, leading to the characteristic proteolytic reactions of apoptosis (8, 13, 16). Fas has also been shown to induce apoptosis via a FADD-independent mechanism, activating the Jun N-terminal protein kinase pathway through DAXX (19). Furthermore, Fas has been shown to act synergistically with T-cell receptor signaling in the induction of T-cell apoptosis (18). The role of Fas in vivo has been documented through the characterization of Fas-deficient mice (1, 3). Fas-deficient mice display marked lymphadenopathy, splenomegaly, and lymphocytosis in immune tissues as well as infiltration of lymphocytes into lung and liver tissue. Although the mechanism and physiological significance of Fas-mediated apoptosis have been well documented, comparatively little is known about the regulation of Fas gene expression.
We have previously shown that T-cell death-associated gene 51 (TDAG51) regulates Fas expression and activation-induced apoptosis of T cells, suggesting that TDAG51 might be important for the regulation of the immune response (14). The TDAG51 protein contains both a proline-glutamine (PQ) repeat domain and a proline-histidine (PH) repeat domain in its C-terminal region. Proteins containing the PQ domain might be important for transcriptional regulation and apoptosis in cells (5, 6, 10). However, there is no clear evidence implicating TDAG51 in these processes. A recent report suggested that TDAG51 mediates Fas expression through protein kinase C activation (17). Another candidate transcriptional activator of Fas expression is p53, which has been recently reported to bind to intron 1 of Fas and up-regulate its expression (12). Although there appears to be a relationship between Fas expression and protein kinase C and/or p53 activation in vitro, the mechanism of Fas regulation in vivo is unclear.
In order to investigate the physiological role of TDAG51, we generated TDAG51-deficient mice using gene targeting techniques. We observed no differences between TDAG51−/− mice and wild-type littermates up to 13 months of age. Furthermore, contrary to previous in vitro results, we found that TDAG51 is not essential for Fas expression and T-cell apoptosis in vivo.
MATERIALS AND METHODS
Generation of TDAG51−/− mouse.
The genomic region of TDAG51 was cloned from a 129/Sv mouse genomic lambda phage library by using a full-length TDAG51 cDNA as a probe. To make the gene targeting construct, long and short homology fragments were ligated into the pPNT vector. The long homology fragment was a 10.0-kb portion of the 3′ untranslated sequence, and the short homology fragment was a 3.2-kb portion of the 5′ flanking sequence. Homologous recombination in embryonic stem (ES) cells produced a deletion of approximately 3.0 kb that contained the entire coding region of TDAG51. The CJ7 ES cells (7) were cultured on mouse embryonic fibroblast feeder layers in Dulbecco's modified Eagle medium containing 15% fetal calf serum and 1,000 U of leukemia inhibitory factor per ml. The ES cells were electroporated with 50 μg of linearized targeting vector using a Bio-Rad electroporater (220 V and 960 μF). Transfected cells were cultured with 200 μg of G418 (GIBCO/BRL) per ml and 0.2 μM ganciclovir (Roche Laboratories) for 7 to 9 days. After selection, 150 colonies were picked and further analyzed by Southern blotting. Five correctly targeted clones were obtained, and two of them were microinjected into blastocysts from C57BL/6J mice. Founders were bred with C57BL/6J mice to test for germ line transmission.
Northern hybridization and quantitative reverse transcription (RT)-PCR.
RNAs were prepared using TRIZOL reagent (GIBCO/BRL). Northern blots containing 20 μg of total RNA of each sample were incubated with hybridization solution (50% formamide, 1× Denhardt reagent, 0.5% sodium dodecyl sulfate [SDS], 50 mM phosphate buffer, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], and single-stranded DNA [100 μg/ml]) containing [α-32P]dCTP-labeled probe for 16 h at 42°C. The probe was a fragment of the TDAG51 cDNA corresponding to the N-terminal region (amino acids 1 to 100). For high-stringency blots, the filters were rinsed in 2× SSC–0.5% SDS at room temperature and then in 0.5× SSC–0.5% SDS at 55°C, with a final wash in 0.1× SSC–0.5% SDS at 65°C. For low-stringency blots, the filters were washed in 2× SSC–0.5% SDS at room temperature and then in 0.5× SSC–0.5% SDS at 50°C, with a final wash in 0.1× SSC–0.5% SDS at 50°C.
For RT-PCR, total RNAs (10 μg) were used for the RT reactions. Quantitative PCR was performed as described previously (14).
Flow cytometry.
Cells were stained and analyzed for expression of T- or B-cell surface markers on a FACScan flow cytometer (Becton Dickinson) as described previously (14). Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated antibodies were purchased from Pharmingen and GIBCO/BRL.
Proliferation assay and apoptosis assay.
For proliferation assay, lymph node (LN)-derived T cells (5 × 105 cells/well) were incubated with concanavalin A (ConA) (0 to 5 μg/ml) for 72 h and then further cultured with [3H]thymidine (Amersham-Pharmacia) for 8 h. Spleen-derived cells (5 × 105 cells/well) were incubated with lipopolysaccharide (LPS) (0 to 25 μg/ml) for 72 h and then further cultured with [3H]thymidine for 16 h. The apoptosis assay was described previously (14). In brief, LN-derived cells were cultured with ConA (5 μg/ml) and interleukin 2 (IL-2) (10 U/ml) for 48 h, washed, and then further incubated with IL-2 (50 U/ml) for 48 h. These LN-derived T cells were subjected to activation-induced apoptosis on 96-well plates coated with mouse anti-CD3 antibodies (0 to 10 μg/ml) and further cultured for 48 h. After culture, the cells were incubated with propidium iodide (1 μg/ml), and dead cells were counted with a FACScan flow cytometer.
RESULTS
Generation of TDAG51−/− mice.
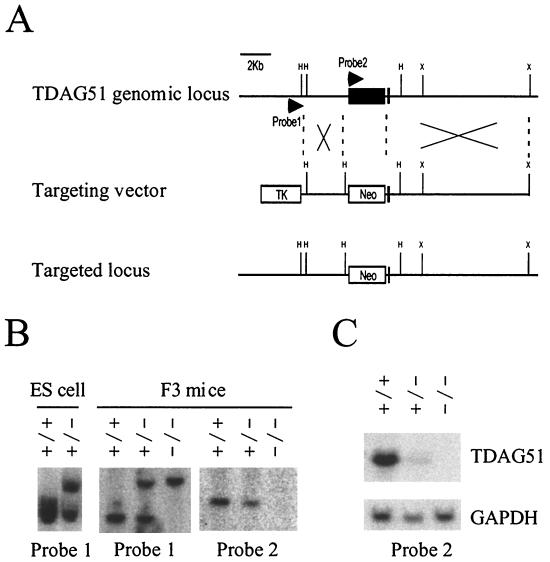
To investigate the role of TDAG51 in vivo, we cloned mouse genomic DNA of TDAG51. The TDAG51 gene consists of two exons. The first exon contains the entire open reading frame, and the second exon is a very short fragment located in the 3′ untranslated region of TDAG51 (Fig. 1A). We constructed a replacement vector that would delete the entire first exon of TDAG51 (Fig. 1A). The TDAG51 gene-targeting vector was electroporated into ES cells and selected for resistance to G418 and ganciclovir. After selection, 150 drug-resistant colonies were analyzed by Southern blotting. We obtained five independent clones that had correctly targeted the TDAG51 genomic locus (Fig. 1B, left panel). To test for single-copy gene integration, the five clones were analyzed by Southern blotting with a neo gene probe (data not shown). Two positive ES clones were microinjected into blastocysts from C57BL/6J mice. The offspring carrying the construct were crossed with C57BL/6J mice. In crosses of heterozygous mutant mice, there were offspring of all three possible genotypes in normal Mendelian ratios (Fig. 1B, middle panel and right panel). To check for TDAG51 expression in the targeted mice, we carried out Northern blot analysis with total RNA derived from liver that expresses a high level of endogenous TDAG51 (14). The TDAG51−/− mice had no expression of TDAG51 mRNA, but the wild-type and heterozygous mice had high levels of expression (Fig. 1C). Thus, TDAG51 was specifically disrupted, and the TDAG51−/− mice were correctly generated. The TDAG51−/− mice did not show any embryonic abnormality, and the overall morphological phenotype of the adult mice was indistinguishable from that of their wild-type littermates (data not shown).
FIG. 1.
Targeted disruption of the TDAG51 gene. (A) The TDAG51 genomic locus and the structure of targeting vector are shown. The pPNT targeting vector was used to delete approximately 3 kb of genomic DNA, including the entire coding region of TDAG51 (exons shown as black boxes). Abbreviations: H, HincII; X, XhoI; TK, thymidine kinase; Neo, neomycin resistance. (B) Southern analysis of TDAG51−/− mutants. Probe 1 is a 0.2-kb fragment of the 5′ untranslated region, and probe 2 is a 0.2-kb fragment from the coding region, including the ATG start codon. The wild-type allele was detected as a 3.2-kb HincII fragment with probe 1 and as a 1.6-kb HincII fragment with probe 2. The targeted allele was detected as a 4.5-kb HincII fragment with probe 1. Symbols: +/+, wild-type; +/−, heterozygote; −/−, homozygote. (C) Northern analysis of TDAG51−/− mice. Total RNAs (20 μg) derived from liver were electrophoresed, transferred to nylon membrane, and hybridized with probe 1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Analysis of immune responsive cells in TDAG51−/− mice.
We examined the lymphoid organs of TDAG51−/− mice and found that they had no detectable abnormalities of the LN and spleen compared to their wild-type littermates. Furthermore, the counts of total white blood cells (WBC) were the same for TDAG51−/− mice and controls (Table 1). To examine the possibility that there were abnormalities in specific subpopulations of T or B cells in TDAG51−/− mice, we stained cells derived from immune responsive organs with fluorescence-conjugated mouse antibodies and analyzed them by flow cytometry. When we stained cells derived from the spleens and LNs of TDAG51−/− mice with PE-conjugated B220 antibody and FITC-conjugated CD3 antibody, the ratios of T cells (CD3+) and B cells (B220+) were similar to those of the wild-type and heterozygous mice (Table 1). We also examined T-cell subpopulations based on their expression of CD4 and CD8. There were no differences between the double-positive (CD4+ CD8+), double-negative (CD4− CD8−), and single-positive (CD4+ CD8− or CD4− CD8+) T cells of TDAG51−/− mice and controls (Table 1). In addition, we examined populations of CD46+, T-cell receptor αβ+, CD11b+, CD11c+, immunoglobulin M+, and immunoglobulin D+ cells, but these cells yielded similar results (data not shown). Taken together, we did not find any differences in the patterns of surface markers in TDAG51−/− mice.
TABLE 1.
Expression patterns of surface markers on T or B cells of TDAG51−/− mice
| Cell type | % Exhibiting expression pattern on indicated cell typea
|
|||||
|---|---|---|---|---|---|---|
| Thymus
|
Spleen
|
LN
|
||||
| +/+ | −/− | +/+ | −/− | +/+ | −/− | |
| WBC | 9.7 ± 1 | 10.1 ± 2 | 6.0 ± 1 | 7.8 ± 1 | 1.5 ± 0 | 1.4 ± 0 |
| CD3+ | NT | NT | 29.8 ± 7 | 27.7 ± 7 | 66.7 ± 6 | 70.0 ± 9 |
| B220+ | NT | NT | 55.1 ± 1 | 53.3 ± 6 | 31.0 ± 7 | 28.3 ± 9 |
| CD4+ CD8− | 10.0 ± 2 | 11.0 ± 3 | 19.7 ± 5 | 19.3 ± 9 | 45.0 ± 8 | 47.7 ± 8 |
| CD8+ CD4− | 2.3 ± 1 | 2.3 ± 1 | 8.3 ± 2 | 8.7 ± 2 | 14.2 ± 2 | 18.0 ± 5 |
| CD4+ CD8+ | 86.7 ± 1 | 85.7 ± 2 | 0.1 ± 2 | 0.1 ± 2 | 0.2 ± 2 | 0.2 ± 5 |
| CD4− CD8− | 0.7 ± 0 | 0.7 ± 0 | 72.0 ± 6 | 71.3 ± 9 | 40.3 ± 9 | 39.7 ± 1 |
| CD4+ Fas+ | NT | NT | 1.7 ± 1 | 1.5 ± 0 | 0.2 ± 2 | 0.3 ± 1 |
| CD8+ Fas+ | NT | NT | 0.5 ± 1 | 0.4 ± 1 | 0.3 ± 1 | 0.2 ± 1 |
The values are given as a percentage of cells positive for antibody staining except those for WBC, which are given as number of WBC (107) positive for antibody staining. NT, not tested.
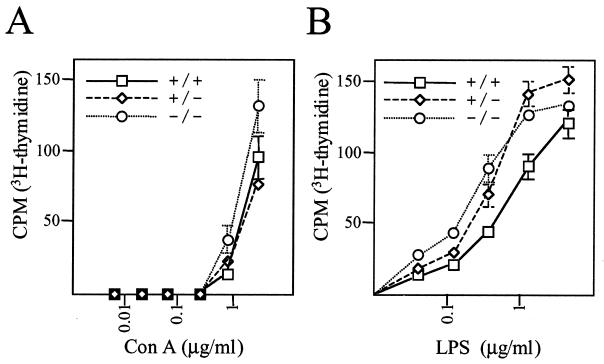
We further analyzed the proliferation of T and B cells activated by ConA or LPS, respectively, in the presence of [3H]thymidine. The T cells derived from LNs were activated by ConA in a dose-dependent manner, and the proliferation of T cells was comparable in TDAG51−/− mice and control littermates (Fig. 2A). The proliferation of B cells was also not significantly different compared to that in their wild-type littermates (Fig. 2B). We concluded that the subpopulations and proliferation of T and B cells are not affected by loss of TDAG51.
FIG. 2.
Proliferation assay. (A) T-cell proliferation assay. LN-derived T cells were incubated with ConA (0 to 5 μg/ml) for 72 h, treated with [3H]thymidine for 6 h, harvested, and counted. (B) B-cell proliferation assay. Spleen-derived cells were incubated with LPS (0 to 25 μg/ml) for 72 h, further cultured with [3H]thymidine for 16 h, harvested, and counted. Experiments were repeated three times; representative results from one experiment are shown. Error bars, standard deviations. Symbols: +/+, wild-type; +/−, heterozygote; −/−, homozygote.
Effect of TDAG51 on Fas expression.
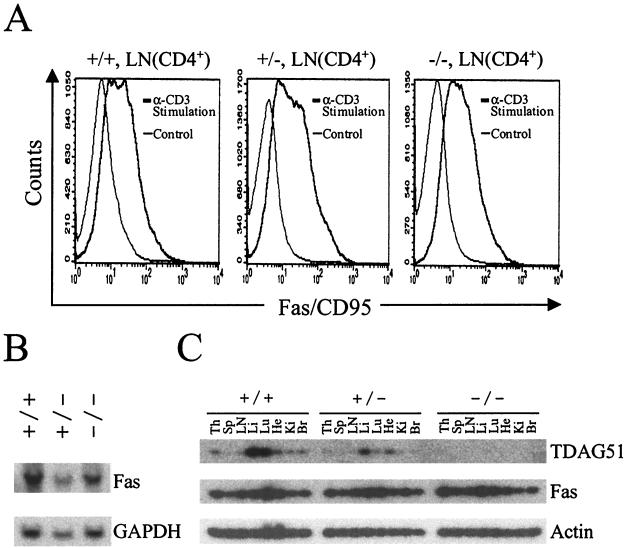
To determine whether TDAG51 is essential for Fas expression, we analyzed the level of Fas expression in TDAG51−/− mice (Fig. 3). Cells derived from LNs and spleens were stained with PE-conjugated anti-Fas antibody or FITC-conjugated anti-CD4 and -CD8 antibodies and analyzed using FACScan flow cytometry (Table 1). Although TDAG51 is a critical factor for the expression of Fas in vitro, the level of Fas expression was not significantly altered in TDAG51−/− mice. Therefore, we further analyzed the Fas expression level in T cells activated by anti-CD3 antibody. However, when LN-derived T cells were activated by anti-CD3 antibody, we did not find any significant difference in Fas expression (Fig. 3A). To test the transcriptional regulation of Fas in TDAG51−/− mice, we analyzed Fas expression by Northern blot analysis (Fig. 3B). The levels of Fas expression in the liver, which is the predominant site of endogenous Fas expression, were very similar in TDAG51−/− mice and wild-type littermates. In addition, we used quantitative RT-PCR to analyze the expression patterns of Fas in other tissues. Fas expression in TDAG51−/− and wild-type mice showed no significant differences in thymus, spleen, LN, liver, lung, kidney, and brain tissues (Fig. 3C). Taken together, we conclude that TDAG51 is not essential for the expression of Fas in vivo.
FIG. 3.
Fas expression in TDAG51−/− mice. (A) Fas expression of T cells stimulated by anti-CD3. LN-derived T cells were stimulated with mouse anti-CD3 antibody for 6 h and stained with PE-conjugated anti-CD95 antibody and FITC-conjugated anti-CD4 antibody. Symbols: +/+, wild-type; +/−, heterozygote; −/−, homozygote. (B) Northern blot analysis of Fas expression. Total RNAs (20 μg) derived from liver were electrophoresed, transferred to nylon membrane, and hybridized with the entire mouse Fas cDNA probe and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe, respectively. (C) Quantitative RT-PCR analysis of Fas expression in tissues. Total RNAs (10 μg) derived from each tissue were prepared and incubated with reverse transcriptase. The cDNA was subjected to quantitative PCR using primers for TDAG51, Fas, or actin. Abbreviations: Th, thymus; Sp, spleen; Li, liver; Lu, lung; He, heart; Ki, kidney; Br, brain.
Apoptosis in TDAG51−/− mice.
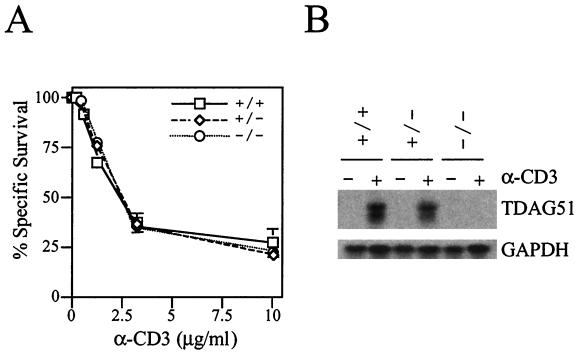
We previously demonstrated that KCIT1-8.5 cells, which express very little TDAG51, are resistant to activation-induced apoptosis, suggesting that TDAG51 might be important for activation-induced apoptosis of T cells (14). To determine the role of TDAG51 in activation-induced apoptosis of T cells in vivo, cells derived from LN were activated with ConA and IL-2 for 48 h and further incubated with IL-2 for 48 h. These T cells are cultured on anti-CD3-coated plates for 48 h and subjected to a cell death assay based on propidium iodide staining. We found no significant differences between TDAG51−/− mice and their wild-type littermates (Fig. 4A), even though TDAG51 was highly activated by anti-CD3 stimulation in wild-type mice but not in TDAG51−/− mice (Fig. 4B). In summary, TDAG51 is not essential for T-cell apoptosis in vivo, even though it plays a key role in apoptosis in vitro.
FIG. 4.
Effect of TDAG51 on anti-CD3 (α-CD3)-induced apoptosis. (A) Apoptosis assay. LN-derived cells were cultured with ConA (5 μg/ml) and IL-2 (10 U/ml) for 48 h, washed, and then further incubated with IL-2 (50 U/ml) for 48 h. These LN-derived T cells were subjected to activation-induced apoptosis on 96-well plates coated with mouse anti-CD3 antibodies (0 to 10 μg/ml) and further cultured for 48 h. After culture, the cells were incubated with propidium iodide (1 μg/ml), and dead cells were counted with a FACScan flow cytometer. Cell viability was measured by a FACScan flow cytometer with propidium iodide solution (1 μg/ml). (B) Northern analysis of TDAG51 activation by anti-CD3 antibody. Total RNA (20 μg) derived from anti-CD3 activated T cells were analyzed with probe 1 (described in the legend to Fig. 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control.
DISCUSSION
We previously cloned the TDAG51 gene cloned by differential display between KMls-8.3.5 T-cell hybridoma and a KCIT1-8.5 mutant that is not susceptible to activation-induced apoptosis due to decreased Fas expression (14). We demonstrated that this KCIT1-8.5 mutant, when transfected with wild-type TDAG51, becomes susceptible to Fas expression and activation-induced apoptosis of T cells, indicating that TDAG51 is a critical regulator of Fas expression and activation-induced apoptosis of T cells in vitro. Evidence from other in vitro studies also suggested that TDAG51 plays a key role in Fas up-regulation and apoptosis in T cells (6, 17). Although these results strongly suggest that TDAG51 has a role in activating Fas expression in vitro, the specific inductive mechanism in vivo had not been identified.
To address this issue, we generated TDAG51−/− mice and tested whether the TDAG51 gene is a key factor in the regulation of Fas expression and apoptosis. TDAG51−/− mice had no gross abnormalities. In contrast, Fas-deficient mice showed dramatic phenotypes in the LN and spleen, with the development of lymphadenopathy and splenomegaly (1, 3, 13). The abnormal T cells, which include Thy1+, B220+, CD4−, and CD8− populations, accumulated in these immune responsive organs of Fas-deficient mice. Based on these results, we expected that TDAG51−/− mice would show a phenotype similar to that of Fas-deficient mice, because TDAG51 is a critical regulator of Fas expression in vitro. Contrary to our expectation, TDAG51−/− mice had no detectable abnormalities in their LN, spleen, or WBC counts. Thus, in vivo, TDAG51 is not necessary for Fas expression and T-cell apoptosis. Therefore, we report here that TDAG51-deficient mice exhibit no obvious phenotype, and TDAG51 is not a critical factor for Fas expression and T-cell apoptosis.
Several possibilities could account for the absence of phenotype in TDAG51−/− mice. One simple explanation is that loss of TDAG51 may be compensated for by other TDAG51 homologs. Recent reports have identified a TDAG51 homolog (IPL/Tssc3/BWR1C) related to parental imprinting (9, 11, 15), which shows ∼44% amino acid identity to TDAG51. IPL (imprinted in placenta and liver), also known as Tssc3, was identified as a parental imprinted gene. The same gene was also identified as BWR1C, as it was identified in a screen of the human chromosome region 11p15.5, near the Beckwith-Wiedemann syndrome locus. Another TDAG51 homolog, Tih1 (TDAG51/IPL homolog 1), which shows ∼44% amino acid identity to TDAG51, is not an imprinted gene (4) and has no clearly identified role in vivo. When the newly finished human genome sequence was blasted with TDAG51, we failed to find additional members other than IPL and Tih1. The answer to whether there are additional members in the TDAG51/IPL/Tih1 family requires, however, further investigation. The generation of mice deficient in other TDAG51 homologs in the future will enable us to ascertain whether TDAG51 is genetically redundant to other homologs that control Fas expression and apoptosis in vivo.
ACKNOWLEDGMENTS
S. Gong and N. Kim contributed equally to this work.
We thank C. G. Park and A. Santana for assistance with plasmid construction and mouse line maintenance, H. W. Lee and D. G. Kim for technical assistance with ES cell culture and flow cytometry, and Jennifer Macke for assistance preparing the text.
This work was supported in part by NIH grant (AI41082 to Y.C.).
REFERENCES
- 1.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 2.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 3.Cohen P L, Eisenberg R, A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 4.Frank D, Mendelsohn C L, Ciccone E, Svensson K, Ohlsson R, Tycko B. A novel pleckstrin homology-related gene family defined by Ipl/Tssc3, TDAG51, and Tih1: tissue-specific expression, chromosomal location, and parental imprinting. Mamm Genome. 1999;10:1150–1159. doi: 10.1007/s003359901182. [DOI] [PubMed] [Google Scholar]
- 5.Gizard F, Lavallee B, DeWitte F, Hum D W. A novel zinc finger protein trep-132 interacts with cbp/p300 to regulate human cyp11a1 gene expression. J Biol Chem. 2001;276:33881–33892. doi: 10.1074/jbc.M100113200. [DOI] [PubMed] [Google Scholar]
- 6.Gomes I, Xiong W, Miki T, Rosner M R. A proline- and glutamine-rich protein promotes apoptosis in neuronal cells. J Neurochem. 1999;73:612–622. doi: 10.1046/j.1471-4159.1999.0730612.x. [DOI] [PubMed] [Google Scholar]
- 7.Gong S, Nussenzweig M C. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 8.Gravestein L A, Borst J. Tumor necrosis factor receptor family members in the immune system. Semin Immunol. 1998;10:423–434. doi: 10.1006/smim.1998.0144. [DOI] [PubMed] [Google Scholar]
- 9.Lee M P, Feinberg A P. Genomic imprinting of a human apoptosis gene homologue, TSSC3. Cancer Res. 1998;58:1052–1056. [PubMed] [Google Scholar]
- 10.Li R. Stimulation of DNA replication in Saccharomyces cerevisiae by a glutamine- and proline-rich transcriptional activation domain. J Biol Chem. 1999;1999 274:30310–30314. doi: 10.1074/jbc.274.42.30310. [DOI] [PubMed] [Google Scholar]
- 11.Muller S, van den Boom D, Zirkel D, Koster H, Berthold F, Schwab M, Westphal M, Zumkeller W. Retention of imprinting of the human apoptosis-related gene TSSC3 in human brain tumors. Hum Mol Genet. 2000;9:757–763. doi: 10.1093/hmg/9.5.757. [DOI] [PubMed] [Google Scholar]
- 12.Munsch D, Watanabe-Fukunaga R, Bourdon J C, Nagata S, May E, Yonish-Rouach E, Reisdorf P. Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J Biol Chem. 2000;275:3867–3872. doi: 10.1074/jbc.275.6.3867. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Park C G, Lee S Y, Kandala G, Lee S Y, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;6:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- 15.Qian N, Frank D, O'Keefe D, Dao D, Zhao L, Yuan L, Wang Q, Keating M, Walsh C, Tycko B. The IPL gene on chromosome 11p15.5 is imprinted in humans and mice and is similar to TDAG51, implicated in Fas expression and apoptosis. Hum Mol Genet. 1997;12:2021–2029. doi: 10.1093/hmg/6.12.2021. [DOI] [PubMed] [Google Scholar]
- 16.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Zhang L, Yin D, Mufson R A, Shi Y. Protein kinase C regulates Fas (CD95/APO-1) expression. J Immunol. 1998;161:2201–2207. [PubMed] [Google Scholar]
- 18.Wong B, Arron J, Choi Y. T cell receptor signals enhance susceptibility to Fas-mediated apoptosis. J Exp Med. 1997;186:1939–1944. doi: 10.1084/jem.186.11.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]