Abstract
Simple Summary
The impact of sarcopenia (i.e., progressive and generalised loss of skeletal muscle mass) and obesity on survival are substantially investigated in cancer patients. However, the relationship between sarcopenia and mortality is quite unclear in patients with lung cancer treated with immunotherapy, while the prognostic value of obesity remains controversial. These issues are potentially related to the obesity paradox and lack of precise measures of body composition on survival. As a result, we aimed to explore the associations between measures of skeletal muscle mass and adiposity (i.e., intramuscular, visceral and subcutaneous adipose tissue) and changes during treatment with disease progression and overall survival in patients with advanced lung cancer receiving immunotherapy. Our results demonstrated that rather than sarcopenia, higher intramuscular and subcutaneous adipose tissue are associated with better prognosis during immunotherapy. These findings are of great importance for clinical practice and may inform specific and tailored therapies to improve immunotherapy prognosis.
Abstract
To investigate the association between skeletal muscle mass and adiposity measures with disease-free progression (DFS) and overall survival (OS) in patients with advanced lung cancer receiving immunotherapy, we retrospectively analysed 97 patients (age: 67.5 ± 10.2 years) with lung cancer who were treated with immunotherapy between March 2014 and June 2019. From computed tomography scans, we assessed the radiological measures of skeletal muscle mass, and intramuscular, subcutaneous and visceral adipose tissue at the third lumbar vertebra. Patients were divided into two groups based on specific or median values at baseline and changes throughout treatment. A total number of 96 patients (99.0%) had disease progression (median of 11.3 months) and died (median of 15.4 months) during follow-up. Increases of 10% in intramuscular adipose tissue were significantly associated with DFS (HR: 0.60, 95% CI: 0.38 to 0.95) and OS (HR: 0.60, 95% CI: 0.37 to 0.95), while increases of 10% in subcutaneous adipose tissue were associated with DFS (HR: 0.59, 95% CI: 0.36 to 0.95). These results indicate that, although muscle mass and visceral adipose tissue were not associated with DFS or OS, changes in intramuscular and subcutaneous adipose tissue can predict immunotherapy clinical outcomes in patients with advanced lung cancer.
Keywords: immunotherapy, cancer, muscle mass, fat mass, overall survival
1. Introduction
In recent years, immune checkpoint inhibitors (ICIs) or immunotherapies, such as nivolumab, pembrolizumab and ipilimumab, have evolved rapidly in medical oncology. The utilisation of ICIs has become a key component for managing a variety of malignancies including lung cancer, resulting in an unprecedented survival advantage over standard therapies such as radiation therapy and chemotherapy. While chemotherapy acts directly on cancer cells inhibiting the cell cycle, ICIs are antibodies targeting the programmed death 1 (PD-1), programmed death-ligand (PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), blocking key regulatory signals that dampen immune responses in the tumour microenvironment. As a result, ICIs counteract immune suppression allowing for tumour reactive T cells to mount an antitumour response utilising the patient’s immune system to target the malignancy [1]. These therapies have shown promising effects in the treatment of lung cancer, as well as a selection of other solid tumours and haematologic malignancies [2,3,4,5].
Several studies have pointed out a significant relationship between immunotherapy and multiple variables on overall survival. Among potential factors, sarcopenia (i.e., progressive and generalised loss of skeletal muscle mass [6]) has emerged as an important prognostic factor in different groups of cancer patients [7]. However, the relationship between sarcopenia and overall survival in patients treated with immunotherapy is still unclear [8,9]. While studies present a significant association between sarcopenia and shorter overall survival [8], others have no significant relationship [9]. For example, in a previous study with small-cell lung cancer patients receiving salvage anti-PD-1 immunotherapy (n = 105), patients presenting with low levels of muscle mass (i.e., sarcopenic patients) had a ~200% greater risk of all-cause mortality compared to those with higher levels of muscle mass [8]. In contrast, there was no difference in overall survival between sarcopenic and non-sarcopenic patients with solid metastatic tumours treated with ICIs (n = 261) [9]. Moreover, the prognostic value of obesity in various malignancies is unknown and remains controversial for the survival of various malignancies [10]. Although previous studies indicated a potential association between body mass index (BMI) and overall survival in advanced cancer patients treated with immunotherapy [11,12], others have demonstrated no significant association between BMI and clinical endpoints [13]. These conflicting results, potentially related to the obesity paradox (i.e., inconsistency concerning the role of obesity on survival), preclude us from understanding the role of fat mass components (i.e., visceral adipose tissue or subcutaneous adipose tissue) on survival in this population [14,15]. For example, while visceral adipose tissue (VAT) secretes various cytokines and cytokine-like factors, which potentially enhance cancer progression [16,17], derived factors from the subcutaneous adipose tissue can increase insulin sensitivity and lipid metabolism potentially resulting in an improved survival [18]. Therefore, although BMI is a much simpler and widely used tool in clinical practice, it does not reflect individual components of body weight such as fat distribution or muscle quantity and quality.
As a result, this study aims to investigate the associations between measures of skeletal muscle mass, intramuscular adipose tissue, subcutaneous adipose tissue, visceral adipose tissue and visceral-to-subcutaneous adipose tissue index and changes throughout treatment with disease progression and overall survival in patients with advanced lung cancer receiving immunotherapy.
2. Materials and Methods
2.1. Study Population
Retrospective analyses of computerised tomography (CT) imaging and electronic medical record data were performed for all patients treated with immunotherapy who presented to Fiona Stanley Hospital, Western Australia between March 2014 and June 2019. A total of 124 patients with lung cancer were identified on immunotherapy. Patients without CT imaging data were excluded from the final cohort, resulting in a total of 97 patients included for further analyses. Demographic, pathological and survival information were obtained via electronic medical record review. The duration of follow-up was 60 months from the first presentation to the date of death for deceased patients or the date of last documented encounter for surviving patients. Demographic and clinical data such as sex, age, BMI, smoking habits, Eastern Cooperative Oncology Group (ECOG) performance status (PS), distant metastases, cancer type, treatment regimens, progression-free survival (PFS) and overall survival (OS) were collected by self-report and medical records, respectively. Our study was approved by the Hospital Ethics Committee (RGS0000003289) and conducted in compliance with the Helsinki Declaration.
2.2. Assessment of Muscle Mass and Fat Mass Parameters
CT scans were at a median of 20 [interquartile range (IQR): 8 to 31] days before commencing immunotherapy treatment. CT scans of the abdomen/pelvis were performed as part of recommended staging pathway and retrieved from the hospital imaging PACS/RIS system (version 6.7.0.6011; Agfa, Mortsel, Belgium). A single 3 mm axial slice through the middle of the L3 vertebral body was retrieved using the sagittal reformatted images with the morphologic L5/S1 junction as reference. These images were imported into SliceOmatic (version 5.0 Rev 12; TomoVision, Magog, QC, Canada) and analysed using the ABACS mode (version 6 Rev-7b; Voronoi Health Analytics, Coquitlam, BC, Canada). If there was an artifact at this level, the nearest artifact-free contiguous slice above or below this level was utilised. A visual colour-coded overlay was reviewed to assess for correct segmentation; any errors were manually corrected using Edit mode and following standard anatomic boundaries. Area measurements (cm2) were obtained by auto-segmentation using the default Hounsfield unit (HU) thresholds and skeletal muscle was determined in the range of −29 to 150 HU, including the skeletal muscle compartment of psoas, paraspinal and abdominal wall musculature. Intramuscular adipose tissue (IMAT) was determined in the range of −190 to −30 HU, visceral adipose tissue (VAT) in the range of −150 to −50 HU and subcutaneous adipose tissue (SAT) in the range of −190 to −30 HU. Visceral-to-subcutaneous adipose tissue ratio was defined as the ratio between VAT and SAT values. Values were normalised to height squared (m2) to derive skeletal muscle, IMAT, VAT, SAT and VAT/SAT indexes.
For further analysis, the skeletal muscle index was analysed as a categorical variable with two levels corresponding to sarcopenia (skeletal muscle index < 43 cm2·m−2 and BMI < 25 kg·m−2, or skeletal muscle index < 53 cm2·m−2 and BMI ≥ 25 kg·m−2) and non-sarcopenia (skeletal muscle index ≥ 43 cm2·m−2 and BMI ≥ 25 kg·m−2, or skeletal muscle index ≥ 53 cm2·m−2 and BMI < 25 kg·m−2), as previously established [19]. Considering the lack of cut-off values for adiposity measures, median values based on our sample were used to categorise patients with higher and lower levels of IMAT, VAT, SAT and VAT/SAT indexes. Relative changes (%) were calculated as , with a threshold of 10% utilised to categorise groups with the lowest and highest index changes throughout treatment.
2.3. Assessment of Outcomes
The primary outcome was overall survival, defined as deaths as a result of any cause, while disease progression defined as an increase in the size of the tumour by 20% was secondary. Vital causes and causes of death were obtained via electronic medical record review. Follow-up time for overall mortality was calculated as the time from CT scans to death from any cause or the end of follow-up (i.e., 60 months following the time of the first scan).
2.4. Statistical Analyses
Analyses were performed using SPSS v.27 (Armonk, IBM Corp., NY, USA) and R Core Team (2013). Differences in overall mortality between groups based on sarcopenia, IMAT, SAT, VAT and VAT/SAT variables were assessed using the Kaplan–Meier method and the log-rank test. Paired-sample t-test was used to compare values between the first and second CT scans during immunotherapy. The hazard ratios (HRs) for the associations of skeletal muscle index, IMAT, SAT, VAT and VAT/SAT ratio indexes with overall mortality and disease progression were estimated in separate models using Cox proportional hazards regression. Logistic regression was used to determine the impact of body composition components on the occurrence of adverse events ≥ grade 2. Odds ratios (ORs) and 95% CIs were reported. Models were adjusted for age, BMI, cancer type and stage. A p-value of ≤0.05 was considered statistically significant and point estimates were presented with 95% confidence interval.
3. Results
3.1. Patient Characteristics
Patient characteristics are presented in Table 1. Patients were 67.5 ± 10.2 years of age (mean ± standard deviation) with a BMI of 26.1 ± 4.9 kg·m−2. Most patients were overweight/obese (60.8%). The majority of patients had adenocarcinoma (62.9%), followed by squamous cell carcinoma (29.9%). Most patients were treated with second line immunotherapy (75.3%). A total of 81 patients were stage IV (84.4%) and had metastatic disease present in more than two sites (22.9%), bone (17.1%), lymph node (8.6%), liver (5.7%), adrenal (2.9%) and brain (2.9%). In this cohort, the most common immunotherapy agent was Nivolumab (58.8%), followed by Pembrolizumab (24.7%) and Atezolumab (16.5%). A total number of 96 patients had disease progression and died during follow-up (99.0%), with median disease progression of 11.3 (IQR: 4.9 to 20.4) months and 15.4 (IQR: 7.2 to 24.0) months, respectively.
Table 1.
Characteristics of patients with lung cancer.
| Characteristics | Patients (n = 97) |
|---|---|
| Age, mean ± SD, years | 67.5 ± 10.2 |
| Males, n (%) | 55 (56.7%) |
| Height, mean ± SD, cm | 168.1 ± 8.7 |
| Weight, mean ± SD, kg | 73.8 ± 15.6 |
| BMI, mean ± SD, kg·m−2 | 26.1 ± 4.9 |
| BMI, categories, n (%) | |
| Underweight | 4 (4.1%) |
| Normal weight | 34 (35.1%) |
| Overweight | 43 (44.3%) |
| Obese | 16 (16.5%) |
| Current smoker, n (%) a | 74 (83.1%) |
| Tumour type, n (%) | |
| Adenocarcinoma | 61 (62.9%) |
| Adeno-squamous | 1 (1.0%) |
| Large cell carcinoma | 2 (2.1%) |
| Poorly differentiated | 3 (3.1%) |
| Pleomorphic | 1 (1.0%) |
| Squamous cell carcinoma | 29 (29.9%) |
| Cancer stage, n (%) a | |
| III | 15 (15.6%) |
| IV | 81 (84.4%) |
| Metastasis location, n (%) a | |
| No metastasis | 14 (40%) |
| Adrenal | 1 (2.9%) |
| Bone | 6 (17.1%) |
| Brain | 1 (2.9%) |
| Liver | 2 (5.7%) |
| Lymph node | 3 (8.6%) |
| ≥2 sites | 8 (22.9%) |
| Treatment line, n (%) | |
| First line treatment | 24 (24.7%) |
| Second line treatment | 73 (75.3%) |
| Treatment, n (%) | |
| Atezolumab | 16 (16.5%) |
| Nivolumab | 57 (58.8%) |
| Pembrolizumab | 24 (24.7%) |
| ECOG, n (%) a | |
| 0 | 49 (51.0%) |
| 1 | 34 (35.5%) |
| 2 | 12 (12.5%) |
| 3 | 1 (1.0%) |
| Neutrophils, mean ± SD | 6.6 ± 4.4 |
| Lymphocytes, mean ± SD | 1.5 ± 0.8 |
| Haemoglobin, mean ± SD | 125.2 ± 20.4 |
| Platelets, mean ± SD | 306.1 ± 122.0 |
| Neutrophils-to-lymphocytes ratio, mean ± SD | 6.9 ± 11.6 |
| PD-L1 levels, median (IQR), % | 60.0 (1.0 to 80.0) |
BMI, Body mass index; ECOG, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; SD, standard deviation; a, missing data: current smoker, n = 8; cancer stage, n = 1; TNM, n = 16; metastasis location, n = 62; ECOG, n = 1.
3.2. Association of Body Composition Components with Disease Progression and Overall Survival
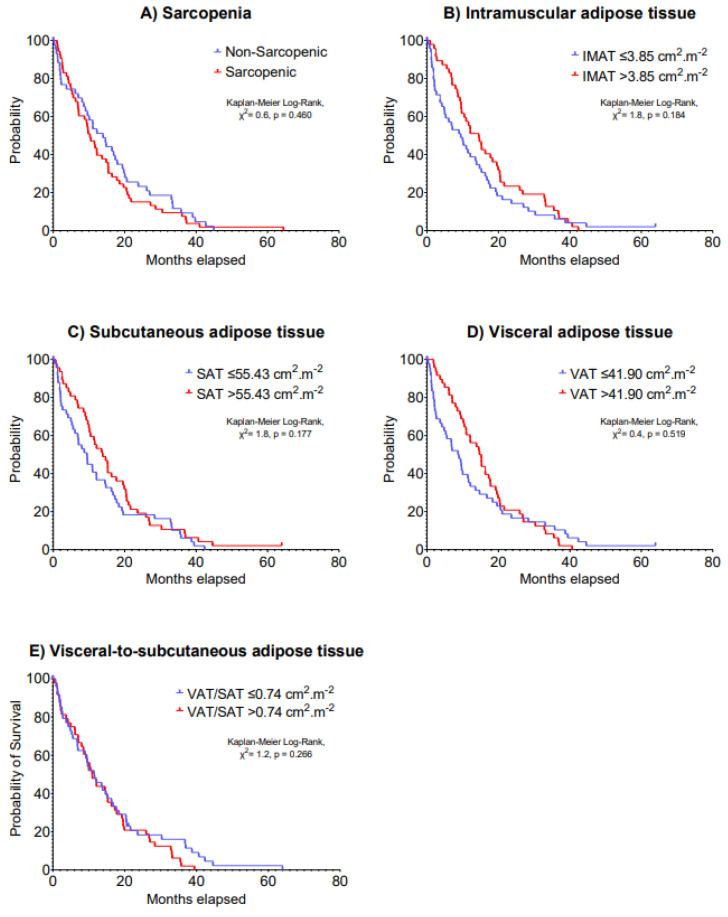
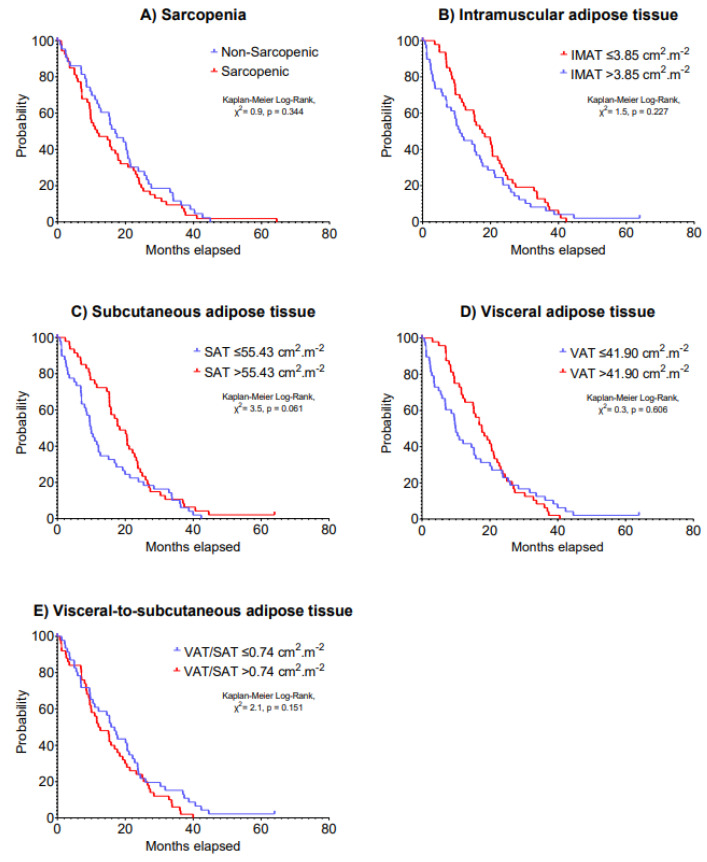
The median IMAT, SAT, VAT and VAT/SAT ratio index values were 3.85, 55.43, 41.90 and 0.74 cm2·m−2, respectively. Multivariable models indicated no significant associations of sarcopenia, IMAT, SAT, VAT and VAT/SAT ratio indexes at baseline with 5-year disease progression (HR: 0.69–1.25, p = 0.199–0.877) and 5-year overall survival (HR: 0.69–1.34, p = 0.123–0.724) in patients with advanced lung cancer undergoing immunotherapy (Table 2). Kaplan–Meier analyses stratifying patients according to body composition components cut-off values on 5-year disease progression and overall survival are presented in Figure 1 and Figure 2, respectively (p = 0.061–0.606).
Table 2.
Hazard ratios and 95% confidence intervals for associations between measures of skeletal muscle, intramuscular adipose tissue, subcutaneous adipose tissue, visceral adipose tissue and visceral-to-subcutaneous adipose tissue indexes and 5-year risk of disease progression and overall survival.
| Variables | N | 5-Year Disease Progression | 5-Year Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Sarcopenia | |||||
| Non-sarcopenic | 44 | Reference | Reference | ||
| Sarcopenic | 53 | 1.22 (0.81 to 1.85) | 0.338 | 1.29 (0.85 to 1.95) | 0.227 |
| Intramuscular adipose tissue index | |||||
| ≤3.85 cm2·m−2 | 49 | Reference | Reference | ||
| >3.85 cm2·m−2 | 48 | 0.83 (0.51 to 1.33) | 0.430 | 0.88 (0.54 to 1.42) | 0.594 |
| Subcutaneous adipose tissue index | |||||
| ≤55.43 cm2·m−2 | 49 | Reference | Reference | ||
| >55.43 cm2·m−2 | 48 | 0.69 (0.43 to 1.10) | 0.199 | 0.69 (0.43 to 1.10) | 0.123 |
| Visceral adipose tissue index | |||||
| ≤41.90 cm2·m−2 | 49 | Reference | Reference | ||
| >41.90 cm2·m−2 | 48 | 0.96 (0.58 to 1.60) | 0.877 | 1.10 (0.66 to 1.83) | 0.724 |
| Visceral-to-Subcutaneous adipose tissue index | |||||
| ≤0.74 cm2·m−2 | 48 | Reference | Reference | ||
| >0.74 cm2·m−2 | 49 | 1.25 (0.82 to 1.90) | 0.305 | 1.34 (0.88 to 2.04) | 0.178 |
All models were adjusted for age, BMI, cancer type and cancer stage. 95% CI, 95% confidence intervals; HR, hazard ratio.
Figure 1.
Kaplan–Meier curves of 5-year disease progression according to (A) sarcopenia, (B) intramuscular adipose tissue, (C) subcutaneous adipose tissue, (D) visceral adipose tissue and (E) visceral-to-subcutaneous adipose tissue at baseline in patients with advanced lung cancer undergoing immunotherapy.
Figure 2.
Kaplan–Meier curves of 5-year overall survival according to (A) sarcopenia, (B) intramuscular adipose tissue, (C) subcutaneous adipose tissue, (D) visceral adipose tissue and (E) visceral-to-subcutaneous adipose tissue at baseline in patients with advanced lung cancer undergoing immunotherapy.
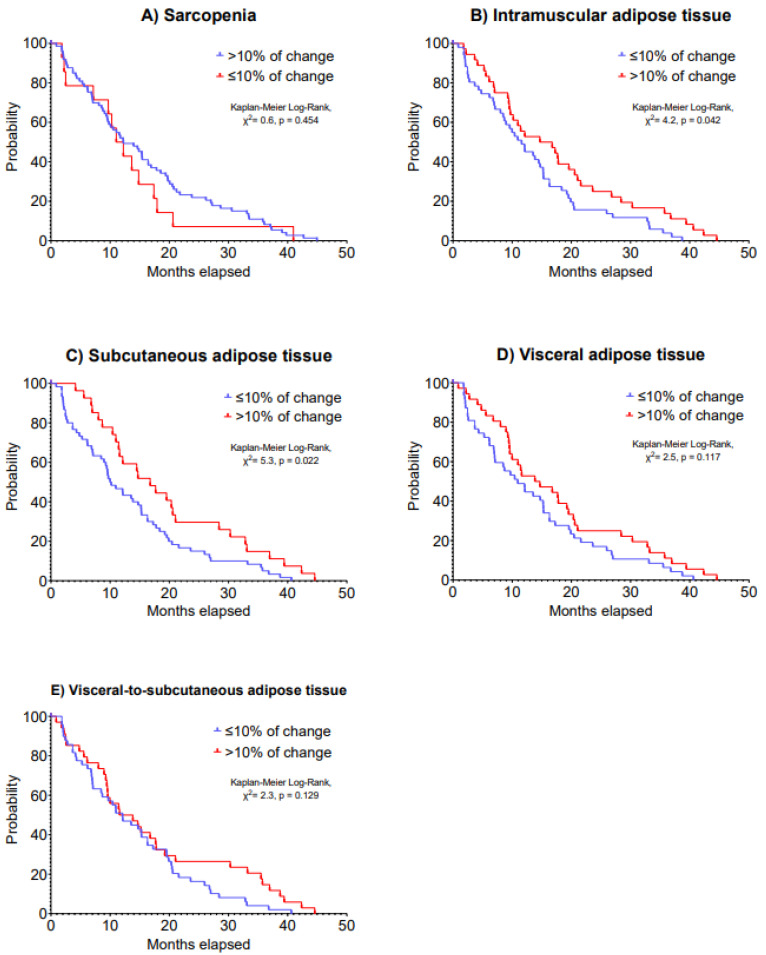
A second CT scan was performed in 88 patients as presented in Table 3. Changes in skeletal muscle, IMAT, SAT, VAT and VAT/SAT ratio indexes were not statistically significant following a median time of 15.4 months after the first CT scan (IQR: 7.1 to 26.5 days). Although changes in sarcopenia, VAT and VAT/SAT ratio indexes were not associated with 5-year disease progression (HR: 0.63–1.24, p = 0.064–0.484), >10% increases in IMAT (HR: 0.60, 95% CI: 0.38 to 0.95) and SAT indexes (HR: 0.59, 95% CI: 0.36 to 0.95) were associated with improved 5-year disease progression (p = 0.028 and 0.029; Table 4). Patients with a >10% increase in IMAT index presented a median disease progression of 15.9 (IQR: 8.8 to 24.6) months vs. 11.7 (IQR: 5.5 to 19.0) months in patients with a ≤10% decrease in IMAT index (Kaplan–Meier Log-Rank, χ2 = 4.2, p = 0.042). Likewise, patients who had a >10% increase in SAT index presented a median disease progression of 16.9 (IQR: 10.8 to 29.6) months vs. 10.2 (IQR: 4.7 to 18.8) months of patients who had a decrease in SAT index (Kaplan–Meier Log-Rank, χ2 = 5.3, p = 0.022). Kaplan–Meier analysis on 5-year disease progression is presented in Figure 3.
Table 3.
Changes in skeletal muscle, intramuscular adipose tissue, subcutaneous adipose tissue, visceral adipose tissue and visceral-to-subcutaneous adipose tissue indexes during immunotherapy.
| Outcomes | n | Baseline | Post-Assessment | Mean Difference |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD (95% CI) |
||
| Skeletal muscle index, cm2·m−2 | 88 | 43.8 ± 8.5 | 43.7 ± 9.4 | −0.1 ± 6.6 (−1.5 to 1.3) |
| Intramuscular adipose tissue index, cm2·m−2 | 88 | 4.5 ± 2.3 | 4.7 ± 2.2 | 0.2 ± 1.3 (−0.1 to 0.5) |
| Subcutaneous adipose tissue index, cm2·m−2 | 88 | 64.2 ± 36.5 | 65.5 ± 38.6 | 1.3 ± 17.5 (−2.4 to 5.0) |
| Visceral adipose tissue index, cm2·m−2 | 88 | 52.5 ± 34.7 | 54.6 ± 36.8 | 2.1 ± 18.9 (−2.0 to 6.2) |
| Visceral-to-subcutaneous adipose tissue ratio, cm2·m−2 | 88 | 1.0 ± 0.7 | 1.0 ± 0.7 | 0.0 ± 0.3 (−0.1 to 0.1) |
Table 4.
Hazard ratios and 95% confidence intervals for associations between changes in measures of skeletal muscle, intramuscular adipose tissue, subcutaneous adipose tissue, visceral adipose tissue and visceral-to-subcutaneous adipose tissue indexes and 5-year risk of disease progression and overall survival.
| Variables | N | 5-Year Disease Progression | 5-Year Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Sarcopenia | |||||
| >10% of change | 74 | Reference | Reference | ||
| ≤10% of change | 14 | 1.24 (0.68 to 2.23) | 0.484 | 1.39 (0.77 to 2.52) | 0.277 |
| Intramuscular adipose tissue index | |||||
| ≤10% of change | 52 | Reference | Reference | ||
| >10% of change | 36 | 0.60 (0.38 to 0.95) | 0.028 | 0.60 (0.37 to 0.95) | 0.031 |
| Subcutaneous adipose tissue index | |||||
| ≤10% of change | 61 | Reference | Reference | ||
| >10% of change | 27 | 0.59 (0.36 to 0.95) | 0.029 | 0.64 (0.39 to 1.03) | 0.066 |
| Visceral adipose tissue index | |||||
| ≤10% of change | 48 | Reference | Reference | ||
| >10% of change | 36 | 0.66 (0.42 to 1.04) | 0.075 | 0.67 (0.43 to 1.07) | 0.093 |
| Visceral-to-Subcutaneous adipose tissue index | |||||
| ≤10% of change | 50 | Reference | Reference | ||
| >10% of change | 34 | 0.63 (0.39 to 1.03) | 0.064 | 0.61 (0.38 to 0.99) | 0.045 |
All models were adjusted for age, BMI, cancer type and cancer stage.
Figure 3.
Kaplan–Meier curves of 5-year disease progression according to changes in (A) sarcopenia, (B) intramuscular adipose tissue, (C) subcutaneous adipose tissue, (D) visceral adipose tissue and (E) visceral-to-subcutaneous adipose tissue in patients with advanced lung cancer undergoing immunotherapy.
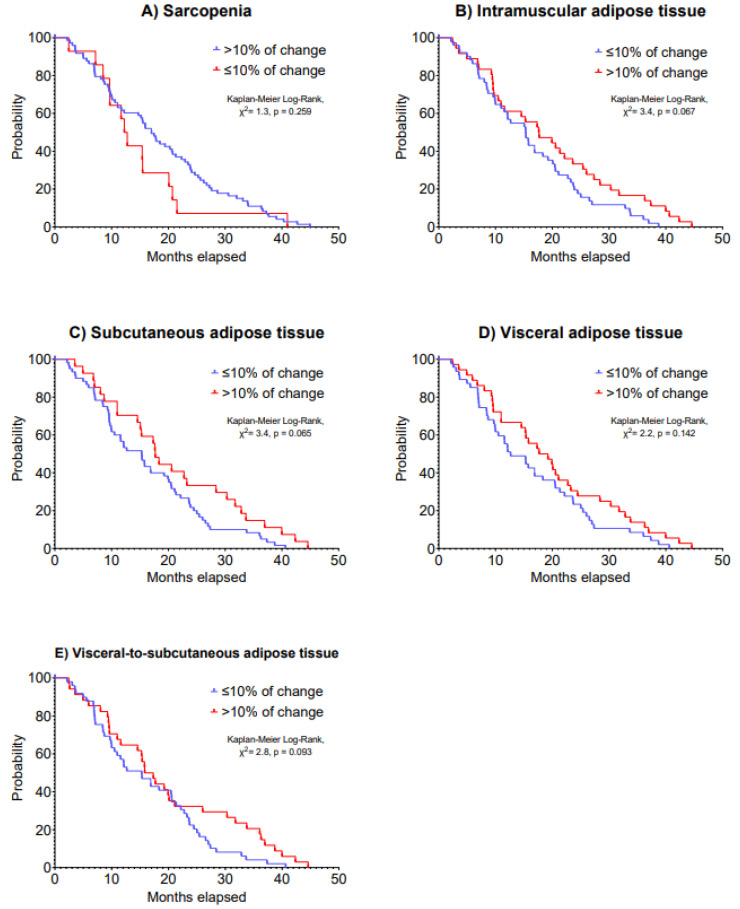
Regarding overall survival, a >10% increase in IMAT was associated with improved 5-year overall survival (HR: 0.60, 95% CI: 0.37 to 0.95, p = 0.031; Table 5). Patients who had an increase of 10% in IMAT presented a median overall survival of 17.8 (IQR: 9.6 to 27.9) months vs. 15.5 (IQR: 8.5 to 23.2) months of patients who had a decrease in this outcome (Kaplan–Meier Log-Rank, χ2 = 3.4, p = 0.067). Kaplan–Meier analysis on 5-year overall survival is presented in Figure 4.
Table 5.
Odds ratio and 95% confidence intervals for associations between measures of skeletal muscle, intramuscular adipose tissue, subcutaneous adipose tissue, visceral adipose tissue and visceral-to-subcutaneous adipose tissue indexes and risk of treatment toxicity.
| Variables | N | Treatment Toxicity | ||
|---|---|---|---|---|
| OR | 95% CI | p-Value | ||
| Sarcopenia | ||||
| Non-sarcopenic | 44 | Reference | ||
| Sarcopenic | 53 | 2.00 | 0.59 to 7.66 | 0.279 |
| Intramuscular adipose tissue index | ||||
| ≤3.85 | 49 | Reference | ||
| >3.85 | 48 | 0.95 | 0.21 to 4.22 | 0.947 |
| Subcutaneous adipose tissue index | ||||
| ≤55.43 | 49 | Reference | ||
| >55.43 | 48 | 1.23 | 0.28 to 5.48 | 0.782 |
| Visceral adipose tissue index | ||||
| ≤41.90 | 49 | Reference | ||
| >41.90 | 48 | 1.35 | 0.30 to 6.50 | 0.696 |
| Visceral-to-Subcutaneous adipose tissue index | ||||
| ≤0.74 | 48 | Reference | ||
| >0.74 | 49 | 1.57 | 0.44 to 5.95 | 0.490 |
All models were adjusted for age, BMI, cancer type and cancer stage.
Figure 4.
Kaplan–Meier curves of 5-year overall survival according to changes in (A) sarcopenia, (B) intramuscular adipose tissue, (C) subcutaneous adipose tissue, (D) visceral adipose tissue and (E) visceral-to-subcutaneous adipose tissue in patients with advanced lung cancer undergoing immunotherapy.
3.3. Association of Body Composition Components with Immune-Related Adverse Events
Thirty-six adverse events (43.4%) were observed during immunotherapy. Of these, a total of 11 grade 2 (13.3%) and 5 grade 3 events (6.0%) were observed. No associations were observed between sarcopenia, IMAT, SAT, VAT and VAT/SAT ratio indexes with high-grade adverse events during immunotherapy (OR: 0.95–2.00, p = 0.279–0.947).
4. Discussion
The present study reported the associations between radiological measures of muscle and adipose tissue with disease progression and overall survival in patients with advanced lung cancer receiving immunotherapy. The main findings were: (i) muscle mass index at the time of or during immunotherapy was not associated with disease progression or overall survival; and (ii) patients with lung cancer presenting with increases of 10% in intramuscular and subcutaneous adipose tissue following treatment were at a ~40% decreased risk of disease progression and overall survival compared to those presenting with lower levels, regardless of age, BMI, cancer type and stage.
The significant association of sarcopenia with poor disease prognosis has been observed in several papers across different types of cancer [20,21,22]. Interestingly, the majority of studies reporting such findings in the field of immunotherapy were undertaken in patients with lung cancer [8,20,23,24,25,26]. As far as we know, this is one of the few studies [25] undertaken in patients with lung cancer mainly with adenocarcinoma and squamous cell carcinoma (~93% of the sample). Our study indicates that sarcopenia is not significantly associated with disease progression or overall survival in this population with advanced cancer receiving immunotherapy. As observed in our results, the presence of sarcopenia at the start of immunotherapy or a reduction of 10% in skeletal muscle mass index were not associated with disease progression and mortality. However, this result disagrees with previous studies undertaken in patients mainly with non-squamous lung cancer [8,26], which indicate that tumour histology may affect the interaction between sarcopenia and immunotherapy in patients with advanced lung cancer. Nevertheless, lower levels of muscle mass may still affect other important components of immunotherapy such as inflammation, cachexia and physical disability. Consequently, more research is required to elucidate the importance of sarcopenia for other important clinical measures.
The investigation of obesity in immunotherapy is challenging given the confounding factors associated with the obesity paradox [15] and its role in cancer dynamics [27,28]. We observed that intramuscular and subcutaneous adipose tissue could be a predictive marker for improved survival when increased throughout the treatment course. The subcutaneous adipose tissue derives a range of factors such as leptin that could act to improve insulin sensitivity and lipid metabolism [17,18,29]. As a result, this could potentially increase overall survival in this group of patients and represent an important measure during the cancer survivorship. However, the result that an increase in intramuscular adipose tissue could improve survival was unexpected. While previous studies identified a significant association of intramuscular adipose tissue with shorter survival in women with non-metastatic breast cancer [30] and men with hormone-sensitive prostate cancer [31], others did not observe a significant association in metastatic breast cancer [32] or advanced non-small-cell lung cancer treated with immunotherapy [33,34]. Moreover, previous studies have demonstrated that increased intramuscular fat is related to increased frailty and sarcopenia [35] and impaired physical function [36]. In addition, others indicate that increased intramuscular fat is associated with poor survival and increased risk of hospitalisation in older adults or critically ill patients [37,38]. Therefore, the interaction between intramuscular fat and immunotherapy is yet to be determined in this setting.
Interestingly, we also observed an unexpectedly longer 5-year disease progression compared to other large immunotherapy randomised controlled studies [39,40,41,42]. While we observed a median disease progression time of 11.3 months, a range of 3.0 to 5.0 months was reported in these trials [39,40,41,42]. The reasons are likely multifactorial and related to our smaller sample size and retrospective nature compared to these large randomised controlled trials [39,40,41,42]. Additionally, we observed high PDL1% values in our sample (median of 60%) and this may also account to a long disease progression as PDL1% is associated with improved survival even when using monotherapy agents in advanced non-small-cell lung cancer. Other factors such as mixed cancer stages (~16% stage III) and treatment line (~25% first treatment line) are different than these previous immunotherapy trials [39,40,41,42] and may affect disease progression. Our cohort also presented more favourable histology (i.e., adenocarcinoma) and tumour burden may be different as 40% did not present distant metastasis. These factors may play a role in disease progression.
Some limitations are worthy of comment. The retrospective nature of the study and the heterogeneity of CT scans may limit our ability to extrapolate our findings to a large scale. Future studies should undertake prospective models to assess the influence of body composition changes on clinical endpoints, as well as reporting the time of body composition assessment. In addition, the lack of standardisation (i.e., cut-off values), and this is due to variability in underlying technique without clear standardisation, makes comparison difficult to assess radiological measures of muscle and adipose tissue and affects our ability to provide more meaningful recommendations based on our findings. Although the use of body composition is promising, critical and technical studies are required to understand the relationship of sarcopenia with clinical endpoints and to inform specific and tailored interventions in patients treated with immunotherapy. Finally, we could not estimate the impact of sarcopenic obesity in our sample. This is an emergent topic in oncology given the high risk of mortality and severe complications experienced by patients during systemic and surgical cancer treatments. Future studies are required to investigate the impact of sarcopenic obesity in lung cancer patients during immunotherapy and identify clinical management strategies for this population.
5. Conclusions
In conclusion, our findings are that rather than muscle mass and visceral adipose tissue, changes in intramuscular and subcutaneous adipose tissue can predict immunotherapy clinical outcomes regardless of age, BMI, cancer type and stage. This result provides new insights into the assessment of body composition in patients with advanced lung cancer undergoing immunotherapy. Consequently, future research should seek to assess a larger sample size of patients undergoing immunotherapy to further elucidate the influence of body composition, specifically monitoring intramuscular and subcutaneous adipose tissues.
Acknowledgments
Pedro Lopez is supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) in Prostate Cancer Survivorship Scholarship. We also acknowledge the Fiona Stanley Hospital Clinic Trials Unit for supporting the imaging software Sliceomatic/ABACS.
Author Contributions
Conceptualization, A.K. (Azim Khan) and A.K. (Adnan Khattak); methodology, A.K. (Azim Khan), C.J.W., P.L., F.S. and A.K. (Adnan Khattak); formal analysis, A.K. (Adnan Khattak), C.J.W., P.L. and F.S.; data curation, A.K. (Azim Khan), C.J.W., A.A., S.O., A.R., S.A., P.L., F.S. and A.K. (Adnan Khattak); writing—original draft preparation, A.K. (Azim Khan), C.J.W., A.A., S.O., A.R., S.A., P.L., F.S. and A.K. (Adnan Khattak); writing—review and editing, A.K. (Azim Khan), C.J.W., A.A., S.O., A.R., S.A., P.L., F.S. and A.K. (Adnan Khattak). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Hospital Ethics Committee of Fiona Stanley Hospital (RGS0000003289).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in the present study will be made available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Role of funding source
Sponsors were not involved in the study design, analysis or interpretation of data, manuscript writing and decision to submit the manuscript for publication.
Funding Statement
No financial support was received to conduct the present study, or for the preparation or publication of this manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 2.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahoney K.M., Freeman G.J., McDermott D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin. Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng W., Fu D., Xu F., Zhang Z. Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis. 2018;7:2. doi: 10.1038/s41389-017-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams G.R., Dunne R.F., Giri S., Shachar S.S., Caan B.J. Sarcopenia in the Older Adult With Cancer. J. Clin. Oncol. 2021;39:2068–2078. doi: 10.1200/JCO.21.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chindapasirt J. Sarcopenia in Cancer Patients. Asian Pac. J. Cancer Prev. 2015;16:8075–8077. doi: 10.7314/APJCP.2015.16.18.8075. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Chen P., Huang J., Liu M., Peng D., Li Z., Chen T., Hong S., Zhou Y. Assessment of sarcopenia as a predictor of poor overall survival for advanced non-small-cell lung cancer patients receiving salvage anti-PD-1 immunotherapy. Ann. Transl. Med. 2021;9:1801. doi: 10.21037/atm-21-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haik L., Gonthier A., Quivy A., Gross-Goupil M., Veillon R., Frison E., Ravaud A., Domblides C., Daste A. The impact of sarcopenia on the efficacy and safety of immune checkpoint inhibitors in patients with solid tumours. Acta Oncol. 2021;60:1597–1603. doi: 10.1080/0284186X.2021.1978540. [DOI] [PubMed] [Google Scholar]
- 10.Xiao J., Mazurak V.C., Olobatuyi T.A., Caan B.J., Prado C.M. Visceral adiposity and cancer survival: A review of imaging studies. Eur. J. Cancer Care. 2018;27:e12611. doi: 10.1111/ecc.12611. [DOI] [PubMed] [Google Scholar]
- 11.Hu J.B., Ravichandran S., Rushing C., Beasley G.M., Hanks B.A., Jung S.H., Salama A.K.S., Ho L., Mosca P.J. Higher BMI, But Not Sarcopenia, Is Associated With Pembrolizumab-related Toxicity in Patients With Advanced Melanoma. Anticancer Res. 2020;40:5245–5254. doi: 10.21873/anticanres.14528. [DOI] [PubMed] [Google Scholar]
- 12.Collet L., Delrieu L., Bouhamama A., Crochet H., Swalduz A., Nerot A., Marchal T., Chabaud S., Heudel P.E. Association between Body Mass Index and Survival Outcome in Metastatic Cancer Patients Treated by Immunotherapy: Analysis of a French Retrospective Cohort. Cancers. 2021;13:2200. doi: 10.3390/cancers13092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crombé A., Kind M., Toulmonde M., Italiano A., Cousin S. Impact of CT-based body composition parameters at baseline, their early changes and response in metastatic cancer patients treated with immune checkpoint inhibitors. Eur. J. Radiol. 2020;133:109340. doi: 10.1016/j.ejrad.2020.109340. [DOI] [PubMed] [Google Scholar]
- 14.Brown J.C., Cespedes Feliciano E.M., Caan B.J. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: Facts and numbers. J. Cachexia Sarcopenia Muscle. 2018;9:1200–1208. doi: 10.1002/jcsm.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D.H., Giovannucci E.L. The obesity paradox in cancer: Epidemiologic insights and perspectives. Curr. Nutr. Rep. 2019;8:175–181. doi: 10.1007/s13668-019-00280-6. [DOI] [PubMed] [Google Scholar]
- 16.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 17.Ebadi M., Baracos V.E., Bathe O.F., Robinson L.E., Mazurak V.C. Loss of visceral adipose tissue precedes subcutaneous adipose tissue and associates with n-6 fatty acid content. Clin. Nutr. 2016;35:1347–1353. doi: 10.1016/j.clnu.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Ebadi M., Martin L., Ghosh S., Field C.J., Lehner R., Baracos V.E., Mazurak V.C. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br. J. Cancer. 2017;117:148–155. doi: 10.1038/bjc.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin L., Birdsell L., Macdonald N., Reiman T., Clandinin M.T., McCargar L.J., Murphy R., Ghosh S., Sawyer M.B., Baracos V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 20.Buentzel J., Heinz J., Bleckmann A., Bauer C., Röver C., Bohnenberger H., Saha S., Hinterthaner M., Baraki H., Kutschka I., et al. Sarcopenia as Prognostic Factor in Lung Cancer Patients: A Systematic Review and Meta-analysis. Anticancer Res. 2019;39:4603–4612. doi: 10.21873/anticanres.13640. [DOI] [PubMed] [Google Scholar]
- 21.Borggreve A.S., den Boer R.B., van Boxel G.I., de Jong P.A., Veldhuis W.B., Steenhagen E., van Hillegersberg R., Ruurda J.P. The Predictive Value of Low Muscle Mass as Measured on CT Scans for Postoperative Complications and Mortality in Gastric Cancer Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020;9:199. doi: 10.3390/jcm9010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez P., Newton R.U., Taaffe D.R., Singh F., Buffart L.M., Spry N., Tang C., Saad F., Galvão D.A. Associations of fat and muscle mass with overall survival in men with prostate cancer: A systematic review with meta-analysis. Prostate Cancer Prostatic Dis. 2022;25:615–626. doi: 10.1038/s41391-021-00442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenuta M., Gelibter A., Pandozzi C., Sirgiovanni G., Campolo F., Venneri M.A., Caponnetto S., Cortesi E., Marchetti P., Isidori A.M., et al. Impact of Sarcopenia and Inflammation on Patients with Advanced Non-Small Cell Lung Cancer (NCSCL) Treated with Immune Checkpoint Inhibitors (ICIs): A Prospective Study. Cancers. 2021;13:6355. doi: 10.3390/cancers13246355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouez M., Delrieu L., Bouleuc C., Girard N., Raynard B., Marchal T. Association between Nutritional Status and Treatment Response and Survival in Patients Treated with Immunotherapy for Lung Cancer: A Retrospective French Study. Cancers. 2022;14:3439. doi: 10.3390/cancers14143439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S., Liu Z., Ren Y., Liu J., Lv S., He P., Yang Y., Sun Y., Chang J., Luo D., et al. Sarcopenia Was a Poor Prognostic Predictor for Patients With Advanced Lung Cancer Treated With Immune Checkpoint Inhibitors. Front. Nutr. 2022;9:900823. doi: 10.3389/fnut.2022.900823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishioka N., Uchino J., Hirai S., Katayama Y., Yoshimura A., Okura N., Tanimura K., Harita S., Imabayashi T., Chihara Y., et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2019;8:450. doi: 10.3390/jcm8040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlandella R.M., Bail J.R., Behring M., Halilova K.I., Johnson R., Williams V., Norian L.A., Demark-Wahnefried W. Body fat indices and survival in immunotherapy-treated patients with cancer. Cancer. 2020;126:3156–3157. doi: 10.1002/cncr.32889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lysaght J. The ‘obesity paradox’ in action with cancer immunotherapy. Nat. Rev. Endocrinol. 2019;15:132–133. doi: 10.1038/s41574-019-0161-2. [DOI] [PubMed] [Google Scholar]
- 29.Porter S.A., Massaro J.M., Hoffmann U., Vasan R.S., O’Donnel C.J., Fox C.S. Abdominal subcutaneous adipose tissue: A protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caan B.J., Cespedes Feliciano E.M., Prado C.M., Alexeeff S., Kroenke C.H., Bradshaw P., Quesenberry C.P., Weltzien E.K., Castillo A.L., Olobatuyi T.A., et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheean P.M., O’Connor P., Joyce C., Vasilopoulos V., Badami A., Stolley M. Clinical Features and Body Composition in Men with Hormone-Sensitive Metastatic Prostate Cancer: A Pilot Study Examining Differences by Race. Prostate Cancer. 2022;2022:9242243. doi: 10.1155/2022/9242243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rier H.N., Jager A., Sleijfer S., van Rosmalen J., Kock M., Levin M.D. Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2018;168:95–105. doi: 10.1007/s10549-017-4574-0. [DOI] [PubMed] [Google Scholar]
- 33.Minami S., Ihara S., Tanaka T., Komuta K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients With Advanced Non-Small Cell Lung Cancer. World J. Oncol. 2020;11:9–22. doi: 10.14740/wjon1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolte F.J., McTavish S., Wakefield N., Shantzer L., Hubbard C., Krishnaraj A., Novicoff W., Gentzler R.D., Hall R.D. Association of sarcopenia with survival in advanced NSCLC patients receiving concurrent immunotherapy and chemotherapy. Front. Oncol. 2022;12:986236. doi: 10.3389/fonc.2022.986236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus R.L., Addison O., Dibble L.E., Foreman K.B., Morrell G., Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012;2012:629637. doi: 10.1155/2012/629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilton T.N., Tuttle L.J., Bohnert K.L., Mueller M.J., Sinacore D.R. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys. Ther. 2008;88:1336–1344. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthon P.M., Fox K.M., Gandra S.R., Delmonico M.J., Chiou C.F., Anthony M.S., Sewall A., Goodpaster B., Satterfield S., Cummings S.R., et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Looijaard W.G., Dekker I.M., Stapel S.N., Girbes A.R., Twisk J.W., Oudemans-van Straaten H.M., Weijs P.J. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care. 2016;20:386. doi: 10.1186/s13054-016-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbst R.S., Baas P., Kim D.W., Felip E., Pérez-Gracia J.L., Han J.Y., Molina J., Kim J.H., Arvis C.D., Ahn M.J., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 41.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazandjian D., Suzman D.L., Blumenthal G., Mushti S., He K., Libeg M., Keegan P., Pazdur R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist. 2016;21:634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the present study will be made available upon request to the corresponding author.