Abstract
Background
To assess the persistence of neighborhood-level lead poisoning disparities in Rhode Island.
Methods
Rhode Island Department of Health blood lead levels (BLL) collected from 2006–2019 were linked to census block group rates of poverty and housing built pre-1950. We computed multivariate logistic regression models of elevated BLLs (≥5 µg/dL and ≥10 µg/dL).
Results
Of the 197,384 study children, 12.9% had BLLs ≥5 µg/dL and 2.3% had BLLs ≥10 µg/dL. The proportion of children with BLL ≥ 5 µg/dL increased across quintiles of poverty and old housing. The odds ratio for highest quintiles was 1.44 (95% CI: 1.29, 1.60) and 1.92 (95% CI: 1.70, 2.17) for poverty and pre-1950 housing, respectively. A significant temporal decline was observed for BLL ≥ 5 µg/dL (2006: 20.5%, 2019: 3.6%). Disparities narrowed over the study period across quintiles of poverty and old housing with a similar trend appearing in the proportion of children with BLL ≥ 10 µg/dL.
Conclusion
Despite tremendous progress in reducing lead exposure, substantial neighborhood disparities in lead poisoning persist. These findings provide valuable considerations for primary childhood lead exposure prevention.
Impact
Through linkage of Rhode Island Department of Health childhood lead poisoning and census data, this study captures neighborhood-level disparities in lead poisoning from 2006–2019.
This study demonstrates that the odds of lead poisoning increased in a stepwise fashion for neighborhood quintiles of poverty and housing built pre-1950. While the magnitude of lead poisoning disparities narrowed across quintiles of poverty and old housing, disparities persist.
Children’s exposure to sources of lead contamination continues to be an important public health concern. The burden of lead poisoning is not equally distributed among all children or communities.
Introduction
Lead exposure continues to be an important public health issue for children, especially those under the age of six. There is robust scientific evidence that elevated blood lead levels (BLL) are associated with adverse effects in infants and children.1–4 However, despite the known dangers posed by lead contamination, an estimated 3.6 million American homes have significant lead-based paint hazards.4,5 Lead laden dust and paint chips from deteriorating lead-based paint on interior surfaces represent the most common source of lead exposure among young children.6 Young children are particularly vulnerable to environmental hazards specifically lead-laden dust in their environment due to frequent hand-to-mouth exploratory behaviors4 On a population level, the social and economic costs of childhood lead poisoning are significant.6,7
The Centers for Disease Control and Prevention (CDC) named the prevention of childhood lead poisoning as one of the “Ten Great Public Health Achievements—United States, 2001–2010.”8 National policies enacted in the 1970s and 1980s focused on primary prevention of lead poisoning by prohibiting lead in gasoline, residential paint, and plumbing.6,9,10 Further national and state policies were implemented in subsequent decades; by 2010, 23 states enacting comprehensive lead prevention laws. These policy initiatives have been a tremendous public health success: national BLLs have decreased dramatically since the 1970s. However, despite these gains, lead poisoning remains a threat to the healthy development of children, and disparities in elevated BLLs persist, with vulnerable groups being disproportionately impacted. Several individual risk factors are attributed to childhood lead poisoning such as earning a household income at or below the poverty threshold or living in housing built pre-1950.11–13 In 2012, the Advisory Council on Childhood Lead Poisoning Prevention convened by the CDC cited new scientific data indicating that there is no known lower threshold for lead-based health effects. In response, the CDC lowered its “level of concern” for lead exposure from ≥10 µg/dL to a reference value of 5 µg/dL, based on the 97.5th percentile of the National Health and Nutrition Examination Survey (NHANES)’s blood lead distribution in children.14 In this action, the CDC recommended that a blood lead level ≥5 µg/dL should prompt public health actions and also emphasized the importance of primary prevention efforts given the lack of an identified threshold for deleterious neurodevelopmental effects.14
The burden of lead poisoning is not equally distributed among all children or communities. Research has demonstrated substantial disparities in risk of lead poisoning across neighborhoods. Specifically, research has shown that children living in poverty and in communities with old housing stock are at greater risk for lead poisoning.5,6,15,16 Less is known about whether these associations are mitigating over time. Given the continuing declines in lead poisoning rates, we undertook a statewide spatial analysis to examine the extent to which neighborhood-level disparities in lead poisoning persist and to assess trends in those disparities over time.
Methods
Study population
We have employed similar methodology of this database and aspects of this lead poisoning analysis in an earlier manuscript detailing lead poisoning rates in Rhode Island from 1993 through 2005.15,17 In brief, the Rhode Island Department of Health (RIDOH) requires universal blood lead testing for children aged 9 to 72 months and records all blood lead test results in the Lead Elimination Surveillance System. Building upon previously reported Rhode Island data, we examined test results for all children up to 72 months of age for January 1, 2006 through December 31, 2019. For analyses, we included the highest test result for each child. In the case of ties, we attributed the results to the age of the first test result.
Lead poisoning rates
In accordance with current national and Rhode Island criteria, any child with a BLL ≥ 5 μg/dL at his or her highest blood lead test, by either capillary or venous testing, was defined as having an elevated BLL. We also categorized those with BLL ≥ 10 μg/dL as having very elevated BLLs. Capillary testing can lead to falsely elevated levels because of environmental contamination of the hand; however, research has shown that capillary testing is highly correlated with venous testing.18–20
Neighborhood-level exposures
We obtained home addresses of patients at the time of testing and geocoded these using ArcGIS v.10.7 (Esri, Redlands, CA) with 80% spelling sensitivity and a composite locator we created using RIGIS’ E911 sites,21 E911 Roads,22 and TIGER Roads.23 A manual review was performed to geocode unmatched addresses. If the address was unable to be geocoded to a Rhode Island address, the child was excluded from the study sample. Based on geographic location, the geocoded addresses were assigned to one of the 812 census block groups in Rhode Island. The census block group is the smallest aggregated geographic unit for which American Community Survey socioeconomic and housing data are available; it generally contains 600 to 3000 people.24 Children living in block groups with fewer than 20 children tested during the study period were excluded from study analyses because of confidentiality concerns.15,17
Block group measures of the percentage of individuals living in poverty and the percentage of housing units built before 1950 were obtained from the 2014–2018 American Community Survey. Block groups were ranked into quintiles based on each of these characteristics. Previous research on childhood lead poisoning25 has demonstrated that the single census variable “percentage of persons below poverty” acts as well as composite measures of economic deprivation. The proportion of housing units built pre-1950 was used due to its validated positive association both on an individual and a neighborhood level with childhood lead poisoning.26 Following regulations in 1978, the lead content of paint was reduced to below trace amounts nationwide. As early as the 1950s, some cities enacted legislation on lead-based paint, and some manufacturers initiated voluntary reduction of lead-based paint concentrations.9 As a result, paint manufactured after the 1950s tended to have lower concentrations of lead compared to those manufactured earlier.
Spatial analysis
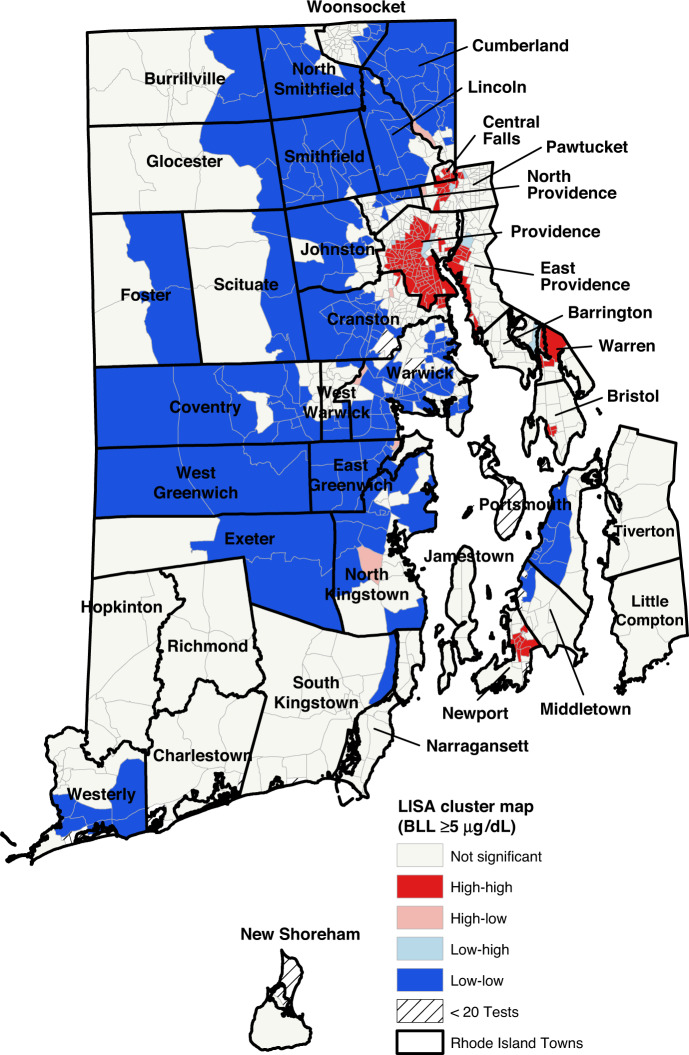
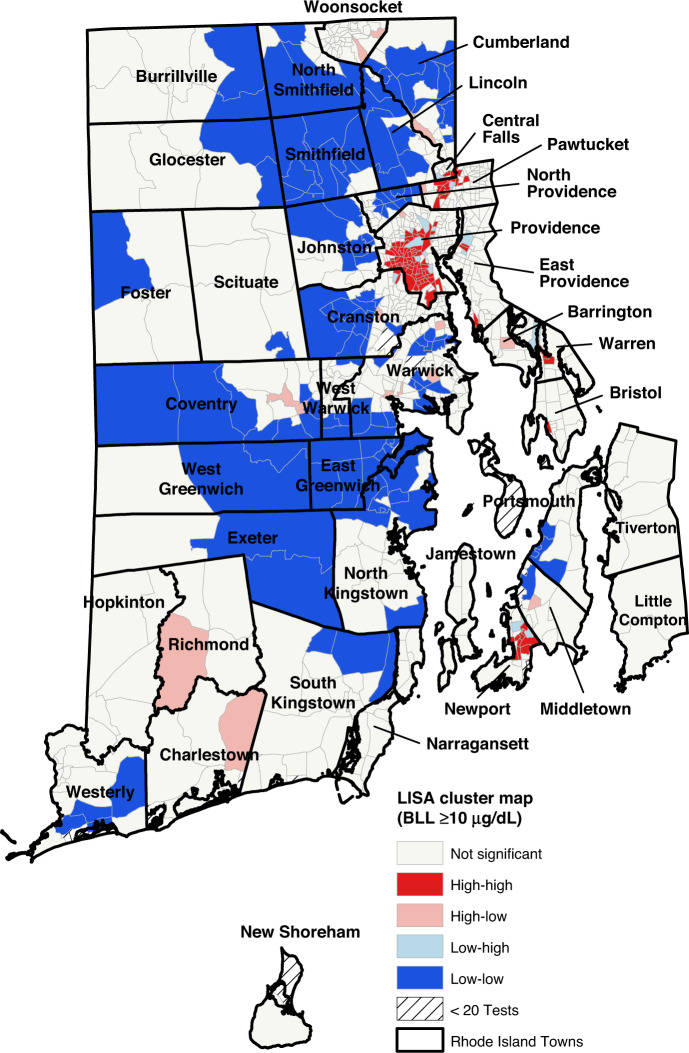
We conducted an exploratory spatial data analysis using ArcGIS and GeoDa v.1.16. We mapped the geographic dissemination of childhood lead poisoning and each neighborhood factor by producing choropleth maps. Choropleth maps use graduated color to represent the block groups in each quintile of neighborhood characteristics. We used a univariate Moran’s I statistic, a global measure of spatial autocorrelation to determine if lead poisoning rates were significantly clustered in a given area versus a random spatial distribution. This statistic of spatial autocorrelation can be interpreted similarly to a correlation coefficient. Values range from −1 (meaning block groups with similar characteristics tend to be uniformly dispersed) to 1 (meaning block groups with similar characteristics tend to be clustered near one another). We created a Local Indicators of Spatial Association (LISA) cluster map, to demonstrate the clustering and dispersion of childhood lead poisoning throughout the state. Block groups designated as high-high or low-low mean that the block group has high (or low) BLL and is neighbored by other block groups with high (or low) BLL. A high-low or low-high designation means that, respectively, a high BLL group is neighbored by low BLL block groups or a low BLL group is neighbored by high BLL block groups. High-high (hot-spotting) and low-low (cool-spotting) zones show areas of clustering, while high-low and low-high zones highlight outliers in the data. Both the LISA cluster map and the univariate Moran’s I statistic used the numerical BLL rather than the quintile values.
To account for the spatial effects of lead exposure across neighboring block groups, a spatial lag term was created using GeoDa. The spatial lag is defined as the value of a variable in a block group multiplied by a weighted average of the values observed in all neighboring locations to that block group. To create these spatial statistics, spatial weights were first calculated for every block group in the data. Queen contiguity (polygons sharing an edge or a corner are included) was used in establishing the weights. To create the spatial lag variable for those with BLLs ≥5 μg/dL, the percent of tests in a block group that had BLLs ≥5 μg/dL was multiplied by the weighted average of the percentages of all neighboring block groups. This process was repeated for tests with BLLs ≥10 μg/dL. It is important to include this spatial lag term in the model, to determine if the results are due to a possible diffusion process – for example, such as in infectious disease processes when events in one place predict an increased likelihood of similar events in neighboring places.
Statistical analysis
We examined the prevalence of elevated BLLs (≥5 μg/dL and ≥10 μg/dL) overall, as well as by the child’s sex and age at the time of the test and the test method. The Cochran-Armitage test was used to analyze overall trends over time and time trends by quintiles of neighborhood attributes. We assessed the proportion of children with elevated BLLs by quintiles of poverty and proportion of pre-1950 housing. We computed crude and adjusted multivariate models of the risk of elevated BLLs. Two sets of multivariate models were computed, the first adjusting for sex, age at test, test method, year, and clustering by block group, the second adding a term for the spatial effects of lead exposure. Models included both population in poverty and pre-1950 housing. All analyses were performed using SAS v.9.4. The Institutional Review Boards of Brown University and RIDOH reviewed and approved this study.
Results
Over the 14-year study period, 199,564 children had one or more blood lead tests. Of these, 11 children were excluded because their lead levels were measured from cord blood (n = 5) or their date of test preceded their date of birth from a data entry error (n = 6). An additional 2071 children (1.0%) were excluded because their addresses could not be geocoded. The primary reasons for not being able to geocode addresses were that the addresses were missing/unknown or incomplete (n = 101); recorded as a post office box (n = 900); not in Rhode Island (n = 29); or listed as an administrative office (n = 357). After manual review, the remaining 683 addresses were unable to be geocoded for unknown reasons. Finally, 13 block groups were excluded as less than 20 children in those block groups had blood lead testing results. Accordingly, an additional 106 individuals were excluded as he or she resided in one of those block groups.
Included in our analysis were 197,384 children (98.9% of the original sample) living in 797 block groups, with 12.9% of whom had a BLLs ≥5 µg/dL and 2.3% of whom had BLLs ≥10 µg/dL (Table 1). Males were more likely than females to have elevated BLLs (BLL ≥ 5 µg/dL: males = 13.6% vs. females = 12.2%, p < 0.0001; BLL ≥ 10 µg/dL: males = 2.6% vs. female = 2.1%, p < 0.0001). Of all tests, 23.0% of all highest tests were capillary, compared to 77% venous. Capillary tests compared to venous tests were more likely to yield elevated results (BLL ≥ 5 µg/dL: capillary = 16.5% vs. venous = 11.8%, p < 0.0001; BLL ≥ 10 µg/dL: capillary = 3.1% vs. venous = 2.1%, p < 0.0001). The proportion of children with elevated BLLs varied by age at the highest blood lead test. The proportion of children with BLL ≥ 5 µg/dL increased from 7.4% among children living in block groups with the lowest quintile of poverty to 18.4% for those in the highest quintile (Table 1). The proportion of children with BLL ≥ 5 µg/dL increased from 6.0% among those living in block groups with the lowest quintile of pre-1950 housing to 19.5% for those in the highest quintile (Table 1).
Table 1.
Percentage of children in Rhode Island’s Statewide Lead Elimination Surveillance System, 2006–2019, with elevated blood lead levels.
| ≥5 µg/dL | ≥10 µg/dL | |||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| N = 171,931 (87.1%) | N = 25,453 (12.9%) | N = 192,752 (97.7%) | N = 4632 (2.3%) | |
| Sex | ||||
| Female | 87.8 | 12.2 | 97.9 | 2.1 |
| Male | 86.4 | 13.6 | 97.4 | 2.6 |
| Test method | ||||
| Capillary | 83.5 | 16.5 | 96.9 | 3.1 |
| Venous | 88.2 | 11.8 | 97.9 | 2.1 |
| Age at test | ||||
| 0–12 months | 92.4 | 7.6 | 98.7 | 1.3 |
| 13–24 months | 86.4 | 13.6 | 97.3 | 2.7 |
| 25–36 months | 82.2 | 17.8 | 96.7 | 3.3 |
| 37–48 months | 83.2 | 16.8 | 97.0 | 3.0 |
| 49–60 months | 84.8 | 15.2 | 97.5 | 2.5 |
| 61–72 months | 86.2 | 13.8 | 97.7 | 2.3 |
| Quintiles of population in poverty | ||||
| Lowest quintile | 92.6 | 7.4 | 98.9 | 1.1 |
| Second quintile | 90.7 | 9.3 | 98.5 | 1.5 |
| Middle quintile | 89.1 | 10.9 | 98.1 | 1.9 |
| Fourth quintile | 84.9 | 15.1 | 97.2 | 2.8 |
| Highest quintile | 81.6 | 18.4 | 96.4 | 3.6 |
| Quintiles of housing built pre-1950 | ||||
| Lowest quintile | 94.0 | 6.0 | 99.3 | 0.7 |
| Second quintile | 91.6 | 8.4 | 98.6 | 1.4 |
| Middle quintile | 87.9 | 12.1 | 98.0 | 2.0 |
| Fourth quintile | 83.7 | 16.3 | 96.9 | 3.1 |
| Highest quintile | 80.5 | 19.5 | 96.0 | 4.0 |
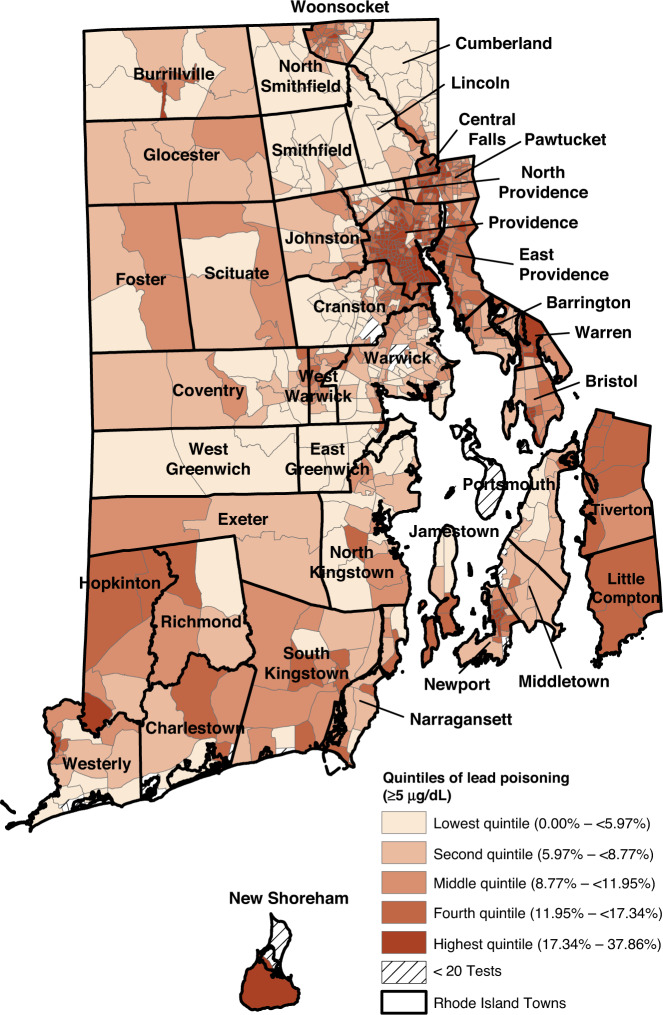
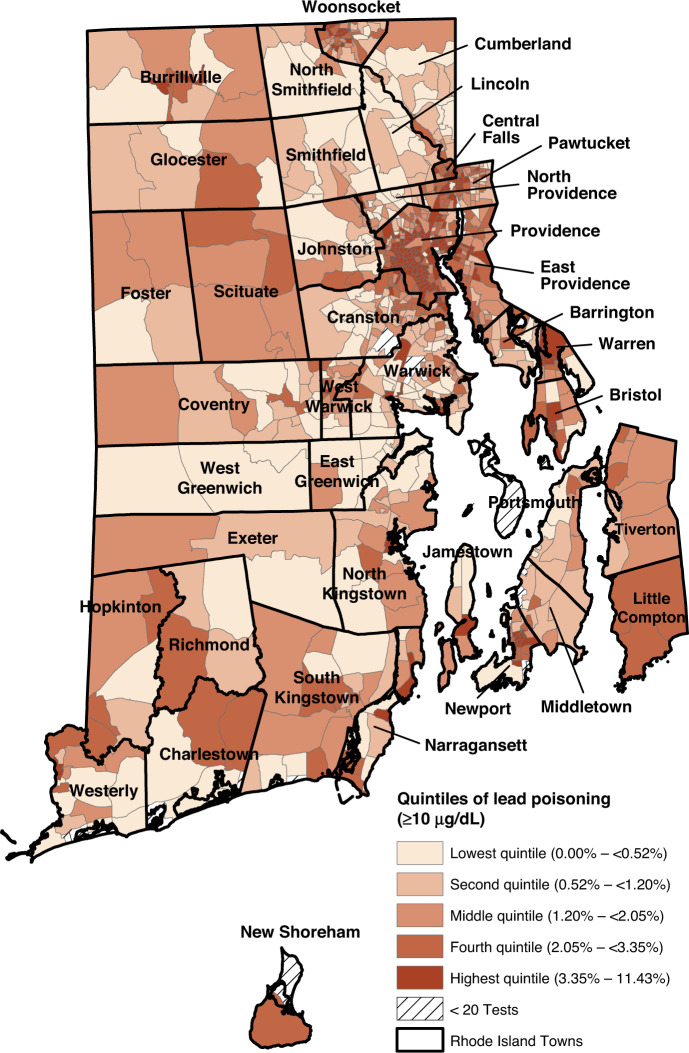
The proportion of children with elevated lead levels (BLL ≥ 5 µg/dL) in the block groups ranged from 0.0 to 37.9%. (Fig. 1) As seen in Figs. 1 and 2, the block groups with the highest proportion of children with BLL ≥ 5 µg/dL and BLL ≥ 10 µg/dL are concentrated in areas that are also characterized by high levels of poverty and pre-1950 housing (Supplementary Figs. 1 and 2). The Moran’s I for both BLLs ≥5 µg/dL (0.68, p < 0.01) and those ≥10 µg/dL (0.52, p < 0.01) indicate that throughout Rhode Island, block groups with elevated rates of lead exposure are near other block groups with elevated rates of lead exposure. These clusters are shown in the LISA clusters maps in Figs. 3 and 4. For both measures, these clusters are located predominately in urban municipalities with the highest levels of children living in poverty.
Fig. 1. Quintiles of percent of children with elevated blood lead levels >5 µg/dL.
Block group proportions of Rhode Island children aged 0–6 years with elevated lead levels (>5 µg/dL) ranged from 0 to 38%. Darker colors reflect higher proportions of children with elevated lead levels.
Fig. 2. Quintiles of percent of children with elevated blood lead levels >10 µg/dL.
Block group proportions of Rhode Island children aged 0–6 years with elevated lead levels (>10 µg/dL) ranged from 0 to 38%. Darker colors reflect higher proportions of children with elevated lead levels.
Fig. 3. LISA cluster maps for block group proportions of children with elevated lead levels (BLL ≥ 5 µg/dL).
Block groups designated as high-high or low-low mean that the block group has high (or low) BLL and is neighbored by other block groups with high (or low) BLL. A high-low or low-high designation means that, respectively, a high BLL group is neighbored by low BLL block groups or a low BLL group is neighbored by high BLL block groups. High-high (hot-spotting) and low-low (cool-spotting) zones show areas of clustering, while high-low and low-high zones highlight outliers in the data.
Fig. 4. LISA cluster maps for block group proportions of children with elevated lead levels (BLL ≥ 10 µg/dL).
Block groups designated as high-high or low-low mean that the block group has high (or low) BLL and is neighbored by other block groups with high (or low) BLL. A high-low or low-high designation means that, respectively, a high BLL group is neighbored by low BLL block groups or a low BLL group is neighbored by high BLL block groups. High-high (hot-spotting) and low-low (cool-spotting) zones show areas of clustering, while high-low and low-high zones highlight outliers in the data.
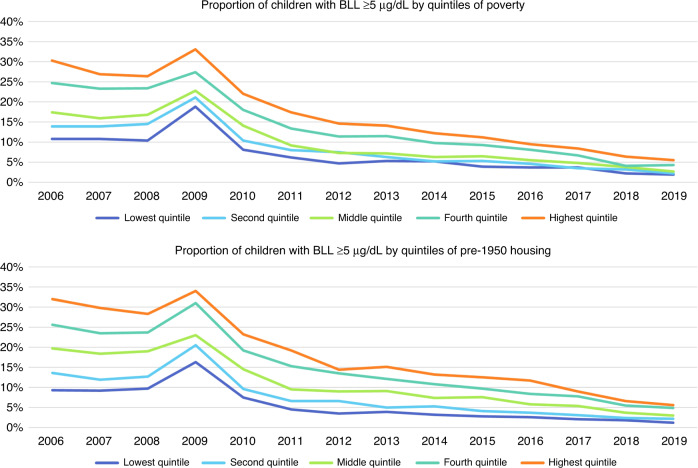
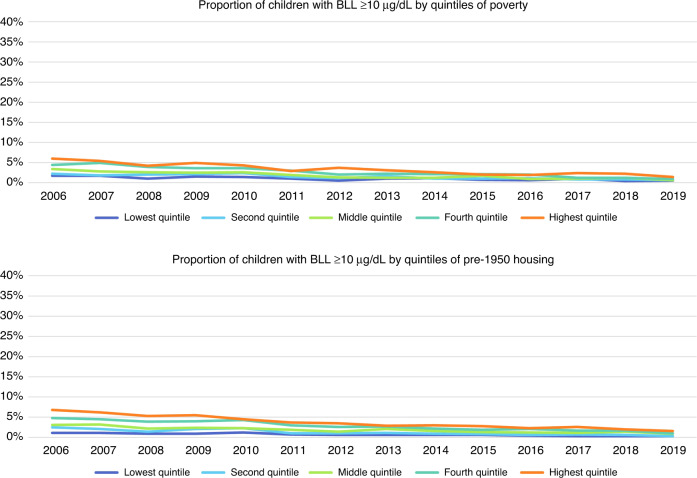
A significant decline in lead exposure in children over the study period was observed: in 2006, 20.5% of the children had BLLs ≥5 µg/dL; by 2019, only 3.6% did. We found significant (p < 0.0001) decreases in BLLs (≥5 µg/dL and ≥10 µg/dL) over the study period. Significant declines over time were also observed across all quintiles of poverty and old housing (Figs. 5 and 6).
Fig. 5. Longitudinal trends of the proportion of children with elevated blood lead levels ≥5 µg/dL by quintiles of poverty and pre-1950 housing, respectively, 2006 through 2019.
The first panel represents the proportion of children with elevated blood lead levels ≥5 µg/dL by quintiles of poverty, where dark blue represents those block groups in the lowest quintile of proportion of population in poverty and orange represents those block groups in the highest quintile of proportion of population in poverty. The second panel represents the proportion of children with elevated blood lead levels ≥5 µg/dL by quintiles of pre-1950 housing, where dark blue represents those block groups in the lowest quintile of proportion of housing units built pre-1950 and orange represents those block groups in the highest quintile of proportion of housing units built pre-1950.
Fig. 6. Longitudinal trends of the proportion of children with elevated blood lead levels ≥10 µg/dL by quintiles of poverty and pre-1950 housing, respectively, 2006 through 2019.
The first panel represents the proportion of children with elevated blood lead levels ≥10 µg/dL by quintiles of poverty, where dark blue represents those block groups in the lowest quintile of proportion of population in poverty and orange represents those block groups in the highest quintile of proportion of population in poverty. The second panel represents the proportion of children with elevated blood lead levels ≥10 µg/dL by quintiles of pre-1950 housing, where dark blue represents those block groups in the lowest quintile of proportion of housing units built pre-1950 and orange represents those block groups in the highest quintile of proportion of housing units built pre-1950.
In multivariate logistic regression models, the odds of BLL ≥ 5 µg/dL increased in a stepwise pattern for quintiles of poverty and housing built pre-1950 (Table 2), with an unadjusted odds ratio of 2.62 (95% CI: 2.30, 2.97) for the highest quintile of poverty and an unadjusted odds ratio of 3.63 (95% CI: 3.24, 4.06) for that of pre-1950 housing compared to the lowest quintile (reference value). These patterns held even when adjusting for potential confounders (sex, age at test, test method, year of test, and clustering within block groups). Adding the term for the spatial effects of lead exposure attenuated the association of poverty and pre-1950 housing with lead exposure, yet they remain significant, indicating these are both important risk factors and this relationship cannot be explained by a diffusion process. Similar results were obtained for models of BLL ≥ 10 µg/dL (Table 2).
Table 2.
Logistic regression models for factors associated with elevated (≥5 µg/dL) and very elevated lead levels (≥10 µg/dL).
| Elevated lead levels (≥5 µg/dL) | Very elevated lead levels (≥10 µg/dL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusteda | Adjustedb | Crude | Adjusteda | Adjustedb | |||||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Quintiles of population in poverty | ||||||||||||
| Lowest quintile | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Second quintile | 1.22 | (1.05, 1.40) | 1.19 | (1.05, 1.35) | 1.13 | (1.02, 1.25) | 1.33 | (1.07, 1.67) | 1.24 | (1.01, 1.52) | 1.20 | (0.996, 1.45) |
| Middle quintile | 1.41 | (1.22, 1.62) | 1.28 | (1.13, 1.46) | 1.17 | (1.05, 1.30) | 1.66 | (1.31, 2.10) | 1.42 | (1.14, 1.76) | 1.31 | (1.08, 1.60) |
| Fourth quintile | 1.98 | (1.74, 2.26) | 1.67 | (1.47, 1.90) | 1.33 | (1.21, 1.48) | 2.39 | (1.95, 2.94) | 1.80 | (1.47, 2.20) | 1.49 | (1.25, 1.79) |
| Highest quintile | 2.62 | (2.30, 2.97) | 2.08 | (1.82, 2.37) | 1.44 | (1.29, 1.60) | 3.33 | (2.73, 4.06) | 2.26 | (1.84, 2.77) | 1.64 | (1.36, 1.99) |
| Quintiles of housing built pre-1950 | ||||||||||||
| Lowest quintile | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Second quintile | 1.42 | (1.25, 1.60) | 1.27 | (1.11, 1.45) | 1.21 | (1.08, 1.35) | 1.82 | (1.44, 2.30) | 1.68 | (1.34, 2.12) | 1.57 | (1.27, 1.95) |
| Middle quintile | 2.06 | (1.83, 2.31) | 1.67 | (1.48, 1.88) | 1.46 | (1.31, 1.62) | 2.64 | (2.18, 3.21) | 2.05 | (1.69, 2.49) | 1.78 | (1.48, 2.15) |
| Fourth quintile | 2.77 | (2.46, 3.10) | 2.22 | (1.94, 2.53) | 1.63 | (1.46, 1.82) | 3.92 | (3.27, 4.71) | 3.11 | (2.57, 3.76) | 2.25 | (1.87, 2.71) |
| Highest quintile | 3.63 | (3.24, 4.06) | 2.88 | (2.53, 3.27) | 1.92 | (1.70, 2.17) | 5.47 | (4.57, 6.54) | 4.18 | (3.46, 5.05) | 2.69 | (2.22, 3.27) |
aAdjusting for gender, age at test, test method, test year, and clustering by block group.
bAdjusting for gender, age at test, test method, test year, spatial effects of lead poisoning, and clustering by block group.
Discussion
This large longitudinal, statewide study, utilizing multivariate analysis and spatial analysis techniques, show the persistence of the neighborhood disparities and its relationship with lead poisoning, an important pediatric health issue with significant economic and societal sequelae. Although lead poisoning rates have declined statewide, significant disparities persist based on where a child lives, even after adjusting for time.
Block group level concentration of poverty and old housing, had a substantial impact on the risk of lead poisoning over this 14-year study period. Homes built before 1950 were painted with high concentrations of lead-containing paint that given the persistence of lead remain in the home today. Children living in these homes or neighborhoods with high proportion of housing built pre-1950 can be exposed to lead, whether in their own homes, in neighboring homes, or in contaminated soil from lead-based paint surrounding these homes. Similar to our findings when analyzing the association of neighborhood factors and childhood lead poisoning rates in Rhode Island from 1993–2005,15,17 block group level poverty rates were also an important risk factor even after adjusting for pre-1950 housing and individual factors. Most likely, housing stock in the high poverty block groups is potentially less well maintained, leading to greater exposure to the lead-dust in the homes; historic redlining practices and systemic racism have been shown to perpetuate these disparities.27 Lastly, children of low socioeconomic status may have a higher risk of nutritional problems such as iron deficiency. Iron deficiency has been associated with a 4- to 5-fold increase in risk of elevated blood lead level due to increased absorption of lead in the gastrointestinal tract.4,28,29 Although a significant decline in lead poisoning rates was found across the study period, across all quintiles of old housing and poverty, disparities still persist that need to be addressed. Lastly, this analysis likely underestimates the rate of children who are affected by lead exposure, as scientific evidence suggests that there are negative health consequences associated with any detectable BLLs.1,2
We have used either a capillary or venous blood test results in the analyses to identify a child’s highest BLL during the study period. The U.S. Preventive Services Task Force found that capillary blood lead testing had a sensitivity of 87–91% and specificity of 92–99% for identifying elevated BLLs compared with venous sampling.19 Furthermore, it is not possible to know whether, among those who are retested, an intervention has been conducted between the time of the capillary test and the venous test.15 An elevated capillary test is indicative of a child with potential lead exposure who may not yet carry a body burden of lead toxicity. By including all testing, regardless of methodology, yielded the most inclusive possible results. Additionally, of those with an elevated test result (n = 25,453), 7497 (29.4%) were capillary samples. Of these, 710 (9.5%) had a venous sample collected within 2 weeks after the capillary sample; 227 (32.0%) again had an elevated BLL.
In terms of the unit of analysis, using the block group has the benefit of defining small geographic units appropriate for targeting as well as the benefit of the ready availability of Census data. The block group level data has the advantage of facilitating targeted resources to places at risk of the greatest lead burden.30,31 Caution should be observed in extending ecological findings to individual children. Although this data minimizes selection bias by including all data that is mandated to be reported to the Rhode Island Department of Health, it still may underestimate the number of children with lead exposure given that not all children are tested. Rhode Island recommends universal lead testing at 1 and 2 years of age. Statewide, by the end of 2019, 75% of 3-year-olds in Rhode Island had received at least one blood test.32 This number is significantly higher than in other states, some of which may recommend only targeted testing of 1- and 2-year-olds who receive Medicaid assistance.33,34 Notably, although many states and Medicaid guidance is limited to 1 and 2 years of age, our data suggests that the highest and second highest proportion of highest BLL ≥ 5 µg/dL and BLL ≥ 10 µg/dL occurred after age 24 months, between 25–36 months and 37–48 months, respectively (Table 1). This finding may have clinical implications, as this study as well as a recent national study has demonstrated increased risk of detectable and elevated blood lead levels after 24 months of age.35 There is currently great variability between individual states and CDC guidance on the frequency and at what ages to conduct universal or targeted blood lead testing.6,35 Lastly, we were not able to identify an etiology for the increase in the proportion of BLL ≥ 5 µg/dL (Fig. 5) in 2009. We did not identify any trends in number of tests, test method, age at test, block group poverty or old housing that were associated with this increase in elevated BLLs. We did not see the same increase in the proportion of BLL ≥ 10 µg/dL in 2009. After final analyses and initial submission of this manuscript were completed, in October 2021, the CDC lowered the blood lead reference level to 3.5 µg/dL further analyses are needed to determine if similar associations were seen with this new benchmark.33
This study demonstrates the power of partnerships between academic institutions and sources of population-level data to inform public health policies and programs. The approach taken here to identify small geographic regions at high risk of lead poisoning can be employed to design a targeted primary prevention lead poisoning program or for population management strategies designed to minimize lead exposure. This approach can also be expanded to guide efforts to address other health conditions such as asthma, injuries, and even SARS-CoV2 infections, that may be exacerbated by housing and community-level risk factors based on readily available data sources. Innovative partnerships between health departments, health care providers, schools of public health, insurers, the Census, and other publicly available population and environmental data sources can and should be used to understand and address health disparities and inequities.
Conclusion
This is one of the first comprehensive statewide longitudinal population-level longitudinal analyses of lead poisoning and its relationship with rates of poverty and older housing within neighborhoods. This study demonstrates that the odds of lead poisoning increased for neighborhood quintiles of poverty and housing built pre-1950. From 2006 through 2019, lead poisoning disparities narrowed across all quintiles of poverty and old housing. While there has been tremendous progress in reducing lead poisoning, substantial neighborhood disparities in lead poisoning persist likely due to historic redlining and systemic racism. These results could inform the design of clinical innovation and primary prevention strategies to proactively identify and mitigate lead hazards in these communities before a child is ever exposed to lead-based paint hazards.
Supplementary information
Funding
Research reported in this publication was supported (in part) by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award Number K23ES031663. This study was also supported (in part) by the Agency for Toxic Substances and Disease Registry (ATSDR), cooperative agreement award number NU61TS000296-02 (to the Region 1 New England Pediatric Environmental Health Specialty Unit (PEHSU). The U.S. Environmental Protection Agency (EPA) supports the Pediatric Environmental Health Specialty Units (PEHSU) by providing funds to ATSDR under Inter-Agency Agreement number DW-75-95877701-02 The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Agency for Toxic Substances and Disease Registry (ATSDR). Neither EPA nor ATSDR endorse the purchase of any commercial products or services mentioned in PEHSU publications. The NIH, ATSDR or EPA did not play any role in the design of this study; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
Data availability
The data that support the findings of this study are available from the Rhode Island Department of Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Rhode Island Department of Health.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Marissa Hauptman, Michelle L. Rogers.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-023-02476-7.
References
- 1.Lanphear BP, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ. Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield RL, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N. Engl. J. Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigg JT, Nikolas M, Mark Knottnerus G, Cavanagh K, Friderici K. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J. Child Psychol. Psychiatry, Allied Discip. 2010;51:58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauptman M, Bruccoleri R, Woolf AD. An update on childhood lead poisoning. Clin. Pediatr. Emerg. Med. 2017;18:181–192. doi: 10.1016/j.cpem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitzman M, et al. Housing and child health. Curr. Probl. Pediatr. Adolesc. Health Care. 2013;43:187–224. doi: 10.1016/j.cppeds.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Council on Environmental Health. Prevention of Childhood Lead Toxicity. Pediatrics138. 10.1542/peds.2016-1493 (2016). [DOI] [PubMed]
- 7.Trasande L, Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff. (Proj. Hope) 2011;30:863–870. doi: 10.1377/hlthaff.2010.1239. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC Ten great public health achievements—United States, 2001-2010. MMWR Morbidity Mortal. Wkly. Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- 9.Dignam T, Kaufmann RB, LeStourgeon L, Brown MJ. Control of lead sources in the United States, 1970–2017: public health progress and current challenges to eliminating lead exposure. J. Public Health Manag. Pract. JPHMP. 2019;25:S13–S22. doi: 10.1097/PHH.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown MJ, Margolis S. Lead in drinking water and human blood lead levels in the United States. MMWR Suppl. 2012;61:1–9. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Populations at Higher Risk; 2021 (accessed 5 August 2022); https://www.cdc.gov/nceh/lead/prevention/populations.htm
- 12.Jacobs DE, et al. The prevalence of lead-based paint hazards in U.S. housing. Environ. Health Perspect. 2002;110:A599–A606. doi: 10.1289/ehp.021100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanphear BP, et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels. A Pool. Anal. 12 Epidemiol. Stud. Environ. Res. 1998;79:51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention.”; 2012 (accessed 5 August 2022); https://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf
- 15.Vivier PM, et al. The important health impact of where a child lives: neighborhood characteristics and the burden of lead poisoning. Matern. Child Health J. 2011;15:1195–1202. doi: 10.1007/s10995-010-0692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClure LF, Niles JK, Kaufman HW. Blood lead levels in young children: US, 2009–2015. J. Pediatr. 2016;175:173–181. doi: 10.1016/j.jpeds.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hauptman, M., Vanderslice, R., Vivier, P.M. State of Lead in Rhode Island: Using GIS Analysis in the Development of a Lead Poisoning Prevention Program. https://search.library.brown.edu/catalog/b4518076 (2007).
- 18.Cantor, A. G. et al. Screening for Elevated Blood Lead Levels in Children: A Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2019 Apr. Report No.: 18-05245-EF-1. [PubMed]
- 19.Parsons PJ, Reilly AA, Esernio-Jenssen D. Screening children exposed to lead: an assessment of the capillary blood lead fingerstick test. Clin. Chem. 1997;43:302–311. doi: 10.1093/clinchem/43.2.302. [DOI] [PubMed] [Google Scholar]
- 20.Schlenker TL, et al. Screening for pediatric lead poisoning. Comp. Simultaneously Draw. Capill. Venous Blood Samples JAMA. 1994;271:1346–1348. doi: 10.1001/jama.271.17.1346. [DOI] [PubMed] [Google Scholar]
- 21.E911_Sites. ArcGIS. Published 2018 (accessed 19 January 2020); https://services2.arcgis.com/S8zZg9pg23JUEexQ/arcgis/rest/services/E911_Sites/FeatureServer
- 22.E911 Road Centerlines. ArcGIS. Published 2019 (accessed 19 January 2021); https://services2.arcgis.com/S8zZg9pg23JUEexQ/arcgis/rest/services/E911_Road_Centerlines/FeatureServer
- 23.TIGER Roads. US Census Bureau. Published 2019 (accessed 19 January 2021); http://www.census.gov/geo/maps-data/data/tiger-line.html%0Asubdirectory = https://www2.census.gov/geo/tiger/TIGER2019/ADDRFEAT/
- 24.Census Block Groups. US Census Bureau. Published 2019 (accessed 20 October 2020); https://www.census.gov/programs-surveys/geography/about/glossary.html#par_textimage_4
- 25.Krieger N, et al. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J. Epidemiol. Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reissman DB, Staley F, Curtis GB, Kaufmann RB. Use of geographic information system technology to aid Health Department decision making about childhood lead poisoning prevention activities. Environ. health Perspect. 2001;109:89–94. doi: 10.1289/ehp.0110989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weintraub M. Racism and lead poisoning. Am. J. Public Health. 1997;87:1871–1872. doi: 10.2105/AJPH.87.11.1871-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright RO, Shannon MW, Wright RJ, Hu H. Association between iron deficiency and low-level lead poisoning in an urban primary care clinic. Am. J. Public Health. 1999;89:1049–1053. doi: 10.2105/AJPH.89.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright RO, Tsaih S-W, Schwartz J, Wright RJ, Hu H. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J. Pediatr. 2003;142:9–14. doi: 10.1067/mpd.2003.mpd0344. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Gotway Crawford CA. The role of geographic scale in testing the income inequality hypothesis as an explanation of health disparities. Soc. Sci. Med. (1982) 2012;75:1022–1031. doi: 10.1016/j.socscimed.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Tatalovich Z, Wilson JP, Milam JE, Jerrett ML, McConnell R. Competing definitions of contextual environments. Int. J. Health Geogr. 2006;5:55. doi: 10.1186/1476-072X-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhode Island Kids Count Factbook. Rhode Island Kids Count. Published 2020 (accessed 1 October 2021); http://www.rikidscount.org/Data-Publications/RI-Kids-Count-Factbook
- 33.National Center for Environmental Health. CDC National Childhood Blood Lead Surveillance Data. Center for Disease Control and Prevention. Published 2022, (accessed 1 August 2022); https://www.cdc.gov/nceh/lead/data/national.htm
- 34.Hauptman M, Niles JK, Gudin J, Kaufman HW. Individual- and community-level factors associated with detectable and elevated blood lead levels in US children: results from a National Clinical Laboratory. JAMA Pediatr. 2021;175:1252–1260. doi: 10.1001/jamapediatrics.2021.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel JJ, Erinoff E, Tsou AY. More Guidelines than states: variations in U.S. lead screening and management guidance and impacts on shareable CDS development. BMC Public Health. 2020;20:127. doi: 10.1186/s12889-020-8225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Rhode Island Department of Health but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Rhode Island Department of Health.