Abstract
In this review we highlight the relevance of biodiversity that inhabit coastal lagoons, emphasizing how species functions foster processes and services associated with this ecosystem. We identified 26 ecosystem services underpinned by ecological functions performed by bacteria and other microbial organisms, zooplankton, polychaetae worms, mollusks, macro-crustaceans, fishes, birds, and aquatic mammals. These groups present high functional redundancy but perform complementary functions that result in distinct ecosystem processes. Because coastal lagoons are located in the interface between freshwater, marine and terrestrial ecosystems, the ecosystem services provided by the biodiversity surpass the lagoon itself and benefit society in a wider spatial and historical context. The species loss in coastal lagoons due to multiple human-driven impacts affects the ecosystem functioning, influencing negatively the provision of all categories of services (i.e., supporting, regulating, provisioning and cultural). Because animals’ assemblages have unequal spatial and temporal distribution in coastal lagoons, it is necessary to adopt ecosystem-level management plans to protect habitat heterogeneity and its biodiversity, ensuring the provision of services for human well-being to multi-actors in the coastal zone.
Keywords: Human well-being, Environmental services, Shallow ecosystems, Brackish waters
Introduction
Coastal lagoons are inland water bodies isolated from the ocean by a physical barrier, which may be intermittently connected to the sea by one or more restricted inlets (Kjerfve, 1994). These estuarine ecosystems are widely distributed across world’s coastlines (Barnes, 1980), usually in human densely populated areas (Gönenç & Wolflin, 2004; El Mahrad et al., 2020). The proximity with urban areas accounts for a variety of non-natural stressors on coastal lagoons, such as eutrophication, input of contaminants, introduction of non-indigenous species and fishery overexploitation (Esteves et al., 2008; Zaldívar et al., 2008; Munaron et al., 2012). Consequently, coastal lagoons are one of the most threatened ecosystems in the world (Newton et al., 2018) and may become more vulnerable with the increasing pressures derived from climate change (Beer & Joyce, 2012).
Although multiple stressors threat coastal lagoons, these ecosystems still underpin human welfare and livelihoods (Newton et al., 2014), providing several ecosystem services, which are functions and products that benefit humans, or yield welfare to society (MEA, 2005). Dozens of provisioning (e.g., food and raw material), regulating (e.g., carbon sequestration and water regulation), supporting (e.g., habitat for species and nutrient cycling) and cultural (e.g., aesthetic and educational values) services are directly associated with these ecosystems (Newton et al., 2018; Pérez-Ruzafa et al., 2019). The importance of services provided by coastal lagoons is clear to experts worldwide (Newton et al., 2018) and has been increasingly recognized by distinct stakeholders (Sy et al., 2018). Broad recognition of services provided by coastal lagoons is crucial to enhance good management practices through ecosystem approaches, such as the Millennium Ecosystem Assessment (MEA, 2005) and the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES, 2019).
Besides the practical progress in ecosystem services science, the theoretical foundations that guide our understanding about how ecosystem functioning provides ecosystem services must be thoroughly discussed and acknowledged, especially the links between biodiversity and ecosystem services (Haines-Young & Potschin, 2010; Mace et al., 2012). In this context, it is essential to understand the term ‘species function’, here defined as the role that each biotic component plays in the environment that surrounds it (biotic and abiotic), so that it can refer to the interactions between two objects by cause within an ecological system (Calow, 1987; Fu et al., 2013). Also, it is important to understand the mechanism in which multiple and interactive species functions generate ecosystem processes (Mace et al., 2012), driving to the provision of ecosystem services (Bennett, 2017; Rullens et al., 2019).
For instance, in coastal lagoons, high biodiversity is driven by the remarkable patterns of spatial–temporal variations (Meerhoff et al., 2013; Bellino et al., 2019) derived from the variability of abiotic factors (Basset et al., 2013) in a mosaic of heterogeneous habitats (e.g., salt marshes, seagrass meadows, and mangroves; see Bloomfield & Gillanders, 2005). Such high biodiversity in coastal lagoons (Newton et al., 2018) may yield different ecosystem functions (Loreau, 2000; Mace et al., 2012) that may influence the ecosystem structure and processes (Tilman, 2001; Petchey & Gaston, 2006; Rullens et al., 2019), and can explain the vast array of services provided by ecosystems (Balvanera et al., 2006; Pérez-Ruzafa et al., 2019). However, even though well-grounded in theory, understanding the relationships between biodiversity, ecological functions and services in coastal lagoons is incipient since efforts in this direction are scarce (see: Danovaro & Pusceddu, 2007).
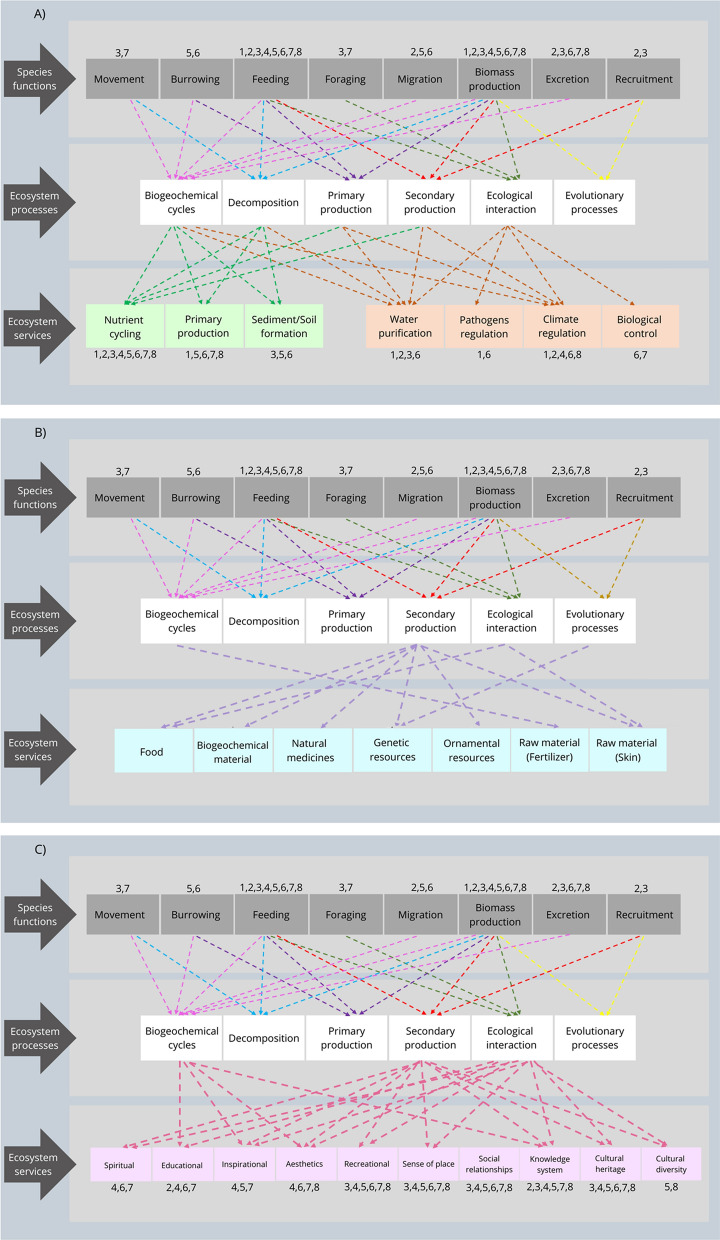
This non-systematic review highlights the main characteristics and ecological functions of the aquatic taxon that inhabit coastal lagoons. We cover the following groups: bacteria and other microbial organisms, zooplankton, polychaete worms, mollusks, macro-crustaceans, fishes, birds, and aquatic mammals. By preference, we extracted information from peer reviewed papers with international scope. In the absence of this possibility, we selected reports, books, and book’s chapters with international scope. To highlight the role of each group (i.e., their species function), we reviewed species/taxon behavioural (i.e., movements, burrowing, feeding, foraging and migration) and population/group processes (i.e., growth = biomass production, excretion, and recruitment). Then, we related their species functions with ecosystem processes and services in coastal lagoons through cause-effects relationships (Jax, 2005). The designations of the ecosystem services follow the United Nations Millennium Ecosystem Assessment (MEA, 2005). To avoid double-counting in MEA's classification, we highlight how a given species functions might through a causal relationship provide an intermediate (i.e., supporting and regulating) or instead a final ecosystem service (i.e., provision or cultural), considering that the outputs of the former flow indirectly to humans and underpin other final services, which provides direct values and benefits to citizens (DeWitt et al., 2020). From this approach, we emphasized how species functions foster ecosystem processes and services associated with costal lagoons (Table 1; Fig. 1).
Table 1.
Ecosystem services provided by biodiversity of coastal lagoons (according to the Millennium Ecosystem Assessment Report, 2003, 2005)
| Biodiversity component | Taxon/Group | Ecological function | Mechanism | Ecosystem processes | Ecosystem service category | Ecosystem service |
|---|---|---|---|---|---|---|
| Microbes | Heterotrophic prokaryotes and Fungi | Feeding | Pollutants degradation |
Decomposition/ Secondary production |
Regulating | Water purification |
| Heterotrophic prokaryotes | Feeding | Toxin degradation |
Decomposition/ Secondary production |
Regulating | Water purification | |
| Eukaryotic and prokariotic predators | Feeding | Predation on pathogenic species | Ecological interactions | Regulating | Disease regulation | |
| Photo and chemo- autotrophic microbes and heterotrophic microbes | Biomass production | Emission or sequestration of greenhouse gases (CO2, CH4, N2) through Photo/chemosynthesis |
Biogeochemical cycles/Primary/ Secondary production |
Regulating | Climate regulation | |
| Bacterivorous protists | Feeding | Predation and Competition | Ecological interactions | Regulating | Biological control | |
| Heterotrophic prokaryotes, fungi, viruses | Feeding | Organic matter degradation | Decomposition/Secondary production | Supporting | Nutrient cycling | |
| Denitrifying bacteria, archaea and protists, Sulfur-oxidizing bacteria and archaea, Sulfide-oxidizing bacteria, Sulfate-reducing bacteria and archaea, DMSP producers and consumers | Feeding | Inorganic nutrient recycling (e.g., nitrogen and sulfur) | Biogeochemical Cycles | Supporting | Nutrient cycling | |
| Photo- and chemo- autotrophic prokaryotes and Photoautotrophic protists | Biomass production | Oxygenic and Anoxygenic Photosynthesys/ Chemoautotrophy | Primary production | Supporting | Primary production | |
| Zooplankton | Zooplankton species | Respiration/Excretion | Vertical distribution of carbon through the system | Biogeochemical Cycles | Supporting | Nutrient cycling |
| Marine zooplankton species | Feeding/Excretion | Detritus from zooplanktons as carbon input to bottom habitats | Biogeochemical Cycles | Regulating | Climate regulation | |
| Rotifera, cladocera and copepoda species | Biomass production | Fossil record in coastal sedimentary layers | Biogeochemical Cycles | Cultural | Formal knowledge system; educational values | |
| Dormant zooplankton species | Recruitment | Re-suspension or re-emerging of dormant stages to restore communities | Secondary production/ Evolutionary processes | Provisioning | Genetic resources | |
| Zooplankton species | Feeding | Recovering energy from protist sink | Biogeochemical Cycles/Secondary production | Supporting | Nutrient cycling | |
| Zooplankton species (e.g., copepods) | Biomass production | Biomass/abundance of prey availability in foraging habitats to pelagic fishes | Secondary production/Ecological interactions | Supporting | Provisioning of habitat | |
| Grazing zooplanktons (e.g., Daphnia spp.) | Feeding | Grazing activity over autotrophs | Biogeochemical Cycles/Ecological interactions | Regulating | Water purification | |
| Daphnia spp., Acartia cf. fancetti Brachionus plicatilis, Cyclophora sp. and Scrippsiella sp. | Biomass production | Biomass/abundance availability of bioindicator species | Secondary production | Cultural | Formal knowledge system | |
| Polychaeta | Polychaeta species | Biomass production | Detrital material converted into biomass | Biogeochemical Cycles/Secondary production | Supporting | Nutrient cycling |
| Polychaeta species | Biomass production | Biomass/abundance prey availability in foraging habitats to macrobenthic fishes | Secondary production/Ecological interactions | Supporting | Provisioning of habitat | |
| Polychaeta species | Moviment (habitat use) | Bioturbation enhancing oxygen penetration in sediment | Biogeochemical cycle/Decomposition | Supporting | Sediment formation | |
| Polychaeta species | Moviment (habitat use) | Bioturbation releasing nutrients to the water column | Biogeochemical Cycles | Supporting | Nutrient cycling | |
| Polychaeta species | Biomass production | Biomass/abundance availability of bioindicator species | Secondary production | Cultural | Formal knowledge system | |
| Arenicolidae, Glyceridae, Lumbrineridae, Nereididae, Nephtyidae, Onuphidae and Eunicidae species | Biomass production | Biomass/abundance availability as fishing baits | Secondary production | Cultural | Sense of place, Social relationships, Recreational values and Cultural heritage values | |
| Mollusks | Deposit-feeders (e.g., A. ovata) | Feeding | Sediment reworking from feeding activity enhancing oxygen penetration and bacterial growth in benthic habitats | Biogeochemical cycle /Decomposition | Supporting | Nutrient cycling, Sediment formation |
| Deposit-feeders (e.g., A. ovata) | Feeding | Sediment oxygenation fostering bacterial activity in the water column | Decomposition/ Secondary production | Regulating | Water purification | |
| Mollusks | Grazing gastropods (e.g., Strombus gigas; M. modulus) | Feeding | Feeding activities enhacing photosynthetic rates in seagrass and its carbon sequestration | Ecological interactions/Primary production | Regulating | Climate regulating |
| Suspension feeder bivalves (e.g. A. tuberculosa; P. rugosa) | Feeding | Feeding activities removing phytoplankton and controling eutrophication | Ecological interactions/Primary production | Regulating | Water purification | |
| Suspension feeder bivalves (e.g. A. tuberculosa; P. rugosa) | Feeding | Feeding activities removing other particles in suspension and influencing on carbon cycling | Biogeochemical Cycles | Supporting | Nutrient cycling | |
| Erodoma mactroides Bosc, 1801 and other mollusks | Biomass production | Prey biomass/abundance availability in nursery area to decapoda species | Secondary production/Ecological interactions | Supporting | Provisioning of habitat | |
| Mollusk's fishery resource | Biomass production | Biomass/abundance availability supporting fishing activity | Secondary production/Ecological interactions | Provisioning | Food | |
| Mollusk's fishery resource | Biomass production | Biomass/abundance availability supporting fishing activity | Secondary production/Ecological interactions | Cultural | Sense of place, Social relationships, Recreational values and Cultural heritage values | |
| Mollusk species | Biomass (shell) production | External shell formation as carbon long-term sink | Secondary production/ Biogeochemical cycle | Regulating | Climate regulating | |
| Mollusks | Mollusk species | Biomass (shell) production | Shells used to craft decoration artefacts or jewels | Secondary production/ Biogeochemical cycle | Cultural | Aesthetic, inspirational values |
| Mollusk species | Biomass production | Mollusk’s species used to classical studies | Secondary production | Cultural | Educational, cultural heritage | |
| Anomalocardia spp; Phacoides spp | Biomass production | Mollusks used as food and to build "Sambaquis" | Secondary production | Cultural | Formal and traditional knowledge system, educational, inspirational, cultural heritage, and recreational and touristic values | |
| Gastropods species | Biomass (shell) production | Shells (named “Búzios”) used in spiritual ceremonies | Secondary production | Cultural | Spiritual | |
| Macro-crustaceans | Isopods; Amphipods species | Biomass production | Prey biomass/abundance availability in nursery area to fish species | Secondary production/Ecological interactions | Supporting | Provisioning of habitat |
| Grapsid crabs; Thalassinidean shrimps; Callinectes spp | Burrowing behaviour | Bioturbation releasing nutrients (e.g. phosporous; nitrogen) to water column | Biogeochemical cycles | Supporting | Nutrient cycling | |
| Grapsid crabs; Thalassinidean shrimps; Callinectes spp. | Burrowing behaviour | Nutrients from bioturbation enhacing primary productors | Primary production | Supporting | Primary production | |
| Grapsid crabs; Thalassinidean shrimps; Callinectes spp. | Burrowing behaviour | Bioturbation enhacing oxygen penetration and bacterial growth in sediment | Decomposition/ Biogeochemical cycle | Supporting | Sediment formation | |
| Macro-crustaceans | Grapsid crabs; Thalassinidean shrimps; Callinectes spp | Burrowing behaviour | Bioturbation enhacing oxygen penetration in sediment (habitat quality to benthic communities) | Sediment formarion/Biogeochemical cycle | Supporting | Provisioning of habitat |
| Penaeids shrimps (and others) | Biomass production | Biomass/abundance availability supporting fishing activity | Secondary production | Provisioning | Food | |
| Crustacean's fisheries resource | Biomass production | Chitin biomass availability | Secondary production | Provisioning | Biochemical material provision | |
| Crustacean's fisheries resource | Biomass production | Biomass/abundance availability supporting fishing activity | Secondary production | Cultural | Sense of place, social relationships, Recreational values; Cultural heritage values | |
| Fish | Bottom-dwelling fish (e.g., Pleutonectiformes) | Feeding | Bioturbation releasing nutrients to the water column | Biogeochemical cycles | Supporting | Nutrient cycling |
| Bottom-dwelling fish (e.g., Pleutonectiformes) | Feeding | Nutrients from bioturbation fostering primary producers | Primary Production | Supporting | Primary production | |
| Fish (all groups) | Excretion | Excretion of mineralized nutrients | Biogeochemical cycles | Supporting | Nutrient cycling | |
| Burrower fish (e.g., Pleutonectiformes) | Burrowing behaviour | Bioturbation releasing nutrients to water column | Biogeochemical cycles | Supporting | Nutrient cycling | |
| Burrower fish (e.g., Pleutonectiformes) | Burrowing behaviour | Nutrients from bioturbation fostering primary producers | Primary Production | Supporting | Primary production | |
| Zooplanktivorous fish | Feeding | Predation on zooplankton in the water column influecing growth of phytoplankton and reducing carbon sequestration | Ecological interactions/Primary production | Regulating | Climate regulation | |
| Fish | Phytoplanktivorous fish | Feeding | Grazing of algae and cyanobacteria controling eutrophication | Ecological interactions/Primary production | Regulating | Water purification |
| Piscivorous fish | Feeding | Predation on zooplanktivorous fishes, growth of zooplankton that consumes phytoplankton (releasing of carbon) | Ecological interactions/Secondary production | Regulating | Climate regulation | |
| Diadromous fish (e.g., G. genidens, P. marinus) | Excretion/migration | Excretion of mineralized nutrients and moviment conecting freshwater and marine systems | Biogeochemical cycles | Supporting | Nutrient cycling | |
| Larvivorous fish (e.g., G. affinis) | Feeding | Predation on mosquito larvae | Ecological interactions/Secondary production | Regulating | Biological control, Disease regulation * | |
| Fish (all groups) | Biomass production | Mutation and natural recombination | Ecological interactions/Evolutionary processes | Provisioning | Genetic resources | |
| Fish (e.g., mullet, sea lamprey) | Biomass production | Increase of biomass and abundance of fish | Secondary production | Provisioning | Food | |
| Fish (e.g., Tilapia species) | Biomass production | Increase of biomass and abundance of fish | Secondary production | Provisioning | Biochemicals, natural medicines and pharmaceuticals | |
| Fish (e.g., N. notopterus and O. gachua) | Biomass production | Increase of biomass and abundance of fish | Secondary production | Provisioning | Ornamental resources | |
| Fish (all groups) | Biomass production | Fish markets underpin relationships among social actors | Secondary production | Cultural | Cultural diversity, social relations | |
| Fish | Fish (e.g., seahorse as amulet) | Biomass production | Availability of biomass and abundance of fish | Secondary production | Cultural | Spiritual and religious values |
| Fish (e.g., sensitive species such as L. saliens, or tolerant species such as P. marmoratus) | Biomass production | Death (or behavior alteration) of sensitive species. Endurance of tolerant species (with dominance in the system) | Secondary production/Ecological interactions | Cultural | Knowledge systems | |
| Fish (all groups) | Biomass production | Biomass/abundance availability supporting fishing and social activities | Ecological interactions/Secondary production | Cultural | Educational,inspirational, aesthetic, cultural heritage, recreational and ecoturism values | |
| Predator birds (N. brasilianus; diving ducks) | Feeding | Biological control of pests, dominant or exotic species populations | Ecological interactions/Secondary production | Regulating | Biological control, Disease regulation | |
| Cormorants, Terns and Gulls | Excretion | Guano and eggshells deposition on land | Biogeochemical cycles | Provisioning | Raw material (Fertilizer) provision | |
| Cormorants, Terns and Gulls | Excretion/moviment | Guano deposition and nutrient enrichment connecting terrestrial and aquatic habitats | Biogeochemical cycles | Supporting | Soil Formation | |
| Cormorants, Terns and Gulls | Excretion | Guano deposition on water influencing nutrients cycling and enhance primary production | Biogeochemical cycles/Primary production | Supporting | Nutrient cycling, Primary production | |
| Birds | Bird species, Flamingos P. roseus, rare and migrating species | Biomass production | Bird diversity promoting recreational activities, as birdwatching | Ecological interactions/Secondary production | Cultural | Formal and informal knowledge system, educational, aesthetics, social relations and recreation and tourism values |
| Geese, Swans and Ducks | Biomass production | Bird diversity promote the creation of conservation actions | Ecological interactions/Secondary production | Cultural | Educational values, social relations, sense of place | |
| Flamingos P. roseus; Sacred Ibis T. aethiopicus | Biomass production | Bird species as cultural values to ancient societies | Ecological interactions/Secondary production | Cultural | Inspirational, formal and traditional knowledge systems, spiritual, cultural heritage | |
| Aquatic mammals | Aquatic and semi-aquatic species | Excretion/movimentation | Mammal's releasing faecal plumes in their foraging habitats | Biogeochemical cycles/Primary production | Supporting | Nutrient cycling, Primary production |
| Aquatic and semi-aquatic species | Biomass production | Mammal's body as carbon sink, and mammal's carcass as carbon source | Secondary production/Biogeochemical cycle | Regulating | Climate regulating | |
| Beluga D. leucas; Harbor seal P. vitulina | Biomass production | Biomass/abundance availability supporting hunting activities | Secondary production/Ecological interactions | Provisioning | Food | |
| Beluga D. leucas; Harbor seal P. vitulina | Biomass production | Mammal's skin and other raw materials used for clothing, equipment, and ornamental | Secondary production/Ecological interactions | Provisioning | Raw material (Skin) | |
| Irrawaddy dolphin O. brevirostris; Lahille`s Bottlenose dolphin T. truncatus gephyreus | Foraging behaviour | Mammals’ species supporting fishing activity | Ecological interactions | Cultural | Recreational, sense of place, social relations, aesthetics, cultural heritage, formal, traditional knowledge, cultural, diversity values |
Fig. 1.
Interactions among species functions (i.e., species processes and behaviors), ecosystem processes and ecosystem services in coastal lagoons. Eco-Evo = Ecological and Evolutionary processes
Furthermore, we reviewed how multiple threats impact biodiversity and how this reverberates in the provision of ecosystem services. This review does not intend to address the whole aquatic biodiversity in coastal lagoons nor to exhaust the ecological functions and their interaction with ecosystem services. Instead, we intend to advance on the understanding of the ecosystem functioning and on the mechanisms that generates the provision of ecosystem services by biodiversity of coastal lagoons.
Bacteria and other microbial organisms
Prokaryotic communities in coastal lagoons occupy different habitats (i.e., water column, sediments, biofilms on different biotic and abiotic substrates) with varying abundance and diversity (Alonso et al., 2009, 2013; Mohit et al., 2014). They show distinct life strategies in coastal lagoons such that communities that live attached to particles are consistently more diverse than their free-living counterparts in the water column (LaMontagne & Holden, 2003; Mohit et al., 2014).
Microbial communities are crucial elements of the food web because they consume and decompose organic matter and foster the nutrient cycling, an important intermediary service to coastal lagoon functioning. Both species functions are performed by a variety of microorganisms, mainly heterotrophic prokaryotes (Amaral et al., 2016). Fungi, for example, can decompose recalcitrant molecules, i.e. lignin and cellulose from leaves and branches (Ortega-Arbulú et al., 2019) and assist with the supply of nutrients otherwise unavailable for other organisms. Microbial communities are also integrated into food webs as resources to many consumers (i.e., grazing and filtering invertebrates), ultimately subsidizing the biomass production of organisms in upper trophic levels (e.g., enabling the provision and cultural services by other taxa; see the following sections). Apart from their small size, viruses are so abundant in the water column and sediment and can also provide organic matter for consumers through viral-induced cell lysis (Weitz et al., 2015).
Microbial communities not only supply mineralized and organic matter to the food webs, but also assist with incorporation of inorganic nutrients from the sediment and water column into the aquatic food web (Mulholland et al., 2009). In particular, the N and S cycles are strongly driven by different prokaryotic functional groups as Nitrogen fixers, Ammonia oxidizing bacteria and archaea, Nitrite-oxidizing bacteria, Commamox bacteria, Denitrifying bacteria, archaea and protists, Sulfur-oxidizing bacteria and archaea, Sulfide-oxidizing bacteria, Sulfate-reducing bacteria and archaea, DMSP producers and consumers (Madigan et al., 2008). Prokaryotic and eukaryotic microbial communities also incorporate nutrients, playing an important role in primary production in coastal lagoons (Fontes et al., 2011).
Microbial communities are key players in Regulating Services. Members of diverse taxonomic groups frequently encountered in coastal lagoons—such as the bacterial genera Acinetobacter, Alcaligenes, Burkholderia, Flavobacterium, Pseudomonas, and Sphingomonas, and the fungal genera Candida, Geotrichum and Rhodotorula—are known to degrade anthropogenic pollutants, and thus provide water purification service (Leahy & Colwell, 1990; Aislabie & Lloyd-Jones, 1995; Seo et al., 2009). Water quality is also maintained by microbial degradation of cyanobacterial toxins by bacterial genera Arthrobacter, Burkholderia, Brevibacterium, Rhodococcus Sphingomonas, and Sphingosinicella (Donovan et al., 2008; Lemes et al., 2008; Kormas & Lymperopoulou, 2013). Bacterivorous protists control microbial pathogens that reach the coastal lagoons through predation (i.e., mainly Ciliates, Mast, 1947) (Staley et al., 2014; Balzano et al., 2015) and competition with the native bacterial community (Korajkic et al., 2013), providing the biological control service. Because microbial communities perform aerobic and anaerobic respiration, aside from nitrogen fixation (i.e., methanogenic Archaea and Cyanobacteria, and autotrophic N fixing prokaryotes), they assist with climate regulation through the production and consumption of greenhouse gases, such as CO2, CH4, and N2O (Hirota et al., 2007; Muduli et al., 2012). Also, changes in carbon availability and DOM quality linked to hydrology strongly affects the activity of different bacterial exoenzymes (Conan et al., 2017), the extent of dissolved organic matter biodegradation and its resulting quality (Alonso et al., 2013; Amaral et al., 2016). Such effects lead to strong spatiotemporal variations in the CO2 emissions (Muduli et al., 2012), and might assist the regulation of carbon exchange in local and regional scales.
As microorganisms occupy the basis of the food web, their functions are integrated within processes that generate intermediary supporting and regulating services. Nonetheless, they are important to subsidize final ecosystem services provided by other organisms from the coastal lagoons.
Zooplankton
Zooplankton pertain to the animal or animal-like heterotrophic organisms comprising the plankton group, a diverse community limited by the water-column interfaces with adjacent aquatic habitats e.g., littoral and sediment zones (Hutchinson, 1959; Margalef, 1983). In high productive and intermittently disturbed environments such as coastal lagoons, zooplankton can show enormous functional complexity (Santangelo et al., 2007). The shallow water columns of coastal lagoons congregate species that spend their entire lives in the pelagic environment (holoplankton) with several occasional planktonic taxa, such as early stages and larvae of shrimps, insects, crabs, and temporary members of the plankton (meroplankton). Therefore, the biological dynamics and ecosystem functioning depend on these benthic–pelagic interactions, connecting higher productive epipelagic zone with bottom communities (Kiljunen et al., 2020).
The importance of zooplankton groups and their contribution in ecosystem services may be the result of the flourishing biodiversity of marine, brackish and freshwater assemblages (Santangelo et al., 2007; Branco et al., 2008; Paturej, 2009), as well as their interactions and functionalities. Due to their short life spans, ubiquiti and high abundances, zooplankton can drive the aquatic biogeochemical cycles of essential elements such as carbon, phosphorus and nitrogen which is respired and converted to inorganic matter, supporting primary producers e.g., phytoplankton and macrophyte banks in coastal lagoons. These biogeochemical processes involve mainly the respiratory carbon (CO2) and particulate organic carbon (POC) through faecal pellets and detritus, not only recycling carbon (i.e., Supporting service), but also defining vertical distribution of carbon through the system. Furthermore, these sinking particles’ movement is related with carbon pool formation in bottom habitats, an important regulating service for the “climate crisis”. Considering that many zooplankton species found in coastal lagoons are from the marine realm (e.g., the oceanic copepod Calanus finmarchicus Gunnerus, 1770, see Jónasdóttir et al., 2015), it is possible to assume that these organisms also play a role in ocean’s biological pump (Lebrato et al., 2019; Robison et al., 2005), the main process that connect atmosphere, pelagic surface layers and deep ocean through the carbon cycle. Besides both intermediary services mentioned above, the fossil record documentation of some groups, although poorly documented, may provide a final ecosystem service to human society, assisting with a formal knowledge system and educational values (cultural services), since they allow us to understand the mechanisms and processes that shape aquatic ecosystems at different time scales. For instance, the fossil record documentation of rotifers (Waggoner & Poinar, 1993), cladocerans (Van Damme & Kotov, 2016) or copepods (Selden et al., 2010) is placed in the context of major historical changes in the aquatic environment, including the appearance of predator–prey relationships and niche availability that have impacted their evolution.
The dormancy is a conspicuous feature of zooplanktonic taxa. In the littoral zone of coastal lagoons, species of certain zooplankton groups leave resting cysts banks (e.g., ephipia in cladocerans, resting eggs in rotifers and copepods and cysts in Artemia spp.), dormant stages that sink to the bottom sediment until they are re-suspended or the right conditions return for them to re-emerge and restore active communities seasonally (Eskinazi-Sant’Anna & Pace, 2018). The dormant stages provide important genetic resources (i.e., provisioning ecosystem service) for the resilience of populations after disturbances (Latta et al., 2010). The restoration of aquatic systems from cyst banks could be further developed in advance of heavily impacted coastal wetlands or coastal lagoons affected by changes in water regimes (Williams et al., 2008; Santangelo et al., 2014; Eskinazi-Sant’Anna & Pace, 2018).
Through grazing and predation, zooplankters play relevant ecological functions by actively participating in ecosystem metabolism. For instance, by grazing on bacteria and very small protists, zooplankton species recover matter and associated energy through the microbial loop that otherwise could be lost to ecosystem sinks (Feitosa et al., 2019). The zooplankton biomass generated may show quite regular patterns and strategically assure fish recruitment and presence of fish stock by acting as a hub of food webs. Copepods represent an important alternative live-feed in marine/brackish fish production, as they improve survival, growth and development of fish larvae (Hansen, 2017). In addition, the vertical migration of microcrustaceans influences migration and consequently food availability to pelagic fishes (Perissinotto & McQuaid, 1992). Thus, this is a suitable example of how zooplankton biomass availability in foraging areas assists with the provision of habitats, intermediating important commercial fisheries in coastal lagoons (Lomartire et al., 2021). The application of large herbivorous zooplankton, especially calanoid copepods and Daphnia spp., in promoting clear-water phases due to its grazing effect on phytoplankton is well documented (Sommer et al., 1986). This has potential application in biological control actions in coastal lagoons, as regulating service to improve water quality.
Since the predicted effects of global climate change, including sea level rise, most likely will intensify saline intrusions into coastal lagoons (Schallenberg et al., 2003; Jeppesen et al., 2007), some zooplankton could be used as biomonitors, providing direct values to human society. The zooplankton abundance and biomass are very sensitive to changes, thus the response to the disturbance will appear in a short-time for the higher trophic level (Jeppesen et al., 2011; Mackas et al., 2012; García-Chicote et al., 2018). Their short time response, their ubiquity and their trophic relationships make them potential bioindicators for different aquatic ecosystems all over the world (Serranito et al., 2016; Kruk et al., 2021), a cultural service which may be framed as formal knowledge system. Examples of zooplankters bioindicators of extreme environmental fluctuations are Daphnia spp., Acartia cf. fancetti McKinnon, Kimmerer & Benzie, 1992, Brachionus plicatilis (Müller, 1786), Cyclophora sp. and Scrippsiella sp. (Attayde & Bozelli, 1998; Branco et al., 2000; Waterkeyn et al., 2008; Souza et al., 2011; Quintana et al., 2018).
Polychaeta worms
Polychaeta are an abundant component in benthic communities in coastal lagoons (Day et al., 2013; Misturini & Colling, 2021). Species of the families Nereididae (e.g., Laeonereis acuta (Treadwell, 1923)), Capitellidae (e.g., Heteromastus similis Southern, 1921), Nephytidae (e.g., Nephtys fluviatilis Monto, 1937) and Spionidae (e.g., Prionospio heterobranchia Moore, 1907) thrive in most of the habitats of coastal lagoons (Netto et al., 2018).
Polychaetes have large variability in their morphological structure, size, and life history, performing different ecological functions by borrowing into sediments, or living within tubes (Hutchings, 1998). They are common components in the estuarine food chain, acting as primary and secondary consumers, contributing to the trophic complexity of shallow bottoms, and often important in determining the structure of the infauna communities (Ambrose, 1984; Muro-Torres et al., 2019). The estuarine worms form one of the bases of the estuarine trophic web, converting part of the detrital material produced in coastal wetlands into biomass, which becomes available to organisms in other trophic levels, including invertebrates, fish, and birds (Iwamatsu et al., 2007; Caron et al., 2004; Palomo & Irbane, 2000). The benthic secondary production drives matter and energy flow along the food web (Sánchez-Moyano et al., 2017), promoting nutrient cycling (i.e., supporting services). Still, polychaeta biomass in coastal lagoons habitats provide a supporting service since polychaetes are key preys to macrobenthic fish predators in coastal lagoons (Vinagre et al., 2008).
Polychaeta worms are also important to the breakdown and remobilization of sediment, particulate organic and inorganic matter and metabolites due to their intense borrowing activities. Intense bioturbation, modification of sediments through particle reworking and burrow ventilation, is a crucial mediator of many geochemical processes in estuaries (Queiros et al., 2013). Filtering and bioturbator polychaetes increase the percolation of water and nutrients in the sediment, both through the pumping process and through the action of feeding, digging and excretion (Díaz-Castañeda & Reish, 2009). The capacity of polychaetes to affect biogeophysical components of the sediment and to be available as a resource to organisms of other trophic levels are dependent on their morphological and life-history traits (Wouters et al., 2018; Otegui et al., 2016). These functions performed by polychaetas assist with sediment formation and nutrient cycling, both supporting services essential to coastal lagoons.
Polychaetes are numerically dominant in macrobenthic components, and they have diverse functional traits in estuarine ecosystems (Hutchings, 1998; Martins & Barros, 2022). The relationships between structure and function of polychaete assemblages might provide a measure of the resilience of estuarine conditions (Magalhães & Barros, 2011) and might be used as bioindicators of environmental disturbance (Pocklington & Wells, 1992; Giangrande et al., 2005), providing an important scientific and formal knowledge service to monitor coastal lagoons. Furthermore, polychaete species from families such as Arenicolidae, Glyceridae, Lumbrineridae, Nereididae, Nephtyidae, Onuphidae and Eunicidae are widely captured as fishing bait (i.e., provision service) worldwide (see Cabral et al., 2019). These organisms are harvested from intertidal flats habitats in coastal ecosystems, such as Ria do Aveiro and Ria Formosa coastal lagoons (Cabral et al., 2019), underpinning recreational fishing and tourism. Since recreational fishing is associated with ecosystem services, such as provisioning (e.g., food) and cultural (e.g., recreation and social relations), it is notorious that the biomass from polychaete supports these goods to fishers.
Mollusks
Gastropod and bivalve’ mollusks are components of the benthic macroinfauna community (Netto et al., 2018), namely animals larger than most of the sediment particles where they inhabit (McLachlan & Defeo, 2017). In coastal lagoons, both groups are abundant in soft-bottom habitats (Warme, 1969; de Jesús-Carrillo et al., 2020) and the composition of these assemblages is heterogeneous (Morelos-Villegas et al., 2018), responding to variations in water conditions and habitat features (Montagna et al., 2008; Davis et al., 2017; Abdelhady et al., 2019).
Similar to freshwater ecosystems (Vaughn, 2018), the functions performed by mollusk in coastal lagoons may underpin processes that benefit human societies. The sediment reworking activity performed by mollusks (Rhoads & Young, 1970) may disrupt sediments and increase the depth of oxygen in benthic habitats. Abra ovata (Philippi, 1836) is an example of primarily surface deposit-feeders (Charles, 1993) in coastal lagoons (Gontikaki et al., 2003). From its feeding activity, A. ovata promotes frequent and small-scale particle displacement, fostering vertical sediment mixing (Maire et al., 2007). This ecological function can support ecosystem services in coastal lagoons because it contributes to nutrient cycling, oxygen penetration and enhances bacterial decomposition (i.e., releasing carbon and nitrogen).
Furthermore, mollusk’s feeding strategies strongly influence soft-bottom species composition (Hunt et al., 1987; Skilleter et al., 1994). Grazing gastropods are a dominant group in coastal lagoons (Almeida et al., 2008) and intertidal estuarine grazing gastropods as Assiminea globulus Connolly, 1939 shapes communities in unequal ways. Such species increases bacterial density and decreases microphytobenthos density not affecting overall meiofaunal density, but affecting meiofaunal community structure (Pillay et al., 2009). In vegetated habitats, grazers such as Strombus gigas (Stoner & Waite, 1991) and Modulus modulus (Mook, 1977) remove epiphyte algae associated with seagrass leaves, enhancing seagrass photosynthetic rates (van Montfrans et al., 1984). In both cases, grazing functions are regulation services since they perform biological control in coastal lagoon. However, worth mentioning the indirectly influence of grazers on organic carbon sequestration and storage by seagrass ecosystems, contributing to climate regulation of the most efficient natural carbon sink on earth (Serrano et al., 2021).
Suspension feeder bivalves are a common component of benthic habitats in coastal lagoons (Dame, 1993; Wall et al., 2008). For instance, in Bahía Magdalena coastal lagoon (Mexico), Anadara tuberculosa (G. B. Sowerby I, 1833) is more abundant in habitats with silt and mangrove sediments, while Pinna rugosa G. B. Sowerby I, 1835 is more abundant in sand-mud benthic habitats (Jiménez-Quiroz et al., 2021). Both species (and others with similar ecological functions) play a key role in water column particles filtration, removing phytoplankton and other particles in suspension (Dame et al., 2002). As a result of this ecological function, suspension feed bivalves may play a role in eutrophication control, promote water purification and influence nutrient cycling (i.e., carbon; Officer et al., 1982; Doering et al., 1986), which can be framed as regulating and supporting ecosystem services, respectively. Also, the suspension-feeding behaviour of bivalve mollusks promote indirectly nutrients and organic matter transfer from the water column to the bottom (Smaal & Prins, 1993), enhancing macroalgae and seagrass growth (Wall et al., 2008).
The secondary production of mollusks species might be associated with several ecosystem services. Bivalves and gastropods are an important food resource for fish and crustaceans in coastal lagoons (Vega-Cendejas & Arreguín-Sánchez, 2001). For instance, Erodoma mactroides Bosc, 1801 is a known prey for decapods, such as Callinectes sapidus Rathbun, 1896, and function as a vector for the cirriped Amphibalanus improvisus, that lives attached to the gastropod shells (Bemvenuti, 1997). The high availability of prey in some habitats might be associated with a nursery function (Liquete et al., 2016) or with provisioning of habitat, a supporting service necessary to produce other ecosystem services, as the provision of fisheries resources (MEA, 2005). Not only are mollusks a food resource to the aquatic food web, but their biomass is also a valuable resource to fisheries in coastal lagoons worldwide (Pérez-Ruzafa & Marcos, 2012; Jiménez-Quiroz et al., 2021), feeding human populations (i.e., provision service).
Mollusks’ secondary production in coastal lagoons generates a significant biomass of external shells. Even though gastropods and bivalves have very distinct morphologies, both groups have calcium carbonate as the main component of their external carapace (Checa, 2018). Therefore, mollusks may influence the CO2 balance between surface waters and the atmosphere and store carbon in a long-term sink (i.e., climate regulation service). Moreover, the shell deposited adds complexity to the sediment structure in bottom habitats (i.e., sediment formation service), promoting an increase in animal population from epibenthic assemblages (Reise, 2002; Petersen et al., 2010).
The morphology and beauty of mollusks’ shells make them a charismatic group (Morris et al., 2016). Many kinds of shells and other structures (e.g., pearls) are used to craft decoration artefacts or jewels (Haszprunar & Wanninger, 2012). These have direct values to human societies, can be framed as cultural ecosystem services and subcategorized as aesthetic and inspiration services. Mollusk’s exploitation is an ancient activity of citizens in coastal zones. Some of them are illustrious, such as Aristotle and Hippocrates, which created classical zoological and medicine work using shellfish (Voultsiadou et al., 2010), contributing to formal and informal education of current human societies. Information extracted from mollusk’s species provided educational services to the aforementioned ancient human societies in the past, while currently their works are a cultural heritage to the global population. In Latin America, there are big mounds (30 m high and hundreds of meters wide) of Anomalocardia and Phacoides (old Lucina) shells (Rohr, 1984) associated with lagoons’ systems. For instance, in the south of Santa Catarina lagoon complex (southern Brazil) there are dozens of these cultural deposits, which are denominated as “Sambaqui” (Deblasis et al., 2007). They have distinct sizes and stratigraphy, encompassing accumulations with several functions and origins (Gaspar et al., 2008). In the Sambaquis the indigenous people buried the corpses of their peers and had other social rituals (Ferreira & Noelli, 2007). Nowadays, the Sambaquis provides arrays of cultural services, such as research, education, inspiration, cultural heritage, and recreation/touristic options. Another interesting service associated with the gastropod’s shells (named as “Búzios”) is their spiritual value in native South American and Afro-Brazilian ceremonies (Léo Neto et al., 2012).
Macro-crustaceans
The shallow vegetated intertidal areas along coastal lagoons (e.g., mangrove, saltmarsh, seagrass bed) shelter several crustaceans, such as shrimps and crabs in post larvae and juveniles’ phases (Minello et al., 2008; Nagelkerken et al., 2015), populations of adult crabs (e.g., Grapsidea, Portunidae and Xanthidae), freshwater prawns (e.g., Palaemonidea), isopods (e.g., Sphaeromatidae) and amphipods (e.g., Talitridae) (Iribarne et al., 1997; Conde & Díaz, 1989; Bloomfield & Gillanders, 2005; Khemaissia et al., 2018). For some species such as penaeids shrimps, non-vegetated areas may support higher densities (Zimmerman & Minello, 1984), while for others, like blue crabs Callinectes sapidus Rathbun, 1896, non-vegetated and vegetated habitats might be equivalent in terms of abundance and biomass (Rodrigues et al., 2019).
Spatiotemporal variations in environmental conditions shape the macro-crustacean assemblage in coastal lagoons (Fortes et al., 2014). For example, the swimming blue crabs Callinectes spp. are represented by dozens of species (see compilation in Mantelatto et al., 2014) that respond to salinity variations in estuarine ecosystems: C. ornatus is abundant in high salinity, C. danae is a dominant species in a wide range of salinity values, while C. exasperatus is less frequent in high salinity locations (De Carvalho & Couto, 2011). Unequal demography in response to environmental conditions may also occur, for example, when the blue crabs’ females migrate to higher salinity areas after matting, while males remain in the low salinity areas (Norse, 1977; Buchanan & Stoner, 1988).
The complex life histories of macro-crustaceans’ species are related to evolutionary adaptation processes and high environmental heterogeneity, playing essential functions in energy flow and nutrient cycling in the food web (Hines, 2007). Because distinct species make different use of the estuarine habitat (Whitfield, 2017), they also contribute to nutrient exchange within the coastal lagoons and between adjacent ecosystems (i.e., freshwater, brackish and marine). Consequently, several ecosystem services provided by coastal lagoons are tied to life history processes of macro-crustacean species (Newton et al., 2018). For instance, the nursery function provided by the estuarine ecosystem (Beck et al., 2001) may be an indicator of ecosystem condition or be directly related with tangible goods that enhance human well-being (Liquete et al., 2016), such as the maintenance of fisheries stocks (Martinho et al., 2007; Barbier et al., 2011). The nursery function may be classified as a provision of habitat, a supporting service necessary for the production of other ecosystem services (MEA, 2005). One important aspect that might modulate the value of habitat as nursery ground is the prey abundance and availability (Peterson, 2003; Adams et al., 2004; Nagelkerken et al., 2015), such as isopods and amphipods species which are important resources in the estuarine food web (Pasquaud et al., 2008; Wouters & Cabral, 2009).
Burrowing behaviour in soft sediments is widespread among decapod crustaceans (Rice & Chapman, 1971), and might be classified as ecological engineering. In coastal lagoons, some species live within a cavity excavated in the substratum, such as the semiterrestrial grapsid crabs Neohelice granulata (or Chasmagnathus granulatus (Dana, 1851)), which inhabit mudflat habitats (Spivak et al., 1994) and the deep-burrowing thalassinidean shrimps, commonly named as mud shrimp or ghost shrimp, that inhabit soft substrates (Felder, 2001). Other crustaceans move into the substratum and encase their body temporally to avoid predators (Bellwood, 2002), as can be observed with Callinectes spp. (Ward, 2012) and penaeid shrimps (Minello, 2017). Independently of the ecological implications to species that perform them, these behaviours are associated with sediment movement and resuspension (Bellwood, 2002). This bioturbation mechanism provides supporting ecosystem services in coastal lagoons because it contributes to nutrient cycling (e.g., nitrogen), which are essential to the energy flow (Forster & Graf, 1995; Webb & Eyre, 2004). Furthermore, the bioturbation mechanism supports oxygen penetration into sediment, enhancing microbial processes and nutrients transport (e.g., phosphorus and nitrogen) through the sediment–water interface (Adámek & Maršálek, 2013), processes that underpin sediment formation and provisioning of habitat (both supporting services) for benthic communities’ maintenance (Rossi et al., 2008) in coastal lagoons.
The secondary production of crustacean species (Zimmerman et al., 2002) provide direct value as food (provision service), sustaining the livelihood of the local human communities that live in coastal areas (García & Le Reste, 1987; Berkes & Seixas, 2005; Ikhwanuddin et al., 2017). For instance, the penaeids shrimp’s species that grow in coastal lagoons (Manzano-Sarabia et al., 2007; Mosha & Gallardo, 2013) are harvested by artisanal fisheries as small juveniles and subadults (D’incao, 1991; Rivera-Velázquez et al., 2009), being an important food item to human societies (Almudi & Kalikoski, 2010). As a commercial resource, shrimps biomass underpins the fishing activity in several tropical and subtropical coastal lagoons worldwide (Pérez-Castañeda & Defeo, 2001; Macia, 2004; Suradi et al., 2017).
The secondary production of crustaceans also generates chitin, present in their exoskeleton (Jayakumar et al., 2011). Because of this, chitin is present in estuarine systems (Gooday et al., 1991), although its degradation and mineralization to CO2 occur rapidly in these ecosystems (Gooday et al., 1991; Boyer, 1994). In areas where crustacean fisheries are intense as coastal lagoons, the waste or by-product of this activity can be hazardous for the environment, increasing risks of biological (e.g., pathogens) and chemical (e.g., oxygen demand and organic matters) pollution (Bruck et al., 2011; Morganti et al., 2011). However, this waste is a potential high value resource, and might be transformed into chitosan (El Knidri et al., 2018), which has several applications in technological and scientific areas (Philibert et al., 2017). The production of chitin and chitosan provide biochemicals (i.e., provision service) even if currently it is not fully explored.
In general, crustacean fisheries in coastal lagoons have large social, economic, cultural and historical values (Pérez-Castañeda & Defeo, 2001, Sunye et al., 2014), delivering direct cultural services to social actors involved in this activity. As for many other small-scale fisheries activities carried out in coastal lagoons (Machado et al., 2019) as well as in other coastal ecosystems (Pellowe & Leslie, 2021), crustacean fisheries may provide several services, such as sense of place, social relationships, recreational, and cultural heritage values. As fisheries in coastal lagoons are widespread and target several biological groups such as prawns and crabs (Pérez-Ruzafa & Marcos, 2012), several crustacean species are potentially related to cultural services worldwide.
Fish
Fish assemblages are composed of species (and life stages) that occupy a wide range of trophic levels in the food chain and assist with the regulation of trophic structure and ecosystem resilience in coastal lagoons (Ocana-Luna & Sanchez-Ramirez, 1998; Pérez-Ruzafa et al., 2007; Muro-Torres et al., 2019). Fish can use coastal lagoons as their primary habitat (i.e., residents, estuarine dependents), may obligatorily pass through it during some life stage (i.e., catadromous and anadromous species), or enter the lagoons to forage (i.e., amphidromous species) (Nagelkerken, 2009; Potts et al., 2014).). Fish movement (use of habitat) and feeding patterns within the lagoon, and between the lagoon and freshwater or marine environments generate many ecological interactions that assist with sediment formation (i.e., bioturbation), nutrient exchange (i.e., migration) and primary production (i.e., excretion and carcass decomposition) (e.g., Supporting Services, according to MEA, 2005) and connect different ecosystems (Murillas et al., 2020). While undertaking these migrations for reproduction purposes (diadromous) and for habitat use (distinct life stages or trophic guilds), all fishes feed and interact with other organisms, generating regulation of food web dynamics and other processes through organisms’ consumption and trophic cascades (Bueno-Pardo et al., 2018; Muro-Torres et al., 2019; Zheng et al., 2020).
By regulating food web dynamics, fish feeding activities can assist with carbon exchange regulation and contribute to global carbon dynamics and climate regulation (i.e., regulating ecosystem service). For example, coastal lagoons can become sources or sinks of carbon because of the feeding activities of fishes. Lagoons with zooplanktivorous fish can become a carbon sink because zooplankton are consumed, and primary producers (carbon fixers) can be released from grazing pressure. Coastal lagoons with piscivorous fishes can become a carbon source because the piscivorous fish suppress the abundance of zooplanktivores, allowing the zooplankton community to grow. Large zooplankton are expected to grow more than small-sized zooplankton and are more efficient grazers on phytoplankton. Hence, phytoplankton abundance decrease, and primary production can be temporarily suppressed (Guariento et al., 2010, 2011).
Fish feeding activity on phytoplankton can assist with the regulation of eutrophication processes (i.e., water purification service) in coastal lagoons, especially because eutrophication is expected to increase due to climate change (Souza et al., 2018). Net productivity regulation in coastal lagoons from Brazil has been hypothesized to be an outcome of fish consumption of periphytic biomass (Guariento et al., 2010). In a eutrophic coastal ecosystem from Japan, Hypomesus nipponensis McAllister, 1963 and Mugil cephalus Linnaeus, 1758 consumed cyanobacteria directly (Fujibayashi et al., 2018). Although previous studies have already shown that cyanobacteria (common group during eutrophication process) are not a preferable carbon source for many aquatic consumers because of the colony formation, filament shape and toxin production (de Bernardi & Giussani, 1990), these brackish fish species can rely on them whenever available (Fujibayashi et al., 2018), providing an important service of biological control.
By consuming other organisms, fishes help the recycling of nutrients within the aquatic ecosystem because they mineralize nitrogen and phosphorus through excretion and defecation, making these nutrients available for primary production (Naiman et al., 2002; Oliveira et al., 2014). Freshwater subsidies to estuarine fish consumers in Lagoa do Patos, a coastal lagoon from south Brazil, was attributed (among other factors) to freshwater fish dispersal during flood pulses, that contributed with fish-derived nutrients from drainage basin to the lagoon (Garcia et al., 2017). On the other way, the anadromous alewife Alosa pseudoharengus (Wilson, 1811) excreted an average of 24.71 µg N g−1 of wet fish mass per hour during their spring spawning, and because they aggregate in high densities within small coastal streams, they contributed substantially with marine-derived nutrients to the freshwater food web (Post & Walters, 2009). Marine-derived carbon and nutrients are also delivered through the production of gametes and fish carcass decomposition, and contribute to the biomass production of algae, insect larvae, microbial decomposers, and other fishes (Boros et al., 2014). For example, the live and carcasses of salmon increased the concentration of NH4+ and phosphorus in the water column of freshwater streams where they spawn (Janetski et al., 2009). The activities and live strategies undertaken by fish that integrate the nutrient cycling (supporting) in coastal lagoons provide important services such as biomass production (provisioning and cultural) and primary production (supporting) in both coastal ecosystems and coastal-influenced freshwaters (Janetski et al., 2009).
There is a historical belief that fish can be used as biological control of diseases because larvivorous fish can consume the aquatic larval stage of mosquitos (Griffin & Knight, 2012). Since some mosquitoes are vectors of diseases such as yellow fever, dengue fever (i.e., Aedes aegypti Linnaeus, 1762) and arboviruses (i.e., Aedes vigilax (Skuse)), fish feeding behaviour could then provide this ecosystem service. For example, Aplocheilus panchax (Hamilton, 1822), Colisa fasciatus (Bloch & Schneider, 1801) and Gambusia affinis (Baird & Girard, 1853) are all species that inhabit brackish waters and are reported as efficient biocontrolers in experimental conditions and their introduction on aquatic ecosystems are encouraged for biological control (Chandra et al., 2008). However, recent evidence has shown that, whenever other prey is available (i.e., annelids, crustacea, rotifers), larvivorous fish consumes only a small proportion of disease-mosquito larvae (Rowe et al., 2008; Kumar et al., 2015) and might even produce a “disservice” to the society because they can also deplete native aquatic fauna, increase primary production (e.g., eutrophication) and alter nitrogen fluxes through the food web (El-Sabaawi et al., 2016).
Since fishes are one of the most diverse assemblages in coastal lagoons, their population growth and reproduction behaviour contribute to the maintenance of genetic variability (Esteves et al., 2008; Gjedrem, 2012). The genetic material of fish populations serves as a source of information for aquaculture production and biological conservation (Esteves et al., 2008; Suplicy et al., 2015; Cossu et al., 2021). For example, Salvelinus fontinalis (Mitchill, 1814), the brook trout, was selected for increased survival to furunculosis, and over three generations survival rate increased from 2 to 69%, protecting the species (for further examples, see Gjedrem, 2012). Genetic manipulation to increase growth rates for aquaculture production has been done for striped bass in the USA, clarid catfish in Thailand and characids in Venezuela (Bartley et al., 2001). Genetic variability among fishes from brackish environments also enhances the diversity of colour patterns and morphologies that draws attention and fosters the ornamental fishing industry (Pouil et al., 2019).
Coastal lagoons provide habitat to immense biodiversity and sustain high abundance and biomass of fishes (Esteves et al., 2008). Because of this availability, fishes play essential Provision Services that benefit society. One of the most recognizable is food provision that can be delivered through fishing of natural populations or aquaculture. The high fish diversity found in lagoons has always represented a source of income and livelihood for human settlements (FAO, 2015). Fishes are one of the main protein sources for 3.3 billion people in the world (FAO, 2020), and for some countries like Bangladesh, Cambodia, the Gambia, Indonesia, Sierra Leone and Sri Lanka, the highest fish productivity derive from coastal lagoons (Pauly & Yáñez-Arancibia, 1994). The typical marine migrants (i.e., diadromous) species are the most important biomass to support fisheries in coastal lagoons (Pérez-Ruzafa & Marcos, 2012). The global average fisheries production in coastal lagoons is around 109.8 (± 11.2 SE) kg ha−1 year−1. The main catches in coastal lagoons worldwide are of Mugilidae and Sparidae (Pérez-Ruzafa & Marcos, 2012, FAO 2015). Mugil cephalus (Linnaeus, 1758) is an important species for fisheries in the Mediterranean and Australian lagoons (Broadhurst et al., 2003), while Mugil liza Valenciennes, 1836, is an important fishery resource in southern Brazil (Simões-Lopes et al., 1998). Sea lamprey, an anadromous species, supports important commercial fisheries in Portugal, Spain and France (Maitland et al., 2015).
Fish biodiversity and genetic variability benefit society because fish tissues (i.e., skin) and other molecules have been processed and improved for medical treatment provision. Researchers in Brazil are experimenting with an innovative treatment using tilapia skin (e.g., glycerol-preserved skin, dermal matrix) in the treatment of burn wounds (Lima Junior et al., 2019) to accelerate tissue recovery after hand surgery (Monte et al., 2022), to repair and protect the palate after the removal of grafts (in dentistry) (Manfredi et al., 2021), and to apply collagen in the cosmetology and nutrition fields (Alves et al., 2018). Many tilapia species (e.g., Oreochromis niloticus (Linnaeus, 1758), Sarotherodon melanotheron Rüppel, 1852 and Tilapia guineensis (Günther, 1862)) already inhabit or shall be introduced in coastal lagoons for aquaculture (Achoh et al., 2018), with potential for this medical provision. Interestingly, the pufferfish (Tetraodontidae) endosymbiotic bacteria produce a potent neurotoxin (tetrodotoxin; Lago et al., 2015), with a potential disservice to humans since it can provoke intoxication (Hagen et al., 2017). However, due to medical research and development, it can now be used in the medical field as a powerful analgesic to treat cancer pain (Hagen et al., 2008, 2017).
All the ecosystem services mentioned above stimulate human development around coastal lagoons and subsidize traditional populations with economic, physical and experimental interactions with the natural environment. The population that directly depends on fish from coastal lagoons and those attracted as consumers and users enhance cultural diversity within these systems. Fish supply Cultural Services via recreational activities such as sport fishing of wild and stocked fishes (Alberini et al., 2007) and snorkelling (e.g., Yal Ku Lagoon, in Mexico and Tambaquis’ Lagoon in Brazil). Sacred and religious rituals are also important for fishers, that ask for safety high catches during fishing trips. In Irakkam island, fishers participate in religious rituals to ask for supernatural powers, good catch, security and prosperity, which include using new fishing boats and worshipping clan deities (Reddy, 2020). In Brazil, records show fishers using sun-dry sea-horse Hippocampus reidi Ginsburg, 1933 as a pendant for good luck and good fishing (Pinto et al., 2015).
Fish biodiversity (i.e., genetic, biomass and abundance) of coastal lagoons promotes socialization areas and provide territorial identity and belonging because fishers and local population use coastal lagoon since childhood, and the attraction to coastal lagoons is often connected to fish. Some of the most harvested species in coastal lagoons in terms of biomass (provisioning services) are also used in local culinary specialty with a rich history of use that is appropriated as cultural heritage (Almeida et al., 2021). For example, during mullet (Mugil liza Valenciennes, 1836) migration in southern Brazil (Lemos et al., 2014), some beaches are closed for other use (i.e., navigation or surfing) to prevent shoals from driving away from the coast, and to facilitate fish access to coastal lagoons. Fishers mobilize, prepare spotting huts and induce the opening of coastal lagoon sand bars to drive mullets inside the lagoons for further fishing. Spotting fish knowledge are passed along to young generations. Fishers commercialize fishes directly on the beach and nearby coastal lagoons, attracting tourists and residents. They also commercialize in fish markets and provide fish for local cultural festivities. Other traditional festivals take place in Europe. In Portugal, species such as the eel (Enguilla anguilla Linnaeus, 1758) and European flounder (Platichthys flesus (Linnaeus, 1758)) are considered “a pride of the local gastronomy” and are the main attraction of the Festival da Enguia da Lagoa de Santo André and Festa das Solhas de Lanhelas, respectively (Caminha, 2022; Santiago do Cacém, 2022). In the Iberian Peninsula, Sea lamprey (Petromyzon marinus Linnaeus, 1758) has been harvested for centuries. Dozens of gastronomic festivals take place every year in Portugal, during which hundreds of thousands of lampreys are consumed and belong to Portuguese and Galician cultural heritage (for further details, see Almeida et al., 2021). These festivals strengthen ties between the community and coastal lagoons, strengthening the symbolic meaning of fishing and increasing the sense of belonging of fishers and local populations.
Another important cultural service is the scientific one (Lillebø et al., 2015). Fish from coastal lagoons are subject of interest for research (Brehmer et al., 2013; Azevedo et al., 2017; Andrade-Tubino et al., 2020). Several scientific topics addressing fish in coastal lagoons are investigated, such as community structure (García-Seoane et al., 2016; Manzo et al., 2016; Petry et al., 2016; Azevedo et al., 2017), fisheries (Pauly & Yáñez-Arancibia 1994, Pérez-Ruzafa & Marcos, 2012, FAO 2015, Haimovici & Cardoso 2017), functional diversity (Mouillot et al., 2007; Franco et al., 2021) and population aspects (Franco et al., 2014), significantly increasing scientific knowledge and attracting researchers for different studies. Although scientific and technical knowledge have been done with fishes from coastal lagoons, their ecology and ecosystem services provisioning still lack recognition among citizens and educators. This situation represents an important gap to be filled by the scientific and educational sectors to introduce relevant concepts in formal education and through Ocean Literacy resources, such as the Environmental Education Network for Ecosystem Services in Portugal (Barracosa et al., 2019). Finally, fishes are already used as bioindicators to assess aquatic habitat degradation in different ecological levels (Santhanam et al., 2010; Verdiell-Cubedo et al., 2012), and this knowledge could be easily translated to formal education activities. Physiological alteration can aid the comprehension of potential risks to wildlife and humans, while population and community alterations can be an early-warning signals of anthropogenic stress that need to be managed by decision-makers (Reis-Filho & Giarrizzo, 2016).
Birds
Birds comprise a diverse group with a broad range of diets and foraging behaviour, from nectar-eating birds (Trochilidae) to piscivorous species consuming almost every type of fish prey (Gheler-Costa et al., 2018; Dalsgaard et al., 2021). In coastal lagoons, the bird assemblages may be organized in foraging habits guilds, such as dabbling birds (ducks), diving birds (cormorants), waders (Ciconiiformes and some Charadriiformes), fishing birds (Kingfishers, gulls and Sterns), filter feeders (Flamingos and some ducks) and insectivorous birds (Flycatchers and some passerines), evidencing the broad range of resources used by birds on coastal landscapes (Tavares et al., 2015). Coastal lagoons support a high diversity of residents and migrating birds due to their high biological productivity and habitat heterogeneity (Kennish & Paerl, 2010), which provides nests, roosting and foraging sites (Sánchez & Rodríguez, 2000; Kularatne et al., 2021). The diversity of birds on coastal lagoons is affected by seasonality, waterbody ecological features and anthropogenic activities, such as habitat degradation, unsustainable hunting and the presence of exotic species (O'Connell, 2000; Dias et al., 2019). Still, stochastic events, such as storms, winds and floods, potentially generate perturbations that may affect birds or the resources they depend upon (Kennish & Paerl, 2010).
Birds feeding over aquatic organisms create a direct link between terrestrial and aquatic systems, assisting with nutrient exchange between habitats through birds’ excretion. Feeding activities of seabird and waterbird guano influence nutrients cycling and enhance primary production (i.e., supporting services) from benthic component in favour of the planktonic pathway in inland coastal aquatic ecosystems (Gagnon et al., 2013; Vizzini et al., 2016). The bird droppings in nesting and roosting sites adjacent to coastal lagoons generate guano deposits, changing soil composition, vegetation, and microbial communities (Kolb et al., 2015), which comprises a supporting service by soil formation and nutrient cycling (Costanza et al., 2017). Seabird and waterbird guano are known as seabird white gold because it has high contents of nitrogen and phosphorus and has been used as a natural fertilizer for over 2000 years (Schnug et al., 2018; de la Peña-Lastra, 2021), providing and important biochemical product to society. The guano production in coastal lagoons is associated mainly with colonial birds, such as cormorants, terns and gulls (Palomo et al., 1999; Gagnon et al., 2013), which is a valuable raw material to agricultural activities (i.e., provisioning service), generating income to local communities.
Bird predation also provides biological control of pests or dominant species in the aquatic ecosystems, especially on closed coastal lagoons (Esteves et al., 2008; Green & Elmberg, 2014). For instance, Nannopterum brasilianus (Gmelin, 1899) (neotropical cormorant) have broad plasticity on feeding habits, and its predation controls the invasive exotic salmonids in Patagonian freshwater ecosystems (Alarcón et al., 2012). Likewise, diving ducks have the potential to control the exotic zebra mussels, reducing the invasive species biomass drastically (Hamilton et al., 1994). Because neotropical cormorants and diving ducks have a wide distribution on Neotropical ecosystems, encompassing coastal lagoons, these species undoubtedly play a role in controlling the abundance of invasive species.
Bird predation generates top-down effects on the food chain that shape fish and invertebrates’ communities in coastal ecosystems (Rodríguez-Pérez & Green, 2012; Gheler-Costa et al., 2018), promoting the coexistence of species from different taxa, like fish, shrimp, and crabs (Rodríguez-Pérez & Green, 2012; Östman et al., 2013). Considering that predation can remove top-predator aquatic animals, secondary consumers abundance and biomass enhances.
Coastal lagoons avifauna can enhance biodiversity-based cultural activities (Sánchez & Rodríguez, 2000; Graves et al., 2019). Birdwatching is an activity that results in high economic benefits, potential conservation management, and collaborative science (Greenwood, 2007; Santos et al., 2019). A very illustrative case is the Celestum Lagoon (Mexico), where Flamingo watching tourism was evaluated in US$16 million/year (Galicia et al., 2018). Birdwatching can be associated with several cultural services, such as knowledge systems, education, aesthetics, social relations, and recreation/tourism. Due to their conspicuousness, beauty, and vocalizations, birds are appreciated and fed in recreational activities, generating benefits to human welfare (Whelan et al., 2008), which might be associated with spiritual and religious, educational, inspiration, aesthetic and recreation/tourism services.
Public interest in some waterbirds such as ducks, swans and geese has opened the door to protection and conservation of many water bodies, including coastal lagoons, as well as the creation of conventions and non-governmental organizations, such as the Ramsar Convention in 1971 and Wildfowl & Wetlands Trust (UK) (Green & Elmberg, 2014). Such initiatives promote education (educational values, social relations) and environmental awareness (sense of place) activities for the protection of wetlands areas, as the Detwah coastal lagoon (Yemen), an important island to biodiversity in the Arabian Sea (Veettil et al., 2020).
Birds on coastal lagoons also have religious importance for ancient and contemporaneous society. Two illustrative examples are the greater flamingos, Phoenicopterus roseus Pallas, 1811, illustrated in Palaeolithic caves in Spain (Lazarich et al., 2019) and the sacre-ibis, Threskiornis aethiopicus (Latham, J 1790), a waterbird species revered that was found mummified and illustrated on Egyptian walls (Wasef et al., 2015). In both cases, it is assumed that these bird species provided inspiration, knowledge systems and spiritual and religious values to ancient civilizations, while currently, besides spiritual values, these illustrations are an important cultural and educational heritage for humanity.
Aquatic mammals
Aquatic and semi-aquatic mammals comprise a diverse group of species that share anatomical (e.g., integument and body shape) and physiological (e.g., thermoregulation, musculoskeletal systems) specializations according to their level of dependence on water (Reidenberg, 2007). Coastal lagoons contain a mosaic of wetlands, mangroves, saltmarshes and seagrass meadows (Basset et al., 2013), which provides vital habitats for a rich diversity of lower trophic level species (i.e., invertebrate and fish) and, consequently, favourable foraging environments for some species of aquatic and semi-aquatic mammals. Interestingly, aquatic and semi-aquatic mammals are not a common component in coastal lagoons. For example, a search in the EcoBase platform (Colléter et al., 2015)—a repository for data from ecosystem models based on the Ecopath framework (Polovina, 1984)—shows that only one from the eighteen models built for coastal lagoons has mammals in its trophic network; see Milessi et al., 2010).
Although the occurrence of aquatic and semi-aquatic mammals in coastal lagoons is occasional, when it happens, they can modulate the structure and stability of these habitats, in particular by their feeding activity. Their high trophic levels and prey consumption rate regulate the abundance of prey populations (Heithaus et al., 2008) in which their depletion can trigger a series of cascading events through lower trophic levels (Terborgh & Estes, 2013). In addition, aquatic mammals can also forage on a variety of food resources, from different trophic levels, which turn it into a challenge to predict their ecological role and consequences for an ecosystem. For instance, the semi-aquatic Lontra longicaudis (Olfers, 1818) is a top predator in the Laguna de Rocha ecosystem that feeds on crabs and freshwater (e.g., Hoplias malabaricus (Bloch, 1794)) and marine (e.g., Flatfishes) fish species (Milessi et al., 2010). The bottlenose dolphin (Tursiops truncates Montagu, 1821) is a generalist predator (Rossman et al., 2015), that feeds on fish species (e.g., Mugilidae) and molluscs (e.g., Sepia officinalis Linnaeus, 1758) in coastal lagoons and estuarine ecosystems (dos Santos et al., 2007).
Due to their relatively high metabolic rates (Bowen, 1997; Estes, 2009), aquatic and semi-aquatic mammals consume a significant amount of biomass and release a high amount of nitrogen in their faecal plumes (Roman & McCarthy, 2010; Roman et al., 2016), enhancing primary production (supporting ecosystem service) on their foraging grounds. They can also provide supporting services in coastal lagoons by increasing the cycling of nutrients through horizontal movements carried out between seascape’s ecosystems and their microhabitats (Katona & Whitehead, 1988; Roman et al., 2014; Kiszka et al., 2022). These large-sized animals also provide regulating services by accumulating tons of carbon during their long lives that will not only generate food and habitat for communities of micro and macro-organisms (Pershing, 2010) but also release all the stored carbon to the atmospheric cycle (Quaggiotto et al., 2022) when their carcasses eventually sink to the bottom habitats.
The biomass of aquatic mammals’ populations is also associated with provision services to several indigenous coastal communities (Reeves & Smith, 2006) where hunting is supported and managed by the International Whaling Comission. The beluga whale Delphinapterus leucas (Pallas, 1776) and harbour seal Phoca vitulina Linnaeus, 1758, for example, are some of the Arctic marine mammal species harvested by Chukchi communities in coastal Arctic lagoons (Neakok, 1985; Wolfe et al., 1999) to be primarily used as food resource, while skin and other raw materials are used for clothing, equipment and ornamental on a local and small scale.
Non-consumptive interactions with aquatic mammals, such as cooperative fishing between fishers and delphinids in coastal lagoons also represent significant cultural services in some regions of Australia, Mauritania, Myanmar and Brazil (Fairholme, 1856; Pryor et al., 1990, Machado et al., 2019; Smith et al., 2009, Kumar et al., 2012). For example, the threatened Irrawaddy dolphin Orcaella brevirostris (Owen in Gray, 1866) population from Ayeyarwady river, in Myanmar, herd fish schools (mainly mullet) from murky or deeper waters towards cast-net fishers standing in small canoes awaiting specific behaviours by the dolphins perceived as cues or ‘signal’ to where and when casting their nets, increasing catching rate (Smith et al., 2009). A subset of small and highly resident Lahille’s Bottlenose dolphin Tursiops truncatus gephyreus Lahille, 1908 populations from Laguna and Barra de Imbé/Tramandaí, southern Brazil, also engage in such cooperative behaviour with local artisanal fishers (Simões-Lopes et al., 1998). Similarly, these dolphins detect, aggregate and drive fish schools (mainly mullet) towards shallow waters where fishers stand in line or on moored canoe. Then, dolphins also display behavioural cues that fishers understand as a signal to cast their nets. For both cases, although the benefits to dolphins remain inconclusive, it is hypothesised that dolphins are more easily able to access fish schools disrupted by barriers or fishing gear (Simões-Lopes et al., 1998). Interestingly, the artisanal fishers from Laguna perceive and recognize that this human-dolphin cooperation provides not only food but also recreation leisure to tourists, as well as a sense of place, social relations, aesthetics, cultural heritage values, knowledge system, and cultural diversity to their community (Machado et al., 2019).
Impacts on costal lagoon biodiversity and consequences in ecosystem services provision
Coastal lagoons provide a wide spectrum of ecosystem services (Newton et al., 2018). In a recent effort, Pérez-Rufafa et al., (2019) highlighted 58 ecosystem services related with processes and attributes from coastal lagoons. Also, the authors highlighted the importance of geomorphological features and ecological processes to coastal lagoons functioning. In our review, we aimed to complement this knowledge, through a more specific understanding of how species functions generate ecosystem processes and services in coastal lagoons. By specific examples, we highlighted how the fauna that inhabits coastal lagoons perform a broad range of ecological functions and may have broad-scale implication for a variety of services. However, both species and ecosystems have been subject to a continuous intensification of human pressures worldwide (Tundisi et al., 2015; He & Silliman, 2019). Coastal areas are the most populated regions of the world (Harley et al., 2006), and are subjected to multiple human-driven impacts associated with regional and local land-use changes (i.e., industrialization, urbanization, agriculture and aquaculture). In addition, the potential future effects of global climate changes and ocean acidification will extensively impact the functioning, survival and spatial distribution of the aquatic (and aquatic-dependent) fauna in coastal lagoons accelerating species loss—a primary and readily worrying threat to ecosystem services provision (Díaz et al., 2006; Cardinale et al., 2012; Loreau & de Mazancourt, 2013).
The stability and resilience of coastal lagoon ecosystems are affected by biodiversity loss (Bec et al., 2011; Loreau & de Mazancourt, 2013; Thibaut & Connolly, 2013), because biodiversity components and ES have an intricate co-dependent complex relationship (Loreau et al., 2001; Hooper et al., 2005; Balvanera et al., 2006; Gamfeldt et al., 2008; Cardinale et al., 2012). Hence, linking socio-economic systems with the uses of ecosystem services necessarily encompasses the historical transformations of human occupations, land use reclamation, and the related impacts—direct or indirect—on biodiversity (He & Silliman, 2019; Pérez-Ruzafa et al., 2019; Thanh et al., 2020).
Direct causes of biodiversity loss in coastal lagoons include nutrient enrichment, pollution with pesticides and heavy metals, alteration of morphology and hydraulics (with consequent physical and chemical water changes), overfishing and the invasion of exotic species (Newton et al., 2014; Vasconcelos et al., 2017; Teichert et al., 2018; Elliott et al., 2019). All of these threats occur simultaneously, with distinct frequency and magnitude depending on the coastal lagoon. So, in this section we intend to highlight general patterns and specific examples, that are not meant to cover all possible impacts to these ecosystems and their related services.
Nutrient enrichment can derive from different human activities and sources (e.g., input of sewage, fertilizer leaching and runoff from agricultural fields, and mariculture). Increases in nutrient concentrations are more intense in shallow lagoons, and in those with intermittent or no connection with the sea, due to little water renewal (Glibert et al., 2007; Wazniak et al., 2007; Fertig et al., 2013). Increased nutrient concentration boost primary production (i.e., a process known as eutrophication) and the consequent outcome are context-dependent (i.e., landscape, proximity to river inputs), because the composition of primary producers may differ following nutrients enrichment and change the energetic base of the food web (Nixon, 2009; Glibert et al., 2010). For example, studies conducted in Florida Bay and the Chincoteague Bay, in the United States, showed that lagoons in which food webs are based on diatoms presented more zooplankton grazers, ultimately supporting a large biomass of secondary producers. In contrast, in lagoons where phytoplankton biomass is dominated by flagellates or cyanobacteria (i.e., Prorocentrum cordatum (Ostenfeld) Dodge, 1976, Microcystis sp.), the system sustains a proportionately greater flow through the microbial loop (Legendre & Rassoulzadegan, 1995; Glibert et al., 2010). Eutrophic conditions may lead to a decrease in fish richness while enhancing fishery yield in coastal lagoons (Pérez-Ruzafa et al., 2007). The higher fishing resource availability may foster food, income, and cultural provision values in coastal lagoons. However, in chronic eutrophication cases, the effects are deleterious, including hypoxia or anaerobiosis, harmful algal blooms, massive animal mortalities and changes in species patterns (Pérez-Rufaza et al., 2012), which may impact longstanding and negatively the provision of ecosystem services.
In some coastal lagoons, rooted macrophytes may be replaced by macroalgae or phytoplankton after nutrient enrichment (Wazniak et al., 2007), and because the structural complexity provided by macrophytes are vital for the recruitment of many coastal fish species, such as Rutilus rutilus (Linnaeus, 1758) and Perca fluviatilis Linnaeus, 1758 (Snickars et al., 2009), their abundance and biomass might be severely reduced, preventing coastal communities of maintaining fisheries, tourism and cultural heritage activities. Eutrophication in coastal lagoons also drives hypoxia because algal blooms form mats in the water surface and reduce light availability underwater, and prevents submerged primary producers to carry out photosynthesis, driving both submerge fauna and flora death (D’Avanzo & Kremer, 1994). In Negombo lagoon, in Sri Lanka, hypoxic events have been frequently registered in both wet and dry seasons, and were related to high seawater temperature, poor water circulation and high nutrient loadings (Hsieh et al., 2021). Raw faces and uncollected solid waste have been dumped into the lagoon’s water, reducing water quality and fishing stocks due to unfavorable ecosystem conditions, imposing real threats to tourism and fisheries in the lagoon (Hsieh et al., 2021). Additionally, cyanobacterial blooms have the potential to release toxins (i.e., microcystin) that are incorporated into the coastal lagoon’s food web (Bukaveckas et al., 2017). Elevated microcystin levels have persisted 7–8 years after cyanobacterial bloom in Curonian lagoon, and extended to several consumers including benthivorous, planktivorous and piscivorous fishes (Bukaveckas et al., 2017), imposing potential risk to human health and economic activities of coastal communities (Ferrão-Filho & Kozlowsky-Suzuki, 2011; El Mahrad et al., 2020).
Contamination of coastal water with pesticides and heavy metals are also a prominent threat because toxic and persistent substances have the potential to bioaccumulate along the food web and be retained in the sediment (Odjer-Bio et al., 2015), posing direct and chronic threats to human health. Amphipod species (i.e., Orchestia montagui Audouin, 1826 and Porcellio scaber Latreille, 1804), for example, grow less in heavy metal-contaminated lagoons in Tunisia because they invest more energy to avoid contamination and are less able to survive due to biochemical alterations and cellular mutations from the intoxication (Jelassi et al., 2020). And while feeding in the sediment, amphipods can make toxic compounds previously retained in the sediment available in the food web. Fish and crustacea are also vulnerable to intoxication in contaminated lagoons, and poses additional risk to coastal communities that relies mostly on the food and income provisioning of fisheries and mariculture within the lagoons (Márquez et al., 2008; Ramírez-Ayala et al., 2021).
Fish and crustaceans, are the two most important resource for the livelihoods of local communities and to regional economies, harvested through fishing and mariculture. Nevertheless, overfishing is a major threat to coastal lagoons due to the removal of biomass of specific taxa (i.e., with commercial values) to attend economic needs, but prevents the ecosystem to maintain the natural balance through species interactions (especially trophic ones). The commercially exploited wild population of the manila clams, Venerupis (Ruditapes) philippinarum (A. Adams & Reeve, 1850) have been declining in the Pialassa Baiona lagoon due to low recruitment and low population recovery due to overfishing (Ponti et al., 2017), reducing water purification service (i.e., through filtering) and food and income provisioning to coastal communities. In Langebaan lagoon, during stock assessment of the southern mullet Chelon richardsonii (Smith, 1846), the authors found that overfishing reduced current spawner-biomass-per-recruit to only 24% of the pristine (unfished) levels, indicating a stock at risk of recruitment failure (Horton et al., 2019).
Alteration of morphology and hydraulics can occur due to embankments for construction purposes and artificial sand bar opening. In Mar Menor lagoon, in Spain, dredging and enlarging of its inlets led to the colonization of the algae Caulerpa prolifera (Forsskal) J.V.Lamouroux, 1809, changing the lagoon’s bottom sediment, that was previously dominated by the phanerogam Cymodocea nodosa (Ucria) Asch. And benthic microalgae. The progressive increase in the organic matter content of the sediments lead to changes in the faunal assemblages and strong decrease in fishing yields, especially of mugilids and sparids (Pérez-Ruzafa et al., 2020). Recreational boating and the support infrastructure (i.e., marinas) can also degrade coastal lagoons habitat through aquatic vegetation removal (Hansen et al., 2019), which in turn reduce sediment stabilization, nutrient uptake and storage (Moksnes et al., 2018), and ultimately reduce densities of fish (i.e., pike [Esox lucius Linnaeus, 1758] and juvenile perch [Perca fluviatilis]) (Diehl & Eklöv, 1995; Craig, 2008). Interestingly, Hansen et al., (2019) compared the effects of boating-macrophyte removal to that of eutrophication.
Artificial sand bar opening justification are manyfold, such as to improve fisheries, to reduce water level to avoid inundation of residences nearby, to improve water quality by exporting nutrients to the sea and to reduce hypoxia risks (Esteves et al., 2008). However, negative consequences include osmo-regulatory stress of species adapted to low salinity gradients (Esteves et al., 2008 and reference therein), reduction of photosynthesis of phytoplankton due to increased UV radiation (Conde et al., 2000), and inefficient effect on eutrophication control, because internal loads of sediment phosphorus regeneration quickly enhance phosphorus concentrations in the water column and maintain eutrophic conditions in some lagoons (Esteves et al., 2008). For example, in Iquipari lagoon, sand bar opening undergone a regime shift from a macrophyte-dominated system to an opportunistic phytoplankton dominated system (Suzuki et al., 2002), altering the entire ecosystem metabolism, changing species biodiversity and composition and ultimately affecting nutrient cycling (i.e., support service), and food and tourism provision.
In addition to the physical habitat and water quality alterations, coastal lagoons can be more susceptible to species invasion because of its connectivity with terrestrial, freshwater and marine ecosystems. Negative effects from invasions on coastal shallow lagoons are expected to rise, because brackish invasive species are stress-tolerant to a broader range of stressors (e.g., Cercopagis pengoi (Ostroumov, 1891); Mnemiopsis leidyi A. Agassiz, 1865; Tilapia sp.; Moina macrocopa (Straus, 1820)) (Katsanevakis et al., 2014; Franco et al., 2021; Macêdo et al., 2022). The introduction of fishes in coastal lagoons, such as Tilapia, can cause both services and disservices to society (Deines et al., 2016). Tilapias can enter the coastal lagoons due to evasion from aquaculture in the vicinity or be introduced for mariculture (Watanabe et al., 2010). These species are of high commercial value to artisanal and sports fisheries, generating income to local communities (Agbekpornu et al., 2016). The “grain-to-feed conversion rates” for fish are equivalent to those of chicken, and far more economical than pork or beef (Canonico et al., 2005). It can cause disservices because it can promote decline in many native fishes, through predation, parasitism or competition (Canonico et al., 2005). It can also cause reduction of aquatic plants and the habitat they provide to native species (Canonico et al., 2005).
In Vistula lagoon, in Poland, the introduction of the alien gammarid species (Gammarus tigrinus Sexton, 1939, Dikerogammarus haemobaphes (Eichwald, 1841), Pontogammarus robustoides (Sars, 1894) and Obesogammarus crassus (G.O. Sars, 1894)) induced nearly total decline of native populations of the marine crayfishes Gammarus zaddachi Sexton, 1912 and Gammarus duebeni Lilljeborg, 1852. The authors attributed it to competitive exclusion, but also to a lowered biotic resistance of the recipient community, mostly associated with increasing pollution and eutrophication of the lagoon (Grabowski et al., 2006). In contrast, in preserved coastal lagoons, such as the Taman Bay, the native macrozoobenthos community was resilient to the alien Anadara kagoshimensis (Tokunaga, 1906). The alien arrived in 2003, and a decade latter macrozoobenthos abundance did not depend on whether the alien or indigenous species were dominant, and the authors attributed this outcome to the absence of persistence disturbances in the lagoon (Kolyuchkina et al., 2019). Intermediate outcomes have also been reported. For example, the introduction of the dark false mussel Mytilopsis leucophaeata (Conrad, 1831) into an urban coastal lagoon in Brazil improved lagoon water quality, and this was perceived as a good outcome. However, it could also increase phosphorus availability through excretion because the species forms dense clusters in newly invaded areas, and might induce additional prejudices to native species in the long term (Neves et al., 2020). The consequences of species invasion in coastal lagoons are context-dependent and data-poor, which limits our ability to draw conclusive inferences about ecosystem services loss due to invasion. However, as an overall pattern, in the long term, alien species are most likely to change ecosystem metabolism and food web structure with direct changes in the supporting capacity of coastal lagoons.
If not enough, beyond local and regional changes to coastal lagoons, we are facing a global changing climate, in which coastal lagoons may experience significant variation in rainfall, temperature, and fluctuations in sea level that could also change the salinity and thermal regime of the habitat (Hawkins, 2012, 2016)—key drivers of biodiversity in coastal lagoons. Nevertheless, if climate change predictions become true (IPCC, 2021), floristic and faunistic composition and ecosystem structure are likely to collapse since future conditions could be unable to accomplish the main processes needed to mitigate impacts or provide ecosystem services. For example, global warming is predicted to indirectly affect coastal lagoons from nutrient uptake and resistance to eutrophication (Lloret et al., 2008) to the timing and route of migrating birds (Gatter, 1992). The combined effects of increased temperature, ocean acidification and sea-level rise (Range et al., 2012; Roberts et al., 2017), combined with local stressors (Mahapatro et al., 2013), will likely stress human communities that value the presence of particular lagoons. Predicted abrupt climatic variability through higher water evaporation leading to extreme shallowness will increase salinity resulting in the loss of nursery and mating habitats, affecting commercially important taxa (e.g., shrimps, crabs, and many fish). These conditions can influence the bioavailability and toxicity of contaminants as metals (Vazquez et al., 1999) and the hydrocarbons derived from petroleum (Ramachandran et al., 2006). Impacts have led to increasing specialization and adaptation to a narrower range of abiotic conditions, such as those that can be experienced within lagoonal habitats (Telesh et al., 2011), there is nevertheless an increasing risk of population declines and local extinctions, considering unknown effects of multiple stressors acting together (Martínez-Megías and Rico, 2021).
Final remarks
Coastal lagoons connect freshwater-brackish-marine and terrestrial habitats and support a high diversity of animal assemblages that ranges from microbes to aquatic mammals. From the review of these assemblages (we did not exhaust all of biodiversity components), we could clarify through a cause-effect perspective how species trophic interactions and habitat use (i.e., species functions) are intrinsically linked with ecosystem processes and services provided by coastal lagoons (Fig. 2). We identified 26 ecosystem services categorized as supporting (5), regulating (4), provisioning (7) and cultural (10) services that are associated with functions performed by several animal groups, highlighting a high functional redundancy between them. Besides, it was notorious that species functions from distinct groups might be complementary to compose ecosystem processes and services, evidencing that the species loss might affect both (i.e., processes and services).
Fig. 2.
Diagram of the pathways from species functions to ecosystem processes and services in coastal lagoons. The most relevant functions assumed for the biological groups described in this study are responsible for a number of processes which in turn are crucial for the provisioning and regulating (A), supporting (B) and cultural (C) services. The numbers from 1 to 8 represent each of the biological groups (1: microbes; 2: zooplankton; 3: polychaetas; 4: mollusks; 5: macro-crustaceans; 6: fishes; 7: birds; 8: aquatic mammals). Dashed arrows represent the proposed links connecting the pathways
Owing to animal assemblages having temporal and spatial asynchrony to use coastal lagoons habitats and that these ecosystems suffer from deleterious impacts from point and nonpoint sources, the conservation and management of coastal lagoons should be carried out through broad lens, to ensure species diversity, habitat heterogeneity and ecosystem functioning. The use of the ecosystem services’ approaches on the watershed scale with multi-actors’ collaboration should be adopted in management plans, to maximize the ecosystem services provision by lagoons and, consequently, the well-being of human societies, especially in coastal zones and densely populated areas worldwide.
Acknowledgements
We would like to thank Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) for providing the financial and infrastructure support to carry out this work: JLRF (PAP2019031000116); SAN (FRH2019091000036); PH (FAB2019121000025). We thank Coordination for the Improvement of Higher Education Personnel (CAPES) for granting postdoctoral funding to VMC and to support the functioning of the authors working group. We thank Brazilian National Council for Scientific and Technological Development (CNPq) to support functioning of the PH working group
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: JL Rodrigues-Filho. Literature search on microbial communities, topic preparation: CA. literature search on zooplankton, topic preparation: RLM; OR; JLR-F. Literature search on mollusk, topic preparation: PRP; JLR-F. Literature search on polychaeta, topic preparation: RMS, JM JLR-F, SAN. Literature search on crustaceans, topic preparation: J.L. Rodrigues-Filho. Literature search on fishes, topic preparation: NCLS; VMC. Literature search on birds, topic preparation: VRAP; JLR-F. Literature search on aquatic mammal, topic preparation: CRT; JLR-F. Literature search on impacts to coastal lagoons: RLM, VMC; JLR-F. draft preparation: JLR-F; RLM; HS; VMC. Draft critical revision: JLR-F; RLM; HS; VRAP; CA; CRT; PRP; SAN; NCLS; FGD-J; OR; PH; JOB; RS; JM; VMC.
Funding
This work was funded by Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) to JLRF (PAP2019031000116), SAN (FRH2019091000036) and PH (FAB2019121000025). This work was supported by a master grant from Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) to RMS. Full time regime funded Comisión Sectorial de Investigación Científica, Universidad de la República to CA. And a postdoctoral grant from Coordination for the Improvement of Higher Education Personnel (CAPES) to VMC.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors reviewed the results and approved the final version of the manuscript.
Footnotes
Guest editors: Verónica Ferreira, Luis Mauricio Bini, Katya E. Kovalenko, Andre A. Padial, Judit Padisák & María de los Ángeles González Sagrario / Aquatic Ecosystem Services.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelhady AA, Khalil MM, Ismail E, Fan J, Zhang S, Xiao J. Water chemistry and substrate type as major determinants of molluscan feeding habit and life-mode in lagoon sediments. Estuarine, Coastal and Shelf Science. 2019;220:120–130. doi: 10.1016/j.ecss.2019.02.019. [DOI] [Google Scholar]
- Achoh ME, Agadjohouede H, Gangbe L, Laleye KRB, Vodougnon MH, Laleye P. Impact of dredging on the ichtyofauna of the lowlands: comparison of the reproduction parameters of Sarotherodon melanotheron populations (Rüppell, 1852) of dredge lowlands and the coastal lagoon at togbin in southern Benin. International Journal of Fisheries and Aquatic Studies. 2018;6:344–350. [Google Scholar]
- Adámek Z, Maršálek B. Bioturbation of sediments by benthic macroinvertebrates and fish and its implication for pond ecosystems: a review. Aquaculture International. 2013;21:1–17. doi: 10.1007/s10499-012-9527-3. [DOI] [Google Scholar]
- Adams AJ, Locascio JV, Robbins BD. Microhabitat use by a post-settlement stage estuarine fish: Evidence from relative abundance and predation among habitats. Journal of Experimental Marine Biology and Ecology. 2004;299:17–33. doi: 10.1016/j.jembe.2003.08.013. [DOI] [Google Scholar]
- Agbekpornu H, Yeboah D, Quaatey S, Pappoe A, Ennin JE. Value chain analysis of captured shrimp and tilapia from Keta lagoon in Ghana. Asian Journal of Agricultural Extension, Economics & Sociology. 2016;14:1–11. doi: 10.9734/AJAEES/2016/30829. [DOI] [Google Scholar]
- Aislabie J, Lloyd-Jones G. A review of bacterial degradation of pesticides. Australian Journal of Soil Research. 1995;33:925–942. doi: 10.1071/SR9950925. [DOI] [Google Scholar]
- Alarcón PAE, Macchi PJ, Trejo A, Alonso MF. Diet of the Neotropical Cormorant (Phalacrocorax brasilianus) in a Patagonian freshwater environment invaded by exotic fish. Waterbirds. 2012;35:149–153. doi: 10.1675/063.035.0115. [DOI] [Google Scholar]
- Alberini A, Zanatta V, Rosato P. Combining actual and contingent behavior to estimate the value of sports fishing in the Lagoon of Venice. Ecological Economics. 2007;61:530–541. doi: 10.1016/j.ecolecon.2006.05.003. [DOI] [Google Scholar]
- Almeida C, Coelho R, Silva M, Bentes L, Monteiro P, Ribeiro J, Erzini KE, Gonçalves JM. Use of different intertidal habitats by faunal communities in a temperate coastal lagoon. Estuarine, Coastal and Shelf Science. 2008;80:357–364. doi: 10.1016/j.ecss.2008.08.017. [DOI] [Google Scholar]
- Almeida PR, Arakawa H, Aronsuu K, Baker C, Blair SR, Beaulaton L, Belo AF, Kitson J, Kucheryavyy A, Kynard B, Lucas MC, Moser M, Potaka B, Romakkaniemi A, Staponkus R, Tamarapa S, Yanai S, Yang G, Zhang T, Zhuang P. Lamprey fisheries: History, trends and management. Journal of Great Lakes Research. 2021;47:S159–S185. doi: 10.1016/j.jglr.2021.06.006. [DOI] [Google Scholar]
- Almudi T, Kalikoski DC. Traditional fisherfolk and no-take protected areas: the Peixe Lagoon National Park dilemma. Ocean and Coastal Management. 2010;53:225–233. doi: 10.1016/j.ocecoaman.2010.04.005. [DOI] [Google Scholar]
- Alonso C, Zeder M, Piccini C, Conde D, Pernthaler J. Ecophysiological differences of betaproteobacterial populations in two hydrochemically distinct compartments of a subtropical lagoon. Environmental Microbiolgy. 2009;11:867–876. doi: 10.1111/j.1462-2920.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Alonso C, Piccini C, Unrein F, Bertoglio F, Conde D, Pernthaler J. Environmental dynamics as a structuring factor for microbial carbon utilization in a subtropical coastal lagoon. Environmental Microbiology. 2013;4:1–19. doi: 10.3389/fmicb.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves APNN, Lima Júnior EM, Piccolo NS, de Miranda MJB, Lima Verde MEQ, Ferreira Júnior AEC, Silva PGB, Feitosa VP, de Bandeira TJPG, Marthor MB, de Moraes MO. Study of tensiometric properties, microbiological and collagen content in nile tilapia skin submitted to different sterilization methods. Cell and Tissue Banking. 2018;19:373–382. doi: 10.1007/s10561-017-9681-y. [DOI] [PubMed] [Google Scholar]
- Ambrose WG. Role of predatory infauna in structuring marine soft-bottom communities. Marine Ecology Progress Series. 1984;17:109–115. doi: 10.3354/meps017109. [DOI] [Google Scholar]
- Andrade-Tubino MF, Azevedo MCC, Franco TP, Araújo FG. How are fish assemblages and feeding guilds organized in different tropical coastal systems? Comparisons among oceanic beaches, bays and coastal lagoons. Hydrobiologia. 2020;847:403–419. doi: 10.1007/s10750-019-04101-3. [DOI] [Google Scholar]
- Attayde JL, Bozelli RL. Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1789–1797. doi: 10.1139/f98-033. [DOI] [Google Scholar]
- Azevedo MCC, Gomes-Gonçalves RS, Mattos TM, Uehara W, Guedes GHS, Araújo FG. Taxonomic and functional distinctness of the fish assemblages in three coastal environments (bays, coastal lagoons and oceanic beaches) in Southeastern Brazil. Marine Environmental Research. 2017;129:180–188. doi: 10.1016/j.marenvres.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology Letters. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- Balzano S, Abs E, Leterme SC. Protist diversity along a salinity gradient in a coastal lagoon. Aquatic Microbial Ecology. 2015;74:263–277. doi: 10.3354/ame01740. [DOI] [Google Scholar]
- Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. The value of estuarine and coastal ecosystem services. Ecological Monographs. 2011;81:169–193. doi: 10.1890/10-1510.1. [DOI] [Google Scholar]
- Barnes RSK. Coastal lagoons: the natural history of a neglected habitat. Cambridge.: Cambridge University Press; 1980. p. 106. [Google Scholar]
- Barracosa H, C. B. de los Santos, M. Martins, C. Freitas & R. Santos, Ocean literacy to mainstream ecosystem services concept in formal and informal education: the example of coastal ecosystems of Southern Portugal. Frontiers in Marine Science. 2019;6:626. doi: 10.3389/fmars.2019.00626. [DOI] [Google Scholar]
- Bartley DM, Rana K, Immink AJ. The use of interspecific hybrids in aquaculture and fisheries. Reviews in Fish Biology and Fisheries. 2001;10:325–337. doi: 10.1023/A:1016691725361. [DOI] [Google Scholar]
- Basset A, Elliott M, West RJ, Wilson JG. Estuarine and lagoon biodiversity and their natural goods and services. Estuarine, Coastal and Shelf Science. 2013;132:1–4. doi: 10.1016/j.ecss.2013.05.018. [DOI] [Google Scholar]
- Bec B, Collos Y, Souchu P, Vaquer A, Lautier J, Fiandrino A, Benau L, Orsoni V, Laugier T. Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquatic Microbial Ecology. 2011;63:29–45. doi: 10.3354/ame01480. [DOI] [Google Scholar]
- Beck MW, Heck KL, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP. The Identification, Conservation, and Management of Estuarine and Marine Nurseries for Fish and Invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. BioScience. 2001;51:633–641. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2. [DOI] [Google Scholar]
- Beer NA, Joyce CB. North Atlantic coastal lagoons: conservation, management and research challenges in the twenty-first century. Hydrobiologia. 2012;701:1–11. doi: 10.1007/s10750-012-1325-4. [DOI] [Google Scholar]
- Bellino A, Mangano MC, Baldantoni D, Russell BD, Mannino AM, Mazzola A, Vizzini S, Sarà G. Seasonal patterns of biodiversity in Mediterranean coastal lagoons. Diversity and Distributions. 2019;25:1512–1526. doi: 10.1111/ddi.12942. [DOI] [Google Scholar]
- Bellwood O. The occurrence, mechanics and significance of burying behaviour in crabs (Crustacea: Brachyura) Journal of Natural History. 2002;36:1223–1238. doi: 10.1080/00222930110048891. [DOI] [Google Scholar]
- Bemvenuti CE. Unvegetated intertidal flats and subtidal bottoms. In: Seeliger U, Odebrecht C, Castello JP, editors. Subtropical convergence marine ecosystem. The coast and the sea in the warm temperate southwestern Atlantic. Heidelberg: Springer; 1997. pp. 78–82. [Google Scholar]
- Bennett EM. Research frontiers in ecosystem service science. Ecosystems. 2017;20:31–37. doi: 10.1007/s10021-016-0049-0. [DOI] [Google Scholar]
- Berkes F, Seixas CS. Building resilience in lagoon social-ecological systems: a local-level perspective. Ecosystems. 2005;8:967–974. doi: 10.1007/s10021-005-0140-4. [DOI] [Google Scholar]
- Bloomfield AL, Gillanders BM. Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries. 2005;28:63–77. doi: 10.1007/BF02732754. [DOI] [Google Scholar]
- Boros G, Takács P, Vanni MJ. The fate of phosphorus in decomposing fish carcassess: a mesocosm experiment. Freshwater Biology. 2014;60:479–489. doi: 10.1111/fwb.12483. [DOI] [Google Scholar]
- Bowen WD. Role of marine mammals in aquatic ecosystems. Marine Ecology Progress Series. 1997;158:267–274. doi: 10.3354/meps158267. [DOI] [Google Scholar]
- Boyer JN. Aerobic and anaerobic degradation and mineralization of 14C-Chitin by water column and sediment inocula of the York River Estuary, Virginia. Applied Environmental Microbiology. 1994;60:174–179. doi: 10.1128/aem.60.1.174-179.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco CWC, Esteves FA, Kozlowski-Suzuki B. The zooplankton and other limnological features of a humic coastal lagoon (Lagoa Comprida, Macaé, R.J.) in Brazil. Hydrobiologia. 2000;437:71–80. doi: 10.1023/A:1026571301811. [DOI] [Google Scholar]
- Branco CWC, Kozlowsky-Suzuki B, Esteves FA, Aguiaro T. Zooplankton distribution and community structure in a Brazilian Coastal lagoon. Vie Et Milieu - Life and Environment. 2008;58:1–9. [Google Scholar]
- Brehmer P, Thierry L, Kantoussan J, Galgani F, Mouillot D. Does coastal lagoon habitat quality affect fish growth rate and their recruitment? Insights from fishing and acoustic surveys. Estuarine, Coastal and Shelf Science. 2013;126:1–6. doi: 10.1016/j.ecss.2013.03.011. [DOI] [Google Scholar]
- Broadhurst MK, Gray CA, Young DJ, Johnson DD. Relative efficiency and size selectivity of bottom-set gillnets for dusky flathead, Platycephalus fuscus and other species in New South Wales, Australia. Archive of Fishery and Marine Research. 2003;50:287–300. [Google Scholar]
- Bruck WM, Slater JW, Carney BF. Chitin and Chitosan from Marine Organisms. In: Kim SK, editor. Chitin, Chitosan, Oligosaccharides and Their Derivatives. New York: CRC- Press; 2011. pp. 11–23. [Google Scholar]
- Buchanan BA, Stoner AW. Distributional patterns of blue crabs (Callinectes sp.) in a tropical estuarine lagoon. Estuaries. 1988;11:231–239. doi: 10.2307/1352009. [DOI] [Google Scholar]
- Bueno-Pardo J, Garcí-Seoane E, Sousa AI, Coelho JP, Morgado M, Frankenbach S, Ezequiel J, Vaz N, Quintino V, Rodrigues AM, Leandro S, Luis A, Serôdio J, Cunha MR, Calado AJ, Lillebo A, Rebelo JE, Queiroga H. Trophic web structure and ecosystem attributes of a temperate coastal lagoon (Ria de Aveiro, Portugal) Ecological Modelling. 2018;378:13–25. doi: 10.1016/j.ecolmodel.2018.03.009. [DOI] [Google Scholar]
- Bukaveckas PA, Lesutienė J, Gasiūnaitė ZR, Ložys L, Olenina I, Pilkaitytė R, Pūtys Ž, Tassone S, Wood J. Microcystin in aquatic food webs of the Baltic and Chesapeake Bay regions. Estuarine, Coastal and Shelf Science. 2017;191:50–59. doi: 10.1016/j.ecss.2017.04.016. [DOI] [Google Scholar]
- Cabral S, Alves AS, Castro N, Chainho P, Sá E, da Fonseca LC, P. F. e Costa, J. Castro, J. Canning-Clode, A. Pombo & J. L. Costa, Polychaete annelids as live bait in Portugal: Harvesting activity in brackish water systems. Ocean & Coastal Management. 2019;181:104890. doi: 10.1016/j.ocecoaman.2019.104890. [DOI] [Google Scholar]
- Calow P. Towards a definition of functional ecology. Functional Ecology. 1987;1:57–61. doi: 10.2307/2389358. [DOI] [Google Scholar]
- Caminha, 2022. Festa das Solhas – Lanhelas. https://www.cm-caminha.pt/pages/1286?event_id=733. Accessed March 6, 2022.
- Canonico GC, Arthington A, Mccrary JK, Thieme ML. The effects of introduced tilapias on native biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems. 2005;15:463–483. doi: 10.1002/aqc.699. [DOI] [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S. Biodiversity loss and its impact on humanity. Nature. 2012;489:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Caron A, Desrosiers G, Olive PJW, Retière C, Nozais C. Comparison of diet and feeding activity of two polychaetes, Nephtys caeca (Fabricius) and Nereis virens (Sars), in an estuarine intertidal environment in Québec, Canada. Journal of Experimental Marine Biology and Ecology. 2004;304:225–242. doi: 10.1016/j.jembe.2003.12.014. [DOI] [Google Scholar]
- Charles F. Utilization of fresh detritus derived from Cystoseira mediterranea and Posidonia oceanica by thedeposit-feeding bivalve Abra ovata. Journal of Experimental Marine Biology and Ecology. 1993;174:43–64. doi: 10.1016/0022-0981(93)90250-R. [DOI] [Google Scholar]
- Chandra G, Bhattacharjee I, Chatterjee SN, Ghosh A. Mosquito control by larvivorous fish. Indian Journal of Medical Research. 2008;127:13–27. [PubMed] [Google Scholar]
- Checa AG. Physical and biological determinants of the fabrication of molluscan shell microstructures. Frontiers in Marine Science. 2018;5:353. doi: 10.3389/fmars.2018.00353. [DOI] [Google Scholar]
- Colléter M, Valls A, Guitton J, Gascuel D, Pauly D, Christense V. Global overview of the applications of the Ecopath with Ecosim modeling approach using the EcoBase models repository. Ecological Modelling. 2015;302:42–53. doi: 10.1016/j.ecolmodel.2015.01.025. [DOI] [Google Scholar]
- Conan, P., M. Pujo-Pay, M. Agab, L. Calva-Benítez, S. Chifflet, P. Douillet, … & J. F. Ghiglione, 2017. Biogeochemical cycling and phyto- and bacterioplankton communities in a large and shallow tropical lagoon (Términos Lagoon, Mexico) under 2009–2010 El Niño Modoki drought conditions. Biogeosciences 14: 959–975.
- Conde JE, Díaz H. The mangrove tree crab Aratus pisonii in a tropical estuarine coastal lagoon. Estuarine, Coastal and Shelf Science. 1989;28:639–650. doi: 10.1016/0272-7714(89)90051-6. [DOI] [Google Scholar]
- Conde D, Aubriot L, Sommaruga R. Changes in UV penetration associated with marine intrusions and freshwater discharge in a shallow coastal lagoon of the Southern Atlantic Ocean. Marine Ecology Progress Series. 2000;207:19–31. doi: 10.3354/meps207019. [DOI] [Google Scholar]
- Cossu P, Mura L, Scarpa F, Lai T, Sanna D, Azzena I, Fois N, Casu M. Genetic patterns in Mugil cephalus and implications for fisheries and aquaculture management. Scientific Reports. 2021;11:2887. doi: 10.1038/s41598-021-82515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza, R., R. De Groot, L. Braat, I. Kubiszewski, L. Fioramonti, P. Sutton, ... & M. Grasso, 2017. Twenty years of ecosystem services: how far have we come and how far do we still need to go? Ecosystem services 28: 1–16.
- Craig JF. A short review of pike ecology. Hydrobiologia. 2008;601:5–16. doi: 10.1007/s10750-007-9262-3. [DOI] [Google Scholar]
- D’Avanzo C, Kremer JN. Diel oxygen dynamics and anoxic events in an eutrophic estuary of Waquoit Bay, Massachusetts. Estuaries. 1994;17:131–139. doi: 10.2307/1352562. [DOI] [Google Scholar]
- D’Incao F. Pesca e biologia de Penaeus paulensis na lagoa dos Patos, RS, Brasil. Atlântica, Rio Grande. 1991;13:159–169. [Google Scholar]
- da Silva Machado AM, Daura-Jorge FG, Herbst DF, Simões-Lopes PC, Ingram SN, de Castilho PV, Peroni N. Artisanal fishers' perceptions of the ecosystem services derived from a dolphin-human cooperative fishing interaction in southern Brazil. Ocean & Coastal Management. 2019;173:148–156. doi: 10.1016/j.ocecoaman.2019.03.003. [DOI] [Google Scholar]
- Dalsgaard, Bo, P. K. Maruyama, J. Sonne, K. Hansen, T. B. Zanata, S. Abrahamczyk, R. Alarcón, A. C. Araujo, F. P. Araújo, S. Buzato, E. Chávez-González, A. G. Coelho, … & A. M. M. González, 2021. Functional Ecology 35: 1120–1133.
- Dame, R. F., 1993. The role of bivalve filter feeder material fluxes in estuarine ecosystems. In: Dame R. F. Bivalve Filter Feeders in Estuarine and Coastal Ecosystem Processes. N.A.T.O., A.S.I. Series, Springer-Verlag, Berlin 33: 245–269.
- Dame R, Bushek D, Allen D, Lewitus A, Edwards D, Koepfler E, Gregory L. Ecosystem response to bivalve density reduction: management implications. Aquatic Ecology. 2002;36:51–65. doi: 10.1023/A:1013354807515. [DOI] [Google Scholar]
- Danovaro R, Pusceddu A. Biodiversity and ecosystem functioning in coastal lagoons: does microbial diversity play any role? Estuarine, Coastal and Shelf Science. 2007;75:4–12. doi: 10.1016/j.ecss.2007.02.030. [DOI] [Google Scholar]
- Davis TR, Harasti D, Kelaher B, Smith SDA. Spatial and temporal variation in subtidal molluscan diversity amongst temperate estuarine habitats. Marine Ecology. 2017;38:1–11. doi: 10.1111/maec.12428. [DOI] [Google Scholar]
- Day, J. W., B. C. Crump, W. M. Kemp & A. Yáñez-Arancibia, 2013. Estuarine ecology. Wiley-Blackwell, Hoboken.
- de Bernardi R, Giussani G. Are blue-green algae a suitable food for zooplankton? An overview. Hydrobiologia. 1990;200:29–41. doi: 10.1007/BF02530326. [DOI] [Google Scholar]
- de Carvalho F, Couto E. Environmental variables influencing the Callinectes (Crustacea: Brachyura: Portunidae) species distribution in a tropical estuary—Cachoeira River (Bahia, Brazil) Journal of the Marine Biological Association of the United Kingdom. 2011;91:793–800. doi: 10.1017/S0025315410001700. [DOI] [Google Scholar]
- de Jesús-Carrillo RM, Ocaña FA, Hernández-Ávila I, Mendoza-Carranza M, Sánchez AJ, Barba-Macías E. Mollusk distribution in four habitats along a salinity gradient in a coastal lagoon from the Gulf of Mexico. Journal of Natural History. 2020;54:1257–1270. doi: 10.1080/00222933.2020.1785030. [DOI] [Google Scholar]
- de La Peña-Lastra S. Seabird droppings: Effects on a global and local level. Science of the Total Environment. 2021;754:142148. doi: 10.1016/j.scitotenv.2020.142148. [DOI] [PubMed] [Google Scholar]
- DeBlasis P, Kneip A, Scheel-Ybert R, Giannini PC, Gaspar MD. Sambaquis e paisagem: dinâmica natural e arqueologia regional no litoral do sul do Brasil. Arqueologia Suramericana. 2007;3:29–61. [Google Scholar]
- Deines AM, Wittmann ME, Deines JM, Lodge DM. Tradeoffs among Ecosystem Services Associated with Global Tilapia Introductions. Reviews in Fisheries Science and Aquaculture. 2016;24:178–191. doi: 10.1080/23308249.2015.1115466. [DOI] [Google Scholar]
- Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Borboroglu PG, Croxall JP. Threats to seabirds: a global assessment. Biological Conservation. 2019;237:525–537. doi: 10.1016/j.biocon.2019.06.033. [DOI] [Google Scholar]
- Díaz S, Fargione J, Chapin FS, Tilman D. Biodiversity loss threatens human well-being. PLoS Biology. 2006;4:e277. doi: 10.1371/journal.pbio.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Castañeda, V. & D. Reish, 2009. Polychaetes in Environmental Studies. Annelids in Modern Biology: 203–27.
- Diehl S, Eklöv P. Piscivore-mediated habitat use in fish: effects on invertebrate resources, diet and growth of perch, Perca fluviatilis. Ecology. 1995;76:1712–1726. doi: 10.2307/1940705. [DOI] [Google Scholar]
- Doering PH, Oviatt CA, Kelly JR. The effects of the filter-feeding clam Mercenaria mercenaria on carbon cycling in experimental marine mesocosms. Journal of Marine Research. 1986;44:839–861. doi: 10.1357/002224086788401611. [DOI] [Google Scholar]
- Donovan CJ, Ku JC, Quilliam MA, Gill TA. Bacterial degradation of paralytic shellfish toxins. Toxicon. 2008;52:91–100. doi: 10.1016/j.toxicon.2008.05.005. [DOI] [PubMed] [Google Scholar]
- dos Santos ME, Coniglione C, Louro S. Feeding behaviour of the bottlenose dolphin, Tursiops truncatus (Montagu, 1821) in the Sado estuary, Portugal, and a review of its prey species. Revista Brasileira De Zoociências. 2007;9:31–39. [Google Scholar]
- Eskinazi-Sant’Anna, E. M & M. L. Pace, The potential of the zooplankton resting-stage bank to restore communities in permanent and temporary waterbodies. Journal of Plankton Research. 2018;40:458–470. doi: 10.1093/plankt/fby023. [DOI] [Google Scholar]
- El Knidri H, Belaabed R, Addaou A, Laajeb A, Lahsini A. Extraction, chemical modification and characterization of chitin and chitosan. International Journal of Biological Macromolecules. 2018;120:1181–1189. doi: 10.1016/j.ijbiomac.2018.08.139. [DOI] [PubMed] [Google Scholar]
- El Mahrad B, Abalansa S, Newton A, Icely JD, Snoussi M, Kacimi I. Social-Environmental Analysis for the Management of Coastal Lagoons in North Africa. Frontiers in Environmental Science. 2020;8:37. doi: 10.3389/fenvs.2020.00037. [DOI] [Google Scholar]
- El-Sabaawi RW, Frauendorf TC, Marques PS, Mackenzie RA, Manna LR, Mazzoni R, Phillip DAT, Warbanski ML, Zandonà E. Biodiversity and ecosystem risks arising from using guppies to control mosquitos. Biology Letters. 2016;12:20160590. doi: 10.1098/rsbl.2016.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, M., J. W. Day, R. Ramachandran & E. Wolanski, 2019. A Synthesis: What Is the Future for Coasts, Estuaries, Deltas and Other Transitional Habitats in 2050 and Beyond? In Wolanski, E., J. W. Day, M. Elliot & R. Ramachandran (eds), Coasts and Estuaries. Elsevier Amsterdam: 1–28.
- Estes, J. A., 2009. Ecological effects of marine mammals. In Encyclopedia of marine mammals. Academic Press: 357–361.
- Esteves FDA, Caliman A, Santangelo JM, Guariento RD, Farjalla VF, Bozelli RL. Neotropical coastal lagoons: an appraisal of their biodiversity, functioning, threats and conservation management. Brazilian Journal of Biology. 2008;68:967–981. doi: 10.1590/S1519-69842008000500006. [DOI] [PubMed] [Google Scholar]
- Fairholme JKE. The blacks of Moreton Bay and the porpoises. Proceedings of the Zoological Society of London. 1856;24:353–354. [Google Scholar]
- Felder DL. Diversity and ecological significance of deepburrowing macrocrustaceans in coastal tropical waters of the Americas (Decapoda: thalassinidea) Interciencia. 2001;26:440–449. [Google Scholar]
- Feitosa IB, Huszar VLM, Domingues CD, Appel E, Paranhos R, Almeida RM, Branco CWC, Bastos WR, Sarmento H. Sarmento. Plankton Community interactions in an Amazonian floodplain lake, from bacteria to zooplankton. Hydrobiologia. 2019;831:55–70. doi: 10.1007/s10750-018-3855-x. [DOI] [Google Scholar]
- Ferrão-Filho A, Kozlowsky-Suzuki B. Cyanotoxins: bioaccumulation and effects on aquatic animals. Marine Drugs. 2011;9:2729–2772. doi: 10.3390/md9122729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LM, Noelli FS. Richard Francis Burton, os sambaquis e a Arqueologia no Brasil Imperial (Com tradução de textos de Burton) Revista Do Museu De Arqueologia e Etnologia. 2007;17:149–168. doi: 10.11606/issn.2448-1750.revmae.2007.89770. [DOI] [Google Scholar]
- Fertig B, O’Neil JM, Beckert KA, Cain CJ, Needham DM, Carruthers TJB, Dennison WC. Elucidating terrestrial nutrient sources to a coastal lagoon, Chincoteague Bay, Maryland, USA. Estuarine, Coastal and Shelf Science. 2013;116:1–10. doi: 10.1016/j.ecss.2012.08.013. [DOI] [Google Scholar]
- Fontes ML, Suzuki MT, Cottrell MT, Abreu PC. Primary Production in a Subtropical Stratified Coastal Lagoon - Contribution of Anoxygenic Phototrophic Bacteria. Microbial Ecology. 2011;61:223–237. doi: 10.1007/s00248-010-9739-x. [DOI] [PubMed] [Google Scholar]
- Fortes WLS, Almeida-Silva PH, Prestrelo L, Monteiro-Neto C. Patterns of fish and crustacean community structure in a coastal lagoon system, Rio de Janeiro, Brazil. Marine Biology Research. 2014;10:111–122. doi: 10.1080/17451000.2013.797645. [DOI] [Google Scholar]
- Forster S, Graf G. Impact of irrigation on oxygen flux into the sediment: intermittent pumping by Callianassa subterranea and “piston-pumping” by Lanice conchilega. Marine Biology. 1995;123:335–346. doi: 10.1007/BF00353625. [DOI] [Google Scholar]
- Franco TP, Araujo CEO, Araújo FG. Length-weight relationships for 25 fish species from three coastal lagoons in Southeastern Brazil. Journal of Applied Ichthyology. 2014;30:248–250. doi: 10.1111/jai.12271. [DOI] [Google Scholar]
- Franco ACS, Araújo FG, Santos AFGN, Andrade-Tubino MF, Santos LN. From meso to hyperhaline: the importance of Neotropical coastal lagoons in supporting the functional fish diversity at regional scale. Hydrobiologia. 2021;849:4101–4118. doi: 10.1007/s10750-021-04683-x. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations, 2015. Mediterranean coastal lagoons: Sustainable management and interactions among aquaculture. Capture fisheries and the environment. Roma: 293.
- Food and Agriculture Organization of the United Nations, 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome: 224.
- Fu B, Wang S, Su C, Forsius M. Linking ecosystem processes and ecosystem services. Current Opinion in Environmental Sustainability. 2013;5:4–10. doi: 10.1016/j.cosust.2012.12.002. [DOI] [Google Scholar]
- Fujibayashi M, Okano K, Takada Y, Mizutani H, Uchida N, Nishimura O, Miyata N. Transfer of cyanobacterial carbon to a higher trophic-level fish community in a eutrophic lake food web: fatty acid and stable isotope analyses. Oecologia. 2018;188:901–912. doi: 10.1007/s00442-018-4257-5. [DOI] [PubMed] [Google Scholar]
- Gagnon K, Rothausler E, Syrjanem A, Yli-Renko M, Jormalainen V. Seabird guano fertilizes Baltic Sea littoral food webs. PLoS One. 2013;8:e61284. doi: 10.1371/journal.pone.0061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia E, Torres-Irineo E, Gasca-Leyva E. Economic value of Caribbean flamingo (Phoenicopterus ruber) at Celestun biosphere reserve, Yucatán, México: a birdwatching-tourism approach. Ornitología Neotropical. 2018;29:135–141. doi: 10.58843/ornneo.v29i1.223. [DOI] [Google Scholar]
- Gamfeldt L, Hillebrand H, Jonsson PR. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology. 2008;89:1223–1231. doi: 10.1890/06-2091.1. [DOI] [PubMed] [Google Scholar]
- García, S., L. Le Reste & FAO., 1987. Ciclos vitales, dinámica, explotación y ordenación de las poblaciones de camarones peneidos costeros. Roma: FAO.
- García-Chicote J, Armengol X, Rojo C. Zooplankton abundance: a neglected key element in the evaluation of reservoir water quality. Limnologica. 2018;69:46–54. doi: 10.1016/j.limno.2017.11.004. [DOI] [Google Scholar]
- García-Seoane E, Dolbeth M, Silva CL, Abreu A, Rebelo JE. Changes in the fish assemblages of a coastal lagoon subjected to gradual salinity increases. Marine Environmental Research. 2016;122:178–187. doi: 10.1016/j.marenvres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Winemiller KO, Hoeinghaus DJ, Claudino MC, Bastos R, Correa F, Huckembeck S, Vieira J, Loebmann D, Abreu P, Ducatti C. Hydrologic pulsing promotes spatial connectivity and food web subsidies in a subtropical coastal ecosystem. Marine Ecology Progress Series. 2017;567:17–28. doi: 10.3354/meps12060. [DOI] [Google Scholar]
- Gaspar MD, DeBlasis P, Fish SK, Fish PR. The handbook of South American archaeology. New York, NY: Springer; 2008. Sambaqui (shell mound) societies of coastal Brazil; pp. 319–335. [Google Scholar]
- Gatter W. Zugzeiten und Zugmuster im Herbst: Einfluß des Treibhauseffekts auf den Vogelzug? Journal Für Ornithologie. 1992;133:427–436. doi: 10.1007/BF01640470. [DOI] [Google Scholar]
- Giangrande A, Licciano M, Musco L. Polychaetes as environmental indicators revisited. Marine Pollution Bulletin. 2005;50:1153–1162. doi: 10.1016/j.marpolbul.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gheler-Costa C, Comin FH, Gilli LC, Verdade LM. Foraging behavior of Brazilian cormorant, Nannopterum brasilianus (Suliformes: Phalacrocoracidae) Zoologia (curitiba) 2018;35:14664. doi: 10.3897/zoologia.35.e14664. [DOI] [Google Scholar]
- Gjedrem T. Genetic improvement for the development of efficient global aquaculture: a personal opinion review. Aquaculture. 2012;21:344–349. [Google Scholar]
- Glibert PM, Wazniak CE, Hall MR, Sturgis B. Seasonal and interannual trends in nitrogen and brown tide in Maryland’s coastal bays. Ecological Applications. 2007;17:S79–S87. doi: 10.1890/05-1614.1. [DOI] [Google Scholar]
- Glibert PM, Boyer JN, Heil CA, Madden CJ, Sturgis B, Wazniak CS. Blooms in Lagoons: Different from those of River-Dominated Estuaries. In: Kennish MJ, Paerl HW, editors. Coastal Lagoons: Critical habitats of Environmental Change. New York: CRC Press; 2010. pp. 91–114. [Google Scholar]
- Gönenç, E. & J. P. Wolflin, 2004. Coastal Lagoons: Ecosystem Processes and Modeling for Sustainable Use and Development. CRC Press.
- Gontikaki E, Antoniadou C, Chintiroglou C. Population structure of Cerastoderma glaucum and Abra ovata in Vouliagmeni Lagoon (Attiki) Journal of the Marine Biological Association of the United Kingdom. 2003;83:1095–1097. doi: 10.1017/S0025315403008312h. [DOI] [Google Scholar]
- Gooday GW, Prosser JI, Hillman K, Cross MG. Mineralization of Chitin in an estuarine sediment: the importance of the Chitosan pathway. Biochemical Systematics and Ecology. 1991;19:395–400. doi: 10.1016/0305-1978(91)90056-6. [DOI] [Google Scholar]
- Grabowski M, Konopacka A, Jazdzewski K, Janowska E. Invasions of alien gammarid species and retreat of natives in the Vistula Lagoon (Baltic Sea, Poland) Helgoland Marine Research. 2006;60:90–97. doi: 10.1007/s10152-006-0025-8. [DOI] [Google Scholar]
- Graves RA, Pearson SM, Turner MG. Effects of bird community dynamics on the seasonal distribution of cultural ecosystem services. Ambio. 2019;48:280–292. doi: 10.1007/s13280-018-1068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AJ, Elmberg J. Ecosystem services provided by waterbirds. Biological Reviews. 2014;89:105–122. doi: 10.1111/brv.12045. [DOI] [PubMed] [Google Scholar]
- Greenwood JD. Citizens, science and bird conservation. Journal of Ornithology. 2007;148:77–124. doi: 10.1007/s10336-007-0239-9. [DOI] [Google Scholar]
- Griffin LF, Knight JM. A review of the role of fish as biological control agents of disease vector mosquitoes in mangrove forests: reducing human health risks while reducing environmental risk. Wetlands Ecology and Management. 2012;20:243–252. doi: 10.1007/s11273-012-9248-4. [DOI] [Google Scholar]
- Guariento RD, Carneiro LS, Caliman A, Bozelli RL, Leal JJF, Esteves FA. Interactive effects of omnivorous fish and nutrient loading on net productivity regulation of phytoplankton and periphyton. Aquatic Biology. 2010;10:273–282. doi: 10.3354/ab00287. [DOI] [Google Scholar]
- Guariento RD, Carneiro LS, Caliman A, Leal JJF, Bozelli RL, Esteves FA. Food Web architecture and basal resources interact to determine biomass and stoichiometric cascades along a benthic food web. PLoS One. 2011;6:e22205. doi: 10.1371/journal.pone.0022205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen NA, Souich P, Lapointe B, Ong-Lam M, Dubuc B, Walde D, Love R, Ngoc AH. Tetrodotoxin for Moderate to Severe Cancer Pain: a Randomized, Double Blind, Parallel Design Multicenter Study. Journal of Pain and Symptom Management. 2008;35:420–429. doi: 10.1016/j.jpainsymman.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Hagen NA, Cantin L, Constant J, Haller T, Blaise G, Ong-Lam M, Souich P, Korz W, Lapointe B. Tetrodotoxin for Moderate to Severe Cancer-Related Pain: a Multicentre, Randomized, Double-Blind, Placebo-Controlled. Parallel-Design Trial. Pain Research and Management. 2017;2017:7212713. doi: 10.1155/2017/7212713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines-Young R, Potschin M. The links between biodiversity, ecosystem services and human well-being. Ecosystem Ecology: a New Synthesis. 2010;1:110–139. doi: 10.1017/CBO9780511750458.007. [DOI] [Google Scholar]
- Haimovici M, Cardoso LG. Long-term changes in the fisheries in the Patos Lagoon estuary and adjacent coastal waters in Southern Brazil. Marine Biology Research. 2017;13:135–150. doi: 10.1080/17451000.2016.1228978. [DOI] [Google Scholar]
- Hamilton DJ, Ankney CD, Bailey RC. Predation of zebra mussels by diving ducks: an exclosure study. Ecology. 1994;75:521–531. doi: 10.2307/1939555. [DOI] [Google Scholar]
- Hansen BW. Advances using Copepods in Aquaculture. Journal of Plankton Research. 2017;39:972–974. doi: 10.1093/plankt/fbx057. [DOI] [Google Scholar]
- Hansen JP, Sundblad G, Bergström U, Austin ÅN, Donadi S, Eriksson BK, Eklöf JS. Recreational boating degrades vegetation important for fish recruitment. Ambio. 2019;48:539–551. doi: 10.1007/s13280-018-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. The impacts of climate change in coastal marine systems. Ecology Letters. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Haszprunar G, Wanninger A. Molluscs. Current Biology. 2012;22:R510–R514. doi: 10.1016/j.cub.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Hawkins SJ. Marine conservation in a rapidly changing world. Aquatic Conservation: Marine and Freshwater Ecosystems. 2012;22:281–287. doi: 10.1002/aqc.2239. [DOI] [Google Scholar]
- Hawkins, S. J., A. J. Evans, L. B. Firth, M. J. Jenner, R. J. H. Herbert, L. C. Adams, P. J. Moore, N. Mieszkowska, R. C. Thompson, M. T. Burrows & P. B. Fenberg, 2016. Impacts and effects of ocean warming on intertidal rocky habitats. In: laffoley, D., and Baxter, J.M. (Eds.) Explaining Ocean warming: Causes, scale, effects and consequences. Full report. Gland, Switzerland: IUCN:147–176.
- He Q, Silliman BR. Climate Change, Human Impacts, and Coastal Ecosystems in the Anthropocene. Current Biology. 2019;29:R1021–R1035. doi: 10.1016/j.cub.2019.08.042. [DOI] [PubMed] [Google Scholar]
- Heithaus MR, Frid A, Wirsing AJ, Worm B. Predicting ecological consequences of marine top predator declines. Trends in Ecology and Evolution. 2008;23:202–210. doi: 10.1016/j.tree.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Hines, A., 2007. Ecology of juvenile and adult blue crabs. The Blue Crab: Callinectes Sapidus: 575–665.
- Hirota M, Y., Senga, Y. Seike, S. Nohara & H. Kunii, Fluxes of carbon dioxide, methane and nitrous oxide in two contrastive fringing zones of coastal lagoon, Lake Nakaumi, Japan. Chemosphere. 2007;68:597–603. doi: 10.1016/j.chemosphere.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Chapin S, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setãlã H, Symstad AJ, Vandermeer J, Wardle DA. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs. 2005;75:3–35. doi: 10.1890/04-0922. [DOI] [Google Scholar]
- Horton M, Parker D, Winker H, Lamberth SJ, Hutchings K, Kerwath SE. Age, growth and per-recruit stock assessment of southern mullet Chelon richardsonii in Saldanha Bay and Langebaan Lagoon, South Africa. African Journal of Marine Science. 2019;41:313–324. doi: 10.2989/1814232X.2019.1657950. [DOI] [Google Scholar]
- Hsieh HH, Chuang MH, Shih YY, Weerakkody WS, Huang WJ, Hung CC, Muller FLL, Ranatunga RRMKP, Wijethunga DS. Eutrophication and Hypoxia in Tropical Negombo Lagoon. Sri Lanka. Frontiers in Marine Science. 2021;8:678832. doi: 10.3389/fmars.2021.678832. [DOI] [Google Scholar]
- Hutchings P. Biodiversity and functioning of polychaetes in benthic sediments. Biodiversity Conservation. 1998;7:1133–1145. doi: 10.1023/A:1008871430178. [DOI] [Google Scholar]
- Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist. 1959;93:145–159. doi: 10.1086/282070. [DOI] [Google Scholar]
- Hunt JH, Ambrose WGJ, Peterson CH. Effects of the gastropod, llyanassa obsoleta (Say), and the bivalve, Mercenaria mercenaria (L.), on larval settlement and juvenile recruitment of infauna. Journal of Experimental Marine Biology and Ecology. 1987;108:229–240. doi: 10.1016/0022-0981(87)90087-6. [DOI] [Google Scholar]
- Ikhwanuddin M, Azmi WA, Borkhanuddin MH, Muhammad SRS, Idris I. Improving the Health of Setiu Wetlands Ecosystems and Productivity of crustacean resources for livelihood enhancement. Journal of Sustainability Science and Management Special. 2017;3:1–7. [Google Scholar]
- IPBES . Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. In: Brondizio ES, Settele J, Díaz S, Ngo HT, editors. IPBES secretariat. Germany: Bonn; 2019. p. 1148. [Google Scholar]
- IPCC, 2021. Climate Change 2021: the Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu & B. Zhou (eds.)]. Cambridge University Press.
- Iribarne O, Bortolus A, Botto F. Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Marine Ecology Progress Series. 1997;155:137–145. doi: 10.3354/meps155137. [DOI] [Google Scholar]
- Iwamatsu S, Akiko S, Sato M. Nereidid Polychaetes as the Major Diet of Migratory Shorebirds on the Estuarine Tidal Flatsat Fujimae-Higata in Japan. Zoological Science. 2007;24:676–685. doi: 10.2108/zsj.24.676. [DOI] [PubMed] [Google Scholar]
- Janetski DJ, Chaloner DT, Tiegs SD, Lamberty GA. Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia. 2009;159:583–595. doi: 10.1007/s00442-008-1249-x. [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Prabaharan M, Kumar PS, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnology Advances. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Jelassi R, Khemaissia H, Ghemari C, Raimond M, Souty-Grosset C, Nasri-Ammar K. The induced damage in the hepatopancreas of Orchestia species after exposure to a mixture of Cu/Zn—An ultrastructural study. Microscopy Research and Technique. 2020;83:148–155. doi: 10.1002/jemt.23397. [DOI] [PubMed] [Google Scholar]
- Jeppesen E, Søndergaard M, Pedersen AR, Jurgens K, Strzelczak A, Lauridsen TL, Johansson LS. Salinity induced regime shift in shallow brackish lagoons. Ecosystems. 2007;10:47–57. doi: 10.1007/s10021-006-9007-6. [DOI] [Google Scholar]
- Jeppesen E, Nõges P, Davidson TA, Haberman J, Nõges T, Blank K, Lauridsen TL, Søndergaard M, Sayer C, Laugaste R, Johansson LS, Bjerring R, Amsink SL. Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD) Hydrobiologia. 2011;676:279–297. doi: 10.1007/s10750-011-0831-0. [DOI] [Google Scholar]
- Jiménez-Quiroz MDC, Barrón-Barraza FJ, Cervantes-Duarte R, Funes-Rodríguez R. Environmental Considerations for the Management of the Bivalve Fisheries of Bahía Magdalena (Mexico), a Coastal Lagoon at the Southern End of the California Current. Frontiers in Marine Science. 2021;8:682148. doi: 10.3389/fmars.2021.682148. [DOI] [Google Scholar]
- Jónasdóttir SH, Visser AW, Richardson K, Heath MR. Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic. Proceedings of the National Academy of Sciences. 2015;112:12122–12126. doi: 10.1073/pnas.1512110112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona S, Whitehead H. Are cetacea ecologically important. Oceanograph Marine Biology Annual Reviews. 1988;26:553–568. [Google Scholar]
- Katsanevakis S, Coll M, Piroddi C, Steenbeek J, Lasram FBR, Zenetos A, Cardoso AC. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Frontiers in Marine Science. 2014;1:32. doi: 10.3389/fmars.2014.00032. [DOI] [Google Scholar]
- Kennish MJ, Paerl HW. Coastal lagoons: critical habitats of environmental change. CRC Press; 2010. [Google Scholar]
- Khemaissia H, Jelassi R, Souty-Grosset C, Nasri-Ammar K. Amphipoda and Isopoda diversity around Tunisian wetlands (North Africa) in relation to environmental conditions. African Journal of Ecology. 2018;56:455–467. doi: 10.1111/aje.12466. [DOI] [Google Scholar]
- Kiljunen M, Peltonen H, Lehtiniemi M, Uusitalo L, Sinisalo T, Norkko J, Kunnasranta M, Torniainen J, Rissanen J, Karjalainen J. Benthic-pelagic coupling and trophic relationships in northern Baltic Sea food webs. Limnology and Oceanography. 2020;65:1706–1722. doi: 10.1002/lno.11413. [DOI] [Google Scholar]
- Kiszka, J. J., M. S. Woodstock & M. R. Heithaus, 2022. Functional Roles and Ecological Importance of Small Cetaceans in Aquatic Ecosystems. Frontiers in Marine Science: 163.
- Kjerfve, B., 1994. Coastal Lagoons, In: B. Kjerfve. Coastal Lagoon Processes. Elsevier Science Publishers, Amsterdam(ed.): 1–8.
- Kolb GS, Palmborg C, Taylor AR, Baath E, Hamback PA. Effects of nesting cormorants (Phalacrocorax carbo) on soil chemistry, microbial communities and soil fauna. Ecosystems. 2015;18:643–657. doi: 10.1007/s10021-015-9853-1. [DOI] [Google Scholar]
- Kolyuchkina GA, Syomin VL, Spiridonov VA, Zalota AK, Biryukova SV, Basin AB, Simakova UV, Nabozhenko MV. The resilience of macrozoobenthos of boreal coastal lagoons to non-indigenous species invasion: a case study of Taman Bay (the Sea of Azov) Regional Studies in Marine Science. 2019;28:100573. doi: 10.1016/j.rsma.2019.100573. [DOI] [Google Scholar]
- Korajkic A, Wanjugi P, Harwood VJ. Indigenous microbiota and habitat influence Escherichia coli survival more than sunlight in simulated aquatic habitats. Applied and Environmental Microbiology. 2013;79:5329–5337. doi: 10.1128/AEM.01362-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AB, Smrithy R, Sathasivam K. Dolphin-assisted cast net fishery in the Ashtamudi Estuary, south-west coast of India. Indian Journal of Fisheries. 2012;59:143–148. [Google Scholar]
- Kormas KA, Lymperopoulou DS. Cyanobacterial toxin degrading bacteria: Who are they? BioMed Research International. 2013;2013:463894. doi: 10.1155/2013/463894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk, M., E. Paturej & K. Obolewski, 2021. Zooplankton predator–prey network relationships indicates the saline gradient of coastal lakes. Machine learning and meta-network approach. Ecological Indicators 125: 107550.
- Kularatne RKA, Harris JM, Vinobaba P, Thanusanth S, Kishoran S, Kankanamge CE. Use of habitats by aquatic and terrestrial avifauna in tropical coastal lagoons. Regional Studies in Marine Science. 2021;47:101926. doi: 10.1016/j.rsma.2021.101926. [DOI] [Google Scholar]
- Lago J, Rodríguez LP, Blanco L, Vieites JM, Cabado AG. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Marine Drugs. 2015;13:6384–6406. doi: 10.3390/md13106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne MG, Holden PA. Comparison of free-living and particle-associated bacterial communities in a coastal lagoon. Microbial Ecology. 2003;46:228–237. doi: 10.1007/s00248-001-1072-y. [DOI] [PubMed] [Google Scholar]
- Latta LC, Fisk DL, Knapp RA, Pfrender ME. Genetic resilience of Daphnia populations following experimental removal of introduced fish. Conservation Genetics. 2010;11:1737–1745. doi: 10.1007/s10592-010-0067-y. [DOI] [Google Scholar]
- Lazarich M, Ramos-Gil A, González-Pérez J. Prehistoric Bird Watching in Southern Iberia? The Rock Art of Tajo de las Figuras Reconsidered. Environmental Archaeology. 2019;24:387–399. doi: 10.1080/14614103.2018.1563372. [DOI] [Google Scholar]
- Leahy, J. G. & R. R. Colwell, 1990. Microbial degradation of hydrocarbons in the environment. Microbiological Reviews 305–315. [DOI] [PMC free article] [PubMed]
- Lebrato M, Pahlow M, Frost JR, Küter M, Jesus-Mendes P, Molinero J, Oschlies A. Sinking of Gelatinous Zooplankton Biomass Increases Deep Carbon Transfer Efficiency Globally. Global Biogeochemical Cycles. 2019;33:1764–1783. doi: 10.1029/2019GB006265. [DOI] [Google Scholar]
- Legendre L, Rassoulzadegan F. Plankton and nutrient dynamics in marine waters. Ophelia. 1995;14:153–172. doi: 10.1080/00785236.1995.10422042. [DOI] [Google Scholar]
- Lemes GAF, Kersanach R, L. da S. Pinto, O. A. Dellagostin, J. S. Yunes & A. Matthiensen, Biodegradation of microcystins by aquatic Burkholderia sp. from a South Brazilian coastal lagoon. Ecotoxicology and Environmental Safety. 2008;69:358–365. doi: 10.1016/j.ecoenv.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Lemos VM, Varela AS, Jr, Schwingel PR, Muelbert JH, Vieira JP. Migration and reproductive biology of Mugil liza (Teleostei: Mugilidae) in south Brazil. Journal of Fish Biology. 2014;85:671–687. doi: 10.1111/jfb.12452. [DOI] [PubMed] [Google Scholar]
- Léo Neto NA, Voeks RA, Dias TLP, Alves RRN. Mollusks of Candomblé: symbolic and ritualistic importance. Journal of Ethnobiology and Ethnomedicine. 2012;8:10. doi: 10.1186/1746-4269-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillebø, A. I., P. Stålnacke & G. D. Gooch, 2015. Coastal lagoons in Europe: Integrated water resource strategies.
- Liquete C, Cid N, Lanzanova D, Grizzetti B, Reynaud A. Perspectives on the link between ecosystem services and biodiversity: the assessment of the nursery function. Ecological Indicators. 2016;63:249–257. doi: 10.1016/j.ecolind.2015.11.058. [DOI] [Google Scholar]
- Lloret J, Marín A, Marín-Guirao L. Is coastal lagoon eutrophication likely to be aggravated by global climate change? Estuarine, Coastal and Shelf Science. 2008;78:403–412. doi: 10.1016/j.ecss.2008.01.003. [DOI] [Google Scholar]
- Lomartire S, Marques JC, Gonçalves AMM. The key role of zooplankton in ecosystem services: a perspective of interaction between zooplankton and fish recruitment. Ecological Indicators. 2021;129:107867. doi: 10.1016/j.ecolind.2021.107867. [DOI] [Google Scholar]
- Loreau M. Are communities saturated? On the relationship between α, β and γ diversity. Ecology Letters. 2000;3:73–76. doi: 10.1046/j.1461-0248.2000.00127.x. [DOI] [Google Scholar]
- Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA. Ecology: Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecology Letters. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Mace GM, Norris K, Fitter AH. Biodiversity and ecosystem services: a multilayered relationship. Trends in Ecology & Evolution. 2012;27:19–26. doi: 10.1016/j.tree.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Macêdo RL, Sousa FDR, Dumont HJ, Rietzler AC, Rocha O, Elmoor-Loureiro LMA. Climate change and niche unfilling tend to favor range expansion of Moina macrocopa Straus 1820, a potentially invasive cladoceran in temporary waters. Hydrobiologia. 2022;849:4015–4027. doi: 10.1007/s10750-022-04835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia A. Juvenile Penaeid Shrimp Density, Spatial Distribution and Size Composition in four adjacent habitats within a Mangrove-Fringed Bay on Inhaca Island, Mozambique. Western Indian Ocean Journal of Marine Science. 2004;3:163–178. [Google Scholar]
- Mackas DL, Greve W, Edwards M, Chiba S, Tadokoro K, Eloire D, Mazzocchi MG, Batten S, Richardson AJ, Johnson C, Head E, Conversi A, Peluso T. Changing zooplankton seasonality in a changing ocean: comparing time series of zooplankton phenology. Progress in Oceanography. 2012;97:31–62. doi: 10.1016/j.pocean.2011.11.005. [DOI] [Google Scholar]
- Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. Brock Biology of Microorganisms. Global. Harlow, United Kingdom: Pearson Education Canada; 2008. [Google Scholar]
- Magalhães WF, Barros F. Structural and functional approaches to describe polychaete assemblages: ecological implications for estuarine ecosystems. Marine and Freshwater Research. 2011;62:918–926. doi: 10.1071/MF10277. [DOI] [Google Scholar]
- Maire O, Duchêne JC, Grémare A, Malyuga VS, Meysman FJR. A comparison of sediment reworking rates by the surface deposit-feeding bivalve Abra ovata during summertime and wintertime, with a comparison between two models of sediment reworking. Journal of Experimental Marine Biology and Ecology. 2007;343:21–36. doi: 10.1016/j.jembe.2006.10.052. [DOI] [Google Scholar]
- Maitland PS, Renaud CB, Quintella BR, Close DA, Docker MF. Conservation of native lampreys. In: Docker MF, editor. Lampreys: Biology, Conservation and Control. Dordrecht: Springer; 2015. pp. 375–428. [Google Scholar]
- Manfredi GGP, Cardoso MV, Stuani VT, Ferreira R, Zangrando MSR, Damante CA, Moraes Filho MO, Alves APNN, Lima Júnior ED, A. C. P. Sant’Ana, The use of Nile tilapia skin as an occlusive biological dressing for palatal wound healing: a case series. Research, Society and Development. 2021;10:e24010817146. doi: 10.33448/rsd-v10i8.17146. [DOI] [Google Scholar]
- Mantelatto FL, Reigada ÁLD, Gatti ACR, Cuesta JA. Morphology of the first zoeal stages of five species of the portunid genus Callinectes (Decapoda, Brachyura) hatched at the laboratory. Anais Da Academia Brasileira De Ciências. 2014;86:755–767. doi: 10.1590/0001-3765201420130030. [DOI] [PubMed] [Google Scholar]
- Manzano-Sarabia MM, Aragón-Noriega EA, Salinas-Zavala CA, Lluch-Cota DB. Distribution and abundance of penaeid shrimps in a hypersaline lagoon in northwestern Mexico, emphasizing the brown shrimp Farfantepenaeus californiensis life cycle. Marine Biology. 2007;152:1021–1029. doi: 10.1007/s00227-007-0763-4. [DOI] [Google Scholar]
- Manzo C, Fabbrocini A, Roselli L, D’Adamo R. Characterization of the fish assemblage in a Mediterranean coastal lagoon: lesina Lagoon (central Adriatic Sea) Regional Studies in Marine Science. 2016;8:192–200. doi: 10.1016/j.rsma.2016.04.003. [DOI] [Google Scholar]
- Margalef, R. 1983. Limnologia. Barcelona. Ediciones Omega S.A.: 1010. IN: Moss, B., 1980. Ecology of fresh waters: man and medium. Oxford.
- Márquez A, Senior W, Fermín I, Martínez G, Castañeda J, González Á. Quantification of the Concentrations of Heavy Metals in Tissues of Fish and Crustaceans of the Unare Lagoon, Anzoateguí State, Venezuela. Revista Científica. 2008;18:73–86. [Google Scholar]
- Martinho F, Leitão R, Neto JM, Cabral HN, Marques JC, Pardal MA. The use of nursery areas by juvenile fish in a temperate estuary, Portugal. Hydrobiologia. 2007;587:281–290. doi: 10.1007/s10750-007-0689-3. [DOI] [Google Scholar]
- Martins AD, Barros F. Ecological Functions of Polychaetes Along Estuarine Gradients. Frontiers in Marine Science. 2022;9:780318. doi: 10.3389/fmars.2022.780318. [DOI] [Google Scholar]
- Mast SO. The food vacuole in Paramecium. Biological Bulletin. 1947;92:31–72. doi: 10.2307/1537967. [DOI] [PubMed] [Google Scholar]
- McLachlan, A. & O. Defeo, 2017. The ecology of sandy shores. Academic press.
- Meerhoff E, Rodríguez-Gallego L, Giménez L, Muniz P, Conde P. Spatial patterns of macrofaunal community structure in coastal lagoons of Uruguay. Marine Ecology Progress Series. 2013;492:97–110. doi: 10.3354/meps10472. [DOI] [Google Scholar]
- Milessi AC, Danilo C, Laura RG, Daniel C, Javier S, Rodríguez-Gallego L. Trophic mass-balance model of a subtropical coastal lagoon, including a comparison with a stable isotope analysis of the food-web. Ecological Modelling. 2010;221:2859–2869. doi: 10.1016/j.ecolmodel.2010.08.037. [DOI] [Google Scholar]
- Millennium Ecosystem Assessment . Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press; 2005. [Google Scholar]
- Minello TJ, Matthews GA, Caldwell PA, Rozas LP. Population and production estimates for decapod crustaceans in wetlands of Galveston Bay, Texas. Transactions of the American Fisheries Society. 2008;137:129–146. doi: 10.1577/T06-276.1. [DOI] [Google Scholar]
- Minello TJ. Environmental factors affecting burrowing by brown shrimp Farfantepenaeus aztecus and white shrimp Litopenaeus setiferus and their susceptibility to capture in towed nets. Journal of Experimental Marine Biology and Ecology. 2017;486:265–273. doi: 10.1016/j.jembe.2016.10.010. [DOI] [Google Scholar]
- Misturini D, Colling LA. Can short-term meteorological events alter subtropical estuarine macrobenthic assemblages in seagrass meadows (Patos Lagoon Estuary - Southern Brazil)? Estuarine. Coastal and Shelf Science. 2021;261:107532. doi: 10.1016/j.ecss.2021.107532. [DOI] [Google Scholar]
- Mohit V, Archambault P, Toupoint N, Lovejoy C. Phylogenetic differences in attached and free-living bacterial communities in a temperate coastal lagoon during summer, revealed via high-throughput 16S rRNA gene sequencing. Applied and Environmental Microbiology. 2014;80:2071–2083. doi: 10.1128/AEM.02916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moksnes PO, Eriander L, Infantes E, Holmer M. Local Regime Shifts Prevent Natural Recovery and Restoration of Lost Eelgrass Beds Along the Swedish West Coast. Estuaries and Coasts. 2018;41:1712–1731. doi: 10.1007/s12237-018-0382-y. [DOI] [Google Scholar]
- Montagna PA, Esteves ED, Palmer TA, Flannery MS. Meta-analysis of the relationship between salinity and molluscs in tidal river estuaries of southwest Florida, U.S.A. American Malacological Bulletin. 2008;24:101–115. doi: 10.4003/0740-2783-24.1.101. [DOI] [Google Scholar]
- Monte TM, Lima Júnior EM, de Moraes Filho MO, Paier CRK, Rodrigues FAR, Alves APNN, Mathor MB, Raposo-Amaral CE. Apert hand reconstruction with tilapia skin. Journal of Craniofacial Surgery. 2022;33:1939. doi: 10.1097/SCS.0000000000008522. [DOI] [PubMed] [Google Scholar]
- Mook D. Studies on fouling invertebrates in the Indian River, Florida 2: Effects of Modulus modulus (Prosobranchia: Modulidae) Nautilus. 1977;91:134–136. [Google Scholar]
- Morelos-Villegas A, Condal AR, Ardisson PL. Spatial heterogeneity and seasonal structure of physical factors and benthic species in a tropical coastal lagoon, Celestun, Yucatán Peninsula. Regional Studies in Marine Science. 2018;22:136–146. doi: 10.1016/j.rsma.2018.06.008. [DOI] [Google Scholar]
- Morganti P, Morganti G, Morganti A. Transforming nanostructured chitin from crustacean waste into beneficial health products: a must for our society. Nanotechnology, Science and Applications. 2011;4:123–129. doi: 10.2147/NSA.S22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, Wang Y, Backeljau T, Chapelle G. Biomimetic and bio-inspired uses of mollusc shells. Marine Genomics. 2016;27:85–90. doi: 10.1016/j.margen.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Mosha EJ, Gallardo WG. Distribution and size composition of penaeid prawns, Penaeus monodon and Penaeus indicus in Saadan estuarine area, Tanzania. Ocean and Coastal Management. 2013;82:51–63. doi: 10.1016/j.ocecoaman.2013.05.003. [DOI] [Google Scholar]
- Mouillot D, Dumay O, Tomasini JA. Limiting similarity, niche filtering and functional diversity in coastal lagoon fish communities. Estuarine, Coastal and Shelf Science. 2007;71:443–456. doi: 10.1016/j.ecss.2006.08.022. [DOI] [Google Scholar]
- Muduli PR, Kanuri VV, Robin RS, Charan Kumar B, Patra S, Raman AV, Rao GN, Subramanian BR. Spatio-temporal variation of CO2 emission from Chilika Lake, a tropical coastal lagoon, on the east coast of India. Estuarine, Coastal and Shelf Science. 2012;113:305–313. doi: 10.1016/j.ecss.2012.08.020. [DOI] [Google Scholar]
- Mulholland MR, Boneillo GE, Bernhardt PW, Minor EC. Comparison of Nutrient and Microbial Dynamics over a Seasonal Cycle in a Mid-Atlantic Coastal Lagoon Prone to Aureococcus anophagefferens (Brown Tide) Blooms. Estuaries and Coasts. 2009;32:1176–1194. doi: 10.1007/s12237-009-9218-0. [DOI] [Google Scholar]
- Munaron D, Tapie N, Budzinski H, Andral B, Gonzalez JL. Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: results from a pilot survey using passive samplers. Estuarine Coastal Shelf Sciences. 2012;114:82–92. doi: 10.1016/j.ecss.2011.09.009. [DOI] [Google Scholar]
- Murillas, A., M. Ashley, C. Marta-Pedroso, A. Muench, L. Rodwell, S. Rees, T. Basic, E. Díaz, P. Lambert, G. Lassalle, C. Mateus, P. R. Almeida, D. J. Nachon, F. G. Cobo, R. Vieira, A. Lecuona, G. H. Copp, J. King, W. Roche, C. Antunes, T. Trancart & E. Feunteun, 2020. Assessing and enhancing ecosystem services provided by diadromous fish in a climate change context: the full list of ecosystem services provided by diadromous fish. Interreg Atlantic Area: 40.
- Muro-Torres VM, Soto-Jiménez MF, Green L, Quintero J, Amezcua F. Food web structure of a subtropical coastal lagoon. Aquatic Ecology. 2019;53:407–430. doi: 10.1007/s10452-019-09698-0. [DOI] [Google Scholar]
- Nagelkerken I. Ecological connectivity among tropical coastal ecosystems. Dordrecht: Springer; 2009. Evaluation of nursery function of mangroves and seagrass beds for tropical decapods and reef fishes: patterns and underlying mechanisms; pp. 357–399. [Google Scholar]
- Nagelkerken I, Sheaves M, Baker R, Connolly RM. The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish and Fisheries. 2015;16:362–371. doi: 10.1111/faf.12057. [DOI] [Google Scholar]
- Naiman RJ, Bilby RE, Schindler DE, Helfield JM. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems. 2002;5:399–417. doi: 10.1007/s10021-001-0083-3. [DOI] [Google Scholar]
- Neakok, W., 1985. To keep the past alive: the Point Lay cultural resource site survey. North Slope Borough.
- Netto, S. A., P. R. Pagliosa, A. Colling, A. L. Fonseca & K. M. Brauko, 2018. Benthic Estuarine Assemblages from the Southern Brazilian Marine Ecoregion. In: lana, P.C; Bernardino, A.F. (Org.). Brazilian Marine Biodiversity. 1ed.: Springer International Publishing 1: 177–212.
- Neves RAF, Naveira C, Miyahira IC, Portugal SGM, Krepsky N, Santos LN. Are invasive species always negative to aquatic ecosystem services? The role of dark false mussel for water quality improvement in a multi-impacted urban coastal lagoon. Water Research. 2020;184:116108. doi: 10.1016/j.watres.2020.116108. [DOI] [PubMed] [Google Scholar]
- Newton A, Icely J, Cristina S, Brito A, Cristina A, Colijn F, Dalla S, Gertz F, Hansen J, Holmer M, Ivanova K, Leppäkoski E, Mocenni C, Mudge S, Murray N, Pejrup M, Razinkovas A, Pérez-ruzafa A, Schernewski G, Schubert H, Seeram L, Solidoro C, Zaldívar J. An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuarine, Coastal and Shelf Science. 2014;140:95–122. doi: 10.1016/j.ecss.2013.05.023. [DOI] [Google Scholar]
- Newton, A., A. C. Brito, J. D. V. Derolez, I. Clara, S. Angus, G. Scherneski, M. Inácio, A. I. Lillebo, A. I. Sousa, B. Béjaoui, C. Solidoro, M. Tosic, M. Cañedo-Arguelles, M. Yamamuro, S. Reizopoulou, H. Tseng, D. Canu, L. Roselli, M. Maanan, … & V. Khokhlov, 2018. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. Journal for Nature Conservation 44: 50–65.
- Nixon SW. Eutrophication and the macroscope. Eutrophication in Coastal Ecosystems. 2009;629:5–19. doi: 10.1007/978-90-481-3385-7_2. [DOI] [Google Scholar]
- Norse E. Aspects of the Zoogeographic Distribution of Callinectes (Brachyura: Portunidae) Bulletin of Marine Science. 1977;27:440–447. [Google Scholar]
- Ocana-Luna A, Sanchez-Ramirez M. Feeding of Sciaenid (Pisces: Sciaenidae) Larvae in Two Coastal Lagoons of the Gulf of Mexico. Gulf Research Reports. 1998;10:1–9. doi: 10.18785/grr.1001.01. [DOI] [Google Scholar]
- Odjer-Bio NT, Belford EJD, Ansong M. What is happening to our Lagoons? The example of Butuah Lagoon in Ghana. International Journal of Energy and Environmental Engineering. 2015;6:183–193. doi: 10.1007/s40095-015-0165-1. [DOI] [Google Scholar]
- Officer CB, Smayd TJ, Mann R. Benthic filter feeding: a natural eutrophication control. Marine Ecology Progress Series. 1982;9:203–210. doi: 10.3354/meps009203. [DOI] [Google Scholar]
- Oliveira MCLM, Bastos RF, Claudino MC, Assumpção CM, Garcia AM. Transport of marine-derived nutrients to subtropical freshwater food webs by juvenile mullets: a case study in southern Brazil. Aquatic Biology. 2014;20:91–100. doi: 10.3354/ab00544. [DOI] [Google Scholar]
- Ortega-Arbulú AS, Pichler M, Vuillemin A, Orsi WD. Effects of organic matter and low oxygen on the mycobenthos in a coastal lagoon. Environmental Microbiology. 2019;21:374–388. doi: 10.1111/1462-2920.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östman Ö, Bostrom MK, Berstrom U, Anderson J, Lunneryd S. Estimating competition between wildlife and humans–a case of cormorants and coastal fisheries in the Baltic Sea. PLoS One. 2013;8:e83763. doi: 10.1371/journal.pone.0083763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MBP, Brauko KM, Pagliosa PR. Matching ecological functioning with polychaete morphology: Consistency patterns along sedimentary habitats. Journal of Sea Research. 2016;114:13–21. doi: 10.1016/j.seares.2016.05.001. [DOI] [Google Scholar]
- Palomo G, Iribarne O, Martinez MM. The effect of migratory seabirds guano on the soft bottom community of a SW Atlantic coastal lagoon. Bulletin of Marine Science. 1999;65:119–128. [Google Scholar]
- Palomo G, Iribarne O. Sediment bioturbation by polychaete feeding may promote sediment stability. Bulletin of Marine Science. 2000;67:249–257. [Google Scholar]
- Pasquaud S, Elie P, Jeantet C, Billy I, Martinez P, Girardin M. A preliminary investigation of the fish food web in the Gironde estuary, France, using dietary and stable isotope analyses. Estuarine, Coastal and Shelf Science. 2008;78:267–279. doi: 10.1016/j.ecss.2007.12.014. [DOI] [Google Scholar]
- Paturej E. A zooplankton-based study of coastal lakes. Baltic Coastal Zone. Journal of Ecology and Protection of the Coastline. 2009;13:25–32. [Google Scholar]
- Pauly D, Yáñez-Arancibia A. Fisheries In Coastal Lagoons. Elsevier Oceanography Series. 1994;60:377–399. doi: 10.1016/S0422-9894(08)70018-7. [DOI] [Google Scholar]
- Pellowe KE, Leslie HM. Ecosystem service lens reveals diverse community values of small-scale fisheries. Ambio. 2021;50:586–600. doi: 10.1007/s13280-020-01405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto R, McQuaid CD. Land-based predator impact on vertically migrating zooplankton and micronekton advected to a Southern Ocean archipelago. Marine Ecology Progress Series. 1992;80:15–27. doi: 10.3354/meps080015. [DOI] [Google Scholar]
- Pershing AJ, Christensen LB, Record NR, Sherwood GD, Stetson PB. The impact of whaling on the ocean carbon cycle: why bigger was better. PloS One. 2010;5:e12444. doi: 10.1371/journal.pone.0012444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Castañeda R, Defeo O. Population variability of four sympatric penaeid shrimps (Farfantepenaeus spp.) in a Tropical coastal lagoon of Mexico. Estuarine, Coastal and Shelf Science. 2001;52:631–641. doi: 10.1006/ecss.2001.0771. [DOI] [Google Scholar]
- Pérez-Ruzafa A, Marcos C, Pérez-Ruzafa IM, Barcala E, Hegazi MI, Quispe J. Detecting changes resulting from human pressure in a naturally quick-changing and heterogeneous environment: Spatial and temporal scales of variability in coastal lagoons. Estuarine, Coastal and Shelf Science. 2007;75:175–188. doi: 10.1016/j.ecss.2007.04.030. [DOI] [Google Scholar]
- Pérez-Ruzafa A, Marcos C. Fisheries in coastal lagoons: an assumed but poorly researched aspect of the ecology and functioning of coastal lagoons. Estuarine, Coastal and Shelf Science. 2012;110:15–31. doi: 10.1016/j.ecss.2012.05.025. [DOI] [Google Scholar]
- Pérez-Ruzafa A, Campillo S, Fernández-Palacios JM, García-Lacunza A, García-Oliva M, Ibañez H, Navarro-Martínez PC, Pérez-Marcos M, Pérez-Ruzafa IM, Quispe-Becerra JI, Sala-Mirete A, Sánchez O, Marcos C. Long-term dynamic in nutrients, chlorophyll a, and water quality parameters in a coastal lagoon during a process of eutrophication for decades, a sudden break and a relatively rapid recovery. Frontiers in Marine Science. 2019;6:26. doi: 10.3389/fmars.2019.00026. [DOI] [Google Scholar]
- Pérez-Ruzafa A, Morkune R, Marcos C, Pérez-Ruzafa IM, Razinkovas-Baziukas A. Can an oligotrophic coastal lagoon support high biological productivity? Sources and pathways of primary production. Marine Environmental Research. 2020;153:104824. doi: 10.1016/j.marenvres.2019.104824. [DOI] [PubMed] [Google Scholar]
- Petchey OL, Gaston KJ. Functional diversity: back to basics and looking forward. Ecology Letters. 2006;9:741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Petersen, C., B. A. Costa-Pierce, B. R. Dumbauld, C. Friedman, E. E. Hoffmann, H. Kite-Powell, D. T. Manahan, F. O'Beirn, R. T. Paine, P. M. Thompson, R. Whitlach & Committee on Best Practices for Shellfish Mariculture and the Effects of Commercial Activities in Drakes Estero, 2010. Ecosystem Concepts for Sustainable Bivalve Mariculture. The National Academies Press.
- Peterson MS. A conceptual view of environment-habitat-production linkages in tidal river estuaries. Reviews in Fisheries Science. 2003;11:291–313. doi: 10.1080/10641260390255844. [DOI] [Google Scholar]
- Petry AC, Guimarães TF, Vasconcellos FM, Hartz SM, Becker FG, Rosa RS, Goyenola G, Caramaschi EP, Astarloa JD, Sarmento-Soares LM, Vieira JP, Garcia AM, Mello FT, Melo F, Meerhoff M, Attayde J, Menezes R, Mazzeo N, Di Dario F. Fish composition and species richness in eastern South American coastal lagoons: additional support for the freshwater ecoregions of the world. Journal of Fish Biology. 2016;89:280–314. doi: 10.1111/jfb.13011. [DOI] [PubMed] [Google Scholar]
- Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Applied Biochemistry and Biotechnology. 2017;181:1314–1337. doi: 10.1007/s12010-016-2286-2. [DOI] [PubMed] [Google Scholar]
- Pillay D, Branch GM, Steyn A. Complex effects of the gastropod Assiminea globulus on benthic community structure in a marine-dominated lagoon. Journal of Experimental Marine Biology and Ecology. 2009;380:47–52. doi: 10.1016/j.jembe.2009.08.016. [DOI] [Google Scholar]
- Pinto MF, Mourão JS, Alves RRN. Use of ichthyofauna by artisanal fishermen at two protected areas along the coast of Northeast Brazil. Journal of Ethnobiology and Ethnomedicine. 2015;11:20. doi: 10.1186/s13002-015-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocklington P, Wells PG. Polychaetes key taxa for marine environmental quality monitoring. Marine Pollution Bulletin. 1992;24:593–598. doi: 10.1016/0025-326X(92)90278-E. [DOI] [Google Scholar]
- Ponti M, Castellini A, Ragazzoni A, Gamba E, Ceccherelli VU, Abbiati M. Decline of the Manila clams stock in the northern Adriatic lagoons: a survey on ecological and socio-economic aspects. Acta Adriatica: International Journal of Marine Sciences. 2017;58:89–104. doi: 10.32582/aa.58.1.7. [DOI] [Google Scholar]
- Post DM, Waters AW. Nutrient excretion rates of anadromous alewives during their spawning migration. Transactions of the American Fisheries Society. 2009;138:264–268. doi: 10.1577/T08-111.1. [DOI] [Google Scholar]
- Potts T, Burdon D, Jackson E, Atkins J, Saunders J, Hastings E, Langmead O. Do marine protected areas deliver flows of ecosystem services to support human welfare? Marine Policy Elsevier. 2014;44:139–148. doi: 10.1016/j.marpol.2013.08.011. [DOI] [Google Scholar]
- Pouil S, Tlusty MF, Rhyne AL, Metian M. Aquaculture of marine ornamental fish: overview of the production trends and the role of academia in research progress. Reviews in Aquaculture. 2019;12:1217–1230. doi: 10.1111/raq.12381. [DOI] [Google Scholar]
- Pryor K, Lindbergh J. A dolphin-human fishing cooperative in Brazil. Marine Mammal Science. 1990;6:77–82. doi: 10.1111/j.1748-7692.1990.tb00228.x. [DOI] [Google Scholar]
- Quaggiotto MM, Sánchez-Zapata JA, Bailey DM, Payo-Payo A, Navarro J, Brownlow A, Deaville R, Lambertucci SA, Selva N, Cortéz-Avizanda A, Hiraldo F, Donázar JA, Moleón M. Past, present and future of the ecosystem services provided by cetacean carcasses. Ecosystem Services. 2022;54:101406. doi: 10.1016/j.ecoser.2022.101406. [DOI] [Google Scholar]
- Queiros AM, Birchenough SNR, Bremner J, Godbold JA, Parker RE, Romero-Ramirez A, Reiss H, Solan M, M., P. J. Somerfield, C. Van Colen, G. Van Hoey & S. Widdicombe, A bioturbation classification of European marine infaunal invertebrates. Ecology and Evolution. 2013;3:3958–3985. doi: 10.1002/ece3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana X, Boix D, Casamitjana X, Colomer A, Compte J, Cunillera-Montcusí D, Gáscon S, Gich F, Menció A, Martinoy M, Montaner J, Pascual J, Sala J, Solà J, Tornero I. Management and Restoration Actions of Confined Mediterranean Coastal Lagoons in the Empord`a and Baix Ter Wetlands. Management and Restoration of Mediterranean Coastal Lagoons in Europe. 2018;10:173–192. [Google Scholar]
- Ramachandran SD, Sweezey MJ, Hodson PV, Boudreau M, Courtenay SC, Lee K, King T, Dixon JA. Influence of salinity and fish species on PAH uptake from dispersed crude oil. Marine Pollution Bulletin. 2006;52:1182–1189. doi: 10.1016/j.marpolbul.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Ramírez-Ayala E, Arguello-Pérez MA, Tintos-Gómez A, Mendoza Pérez JA, Díaz-Gómez JA, Pérez-Rodríguez RY, Núñez-Nogueira G, Sepúlveda-Quiroz CA, Zepeda-González FA, Lezama-Cervantes C. Heavy metals in sediment and fish from two coastal lagoons of the Mexican Central Pacific. Latin American Journal of Aquatic Research. 2021;49:818–827. doi: 10.3856/vol49-issue4-fulltext-2639. [DOI] [Google Scholar]
- Range P, Piló D, Ben-Hamadou R, Chícharo MA, Matias D, Joaquim S, Oliveira AP, Chícharo L. Seawater acidification by CO2 in a coastal lagoon environment: effects on life history traits of juvenile mussels Mytilus galloprovincialis. Journal of Experimental Marine Biology and Ecology. 2012;424:89–98. doi: 10.1016/j.jembe.2012.05.010. [DOI] [Google Scholar]
- Reddy CS. The role of religious rituals in fishing community: a study of Irakkam Fishermen in Pulicat Lake. The Oriental Anthropologist. 2020;20:202–216. doi: 10.1177/0972558X20913755. [DOI] [Google Scholar]
- Reeves, R. R. & T. D. Smith, 2006. A taxonomy of world whaling. IN: Estes, J. A., D. P. DeMaster, D. F. Doak, T.M. Williams & R. L. Brownell Jr. Whales, whaling, and ocean ecosystems (eds.). University of California Press, Berkeley: 82–101.
- Reidenberg JS. Anatomical adaptations of aquatic mammals. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology: Advances in Integrative Anatomy and Evolutionary Biology. 2007;290:507–513. doi: 10.1002/ar.20541. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JA, Giarrizzo T. Microgobius meeki as a potential bio-indicator of habitat disturbance in shallow estuarine areas: a useful tool for the assessment of estuarine quality. Journal of Fish Biology. 2016;89:713–734. doi: 10.1111/jfb.13007. [DOI] [PubMed] [Google Scholar]
- Reise K. Sediment mediated species interactions in coastal waters. Journal of Sea Research. 2002;48:127–141. doi: 10.1016/S1385-1101(02)00150-8. [DOI] [Google Scholar]
- Rhoads DC, Young DK. Influence of deposit-feeding organisms on sediment stability and community trophic structure. Journal of Marine Research. 1970;28:150–178. [Google Scholar]
- Rice AL, Chapman CJ. Observations on the burrows and burrowing behaviour of two mud-dwelling decapod crustaceans, Nephrops norvegicus and Goneplax rhomboides. Marine Biology. 1971;10:4. doi: 10.1007/BF00368093. [DOI] [Google Scholar]
- Rivera-Velázquez G, Soto LA, Salgado-Ugarte IH, Naranjo EJ. Assessment of an artisanal shrimp fishery of Litopenaeus vannamei in a lagoon-estuarine system based on the concept of maximum sustainable yield. Revista De Biología Marina y Oceanografía. 2009;44:635–646. doi: 10.4067/S0718-19572009000300010. [DOI] [Google Scholar]
- Roberts CM, O’Leary BC, Mccauley DJ, Cury PM, Duarte CM, Lubchenco J, Pauly D, Sáenz-Arroyo A, Sumaila UR, Wilson RW, Worm B, Castilla JC. Marine reserves can mitigate and promote adaptation to climate change. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:6167–6175. doi: 10.1073/pnas.1701262114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison BH, Reisenbichler KR, Sherlock RE. Giant larvacean houses: Rapid carbon transport to the deep-sea floor. Science. 2005;308:1609–1611. doi: 10.1126/science.1109104. [DOI] [PubMed] [Google Scholar]
- Rodrigues MA, Ortega I, D’Incao F. The importance of shallow areas as nursery grounds for the recruitment of blue crab (Callinectes sapidus) juveniles in subtropical estuaries of Southern Brazil. Regional Studies in Marine Science. 2019;25:100492. doi: 10.1016/j.rsma.2018.100492. [DOI] [Google Scholar]
- Rodríguez-Pérez H, Green AJ. Strong seasonal effects of waterbirds on benthic communities in shallow lakes. Freshwater Science. 2012;31:1273–1288. doi: 10.1899/11-129.1. [DOI] [Google Scholar]
- Rohr, J. A., 1984. Sítios Arqueológicos de Santa Catarina. Anais do Museu de Antropologia da UFSC. Florianópolis: MA/UFSC 17: 77–168.
- Roman J, McCarthy JJ. The whale pump: marine mammals enhance primary productivity in a coastal basin. PloS One. 2010;5:e13255. doi: 10.1371/journal.pone.0013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J, Estes JA, Morissette L, Smith C, Costa D, McCarthy J, Nation JB, Nicol SN, Pershing A, Smetacek V. Whales as marine ecosystem engineers. Frontiers in Ecology and the Environment. 2014;12:377–385. doi: 10.1890/130220. [DOI] [Google Scholar]
- Roman J, Nevins J, Altabet M, Koopman H, McCarthy J. Endangered right whales enhance primary productivity in the Bay of Fundy. PLoS One. 2016;11:e0156553. doi: 10.1371/journal.pone.0156553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Gribsholt B, Middelburg JJ, Heip C. Context-dependent effects of suspension feeding on intertidal ecosystem functioning. Marine Ecology Progress Series. 2008;354:47–57. doi: 10.3354/meps07213. [DOI] [Google Scholar]
- Rossman S, McCabe EB, Barros NB, Gandhi H, Ostrom PH, Stricker CA, Wells RS. Foraging habits in a generalist predator: Sex and age influence habitat selection and resource use among bottlenose dolphins (Tursiops truncatus) Marine Mammal Science. 2015;31:155–168. doi: 10.1111/mms.12143. [DOI] [Google Scholar]
- Rowe, D., A. Moore, A. Giorgetti, C. Maclean, P. Grace, S. Wadhwa & J. Cooke, 2008. Review of the impacts of gambusia, redfin perch, tench, roach, yellowfin goby and streaked goby in Australia. Department of the Environment, Water, Heritage and the Arts: 245.
- Rullens V, Lohrer AM, Townsend M, Pilditch CA. Ecological mechanisms underpinning ecosystem service bundles in marine environments–a case study for shellfish. Frontiers in Marine Science. 2019;6:409. doi: 10.3389/fmars.2019.00409. [DOI] [Google Scholar]
- Sánchez B, Rodríguez D. Avifauna associated with the aquatic and coastal ecosystems of Cayo Coco. Cuba. Journal of Caribbean Ornithology. 2000;13:68–75. [Google Scholar]
- Sánchez-Moyano JE, García-Asencio I, Donázar-Aramendía I, Miró JM, Megina C, García-Gómez JC. BENFES, a new biotic index for assessing ecological status of soft-bottom communities. Towards a lower taxonomic complexity, greater reliability, and less effort. Marine Environmental Research. 2017;132:41–50. doi: 10.1016/j.marenvres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Santangelo JM, Rocha ADM, Bozelli RL, Carneiro LS, Esteves FDA. Zooplankton responses to sandbar opening in a tropical eutrophic coastal lagoon. Estuarine Coastal and Shelf Science. 2007;71:657–668. doi: 10.1016/j.ecss.2006.09.021. [DOI] [Google Scholar]
- Santiago do Cacém, 2022. 7º Festival da Enguia da Lagoa de Santo André. https://www.cm-santiagocacem.pt/evento/o-festival-da-enguia-da-lagoa-de-santo-andre/. Accessed March 6, 2022.
- Santhanam H, Amal RS, Thanasekaran K. Selection of Suitable Ecosystem Indicators as Tools to Assess the Ecosystem Health of Coastal Lagoons and Their Implication in Management. 2010;7:59–64. [Google Scholar]
- Santos FCV, Lima LB, M. dos S. Nascimento & A. Guzzi, O potencial do Birdwatching na área de proteção ambiental do Delta do Parnaíba (Piauí, Brasil) Revista Brasileira De Ecoturismo. 2019;12:854–865. [Google Scholar]
- Schallenberg M, Hall CJ, Burns CW. Consequences of climate-induced salinity increases on zooplankton abundance and diversity in coastal lakes. Marine Ecology Progress Series. 2003;251:181–189. doi: 10.3354/meps251181. [DOI] [Google Scholar]
- Schnug E, Jacobs F, Stoven K. Guano: the white gold of the seabirds. Seabirds. 2018;1:79–100. [Google Scholar]
- Selden P, Huys R, Stephenson M, Herward AP, Taylor PN. Crustaceans from bitumen clast in Carboniferous glacial diamictite extend fossil record of copepods. Nature Communications. 2010;1:1–6. doi: 10.1038/ncomms1049. [DOI] [PubMed] [Google Scholar]
- Seo JS, Keum YS, Li QX. Bacterial degradation of aromatic compounds. International Journal of Environmental Research and Public Health. 2009;6:278–309. doi: 10.3390/ijerph6010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serranito B, Aubert A, Stemmann L, Rossi N, Jamet JL. Proposition of indicators of anthropogenic pressure in the Bay of Toulon (Mediterranean Sea) based on zooplankton time-series. Continental Shelf Research. 2016;121:3–12. doi: 10.1016/j.csr.2016.01.016. [DOI] [Google Scholar]
- Serrano O, Gómez-López DI, Sánchez-Valencia L, Acosta-Chaparro A, Navas-Camacho R, González-corredor J, Salinas C, Masque P, Bernal CA, Marbà N. Seagrass blue carbon stocks and sequestration rates in the Colombian Caribbean. Scientific Reports. 2021;11:11067. doi: 10.1038/s41598-021-90544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Lopes PC, Fabián ME, Menegheti JO. Dolphin interactions with the mullet artisanal fishing on southern Brazil: a qualitative and quantitative approach. Revista Brasileira De Zoologia. 1998;15:709–726. doi: 10.1590/S0101-81751998000300016. [DOI] [Google Scholar]
- Smith BD, Tun MT, Chit AM, Win H, Moe T. Catch composition and conservation management of a human–dolphin cooperative cast-net fishery in the Ayeyarwady River, Myanmar. Biological Conservation. 2009;142:1042–1049. doi: 10.1016/j.biocon.2009.01.015. [DOI] [Google Scholar]
- Skilleter GA, Peterson CH. Control of foraging behavior of individuals within an ecosystem context: the clam Macoma balthica and interactions between competition and siphon cropping. Oecologia. 1994;100:268–278. doi: 10.1007/BF00316954. [DOI] [PubMed] [Google Scholar]
- Smaal AC, Prins TC. The uptake of organic matter and the release of inorganic nutrients by bivalve suspension feeder beds. In: Dame RF, editor. Bivalve filter feeders in estuarine and coastal ecosystem processes. Berlin: Springer- Verlag; 1993. pp. 271–298. [Google Scholar]
- Snickars M, Sandström A, Lappalainen A, Mattila J, Rosqvist K, Urho L. Fish assemblages in coastal lagoons in land-uplift succession: the relative importance of local and regional environmental gradients. Estuarine, Coastal and Shelf Science. 2009;81:247–256. doi: 10.1016/j.ecss.2008.10.021. [DOI] [Google Scholar]
- Sommer U, Gliwicz M, Lampert W, Duncan A. The PEG-model of seasonal succession of planktonic eventsin fresh waters. Archiv Für Hydrobiologie. 1986;106:433–471. doi: 10.1127/archiv-hydrobiol/106/1986/433. [DOI] [Google Scholar]
- Souza LC, e, C. W. C. Branco, P. Domingos & S. L. C. Bonecker, Zooplankton of an urban coastal lagoon: composition and association with environmental factors and summer fish kill. Zoologia. 2011;28:357–364. doi: 10.1590/S1984-46702011000300010. [DOI] [Google Scholar]
- Souza MS, Muelbert JH, Costa LDF, Eliana VK, Yunes JS. Environmental variability and cyanobacterial blooms in a subtropical coastal lagoon: searching for a sign of climate change effects. Frontiers in Microbiology. 2018;9:1727. doi: 10.3389/fmicb.2018.01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak E, Anger K, Luppi T, Bas C, Ismael D. Distribution and habitat preferences of two grapsid crab species in Mar Chiquita Lagoon (Province of Buenos Aires, Argentina) Helgolander Meeresunters. 1994;48:59–78. doi: 10.1007/BF02366202. [DOI] [Google Scholar]
- Staley ZR, Rohr JR, Senkbeil JK, Harwood VJ. Agrochemicals indirectly increase survival of E. coli O157:H7 and indicator bacteria by reducing ecosystem services. Ecological Applications. 2014;24:1945–1953. doi: 10.1890/13-1242.1. [DOI] [PubMed] [Google Scholar]
- Stoner AW, Waite JM. Trophic biology of Strombus gigas in nursery habitats: diets and food sources in seagrass meadows. Journal of Molluscan Studies. 1991;57:451–460. doi: 10.1093/mollus/57.4.451. [DOI] [Google Scholar]
- Sunye, P, T. J Pereira, A. D. P Russo & S. A. Netto, 2014. Pesca do camarão-rosa no Sistema Estuarino de Laguna, SC: História e acasos das políticas de manejo. In Haimovici, M., J. M. Andriguetto-Filho & P. S. Sunye, (org.). A pesca marinha e estuarina no Brasil: estudos de caso multidisciplinares. Rio Grande, Editora da FURG: 191.
- Suplicy, F. M., L. F. N. Vianna, G. S. Rupp, A. L. T. Novaes, L. H. P. Garbossa, R. V. de Souza, J. Guzenski, S. W. da Costa, F. M. Silva & A. A. dos Santos. Planning and management for sustainable coastal aquaculture development in Santa Catarina, south Brazil. Reviews in Aquaculture 9: 107–124.
- Suradi WS, Solichin A, Taufani WT, Sabdono A. Population dynamics of exploited species west shrimps Parapenaeopsis coromandelica H. Milne. Edwards 1837 from the Teluk Penyu coastal waters, Indonesian ocean. The Egyptian Journal of Aquatic Research. 2017;43:307–312. doi: 10.1016/j.ejar.2017.12.002. [DOI] [Google Scholar]
- Suzuki MS, Figueiredo RO, Castro SC, Silva CE, Pereira EA, Silva JA, Aragon GT. Sand bar opening in a coastal Lagoon (Iquipari) in the northern region of Rio de Janeiro state: Hydrological and hydrochemical changes. Brazilian Journal of Biology. 2002;62:51–62. doi: 10.1590/S1519-69842002000100007. [DOI] [PubMed] [Google Scholar]
- Sy MM, Rey-Valette H, Simier M, Pasqualini V, Figuières C, De Wit R. Identifying consensus on coastal lagoons ecosystem services and conservation priorities for an effective decision making: a Q approach. Ecological Economics. 2018;154:1–13. doi: 10.1016/j.ecolecon.2018.07.018. [DOI] [Google Scholar]
- Tavares DC, Guadagnin DL, de Moura JF, Siciliano S, Merico A. Environmental and anthropogenic factors structuring waterbird habitats of tropical coastal lagoons: implications for management. Biological Conservation. 2015;186:12–21. doi: 10.1016/j.biocon.2015.02.027. [DOI] [Google Scholar]
- Teichert N, Lepage M, Lobry J. Beyond classic ecological assessment: the use of functional indices to indicate fish assemblages sensitivity to human disturbance in estuaries. Science of the Total Environment. 2018;639:465–475. doi: 10.1016/j.scitotenv.2018.05.179. [DOI] [PubMed] [Google Scholar]
- Telesh IV, Schubert H, Skarlato SO. Revisiting Remane’s concept: Evidence for high plankton diversity and a protistan species maximum in the horohalinicum of the Baltic Sea. Marine Ecology Progress Series. 2011;421:1–11. doi: 10.3354/meps08928. [DOI] [Google Scholar]
- Terborgh J, Estes JA. Trophic cascades: predators, prey, and the changing dynamics of nature. New York: Island Press; 2013. [Google Scholar]
- Thanh HT, Tschakert P, Hipsey MR. Tracing environmental and livelihood dynamics in a tropical coastal lagoon through the lens of multiple adaptive cycles. Ecology and Society. 2020;25:31. doi: 10.5751/ES-11489-250131. [DOI] [Google Scholar]
- Thibaut LM, Connolly SR. Understanding diversity-stability relationships: towards a unified model of portfolio effects. Ecology Letters. 2013;16:140–150. doi: 10.1111/ele.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D. Functional diversity. In: Levin SA, editor. Encyclopaedia of Biodiversity. San Diego, CA: Academic Press; 2001. pp. 109–120. [Google Scholar]
- Tundisi JG, Matsumura-Tundisi T, Ciminelli VS, Barbosa FA. Water availability, water quality water governance: the future ahead. IAHS-AISH Proceedings and Reports. 2015;366:75–79. doi: 10.5194/piahs-366-75-2015. [DOI] [Google Scholar]
- van Damme K, Kotov AA. The fossil record of the Cladocera (Crustacea: Branchiopoda): Evidence and hypotheses. Earth-Science Reviews. 2016;163:162–189. doi: 10.1016/j.earscirev.2016.10.009. [DOI] [Google Scholar]
- van Montfrans J, Wetzel RL, Orth RJ. Epiphyte-grazer relationships in seagrass meadows: consequences for seagrass growth and production. Estuaries. 1984;7:289–309. doi: 10.2307/1351615. [DOI] [Google Scholar]
- Vaughn CC. Ecosystem services provided by freshwater mussels. Hydrobiologia. 2018;810:15–27. doi: 10.1007/s10750-017-3139-x. [DOI] [Google Scholar]
- Vasconcelos RP, Batista MI, Henriques S. Current limitations of global conservation to protect higher vulnerability and lower resilience fish species. Scientific Reports. 2017;7:7702. doi: 10.1038/s41598-017-06633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez GF, Sharma VK, Magallanes VR, Marmolejo AJ. Heavy metals in a coastal lagoon of the Gulf of Mexico. Marine Pollution Bulletin. 1999;38:479–485. doi: 10.1016/S0025-326X(98)00173-8. [DOI] [Google Scholar]
- Veettil BK, Van DD, Quang NX. Biodiversity and nature conservation in island ecosystems: spatiotemporal changes in Socotra archipelago (Yemen) Journal of Coastal Conservation. 2020;24:1–9. doi: 10.1007/s11852-020-00774-4. [DOI] [Google Scholar]
- Vega-Cendejas ME, Arreguín-Sánchez F. Energy fluxes in a mangrove ecosystem from a coastal lagoon in Yucatan Peninsula, Mexico. Ecological Modelling. 2001;137:119–133. doi: 10.1016/S0304-3800(00)00421-X. [DOI] [Google Scholar]
- Verdiell-Cubedo D, Torralva M, Andreu-Soler A, Oliva-Paterna FJ. Effects of shoreline urban modification on habitat structure and fish community in littoral areas of a Mediterranean coastal lagoon (Mar Menor, Spain) Wetlands. 2012;32:631–641. doi: 10.1007/s13157-012-0296-6. [DOI] [Google Scholar]
- Vinagre C, Cabral H, Costa MJ. Prey selection by flounder, Platichthys flesus, in the Douro estuary, Portugal. Journal of Applied Ichthyology. 2008;24:238–243. doi: 10.1111/j.1439-0426.2008.01055.x. [DOI] [Google Scholar]
- Vizzini S, Signa G, Mazzola A. Guano-derived nutrient subsidies drive food web structure in coastal ponds. PLoS One. 2016;11:e0151018. doi: 10.1371/journal.pone.0151018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voultsiadou E, Koutsoubas D, Achparaki M. Bivalve mollusc exploitation in Mediterranean coastal communities: an historical approach. Journal of Biological Research. 2010;13:35. [Google Scholar]
- Wall CC, Peterson BJ, Gobler CJ. Facilitation of seagrass Zostera marina productivity by suspension-feeding bivalves. Marine Ecology Progress Series. 2008;357:165–174. doi: 10.3354/meps07289. [DOI] [Google Scholar]
- Waggoner BM, Poinar GO., Jr Fossil habrotrochid rotifers in Dominican amber. Experientia (basel) 1993;49:354–357. doi: 10.1007/BF01923421. [DOI] [Google Scholar]
- Ward, G. H., 2012. The Blue Crab: a Survey with Application to San Antonio Bay. Center for Research in Water Resources. https://www.twdb.texas.gov/publications/reports/contracted_reports/
- Warme JE. Live and dead molluscs in a coastal lagoon. Journal of Paleontology. 1969;43:141–150. [Google Scholar]
- Wasef S, Wood R, El Merghani S, Ikram S, Curtis C, Holland B, Willerslev E, Millar CD, Lambert DM. Radiocarbon dating of Sacred Ibis mummies from ancient Egypt. Journal of Archaeological Science: Reports. 2015;4:355–361. [Google Scholar]
- Watanabe WO, Losordo TM, Fitzsimmons K, Hanley F. Tilapia production systems in the Americas: technological Advances, Trends and Challenges. Reviews in Fisheries Science. 2010;10:465–498. doi: 10.1080/20026491051758. [DOI] [Google Scholar]
- Waterkeyn A, Grillas P, Vanschoenwinkel B, Brendonck L. Invertebrate community patterns in Mediterranean temporary wetlands along hydroperiod and salinity gradients. Freshwater Biology. 2008;53:1808–1822. doi: 10.1111/j.1365-2427.2008.02005.x. [DOI] [Google Scholar]
- Wazniak CE, Hall MR, Carruthers TJB, Sturgis B, Dennison WC, Orth RJ. Linking water quality to living resources in a Mid-Atlantic Lagoon System, USA. Ecological Applications. 2007;17:S64–S78. doi: 10.1890/05-1554.1. [DOI] [Google Scholar]
- Webb AP, Eyre BD. Effect of natural populations of burrowing thalassinidean shrimp on sediment irrigation, benthic metabolism, nutrient fluxes and denitrification. Marine Ecology Progress Series. 2004;268:205–220. doi: 10.3354/meps268205. [DOI] [Google Scholar]
- Weitz JS, Stock CA, Wilhelm SW, Bourouiba L, Coleman ML, Buchan A, Follows MJ, Fuhrman JA, Jover LF, Lennon JT, Middelboe M, Sonderegger DL, Suttle CA, Taylor BP, Thingstad TF, Wilson WH, Wommack KE. A multitrophic model to quantify the effects of marine viruses on microbial food webs and ecosystem processes. ISME Journal. 2015;9:1352–1364. doi: 10.1038/ismej.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan CJ, Wenny DG, Marquis RJ. Ecosystem services provided by birds. Annals of the New York Academy of Sciences. 2008;1134:25–60. doi: 10.1196/annals.1439.003. [DOI] [PubMed] [Google Scholar]
- Whitfield AK. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Reviews in Fish Biology and Fisheries. 2017;27:75–110. doi: 10.1007/s11160-016-9454-x. [DOI] [Google Scholar]
- Williams PM, Whitfield M, Biggs J. How can we make new ponds more biodiverse? A case study monitored over 7 years. Hydrobiologia. 2008;597:137–148. doi: 10.1007/s10750-007-9224-9. [DOI] [Google Scholar]
- Wolfe, R. J. & C. Mishler, 1999. The subsistence harvest of harbor seal and sea lion by Alaska Natives in 1998. Alaska Department of Fish and Game, Division of Subsistence.
- Wouters N, Cabral HN. Are flatfish nursery grounds richer in benthic prey? Estuarine, Coastal and Shelf Science. 2009;83:613–620. doi: 10.1016/j.ecss.2009.05.011. [DOI] [Google Scholar]
- Wouters JM, Gusmão JB, Mattos G, Lana PC. Polychaete functional diversity in shallow habitats: Shelter from the storm. Journal of Sea Research. 2018;135:18–30. doi: 10.1016/j.seares.2018.02.005. [DOI] [Google Scholar]
- Zaldívar JM, Cardoso AC, Viaroli P, Newton A, De Wit R, Ibañez C, Reizopoulou S, Somma F, Razinkovas A, Basset A, Holmer M, Murray N. Eutrophication in transitional waters: an overview. Transitional Water MoNographs. 2008;2:1–78. [Google Scholar]
- Zheng X, Como S, Huang L, Magni P. Temporal changes of a food web structure driven by different primary producers in a subtropical eutrophic lagoon. Marine Environmental Research. 2020;161:105128. doi: 10.1016/j.marenvres.2020.105128. [DOI] [PubMed] [Google Scholar]
- Zimmerman RJ, Minello TJ. Densities of Penaeus aztecus, Penaeus setiferus, and other natant macrofauna in a Texas salt marsh. Estuaries. 1984;7:421–433. doi: 10.2307/1351623. [DOI] [Google Scholar]
- Zimmerman RJ, Minello TJ, Rozas LP. Salt Marsh Linkages to Productivity of Penaeid Shrimps and Blue Crabs in the Northern Gulf of Mexico. In: Weinstein MP, Kreeger DA, editors. Concepts and Controversies in Tidal Marsh Ecology. Dordrecht: Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.