Abstract
As one of the most promising wine regions in China, the eastern foothills of the Helan Mountain (EFHM) in the Ningxia Hui Autonomous Region has attracted great attention recently. Geographically, EFHM is divided into six sub-regions, namely Shizuishan, Xixia, Helan, Qingtongxia, Yongning and Hongsipu. However, there have been few reports on the character and differences between wines in the six sub-regions. In this experiment, a total of 71 commercial Cabernet Sauvignon wines from six sub-regions were collected, and their phenolic compounds, visual properties and mouthfeel were investigated. The results showed that wines from the six sub-regions of EFHM showed distinctive phenolic profiles and could be distinguished through the OPLS-DA mode using 32 potential markers. In terms of color, Shizuishan wines showed higher a* values and lower b* values. The sensory evaluation showed that Hongsipu wines had higher astringency strength and lower tannin texture. The overall results implied that the phenolic compounds of wines in different sub-regions were affected by terroir conditions. To the best of our knowledge, this is the first time that a wide coverage of phenolic compounds has been analysed for wines from the sub-regions of EFHM, which could provide valuable information in deciphering the terroir of EFHM.
Keywords: sub-region, Cabernet Sauvignon, phenolic compounds, CATA, QDA
1. Introduction
Wine is the most popular alcoholic drink across the world because of its unique culture and complex flavor. The term ‘terroir’ appears to have first been applied in the 14th century in France and encompasses the key natural elements of landscape features, soil characteristics, climate and human factors (socio-economics, history, genotype, variety and rootstock selection, winemaking technologies and vineyard management practices) that result in the production of unique, site-specific terroir wines [1,2].
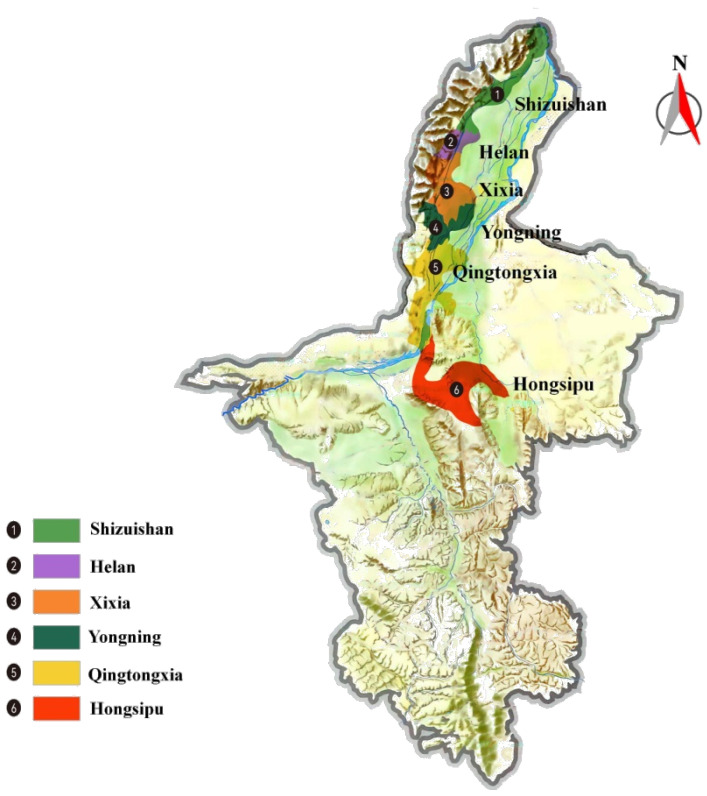
The eastern foothills of the Helan Mountain (EFHM) in the Ningxia Hui Autonomous Region, with latitude of 34°14′–39°23′ and longitude of 104°17′–107°39′, is located in the flood plain zone between the alluvial plain of the Yellow River and the alluvial fan of Helan Mountain (Figure 1). EFHM has a classic continental climate with sufficient sunlight, heat and suitable rainfall (2850–3110 h of sunshine, 3100–3500 °C of active accumulated temperature and 150–200 mm of rainfall). Helan Mountain obstructs cold air from the northwest, and the irrigation canals of the Yellow River in the east provide sufficient water, making EFHM a suitable region for wine grapes [3,4]. In 2011, the General Administration of Quality Supervision, Inspection and Quarantine of China ratified the protection of place of origin (POD) of EFHM in Ningxia Hui Autonomous Region, making it the third Appellation wine region, after Changli in Hebei province and Yantai in Shandong province.
Figure 1.
The topographic map of the six sub-regions from EFHM in Ningxia Hui Autonomous Region.
High in the north and low in the south, the Helan Mountain borders the desert in the south, creating a microclimate that varies from north to south. In addition, the wine-producing region in EFHM is located along the mountain and river, and this provides different soil types due to various distances from the mountain, thus creating a variety of vineyard conditions. After 10 years of development, delicate sub-regions in EFHM have been formed and recognised officially, namely Hongsipu region, Qingtongxia region, Yongning region, Xixia region, Helan region and Shizuishan region (Figure 1). Although EFHM is the first appellation in China with geographical indication for sub-regions, the comprehensive understanding of the terroir in these sub-regions is far from adequate due to a short development period.
Cabernet Sauvignon is the offspring of a cross between Cabernet Franc and Sauvignon Blanc [5], originated from the Bordeaux region in France, and is regarded as one of the most important grape varieties for making high quality red wines [6]. It has pronounced influences on the wine making regime, given its aptitude to vinification by itself, but particularly when blended with other grape varieties [7]. It is also one of the most popular grape varieties in China and has a sizable production in EFHM. EFHM contains 4000 hectares of Cabernet Sauvignon vine, accounting for 63% of the total wine grape cultivation area in the region. In addition, Cabernet Sauvignon is grown in all sub-regions, accounting for 50% or more of the total wine grape area in each. Cabernet Sauvignon is a late maturing variety with a very thick pericarp, resulting in abundant accumulation of phenolic compounds. Color and properties are important organoleptic aspects of wines, and phenolic compounds contribute to color [8,9], bitterness and astringency [10,11]. The phenolic composition of a wine is dependent on cultivar [12,13], climate [14], soil type [15], viticulture practice and vinification techniques [16], all of which are factors of terroir.
Different wine regions can have different terroir conditions which can lead to great differences in the quality, style and composition of wine. This difference could be reflected in the profile of secondary metabolites especially aromatic and phenolic compounds, as well as sensory characteristics. For instance, regional variation was characterized in 14 commercial Canadian Riesling wines using descriptive analysis (DA), and significant differences in the key aroma compounds of the wines were found [17]. Similarly, regional variation was notably observed based on total polyphenols, trans and cis-resveratrol and biogenic amines in 73 wines from four Southern Italy regions [18]. Using 18 non-flavonoid phenolic compounds, the origin of 43 Riesling wines from five regions in the Czech Republic could be successfully distinguished [19]. Li et al. analysed the phenolic compounds in Cabernet Sauvignon wines from five distinct regions across China and found different phenolic profile in wines from hot and arid regions in northwest China and warm and humid regions in eastern China, respectively. Compared with wines from the other four regions selected for the experiment, wines from Deqin in Yunnan province (a highland valley in southwest China) contained extremely high concentrations of cyanidin derivatives and quercetin derivatives, but extremely low concentration of epicatechin, reflecting the terroir effect of the wines [20].
However, the characteristics of phenolic compounds in Cabernet Sauvignon wines from different sub-regions in EFHM have not been reported.
With the aim of studying the delicate regional variation of Cabernet Sauvignon wines, 71 wines from six sub-regions of EFHM were selected. Primary phenolic compounds, including non-anthocyanin phenolics, anthocyanins and their derivatives, were analysed by high-performance liquid chromatography-triple-quadrupole (HPLC-QqQ-MS/MS). The colors of the wines were quantified using the CIELAB method. At the same time, the mouthfeel of the wines was evaluated using Check-All-That-Apply (CATA) and Quantitative Descriptive Analysis (QDA). To the best of our knowledge, this is the first time that such a wide range of wines from six sub-regions of EFHM has been collected. The phenolic profile of these samples from such a wide coverage could provide valuable information in deciphering the terroir of EFHM, which in turn could provide an academic basis for the better development of the wine industry in China.
2. Materials and Methods
2.1. Wine Samples
A total of 71 commercial wines from six different sub-regions of EFHM were collected. These wines were all Cabernet Sauvignon or Cabernet Sauvignon-dominant blends (>75%), with vintages ranging from 2015 to 2021. The basic wine compositions (residual sugar, alcohol level, pH, volatile acidity and total acidity) were measured using a WineScan (FT 120) rapid-scanning infrared Fourier-transform spectrometer with FOSS WineScan software version 2.2.1 (Foss Electric, Hillerød, Denmark). Information for all wine samples is given in Table S1A.
2.2. Chemicals and Standards
Methanol, formic acid and acetonitrile (HPLC grade) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Deionised water was purchased from Wahaha Co., Ltd. (Hangzhou, China). The standard compounds of anthocyanin and non-anthocyanin phenolics were purchased from Sigma-Aldrich (St. Louis, MO, USA), ChromaDex (Irvine, CA, USA), and Extrasynthese (Genay, France).
2.3. Analysis of Phenolic Compounds
Phenolic compounds in wines were analysed according to various published methods. All wines were filtered through a 0.22 μm inorganic polyether-sulfone membrane prior to HPLC-MS analysis. An Agilent 1200 series high-performance liquid chromatographer equipped with an Agilent 6410B triple-quadrupole (QqQ) mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) was used. The column was a Poroshell 120 EC-C18 column (150 mm × 2.1 mm, 2.7 µm; Agilent Technologies, Santa Clara, CA, USA). The mobile phases A were 0.1% formic acid in water; the mobile phases B were 0.1% formic acid in methanol and acetonitrile (50/50 v/v).
2.3.1. Analysis of Non-Anthocyanin Phenolic Compounds [21]
The gradient elution was: (1) from 10% to 46% B in 28 min; (2) from 46% to 10% B in 1 min. The post time was 5 min. The injection volume was 5 µL and the flow rate was 0.4 mL/min. The column was thermostatically controlled at 55 °C. An electrospray ionization source was used with 4 kV voltage and in the negative mode. The temperatures of the ion source and the drying gas (N2) were 150 °C and 350 °C, respectively. The drying gas flow rate was 12 L/min and the nebulizer pressure was 35 psi. The precursor ions and product ions of each phenolic compound were set in the multiple reaction monitoring (MRM) mode according to the published method [22]. The quantification of each phenolic compound was achieved through a calibration curve for each commercially available phenolic standard.
2.3.2. Analysis of Anthocyanins [23]
The HPLC conditions for the analysis of anthocyanins were the same as for non-anthocyanin phenolic compounds. The ion source parameters were the same as for the non-anthocyanin phenolics, except that the positive mode was used. The MRM mode was also selected for the detection of anthocyanins according to a previous publication [23]. The quantification of each anthocyanin compound was achieved through the malvidin-3-O-glucoside calibration curve.
2.3.3. Analysis of Anthocyanin Derivatives [24]
The mobile phase for the analysis of anthocyanin derivatives was the same as the above. The gradient elution started with the isocratic elution of 100% A for 1 min, then linearly increased B to 25% at 3 min, to 30% B at 15 min, to 100% B at 20 min, when the column was maintained by eluting with 100% B for an additional 5 min. The post time was 5 min. The injection volume was 10 µL and the flow rate was 0.3 mL/min. The ion source conditions were the same as for the analysis of anthocyanins. The MRM parameters for the anthocyanin derivatives were set according to a previous publication [24]. The semi-quantification of each anthocyanin derivative was calculated from the basis of the calibration curve of malvidin-3-O-glucoside measured by the same method.
2.4. Color Measurement
The chromatic characteristics of all wines were quantified using the CIELAB approach [25]. All wines were first filtered through a 0.22 μm inorganic polyether-sulfone membrane and then placed in a 2-mm-optical-path glass cuvette. The absorbances at wavelengths ranging from 400 nm to 700 nm (at 1 nm intervals) were measured using a UV-visible spectrophotometer (Shimadzu UV-2450, Shimadzu Co., Kyoto, Japan). The values of lightness (L*), red-greenness (a*), and yellow-blueness (b*) were calculated accordingly.
2.5. Sensory Analysis
It should be emphasized that this study complied with The Code of Ethics of the World Medical Association (Declaration of Helsinki), and all sensory evaluators provided informed consent to participate in the study. The Research Ethics Committee of China Agricultural University gave its approval for human subjects to be involved in this study, reference number CAUHR-20220901.
2.5.1. CATA
CATA is a rapid descriptive analysis method for consumers to select all sensory properties from a given list of sensory descriptors. CATA chooses consumers to replace professional sensory evaluators, without the need for professional training and maintenance [26]. Prior to the formal experiment, all 71 wine samples were evaluated by 16 experienced experts (professional sommeliers, winemakers and faculty), including 10 males and 6 females, aged between 26 and 58 years. Firstly, twelve experts were asked to participate in two sessions, each of which was divided into two rounds (approximately 50 min each). A glossary was then created. In the subsequent session, the appropriate terms were then agreed on by another four experts who had evaluated 71 wines. The final list consists of the 18 descriptors in Table S2A.
Forty people were randomly recruited to participate in the CATA, 14 males and 26 females, aged between 20 and 30 years old. All sensory evaluators had experience of wine tasting and were selected on the basis of their interest. Prior to the formal CATA, each evaluator was trained to successfully describe the astringency and tannin texture of wines. For the training session, gradient solutions of skin tannin extract (0.1, 0.5, 1.0, 1.5 and 2.0 g/L) were used, and the scales for perceived astringency strength were set as weak, moderately weak, moderate, moderately strong and strong, respectively. For the descriptors describing the tannin texture sensations (satin, velvet, fine emery and abrasive), the touch of a physical standard with the fingertips could be used as a reference, as recommended by Gawel et al. [27], e.g., the touch of a velvet cloth to represent the mouth surface sensation labelled velvet. The detailed sensory options are shown in Table S2A. After the training, 40 sensory evaluators were instructed to taste prepared wine samples and then check the appearance, astringency strength and tannin texture options using a pre-designed questionnaire. Water and tasteless biscuits were prepared for each evaluator, and they were requested to relieve their mouths after each tasting. The entire CATA was conducted in 3 sessions, and each session consisted of 4 rounds. In each round, 6 wines were served and the evaluator was requested to complete the questionnaire within 25 min. A 10-min break was provided after the first two rounds. All wines were prepared in International Standards Organisation (ISO) wine tasting glasses (ISO 3591:1977) containing approximately 30 mL of wine and presented in a random order. All sensory evaluators worked in individual booths at a controlled temperature (20 °C).
2.5.2. QDA
QDA is one of the classical descriptive sensory techniques to describe the characteristic and the intensity of sensory properties from a single evaluation of a product [28,29]. It has been widely applied to vegetables [30], milk [31], wine [32], etc. CATA can only identify the characteristics of wines from different sub-regions, but cannot quantify these characteristics, especially when comparing the differences between wines from different sub-regions. Therefore, based on the results of CATA, wines with typical characteristics of each sub-region were selected for QDA. Seventeen experienced sensory evaluators were invited to participate in the QDA, 12 females and 5 males, aged between 25 and 32.
Each sensory evaluator had passed the selection, training and periodic testing stipulated in the national standards of China (GB/T 16291.1-2012). Prior to the formal experiment, two Cabernet Sauvignon wines were used to standardize the scoring criteria and all sensory evaluators were requested to evaluate and discuss the body, finish, astringency strength and tannin texture of the wines until they reached a consensus. They were then asked to rate the selected wines on a scale of 0 to 10 for the four mouthfeel characteristics, with 0 being very weak and 10 being very strong. The wines for the QDA were divided into four rounds for scoring, lasting a total of two hours, with a 10-min break at the end of each session. The environment and supplies of QDA are the same as for CATA conditions.
2.6. Statistical Analysis
The identification and quantification of all phenolic compounds in the wines were achieved using Mass Hunter workstation software (version 10.0) (Agilent Technologies, Santa Clara, CA, USA). One-way analysis of variance (ANOVA) was conducted and Duncan’s post-hoc test with a significance level of 0.05 was performed in SPSS Statistics software (version 25.0) (IBM, Chicago, IL, USA). Soft Independent Modeling of Class Analogy (SIMCA, version 14.1 from Umetrics) was used for Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA). Hierarchical cluster analysis was achieved through “MetaboAnalyst 5.0” (http://www.metaboanalyst.ca/, (accessed on 14 October 2022)). CATA data were analysed using XLSTAT 2019 (Addinsoft, New York, NY, USA).
3. Results and Discussion
3.1. Basic Wine Compositions
The basic compositions of all the wines, including ethanol level, residual sugar, pH, total acidity and volatile acidity are listed in Table S1A. The alcohol level of all the wines ranged from 13.14% to 15.74% (v/v), the residual sugar ranged from 1.7 to 10.3 (g/L), the pH ranged from 3.54 to 4.23, the total acidity ranged from 4.9 to 7.6 (g/L, tartaric acid equivalent), and the volatile acidity ranged from 0.5 to 1.0 (g/L). All the basic parameters of the wines conformed to the national standards of China (GB/T 15037-2006) and can be used for subsequent analysis.
3.2. OPLS-DA Analysis
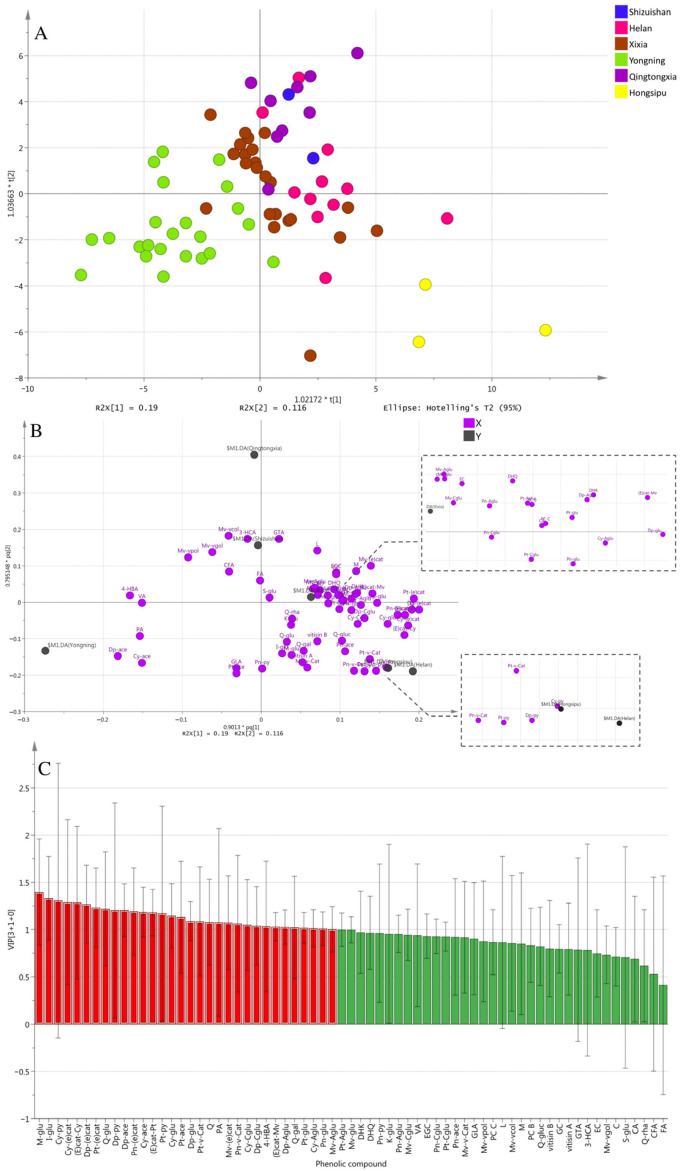
A total of 67 phenolic compounds were identified in all wines by HPLC-QqQ-MS/MS. Detailed information is shown in Table 1. The OPLS-DA model was used to differentiate and characterize six sub-regions in EFHM, as it has shown good performance in wines with subtle regional and vintage variations [33]. In the model, R2 is a measure of fitness, i.e., how well the model fits the data. Q2 indicates the predictability of the model. As shown in Figure 2A, a separation was obtained by a reliable OPLS-DA model (R2X = 0.662, R2Y = 0.331, Q2 = 0.159) based on the concentration of phenolic compounds. The further validation of the model was tested by 7-fold internal cross-validation and 200-time permutation tests. The prediction result of the cross-validated score plot was basically consistent with the actual score plot (Figure S1 and Figure 2A), indicating a good predictive effect. The results of 200-time permutation tests showed that the OPLS-DA model did not overfit (Figure S2). According to the model, it can be clearly seen that the wines from Yongning, Qingtongxia and Hongsipu region were all well separated. Meanwhile, there was some overlap between the wines from Helan and Xixia region (Figure 2A). We speculated that this may be due to the fact that Helan and Xixia regions are adjacent to each other (region 2 and 3 in Figure 1), leading to more similarities in topographical features. Interestingly, wines from Shizuishan and Qingtongxia regions also overlap in the model, but the two sub-regions have nothing in common other than the same altitude (Table S3).
Table 1.
Abbreviation, MRM information, and calibration curves of phenolic compounds.
| Phenolic Compounds | Abbreviation | MRM Transition Ions (m/z) | Retention Time (Min) | Quantitative Standards | Calibration Curves (mg/L) | R2 |
|---|---|---|---|---|---|---|
| Procyanin B | PC B | 577–407 | 7.6 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Procyanin C1 | PC C | 865–407 | 10.36 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Epigallocatechin | EGC | 305–125 | 5.2 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Catechin | C | 289–123 | 5.8 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Epicatechin | EC | 289–123 | 9.3 | EC | y = 0.002x − 2.4286 | R² = 0.9929 |
| Gallo-catechin | GC | 305–125 | 2.5 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Caffeic acid | CFA | 179–135 | 7.2 | CFA | y = 0.00008x + 0.8751 | R² = 0.9921 |
| 3-hydroxycinnamic acid | 3-HCA | 163–119 | 10.58 | 3-HCA | y = 0.000006x + 0.066 | R² = 0.9966 |
| Ferulic acid | FA | 193–134 | 12.72 | FA | y = 0.0003x + 0.3138 | R² = 0.9991 |
| Chlorogenic acid | CA | 353–191 | 6.3 | CA | y = 0.00009x + 0.3061 | R² = 0.9969 |
| Gallic acid | GLA | 169–125 | 1.7 | GLA | y = 0.0002x + 0.4347 | R² = 0.9972 |
| Protocatechuic acid | PA | 153–109 | 3.0 | PA | y = 0.0002x − 0.0369 | R² = 0.9996 |
| 4-hydroxybenzoic acid | 4-HBA | 137–93 | 5.02 | 4-HBA | y = 0.0003x − 0.0372 | R² = 0.9909 |
| Gentisic acid | GTA | 153–109 | 5.0 | GTA | y = 0.0002x + 0.045 | R² = 0.9996 |
| Vanillic acid | VA | 167–152 | 6.9 | GTA | y = 0.0002x + 0.045 | R² = 0.9996 |
| Myricetin-glucoside | M-glu | 479–316 | 13.3 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Dihydro-quercetin | DHQ | 303–125 | 13.5 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Dihydro-kampferol | DHK | 287–259 | 17.13 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-glucoside | Q-glu | 463–300 | 16.3 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-galactoside | Q-gal | 463–300 | 15.75 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-glucuronide | Q-gluc | 477–301 | 15.9 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin | Q | 301–151 | 23.9 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Larictrin | L | 331–151 | 24.8 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Myricetin | M | 317–151 | 19.02 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Isorhamnetin-glucoside | I-glu | 477–314 | 19.77 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Kaempferol-3-O-glucoside | K-glu | 447–255 | 18.9 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Syringetin-glucoside | S-glu | 507–344 | 20.1 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-rhamnoside | Q-rha | 447–300 | 19.0 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Cyanidin-3-O-glucoside | Cy-glu | 449–287 | 4.5 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-acetylglucoside | Cy-Aglu | 491–287 | 5.69 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-coumaroylglucoside (cis+trans) | Cy-Cglu | 595–287 | 6.43 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside | Dp-glu | 465–303 | 4.6 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-acetylglucoside | Dp-Aglu | 507–303 | 5.39 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-coumaroylglucoside (cis+trans) | Dp-Cglu | 611–303 | 6.16 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside | Pn-glu | 463–301 | 5.07 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-acetylglucoside | Pn-Aglu | 505–301 | 6.08 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-coumaroylglucoside (cis+trans) | Pn-Cglu | 609–301 | 6.76 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside | Pt-glu | 479–317 | 4.7 | Mv-glu | y = 0.00002 x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-acetylglucoside | Pt-Aglu | 521–317 | 5.76 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-coumaroylglucoside (cis + trans) | Pt-Cglu | 625–317 | 6.47 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside | Mv-glu | 493–331 | 5.15 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-acetylglucoside | Mv-Aglu | 535–331 | 6.08 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-coumaroylglucoside (cis + trans) | Mv-Cglu | 639–331 | 6.74 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-(epi)catechin (A type) | Mv-(e)cat | 783–343 | 10.53 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-(epi)catechin (A type) | Pn-(e)cat | 753–313 | 10.29 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside-(epi)catechin (A type) | Dp-(e)cat | 755–315 | 8.08 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-(epi)catechin (A type) | Pt-(e)cat | 769–329 | 9.1 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-glucoside-(epi)catechin (A type) | Cy-(e)cat | 739–299 | 8.9 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| (Epi)catechin-cyanidin-3-O-glucoside (B type) | (E)cat-Cy | 737–575 | 6.39 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| (Epi)catechin-malvidin-3-O-glucoside (B type) | (E)cat-Mv | 781–619 | 6.97 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| (Epi)catechin-petunidin-3-O-glucoside (B type) | (E)cat-Pt | 767–605 | 6.5 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-glucoside-acetaldehyde | Cy-ace | 473–311 | 8.5 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside-acetaldehyde | Dp-ace | 489–327 | 7.2 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-acetaldehyde | Vitisin B | 517–355 | 10.7 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-acetaldehyde | Pt-ace | 503–341 | 10.1 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-acetaldehyde | Pn-ace | 487–325 | 10.14 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-glucoside-pyruvic acid | Cy-py | 517–355 | 8.36 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside-pyruvic acid | Dp-py | 533–371 | 7.6 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-pyruvic acid | Vitisin A | 561–399 | 10.415 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-pyruvic acid | Pt-py | 547–385 | 8.7 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-pyruvic acid | Pn-py | 532–369 | 9.86 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinyl(epi)catechin | Mv-v-Cat | 805–643 | 20.69 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-4-vinyl(epi)catechin | Pn-v-Cat | 775–613 | 20.51 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-4-vinyl(epi)catechin | Pt-v-Cat | 791–629 | 19.34 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinylcatechol | Mv-vcol | 625–463 | 20.9 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinylphenol | Mv-vpol | 609–447 | 21.21 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinylguaiacol | Mv-vgol | 639–477 | 21.31 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
Figure 2.
OPLS-DA model based on the concentrations of phenolic compounds in Cabernet Sauvignon wines from six sub-regions of EFHM. (A) Score plot (B) Loading plot (C) VIP plot of the OPLS-DA model.
Figure 2B shows the correlation between the explanatory variables, i.e., the concentration of phenolic compounds (in purple dots) and the dependent variables, i.e., the sub-regions (in black dots) in the first and second principal components. These compounds, located close to the sub-regions in Figure 2B, could be considered as potential features with these sub-regions. Figure 2C shows the Variable Importance for the Projection (VIP) plot of the OPLS-DA model. A higher VIP value indicates a greater contribution of the explanatory variable to the discriminative ability in OPLS-DA. Normally, a VIP value above 1 could be considered as a threshold for selecting potential markers [34]. A total of 32 phenolic compounds with VIP above 1 were screened (Figure 2C).
Combined with Figure 2B,C, it can be seen that cyanidin-3-O-acetylglucoside (Cy-Aglu), malvidin-3-O-glucoside-(epi)catechin (A type) (Mv-(e)cat) and (epi)catechin-malvidin-3-O-glucoside (B type) ((E)cat-Mv) were the potential markers in wines from Xixia region. Delphinidin-3-O-glucoside-pyruvic acid (Dp-py), petunidin-3-O-glucoside-pyruvic acid (Pt-py) and cyanidin-3-O-glucoside (Cy-glu) were the potential markers in wines from Helan region and Hongsipu region. Delphinidin-3-O-glucoside-acetaldehyde (Dp-ace), cyanidin-3-O-glucoside-acetaldehyde (Cy-ace) and protocatechuic acid (PA) were the potential markers in wines from Yongning region.
3.3. Sub-Regional Variation of Phenolic Compounds
3.3.1. Comparison of Non-Anthocyanin Phenolic Compounds
A total of 28 non-anthocyanin phenolic compounds were identified in all wines via HPLC-QqQ-MS/MS (Table S4), including six flavan-3-ols, nine phenolic acids (three hydroxycinnamic acids and six hydroxybenzoic acids) and 13 flavonols.
It was found that the concentration of the total detectable flavan-3-ols in wines from six sub-regions ranged from 145.12 to 673.67 mg/L. Wines from the Yongning region had the lowest flavan-3-ol concentration, while those from the Hongsipu region had the highest. (Table S4). Previous studies had shown that the type of soil has a significant effect on phenolic compounds in grape [35,36]. The soil of Hongsipu region is a sierozem soil with loose texture and good aeration, which makes the soil have strong water and fertilizer holding capacity and rich calcium concentration [37,38]. Such soil provided favourable conditions for tannin accumulation [39]. This may be one of the reasons accounting for the higher concentration of flavan-3-ols in wines from Hongsipu region.
The total detectable flavonol concentrations in wines from the six sub-regions ranged from 21.74 to 118.69 mg/L. The concentrations of myricetin-glucoside (M-glu), quercetin-glucoside (Q-glu) and isorhamnetin-glucoside (I-glu) in Helan wines were significantly higher than those in other sub-regions, resulting overall in the highest total flavonol concentrations. In contrast, the Hongsipu wines had the lowest flavonol concentrations (Table S4). Both genotype (variety) and environment are critical factors in controlling the production of flavonols [40]. Furthermore, in the case of wines, even common wine-making processes, including grape skin contact, stabilization processes and ageing, have been shown to cause significant changes in flavonols [41]. However, the reason for the high concentration of flavonols in Helan wines is still unclear and further studies are required.
The concentration of total detectable hydroxybenzoic acids ranged from 10.16 to 51.38 mg/L, with the highest concentration in Shizuishan wines and the lowest in Qingtongxia wines. Gallic acid (GLA) was the predominant hydroxybenzoic acid (6.77 to 38.12 mg/L), which was consistent with previous reports [42]. Compared with the wines from other sub-regions, the wines from the Hongsipu region contained a significantly higher concentration of GLA but a lower concentration of vanillic acid (VA), 4-hydroxybenzoic acid (4-HBA) and PA. In contrast, more 4-HBA and PA were detected in Shizuishan wines, even up to about twice as much as in wines from other sub-regions (Table S4).
In terms of hydroxycinnamic acids, concentration ranged from 4.47 to 29.19 mg/L. In addition, as the predominant hydroxycinnamic acid [43,44], caffeic acid (CFA) showed no significant difference among the six sub-regions. More generally, the remaining hydroxycinnamic acids in the six sub-regions of wines were not significantly different except for 3-hydroxycinnamic acid (3-HCA) (Table S4).
3.3.2. Comparison of Anthocyanins
A total of 15 anthocyanins were identified by HPLC-QqQ-MS/MS in all wines, including non-acylated anthocyanins, acylated anthocyanins and coumaroylated anthocyanins (Table S5). In general, there were no significant differences in the total concentration of detectable anthocyanins between the six sub-regions. Nonetheless, wines from Helan and Shizuishan region showed a higher and lower concentration of total detectable anthocyanins, respectively. The biosynthesis of anthocyanins in grape was influenced by temperature, with a lower temperature promoting the expression levels of anthocyanin biosynthesis genes such as VIMYBA2, while a higher temperature may suppress them [45,46]. Although climate data for recent years were not available, a higher effective accumulated temperature from July to October was observed in the Shizuishan region from historical data (Table S3). This may be one of the reasons that account for a lower anthocyanin concentration in this region.
All five types of anthocyanins were detected: cyanidin, delphinidin, peonidin, petunidin and malvidin. The proportion of the five types of anthocyanins in wines from the six sub-regions was almost the same (Table S5). Cyanidin-type anthocyanins had the lowest concentration and malvidin-type anthocyanins had the highest concentration, suggesting that the anthocyanin composition was not influenced by sub-regional factor but might be inherently controlled by a genetic factor, such as cultivar [47]. Among all the anthocyanins, malvidin-3-O-glucoside (Mv-glu) showed the highest concentration in wine, in agreement with previous results [48,49].
3.3.3. Comparison of Anthocyanin Derivatives
A total of 24 anthocyanin derivatives were identified in all wines by HPLC-QqQ-MS/MS (Table S6), including three direct flavanol-anthocyanin condensation products (F-A), five direct anthocyanin-flavanol condensation products (A-F) and 16 pyrano-anthocyanins (10 vitisins, three flavanyl-pyrano-anthocyanins and three pinotins). Overall, there was no significant difference in the concentration of total detectable anthocyanin derivatives in different sub-regions, but it could be seen that the wines from Hongsipu region had the highest concentration of total detectable anthocyanin derivatives, followed by the wines from Helan region, and the wines from Shizuishan region had the lowest concentration of total detectable anthocyanin derivatives (Table S6). For most anthocyanin derivatives, such as delphinidin-3-O-glucoside-pyruvic acid (Dp-py) and petunidin-3-O-glucoside-pyruvic acid (Pt-py), their concentrations were significantly higher in wines from Hongsipu, which was largely consistent with the concentration conditions of their anthocyanin precursors. For those anthocyanin derivatives with no significant difference, a reasonable explanation was that there were also no significant differences in the concentrations of their anthocyanin precursors. In addition, anthocyanin derivatives were mostly formed during the process of alcoholic fermentation, malolactic fermentation and aging of wine [50]. For example, the precursors of vitisins were pyruvic acid and acetaldehyde derived from yeast metabolism during alcoholic fermentation [51]. Therefore, the accumulation of anthocyanin derivatives in wines from the six sub-regions was a complex process influenced by many factors, such as wine-making technology and grape variety.
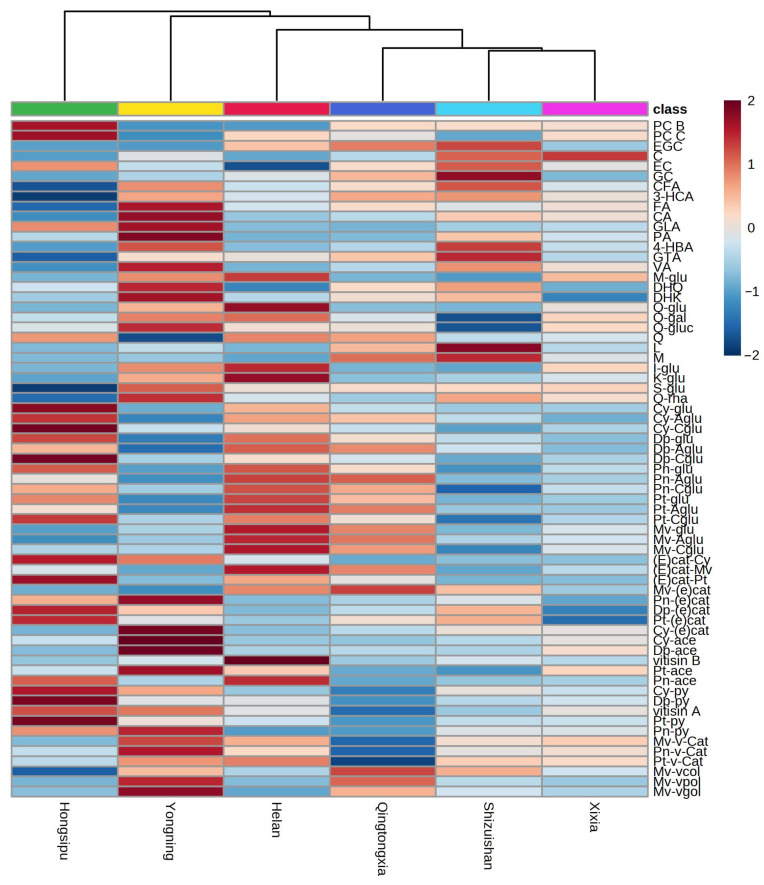
3.4. Hierarchical Cluster Analysis
The phenolic compounds of the 71 EFHM wines were analyzed using hierarchical cluster analysis to determine the similarities for these sub-regions (Figure 3). Yongning region, Helan region, Qingtongxia region, Shizuishan region and Xixia region were consecutively grouped into one category, which may be due to the fact that these sub-regions are all adjacent to the foothill of Helan Mountain and therefore share similar climate and soil type. As the southernmost sub-region of EFHM, Hongsipu region is far away from other sub-regions and less protected by Helan Mountain (Figure 1). Moreover, its high altitude makes it more susceptible to the northwesterly cold flow. It also received more rainfall than most of the sub-regions (Table S3). These factors led to large differences in terroir, so Hongsipu region was divided into a separate category, which is consistent with the clustering results of different sub-regions in EFHM by Zhang et al. [52].
Figure 3.
Hierarchical cluster analysis based on the concentrations of phenolic compounds in Cabernet Sauvignon wines in the six sub-regions of EFHM.
3.5. Comparison of Color of Wines
The chromatic properties of wines from different sub-regions were quantified using the CIELAB approach (Table 2). The color of wine is originally derived from anthocyanins extracted from grape skins during winemaking. Anthocyanins showed a negative correlation with L* and b* values but a positive correlation with a* values [53]. The results of the chromatic characteristics of all wines showed no significant differences in L* values between wines from different sub-regions, indicating no significant variation in color intensity. Wines from Shizuishan had significantly higher b* values (more yellowness) and lower a* values (less redness) than those from other sub-regions. Based on the quantification of phenolic compounds, we found that this might be due to the lowest concentration of total anthocyanins in Shizuishan wines (Table S5). In addition, wines from Shizuishan region had a significantly higher pH than those from other sub-regions (Table S1B), while higher pH could result in wine losing its color intensity and redness [54,55].
Table 2.
Color parameters of Cabernet Sauvignon wines from the six sub-regions of EFHM.
| Shizuishan | Helan | Xixia | Yongning | Qingtongxia | Hongsipu | |
|---|---|---|---|---|---|---|
| L* | 51.38 ± 2.36 a | 44.06 ± 9.66 a | 48.02 ± 10.08 a | 48.25 ± 8.85 a | 50.03 ± 8.95 a | 47.46 ± 12.14 a |
| a* | 40.84 ± 0.34 b | 47.24 ± 7.52 a | 43.92 ± 7.71 ab | 43.09 ± 6.94 ab | 41.98 ± 5.82 ab | 45.22 ± 7.68 ab |
| b* | 28.52 ± 5.22 a | 24.05 ± 4.91 b | 24.36 ± 5.85 b | 21.82 ± 5.11 bc | 22.16 ± 3.60 bc | 19.78 ± 5.15 c |
* Expressed as average value plus and minus standard deviation; different letters in the same row indicate significant difference (p < 0.05) using Duncan’s multiple range test.
3.6. Sensory Characteristics of Wines
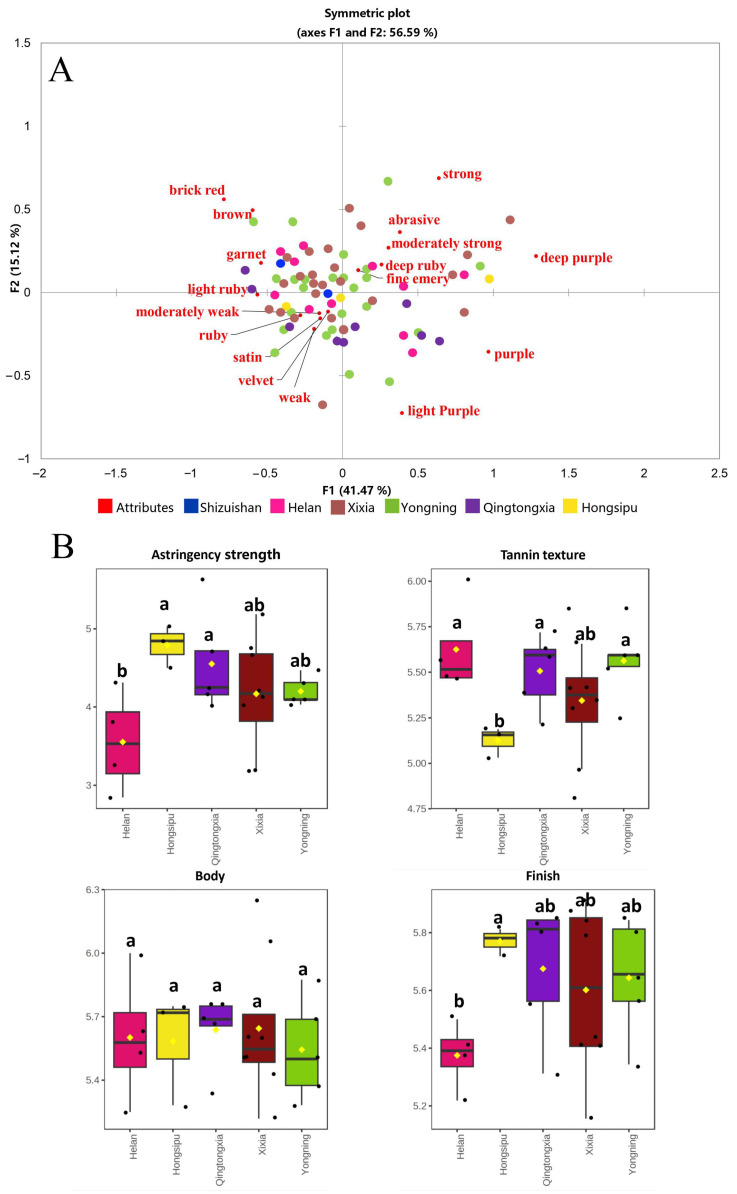
CATA was used to characterize the appearance, astringency strength and tannin texture of Cabernet Sauvignon wines from the six sub-regions of EFHM. Correspondence analysis (CA) is a multivariate statistical technique which is applicable to tables of categorical data [56]. In the study, CA was used to explore the visual and mouthfeel characteristics of wines in descriptors of specific characteristics such as appearance, astringency strength and tannin texture. In CA, there was a significant difference in frequency for 17 of the 18 descriptors (p < 0.05) and their correlation with the wines is shown in Figure 4A. F1 and F2 explained a 56.59% of the total variance. Wines in the first quadrant were deep ruby or deep purple in appearance, with strong to moderately strong astringency strength and fine emery or abrasive tannin texture. Wines in the second quadrant were brick red, brown or garnet in appearance. In the third quadrant, the wines were mainly light ruby or ruby in appearance, with moderately weak or weak astringency strength and satin or velvet tannin texture. In the fourth quadrant, the wines were mainly light purple and purple in appearance.
Figure 4.
Sensory characteristics of Cabernet Sauvignon wines from the six sub-regions of EFHM. (A) Correspondence analysis of wines based on CATA; (B) Box plot of wines based on QDA. Different letters in the same area indicate significant difference (p < 0.05) using Duncan’s multiple range test.
In addition, Table S2B shows the frequencies of the descriptors used to describe the wines, from which frequencies above 20% were selected as representative sensory characteristics. The typical visual and mouthfeel characteristics of wines from different sub-regions were obtained. The results showed that the wines from Shizuishan region were ruby, while the astringency strength was moderately weak, with velvet or fine emery tannin texture. However, moderately strong astringency strength was also selected by some sensory evaluators for Shizuishan wines. Most Helan wines were deep ruby or ruby, and their astringency strength was considered as moderately weak, with velvet or fine emery tannin texture. Wines from Xixia region were ruby or deep ruby in appearance, with moderately weak astringency strength and velvet or fine emery tannin texture. Yongning wines were light ruby or deep ruby, some wines were described as garnet, and the astringency strength was moderately weak, with velvet or fine emery tannin texture. Qingtongxia wines were mostly ruby, and the astringency strength was moderately weak or weak, felt satin or velvet, and a few were described as fine emery. Hongsipu wines were purple or ruby in appearance, with moderately weak astringency strength and fine emery tannin texture.
Using the above-mentioned criteria (frequency > 0.2), 27 representative wines from different sub-regions (excluding Shizuishan region) were used for QDA. The box plot in Figure 4B shows the profile of astringency strength, tannin texture, body and finish of wines from the different sub-regions. The results shows that the astringency strength of Hongsipu wines is significantly higher (Table S2C). It has been confirmed that flavan-3-ols are the most important compounds in determining the astringency strength of wine [10,57]; in this case, however, flavan-3-ols were more pronounced in Hongsipu wines, albeit no significant difference was observed (Table S4). Hydroxybenzoic acids were also proved to contribute to astringency [58], and this might be one of the reasons for the stronger astringency of Hongsipu wines. Lower pH and ethanol in Hongsipu wines (Table S1B) could also accentuate the astringent sensation in month [59], and could lead to a higher astringency strength result. In addition, Hongsipu wines showed lower tannin texture than the others, which was basically consistent with the CATA results, possibly due to more flavan-3-ols and hydroxybenzoic acids, as well as lower pH and ethanol level, as suggested [60].
4. Conclusions
In this study, primary phenolic compounds, visual properties and mouthfeel of 71 Cabernet Sauvignon wines from the six sub-regions of EFHM were analysed. Through the mining of the OPLS-DA model, it was found that 32 phenolic compounds could be used as characteristic compounds to distinguish wines from different sub-regions. The quantitative analysis results showed that the concentration of phenolic compounds in wines from different sub-regions had their own characteristics; especially, the Hongsipu wines showed great differences in phenolic compounds compared with others. In addition, these characteristics are also reflected in the senses, forming the unique visual properties and mouthfeel of the wines of different sub-regions.
Thus, a terroir effect was observed for phenolic compounds and detailed studies on the effects of terroir on the phenolic compounds in wines from different sub-regions in EFHM should be further investigated. It should also be noted that the exploration of terroir conditions in different sub-regions of EFHM is still limited, and the typical characteristics of wines in different regions are not well understood. In the future, further probing could be carried out in this respect.
Acknowledgments
The authors are grateful to China Agricultural University for providing sensory evaluation conditions and the help of all sensory evaluation experts invited in this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12051081/s1. Table S1A: The information and basic composition and L*, a*, and b* values of all wine samples; Table S1B: The statistical summary of basic physical and chemical indexes of Cabernet Sauvignon wines from six sub-regions of EFHM; Table S2A: The materials and scales for training in CATA; Table S2B: The CATA frequency of 71 Cabernet Sauvignon wines from the six sub-regions of EFHM; Table S2C: The QDA result of 71 Cabernet Sauvignon wines from the six sub-regions of EFHM; Table S3: The historic terroir parameter of the six sub-regions of EFHM; Figure S1: Cross-validated score plot for the OPLS-DA model based on the concentrations of phenolic compounds in Cabernet Sauvignon wines from six sub-regions of EFHM; Figure S2: Validation plot obtained from 200-time permutation tests for the OPLS-DA model based on the concentrations of phenolic compounds in Cabernet Sauvignon wines from six sub-regions of EFHM; Table S4: Concentration of non-anthocyanin phenolic compound in Cabernet Sauvignon wines from six sub-regions of EFHM; Table S5: Concentration of anthocyanin in Cabernet Sauvignon wines from six sub-regions of EFHM; Table S6: Concentration of anthocyanin derivative in Cabernet Sauvignon wines from six sub-regions of EFHM.
Author Contributions
B.-Y.Z. contributed to investigation, formal analysis, data curation and writing of the original draft. X.-K.Z. contributed to formal analysis, data curation, methodology and review & editing. Y.-B.L. contributed to methodology and supervision. C.-Q.D. contributed to conceptualization, supervision and project administration. B.-Q.Z. contributed to methodology and supervision. D.-M.L. contributed to conceptualization, resources, supervision and review & editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Key Project of R&D Program of Ningxia Hui Autonomous Region, China (grant number 2021BEF02014) and the National Natural Science Foundation of China (grant number U20A2042).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Whalen P. ‘Insofar as the Ruby Wine Seduces Them’: Cultural Strategies for Selling Wine in Inter-war Burgundy. Contemp. Eur. Hist. 2009;18:67–98. doi: 10.1017/S0960777308004839. [DOI] [Google Scholar]
- 2.Van Leeuwen C., Seguin G. The concept of terroir in viticulture. J. Wine Res. 2006;17:1–10. doi: 10.1080/09571260600633135. [DOI] [Google Scholar]
- 3.Li Y.-D., Zhang J.-X., Wang Z.-D., Cai X.-Q., Yu H.-M., Ma Y.-M., Wang F.-Q., Zhang G.-L. Grape Vintages and Climate at the Eastern Foothills of Helan Mountain in Ningxia. Vitic. Winemak. 2004;2:54–57. (In Chinese) [Google Scholar]
- 4.Qi Y., Wang R., Qin Q., Sun Q. Soil affected the variations in grape and wine properties along the eastern foot of Helan Mountain, China. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019;69:494–502. doi: 10.1080/09064710.2019.1611914. [DOI] [Google Scholar]
- 5.Bowers J.E., Meredith C.P. The parentage of a classic wine grape, Cabernet Sauvignon. Nat. Genet. 1997;16:84–87. doi: 10.1038/ng0597-84. [DOI] [PubMed] [Google Scholar]
- 6.Robinson J., Harding J. The Oxford Companion to Wine. American Chemical Society; Washington, DC, USA: 2015. [Google Scholar]
- 7.Gatti M., Civardi S., Ferrari F., Fernandes N., Goncaves M.V.Z.D.M.B., Bavaresco L. Viticultural performances of different ‘cabernet sauvignon’ clones. Acta Hortic. 2014;1046:659–664. doi: 10.17660/ActaHortic.2014.1046.90. [DOI] [Google Scholar]
- 8.Gómez-Plaza E., Gil-Muñoz R., López-Roca J., Martínez-Cutillas A., Fernández-Fernández J. Phenolic Compounds and Color Stability of Red Wines: Effect of Skin Maceration Time. Am. J. Enol. Vitic. 2001;52:266–270. doi: 10.5344/ajev.2001.52.3.266. [DOI] [Google Scholar]
- 9.Lorenzo C., Pardo F., Zalacain A., Alonso G.L., Salinas M.R. Effect of Red Grapes Co-winemaking in Polyphenols and Color of Wines. J. Agric. Food Chem. 2005;53:7609–7616. doi: 10.1021/jf050848c. [DOI] [PubMed] [Google Scholar]
- 10.Peleg H., Gacon K., Schlich P., Noble A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999;79:1123–1128. doi: 10.1002/(SICI)1097-0010(199906)79:8<1123::AID-JSFA336>3.0.CO;2-D. [DOI] [Google Scholar]
- 11.Gawel R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998;4:74–95. doi: 10.1111/j.1755-0238.1998.tb00137.x. [DOI] [Google Scholar]
- 12.Xu C., Zhang Y., Cao L., Lu J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010;119:1557–1565. doi: 10.1016/j.foodchem.2009.09.042. [DOI] [Google Scholar]
- 13.Mazza G., Fukumoto L., Delaquis P., Girard B., Ewert B. Anthocyanins, Phenolics, and Color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999;47:4009–4017. doi: 10.1021/jf990449f. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer-Gallego R., Hernández-Hierro J.M., Rivas-Gonzalo J.C., Escribano-Bailón M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta. 2012;732:73–77. doi: 10.1016/j.aca.2011.12.072. [DOI] [PubMed] [Google Scholar]
- 15.Gutiérrez-Gamboa G., Carrasco-Quiroz M., Verdugo-Vásquez N., Díaz-Gálvez I., Garde-Cerdán T., Moreno-Simunovic Y. Characterization of grape phenolic compounds of ‘Carignan’ grapevines grafted onto ‘País’ rootstock from Maule Valley (Chile): Implications of climate and soil conditions. Chil. J. Agric. Res. 2018;78:310–315. doi: 10.4067/S0718-58392018000200310. [DOI] [Google Scholar]
- 16.Downey M.O., Dokoozlian N.K., Krstic M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006;57:257–268. doi: 10.5344/ajev.2006.57.3.257. [DOI] [Google Scholar]
- 17.Douglas D., Cliff M.A., Reynolds A.G. Canadian terroir: Characterization of Riesling wines from the Niagara Peninsula. Food Res. Int. 2001;34:559–563. doi: 10.1016/S0963-9969(01)00071-0. [DOI] [Google Scholar]
- 18.Galgano F., Caruso M., Perretti G., Favati F. Authentication of Italian red wines on the basis of the polyphenols and biogenic amines. Eur. Food Res. Technol. 2011;232:889–897. doi: 10.1007/s00217-011-1457-1. [DOI] [Google Scholar]
- 19.Pavloušek P., Kumšta M. Authentication of Riesling wines from the Czech Republic on the basis of the non-flavonoid phenolic compounds. Czech J. Food Sci. 2013;31:474–482. doi: 10.17221/40/2013-CJFS. [DOI] [Google Scholar]
- 20.Li Z., Pan Q., Jin Z., Mu L., Duan C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011;125:77–83. doi: 10.1016/j.foodchem.2010.08.039. [DOI] [Google Scholar]
- 21.Lan Y., Liu M., Zhang X., Li S., Shi Y., Duan C. Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China. Foods. 2022;11:787. doi: 10.3390/foods11060787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S.-Y., He F., Zhu B.-Q., Wang J., Duan C.-Q. Comparison of phenolic and chromatic characteristics of dry red wines made from native Chinese grape species and vitis vinifera. Int. J. Food Prop. 2017;20:2134–2146. doi: 10.1080/10942912.2016.1233117. [DOI] [Google Scholar]
- 23.Li S.-Y., He F., Zhu B.-Q., Xing R.-R., Reeves M.J., Duan C.-Q. A systematic analysis strategy for accurate detection of anthocyanin pigments in red wines. Rapid Commun. Mass Spectrom. 2016;30:1619–1626. doi: 10.1002/rcm.7584. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X.-K., Li S.-Y., Zhao X., Pan Q.-H., Shi Y., Duan C.-Q. HPLC-MS/MS-based targeted metabolomic method for profiling of malvidin derivatives in dry red wines. Food Res. Int. 2020;134:109226. doi: 10.1016/j.foodres.2020.109226. [DOI] [PubMed] [Google Scholar]
- 25.Ayala F., Echávarri J.F., Negueruela A.I. A New Simplified Method for Measuring the Color of Wines. I. Red and Rosé Wines. Am. J. Enol. Vitic. 1997;48:357–363. doi: 10.5344/ajev.1997.48.3.357. [DOI] [Google Scholar]
- 26.Dooley L., Lee Y.-S., Meullenet J.-F. The application of check-all-that-apply (CATA) consumer profiling to preference mapping of vanilla ice cream and its comparison to classical external preference mapping. Food Qual. Prefer. 2010;21:394–401. doi: 10.1016/j.foodqual.2009.10.002. [DOI] [Google Scholar]
- 27.Gawel R., Oberholster A., Francis I.L. A ‘Mouth-feel Wheel’: Terminology for communicating the mouth-feel characteristics of red wine. Aust. J. Grape Wine Res. 2000;6:203–207. doi: 10.1111/j.1755-0238.2000.tb00180.x. [DOI] [Google Scholar]
- 28.Stone H., Sidel J., Oliver S., Woolsey A., Singleton R.C. Sensory evaluation by quantitative descriptive analysis. In: Gacular M.C. Jr., editor. Descriptive Sensory Analysis in Practice. Food & Nutrition Press, Inc.; Trumbull, CT, USA: 2004. pp. 23–34. [Google Scholar]
- 29.Ng M., Lawlor J., Chandra S., Chaya C., Hewson L., Hort J. Using quantitative descriptive analysis and temporal dominance of sensations analysis as complementary methods for profiling commercial blackcurrant squashes. Food Qual. Prefer. 2012;25:121–134. doi: 10.1016/j.foodqual.2012.02.004. [DOI] [Google Scholar]
- 30.Krumbein A., Peters P., Brückner B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004;32:15–28. doi: 10.1016/j.postharvbio.2003.10.004. [DOI] [Google Scholar]
- 31.Chapman K., Lawless H., Boor K. Quantitative Descriptive Analysis and Principal Component Analysis for Sensory Characterization of Ultrapasteurized Milk. J. Dairy Sci. 2001;84:12–20. doi: 10.3168/jds.S0022-0302(01)74446-3. [DOI] [PubMed] [Google Scholar]
- 32.Castiñeira M.D.M., Feldmann I., Jakubowski N., Andersson J.T. Classification of German White Wines with Certified Brand of Origin by Multielement Quantitation and Pattern Recognition Techniques. J. Agric. Food Chem. 2004;52:2962–2974. doi: 10.1021/jf035120f. [DOI] [PubMed] [Google Scholar]
- 33.Li S.-Y., Zhu B.-Q., Reeves M.J., Duan C.-Q. Phenolic Analysis and Theoretic Design for Chinese Commercial Wines’ Authentication. J. Food Sci. 2017;83:30–38. doi: 10.1111/1750-3841.13961. [DOI] [PubMed] [Google Scholar]
- 34.Chong I.-G., Jun C.-H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005;78:103–112. doi: 10.1016/j.chemolab.2004.12.011. [DOI] [Google Scholar]
- 35.Prado R.D.A.-D., Yuste-Rojas M., Sort X., Andrés-Lacueva C., Torres M., Lamuela-Raventós R.M. Effect of Soil Type on Wines Produced from Vitis vinifera L. Cv. Grenache in Commercial Vineyards. J. Agric. Food Chem. 2007;55:779–786. doi: 10.1021/jf062446q. [DOI] [PubMed] [Google Scholar]
- 36.Van Leeuwen C., Roby J.-P., De Rességuier L. Soil-related terroir factors: A review. OENO One. 2018;52:173–188. doi: 10.20870/oeno-one.2018.52.2.2208. [DOI] [Google Scholar]
- 37.Li W., Sun P., Wang Z. Effects of different soil condition on physiology and fruit quality of wine grapes. J. Fruit Sci. 2012;29:837–842. (In Chinese) [Google Scholar]
- 38.Wang L., Zhang Z.J.N.H. Research on quality of wine grape of main cultivated in Ningxia in 2009. North. Hortic. 2011;3:4–8. (In Chinese) [Google Scholar]
- 39.Wang R., Sun Q., Chang Q. Soil Types Effect on Grape and Wine Composition in Helan Mountain Area of Ningxia. PLoS ONE. 2015;10:e0116690. doi: 10.1371/journal.pone.0116690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang N.-N., He F., Bi H.-Q., Duan C.-Q., Reeves M.J., Wang J. Evolution of flavonols in berry skins of different grape cultivars during ripening and a comparison of two vintages. Eur. Food Res. Technol. 2012;235:1187–1197. doi: 10.1007/s00217-012-1850-4. [DOI] [Google Scholar]
- 41.Makris D.P., Kallithraka S., Kefalas P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006;19:396–404. doi: 10.1016/j.jfca.2005.10.003. [DOI] [Google Scholar]
- 42.Cadenas E., Packer L. Handbook of Antioxidants. Volume 712 Marcel Dekker; New York, NY, USA: 2002. [Google Scholar]
- 43.Cantos E., Espín J.C., Tomás-Barberán F.A. Varietal Differences among the Polyphenol Profiles of Seven Table Grape Cultivars Studied by LC−DAD−MS−MS. J. Agric. Food Chem. 2002;50:5691–5696. doi: 10.1021/jf0204102. [DOI] [PubMed] [Google Scholar]
- 44.Magro L.D., Goetze D., Ribeiro C.T., Paludo N., Rodrigues E., Hertz P., Klein M.P., Rodrigues R.C. Identification of Bioactive Compounds From Vitis labrusca L. Variety Concord Grape Juice Treated With Commercial Enzymes: Improved Yield and Quality Parameters. Food Bioprocess. Technol. 2015;9:365–377. doi: 10.1007/s11947-015-1634-5. [DOI] [Google Scholar]
- 45.Azuma A., Yakushiji H., Koshita Y., Kobayashi S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta. 2012;236:1067–1080. doi: 10.1007/s00425-012-1650-x. [DOI] [PubMed] [Google Scholar]
- 46.Gao-Takai M., Katayama-Ikegami A., Matsuda K., Shindo H., Uemae S., Oyaizu M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant Sci. 2019;283:165–176. doi: 10.1016/j.plantsci.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Romero-Cascales I., Fernández-Fernández J.I., López-Roca J.M., Gómez-Plaza E. The maceration process during winemaking extraction of anthocyanins from grape skins into wine. Eur. Food Res. Technol. 2005;221:163–167. doi: 10.1007/s00217-005-1144-1. [DOI] [Google Scholar]
- 48.Wang H., Race E.J., Shrikhande A.J. Anthocyanin Transformation in Cabernet Sauvignon Wine during Aging. J. Agric. Food Chem. 2003;51:7989–7994. doi: 10.1021/jf034501q. [DOI] [PubMed] [Google Scholar]
- 49.Gao L., Girard B., Mazza G., Reynolds A.G. Changes in Anthocyanins and Color Characteristics of Pinot Noir Wines during Different Vinification Processes. J. Agric. Food Chem. 1997;45:2003–2008. doi: 10.1021/jf960836e. [DOI] [Google Scholar]
- 50.Rentzsch M., Schwarz M., Winterhalter P., Hermosín-Gutiérrez I. Formation of Hydroxyphenyl-pyranoanthocyanins in Grenache Wines: Precursor Levels and Evolution during Aging. J. Agric. Food Chem. 2007;55:4883–4888. doi: 10.1021/jf0702491. [DOI] [PubMed] [Google Scholar]
- 51.He F., Liang N.-N., Mu L., Pan Q.-H., Wang J., Reeves M.J., Duan C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules. 2012;17:1483–1519. doi: 10.3390/molecules17021483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X., Wang K., Gu X., Sun X., Jin G., Zhang J., Ma W. Flavor Chemical Profiles of Cabernet Sauvignon Wines: Six Vintages from 2013 to 2018 from the Eastern Foothills of the Ningxia Helan Mountains in China. Foods. 2021;11:22. doi: 10.3390/foods11010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han F.-L., Zhang W.-N., Pan Q.-H., Zheng C.-R., Chen H.-Y., Duan C.-Q. Principal Component Regression Analysis of the Relation Between CIELAB Color and Monomeric Anthocyanins in Young Cabernet Sauvignon Wines. Molecules. 2008;13:2859–2870. doi: 10.3390/molecules13112859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czibulya Z., Horváth I., Kollár L., Pour Nikfardjam M., Kunsági-Máté S. The effect of temperature, pH, and ionic strength on color stability of red wine. Tetrahedron. 2015;71:3027–3031. doi: 10.1016/j.tet.2015.01.036. [DOI] [Google Scholar]
- 55.Sims C.A., Morris J.R. Effects of pH, Sulfur Dioxide, Storage Time, and Temperature on the Color and Stability of Red Muscadine Grape Wine. Am. J. Enol. Vitic. 1984;35:35–39. doi: 10.5344/ajev.1984.35.1.35. [DOI] [Google Scholar]
- 56.Greenacre M. Correspondence analysis in medical research. Stat. Methods Med. Res. 1992;1:97–117. doi: 10.1177/096228029200100106. [DOI] [PubMed] [Google Scholar]
- 57.Huang R., Xu C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2021;20:1036–1074. doi: 10.1111/1541-4337.12679. [DOI] [PubMed] [Google Scholar]
- 58.Ferrer-Gallego R., Hernández-Hierro J.M., Rivas-Gonzalo J.C., Escribano-Bailón M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Res. Int. 2014;62:1100–1107. doi: 10.1016/j.foodres.2014.05.049. [DOI] [Google Scholar]
- 59.Fontoin H., Saucier C., Teissedre P.-L., Glories Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer. 2008;19:286–291. doi: 10.1016/j.foodqual.2007.08.004. [DOI] [Google Scholar]
- 60.González-Muñoz B., Garrido-Vargas F., Pavez C., Osorio F., Chen J., Bordeu E., A O’Brien J., Brossard N. Wine astringency: More than just tannin–protein interactions. J. Sci. Food Agric. 2021;102:1771–1781. doi: 10.1002/jsfa.11672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.