Abstract
Atypical protein kinase C (PKC) isoforms are required for nerve growth factor (NGF)-initiated differentiation of PC12 cells. In the present study, we report that PKC-ι becomes tyrosine phosphorylated in the membrane coincident with activation posttreatment with nerve growth factor. Tyrosine phosphorylation and activation of PKC-ι were inhibited in a dose-dependent manner by both PP2 and K252a, src and TrkA kinase inhibitors. Purified src was observed to phosphorylate and activate PKC-ι in vitro. In PC12 cells deficient in src kinase activity, both NGF-induced tyrosine phosphorylation and activation of PKC-ι were also diminished. Furthermore, we demonstrate activation of src by NGF along with formation of a signal complex including the TrkA receptor, src, and PKC-ι. Recruitment of PKC-ι into the complex was dependent on the tyrosine phosphorylation state of PKC-ι. The association of src and PKC-ι was constitutive but was enhanced by NGF treatment, with the src homology 3 domain interacting with a PXXP sequence within the regulatory domain of PKC-ι (amino acids 98 to 114). Altogether, these findings support a role for src in regulation of PKC-ι. Tyrosine 256, 271, and 325 were identified as major sites phosphorylated by src in the catalytic domain. Y256F and Y271F mutations did not alter src-induced activation of PKC-ι, whereas the Y325F mutation significantly reduced src-induced activation of PKC-ι. The functional relevance of these mutations was tested by determining the ability of each mutant to support TRAF6 activation of NF-κB, with significant impairment by the Y325F PKC-ι mutant. Moreover, when the Y352F mutant was expressed in PC12 cells, NGF's ability to promote survival in serum-free media was reduced. In summary, we have identified a novel mechanism for NGF-induced activation of atypical PKC involving tyrosine phosphorylation by c-Src.
Nerve growth factor (NGF) induces differentiation of PC12 cells, a rat pheochromocytoma cell line, so that they express a phenotype that closely resembles that of neurons. Differentiation occurs via the TrkA neurotrophin receptor family, e.g., TrkA, TrkB, and TrkC (reviewed in references 25 and 54). The cytoplasmic tyrosine kinase domain of TrkA recruits signaling complexes, which activate mitogen-activated protein (MAP) kinase leading to differentiation of the cells. In contrast, the gp75NFGR receptor, a member of the TNFR/Fas receptor family, has no intrinsic enzymatic activity but does contain a death homology domain, hypothesized to initiate apoptosis (25).
Various members of the protein kinase C (PKC) family have been implicated in mediating NGF responses of PC12 cells (6, 19, 42, 64). PKC comprises a family of 12 isoforms that can be divided into three groups based on structural differences and cofactor dependency (reviewed in references 37 and 40). Classical (cPKC) isoforms (e.g., α, βI, βII, and γ) are sensitive to calcium and diacylglycerol or phorbol ester, whereas novel (nPKC) isoforms (e.g., δ, ɛ, η, θ, and μ) are sensitive to diacylglycerol and phorbol ester but insensitive to calcium. The atypical (aPKC) isoforms (e.g., ζ and λ/ι) possess only one zinc finger and lack the characteristic C2 domain; hence they are insensitive to activation by Ca2+, diacylglycerol, and phorbol esters. The various isoforms have a unique tissue distribution, with high expression in neural tissue (55). PC12 cells, which are frequently used to examine the trophic effect of NGF, express isoforms of each group and specifically express both aPKC isoforms, PKC-ζ and PKC-ι (62, 63). NGF treatment of these cells results in translocation and activation of all PKC isoforms expressed (63). Several studies support a specific role for aPKC isoforms in NGF-mediated differentiation of PC12 cells. (i) Phorbol ester-induced depletion of cPKC and nPKC enhances NGF-induced neurite outgrowth without altering aPKC (ζ) levels (8, 63). (ii) Depletion of cPKC and nPKC by prolonged phorbol ester treatment does not prevent NGF-induced differentiation (8). (iii) Inhibition of PKC-ζ with antisense oligonucleotides prevents NGF-induced neurite outgrowth of PC12 cells (8). (iv) Overexpression of aPKC isoforms in PC12 cells enhances NGF-mediated differentiation along with activation of the transcription factor NF-κB (66).
The mechanisms responsible for activation of aPKC isoforms are not fully understood, and it is becoming more apparent that protein-protein interactions play a major role in restricting the localization as well as the activation of the aPKCs (38). Alterations in the cellular lipid environment have also been implicated in the activation of aPKCs, involving generation of phosphatidylinositol 3,4,5-triphosphate through phosphatidylinositol 3-kinase activation and release of arachidonic acid due to lipid turnover. In vivo data indicate that PIP3, ceramide, and arachidonic acid are potent activators of aPKC isoforms (39). Direct interaction with specific binding proteins also contributes to modulation of the aPKC isoforms. In this regard λ-interacting protein (LIP) binding potentiates PKC-λ/ι activity (14), whereas binding of aPKC isoforms to Par4 inhibits activity of the enzyme (15). Other proteins that bind to aPKC isoforms include ras (12), tubulin (16), zeta-interacting protein (ZIP/p62) (44, 48), fasciculation and elongation protein zeta 1 (FEZ1) (28), ASIP (21), src (50), and cell polarity protein Par6 (23). The exact function of the binding proteins in the context of specific signaling pathways is not yet clear, although binding proteins participate in transport or shuttling aPKCs to distinct subcellular sites or serve as anchors to scaffold the enzyme, resulting in the formation of oligomeric signaling complexes.
Phosphorylation of PKC isoforms, as a consequence of the action of other serine/threonine kinases as well as autophosphorylation, has also been implicated in activation of these enzymes (40). Moreover, tyrosine phosphorylation of PKC isoforms in response to H202 has been shown to induce activation of the enzyme (27). However, ligand-induced tyrosine phosphorylation of aPKC isoforms has not yet been demonstrated. Previously we observed coassociation between PKC-ζ and v-Src (50). Since v-Src expression induces neuritogenesis (45), whereas removal of PKC-ι has the opposite effect on NGF-induced neurite outgrowth (8), we reasoned that c-src may thus regulate PKC-ι via tyrosine phosphorylation upon NGF treatment. Moreover, src and aPKC are known to regulate transcription factor NF-κB (1, 49, 60). Thus, src-induced phosphorylation of aPKC may serve as a means to link PKC-ι–src to the Ras/MAP kinase signal cassette required for differentiation as well as activation of NF-κB in PC12 cells (4, 13, 66). Here we demonstrate for the first time that PKC-ι is tyrosine phosphorylated by src and that, as a functional consequence, impairment of this phosphorylation in vivo leads to reduction in activation of NF-κB and NGF-mediated cell survival.
MATERIALS AND METHODS
Materials.
Synthetic peptides containing the various tyrosine residues spanning the primary sequence of PKC-ι were synthesized by Research Genetics (Huntsville, Ala.). Enhanced chemiluminescence (ECL) reagents, a horseradish peroxidase (HRP)-conjugated secondary antibody, Hyperfilm, an ECL kit, and [γ-32P]ATP (3,000 Ci/mmol) were purchased from Amersham Pharmacia Biotech, Inc. (Piscataway, N.J.). The anti-PKC-ι and antiphophotyrosine PY20 monoclonal antibodies were from Transduction Laboratories (Lexington, Ky.), while polyclonal anti-PKC-ι, polyclonal anti-hemagglutinin (HA), polyclonal src, and polyclonal anti-TrkA (C-14) were from Santa Cruz Biotechnology (Santa Cruz, Calif.). The anti-src antibody, agarose-coupled 4G10 (antiphosphotyrosine) antibody, c-Src, and c-Abl were purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Inhibitors K252a, genistein, and herbimycin A were bought from Biomol Research Laboratories Inc. (Plymouth Meeting, Pa.). Active PKC-ι and PKC-ζ isoforms were isolated from baculovirus-infected Sf-9 cells expressing the various PKC isoforms as previously described (73). Reagents for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protein molecular weight standards were bought from Bio-Rad (Hercules, Calif.). The luciferase assay kit was purchased from Promega (Madison, Wis.). Agarose-coupled secondary antibodies used for immunoprecipitation and all other chemicals and reagents were purchased from Sigma (St. Louis, Mo.). The peptide (PXXP) spanning the putative PKC-ι SH3 domain and the scramble peptide were synthesized by Carol M. Beach, University of Kentucky Macromolecular Structure Analysis Facility (Lexington, Ky.). c-Src-deficient cells were obtained from Simon Halegoua (Stony Brook, N.Y.), while David Shalloway (Ithaca, N.Y.) generously donated the bacterial vectors expressing the glutathione S-transferase (GST)-src fusion constructs.
Cell culture, stimulation, and subcellular fractionation.
PC12 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated horse serum, 5% heat-inactivated fetal calf serum, 50 μg of streptomycin/ml, and 50 U of penicillin/ml as previously described (8). To induce quiescence, the medium was replaced with medium containing reduced serum (1 part complete medium/5 parts serum-free medium) 24 to 48 h prior to stimulation. Poststimulation the cells were gently washed once with ice-cold phosphate-buffered saline (PBS) and removed from the petri dishes by repeated pipetting of 10 ml of ice-cold PBS and the cells were harvested by centrifugation. The cell pellets were disrupted by sonication for 5 to 10 s in the appropriate buffer, and large cellular debris was removed by centrifugation at 12,000 × g in a microcentrifuge at 4oC for 3 min. The protein content of the harvested supernatants was determined by the Bradford method using Bio-Rad protein assay reagent with bovine serum albumin as the standard.
For subcellular fractionation, the sonicated cell lysates were centrifuged at 100,000 × g for 1 h in a Sorvall ultracentrifuge at 4oC (63). The supernatant containing the cytosolic material was harvested, and the pellet was resuspended in 500 μl of the original lysis buffer and ultracentrifuged for an additional 30 min to remove any cytosolic material contaminating the pellet. The pellet containing membrane-associated material was resuspended in lysis buffer containing 1% Triton X-100 (TX-100) for 30 min at 4oC with gentle mixing. The TX-100-insoluble material was removed by ultracentrifugation. The TX-100-soluble material, containing membrane-associated protein, was used as a source of membrane-associated PKC.
Immunoprecipitation, immunoblotting, and immune complex kinase assays.
Immunoprecipitation with 4G10 and anti-PKC antibodies was carried out essentially as previously described (32). Briefly, cells were lysed in a solution containing 20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1 mM MgCl2, 0.1 mM CaCl2, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10% glycerol, 10 μg of leupeptin/ml, 5 μg of aprotinin/ml, 10 mM β-glycerophosphate, 100 μM NaF, and 0.1% TX-100. The agarose-coupled 4G10 was added directly to the cell lysate at a final concentration of 2 μg of antibody/400 μg of protein. The samples were rotated end-over-end for 4 h at 4oC, and the immune complexes were collected by centrifugation in a microcentrifuge. For immunoprecipitation of PKC-ι and TrkA, an anti-PKC-ι antibody or an anti-TrkA antibody was added to a final concentration of 2 μg/400 μg of protein and the microcentrifuge tubes were rotated overnight at 4oC prior to the addition of the appropriate agarose-coupled secondary antibody (e.g., rabbit anti-mouse immunoglobulin G [IgG]). Rotation of the tubes was continued for an additional 3 h prior to collection of the immune complexes. All immune complexes were washed six times with 500 μl of immunoprecipitation wash buffer containing 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 1 mM MgCl2, 0.1 mM CaCl2, 1 mM Na3VO4, 1 mM PMSF, 10 mM β-glycerophosphate, and 100 μM NaF. The immunoprecipitated proteins were released by boiling for 2 min in SDS-PAGE sample buffer. The agarose beads were removed by centrifugation prior to loading the samples on SDS–7.5 or 10% polyacrylamide gels.
Immunoblotting was performed as described previously (60). Briefly, proteins were transferred from the resolved SDS-PAGE gels to nitrocellulose membranes by electroblotting at 80 V overnight. The filters were stained with 0.5% Ponceau S–5% trichloroacetic acid to identify the molecular weight markers and proteins prior to blocking with blot wash (PBS containing 0.1% TX-100 and 0.1% Tween 20) containing 7% nonfat milk at 4oC for 4 h. The diluted primary antibody (PKC-ι, 1:1,000; 4G10, 1:1,000) was incubated overnight with the filters in the same solution. The filters were then washed in multiple changes of blot wash prior to the addition of the HRP-conjugated secondary antibody for 2 h at room temperature. The filters were washed with frequent buffer changes and developed using ECL reagent prior to being exposed to X-ray film.
Activity of tyrosine phosphorylated and non-tyrosine-phosphorylated aPKC isoforms from NGF-stimulated PC12 cells was determined by immune complex kinase assay as described previously (10, 11). Briefly, control and stimulated cells were harvested, and TX-100-soluble membrane material was generated as described above. The protein concentration was determined, and triplicate antiphosphotyrosine immunoprecipitations (500 μg of protein) were performed with 20 μl of 50% 4G10-agarose at 4oC for 4 h. The immune complexes containing tyrosine-phosphorylated proteins were separated from non-phosphotyrosine-containing proteins by centrifugation at 12,000 × g in a microcentrifuge for 5 min at 4oC. The immune complexes were washed six times with immunoprecipitation wash buffer to remove any contaminating non-tyrosine-phosphorylated protein. The immune complexes were resuspended in 500 μl of immunoprecipitation lysis buffer containing 30 mM para-nitrophenol phosphate (pNPP) to release the phosphotyrosine-containing protein. Simultaneously, supernatants containing non-tyrosine-phosphorylated proteins were adjusted to 30 mM with pNPP. The anti-PKC-ι antibody (2 μg) was added to each tube, and the tubes were incubated at 4oC overnight with gentle rocking. The agarose-conjugated rabbit anti-mouse IgG antibody (15 μl of 50% slurry) was added, and the 4oC incubation continued for 1.5 h. Immune complexes containing PKC-ι were harvested by centrifugation, washed 5 times with 500 μl of immunoprecipitation kinase wash buffer (35 mM Tris-HCl [pH 7.4], 150 mM NaCl, 15 mM MgCl2, 1 mM MnCl2, 0.5 mM EGTA, 0.1% TX-100, 25 μg of leupeptin/ml, 25 μg of aprotinin/ml) and twice with immunoprecipitation kinase assay buffer (35 mM Tris-HCl [pH 7.4], 5 mM MgCl2, 1 mM MnCl2, 0.5 mM EGTA, 25 μg of leupeptin/ml, 25 μg of aprotinin/ml, 1 mM Na3VO4). Immune complex kinase assays were performed for 10 min at 30oC as previously described (65). At the end of the incubation, the assay was stopped by placing the tubes into an ice bath. The immune complexes containing the bound PKC-ι were collected by microcentrifuge centrifugation at 4oC. The phosphorylated substrate in the supernatant was collected into a tube containing 20 μl of ice-cold 280 mM H3PO4, and the immune complex pellet was washed once with 50 μl of ice-cold immunoprecipitation assay buffer. The acid-precipitated supernatant was spotted onto Whatman P81 filter disks, which were washed four times for 10 min in 75 mM H3PO4 and once with ethanol before being counted in a liquid scintillation counter. The immune complexes were boiled in 50 μl of 1× SDS-sample buffer, and the amount of PKC-ι in each complex was determined by immunoblotting as described above. The results of the immunoblotting were used to normalize for the amount of PKC-ι in each individual immunoprecipitation kinase assay after densitometric scanning of the immunoblot autoradiogram.
In vitro phosphorylation of PKC-ι by c-Src.
Purified, recombinant PKC-ι (1.8 μg) was incubated with c-Src at 30oC for 10 min in a 50-μl reaction mixture containing 25 mM Tris-HCl, pH 7.2, 31.25 mM MgCl2, 6.25 mM MnCl2, 0.5 mM EGTA, 0.5 mM dithiothreitol (DTT), and 0.25 mM Na3VO4 (17). The reaction was initiated by adding 20 μM cold ATP and [γ-32P]ATP and terminated by the addition of 50 μl of 2× SDS-PAGE sample buffer and boiling for 2 min. The labeled proteins were resolved by SDS–10% PAGE, the gel was dried, and 32P-labeled proteins were visualized by autoradiography. Thereafter, the gel was rehydrated and incubated in 1 M KOH at 60oC for 1 h to destroy the alkali-sensitive serine/threonine phosphorylated residues. The gel was redried, and reexposed to X-ray film to visualize tyrosine-phosphorylated proteins. Assays performed in the presence of PKC-ι alone showed no incorporation of alkali-resistant label into PKC-ι.
The influence of c-Src phosphorylation on PKC-ι activity was determined using the same reaction as above, with protamine sulfate, myelin basic protein (MBP), ɛ-peptide, and histone IIIs as PKC substrates. All assays were performed in triplicate. After incubation at 30oC for 10 min, the reactions were stopped by the addition of 10 μl of ice-cold 280 mM H3PO4. The reaction mixture was spotted onto Whatman P81 paper disks, the disks were washed four times for 10 min in large volumes of 75 mM H3PO4, and the radioactivity incorporated in the substrates was determined by Cerenkov counting.
Measurement of intracellular c-Src activity.
The influence of NGF on c-Src activity was determined using previously described assays with acid-treated enolase as the substrate (9). The cells were stimulated with NGF, harvested, and lysed in immunoprecipitation lysis buffer. The anti-src antibody (2 μg) was added to 500 μg of cell lysate, and the mixture was incubated at 4oC for 3 h before addition of 15 μl of 50% anti-mouse IgG agarose for an additional 1.5 h. The complexes were washed five times with 500 μl of immunoprecipitation kinase wash buffer and twice with src immunoprecipitation kinase assay buffer (20 mM HEPES [pH 7.0], 10 mM MnCl2, 0.05% TX-100). Finally, the immune complex beads were suspended in 45 μl of src immunoprecipitation kinase assay buffer containing 1 μg of acid-treated enolase. The kinase reaction was initiated by the addition of 5 μl of [γ-32P]ATP, and the reaction mixture was incubated at 30oC for 10 min. The reaction was stopped by the addition of 50 μl of 2× SDS-PAGE sample buffer. Radiolabeled proteins were resolved by SDS–10% PAGE. The resolved gels were dried and exposed to Kodak XAR film.
Mapping of c-Src–PKC-ι interaction.
Full-length c-Src, src-SH2, and src-SH3 domains were expressed as GST fusion constructs in Escherichia coli. Bacteria were grown, and the GST constructs were isolated by binding to GST-agarose as previously described (50). To conduct cobinding assays, GST-agarose beads were washed extensively with PBS, pH 7.4, containing 1 mM PMSF, 10 μg of leupeptin/ml, and 10 μg of aprotinin/ml. Nonspecific binding sites were blocked by incubation in PBS containing 1% nonfat milk and 1% bovine serum albumin. PKC-ι (2 μg) was autophosphorylated by addition of 20 μM ATP at 30oC for 10 min. GST-src constructs were added at a ratio of 5 μg of construct/μg of PKC-ι and incubated for 3 h at 4oC in the presence of the GST beads. The GST–PKC-ι complexes were then washed five times in MTPBS (150 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4, pH 7.3) and suspended in 80 μl of SDS-PAGE sample buffer. The complexes were resolved by SDS–7.5% PAGE, followed by immunoblotting with the PKC-ι antibody. The blots were scanned, and the data were normalized to percent binding of full-length c-Src to PKC-ι.
To map the site of interaction between PKC-ι and c-Src, purified src enzyme and purified PKC-ι were incubated with a proline-rich peptide (PKC-ι: 98VFPSIPEQPGMPCPGE114; proline residues are in boldface) as follows: PKC-ι (2 μg) was combined with increasing concentrations of the peptide (PXXP) [25, 75, and 150 μM], or was not combined with the peptide, in a 50-μl reaction mixture containing 25 mM Tris-HCl, pH 7.5, 31.25 mM MgCl2, 6.25 mM MnCl2, 0.5 mM EGTA, 0.5 mM DTT, 0.0625 mM Na3VO4 and incubated on ice for 30 min. Subsequently, PKC-ι was activated by addition of 20 μM ATP at 30oC for 30 min followed by addition of 3 U of purified src for an additional 5 min at 30oC. src–PKC-ι complexes were incubated for an additional 2 h at 4oC. The resulting src–PKC-ι complexes were captured by addition of 2.5 μg of anti-PKC-ι polyclonal antibody coupled to anti-rabbit IgG agarose by binding at 4oC for 2 h. The beads were washed three times in a solution containing 20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1 mM MgCl2, 1 mM Na3VO4, 100 μM NaF, 1 mM PMSF, 10 μg of leupeptin/ml, and 10 μg of aprotinin/ml before 80 μl of SDS-PAGE sample buffer was added. The complexes were resolved by SDS–7.5% PAGE with both purified c-Src and PKC-ι included in the gels as standards. The proteins were immunoblotted with the src antibody, resultant autoradiographs were scanned, and binding was normalized to the moles of each protein input into the assay. The values obtained in the pull-down assay with full-length c-Src and PKC-ι represented maximum binding.
Identification of phosphorylation sites, site-directed mutagenesis, and expression.
Individual peptides spanning the primary sequence of PKC-ι were synthesized by Research Genetics and spotted onto an activated polymeric membrane essentially as described by Li et al. (31). Two control peptides were synthesized as well, a peptide which is a general substrate for src family tyrosine kinases and a src family kinase substrate derived from cdc2. Each spot yielded approximately 5 nmol of peptide on average. The membrane was dried and stored at −20oC until use. Phosphorylation of the membrane-associated peptides was carried out as previously described (53) in a buffer containing 50 mM Tris-HCl (pH 7), 25 mM MgCl2, 5 mM MnCl2, mM Na3VO4, 80 mM HEPES (pH 7), 0.15% NP-40, and 100 μM ATP in the presence or absence of 6 U of purified src kinase (Upstate Biotechnology Inc.)/ml at 30oC for 30 min. The membranes were washed twice in 50 mM phosphoric acid and twice in 100 mM KOH to remove the non-covalently bound phosphate; then the membranes were rinsed several times in PBS. The extent of tyrosine phosphorylation of the peptides was determined by using a monoclonal antiphosphotyrosine antibody at a dilution of 1:1,000, followed by an HRP secondary antibody (1:1,000). The degree of peptide phosphorylation was visualized using the ECL system. The blots were scanned with a computer-interfaced densitometer. The degree of peptide phosphorylation by src was normalized to that obtained with the control peptides.
PKC-ι in pCDNA-HA was used as template to create single mutations Y256F, Y271F, and Y325F using the QuickChange kit (Stratagene, La Jolla, Calif.) in accordance with instructions by the manufacturer. The mutations were verified by DNA sequencing. The wild type along with each mutant was transiently transfected into HEK293 cells using calcium phosphate precipitation followed by immunoprecipitation as previously described (49, 67). Lysates were prepared in PD buffer (40 mM Tris-HCl [pH 8], 500 mM NaCl, 0.1% NP-40, 6 mM EDTA, 6 mM EGTA, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM phenylphosphate, 300 μM Na3V04, 1 mM benzamidine, 2 mM PMSF, 10 μg of aprotinin/ml, 1 μg of leupeptin/ml, 1 μg of pepstatin/ml, 1 mM DTT) followed by immunoprecipitation of HA-tagged PKC-ι, wild type and mutants. To examine the phosphorylation state of PKC-ι, the immunoprecipitates were separated by SDS–10% PAGE followed by blotting with the antiphosphotyrosine antibody (PY20; Transduction Labs). A fraction of the lysate was blotted with the HA antibody to check the expression of the HA constructs. Alternatively, to assess the activity of PKC-ι, the immune complexes were captured on anti-rabbit IgG coupled to agarose (Sigma). The beads were extensively washed in lysis buffer followed by two washes in immune complex kinase buffer (66). To assess the activity of PKC-ι, MBP-hnRNPA1 (2 μg) was included in the kinase assay as the substrate. The assay was initiated by addition of [γ-32P]ATP for 30 min at 30oC. Phosphorylation of hnRNPA1 was monitored by SDS–10% PAGE followed by autoradiography.
Reporter assay.
For reporter assays, HEK293 cells were seeded into six-well plates. Cells were then transfected the following day employing the calcium phosphate precipitation method with 1 ng of κB luciferase reporter gene plasmid (pGL3; Promega) and various amounts of each expression construct. The total DNA transfected was kept constant by supplementation with control vector pCDNA3. After 24 h, extracts were prepared, and luciferase activity was determined as previously described (15).
Cell survival assay.
PC12 cells were transfected with Lipofectamine 2000. Twenty-four hours posttransfection the cells were washed five times in serum-free medium and NGF (50 ng/ml) was added. After an additional 36 h, the cells were incubated for 2 h with MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] reagent (Promega). In the presence of phenozine methosulfate, MTS is converted to a water-soluble formazan by a dehydrogenase enzyme found in metabolically active cells. The quantity of formazan product was determined by spectrophotometry (Dynatech microplate reader) at 490 nm. Values are the means and standard errors of the means (SEM; n = 3).
RESULTS
NGF induces tyrosine phosphorylation of membrane-associated PKC-ι in PC12 cells.
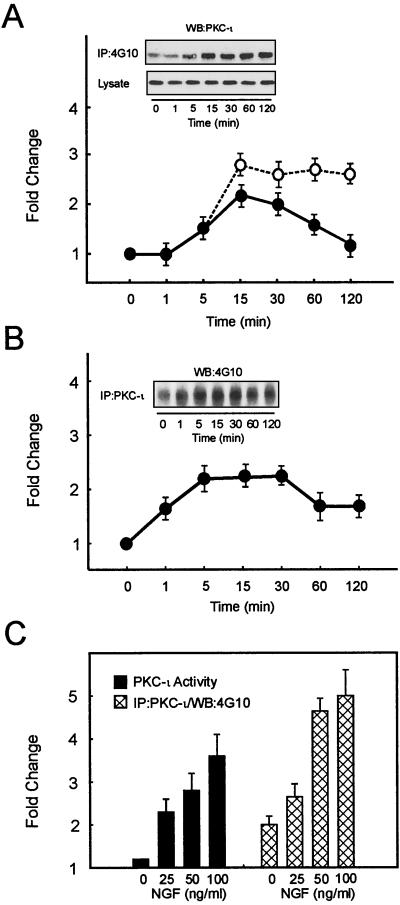
Since v-src was observed to specifically associate with PKC-ι both in vitro and in vivo (50), we elected to examine in a more physiological setting whether treatment of PC12 cells with NGF would lead to tyrosine phosphorylation of PKC-ι. To explore this possibility, PC12 cells were treated with NGF followed by immunoprecipitation of tyrosine-phosphorylated proteins employing 4G10-coupled agarose and Western blot analysis with the PKC-ι antibody. In parallel, the activity of PKC-ι was measured by immune complex kinase assay. Treatment of PC12 cells with NGF leads to rapid activation of aPKC, which reaches a maximum at 15 min and slowly declines with increased exposure time (Fig. 1A). Analysis of immunoblots revealed that, coincident with enzyme activation, NGF-mediated tyrosine phosphorylation of PKC-ι reached a maximum after 15 min as well. Since PKC-ι might be recruited to complexes that contain phosphotyrosine, an alternative approach was undertaken to examine changes in PKC-ι's phosphorylation state. Cells were treated with NGF followed by immunoprecipitation of PKC-ι followed by blotting with an antibody to phosphotyrosine (Fig. 1B). Likewise, cells were treated with various doses of NGF followed by measurement of PKC-ι activity and immunoprecipitation with the PKC-ι antibody along with blotting employing the 4G10 antibody (Fig. 1C). The results obtained by immunoprecipitation of phosphotyrosine-containing protein and by immunoprecipitation of PKC-ι and Western blotting with antiphosphotyrosine showed similar kinetics and magnitudes. Collectively, these results reveal that changes in the tyrosine phosphorylation state of PKC-ι are an NGF-regulatable event.
FIG. 1.
NGF treatment of PC12 cells increases activation and tyrosine phosphorylation of PKC-ι. An equivalent amount of protein (500 μg) was used to determine PKC-ι activity by immune complex kinase assay, and the tyrosine phosphorylation of PKC-ι was examined by immunoprecipitation of PKC-ι followed by blotting with the 4G10 antiphosphotyrosine (anti-pTyr) antibody or immunoprecipitation (IP) of proteins containing phosphotyrosine and Western blotting with the PKC-ι antibody. The autoradiographs were scanned densitometrically and plotted to show the fold change. (A) PC12 cells were stimulated with NGF (100 ng/ml) for the indicated times. PKC-ι activity (●) and tyrosine phosphorylation of PKC-ι (○) were determined. Lysate used for immunoprecipitation was also probed with anti-PKC-ι. PKC-ι Western blots of the immunoprecipitate and the lysate are shown as insets. (B) PC12 cells were stimulated with NGF (50 ng/ml) and immunoprecipitated with the PKC-ι antibody, followed by blotting with the 4G10 anti-pTyr antibody. (C) PC12 cells were stimulated with various doses of NGF as shown for 15 min. PKC-ι activity was examined. The tyrosine phosphorylation of PKC-ι was examined by immunoprecipitation of PKC-ι followed by blotting with the 4G10 anti-pTyr antibody. The results (means ± SEM) are representative of three other independent experiments.
Translocation of PKC isoforms from cytosol to the cell membrane is frequently used as a measure of PKC activation (43, 63). The data in Fig. 2A reveal that NGF treatment of PC12 cells induced rapid tyrosine phosphorylation of PKC-ι in the cell membrane without significant change in the amount of PKC-ι found in the membrane fraction. In contrast, the cytosol did not contain tyrosine-phosphorylated PKC-ι at any time point. Even prolonged exposure of the immunoblot failed to reveal the presence of tyrosine-phosphorylated PKC-ι in the cytosol. Furthermore, NGF-induced translocation and tyrosine phosphorylation of PKC-ι occurred in the same period as NGF-induced activation of enzyme activity (Fig. 1). In addition, there was consistently a preexistent pool of tyrosine-phosphorylated, membrane-associated PKC-ι (time zero; Fig. 1 and 2). These findings indicate that the tyrosine phosphorylation of PKC-ι selectively occurs within the membrane microenvironment. To specifically assess the influence of tyrosine phosphorylation on the activity of membrane-associated PKC-ι, PC12 cells were stimulated for 15 min with NGF and the activity of non-tyrosine-phosphorylated PKC-ι compared to that of tyrosine-phosphorylated PKC-ι in the membrane fraction was determined (Fig. 2B). There was a significant increase in the activity of the phosphotyrosine-containing enzyme relative to that of the non-phosphotyrosine-containing PKC-ι, demonstrating that maximal tyrosine phosphorylation of PKC-ι at 15 min during NGF treatment is associated with activation of the enzyme that occurs within the membrane compartment.
FIG. 2.
NGF induces tyrosine phosphorylation and activity of membrane-associated PKC-ι. (A) Cells were stimulated with NGF (100 ng/ml) for the indicated times and subfractionated into cytosol and TX-100-soluble material (membrane). To examine NGF-mediated translocation of PKC-ι, equal amounts of protein from cell lysates were resolved by SDS-PAGE, blotted onto nitrocellulose membranes, and immunoblotted for PKC-ι. Alternatively, equal protein concentrations were immunoprecipitated (IP) with 4G10 and analyzed for PKC-ι by Western blotting (WB). WCL and S, standards of PC12 whole-cell lysates (60 μg) and purified PKC-ι, respectively, included as controls. These results are representative of two other independent experiments. (B) Activity of tyrosine-phosphorylated and non-tyrosine-phosphorylated membrane-associated PKC-ι. PC12 cells were stimulated with 100 ng of NGF/ml for 0 and 15 min. TX-100-soluble membrane material was isolated and tyrosine phosphorylated (solid bars), and non-tyrosine-phosphorylated PKC-ι (open bars) was prepared by sequential immunoprecipitation with 4G10 and anti-PKC-ι antibodies. PKC activity was determined in triplicate, and the amount of PKC in each immune complex kinase assay was normalized by Western blotting with the anti-PKC-ι antibody. These findings are means ± SEM (n = 3).
We reasoned that the high-affinity NGF receptor, TrkA, itself a tyrosine kinase, may participate either directly or indirectly in the tyrosine phosphorylation of PKC-ι. To explore this possibility, we used various kinase inhibitors to explore regulation of PKC-ι by phosphorylation: K252a, a highly specific inhibitor of TrkA tyrosine kinase activity (41, 56), PP2, a specific src-kinase inhibitor, and genistein, a tyrosine kinase inhibitor. Pretreatment of NGF-stimulated PC12 cells with either genistein, PP2, or K252a reduced the tyrosine phosphorylation of PKC-ι (Fig. 3A). Other src-specific tyrosine kinase inhibitors herbimycin A and radicicol (3, 57, 68) likewise inhibited NGF-induced PKC-ι tyrosine phosphorylation. These findings suggest that src, a non-TrkA receptor-associated tyrosine kinase, may be responsible for the phosphorylation of PKC-ι. Additionally, these results reveal that TrkA participates in the activation cascade, since the tyrosine phosphorylation of PKC-ι was inhibited in the presence of K252a. The effects of genistein on NGF-induced activation of PKC-ι (Fig. 3B) were likewise examined. Impairment of tyrosine phosphorylation led to a dose-dependent reduction in the activity of PKC-ι, thus further suggesting that tyrosine phosphorylation contributes to activity changes.
FIG. 3.
Influence of kinase inhibitors on PKC-ι's tyrosine phosphorylation state and activity. (A) Cells were pretreated with genistein (0 to 20 μM), PP2 (0 to 40 μM), or K252a (0 to 300 nM) for 1 h prior to stimulation with 100 ng of NGF/ml for 15 min. Tyrosine phosphorylation of PKC-ι was determined by immunoprecipitation (IP) with 4G10 followed by PKC-ι Western blotting (WB). (B) Activity of PKC-ι was assayed in triplicate by immune complex kinase assay. PKC activity was normalized after adjusting NGF-induced activity to 100%. Values are means ± SEM (n = 3).
PKC-ι is phosphorylated and activated by c-Src both in vitro and in vivo.
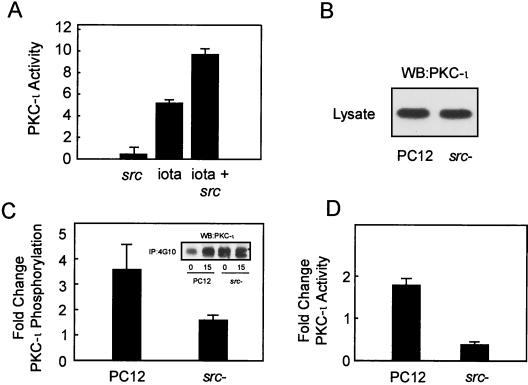
To establish a role for src in the phosphorylation of PKC-ι, we first determined whether c-Src would phosphorylate purified PKC-ι (Fig. 4A). PKC-ι at increasing concentrations was incubated in an in vitro kinase assay followed by SDS-PAGE and KOH hydrolysis of Ser/Thr-containing phosphoamino acids. Phosphorylation of PKC-ι was observed to be dependent on the concentrations of PKC-ι (Fig. 4A) and alkali resistance, indicating an increase in the phosphotyrosine content of PKC-ι. Furthermore, upon maximum phosphorylation of PKC-ι, a parallel reduction in the autophosphorylation of c-Src was observed. Alternatively, increases in the concentration of c-Src with a fixed amount of PKC-ι promoted a dose-dependent increase in PKC-ι phosphorylation (data not shown). Similar findings were observed with PKC-ζ, which is a member of the aPKC family and which is highly homologous to PKC-ι. In contrast, tyrosine kinase c-Abl, an enzyme that phosphorylates PKC-δ in vitro and that lies upstream of aPKC activation in K562 cells (22), did not phosphorylate PKC-ι directly (data not shown). To further confirm tyrosine-induced phosphorylation of PKC-ι by src, parallel kinase reactions were conducted by incubating PKC-ι in the presence of increasing c-Src with or without ATP, conditions which promote activation of c-Src, followed by SDS-PAGE and immunoblotting with the antiphosphotyrosine antibody (Fig. 4B). There was substantial tyrosine phosphorylation of PKC-ι only when ATP was present. Last, a separate cotransfection approach was likewise undertaken to demonstrate src phosphorylation of PKC-ι in vivo. HA-tagged PKC-ι and c-Src or a kinase-dead c-Src mutant (71) were transfected into HEK293 cells followed by immunoprecipitation of HA-tagged PKC-ι followed by Western blotting with the antiphosphotyrosine antibody (Fig. 4C). Although all transfectants expressed equal HA-tagged proteins, only those cotransfected with active c-Src were tyrosine phosphorylated. Collectively, these findings demonstrate that PKC-ι is phosphorylated by c-Src.
FIG. 4.
c-Src phosphorylates PKC-ι in vitro. (A) Purified c-Src was used to phosphorylate purified PKC-ι in increasing concentrations. Proteins were resolved by SDS–10% PAGE and stained, and the gel was treated for 1 h at 60°C in 1 M KOH to destroy alkali phosphate attached to Ser and Thr residues. The gel was dried and exposed to X-ray film for 1 to 2 days. Note that increasing PKC-ι phosphorylation is paralleled by decreasing src autophosphorylation. Similar results were generated in three additional experiments. (B) The experiment in panel A was replicated in the presence or absence of cold ATP in the in vitro assay. The proteins were resolved by SDS-PAGE followed by immunoblotting with the antiphosphotyrosine antibody. Increased phosphorylation by src (A) was paralleled by an increase in the tyrosine phosphorylation state of PKC-ι. (C) src, constitutively active or kinase dead, along with HA-tagged PKC-ι, was transiently coexpressed in HEK293 cells. The cell lysates were immunoprecipitated (IP) with anti-HA followed by immunoblotting with the antiphosphotyrosine antibody. Additionally, an equal amount of protein (60 μg) from whole-cell lysate (WCL) was immunoblotted with anti-HA to check for expression of PKC-ι. These results were obtained in three other identical experiments. WB, Western blotting.
Since tyrosine phosphorylation of PKC-ι correlated with a change in the activity of the enzyme (Fig. 1), we sought to examine whether c-Src phosphorylation would likewise induce activation of PKC-ι. Thus, PKC-ι activity was measured by employing aPKC-specific substrate ɛ-peptide (26). Phosphorylation of PKC-ι by src in vitro enhanced the activity of the enzyme (Fig. 5A). Because ligand-mediated tyrosine phosphorylation of PKC reportedly alters the substrate specificity of the enzymes (18), we included either protamine sulfate, MBP, or histone IIIs as an alternative PKC substrate. No differences in substrate phosphorylation by c-Src-activated PKC-ι were identified (data not shown). To explore the role of src as a modulator of aPKC activity in an in vivo setting, PC12 cells deficient in src activity due to the dominant-inhibitory src allele were utilized (46). The levels of PKC-ι in both the parental and src-deficient cells were examined (Fig. 5B). No differences in the levels of PKC-ι were detected. NGF treatment of c-Src-deficient PC12 cells resulted in reduced tyrosine-phosphorylated PKC-ι compared to that for the parental PC12 cell line (Fig. 5C), with a parallel reduction in NGF-induced activation of PKC-ι (Fig. 5D). These findings can be reconciled by the observation that the src-deficient cells possess higher levels of basal phosphorylation and activity, thus suggesting that some compensatory mechanism takes places due to the reduction in src activity. Altogether, these findings indicate that c-Src is essential for early induction and NGF-mediated tyrosine phosphorylation and activation of PKC-ι.
FIG. 5.
c-Src modulates PKC-ι activity. (A) In vitro activation of purified PKC-ι by c-Src. The PKC-specific substrate ɛ-peptide was phosphorylated in vitro by src, PKC-ι, or src-activated PKC-ι in triplicate assays, and incorporation of the 32P label into ɛ-peptide was determined by Cerenkov counting. The relative activity of PKC-ι (iota) in the presence of each of the two kinases separately or in combination is plotted. Values are means ± SEM (n = 3). (B) The levels of PKC-ι in parental and src-deficient PC12 cells were determined by Western blotting (WB) with PKC-ι antibody. (C) Parental and src-deficient PC12 cells were stimulated with 100 ng of NGF/ml for 15 min and the change in tyrosine phosphorylation of PKC-ι was determined by immunoprecipitation of tyrosine-phosphorylated proteins with 4G10 followed by PKC-ι Western blotting. The fold change in the tyrosine phosphorylation state was normalized to control for the absence of NGF. Values are means ± SEM (n = 3). (Inset) Western blots of immunoprecipitates obtained from each cell line. (C) Parental and src-deficient PC12 cells were stimulated with NGF, and PKC-ι activity was determined by immune complex kinase assay. The fold change in activity was normalized to control for the absence of NGF. Values are means ± SEM (n = 3). Values for parental and src-deficient cells are significantly different (P < 0.05).
NGF activates c-Src, leading to formation of a signaling complex.
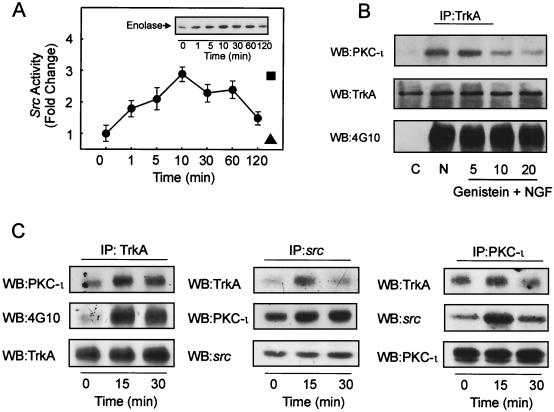
c-Src was capable of tyrosine phosphorylating PKC-ι along with activation of enzyme activity; therefore we postulated that NGF may activate c-Src. To test this hypothesis, PC12 cells were stimulated with NGF followed by immunoprecipitation of c-src and measurement of its activity by an immune complex kinase assay with acid-treated enolase as the substrate (Fig. 6A). These experiments indicated that the time of maximum NGF stimulation of c-Src activity in PC12 cells (10 min) precedes the time of tyrosine phosphorylation and activation of PKC-ι (Fig. 1). Other proteins which may associate with src might phosphorylate enolase and hence contribute to activity changes in the immune complex kinase assay. To exclude this possibility, the cells were pretreated with PP2, a src kinase inhibitor, which completely abolished NGF-stimulated changes in src activity. On the other hand, PP3, an inactive form of PP2 and negative control, had little effect on NGF-induced src activity (Fig. 6A). Collectively, these findings indicate that the majority of the NGF-stimulated activity present in the immune complex kinase assay is attributed to src.
FIG. 6.
NGF treatment of PC12 cells activates c-Src and induces formation of a signal complex. (A) Time course of c-Src activation by NGF. Cells were stimulated with 100 ng of NGF/ml for the indicated times, and c-Src activity was determined by immune complex kinase assay with acid-treated enolase as the substrate. Values are means ± SEM (n = 3). (Inset) Autoradiogram of the labeled enolase. The enolase band on the autoradiograph was scanned and plotted as fold change (●). As a control, cells were treated with PP2 (40 μM; ▴) or PP3 (40 μM; ▪) prior to treatment with NGF. (B) PC12 cells were pretreated with genistein (5, 10, or 20 μM) followed by addition of NGF (100 ng/ml) for 15 min. Thereafter, TrkA was immunoprecipitated (IP), followed by Western blot (WB) analysis for PKC-ι, TrkA, and phosphotyrosine-containing TrkA as indicated.(C) TrkA, src, and PKC-ι were immunoprecipitated from NGF-stimulated cells, and immune complexes were probed for the presence of TrkA, PKC-ι, c-Src, or 4G10 as indicated. These data are representative of three other experiments.
Since activation of c-Src took place upon NGF stimulation, we reasoned that NGF may promote formation of a signal complex between PKC-ι and the NGF receptor, TrkA. Thus, we hypothesized that tyrosine phosphorylation of PKC-ι by src may assist in recruitment of PKC-ι to the TrkA receptor complex. To test this idea, PC12 cells were treated with various concentrations of genistein, a tyrosine kinase inhibitor previously shown to reduce both the activity and tyrosine phosphorylation of PKC-ι (Fig. 3), followed by NGF treatment and immunoprecipitation of TrkA receptor complexes (Fig. 6B). Inhibition of PKC-ι's tyrosine phosphorylation state (Fig. 3) resulted in a dose-dependent reduction of PKC-ι recruited to the TrkA receptor complex. As a control, the amount of TrkA remained constant in the complex and its tyrosine phosphorylation state remained unchanged, demonstrating the specificity of this inhibitor for tyrosine kinases such as src. To explore the existence of a signal complex between TrkA-src and PKC-ι coimmunoprecipitation was undertaken. Our results (Fig. 6C) document the presence of PKC-ι in TrkA immunoprecipitates. As a control, the tyrosine phosphorylation of TrkA and the levels of TrkA were examined. Coincident with TrkA phosphorylation, PKC-ι is recruited to the receptor complex. In the src immunoprecipitates, a small amount of TrkA was detected; in comparison the amount of PKC-ι was much greater, whereas the levels of src were constant. Upon immunoprecipitation of PKC-ι a relatively minor amount of TrkA could be detected; however, the complex contained a significant amount of src, whereas the levels of PKC-ι were constant (Fig. 6C). Collectively, these findings document the formation of a signal complex containing TrkA-src and PKC-ι. These findings suggest that both src and PKC-ι only associate with TrkA in their activated, tyrosine-phosphorylated states, since only a small fraction of the total TrkA pool coassociated with either protein upon NGF stimulation.
The SH3 domain of c-Src interacts with a proline-rich motif in PKC-ι.
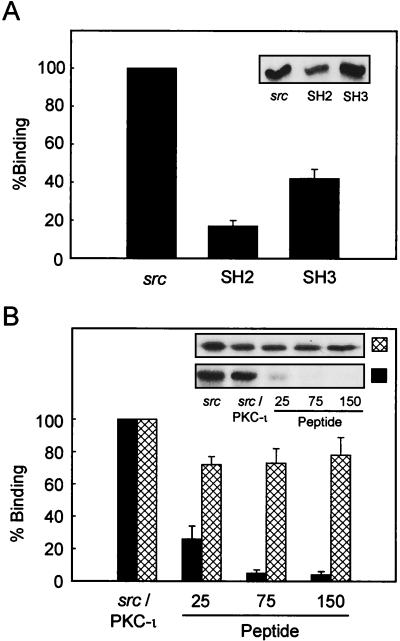
c-Src is a modular protein divided into four domains. Amino acids 1 to 90 contain the myristoylation site and unique domain, amino acids 91 to 150 contain the SH3 interaction domain, amino acids 151 to 249 contain the SH2 interaction domain, and amino acids 250 to 536 contain the SH1 catalytic domain. Since PKC-ι could bind and interact with src both in vitro (50) and in vivo, as determined herein, we sought to specifically map the means by which PKC-ι could bind src. To map the association between PKC-ι and src, purified PKC-ι was incubated with a c-Src–GST fusion protein prepared from either the entire protein or the SH2 or SH3 domain of c-Src (71). Analysis of the protein captured in the GST pull-down assay by immunoblotting with the anti-PKC-ι antibody confirmed direct interaction between these two proteins (Fig. 7A). Furthermore, PKC-ι was observed to preferentially bind to the SH3 domain rather than the SH2 domain (Fig. 7A). The c-Src SH3 domain binds to proline-rich sequences with the PXXP consensus (69). Since PKC-ι bound to src through the regulatory domain (50), the sequence was analyzed for a proline-rich motif which could serve as an SH3 binding domain. Amino acid residues 98 to 114 (VFPSIPEQPGMPCPGE; proline residues are in boldface) of PKC-ι possess characteristics which would make them a potential SH3 binding site. We therefore determined whether this region was required for mediating the interaction between c-Src and PKC-ι by synthesizing a peptide of this sequence. Inclusion of this peptide (amino acids 98 to 114) during c-Src–PKC-ι coassociation experiments resulted in a dose-dependent inhibition of the interaction between PKC-ι and c-Src (Fig. 7B). As a control a scrambled peptide of the same length where alanine had been substituted for proline was also included in the assay. The control peptide had little or no effect on the interaction of src with PKC-ι. These data indicate that the SH3 domain of c-Src binds the regulatory domain of PKC-ι through a proline-rich motif, amino acids 98 to 114.
FIG. 7.
Mapping of the interaction between c-Src and PKC-ι. (A) PKC-ι was autophosphorylated and interacted in a cobinding assay with either full-length src, the src SH2 domain, or src-SH3 domain as a GST fusion construct. The data are normalized to the ratio of the moles of the proteins included in the assay. Results are presented as percent binding obtained between full-length src and PKC-ι. Values are means ± SEM (n = 3). (B) PKC-ι was phosphorylated by c-Src followed by addition, at increasing concentrations (25, 75, and 150 μM), of the PKC-ι PXXP (solid bars) or scrambled (cross-hatched bars) peptide. The resulting c-Src–PKC-ι complexes were captured using the anti-PKC-ι antibody coupled to anti-rabbit IgG-agarose. Immune complexes were resolved by SDS-PAGE and immunoblotted with the anti-c-Src antibody. As a control, purified c-Src was included at concentrations used in the cobinding assay. Results are percentages of binding of c-Src to PKC-ι, with the amount of c-src input into the assay representing 100% binding. (Insets) Autoradiographs of the respective immunoblots. These experiments were repeated three times.
Tyr 256, 271, and 325 of PKC-ι are the major sites phosphorylated by src.
To map the site(s) in PKC-ι phosphorylated by src, we first determined if the regulatory domain, catalytic domain, or both could be phosphorylated by src. Purified GST fusion constructs of PKC-ι were included in an in vitro kinase assay with c-Src. Under these conditions, PKC-ι was prominently phosphorylated within the catalytic domain, whereas only a minor degree of tyrosine phosphorylation was observed for the regulatory domain (data not shown). In addition, purified PKC-ι was cleaved into regulatory and catalytic domains by incubation with trypsin, followed by src-induced tyrosine phosphorylation. The tyrosine-phosphorylated fragments were blotted with the antiphosphotyrosine antibody, which revealed that the catalytic domain was the most heavily phosphorylated (data not shown). To map the specific site within PKC-ι phosphorylated by c-src, a series of PKC-ι-derived peptides spanning the entire length of PKC-ι were synthesized and immobilized onto polyvinylidene difluoride (Table 1). These sites are highly conserved among both members of the aPKC family, ι/λ and ζ. In addition two control src kinase substrate peptides were also synthesized, a general substrate for src family tyrosine kinases and a peptide substrate derived from cdc2 (53). Each peptide contained the tyrosine in the center and were used as substrates in an in vitro src kinase assay conducted directly on the filter. The tyrosine phosphorylation of each peptide was compared to that of the src peptide control via densitometric scan of the blots. Peptides containing tyrosine 116, 127, 256, 271, and 325 were observed to be good substrates for src (Table 1).
TABLE 1.
Sequences of the synthetic peptides containing tyrosine residues spanning the entire region of PKC-1 (1 to 14)
| Peptide | Sequencea | Site(s) | Degree of phosphorylation |
|---|---|---|---|
| 1 | SHQVRVKAYYRGDIMITH | Y22, Y23 | 1+ |
| 2 | ELEEAFRLYELNKDSEL | Y84 | 0 |
| 3 | SIYRRGARRWRKLYCAN | Y116, Y127 | 3+ |
| 4 | IWGLGRQGYKCINCKLL | Y160 | 0 |
| 5 | DHAQTVIPYNEIDGIAY | Y210 | 0 |
| 6 | LRVIGRGSYAKVLLVR | Y256 | 3+ |
| 7 | RLKKTDTIYAMKVVKKE | Y271 | 3+ |
| 8 | SRLFFVIEYVNGGDLMF | Y325 | 3+ |
| 9 | LPEEHARFYSAEISALAL | Y349 | 1+ |
| 10 | LNYLHERGIYRDLKLD | Y359, Y367 | 1+ |
| 11 | EGHIKLTDYGMCKEGLR | Y388 | 0 |
| 12 | GTPNYIAPEILRGEDYGFSV | Y410, Y421 | 0 |
| 13 | NPDQUNTEDYLFQVILEK | Y460 | 0 |
| 14 | QSEFEGFEYINPLLMSA | Y575 | 0 |
| srcb | RRLIEDAEYAARG | 3+ | |
| cdc2b | KVRKIGEGTYGVVKK | 3+ |
Tyrosine residues are in boldface.
Control.
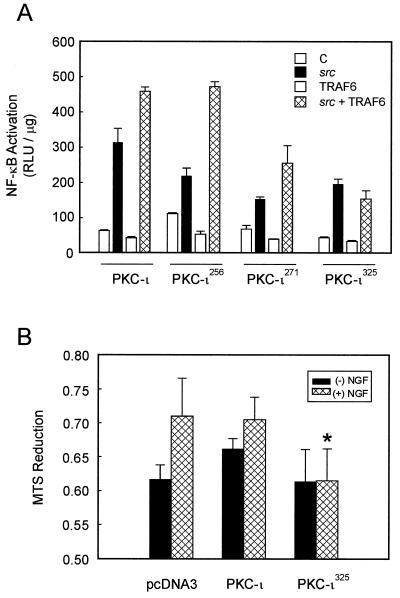
To assess the functional relevance of sites within the catalytic domain (amino acids 256, 271, and 325), each of the three tyrosines were replaced with phenylalanine by site-directed mutagenesis (Fig. 8). Each of the HA-tagged mutants and normal PKC-ι were cotransfected along with constitutively active src into HEK293 cells followed by HA immunoprecipitation and immunoblotting with the PY20 antiphosphotyrosine antibody. The relative levels of the HA-tagged constructs expressed in HEK293 cells were similar (Fig. 8A). In the absence of src little or no tyrosine phosphorylation of PKC-ι was observed. Inclusion of src resulted in potent stimulation of tyrosine phosphorylation. Neither of the single-tyrosine substitutions significantly altered the tyrosine phosphorylation state of PKC-ι, thus indicating that src did not stimulate processive phosphorylation. When HA-tagged immunoprecipitates were included in an immune complex kinase assay with hnRNPA1 as the substrate, differences between the various mutations were observed (Fig. 8B). Inclusion of PP2 reduced the activity of PKC-ι, thereby indicating that changes in the activity are attributed to PKC-ι phosphorylation of hnRNPA1. Moreover, src alone failed to phosphorylate hnRNPA1 (data not shown). The change Y325F was observed to significantly diminish src-induced activation of PKC-ι enzyme activity, although not to basal levels, indicating cooperative interaction between the phosphotyrosine residues.
FIG. 8.
The effect of mutating tyrosine 256, 271, and 325 in PKC-ι on its tyrosine phosphorylation and activation. HEK293 cells were untransfected (C) or transiently transfected by calcium phosphate with HA-tagged vector pcDNA3 (V) or HA-tagged wild-type PKC-ι or Y256F, Y271F or Y325F mutants along with constitutively active src. (A) Transfected cell lysates were prepared (1 mg) and immunoprecipitated (IP) with the polyclonal anti-HA antibody followed by Western blot (WB) analysis with the PY20 antiphosphotyrosine antibody. Whole-cell lysates (lysate) were blotted (60 μg) to check for the expression of HA-tagged constructs. (B) Transfected cell lysates were prepared (500 μg), immunoprecipitated with the polyclonal HA antibody, and subjected to an immune complex kinase assay with recombinant hnRNPA1 as the substrate (2 μg). The results are the means ± SEM (n = 3).
Atypical PKCs have previously been shown to lie upstream of NF-κB (29), where they form a complex with IκB kinase, leading to its phosphorylation and subsequent activation. We therefore tested the ability of each of the PKC-ι mutants to activate NF-κB. HEK293 cells were transiently cotransfected with each mutant PKC-ι, src, the TRAF6 adapter protein, and a κB reporter plasmid. NF-κB activity was measured by luciferase assay (Fig. 9A). src was observed to induce NF-κB activity, which is consistent with previous results obtained by gel shift analysis (66). TRAF6 synergized with src, leading to potent induction of NF-κB. Each mutant PKC-ι was likewise tested, the Y256F mutant had little effect on activation of NF-κB, whereas the Y271F mutant had a modest effect and the Y325F mutant completely eliminated TRAF6's ability to induce NF-κB. This mutant was likewise impaired in its enzyme activity (Fig. 8B), which is consistent with a requirement for PKC-ι activity in the activation of NF-κB (29). Since the NF-κB pathway plays a role in neuronal survival signaling (65, 67), we further tested the functional relevance of the Y352F mutant in PC12 cells (Fig. 9B). Expression of the Y325F mutant reduced NGF's ability to stimulate cell survival, which is in keeping with a requirement for aPKC and NF-κB in mediating NGF survival (65–67).
FIG. 9.
Role of PKC-ι mutants in NF-κB activation and cell survival. (A) Subconfluent cultures of HEK293 cells were transfected with 1 ng of the κB luciferase reporter gene plasmid along with PKC-ι (1 μg), src (100 ng), or TRAF6 (100 ng) alone or in combination and enough empty vector to give 2.5 μg of total DNA. After 24 h, cell extracts were prepared and the levels of luciferase activity were determined. Results are the means ± SEM of three independent experiments with duplicates. (B) PC12 cells were transfected with vector, PKC-ι or the PKC-ι Y325F mutant. The cells were switched to a serum-free environment, and cell survival was measured by MTS reduction. The PKC-ι Y325F mutant reduced NGF-dependent survival compared to either PKC-ι or vector alone (∗, P < 0.05).
DISCUSSION
In the present study we provide evidence for concomitant NGF-mediated tyrosine phosphorylation and activation of PKC-ι via activation of src in PC12 cells. A growing number of agents and cytokines have been shown to induce tyrosine phosphorylation of PKC isoforms. Peroxide treatment of COS-7 cells induces tyrosine phosphorylation of PKC isoforms along with an increase in enzyme activity, particularly of aPKC isoforms (27). The most extensive study on the role of tyrosine phosphorylation of PKC involved PKC-δ, a novel PKC isoform. These studies demonstrated that PKC-δ becomes phosphorylated on tyrosine residues in response to diverse stimuli, leading to either activation, inhibition, or a change in substrate specificity and cofactor dependency of PKC-δ depending on the cell type and stimulus employed (11, 18, 24, 32, 70). Collectively these findings thus set a precedent for the importance of tyrosine phosphorylation in regulating the activity and function of PKC isoforms (7, 34).
We have recently demonstrated in a preliminary study an interaction between src and aPKC (50). Altogether our findings reveal a direct interaction between src and aPKC. (i) Employing the regulatory domain of aPKC coupled to GST-Sepharose we observed interaction with src from lysates prepared from PC12 cells. (ii) aPKC interacted with src in blot overlay assays. (iii) aPKC interacted with active but not inactive v-src. Herein we extend these initial observations demonstrating that the regulatory domain of PKC-ι binds to the SH3 domain of src through a proline-rich motif located within the regulatory domain (amino acids 98 to 114), thereby allowing src to phosphorylate specific tyrosine residues within both the regulatory and catalytic domains of PKC-ι. There is a similar mechanism which defines the interaction between PKC-δ and c-Abl tyrosine kinase (70). In an analogous fashion, PKC-δ binds to the SH3 domain of c-Abl through a putative PXXP motif located in the C terminus of PKC-δ, thereby allowing c-Abl to phosphorylate PKC-δ.
Various tyrosine residues in PKC-δ have been implicated in mediating distinct biological effects. Tyrosine phosphorylation of two tyrosine residues (tyrosine 512 and tyrosine 523) in the C-terminal domain of PKC-δ is required for H2O2-induced activation of nPKC, though these studies show that other tyrosine residues are also phosphorylated (27). These alternative residues include tyrosine 52 and tyrosine 187 of PKC-δ (33, 51), and src has been shown to play a role in phosphorylation of tyrosine 311 during platelet-derived growth factor (PDGF)-induced degradation of PKC-δ (5). Mutation of tyrosine 52 to phenylalanine prevents receptor-induced tyrosine phosphorylation and association of PKC-δ with src-related kinase Lyn in mast cells (51). Thus, diverse effects of tyrosine phosphorylation on PKC-δ may be mediated through the phosphorylation of distinct tyrosine residues in the enzyme by distinct tyrosine kinases such c-Abl, c-Src, Lyn, PDGF-βR, and insulin receptor (11, 18, 70). We mapped the sites of src phosphorylation within PKC-ι to tyrosines 116,127, 256, 271, and 325 by employing peptide analysis (Fig. 10; Table 1). In the present study we opted to focus our functional analysis on the effects which mutation within the catalytic domain have. The temporal kinetics of phosphorylation at these sites as well as those in the regulatory domain, in addition to the hierarchy of phosphorylation, warrant further study. Interestingly, the sites induced by peroxide-mediated tyrosine phosphorylation of PKC-δ, Tyr 451, 469, 512, and 523, are conserved among all members of the PKC family (27). However, none of the sites within PKC-ι were phosphorylated by src. Collectively these findings suggest that diverse stimuli lead to activation of specific, potentially nonoverlapping pathways, which mediate tyrosine phosphorylation of specific residues. The importance of individual sites remains to be determined via mutagenesis of all possible sites.
FIG. 10.
Localization of PKC-ι tyrosine phosphorylation sites. Shown is a schematic outline of the structural domains of PKC-ι including the regulatory domain, hinge region, catalytic kinase core, cysteine-rich domain (CR), and pseudosubstrate motif (PSD). Open circles, phosphorylation sites. Proteins that associate with aPKCs are listed under the region in aPKC with which they have been shown to interact: LIP (λ-interacting protein), activator of aPKCs (14); Par4, inhibitor of aPKC enzyme activity (15); tubulin (16); ZIP/p62 (44, 48); fasciculation and elongation protein zeta 1 (FEZ1) (28); ASIP (21); src (50); and cell polarity protein Par6 (23).
Phosphorylation of PKC-ι at particular sites may be an intricate mechanism for the selective control of its biological function. It can be speculated that these phosphorylation sites may induce some conformational change that facilitates protein-protein interactions. Phosphorylation of the C terminus may provide an electrostatic anchor that structures the kinase and/or alters its surface to promote or disrupt protein-protein interactions (59). The atypical PKCs have been reported to specifically bind several proteins (Fig. 10) through their regulatory domains; these proteins include LIP (14), Par4 (15), tubulin (16), zeta-interacting protein (ZIP/p62) (44, 48), fasciculation and elongation protein zeta 1 (FEZ1) (28), ASIP (21), src (50), and cell polarity protein Par6 (23). It is possible that multisite phosphorylation of atypical PKCs induced by src or related tyrosine kinase family members may play a role in dictating interaction with the regulatory domain directly or may induce a favorable conformation that exposes the regulatory domain to interaction with other proteins. Since ASIP binds to the aPKCs by interacting with the catalytic domain (21), it is possible that ASIP binding may be modulated by the tyrosine phosphorylation state at one or multiple sites within the catalytic domain. Thus, tyrosine phosphorylation of specific aPKC tyrosine residues at various times after cellular activation may influence the nature of the interaction between aPKC and aPKC binding proteins. In support of this mechanism, we have recently demonstrated that tyrosine phosphorylation of aPKC affects its interaction with ZIP/p62 (47). In addition, the tyrosine phosphorylation state of PKC-ι may also affect its intracellular localization. We have previously demonstrated movement of aPKC to the nucleus (64), where it phosphorylates its substrate nucleolin (73), thereby potentially altering nuclear events involved in PC12 cell differentiation. PKC-ι possesses a bipartite motif (64), which may be exposed via conformational changes brought about by tyrosine phosphorylation. Interestingly, phosphorylation has also been shown to regulate interaction of proteins with the nuclear import machinery (20). Studies are under way to address the role of tyrosine phosphorylation in regulation of aPKC at the level of the nucleus. Interestingly, Y256 and Y271 are conserved among members of the aPKCs, whereas Y325 is also found in α-PKC, thus suggesting specific regulatory roles for tyrosine residues 256 and 271 in specific aPKC functions, whereas Y325 may possess a more global role.
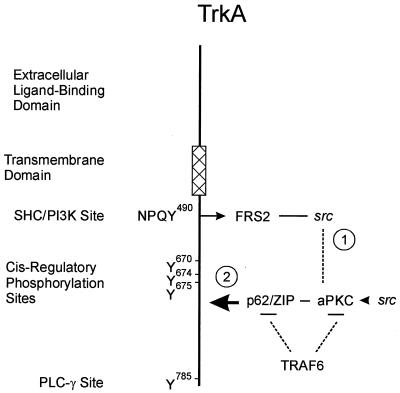
Both active c-Src and PKC-ι physically associate in a signal complex with the NGF receptor, TrkA. While the association of c-Src and PKC-ι is dependent on the c-Src SH3 domain and the PXXP motif in PKC-ι, the processes responsible for recruitment of these two enzymes to TrkA likely involve adapter proteins capable of interacting with either the receptor and PKC-ι or src. In this regard, FRS-2/SNT has been shown to bind src (36). FRS-2/SNT is an adapter molecule associated with a neurotrophic factor target and has been shown to bind TrkA at the Shc site, tyrosine 490, within the receptor. Therefore, FRS-2/SNT may serve to recruit src to the TrkA receptor, thereby leading to recruitment of PKC-ι. However, ZIP/p62, the selective interactor of the aPKCs (44, 48), also directly binds to TrkA in an NGF-dependent fashion (67). The binding site for ZIP/p62 might overlap with FRS-2/SNT within the TrkA receptor, or p62 may possess a separate binding site within TrkA. Therefore, TrkA may be capable of recruiting both FRS-2/src and ZIP/p62 independently (Fig. 11). Thus, src could be recruited and interact directly with PKC-ι in the absence of any interaction with FRS-2/SNT. src has been reported to phosphorylate dynamin (2), which may account for src's ability to regulate TrkA internalization and differentiation responses (72). The complex interaction between these various proteins warrants further study. The mapping of TrkA binding to p62 and the mapping of p62 binding with TrkA are in progress.
FIG. 11.
Model for formation of TrkA–src-aPKC complexes. TrkA binds the signaling adapter FRS2/SNT at tyrosine 490, which would enable recruitment of c-src to the receptor (1) (36). On the other hand, TrkA also directly binds ZIP/p62, which recruits aPKC to the receptor (2) (67). A ternary complex between TrkA-FRS2/SNF and p62 can be formed via the binding of aPKC and src. In addition, ZIP/p62 and aPKC are capable of binding to TRAF6, the critical regulator of the NF-κB pathway, thereby directly linking components of the receptor complex to activation of the κB pathway. Therefore, the NGF-induced activation of NF-κB may be modulated by any one of the following upstream critical regulatory elements: FRS2, src, p62/ZIP, and aPKC.
A functional role for tyrosine phosphorylation of aPKC is provided by studies which have employed the inhibitors genistein and PP2. These agents, which specifically inhibit c-src activity (3), block the tyrosine phosphorylation of aPKC and its activity, impede recruitment of PKC-ι to TrkA, and also abolish NGF-induced differentiation of PC12 cells (35). Thus, tyrosine phosphorylation of aPKC by NGF-activated c-Src represents one potential mechanism for mediating NGF-initiated src responses. The combined requirement of c-Src and aPKC activation for NGF-mediated differentiation may function initially through a cascade requiring both enzymes. Evidence for such a c-Src- and PKC-ι-dependent cascade is provided by data showing that both aPKC isoforms and c-Src activate components of the Ras/MAP kinase and NF-κB pathways. Atypical PKC isoforms are able to activate MEK in PC12 (66) and other cells (4, 13), while c-Src is able to activate the Ras/MAP kinase pathway in mammary epithelial cells and Xenopus oocytes (30, 58). NGF-induced differentiation of PC12 cells requires NF-κB activation and, indeed, aPKC is involved in activating NF-κB in PC12 and other cells (13, 60). Similarly, src has been shown to directly phosphorylate IκBα, thereby leading to activation of NF-κB (1). Thus, NGF-induced activation of aPKC through c-Src-dependent tyrosine phosphorylation may be sufficient to activate both pathways involved in PC12 cell differentiation. This would be in keeping with the ability of specific tyrosine residues, such as PKC-ι 325, to play a role in NF-κB activation. Alternatively, this site may represent a binding site for another signaling intermediate that contains a phosphotyrosine binding domain (SH2) and hence regulates function. In this regard, active PKC-ι serves as an IKK kinase leading to its activation and phosphorylation (29). Moreover, during activation of NF-κB, PKC-ι dissociates from IkB (66). However, upon inhibition of PKC-ι's tyrosine phosphorylation state, PKC-ι fails to dissociate from IkB and is not activated by NF-κB. Impaired c-src-mediated PKC-ι activity via the tyrosine 325 mutation is in keeping with the requirement of PKC-ι for activation of NF-κB. Therefore, NGF-induced activation of NF-κB may likely be modulated by any one of the upstream critical regulatory elements: FRS2, src, ZIP/p62, or aPKC (Fig. 11). This model would be in keeping with a recent study demonstrating regulation of TRAF6 by c-src (61).
Last, PKC-ι can be negatively regulated by Par4, which binds to the regulatory domain, thereby leading to inhibition of aPKC activity. However, cells are capable of surviving with high levels of Par4 not bound to aPKCs (15), yet some trigger leads to the association of preexisting Par4 with aPKC. We speculate that tyrosine-phosphorylated aPKCs may not bind Par4, possibly due to steric hindrance. However, activation of tyrosine phosphatases may cause inhibition of src along with dephosphorylation of aPKC. Thus, inactivation of aPKC, renders aPKC capable of binding Par4 under apoptotic conditions. Therefore, aPKC may function in both pro- and antiapoptotic pathways, as has been postulated for the function of PKC-δ (52). On the one hand, aPKC may possess an antiapoptotic fate in its tyrosine-phosphorylated state, whereas on the other it would serve a proapoptotic role in its dephosphorylated state. Preliminary evidence for such a mechanism comes from studies demonstrating that PC12 cells deficient in src and hence NF-κB activity are extremely sensitive to trophic factor withdrawal, whereas cells which overexpress c-src and which possess enhanced NF-κB display enhanced survivability upon withdrawal of trophic factor support (65). In conclusion, this study underscores the importance of tyrosine phosphorylation as a critical and common regulator for the function of the aPKCs.
ACKNOWLEDGMENTS
We are indebted to Simon Halegoua for providing us with the c-Src-deficient PC12 cells and David Shalloway for the bacteria expressing the GST-src fusion constructs as well as members of our laboratory for many fruitful discussions. We thank Jorge Moscat for reading the manuscript.
This study was supported by the National Institutes of Health (M.W.W.). This study was also supported in part by a fellowship from the government of Spain (M.W.W., J.M.).
REFERENCES
- 1.Abu-Amer Y, Ross F P, McHugh K P, Livolsi A, Peyron J F, Teitelbaum S L. Tumor necrosis factor alpha activation of nuclear transcription factor kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of IkBalpha. J Biol Chem. 1998;273:29417–29423. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S, Maudsley S, Luttrell L M, Lefkowitz R J, Daaka Y. Src-medicated tyrosine phosphorylation of dynamin is required for β-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Ogawara H. Use and specificity of genistein as an inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- 4.Berra E, Diaz-Meco M T, Lozano J, Frutos S, Munico M M, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake R A, Garcia-Paramio P, Parker P J, Courtneidge S A. Src promotes PKC-δ degradation. Cell Growth Differ. 1999;10:231–241. [PubMed] [Google Scholar]
- 6.Brodie C, Bogi K, Acs P, Lazarovici P, Petrovics G, Anderson W B, Blumberg P M. Protein kinase epsilon plays a role in neurite outgrowth in response to epidermal growth factor and nerve growth factor in PC12 cells. Cell Growth Differ. 1999;10:183–191. [PubMed] [Google Scholar]
- 7.Brodie C, Bogi K, Acs P, Lorenzo P S, Baskin L, Blumberg P M. Protein kinase C delta inhibits the expression of glutamine synthetase in glial cells via the PKC delta regulatory domain and its tyrosine phosphorylation. J Biol Chem. 1998;273:30713–30718. doi: 10.1074/jbc.273.46.30713. [DOI] [PubMed] [Google Scholar]
- 8.Coleman E S, Wooten M W. Nerve growth factor-induced differentiation of PC12 cells employs the PMA-insensitive protein kinase C zeta isoform. J Mol Neurosci. 1994;5:39–57. doi: 10.1007/BF02736693. [DOI] [PubMed] [Google Scholar]
- 9.Cooper J A, Esch F S, Taylor S S, Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine kinases in vivo and in vitro. J Biol Chem. 1984;259:7835–7841. [PubMed] [Google Scholar]
- 10.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 11.Denning M F, Dlugosz A A, Threadgill D W, Magnuson T, Yuspa S H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Meco M T, Lozano J, Municio M M, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zeta. J Biol Chem. 1994;269:31706–31710. [PubMed] [Google Scholar]
- 13.Diaz-Meco M T, Dominguez I, Sanz L, Dent P, Lozano J, Municio M M, Berra E, Hay R T, Sturgill T W, Moscat J. ZetaPKC induces phosphorylation and inactivation of IkBalpha in vitro. EMBO J. 1994;13:2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Meco M T, Municio M M, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ι and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/mcb.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Meco M T, Munico M M, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of Par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Rocha M, Avila J, Lozano J. The zeta-isozyme of protein kinase C binds to tubulin through the pseduosubstrate domain. Exp Cell Res. 1997;230:1–8. doi: 10.1006/excr.1996.3364. [DOI] [PubMed] [Google Scholar]
- 17.Gschwendt M, Kielbassa K, Kittstein W, Marks M. Tyrosine phosphorylation and stimulation of protein kinase C delta from porcine spleen by src in vitro. Dependence of the activated state of protein kinase C delta. FEBS Lett. 1994;347:85–89. doi: 10.1016/0014-5793(94)00514-1. [DOI] [PubMed] [Google Scholar]
- 18.Haleem-Smith H, Chang E Y, Szallasi Z, Blumberg P, Rivera J. Tyrosine phosphorylation of protein kinase C delta in response to the activation of the high-affinity receptor for immunoglobulin E modifies its substrate recognition. Proc Natl Acad Sci USA. 1995;92:9112–9116. doi: 10.1073/pnas.92.20.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hama T, Huang K P, Guroff G. Protein kinase C as a component of a nerve growth factor-sensitive phosphorylation system in PC12 cells. Proc Natl Acad Sci USA. 1986;83:2353–2357. doi: 10.1073/pnas.83.8.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubner S, Xiao C, Jans D A. The protein kinase CK2 site enhances recognition of the simian virus 40 large T-antigen nuclear localization sequence by importin. J Biol Chem. 1997;272:17191–17195. doi: 10.1074/jbc.272.27.17191. [DOI] [PubMed] [Google Scholar]
- 21.Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues K J, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson L, Carpenter L, Biden T J, Fields A P. Protein kinase C iota activity is necessary for Bcr-Abl mediated resistance to drug-induced apoptosis. J Biol Chem. 1999;274:3927–3930. doi: 10.1074/jbc.274.7.3927. [DOI] [PubMed] [Google Scholar]
- 23.Joberty G, Petersen C, Gao L, Macara I G. The cell-polarity protein Par5 links Par3 and atypical protein kinase C to cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 24.Kadotani M, Nishiuma T, Nanahoshi M, Tsujishita Y, Ogita K, Nakamura S I, Kikkawa U, Asaoka Y. Characterization of tyrosine-phosphorylated delta isoform of protein kinase C isolated from Chinese hamster ovary cells. J Biochem. 1997;121:1047–1053. doi: 10.1093/oxfordjournals.jbchem.a021693. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan D R, Miller F A. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 26.Kazanietz M G, Areces L B, Bahador A, Mischak H, Goodnight J, Mushinski J F, Blumberg P M. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1993;44:298–307. [PubMed] [Google Scholar]
- 27.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H202. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K. Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta interacting protein. J Cell Biol. 1999;144:403–411. doi: 10.1083/jcb.144.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lallena M J, Diaz-Meco M T, Bren G, Paya C V, Moscat J. Activation of IkB kinase B by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee R J, Albanese C, Stenger R J, Watanabe G, Inghirami G, Haines G K, Webster M, Muller W J, Brugge J S, Davis R J, Pestell R G. pp60v-src induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and jun kinase pathways. J Biol Chem. 1999;274:7341–7350. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Seitz R, Lisanti M P. Phosphorylation of caveolin by src tyrosine kinases. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 32.Li W, Mischak H, Yu J C, Wang L M, Mushinski J F, Heidaran M A, Pierce J H. Tyrosine phosphorylation of protein kinase C delta in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- 33.Li W, Chen X H, Kelley C A, Alimandi M, Zhang J, Chen Q, Bottaro D P, Pierce J H. Identification of tyrosine 187 as a protein kinase C delta phosphorylation site. J Biol Chem. 1996;271:26404–26409. doi: 10.1074/jbc.271.42.26404. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Witte S, Liu Y C, Doyle M, Elly C, Altman A. Regulation of protein kinase C theta function during T cell activation by Lck-mediated tyrosine phosphorylation. J Biol Chem. 2000;275:3603–3609. doi: 10.1074/jbc.275.5.3603. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd E D, Wooten M W. MAP kinase is a component of the neurogenic pathway utilized by nerve growth factor in PC12 cells. J Neurochem. 1992;59:1099–1109. doi: 10.1111/j.1471-4159.1992.tb08352.x. [DOI] [PubMed] [Google Scholar]
- 36.Meakin S O, MacDonald J I S, Gryz E A, Kubu C J, Verdi J M. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 37.Mellor H, Parker P J. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moscat J, Diaz-Meco M T. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKCζ is a molecular switch in signal transduction of the TNF-a bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newton A C. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 41.Nye S H, Squinto S P, Glass D, Stitt T N, Hantzopoulos P, Macchi M J, Lindsay N S, Ip N Y, Yancopoulos G D. K252a and staurosporine selectively block autophosphorylation of neurotrophin-mediated responses. Mol Biol Cell. 1992;3:677–686. doi: 10.1091/mbc.3.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Driscoll K R, Teng K K, Fabbro D, Greene L A, Weinstein I B. Selective translocation of protein kinase C delta in PC12 cells during nerve growth factor-induced neuritogenesis. Mol Biol Cell. 1995;6:449–458. doi: 10.1091/mbc.6.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivier A R, Parker P J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem. 1994;269:2758–2763. [PubMed] [Google Scholar]
- 44.Puls A, Schimdt S, Grawe F, Stabel S. Interaction of protein kinase C zeta with ZIP, a novel protein kinase C binding protein. Proc Natl Acad Sci USA. 1997;94:6191–6196. doi: 10.1073/pnas.94.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rausch D M, Dickens G, Doll S, Fujita K, Koizumi S, Rudkin B B, Tocco M, Eiden L E, Guroff G. Differentiation of PC12 cells with v-src: comparison with nerve growth factor. J Neurosci Res. 1989;24:49–58. doi: 10.1002/jnr.490240108. [DOI] [PubMed] [Google Scholar]
- 46.Rusanescu G, Qi H, Thomas S M, Brugge J S, Halegoua S. Calcium influx induces neurite outgrowth through a Src-Ras signaling cassette. Neuron. 1995;15:1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 47.Samuels I S, Seibenhener M L, Neidigh K B W, Wooten M W. Nerve growth factor stimulates the interaction of ZIP/p62 with atypical protein kinase C and targets endosomal localization: evidence for regulation of nerve growth factor-induced differentiation. J Cell Biochem. 2001;82:452–466. doi: 10.1002/jcb.1177. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez P, De Carcer G, Sandoval I V, Moscat J, Diaz-Meco M T. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol Cell Biol. 1998;18:3069–3080. doi: 10.1128/mcb.18.5.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanz L, Sanchez P, Lallena M J, Diaz-Meco M T, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seibenhener M L, Roehm J, White W O, Neidigh K B W, Vandenplas M L, Wooten M W. Identification of src as a novel atypical protein kinase C interacting protein. Mol Cell Biol Res Commun. 1999;2:28–31. doi: 10.1006/mcbr.1999.0140. [DOI] [PubMed] [Google Scholar]
- 51.Song J S, Swann P G, Szallasi Z, Blank U, Blumberg P M, Rivera J. Tyrosine phosphorylation-dependent and independent association of protein kinase C delta with Src family kinases in RBL-2H3 mast cell line: regulation of src family kinase activity by protein kinase C delta. Oncogene. 1998;16:3357–3368. doi: 10.1038/sj.onc.1201886. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Wu F, Datta R, Kharbanda S, Kufe D. Interaction between protein kinase C delta and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J Biol Chem. 2000;275:7470–7473. doi: 10.1074/jbc.275.11.7470. [DOI] [PubMed] [Google Scholar]
- 53.Szallasi Z, Denning M F, Chang E, Rivera J, Yuspa S H, Lehel C, Olah Z, Anderson W B, Blumberg P M. Development of rapid approach to identification of tyrosine phosphorylation sites: application to PKCdelta phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells. Biochem Biophys Res Commun. 1995;214:888–894. doi: 10.1006/bbrc.1995.2370. [DOI] [PubMed] [Google Scholar]
- 54.Szeberenyi J, Erhardt P. Cellular components of nerve growth factor signaling. Biochim Biophys Acta. 1994;1222:87–202. doi: 10.1016/0167-4889(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 56.Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- 57.Uehara Y, Fukazawa H. Use and selectivity of herbimycin A as an inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- 58.VanRenterghem B, Browning M D, Maller J L. Regulation of mitogen-activated protein kinase activation by protein kinase A and C in a cell-free system. J Biol Chem. 1994;269:24666–24672. [PubMed] [Google Scholar]
- 59.Vertommen D, Rider M, Ni Y, Waelkens E, Merlevede W, Vanderheede J R, Van Lin J. Regulation of protein kinase D by multisite phosphorylation. J Biol Chem. 2000;275:19567–19576. doi: 10.1074/jbc.M001357200. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Seibenhener M L, Vandenplas M L, Wooten M W. Atypical PKC zeta is activated by ceramide, resulting in co-activation of NF-kappaB/JNK kinase and cell survival. J Neurosci Res. 1999;55:293–302. doi: 10.1002/(SICI)1097-4547(19990201)55:3<293::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 61.Wong B R, Besser D, Kim N, Arron J R, Vologodskai M, Hanafusa H, Choi V. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 62.Wooten M W, Seibenhener M L, Soh Y, Ewald S, White K R, Lloyd E D, Olivier A, Parker P J. Characterization and differential expression of protein kinase C isoforms in PC12 cells. FEBS Lett. 1992;298:74–78. doi: 10.1016/0014-5793(92)80025-c. [DOI] [PubMed] [Google Scholar]
- 63.Wooten M W, Zhou G, Seibenhener M L, Coleman E S. A role for zeta protein kinase C in nerve growth factor-induced differentiation of PC12 cells. Cell Growth Differ. 1994;5:395–403. [PubMed] [Google Scholar]
- 64.Wooten M W, Zhou G, Wooten M C, Siebenhener M L. Transport of protein kinase C isoforms to the nucleus of PC12 cells by nerve growth factor: association of atypical PKC-zeta with the nuclear matrix. J Neurosci Res. 1997;49:393–403. doi: 10.1002/(sici)1097-4547(19970815)49:4<393::aid-jnr1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Wooten M W, Seibenhener M L, Zhou G, Vandenplas M L, Tan T H. Overexpression of atypical PKC in PC12 cells enhances NGF responsiveness and survival through an NF-kappaB dependent pathway. Cell Death Differ. 1999;6:753–764. doi: 10.1038/sj.cdd.4400548. [DOI] [PubMed] [Google Scholar]
- 66.Wooten M W, Seibenhener M L, Neidigh K B W, Vandenplas M L. Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells. Mol Cell Biol. 2000;20:4494–4504. doi: 10.1128/mcb.20.13.4494-4504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wooten M W, Seibenhener M L, Mamidipudi V, Diaz-Meco M T, Barker P, Moscat J. The atypical protein kinase C interacting protein p62 is a scaffold for NF-kappaB activation by nerve growth factor. J Biol Chem. 2001;276:7709–7712. doi: 10.1074/jbc.C000869200. [DOI] [PubMed] [Google Scholar]
- 68.Yen A, Soong S, Kwon H J, Yoshida M, Beppu T, Varvayanis S. Enhanced cell differentiation when RB is hypophosphorylated and down-regulated by radicicol, a src-kinase inhibitor. Exp Cell Res. 1994;214:163–171. doi: 10.1006/excr.1994.1245. [DOI] [PubMed] [Google Scholar]
- 69.Yu H, Chen J K, Feng S, Dalgarno D C, Brauer A, Schreiber S L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- 70.Yuan Z-M, Utsugisawa T, Ishiko T, Nakada S, Huang Y, Kharbanda S, Weichselbaum R, Kufe D. Activation of protein kinase C delta by the c-abl tyrosine kinase in response to ionizing radiation. Oncogene. 1998;16:1643–1648. doi: 10.1038/sj.onc.1201698. [DOI] [PubMed] [Google Scholar]
- 71.Zang Q, Lu Z, Curto M, Barile N, Shalloway D, Foster D A. Association between v-src and protein kinase C delta in v-src transformed fibroblasts. J Biol Chem. 1997;272:13275–13280. doi: 10.1074/jbc.272.20.13275. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Moheban D B, Conway B R, Bhattacharyya A, Segal R A. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou G, Seibenhener M L, Wooten M W. Nucleolin is a protein kinase C zeta substrate. J Biol Chem. 1997;272:31130–31137. doi: 10.1074/jbc.272.49.31130. [DOI] [PubMed] [Google Scholar]