Abstract
Bcl-3 is a distinctive member of the IκB family of NF-κB inhibitors because it can function to coactivate transcription. A potential involvement of Bcl-3 in oncogenesis is highlighted by the fact that it was cloned due to its location at a breakpoint junction in some cases of human B-cell chronic lymphocytic leukemia and that it is highly expressed in human breast tumor tissue. To analyze the effects of Bcl-3 dysregulation in breast epithelial cells, we created stable immortalized human breast epithelial cell lines either expressing Bcl-3 or carrying the corresponding vector control plasmid. Analysis of the Bcl-3-expressing cells suggests that these cells have a shortened G1 phase of the cell cycle as well as a significant increase in hyperphosphorylation of the retinoblastoma protein. Additionally, the cyclin D1 gene was found to be highly expressed in these cells. Upon further analysis, Bcl-3, acting as a coactivator with NF-κB p52 homodimers, was demonstrated to directly activate the cyclin D1 promoter through an NF-κB binding site. Therefore, our results demonstrate that dysregulated expression of Bcl-3 potentiates the G1 transition of the cell cycle by stimulating the transcription of the cyclin D1 gene in human breast epithelial cells.
The NF-κB family of transcription factors regulates a wide variety of cellular processes, including immune responses, cellular growth and differentiation, and apoptosis (2, 11). In mammals, there are five members of the NF-κB family, p50, p65 (RelA), p52, c-Rel, and RelB, all of which share a conserved Rel homology domain allowing dimerization and DNA binding. Classic NF-κB, a heterodimer composed of p65 and p50 subunits, is normally found in the cytoplasm complexed with inhibitory IκB molecules. Stimulation with a variety of inducers causes IκB degradation, NF-κB nuclear translocation and transcriptional activation through the transactivation domain of p65. The IκB family, sharing a conserved domain of six to seven ankyrin repeats, is composed of p105 and p100 (precursors to p50 and p52, respectively), IκBα, IκBβ, IκBɛ, and Bcl-3.
Bcl-3, a candidate proto-oncogene, is upregulated transcriptionally in some cases of human B-cell chronic lymphocytic leukemia due to its location next to the breakpoint junction of a t(14;19) translocation (20, 21, 26). Bcl-3 binds to p50 or p52 NF-κB homodimers (10, 25, 38). Despite its homology to IκB, Bcl-3 can function as a coactivator when complexed with p50 or p52, which lack activation domains (4, 10). When bound to NF-κB sites as homodimers, p50 and p52 can competitively inhibit binding of transactivating NF-κB heterodimers, thus functioning as transcriptional repressors (9). However, upon association with Bcl-3, p50 and p52 homodimers can activate transcription through the transactivation domain of Bcl-3 (4, 10).
Bcl-3 has properties of a transcriptional coactivator, bridging transcription factors with the basal transcription machinery. Bcl-3 associates with the general transcription factors TFIIB, TATA-binding protein (TBP), and TFIIA (22). Bcl-3 also interacts with other coactivators, including CBP/p300, the steroid receptor coactivator 1 (SRC-1), and the Tip60 histone acetyltransferase (7, 23). In addition to p50 and p52 homodimers, Bcl-3 has been shown to bind to the AP-1 and RXR transcription factors, potentiating their activities (22, 23).
Recent findings correlate Bcl-3 expression with increased cellular proliferation and survival. Thus, transgenic mice expressing Bcl-3 were found to have an expansion of B cells in vivo, suggesting a role for Bcl-3 in B-cell proliferation (27). Consistent with a role for Bcl-3 in proliferation, Bcl-3 is positively regulated by many growth factors (5, 29, 30, 40). Bcl-3 was also shown to cause an increased rate of DNA synthesis when microinjected into Rat-1 cells (23). Additionally, transgenic mice expressing a dominant-negative NcoR corepressor targeted to the liver showed increased levels of hepatocyte proliferation as well as increased levels of Bcl-3 expression, showing a correlation between Bcl-3 levels and proliferation rates (8). However, in T cells, Bcl-3 expression does not alter cell growth, but instead promotes cell survival. Bcl-3 expression in interleukin 4 (IL-4)-deprived T cells protected the cells from apoptosis (29). In T cells activated by antigenic peptides, the addition of adjuvant increases expression of Bcl-3. Further study showed that overexpressed Bcl-3 increased the survival rates of the activated T cells (29). The mechanisms of Bcl-3 action in cell proliferation and cell survival have not been described.
An important factor involved in regulating cellular proliferation is cyclin D1 (32). The association of cyclin D1 with the cyclin-dependent kinases CDK4 and CDK6 results in phosphorylation of the retinoblastoma protein (Rb), thus releasing the transcription factor E2F (3). E2F is then able to activate S-phase-specific genes (16). Cyclin D1 is upregulated in the majority of human breast cancer (37). Importantly, it has been shown that transgenic expression of cylin D1 is sufficient to generate mammary hyperplasia and carcinoma (36), and cyclin D1 has been shown to be required for transformation by Her-2/Neu, a member of the epidermal growth factor (EGF) receptor family found overexpressed in a subset of breast tumors (17). In addition, cyclin D1 is required for the malignant transformation of human mammary epithelial cells by Her-2/Neu and Ras (39). Recent data have demonstrated elevated levels of Bcl-3, p52, and cyclin D1 in human breast cancer (6).
In this study, we investigated the effects of increased expression of Bcl-3 in immortalized human breast epithelial cells. Our data suggest that expression of Bcl-3 leads to a shortened G1 phase of the cell cycle and to a corresponding hyperphosphorylation of Rb. We also show that endogenous levels of cyclin D1 mRNA and cyclin D1 protein are increased in these cells as well as in cells transiently expressing Bcl-3. Furthermore, we demonstrate that Bcl-3, in cooperation with p52, can strongly activate the cyclin D1 promoter in transient transfection assays and that a p52–Bcl-3 complex can bind to the proximal NF-κB site of the cyclin D1 promoter. Dysregulation of Bcl-3 may promote oncogenesis through the upregulation of cyclin D1 and the subsequent stimulation of the G1 transition.
MATERIALS AND METHODS
Cell culture and reagents.
Murine NIH 3T3 fibroblasts were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Rockland, Md.) supplemented with 10% calf serum (HyClone Laboratories, Logan, Utah) and penicillin-streptomycin. Immortalized human 293T kidney cells and monkey COS-7 kidney cells were grown in DMEM supplemented with 10% fetal bovine serum (HyClone Laboratories) and penicillin-streptomycin. H16N2 immortalized human mammary epithelial cells were grown in Ham's F-12 medium (Life Technologies, Rockland, Md.) supplemented with hormones and growth factors (1 μg of hydrocortisone per ml, 10 ng of EGF per ml, 0 0.5 μg of Fungizone per ml, 5 mg of gentamicin per ml, 5 mM ethanolamine, 10 mM HEPES, 5 μg of transferrin per ml, 10 mM T3, 50 μM selenite, 1 g of bovine serum albumin per liter) or supplemented with hormones ± 10% fetal bovine serum. Cells expressing Bcl-3 were generated by transfecting the expression construct pFlag-Bcl-3 into H16N2 cells. Three H16N2:Bcl-3 stable clones were generated in medium containing 1 μg of puromycin per ml (Sigma, St. Louis, Mo.). Clones were verified by Western blotting with a Bcl-3-specific antibody (Bcl-3 C-14; Santa Cruz Biotechnology, Santa Cruz, Calif.).
Plasmid constructs.
The p50, p52, and pBcl-3 expression constructs were made by cloning PCR products in frame into the HindIII and EcoRV sites of the pFlag-CMV2 expression vector. Plasmid integrity was verified by sequencing. The p65 expression construct has been described previously (35). The cyclin D1 promoter reporter constructs CD1 −963 WT-Luc, CD1 −66 WT-Luc, and CD1 −66 Mut-Luc have been previously described (1). pPCMVEGFP-spectrin (15), pBPSTR-1 (17), and pBPSTR-1 CD1AS (17) have been described previously.
Transfection and luciferase reporter assays.
Cells were transiently transfected in six-well plates at 70% confluence with the Superfect reagent (Qiagen, Valencia, Calif.) according to the manufacturer's instructions. Briefly, plasmid constructs (3 μg of total DNA) were diluted in serum-free medium and mixed with the Superfect reagent. Complexes were allowed to form for 10 min before serum-containing medium was added to the mixture. The cells were washed once in 1× phosphate-buffered saline (PBS), and Superfect-DNA complexes were added to the cells and placed in a humidified incubator at 37°C with 5% CO2. Three hours posttransfection, cells were washed with 1× PBS and replenished with fresh serum-containing medium. Forty-eight hours posttransfection, cells were washed once in 1× PBS and lysed in Reporter lysis buffer (Promega, Madison, Wis.) for 10 min at room temperature. Extracts were collected and cleared by centrifugation. Protein concentration was determined with the Bio-Rad (Hercules, Calif.) protein assay dye reagent. Luciferase assays were performed with 50 μg of protein per sample. d-Luciferin (Sigma, St. Louis, Mo.) was used as a substrate, and relative light units were measured with an AutoLumat LB953 luminometer (Berthold Analytical Instruments, Bad Wildbad, Germany). For assays with the stable H16N2 cell lines, transfections were also done with pCMV-LacZ to assay for transfection efficiency by counting β-galactosidase-positive cells as described previously (19).
Western analysis.
Total cellular protein (50 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Membranes were blocked and incubated for 1 h with primary antibody. Proteins were visualized by incubation with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL) reagents (Amersham, Piscataway, N.J.). A Bcl-3-specific antibody (Bcl-3 C-14; Santa Cruz Biotechnology), Flag-specific antibody (anti-Flag M2; Sigma), and tubulin-specific antibody (Sigma) were used. The cyclin D1 and Rb antibodies are from Pharmingen (Franklin Lakes, N.J.).
FACS analysis.
H16N2:Puro or H16N2:Bcl-3 cells were grown in Ham's F-12 medium (Life Technologies, Rockland, Md.) supplemented with hormones and growth factors and harvested at 70% confluence. A total of 106 cells per sample were pelleted at 500 × g for 5 min and resuspended in 200 μl of PBS. Cells were fixed by adding 2 volumes of cold absolute ethanol by incubating for 1 h at 4°C. After centrifugation at 500 × g for 10 min and resuspension in PBS, RNase A, and propidium iodide were added to final concentrations of 0.1 and 40 μg/ml, respectively. Samples were incubated at 37°C for 30 min and then stored at 4°C. The percentage of cells in each cell cycle phase was determined by flow cytometry. To show that cyclin D1 is required for the induction of the G1 phase by Bcl-3, H16N2:Puro or H16N2:Bcl-3 cells were transfected with pBPSTR-1 or pBPSTR-1 CD1AS plasmids along with pPCMVEGFP-spectrin at a ratio of 10:1. Forty-eight hours after transfection, cells were harvested as described above for fluorescence-activated cell sorting (FACS). The cell cycle status of over 1,000 green fluorescent protein (GFP)-positive cells for each sample was then examined.
Cell proliferation assay.
The cell proliferation enzyme-linked immunosorbent assay (ELISA) system from Amersham Pharmacia (Peapack, N.J.) was used according to the manufacturer's instructions. Briefly, 5 × 104 cells were plated per well of a 96-well tissue culture plate in Ham's F-12 medium (Life Technologies) supplemented with hormones ± 10% fetal bovine serum. The cells were allowed to grow for 48 h and then labeled with bromodeoxyuridine (BrdU) for 6 h. After labeling, the medium was removed, cells were fixed, and DNA was denatured. The cells were then incubated with a blocking solution, followed by incubation with peroxidase-labeled anti-BrdU antibody. After washing and addition of substrate, the cells were incubated for 10 min before measurement of optical density at 450 nm.
Northern analysis.
Total RNA was isolated with Trizol reagent as recommended by the manufacturer (Life Technologies, Rockville, Md.). RNA samples (10 μg each) were run on an agarose gel and transferred to a nylon filter overnight. RNA was cross-linked to the filter with a UV cross-linker (Stratagene, La Jolla, Calif.). Filters were hybridized in QuickHyb buffer (Stratagene) in the presence of radioactive probes following the manufacturer's protocol. Probes were generated from PCR fragments with a random-primed labeling kit (Life Technologies) and with [α-32P]dCTP.
EMSAs.
Nuclear extracts were prepared and electrophoretic mobility shift assays (EMSAs) were performed as previously described (18). Briefly, COS-7 cells were transfected with 1 μg each of pFlag-Bcl-3, pFlag-p52, or vector control expression constructs in various combinations. Nuclear extracts were prepared 48 h posttransfection from COS-7 cells and incubated with [α-32P]dCTP-labeled, double-stranded probes containing sequences corresponding to both wild-type and mutant versions of the proximal NF-κB site within the cyclin D1 promoter (12). Labeled probe-nuclear extract complexes were incubated for 20 min at room temperature and separated on a 5% polyacrylamide gel. The gel was dried and exposed to film. For supershift analysis, p52 or Bcl-3 antibodies were incubated with the nuclear extract for 10 min prior to the addition of probe. Nancy Rice provided the human p52 antibody (no. 1267) and Timothy McKeithan provided the Bcl-3 antibody (41) used in the supershift reactions.
RESULTS
Stable expression of Bcl-3 in breast epithelial cells leads to enhanced progression through the G1 phase of the cell cycle.
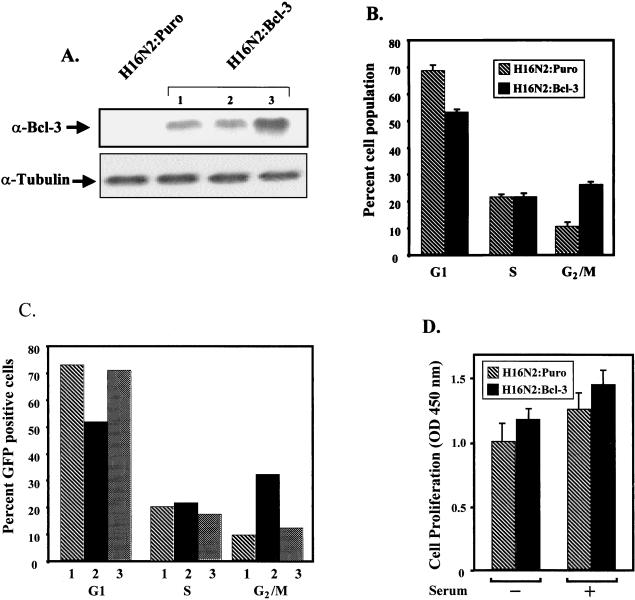
To begin to study a potential role for Bcl-3 in oncogenesis, we generated immortalized breast epithelial cell lines containing either Bcl-3 (H16N2:Bcl-3) or vector control (H16N2:Puro) plasmids (Fig. 1A). Like parental cells, H16N2:Puro cells do not express Bcl-3, while H16N2:Bcl-3 cells express Bcl-3, as shown by Western blot analysis (Fig. 1A). Using the H16N2:Bcl-3 clone 3, we then performed FACS analysis on actively dividing cells to observe possible differences in the ability of these cell lines to transition through the cell cycle. As shown in Fig. 1B, a significantly lower percentage of the H16N2:Bcl-3 cell population was observed in the G1 phase of the cell cycle, as compared to H16N2:Puro cells. Although similar percentages of H16N2:Puro and H16N2:Bcl-3 cells were observed in the S phase of the cell cycle, H16N2:Bcl-3 displayed a higher percentage of cells in the G2/M phase of the cell cycle. Therefore, these data suggest that the G1 phase of the cell cycle is shortened in the H16N2:Bcl-3 cells compared to that in H16N2:Puro cells. Other less likely alternatives are that an increase in the growth fraction or a decrease in cell death together with a G2/M delay could explain the results.
FIG. 1.
Stable expression of Bcl-3 in breast epithelial cells promotes the G1 transition. (A) Creation o f stable cell lines expressing Bcl-3. H16N2 immortalized human breast epithelial cells were transfected with pFlag-CMV2 or pFlag-Bcl-3, and stable cell lines were generated (H16N2:Puro and H16N2:Bcl-3). Western analysis with anti-Bcl-3 shows expression of Bcl-3 protein in H16N2:Bcl-3 clones 1, 2, and 3. (B) FACS analysis shows that Bcl-3 expression leads to an accelerated G1 phase. Proliferating cells were harvested and analyzed by FACS. The percentage of cells in each stage of the cell cycle is indicated. Data represent the mean ± standard deviation of three independent experiments. (C) Cyclin D1 is required for the induction of G1 phase in H16N2:Bcl-3 cells. Cells were cotransfected with pPCMVEGFP-spectrin and either vector control plasmid (pBPSTR-1) or a cyclin D1 antisense plasmid (pBPSTR-1 CD1 AS). GFP-positive cells were then analyzed by FACS. In sample 1, H16N2:Puro cells were transfected with a vector control plasmid. In sample 2, H16N2:Bcl-3 cells were transfected with a vector control plasmid. In sample 3, H16N2:Bcl-3 cells were transfected with a cyclin D1 antisense plasmid. (D) Expression of Bcl-3 does not lead to increased cellular proliferation in H16N2 cells. H16N2:Puro or H16N2:Bcl-3 cells were grown in the presence or absence of serum in triplicate wells of a 96-well plate. Twenty-four hours after plating, the cells were incubated for 4 h with BrdU and analyzed by ELISA for BrdU incorporation. OD, optical density. Results represent the mean ± standard deviation of three independent experiments.
To show that cyclin D1 is required for the induction of the G1 phase in H16N2:Bcl-3 cells, H16N2:Puro or H16N2:Bcl-3 cells were cotransfected with pPCMVEGFP-spectrin and either a vector control plasmid (pBPSTR-1) or a cyclin D1 antisense plasmid (pBPSTR-1 CD1AS). GFP-positive cells were then analyzed by FACS (Fig. 1C). When transfected into H16N:Bcl-3 cells, the cyclin D1 antisense construct caused the percentage of cells in G1 to return to the levels seen in H16N2:Puro cells, demonstrating a requirement for cyclin D1 in promoting the G1 transition.
To determine whether the ability of Bcl-3 to potentiate transition through G1 correlates with enhanced proliferation of the H16N2 cells, cell proliferation studies were performed by measuring BrdU incorporation. To quantitate differences in proliferation rates, H16N2:Puro and H16N2:Bcl-3 cells were grown in complete media or were cultured in serum-free media and assayed in a cellular proliferation assay. As shown in Fig. 1D, the H16N2:Bcl-3 cells did not show a significant increase in BrdU incorporation above levels observed for H16N2:Puro. Our inability to detect significant differences in BrdU incorporation was also consistent with our inability to detect differences in cell growth rates (data not shown). Although Bcl-3 expression has been shown to enhance proliferation in certain cell types (8, 23), in the immortalized breast epithelial cell line H16N2, Bcl-3 expression alone is not sufficient to potentiate proliferation. Additionally, the Bcl-3-expressing H16N2 cells were not tumorigenic in nude mice (unpublished observations). These results suggest that other oncogenic events in addition to Bcl-3 overexpression would need to take place to facilitate dysregulated growth of breast epithelial cells.
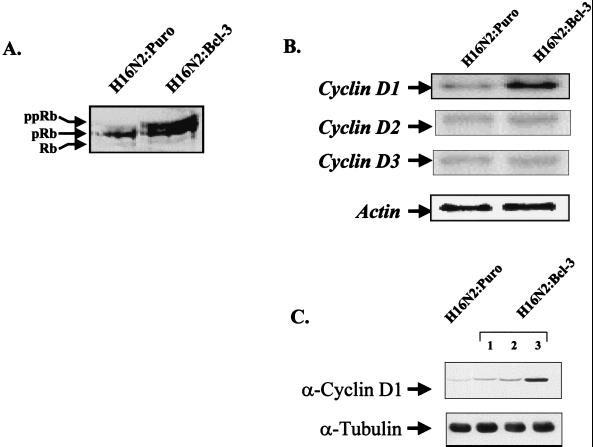
Bcl-3 expression leads to hyperphosphorylation of Rb and upregulation of endogenous levels of cyclin D1.
Because of the accelerated G1 transition observed in H16N2:Bcl-3 cells, we analyzed the phosphorylation status of Rb, a key cellular regulator of the G1 transition. Once Rb becomes phosphorylated by the cyclin D–cyclin-dependent kinase complex, it releases the transcription factor E2F, allowing the transcription of S-phase-specific genes and progression of the cell cycle (32). Protein extracts isolated from H16N2:Puro and H16N2:Bcl-3 cells were resolved by SDS-PAGE, and Western blots were probed with anti-Rb antibody. Both H16N2:Puro and H16N2:Bcl-3 cells displayed nearly equal amounts of hypophosphorylated Rb (Fig. 2A, pRb and Rb bands). However, H16N2:Bcl-3 cells displayed a higher-molecular-weight hyperphosphorylated band, which was not observed in H16N2:Puro control cells (Fig. 2A, ppRb band). Therefore, expression of Bcl-3 in H16N2 cells causes increased levels of Rb phosphorylation, consistent with our data suggesting a shortening of the G1 phase of the cell cycle.
FIG. 2.
Bcl-3 expression leads to hyperphosphorylation of Rb and induction of endogenous cyclin D1 levels. (A) Fifty micrograms of total cellular protein from the H16N2:Puro and H16N2:Bcl-3 cell lines was run on SDS-PAGE. After transfer to nitrocellulose, the membrane was probed with an anti-Rb antibody. Of the three Rb bands detected, the upper-molecular-weight band represents hyperphosphorylated Rb (ppRB). (B) The H16N2:Bcl-3 cell line shows increased levels of cyclin D1 RNA by Northern analysis. Cyclin D2 and cyclin D3 mRNA levels are unchanged. Equal RNA loading was verified by using a probe for actin. (C) Cell extracts were isolated from the H16N2:Puro and H16N2:Bcl-3 cell lines and run on SDS-PAGE. After transfer to nitrocellulose, the membranes were probed with an anti-cyclin D1 antibody. Equal protein loading was verified with antitubulin antibody.
Because the phosphorylation of Rb is regulated by the cyclin D–cyclin-dependent kinase complex, we also analyzed the levels of cyclin D present in our cell lines. RNAs were isolated, and Northern blot analysis was performed. As shown in Fig. 2B, the levels of endogenous cyclin D1 transcripts were elevated in the H16N2:Bcl-3 cells, as compared to H16N2:Puro control cells. However, the levels of cyclin D2 and cyclin D3 mRNAs were the same in the two cell lines. Therefore, stable expression of Bcl-3 in breast epithelial cells results in specifically higher levels of cyclin D1 RNA. A Western blot was also performed that showed increased cyclin D1 protein expression in the H16N2:Bcl-3 cells (Fig. 2C). For this experiment, we analyzed all three of our H16N2:Bcl-3 clones and found that cyclin D1 protein levels correlate with the expression level of Bcl-3, providing further evidence that Bcl-3 regulates cyclin D1 expression.
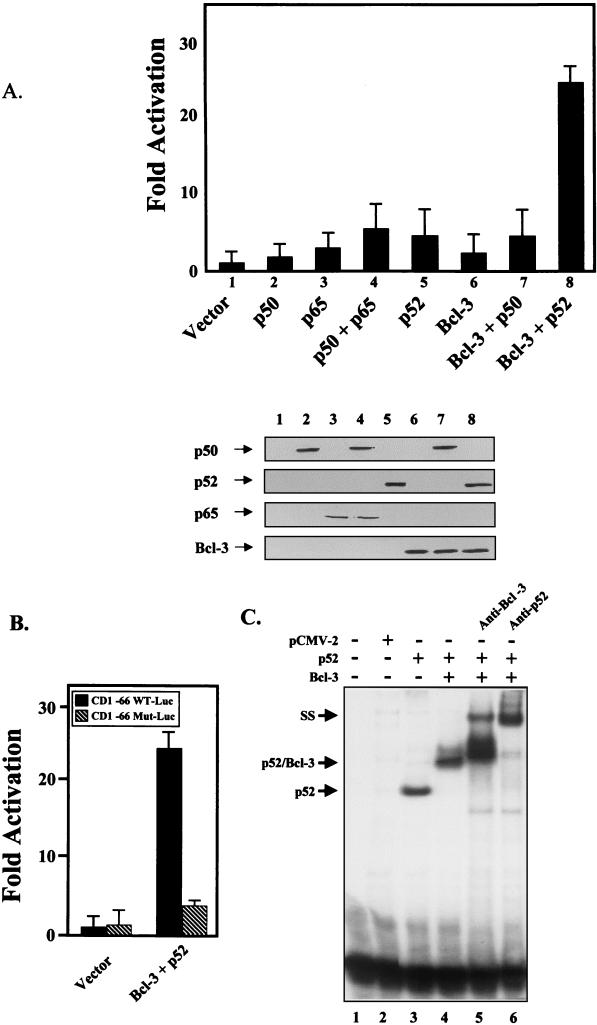
Bcl-3 and p52 synergistically activate the cyclin D1 promoter through the proximal NF-κB site.
Cyclin D1 has previously been shown to be regulated by classic p65-p50 NF-κB (12–14). However, in breast tumor tissues that overexpress cyclin D1, nuclear levels of p65 are not elevated typically, while nuclear levels of p50, p52, and Bcl-3 are increased (6). Therefore, we wanted to determine if the expression of Bcl-3 could transcriptionally regulate the cyclin D1 promoter. In order to see if Bcl-3, complexed with p50 or p52, could activate transcription of the cyclin D1 gene, NIH 3T3 cells were transiently cotransfected with a cyclin D1 promoter luciferase construct containing three putative NF-κB sites and an AP-1 site (CD1 −963 WT-Luc) and with expression vectors encoding p50, p65, p52, and Bcl-3 proteins alone or in combination (Fig. 3A). NIH 3T3 fibroblasts were used in these studies, since these cells normally express undetectable levels of endogenous Bcl-3 protein (unpublished observations). Although classic p65-p50 NF-κB dimers activate the cyclin D1 promoter approximately fivefold over the vector control, Bcl-3 and p52 together synergistically activated the cyclin D1 promoter 24-fold over the vector control. Interestingly, p52 and Bcl-3 activated the cyclin D1 promoter more efficiently than p50 and Bcl-3 (Fig. 3A). The differences in reporter activation were not due to differences in transgene expression, since similar levels of proteins were expressed as verified by Western analysis (Fig. 3A, lower panel).
FIG. 3.
Bcl-3 and p52 synergistically activate the cyclin D1 promoter. (A) NIH 3T3 cells were transiently cotransfected with the full-length cyclin D1 promoter luciferase construct (CD1 −963 WT-Luc) and with expression vectors encoding p50, p65, p52, and Bcl-3 proteins alone or in combination. Forty-eight hours posttransfection, cells were harvested and luciferase assays were performed. The data presented represent the mean ± standard deviation of three independent experiments performed in triplicate. The fold inductions, as compared to the vector control, were plotted. (Lower panel) Western analysis showing equivalent expression of transiently transfected constructs. (B) Bcl-3 and p52 activate transcription through the proximal NF-κB site in transient transfection assays. NIH-3T3 cells were transiently cotransfected with a cyclin D1 promoter luciferase construct containing the wild-type proximal NF-κB site (CD1 −66 WT-Luc) or a mutated site (CD1 −66 Mut-Luc) together with expression vectors encoding p52 and Bcl-3 proteins and assayed as described above. (C) Bcl-3 and p52 proteins bind to the proximal NF-κB site of the cyclin D1 promoter. COS-7 cells were transfected with various combinations of Bcl-3, p52, or vector control expression constructs as indicted. Nuclear extracts were analyzed by gel shift. The identities of the bound proteins were verified by supershift (SS) with antibodies against Bcl-3 and p52, as indicated.
Previously, NF-κB activation of the cyclin D1 promoter has been shown to function mainly through the proximal NF-κB site located 39 nucleotides upstream of the major start of transcription (12–14). To determine if Bcl-3 and p52 also function through this site, NIH 3T3 cells were transiently cotransfected with a cyclin D1 promoter luciferase construct containing the wild-type proximal NF-κB site (CD1 −66 WT-Luc) or a mutated site (CD1 −66 Mut-Luc), together with expression vectors encoding p52 and Bcl-3 proteins (Fig. 3B). CD1 –66 does not contain the upstream AP-1 site (1). Bcl-3 and p52 were able to activate the reporter containing only the proximal wild-type NF-κB site as well as they were able to activate the full-length reporter. Only minimal activation of the reporter with the mutant NF-κB site was observed (Fig. 3B). Therefore, like classic NF-κB, Bcl-3 also activates the cyclin D1 promoter through the proximal NF-κB site.
To confirm that Bcl-3 upregulated the cyclin D1 promoter by directly interacting with the proximal NF-κB cis element, EMSAs were performed (Fig. 3C). Nuclear extracts isolated from COS-7 cells transfected with an expression plasmid encoding p52 alone or p52 and Bcl-3 proteins together displayed DNA binding activity that recognized the proximal NF-κB element from the cyclin D1 promoter (Fig. 3C). The observed DNA binding activity was specific and could be supershifted with antibodies against either Bcl-3 or p52 (Fig. 3C). Therefore, Bcl-3 tethered to a p52 homodimer complex is specifically targeted to the proximal NF-κB cis element located in the cyclin D1 promoter region. Bcl-3–p52 DNA complexes could also be detected by using extracts from the H16N2:Bcl-3 cell line (data not shown).
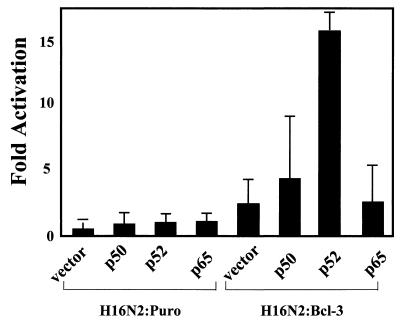
p52 activates the cyclin D1 promoter in a Bcl-3 stable transfectant.
While Bcl-3 alone is sufficient to activate the cyclin D1 gene in H16N2:Bcl-3 cells, presumably through association with endogenous p52, we wanted to test whether expression of p52 would increase transcription from the cyclin D1 promoter. H16N2:Puro and H16N2:Bcl-3 cell lines were transiently transfected with the cyclin D1 promoter luciferase construct (CD1 −963 WT-Luc), a lacZ expression vector, and with p50, p52, or p65 alone or in combination, as indicated (Fig. 4). Transfection efficiency for the two cell lines was found to be equivalent, as determined by counting β-galactosidase-positive cells (data not shown). In the H16N2:Puro cell line, addition of p50, p52, or p65 did not significantly increase activity from the cyclin D1 reporter. However, in the H16N2:Bcl-3 cell line, p52 expression dramatically increased the activation of the cyclin D1 reporter. In contrast, overexpression of p50 or p65 did not show a strong activation over vector control. These results provide evidence for the cooperation of Bcl-3 and p52 in the regulation of cyclin D1 in human breast epithelial cells.
FIG. 4.
Activation of the cyclin D1 promoter by p52 in Bcl-3 stable breast epithelial cells. H16N2:Puro and H16N2:Bcl-3 cell lines were transiently transfected with the cyclin D1 promoter luciferase construct (CD1 −963 WT-Luc), a lacZ expression vector, and with expression vectors encoding p50, p52, and p65 proteins alone or in combination. Forty-eight hours posttransfection, cells were harvested and luciferase assays were performed. Transfection efficiency for the two cell lines was found to be equivalent, as determined by counting β-galactosidase-positive cells (data not shown). The data presented represent the mean ± standard deviation of luciferase expressions of three independent experiments performed in triplicate. The fold inductions, as compared to that of the vector control, were plotted.
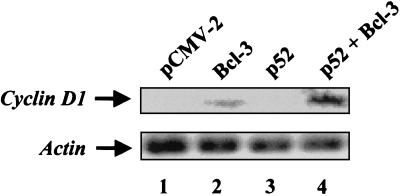
Transient expression of Bcl-3 alone or with p52 leads to increased levels of endogenous cyclin D1 mRNA in 293T cells.
To determine whether increased expression of Bcl-3 and p52 could lead to activation of endogenous cyclin D1 mRNA levels in a different cell type other than breast cells, HEK 293T cells were transfected with expression vectors encoding Bcl-3, p52, or both, and Northern blot analysis was performed (Fig. 5). This approach was feasible, since the transfection efficiency of the HEK 293T cells was over 80%. Northern blots were analyzed for changes in endogenous cyclin D1 mRNA levels following the transient expression of Bcl-3. Bcl-3 expression alone increased levels of cyclin D1 transcripts (Fig. 5, lane 2), as compared to the vector control (Fig. 5, lane 1). Although p52 alone did not affect cyclin D1 expression (Fig. 5, lane 3), cells expressing both p52 and Bcl-3 proteins displayed a significant increase in cyclin D1 mRNA levels over that attained with Bcl-3 alone. Equal RNA loading was verified by stripping the blot and reprobing with an actin probe. Therefore, the regulation of endogenous cyclin D1 mRNA levels by Bcl-3 is not restricted to human breast cells.
FIG. 5.
Bcl-3 and p52 proteins upregulate endogenous cyclin D1 mRNA in 293T cells. 293T cells were transfected with various combinations of Bcl-3, p52, or vector control expression constructs as indicated. RNA was isolated, and Northern blot analysis was used to probe for levels of cyclin D1 mRNA. An actin probe was used to verify equal RNA loading.
DISCUSSION
In order to understand the potential oncogenic properties of Bcl-3 in human breast cancer and presumably other cancers, we have analyzed the biological consequences of Bcl-3 expression in H16N2 immortalized human breast epithelial cells. We find that the H16N2:Bcl-3 cells have a shorter G1 phase of the cell cycle than H16N2:Puro cells. H16N2:Bcl-3 cells also have hyperphosphorylated Rb, which led us to test whether cyclin D1 levels were altered. We found that endogenous levels of cyclin D1 mRNA and cyclin D1 protein were indeed upregulated in the H16N2:Bcl-3 cells.
Most NF-κB-regulated genes studied to date have been analyzed in terms of their transcriptional activation by classic p65-p50 NF-κB. Little is known about the specificity of different NF-κB family members in gene regulation. Previously, several groups have implicated NF-κB p65-p50 as a positive regulator of the cyclin D1 gene (12–14). Here we show that Bcl-3–p52 complexes regulate the cyclin D1 promoter more strongly than classic p65-p50 NF-κB. Cyclin D1 may in fact be a Bcl-3-responsive gene, since the expression of Bcl-3 and p52 upregulates the cyclin D1 promoter approximately fivefold higher than classic NF-κB. We further demonstrate that the proximal NF-κB site is able to bind p52–Bcl-3 complexes in EMSAs. Bcl-3–p52 complexes have also been shown through EMSA and reporter assays to specifically regulate the human P-selectin promoter (28). Thus, there may be a select group of NF-κB-regulated genes that are transcriptionally controlled by Bcl-3–p52 complexes.
Interestingly, we had found that human breast tumors contain enhanced nuclear levels of p52, Bcl-3, and cyclin D1 compared to normal adjacent tissue (6). Surprisingly, the same tumors do not show elevated nuclear levels of p65. Therefore, our finding that Bcl-3 and p52 can activate the cyclin D1 promoter may have relevance in the progression of human breast cancer. We do note, however, that other studies have found upregulation of the p65 subunit of NF-κB in some cases of breast cancer (24, 34). Thus, classic NF-κB may also contribute to cyclin D1 upregulation in certain breast cancers.
The cyclin D1 gene is overexpressed as a result of gene amplification in many human tumors (31). However, cyclin D1 protein overexpression often occurs without a corresponding amplification of the cyclin D1 locus. In breast cancer, for example, the frequency of cyclin D1 gene amplification is low, while most breast cancers overexpress the cyclin D1 protein (31). Therefore, dysregulation of the regulatory pathways that control cyclin D1 expression is likely to play a role in breast cancer. Cyclin D1 has been shown to contribute directly to breast oncogenesis in transgenic mice. For example, a targeted overexpression of cyclin D1 in murine mammary epithelial cells results in ductal hyperproliferation and tumor formation (36). Mice null for cyclin D1, on the other hand, exhibit defects in mammary lobuloalveolar development during pregnancy (33). Furthermore, cyclin D1 has been shown to be required for transformation induced by Her-2/Neu (17). Therefore, the critical role that cyclin D1 plays in normal breast development may be coupled to its tumorigenic properties when aberrantly expressed.
In most systems studied so far, Bcl-3 has been found to be a proliferative factor (8, 23). This is logical, since Bcl-3 is induced by a variety of growth-promoting cytokines in multiple cell types (5, 29, 30, 40). We have shown that this is also true in breast cells. In MCF7 breast carcinoma cells, Bcl-3 mRNA is induced by tumor necrosis factor alpha (TNF-α), IL-1β, and platelet-derived growth factor (PDGF-β) (unpublished observations). In the H16N2 normal breast epithelial cell line expressing Bcl-3, however, we did not observe a significant increase in cell proliferation, even though the level of cyclin D1 mRNA is increased. However, we demonstrate an increase in hyperphosphorylated Rb, the biological target of cyclin D1 and associated kinases. This correlates with a potentially shorter G1 phase of the cell cycle, as measured by FACS. However, the overall cell cycle time is not changed in the H16N2:Bcl-3 cells, because the cells do not grow significantly faster than the H16N2:Puro cells (data not shown). Our FACS data suggest that the G2/M phase of the cell cycle is lengthened, thus making up for the shortened G1 phase and allowing total cell division time to remain the same. At present, we do not know the reason for the lengthened G2/M phase. In our system, we hypothesize that other genes may be required to act in concert with Bcl-3 to increase the overall rate of cellular proliferation and/or oncogenic potential.
Taken together, our data establish a link between upregulated nuclear levels of Bcl-3 and p52 in breast cancer cells with upregulated levels of cyclin D1. We show that cyclin D1 is transcriptionally activated by Bcl-3–p52 complexes more efficiently than by p65-p50 complexes. The increased levels of cyclin D1 in the Bcl-3-expressing cells led to hyperphosphorylated Rb and a shortened G1 transition, but not to significantly increased levels of cellular proliferation. We note that the H16N2:Bcl-3 cells do not form tumors in nude mice (unpublished observations). Because cancer is a multistep process, we propose that Bcl-3 may promote increased cell growth in combination with other oncogenes. Bcl-3 has been shown by EMSA and reporter assays to specifically regulate the human P-selectin promoter (28). To our knowledge, cyclin D1 is the first endogenous gene shown to be regulated by Bcl-3. In addition to cyclin D1, it will also be important to study other proto-oncogenes that may be overexpressed by aberrant activation of Bcl-3 or that may cooperate with Bcl-3 to promote oncogenesis.
ACKNOWLEDGMENTS
We are grateful to Carolyn Sartor for providing us with the H16N2 cells. We also thank Nancy Rice for kindly providing the p52 antibody and Timothy McKeithan for kindly providing the Bcl-3 antibody used in the EMSA reactions. The cyclin D1 promoter reporter constructs and cyclin D1 antisense expression vector were provided by Richard Pestell. We thank Denis Guttridge for providing reagents and experimental advice. We also thank Jayne Keifer, Denis Guttridge, and Raquel Sitcheran for a critical reading of the manuscript and the members of the Baldwin laboratory for helpful discussions.
This work was supported by grants from the American Cancer Society (PF-00–023-01-MGO) to S.D.W. and from the NIH (CA73756 and CA75080) and the Leukemia and Lymphoma Society to A.S.B.
REFERENCES
- 1.Albanese C, Johnson G, Watanabe N, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 5.Brasier A R, Lu M, Hai T, Lu Y, Boldogh I. NF-κB inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-κB1 residence. J Biol Chem. 2001;276:32080–32093. doi: 10.1074/jbc.M102949200. [DOI] [PubMed] [Google Scholar]
- 6.Cogswell P C, Guttridge D C, Funkhouser W K, Baldwin A S., Jr Selective activation of NF-κB subunits in human breast cancer: potential roles for NF-κB2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 7.Dechend R, Hirano F, Lehmann K, Heissmeyer V, Ansieau S, Wulczyn F, Scheidereit C, Leutz A. The Bcl-3 oncoprotein acts as a bridging factor between NF-κB/Rel and nuclear co-regulators. Oncogene. 1999;18:3316–3323. doi: 10.1038/sj.onc.1202717. [DOI] [PubMed] [Google Scholar]
- 8.Feng X, Jiang Y, Meltzer P, Yen P M. Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by the thyroid hormone receptor and increases cell proliferation. J Biol Chem. 2001;276:15066–15072. doi: 10.1074/jbc.m011027200. [DOI] [PubMed] [Google Scholar]
- 9.Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Nolan G P, Liou H C, Scott M L, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-κB p50 homodimers. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S, May M J, Kopp E B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 12.Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein J U, Lee R J, Segall J E, Westwick J K, Der C J, Pestell R G. Integration of Rac-dependent regulation of cyclin D1 transcription through a NF-κB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 15.Kalejta R F, Shenk T, Beavis A J. Use of a membrane-localized green fluorescent protein allows simultaneous identification of transfected cells and cell cycle analysis by flow cytometry. Cytometry. 1997;29:286–291. doi: 10.1002/(sici)1097-0320(19971201)29:4<286::aid-cyto4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.La Thangue N B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994;19:108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee R J, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, Haines III G K, Siegel P M, Hung M C, Yarden Y, Horowitz J M, Muller W J, Pestell R G. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayo M W, Norris J L, Baldwin A S. Ras regulation of NF-κB and apoptosis. Methods Enzymol. 2001;333:73–87. doi: 10.1016/s0076-6879(01)33046-x. [DOI] [PubMed] [Google Scholar]
- 19.Mayo M W, Wang C Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S., Jr Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 20.McKeithan T W, Rowley J D, Shows T B, Diaz M O. Cloning of the chromosome translocation breakpoint junction of the t(14;19) in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 1987;84:9257–9260. doi: 10.1073/pnas.84.24.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeithan T W, Takimoto G S, Ohno H, Bjorling V S, Morgan R, Hecht B K, Dube I, Sandberg A A, Rowley J D. BCL3 rearrangements and t(14;19) in chronic lymphocytic leukemia and other B-cell malignancies: a molecular and cytogenetic study. Genes Chromosomes Cancer. 1997;20:64–72. [PubMed] [Google Scholar]
- 22.Na S Y, Choi H S, Kim J W, Na D S, Lee J W. Bcl3, an IκB protein, as a novel transcription coactivator of the retinoid X receptor. J Biol Chem. 1998;273:30933–30938. doi: 10.1074/jbc.273.47.30933. [DOI] [PubMed] [Google Scholar]
- 23.Na S Y, Choi J E, Kim H J, Jhun B H, Lee Y C, Lee J W. Bcl3, an IκB protein, stimulates activating protein-1 transactivation and cellular proliferation. J Biol Chem. 1999;274:28491–28496. doi: 10.1074/jbc.274.40.28491. [DOI] [PubMed] [Google Scholar]
- 24.Nakshatri H, Bhat-Nakshatri P, Martin D A, Goulet R J, Jr, Sledge G W., Jr Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolan G P, Fujita T, Bhatia K, Huppi C, Liou H-C, Scott M L, Baltimore D. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NF-κB p50 and p52 in a phosphorylation-dependent manner. Mol Cell Biol. 1993;13:3557–3566. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno H, Doi S, Yabumoto K, Fukuhara S, McKeithan T W. Molecular characterization of the t(14;19)(q32;q13) translocation in chronic lymphocytic leukemia. Leukemia. 1993;7:2057–2063. [PubMed] [Google Scholar]
- 27.Ong S T, Hackbarth M L, Degenstein L C, Baunoch D A, Anastasi J, McKeithan T W. Lymphadenopathy, splenomegaly, and altered immunoglobulin production in BCL3 transgenic mice. Oncogene. 1998;16:2333–2343. doi: 10.1038/sj.onc.1201771. [DOI] [PubMed] [Google Scholar]
- 28.Pan J, McEver R P. Regulation of the human P-selectin promoter by Bcl-3 and specific homodimeric members of the NF-κB/Rel family. J Biol Chem. 1995;270:23077–23083. doi: 10.1074/jbc.270.39.23077. [DOI] [PubMed] [Google Scholar]
- 29.Rebollo A, Dumoutier L, Renauld J-C, Zaballos A, Ayllón V, Martinez-A C. Bcl-3 expression promotes cell survival following interleukin-4 deprivation and is controlled by AP1 and AP1-like transcription factors. Mol Cell Biol. 2000;20:3407–3416. doi: 10.1128/mcb.20.10.3407-3416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard M, Louahed J, Demoulin J B, Renauld J C. Interleukin-9 regulates NF-κB activity through BCL3 gene induction. Blood. 1999;93:4318–4327. [PubMed] [Google Scholar]
- 31.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 32.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 33.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 34.Sovak M A, Bellas R E, Kim D W, Zanieski G J, Rogers A E, Traish A M, Sonenshein G E. Aberrant NF-κB/Rel expression and the pathogenesis of breast cancer. J Clin Investig. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Baldwin A S., Jr Activation of NF-κB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 36.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 37.Weinstat-Saslow D, Merino M J, Manrow R E, Lawrence J A, Bluth R F, Wittenbel K D, Simpson J F, Page D L, Steeg P S. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med. 1995;1:1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- 38.Wulczyn F G, Naumann M, Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-κB. Nature. 1992;358:597–599. doi: 10.1038/358597a0. [DOI] [PubMed] [Google Scholar]
- 39.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M Y, Harhaj E W, Bell L, Sun S C, Miller B A. Bcl-3 expression and nuclear translocation are induced by granulocyte-macrophage colony-stimulating factor and erythropoietin in proliferating human erythroid precursors. Blood. 1998;92:1225–1234. [PubMed] [Google Scholar]
- 41.Zhang Q, Didonato J A, Karin M, McKeithan T W. BCL3 encodes a nuclear protein which can alter the subcellular location of NF-κB proteins. Mol Cell Biol. 1994;14:3915–3926. doi: 10.1128/mcb.14.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]