Abstract
Background. Eyes shut homolog (EYS) gene mutations are estimated to affect at least 5% of patients with autosomal recessive retinitis pigmentosa. Since there is no mammalian model of human EYS disease, it is important to investigate its age-related changes and the degree of central retinal impairment. Methods. A cohort of EYS patients was studied. They underwent full ophthalmic examination as well as assessment of retinal function and structure, by full-field and focal electroretinograms (ERGs) and spectral domain optical coherence tomography (OCT), respectively. The disease severity stage was determined by the RP stage scoring system (RP-SSS). Central retina atrophy (CRA) was estimated from the automatically calculated area of the sub-retinal pigment epithelium (RPE) illumination (SRI). Results. The RP-SSS was positively correlated with age, showing an advanced severity score (≥8) at an age of 45 and a disease duration of 15 years. The RP-SSS was positively correlated with the CRA area. LogMAR visual acuity and ellipsoid zone width, but not ERG, were correlated with CRA. Conclusions. In EYS-related disease, the RP-SSS showed advanced severity at a relative early age and was correlated with the central area of the RPE/photoreceptor atrophy. These correlations may be relevant in view of therapeutic interventions aimed at rescuing rods and cones in EYS-retinopathy.
Keywords: retinal degeneration, EYS gene, disease staging, multimodal imaging, OCT, subretinal illumination, electroretinography
1. Introduction
Pathogenic variants in the eyes shut homolog (EYS) gene are estimated to affect at least 5% of patients with autosomal recessive retinitis pigmentosa (arRP) [1]. The gene is defective in several arRP populations worldwide [2,3,4,5]. EYS encodes a large extracellular protein [6] that in Drosophila promotes the formation of epithelial lumina, a selective space inside the rhabdomeres which isolates individual photoreceptor cells and is useful for their development [7]. In humans, the function of EYS is not yet fully understood. However, the protein product is thought to play a role in stabilizing ciliary axonemes in rods and cones and it is involved in the maintenance of photoreceptor cells [8]. Since there is no mammalian model of human EYS disease, it is important to investigate its age-related retinal changes in both structure and function, as well as the degree of central retina involvement.
Recently, Iftikhar et al. [9] developed a simple and easily applicable classification of disease severity in RP patients, with a score based on best corrected visual acuity (BCVA), Goldmann visual field diameter and ellipsoid zone (EZ) width. This RP stage score system (RP-SSS) appeared to be readily applicable to different subtypes of RP. In particular, our group [10] recently applied the RP-SSS to the clinical and morphological features of USH2A related retinal degeneration. The USH2A severity score was reliably correlated with patient age as well as with several morphologic and functional parameters of retinal disease [10].
In a previous study by Mcguigan et al. [11], EYS patients showed typically RP features. Although a residual foveal cone function could still be detected into the fourth, fifth or sixth decade of life, the outer nuclear layer (ONL) thickness and EZ width progressively decreased. Determining the degree of retinal atrophy involving retinal pigment epithelium (RPE)/photoreceptors in the macular region may be particularly relevant either for visual prognosis or for the effects of potential treatments rescuing photoreceptors. An estimate of the area of central macular atrophy (CRA) can be obtained by the automatic determination of the area of sub-RPE illumination (SRI). The SRI identifies bright areas of increased light transmission beneath the RPE, indicating RPE/outer retina atrophy, averaged over a circular area of 5 mm around the fovea by the automated spectral domain optical coherence tomography (SD-OCT) software. In age-related macular degeneration, the SRI was used to measure the area of RPE and outer retinal atrophy (RORA), established from an international consensus by Guymer et al. [12].
The aim of the present study was to determine the disease severity stage in a cohort of ArRP patients carrying pathogenic variants in the EYS gene and to estimate, in these patients, the extent of CRA by using the automated SRI measurements.
2. Materials and Methods
This clinical study was performed in compliance with the ICH Guidelines for Good Clinical Practice, adhered to the tenets of the Declaration of Helsinki (1991) and was approved by the Ethics Committee/Institutional Review Board of the Catholic University of Rome, Italy (protocol #8383/15).
After a detailed explanation regarding the study procedures, written informed consents for clinical and molecular analyses were obtained from all the adult subjects or relatives when the patient was a minor.
All the reported clinical data were retrospectively re-evaluated.
2.1. Subjects
We enrolled 17 patients (8 male, 9 female; mean age: 50.5 years.; SD: ±14.7) affected by RP due to variants in the EYS gene and followed at the Center for Inherited Retinal Degenerations of Fondazione Policlinico A. Gemelli, IRCCS. All patients were evaluated between November 2013 and December 2021 and met the following inclusion criteria: (1) clinical and genetic diagnosis of EYS-related RP; (2) good cooperation in psychophysical testing; (3) dioptric media clean enough to perform Spectral Domain-Optical Coherence Tomography (SD-OCT). Exclusion criteria were the presence of: (1) concomitant ocular (e.g., amblyopia, glaucoma) and systemic diseases; (2) poor cooperation in psychophysical testing; (3) severe ocular media opacities. EYS-related RP diagnosis was confirmed through a genetic test and, whenever possible, a segregation analysis on available family members was performed. Seven patients were homozygous and the remaining eight were compound heterozygous for EYS variants. Molecular genetic data are reported in Table 1. The pathogenic variants in the EYS gene were detected using the Next Generation Sequencing (NGS) technology. NGS was performed on a MiSeq personal sequencer (Illumina, San Diego, CA), following the molecular and bioinformatic strategy that we previously published [13,14]. Multiplex ligation-dependent probe amplification (MLPA) (www.mrc-holland.com, (accessed on 1 December 2017) was also performed in one patient using the Beckman Coulter CEQ 8000 sequencer, and revealed the presence of a deletion in heterozygosity (c. (2135 _2204) _ (2351_2469) del) resulting in the loss of the exons 14 and 15. All variants identified were evaluated according to American College of Medical Genetics and Genomics (ACMG) guidelines [15] with the help of the VarSome online tool (https://varsome.com/, (accessed on 24 May 2022) [16]. Molecular genetic data for each patient are detailed in Table 1.
2.2. Clinical Assessment and Functional Evaluation
All patients underwent a full ophthalmologic examination including detailed family history, anterior segment biomicroscopy, intraocular pressure measurement, BCVA measured with ETDRS charts, Goldmann visual field using the V/4e target, SD-OCT with measurement of the EZ extension, scotopic and photopic full-field electroretinogram (ERG) recordings and direct and indirect ophthalmoscopy. All patients had a typical RP phenotype. The data collected allowed the determination of the disease severity stage of all enrolled patients according to the cumulative score (CS) and grade indicated by Iftikhar et al [9]. Sub-groups of patients underwent a more detailed comprehensive electro-functional study. A total of 12 out of 17 patients performed 30 Hz submicrovolt flicker ERG, (Retimax Advanced Plus, CSO, Scandicci, Italy)) with the assessment of response variability and signal-to-noise ratio (S/N), as already described in a previous article by Falsini et al. [10].
Macular cone-mediated focal ERG (FERG) was recorded in 7 out of 17 patients using a published technique [17,18,19,20]. Briefly, FERGs were recorded monocularly in response to a flickering uniform red field stimulus superimposed on an equiluminant steady adapting background. Off-line discrete Fourier analysis quantified the peak-to-peak amplitude of the response first harmonic at 41 Hz.
2.3. Morphological Analysis Using Retinal Advanced Multimodal Imaging
All patients underwent advanced retinal analysis by SD-OCT using Zeiss Cirrus 5000-HD-OCT Angioplex, sw version 10.0, (CarlZeiss, Meditec, Inc., Dublin, CA, USA). In two patients, the advanced imaging was not possible due to unstable fixation. A High-Definition 5-Line Raster and a macular map (6 × 6 mm Macular Cube 512 × 128) were acquired. In order to obtain the most reliable measurements, two independent operators (M.C.S. and M.M.) measured the residual EZ extension on OCT horizontal scans using a caliper, as previously described [21]. The agreement rate between the two independent experts was equal to 89% (95% confidence interval = 79–98%).
The EZ extension was determined by the retinal points where the temporal and nasal EZ borders met the RPE becoming indistinguishable.
The Advanced RPE post-processing analysis was used to automatically determine areas of sub-RPE illumination (SRI, measured in mm2) for increased light penetration through atrophic OR, RPE and choriocapillaris, by means of the sub-RPE algorithm. An automated SD-OCT software allowed detection of RPE atrophy in a 5 mm circular area around the fovea. The SRI was used to measure the RPE and outer retinal atrophy (RORA), as established in an international consensus by Guymer et al. [12].
RORA corresponds to a region of signal hyper-transmission into the choroid resulting from the interruption of the RPE and OR and can be classified as complete and incomplete. If the signal hyper-transmission into the choroid does not exceed an area of 250 microns, RORA is defined as incomplete (iRORA). RORA is complete (cRORA) when this value is higher and corresponds to geographic atrophy (GA).
2.4. Statistical Analysis
We analyzed both right and left eyes. In this study, we considered only the results from the right eyes for statistical analysis in order not to overestimate the statistical significance and p-values. Analysis from right and left eyes showed substantially similar results.
The data were analyzed by parametric or non-parametric analyses, depending on their distribution. Both Pearson’s correlation and Spearman rank order correlation were used. ERG data were log transformed to better approximate normal distribution. A p value of less than 0.05 was considered statistically significant.
Table 1.
Molecular Genetic Data of EYS patients.
| ID | Sex | Nucleotide Change | Amino Acid Change | Allele State | Varsome | ACMG Criteria | dbSNP rs | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | c.8411_8412insTT | p.(Thr2805*) | HOM | LP | PVS1 | PM2 | NA | [11] | ||
| 2 | F | c.8598del | p.(Gly2867Valfs*5) | HOM | P | PVS1 | PM2 | PP5 | rs1050742628 | NA | |
| 3 | M | c.8161_8165del | p.(Gln2721Alafs*24) | HET | LP | PVS1 | PM2 | NA | NA | ||
| c.9405T>A | p.(Tyr3135*) | HET | P | PVS1 | PM2 | PP5 | rs137853190 | [6] | |||
| 4 | M | c.5928-2A>G | HOM | P | PVS1 | PM2 | PP5 | rs181169439 | [2] | ||
| 5 | M | c.5621dup | p.(Pro1875Thrfs*8) | HET | LP | PVS1 | PM2 | NA | [22] | ||
| c.8411_8412insTT | p.(Thr2805*) | HET | LP | PVS1 | PM2 | NA | [11] | ||||
| 6 | M | c.8565_8568del | p.(Asn2855Lysfs*5) | HET | P | PVS1 | PM2 | PP5 | rs1216993077 | NA | |
| c.4073del | p.(Pro1358Glnfs*23) | HET | LP | PVS1 | PM2 | NA | NA | ||||
| 7 | F | c.5644+5G>T | HOM | LP | PM2 | PP3 | NA | NA | |||
| 8 | F | c.4045C>T | p.(Arg1349*) | HET | P | PVS1 | PM2 | PP5 | rs930421180 | [2] | |
| c.4350_4356del | p.(Ile1451Profs*3) | HET | PVS1 | PM2 | PP5 | rs761238771 | [2] | ||||
| 9 | F | c.7919G>A | p.(Trp2640*) | HOM | P | PVS1 | PM2 | PP5 | rs527236066 | [2] | |
| 10 | F | c.403_423delinsCTTTT | p.(Thr135Leufs*26) | HET | P | PVS1 | PM2 | PP5 | rs1582376398 | [23] | |
| c.(2135_2204)_(2351_2469)del | HET | LP | PVS1 | PM2 | NA | NA | |||||
| 11 | F | c.(2137+1_2138-1)_(2259+1_2260-1)del | HOM | LP | PVS1 | PM2 | NA | [24] | |||
| 12 | M | c.4045C>T | p.(Arg1349*) | HET | P | PVS1 | PM2 | PP5 | rs930421180 | [25] | |
| c.9299_9302del | p.(Thr3100Lysfs*26) | HET | P | PVS1 | PM2 | PP5 | rs769824975 | [26] | |||
| 13 | F | c.9328G>A | p.(Gly3110Ser) | HOM | VUS | PM2 | PM5 | PP3 | NA | NA | |
| 14 | c.5621dup | p.(Pro1875Thrfs*8) | HET | LP | PVS1 | PM2 | NA | [22] | |||
| c.8411_8412insTT | p.(Thr2805*) | HET | LP | PVS1 | PM2 | NA | [11] | ||||
| 15 | M | c.5928-2A>G | HOM | P | PVS1 | PM2 | PP5 | rs181169439 | [2] | ||
| 16 | F | c.4219C>T | p.(Gln1407*) | HET | LP | PVS1 | PM2 | rs1421392730 | NA | ||
| del ex32-35 | HET | LP | PVS1 | PM2 | NA | NA | |||||
| 17 | M | c.1852G>A | p.(Gly618Ser) | HET | VUS | PM2 | PP5 | BP4 | rs142450703 | [2] | |
| c.1561_1563del | p.(Asn521del) | HET | VUS | PM2 | PM4 | PP5 | rs747069281 | NA | |||
| c.2309A>C | p.(Gln770Pro) | HET | VUS | PM2 | BP4 | rs398123574 | [27] | ||||
Legend: F, female; M, male; HET, heterozygous; HOM, homozygous; in italics, in cis variants; NA, not available; VUS, variant of unknown significance; LP, likely pathogenic; P, pathogenic.
3. Results
Clinical results of individual patients are reported in Table 2. The results of RP-SSS, ERGs and iRORA area are reported in Supplementary Material (Table S1).
Table 2.
Demographic and clinical data of studied patients.
| Nr | Sex | Onset | Age of Assessment |
Disease Duration |
RE | LE | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCVA (LogMAR) |
VF | EZ | BCVA (LogMAR) |
VF | EZ | |||||
| 1 | M | 25 | 46 | 21 | −0.50 | 21 | 0 | −1.0 | 25 | 0 |
| 2 | F | 42 | 75 | 33 | −2.70 | 0 | 0 | −0.50 | 10 | 0 |
| 3 | M | 39 | 43 | 4 | 0.00 | 124 | 5251 | −0.10 | 131 | 5053 |
| 4 | M | 18 | 42 | 24 | −3.70 | 0 | 0 | −3.70 | 0 | 0 |
| 5 | M | 17 | 30 | 13 | −0.50 | 50 | 3321 | −0.18 | 43 | 641 |
| 6 | M | 22 | 26 | 4 | 0.00 | 129 | 3576 | 0.00 | 133 | 3525 |
| 7 | F | 20 | 62 | 42 | −0.92 | 17 | 2084 | −0.80 | 18 | 1337 |
| 8 | F | 14 | 26 | 12 | 0.00 | 79 | 2197 | 0.00 | 80 | 2577 |
| 9 | F | 25 | 49 | 24 | −0.70 | 120 | 2085 | −0.50 | 107 | 1556 |
| 10 | F | 25 | 46 | 21 | −0.30 | 62 | 1549 | −0.30 | 60 | 1581 |
| 11 | F | 43 | 68 | 25 | −2.70 | 17 | 0 | −2.70 | 0 | 0 |
| 12 | M | 39 | 49 | 10 | −0.50 | 30 | 4075 | −0.40 | 16 | 4313 |
| 13 | F | 30 | 65 | 35 | −0.70 | 18 | 0 | −0.50 | 16 | 0 |
| 14 | F | 30 | 51 | 21 | −0.10 | 22 | 2018 | 0.00 | 25 | 2448 |
| 15 | M | 14 | 69 | 55 | −3.70 | 0 | 0 | −3.70 | 0 | N/A |
| 16 | F | 26 | 51 | 25 | −0.30 | 19 | 0 | −0.18 | 20 | 3395 |
| 17 | M | 45 | 61 | 16 | −2.70 | 0 | 0 | −2.70 | 0 | 0 |
Legend: F, female; M, male; RE, right eye; LE, left eye; BCVA, best corrected visual acuity; VF, visual field; EZ, ellipsoid zone.
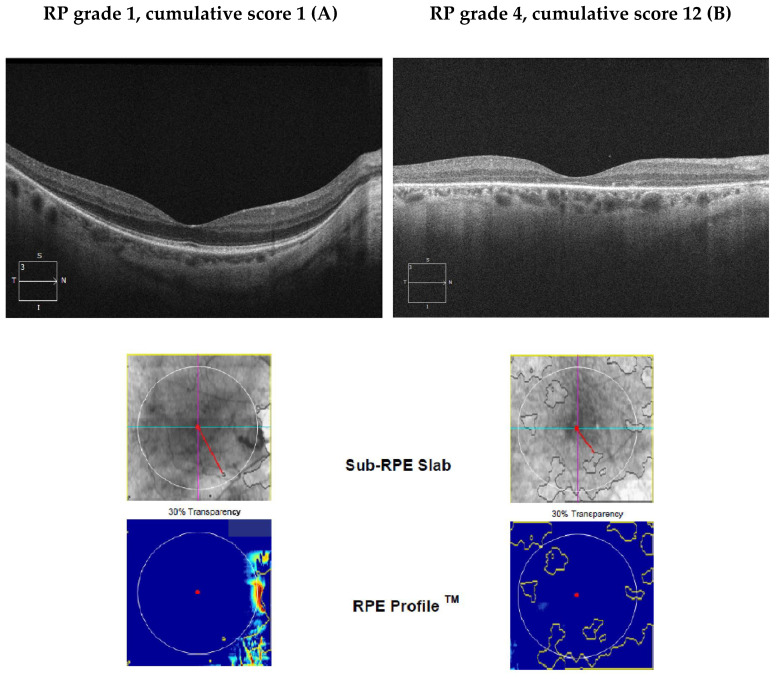
Figure 1 shows typical macular appearance in an EYS patient with a mild (Pt #3) and an advanced RP-SSS (Pt #1) according to the staging classification by Iftkhar [9]. In the advanced patient EZ is not detectable and ONL thickness is markedly reduced in comparison to the patient with an early stage of the disease. Figure 1 shows the SRI analysis in the macula obtained from the moderate (A) and the advanced (B) EYS patient. SRI area increases with disease severity.
Figure 1.
Images OCT B scan, sub-RPE slab and RPE profile of two patients with early stage RP (A) and advanced stage RP (B). In early stage RP (A), the ellipsoid zone is preserved in the foveal and parafoveal regions. In advanced RP (B) the almost complete loss of the ellipsoid zone and marked reduction in the outer nuclear layer are observable.
Patient A has a RP-SSS of 1 and 12 corresponding to grade 1 and 4, respectively.
This picture is associated with changes in the sub-RPE slab (areas of increased SRI) within the 5 mm circle outlined in white. The red line shows the atrophic area closest to the fovea.
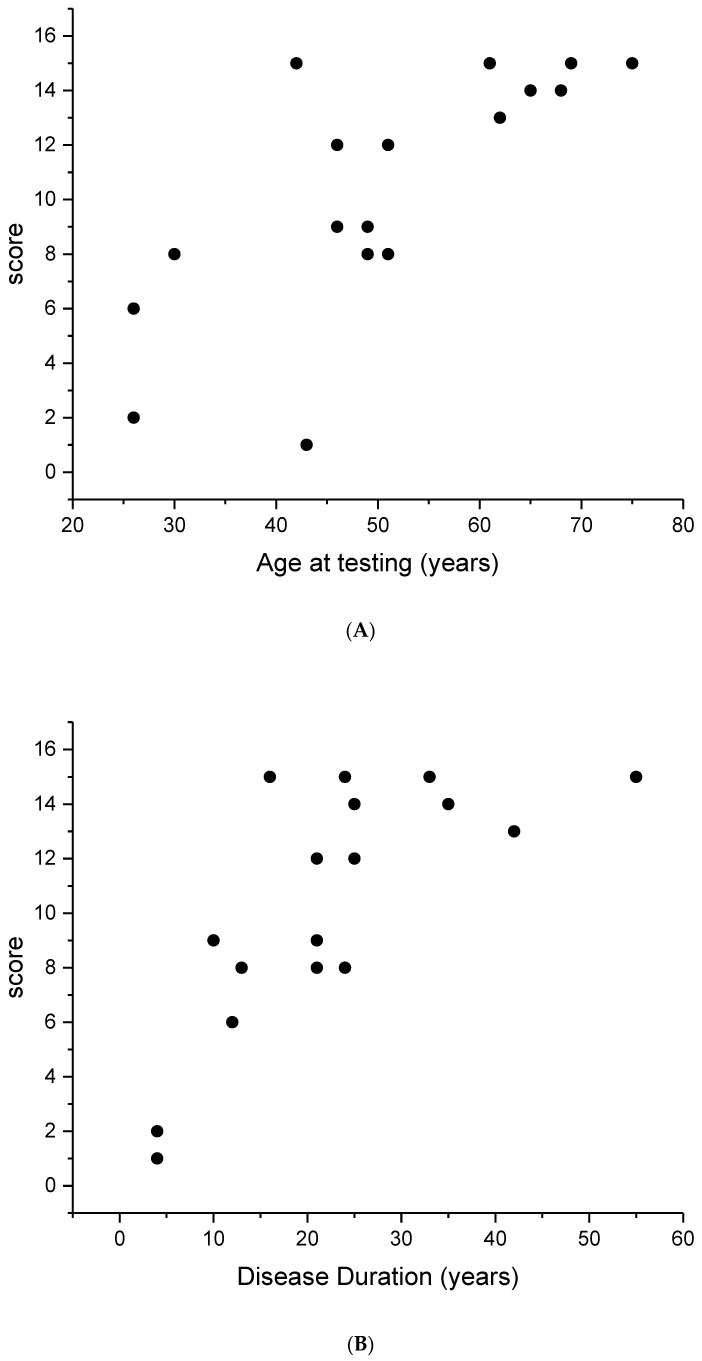
The staging score was significantly correlated with age and disease duration (r = 0.54, p < 0.01) as shown in Figure 2A,B. An age of 45 years and a disease duration of 15 years corresponded to the appearance of an advanced RP score (≥8). The data showed a trend to saturate after the age of 50, indicating a ceiling effect.
Figure 2.
Cumulative score (CS) from the right eye of each EYS patient plotted as a function of age at testing (A) and disease duration (B). It can be noted that the score increases linearly with both parameters. The r value is 0.54 (p < 0.01).
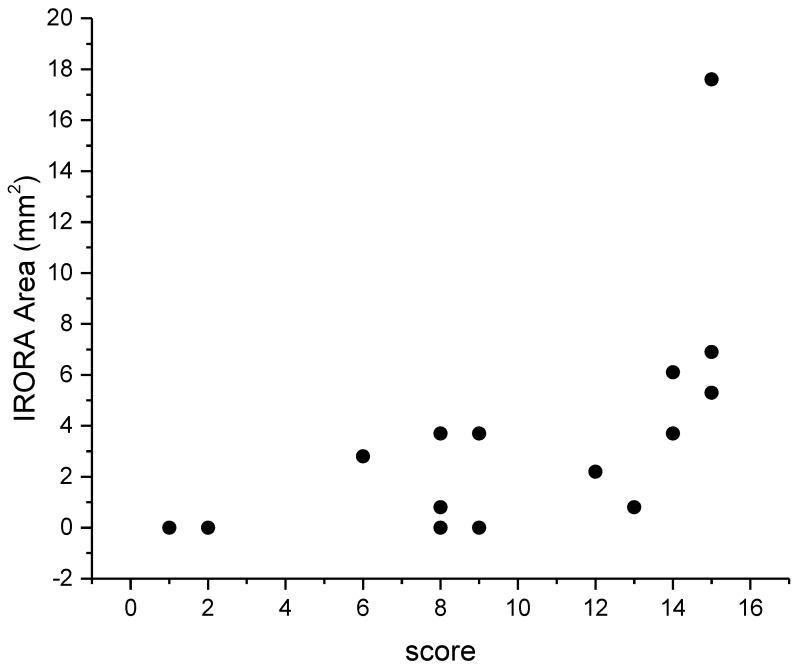
Figure 3 shows a scattergram of SRI area as a function of staging score. It can be noted that SRI area, and consequently the CRA severity, was positively correlated with severity score (r = 0.5, p < 0.05).
Figure 3.
Results of correlation analysis between SRI and CS. SRI showed a positive correlation with CS. The r value is 0.5 (p < 0.05).
SRI did not show any significant correlation with 30 Hz Flicker or focal ERG amplitude.
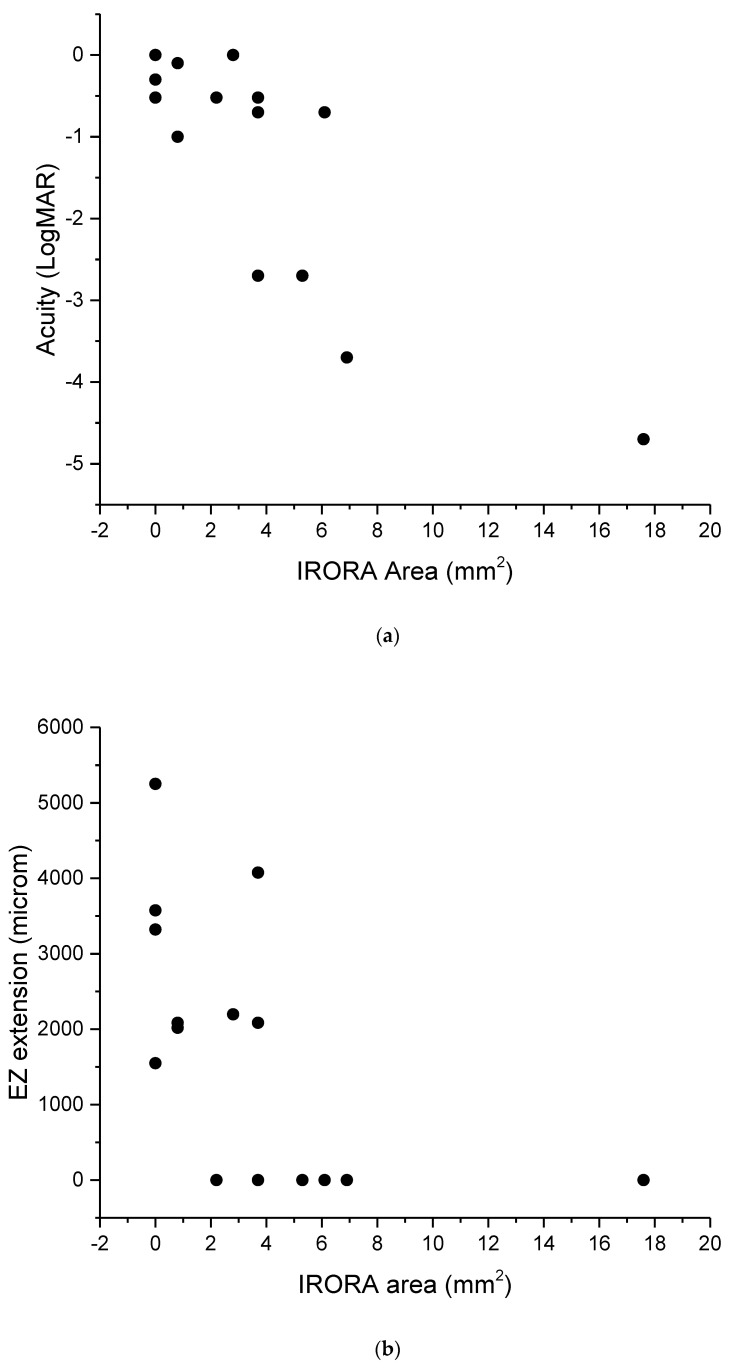
Figure 4A,B shows scattergrams of SRI area as a function of LogMAR acuity and EZ extension. SRI area was significantly correlated with both measurements (r = 0.5, p < 0.01).
Figure 4.
Correlation analysis between SRI and BCVA (a) and EZ extension (b). SRI area gradually increases as visual acuity and/or ellipsoid zone extension decreases.
4. Discussion
The present study was designed to evaluate the severity stage of EYS disease patients, as determined by the RP-SSS [9], and to estimate the area of CRA (from a standardized method measuring the area of SRI in the macula) in relation to disease stage in the same patients. The results of our study showed a correlation between the stage of RP and the age and disease duration of patients. Such correlation revealed a severe stage at a relatively early age of 45, supporting the severity of the molecular pathology underlying this sub-type of RP.
Previous scientific investigations concerning structure and function correlations in patients with biallelic mutations in the EYS gene support a rapid disease progression as a function of time. McGuigan et al. [11] evaluated a cohort of 15 patients by chromatic static perimetry, SD-OCT, and enface autofluorescence imaging reporting anomalies in the outer nuclear retinal layer of the central retina with some exceptions in the foveal region. Furthermore, an analysis of the perifoveal region as a function of time showed that photoreceptor structural loss was followed by dysmorphology of the inner retina and loss of retinal pigment epithelial integrity. Based on their results, arRP caused by EYS mutation was considered a more rapidly progressive disease compared to other gene mutations causing arRP, such as the USH2A and MAK variants. Some authors suggested that a different phenotype and progression in EYS-related retinal degeneration could be related to the specific EYS genotype [26,28,29,30,31]. However, due to the limited number of patients examined, such correlations could not be verified and confirmed in the present study.
An important finding of this study is represented by the correlation of CRA area with RP-SSS. The increase in CRA area was positively correlated with a greater RP-SSS value, indicating an increase in the disease severity. In addition, it was correlated inversely with the EZ width and positively with LogMAR acuity. Although other morphological predictor factors of atrophy progression in OCT analysis have been reported [32], RORA is the result of multiple information on the status of EZ, outer segments of the photoreceptors, interdigitation zone and RPE-Bruch’s membrane complex [33,34,35]. Recently, RORA assessment has been considered not only for early diagnosis but also as a prognostic factor of AMD progression [21]. Indeed, the possibility of evaluating the progression of central atrophy growth could become a key element in the evolutionary study of RP. Currently, the detection of RORA finds an important application in eyes with age-related macular degeneration (AMD) [36]. Automated identification of RORA using machine learning has also been described [37]. Extending the present approach to inherited retinal diseases would be important for predicting their natural history. Indeed, we already demonstrated in other studies how the SRI area, as a result of RORA, was correlated with the disease severity stage in patients with USH2A-related Retinitis Pigmentosa [10].
Although EYS-related RP represents a rare disease, our sample did not include a high number of patients. This constitutes a limitation to our study. Further comparative studies using a similar methodology would be desirable in order to identify useful diagnostic and prognostic criteria to study the natural history of the EYS-related disease, which is currently still difficult to predict.
In our study population, no correlations were found between the stage of the disease and the ERG, both focal and 30 Hz flicker ERG. Although in most cases the degenerative evolution of the disease proceeds from rod to cone photoreceptors and the cone loss is considered a secondary occurrence, the obtained ERG data show that a severe cone dysfunction may be present early in EYS patients. Similar to what is observed in patients with USH2A mutations [20], our study patients showed severely abnormal 30 Hz flicker ERG and fERG, well in advance of other cone-related visual tests, such as visual acuity. This finding supports an early involvement of cone driven responses in EYS-related RP. This hypothesis is in agreement with McGuigan et al. [11] who reported the constant presence of anomalies in the outer nuclear retinal layer of the central retina in a cohort of patients with EYS mutations, except in some where only the foveal region appeared still preserved. It is important to specify that, although the number of patients who underwent a FERG in this study was 7 out of 15, all patients had abnormal ERG responses. Additionally, 30 Hz Flicker ERG showed markedly reduced responses in all patients and was unrelated to other parameters. This latter finding indicates that in our patients a severe extrafoveal cone dysfunction was present, too.
Although no treatments are available for EYS-related RP, many novel therapeutic approaches could represent potential therapeutic options in the future [38]. In view of the above, the correct identification of useful outcome measures to evaluate the effectiveness of potential therapies in upcoming clinical trials is a goal to be achieved. Studies in this regard are still few and more will have to be conducted to strengthen our hypothesis.
The data reported in this study qualifies the SRI in the detection of iRORA as an important parameter concerning the rate of progression of retinal degeneration caused by biallelic mutations in the EYS gene.
5. Conclusions
This research has provided new information concerning patients affected by Retinitis Pigmentosa associated with EYS gene mutations. We used a known RP severity score and validated techniques for the detection of morpho-functional correlations. In particular, in EYS-related RP the severity score was positively correlated with the central retinal atrophy, the central visual acuity and the EZ extension and increases with age and disease duration. In contrast, due to the intrinsic characteristics of the disease, functional measures did not appear sensitive enough to reveal staging-based changes. Given the small size of our sample, further studies concerning the natural history of this kind of retinal dystrophy would be desirable.
Acknowledgments
Supported by an unrestricted grant from Retina Italia ODV to BF Supported by ERN-eye (BF and SR). The contribution of Fondazione Bietti in this paper was supported by the Ministry of Health and Fondazione Roma.
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| AMD | Age-related macular degeneration |
| arRP | Autosomal recessive retinitis pigmentosa |
| BCVA | Best corrected visual acuity |
| CRA | Central retina atrophy |
| cRORA | Complete RORA |
| CS | Cumulative score |
| ERG | Electroretinogram |
| EYS | Eyes shut homolog |
| EZ | Ellipsoid zone |
| F | Female |
| FERG | Focal ERG |
| GA | Geographic atrophy |
| HET | Heterozygous |
| HOM | Homozygous |
| iRORA | Incomplete RORA |
| LE | Left eye |
| LogMAR | Logarithm of minimum angle of resolution |
| LP | Likely pathogenic |
| M | Male |
| MLPA | Multiplex ligation-dependent probe amplification |
| NA | Not available |
| NGS | Next Generation Sequencing |
| OCT | Optical coherence tomography |
| ONL | Outer nuclear layer |
| P | Pathogenic |
| RE | Right eye |
| RORA | RPE and outer retinal atrophy |
| RPE | Retinal pigment epithelium |
| RP-SSS | RP stage scoring system |
| SD-OCT | Spectral domain optical coherence tomography |
| SRI | Sub-retinal pigment epithelium illumination |
| VF | Visual Field |
| VUS | Variant of unknown significance |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13050850/s1, Table S1: Results of RP-SSS, ERGs and iRORA area of individual patients.
Author Contributions
Conceptualization, B.F., G.P., S.R. and M.C.S.; methodology, B.F., P.C., M.B. and P.E.M.; software, P.E.M., B.F. and G.P.; formal analysis, B.F. and M.C.S.; investigation, B.F., G.P., E.D., V.C., A.M.M. and M.M.; data curation, G.P., E.D. and V.C.; writing—original draft preparation, G.P., M.C.S. and B.F.; writing—review and editing, B.F., G.P., M.C.S., P.E.M., A.M.M., M.M., L.Z. and V.P.; supervision, S.R. and P.C.; project administration, B.F., S.R. and G.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Ethics Committee/Institutional Review Board of the Catholic University of Rome, Italy (protocol #8383/15). This research adhered to the tenets of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all patients, after full and detailed explanation of the goals and procedures of the study.
Data Availability Statement
Data available from authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
Unrestricted grant for Retinitis Pigmentosa studies from Retina Italia ODV.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Littink K.W., van den Born L.I., Koenekoop R.K., Collin R.W.J., Zonneveld M.N., Blokland E.A.W., Khan H., Theelen T., Hoyng C.B., Cremers F.P.M., et al. Mutations in the EYS Gene Account for Approximately 5% of Autosomal Recessive Retinitis Pigmentosa and Cause a Fairly Homogeneous Phenotype. Ophthalmology. 2010;117:2026–2033.e7. doi: 10.1016/j.ophtha.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Audo I., Sahel J.-A., Mohand-Saïd S., Lancelot M.-E., Antonio A., Moskova-Doumanova V., Nandrot E.F., Doumanov J., Barragan I., Antinolo G., et al. EYS Is a Major Gene for Rod-Cone Dystrophies in France. Hum. Mutat. 2010;31:E1406–E1435. doi: 10.1002/humu.21249. [DOI] [PubMed] [Google Scholar]
- 3.Numa S., Oishi A., Higasa K., Oishi M., Miyata M., Hasegawa T., Ikeda H.O., Otsuka Y., Matsuda F., Tsujikawa A. EYS Is a Major Gene Involved in Retinitis Pigmentosa in Japan: Genetic Landscapes Revealed by Stepwise Genetic Screening. Sci. Rep. 2020;10:20770. doi: 10.1038/s41598-020-77558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai Y., Maeda A., Hirami Y., Ishigami C., Kosugi S., Mandai M., Kurimoto Y., Takahashi M. Retinitis Pigmentosa with EYS Mutations Is the Most Prevalent Inherited Retinal Dystrophy in Japanese Populations. J. Ophthalmol. 2015;2015:1–10. doi: 10.1155/2015/819760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y.N., Kim Y.J., Seol C.A., Seo E.-J., Lee J.Y., Yoon Y.H. Genetic Profile and Associated Characteristics of 150 Korean Patients with Retinitis Pigmentosa. J. Ophthalmol. 2021;2021:1–15. doi: 10.1155/2021/5067271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin R.W.J., Littink K.W., Klevering B.J., van den Born L.I., Koenekoop R.K., Zonneveld M.N., Blokland E.A.W., Strom T.M., Hoyng C.B., den Hollander A.I., et al. Identification of a 2 Mb Human Ortholog of Drosophila Eyes Shut/Spacemaker That Is Mutated in Patients with Retinitis Pigmentosa. Am. J. Hum. Genet. 2008;83:594–603. doi: 10.1016/j.ajhg.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain N., Pellikka M., Hong H., Klimentova T., Choe K.-M., Clandinin T.R., Tepass U. The Agrin/Perlecan-Related Protein Eyes Shut Is Essential for Epithelial Lumen Formation in the Drosophila Retina. Dev. Cell. 2006;11:483–493. doi: 10.1016/j.devcel.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Alfano G., Kruczek P.M., Shah A.Z., Kramarz B., Jeffery G., Zelhof A.C., Bhattacharya S.S. EYS Is a Protein Associated with the Ciliary Axoneme in Rods and Cones. PLoS ONE. 2016;11:e0166397. doi: 10.1371/journal.pone.0166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iftikhar M., Lemus M., Usmani B., Campochiaro P.A., Sahel J.A., Scholl H.P.N., Shah S.M.A. Classification of Disease Severity in Retinitis Pigmentosa. Br. J. Ophthalmol. 2019;103:1595–1599. doi: 10.1136/bjophthalmol-2018-313669. [DOI] [PubMed] [Google Scholar]
- 10.Falsini B., Placidi G., De Siena E., Savastano M.C., Minnella A.M., Maceroni M., Midena G., Ziccardi L., Parisi V., Bertelli M., et al. USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies. Diagnostics. 2021;11:213. doi: 10.3390/diagnostics11020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuigan D., Heon E., Cideciyan A., Ratnapriya R., Lu M., Sumaroka A., Roman A., Batmanabane V., Garafalo A., Stone E., et al. EYS Mutations Causing Autosomal Recessive Retinitis Pigmentosa: Changes of Retinal Structure and Function with Disease Progression. Genes. 2017;8:178. doi: 10.3390/genes8070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guymer R.H., Rosenfeld P.J., Curcio C.A., Holz F.G., Staurenghi G., Freund K.B., Schmitz-Valckenberg S., Sparrow J., Spaide R.F., Tufail A., et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration. Ophthalmology. 2020;127:394–409. doi: 10.1016/j.ophtha.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltese P.E., Orlova N., Krasikova E., Emelyanchik E., Cheremisina A., Kuscaeva A., Salmina A., Miotto R., Bonizzato A., Guerri G., et al. Gene-Targeted Analysis of Clinically Diagnosed Long QT Russian Families. Int. Heart J. 2017;58:81–87. doi: 10.1536/ihj.16-133. [DOI] [PubMed] [Google Scholar]
- 14.Marceddu G., Dallavilla T., Guerri G., Manara E., Chiurazzi P., Bertelli M. PipeMAGI: An Integrated and Validated Workflow for Analysis of NGS Data for Clinical Diagnostics. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6753–6765. doi: 10.26355/eurrev_201908_18566. [DOI] [PubMed] [Google Scholar]
- 15.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopanos C., Tsiolkas V., Kouris A., Chapple C.E., Albarca Aguilera M., Meyer R., Massouras A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics. 2019;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli-Resta L., Piccardi M., Ziccardi L., Fadda A., Minnella A., Marangoni D., Placidi G., Resta G., Falsini B. Early Detection of Central Visual Function Decline in Cone–Rod Dystrophy by the Use of Macular Focal Cone Electroretinogram. Invest. Ophthalmol. Vis. Sci. 2013;54:6560. doi: 10.1167/iovs.13-12676. [DOI] [PubMed] [Google Scholar]
- 18.Galli-Resta L., Falsini B., Rossi G., Piccardi M., Ziccardi L., Fadda A., Minnella A., Marangoni D., Placidi G., Campagna F., et al. Bilateral Symmetry of Visual Function Loss in Cone–Rod Dystrophies. Invest. Ophthalmol. Vis. Sci. 2016;57:3759. doi: 10.1167/iovs.15-18313. [DOI] [PubMed] [Google Scholar]
- 19.Abed E., Placidi G., Calandriello L., Piccardi M., Campagna F., Bertelli M., Minnella A.M., Savastano M.C., Falsini B. Correlation of Macular Focal Electroretinogram with Ellipsoid Zone Extension in Stargardt Disease. J. Ophthalmol. 2017;2017:1–7. doi: 10.1155/2017/3643495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli-Resta L., Placidi G., Campagna F., Ziccardi L., Piccardi M., Minnella A., Abed E., Iovine S., Maltese P., Bertelli M., et al. Central Retina Functional Damage in Usher Syndrome Type 2: 22 Years of Focal Macular ERG Analysis in a Patient Population from Central and Southern Italy. Invest. Ophthalmol. Vis. Sci. 2018;59:3827. doi: 10.1167/iovs.17-23703. [DOI] [PubMed] [Google Scholar]
- 21.Savastano M.C., Falsini B., Cozzupoli G.M., Savastano A., Gambini G., De Vico U., Minnella A.M., Placidi G., Piccardi M., Rizzo S. Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Correlation with Macular Function. JCM. 2020;9:2973. doi: 10.3390/jcm9092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunghi C., Galli-Resta L., Binda P., Cicchini G.M., Placidi G., Falsini B., Morrone M.C. Visual Cortical Plasticity in Retinitis Pigmentosa. Invest. Ophthalmol. Vis. Sci. 2019;60:2753. doi: 10.1167/iovs.18-25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandah-Rozenfeld D., Littink K.W., Ben-Yosef T., Strom T.M., Chowers I., Collin R.W.J., den Hollander A.I., van den Born L.I., Zonneveld M.N., Merin S., et al. Novel Null Mutations in the EYS Gene Are a Frequent Cause of Autosomal Recessive Retinitis Pigmentosa in the Israeli Population. Invest. Ophthalmol. Vis. Sci. 2010;51:4387. doi: 10.1167/iovs.09-4732. [DOI] [PubMed] [Google Scholar]
- 24.Jespersgaard C., Fang M., Bertelsen M., Dang X., Jensen H., Chen Y., Bech N., Dai L., Rosenberg T., Zhang J., et al. Molecular Genetic Analysis Using Targeted NGS Analysis of 677 Individuals with Retinal Dystrophy. Sci. Rep. 2019;9:1219. doi: 10.1038/s41598-018-38007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberger T., Neuhaus C., Khan A.O., Decker C., Preising M.N., Friedburg C., Bieg A., Gliem M., Issa P.C., Holz F.G., et al. Increasing the Yield in Targeted Next-Generation Sequencing by Implicating CNV Analysis, Non-Coding Exons and the Overall Variant Load: The Example of Retinal Dystrophies. PLoS ONE. 2013;8:e78496. doi: 10.1371/journal.pone.0078496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengillo J.D., Lee W., Nagasaki T., Schuerch K., Yannuzzi L.A., Freund K.B., Sparrow J.R., Allikmets R., Tsang S.H. A Distinct Phenotype of Eyes Shut Homolog (EYS)-Retinitis Pigmentosa Is Associated with Variants Near the C-Terminus. Am. J. Ophthalmol. 2018;190:99–112. doi: 10.1016/j.ajo.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neveling K., Collin R.W.J., Gilissen C., van Huet R.A.C., Visser L., Kwint M.P., Gijsen S.J., Zonneveld M.N., Wieskamp N., de Ligt J., et al. Next-generation Genetic Testing for Retinitis Pigmentosa. Hum. Mutat. 2012;33:963–972. doi: 10.1002/humu.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo J.-E., Cheng C.-Y., Yang C.-H., Yang C.-M., Chen Y.-C., Huang Y.-S., Chen P.-L., Chen T.-C. Genotypes Influence Clinical Progression in EYS -Associated Retinitis Pigmentosa. Trans. Vis. Sci. Tech. 2022;11:6. doi: 10.1167/tvst.11.7.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katagiri S., Akahori M., Hayashi T., Yoshitake K., Gekka T., Ikeo K., Tsuneoka H., Iwata T. Autosomal Recessive Cone–Rod Dystrophy Associated with Compound Heterozygous Mutations in the EYS Gene. Doc. Ophthalmol. 2014;128:211–217. doi: 10.1007/s10633-014-9435-0. [DOI] [PubMed] [Google Scholar]
- 30.Littink K.W., Koenekoop R.K., van den Born L.I., Collin R.W.J., Moruz L., Veltman J.A., Roosing S., Zonneveld M.N., Omar A., Darvish M., et al. Homozygosity Mapping in Patients with Cone–Rod Dystrophy: Novel Mutations and Clinical Characterizations. Invest. Ophthalmol. Vis. Sci. 2010;51:5943. doi: 10.1167/iovs.10-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suvannaboon R., Pawestri A.R., Jinda W., Tuekprakhon A., Trinavarat A., Atchaneeyasakul L. Genotypic and Phenotypic Profiles of EYS Gene-Related Retinitis Pigmentosa: A Retrospective Study. Sci. Rep. 2022;12:21494. doi: 10.1038/s41598-022-26017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majander A., Sankila E., Falck A., Vasara L.K., Seitsonen S., Kulmala M., Haavisto A., Avela K., Turunen J.A. Natural History and Biomarkers of Retinal Dystrophy Caused by the Biallelic TULP1 Variant c.148delG. Acta Ophthalmol. 2022:aos.15252. doi: 10.1111/aos.15252. [DOI] [PubMed] [Google Scholar]
- 33.Pfau M., von der Emde L., de Sisternes L., Hallak J.A., Leng T., Schmitz-Valckenberg S., Holz F.G., Fleckenstein M., Rubin D.L. Progression of Photoreceptor Degeneration in Geographic Atrophy Secondary to Age-Related Macular Degeneration. JAMA Ophthalmol. 2020;138:1026. doi: 10.1001/jamaophthalmol.2020.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleckenstein M., Mitchell P., Freund K.B., Sadda S., Holz F.G., Brittain C., Henry E.C., Ferrara D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125:369–390. doi: 10.1016/j.ophtha.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Erfurth U., Waldstein S.M., Klimscha S., Sadeghipour A., Hu X., Gerendas B.S., Osborne A., Bogunovic H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Invest. Ophthalmol. Vis. Sci. 2018;59:3199. doi: 10.1167/iovs.18-24106. [DOI] [PubMed] [Google Scholar]
- 36.Gigon A., Mosinska A., Montesel A., Derradji Y., Apostolopoulos S., Ciller C., De Zanet S., Mantel I. Personalized Atrophy Risk Mapping in Age-Related Macular Degeneration. Trans. Vis. Sci. Tech. 2021;10:18. doi: 10.1167/tvst.10.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang J.N., Corradetti G., Nittala M.G., Corvi F., Rakocz N., Rudas A., Durmus B., An U., Sankararaman S., Chiu A., et al. Automated Identification of Incomplete and Complete Retinal Epithelial Pigment and Outer Retinal Atrophy Using Machine Learning. Ophthalmol. Retin. 2022;7:S246865302200392X. doi: 10.1016/j.oret.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Delgado A.B., Valdes-Sanchez L., Morillo-Sanchez M.J., Ponte-Zuñiga B., Diaz-Corrales F.J., de la Cerda B. Dissecting the Role of EYS in Retinal Degeneration: Clinical and Molecular Aspects and Its Implications for Future Therapy. Orphanet. J. Rare Dis. 2021;16:222. doi: 10.1186/s13023-021-01843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from authors.