Abstract
Colorectal cancer (CRC) burden across the world is expected to increase by ~2.2 million new cases and ~1.1 million deaths by 2030. Regular physical exercise is recommended to prevent CRC, but the myriad of protocols preclude further discussion on how to manage its variables for this population. Home-based exercise guided by remote monitoring provides an alternative to surpass the barriers of supervised exercise. However, no meta-analysis was conducted to verify the effectiveness of this intervention for improving physical activity (PA). We performed a systematic review of remote and unsupervised strategies imposed on CRC patients for improving PA and compared, via a meta-analysis, their effectiveness against CRC patients submitted to usual care or no intervention. The databases PubMed, Scopus, and Web of Science were searched on 20 September 2022. Eleven studies attained the criteria for eligibility in the qualitative approach, and seven were included in the meta-analysis. No significant effect (p = 0.06) of remote and unsupervised exercise intervention was observed. However, a sensitivity analysis including three studies that only considered CRC patients was performed, demonstrating a significant effect in favor of exercise (p = 0.008). Based on our sensitivity analysis, remote and unsupervised exercise strategies were effective to improve the PA of CRC patients.
Keywords: neoplasm, physical exercise, rehabilitation, colon, cancer
1. Introduction
Healthy behaviors, like engaging in physical exercise, benefit colorectal cancer (CRC) patients by improving their quality of life [1,2,3,4,5,6]. Previous meta-analyses have focused on the benefits of physical exercise for CRC patients, including improvements in aerobic power and metabolism [7], quality of life, and functional capacity [8], in addition to being feasible [9]. Further, a large body of scientific data has concluded that physical activity (PA) in distinct ages or conditions can improve, even during the treatment or in long-term follow-up, both the survival rates and mental health of CRC patients [3,4,10,11,12,13,14,15]. In this scenario, new strategies to improve the PA in this population are paramount.
Programs to improve PA can be trialed in hospital settings, especially for a patient awaiting surgery. However, hospital-based initiatives may not be suitable for everyone due to other commitments, travel difficulties, distance and costs, multi-morbidity, and discomfort in group settings. Therefore, health care can be provided at home, mainly for immunocompromised patients at risk of infections [16]. In this sense, home-based interventions could be a promising alternative to hospital-based rehabilitation. To assess compliance, strategies based on a daily diary or logbook are valid to record all activities, besides the possibility of remote monitoring via telephone calls, for instance [17,18].
Exercise practice guided by new technologies had great momentum during the pandemic period [16] and was strongly recommended to maintain or improve the PA of cancer patients, regardless of the cancer type [19]. However, diverse strategies with the same goal have been applied well before the coronavirus outbreak. Approaches using health coaching delivered by telephone, internet, or combined methods were associated with improved quality of life, mood, and PA of patients with any type of cancer, but not self-efficacy [20]. Regarding CRC patients, most studies in a systematic review reported a positive effect of using eHealth, a system that incorporates a wide range of applications facilitating communication between patients and healthcare professionals and monitoring patients’ health status, providing useful services for supporting this population [21]. On the other hand, delivering a digital versatile disk (DVD) containing exercises or pedometers recorded monthly showed no improvement in the quality of life of these patients [22].
Overall, controversial data is available regarding the effectiveness of remote and non-presential supervision exercise strategies for CRC patients, including the improvements in PA. In this context, a myriad of strategies based on workbooks, booklets, newsletters, motivational interviews, and telephone counseling was applied in the specialized literature. However, the scientific community lacks some systematic review along with a meta-analysis to explore these approaches and verify their efficiency to improve the PA of CRC patients. Since regular physical exercise has been associated with CRC-specific and all-cause mortality [23], gathering information on these approaches as well as their benefits on PA becomes relevant. Therefore, we performed a systematic review of remote and unsupervised strategies imposed on CRC patients for improving PA and compared, via a meta-analysis, their effectiveness against CRC patients submitted to usual care or no intervention. Given the importance of physical exercise for this population and the available technological and remote resources to increase PA, we hypothesized that these strategies will improve this outcome of CRC patients.
2. Materials and Methods
2.1. Search Strategy
The databases PubMed, Scopus, and Web of Science were searched on 20 September 2022. One researcher (LHDM) with experience in advanced searches retrieved the studies for the initial screening. The Boolean operators “AND” and “OR” were used along with the terms “physical activity OR physical exercise OR physical training” AND “colorectal neoplasm OR colorectal cancer”. The “English language” and “human” filters were activated whenever possible. The titles were transposed to a Microsoft Excel datasheet with three tabs, one per database. The screening supervisor (LHDM) checked all titles and excluded duplicates or triplicates manually. Subsequently, four of these Excel files were generated, each one with the name of the reviewer. This procedure secured the blind revision.
2.2. Eligibility Criteria
The screening was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [24]. Furthermore, the Population (P), Intervention (I), Comparator (C), Outcomes (O), and Study design (S) (PICOS) [25] structure guided reviewers to include or exclude the studies. Therefore, the PICOS consisted of CRC patients (P) submitted to remote and unsupervised exercise (I) and compared to CRC patients without exercise (C) to verify the intervention’s effect on the PA levels (O) in randomized controlled trials (S).
The meticulous inclusion consisted of: (i) studies showing the effects of the intervention of PA levels rather than only associations between this parameter with other outcomes; (ii) interventions without presential supervision, but that included some presential visits through the intervention for resolving doubts of asses the outcomes, including the PA; (iii) interventions with some level of follow-up during the period, including phone calls, instruction books, pamphlets, DVDs, letters, home logs, trackers, or any way to instruct the CRC patients; (iv) the length of the intervention was clearly presented; (v) the remote and unsupervised strategy was clearly presented, allowing reproduction; (vi) some PA outcome was measured, including questionnaires, pedometers or other equipment that retrieved this parameter; and (vii) studies in English. Manuscripts were not included if: (i) only the abstract was available; (ii) no CRC patient was included; (iii) mean or variance was not provided; (iv) missed control or usual care group, (v) were transversal intervention, and (vi) provided supervision during the physical exercise.
2.3. Data Extraction and Quality Assessment
Four reviewers (GCR, JSP, MERF, and MSPO) independently screened the studies. In each screening round (i.e., title, abstract, and full text) another researcher (LHDM) retrieved the Excel sheets and verified the discrepancies. The same researcher brought the reviewers together to discuss disagreements, and a resolution was reached in all cases. Information on the sample size, age, type and stage of cancer, intervention strategy, and PA results were retrieved. Another researcher (KCS) double-checked the information retrieved. The corresponding authors were contacted when the information presented in the studies was insufficient or inconclusive. If no response was provided, the study was not included. The quality assessment was performed by one reviewer (LHDM) using the Physiotherapy Evidence Database (PEDro) scale [26]. Studies were classified from fair to substantial or fair to good [27].
2.4. Meta-Analysis
The PA (dependent variable) retrieved from seven studies [28,29,30,31,32,33,34] was considered a continuous variable. Four studies [35,36,37,38] were not included since no variance or mean was provided. The Review Manager 5 software (version 5.3, The Nordic Cochrane Centre, Copenhagen, Denmark) was used for the meta-analysis. A random effects model was used for the analysis and post-intervention means and standard deviations (SDs) were adopted for comparisons. The standardized mean difference (SMD) was considered as the effect size index along with 95% confidence intervals (CI). Significance analysis, proportion estimation of variance, and variation in the treatment effects were based on the Z-value, I2, and prediction interval (PI), respectively. The PI was calculated by the comprehensive meta-analysis software (CMA) [39]. The same software was used to calculate Egger’s regression intercept and the Fail-safe N. Since several studies included in the qualitative search also considered patients with other cancers, we performed a sensitivity analysis with those that only considered CRC patients. The statistical significance was set at p < 0.05.
3. Results
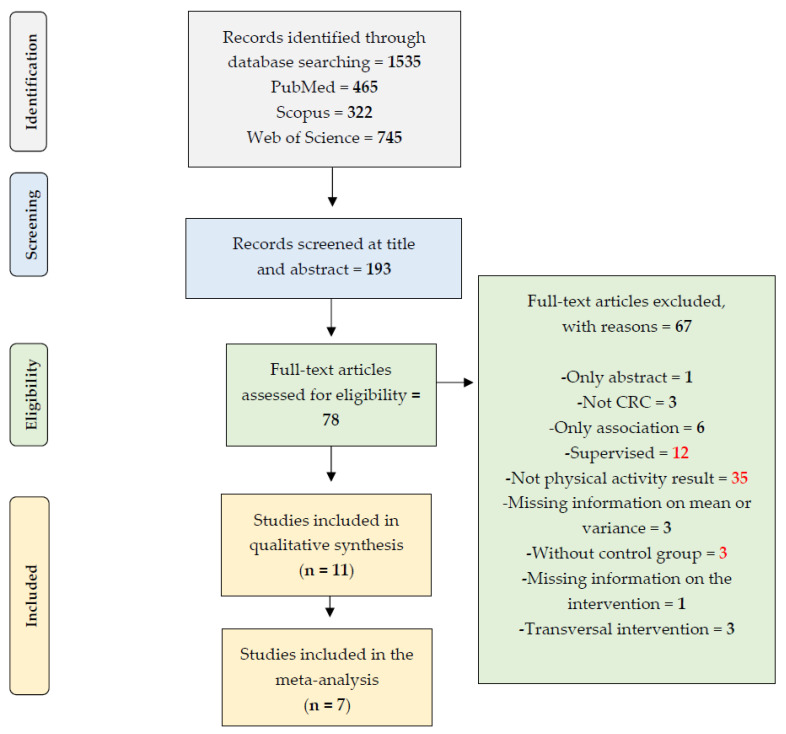
The initial search retrieved 1535, of which 11 attained the criteria for eligibility in the qualitative approach, andseven provided enough data to be included and the meta-analysis (Figure 1). The studies were published between 2009 and 2019. The PEDro score was 7.27 ± 0.90, with three studies classified as six, two with seven, and the remaining attained eight (Table A1).
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) used to guide the systematic review.
Information on sample size, age, type, and stage of cancer was synthesized in Table 1. A total of 2805 cancer patients were enrolled in randomized controlled trials, of which 1405 initiated remote and unsupervised exercise intervention and 1400 received usual care or no intervention. The raw comparison showed that male cancer patients were higher (1462 interventions = 735; control = 727) than females (1343 interventions = 670; control = 673) in the overall sample. Exclusively for CRC patients, 682 were randomized in the control or usual care group, while 672 initiated the intervention. Similar age was observed for both exercised and controls (63 ± 7 years in both groups). Six studies [28,29,32,34,35,37] considered patients with cancers more than CRC, while the remaining five [30,31,33,36,38] only considered patients with this neoplasm.
Table 1.
Summary of the included studies in the qualitative synthesis.
| Study | Sample Size | Age | Only CRC Patients? Stage of Cancer or Condition |
|---|---|---|---|
| Morey et al., (2009) [34] |
Control—n = 322 Female—n = 177 Male—n = 145 Intervention—n = 319 Female—n = 172 Male—n = 147 |
Control = 73 ± 5 yrs Intervention = 73 ± 5 yrs |
No Control Breast—n = 146 Prostate—n = 130 Colorectal—n = 46 Years since cancer diagnosis = 8 ± 2 Intervention Breast—n = 143 Prostate—n = 131 Colorectal—n = 45 Years since cancer diagnosis = 8 ± 2 |
| Ligibel et al., (2012) [32] |
Control—n = 60 Female—n = 56 Male—n = 4 Intervention—n = 61 Female—n = 56 Male—n = 5 |
Control = 55 ± 10 yrs Intervention = 53 ± 10 yrs |
No Control Breast—n = 50 Colorectal—n = 10 Stage 1 = 21; Stage 2 = 23; Stage 3 = 16 Intervention Breast—n = 50 Colorectal—n = 11 Stage 1 = 20; Stage 2 = 19; Stage 3 = 22 |
| # Demark-Wahnefried et al., (2012) [28] |
Control—n = 245 Female—n = 138 Male—n = 107 Intervention—n = 243 Female—n = 132 Male—n = 111 |
Control = 72 ± 5 yrs Intervention = 73 ± 5 yrs |
No Control Breast—n = 110 Prostate—n = 94 Colorectal—n = 41 Intervention Breast—n = 111 Prostate—n = 99 Colorectal—n = 33 ≥5 years from diagnosis |
| Hawkes et al., (2013) [30] |
Control—n = 205 Female—n = 90 Male—n = 115 Intervention—n = 205 Female—n = 99 Male—n = 106 |
Control = 67 ± 9 yrs Intervention = 64 ± 10 yrs |
Yes Control—Dukes’ staging—A = 39; B = 53; C = 48; Unknown = 65; Intervention—Dukes’ staging—A = 36; B = 65; C = 45; Unknown = 59 |
| Pinto et al., (2013) [38] |
Control—n = 26 Female—n = 14 Male—n = 12 Intervention—n = 20 Female—n = 12 Male—n = 8 |
Control = 55 ± 8 yrs Intervention = 59 ± 11 yrs |
Yes Stages 1, 2 or 3 |
| Park et al., (2015) [37] |
Control—n = 59 Female—n = 50 Male—n = 9 Intervention—n = 50 Female—n = 45 Male—n = 5 |
Control = 53 ± 8 yrs Intervention = 50 ± 8 yrs |
No Control Breast—n = 41 Colorectal—n = 18 Stage 1 = 22; Stage 2 = 21; Stage 3 = 12 Intervention Breast—n = 39 Colorectal—n = 11 Stage 1 = 19; Stage 2 = 15; Stage 3 = 8 |
| Mayer et al., (2018) [33] |
Control—n = 140 Female—n = 73 Male—n = 67 Intervention—n = 144 Female—n = 74 Male—n = 70 |
Control = 57 ± 14 yrs (n = 104) Intervention = 59 ± 13 yrs (n = 115) |
Yes Control—Stage 1 = 27; Stage 2 = 82; Stage 3 = 29 Intervention—Stage 1 = 39; Stage 2 = 63; Stage 3 = 41 |
| Lee et al., (2018) [31] |
Control—n = 56 Female—n = 26 Male—n = 30 Intervention—n = 56 Female—n = 16 Male—n = 40 |
Control = 64 ± 9 yrs Intervention =66 ± 9 yrs |
Yes Control—Stage 1 = 12; Stage 2 = 24; Stage 3 or 4= 20 Intervention—Stage 1 = 8; Stage 2 = 27; Stage 3 or 4= 20 |
| Golsteijn et al., (2018) [29] |
Control—n = 229 Female—n = 25 Male—n = 204 Intervention—n = 249 Female—n = 37 Male—n = 212 |
Control = 66 ± 8 yrs Intervention = 66 ± 7 yrs |
No Control Prostate—n = 143 Colorectal—n = 86 Intervention Prostate—n = 149 Colorectal—n = 100 At least 6 weeks post-surgery |
| Maxwell-Smith et al., (2018) [40] |
Control—n = 34 Female—n = 13 Male—n = 21 Intervention—n = 34 Female—n = 21 Male—n = 13 |
Control = 62 ± 8 yrs Intervention = 65 ± 7 yrs |
No Control Gynaecologic—n = 4 Colorectal—n = 30 Intervention Gynaecologic—n = 11 Colorectal—n = 23 Stages 1 or 2 |
| Moug et al., (2019) [36] |
Control—n = 24 Female—n = 11 Male—n = 13 Intervention—n = 24 Female—n = 6 Male—n = 18 |
Control = 66 ± 9 yrs Intervention = 65 ± 11 yrs |
Yes New diagnosis |
The “n” inserted in the table refers to the initial. Some studies only reported the participant’s status at the baseline of the total “n”, but did not present the characteristic of the final “n”.
The remote and unsupervised exercise strategies, the instruments for measuring PA, and the results of the trials are shown in Table 2. Studies presented large heterogeneity regarding the intervention, including workbooks, letters, phone calls, health coach sessions, trackers, and distinct motivational approaches. Likewise, different questionnaires were adopted in eight studies [28,29,30,32,33,34,37,38] for measuring the PA, while accelerometers retrieved this outcome in three randomized controlled trials [31,35,36]. The mean length of interventions was 31 ± 26 weeks. Short interventions (four weeks) were observed in only one study [37], while others submitted cancer patients to 12 [35,36,38], 16 [32], 24 [29,33], 48 [28,30,34], and 96 [31] weeks of physical exercise or follow-up.
Table 2.
Intervention strategy, length, and the results of the included studies in the qualitative synthesis.
| Study | -Intervention Strategy -Control -Length |
Physical Activity Parameter (Dependent Variable) and Results |
|---|---|---|
| Morey et al., (2009) [34] |
|
Community Health Activities Model Program for Seniors questionnaire § Control Baseline = 28.7 ± 2.3 min/wk 48 wk = 23.4 ± 5.6 min/wk Intervention Baseline = 24.6 ± 2.1 min/wk 48 wk = 36.3 ± 4.9 min/wk |
| Ligibel et al., (2012) [32] |
|
7-Day Physical Activity Recall Control Baseline = 65.7 ± 84.1 min/wk 16 wk = 14.6 ± 117.2 min/wk Intervention Baseline = 44.9 ± 58.5 min/wk 16 wk = 54.5 ± 142.0 min/wk |
| Demark-Wahnefried et al., (2012) [28] |
|
Community Health Activities Model Program for Seniors questionnaire Control Baseline = 37.5 ± 3.2 min/wk 48 wk = 69.0 ± 7.8 min/wk Intervention Baseline = 33.3 ± 2.9 min/wk 48 wk = 101.1 ± 7.3 min/wk |
| Hawkes et al., (2013) [30] |
|
Godin Leisure-Time Exercise Questionnaire Control Baseline = 52.0 ± 112.5 min/wk 48 wk = 54.3 ± 120.0 min/wk Intervention Baseline = 58.9 ± 132.9 min/wk 48 wk = 85.2 ± 181.0 min/wk |
| Pinto et al., (2013) [38] |
|
Seven-day Physical Activity Recall Control §§ Baseline = 30 min/wk 12 wk = 146 min/wk Intervention §§ Baseline = 30 min/wk 12 wk = 88 min/wk |
| Park et al., (2015) [37] |
|
Godin Leisure-Time Exercise Questionnaire Control §§ Baseline = 254.56 min/wk 4 wk = −39.05 min/wk Intervention §§ Baseline = 187.85 min/wk 4 wk = +47.57 min/wk |
| Mayer et al., (2018) [33] |
|
Godin Leisure-Time Exercise Questionnaire Control Baseline = 15.49 ± 27.6 min/wk 24 wk = 40.27 ± 42.22 min/wk Intervention Baseline = 19.43 ± 27.07 min/wk 24 wk = 49.98 ± 45.28 min/wk |
| Lee et al., (2018) [31] |
|
Accelerometer §§§ Control Baseline = 473.3 ± 267.3 min/wk 96 wk = 642.4 ± 294.7 min/wk Intervention Baseline = 460.8 ± 239.6 min/wk 96 wk = 680.5 ± 259.8 min/wk |
| Golsteijn et al., (2018) [29] |
|
Short Questionnaire to Assess Health Enhancing Physical Activity Control Baseline = 873 ± 764 min/wk 24 wk = 943 ± 769 min/wk Intervention Baseline = 780 ± 721 min/wk 24 wk = 1145 ± 883 min/wk |
| Maxwell-Smith et al., (2018) [40] |
|
Accelerometer Control §§ Baseline = 158 min/wk 12 wk = 138 min/wk Intervention §§ Baseline = 170 min/wk 12 wk = 186 min/wk |
| Moug et al., (2019) [36] |
|
Accelerometer Control Baseline = 7773 ± 3975 median steps/day 12 wk = 5920 ± 3152 median steps/day Intervention Baseline = 7779 ± 4045 steps/day §§§§ 12 wk = 6675 ± 3100 steps/day §§§§ |
§ The retrieved data refers to the duration of the endurance exercise; §§ The variance was not presented. §§§ The data refers to groups C and D, considering only the baseline and 12-month results. §§§§ Median. RENEW—Reach out to Enhance Wellness. CHESS—Comprehensive Health Enhancement Support System. WATAAP—Wearable Activity Technology and Action Planning.
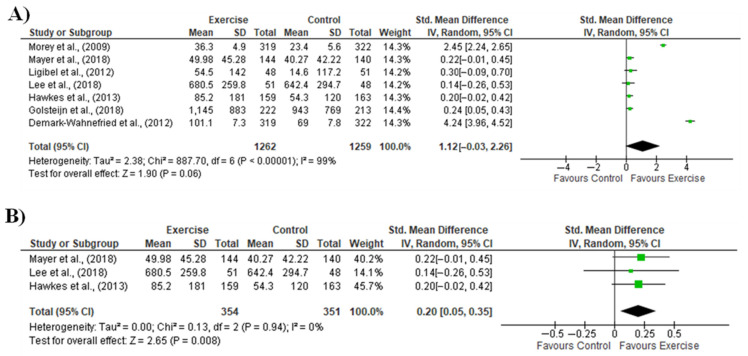
The meta-analysis including 1262 cancer patients and 1259 controls showed no significant effect of remote and unsupervised exercise intervention for promoting PA improvement (Figure 2A). Additionally, a high proportion of variance was observed along with PI between −3.12 to 5.36. Egger’s regression intercept was observed at 2.02 and a p-value of 0.46. Regarding the Fail-safe N, Z-value was obtained at 23.3 with a p-value of 0.000, and the number of missing studies that would bring the p-value to > alpha was 983. The sensitivity analysis including studies that only considered CRC patients (i.e., 354 patients and 351 controls) demonstrated a significant effect in favor of exercise (Figure 2B). Further, the CMA returned a common effect size.
Figure 2.
Meta-analysis of physical activity (dependent variable) measured in randomized controlled trials that conducted remote and unsupervised exercise for cancer patients; (A) Comparison of the physical activity levels between cancer patients—including colorectal—submitted to remote and unsupervised exercise and cancer patients in the control group; (B) Sensitivity analysis considering the studies that only included patients diagnosed with colorectal cancer [28,29,30,31,32,33,34].
4. Discussion
This meta-analysis demonstrated large variability in terms of sample size, interventions, and instruments to measure PA in remote and unsupervised exercise interventions for cancer patients. These factors may have contributed to the non-significant effect of the intervention. On the other hand, our sensitivity analysis suggests that exercise interventions with remote monitoring can improve the PA of CRC patients.
The better approach regarding exercise prescription for CRC patients—as for other neoplasms—are in course of discussion. Supervised exercise training improves the functional capacity following colorectal cancer resection, also promoting faster recovery to regular patients’ activities [41]. Using a qualitative approach, Hatlevoll et al., [42] concluded that presential appointments with a physiotherapist serve as an important external motivational factor. Further, the CRC patients in this study reported improvements in muscle strength and mental health, along with a reduction in sensory neuropathic symptoms.
Despite the advantages of supervised exercise, patients with cancer may face barriers that limit their adherence to this intervention, including, for instance, time and cost [40]. On the other hand, a myriad of approaches and definitions of the “home-based” setting is available [43], leading to distinct conclusions regarding this approach. Such a perspective was also observed in this study. From the seven studies included in the meta-analysis, five [29,30,31,32,33] reported a large variance in PA after the exercise program. Another factor that may contribute to the non-significant effect of this analysis is the usual care adopted in many of the included studies. Although it is merely speculative, the fact that some interventions also included motivational implements or workbooks with guidance for healthy behaviors may have improved the PA of controls. On the other hand, these approaches are fully expected, since it is not ethically acceptable to prevent control patients from receiving materials to improve their health.
Both supervised and home-based approaches have advantages and disadvantages. However, a recent meta-analysis by our group has suggested that adherence to the intervention, regardless of the supervision level, is relevant for improving the functional capacity and quality of life of CRC patients [8]. In this scenario, new technologies and instruments are valid alternatives to inform, motivate, and remember the PA benefits for these patients, ultimately improving their intervention adherence. Such a suggestion is supported by the findings of Fisher et al., [44], who observed that recall of PA advice after a diagnosis of CRC was associated with higher levels of this outcome.
The systematic review by Wang et al., [5] of behavioral interventions using the web and mobile technology enrolled patients with several kinds of cancer, among them, CRC. These patients were submitted to mobile health (mHealth), defined by the World Health Organization (WHO) as medical and public health practices supported by a mobile device, such as a mobile phone, patient monitoring devices, personal digital assistants, and other wireless devices. The results show that mHealth interventions are a promising approach to improving PA and dietary behaviors in cancer survivors.
Despite the promising results, this meta-analysis must be comprehended along with its limitations. Specifically for the included studies, it is possible to observe a lack of standardization regarding the period of interventions. This prevents a conclusion about the minimum time needed to observe an increase in PA in the studied population. A similar limitation resides in the format of the interventions. Although all are part of remote and unsupervised interventions, each study used a different strategy. Additionally, only three studies presented specific results for CRC patients, which is a limiting factor for increasing the power of the meta-analysis.
Based on our eligibility criteria, a few studies were inserted in both qualitative and quantitative analysis. The main issue in this context is the absence of PA parameters in randomized and controlled trials. This factor accounted for 46% of the reasons for exclusion. Further, the heterogeneity observed precludes deep interpretations regarding the effectiveness of remote and unsupervised exercise interventions for improving the PA of patients with cancer, including CRC. In this sense, we opted to include studies with other neoplasms than CRC to strengthen the qualitative analysis, also improving the understanding of remote and unsupervised exercise strategies for CRC patients. To solve this limitation, future studies must present the separated results according to the cancer type. Apart from the limitations, we could perform the sensitivity analysis and explore the effects of these interventions to improve the PA of CRC patients. This factor can be comprehended as the main strength and innovation of this meta-analysis.
5. Conclusions
This meta-analysis verified the effects of remote and unsupervised exercise strategies on the PA of CRC patients. When the analysis was performed with studies that considered other cancers, no significant effect of the exercise intervention was observed, along with large heterogeneity. However, based on our sensitivity analysis, remote and unsupervised exercise strategies were effective in improving the PA of CRC patients. This result demonstrated the relevance of PA for this population, promoting the benefits derived from physical exercise and acting as an adjuvant in the treatment of this disease.
Acknowledgments
We would like to thank the Postgraduate Program in Health Sciences of the University of São Francisco.
Appendix A
Table A1.
The score in each item of the included studies based on the Physiotherapy Evidence Database (PEDro).
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Morey et al., 2009 [34] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Ligibel et al., 2012 [32] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes |
| Demark-Wahnefried et al., 2012 [28] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Hawkes et al., 2013 [30] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Pinto et al., 2013 [38] |
Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Park et al., 2015 [37] |
Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Mayer et al., 2018 [33] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Lee et al., 2018 [31] |
Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Golsteijn et al., 2018 [29] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes |
| Maxwell-Smith et al., 2018 [40] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Moug et al., 2019 [36] |
Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
1. Eligibility criteria were specified. 2. Subjects were randomly allocated to groups. 3. Allocation was concealed. 4. The groups were similar at baseline regarding the most important prognostic indicators. 5. There was blinding of all subjects. 6. There was blinding of all therapists who administered the therapy. 7. There was blinding of all assessors who measured at least one key outcome. 8. Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups. 9. All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat”. 10. The results of between-group statistical comparisons are reported for at least one key outcome. 11. The study provides both point measures and measures of variability for at least one key outcome.
Author Contributions
Conceptualization, L.H.D.M. and I.G.M.d.R.; methodology, A.C.P., G.C.R., J.S.P., K.C.S., M.E.R.F., M.S.P.O., I.G.M.d.R. and L.H.D.M.; software, L.H.D.M.; validation, L.H.D.M. and I.G.M.d.R.; formal analysis, A.C.P., G.C.R., J.S.P., K.C.S., M.E.R.F., M.S.P.O., L.H.D.M. and I.G.M.d.R.; investigation, A.C.P. and L.H.D.M.; resources, L.H.D.M.; data curation, A.C.P., G.C.R., J.S.P., K.C.S., M.E.R.F., M.S.P.O., L.H.D.M. and I.G.M.d.R.; writing—original draft preparation, A.C.P. and L.H.D.M.; writing—review and editing, A.C.P., I.G.M.d.R., and L.H.D.M.; visualization, A.C.P. and L.H.D.M.; supervision, L.H.D.M.; project administration L.H.D.M.; funding acquisition, L.H.D.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Finance Code 001 and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–grant # 408680/2021-0).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jung Y.M., Chung J.P., Son H.P. Physical Activity Interventions for Colorectal Cancer Survivors: A Systematic Review and Meta- analysis of Randomized Controlled Trials. Cancer Nurs. 2021;44:E414–E428. doi: 10.1097/NCC.0000000000000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mbous Y.P., Patel J., Kelly K.M. A systematic review and meta-analysis of physical activity interventions among colorectal cancer survivors. Transl. Behav. Med. 2020;10:1134–1143. doi: 10.1093/tbm/ibz176. [DOI] [PubMed] [Google Scholar]
- 3.Schmid D., Leitzmann M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 4.Walter V., Jansen L., Knebel P., Chang-Claude J., Hoffmeister M., Brenner H. Physical activity and survival of colorectal cancer patients: Population-based study from Germany. Int. J. Cancer. 2017;140:1985–1997. doi: 10.1002/ijc.30619. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Langlais C.S., Kenfield S.A., Chan J.M., Graff R.E., Allen I.E., Atreya C.E., Van Blarigan E.L. mHealth Interventions to Promote a Healthy Diet and Physical Activity among Cancer Survivors: A Systematic Review of Randomized Controlled Trials. Cancers. 2022;14:3816. doi: 10.3390/cancers14153816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiltink L.M., White K., King M.T., Rutherford C. Systematic review of clinical practice guidelines for colorectal and anal cancer: The extent of recommendations for managing long-term symptoms and functional impairments. Support. Care Cancer. 2020;28:2523–2532. doi: 10.1007/s00520-020-05301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao R., Yu T., Liu L., Bi J., Zhao H., Tao Y., Li F., Guo L. Exercise intervention for post-treatment colorectal cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2020;14:878–893. doi: 10.1007/s11764-020-00900-z. [DOI] [PubMed] [Google Scholar]
- 8.Kraemer M.B., Priolli D.G., Reis I.G.M., Pelosi A.C., Garbuio A.L.P., Messias L.H.D. Home-based, supervised, and mixed exercise intervention on functional capacity and quality of life of colorectal cancer patients: A meta-analysis. Sci. Rep. 2022;12:2471. doi: 10.1038/s41598-022-06165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B., Hayes S.C., Spence R.R., Steele M.L., Millet G.Y., Gergele L. Exercise and colorectal cancer: A systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int. J. Behav. Nutr. Phys. Act. 2020;17:1–14. doi: 10.1186/s12966-020-01021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy K.T., Lam K., Kong J.C. Exercise and colorectal cancer survival: An updated systematic review and meta-analysis. Int. J. Color. Dis. 2022;37:1751–1758. doi: 10.1007/s00384-022-04224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyl R.E., Xie K., Koch-Gallenkamp L., Brenner H., Arndt V. Quality of life and physical activity in long-term (≥5 years post-diagnosis) colorectal cancer survivors—systematic review. Health Qual. Life Outcomes. 2018;16:112. doi: 10.1186/s12955-018-0934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschey R., Nance J., Hoover R., Triglianos T., Coffman E., Horrell L.N., Walker J.S., Bryant A.L., Valle C.G. Physical Activity: A Systematic Review to Inform Nurse Recommendations During Treatment for Colorectal Cancer. Clin. J. Oncol. Nurs. 2021;25:697–705. doi: 10.1188/21.CJON.697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong J., Park J. Systematic Review: Recommendations of Levels of Physical Activity among Colorectal Cancer Patients (2010–2019) Int. J. Environ. Res. Public Health. 2021;18:2896. doi: 10.3390/ijerph18062896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGettigan M., Cardwell C.R., Cantwell M.M., Tully M.A. Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst. Rev. 2020;5:CD012864. doi: 10.1002/14651858.CD012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu S., Jiang C., Zhou L. Physical activity and mortality in patients with colorectal cancer: A meta-analysis of prospective cohort studies. Eur. J. Cancer Prev. 2020;29:15–26. doi: 10.1097/CEJ.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 16.van Gestel T., Groen L., Puik J., van Rooijen S., van der Zaag-Loonen H., Schoonmade L., Danjoux G., Daams F., Schreurs W., Bruns E. Fit4Surgery for cancer patients during covid-19 lockdown—A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022;48:1189–1197. doi: 10.1016/j.ejso.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandenbarg D., Korsten J.H.W.M., Berger M.Y., Berendsen A.J. The effect of physical activity on fatigue among survivors of colorectal cancer: A systematic review and meta-analysis. Support. Care Cancer. 2018;26:393–403. doi: 10.1007/s00520-017-3920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huizinga F., Westerink N.-D.L., Berendsen A.J., Walenkamp A.M.E., de Greef M.H.G., Nijeweeme J.K.O., de Bock G.H., Berger M.Y., Brandenbarg D. Home-based Physical Activity to Alleviate Fatigue in Cancer Survivors: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2021;53:2661. doi: 10.1249/MSS.0000000000002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezende L.F.M., Lee D.H., Ferrari G., Eluf-Neto J., Giovannucci E.L. Physical activity for cancer patients during COVID-19 pandemic: A call to action. Cancer Causes Control. 2020;32:1–3. doi: 10.1007/s10552-020-01367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barakat S., Boehmer K., Abdelrahim M., Ahn S., Al-Khateeb A.A., Villalobos N., Prokop L., Erwin P.J., Fleming K., Serrano V., et al. Does Health Coaching Grow Capacity in Cancer Survivors? A Systematic Review. Popul. Health Manag. 2018;21:63–81. doi: 10.1089/pop.2017.0040. [DOI] [PubMed] [Google Scholar]
- 21.Ayyoubzadeh S.M., Kalhori S.R.N., Shirkhoda M., Mohammadzadeh N., Esmaeili M. Supporting colorectal cancer survivors using eHealth: A systematic review and framework suggestion. Support. Care Cancer. 2020;28:3543–3555. doi: 10.1007/s00520-020-05372-6. [DOI] [PubMed] [Google Scholar]
- 22.Bezerra K.H.D.S., de Oliveira M.V.L., Nascimento I.J.B.D., Rocha L.D.B., Filho L.E.C.D.S., Rocha R.S.B., da Silva M.L., Cunha K.D.C. Physical Exercise and Quality of Life of Patients Diagnosed with Colorectal Cancer: Systematic Literature Review. J. Gastrointest. Cancer. 2021;52:17–22. doi: 10.1007/s12029-020-00506-9. [DOI] [PubMed] [Google Scholar]
- 23.Je Y., Jeon J.Y., Giovannucci E.L., Meyerhardt J.A. Association between physical activity and mortality in colorectal cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer. 2013;133:1905–1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 24.Liberati M., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Systematica Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. [(accessed on 10 November 2022)]. Available online: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- 26.Database PE. [(accessed on 10 November 2022)]. Available online: https://pedro.org.au/
- 27.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 28.Demark-Wahnefried W., Morey M.C., Sloane R., Snyder D.C., Miller P.E., Hartman T.J., Cohen H.J. Reach Out to Enhance Wellness Home-Based Diet-Exercise Intervention Promotes Reproducible and Sustainable Long-Term Improvements in Health Behaviors, Body Weight, and Physical Functioning in Older, Overweight/Obese Cancer Survivors. J. Clin. Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golsteijn R.H.J., Bolman C., Volders E., Peels D.A., De Vries H., Lechner L. Short-term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2018;15:1–14. doi: 10.1186/s12966-018-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkes A.L., Chambers S.K., Pakenham K.I., Patrao T.A., Baade P.D., Lynch B.M., Aitken J.F., Meng X., Courneya K.S. Effects of a Telephone-Delivered Multiple Health Behavior Change Intervention (CanChange) on Health and Behavioral Outcomes in Survivors of Colorectal Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2013;31:2313–2321. doi: 10.1200/JCO.2012.45.5873. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.F., Ho J.W.C., Fong D.Y.T., Macfarlane D.J., Cerin E., Lee A.M., Leung S., Chan W.Y.Y., Leung I.P.F., Lam S.H.S., et al. Dietary and Physical Activity Interventions for Colorectal Cancer Survivors: A Randomized Controlled Trial. Sci. Rep. 2018;8:5731. doi: 10.1038/s41598-018-24042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ligibel J.A., Meyerhardt J., Pierce J.P., Najita J., Shockro L., Campbell N., Newman V.A., Barbier L., Hacker E., Wood M., et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res. Treat. 2012;132:205–213. doi: 10.1007/s10549-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer D.K., Landucci G., Awoyinka L., Atwood A.K., Carmack C.L., Demark-Wahnefried W., McTavish F., Gustafson D.H. SurvivorCHESS to increase physical activity in colon cancer survivors: Can we get them moving? J. Cancer Surviv. 2017;12:82–94. doi: 10.1007/s11764-017-0647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morey M.C., Snyder D.C., Sloane R., Cohen H.J., Peterson B., Hartman T.J., Miller P., Mitchell D.C., Demark-Wahnefried W. Effects of Home-Based Diet and Exercise on Functional Outcomes Among Older, Overweight Long-term Cancer Survivors: RENEW: A randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maxwell-Smith C., Hince D., Cohen P., Bulsara M.K., Boyle T., Platell C., Tan P., Levitt M., Salama P., Tan J., et al. A randomized controlled trial of WATAAP to promote physical activity in colorectal and endometrial cancer survivors. Psycho-Oncology. 2019;28:1420–1429. doi: 10.1002/pon.5090. [DOI] [PubMed] [Google Scholar]
- 36.Moug S.J., Mutrie N., Barry S.J.E., Mackay G., Steele R.J.C., Boachie C., Buchan C., Anderson A.S. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: Results from the REx trial. Color. Dis. 2019;21:548–562. doi: 10.1111/codi.14560. [DOI] [PubMed] [Google Scholar]
- 37.Park J.-H., Lee J., Oh M., Park H., Chae J., Kim D.-I., Lee M.K., Yoon Y.J., Lee C.W., Park S., et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomized controlled trial. Cancer. 2015;121:2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinto B.M., Papandonatos G., Goldstein M.G., Marcus B.H., Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psycho-Oncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 39.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Biostat; Englewood, NJ, USA: 2006. Comprehensive Meta-Analysis (Version 2.2. 027) [Google Scholar]
- 40.Hardcastle S.J., Maxwell-Smith C., Kamarova S., Lamb S., Millar L., Cohen P.A. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support. Care Cancer. 2018;26:1289–1295. doi: 10.1007/s00520-017-3952-9. [DOI] [PubMed] [Google Scholar]
- 41.Awasthi R., Minnella E.M., Ferreira V., Ramanakumar A.V., Scheede-Bergdahl C., Carli F. Supervised exercise training with multimodal pre-habilitation leads to earlier functional recovery following colorectal cancer resection. Acta Anaesthesiol. Scand. 2019;63:461–467. doi: 10.1111/aas.13292. [DOI] [PubMed] [Google Scholar]
- 42.Hatlevoll I., Skolbekken J., Oldervoll L.M., Wibe A., Hofsli E. Colorectal cancer patients’ experiences with supervised exercise during adjuvant chemotherapy—A qualitative study. Scand. J. Med. Sci. Sports. 2021;31:2300–2309. doi: 10.1111/sms.14048. [DOI] [PubMed] [Google Scholar]
- 43.Lopez C., McGarragle K., Pritlove C., Jones J.M., Alibhai S.M.H., Lenton E., Mina D.S. Variability and limitations in home-based exercise program descriptions in oncology: A scoping review. Support. Care Cancer. 2020;28:4005–4017. doi: 10.1007/s00520-020-05453-6. [DOI] [PubMed] [Google Scholar]
- 44.Fisher A., Williams K., Beeken R., Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ Open. 2015;5:e006853. doi: 10.1136/bmjopen-2014-006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.